Abstract
Increasing studies have revealed the importance of mechanical cues in tumor progression, invasiveness and drug resistance. During malignant transformation, changes manifest in either the mechanical properties of the tissue or the cellular ability to sense and respond to mechanical signals. The major focus of the review is the subtle correlation between mechanical cues and apoptosis in tumor cells from a mechanobiology perspective. To begin, we focus on the intracellular force, examining the mechanical properties of the cell interior, and outlining the role that the cytoskeleton and intracellular organelle-mediated intracellular forces play in tumor cell apoptosis. This article also elucidates the mechanisms by which extracellular forces guide tumor cell mechanosensing, ultimately triggering the activation of the mechanotransduction pathway and impacting tumor cell apoptosis. Finally, a comprehensive examination of the present status of the design and development of anti-cancer materials targeting mechanotransduction is presented, emphasizing the underlying design principles. Furthermore, the article underscores the need to address several unresolved inquiries to enhance our comprehension of cancer therapeutics that target mechanotransduction.
Keywords: mechanotransduction, cancer cell, apoptosis, mechanical stimulation, anticancer materials
Graphical Abstract
Introduction
Cells are highly sensitive to mechanical signals from the extracellular microenvironment, as to chemical signals. Cells can maintain their mechanical stability through tensegrity structures [1]. The linker of nucleoskeleton and cytoskeleton (LINC) complex-cytoskeleton-focal adhesion-extracellular matrix (ECM) connection facilitates the transmission of extracellular mechanical information, including external stresses such as shear stress, circumferential stretch, ECM ligation and interstitial pressure, as well as intrinsic stresses resulting from cellular tractions through adhesions with neighboring cells and the ECM. This transmission occurs through mechano-chemical transduction, ultimately influencing cell activation, transcription regulation and metabolism. During this process, effector molecules, such as Yes-associated protein (YAP) and tafazzin protein (TAZ), may become activated, rendering cells more adaptive to survival in complex mechanical environments or expediting cell death [2, 3].
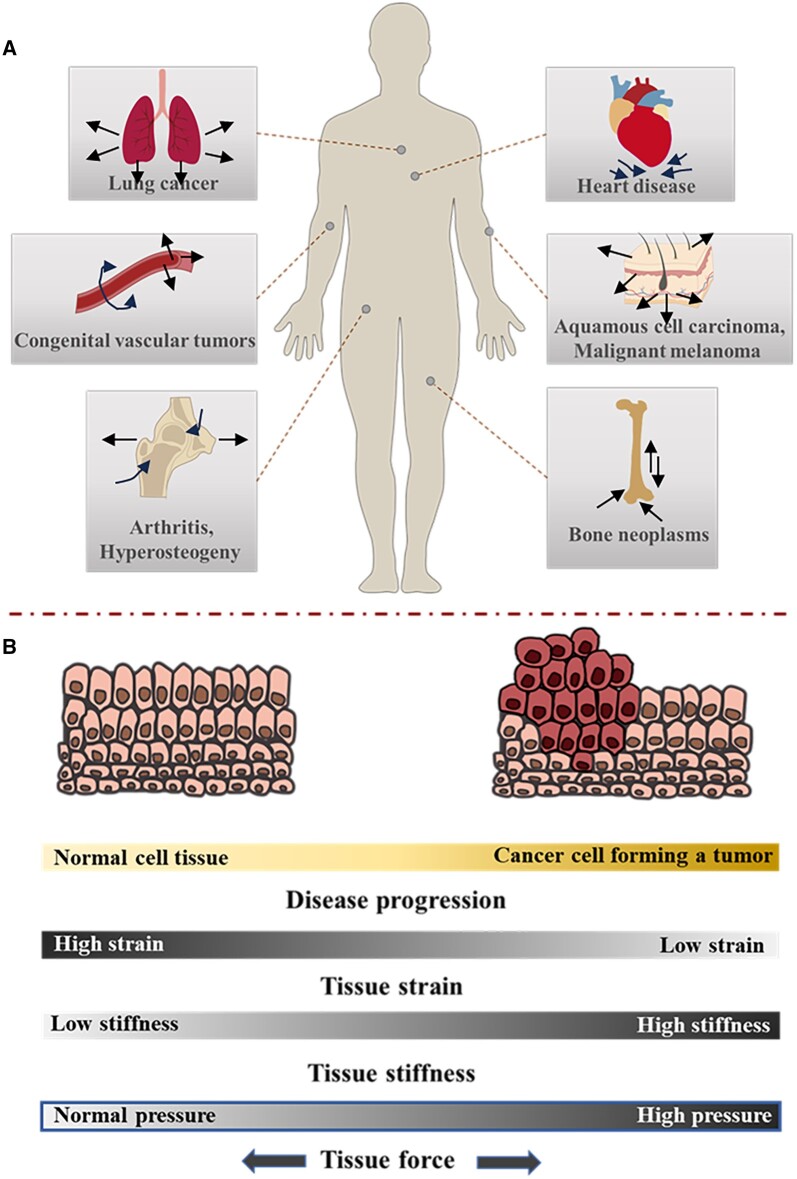
How cells sense and respond to the mechanical signals of the extracellular microenvironment are closely related to the occurrence and development of various cancers and diseases [2] (Figure 1). The viscoelasticity of cells is expected to undergo adaptive alterations in response to the mechanical properties of the ECM. For instance, non-malignant mammary epithelial cells grown in a 3D laminin ECM show their viscoelasticity similar to that of the local ECM, while breast cancer cells cultured in these environments are more rigid [4]. Several changes occur in the microenvironment during tumor development, including the significant increases in matrix stiffness, interstitial flow shear force, interstitial fluid pressure and solid stress of tumor growth. These mechanical changes are mutually associated with the occurrence and progression of cancer [5]. Meanwhile, the change in the tumor microenvironment affects the behavior of normal cells around the tumor, such as fibroblasts and macrophages, further reshaping the ECM of tumor cells and promoting tumor growth and invasion.
Figure 1.
Mechanical stimuli in mechano-associated cancer and diseases in the human body. Cells in the different tissues of the human body experience mechanical stimuli and deformation. (A) There are examples of common forces, stresses, loads, and deformations that are shown, as well as possible mechano-associated diseases. (B) The conversion of a normal cell to a cancer cell is frequent along with alterations in mechanical properties.
Tumor development can be counteracted by the stimulation of apoptosis or other programmed cell death [6–8]. Considering that cancer is marked by uncontrolled and uncoordinated cell proliferation and survival, implementing strategies to inhibit cell proliferation and promote apoptotic cell death within tumors has proven effective in cancer treatment [9, 10]. Recently, it has been recognized that physical forces such as stretch, strain and tension play an important role in regulating the process of cell apoptosis.
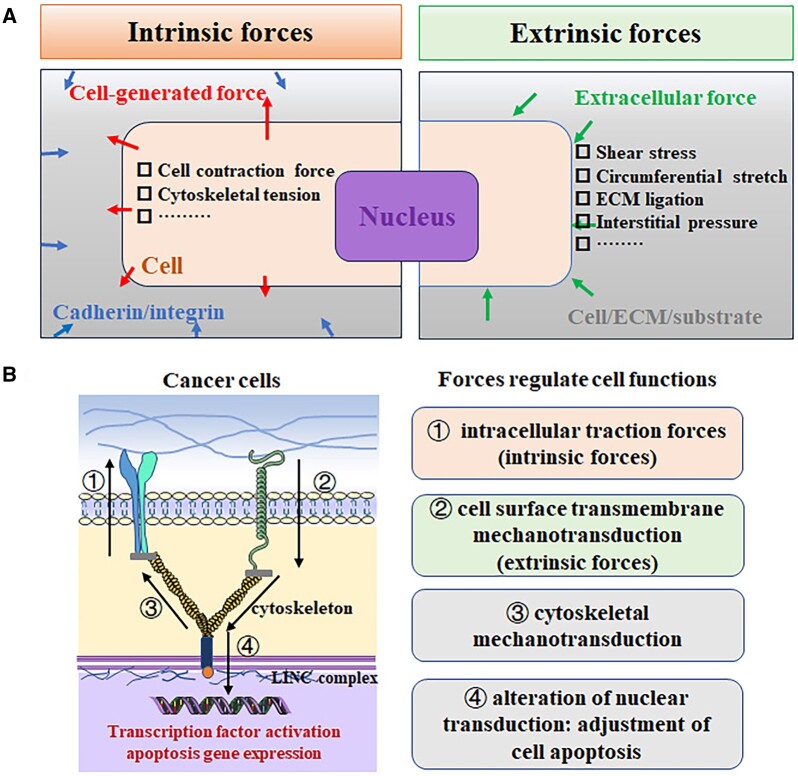
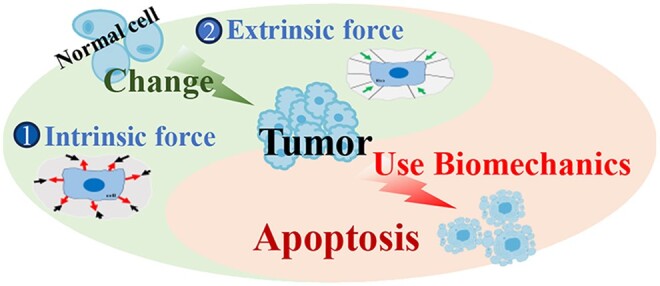
The mechanical cues to which cells are subjected can be broadly divided into two categories (Figure 2). One class, extrinsic forces are from blood flow-induced shear stress and circumferential stretch, externally applied mechanical strain, fluid flow-induced shear stress and ECM ligation [11–15]. Another class, intrinsic forces are from applied cellular traction through cell–cell and cell–ECM adhesions, as well as cell migration. The endogenous machinery of the cell, including tensile (actin) and compressive (microtubule) elements, and FA attachment complexes, participate in intracellular signaling [16]. Through the regulation of intracellular contractile forces and anchoring forces to the underlying substrate, cells achieve the preservation of a nuanced mechanical equilibrium [17]. This intricate and dynamic balance is governed by structures of cell-substrate adhesion, cytoskeletal configurations and the spatiotemporally controlled magnitude and distribution of internal contractile forces [18, 19]. Moreover, the interplay between the dynamic equilibrium and disequilibrium of forces exerts a significant impact on tissue functionality, regeneration, angiogenesis and the metastasis of cancer. This phenomenon holds paramount importance in the advancement of research and treatment methodologies for patients afflicted with various ailments, including cardiovascular disease, cancer and aging. Furthermore, it serves as a driving force for progress in the realms of regenerative medicine and the production of artificial organs [20, 21].
Figure 2.
Cancer cells exert forces and are subject to external forces, which modulate their intracellular signaling pathway and thereby influence their apoptosis. (A) Within cells, intrinsic or cell-generated forces are generated and transmitted to other cells through cell–cell junctions, like cadherin receptors or through traction on adhesion ligands bound to integrins in the ECM. Extrinsic forces are the external forces applied to cells by shear or tension and/or compression. (B) Force is applied to cancer cell mechanotransduction mechanisms allowing them to detect physical cues from their microenvironment and to transduce and biochemically amplify these signals to regulate cancer cell apoptosis.
In prior studies, mechanical signals have been identified as critical regulators of cell behavior, tissue regeneration, and disease occurrence and treatment [22–24]. This study delves into the fundamental mechanism of mechanical signal transduction and its influence on tumor cell behavior, exploring three aspects: extrinsic force, intrinsic force and the effect of forces on tumor therapy, mainly focusing on cancer cell apoptosis. Through this review, we aim to provide readers with a deeper understanding of mechanics and their potential applications in tumor therapy.
Intrinsic force-mediated tumor cell apoptosis
Intrinsic force generated from cell–cell and cell–ECM adhesion and migration
In response to the local stiffness of 2D substrates or 3D microenvironments, cells regulate their force generation by pulling on the ECM, i.e. ‘inside-out’ mechanical stimulation. These processes depend on ECM adhesions, which transmit force between the ECM and the cellular cytoskeleton to transmit force, with myosin-based contractility serving as an important regulator of cellular traction (contractile) forces [25]. Indeed, these intracellular forces, including cell contraction force and cytoskeletal tension, play a regulatory role in signaling pathways involving in fundamental aspects of cell, such as cellular transformation to neoplasm, tumor formation and progression.
Contractile force refers to the force generated by cells in a 3D environment, which is resisted by the mechanical properties of the microenvironment. During the process of cellular migration, it actively engages in various cellular events, including adhesion with the substrate, formation of leading-edge protrusions, generation of cell body protrusions, translocation of the cell body and establishment of cell polarity. For example, cells need to exert forces against the substrate to move forward [26]. In migration, the cell pulls continuously on the matrix and neighboring cells, causing the contractile forces to be dynamically generated by the action of myosin II on the cytoskeleton, thus providing a lasting mechanical stimulus [27].
Cellular tension is governed by the cytoskeletal structure, influencing both cell shape and mechanical load bearing. Subcellular organelles are influenced by the forces within this tensed actin cytoskeleton, the stiffest and largest of which is the nucleus. Bonse et al. [28] demonstrated that cytoskeletal tension could regulate YAP nuclear localization, especially under pathological condition . YAP nuclear accumulation and transcriptional activity are enhanced by high cytoskeletal tension in cancer-associated myofibroblasts [29].
In order to transmit force to the ECM, a functional force linkage is formed between intracellular, contractile, force-generating motor proteins, the cytoskeleton and transcellular adhesions (e.g. integrins). As well as being closely linked to the actin cytoskeleton, cadherin-mediated cell contacts between neighboring cells are also subject to and respond to cell-to-cell forces. In the context of cancer, the disruption of tensional homeostasis can dysregulate integrin expression and activation, focal adhesion protein assembly, cytoskeletal structure and cell-to-cell and cell-to-ECM adhesions, thereby promoting cancerization.
Intrinsic cytoskeleton force
Internal forces are generated by the cytoskeleton and then transmitted to the nucleus by LINC complexes or to other cells and the ECM through cytoskeleton-transmembrane receptors. The cytoskeleton is a network of biopolymers, including actin filaments, microtubules, intermediate filaments, motility proteins, cross-linked proteins, actomyosin complexes and regulatory proteins, within living cells that confers mechanical structure to cell, and transmits physical forces to and from the ECM surrounding them [30]. The actin filaments, microtubules and intermediate filaments comprise the main components of cellular rigidity [31]. Interference with the synthesis and assembly of actin, myosin or microtubule can severely weaken the tension generated by the cell, affecting cell spread and migration [32]. Actin filaments are closely bound to all components of the cell, forming a huge network connecting the nucleus, various organelles, and cell membrane, which are the largest contributor to the elastic modulus of the cell [31, 33]. A well-developed actin cytoskeleton produces a higher tension level, which gives the cells a wider adhesion to the substrate and a higher resistance to shear deformation [34]. It is becoming clear that the cytoskeleton plays an important role in mechanical force transduction to the cell interior [35–37].
