Abstract
BACKGROUND
Multiple Sclerosis (MS) is a chronic inflammatory, demyelinating, degenerative disease of the central nervous system and the second most frequent cause of permanent disability in young adults. One of the most common issues concerns the ability to perform postural and gait tasks while simultaneously completing a cognitive task (namely, dual-task DT).
AIM
Assessing cognitive-motor dual-task training effectiveness in patients with Multiple Sclerosis (PwMS) for dynamic gait quality when walking on straight, curved, and blindfolded paths.
DESIGN
Two-arm single-blind randomized controlled trial. Follow-up at 8 weeks.
SETTING
Neurorehabilitation Hospital.
POPULATION
A sample of 42 PwMS aged 28-71, with a score of 4.00±1.52 on the Expanded Disability Status Scale were recruited.
METHODS
Participants were randomized in conventional (CTg) neurorehabilitation and dual-task training (DTg) groups and received 12 sessions, 3 days/week/4 weeks. They were assessed at baseline (T0), after the treatment (T1), and 8 weeks after the end of the treatment (T2) through Mini-BESTest, Tinetti Performance Oriented Mobility Assessment, Modified Barthel Index, and a set of spatiotemporal parameters and gait quality indices related to stability, symmetry, and smoothness of gait extracted from initial measurement units (IMUs) data during the execution of the 10-meter Walk Test (10mWT), the Figure-of-8 Walk Test (Fo8WT) and the Fukuda Stepping Test (FST).
RESULTS
Thirty-one PwMS completed the trial at T2. Significant improvement within subjects was found in Mini-BESTest scores for DTg from T0 to T1. The IMU-based assessment indicated significant differences in stability (P<0.01) and smoothness (P<0.05) measures between CTg and DTg during 10mWT and Fo8WT. Substantial improvements (P<0.017) were also found in the inter-session comparison, primarily for DTg, particularly for stability, symmetry, and smoothness measures.
CONCLUSIONS
This study supports the effectiveness of DT in promoting dynamic motor abilities in PwMS.
CLINICAL REHABILITATION IMPACT
Cognitive-motor DT implemented into the neurorehabilitation conventional program could be a useful strategy for gait and balance rehabilitation.
Key words: Neurorehabilitation, Postural balance, Multiple sclerosis
Multiple Sclerosis (MS) is a chronic inflammatory, demyelinating, degenerative disease of the central nervous system and the second most frequent cause of permanent disability in young adults.1 The clinical features of MS vary widely from one patient to another but 60% of cases manifest both walking and balance difficulties and cognitive deficits.2, 3 One of the most common issues concerns the ability to perform postural and gait tasks while simultaneously completing a cognitive task (namely, dual-task DT).4 Indeed, in daily life activities it is frequent that, while walking, it is needed to pay attention to environmental/external stimuli, such as talking on the mobile phone. Considering that attention is a function with limited capacity, dividing it between two simultaneous tasks (motor and cognitive) generates motor-cognitive interference with motor performance remaining stable while cognitive performance deteriorates, or the opposite.5 This deterioration in terms of cognitive/motor performance could result, consequently, in frequent falls,6 home and work accidents,7, 8 driving accidents,9 and many other adverse events that can even frequently occur in people with MS (PwMS).10 Balance and gait training programs, historically focused on deficiencies related to compromised visual, somatosensory, or vestibular sensory systems,11, 12 as well as muscle weakness and spasticity,13 are known to produce significant beneficial effects in PwMS in terms of cognitive and motor performances. Indeed, conventional rehabilitation programs still suggest separate treatments of motor and cognitive deficits,13 although task-oriented training focused on the DT paradigm demonstrated superior benefit to single-task interventions in different neurological disorders in improving dynamic motor abilities.14, 15 A recent systematic review reported that just a few studies investigated the effects of DT training in PwMS with inconsistent evidence in improving balance and gait.16 In fact, despite several encouraging findings, DT treatments seem to have little effect on clinical balance and gait measurements in PwMS. Clinical measures of balance and gait, as they do not measure dual-task components, may lack the sensitivity to identify dual-task performance improvements. Indeed, Morelli and colleagues16 suggested that future research using objective measures of postural sway, during static and dynamic balance tasks for single and dual tasks, may show important variations that are not detected by conventional clinical scales. Furthermore, an increasing number of researchers propose to introduce more objective measures of motor performance during dynamic tasks.17-19 In fact, while motor ability assessment is typically performed using clinical scales, promising results were obtained using instrumental assessments, giving a more accurate and detailed evaluation of gait alterations.20 Nowadays, many technologies have been used to quantify human motion,21 but an increasing interest has been directed toward inertial measurement units (IMUs).22, 23 IMU-based evaluation permits the quantitative estimation of spatiotemporal and gait quality parameters, the latter providing information such as dynamic postural stability and gait smoothness.24, 25 Indeed, straight walking tests, like the 10-meter Walk Test (10mWT), are frequently used to assess gait impairments,26 however, these tests are sometimes not sensitive enough to detect motor ability disorders in the activities of daily living.27 Recently, further dynamic motor tasks have been developed for patients with neurological disorders to test biomechanical characteristics related to gait stability, symmetry, and smoothness while walking following curvilinear paths or stepping while blindfolded.22, 28, 29 In the literature, an integrated clinical and instrumented-based assessment has been proven to provide quantitative and objective differentiation of various walking levels as well as the correlation between the clinical scale scores and gait stability indices in different neurological populations. This integrated approach has also already been used to assess motor changes following dynamic rehabilitation training in different neurological disorders30 and could be more sensitive in detecting changes in gait stability with respect to more traditional clinical evaluation. We hypothesize that DT-based training could be useful in improving dynamic stability during dynamic tasks assessed through a combined clinical and instrumental evaluation. Therefore, the aim of this study is to evaluate the effectiveness of cognitive-motor DT training in PwMS on dynamic gait stability during straight, curved, and blindfolded paths.
Materials and methods
Study design
This study was a two-arm single-blind randomized controlled trial. All procedures contributing to this work comply with the ethical standards of the relevant national and institutional guidelines on human experimentation and with the World Medical Association Declaration of Helsinki.31 This trial was approved by the Local Ethic Committee of “Santa Lucia” Foundation (FSL) Institute for Research and Health Care (IRCCS) (Protocol number CE/PROG.812) and carried out at the FSL IRCCS (Rome, Italy); all participants were included in the study after providing their informed consent. The trial was registered before enrollment on ClinicalTrials.gov with the ID number NCT04619953.
