Abstract
Background: Early-onset myopia increases the risk of irreversible high myopia. Methods: This study systematically evaluated the efficacy and safety of low-dose atropine for myopia control in children with premyopia through meta-analysis using random-effects models. Effect sizes were calculated using risk ratios (RRs) with 95% confidence intervals (CIs). Comprehensive searches of PubMed, EMBASE, Cochrane CENTRAL, and ClinicalTrials.gov were conducted until 20 December 2023, without language restrictions. Results: Four studies involving 644 children with premyopia aged 4–12 years were identified, with atropine concentrations ranging from 0.01% to 0.05%. The analysis focused on myopia incidence and atropine-related adverse events. Lower myopia incidence (RR, 0.62; 95% CI, 0.40–0.97 D/y; p = 0.03) and reduction in rapid myopia shift (≥0.5 D/1y) (RR, 0.50; 95% CI, 0.26–0.96 D/y; p < 0.01) were observed in the 12–24-month period. Spherical equivalent and axial length exhibited attenuated progression in the atropine group. No major adverse events were detected in either group, whereas the incidence of photophobia and allergic conjunctivitis did not vary in the 12–24-month period. Conclusions: Our meta-analysis supports atropine’s efficacy and safety for delaying myopia incidence and controlling progression in children with premyopia. However, further investigation is warranted due to limited studies.
Keywords: myopia, myopia control, atropine, premyopia, systematic review, meta-analysis, efficacy, safety
1. Introduction
Myopia, which is a prevalent refractive error [1], has emerged as a growing global concern, with projections indicating that nearly half of the world’s population may be affected by 2050 [2], particularly in East and Southeast Asia [3]. The irreversible nature of myopia, together with its propensity to manifest early in life, underscores the urgency of addressing this issue. The onset of myopia at an early age is associated with an increased risk of development of high myopia [4,5], leading to a spectrum of ocular pathologies, including myopic maculopathy [6,7], cataracts [8], open-angle glaucoma [9], and progressive visual debilitation over time [10]. Therefore, the delay or prevention of myopia becomes imperative for the optimization of long-term visual outcomes.
Among school-age children, refraction typically undergoes gradual changes over a span of several years. As pointed out by Schmid, approximately 80% of young children exhibit hyperopia, which tends to increase until around the age of 8 years, before gradually decreasing until approximately age 20 [11]. In contrast with the traditional perception of emmetropia as being a stable state, this condition can be transient in this age group [12]. Moreover, previous studies have reported that young children with a low level of hyperopia may be at a greater risk of developing myopia later in life; individuals with hyperopia often experience slow myopic shifts, whereas individuals with myopia undergo more rapid changes [13,14]. This underscores the potential importance of preserving hyperopia for understanding and predicting the development of myopia in children.
The concept of premyopia, which was introduced by the International Myopia Institute, outlines a refractive state that is characterized by an eye power of ≤0.75 D and >−0.50 D in children. This condition is diagnosed by considering the baseline refraction, age, and other quantifiable risk factors, which collectively suggest a significant likelihood of the future development of myopia and justify preventive interventions [15]. Despite its significance, a lack of consensus remains regarding the precise definition of premyopia. In addition, the lack of standardized tools for risk stratification and management in these children complicates this scenario [16,17,18].
Among the pharmacological approaches that are available for controlling the progression of myopia, low-dose atropine has demonstrated efficacy in children with this condition in various studies [19,20,21], emerging as a main method [22]. However, the extent of its effectiveness in children with premyopia remains unclear. Recent findings from the LAMP2 trials revealed that, compared with a placebo, 0.05% atropine significantly reduced the cumulative incidence of myopia by 24.6% over a 2-year period, whereas no notable difference was observed between the 0.01% atropine and the placebo groups [18]. Conversely, another study reported that 0.01% atropine significantly prevented the myopic shift in early childhood, resulting in a 24% reduction in the incidence of myopia over 6 years compared with the placebo [17]. Moreover, several studies reported a higher rate of side effects and adverse events for topical 0.05% atropine prescribed for myopia control in children [23,24]. Therefore, the critical question regarding the effectiveness and safety of atropine in children with premyopia warrants a thorough investigation.
Therefore, this study aimed at conducting a comprehensive systematic review and meta-analysis to address this gap in the literature, focusing on the efficacy and safety of low-dose atropine for the management of children with premyopia. By meticulously reviewing the existing literature and analyzing the available data, the outcomes of this study have the potential to significantly impact the clinical decision-making and elevate the overall quality of care in the management of the progression of myopia.
2. Methods
2.1. Study Design
This meta-analysis aimed at investigating the efficacy and safety of low-dose atropine eye drops for controlling myopia in children with premyopia. This study adhered strictly to the guidelines provided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [25]. The methodology was pre-specified and registered on the PROSPERO website on 15 January 2024 (Registration No. PROSPERO CRD42024498463).
2.2. Eligibility Criteria
To ensure the validity of our analysis, we included studies that met the following criteria: (1) randomized controlled trials (RCTs) or other interventional studies; (2) studies reporting data on myopia incidence, axial length, or spherical equivalent measurements; and (3) studies involving children with premyopia, defined as an SE ranging between +1 D and −1 D, who were treated with atropine eye drops for at least 6 months.
Studies that met any of the following criteria were excluded from the analysis: (1) those including patients with myopia (SE < −1 D); (2) those that involved participants with congenital or severe ophthalmological conditions or surgical history, which may influence the outcome; and (3) those with overlapping participants.
2.3. Outcome Measures
The outcomes were categorized based on time, either within 1 year or over 1 year:
-
▪
Myopia incidence;
-
▪
Fast myopic shift: myopia progression exceeding 0.5 D within 1 year or at an equivalent rate;
-
▪
Axial length (AL);
-
▪
Spherical equivalent (SE);
-
▪
Pupil size;
-
▪
Accommodative amplitude;
-
▪
Adverse effect (photophobia, allergic conjunctivitis).
2.4. Data Sources and Literature Searches
Our study employed a rigorous search methodology that was carried out independently by two authors (Ssu-Hsien Lee and Bor-Yuan Tseng) across multiple databases, including PubMed, Cochrane CENTRAL, Embase, and ClinicalTrials.gov, up to 20 December 2023. We utilized a combination of keywords, such as “premyopia” and “nonmyopia”, with Medical Subject Headings to identify pertinent studies. The detailed search methods used here are outlined in Table S1. Furthermore, language restrictions were not imposed, and we meticulously reviewed reference lists, to ensure the inclusion of all relevant research.
2.5. Risk-of-Bias Assessment
To assess the methodological quality of the RCTs included in this analysis, we utilized the Cochrane risk-of-bias tool for randomized trials version 2 (RoB 2.0). This tool comprises five main items: the randomization process, intervention adherence, missing outcome data, outcome measurement, and selective reporting. For other study designs, we employed the Newcastle–Ottawa Scale (NOS) tool to assess potential biases; this tool consists of three domains, with up to nine stars: assessment of selection bias, comparability bias, and outcome bias.