The reciprocity between cancer cells and their intrinsic mechanical surroundings functions as a feedback system, empowering cancer with considerable adaptability across progressive stages. At the core of this feedback system lies the mechanical program involving F-actin and mechanoresponsive proteins, such as non-muscle myosin II, actinin, filamin and potentially others [38]. Studies indicate minimal changes in the mechanical properties of cells upon carcinogenesis, with preserved regular arrangements of cytoskeletal structures [39]. Nevertheless, heightened migration and invasion potential in cancer cells lead to pronounced changes in the expression of this mechanical network, facilitating significant spatial and temporal reorganization of the cytoskeleton in metastatic cells. Unsurprisingly, diverse protein levels of critical mechanobiome components and the broader actin cytoskeleton have been observed across various cancers. In addition, major cancer drivers and signaling proteins exhibit altered expression patterns, influencing cell mechanics. YAP, overexpressed in numerous cancers, modulates cellular actin architecture, nonmuscle myosin II regulatory light chain expression and phosphorylation, thereby impacting mechanical parameters, notably cortical tension, and deformability. Early activating KRAS mutations, prevalent in over 90% of pancreatic cancers and at high rates in colorectal and lung cancers, result in increased deformability and altered contractility [40]. Tumor cells with a high metastatic propensity manifest augmented softness, deformability and heightened intracellular molecular mobility, leading to increased diffusion [41–43]. This phenomenon is attributed to the necessity for cells to disseminate from the primary tumor and undergo intravasation to the systemic circulation, prompting the remodeling of their actomyosin cytoskeleton and increased deformability [44]. Tumor cells are frequently softer than normal cells, suggesting an adaptive softening during invasion and metastasizing [45, 46]. RAS/MAPK signaling pathway and EGFR signaling pathway mediate cytoskeletal remodeling and increase cell hardness. Restoring the mechanical properties of cancer cells by blocking these signaling pathways emerges as a reliable strategy to inhibit metastasis and spread of cancer cells [45, 47].
The alteration of cytoskeleton-mediated cellular forces plays an important role in the process of cell apoptosis. The manipulation of actin cytoskeleton recombination, achieved through the expression of Maspin protein in tumor cells, leads to the manifestation of epithelioid morphology in these cells. Additionally, this manipulation results in reduced cell migration, enhanced intercellular adhesion, and increased occurrence of apoptosis [48]. The relationship between cell structure, cellular force, and the expressions of apoptotic proteins is closely intertwined. For example, following exposure to carbon ion irradiation, the cancer cell cytoskeleton undergoes gradual disintegration, resulting in a decrease in cell hardness and volume, along with the formation of apoptotic bodies. Meantime, the expression of caspase-3 is negatively correlated with the increase of cell hardness, and the ratio of Bax/Bcl-2 is also gradually increased [36]. Studies have shown that elevated temperature (40°C) disrupts mechanical equilibrium in tumor cells, resulting in G1 phase arrest, senescence and apoptosis. This effect is attributed to the downregulation of F-actin expression and its associated generation of cell traction [49]. Besides, changes in cell mechanics prompt a more rapid apoptosis of tumor cells compared to biological signals [50]. Before the activation of the death receptor CD95/Fas, cytorelaxin B induces initial filamentous actin depolymerization, leading to decreased cell hardness, volume and intracellular molecular movement space. This process results in increased membrane prominence and roughness, ultimately culminating in the apoptosis of HeLa cells [50]. In addition, cytoskeletal deformation and recombination also play important roles in cell division and lysis [51, 52]. When external forces are excessive to the point where cytoskeletal remodeling and deformation cannot be fully offset, or when they remain unrelieved for an extended period of time, other cellular structures may become damaged, which would eventually lead to cell apoptosis or necrosis [53].
Nuclear mechanics and nuclear mechanotransduction
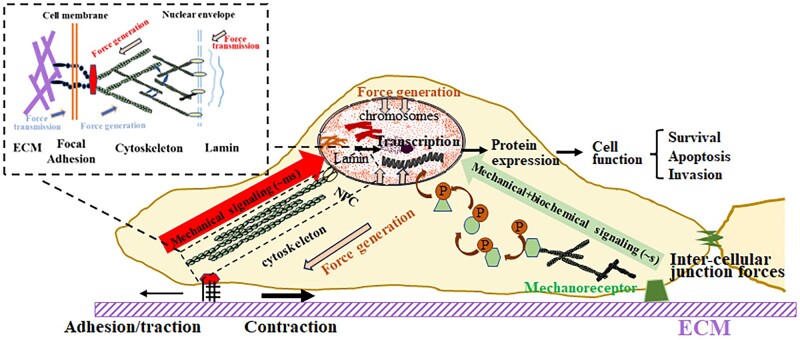
Commonly regarded as the cellular ‘brain’, the nucleus governs the comprehensive mechanical response of the cell. There are roughly two parts to the nucleus, the nuclear envelope and the nuclear interior. Typically, it is 2–10 times stiffer than the surrounding cytoplasm and exhibits both elastic (the nuclear lamina and nuclear envelope) and viscoelastic (the nuclear interior) behaviors [54–56]. In recent years, several studies have documented alterations in nuclear envelope composition in cancer. Denais and Lammerding [57] have reported the pivotal role of the nuclear envelope in cellular mechanics and function, encompassing the regulation of nuclear deformability, fragility and involvement in mechanotransduction signaling. There are two main ways in which extracellular and cytoplasmic forces are transmitted across the nuclear envelope to the nuclear interior: direct FA-cytoskeleton-LINC and indirect signaling cascades (Figure 3) [58].
Figure 3.
The extracellular and cytoplasmic force transduction by direct FA-cytoskeleton-LINC and indirect signaling cascades.
Direct force transduction serves as a rapid and efficient mechanism transmitted to the nucleus at a considerably higher speed compared to chemical signals and molecular motors. This transmission occurs through a high-speed network established by the physical coupling of FA, cytoskeleton and nuclear membrane complex [59, 60]. Upon cellular stretching, tensile force is directly conveyed to the nucleus through stress fibers, which leads to nuclear deformation and the generation of non-uniform stiffness, viscoelasticity and strain within the perinuclear region and the nucleus [61–64]. Upon reaching a certain threshold of extracellular shear force or compression stress, chromatin movement is enhanced through the actin network, which causes alterations or depolymerization of chromatin conformation, ultimately promoting specific gene expressions [65–69]. Alternatively, evidence suggests that Lamin type A, a major contributor to nuclear mechanics, is physically connected to the perinuclear actin cap via the LINC (the link between the nuclear skeleton and cytoskeleton) complex, further transmitting cytoskeletal forces to the nucleus, inducing chromatin remodeling, transcription activation and gene expression with important implications in cancer progression [70, 71]. Cancer cells exhibit abnormal nuclear morphologies, such as invaginations, irregularities in volume and shape, as well as aberrant chromatin regions, in contrast to normal cells. These morphological variations contribute to alterations in the mechanical properties of cancer cell nuclei. For example, abnormal nuclear mechanics in MDA-MB-231 breast cancer cells lead to “cap” bumps in the cell and nucleus. This could be inhibited by Lamin A/C overexpression [72]. The overexpression or silencing of Lamin A would effectively regulate multiple oncogenic processes, including proliferation, apoptosis, embryonic development, tumorigenesis, epithelial-to-mesenchymal transition (EMT) and metastasis [73]. Meanwhile, overexpression of Lamin B1 inhibits the connection between the nuclear membrane and actin filaments, promoting the migration of melanoma cells [74].
Moreover, the nucleus can indirectly sense extracellular and cytoplasmic forces through signaling cascades. Various studies have demonstrated the presence of mechanical receptors on the surface of cell membrane. Following mechanical stimulation, post-translational modification or configuration changes open the cellular cascade signal network, facilitating the transmission of mechanical signals to regulate gene transcription in the nucleus [75]. Among them, the exploration and discovery of the new mechanical receptor and nuclear stress signal transduction mechanism have become a research hotspot. For example, Plexin D1, a member of the semaphorin family of stress receptors associated with both shear stress and tensile strain on the surface of vascular endothelial cells, forms a complex with the transmembrane glycoprotein neuropilin-1 and vascular endothelial growth factor (VEGF) receptor type 2, thus leading to the activation of the AKT and ERK signaling pathways [76].
Overall, the process of mechanotransduction does not operate in ‘one-way street’ and signals from the nucleus can be routed back to the cytoskeleton to change the way a cell perceives mechanical stimuli, creating a feedback loop in transcription. A deeper understanding of how nuclear mechanics and tumor progression interact may have profound implications for cancer diagnostics and treatment, and reveal new therapeutic targets for pharmacologically inhibiting cancer cell invasion.
Cell membrane mechanics
The cell membrane is the most important part of the biofilm system, which is the barrier that keeps the cell isolated from the outside world. The cell membrane can be involved in the effects of cell mechanics on the cell behavior and function through the changes in membrane hardness, membrane tension, various force receptors and force-sensitive ion channels on the membrane surface. Lipid rafts are important structures in cell membranes that mediate cellular mechanics to regulate cancer metastasis and progression. They are rich in cholesterol and mechanically sensitive membrane proteins (such as Rho-type GTPase and integrin) [77]. The decrease in lipid rafts and the fatty acid chain length of phospholipid molecules cause a decrease in the stiffness of the cancer cell membrane, thus promoting the aggressiveness of cancer cells [78, 79]. Conversely, the increased stiffness of cell membranes can inhibit the invasion of cancer cells [79].
Membrane tension is the main factor regulating membrane deformation. Changes in membrane tension can be rapidly transmitted to the nucleus and various organelles, affecting the genetic and cellular events that can accelerate or inhibit cancer induction expression, movement, endocytosis and cytoskeletal remodeling [80–82]. The decreased membrane tension can improve the ability of membrane deformation, which is in favor of the metastasis of ovarian cancer cells to a certain extent [83]. Despite its functional significance, the membrane itself is relatively fragile. Tension in the plasma membrane primarily arises from attachment to the underlying actin cortex and osmotic pressure. The membrane is supported by the cell cortex, an F-actin scaffolding. The cell cortex is a thin shell of actin that lies underneath the plasma membrane and is part of the cytoskeleton. In mammalian cells, this cortex is approximately 1 μm thick and connects to the plasma membrane through specific protein links, as well as active proteins polymerizing actin locally. The cortex is a substrate for myosin attachment and tension buildup. Many of the ‘mechanosensitive elements’ in the membrane can be mechanosensitive because they are a part of the membrane-cortex complex, and in many cases, directly linked to the underlying cytoskeletal machinery and/or the ECM. For instance, the classical model of Piezo1’s physiological function posits its diffusion in the plasma membrane, locally activated by membrane bilayer tension or curvature changes resulting from cytoskeletal-dependent mechanical events [84].
Cell membrane itself is also a force sensor [85]. When cells are exposed to shear or tensile stress, the lipid order, fluidity, and cholesterol content of the plasma membrane would produce specific changes and guide cell behavior [86]. This may be related to the thinning of lipid bilayer in plasma membrane under stress, which leads to the exposure of force-sensitive channels or proteins such as TRPC6 [87].
Taken together, living cells, as complex soft materials, exhibit intriguing mechanical properties. An inherent mechanical program empowers cancer cells to continually sense and adapt to their environment, influencing tumorigenesis and development. The interactions between the cytoskeleton and membranes extend beyond mere structural or mechanical linkages, evolving into dynamic interactions that encompass newly identified functions related to signal transduction pathways. Exploring the mechanical system as a therapeutic target holds promise for more effective treatments in the future.
Extrinsic force-mediated tumor cell apoptosis
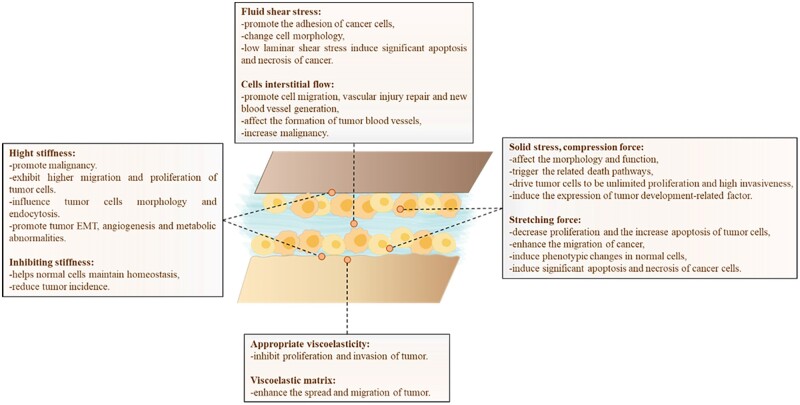
Extrinsic forces, such as elasticity and viscoelasticity of ECM, shear force and pressure of fluid, compression force and tension force of tissue, directly or indirectly participate in the regulation of cell life activities. It has been demonstrated that mechanical stimuli, in addition to disrupting cellular homeostasis, establish significant connections with cancer [88, 89]. Cells are compelled to respond to these stimuli, either to preserve their integrity or initiate an appropriate response (Figure 4).
Figure 4.
The extrinsic forces modulate the development and progression of tumors, including elasticity and viscoelasticity, shear force, interstitial flow, solid stress, compression force and stretching force.
Mechanical stresses generated from cells surrounding the extracellular matrix
The mechanical stress generated from cell surrounding the ECM is essential for cell adhesion, cell function and tissue development. The close connection between cells and their surrounding ECM allows them to keenly sense changes in their external environment. Abnormal metabolism of the ECM leads to changes in its structure and mechanical properties of ECM, contributing to the onset of various diseases, including tissue fibrosis, cancer cell proliferation, migration, EMT and so on [90, 91]. Therefore, the mechanical microenvironment of cells plays an important role in the generation, migration and progression of cancer. An ECM extends the cytoskeleton through integrins and convergent proteins, establishing a physical link between the ECM and the microfilaments of the cell. Studying the mechanical stresses between cells and their surrounding ECM can offer valuable insights into cancer development and aid in enhancing treatment options.
Matrix stiffness and elasticity
The stiffness observed in the ECM arises from the interactions between the ECM and cells, commonly understood as the mechanical forces exerted by the ECM on the cells. Deviations in stiffness can significantly impact various cellular processes such as differentiation, proliferation, tumor metastasis and drug resistance [92–94]. Tissue cells transmit forces to substrates as they exert pushing and pulling actions on their surroundings, facilitated by adhesion molecules like integrins and cadherins. Accordingly, the substrate’s resistance determines how well the cell can contract and migrate, causing the cell to adjust adhesion and reorganize cytoskeletal structures [64, 95–97]. It is complex mechanisms that allow cells to communicate and probe their microenvironment while also being sensitive to changes in substrate stiffness.