Based on the inclusion and exclusion criteria, a researcher who was not participating in the intervention sessions determined the patients’ eligibility to participate. Participants were randomized into one of two groups: a conventional therapy group (CTg) and a dual-task training group (DTg).
Participants
Participants with a diagnosis of MS according to the McDonald Criteria32, 33 were recruited and enrolled on the basis of consecutive sampling at the FSL between November 2020 and October 2022.
This sample size met the minimum requirements set by an a priori power analysis for nonparametric between-group comparisons conducted on preliminary data (α=0.05; β=0.8; ES=0.6).34 According to this sample size estimation procedure, each group should have had at least 15 patients.
The following inclusion criteria were applied: 1) diagnosis of relapsing–remitting (RRMS) or secondary-progressive (SPMS) MS diagnosed by a certified neurologist; 2) to be native Italian speakers; 3) aged between 28 and 71 years, 4) Expanded Disability Status Scale (EDSS) score35 between 0 and 6.5; 5) the ability to walk independently for at least 50 meters. Exclusion criteria consisted of: 1) the presence of psychiatric and neurological disorders (other than MS) and other pathological conditions and/or clinical disorders severe enough to interfere with cognitive functioning and/or the performance of motor or cognitive tasks; 2) the occurrence of a clinical relapse in the three months prior to enrollment; 3) steroid therapies in the 30 days prior to enrolment; 4) the occurrence of a lower extremity fracture within three months prior to enrolment.
Interventions
The enrolled patients underwent 12 individual sessions of conventional or DT intervention, 3 days per week/4 week. Each session lasted 50 min.
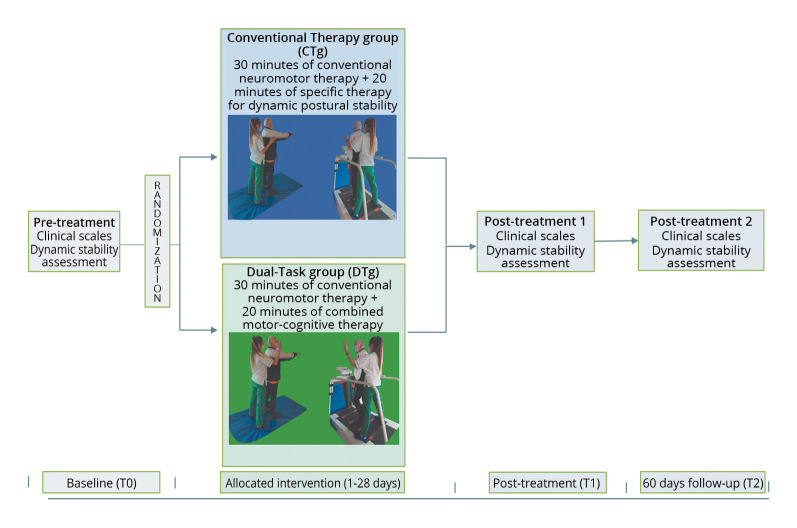
All the interventions were performed at the neurorehabilitation gym of the FSL, by physiotherapists with at least 5 years of experience in neurorehabilitation. A Schematic representation of the experimental design is reported in Figure 1.
Figure 1.
—Schematic representation of the experimental design.
Conventional therapy
Conventional neuromotor rehabilitation consisted of 30 minutes of muscle stretching, active-assisted mobilizations, neuromuscular facilitation, gait training, and balance exercises using swinging platforms,11 plus 20 minutes of dynamic postural stability consisted of marching on unstable surfaces and walking on the treadmill both with open and closed eyes.
Dual-task training
Twenty minutes of cognitive-motor training was added to conventional therapy and consisted of a dual-task paradigm in which each patient was asked to walk without stopping and was explained that, during the task, they might hear a sound, and in that case, they should have turned their head towards the stimulus’ side and recognized a visual target.36 This dual task was performed both by marching on an unstable surface and walking on the treadmill at different velocities.
Blinding randomization
The randomization was performed by an independent researcher not involved in the decision of the patient’s eligibility nor in the intervention sessions. A computer-generated randomization list was used to produce block randomization using a block size. The researcher in charge of randomization stored the list in secure web-based storage. Allocation concealment was ensured by using an automatic random number generator.
Clinical outcome measures
At enrollment, clinical and demographic data were collected. A researcher not involved in the interventions, blinded to allocation, assessed primary and secondary outcomes at baseline (T0), after 4 weeks of training (T1), and 8 weeks after the end of the training (T2).
The primary outcome measure was the Mini-BESTest37 to assess dynamic balance, postural responses, anticipatory postural adjustments, sensory orientation as well as the ability to modify the gait in response to changing task demands. Secondary outcome measures were the Tinetti Performance Oriented Mobility Assessment (POMA)38 to evaluate static balance and gait, the Modified Barthel Index (MBI) to evaluate the performance during the activities of daily living (ADL),39 and an instrumental sensor-based assessment during dynamic motor tasks.
Instrumental assessment
All participants were asked to perform three different motor tasks in a randomized order: the 10-meter Walk Test (10mWT) (Figure 2A), the Figure-of-8 Walk Test (Fo8WT) (Figure 2B)22 performed both in clockwise and counterclockwise directions, and the Fukuda Stepping Test (FST) (Figure 2C).40 Each task was performed three times, of which the mean value between trials was subsequently calculated and used for the statistical analysis.
Figure 2.
