Abstract
Background: Cardiovascular magnetic resonance (CMR) has emerged as the most accurate, non-invasive method to support the diagnosis of clinically suspected myocarditis and as a risk-stratification tool in patients with cardiomyopathies. We aim to assess the diagnostic and prognostic role of CMR at diagnosis in patients with myocarditis. Methods: We enrolled consecutive single-center patients with 2013 ESC consensus-based endomyocardial biopsy (EMB)-proven or clinically suspected myocarditis undergoing CMR at diagnosis. The pre-specified outcome was defined as NYHA class > I and echocardiographic left ventricular ejection fraction (LVEF) < 50% at follow-up. Results: We included 207 patients (74% male, median age 36 years; 25% EMB-proven). CMR showed the highest sensitivity in myocarditis with infarct-like presentation. Patients with EMB-proven myocarditis were more likely to have diffuse LGE and right ventricular LGE (p < 0.001), which was also more common among patients with arrhythmic presentation (p = 0.001). The outcome was met in 17 patients at any follow-up time point, more commonly in those with larger biventricular volumes (p < 0.001), CMR-based diagnosis of dilated cardiomyopathy (p < 0.001), and ischemic LGE (p = 0.005). Higher biventricular systolic function (p < 0.001) and greater LGE extent (p = 0.033) at diagnosis had a protective effect. Conclusions: In our single-center cohort of rigorously defined myocarditis patients, higher biventricular systolic function and greater LGE extent on CMR at diagnosis identified patients with better functional class and higher left ventricular ejection fraction at follow-up. Conversely, larger biventricular volumes, CMR-based DCM features, and the presence of an ischemic LGE pattern at diagnosis were predictors of worse functional class and LV systolic dysfunction at follow-up. Larger prospective studies are warranted to extend our findings to multi-center cohorts.
Keywords: myocarditis, cardiovascular magnetic resonance, endomyocardial biopsy, outcome
1. Introduction
Myocarditis is an inflammatory disease of the myocardium with variable clinical presentation and outcome [1,2,3]. Endomyocardial biopsy (EMB) provides diagnosis and defines etiology, key to a tailored treatment [1,4,5,6]. Despite not replacing EMB, cardiovascular magnetic resonance (CMR) has become the non-invasive gold standard for diagnosing clinically suspected myocarditis [1,7,8]. CMR diagnosis was originally based on the presence of edema and/or early or late gadolinium enhancement (Lake Louise criteria [7]). These criteria have been recently updated to include parametric mapping and are expected to increase CMR diagnostic accuracy [7,8]. Several studies have addressed the prognostic role of CMR in myocarditis, but results have been conflicting [9,10,11,12,13,14,15,16].
We assessed the diagnostic and prognostic role of CMR in a single-center cohort of biopsy-proven or clinically suspected myocarditis, strictly defined following the 2013 ESC consensus criteria.
2. Materials and Methods
2.1. Study Participants
We retrospectively enrolled consecutive patients with myocarditis undergoing CMR at diagnosis during the index hospital admission. All patients in our study fulfilled the diagnosis of suspected myocarditis irrespective of CMR results. Clinically suspected (CS) myocarditis was defined according to the 2013 ESC position statement [1] as follows: ≥1 clinical presentation suggestive of myocarditis and ≥1 diagnostic criterion from different categories (ECG, increased myocytolitic enzymes, morpho-functional abnormalities at cardiac imaging, tissue characterization by CMR) in the absence of angiographically detectable coronary artery disease (CAD) and known pre-existing cardiovascular disease or extra-cardiac causes that could explain the syndrome; in asymptomatic patients ≥ 2 diagnostic tests were needed to diagnose clinically suspected myocarditis. According to the 2013 ESC consensus [1] clinical presentation of myocarditis was defined as follows: (1) infarct-like (in the absence of CAD), characterized by acute chest pain (usually starting 1–4 weeks following respiratory or gastrointestinal infection) and evidence of ST-segment elevation/depression and/or T waves inversion on ECG, with or without normal global or regional left ventricular (LV) and/or right ventricular (RV) dysfunction on echocardiography or CMR; (2) heart failure (HF), new onset or worsening (2 weeks–3 months symptoms duration, possibly started after a respiratory or gastrointestinal infection, or in the peri-partum period) or chronic HF (>3 months symptoms duration), after exclusion of CAD and other known causes of HF, with impaired systolic LV and/or RV function, with or without an increase in wall thickness, with or without dilated LV and/or RV on echocardiography or CMR, with or without increased troponin and non-specific ECG signs (bundle branch block, atrio-ventricular block, and/or ventricular arrhythmias); (3) arrhythmias, life-threatening arrhythmias and/or aborted sudden death, in the absence of CAD and known causes of HF.
Endomyocardial biopsy (EMB) was performed as clinically indicated according to current expert position papers [4,5,6] by obtaining 4–6 myocardial samples, 1–2 mm in size, from the right ventricle [4,5]; one or two frozen EMB specimens per patient were used for polymerase chain reaction (PCR) and reverse transcriptase PCR analysis and for detection of cardiotropic viruses’ genome simultaneously to histological analysis [4,17]. EMB-proven myocarditis was defined by histological (Dallas criteria), immunohistochemical (≥14 leucocytes/mm2 including up to 4 monocytes/mm2 with the presence of CD 3 positive T-lymphocytes ≥ 7 cells/mm2), and molecular criteria (search of cardiotropic viruses’ genome) [1].
Clinical, laboratory, and imaging data were collected at diagnosis and during follow-up. All patients were followed up at the outpatient Cardio–Immunology Clinic of the Padua University Hospital (Italy) every six months unless otherwise clinically indicated; functional status, ECG, and transthoracic echocardiography (TTE) were assessed at each visit. The pre-specified outcome was a composite of NYHA class > I and echocardiographic LV systolic dysfunction (defined as LVEF < 50%) at follow-up [6,18]. The study was approved by our Ethics Committee (protocol number 0021857); all patients provided informed consent. Patients or the public were not involved in the design, conduct, reporting, or dissemination plans of our research.
2.2. CMR Protocol and Analysis
Patients underwent a 1.5T CMR scan including long and short-axis cine sequences, T2-weighted (T2w), early gadolinium enhancement (EGE), and late gadolinium enhancement (LGE) sequences. EGE and LGE images were respectively acquired 1–3 min and 10–15 min after intravenous injection of 0.2 mmol/kg of gadolinium-based contrast agent (GBCA), in identical planes to cine images.
Biventricular volumes and function were assessed with dedicated software (Circle CVI42®, Calgary, AB, Canada, version 5.11), and all measurements were indexed to body surface area. Myocardial edema on T2w images was defined by a ratio of signal intensity (SI) between the myocardium and the mean SI of the skeletal muscle ≥ 2 [7]; in order to obtain the T2 SI ratio, we first outlined left ventricular endocardial and epicardial contours on each short axis T2w slice and then we drew the contour for a region of interest (ROI) in a large area of the skeletal muscle closest to the heart and to the center of the reception field of the coil (usually in the M. serratus anterior). LGE was defined as subendocardial or transmural (ischemic pattern) if involving <50% or ≥50 of wall thickness, respectively, and mid-wall/epicardial (non-ischemic pattern). LGE distribution was defined as diffuse when involving multiple territories. LGE extent was quantified, blinded to EMB results, using the full width at half maximum method by dedicated software (Circle CVI 42®, Calgary, AB, Canada, version 5.11) [19]. As CMR scans were performed over the last decade, for data uniformity, CMR findings were considered consistent with myocarditis if at least 2 of the following criteria were present: (a) regional or global myocardial signal intensity (SI) increase on T2w images, (b) increased global myocardial EGE ratio between myocardium and skeletal muscle in gadolinium-enhanced sequences, (c) at least one focal lesion with non-ischemic regional distribution on LGE sequences [7]. Analysis was carried out according to recommendations of the Society for Cardiovascular Magnetic Resonance [20].