Each cell type specifically exists in the environment with a different modulus of elasticity or plays its function in the environment within a certain range of modulus of elasticity. For example, spinal cord neurons exhibit stellate spread at 50–550 Pa, myocytes exhibit myosin/actin stripes only at about 12 kPa of gel, and osteoblasts exhibit high cellular activity at 110 kPa [98]. The elasticity of the ECM determines the extent to which cells can influence their surroundings. The expression profile of ECM protein in tumor is significantly different from that in normal tissue. In response to increasing ECM elasticity, cells undergo a variety of changes at the molecular and cellular scales. Mammary epithelial cells exhibit loss of apicobasal polarity, enhanced focal adhesion assembly and increased proliferation [99]. Application of tensile forces to integrins triggers Rho signaling, leading to actin filament assembly, nuclear translocation of myocardin-related transcription factor A, and the expression of smooth muscle α-actin in fibroblasts [100]. ECM elasticity also induces shifts in protein localization, fostering malignant cellular behaviors. For instance, integrin clustering and activation on stiff matrices cause Rac1b to localize to the plasma membrane in mammary epithelial cells, resulting in upregulation of Snail and EMT [101]. YAP and transcriptional coactivator with PDZ-binding motif localize to the nucleus in mesenchymal stem cells cultured on stiff substrata, while they remain in the cytoplasm in cells cultured on the soft substrate [102]. Once in the nucleus, YAP and TAZ promote proliferation, and at elevated levels, this can result in neoplasia. Changes in the elastic modulus of the ECM are associated with the progression of many diseases, including cancer, fibrosis, atherosclerosis and so on (Table 1). The elasticity of tumor tissue is significantly higher than that of normal tissue. While type I collagen in normal tissue forms a loose network structure, collagen fibers in tumor tissue have a more consistent orientation. Historically, breast tumor macrohardness has been used to diagnose the disease and has also been strongly associated with local recurrence and metastasis [109].
Table 1.
The mechanical properties of normal tissue and cancer tissue
| Normal tissue (kPa) | Cancer tissue (kPa) | Ref. | |
|---|---|---|---|
| Breast | 0.1–4 | 10–42 | [103] |
| Brain | 7.3 ± 3.6 | ∼35 (low-grade gliomas) | [104] |
| ∼50 (high-grade gliomas) | |||
| Liver | <7.3 | >17.5 (cirrhosis and fibrosis) | [105] |
| Prostate | 16.0 ± 5.7 | 40.6 ± 15.9 | [106] |
| Mammary gland | 167 ± 31 Pa | 4049 ± 938 Pa | [99] |
| Pancreas | <15 | >40 | [107] |
| Bladder | <5 | 5–15 | [108] |
Enhancing ECM stiffness promotes the transition from pre-malignancy to invasive malignancy, whereas inhibiting matrix stiffness reduces tumor incidence and improves cancer treatment effects. The increased stiffness of ECM causes the deeper folds at the nuclear membrane of breast cancer cells and alters the chromatin accessibility, which in turn promotes the malignant development of breast cancer through the Sp1-HDAC3/8 pathway [110]. ECM stiffness and laminin content control the expression of β-casein in normal mammary epithelial cells. The soft matrix helps cells maintain homeostasis, while stiff ECM and the loss of laminin signal promote the occurrence and development of breast cancer [111]. A similar phenomenon has been observed in glial cells within the human brain, where tumor glioma cells exhibit heightened migration and proliferation in response to increased matrix stiffness [112]. The increase of matrix elastic modulus in a 3D environment reduces the expression of normal hyaluronic acid membrane receptor CD44s and increases the mutant CD44v6, thereby promoting the EMT, angiogenesis and metabolic abnormalities of gastric cancer cells [113].
When examining the impact of ECM stiffness on various tumor cells, there are indeed some commonalities between different tumor cells. Specifically, tumor cells from different origins exhibit an enhanced migratory and invasive capacity with increased ECM stiffness, indicating a shared sensitivity to stiffness alterations and a more efficient adaptation to diverse tissue environments. Variations in ECM stiffness can activate common signaling pathways, including Rho GTPases and MAPK, influencing tumor cell survival, proliferation and migration. Activation of these pathways appears to be a shared response mechanism among tumor cells. Furthermore, alterations in ECM stiffness may collectively enhance adhesion and matrix interaction in tumor cells, representing a shared pathway for improved adaptation and execution of migration and invasion in stiffer environments. Despite these shared characteristics, notable distinctions exist among tumor cells. Consequently, a thorough examination of the collective response of tumor cells to ECM stiffness should appropriately acknowledge these diversities, fostering a more comprehensive comprehension of tumor cell behavior in mechanical environments.
Cytoskeleton-integrin pathway mediates the regulation of ECM stiffness on cell behavior. β4 integrin/Ras-related C3 botulinum toxin substrate 1(Rac1)/phosphoinositide 3-kinase (PI3K) is the pathway by which mammary epithelial cells sense matrix rigidity and adhesion. Normal mammary epithelial cells exhibit malignant phenotypes as a result of increased matrix stiffness, which inhibits the expression of α6β4 integrin in the semi-desmosomes. This malignant phenotype would disappear with the increase of ligands on the matrix [114]. In addition, within 3D matrices, prostate cancer cells exhibit a response to increased matrix stiffness characterized by a reduction in cell elasticity and an increase in intracellular viscoelasticity. This behavior may be linked to an increase in adhesion sites and integrin mediations in the 3D matrix [115]. Matrix stiffness also influences cell morphology and endocytosis, which is regulated by actin cytoskeletal recombinant proteins (phosphorylated cofilin, profilin, adhesion plaque kinase and vinculin) and intracellular proteins (Caveolin 1 and Rab 11) [116]. Adhesion plaque kinase (FAK) and PI3K are also involved in the regulation of matrix stiffness on cell behavior [117]. Notably, talin and actin cytoskeleton-integrin linking molecule can only grow to a sufficient length to bind to vinculin when the substrate stiffness is greater than 1 kPa, forming a stable actin-integrin connection, which can play a role in cell sensing ECM stiffness [118].
Matrix viscoelasticity
Biological tissues and ECMs are not purely elastic materials, like a rubber ball or a spring, since they exhibit complex, time- and rate-dependent mechanical behaviors, a property called viscoelasticity or poroelasticity [119, 120]. Viscoelastic materials have three common features: stress relaxation (constant strain resulting in time-dependent decreasing stress), creep (constant stress resulting in time-dependent decreasing strain) and hysteresis (the difference between loading and unloading processes) [121, 122]. Viscoelasticity as a mechanical property influences disease progression, together with or independently of stiffness. Matrix viscoelasticity appears to govern essential cellular processes, including behaviors not observed in cells cultured in purely elastic hydrogels, both in two- and three-dimensional culture surroundings [123]. Specifically, by considering the tumor ECM as a standard linear viscoelastic solid, there is evidence that an intermediate viscosity level can facilitate cancer cell spreading when the ECM stiffness is relatively weak, which mirrors the circumstance that the substrate relaxation time under such conditions is somewhere between the coupling bond time scale and its typical bond lifetime. In other words, viscosity serves to stiffen soft substrates, which encourages cell adhesion to the ECM and facilitates consequently cell spreading. As with high stiffness, the large stress carried by the couplings elicits an enhancement of their binding levels as well as an augmentation of integrin tightness (clutch amplification), thereby rendering the cell contribution to substrate stiffness to become a saturated response, and viscosity no longer to be an issue [124].
Malignant and benign cells have significant differences in their viscoelastic response [125, 126]. For instance, human hepatocellular carcinoma cells (Huh7) demonstrate enhanced spread and migration on viscoelastic substrates, while normal hepatocellular cells (PHH and LX-2) exhibit reduced spread and adhesion. This difference is related to the ineffective assembly of actin stress fibers in the viscoelastic matrix of normal hepatocytes [127, 128]. Additionally, this is accompanied by a shift in cellular morphology, as viscoelasticity induces an EMT phenotype. The migratory ability of MDA-MB-231 triple-negative breast cancer cells increases with a decreasing damping coefficient [129]. Appropriate viscoelasticity can also inhibit the proliferation and invasion of breast cancer cells by influencing normal bone cells to secrete more pro-inflammatory factors and chemokines [130]. Cell behaviors, including spreading, conformation, division and movement, are largely dependent on viscoelasticity, primarily determined by the supporting cytoskeletal systems [131]. Upon mechanical deformation, these mechanical loads from the microenvironment are transmitted to the nucleus via filament linkages, altering gene expression and consequently biochemical signaling as a result [132]. Therefore, the intricate interplay between cancer cells and the viscoelastic nature of microenvironment in which these cells reside is crucial for cellular mechanotransduction. This interplay guides cancer cell behavior and, in turn, prevents tumors from experiencing excessive growth and metastasis.
Solid stress, compressive, tensile and shear
The rapid growth of tumors produces a large compression force on the cells inside the tumor, which can affect the morphology and function of cells and trigger the related death pathways [133, 134]. However, at the peripheral region of the tumor, the effect of compression force on cells is relatively small, promoting unlimited proliferation and high invasiveness of tumor cells [134]. The alterations in stress, interstitial fluid pressure and permeability in tumor are closely related to the compressible remodeling of ECM and collagen densification induced by excessive proliferation of cancer cells [135]. Rho/ROCK signaling pathway mediated F-actin activity and cyclin-dependent kinase inhibitor p21 are involved in the enhanced hardness of compressive stress-induced tumor spheroids, and the accumulation of compressive stress [136]. In a 2D environment, human fibrosarcoma cells sense compression force through Ca2+, TRPV4, Rho and YAP signaling pathways [137]. Meantime, intratumor compression forces also affect normal cells in and around the tumor. For example, intratumor compression forces would induce the expression of normal fibroblast growth differentiation factor-15 (GDF15) and ECM protein secretion in normal epithelial cells, as well as the activation of the β-catenin pathway, which further exacerbates tumor development [138, 139]. It is noteworthy that compression force promotes autophagy in HeLa cells via the lipid raft-mediated phosphorylation of p38MAPKs. Additionally, it enhances the invasiveness of HeLa cells by increasing paxlin turnover and MMP-2 secretion [140].
In addition, stretching force also plays an important role in the migration, apoptosis, and necrosis of cancer cells, and can play a tumor-killing role by enhancing the M1 polarization of macrophages. The stretching force promotes the expressions of Akt and caspase-3 in skin melanoma cells (B16F10) that are co-cultured with macrophages, leading to the decreased proliferation and the increased apoptosis of tumor cells. However, it has also been shown that stretching force enhances the migration of human ovarian epithelial cancer cells (SKOV-3). When cancer cells are exposed to periodic stretching, the cell would quickly respond via integrin α5β1 or ανβ3 independently mediated actin cytoskeletal remodeling [141]. The integrin α5β1 responds to stretching by adjusting the centripetal traction generated by long actin filaments, while integrin ανβ3 responds to stretching by adjusting short actin filaments to generate more flexible and dynamic random forces [141]. In ovarian cancer cells, actin also contributes to the response of G protein-coupled receptor 1 (OGR1) to acidification of the extracellular microenvironment under stretching conditions [142]. Simultaneously, stretching force induces normal tissue-associated fibroblasts (NAFS) to adopt a phenotype similar to that of cancer-associated fibroblasts (CAF) via the organization of the ECM fiber arrangement, which enhances the invasion and aggression of tumor cells [143]. Additionally, studies have shown that stretching can promote the spread and migration of breast cancer cells only in the initial cycle, while tumor cells would undergo significant apoptosis and necrosis after more stretching cycles [144]. Overall, reasonable control of stretching force may play a role in cancer treatment and prevention. Animal experiments have shown that stretching therapy in mice alone can effectively limit the growth of implanted tumors, with the stretching group showing a 52% smaller tumor volume than the non-stretching group [145].
Fluid pressure
Human cells are usually immersed in a fluid environment. When the fluid flows, shear stress occurs. According to recent research, fluid shear stress also affects cancer cell proliferation, metastasis, adhesion and apoptosis [146]. For instance, the presence of elevated wall shear stress at the arterial bifurcation promotes the development of intracranial aneurysms [147]. Under the shear force of 13 dynes/cm2, endothelial cells secret more von Willebrand factor and thrombocytopresin-1 into ECM, which promotes the adhesion of breast cancer cells to ECM through integrin [148]. The incremental elevation of fluid shear stress within the microfluidic channel (ranging from 0.12–1.8 dynes/cm2) elicits a progressive transformation in the morphology of human glioma cells. This metamorphosis is characterized by an augmentation in cortical rigidity and nuclear softness. Concurrently, the cells exhibit a gradual shift in motility behavior from crawling to rolling, coupled with an observed increase in adhesion forces [149]. Conversely, it is observed that low laminar shear stress results in substantial apoptosis and necrosis of cancer cells [150, 151]. However, investigations have revealed variability in the apoptosis mechanisms among different types of tumor cells. Melanoma is associated with integrin avb3 and AKT, while breast cancer cells are associated with concave-1. In liver cancer cells, FAK and integrin are involved together [152]. An additional study indicates a close association between the apoptosis of tumor cells in blood circulation and the generation and buildup of superoxide in mitochondria induced by fluid shear stress. Notably, cancer cells with high metastatic potential exhibit the ability to transform these superoxides into hydrogen peroxide through increased expression of MnSOD. This conversion facilitates the migration and adhesion of tumor cells, presenting a contrasting effect on their behavior [153].
In addition to the shear stress caused by the flow of blood and lymph fluid, the extensive flow of small interstitial fluid between tissues can also produce shear force and thereby affect the life activities of tissue cells [154]. The shear stress generated by interstitial flow is typically modest, yet it proves adequate for detection by the glycocalyx (the proteoglycan/glycoprotein layer located on the cell surface). This sensing mechanism outside the cell membrane initiates intracellular mechanotransduction processes [155]. The interstitial fluid flow induces endothelial cells and fibroblasts to produce MMP-1 and VEGF, which promotes cell migration, vascular injury repair and new blood vessel generation [156, 157]. For tumor cells, interstitial flow can also work in concert with other physical properties of ECM, such as matrix hardness, to promote cell migration [158]. Interstitial flow can cause stromal fibers to be arranged parallel to the flow direction and the uneven concentration of chemokines secreted by tumor cells such as tumor angiogenic factor TAFs, thereby inducing cell migration along the flow direction of the interstitial, affecting the formation of tumor blood vessels, and increasing malignancy [159, 160]. In addition, the tumor tissue has a higher interstitial fluid flow (∼3 μm/s) than the normal tissue, and the interstitial fluid continuously flows out from the tumor [161, 162]. This would induce the localization of FA-related proteins near the upstream membrane of the cell such as β1 integrin, nestin and FAK, leading to adhesion activation, polarization and countercurrent migration to the upstream direction of the interstitial flow of the breast cancer cells [161]. Under shear forces, normal cells also assist tumor development. Macrophages differentiate toward M2-type macrophages via β1 integrin/Src/Akt/FAK signal cascades upon exposure to tumor interstitial flow, which releases a larger number of factors associated with the EMT, migration, and invasion of cancer cells [162]. Furthermore, in co-culture with breast cancer cells MDA-MB-435S, interstitial flow triggers fibroblast migration and the remodeling of collagen matrix. This process leads to increased matrix density surrounding the cells, consequently enhancing the invasion ability of the tumor cells [163]. In tumor spheres formed through the co-culture of normal mammary epithelial cells (MCF-10A) and breast cancer cells (MDA-MB-231), the down-regulation of the intercellular adhesion protein E-cadherin in normal epithelial cell occurs due to the collaborative influence of mesenchymal flow and tumor cells, which promotes the invasion of the tumor [164].