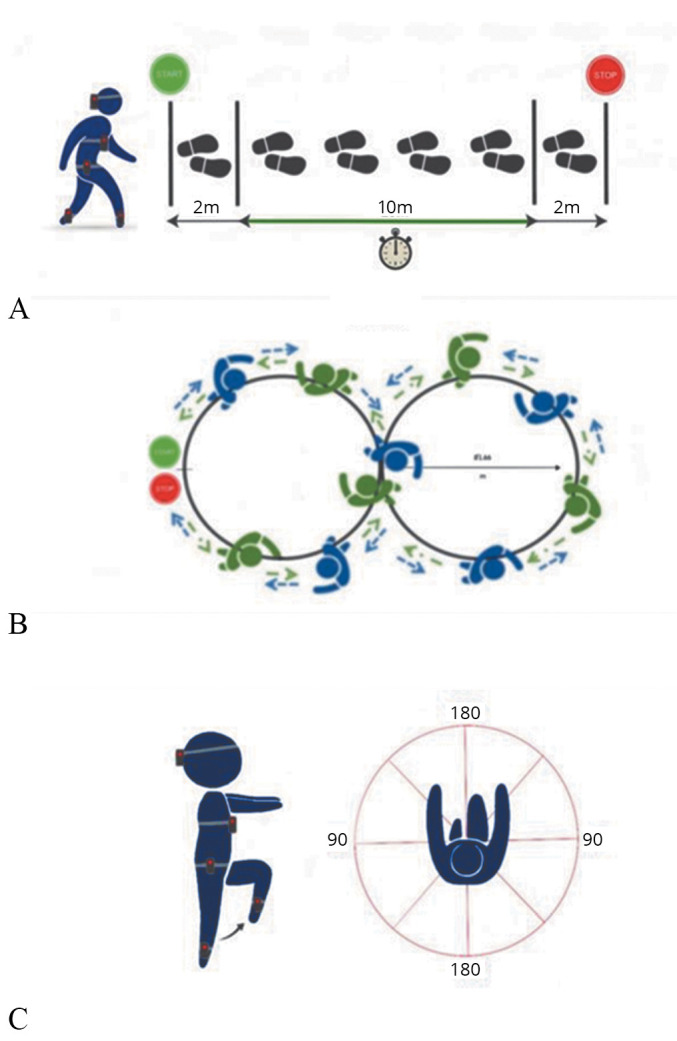
—Schematic representation of the three motor tasks performed: A) 10mWT, patients were asked to walk at their preferred speed on a 14 m trail; B) Fo8WT, clockwise and counterclockwise directions are indicated with blue and green arrows, respectively; C) FST, patients were asked to walk on the spot with eyes closed and arms in front of them.
The instrumental assessments were performed by two physiotherapists specifically trained in gait analysis with inertial sensors.
During the performance of each task (10mWT, Fo8WT, and FST), participants were equipped with five synchronized IMUs (128 Hz, Opal, APDM, Portland, OR, USA), measuring three-dimensional linear accelerations and angular velocities. IMUs were located on the occipital cranium bone, near the lambdoid suture of the head (H), at the center of the sternum (S), and at the L4/L5 level, just above the pelvis (P). For step and stride segmentation, one IMU was placed on each shank, just above the lateral malleoli. All IMUs were attached to the participant’s body with Velcro straps. The data were processed in the Matlab® environment (MATLAB R2022b, MathWorks) for the extraction of spatiotemporal and gait quality parameters. The following spatiotemporal parameters and gait quality indices were obtained for the three tasks:
spatiotemporal:
for the gait tasks: 1) average walking speed (WS) as the ratio between total distance and time to complete the test; 2) average stride duration (ADstride) as the ratio between time to complete the test and the number of strides; 3) average stride frequency (Freqstride) as the total number of strides divided by the time needed to complete the test. The number of strides was automatically obtained through a peak detection algorithm on the ML angular velocity signals measured by the two IMUs on the shanks;41, 42
for the FST, the number of steps (Nrstep), step frequency (Freqstep), and step duration (ADstep) were taken into consideration;
stability:
- normalized root mean square (RMS) of the acceleration measured at the pelvis, trunk, and head levels. The RMS values of each stride acceleration were obtained for the AP, ML, and CC components. To take the influence of the walking speed into account, the AP and ML components were then divided by the CC component, as suggested by.43 High nRMS values have been associated with higher levels of acceleration, and hence, decreased stability:18
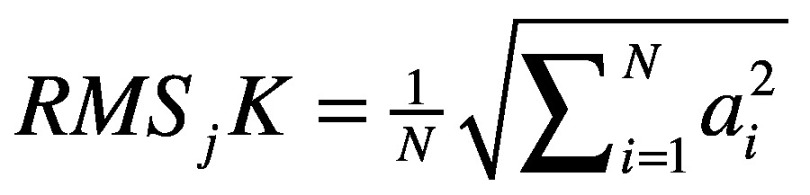 ;
;
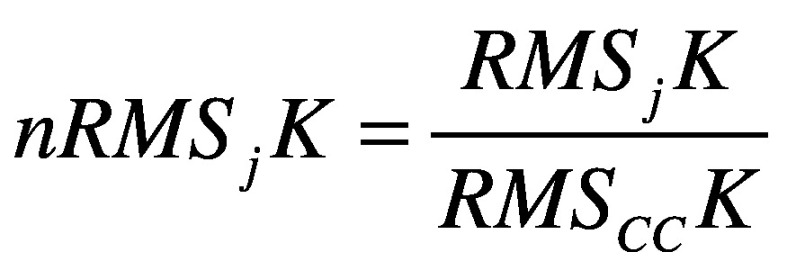
where j represents the component (AP and ML), K represents the upper body level (Pelvis, Sternum, Head), N is the number of data sample and a is the acceleration signal.