2.6. Data Extraction
Two authors (Ssu-Hsien Lee and Bor-Yuan Tseng) independently performed data extraction from the selected studies. The extracted data included demographic information; study design details; atropine treatment specifics; and measurements of myopia incidence, fast myopic shift, axial length, spherical equivalent, pupil size, accommodative amplitude, adverse effects, and relevant outcomes. If essential data were absent in published articles, we contacted the corresponding authors to secure the original data.
2.7. Data Synthesis and Analysis
In our meta-analysis, we used a random-effects model to account for the inherent heterogeneity among the included studies via Review Manager, version 5.4. Statistical significance was set at a two-tailed p-value < 0.05. Effect sizes were measured based on the weighted mean difference (WMD) with 95% confidence intervals (CIs), as well as risk ratios (RRs) with 95% CIs. Heterogeneity among the studies was assessed using I2 statistics, using I2 values of 25%, 50%, and 75% as being indicative of low, moderate, and high heterogeneity, respectively. To assess the publication bias, we generated and examined funnel plots and performed Egger’s test of asymmetry.
3. Results
3.1. Literature Search
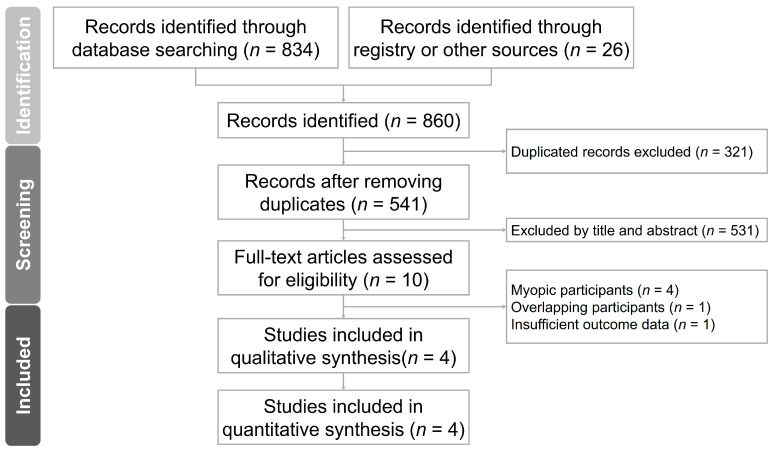
The process used for our literature search and article selection is illustrated in Figure 1. Initially, 860 studies were retrieved through the database search in accordance with the PRISMA guidelines [25]. After the removal of duplicate records, a thorough examination of the titles and abstracts of the articles led to the identification of 10 studies for full-text screening. Ultimately, four studies were included in our meta-analysis. Tables S1 and S2 outline the keywords used in the search and the rationale for study exclusions, respectively. Figure S1 illustrates the funnel plot.
Figure 1.
PRISMA flow chart.
3.2. Characteristics of the Included Studies
Table 1 presents the detailed characteristics of the included studies. Our search encompassed four atropine-related studies, consisting of three RCTs and one non-RCT, involving a total of 644 children with premyopia with an average age of 7.13 ± 1.60 years. The baseline cycloplegic SE was 0.38 ± 0.44 D, and the baseline AL was 22.72 ± 0.94 mm. All studies had been conducted in Asian countries, such as India, China, and Taiwan. The atropine dosages ranged from 0.01% to 0.05%, with three studies utilizing 0.01% atropine and the others using 0.05% and 0.25% atropine. Treatment durations varied from 6 months to 2 years, with two studies lasting 2 years, one averaging 18.4 months, and only one study spanning 6 months.
Table 1.
Characteristics of the included studies.
| Study | Study Design | Atropine %, Treatment Time |
n | Age (years) | Baseline SE (D) | Baseline AL (mm) | Country |
|---|---|---|---|---|---|---|---|
| J.C. Yam 2023 [18] | RCT | 0.05%, 2 y | 160 | 6.86 ± 1.42 | 0.50 ± 0.33 | 22.82 ± 0.72 | Hong Kong |
| 0.01%, 2 y | 159 | 6.88 ± 1.35 | 0.51 ± 0.33 | 22.89 ± 0.70 | |||
| Placebo, 2 y | 155 | 6.75 ± 1.27 | 0.53 ± 0.31 | 22.80 ± 0.64 | |||
| W. Wang 2023 [17] | RCT | 0.01%, 6 m | 30 | 8.60 ± 1.72 | −0.19 ± 0.28 | 23.59 ± 0.77 | China |
| Placebo, 6 m | 30 | 8.50 ± 1.74 | −0.21 ± 0.32 | 23.61 ± 0.75 | |||
| J. Jethani 2022 [26] | RCT | 0.01%, 2 y | 30 | 7.70 ± 2.10 | N/A | 20.80 ± 0.60 | India |
| Placebo, 2 y | 30 | 7.20 ± 1.90 | 21.00 ± 0.50 | ||||
| P.C. Fang 2010 [16] | Non-RCT | 0.025%, 18.4 m * | 24 | 7.60 ± 1.70 | −0.31 ± 0.45 | N/A | Taiwan |
| Placebo, 16.3 m * | 26 | 8.20 ± 2.10 | −0.17 ± 0.50 |
Note: m: months; y: years; N/A: not available. All data are reported as the mean ± SD, if not marked otherwise. * Average follow-up time.
Our analysis revealed consistent evidence across all four studies indicating the efficacy of low-dose atropine in children with premyopia. These findings encompassed a reduction in myopia incidence and a deceleration in the rate of myopia progression, coupled with a limitation in the elongation of AL and SE. Notably, adverse events reported across the studies primarily comprised incidences of photophobia and allergic conjunctivitis. Furthermore, investigations into secondary outcomes such as accommodation amplitude and pupil size post-atropine administration were prevalent.
Regarding the variability in atropine concentrations, the LAMP2 study delineated three distinct groups: 0.05% atropine, 0.01% atropine, and a placebo cohort. Notably, a dose-dependent effect was observed, with the 0.05% atropine group demonstrating superior efficacy in reducing myopia incidence and decelerating myopia progression compared to the 0.01% atropine group. Nevertheless, the 0.05% atropine cohort exhibited a reduction in accommodation amplitude and an increase in pupil size. However, incidences of photophobia and allergic conjunctivitis remained comparable between the two atropine concentrations.
3.3. Risk-of-Bias Assessment
The results of the RoB2.0 and NOS assessments are summarized in Tables S3 and S4, respectively. Most of the studies exhibited a low bias across all domains. However, in one RCT, we observed a performance bias without information about blinding, and a reporting bias because of the lack of registration of the trial. Nonetheless, the remaining studies exhibited a low risk of bias in the other domains.