2.3. Statistical Analysis
Descriptive variables were reported as mean ± SD or as median (IQR); categorical variables were reported as absolute numbers and percentages. The Kruskal–Wallis test and Pearson chi-square tests were used to evaluate differences in distribution according to myocarditis diagnosis (EMB vs. CS) and to clinical presentation, respectively, for continuous and categorical variables. The Spearman correlation was used to test the correlation between echocardiographic LVEF and CMR LVEF. Given the low number of events at follow-up, only univariate analysis was performed to assess the effect of potential outcome predictors using Cox Proportional Hazard models. Results were reported as Hazard Ratio (HR), 95% Confidence Interval (CI), and p-value. Outcome predictors were assessed at pre-defined follow-up time points (0–6, 6–12, 12–24 > 24 months, and at any time point). A p-value < 0.05 was considered significant. The analyses were performed using R software (version 4.0.2) with the packages rms and survival [21,22,23,24].
3. Results
3.1. Baseline Patient Characteristics
We retrospectively included 207 patients, 74% male, median age 36 (24–47) years, 156 (75%) with CS, and 51 (25%) with EMB-proven myocarditis (Table 1).
Table 1.
Patients’ characteristics at diagnosis.
| All n = 207 |
EMB-Proven Myocarditis n = 51 |
Clinically Suspected Myocarditis n = 156 |
p-Value | |
|---|---|---|---|---|
| Age, years | 36 (24–47) | 41 ± 15 | 35 ± 14 | 0.01 |
| Female | 54 (26%) | 20 (39%) | 34 (22%) | 0.014 |
| FHx ID * | 23 (11%) | 7 (14%) | 16 (10%) | 0.52 |
| FHx CAD † | 42 (21%) | 12 (25%) | 30 (20%) | 0.45 |
| Previous viral infection * | 91 (45%) | 12 (24%) | 79 (52%) | <0.001 |
| ID | 25 (12%) | 11 (22%) | 14 (9%) | 0.017 |
| Arrhythmic presentation | 16 (8%) | 8 (16%) | 8 (5%) | 0.014 |
| HF presentation | 41 (20%) | 32 (63%) | 9 (6%) | <0.001 |
| Infarct-like presentation | 144 (70%) | 9 (18%) | 135 (87%) | <0.001 |
| Symptom duration before diagnosis (mo) | 1.00 (0.17–6.00) | 2.00 (1.00–11.25) | 0.15 (0.03–1.25) | <0.001 |
| NYHA class at diagnosis ‡, I |
167 (81%) | 18 (36%) | 149 (96%) | <0.001 |
| II–IV | 39 (19%) | 32 (64%) | 7 (4%) | |
| Left HF | 37 (18%) | 31 (61%) | 6 (4%) | <0.001 |
| Right HF | 14 (7%) | 12 (24%) | 2 (1%) | <0.001 |
| Sinus rhythm * | 198 (97%) | 47 (92%) | 151 (99%) | 0.057 |
| Atrial fibrillation * | 3 (1%) | 2 (4%) | 1 (1%) | |
| Left bundle branch block § | 9 (4%) | 8 (16%) | 1 (1%) | <0.001 |
| Right bundle branch block § | 13 (6%) | 6 (12%) | 7 (5%) | <0.001 |
| Troponin I peak, ng/L ? | 3350 (276–10,575) | 210 (0–7930) | 4963 (1100–11,100) | <0.001 |
| CRP peak, mg/dL # | 19.2 (4.9–50.0) | 6.8 (3.1–21.8) | 25.5 (7.0–51.5) | 0.004 |
| LV diastolic diameter, mm ** | 51 ± 7 | 56.2 ± 9.8 | 49.6 ± 4.9 | <0.001 |
| Left atrial volume mL/m2 ‡‡ | 33 ± 13 | 43 ± 18 | 30 ± 10 | <0.001 |
| LVEDVi, mL/m2 ? | 71 ± 25 | 92 ± 39 | 64 ± 12 | <0.001 |
| LVEF, % §§ | 52 ± 14 | 36.9 ± 16.2 | 57.0 ± 7.3 | <0.001 |
| RVEDA, cm2 ?? | 21.3 ± 5.2 | 23.0 ± 7.3 | 20.8 ± 4.1 | 0.21 |
| RVFAC, % ## | 42 ± 10 | 33.6 ± 10.2 | 45.4 ± 7.1 | <0.001 |
Data are n (%), mean ± SD, or median (IQR). FHx, family history; ID, immune-mediated disease; CAD, coronary artery disease; HF, heart failure; NYHA, New York Heart Association; CRP, C-reactive protein; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; RVEDA, right ventricular end-diastolic area; RVFAC, right ventricular fractional area change. Data available in * 204, † 199, ‡ 206, § 202, ? 178, # 173, ** 154, ‡‡ 144, §§ 191, ?? 139, ## 133 patients.
Of the overall cohort, 63% of patients reported symptoms before diagnosis; 47% had chest pain. The majority of patients had an acute presentation, as shown by the median symptoms’ duration in the overall cohort. Anti-heart auto-antibodies (AHA), tested in 182 patients, were positive in 107 (59%), the most prevalent being organ-specific AHA (92%). All patients received standard care. Previous respiratory or gastrointestinal viral infection (1–4 weeks prior to symptoms’ onset) was more frequent, and troponin I was higher in patients with CS myocarditis.
Patients with EMB-proven myocarditis were mainly older females with immune diseases, longer symptom duration, more advanced NYHA, arrhythmias, signs of left and right HF, and lower ventricular function on TTE. Histological types of EMB-proven myocarditis were lymphocytic (n = 48), giant-cell (n = 2), and polymorphic (n = 1); six patients (3%) had a fulminant presentation. Viral genomes were detected in eight biopsy specimens: Parvovirus B19 (PVB19) (n = 5), Epstein–Barr virus (n = 1), Cytomegalovirus (n = 1), and Influenza A virus (n = 1). All patients received standard optimal medical therapy (OMT) according to international guidelines; a third of patients were treated with beta-blockers (more frequently biopsy-proven patients), 40% were treated with renin–angiotensin–aldosterone system inhibitors (more frequently biopsy-proven patients), and a few patients received anti-arrhythmic treatment, mainly amiodarone. Twenty-two EMB-proven virus-negative patients (11%) started immunosuppressive therapy on top of standard OMT, 12 for worsening/unremitting HF; LVEF improved in all but one.
3.2. CMR Findings—Overall Cohort
CMR findings are summarized in Table 2.
Table 2.
