Abstract
Pulmonary embolism (PE) represents mechanical obstruction of one or more branches of pulmonary vasculature due to thromboembolism from a deep vein thrombosis. Hormonal contraceptives, hormonal replacement therapy, antipsychotics and fibrates are medications that are associated with increased risk of venous thromboembolism(VTE). Etanercept is a TNF alpha and beta receptor blocker. Rarely this medication has been identified as a risk factor for VTE. There is a possible correlation between rheumatoid arthritis(RA) and risk of VTE in patients with RA. Use of medication that can increase risk of VTE in a patient with systemic inflammatory condition that in itself may be a risk factor for VTE requires shared decision and risk versus benefit discussion with the patient. We present a case of unprovoked PE in a patient on Etanercept treatment for RA. Though in this case, a causal relationship between the drug use and the event could not be proved, the patient preferred to discontinue the medication. In newer classes of medications, when the mechanism of side effects is unclear, we strongly recommend extensive discussion with patients about available scientific literature and encourage shared decision making with the patient.
Keywords: Pulmonary embolism, Venous thromboembolism, Etanercept, Shared decision making
1. Introduction
RA is the most common inflammatory arthritis with estimated prevalence of 0.6% in the United states.1 It is a systemic inflammatory condition causing symmetric, relapsing or chronic synovitis. Disease modifying antirheumatic drugs (DMARDS) are recommended first line therapy. Biological DMARDS such as abatacept, anakinra, rituximab, tocilizumab and TNF inhibitors such as etanercept are recommended after failure of nonbiologic DMARDS. Injection site reaction, upper respiratory infections and diarrhea are the most common side effects of etanercept. Rarely this medication has been associated with increasing the risk of VTE.
2. Case presentation
A 67-year-old male with a past medical history of rheumatoid arthritis, emphysema, coronary artery disease with history of coronary artery bypass grafting (CABG) 6 months ago presented with chest pain. Patient had severe COPD with FEV1 (Forced Expiratory Volume) of 33%. Chest pain was acute in onset, left-sided, sharp, nonradiating, not associated with nausea vomiting or diaphoresis. Patient reported no change in his ambulatory status prior to this admission. Patient did not have personal or family history of blood clots. No recent long-distance travel. Patient was diagnosed with rheumatoid arthritis(RA) around 20 years prior to presentation and family history was positive for RA in his mother, without any history of VTE in the mother. Other family history was positive for congestive heart failure and diabetes in mother and hypertension in father. Patient reported a 12 pack-year smoking history. Patient reported quitting smoking after his CABG 6 months ago. He denied alcohol use. He denied any other illicit drug use. At baseline, he was able to perform his activities of daily living and walk one block. His body mass index (BMI) was 19.66 kg/m2. The patient’s Well’s score (Well’s criteria for Pulmonary embolism) was 3, placing patients in the moderate risk group for VTE.
The patient had been on etanercept therapy for treatment of methotrexate resistant RA for approximately 2 years prior to presentation. His symptoms of RA were well controlled with no worsening joint symptoms prior to this presentation.
On physical examination, the patient was noted to be tachypnic with respiratory rate of 25 per minutes, tachycardic at heart rate of 110 per minute and was hypoxic with pulse oximetry reading of 86% on room air. No lung crackles or wheezing was noted on auscultation. No pedal edema or jugular venous distension was noted.