3.4. Myopia Incidence and Fast Myopia Shift
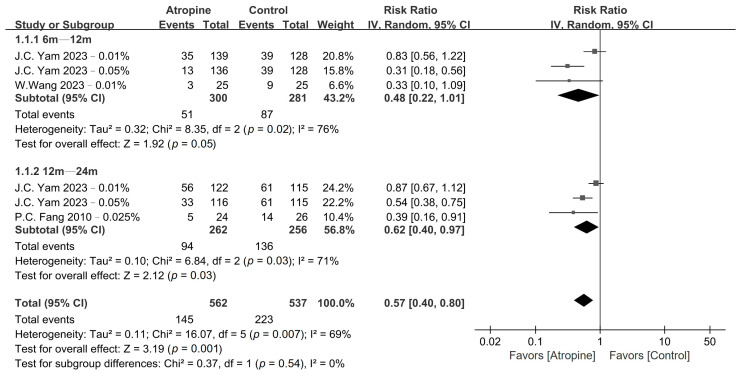
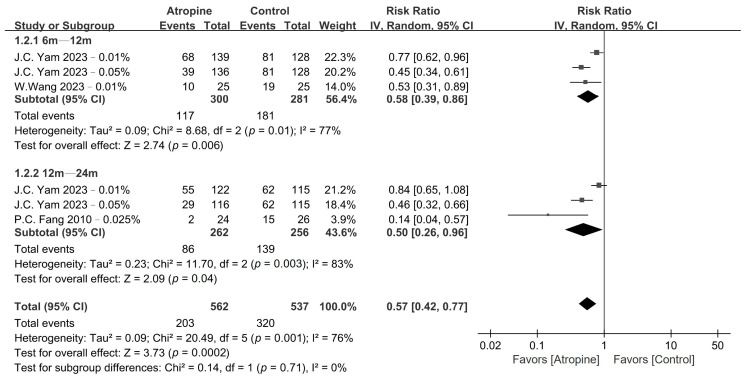
The pooled results obtained for myopia incidence and fast myopia shift are presented in Figure 2 and Figure 3, respectively, whereas the GRADE summary of findings is provided in Table S5. Low-dose atropine exhibited a tendency to reduce myopia incidence in the 6–12-month period (RR, 0.48; 95% CI, 0.22–1.01; p = 0.05), with a more significant reduction over the 12–24-month period (RR, 0.62; 95% CI, 0.40–0.97; p = 0.03). Regarding the fast myopia shift, atropine yielded a significant reduction in this phenomenon in the 6–12-month period (RR, 0.58; 95% CI, 0.39–0.86; p < 0.01) and the 12–24-month period (RR, 0.50; 95% CI, 0.26–0.96; p = 0.04).
Figure 2.
Forest plot of the risk ratio of myopia incidence between the atropine and placebo groups.
Figure 3.
Forest plot of the risk ratio of fast myopia shift between the atropine and placebo groups.
3.5. Spherical Equivalent and Axial Length
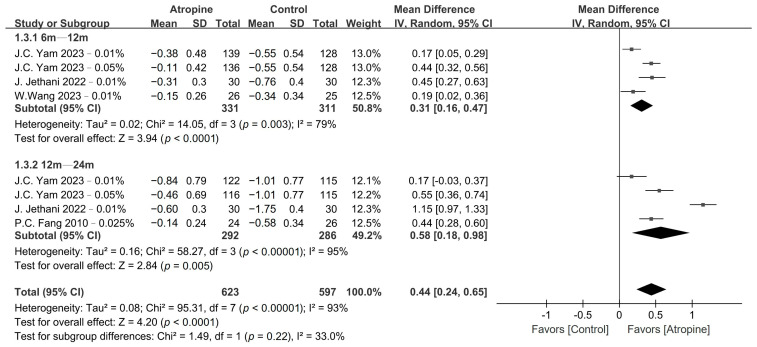
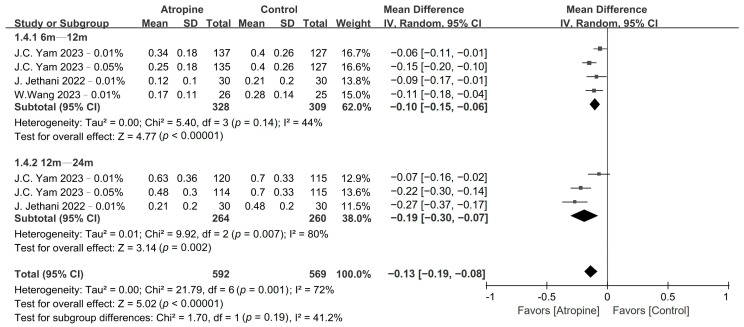
Atropine also had a significant impact on SE and AL progression, as shown in Figure 4 and Figure 5, respectively. Low-dose atropine resulted in a significantly slower SE progression vs. the placebo in the 6–12-month period (WMD, 0.31 D; 95% CI, 0.16–0.47 D; p < 0.01), and an even more significant slowing of SE progression over the 12–24-month period (WMD, 0.58 D; 95% CI, 0.18–0.98 D; p < 0.01). The axial length also exhibited a similar trend, with AL progression being significantly slower in the atropine group in the 6–12-month period (WMD, −0.10 mm; 95% CI, −0.15 to −0.06 mm; p < 0.01) and even more so over the 12–24-month period (WMD, −0.19 mm; 95% CI, −0.30 to −0.07 mm; p < 0.01).
Figure 4.
Forest plot of the mean difference in spherical equivalent between the atropine and placebo groups.
Figure 5.
Forest plot of the mean difference in axial length between the atropine and placebo groups.