CMR findings.
| All n = 207 |
EMB-Proven Myocarditis n = 51 |
Clinically Suspected Myocarditis n = 156 |
p-Value | |
|---|---|---|---|---|
| LVEDVi mL/m2 | 90 (79–106) | 110 (92–156) | 86 (78–98) | <0.001 |
| LVESVi mL/m2 | 38 (31–48) | 69 (41–118) | 36 (30–42) | <0.001 |
| LVSV mL | 93 (76–106) | 67 (53–86) | 96 (83–111) | <0.001 |
| LVEF % | 57 (51–62) | 33 (23–51) | 59 (55–62) | <0.001 |
| LV mass, g/m2 | 61 (52–74) | 76 (58–85) | 59 (51–69) | <0.001 |
| LV regional WMA | 61 (29%) | 21 (41%) | 40 (26%) | 0.035 |
| LV diffuse WMA | 33 (16%) | 28 (55%) | 5 (3%) | <0.001 |
| RVEDVi mL/m2 | 83 (74–96) | 84 (70–100) | 83 (74–94) | 0.86 |
| RVESVi mL/m2 | 35 (28–44) | 45 (31–57) | 34 (27–40) | <0.001 |
| RVSV mL | 90 (72–106) | 68 (56–86) | 96 (81–109) | <0.001 |
| RVEF % | 58 (53–63) | 47 (36–56) | 59 (56–60) | <0.001 |
| Edema | 129 (62%) | 19 (37%) | 110 (70%) | <0.001 |
| LV segments with edema | 3 (2–5) | 2 (2–6) | 3 (2–4) | 0.53 |
| Transmural edema | 48 (38%) | 7 (37%) | 41 (38%) | 0.9 |
| EGE | 83 (40%) | 13 (25%) | 70 (45%) | 0.015 |
| LGE | 193 (93%) | 45 (88%) | 147 (94%) | 0.15 |
| LV segments with LGE | 3 (2–5) | 2 (2–4) | 3 (2–5) | 0.24 |
| Transmural LGE | 41 (21%) | 14 (30%) | 27 (18%) | 0.081 |
| LGE mass, g | 3.5 (1.7–7.5) | 3.3 (1.6–7.6) | 3.5 (1.7–7.5) | 0.88 |
| LGE mass, % of LV | 3.0 (1.5–6.1) | 2.5 (1.0–6.2) | 3.2 (1.6–5.9) | 0.29 |
Data are n (%), mean ± SD, and median (IQR). LVEDVi, indexed left ventricular end-diastolic volume; LVESVi, indexed left ventricular end-systolic volume; LVSV, left ventricular stroke volume; LVEF, left ventricular ejection fraction; WMA, wall motion abnormality; RVEDVi, indexed right ventricular end-diastolic volume; RVESVi, indexed right ventricular end-systolic volume; RVSV, right ventricular stroke volume; EGE, early gadolinium enhancement; LGE, late gadolinium enhancement.
In the overall cohort, biventricular volumes and function were preserved on CMR, and there was a positive correlation between LVEF on CMR and on TTE at diagnosis (r = 0.65, p < 0.001). Myocardial edema was found in more than half of patients, affecting more than one LV wall (57%), the lateral wall (29%), the anterior wall (1%), the septum 6%, and the inferior wall (5%). Diffuse edema was present in 2% of cases.
Nearly all patients showed LGE on post-contrast images, with a non-ischemic pattern in 93% of cases. LGE was more commonly found in more than one site (69%) and in the lateral wall (22%), while the remaining walls were less commonly affected (inferior wall 3%, septum 5%, diffuse distribution 1%). Pericardial and pleural effusion were found in 48 (23%) and 37 (18%) patients.
The CMR-based diagnosis was consistent with myocarditis in 78% of patients, dilated cardiomyopathy (DCM) in 11%, structurally normal heart in 3%, ischemic necrosis in 1%, and another final diagnosis in 15 (7%). CMR showed the highest sensitivity (CMR features of myocarditis among EMB-proven myocarditis cases) in diagnosing myocarditis with infarct-like presentation (100%) but lower sensitivity in those with arrhythmias (50%) and heart failure (28%). Only three EMB-proven patients were asymptomatic; CMR did not show myocarditis features in any of them.
Patients presenting with HF (as compared to the remaining population with other types of clinical presentation) had larger LV volumes (LVEDV 132 ± 54 mL/m2 vs. 89 ± 18 mL/m2, p < 0.001; LVESV 92 ± 57 mL/m2 vs. 38 ± 14 mL/m2, p < 0.001) and lower biventricular systolic function (LVEF 35 ± 16% vs. 58 ± 8%, p < 0.001; RVEF 46 ± 14% vs. 59 ± 7, p < 0.001). They more likely showed a diffuse myocardial edema pattern (7% vs. 2%, p = 0.022). LGE was more likely to be absent in patients with HF presentation (LGE absence 17% vs. 5%, p = 0.007) and affected fewer myocardial segments (2.7 ± 1.8 vs. 4.1 ± 3.0, p = 0.014); LGE had a lower extent in HF presentation (3.6 ± 3.9 g vs. 5.9 ± 5.9 g, p = 0.029) and more commonly a diffuse distribution (3% vs. 1%, p = 0.046). Patients with arrhythmic presentation more commonly showed right ventricular LGE (12% vs. 1%, p = 0.001).
3.3. CMR Findings—CS vs. EMB-Proven Cohort
The median time between EMB and CMR was 5 days (2.25–6). A CMR diagnosis of myocarditis was more common among patients with CS myocarditis (87% vs. 47%), while a CMR diagnosis of DCM was more common among EMB-proven myocarditis patients (42% vs. 1%, p < 0.001).
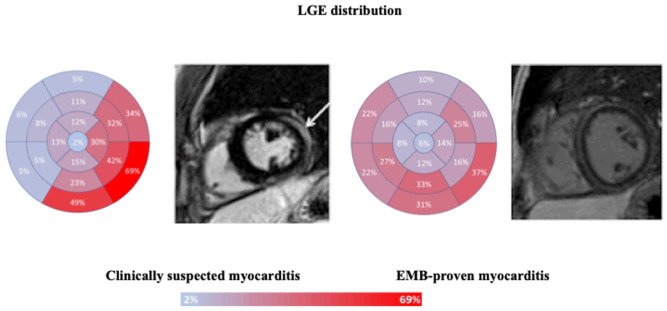
CS myocarditis more commonly showed myocardial edema in the inferior (34% vs. 14%, p = 0.006) and lateral walls (38% vs. 14%, p = 0.001). Despite no difference in LGE prevalence and extent between the two groups, CS myocarditis more frequently had LGE in the lateral (66% vs. 45%, p = 0.008) and inferior walls (61% vs. 41%, p = 0.014), while EMB-proven myocarditis had a more diffuse LGE distribution and a higher prevalence of right ventricular LGE (8% vs. 0, p < 0.001) (Figure 1).
Figure 1.
LGE distribution, according to the American Heart Association bull’s eye plot, in patients with clinically suspected or EMB-proven myocarditis. Focal LGE distribution, mainly located in inferior and lateral walls in clinically suspected myocarditis, as shown by the short axis mid-cavity post-contrast sequence displaying epicardial LGE of the lateral wall (left, white arrow). Diffuse LGE distribution in EMB-proven myocarditis, with evidence of circumferential mid-wall/subepicardial LGE (right).
3.4. Prognosis in the Overall Cohort
Follow-up data were available for 201 patients with a median follow-up of 32 months (IQR 14–61). At the last follow-up, patients with EMB-proven myocarditis, compared to those with CS myocarditis, had worse clinical status and lower LVEF on TTE and were more likely to be on HF and/or anti-arrhythmic therapy (Table 3).