Other
In addition to elasticity, viscoelasticity and shear stress, interstitial pressure, hydrostatic pressure and osmotic pressure also have important effects on cell behavior. The competitive growth of tumor cells induces high interstitial pressure within the tumor, accelerating the outflow of interstitial fluid from the tumor and concurrently slowing the inflow from the external environment [165, 166]. Under high interstitial pressure, the overexpression of caveolin-1 and CAVIN1 in glioblastoma (GBM) mediates the upregulation of EMT-related proteins (such as MMPs), resulting in the promotion of tumor cell invasion [167, 168]. Through the downregulation of RYBP leading to p53 degradation, high interstitial pressure can promote the occurrence and development of breast cancer [169]. Tumor interstitial fluid and its pressure can activate the expressions of CLIC4 and integrin α11β1 in fibroblasts, promoting collagen secretion and ECM hardening, thereby increasing the proliferation and invasion abilities of breast cancer cells [166, 169]. However, exposure to higher osmotic pressure leads to reduced cell proliferation, migration, and dispersion in metastatic cancer cells [170].
In addition, increased hydrostatic pressure can induce the stiffening of endothelial cell membranes under constant tension, thereby activating swelling-associated sensitive ion channels and signal transduction pathways [171]. The leukemia cell line K562 and normal leukocyte exhibit divergent responses to sudden increases or decreases in hydrostatic pressure. Specifically, the sodium and potassium channels in K562 cells are activated, leading to reversible volume deformation. In contrast, normal leukocytes do not undergo such changes [172]. Hydrostatic pressure below 80 mmHg can promote the proliferation of human mesangial cells, while hydrostatic pressure above 100 mmHg can induce apoptosis and ECM deposition of human mesangial cells [173]. Besides, high osmotic pressure can synergistically interact with the acidic environment of cancer, affecting the cross-presentation of tumor-specific antigens by dendritic cells to allow tumor cells to escape the recognition and attack of immune cells [174].
Overall, in the tumor microenvironment, mechanical factors are pivotal. Tumor growth and development coincide with alterations in these mechanical factors, such as stress from uncontrolled tumor proliferation, augmented stromal stiffness, elevated interstitial hydraulic pressure and intensified interstitial fluid flow. Simultaneously, changes in mechanical factors exert influence on the tumor microenvironment, as evidenced by residual stresses compressing blood and lymphatic vessels, resulting in functional impairment and consequent perturbation of the metabolic milieu within the tumor, leading to interstitial hypertension. Moreover, mechanical factors have implications for tumor treatment and the metastasis of tumor cells. Notably, the rapid proliferation of tumor cells may compress the interstitial stroma into a convoluted and dense network, posing challenges for drug delivery. Additionally, the flow of interstitial fluid can influence the directional aspects of cell metastasis.
Furthermore, organizations exhibit intricate mechanical landscapes, wherein extrinsic and intrinsic cues frequently interact and cannot be easily separated. Forces acting on cells and those imposed on the extracellular environment contribute to stresses and deformations. Consequently, gene regulatory circuits may be redirected, leading to abnormal reactivation of embryonic developmental programs, which can drive cells to transform and cancer to initiate, as well as cause cancer progression and metastasis later during the course of the disease.
Biomechanics guide tumor therapy
A potential application for the above finding lies in the tumor therapy. Tumor therapy and drug resistance are highly influenced by changes in mechanical properties of cell microenvironment. Tumor ECMs can protect tumor cells from apoptosis, either directly by physical restriction or indirectly by binding to cellular ECM receptors (such as integrins), thus forming resistance to chemotherapy drugs [175, 176]. Accordingly, modulating the mechanical properties of tumor ECM or blocking/affecting cell response to ECM mechanics may be an effective strategy to induce tumor cell apoptosis and overcome drug resistance (Figure 5).
Figure 5.
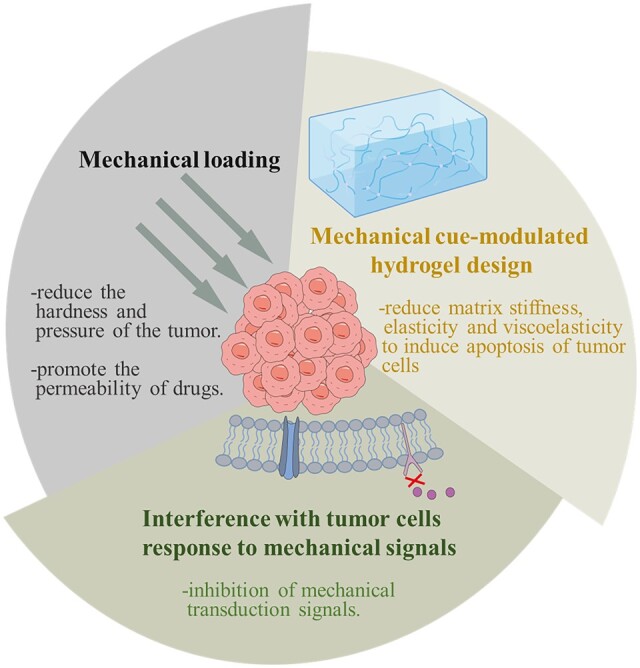
Biomechanics guide tumor therapy, including mechanical loading, mechanical cue-modulated hydrogel design and interference with tumor cell’s response to mechanical signals.
Mechanical cue-modulated hydrogel design
The multiscale biomechanics studies mentioned above offer valuable insights for the development of novel cancer therapies. The therapeutic strategy for tumor, especially solid tumor, has shifted toward targeting the tumor microenvironment, rather than the tumor itself, exerting stronger anti-tumor effects. In recent years, modulating the mechanical properties of the tumor microenvironment is a promising new therapeutic strategy in the treatment of cancer. Despite the limited research reports, it has promising clinical application prospects. Hydrogels can effectively simulate ECM, and their mechanical properties are easy to control, which has attracted extensive attention from scholars [177–182]. Currently, researchers are striving to manipulate the mechanical properties of hydrogels, including elasticity, viscoelasticity and so on, thus accelerating the apoptosis of tumor cells and inhibiting tumor progression.
Improved tumor cell apoptosis can be achieved by engineering appropriate mechanical microenvironments of tumor cells [183–186]. Reducing matrix elasticity to induce apoptosis of tumor cells is considered as an effective strategy. For example, Guo et al. [187] manipulate the mechanical strength of the matrix material. With the mechanical strength decreasing from 62 to 1.4 Pa, the apoptosis rate of ovarian cancer cells increases from 1.85% to 52.68%. Moreover, in the treatment of liver cancer, as the matrix stiffness gradually decreases from 500 to 100 kPa, caspase-3 and caspase-9 are activated in combination with the anticancer drug oxaliplatin, and the apoptosis rate increases from 20% to 35% [188]. While the significance of the mechanical microenvironment in tumor progression is gaining increased attention, understanding chemoradiotherapy resistance driven by mechanical microenvironment is still in its early stages. Further studies are required to elucidate the related mechanisms. For example, the establishment of hepatocellular carcinoma microspheres with varying microenvironmental stiffness is achieved by adjusting the mechanical strength of sodium alginate microspheres. The results indicate that, under the treatment of paclitaxel, cisplatin and 5-fluorouracil, respectively, the survival rate of hepatocellular carcinoma cells in the high stiffness environment (105 kPa) is significantly higher than that in the low stiffness environment (21 kPa) and the medium stiffness environment (70 kPa). This suggests that the high-stiffness environment significantly enhances chemotherapy resistance in liver cancer, possibly linked to stiffness-induced endoplasmic reticulum stress response [189]. In the context of cancer therapy, in situ gels represent a novel type of environmentally responsive hydrogel. These gels can serve not only as drug delivery carriers but also as providers of a mechanical microenvironment. Hence, through the integration of engineering and biology expertise, it becomes feasible to spatially control the stiffness microenvironment, potentially inducing tumor cell apoptosis and mitigating drug resistance in tumor cells.
Reproducing tissue elasticity and dissipative properties represents an emerging strategy in cancer treatment [190]. Most hydrogels, especially biopolymer-based hydrogels, exhibit both elastic and viscous properties. However, the progress in developing matrix materials capable of independently regulating various viscoelastic properties (such as viscosity damping coefficient, loss modulus, stress relaxation, loss angle, creep, viscous dissipation, etc.) has been slow for a considerable period, limiting our exploration of the effects of viscoelasticity on cells [191]. Recently, advances in materials processing techniques have been made in the development of viscoelastic matrix materials with good biocompatibility. Several dissipating processes may lead to the viscous properties of hydrogels, including the unbinding of weak bonds, polymer disentanglement, protein unfolding and molecule slipping [192, 193]. It has been demonstrated that the viscoelasticity of hydrogels can be modulated through polymer molecular weight or network chain length, crosslink type and density, hydrogel composition or concentration and degradation, and some combination of varying the above parameters [123, 194–197]. Recent studies have demonstrated that hydrogel viscoelasticity significantly affects cell spreading and apoptosis. For instance, Mooney et al. [198] conducted a study revealing that U2OS cells exhibit increased spreading when placed on soft substrates with stress relaxation, compared to cells on elastic substrates with an equivalent modulus. However, the spreading of these cells resembled that of cells on stiffer elastic substrates. Additionally, our latest findings observe variations in the apoptosis rate of human osteosarcoma MG63 cells under different viscoelastic stimulation conditions [199]. Therefore, it is important to take into account hydrogel viscoelasticity when engineering 3D mechanical microenvironment for tumor cells.
It is noteworthy that the roles of elasticity and viscoelasticity in regulating the biology of the various cell types within the tumor microenvironment-including fibroblasts, tissue-resident stem and differentiated cells, and immune cells cannot be ignored. This consideration is essential for the rational design of materials aimed at enhancing cancer cell apoptosis. In the design of biomaterials to achieve mechanical stability in regenerating or engineered tissue, it may also be necessary to decouple the local viscoelastic or elastic properties that cells sense from the larger, tissue-scale properties [200]. Therefore, the introduction of biomaterials with controlled mechanical properties, encompassing viscoelasticity or elasticity, may have a transformative effect on therapy.
Mechanical loading
The dense ECM and high interstitial fluid pressure in the tumor limit the diffusion of drugs into the tumor sphere, which affects the therapeutic effect of chemotherapy drugs. By mechanical loading on the tumor, it may reduce the hardness and pressure of the tumor and improve the penetration efficiency of the drug, which exerts a direct killing effect on tumor cells. The combined treatment of unfocused ultrasound and microbubble can change the interstitial fluid pressure in rabbit VX2 tumor. When the ultrasound intensity reaches 3 or 5 MPa, the interstitial fluid pressure decreases steadily [201]. Fluid shear force can promote the permeability of doxorubicin to tumors, thereby reducing colorectal cancer spheroid survival [202]. Meantime, ultrasound induces significant apoptosis and necrosis of human histiocytic lymphoma cell (U937) in a non-lethal hypotonic pressure environment [203]. Furthermore, simultaneously reducing ECM hardness and intratumor pressure to promote drug penetration into the tumor is also an important idea of multi-target combination drug delivery [204–206].
The perturbation in tumor cell response to mechanics
Interference with tumor cells response to mechanical signals is another way to regulate the mechanical microenvironment. Inhibition of mechanical transduction signals mediated by the specific membrane receptor, integrin family, FAK, Rho-associated kinase (ROCK) and YAP/TAZ may prevent cancer initiation and development, as well as reverse tumor drug resistance.
Cells can sense the mechanical information of ECM and regulate their own behavior by anchoring ECM with receptors on the surface of cell membrane [207]. For example, Tetraspanin CD82 is a membrane protein in mammary epithelial cell membranes that mediates adhesion and migration. The activation of CD82 inhibits the metastasis of breast cancer cells by regulating cell membrane tension through the litavin-1 and YAP pathways [208]. Therefore, designing some chemical ligands to bind to the force-sensitive receptors on the cell membrane to affect/hinder the interaction between tumor cell receptors and ECM would be an effective strategy to inhibit the development of cancer.
The additional efforts aim to target mechanotransduction pathways at their downstream components, such as Rho, ROCK and YAP/TAZ signaling (Table 2). Metastatic progression necessitates cancer cells to remodel their cytoskeletons, enabling them to cope with increased motility and interaction with ECM [216–219]. It is primarily accomplished through the activation of the RhoA main effectors ROCK [220]. There is evidence indicating a regulation of ROCK expression in several cancers, which is associated with a poor prognosis. Moreover, several studies have demonstrated that their activity contributes substantially to the progression of cancer [221–224]. Thus, ROCK is generally considered an important player in the progression and development of cancer, which results in considering the use of ROCK inhibitors in the treatment of cancers, such as small-molecule inhibitors against ROCK (Y27632 and Fasudil). Moreover, YAP and TAZ present attractive targets for cancer therapy, given that their elevated nuclear levels are associated with a broad range of aggressive cancers [225–227]. In addition, a direct inhibitor of YAP/TAZ, verteporfin, can suppress the communication between YAP and its binding transcription factor TEAD, therefore inhibiting YAP/TEAD downstream signaling in various cancers, including retinoblastoma, malignant pleural mesothelioma, endometrial cancer cells and so on [228–232]. Hence, the development of ROCK and YAP/TAZ inhibition would be the efficient strategy for cancer treatment, but the use of these inhibitors for anticancer purposes should be tightly controlled to avoid adverse effects.
Table 2.
Effects of mechanotransduction-targeted drugs in cell treatment
| Agent/material | Action mechanism | Cells | Outcomes | Mechanical cues | Ref. |
|---|---|---|---|---|---|
| Gsmtx4 | Block CaN/NFAT1 signaling axis | Rat primary chondrocytes | Protect chondrocytes from apoptosis and anabolic/catabolic imbalance under mechanical strain | Inhibited the deleterious effects of mechanical strain | [209] |
| Andrographolide | Activating the MAPK/Nrf2/HO-1 signaling pathway | Nucleus pulposus cells | Inhibits static mechanical pressure-induced apoptosis and improves cell viability | Suppress static mechanical pressure-induced ROS accumulation in the NPCs | [210] |
| γ-Fe2O3 SPIONPs | The opening of the Piezol mechanosensor | Neural stem cells | Regulates the directional differentiation of NSCs and neuron regeneration | Increase the elastic modulus of the NSCs | [211] |
| Y-27632 | Inhibit RhoA/ROCK pathway | Unknown | Reduce internal resistance | Stiffness-induced cell activation | [212] |
| Morin | Inhibit Hippo/YAP and TGF-β1/Smad pathways | Hepatic stellate cells (HSCs) | Show antifibrotic effect | Stiffness-induced HSC activation | [213] |
| Xanthohumol | Mediate the GAS5/miR-27a signaling pathway | Chondrocytes | Protected chondrocytes and increased viability | Protective effects against mechanical stimulation-induced ECM degradation | [214] |
| Metuzumab | Decrease β1 integrin/FAK/Akt activation via CD147 blockade |
|
Inhibit tumor growth and metastatic potentials | Stiffness, shear stress or IFP-induced proliferation and metastasis of HCC cells | [215] |
Taken together, tumor biophysics offers novel insights for an enhanced comprehension of cancer, playing a pivotal role in the exploration of innovative therapeutic approaches. The emerging domain of mechanics-based design in oncology therapeutics focuses on harnessing the mechanical attributes of the tumor microenvironment to optimize treatment effectiveness. This encompasses the modulation of matrix mechanical properties to influence tumor cell behavior, the development of mechanosensitive drug delivery systems and direct mechanical interventions on tumor tissue. The future trajectory of this field anticipates heightened individualization and precision, fostering breakthroughs in cancer treatment to elevate therapeutic efficacy while mitigating adverse effects.