3.6. Adverse Events
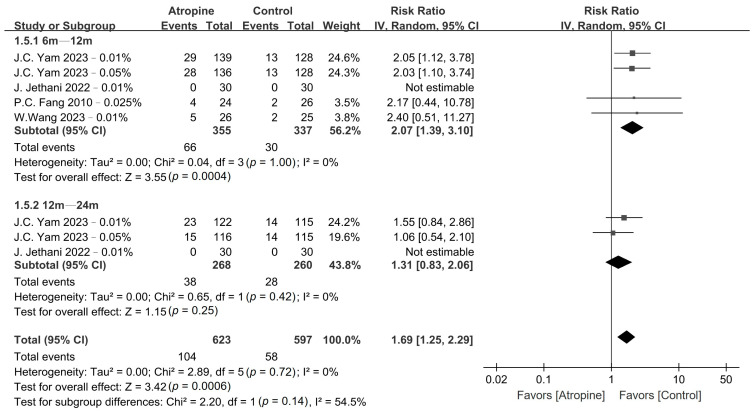
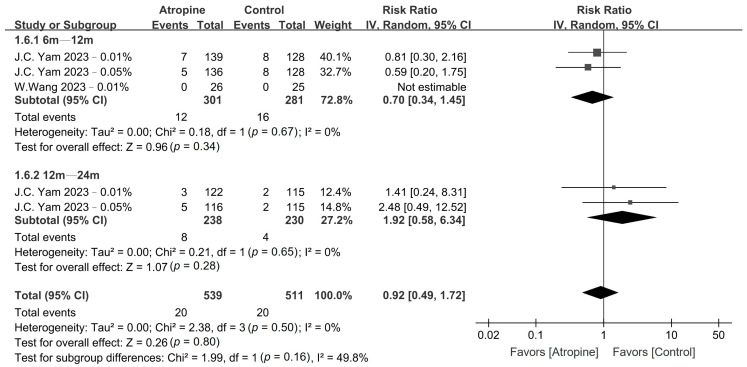
No major adverse events were observed in either the atropine or the placebo group. However, the patients in the atropine group exhibited a significantly higher incidence of photophobia compared with those in the placebo group in the 6–12-month period (RR, 2.07; 95% CI, 1.39–3.10; p < 0.01); however, no such difference was observed over the 12–24-month period (RR, 1.31; 95% CI, 0.83–2.06; p = 0.25), as shown in Figure 6. Conversely, there was no difference in the incidence of allergic conjunctivitis between the atropine and placebo groups in the 6–12-month period (RR, 0.70; 95% CI, 0.34–1.45; p = 0.34) or the 12–24-month period (RR, 1.92; 95% CI, 0.58–6.34; p = 0.28), as depicted in Figure 7.
Figure 6.
Forest plot of the risk ratio of photophobia incidence between the atropine and placebo groups.
Figure 7.
Forest plot of the risk ratio of allergic conjunctivitis incidence between the atropine and placebo groups.
3.7. Accommodation Amplitude and Pupil Size
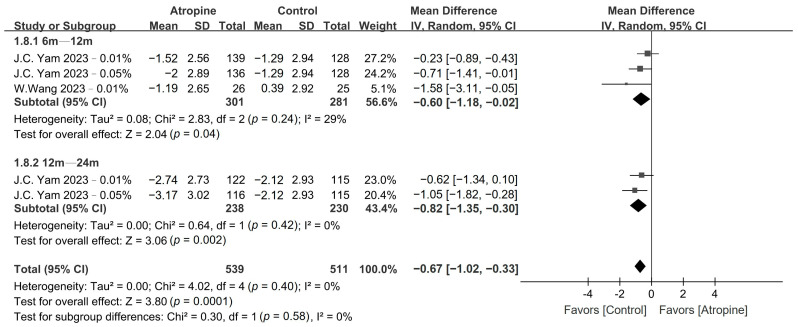
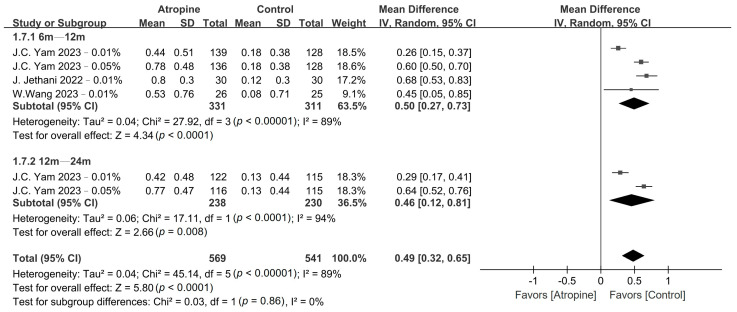
The patients who were treated with low-dose atropine exhibited a significantly lower accommodation amplitude compared with the placebo group in the 6–12-month period (WMD, −0.60 D; 95% CI, −1.18 to −0.02 D; p = 0.04), and even more so over the 12–24-month period (WMD, −0.82 D; 95% CI, −1.35 to −0.30 D; p < 0.01), as shown in Figure 8. Moreover, children in the atropine group also exhibited a larger pupil size than did those in the placebo group in the 6–12-month period (WMD, 0.50 mm; 95% CI, 0.27–0.73 mm; p < 0.01) and in the 12–24-month period (WMD, 0.46 mm; 95% CI, 0.12–0.81 mm; p < 0.01), as depicted in Figure 9.
Figure 8.
Forest plot of the mean difference in accommodation amplitude incidence between the atropine and placebo groups.
Figure 9.
Forest plot of the mean difference in pupil size incidence between the atropine and placebo groups.
4. Discussion
To the best of our knowledge, this study represents a pioneering systematic review and meta-analysis that evaluated the efficacy and safety of low-dose atropine in children with premyopia. Our findings suggest that low-dose atropine is effective in reducing the incidence of myopia, slowing down the shift toward fast myopia, and curbing the progression of SE and AL. These results agree with observations performed in children with myopia [24,27,28]. Moreover, there were no discernible differences in the incidence of photophobia and allergic conjunctivitis over a period exceeding 1 year. Conversely, although children in the atropine group exhibited a lower accommodation amplitude and a larger pupil size, these differences may not be clinically significant. Overall, the results of our study suggest that low-dose atropine is both effective and safe, even in children without pre-existing myopia. Nevertheless, due to the limited number of studies, some confidence intervals of RR are close to 1, warranting further investigation to validate our findings and provide more robust evidence.
Premyopia, alternatively termed low hyperopia reserve [12], is associated with various factors, including parental and environmental myopiogenic effects [29]. The definitions of premyopia are highly divergent, with some researchers describing it as a child experiencing a change in glass power greater than 0.5 D per year on the myopic side and a spherical equivalent lower than +1.00 D [30]. Various studies, such as those reported by Fang et al. [16] and Wang et al. [17], employed distinct SE criteria for premyopia, underscoring the need for standardized criteria. Larger research studies, such as LAMP2 [18] and ATOM3 [31], have contributed to this ongoing debate. The LAMP2 study defined premyopia as an SE of +1–0 D in 4–9-year-old children. In addition, the design of the ATOM3 study included patients with a family history of myopia presenting with low hyperopia or low myopia, encompassing 5–9-year-old children with an SE range of +1.00 to −1.50 D. The ongoing debate regarding the definition of premyopia necessitates further exploration, to identify genuine factors associated with this condition and distinguish young individuals with premyopia from their peers with stable emmetropia.