Table 3.
Patients’ characteristics at the last follow-up.
| All n = 201 |
EMB-Proven Myocarditis n = 49 |
Clinically Suspected Myocarditis n = 152 |
p-Value | |
|---|---|---|---|---|
| Duration of the follow-up (months) | 32 (14–61) | 42 (17–64) | 30 (14–57) | 0.16 |
| Dead or transplanted | 1 (0%) | 1 (2%) | 0 (0%) | 0.074 |
| NYHA class, I | 188 (94%) | 40 (85%) | 148 (97%) | 0.001 |
| II–IV | 11 (6%) | 7 (15%) | 4 (3%) | |
| Sinus rhythm | 187 (96%) | 40 (87%) | 147 (99%) | <0.001 |
| Atrial fibrillation | 4 (2%) | 4 (9%) | 0 (0%) | |
| Beta-blockers | 63 (31%) | 33 (67%) | 30 (20%) | 0.002 |
| Ivabradine | 6 (3%) | 4 (8%) | 2 (1%) | 0.014 |
| ACE inhibitors * | 59 (32%) | 20 (44%) | 39 (27%) | 0.033 |
| ARB † | 10 (9%) | 8 (22%) | 2 (3%) | <0.001 |
| Amiodarone | 2 (1%) | 2 (4%) | 0 (0%) | 0.012 |
| Anticoagulants * | 7 (4%) | 7 (16%) | 0 (0%) | <0.001 |
| LV end-diastolic diameter, mm ‡ | 49.4 ± 6.3 | 52.1 ± 8.8 | 48.6 ± 5.1 | 0.005 |
| Left atrial volume, mL/mq § | 23.3 ± 10.1 | 26.0 ± 13.5 | 22.0 ± 7.6 | 0.32 |
| LVEDVi, mL/mq ? | 59 ± 17 | 69 ± 28 | 56 ± 11 | <0.001 |
| LVEF, % # | 62.4 ± 8.1 | 57.4 ± 11.3 | 64.0 ± 6.0 | <0.001 |
| RVEDA, cm ‡ | 18.5 ± 4.6 | 18.8 ± 6.5 | 18.5 ± 3.9 | 0.50 |
| RVFAC, % ** | 48.9 ± 8.0 | 48.3 ± 10.1 | 49.2 ± 7.2 | 0.68 |
Data are n (%), mean ± SD, or median (IQR). ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; for other abbreviations see Table 1. Data available in * 187, † 110, ‡ 189, § 108, ? 195, # 198, ** 188 patients.
One EMB-proven patient underwent a heart transplant, but no patient died. The pre-specified composite outcome was met in 17 patients. As shown in Table 4, across each time point at follow-up, larger biventricular volumes and diffuse wall motion abnormalities at diagnosis increased the risk of the end-point, while higher baseline biventricular systolic function was associated with favorable outcomes.
Table 4.
Univariate Cox-predictors of the surrogate end-point at different follow-up visits.
| 0–6 Months | 6–12 Months | 12–24 Months | >24 Months | Any Time Point | |
|---|---|---|---|---|---|
| Number of Events | 10 | 9 | 9 | 3 | 17 |
| LVEDVi mL/m2 | HR 1.01 (95% CI 1.01–1.02, p < 0.001) | HR 1.02 (95% CI 1.01–1.03, p < 0.001) | HR 1.02 (95% CI 1.01–1.03, p < 0.001) | HR 1.05 (95% CI 1.01–1.08, p = 0.006) | HR 1.02 (95% CI 1.02–1.03, p < 0.001) |
| LVESVi mL/m2 | HR 1.01 (95%CI 1.01–1.02, p < 0.001) | HR 1.01 (95% CI 1.01–1.02, p < 0.001) | HR 1.02 (95% CI 1.01–1.03, p < 0.001) | HR 1.06 (95% CI 1.01–1.12, p = 0.013) | HR 1.02 (95% CI 1.02–1.03, p < 0.001) |
| LVEF % | HR 0.93 (95% CI 0.89–0.97, p < 0.001) | HR 0.92 (95% CI 0.88–0.96, p < 0.001) | HR 0.91 (95% CI 0.86–0.97, p = 0.002) | HR 0.90 (95% CI 0.82–0.98, p = 0.013) | HR 0.89 (95% CI 0.88–0.94, p < 0.001) |
| LV mass, g/m2 | NS | HR 1.02 (95% CI 1.00–1.04, p = 0.027) | HR 1.03 (95% CI 1.01–1.04, p = 0.005) | NS | HR 1.04 (95% CI 1.02–1.05, p < 0.001) |
| LV regional WMA | NS | NS | NS | NS | NS |
| LV diffuse WMA | HR 10.8 (95% CI 2.26–51.3, p = 0.003) | HR 7.62 (95% CI 1.87–30.7, p = 0.004) | HR 6.79 (95% CI 1.12–41.3, p = 0.037) | NS | HR 22.4 (95% CI 6.41–78, p < 0.001) |
| RVEDVi mL/m2 | HR 1.03 (95% CI 1.01–1.06, p = 0.002) | HR 1.04 (95% CI 1.02–1.07, p < 0.001) | HR 1.06 (95% CI 1.03–1.08, p < 0.001) | HR 1.07 (95% CI 1.00–1.14, p = 0.038) | HR 1.04 (95% CI 1.02–1.06, p < 0.001) |
| RVESVi mL/m2 | HR 1.04 (95% CI 1.02–1.06, p < 0.001) | HR 1.03 (95% CI 1.03–1.07, p < 0.001) | HR 1.05 (95% CI 1.03–1.07, p < 0.001) | HR 1.10 (95% CI 1.02–1.19, p = 0.015) | HR 1.04 (95% CI 1.03–1.06, p < 0.001) |
| RVEF % | HR 0.9 (95%CI 0.86–0.95, p < 0.001) | HR 0.90 (95% CI 0.86–0.94, p < 0.001) | HR 0.90 (95% CI 0.85–0.95, p < 0.001) | HR 0.88 (95% CI 0.80–0.98, p = 0.014) | HR 0.91 (95% CI 0.88–0.94, p < 0.001) |
| Edema | NS | NS | NS | NS | NS |
| Transmural edema | NS | NS | NS | NS | NS |
| EGE | NS | NS | NS | NS | NS |
| LGE | NS | NS | NS | NS | NS |
| LGE > 1 wall | NS | HR 0.21 (95% CI 0.05–0.86, p = 0.029) | NS | NS | HR 0.03 (95% CI 0.01–0.13, p < 0.001) |
| Transmural LGE | NS | HR 7.26 (95% CI 1.84–28.7, p = 0.005) | NS | NS | NS |
| LGE mass, g | NS | NS | NS | NS | NS |
| LGE mass, % of LV | NS | NS | NS | NS | HR 0.73 (95% CI 0.55–0.97, p = 0.033) |
For abbreviations, see Table 2. NS, non-significant.