2.1. Investigations
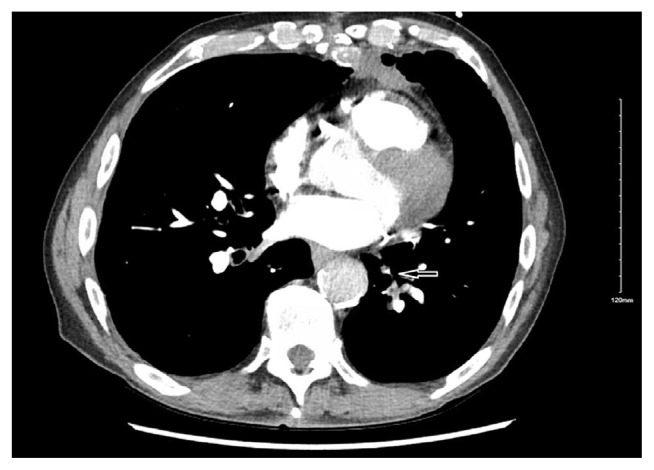
Complete blood count showed no leukocytosis. Patient had chronic anemia and hemoglobin was noted to be at baseline. He did not have any thrombocytosis. Comprehensive metabolic profile showed mild hypokalemia otherwise was within normal limits. B-natriuretic peptide was not elevated. Antinuclear antibody screen was negative. Anti phospholipid antibody was negative. A computed tomographic angiogram of the chest showed segmental and subsegmental pulmonary embolism involving bilateral lower lobes of lungs [Fig. 1].
Fig. 1.
Axial computed tomographic image of lung showing pulmonary embolism (leftward arrow).
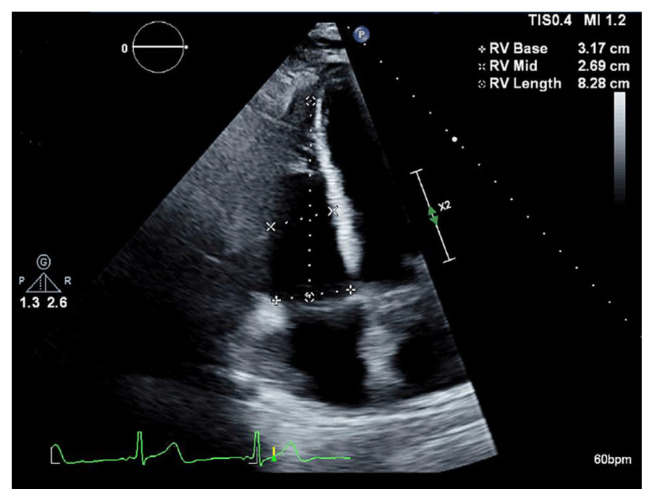
Duplex venous ultrasound of bilateral lower extremity was negative. Echocardiogram showed mildly dilated right ventricle without compromise of systolic function [Fig. 2].
Fig. 2.
Transthoracic echocardiogram of heart showing mild right ventricle dilation (marked).
2.2. Treatment
Patient was initiated on heparin drip for acute pulmonary embolism and was transitioned to apixaban for treatment of acute pulmonary embolism. The patient was on dual antiplatelet treatment with his recent history of coronary artery bypass grafting. As his CABG was more than 6 months prior to this presentation his aspirin was discontinued, and the patient was continued on clopidogrel and apixaban on discharge.
3. Discussion
Etanercept is a recombinant DNA-derived protein composed of tumor necrosis factor receptor (TNFR) linked to the Fc portion of human IgG1. TNF is a naturally occurring cytokine involved in normal inflammatory response, plays a key role in the inflammatory processes and the resulting joint pathology of rheumatoid arthritis (RA), polyarticularcourse juvenile idiopathic arthritis (JIA), ankylosing spondylitis (AS), and plaque psoriasis.2 It is used as an alternative to methotrexate in disease-modifying antirheumatic drug-naive patients with moderate to high disease activity in RA, or as adjunctive therapy in patients who have not met treatment goals despite maximally tolerated methotrexate therapy.