Challenges and future perspectives
Major challenges persist in understanding the mechanical characteristics of the tumor microenvironment and the concept of mechanomedicine in cancer therapy. First, the multiscale modeling of complex mechanical microenvironments in humans and animals remains a formidable task. Researchers often study a single type of mechanical stimuli on cancer cells in vitro, while these stimuli are often combined in vivo to modulate cellular and molecular events. Replicating multiple types of mechanical stimuli in vitro is essential for comprehending cellular responses and understanding cancer development from a global perspective. Second, there is an attractive prospect of integrating quantitative data obtained from multiscale biomechanics measurements and predictions to create a virtual mechanical tumor. This integration involves several steps such as data pre-processing, feature proposal and model training, which may vary depending on the virtual mechanical organ/tissue and the type of inspection data. The development of state-of-the-art biomechanical experimental techniques and computer-based simulation approaches or even artificial intelligence algorithms will provide further information with high spatiotemporal resolution strategies, offering insights into molecular and cellular mechanisms from the perspective of mechanobiology or mechanomedicine. Third, numerous cancer mechanotherapy strategies target nuclear mechanics (e.g. high-frequency LIPU therapy, shock-wave therapy) or mechanotransduction (e.g. mechanical stretch therapy, low-frequency LIPU therapy) due to the mismatched mechanophenotyping between cancer cells and normal cells. However, there are still many problems to be solved. For example, the synergistic participation of mechanical signals and biochemical signals in cancer mechanotherapy remains unclear. It is unclear whether commonalities exist among different cancer species in mechanical signals mediating tumor cell apoptosis and the mechanisms of chemoradiotherapy resistance. With the progress of cutting-edge technologies such as three-dimensional organoid models, laser capture microcutting, single-cell multiomics sequencing and artificial intelligence, it is possible to identify the characteristics of different local microenvironments within the same cancer species and the common characteristics of microenvironments among different cancer species. This is expected to bring breakthrough changes to the reversal of tumor progression and chemoradiotherapy resistance in clinical tumors. Alongside conventional cancer treatments, mechanotherapy-based therapeutic systems will be developed for use in clinics.
Conclusions
In conclusion, this review emphasizes the significance of mechanical cues in tumor cell apoptosis, including both extracellular force and intracellular force. Throughout the cancer development process, alterations in the extracellular mechanical microenvironment elevate the risk of malignancy, foster tumor progression and induce tumor cell phenotypes resembling stem cells to exacerbate tumor aggression and concurrently hinder drug delivery and immunotherapy. On the other hand, intracellular force can modulate mechanotransduction and intracellular force transmission, initiating a positive feedback loop that further shapes mechanical microenvironment and facilitates carcinogenesis. Clearly, mechanical cue has a critical role in regulating tumor development and treatment. Therefore, the application of the mechanomedicine concept in cancer holds the promise of designing scaffolds and delivering drugs for more effective therapeutic interventions.
Contributor Information
Jun Shu, National Engineering Research Center for Biomaterials, College of Biomedical Engineering, Sichuan University, Chengdu 610064, PR China.
Huan Deng, National Engineering Research Center for Biomaterials, College of Biomedical Engineering, Sichuan University, Chengdu 610064, PR China.
Yu Zhang, College of Food and Biological Engineering, Chengdu University, Chengdu 610106, PR China.
Fang Wu, National Engineering Research Center for Biomaterials, College of Biomedical Engineering, Sichuan University, Chengdu 610064, PR China.
Jing He, National Engineering Research Center for Biomaterials, College of Biomedical Engineering, Sichuan University, Chengdu 610064, PR China.
Funding
This work was financially supported by the National Natural Science Foundation of China (grant no. 31971257) and Natural Science Foundation of Sichuan Province (grant no. 2022NSFSC0360).
Conflicts of interest statement. None declared.
References
- 1. Ingber DE. Tensegrity-based mechanosensing from macro to micro. Prog Biophys Mol Biol 2008;97:163–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Martino F, Perestrelo AR, Vinarsky V, Pagliari S, Forte G.. Cellular mechanotransduction: from tension to function. Front Physiol 2018;9:824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yu H, Hou Z, Chen N, Luo R, Yang L, Miao M, Ma X, Zhou L, He F, Shen Y, Liu X, Wang Y.. Yes-associated protein contributes to magnesium alloy-derived inflammation in endothelial cells. Regen Biomater 2022;9:rbac002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Staunton JR, So WY, Paul CD, Tanner K.. High-frequency microrheology in 3D reveals mismatch between cytoskeletal and extracellular matrix mechanics. Proc Natl Acad Sci USA 2019;116:14448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shieh AC. Biomechanical forces shape the tumor microenvironment. Ann Biomed Eng 2011;39:1379–89. [DOI] [PubMed] [Google Scholar]
- 6. Zhang F, Zhang A, Xie Y, Wen H, Kankala RK, Huang J, Zhang A, Wang Q, Chen B, Dong H, Guo Z, Chen A, Yang D.. Nanocarrier of Pin1 inhibitor based on supercritical fluid technology inhibits cancer metastasis by blocking multiple signaling pathways. Regen Biomater 2023;10:rbad014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. He Z, Wang Q, Zhang N, Yan J, Li L, Cao J, He B.. Gold nanorods/tetrahedral DNA composites for chemo-photothermal therapy. Regen Biomater 2022;9:rbac032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Knap K, Kwiecień K, Reczyńska-Kolman K, Pamuła E.. Inhalable microparticles as drug delivery systems to the lungs in a dry powder formulations. Regen Biomater 2023;10:rbac099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peng C, Zeng X, Cai J, Huang H, Yang F, Jin S, Guan X, Wang Z.. Albumin-based nanosystem for dual-modality imaging-guided chem-phototherapy against immunecold triple-negative breast cancer. Regen Biomater 2023;10:rbad073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meng Z, Wang B, Liu Y, Wan Y, Liu Q, Xu H, Liang R, Shi Y, Tu P, Wu H, Xu C.. Mitochondria-targeting plydopamine-coated nanodrugs for effective photothermal- and chemo-synergistic therapies against lung cancer. Regen Biomater 2022;9:rbac051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pelham RJ, Wang YL.. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci USA 1997;94:13661–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saez A, Ghibaudo M, Buguin A, Silberzan P, Ladoux B.. Rigidity-driven growth and migration of epithelial cells on microstructured anisotropic substrates. Proc Natl Acad Sci USA 2007;104:8281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bian L, Hou C, Tous E, Rai R, Mauck RL, Burdick JA.. The influence of hyaluronic acid hydrogel crosslinking density and macromolecular diffusivity on human MSC chondrogenesis and hypertrophy. Biomaterials 2013;34:413–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kapur S, Baylink DJ, Lau KHW.. Fluid flow shear stress stimulates human osteoblast proliferation and differentiation through multiple interacting and competing signal transduction pathways. Bone 2003;32:241–51. [DOI] [PubMed] [Google Scholar]
- 15. Guo Y, Zhang CQ, Zeng QC, Li RX, Liu L, Hao QX, Shi CH, Zhang XZ, Yan YX.. Mechanical strain promotes osteoblast ECM formation and improves its osteoinductive potential. Biomed Eng Online 2012;11:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Galbraith CG, Sheetz MP.. Forces on adhesive contacts affect cell function. Curr Opin Cell Biol 1998;10:566–71. [DOI] [PubMed] [Google Scholar]
- 17. Lee G, Han SB, Lee JH, Kim HW, Kim DH.. Cancer mechanobiology: microenvironmental sensing and metastasis. ACS Biomater Sci Eng 2019;5:3735–52. [DOI] [PubMed] [Google Scholar]
- 18. Dumbauld DW, Shin H, Gallant ND, Michael KE, Radhakrishna H, García AJ.. Contractility modulates cell adhesion kinase and assembly of vinculin-containing focal adhesions. J Cell Physiol 2010;223:746–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Halder G, Dupont S, Piccolo S.. Transduction of mechanical and cytoskeletal cues by Yap and TAZ. Nat Rev Mol Cell Biol 2012;13:591–600. [DOI] [PubMed] [Google Scholar]
- 20. Lim CT, Bershadsky A, Sheetz MP.. Mechanobiology. J Royal Soc Interface 2010;7:S291–S293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sanz-Herrera JA, Reina-Romo E.. Cell-biomaterial mechanical interaction in the framework of tissue engineering: insights, computational modeling and perspectives. Int J Mol Sci 2011;12:8217–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li J, Liu X, Tao W, Li Y, Du Y, Zhang S.. Micropatterned composite membrane guides oriented cell growth and vascularization for accelerating wound healing. Regen Biomater 2023;10:rbac108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li H, Xu X, Wu L, Chen X, Akhter H, Wang Y, Song P, Liao X, Zhang Z, Li Z, Zhou C, Cen Y, Ai H, Zhang X.. Recent progress and clinical applications of advanced biomaterials in cosmetic surgery. Regen Biomater 2023;10:rbad005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li Y, Zhou M, Zheng W, Yang J, Jiang N.. Scaffold-based tissue engineering strategies for soft-hard interface regeneration. Regen Biomater 2023;10:rbac091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Provenzano PP, Keely PJ.. Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and Rho GTPase signaling. J Cell Sci 2011;124:1195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. du Roure O, Saez A, Buguin A, Austin RH, Chavrier P, Silberzan P, Ladoux B.. Force mapping in epithelial cell migration. Proc Natl Acad Sci USA 2005;102:2390–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y, He J, Zhang J, Zhang N, Zhou Y, Wu F.. Cell migration induces apoptosis in osteosarcoma cell via inhibition of Wnt-β-catenin signaling pathway. Colloids Surf B Biointerfaces 2023;223:113142. [DOI] [PubMed] [Google Scholar]
- 28. Bonse J, Wennmann DO, Kremerskothen J, Weide T, Michgehl U, Pavenstädt H, Vollenbröker B.. Nuclear Yap localization as a key regulator of podocyte function. Cell Death Dis 2018;9:850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hu C, Yang J, Su HY, Waldron RT, Zhi M, Li L, Xia Q, Pandol SJ, Lugea A.. Yes-associated protein 1 plays major roles in pancreatic stellate cell activation and fibroinflammatory responses. Front Physiol 2019;10:1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pullarkat PA, Fernandez PA, Ott A.. Rheological properties of the eukaryotic cell cytoskeleton. Phys Rep Rev Sec Phys Lett 2007;449:29–53. [Google Scholar]
- 31. Grady ME, Composto RJ, Eckmann DM.. Cell elasticity with altered cytoskeletal architectures across multiple cell types. J Mech Behav Biomed Mater 2016;61:197–207. [DOI] [PubMed] [Google Scholar]
- 32. Kraning-Rush CM, Carey SP, Califano JP, Smith BN, Reinhart-King CA.. The role of the cytoskeleton in cellular force generation in 2D and 3D environments. Phys Biol 2011;8:015009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim Y, Kim M, Shin JH, Kim J.. Characterization of cellular elastic modulus using structure based double layer model. Med Biol Eng Comput 2011;49:453–62. [DOI] [PubMed] [Google Scholar]
- 34. Dowling EP, McGarry JP.. Influence of spreading and contractility on cell detachment. Ann Biomed Eng 2014;42:1037–48. [DOI] [PubMed] [Google Scholar]
- 35. Fu Q, Wu C, Shen Y, Zheng S, Chen R.. Effect of LIMK2 RNAi on reorganization of the actin cytoskeleton in osteoblasts induced by fluid shear stress. J Biomech 2008;41:3225–8. [DOI] [PubMed] [Google Scholar]
- 36. Zhang B, Li L, Li Z, Liu Y, Zhang H, Wang J.. Carbon ion-irradiated hepatoma cells exhibit coupling interplay between apoptotic signaling and morphological and mechanical remodeling. Sci Rep 2016;6:3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ketene AN, Roberts PC, Shea AA, Schmelz EM, Agah M.. Actin filaments play a primary role for structural integrity and viscoelastic response in cells. Integr Biol (Camb) 2012;4:540–9. [DOI] [PubMed] [Google Scholar]
- 38. Nguyen LTS, Jacob MAC, Parajón EP, Robinson DN.. Cancer as a biophysical disease: targeting the mechanical-adaptability program. Biophys J 2022;121:3573–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhou Z, Zheng C, Li S, Zhou X, Liu Z, He Q, Zhang N, Ngan A, Tang B, Wang A.. AFM nanoindentation detection of the elastic modulus of tongue squamous carcinoma cells with different metastatic potentials. Nanomedicine 2013;9:864–74. [DOI] [PubMed] [Google Scholar]
- 40. Surcel A, Schiffhauer ES, Thomas DG, Zhu Q, DiNapoli KT, Herbig M, Otto O, West-Foyle H, Jacobi A, Kräter M, Plak K, Guck J, Jaffee EM, Iglesias PA, Anders RA, Robinson DN.. Targeting mechanoresponsive proteins in pancreatic cancer: 4-hydroxyacetophenone blocks dissemination and invasion by activating MYH14. Cancer Res 2019;79:4665–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gal N, Weihs D.. Intracellular mechanics and activity of breast cancer cells correlate with metastatic potential. Cell Biochem Biophys 2012;63:199–209. [DOI] [PubMed] [Google Scholar]
- 42. Klymenko O, Wiltowska-Zuber J, Lekka M, Kwiatek WM.. Energy dissipation in the AFM elasticity measurements. Acta Phys Pol A 2009;115:548–51. [Google Scholar]
- 43. Nagayama K, Ohata S, Obata S, Sato A.. Macroscopic and microscopic analysis of the mechanical properties and adhesion force of cells using a single cell tensile test and atomic force microscopy: remarkable differences in cell types. J Mech Behav Biomed Mater 2020;110:103935. [DOI] [PubMed] [Google Scholar]
- 44. Bryan DS, Stack M, Krysztofiak K, Cichoń U, Thomas DG, Surcel A, Schiffhauer ES, Beckett MA, Khodarev NN, Xue L, Poli EC, Pearson AT, Posner MC, Robinson DN, Rock RS, Weichselbaum RR.. 4-Hydroxyacetophoenone modulates the actomyosin cytoskeleton to reduce metastasis. Proc Natl Acad Sci USA 2020;117:22423–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rudzka DA, Spennati G, McGarry DJ, Chim YH, Neilson M, Ptak A, Munro J, Kalna G, Hedley A, Moralli D, Green C, Mason S, Blyth K, Mullin M, Yin H, Olson MF.. Migration through physical constraints is enabled by MAPK-induced cell softening via actin cytoskeleton re-organization. J Cell Sci 2019;132:jcs224071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rianna C, Radmacher M, Kumar S.. Direct evidence that tumor cells soften when navigating confined spaces. Mol Biol Cell 2020;31:1726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Azadi S, Omidvar R, Tafazzoli-Shadpour M, Habibi-Anbouhi M.. IEEE. Restoring Elastic Properties of Breast Cancer Cells by EGFR Targeting: Atomic Force Microscopy Measurement. In: Biomedical Engineering & International Iranian Conference on Biomedical Engineering, Tehran, Iran, 2016,7–10. [Google Scholar]
- 48. Al-Mamun MA, Ravenhill L, Hossain MA, Farid DM, Bass R, IEEE. Fractal and image analysis of cytoskeletal changes in tumour cells due to the effects of maspin. In: IEEE-EMBS International Conference on Biomedical and Health Informatics (BHI), Valencia, Spain,2014, pp. 209–12.