While edema and EGE at baseline were never associated with the end-point, transmural LGE was associated with the end-point at 6–12 months follow-up. Pericardial and pleural effusion were associated with the end-point at each time point, while an epicardial stria of LGE showed a protective effect both at 6–12 months (HR 0.04, 95% CI 0–0.68, p = 0.026) and at “any time point” (HR 0.07, 95% CI 0.01–0.53, p = 0.01). CMR diagnoses of DCM and of ischemic LGE were strongly associated with the end-point (0–6 months: DCM HR 14.8, 95% CI 1.81–122, p = 0.012, ischemic LGE HR 51.8, 95% CI 3.05–878, p = 0.006; at any time point: DCM HR 40.7, 95% CI 5.31–312, p < 0.001, ischemic LGE HR 56.4, 95% CI 3.41–932, p = 0.005).
4. Discussion
4.1. Diagnostic Role of CMR in Suspected Myocarditis
CMR confirmed the clinical suspicion of myocarditis in 78% of our overall cohort, but in 53% of the biopsy-proven patients, CMR failed to confirm the diagnosis, mainly reporting DCM features, as previously described [25]. In keeping with previous findings from Francone et al. [25], in our cohort, CMR showed the highest sensitivity in patients with infarct-like presentation. This may reflect a higher accuracy of the old Lake Louise CMR criteria in detecting focal myocardial disease, typical of infarct-like presentation, as compared to diffuse myocardial processes, more common in myocarditis presenting as HF [8]. Indeed, in our cohort, EMB-proven myocarditis patients with HF were more likely to have diffuse edema and LGE, as compared to patients with an infarct-like presentation, who mainly showed edema and LGE of the inferior and lateral LV walls. Moreover, the frequency of edema was lower in our EMB-proven patients, suggesting a possible underestimation of this feature by standard T2-weighted sequences. To overcome some of these limitations, the revised Lake Louise criteria now include parametric mapping that does not rely on the presence of remote, normal myocardium [8] and is ideal for detecting diffusely diseased myocardium. Preliminary data are promising in this regard [26]; in a cohort of 102 patients with suspected myocarditis, of whom 30 were biopsy-proven, the addition of parametric mapping increased the diagnostic accuracy of CMR in patients with heart failure while showing no significant diagnostic improvement in those with infarct-like or arrhythmic presentation. Interestingly, this was also confirmed when considering only patients with a biopsy-proven diagnosis.
4.2. CMR Features in EMB-Proven versus CS Myocarditis
In our study, EMB-proven myocarditis showed more advanced reverse remodeling and lower biventricular systolic function, likely reflecting the more frequent use of EMB in most severe presentations [2,5,6,27]. Although we confirmed in the overall cohort a high prevalence of edema and LGE involving three LV myocardial segments [9,15,16], we found no difference in LGE frequency and extent between CS and EMB-proven groups.
However, LGE was less frequent among our EMB-proven patients with HF, and its extent was lower as compared to those without HF presentation. This is in keeping with a previous study on CS myocarditis describing higher LGE prevalence and a higher extent among patients with infarct-like as compared to those without infarct-like presentation [13]. In our cohort, right ventricular LGE was only found in EMB-proven myocarditis less frequently than previously reported [28], possibly due to the higher prevalence of CS myocarditis with an infarct-like presentation in our cohort. Our patients with an arrhythmic presentation more commonly had RV LGE on CMR, in keeping with the known arrhythmogenicity of right ventricular scars in different non-ischemic cardiomyopathies [29,30].
4.3. Prognostic Role of CMR in Myocarditis
The main prognostic implication of our study is that in the overall cohort, higher biventricular systolic function and greater LGE extent on CMR at diagnosis predict more favorable outcomes in myocarditis. Conversely, larger biventricular volumes, CMR-based DCM features, and the presence of an ischemic LGE pattern at diagnosis were predictors of worse outcomes. The very low rate of major adverse cardiovascular events (MACE) in our cohort (only one patient underwent a heart transplant and no one died) might relate to the relatively preserved LVEF at baseline in the CS cohort, to a potential selection bias as the more severely ill patients, known to have a worse outcome, may not receive CMR and to the favorable effect of immunosuppression in the biopsy-proven group by improving patients’ clinical status and left ventricular function. Indeed, left and/or right ventricular dysfunction at diagnosis is known to be the main predictor of dismal prognosis in myocarditis [1,2,3,11,17,31,32,33,34] and of lower LVEF at follow-up, in keeping with our findings. In addition, autoimmune etiopathogenesis was associated with worse outcomes in the pre-immunosuppression era [35], and immunosuppression has a beneficial effect on LVEF and life-threatening arrhythmia, both surrogate end-points of outcome [36,37,38,39].
The most novel finding of our study was that the extent of myocardial LGE on CMR at diagnosis predicted more favorable outcomes when assessed at any time point of follow-up. It is well known that LGE represents expanded interstitial space, rather than peremptorily indicating irreversibly damaged myocardium [8], and, especially in the acute setting, it may also represent myocardial edema, as evidenced by complete LGE resolution on follow-up CMR in some myocarditis cases [40]. The protective role of LGE in our cohort may, therefore, reflect the presence or co-existence of myocardial edema, a marker of a reversible process, thus explaining the better outcome, leading to higher LVEF and better functional status at follow-up. Edema did not correlate with outcome in our cohort, but this may be due to the known lower sensitivity of T2w sequences to detect edema, as compared to other sequences (i.e., T2 mapping). Moreover, the non-ischemic distribution of LGE typical of myocarditis patients, which spares the sub-endocardium, may as well explain why our myocarditis patients with non-ischemic LGE at baseline CMR showed higher LVEF at follow-up, as opposed to the negative predictive role of ischemic LGE on follow-up LVEF in our cohort, in keeping with previous studies [13]. It has to be reminded, among the possible explanations, that in the absence of EMB confirmation, an infarct-like LGE pattern, rather than reflecting myocarditis, may represent myocardial infarction with non-obstructive coronary arteries, a clinical entity known to have a worse outcome. The prognostic role of LGE in myocarditis is presently unclear. Two recent meta-analyses on more than 2000 patients with CS myocarditis reported the association of LGE and reduced LVEF with worse outcomes [41,42], although it is unclear whether LGE had an independent prognostic role over LVEF. Similarly, a study on 670 patients with suspected myocarditis and mildly impaired LVEF (50%) found that LGE, especially septal and mid-wall, doubled the risk of MACE [12]; however, among patients with LVEF < 40% (who had a worse outcome as compared to those with higher LVEF), LGE presence did not provide additional prognostic stratification. Therefore, the univocal, independent prognostic role of LGE over LVEF is unclear. The ITAMY study also found that anteroseptal LGE predicted worse outcomes in infarct-like CS myocarditis with normal LVEF [9]. On the other hand, in keeping with our findings, other studies have found that LGE does not add prognostic value to reduced baseline LVEF [10,16], although end-points were different. A recent study has also shown low long-term sudden cardiac death risk in patients with non-ischemic LGE and normal LV volumes and LVEF, with mortality mainly driven by age and not by LGE presence, location, or extent [43]. What seems to emerge from available research studies is that the prognostic capability of imaging features in myocarditis may rather derive from their incremental value when considered together than considering each of them separately; in a recent study on 455 patients with myocarditis (defined according to ESC criteria [1]), the addition of imaging markers, such as LVEF, LGE (when considering both its extent and location, i.e., mid-wall distribution), and left ventricular strain, to clinical characteristics and symptoms improved the overall prognostic accuracy [44]. It may be supposed that the heterogeneous nature of myocarditis may also account for heterogeneity in its risk predictors.