- attenuation coefficients (AC)42 between each level pair of the upper body (pelvis-sternum, pelvis-head, sternum-head), for each acceleration component (AP, ML, and CC) defined as:
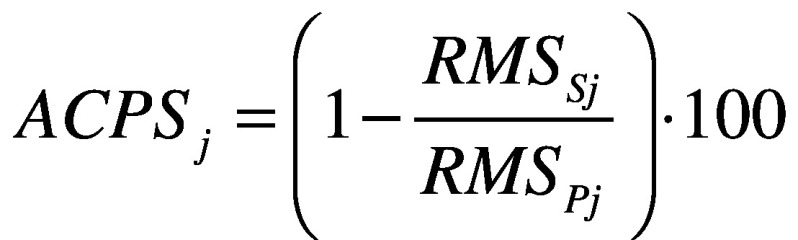 ;
;
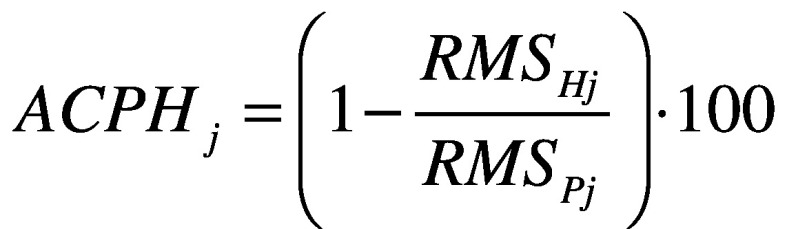 ;
;
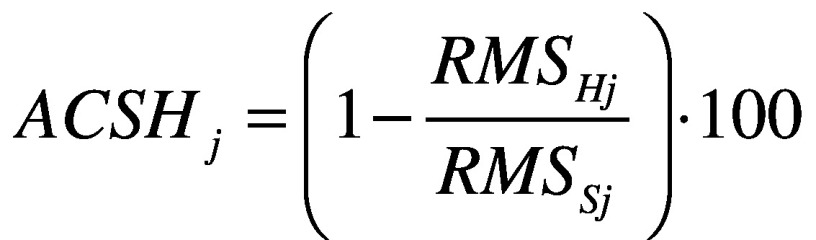
where j represents the direction AP, ML and CC. Each coefficient represents the variation of the acceleration from lower to upper-body levels. A positive coefficient indicates an attenuation of the accelerations, while a negative coefficient indicates an amplification of the accelerations from the lower to the upper body level. It has been demonstrated that, in typical walking, accelerations are attenuated from the pelvis to the head to stabilize the optic flow and increase the head stability;44
- symmetry: improved harmonic ratio (iHR)45 measured at the level of the pelvis, for each acceleration component (AP, ML and CC). This parameter ranges from 0% (total asymmetry) to 100% (total symmetry). It is calculated as:
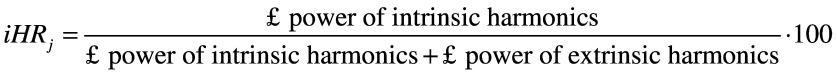
where j represents the direction AP, ML and CC.
- smoothness: log dimensionless jerk (LDLJ) measured at the pelvis level, calculated from the linear acceleration and angular velocity signals, LDLJ(a) and LDLJ(v), respectively. With reference to LDLJ(v), it is defined as:46
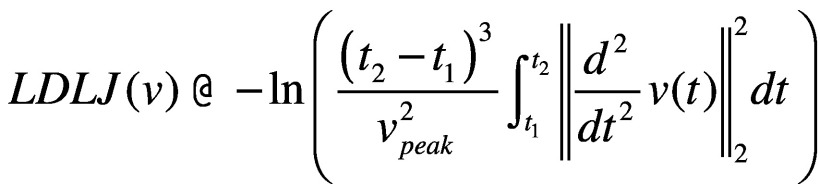 ;
;
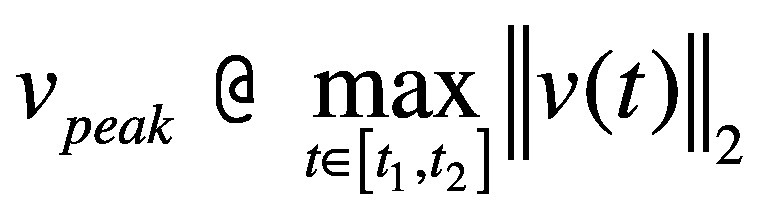
where υ(t) represents the angular velocity of the movement in the time domain; t1 and t2 represent the beginning and end of the movement, respectively. Lower LDLJ values have been associated with a higher level of smoothness of a translational/rotational movement.
Statistical analysis
Statistical analysis was performed using the IBM SPSS Statistics software (v23, IBM Corp., Armonk, NY, USA). Statistical level of significance was set at alpha = 0.05. The normal distribution of each parameter and each clinical scale was verified using the Shapiro-Wilk test. Non-parametric tests were performed for both within and between groups, after the distribution analysis. To evaluate significant differences between the two groups, the Mann-Whitney U-test was used on all estimated parameters. For the within-group analysis, a Friedman test was performed, to investigate if there were significant differences for each group across the three temporal evaluations (T0-T2). When a significant effect was found, a post-hoc test with the Wilcoxon rank-sign test with Holm-Bonferroni correction was performed.
Data availability
The data associated with the paper are not publicly available but are available from the corresponding author on reasonable request.
Results
Thirty-nine patients (CTg: 51.60±9.4 years, DTg: 50.40± 10.27 years) were enrolled and allocated to the CTg and DTg. The two groups were homogeneous with respect to demographic and anthropometric characteristics, and clinical status as reported in Table I.
Table I. —Demographic and clinical characteristics at the baseline.
| Characteristics | CTg (N.=18) | DTg (N.=21) | P value |
|---|---|---|---|
| Age (years) | 51.60±9.42 | 50.40±10.27 | 0.298 |
| Gender, female | 8 | 14 | |
| Time since MS diagnosis, years | 11±10 | 14±9.55 | 0.530 |
| EDSS | 4.00±1.51 | 4.00±1.53 | 0.837 |
| Mini-BESTest | 18.15±6.51 | 18.49±6.59 | 0.443 |
| POMA | 22.61±6.19 | 22.67±6.29 | 0.410 |
| MBI | 96.92±4.49 | 96.86±4.52 | 0.530 |
Data presented as mean±SD. CTg: conventional therapy group; DTg: dual-task training group; MS: multiple Sclerosis; EDSS: Expanded Disability Status Scale; POMA: Tinetti Performance Oriented Mobility Assessment; MBI: Modified Barthel Index.
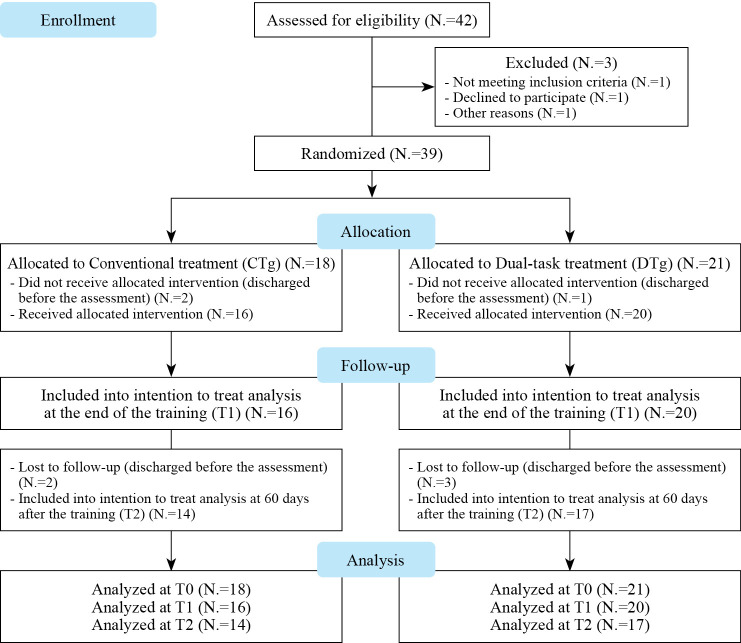
Sixteen CTg and twenty DTg patients completed the evaluation at T1 (after the end of the training) and fourteen CTg and seventeen DTg completed the evaluation at T2 (60 days after the evaluation at T1) as reported in Figure 3. The within-subjects comparison over time, showed a significant improvement in Mini-BESTest scores only for the DTg at T1 with respect to T0 (P=0.013) (Table II).