Common side effects include skin rash, diarrhea and upper respiratory infections. The medication can cause antinuclear antibody(ANA) positivity in approximately 11% of patients. It can cause induction of antiphospholipid antibody (APL), anticardiolipin antibody and lupus anticoagulant.3 Anti double stranded antibody can be found in 3–15% of cases treated with etanercept.4 As a result of induction of autoantibodies, the medication is suspected to increase risk of venous thromboembolic event (VTE), though the exact mechanism remains unclear.4 Makol et al. in their case series of 3 patients with rheumatoid arthritis treated with etanercept, reported DVT or pulmonary embolism 1–3 years after initiation of medication.5 In this case series, all 3 patients had elevated APTT (activated Partial thromboplastin time) at time of presentation. Interestingly in our patient the APTT prior to initiation of heparin drip was normal. Another case series of 5 patients by Virupannavar et al. supported the correlation between etanercept, antiphospholipid syndrome and VTE.6 The authors, based on their study, recommended checking APL prior to initiation of medications.6
The mechanism behind etanercept causing thromboembolic events is unclear. Medication induced autoantibody leading to hypercoagulable state is thought to be the underlying pathogenesis. Interestingly, our patient tested negative for lupus anticoagulant antibody. Our patient did not report any weight loss or type B symptoms to suggest a possible active malignancy process leading to hypercoagulable state. Also, he was not on any hormonal supplement or other medication that could have led to hypercoagulable state. Our patient had his first event of unprovoked VTE at an advanced age, with no family history of thromboembolism, therefore workup for inherited thrombophilia was not performed based on guidelines.7
RA itself is a systemic inflammatory condition, production of cytokines such as TNF-alpha and IL-1 is thought to lead to downregulation of protein C, inhibition of fibrinolysis and endothelial dysfunction.8,9 There is data showing possible correlation between RA and risk of VTE. A large retrospective study performed by Kim et al. comparing the risk of VTE in RA patients and new VTE on post hospital discharge, concluded that these patients have 2.4 times higher risk of VTE.10 But, the authors also reported that one third of the patients had at least one major VTE risk factor such as acute hospitalization, surgery and malignancy diagnosis 90 days before and after the VTE event that could have confounded the result.10 Choi et al. performed another study in the UK showing higher risk of VTE in patients with RA.11 In this study 84.5% did not have a history of hospitalization within the last year. Holmqvist et al. performed a study in Sweden that showed a higher rate of VTE in the first year following hospitalization in RA patients as compared to the general population. 12 Body mass index and its regional variability due to lifestyle, immobility and other medical comorbidities can contribute to confounding these results or association between RA and VTE. The Wells score is a predictive score based on clinical findings of signs or symptoms of deep vein thrombosis, tachycardia, immobilization, prior history of VTE, hemoptysis and active malignancy. Rheumatoid arthritis is one of the autoimmune disorders, associated with systemic inflammation and few studies have identified a higher risk of VTE in patients with RA. Addition of RA and other autoimmune disorders to the Wells criteria is a topic for further exploration, as this will place several low risk patients into a higher risk category.
As both RA and use of etanercept can lead to VTE, a shared decision making between patient and provider, with discussion of risk versus benefit is suggested. Literature review of etanercept leading to possible VTE was discussed with the patient and he expressed his wishes to discontinue the medication at time of discharge. Patients’ symptoms of RA were under control. On outpatient follow up with rheumatologist, the patient was not initiated on other biologics. He was on opioids for pain control that were continued. Other biologics such as infliximab and adalimumab have been associated with increased risk of VTE, though causative relationship still needs to be studied further.13–15
4. Limitations
We would like to reiterate that our patient had multiple medical comorbidities including severe COPD, RA and history of fairly recent CABG six months prior to this presentation. His recent CABG could have contributed to the risk of PE. As the etiology of VTE was considered provoked, he was discharged with plan to continue anticoagulation for 6 months, while holding the etanercept. Our patient was on etanercept for about 2 years prior to this presentation, but, based on a case series by Makol et al. the side effect can be seen 1–3 years after initiation of the medication.5 As this side effect of medication is still being studied, one may argue on the temporal correlation between the drug initiation and the noted side effect.
5. Conclusions
Though venous thromboembolism while on TNF treatment is a rare side effect of etanercept, it should be considered as a trigger for hypercoagulable state. Etanercept can cause auto antibody formation that may explain the pathogenesis of VTE while on this medication, though no larger studies exist to confirm this correlation. Also, study of independent markers which could predict the higher risk of VTE in such cases may be considered. Due to risk of thromboembolism while on the medication we recommend discontinuation of medication in patients who have had unprovoked pulmonary embolism. More research is needed to study this medication’s effect on pro coagulant proteins.
Footnotes
Disclaimers: The article has NOT been submitted to other publications and/or presented at a conference or meeting.