As previously reported, in our cohort, myocardial edema on CMR at diagnosis did not show a prognostic association with the outcome, in keeping with previous studies [45,46], and this may be due to the reversible myocardial injury that edema represents. Other studies have instead shown the negative prognostic role of persisting edema on follow-up CMR [40], likely as an expression of ongoing myocardial inflammation and, therefore, of an active disease process. The advent of parametric mapping has shown the incremental prognostic role of T2 mapping in risk stratification, as compared to standard T2-weighted sequences that are subject to artifact and may therefore be less sensitive [45]. Moreover, both T1 and T2 mapping appeared promising as tools to monitor the inflammatory state [46].
4.4. Limitations
We acknowledge that EMB was performed as clinically indicated, according to current expert position papers [4,5,6], only in a minority of patients; however, the remaining clinically suspected myocarditis cases were strictly defined following the 2013 ESC consensus criteria [1]. We, therefore, believe that our cohort represents a real-life description of myocarditis patients. CMR was performed at the clinician’s discretion in hemodynamically stable patients, potentially introducing a selection bias, as patients with worse presentations are less likely to receive CMR. The higher prevalence of infarct-like CS myocarditis, with a more favorable disease course, the absence of hard MACE at follow-up, and the small number of patients meeting the surrogate end-point may have blunted the additional predictive role of CMR features. Moreover, the EMB-proven group had more diffuse myocardial involvement, which is less accurately detected by standard CMR tissue characterization sequences, and parametric mapping sequences were not systematically acquired in our cohort. Although the prognostic value of parametric mapping sequences has been less explored, T2 mapping has been shown to have a role in predicting MACE and HF hospitalization in patients with myocarditis [45], as long as an extracellular volume that, when increased, portends a worse prognosis, irrespective of the presence of LGE [47]; further studies are therefore needed to clarify the role of parametric mapping as a risk stratification tool. We used the FWHM method to quantify LGE extent, and we cannot exclude that results may have been different by using alternative LGE quantification methods (i.e., n-Standard Deviation techniques); however, the FWHM method has shown to be the optimal semi-automated quantification method in risk-stratifying patients with suspected myocarditis, showing the strongest association with MACE and the highest technical consistency [19]. Finally, a follow-up CMR could have provided further insights into its prognostic role [40].
5. Conclusions
In our single-center cohort of rigorously defined myocarditis patients, higher biventricular systolic function and greater LGE extent on CMR at diagnosis identified patients with better functional class and higher left ventricular ejection fraction at follow-up. Conversely, larger biventricular volumes, CMR-based DCM features, and the presence of an ischemic LGE pattern at diagnosis were predictors of worse functional class and LV systolic dysfunction at follow-up. Larger prospective studies are warranted to extend our findings to multi-center cohorts.
Author Contributions
Conceptualization, A.B., R.M. (Renzo Marcolongo) and A.L.P.C.; methodology, A.B., C.-Y.C., G.S., R.M. (Renz Marcolongo) and A.L.P.C.; formal analysis, H.O., G.L. and D.G.; data curation, C.-Y.C., G.S. and A.S.G.; writing—original draft preparation, A.B., C.-Y.C. and G.S.; writing—review and editing, C.B., S.R., M.D.G., R.M. (Raffaella Motta), G.D.C., M.P.M., G.T., S.I. and R.M. (Renzo Marcolongo). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by Comitato Etico per la Sperimentazione Clinica della Provincia di Padova, protocol code RF-2019-12370183, study protocol number 0021857, date of approval 2 April 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data will be shared upon reasonable request to the Corresponding Author.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
A.L.P.C. acknowledges the support of Budget Integrato per la Ricerca dei Dipartimenti (BIRD, year 2019), Padova University, Padova, Italy (project Title: Myocarditis: genetic background, predictors of dismal prognosis and of response to immunosuppressive therapy) and of the Italian Ministry of Health, Target Research, Rome, Italy, year 2019, RF-2019-12370183 (project Title: Biopsy-Proven Myocarditis: Genetic Background, Predictors of Dismal Prognosis and of Response To Immunosuppressive Therapy And Preclinical Evaluation of Innovative Immunomodulatory Therapies).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Caforio A.L., Pankuweit S., Arbustini E., Basso C., Gimeno-Blanes J., Felix S.B., Fu M., Heliö T., Heymans S., Jahns R., et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
- 2.Tschöpe C., Ammirati E., Bozkurt B., Caforio A.L.P., Cooper L.T., Felix S.B., Hare J.M., Heidecker B., Heymans S., Hübner N., et al. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat. Rev. Cardiol. 2021;18:169–193. doi: 10.1038/s41569-020-00435-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinagra G., Anzini M., Pereira N.L., Bussani R., Finocchiaro G., Bartunek J., Merlo M. Myocarditis in clinical practice. Mayo. Clin. Proc. 2016;91:1256–1266. doi: 10.1016/j.mayocp.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Cooper L.T., Baughman K.L., Feldman A.M., Frustaci A., Jessup M., Kuhl U., Levine G.N., Narula J., Starling R.C., Towbin J., et al. The role of endomyocardial biopsy in the management of cardiovascular disease: A scientific statement from American Heart Association, the American College of Cardiology, and the European Society of Cardiology. J. Am. Coll. Cardiol. 2007;50:1914–1931. doi: 10.1016/j.jacc.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Seferović P.M., Tsutsui H., McNamara D.M., Ristić A.D., Basso C., Bozkurt B., Cooper L.T., Jr., Filippatos G., Ide T., Inomata T., et al. Heart Failure Association of the ESC, Heart Failure Society of America and Japanese Heart Failure Society Position statement on endomyocardial biopsy. Eur. J. Heart Fail. 2021;23:854–871. doi: 10.1002/ejhf.2190. [DOI] [PubMed] [Google Scholar]