Our research underscores the substantial efficacy of low-dose atropine in reducing myopia incidence and fast myopia shift, as well as diminishing SE and AL progression in children with premyopia. The significance of delaying the onset of myopia is paramount in mitigating the development of high myopia [32,33], thus emphasizing the importance of premyopia control, which may prove to be more effective than interventions in individuals who already have myopia. The varying efficacy of low-dose atropine observed in patients with myopia and premyopia may be attributed to the differing rates of SE and AL progression recorded over time between these two conditions. Previous research indicates that children with premyopia undergo more rapid changes in refraction and axial length, with the pace gradually slowing down after the onset of myopia [34,35]. Nevertheless, the mechanism underlying the observation that children with premyopia with rapid AL and SE progression exhibited a better response to low-dose atropine remains unclear. In the realm of myopia research, an association is often established between this condition and choroidal thickness [36,37]. The possible underlying mechanisms include an atropine-induced choroidal thickening through the stimulation of dopamine release [38]. In addition, atropine may mediate choroidal blood vessel relaxation, potentially increasing capillary permeability, leading to choroidal thickening [39]. We hypothesized that, in individuals with premyopia, the mechanism of action of atropine is similar to that observed in patients with myopia. Nevertheless, beyond lifestyle modifications, atropine emerges as a pivotal method for controlling myopia, especially in patients with identifiable myopia risks [40].
Because of the limited number of available studies, in this study, we were unable to conduct subgroup analyses regarding the concentration of atropine. However, the efficacy of atropine for myopia control exhibited a dose-dependent relationship, with higher concentrations enhancing effectiveness but also resulting in more severe adverse events [41]. The debate on the optimal concentration of atropine for children with myopia persists. Notably, although 0.01% atropine has proven to be effective in slowing myopia progression down in Asian children [41], its efficacy has not been replicated in American children compared with a placebo [42]. Moreover, the LAMP study designates 0.05% atropine as the optimal concentration, striking a balance between efficacy and adverse events [41]. However, the confirmation of the optimal concentration for use in children with premyopia requires further research.
In terms of adverse events, our analysis revealed a higher incidence of photophobia in the atropine group during the 6–12-month period, with no significant differences in allergic conjunctivitis and photophobia detected between the groups in the 12–24-month period. Consistent with the existing myopia studies, photophobia and blurred near vision are adverse effects that are commonly reported to be associated with atropine therapy [24,32]. Despite the initial adverse effects related to atropine usage, the prolonged use of this medication appears to mitigate these effects [43]. This mitigation is likely caused by drug-tolerance and compensation mechanisms. Our analysis further demonstrated that the atropine group exhibited a lower accommodation amplitude and a larger pupil size; however, these findings appeared to be subclinical. More specifically, low-dose atropine resulted in a loss of less than 1 D of accommodation and an increase of about 0.5 mm in pupil size. However, these mild side effects were deemed to be clinically insignificant, as observed in studies such as ATOM2, in which no change or loss in distance or near visual acuity was observed. Notably, pupil size and accommodation returned to the baseline levels at approximately 2 months after the discontinuation of atropine [21]. Consequently, in children with myopia, as these changes are subclinical and reversible, adherence to low-dose atropine drop therapy is generally good. Previous reports indicate that over 90% of children adhere to the treatment more than six times per week [44]. Similarly, we infer that, due to the low impact on the quality of life, there might be high adherence to low-dose atropine in premyopic children.
Beyond atropine intervention, various strategies are employed to impede the progression to myopia among children with premyopia. A noteworthy approach involves the repeated application of low-level red light, utilizing a device emitting 650 nm visible red light. When administered twice daily in sessions of 3 min, this novel therapy has demonstrated efficacy in children with myopia [45,46], and recent studies have corroborated its effectiveness in children with premyopia [47,48]. The 12-month incidence of myopia was 40.8% in the intervention group and 61.3% in the control group, whereas SE and AL progression was also reduced in the intervention group [48]. However, its susceptibility to a rebound effect is a notable drawback [49]. Another viable method consists of increasing the time spent outdoors [50,51], particularly under robust sunlight exposure [52,53], which has proven to be effective in controlling myopia progression both in children with myopia and in those with premyopia. Furthermore, family health education serves as an additional avenue for myopia control [54,55,56]. In contrast to pharmacological approaches, there is a paucity of optical methods, such as orthokeratology, for the cohort with premyopia. Additional research is imperative to ascertain the effectiveness of these methodologies and explore potential synergies arising from their combination.
5. Limitations
Our study had several limitations. Firstly, the diverse definitions of myopia and premyopia used across the studies included in the present analysis may have introduced variability in our results. Fortunately, the fast myopia shift, which remains unaffected by the definitions, exhibited a similar trend to that observed for myopia incidence, supporting the robustness of our findings. Secondly, the limited number of studies available may have impacted the statistical power and hindered our ability to conduct subgroup analyses, such as exploring the effects of different atropine concentrations and treatment durations. Therefore, further investigations are imperative to determine the optimal atropine concentration and treatment duration for children with premyopia. Thirdly, the rebound effect of atropine was not assessed because of the scarcity of relevant studies. Future research should focus on designing studies specifically addressing the rebound effect of atropine in children with premyopia. Finally, the fact that all the studies included here were conducted in Asia may restrict the generalizability of our findings to populations with diverse genetic, environmental, or lifestyle factors influencing myopia progression. Therefore, it is essential to conduct further studies involving diverse ethnic groups to enhance the external validity of our results.
6. Conclusions
Our meta-analysis highlighted the effectiveness of low-dose atropine in controlling myopia among children with premyopia, as evidenced by a decrease in myopia incidence and fast myopia shift, together with a notable reduction in SE and AL progression. In terms of safety, our findings revealed the absence of major adverse events, and the minor differences in adverse events observed between the atropine and placebo groups lacked clinical significance. In summary, our findings support the safety and efficacy of low-dose atropine for individuals with premyopia. Nevertheless, due to the current limitations in evidence, further research is imperative to determine the optimal dosage and treatment duration, for the establishment of more conclusive recommendations.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jcm13051506/s1, Table S1: keywords and search results in different databases, Table S2: excluded studies and reasons [57,58,59,60,61,62], Table S3: detailed quality assessment of included studies using Cochrane risk-of-bias 2 tool (RoB 2.0) [17,18,26], Table S4: detailed quality assessment of included studies using Newcastle–Ottawa Scale (NOS) [16], Table S5: GRADE summary of findings, Table S6: PRISMA checklist, Figure S1: funnel plot of myopia incidence.