Figure 3.
—CONSORT flow diagram of patient enrollment, randomization, and procedures.
Table II. —Clinical scales scores at time T0, T1 and T2.
| Clinical scales | Group | T0 | T1 | T2 |
|---|---|---|---|---|
| Mini-BESTest | CTg | 18.15±6.51 | 19.18±7.28 | 17.71±8.10 |
| DTg | 18.49±6.59 | 21.75±5.80* | 19.05±8.89 | |
| POMA | CTg | 22.61±6.19 | 21.81±7.42 | 21.35±6.10 |
| DTg | 22.67±6.29 | 25.75±5.80 | 24.05±5.55 | |
| MBI | CTg | 96.92±4.49 | 96.12±5.45 | 95.35±5.67 |
| DTg | 96.86±4.52 | 97.6±5.24 | 96.23±7.25 |
Data presented as mean±SD. CTg: conventional therapy group; DTg: dual-task training group; POMA: Tinetti Performance Oriented Mobility Assessment; MBI: Modified Barthel Index; T0: baseline; T1: end of the training; T2: 60 days after the end of the training. *Statistically significant differences with respect to T0 (P<0.017).
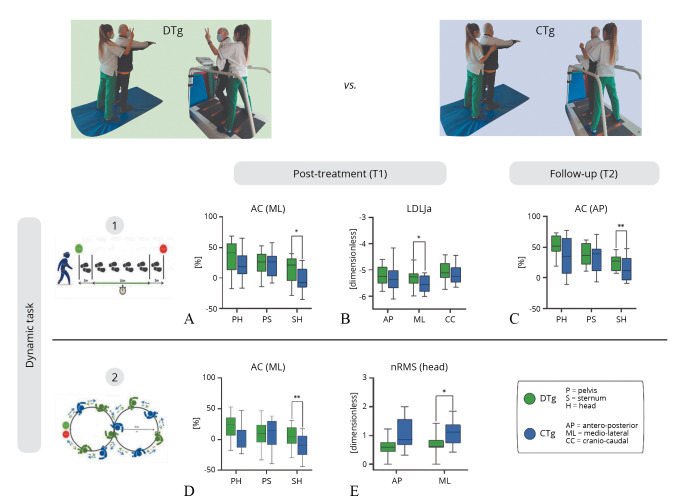
The IMU-based assessment of linear walking during the 10mWT revealed significant differences between the CTg and DTg in the ML axis for both AC from sternum to head level (U=88.00, z=-2.29, P=0.021) and LDLJa from pelvis (U=98.00, z=-1.97, P=0.049) at T1 (Figure 4A, B). Also, at time T2, the AC from sternum to head in the AP (U=22.00, z=-2.53, P=0.01) and CC axis (U=60.00, z=-2.54, P=0.01) was found significantly different between the groups (Figure 4C). A main effect of the session was highlighted for the iHR parameter in the AP and CC components for CTg, showing a difference in gait symmetry from T1 to T2 (P<0.017).
Figure 4.
—IMU-based assessment of 10mWT and Fo8WT, between groups analysis at T1 and T2. Normalized root mean square (nRMS), attenuation coefficients (AC) and Log Dimensionless Jerk on acceleration (LDLJa) values for DTg and CTg. Medians and interquartile ranges are reported. AP: antero-posterior; ML: medio-lateral; CC: cranio-caudal; P: pelvis; S: sternum; H: head. The asterisks indicate statistically significant between-group differences (* P<0.05; **P<0.01).
Significant differences between the CTg and DTg emerged during the curvilinear walking (Fo8WT) at time T1 (Figure 4D, E) in the ML axis for both the nRMS at head level (U=89.00, z=-2.26, P=0.023) and for the AC from sternum to head level (U=62.00, z=-3.11, P=0.001). Furthermore, significant differences (P<0.017) in the within-analysis were found for DTg from T0 to T1 (Freqstride, ADstride, WS, nRMS_H_ML) and from T1 to T2 (WS, LDLJv_AP). Whereas for the CTg, for the same task, significant results emerged from T0 to T1 and from T0 to T2 for iHR_CC.
During the FST, in the within-analysis, significant differences (P<0.017) for the DTg were identified from T0 to T2 for Freqstep. While for the CTg, significant results emerged for Nrstep, Freqstep, ADstep from T0 to T1, and from T0 to T2 for Nrstep, Freqstep, ADstep, LDLJa_AP, LDLJa_ML, LDLJa_CC parameters. No side effects were reported from all the included participants who completed the study.