- 6.McDonagh T.A., Metra M., Adamo M., Gardner R.S., Baumbach A., Böhm M., Burri H., Butler J., Čelutkienė J., Chioncel O., et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 7.Friedrich M.G., Sechtem U., Schulz-Menger J., Holmvang G., Alakija P., Cooper L.T., White J.A., Abdel-Aty H., Gutberlet M., Prasad S., et al. Cardiovascular Magnetic Resonance in Myocarditis: A JACC White Paper. J. Am. Coll. Cardiol. 2009;53:1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferreira V.M., Schulz-Menger J., Holmvang G., Kramer C.M., Carbone I., Sechtem U., Kindermann I., Gutberlet M., Cooper L.T., Liu P., et al. Cardiovascular Magnetic Resonance in nonischemic myocardial inflammation: Expert Recommendations. J. Am. Coll. Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 9.Aquaro G.D., Perfetti M., Camastra G., Monti L., Dellegrottaglie S., Moro C., Pepe A., Todiere G., Lanzillo C., Scatteia A., et al. Cardiac MR with late gadolinium enhancement in acute myocarditis with preserved systolic function. ITAMY study. J. Am. Coll. Cardiol. 2017;70:1977–1987. doi: 10.1016/j.jacc.2017.08.044. [DOI] [PubMed] [Google Scholar]
- 10.Schumm J., Greulich S., Wagner A., Grün S., Ong P., Bentz K., Klingel K., Kandolf R., Bruder O., Schneider S., et al. Cardiovascular magnetic resonance risk stratification in patients with clinically suspected myocarditis. J. Cardiovasc. Magn. Reson. 2014;16:14. doi: 10.1186/1532-429X-16-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anzini M., Merlo M., Sabbadini G., Barbati G., Finocchiaro G., Pinamonti B., Salvi A., Perkan A., Di Lenarda A., Bussani R., et al. Long-term evolution and prognostic stratification of biopsy-proven active myocarditis. Circulation. 2013;128:2384–2394. doi: 10.1161/CIRCULATIONAHA.113.003092. [DOI] [PubMed] [Google Scholar]
- 12.Gräni C., Eichhorn C., Bière L., Murthy V.L., Agarwal V., Kaneko K., Cuddy S., Aghayev A., Steigner M., Blankstein R., et al. Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J. Am. Coll. Cardiol. 2017;70:1964–1976. doi: 10.1016/j.jacc.2017.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chopra H., Arangalage D., Bouleti C., Zarka S., Fayard F., Chillon S., Laissy J.-P., Henry-Feugeas M.-C., Steg P.-G., Vahanian A., et al. Prognostic value of the infarct- and non-infarct like patterns and cardiovascular magnetic resonance parameters on long-term outcome of patients after acute myocarditis. Int. J. Cardiol. 2016;212:63–69. doi: 10.1016/j.ijcard.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Grün S., Schumm J., Greulich S., Wagner A., Schneider S., Bruder O., Kispert E.-M., Hill S., Ong P., Klingel K., et al. Long-term follow-up of biopsy-proven viral myocarditis. Predictors of mortality and incomplete recovery. J. Am. Coll. Cardiol. 2012;59:1604–1615. doi: 10.1016/j.jacc.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Pommier T., Leclercq T., Guenancia C., Tisserand S., Lairet C., Carré M., Lalande A., Bichat F., Maza M., Zeller M., et al. More than 50% of persistent myocardial scarring at one year in “infarct-like” acute myocarditis evaluated by CMR. J. Clin. Med. 2021;10:4677. doi: 10.3390/jcm10204677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanguineti F., Garot P., Mana M., O’h-Ici D., Hovasse T., Unterseeh T., Louvard Y., Troussier X., Morice M.-C., Garot J. Cardiovascular magnetic resonance predictors of clinical outcome in patients with suspected acute myocarditis. J. Cardiovasc. Magn. Reson. 2015;17:78. doi: 10.1186/s12968-015-0185-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caforio A.L., Calabrese F., Angelini A., Tona F., Vinci A., Bottaro S., Ramondo A., Carturan E., Iliceto S., Thiene G., et al. A prospective study of biopsy-proven myocarditis: Prognostic relevance of clinical and aetiopathogenetic features at diagnosis. Eur. Heart J. 2007;28:1326–1333. doi: 10.1093/eurheartj/ehm076. [DOI] [PubMed] [Google Scholar]
- 18.Merlo M., Ammirati E., Gentile P., Artico J., Cannatà A., Finocchiaro G., Barbati G., Sormani P., Varrenti M., Perkan A., et al. Persistent left ventricular dysfunction after acute lymphocytitc myocarditis: Frequency and predictors. PLoS ONE. 2019;14:e0214616. doi: 10.1371/journal.pone.0214616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gräni C., Eichhorn C., Bière L., Kaneko K., Murthy V.L., Agarwal V., Aghayev A., Steigner M., Blankstein R., Jerosch-Herold M., et al. Comparison of myocardial fibrosis quantification methods by cardiovascular magnetic resonance imaging for risk stratification of patients with suspected myocarditis. J. Cardiovasc. Magn. Reson. 2019;21:14. doi: 10.1186/s12968-019-0520-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulz-Menger J., Bluemke D.A., Bremerich J., Flamm S.D., Fogel M.A., Friedrich M.G., Kim R.J., von Knobelsdorff-Brenkenhoff F., Kramer C.M., Pennell D.J., et al. Standardized image interpretation and post-processing in cardiovascular magnetic resonance–2020 update. J. Cardiovasc. Magn. Reson. 2020;22:19. doi: 10.1186/s12968-020-00610-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.R Development Core Team 3.0.1 . A Language and Environment for Statistical Computing [Internet] Volume 2. R Foundation for Statistical Computing; Vienna, Austria: 2019. [(accessed on 1 December 2020)]. Available online: https://www.R-project.org. [Google Scholar]
- 22.Harrell F.E., Jr. rms: Regression Modeling Strategies. 2019. [(accessed on 1 December 2020)]. pp. 1–246. Available online: https://cran.r-project.org/web/packages/rms/rms.pdf.
- 23.Therneau T.M., Grambsch P.M. Modeling Survival Data: Extending the Cox Model. Springer; Berlin/Heidelberg, Germany: 2000. 350p [Google Scholar]
- 24.Alboukadel K., Marcin K., Przemyslaw B., Scheipl F. Drawing Survival Curves Using ‘ggplot2’. R Foundation for Statistical Computing; Vienna, Austria: 2018. R Package Survminer Version 0.4.3. [Google Scholar]
- 25.Francone M., Chimenti C., Galea N., Scopelliti F., Verardo R., Galea R., Carbone I., Catalano C., Fedele F., Frustaci A. CMR sensitivity varies with clinical presentation and extent of cell necrosis in ciopsy-proven acute myocarditis. J. Am. Coll. Cardiol. 2014;7:254–263. doi: 10.1016/j.jcmg.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Cundari G., Galea N., De Rubeis G., Frustaci A., Cilia F., Mancuso G., Marchitelli L., Catapano F., Carbone I., Catalano C., et al. Use of the new Lake Louise Criteria improves CMR detection of atypical forms of acute myocarditis. Int. J. Cardiovasc. Imaging. 2021;37:1395–1404. doi: 10.1007/s10554-020-02097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bozkurt B., Colvin M., Cook J., Cooper L.T., Deswal A., Fonarow G.C., Francis G.S., Lenihan D., Lewis E.F., McNamara D.M., et al. Current Diagnostic and Treatment Strategies for Specific Dilated Cardiomyopathies: A Scientific Statement From the American Heart Association. Circulation. 2016;134:e579–e646. doi: 10.1161/CIR.0000000000000455. [DOI] [PubMed] [Google Scholar]
- 28.Aquaro G.D., Negri F., De Luca A., Todiere G., Bianco F., Barison A., Camastra G., Monti L., Dellegrottaglie S., Moro C., et al. Role of right ventricular involvement in acute myocarditis, assessed by cardiac magnetic resonance. Int. J. Cardiol. 2018;271:359–365. doi: 10.1016/j.ijcard.2018.04.087. [DOI] [PubMed] [Google Scholar]
