Abstract
Background
Transarterial chemoembolization (TACE) and hepatic arterial infusion chemotherapy (HAIC) are two new treatments for hepatocellular carcinoma (HCC). Previous studies had reported that TACE combined with HAIC conferred better survival benefit than TACE alone. The study was to evaluate the availability and safety of TACE combined with HAIC for the treatment of large HCC.
Methods
Patients with unresectable large HCC who underwent TACE combined with HAIC (TACE-HAIC group) and HAIC alone (HAIC group) at the Department of Interventional Radiology between August 2018 and September 2022 were retrospectively enrolled in this study. Overall survival (OS), progression-free survival (PFS), tumor response, and adverse events (AEs) were used to evaluate the efficacy and safety of the two groups by using log-rank test. The independent factors of OS of large HCC patients were investigated by Cox regression model.
Results
A total of 73 patients (mean age, 59.8±8.8; 60 men) with unresectable large HCC were finally screened in the current study, including 32 who received TACE combined with HAIC and 41 who received HAIC alone. Compared with patients in HAIC group, TACE-HAIC group had higher median OS (37.1 vs. 14.9 months, P=0.0014). Similarly, PFS in the TACE-HAIC group was longer than that in the HAIC group (16.5 vs. 6.9 months, P=0.0037). The objective response rate (ORR) was 65.6% vs. 53.7% and the disease control rate (DCR) was 90.6% vs. 78.0% in the two groups, neither was statistically significant (P=0.345 and 0.208, respectively). All AEs related to therapy were manageable, and there were no significant differences in the incidence of any grade and grade 3/4 AEs between the two groups (P>0.05).
Conclusions
TACE combined with HAIC yielded a promising prognosis in treating patients with large HCC compared with HAIC alone, with tolerable toxicity.
Keywords: Transarterial chemoembolization (TACE), hepatic arterial infusion chemotherapy (HAIC), hepatocellular carcinoma (HCC), large, survival prognosis
Highlight box.
Key findings
• Transarterial chemoembolization (TACE) combined with hepatic arterial infusion chemotherapy (HAIC) yielded a promising prognosis in treating patients with large hepatocellular carcinoma (HCC) compared with HAIC alone, with tolerable toxicity.
What is known and what is new?
• TACE and HAIC are two new treatments for HCC. Previous studies had reported that TACE combined with HAIC conferred better survival benefit than TACE alone.
• This study is the first to compare the efficacy and safety of TACE combined with HAIC and HAIC alone in the treatment of large HCC.
What is the implication, and what should change now?
• The treatment of TACE combined with HAIC needs to be emphasized.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide and the third most common cause of cancer-associated mortality (1,2). Surgical resection is the preferred treatment for HCC, but 50% patients are referred to as unresectable when they are first diagnosed (3). As a widely used treatment strategy for unresectable HCC, transarterial chemoembolization (TACE) can block the blood supply artery of HCC, cause cancer tissue shrinkage and necrosis, and finally eliminate tumors (4,5). However, TACE alone may not be suitable for all patients, such as those with large HCC, which may be of limited benefit (6). Therefore, there is a long way to seek more effective treatments for such populations.
Hepatic arterial infusion chemotherapy (HAIC), an important local therapy for HCC, can directly deliver chemotherapeutic drugs to tumor-feeding arteries and increase the local drug concentration in the liver to achieve stronger antitumor effects. It can also pass through the first-pass effect of the liver to generate minimal systemic toxicity (7-9). HAIC is widely used in Asia, particularly in Japan (10). A retrospective study showed that HAIC with modified FOLFOX (mFOLFOX), composed of 5-fluorouracil (5-FU), leucovorin, and oxaliplatin (OXA), had a relatively high response and acceptable toxicity (11). Moreover, he reported that HAIC with mFOLFOX yielded significantly better clinical effect than did TACE in large HCC (12). These studies revealed the wide prospect of HAIC for large HCC.
A previous study reported that TACE combined with HAIC conferred better survival benefit than TACE alone (13). However, to date, few studies have compared TACE plus HAIC and HAIC alone as the initial treatments for unresectable large HCC. Here, we carried out a retrospective study to evaluate the safety and effectiveness of TACE plus HAIC compared to HAIC alone in large HCC patients. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-821/rc).