Discussion
The aim of this study was to explore the effectiveness of combined motor and cognitive therapy through a dual-task paradigm in PwMS when compared to dynamic postural stability rehabilitation, and to assess the maintenance of possible improvements with an 8- week follow-up. DT, when integrated into a neurorehabilitation program, significantly improved (P<0.017) dynamic balance, postural responses, and adjustments assessed by the Mini-BESTest after the end of the training. Although significant improvements were observed only for the Mini-BESTest score from T0 to T1; an increase in clinical scale scores was found also from T0 to T2 in the Mini-BESTest, and from T0 to T1 in the POMA for the DTg with respect to the CTg. A wearable sensor-based protocol was adopted to evaluate gait stability, symmetry, and smoothness of gait during linear (10mWT), curved (Fo8WT), and blindfolded paths (FST). The sensor-based assessment during the dynamic tasks, showed significant improvements in both groups CTg and DTg at T1 during the 10mWT for the AC from sternum to head in AP and ML component (Figure 4A, C) and for the LDLJa in ML component (Figure 4B), implying greater stability and smoothness during straight walking after the rehabilitation program. Significant differences between groups were found in the ML axis for both AC from sternum to head level (P=0.021) and LDLJa (P=0.049) at T1, and for the AC from sternum to head in the AP (P=0.010) and CC axis (P=0.01) at T2. These results suggest that patients who performed the DT training showed more pronounced improvements in the stability and smoothness indices during the linear path when compared to CTg 60 days after the end of the program (T2). Interestingly, significant differences between groups emerged during the curvilinear walking (Fo8WT) at T1 in the ML axis for both the nRMS at head level (P=0.023) and for the AC from sternum to head level (P=0.001). These findings suggest that undergoing a more dynamic and challenging 4-week program focused on cognitive-motor training allowed PwMS to perform more stable curved paths with respect to a conventional postural stability training (Figure 4D, E). Furthermore, significant improvements were found during the Fo8WT at the end of the training in spatiotemporal (Freqstride, ADstride, WS), smoothness (LDLJv in AP direction), and stability (nRMS, head level in the ML component) parameters. Conversely for the CTg, significant results emerged only for symmetry in CC direction. The LDLJv is a significant supplementary metric to measure the effectiveness of rotational movement, showing postural reactions as a result of specific rehabilitation treatment. Because it can quantify motor recovery47, 48 and forecast the degree of motor independence,49, 50 the evaluation of smoothness is a helpful index in neurorehabilitation. The increase in LDLJv values during the curvilinear walking test indicates that a combined cognitive-motor training could provide an effective complementary strategy for enhancing dynamic motor abilities when walking along a curved path, especially for a disease that is inherently progressive. Furthermore, during the FST significant differences were found for both DTg and CTg in the spatiotemporal parameters, although LDLJa in all three directions was significant only for the CTg. Indeed, both trainings improved motor abilities during visual deprivation conditions. These results are interesting because only the CTg performed a specific training blindfolded but also the patients who performed a cognitive-motor training reported positive results during this task, suggesting that DT therapy could reduce the visual dependence in PwMS.49 To the best of our knowledge, this is the first study carried out on PwMS to evaluate the effects of a specific DT training using five synchronized IMUs to measure stability, symmetry, and smoothness of gait with a 2-month follow-up. Similar to previous studies,51-53 our results support the benefits of complementary integrated cognitive-motor training in PwMS in gait and balance recovery.
Limitations of the study
We acknowledge some limitations in the interpretation of the results. Indeed, this clinical trial was carried out during the COVID-19 pandemic, and this affected the final sample size. Furthermore, we did not report the fatigue and psychological assessment after the training to evaluate the possible effects also on neuropsychological disorders. In fact, as the DT training involves the enhancement of both motor and cognitive functions together, we may expect an improvement in some aspects related to attention and cognition in general. Future research will overcome this limitation.
Conclusions
A combined cognitive-motor DT training integrated into the neurorehabilitation conventional program could enhance the smoothness of gait during the 10mWT and dynamic postural stability during linear and curved paths when compared to conventional postural stability training.
Acknowledgements
The authors would like to thank Giordano Guredda and Stefano Turchetti for their clinical support.
Footnotes
Conflicts of interest: The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
Funding: This work was supported by the Italian Ministry of Health as part of the “Ricerca Corrente” and by Fondazione Baroni with a grant.
References
- 1.Reich DS, Lucchinett CF, Calabresi PA. Imag(in)ing multiple sclerosis: time to take better pictures. J Neuroimmunol 2017;304:72–80. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27742080&dopt=Abstract 10.1016/j.jneuroim.2016.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Argento O, Piacentini C, Bossa M, Caltagirone C, Santamato A, Saraceni V, et al. Motor, cognitive, and combined rehabilitation approaches on MS patients’ cognitive impairment. Neurol Sci 2023;44:1109–18. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=36542204&dopt=Abstract 10.1007/s10072-022-06552-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kasser SL, Jacobs JV, Foley JT, Cardinal BJ, Maddalozzo GF. A prospective evaluation of balance, gait, and strength to predict falling in women with multiple sclerosis. Arch Phys Med Rehabil 2011;92:1840–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21840497&dopt=Abstract 10.1016/j.apmr.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 4.Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol 2008;7:1139–51. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19007738&dopt=Abstract 10.1016/S1474-4422(08)70259-X [DOI] [PubMed] [Google Scholar]
- 5.Plummer P, Eskes G, Wallace S, Giuffrida C, Fraas M, Campbell G, et al. American Congress of Rehabilitation Medicine Stroke Networking Group Cognition Task Force . Cognitive-motor interference during functional mobility after stroke: state of the science and implications for future research. Arch Phys Med Rehabil 2013;94:2565–2574.e6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23973751&dopt=Abstract 10.1016/j.apmr.2013.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Etemadi Y. Dual task cost of cognition is related to fall risk in patients with multiple sclerosis: a prospective study. Clin Rehabil 2017;31:278–84. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26951347&dopt=Abstract 10.1177/0269215516637201 [DOI] [PubMed] [Google Scholar]
- 7.Argento O, Incerti CC, Pisani V, Magistrale G, Di Battista G, Romano S, et al. Domestic accidents and multiple sclerosis: an exploratory study of occurrence and possible causes. Disabil Rehabil 2014;36:2205–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24588071&dopt=Abstract 10.3109/09638288.2014.895429 [DOI] [PubMed] [Google Scholar]