Author Contributions
Conceptualization, C.-J.C.; methodology, C.-J.C. and J.-H.W.; software, S.-H.L., B.-Y.T. and J.-H.W.; validation, C.-J.C. and J.-H.W.; formal analysis, S.-H.L. and B.-Y.T.; investigation, S.-H.L., B.-Y.T. and C.-J.C.; resources, C.-J.C. and J.-H.W.; data curation, S.-H.L. and B.-Y.T.; writing—original draft preparation, S.-H.L. and B.-Y.T.; writing—review and editing, S.-H.L., B.-Y.T., J.-H.W. and C.-J.C.; visualization, S.-H.L., B.-Y.T., J.-H.W. and C.-J.C.; supervision, C.-J.C. and J.-H.W.; project administration, C.-J.C. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Data are contained within the article or Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by the Buddhist Tzu Chi Medical Foundation grant number TCMF-P 113-07.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Morgan I.G., Ohno-Matsui K., Saw S.M. Myopia. Lancet. 2012;379:1739–1748. doi: 10.1016/S0140-6736(12)60272-4. [DOI] [PubMed] [Google Scholar]
- 2.Holden B.A., Fricke T.R., Wilson D.A., Jong M., Naidoo K.S., Sankaridurg P., Wong T.Y., Naduvilath T.J., Resnikoff S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology. 2016;123:1036–1042. doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Dolgin E. The myopia boom. Nature. 2015;519:276–278. doi: 10.1038/519276a. [DOI] [PubMed] [Google Scholar]
- 4.Fang Y., Yokoi T., Nagaoka N., Shinohara K., Onishi Y., Ishida T., Yoshida T., Xu X., Jonas J.B., Ohno-Matsui K. Progression of Myopic Maculopathy during 18-Year Follow-up. Ophthalmology. 2018;125:863–877. doi: 10.1016/j.ophtha.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Chua S.Y., Sabanayagam C., Cheung Y.B., Chia A., Valenzuela R.K., Tan D., Wong T.Y., Cheng C.Y., Saw S.M. Age of onset of myopia predicts risk of high myopia in later childhood in myopic Singapore children. Ophthalmic Physiol. Opt. 2016;36:388–394. doi: 10.1111/opo.12305. [DOI] [PubMed] [Google Scholar]
- 6.Liu H.H., Xu L., Wang Y.X., Wang S., You Q.S., Jonas J.B. Prevalence and progression of myopic retinopathy in Chinese adults: The Beijing Eye Study. Ophthalmology. 2010;117:1763–1768. doi: 10.1016/j.ophtha.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 7.Wong Y.L., Sabanayagam C., Ding Y., Wong C.W., Yeo A.C., Cheung Y.B., Cheung G., Chia A., Ohno-Matsui K., Wong T.Y., et al. Prevalence, Risk Factors, and Impact of Myopic Macular Degeneration on Visual Impairment and Functioning among Adults in Singapore. Investig. Ophthalmol. Vis. Sci. 2018;59:4603–4613. doi: 10.1167/iovs.18-24032. [DOI] [PubMed] [Google Scholar]
- 8.Pan C.W., Cheng C.Y., Saw S.M., Wang J.J., Wong T.Y. Myopia and age-related cataract: A systematic review and meta-analysis. Am. J. Ophthalmol. 2013;156:1021–1033.e1. doi: 10.1016/j.ajo.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Marcus M.W., de Vries M.M., Junoy Montolio F.G., Jansonius N.M. Myopia as a risk factor for open-angle glaucoma: A systematic review and meta-analysis. Ophthalmology. 2011;118:1989–1994.e2. doi: 10.1016/j.ophtha.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 10.Tideman J.W., Snabel M.C., Tedja M.S., van Rijn G.A., Wong K.T., Kuijpers R.W., Vingerling J.R., Hofman A., Buitendijk G.H., Keunen J.E., et al. Association of Axial Length with Risk of Uncorrectable Visual Impairment for Europeans with Myopia. JAMA Ophthalmol. 2016;134:1355–1363. doi: 10.1001/jamaophthalmol.2016.4009. [DOI] [PubMed] [Google Scholar]
- 11.Schmid K. Myopia Manual: An Impartial Documentation of All the Reasons, Therapies and Recommendations. Pagefree Publishing; Portage, MI, USA: 2018. [Google Scholar]
- 12.Sankaridurg P., Berntsen D.A., Bullimore M.A., Cho P., Flitcroft I., Gawne T.J., Gifford K.L., Jong M., Kang P., Ostrin L.A., et al. IMI 2023 Digest. Investig. Ophthalmol. Vis. Sci. 2023;64:7. doi: 10.1167/iovs.64.6.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zadnik K., Sinnott L.T., Cotter S.A., Jones-Jordan L.A., Kleinstein R.N., Manny R.E., Twelker J.D., Mutti D.O. Prediction of Juvenile-Onset Myopia. JAMA Ophthalmol. 2015;133:683–689. doi: 10.1001/jamaophthalmol.2015.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma Y., Zou H., Lin S., Xu X., Zhao R., Lu L., Zhao H., Li Q., Wang L., Zhu J., et al. Cohort study with 4-year follow-up of myopia and refractive parameters in primary schoolchildren in Baoshan District, Shanghai. Clin. Exp. Ophthalmol. 2018;46:861–872. doi: 10.1111/ceo.13195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flitcroft D.I., He M., Jonas J.B., Jong M., Naidoo K., Ohno-Matsui K., Rahi J., Resnikoff S., Vitale S., Yannuzzi L. IMI—Defining and Classifying Myopia: A Proposed Set of Standards for Clinical and Epidemiologic Studies. Investig. Ophthalmol. Vis. Sci. 2019;60:M20–M30. doi: 10.1167/iovs.18-25957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang P.C., Chung M.Y., Yu H.J., Wu P.C. Prevention of myopia onset with 0.025% atropine in premyopic children. J. Ocul. Pharmacol. Ther. 2010;26:341–345. doi: 10.1089/jop.2009.0135. [DOI] [PubMed] [Google Scholar]
- 17.Wang W., Zhang F., Yu S., Ma N., Huang C., Wang M., Wei L., Zhang J., Fu A. Prevention of myopia shift and myopia onset using 0.01% atropine in premyopic children—A prospective, randomized, double-masked, and crossover trial. Eur. J. Pediatr. 2023;182:2597–2606. doi: 10.1007/s00431-023-04921-5. [DOI] [PubMed] [Google Scholar]