Methods
Study population
This retrospective study enrolled patients with large HCC at the Department of Interventional Radiology between August 2018 and September 2022 from the Affiliated Hospital of Xuzhou Medical University, who were treated with TACE and HAIC in combination (TACE-HAIC group, 32 patients) or HAIC alone (HAIC group, 41 patients). HCC was diagnosed based on the guidelines of the European Association for the Study of Liver (EASL) and the American Association for the Study of Liver Disease (AASLD) (14,15).
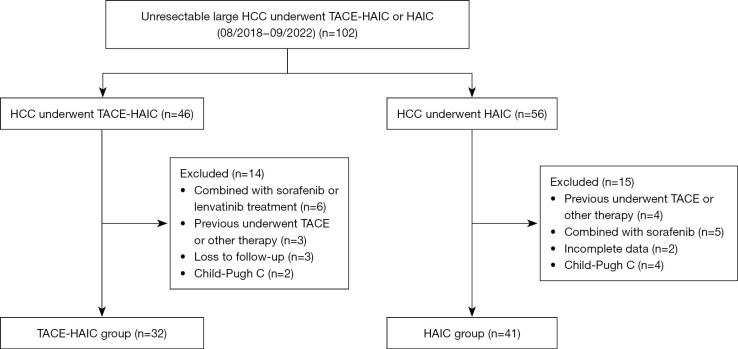
The inclusion criteria were as follows: (I) maximum cross section of tumors >5 cm on computed tomography (CT) or magnetic resonance imaging (MRI); (II) with no indication for surgical resection after a multidisciplinary discussion; (III) Eastern Cooperative Oncology Group (ECOG) performance status ≤1; and (IV) Child-Pugh (CP) class A or B. Exclusion criteria: (I) previously received radical surgery, chemotherapy, radiotherapy, or other therapeutic methods; (II) combined with other malignant tumors; (III) CP class C; (IV) combined with sorafenib, lenvatinib, other immunotherapy or systemic chemotherapy; and (V) incomplete admission or follow-up data (Figure 1). This study was approved by the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (No. XYFY2023-KL116-01), and the procedures were performed in accordance with the principles of the Declaration of Helsinki (as revised in 2013). Informed consent was not required owing to the retrospective nature of the study.
Figure 1.
Flow diagram of the enrolled population. HCC, hepatocellular carcinoma; TACE, transarterial chemoembolization; HAIC, hepatic arterial infusion chemotherapy.
Therapeutic procedure
The protocol was completed in digital subtraction angiography room by experienced interventionalists. A 4F-sheath was applied to puncture the femoral artery, according to the modified Seldinger method. Next, a 4F MPA catheter was inserted into the celiac trunk and superior mesenteric artery, as well as into the right diaphragm artery. Arteriography was routinely performed to assess the tumor-supplying arteries. In the TACE-HAIC group, the microcatheter was super-selectively catheterized to the feeding artery of the tumor and a mixed suspension of iodized oil (3–15 mL) and idarubicin (20–40 mg) was injected under fluoroscopic guidance. For particularly large tumors, gelatin sponge particles were embolized to the proximal end of the tumor-feeding artery. The endpoint of TACE was that arteriography showed the stasis of tumor’s blood flow. The patients then returned to the wards to complete HAIC treatment. HAIC was administered using the modified FOLFOX regimen: OXA 85 mg/m2 for 4 h, leucovorin 200 mg/m2 for 2 h, and 5-FU, 1.5 g/m2 for 20 h. The sheath and catheter were pulled when the HAIC treatment was completed. Repeated therapy was administered every 3–4 weeks for 4–6 cycles generally, unless the tumor progressed or due to personal intolerability.
In the HAIC group, arteriography was routinely performed to assess the tumor-supplying arteries. The microcatheter was then superselectively catheterized to the feeding artery of the tumor and retained to perform HAIC in the ward. The HAIC procedure has been described as above.