- 49. Mundhara N, Majumder A, Panda D.. Hyperthermia induced disruption of mechanical balance leads to G1 arrest and senescence in cells. Biochem J 2021;478:179–96. [DOI] [PubMed] [Google Scholar]
- 50. Su X, Zhang L, Kang H, Zhang B, Bao G, Wang J.. Mechanical, nanomorphological and biological reconstruction of early-stage apoptosis in HeLa cells induced by cytochalasin B. Oncol Rep 2019;41:928–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Takamatsu H, Takeya R, Naito S, Sumimoto H.. On the mechanism of cell lysis by deformation. J Biomech 2005;38:117–24. [DOI] [PubMed] [Google Scholar]
- 52. Chen YQ, Kuo CY, Wei MT, Wu K, Su PT, Huang CS, Chiou A.. Intracellular viscoelasticity of HeLa cells during cell division studied by video particle-tracking microrheology. J Biomed Opt 2014;19:011008. [DOI] [PubMed] [Google Scholar]
- 53. Gefen A, Weihs D.. Cytoskeleton and plasma-membrane damage resulting from exposure to sustained deformations: a review of the mechanobiology of chronic wounds. Med Eng Phys 2016;38:828–33. [DOI] [PubMed] [Google Scholar]
- 54. Kim DH, Hah J, Wirtz D.. Mechanics of the cell nucleus. Adv Exp Med Biol 2018;1092:41–55. [DOI] [PubMed] [Google Scholar]
- 55. Krause M, te Riet J, Wolf K.. Probing the compressibility of tumor cell nuclei by combined atomic force-confocal microscopy. Phys Biol 2013;10:065002. [DOI] [PubMed] [Google Scholar]
- 56. Wei F, Lan F, Liu B, Liu L, Li G.. Poroelasticity of cell nuclei revealed through atomic force microscopy characterization. Appl Phys Lett 2016;109:213701. [Google Scholar]
- 57. Denais C, Lammerding J.. Nuclear mechanics in cancer. Adv Exp Med Bio 2014;773:435–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Newberg J, Schimpf J, Woods K, Loisate S, Davis PH, Uzer G.. Isolated nuclei stiffen in response to low intensity vibration. J Biomech 2020;111:110012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Balikov DA, Brady SK, Ko UH, Shin JH, de Pereda JM, Sonnenberg A, Sung HJ, Lang MJ.. The nesprin-cytoskeleton interface probed directly on single nuclei is a mechanically rich system. Nucleus 2017;8:534–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tang XW, Ding MX.. Hypothesis of intracellular signaling based on the cytoskeleton network. Chinese Phys Lett 1998;15:849–50. [Google Scholar]
- 61. Deguchi S, Yano M, Hashimoto K, Fukamachi H, Washio S, Tsujioka K.. Assessment of the mechanical properties of the nucleus inside a spherical endothelial cell based on microtensile testing. J Mech Mater Struct 2007;2:1087–102. [Google Scholar]
- 62. Wakhloo NT, Anders S, Badique F, Eichhorn M, Brigaud I, Petithory T, Vassaux M, Milan JL, Freund JN, Rühe J, Davidson PM, Pieuchot L, Anselme K.. Actomyosin, vimentin and LINC complex pull on osteosarcoma nuclei to deform on micropillar topography. Biomaterials 2020;234:119746. [DOI] [PubMed] [Google Scholar]
- 63. Tsukamoto S, Asakawa T, Kimura S, Takesue N, Mofrad MRK, Sakamoto N.. Intranuclear strain in living cells subjected to substrate stretching: a combined experimental and computational study. J Biomech 2021;119:110292. [DOI] [PubMed] [Google Scholar]
- 64. Driscoll TP, Cosgrove BD, Heo SJ, Shurden ZE, Mauck RL.. Cytoskeletal to nuclear strain transfer regulates Yap signaling in mesenchymal stem cells. Biophys J 2015;108:2783–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nagayama K, Yahiro Y, Matsumoto T.. Stress fibers stabilize the position of intranuclear DNA through mechanical connection with the nucleus in vascular smooth muscle cells. FEBS Lett 2011;585:3992–7. [DOI] [PubMed] [Google Scholar]
- 66. Jean RP, Chen CS, Spector AA.. Finite-element analysis of the adhesion-cytoskeleton-nucieus mechanotransduction pathway during endothelial cell rounding: axisymmetric model. J Biomech Eng 2005;127:594–600. [DOI] [PubMed] [Google Scholar]
- 67. Dahl KN, Booth-Gauthier EA, Ladoux B.. In the middle of it all: mutual mechanical regulation between the nucleus and the cytoskeleton. J Biomech 2010;43:2–8. [DOI] [PubMed] [Google Scholar]
- 68. Booth-Gauthier EA, Alcoser TA, Yang G, Dahl KN.. Force-induced changes in subnuclear movement and rheology. Biophys J 2012;103:2423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Iyer KV, Pulford S, Mogilner A, Shivashankar GV.. Mechanical activation of cells induces chromatin remodeling preceding MKL nuclear transport. Biophys J 2012;103:1416–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fruleux A, Hawkins RJ.. Physical role for the nucleus in cell migration. J Phys Condens Matter 2016;28:363002. [DOI] [PubMed] [Google Scholar]
- 71. Kim DH, Wirtz D.. Cytoskeletal tension induces the polarized architecture of the nucleus. Biomaterials 2015;48:161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kent IA, Zhang Q, Katiyar A, Li Y, Pathak S, Dickinson RB, Lele TP.. Apical cell protrusions cause vertical deformation of the soft cancer nucleus. J Cell Physiol 2019;234:20675–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zwerger M, Ho CY, Lammerding J.. Nuclear mechanics in disease. Annu Rev Biomed Eng 2011;13:397–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fracchia A, Asraf T, Salmon-Divon M, Gerlitz G.. Increased lamin B1 levels promote cell migration by altering perinuclear actin organization. Cells 2020;9:2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jiang Y, Yang X, Jiang J, Xiao B.. Structural designs and mechanogating mechanisms of the mechanosensitive piezo channels. Trends Biochem Sci 2021;46:472–88. [DOI] [PubMed] [Google Scholar]
- 76. Mehta V, Pang KL, Rozbesky D, Nather K, Keen A, Lachowski D, Kong Y, Karia D, Ameismeier M, Huang J, Fang Y, Hernandez ADR, Reader JS, Jones EY, Tzima E.. The guidance receptor plexin D1 is a mechanosensor in endothelial cells. Nature 2020;578:290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Levitan I, Gooch KJ.. Lipid rafts in membrane-cytoskeleton interactions and control of cellular biomechanics: actions of oxLDL. Antioxid Redox Signal 2007;9:1519–34. [DOI] [PubMed] [Google Scholar]
- 78. Händel C, Schmidt BUS, Schiller J, Dietrich U, Möhn T, Kießling TR, Pawlizak S, Fritsch AW, Horn L-C, Briest S, Höckel M, Zink M, Käs JA.. Cell membrane softening in human breast and cervical cancer cells. New J Phys 2015;17:083008. [Google Scholar]
- 79. Braig S, Sebastian Schmidt BU, Stoiber K, Händel C, Möhn T, Werz O, Müller R, Zahler S, Koeberle A, Käs JA, Vollmar AM.. Pharmacological targeting of membrane rigidity: implications on cancer cell migration and invasion. New J Phys 2015;17:083007. [Google Scholar]
- 80. Sens P, Plastino J.. Membrane tension and cytoskeleton organization in cell motility. J Phys Condens Matter 2015;27:273103. [DOI] [PubMed] [Google Scholar]
- 81. Keren K. Cell motility: the integrating role of the plasma membrane. Eur Biophys J Biophys Lett 2011;40:1013–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Nambiar R, McConnell RE, Tyska MJ.. Control of cell membrane tension by myosin-I. Proc Natl Acad Sci USA 2009;106:11972–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lu T, Anvari B.. Characterization of the viscoelastic properties of ovarian cancer cells membranes by optical tweezers and quantitative phase imaging. Front Phys 2020;8:582956. [Google Scholar]
- 84. Yao M, Tijore A, Cheng D, Li JV, Hariharan A, Martinac B, Nhieu GTV, Cox CD, Sheetz M.. Force-and cell state-dependent recruitment of Piezo 1 drives focal adhesion dynamics and calcium entry. Sci Adv 2022;8:eabo1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Le Roux AL, Quiroga X, Walani N, Arroyo M, Roca-Cusachs P.. The plasma membrane as a mechanochemical transducer. Philos Trans R Soc Lond B Biol Sci 2019;374:20180221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yamamoto K, Ando J.. Emerging role of plasma membranes in vascular endothelial mechanosensing. Circ J 2018;82:2691–8. [DOI] [PubMed] [Google Scholar]
- 87. Huber TB, Schermer B, Benzing T.. Podocin organizes ion channel-lipid supercomplexes: implications for mechanosensation at the slit diaphragm. Nephron Exp Nephrol 2007;106:E27–31. [DOI] [PubMed] [Google Scholar]
- 88. Northey JJ, Przybyla L, Weaver VM.. Tissue force programs cell fate and tumor aggression. Cancer Discov 2017;7:1224–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Faurobert E, Bouin AP, Albiges-Rizo C.. Microenvironment, tumor cell plasticity, and cancer. Curr Opin Oncol 2015;27:64–70. [DOI] [PubMed] [Google Scholar]
- 90. Roomi MW, Kalinovsky T, Niedzwiecki A, Rath M.. Modulation of u-PA, MMPs and their inhibitors by a novel nutrient mixture in human lung cancer and mesothelioma cell lines. Int J Oncol 2013;42:1883–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Woodcock HV, Eley JD, Guillotin D, Plate M, Nanthakumar CB, Martufi M, Peace S, Joberty G, Poeckel D, Good RB, Taylor AR, Zinn N, Redding M, Forty EJ, Hynds RE, Swanton C, Karsdal M, Maher TM, Fisher A, Bergamini G, Marshall RP, Blanchard AD, Mercer PF, Chambers RC.. The mTORC1/4E-BP1 axis represents a critical signaling node during fibrogenesis. Nat Commun 2019;10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bai M, Zhang Z, Chen H, Liu X, Xie J.. Paxillin tunes the relationship between cell-matrix and cell-cell adhesions to regulate stiffness-dependent dentinogenesis. Regen Biomater 2023;10:rbac100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Bu W, Wu Y, Ghaemmaghami AM, Sun H, Mata A.. Rational design of hydrogels for immunomodulation. Regen Biomater 2022;9:rbac009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Meyer N, Bax DV, Beck J, Cameron RE, Best SM.. Adjusting the physico-chemical properties of collagen scaffolds to accommodate primary osteoblasts and endothelial cells. Regen Biomater 2023;10:rbad15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Todorovski V, Fox AH, Choi YS.. Matrix stiffness-sensitive long noncoding RNA NEAT1 seeded paraspeckles in cancer cells. Mol Biol Cell 2020;31:1654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Sugimoto W, Itoh K, Mitsui Y, Ebata T, Fujita H, Hirata H, Kawauchi K.. Substrate rigidity-dependent positive feedback regulation between Yap and ROCK2. Cell Adhes Migr 2018;12:101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Molina ER, Chim LK, Salazar MC, Mehta SM, Menegaz BA, Lamhamedi-Cherradi SE, Satish T, Mohiuddin S, McCall D, Zaske AM, Cuglievan B, Lazar AJ, Scott DW, Grande-Allen JK, Ludwig JA, Mikos AG.. Mechanically tunable coaxial electrospun models of Yap/TAZ mechanoresponse and IGF-1R activation in osteosarcoma. Acta Biomater 2019;100:38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Xie K, Yang Y, Jiang H.. Controlling cellular volume via mechanical and physical properties of substrate. Biophys J 2018;114:675–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM.. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005;8:241–54. [DOI] [PubMed] [Google Scholar]
- 100. Zhao XH, Laschinger C, Arora P, Szászi K, Kapus A, McCulloch CA.. Force activates smooth muscle α-actin promoter activity through the Rho signaling pathway. J Cell Sci 2007;120:1801–9. [DOI] [PubMed] [Google Scholar]
- 101. Lee K, Chen QK, Lui C, Cichon MA, Radisky DC, Nelson CM.. Matrix compliance regulates Rac1b localization, NADPH oxidase assembly, and epithelial-mesenchymal transition. Mol Biol Cell 2012;23:4097–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Péchoux C, Gudjonsson T, Ronnov-Jessen L, Bissell MJ, Petersen OW.. Human mammary luminal epithelial cells contain progenitors to myoepithelial cells. Dev Biol 1999;206:88–99. [DOI] [PubMed] [Google Scholar]
- 103. Ruud KF, Hiscox WC, Yu I, Chen RK, Li W.. Distinct phenotypes of cancer cells on tissue matrix gel. Breast Cancer Res 2020;22:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Bhargav AG, Domino JS, Chamoun R, Thomas SM.. Mechanical properties in the glioma microenvironment: emerging insights and theranostic opportunities. Front Oncol 2021;11:805628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ravichandran A, Murekatete B, Moedder D, Meinert C, Bray LJ.. Photocrosslinkable liver extracellular matrix hydrogels for the generation of 3D liver microenvironment models. Sci Rep 2021;11:15566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hoyt K, Castaneda B, Zhang M, Nigwekar P, di Sant’Agnese PA, Joseph JV, Strang J, Rubens DJ, Parker KJ.. Tissue elasticity properties as biomarkers for prostate cancer. Cancer Biomark 2008;4:213–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Nabavizadeh A, Payen T, Iuga AC, Sagalovskiy IR, Desrouilleres D, Saharkhiz N, Palermo CF, Sastra SA, Oberstein PE, Rosario V, Kluger MD, Schrope BA, Chabot JA, Olive KP, Konofagou EE.. Noninvasive Young’s modulus visualization of fibrosis progression and delineation of pancreatic ductal adenocarcinoma (PDAC) tumors using harmonic motion elastography (HME) in vivo. Theranostics 2020;10:4614–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ghasemi H, Mousavibahar SH, Hashemnia M, Karimi J, Khodadadi I, Mirzaei F, Tavilani H.. Tissue stiffness contributes to Yap activation in bladder cancer patients undergoing transurethral resection. Ann N Y Acad Sci 2020;1473:48–61. [DOI] [PubMed] [Google Scholar]
- 109. Bian J, Zhang J, Hou X.. Diagnostic accuracy of ultrasound shear wave elastography combined with superb microvascular imaging for breast tumors: a protocol for systematic review and meta-analysis. Medicine (Baltimore) 2021;100:E26262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Stowers RS, Shcherbina A, Israeli J, Gruber JJ, Chang J, Nam S, Rabiee A, Teruel MN, Snyder MP, Kundaje A, Chaudhuri O.. Matrix stiffness induces a tumorigenic phenotype in mammary epithelium through changes in chromatin accessibility. Nat Biomed Eng 2019;3:1009–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Alcaraz J, Xu R, Mori H, Nelson CM, Mroue R, Spencer VA, Brownfield D, Radisky DC, Bustamante C, Bissell MJ.. Laminin and biomimetic extracellular elasticity enhance functional differentiation in mammary epithelia. EMBO J 2008;27:2829–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Pogoda K, Chin L, Georges PC, Byfield FJ, Bucki R, Kim R, Weaver M, Wells RG, Marcinkiewicz C, Janmey PA.. Compression stiffening of brain and its effect on mechanosensing by glioma cells. New J Phys 2014;16:075002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. da Cunha CB, Klumpers DD, Koshy ST, Weaver JC, Chaudhuri O, Seruca R, Carneiro F, Granja PL, Mooney DJ.. CD44 alternative splicing in gastric cancer cells is regulated by culture dimensionality and matrix stiffness. Biomaterials 2016;98:152–62. [DOI] [PubMed] [Google Scholar]