- 18.Yam J.C., Zhang X.J., Zhang Y., Yip B.H.K., Tang F., Wong E.S., Bui C.H.T., Kam K.W., Ng M.P.H., Ko S.T., et al. Effect of Low-Concentration Atropine Eyedrops Vs Placebo on Myopia Incidence in Children: The LAMP2 Randomized Clinical Trial. JAMA. 2023;329:472–481. doi: 10.1001/jama.2022.24162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tong L., Huang X.L., Koh A.L., Zhang X., Tan D.T., Chua W.H. Atropine for the treatment of childhood myopia: Effect on myopia progression after cessation of atropine. Ophthalmology. 2009;116:572–579. doi: 10.1016/j.ophtha.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 20.Chia A., Chua W.H., Cheung Y.B., Wong W.L., Lingham A., Fong A., Tan D. Atropine for the treatment of childhood myopia: Safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2) Ophthalmology. 2012;119:347–354. doi: 10.1016/j.ophtha.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 21.Chia A., Lu Q.S., Tan D. Five-Year Clinical Trial on Atropine for the Treatment of Myopia 2: Myopia Control with Atropine 0.01% Eyedrops. Ophthalmology. 2016;123:391–399. doi: 10.1016/j.ophtha.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Jonas J.B., Ang M., Cho P., Guggenheim J.A., He M.G., Jong M., Logan N.S., Liu M., Morgan I., Ohno-Matsui K., et al. IMI Prevention of Myopia and Its Progression. Investig. Ophthalmol. Vis. Sci. 2021;62:6. doi: 10.1167/iovs.62.5.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joachimsen L., Farassat N., Bleul T., Böhringer D., Lagrèze W.A., Reich M. Side effects of topical atropine 0.05% compared to 0.01% for myopia control in German school children: A pilot study. Int. Ophthalmol. 2021;41:2001–2008. doi: 10.1007/s10792-021-01755-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong Q., Janowski M., Luo M., Wei H., Chen B., Yang G., Liu L. Efficacy and Adverse Effects of Atropine in Childhood Myopia: A Meta-analysis. JAMA Ophthalmol. 2017;135:624–630. doi: 10.1001/jamaophthalmol.2017.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jethani J. Efficacy of low-concentration atropine (0.01%) eye drops for prevention of axial myopic progression in premyopes. Indian J. Ophthalmol. 2022;70:238–240. doi: 10.4103/ijo.IJO_1462_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ha A., Kim S.J., Shim S.R., Kim Y.K., Jung J.H. Efficacy and Safety of 8 Atropine Concentrations for Myopia Control in Children: A Network Meta-Analysis. Ophthalmology. 2022;129:322–333. doi: 10.1016/j.ophtha.2021.10.016. [DOI] [PubMed] [Google Scholar]
- 28.Huang J., Wen D., Wang Q., McAlinden C., Flitcroft I., Chen H., Saw S.M., Chen H., Bao F., Zhao Y., et al. Efficacy Comparison of 16 Interventions for Myopia Control in Children: A Network Meta-analysis. Ophthalmology. 2016;123:697–708. doi: 10.1016/j.ophtha.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Wang C.Y., Hsu N.W., Yang Y.C., Chen Y.L., Shyong M.P., Tsai D.C. Premyopia at Preschool Age: Population-based Evidence of Prevalence and Risk Factors from a Serial Survey in Taiwan. Ophthalmology. 2022;129:880–889. doi: 10.1016/j.ophtha.2022.03.017. [DOI] [PubMed] [Google Scholar]
- 30.Drobe B., de Saint-André R. The pre-myopic syndrome. Ophthalmic Physiol. Opt. 1995;15:375–378. doi: 10.1046/j.1475-1313.1995.9500075o.x. [DOI] [PubMed] [Google Scholar]
- 31.The Use of Atropine 0.01% in the Prevention and Control of Myopia (ATOM3) [(accessed on 1 January 2024)]; Available online: https://classic.clinicaltrials.gov/show/NCT03140358.
- 32.Wu P.C., Chuang M.N., Choi J., Chen H., Wu G., Ohno-Matsui K., Jonas J.B., Cheung C.M.G. Update in myopia and treatment strategy of atropine use in myopia control. Eye. 2019;33:3–13. doi: 10.1038/s41433-018-0139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Modjtahedi B.S., Abbott R.L., Fong D.S., Lum F., Tan D. Reducing the Global Burden of Myopia by Delaying the Onset of Myopia and Reducing Myopic Progression in Children: The Academy’s Task Force on Myopia. Ophthalmology. 2021;128:816–826. doi: 10.1016/j.ophtha.2020.10.040. [DOI] [PubMed] [Google Scholar]
- 34.Mutti D.O., Hayes J.R., Mitchell G.L., Jones L.A., Moeschberger M.L., Cotter S.A., Kleinstein R.N., Manny R.E., Twelker J.D., Zadnik K. Refractive error, axial length, and relative peripheral refractive error before and after the onset of myopia. Investig. Ophthalmol. Vis. Sci. 2007;48:2510–2519. doi: 10.1167/iovs.06-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiang F., He M., Morgan I.G. Annual changes in refractive errors and ocular components before and after the onset of myopia in Chinese children. Ophthalmology. 2012;119:1478–1484. doi: 10.1016/j.ophtha.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 36.Xu H., Ye L., Peng Y., Yu T., Li S., Weng S., Huang Y., Chen Y., Fan Y., Zou H., et al. Potential Choroidal Mechanisms Underlying Atropine’s Antimyopic and Rebound Effects: A Mediation Analysis in a Randomized Clinical Trial. Investig. Ophthalmol. Vis. Sci. 2023;64:13. doi: 10.1167/iovs.64.4.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yam J.C., Jiang Y., Lee J., Li S., Zhang Y., Sun W., Yuan N., Wang Y.M., Yip B.H.K., Kam K.W., et al. The Association of Choroidal Thickening by Atropine with Treatment Effects for Myopia: Two-Year Clinical Trial of the Low-concentration Atropine for Myopia Progression (LAMP) Study. Am. J. Ophthalmol. 2022;237:130–138. doi: 10.1016/j.ajo.2021.12.014. [DOI] [PubMed] [Google Scholar]
- 38.Nickla D.L., Totonelly K., Dhillon B. Dopaminergic agonists that result in ocular growth inhibition also elicit transient increases in choroidal thickness in chicks. Exp. Eye Res. 2010;91:715–720. doi: 10.1016/j.exer.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nickla D.L., Wallman J. The multifunctional choroid. Prog. Retin. Eye Res. 2010;29:144–168. doi: 10.1016/j.preteyeres.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morgan I.G., French A.N., Ashby R.S., Guo X., Ding X., He M., Rose K.A. The epidemics of myopia: Aetiology and prevention. Prog. Retin. Eye Res. 2018;62:134–149. doi: 10.1016/j.preteyeres.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Yam J.C., Zhang X.J., Zhang Y., Wang Y.M., Tang S.M., Li F.F., Kam K.W., Ko S.T., Yip B.H.K., Young A.L., et al. Three-Year Clinical Trial of Low-Concentration Atropine for Myopia Progression (LAMP) Study: Continued Versus Washout: Phase 3 Report. Ophthalmology. 2022;129:308–321. doi: 10.1016/j.ophtha.2021.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Repka M.X., Weise K.K., Chandler D.L., Wu R., Melia B.M., Manny R.E., Kehler L.A.F., Jordan C.O., Raghuram A., Summers A.I., et al. Low-Dose 0.01% Atropine Eye Drops vs Placebo for Myopia Control: A Randomized Clinical Trial. JAMA Ophthalmol. 2023;141:756–765. doi: 10.1001/jamaophthalmol.2023.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yam J.C., Li F.F., Zhang X., Tang S.M., Yip B.H.K., Kam K.W., Ko S.T., Young A.L., Tham C.C., Chen L.J., et al. Two-Year Clinical Trial of the Low-Concentration Atropine for Myopia Progression (LAMP) Study: Phase 2 Report. Ophthalmology. 2020;127:910–919. doi: 10.1016/j.ophtha.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 44.Polling J.R., Kok R.G., Tideman J.W., Meskat B., Klaver C.C. Effectiveness study of atropine for progressive myopia in Europeans. Eye. 2016;30:998–1004. doi: 10.1038/eye.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dong J., Zhu Z., Xu H., He M. Myopia Control Effect of Repeated Low-Level Red-Light Therapy in Chinese Children: A Randomized, Double-Blind, Controlled Clinical Trial. Ophthalmology. 2023;130:198–204. doi: 10.1016/j.ophtha.2022.08.024. [DOI] [PubMed] [Google Scholar]