Evaluating indicator and follow-up
This study was followed up until January 2023. Each follow-up comprised routine hematological examinations, such as tumor marker detection, liver function tests, and imaging tests, including contrast-enhanced CT or MRI. Follow-up was conducted every two months until the cut-off date of follow-up or death. The primary outcome measures were overall survival (OS) and progression-free survival (PFS). OS was defined as the period from the date of the first treatment to death or the deadline of the last visit, whereas PFS was measured as the time between the first treatment and tumor progression, death, or the last follow-up. The best observed treatment responses were evaluated by CT or MRI according to the modified Response Evaluation Criteria for Solid Tumors (mRECIST, version 1.1) (16) recommended by the American Society of Hepatology, including complete response (CR), partial response (PR), stable disease (SD), and disease progression (PD). objective response rate (ORR) was calculated as CR + PR and disease control rate (DCR) was computed as CR + PR + SD. The Common Adverse Event Evaluation Criteria (CTCAE) version 5.0 (17) was adopted to assess treatment-related adverse reactions.
Statistical analysis
SPSS software (version 24.0) and R software (version 4.1.3, http://www.r-project.org/) were used for data analysis. The variables in the baseline table were grouped according to the optimum cutoff values. The independent sample t-test was used to assess continuous variables, which were presented as the mean ± standard deviation. Classified variables were expressed as frequencies and percentages and analyzed by the χ2 test. The Kaplan-Meier method was used to estimate OS and PFS, which were compared using the log-rank test. A Cox proportional hazards regression model was used to perform univariate and multivariate analyses to evaluate clinical factors associated with OS. Statistical significance was defined as a two-sided P value of <0.05.
Results
Baseline patient characteristics
A total of 73 patients with unresectable large HCC were finally enrolled in this study, of whom 32 were in the TACE-HAIC group and 41 were in the HAIC group. No significant differences were found in baseline characteristics between the two groups (Table 1). The median follow-up period was 17.8 months [95% confidence interval (CI): 13.5–22.8] in the TACE-HAIC group, while 14.9 months (95% CI: 9.4–18.2) in the HAIC group (P=0.231). All patients received repeated TACE-HAIC or HAIC therapy, with an average number of treatments of 2.8 (range, 2–4) and 3.5 (range, 2–5) in the two groups, respectively (P=0.125).
Table 1. Baseline clinical characteristics of enrolled patients in two groups.
| Variables | All (N=73) | TACE-HAIC group (N=32) | HAIC group (N=41) | P value |
|---|---|---|---|---|
| Age (years) | 59.8±8.8 | 60.4±7.7 | 59.4±9.7 | 0.570 |
| Sex | 0.365 | |||
| Male | 60 (82.2) | 28 (87.5) | 32 (78.0) | |
| Female | 13 (17.8) | 4 (12.5) | 9 (22.0) | |
| HBsAg | 0.242 | |||
| Presence | 42 (57.5) | 21 (65.6) | 21 (51.2) | |
| Absence | 31 (42.5) | 11 (34.4) | 20 (48.8) | |
| Cirrhosis | 0.095 | |||
| Presence | 41 (56.2) | 18 (56.3) | 14 (34.1) | |
| Absence | 32 (43.8) | 14 (43.8) | 27 (65.9) | |
| Ascites | 0.053 | |||
| Presence | 27 (37.0) | 16 (50.0) | 11 (26.8) | |
| Absence | 46 (63.0) | 16 (50.0) | 30 (73.2) | |
| Tumor size (cm) | 9.7±3.2 | 9.2±3.2 | 10.1±3.2 | 0.245 |
| Tumor number | 0.095 | |||
| Single | 32 (43.8) | 18 (56.3) | 14 (34.1) | |