- 114. Chaudhuri O, Koshy ST, da Cunha CB, Shin JW, Verbeke CS, Allison KH, Mooney DJ.. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat Mater 2014;13:970–8. [DOI] [PubMed] [Google Scholar]
- 115. Baker EL, Bonnecaze RT, Zaman MH.. Extracellular matrix stiffness and architecture govern intracellular rheology in cancer. Biophys J 2009;97:1013–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Amit C, Padmanabhan P, Elchuri SV, Narayanan J.. Probing the effect of matrix stiffness in endocytic signalling pathway of corneal epithelium. Biochem Biophys Res Commun 2020;525:280–5. [DOI] [PubMed] [Google Scholar]
- 117. Huang J, Zhang L, Wan D, Zhou L, Zheng S, Lin S, Qiao Y.. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct Target Ther 2021;6:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hirata H, Chiam K-H, Lim CT, Sokabe M.. Actin flow and talin dynamics govern rigidity sensing in actin -integrin linkage through talin extension. J R Soc Interface 2014;11:20140734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Storm C, Pastore JJ, MacKintosh FC, Lubensky TC, Janmey PA.. Nonlinear elasticity in biological gels. Nature 2005;435:191–4. [DOI] [PubMed] [Google Scholar]
- 120. Zhang S, Jia Y, Liu J, Feng F, Wei Z, Zhang M, Xu F.. A viscoelastic alginate-based hydrogel network coordinated with spermidine for periodontal ligament regeneration. Regen Biomater 2023;10:rbad009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Khalilgharibi N, Mao Y.. To form and function: on the role of basement membrane mechanics in tissue development, homeostasis and disease. Open Biol 2021;11:200360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Mierke CT. Viscoelasticity, like forces, plays a role in mechanotransduction. Front Cell Dev Biol 2022;10:789841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Charbonier F, Indana D, Chaudhuri O.. Tuning viscoelasticity in alginate hydrogels for 3D cell culture studies. Curr Protoc 2021;1:e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Mierke CT. Visoelasticity acts as a marker for tumor extracellular matrix characteristics. Front Cell Dev Biol 2021;9:785138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Tang S, Ma H, Tu HC, Wang HR, Lin PC, Anseth KS.. Adaptable fast relaxing boronate-based hydrogels for probing cell-matrix interactions. Adv Sci 2018;5:1800638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Indana D, Agarwal P, Bhutani N, Chaudhuri O.. Viscoelasticity and adhesion signaling in biomaterials control human pluripotent stem cell morphogenesis in 3D culture. Adv Mater 2021;33:e2101966. [DOI] [PubMed] [Google Scholar]
- 127. Mandal K, Gong Z, Rylander A, Shenoy VB, Janmey PA.. Opposite responses of normal hepatocytes and hepatocellular carcinoma cells to substrate viscoelasticity. Biomater Sci 2020;8:2040–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Hui E, Gimeno KI, Guan G, Caliari SR.. Spatiotemporal control of viscoelasticity in phototunable hyaluronic acid hydrogels. Biomacromolecules 2019;20:4126–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Ishikawa Y, Sasaki R, Domura R, Okamoto M.. Cellular morphologies, motility, and epithelial-mesenchymal transition of breast cancer cells incubated on viscoelastic gel substrates in hypoxia. Mater Today Chem 2019;13:8–17. [Google Scholar]
- 130. Nguyen HD, Sun X, Yokota H, Lin CC.. Probing osteocyte functions in gelatin hydrogels with tunable viscoelasticity. Biomacromolecules 2021;22:1115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Pegoraro AF, Janmey P, Weitz DA.. Mechanical properties of the cytoskeleton and cells. Cold Spring Harb Perspect Biol 2017;9:a022038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Paluch EK, Nelson CM, Biais N, Fabry B, Moeller J, Pruitt BL, Wollnik C, Kudryasheva G, Rehfeldt F, Federle W.. Mechanotransduction: use the force(s). BMC Biol 2015;13:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Valon L, Levayer R.. Dying under pressure: cellular characterisation and in vivo functions of cell death induced by compaction. Biol Cell 2019;111:51–66. [DOI] [PubMed] [Google Scholar]
- 134. Dolega ME, Delarue M, Ingremeau F, Prost J, Delon A, Cappello G.. Cell-like pressure sensors reveal increase of mechanical stress towards the core of multicellular spheroids under compression. Nat Commun 2017;8:14056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Ferruzzi J, Sun M, Gkousioudi A, Pilvar A, Roblyer D, Zhang Y, Zaman MH.. Compressive remodeling alters fluid transport properties of collagen networks-implications for tumor growth. Sci Rep 2019;9:17151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Taubenberger AV, Girardo S, Träber N, Fischer-Friedrich E, Kräter M, Wagner K, Kurth T, Richter I, Haller B, Binner M, Hahn D, Freudenberg U, Werner C, Guck J.. 3D microenvironment stiffness regulates tumor spheroid growth and mechanics via p21 and ROCK. Adv Biosyst 2019;3:e1900128. [DOI] [PubMed] [Google Scholar]
- 137. He L, Tao J, Maity D, Si F, Wu Y, Wu T, Prasath V, Wirtz D, Sun SX.. Role of membrane-tension gated Ca2+ flux in cell mechanosensation. J Cell Sci 2018;131:jcs208470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Fernández-Sánchez ME, Barbier S, Whitehead J, Béalle G, Michel A, Latorre-Ossa H, Rey C, Fouassier L, Claperon A, Brullé L, Girard E, Servant N, Rio-Frio T, Marie H, Lesieur S, Housset C, Gennisson J-L, Tanter M, Ménager C, Fre S, Robine S, Farge E.. Mechanical induction of the tumorigenic beta-catenin pathway by tumour growth pressure. Nature 2015;523:92–5. [DOI] [PubMed] [Google Scholar]
- 139. Kalli M, Papageorgis P, Gkretsi V, Stylianopoulos T.. Solid stress facilitates fibroblasts activation to promote pancreatic cancer cell migration. Ann Biomed Eng 2018;46:657–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Das J, Agarwal T, Chakraborty S, Maiti TK.. Compressive stress-induced autophagy promotes invasion of HeLa cells by facilitating protein turnover in vitro. Exp Cell Res 2019;381:201–7. [DOI] [PubMed] [Google Scholar]
- 141. Balcioglu HE, van Hoorn H, Donato DM, Schmidt T, Danen EHJ.. The integrin expression profile modulates orientation and dynamics of force transmission at cell-matrix adhesions. J Cell Sci 2015;128:1316–26. [DOI] [PubMed] [Google Scholar]
- 142. Wei WC, Bianchi F, Wang YK, Tang MJ, Ye H, Glitsch MD.. Coincidence detection of membrane stretch and extracellular pH by the proton-sensing receptor OGR1 (GPR68). Curr Biol 2018;28:3815–23.e4. [DOI] [PubMed] [Google Scholar]
- 143. Ao M, Brewer BM, Yang L, Coronel OEF, Hayward SW, Webb DJ, Li D.. Stretching fibroblasts remodels fibronectin and alters cancer cell migration. Sci Rep 2015;5:8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Yadav S, Vadivelu R, Ahmed M, Barton M, Nam-Trung N.. Stretching cells - an approach for early cancer diagnosis. Exp Cell Res 2019;378:191–7. [DOI] [PubMed] [Google Scholar]
- 145. Berrueta L, Bergholz J, Munoz D, Muskaj I, Badger GJ, Shukla A, Kim HJ, Zhao JJ, Langevin HM.. Stretching reduces tumor growth in a mouse breast cancer model. Sci Rep 2018;8:7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Novak C, Horst E, Mehta G.. Mechanotransduction in ovarian cancer: shearing into the unknown. APL Bioeng 2018;2:031701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Dolan JM, Meng H, Sim FJ, Kolega J.. Differential gene expression by endothelial cells under positive and negative streamwise gradients of high wall shear stress. Am J Physiol Cell Physiol 2013;305:C854–C866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Gomes N, Legrand C, Fauvel-Lafève F.. Shear stress induced release of von Willebrand factor and thrombospondin-1 in HUVEC extracellular matrix enhances breast tumour cell adhesion. Clin Exp Metastasis 2005;22:215–23. [DOI] [PubMed] [Google Scholar]
- 149. Li W, Mao S, Khan M, Zhang Q, Huang Q, Feng S, Lin JM.. Responses of cellular adhesion strength and stiffness to fluid shear stress during tumor cell rolling motion. ACS Sens 2019;4:1710–5. [DOI] [PubMed] [Google Scholar]
- 150. Tzukert K, Shimony N, Krasny L, Urieli-Shoval S, Gorodetsky R, Avrahami I, Nettelbeck DM, Haviv YS.. Human melanoma cells expressing the alpha v beta 3 integrin are partially protected from necrotic cell death induced by dynamic matrix detachment. Cancer Lett 2010;290:174–81. [DOI] [PubMed] [Google Scholar]
- 151. Shimony N, Avrahami I, Gorodetsky R, Elkin G, Tzukert K, Zangi L, Levdansky L, Krasny L, Haviv YS.. A 3D rotary renal and mesenchymal stem cell culture model unveils cell death mechanisms induced by matrix deficiency and low shear stress. Nephrol Dial Transplant 2008;23:2071–80. [DOI] [PubMed] [Google Scholar]
- 152. von Sengbusch A, Gassmann P, Fisch KM, Enns A, Nicolson GL, Haier J.. Focal adhesion kinase regulates metastatic adhesion of carcinoma cells within liver sinusoids. Am J Pathol 2005;166:585–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Ma S, Fu A, Lim S, Chiew GGY, Luo KQ.. MnSOD mediates shear stress-promoted tumor cell migration and adhesion. Free Radic Biol Med 2018;129:46–58. [DOI] [PubMed] [Google Scholar]
- 154. Rutkowski JM, Swartz MA.. A driving force for change: interstitial flow as a morphoregulator. Trends Cell Biol 2007;17:44–50. [DOI] [PubMed] [Google Scholar]
- 155. Tarbell JM, Shi ZD.. Effect of the glycocalyx layer on transmission of interstitial flow shear stress to embedded cells. Biomech Model Mechanobiol 2013;12:111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Shi Z-D, Ji X-Y, Qazi H, Tarbell JM.. Interstitial flow promotes vascular fibroblast, myofibroblast, and smooth muscle cell motility in 3-D collagen I via upregulation of MMP-1. Am J Physiol Heart Circ Physiol 2009;297:H1225–H1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Shirure VS, Lezia A, Tao A, Alonzo LF, George SC.. Low levels of physiological interstitial flow eliminate morphogen gradients and guide angiogenesis. Angiogenesis 2017;20:493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Li A, Sun M, Spill F, Sun R, Zaman MH.. Are the effects of independent biophysical factors linearly additive? A 3D tumor migration model. Biophys J 2019;117:1702–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Li A, Sun R.. Role of interstitial flow in tumor migration through 3D ECM. Acta Mech Sin 2020;36:768–74. [Google Scholar]
- 160. Vilanova G, Bures M, Colominas I, Gomez H.. Computational modelling suggests complex interactions between interstitial flow and tumour angiogenesis. J R Soc Interface 2018;15:20180415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Polacheck WJ, German AE, Mammoto A, Ingber DE, Kamm RD.. Mechanotransduction of fluid stresses governs 3D cell migration. Proc Natl Acad Sci USA 2014;111:2447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Li R, Serrano JC, Xing H, Lee TA, Azizgolshani H, Zaman M, Kamm RD.. Interstitial flow promotes macrophage polarization toward an M2 phenotype. Mol Biol Cell 2018;29:1927–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Shieh AC, Rozansky HA, Hinz B, Swartz MA.. Tumor cell invasion is promoted by interstitial flow-induced matrix priming by stromal fibroblasts. Cancer Res 2011;71:790–800. [DOI] [PubMed] [Google Scholar]
- 164. Huang YL, Ma Y, Wu C, Shiau C, Segall JE, Wu M.. Tumor spheroids under perfusion within a 3D microfluidic platform reveal critical roles of cell-cell adhesion in tumor invasion. Sci Rep 2020;10:9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Tran QD, Gonzalez-Rodriguez D, Marcos. Quantitative characterization of viscoelastic fracture induced by time-dependent intratumoral pressure in a 3D model tumor. Biomicrofluidics 2019;13:054107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Smeland HYH, Lu N, Karlsen TV, Salvesen G, Reed RK, Stuhr L.. Stromal integrin 11-deficiency reduces interstitial fluid pressure and perturbs collagen structure in triple-negative breast xenograft tumors. BMC Cancer 2019;19:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Pu W, Qiu J, Nassar ZD, Shaw PN, McMahon KA, Ferguson C, Parton RG, Riggins GJ, Harris JM, Parat MO.. A role for caveola-forming proteins caveolin-1 and CAVIN1 in the pro-invasive response of glioblastoma to osmotic and hydrostatic pressure. J Cell Mol Med 2020;24:3724–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Pu W, Qiu J, Riggins GJ, Parat MO.. Matrix protease production, epithelial-to-mesenchymal transition marker expression and invasion of glioblastoma cells in response to osmotic or hydrostatic pressure. Sci Rep 2020;10:2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Kenny TC, Schmidt H, Adelson K, Hoshida Y, Koh AP, Shah N, Mandeli J, Ting J, Germain D.. Patient-derived interstitial fluids and predisposition to aggressive sporadic breast cancer through collagen remodeling and inactivation of p53. Clin Cancer Res 2017;23:5446–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Miermont A, Lee SWL, Adriani G, Kamm RD.. Quantitative screening of the effects of hyper-osmotic stress on cancer cells cultured in 2-or 3-dimensional settings. Sci Rep 2019;9:13782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Ayee MAA, LeMaster E, Teng T, Lee J, Levitan I.. Hypotonic challenge of endothelial cells increases membrane stiffness with no effect on tether force. Biophys J 2018;114:929–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Hui TH, Zhou ZL, Qian J, Lin Y, Ngan AHW, Gao H.. Volumetric deformation of live cells induced by pressure-activated cross-membrane ion transport. Phys Rev Lett 2014;113:118101. [DOI] [PubMed] [Google Scholar]