- 46.Jiang Y., Zhu Z., Tan X., Kong X., Zhong H., Zhang J., Xiong R., Yuan Y., Zeng J., Morgan I.G., et al. Effect of Repeated Low-Level Red-Light Therapy for Myopia Control in Children: A Multicenter Randomized Controlled Trial. Ophthalmology. 2021;129:509–519. doi: 10.1016/j.ophtha.2021.11.023. [DOI] [PubMed] [Google Scholar]
- 47.Tian L., Cao K., Ma D.L., Lu L.X., Zhao S.Q., Li A., Chen C.X., Ma Z.F., Jin Z.B., Ma C.R., et al. Six-month repeated irradiation of 650 nm low-level red light reduces the risk of myopia in children: A randomized controlled trial. Int. Ophthalmol. 2023;43:3549–3558. doi: 10.1007/s10792-023-02762-7. [DOI] [PubMed] [Google Scholar]
- 48.He X., Wang J., Zhu Z., Xiang K., Zhang X., Zhang B., Chen J., Yang J., Du L., Niu C., et al. Effect of Repeated Low-level Red Light on Myopia Prevention Among Children in China With Premyopia: A Randomized Clinical Trial. JAMA Netw. Open. 2023;6:e239612. doi: 10.1001/jamanetworkopen.2023.9612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiong R., Zhu Z., Jiang Y., Kong X., Zhang J., Wang W., Kiburg K., Yuan Y., Chen Y., Zhang S., et al. Sustained and rebound effect of repeated low-level red-light therapy on myopia control: A 2-year post-trial follow-up study. Clin. Exp. Ophthalmol. 2022;50:1013–1024. doi: 10.1111/ceo.14149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He X., Sankaridurg P., Wang J., Chen J., Naduvilath T., He M., Zhu Z., Li W., Morgan I.G., Xiong S., et al. Time Outdoors in Reducing Myopia: A School-Based Cluster Randomized Trial with Objective Monitoring of Outdoor Time and Light Intensity. Ophthalmology. 2022;129:1245–1254. doi: 10.1016/j.ophtha.2022.06.024. [DOI] [PubMed] [Google Scholar]
- 51.Guo Y., Liu L., Lv Y., Tang P., Feng Y., Wu M., Xu L., Jonas J.B. Outdoor Jogging and Myopia Progression in School Children from Rural Beijing: The Beijing Children Eye Study. Transl. Vis. Sci. Technol. 2019;8:2. doi: 10.1167/tvst.8.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu P.C., Chen C.T., Lin K.K., Sun C.C., Kuo C.N., Huang H.M., Poon Y.C., Yang M.L., Chen C.Y., Huang J.C., et al. Myopia Prevention and Outdoor Light Intensity in a School-Based Cluster Randomized Trial. Ophthalmology. 2018;125:1239–1250. doi: 10.1016/j.ophtha.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 53.Hua W.J., Jin J.X., Wu X.Y., Yang J.W., Jiang X., Gao G.P., Tao F.B. Elevated light levels in schools have a protective effect on myopia. Ophthalmic Physiol. Opt. 2015;35:252–262. doi: 10.1111/opo.12207. [DOI] [PubMed] [Google Scholar]
- 54.Zhang J., Wang L., Guo L., Guo Y., Zhao F., Hu Y., Li Q., Du X., Deng X., Deng N., et al. Effects of online family health education on myopia prevention in children by parental myopia: A randomized clinical trial. Clin. Exp. Optom. 2023:1–8. doi: 10.1080/08164622.2023.2216840. [DOI] [PubMed] [Google Scholar]
- 55.Li S.M., Ran A.R., Kang M.T., Yang X., Ren M.Y., Wei S.F., Gan J.H., Li L., He X., Li H., et al. Effect of Text Messaging Parents of School-Aged Children on Outdoor Time to Control Myopia: A Randomized Clinical Trial. JAMA Pediatr. 2022;176:1077–1083. doi: 10.1001/jamapediatrics.2022.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Q., Guo L., Zhang J., Zhao F., Hu Y., Guo Y., Du X., Zhang S., Yang X., Lu C. Effect of School-Based Family Health Education via Social Media on Children’s Myopia and Parents’ Awareness: A Randomized Clinical Trial. JAMA Ophthalmol. 2021;139:1165–1172. doi: 10.1001/jamaophthalmol.2021.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu S., Lv Y., Wang W., Cui C., Wei L., Huang C., Ma N., Zhao B., Zhang J., Fu A. Effects of 0.01% atropine eye drops on the prevention of myopia onset among schoolchildren: A randomized, double-blind, controlled trial. Chin. J. Exp. Ophthalmol. 2022;40:533–540. doi: 10.3760/cma.j.cn115989-20210821-00470. [DOI] [Google Scholar]
- 58.Li J., Hu F., Zhou X., Li C., Hu X. Prevention of myopia onset and progression with 0.01% atropine solution among school-age children. Chin. J. Sch. Health. 2018;39:432–435. [Google Scholar]
- 59.Li Y., Liu J., Qi P. The increasing prevalence of myopia in junior high school students in the Haidian District of Beijing, China: A 10-year population-based survey. BMC Ophthalmol. 2017;17:88. doi: 10.1186/s12886-017-0483-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scheiman M., Gwiazda J., Zhang Q., Deng L., Fern K., Manny R.E., Weissberg E., Hyman L. Longitudinal changes in corneal curvature and its relationship to axial length in the Correction of Myopia Evaluation Trial (COMET) cohort. J. Optom. 2016;9:13–21. doi: 10.1016/j.optom.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wong Y.L., Li X., Huang Y., Yuan Y., Ye Y., Lim E.W., Yang A., Spiegel D., Drobe B., Bao J., et al. Eye growth pattern of myopic children wearing spectacle lenses with aspherical lenslets compared with non-myopic children. Ophthalmic Physiol. Opt. 2024;44:206–213. doi: 10.1111/opo.13232. [DOI] [PubMed] [Google Scholar]
- 62.Lee L.C., Hsieh M.W., Chen Y.H., Chen P.L., Chien K.H. Characteristics of responders to atropine 0.01% as treatment in Asian myopic children. Sci. Rep. 2022;12:7380. doi: 10.1038/s41598-022-10978-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article or Supplementary Materials. Further inquiries can be directed to the corresponding author.