- 29.Corrado D., Basso C., Leoni L., Tokajuk B., Turrini P., Bauce B., Migliore F., Pavei A., Tarantini G., Napodano M., et al. Three-dimensional electroanatomic voltage mapping and histologic evaluation of myocardial substrate in right ventricular outflow tract tachycardia. J. Am. Coll. Cardiol. 2008;51:731–739. doi: 10.1016/j.jacc.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 30.Basso C. Arrhythmogenic right ventricular cardiomyopathy. Lancet. 2009;373:1289–1300. doi: 10.1016/S0140-6736(09)60256-7. [DOI] [PubMed] [Google Scholar]
- 31.Ammirati E., Cipriani M., Lilliu M., Sormani P., Varrenti M., Raineri C., Petrella D., Garascia A., Pedrotti P., Roghi A., et al. Survival and Left Ventricular Function Changes in Fulminant Versus Nonfulminant Acute Myocarditis. Circulation. 2017;136:529–545. doi: 10.1161/CIRCULATIONAHA.117.026386. [DOI] [PubMed] [Google Scholar]
- 32.Ammirati E., Cipriani M., Moro C., Raineri C., Pini D., Sormani P., Mantovani R., Varrenti M., Pedrotti P., Conca C., et al. Clinical Presentation and Outcome in a Contemporary Cohort of Patients With Acute Myocarditis: Multicenter Lombardy Registry. Circulation. 2018;138:1088–1099. doi: 10.1161/CIRCULATIONAHA.118.035319. [DOI] [PubMed] [Google Scholar]
- 33.Ammirati E., Veronese G., Brambatti M., Merlo M., Cipriani M., Potena L., Sormani P., Aoki T., Sugimura K., Sawamura A., et al. Fulminant Versus Acute Nonfulminant Myocarditis in Patients With Left Ventricular Systolic Dysfunction. J. Am. Coll. Cardiol. 2019;74:299–311. doi: 10.1016/j.jacc.2019.04.063. [DOI] [PubMed] [Google Scholar]
- 34.Verdonschot J.A., Merlo M., Dominguez F., Wang P., Henkens M.T., Adriaens M.E., Hazebroek M.R., Mase M., Escobar L.E., Cobas-Paz R., et al. Phenotypic clustering of dilated cardiomyopathy patients highlights important pathophysiological differences. Eur. Heart J. 2021;42:162–174. doi: 10.1093/eurheartj/ehaa841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baritussio A., Schiavo A., Basso C., Giordani A.S., Cheng C., Pontara E., Cattini M.G., Bison E., Gallo N., De Gaspari M., et al. Predictors of relapse, death or heart transplantation in myocarditis before the introduction of immunosuppression: Negative prognostic impact of female gender, fulminant onset, lower ejection fraction and serum autoantibodies. Eur. J. Heart Fail. 2022;24:1033–1044. doi: 10.1002/ejhf.2496. [DOI] [PubMed] [Google Scholar]
- 36.Merken J., Hazebroek M., Van Paassen P., Verdonschot J., Van Empel V., Knackstedt C., Hamid M.A., Seiler M., Kolb J., Hoermann P., et al. Immunosuppressive Therapy Improves Both Short- and Long-Term Prognosis in Patients With Virus-Negative Nonfulminant Inflammatory Cardiomyopathy. Circ. Heart Fail. 2018;11:e004228. doi: 10.1161/CIRCHEARTFAILURE.117.004228. [DOI] [PubMed] [Google Scholar]
- 37.Frustaci A., Russo M.A., Chimenti C. Randomized study on the efficacy of immunosuppressive therapy in patients with virus-negative inflammatory cardiomyopathy: The TIMIC study. Eur. Heart J. 2009;30:1995–2002. doi: 10.1093/eurheartj/ehp249. [DOI] [PubMed] [Google Scholar]
- 38.Chimenti C., Russo M.A., Fristaci A. Immunosuppressive therapy in virus-negative inflammatory cardiomyopathy: 20-year follow-up of the TIMIC trial. Eur. Heart J. 2022;43:3463–3473. doi: 10.1093/eurheartj/ehac348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peretto G., Sala S., De Luca G., Marcolongo R., Campochiaro C., Sartorelli S., Tresoldi M., Foppoli L., Palmisano A., Esposito A., et al. Immunosuppressive therapy and risk stratification of patients with myocarditis presenting with ventricular arrhythmias. JACC Clin. Electrophysiol. 2020;6:1221–1234. doi: 10.1016/j.jacep.2020.05.013. [DOI] [PubMed] [Google Scholar]
- 40.Aquaro G.D., Habtemicael Y.G., Camastra G., Monti L., Dellegrottaglie S., Moro C., Lanzillo C., Scatteia A., Di Roma M., Pontone G., et al. Prognostic value of repeating cardiac magnetic resonance in patients with acute myocarditis. J. Am. Coll. Cardiol. 2019;74:2339–2348. doi: 10.1016/j.jacc.2019.08.1061. [DOI] [PubMed] [Google Scholar]
- 41.Georgiopoulos G., Figliozzi S., Sanguineti F., Aquaro G.D., di Bella G., Stamatelopoulos K., Chiribiri A., Garot J., Masci P.G., Ismail T.F. Prognostic impact of late gadolinium enhancement by cardiovascular magnetic resonance in myocarditis. A systematic review and meta-analysis. Circ. Cardiovasc. Imaging. 2021;14:e011492. doi: 10.1161/CIRCIMAGING.120.011492. [DOI] [PubMed] [Google Scholar]
- 42.Blissett S., Chocron Y., Kovacina B., Afilalo J. Diagnostic and prognostic value of cardiac magnetic resonance in acute myocarditis: A systematic review and meta-analysis. Int. J. Cardiovasc. Imaging. 2019;35:2221–2229. doi: 10.1007/s10554-019-01674-x. [DOI] [PubMed] [Google Scholar]
- 43.Lota A.S., Tsao A., Owen R., Halliday B.P., Auger D., Vassiliou V.S., Tayal U., Almogheer B., Vilches S., Al-Balah A., et al. Prognostic Significance of Nonischemic Myocardial Fibrosis in Patients With Normal LV Volumes and Ejection-Fraction. J. Am. Coll. Cardiol. Imaging. 2021;14:2353–2365. doi: 10.1016/j.jcmg.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fischer K., Obrist S.J., Erne S.A., Stark A.W., Marggraf M., Kaneko K., Guensch D.P., Huber A.T., Greulich S., Aghayev A., et al. Feature tracking myocardial strain incrementally improves prognostication in myocarditis beyond traditional CMR imaging features. J. Am. Coll. Cardiol. Imaging. 2020;13:1891–1901. doi: 10.1016/j.jcmg.2020.04.025. [DOI] [PubMed] [Google Scholar]
- 45.Spieker M., Haberkorn S., Gastl M., Behm P., Katsianos S., Horn P., Jacoby C., Schnackenburg B., Reinecke P., Kelm M., et al. Abnormal T2 mapping cardiovascular magnetic resonance correlates with adverse clinical outcome in patients with suspected acute myocarditis. J. Cardiovasc. Magn. Reson. 2017;19:38. doi: 10.1186/s12968-017-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bohnen S., Radunski U., Lund G., Ojeda F., Looft Y., Senel M., Radziwolek L., Avanesov M., Tahir E., Stehning C., et al. Tissue characterization by T1 and T2 mapping cardiovascular magnetic resonance imaging to monitor myocardial inflammation and healing. Eur. Heart J.-Cardiovasc. Imaging. 2017;18:744–751. doi: 10.1093/ehjci/jex007. [DOI] [PubMed] [Google Scholar]
- 47.Gräni C., Bière L., Eichhorn C., Kaneko K., Agarwal V., Aghayev A., Steigner M., Blankstein R., Jerosch-Herold M., Kwong R.Y. Incremental value of extracellular volume assessment by cardiovascular magnetica resonance imaging in risk stratifying patients with suspected myocarditis. Int. J. Cardiovasc. Imaging. 2019;35:1067–1078. doi: 10.1007/s10554-019-01552-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be shared upon reasonable request to the Corresponding Author.