Conflict of interest: The authors report no conflict of interest.
Source(s) of support: The Authors report NO source(s) of support such as grants, drug(s), equipment, and/or other support that facilitated conduct of the work described in the article or in the writing of the article.
References
- 1. Littlejohn EA, Monrad SU. Early diagnosis and treatment of rheumatoid arthritis. Prim Care. 2018;45:237–255. doi: 10.1016/j.pop.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Product Information: Enbrel, Subcutaneous Injection Solution, Etanercept Subcutaneous Injection Solution. Thousands Oaks, CA: Amgen Inc; 2015. [Google Scholar]
- 3. Bazzoni F, Beutler B. The tumor necrosis factor ligand and receptor families. N Engl J Med. 1996;334:717–725. doi: 10.1056/NEJM199606273342607. [DOI] [PubMed] [Google Scholar]
- 4. Pisetsky DS. Tumor necrosis factor alpha blockers and the induction of anti-DNA autoantibodies. Arthritis Rheum. 2000;43:11–2381. doi: 10.1002/1529-0131(200011)43:11<2381::AID-ANR1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 5. Makol Grover M, Guggenheim C, Hassouna H. Etanercept and venous thromboembolism: a case series. J Med Case Rep. 2010;12:20104. doi: 10.1186/1752-1947-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Virupannavar S, Brandau A, Guggenheim C, Laird-Fick H. Possible association of etanercept, venous thrombosis, and induction of antiphospholipid syndrome. Case Rep Rheumatol. 2014;2014:801072. doi: 10.1155/2014/801072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Institute for Health and Care Excellence. Venous thromboembolic diseases: the management of venous thromboembolic diseases and the role of thrombophilia testing. London: NICE; https://www.nice.org.uk/guidance/ng158 . [Google Scholar]
- 8. Fox EA, Kahn SR. The relationship between inflammation and venous thrombosis. A systematic review of clinical studies. Thromb Haemostasis. 2005;94:362–365. doi: 10.1160/TH05-04-0266. [DOI] [PubMed] [Google Scholar]
- 9. Zöller B, Li X, Sundquist J, et al. Autoimmune diseases and venous thromboembolism: a review of the literature. Am J Cardiovasc Dis. 2012;2:171–183. [PMC free article] [PubMed] [Google Scholar]
- 10. Kim SC, Schneeweiss S, Liu J, Solomon DH. Risk of venous thromboembolism in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken. 2013;10:1600–1607. doi: 10.1002/acr.22039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choi HK, Rho YH, Zhu Y, et al. The risk of pulmonary embolism and deep vein thrombosis in rheumatoid arthritis: a UK population-based outpatient cohort study. Ann Rheum Dis. 2012 doi: 10.1136/annrheumdis-2012-201669. [DOI] [PubMed] [Google Scholar]
- 12. Holmqvist M, Neovius M, Eriksson J, et al. Risk of venous thromboembolism in patients with rheumatoid arthritis and association with disease duration and hospitalization. JAMA. 2012;308:350–356. doi: 10.1001/2012.jama.11741. [DOI] [PubMed] [Google Scholar]
- 13. Jonsdottir T, Forslid J, van Vollenhoven A, et al. Treatment with tumour necrosis factor alpha antagonists in patients with rheumatoid arthritis induces anticardiolipin antibodies. Ann Rheum Dis. 2004;63:075–078. doi: 10.1136/ard.2003.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Korswagen LA, Bartelds GM, Krieckaert CL, et al. Venous and arterial thromboembolic events in adalimumab-treated patients with antiadalimumab antibodies: a case series and cohort study. Arthritis Rheum. 2011;63:877–883. doi: 10.1002/art.30209. [DOI] [PubMed] [Google Scholar]
- 15. Kim SC, Solomon DH, Liu J, Franklin JM, Glynn RJ, Schneeweiss S. Risk of venous thromboembolism in patients with rheumatoid arthritis: initiating disease-modifying antirheumatic drugs, 539-7. Am J Med. 2015;128 doi: 10.1016/j.amjmed.2014.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]