- 8.Argento O, Incerti CC, Pisani V, Magistrale G, Caltagirone C, Nocentini U. Gli incidenti domestici nei pazienti con Sclerosi Multipla: proposta e applicazione di questionari per la loro rilevazione [Domestic accidents in patients with multiple sclerosis: proposal and application of questionnaires for their detection]. G Ital Med Lav Ergon 2017;38:265–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29916608&dopt=Abstract [PubMed] [Google Scholar]
- 9.Lincoln NB, Radford KA. Cognitive abilities as predictors of safety to drive in people with multiple sclerosis. Mult Scler 2008;14:123–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17893114&dopt=Abstract 10.1177/1352458507080467 [DOI] [PubMed] [Google Scholar]
- 10.Piacentini C, Argento O, Nocentini U. Cognitive impairment in multiple sclerosis: “classic” knowledge and recent acquisitions. Arq Neuropsiquiatr 2023;81:585–96. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=37379870&dopt=Abstract 10.1055/s-0043-1763485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tramontano M, Grasso MG, Soldi S, Casula EP, Bonnì S, Mastrogiacomo S, et al. Cerebellar Intermittent Theta-Burst Stimulation Combined with Vestibular Rehabilitation Improves Gait and Balance in Patients with Multiple Sclerosis: a Preliminary Double-Blind Randomized Controlled Trial. Cerebellum 2020;19:897–901. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32681455&dopt=Abstract 10.1007/s12311-020-01166-y [DOI] [PubMed] [Google Scholar]
- 12.Tramontano M, Martino Cinnera A, Manzari L, Tozzi FF, Caltagirone C, Morone G, et al. Vestibular rehabilitation has positive effects on balance, fatigue and activities of daily living in highly disabled multiple sclerosis people: A preliminary randomized controlled trial. Restor Neurol Neurosci 2018;36:709–18. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30412513&dopt=Abstract 10.3233/RNN-180850 [DOI] [PubMed] [Google Scholar]
- 13.Khan F, Amatya B, Turner-Stokes L. Symptomatic therapy and rehabilitation in primary progressive multiple sclerosis. Neurol Res Int 2011;2011:740505. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22013521&dopt=Abstract 10.1155/2011/740505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghai S, Ghai I, Effenberg AO. Effects of dual tasks and dual-task training on postural stability: a systematic review and meta-analysis. Clin Interv Aging 2017;12:557–77. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28356727&dopt=Abstract 10.2147/CIA.S125201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong-Yu IS, Mak MK. Task- and Context-Specific Balance Training Program Enhances Dynamic Balance and Functional Performance in Parkinsonian Nonfallers: A Randomized Controlled Trial With Six-Month Follow-Up. Arch Phys Med Rehabil 2015;96:2103–11. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26299751&dopt=Abstract 10.1016/j.apmr.2015.08.409 [DOI] [PubMed] [Google Scholar]
- 16.Morelli N, Morelli H. Dual task training effects on gait and balance outcomes in multiple sclerosis: A systematic review. Mult Scler Relat Disord 2021;49:102794. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33540278&dopt=Abstract 10.1016/j.msard.2021.102794 [DOI] [PubMed] [Google Scholar]
- 17.Park S, Yoon S. Validity evaluation of an inertial measurement unit (IMU) in gait analysis using statistical parametric mapping (SPM). Sensors (Basel) 2021;21:21. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34070344&dopt=Abstract 10.3390/s21113667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angelini L, Hodgkinson W, Smith C, Dodd JM, Sharrack B, Mazzà C, et al. Wearable sensors can reliably quantify gait alterations associated with disability in people with progressive multiple sclerosis in a clinical setting. J Neurol 2020;267:2897–909. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32468119&dopt=Abstract 10.1007/s00415-020-09928-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carpinella I, Anastasi D, Gervasoni E, Di Giovanni R, Tacchino A, Brichetto G, et al. Balance Impairments in People with Early-Stage Multiple Sclerosis: Boosting the Integration of Instrumented Assessment in Clinical Practice. Sensors (Basel) 2022;22:1–21. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=36502265&dopt=Abstract 10.3390/s22239558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tramontano M, Belluscio V, Bergamini E, Allevi G, De Angelis S, Verdecchia G, et al. Vestibular Rehabilitation Improves Gait Quality and Activities of Daily Living in People with Severe Traumatic Brain Injury: A Randomized Clinical Trial. Sensors (Basel) 2022;22:8553. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=36366250&dopt=Abstract 10.3390/s22218553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zampogna A, Mileti I, Palermo E, Celletti C, Paoloni M, Manoni A, et al. Fifteen years of wireless sensors for balance assessment in neurological disorders. Sensors (Basel) 2020;20:1–32. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32517315&dopt=Abstract 10.3390/s20113247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belluscio V, Bergamini E, Tramontano M, Formisano R, Buzzi MG, Vannozzi G. Does curved walking sharpen the assessment of gait disorders? An instrumented approach based on wearable inertial sensors. Sensors (Basel) 2020;20:1–11. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32937877&dopt=Abstract 10.3390/s20185244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tramontano M, Bergamini E, Iosa M, Belluscio V, Vannozzi G, Morone G. Vestibular rehabilitation training in patients with subacute stroke: A preliminary randomized controlled trial. NeuroRehabilitation 2018;43:247–54. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30040765&dopt=Abstract 10.3233/NRE-182427 [DOI] [PubMed] [Google Scholar]
- 24.Del Din S, Kirk C, Yarnall AJ, Rochester L, Hausdorff JM. Body-Worn Sensors for Remote Monitoring of Parkinson’s Disease Motor Symptoms: Vision, State of the Art, and Challenges Ahead. J Parkinsons Dis 2021;11(s1):S35–47. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33523020&dopt=Abstract 10.3233/JPD-202471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beck Y, Herman T, Brozgol M, Giladi N, Mirelman A, Hausdorff JM. SPARC: a new approach to quantifying gait smoothness in patients with Parkinson’s disease. J Neuroeng Rehabil 2018;15:49. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29914518&dopt=Abstract 10.1186/s12984-018-0398-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia FD, da Cunha MJ, Schuch CP, Schifino GP, Balbinot G, Pagnussat AS. Movement smoothness in chronic post-stroke individuals walking in an outdoor environment-A cross-sectional study using IMU sensors. PLoS One 2021;16:e0250100. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33886640&dopt=Abstract 10.1371/journal.pone.0250100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kieseier BC, Pozzilli C. Assessing walking disability in multiple sclerosis. Mult Scler 2012;18:914–24. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22740603&dopt=Abstract 10.1177/1352458512444498 [DOI] [PubMed] [Google Scholar]
- 28.Hess RJ, Brach JS, Piva SR, VanSwearingen JM. Walking skill can be assessed in older adults: validity of the Figure-of-8 Walk Test. Phys Ther 2010;90:89–99. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19959654&dopt=Abstract 10.2522/ptj.20080121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong SS, Yam MS, Ng SS. The Figure-of-Eight Walk test: reliability and associations with stroke-specific impairments. Disabil Rehabil 2013;35:1896–902. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23600714&dopt=Abstract 10.3109/09638288.2013.766274 [DOI] [PubMed] [Google Scholar]