- 173. Hishikawa K, Oemar BS, Nakaki T.. Static pressure regulates connective tissue growth factor expression in human mesangial cells. J Biol Chem 2001;276:16797–803. [DOI] [PubMed] [Google Scholar]
- 174. Burgdorf S, Porubsky S, Marx A, Popovic ZV.. Cancer acidity and hypertonicity contribute to dysfunction of tumor-associated dendritic cells: potential impact on antigen cross-presentation machinery. Cancers (Basel) 2020;12:2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Sebens S, Schafer H.. The tumor stroma as mediator of drug resistance - a potential target to improve cancer therapy? Curr Pharm Biotechnol 2012;13:2259–72. [DOI] [PubMed] [Google Scholar]
- 176. Ji W, Zhang Y, Deng Y, Li C, Kankala RK, Chen A.. Nature-inspired nanocarriers for improving drug therapy of atherosclerosis. Regen Biomater 2023;10:rbad069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Ellermann E, Meyer N, Cameron RE, Best SM.. In vitro angiogenesis in response to biomaterial properties for bone tissue engineering: a review of the state of the art. Regen Biomater 2023;10:rbad027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Wang B, Chen J, Caserto JS, Wang X, Ma M.. An in situ hydrogel-mediated chemo-immunometabolic cancer therapy. Nat Commun 2022;13:3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Cao D, Ding J.. Recent advances in regenerative biomaterials. Regen Biomater 2022;9:rbac098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Erfani A, Diaz AE, Doyle PS.. Hydrogel-enabled, local administration and combinatorial delivery of immunotherapies for cancer treatment. Mater Today 2023;65:227–43. [Google Scholar]
- 181. Cao Y, Wang L, Zhang X, Lu Y, Wei Y, Liang Z, Hu Y, Huang D.. Double-crosslinked PNIPAM-based hydrogel dressings with adjustable adhesion and contractility. Regen Biomater 2023;10:rbad081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Meng Z, Wang H, Liu Y, Yang M, Zeng H, Han Q.. Evaluation of the effectiveness of alginate-based hydrogels in preventing peritoneal adhesions. Regen Biomater 2023;10:rbad017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Wang S, Yu H, Wan G, Fang H, Mi J, Xu W, Sun K, Zhang K, Yin J, Deng W.. Highly tough and elastic microspheric gel for transarterial catheter embolization in treatment of liver metastasis tumor. Regen Biomater 2023;10:rbad026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Deng H, Shu X, Wang Y, Zhang J, Yin Y, Wu F, He J.. Matrix stiffness regulated endoplasmic reticulum stress-mediated apolptosis of osteosarcoma cell through Ras signal cascades. Cell Biochem Biophys 2023;81:839–50. [DOI] [PubMed] [Google Scholar]
- 185. Sun L, Wang X, He Y, Chen B, Shan B, Yang J, Wang R, Zeng X, Li J, Tan H, Liang R.. Polyurethane scaffold-based 3D lung cancer model recapitulates in vivo tumor biological behavior for nanoparticulate drug screening. Regen Biomater 2023;10:rbad091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Carneiro BA, El-Deiry WS.. Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol 2020;17:395–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Guo Z, Zhang T, Fang K, Liu P, Li M, Gu N.. The effect of porosity and stiffness of glutaraldehyde cross-linked egg white scaffold simulating aged extracellular matrix on distribution and aggregation of ovarian cancer cells. Colloid Surf A Physicochem Eng Asp 2016;504:43–52. [Google Scholar]
- 188. Liu XQ, Chen XT, Liu ZZ, Gu SS, He LJ, Wang KP, Tang RZ.. Biomimetic matrix stiffness modulates hepatocellular carcinoma malignant phenotypes and macrophage polarization through multiple modes of mechanical feedbacks. ACS Biomater Sci Eng 2020;6:3994–4004. [DOI] [PubMed] [Google Scholar]
- 189. Liu C, Liu Y, Xie HG, Zhao S, Xu XX, Fan LX, Guo X, Lu T, Sun GW, Ma XJ.. Role of three-dimensional matrix stiffness in regulating the chemoresistance of hepatocellular carcinoma cells. Biotechnol Appl Biochem 2015;62:556–62. [DOI] [PubMed] [Google Scholar]
- 190. Chaudhuri O, Cooper-White J, Janmey PA, Mooney DJ, Shenoy VB.. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020;584:535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Elosegui-Artola A. The extracellular matrix viscoelasticity as a regulator of cell and tissue dynamics. Curr Opin Cell Biol 2021;72:10–8. [DOI] [PubMed] [Google Scholar]
- 192. Ma Y, Lin M, Huang G, Li Y, Wang S, Bai G, Lu TJ, Xu F.. 3D spatiotemporal mechanical microenvironment: a hydrogel-based platform for guiding stem cell fate. Adv Mater 2018;30:1705911. [DOI] [PubMed] [Google Scholar]
- 193. Müller C, Pompe T.. Distinct impacts of substrate elasticity and ligand affinity on traction force evolution. Soft Matter 2016;12:272–80. [DOI] [PubMed] [Google Scholar]
- 194. Park YD, Tirelli N, Hubbell JA.. Photopolymerized hyaluronic acid-based hydrogels and interpenetrating networks. Biomaterials 2003;24:893–900. [DOI] [PubMed] [Google Scholar]
- 195. Foo CTSWP, Lee JS, Mulyasasmita W, Parisi-Amon A, Heilshorn SC.. Two-component protein-engineered physical hydrogels for cell encapsulation. Proc Natl Acad Sci USA 2009;106:22067–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Zhao X, Huebsch N, Mooney DJ, Suo Z.. Stress-relaxation behavior in gels with ionic and covalent crosslinks. J Appl Phys 2010;107:63509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Nam S, Stowers R, Lou J, Xia Y, Chaudhuri O.. Varying PEG density to control stress relaxation in alginate-PEG hydrogels for 3D cell culture studies. Biomaterials 2019;200:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Chaudhuri O, Gu L, Darnell M, Klumpers D, Bencherif SA, Weaver JC, Huebsch N, Mooney DJ.. Substrate stress relaxation regulates cell spreading. Nat Commun 2015;6:6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Deng H, Wang Y, Yin Y, Shu J, Zhang J, Shu X, Wu F, He J.. Effects of matrix viscoelasticity on cell-matrix interaction, actin cytoskeleton organization, and apoptosis of osteosarcome MG-63 cells. J Mater Chem B 2024;12:222–32. [DOI] [PubMed] [Google Scholar]
- 200. Wang Y, Zhang J, Shu X, Wu F, He J.. Controlled domain gels with a wide stiffness gradient simultaneously promote bone regeneration and suppress tumor recurrence through DAPK activity. Chem Eng J 2024;482:149018. [Google Scholar]
- 201. Zhang Q, Jin H, Chen L, Chen Q, He Y, Yang Y, Ma S, Xiao S, Xi F, Luo Q, Liu J.. Effect of ultrasound combined with microbubble therapy on interstitial fluid pressure and VX2 tumor structure in rabbit. Front Pharmacol 2019;10:716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Bourn MD, Batchelor DVB, Ingram N, McLaughlan JR, Coletta PL, Evans SD, Peyman SA.. High-throughput microfluidics for evaluating microbubble enhanced delivery of cancer therapeutics in spheroid cultures. J Control Release 2020;326:13–24. [DOI] [PubMed] [Google Scholar]
- 203. Feril LB, Kondo T, Takaya K, Riesz P.. Enhanced ultrasound-induced apoptosis and cell lysis by a hypotonic medium. Int J Radiat Biol 2004;80:165–75. [DOI] [PubMed] [Google Scholar]
- 204. Pang N, Li J, Sun A, Yang Z, Cheng S, Qi XR.. Prior anti-CAFs break down the CAFs barrier and improve accumulation of docetaxel micelles in tumor. Int J Nanomedicine 2018;13:5971–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Guo Q, He X, Li C, He Y, Peng Y, Zhang Y, Lu Y, Chen X, Zhang Y, Chen Q, Sun T, Jiang C.. Dandelion-like tailorable nanoparticles for tumor microenvironment modulation. Adv Sci 2019;6:1901430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Fang T, Zhang J, Zuo T, Wu G, Xu Y, Yang Y, Yang J, Shen Q.. Chemo-photothermal combination cancer therapy with ROS scavenging, extracellular matrix depletion, and tumor immune activation by telmisartan and diselenide-paclitaxel prodrug loaded nanoparticles. ACS Appl Mater Interfaces 2020;12:31292–308. [DOI] [PubMed] [Google Scholar]
- 207. Nam S, Lee J, Brownfield DG, Chaudhuri O.. Viscoplasticity enables mechanical remodeling of matrix by cells. Biophys J 2016;111:2296–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Ordas L, Costa L, Lozano A, Chevillard C, Calovoulos A, Kantar D, Fernandez L, Chauvin L, Dosset P, Doucet C, Heron-Milhavet L, Odintsova E, Berditchevski F, Milhiet P-E, Bénistant C.. Mechanical control of cell migration by the metastasis suppressor tetraspanin CD82/KAI1. Cells 2021;10:1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Ren X, Zhuang H, Li B, Jiang F, Zhang Y, Zhou P.. Gsmtx4 alleviated osteoarthritis through Piezo1/Calcineurin/NFAT1 signaling axis under excessive mechanical strain. Int J Mol Sci 2023;24:4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Zhang C, Lu Z, Lyu C, Zhang S, Wang D.. Andrographolide inhibits static mechanical pressure-induced intervertebral disc degeneration via the MAPK/Nrf2/HO-1 pathway. Drug Des Devel Ther 2023;17:535–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Li J, Zhang Y, Lou Z, Li M, Cui L, Yang Z, Zhang L, Zhang Y, Gu N, Yang F.. Magnetic nanobubble mechanical stress induces the Piezo1-Ca2+-BMP2/smad pathway to modulate neural stem cell fate and MRI/ultrasound dual imaging surveillance for ischemic stroke. Small 2022;18:e2201123. [DOI] [PubMed] [Google Scholar]
- 212. Zhou Q, Hennenberg M, Trebicka J, Jochem K, Leifeld L, Biecker E, Sauerbruch T, Heller J.. Intrahepatic upregulation of RhoA and Rho-kinase signalling contributes to increased hepatic vascular resistance in rats with secondary biliary cirrhosis. Gut 2006;55:1296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Li N, Zhang X, Zhou J, Li W, Shu X, Wu Y, Long M.. Multiscale biomechanics and mechanotransduction from liver fibrosis to cancer. Adv Drug Delivery Rev 2022;188:114448. [DOI] [PubMed] [Google Scholar]
- 214. Zheng T, Zhou Q, Huang J, Lai J, Ji G, Kong D.. Xanthohumol inhibited mechanical stimulation-induced articular ECM degradation by mediating lncRNA GAS5/miR-27a axis. Front Pharmacol 2021;12:737552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Wu J, Li Y, Dang YZ, Gao HX, Jiang JL, Chen ZN.. HAb18G/CD147 promotes radioresistance in hepatocellular carcinoma cells: a potential role for integrin beta 1 signaling. Mol Cancer Ther 2015;14:553–63. [DOI] [PubMed] [Google Scholar]
- 216. Chuang HH, Liang SW, Chang ZF, Lee HH.. Ser1333 phosphorylation indicates ROCKI activation. J Biomed Sci 2013;20:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Poisson L, Lopez-Charcas O, Chadet S, Bon E, Lemoine R, Brisson L, Ouaissi M, Baron C, Besson P, Roger S, Moussata D.. Rock inhibition promotes Na(V)1.5 sodium channel-dependent SW620 Colon cancer cell invasiveness. Sci Rep 2020;10:13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Gupta GP, Massague J.. Cancer metastasis: building a framework. Cell 2006;127:679–95. [DOI] [PubMed] [Google Scholar]
- 219. Fidler IJ. Critical factors in the biology of human cancer metastasis: twenty-eighth G.H.A. Clowes memorial award lecture. Cancer Res 1990;50:6130–8. [PubMed] [Google Scholar]
- 220. Wei L, Surma M, Shi S, Lambert-Cheatham N, Shi J.. Novel insights into the roles of rho kinase in cancer. Arch Immunol Ther Exp (Warsz) 2016;64:259–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Morgan-Fisher M, Wewer UM, Yoneda A.. Regulation of ROCK activity in cancer. J Histochem Cytochem 2013;61:185–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Lane J, Martin TA, Watkins G, Mansel RE, Jiang WG.. The expression and prognostic value of ROCK I and ROCK II and their role in human breast cancer. Int J Oncol 2008;33:585–93. [PubMed] [Google Scholar]
- 223. Liu X, Choy E, Hornicek FJ, Yang S, Yang C, Harmon D, Mankin H, Duan Z.. ROCK1 as a potential therapeutic target in osteosarcoma. J Orthop Res 2011;29:1259–66. [DOI] [PubMed] [Google Scholar]
- 224. Wong CCL, Wong CM, Tung EKK, Man K, Ng IOL.. Rho-Kinase 2 is frequently overexpressed in hepatocellular carcinoma and involved in tumor invasion. Hepatology 2009;49:1583–94. [DOI] [PubMed] [Google Scholar]
- 225. Hiemer SE, Szymaniak AD, Varelas X.. The transcriptional regulators TAZ and Yap direct transforming growth factor beta-induced tumorigenic phenotypes in breast cancer cells. J Biol Chem 2014;289:13461–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Harvey KF, Zhang X, Thomas DM.. The hippo pathway and human cancer. Nat Rev Cancer 2013;13:246–57. [DOI] [PubMed] [Google Scholar]
- 227. Lamar JM, Stern P, Liu H, Schindler JW, Jiang ZG, Hynes RO.. The hippo pathway target, Yap, promotes metastasis through its TEAD-interaction domain. Proc Natl Acad Sci USA 2012;109:E2441–E2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Zhang WQ, Dai YY, Hsu PC, Wang H, Cheng L, Yang YL, Wang YC, Xu ZD, Liu S, Chan G, Hu B, Li H, Jablons DM, You L.. Targeting Yap in malignant pleural mesothelioma. J Cell Mol Med 2017;21:2663–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Bang LG, Dasari VR, Kim D, Gogoi RP.. Differential gene expression induced by verteporfin in endometrial cancer cells. Sci Rep 2019;9:3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Noguchi S, Saito A, Mikami Y, Urushiyama H, Horie M, Matsuzaki H, Takeshima H, Makita K, Miyashita N, Mitani A, Jo T, Yamauchi Y, Terasaki Y, Nagase T.. TAZ contributes to pulmonary fibrosis by activating profibrotic functions of lung fibroblasts. Sci Rep 2017;7:42595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Eales KL, Wilkinson EA, Cruickshank G, Tucker JHR, Tennant DA.. Verteporfin selectively kills hypoxic glioma cells through iron-binding and increased production of reactive oxygen species. Sci Rep 2018;8:14358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Allegra A, Pioggia G, Innao V, Musolino C, Gangemi S.. New insights into YES-associated protein signaling pathways in hematological malignancies: diagnostic and therapeutic challenges. Cancers (Basel) 2021;13:1981. [DOI] [PMC free article] [PubMed] [Google Scholar]