- 30.Tramontano M, Russo V, Spitoni GF, Ciancarelli I, Paolucci S, Manzari L, et al. Efficacy of Vestibular Rehabilitation in Patients With Neurologic Disorders: A Systematic Review. Arch Phys Med Rehabil 2021;102:1379–89. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33383031&dopt=Abstract 10.1016/j.apmr.2020.11.017 [DOI] [PubMed] [Google Scholar]
- 31.General Assembly of the World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Coll Dent 2014;81:14–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25951678&dopt=Abstract [PubMed] [Google Scholar]
- 32.Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20332509&dopt=Abstract 10.1136/bmj.c332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21387374&dopt=Abstract 10.1002/ana.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen J. Statistical power analysis for the behavioral sciences. Second edition. New York: Routledge; 1988. [Google Scholar]
- 35.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444–52. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=6685237&dopt=Abstract 10.1212/WNL.33.11.1444 [DOI] [PubMed] [Google Scholar]
- 36.Tramontano M, Morone G, Curcio A, Temperoni G, Medici A, Morelli D, et al. Maintaining gait stability during dual walking task: effects of age and neurological disorders. Eur J Phys Rehabil Med 2017;53:7–13. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27575014&dopt=Abstract 10.23736/S1973-9087.16.04203-9 [DOI] [PubMed] [Google Scholar]
- 37.Franchignoni F, Horak F, Godi M, Nardone A, Giordano A. Using psychometric techniques to improve the Balance Evaluation Systems Test: the mini-BESTest. J Rehabil Med 2010;42:323–31. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20461334&dopt=Abstract 10.2340/16501977-0537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canbek J, Fulk G, Nof L, Echternach J. Test-retest reliability and construct validity of the tinetti performance-oriented mobility assessment in people with stroke. J Neurol Phys Ther 2013;37:14–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23389388&dopt=Abstract 10.1097/NPT.0b013e318283ffcc [DOI] [PubMed] [Google Scholar]
- 39.Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol 1989;42:703–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2760661&dopt=Abstract 10.1016/0895-4356(89)90065-6 [DOI] [PubMed] [Google Scholar]
- 40.Belluscio V, Bergamini E, Iosa M, Tramontano M, Morone G, Vannozzi G. The iFST: an instrumented version of the Fukuda Stepping Test for balance assessment. Gait Posture 2018;60:203–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29277058&dopt=Abstract 10.1016/j.gaitpost.2017.12.010 [DOI] [PubMed] [Google Scholar]
- 41.De Marchis C, Ranaldi S, Varrecchia T, Serrao M, Castiglia SF, Tatarelli A, et al. Characterizing the Gait of People With Different Types of Amputation and Prosthetic Components Through Multimodal Measurements: A Methodological Perspective. Front Rehabil Sci 2022;3:804746. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=36189078&dopt=Abstract 10.3389/fresc.2022.804746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castiglia SF, Trabassi D, De Icco R, Tatarelli A, Avenali M, Corrado M, et al. Harmonic ratio is the most responsive trunk-acceleration derived gait index to rehabilitation in people with Parkinson’s disease at moderate disease stages. Gait Posture 2022;97:152–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35961132&dopt=Abstract 10.1016/j.gaitpost.2022.07.235 [DOI] [PubMed] [Google Scholar]
- 43.Marchetti GF, Whitney SL, Blatt PJ, Morris LO, Vance JM. Temporal and spatial characteristics of gait during performance of the Dynamic Gait Index in people with and people without balance or vestibular disorders. Phys Ther 2008;88:640–51. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18292216&dopt=Abstract 10.2522/ptj.20070130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mazzà C, Iosa M, Pecoraro F, Cappozzo A. Control of the upper body accelerations in young and elderly women during level walking. J Neuroeng Rehabil 2008;5:30. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19014631&dopt=Abstract 10.1186/1743-0003-5-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pasciuto I, Bergamini E, Iosa M, Vannozzi G, Cappozzo A. Overcoming the limitations of the Harmonic Ratio for the reliable assessment of gait symmetry. J Biomech 2017;53:84–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28104246&dopt=Abstract 10.1016/j.jbiomech.2017.01.005 [DOI] [PubMed] [Google Scholar]
- 46.Melendez-Calderon A, Shirota C, Balasubramanian S. Estimating Movement Smoothness From Inertial Measurement Units. Front Bioeng Biotechnol 2021;8:558771. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33520949&dopt=Abstract 10.3389/fbioe.2020.558771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menz HB, Lord SR, Fitzpatrick RC. Acceleration patterns of the head and pelvis when walking on level and irregular surfaces. Gait Posture 2003;18:35–46. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12855299&dopt=Abstract 10.1016/S0966-6362(02)00159-5 [DOI] [PubMed] [Google Scholar]
- 48.Latt MD, Menz HB, Fung VS, Lord SR. Walking speed, cadence and step length are selected to optimize the stability of head and pelvis accelerations. Exp Brain Res 2008;184:201–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17717650&dopt=Abstract 10.1007/s00221-007-1094-x [DOI] [PubMed] [Google Scholar]
- 49.Brach JS, McGurl D, Wert D, Van Swearingen JM, Perera S, Cham R, et al. Validation of a measure of smoothness of walking. Journals Gerontol - Ser A Biol Sci. Med Sci 2011;66:136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duarte E, Marco E, Muniesa JM, Belmonte R, Aguilar JJ, Escalada F. Early detection of non-ambulatory survivors six months after stroke. NeuroRehabilitation 2010;26:317–23. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20555154&dopt=Abstract 10.3233/NRE-2010-0568 [DOI] [PubMed] [Google Scholar]
- 51.Peruzzi A, Zarbo IR, Cereatti A, Della Croce U, Mirelman A. An innovative training program based on virtual reality and treadmill: effects on gait of persons with multiple sclerosis. Disabil Rehabil 2017;39:1557–63. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27808596&dopt=Abstract 10.1080/09638288.2016.1224935 [DOI] [PubMed] [Google Scholar]
- 52.Veldkamp R, Baert I, Kalron A, Tacchino A, D’hooge M, Vanzeir E, et al. Structured Cognitive-Motor Dual Task Training Compared to Single Mobility Training in Persons with Multiple Sclerosis, a Multicenter RCT. J Clin Med 2019;8:2177. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31835502&dopt=Abstract 10.3390/jcm8122177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jonsdottir J, Gervasoni E, Bowman T, Bertoni R, Tavazzi E, Rovaris M, et al. Intensive multimodal training to improve gait resistance, mobility, balance and cognitive function in persons with multiple sclerosis: A pilot randomized controlled trial. Front Neurol 2018;9:800. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30323787&dopt=Abstract 10.3389/fneur.2018.00800 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data associated with the paper are not publicly available but are available from the corresponding author on reasonable request.