| Multiple | 41 (56.2) | 14 (43.8) | 27 (65.9) | |
| Extrahepatic metastasis | 0.801 | |||
| Presence | 22 (30.1) | 9 (28.1) | 13 (31.7) | |
| Absence | 51 (69.9) | 23 (71.9) | 28 (68.3) | |
| Tumor thrombus | >0.999 | |||
| Presence | 33 (45.2) | 14 (43.8) | 19 (46.3) | |
| Absence | 40 (54.8) | 18 (56.3) | 22 (53.7) | |
| ECOG | 0.787 | |||
| 0 | 56 (76.7) | 24 (75.0) | 32 (78.0) | |
| 1 | 17 (23.3) | 8 (25.0) | 9 (22.0) | |
| CP score | >0.999 | |||
| A | 65 (89.0) | 29 (90.6) | 36 (87.8) | |
| B | 8 (11.0) | 3 (9.4) | 5 (12.2) | |
| WBC (109/L) | 0.102 | |||
| <4.8 | 35 (47.9) | 19 (59.4) | 16 (39.0) | |
| ≥4.8 | 38 (52.1) | 13 (40.6) | 25 (61.0) | |
| HGB (g/L) | 0.223 | |||
| <150 | 49 (67.1) | 24 (75.0) | 25 (61.0) | |
| ≥150 | 24 (32.9) | 8 (25.0) | 16 (39.0) | |
| ALT (U/L) | 0.214 | |||
| <69 | 48 (65.8) | 24 (75.0) | 24 (58.5) | |
| ≥69 | 25 (34.2) | 8 (25.0) | 17 (41.5) | |
| AST (U/L) | 0.234 | |||
| <41 | 41 (56.2) | 15 (46.9) | 26 (63.4) | |
| ≥41 | 32 (43.8) | 17 (53.1) | 15 (36.6) | |
| ALP (U/L) | 0.636 | |||
| <191 | 40 (54.8) | 19 (59.4) | 21 (51.2) | |
| ≥191 | 33 (45.2) | 13 (40.6) | 20 (48.8) | |
| GGT (U/L) | 0.237 | |||
| <249 | 39 (53.4) | 20 (62.5) | 19 (46.3) | |
| ≥249 | 34 (46.6) | 12 (37.5) | 22 (53.7) | |
| ALB (g/L) | 0.059 | |||
| <35 | 35 (47.9) | 11 (34.4) | 24 (58.5) | |
| ≥35 | 38 (52.1) | 21 (65.6) | 17 (41.5) | |
| TBIL (μmol/L) | 0.136 | |||
| <25.5 | 50 (68.5) | 25 (78.1) | 25 (61.0) | |
| ≥25.5 | 23 (31.5) | 7 (21.9) | 16 (39.0) | |
| DBIL (μmol/L) | 0.304 | |||
| <12.5 | 52 (71.2) | 25 (78.1) | 27 (65.9) | |
| ≥12.5 | 21 (28.8) | 7 (21.9) | 14 (34.1) | |
| PT (s) | >0.999 | |||
| <12.8 | 45 (61.6) | 20 (62.5) | 25 (61.0) | |
| ≥12.8 | 28 (38.4) | 12 (37.5) | 16 (39.0) | |
| AFP (ng/mL) | 0.812 | |||
| <400 | 32 (43.8) | 15 (46.9) | 17 (41.5) | |
| ≥400 | 41 (56.2) | 17 (53.1) | 24 (58.5) |
Results in the table are presented as mean ± SD or number (%). TACE, transarterial chemoembolization; HAIC, hepatic arterial infusion chemotherapy; HBsAg, hepatitis B surface antigen; ECOG, Eastern Cooperative Oncology Group; CP, Child-Pugh; WBC, white blood cell; HGB, hemoglobin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyl transpeptidase; ALB, albumin; TBIL, total bilirubin; DBIL, direct bilirubin; PT, prothrombin time; AFP, alpha-fetoprotein.
Tumor responses
Tumor responses in the two groups are shown in Table 2. In the TACE-HAIC group, 2 (6.3%) patients had CR, 19 (59.4%) patients had PR, 8 (25.0%) patients achieved SD, and 3 (9.4%) patients had PD, whereas in the HAIC group, 22 (53.7%) patients had PR, 10 (24.4%) patients achieved SD, and 9 (22.0%) patients had PD. The ORR and DCR for the TACE-HAIC group were 65.6% and 90.6%, respectively, which were higher than the 53.7% and 78.0% reflected in the HAIC group, but the difference was not statistically significant (P=0.345 and 0.208, respectively).
Table 2. Tumor responses of the two groups based on mRECIST 1.1.
| Tumor response | TACE-HAIC group (n=32) | HAIC group (n=41) | P value |
|---|---|---|---|
| CR | 2 (6.3%) | 0 | |
| PR | 19 (59.4%) | 22 (53.7%) | |
| SD | 8 (25.0%) | 10 (24.4%) | |
| PD | 3 (9.4%) | 9 (22.0%) | |
| ORR (CR + PR) | 21 (65.6%) | 22 (53.7%) | 0.345 |
| DCR (CR + PR + SD) | 29 (90.6%) | 32 (78.0%) | 0.208 |
mRECIST, modified Response Evaluation Criteria in Solid Tumors; TACE, transarterial chemoembolization; HAIC, hepatic arterial infusion chemotherapy; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; DCR, disease control rate.
Survival analysis
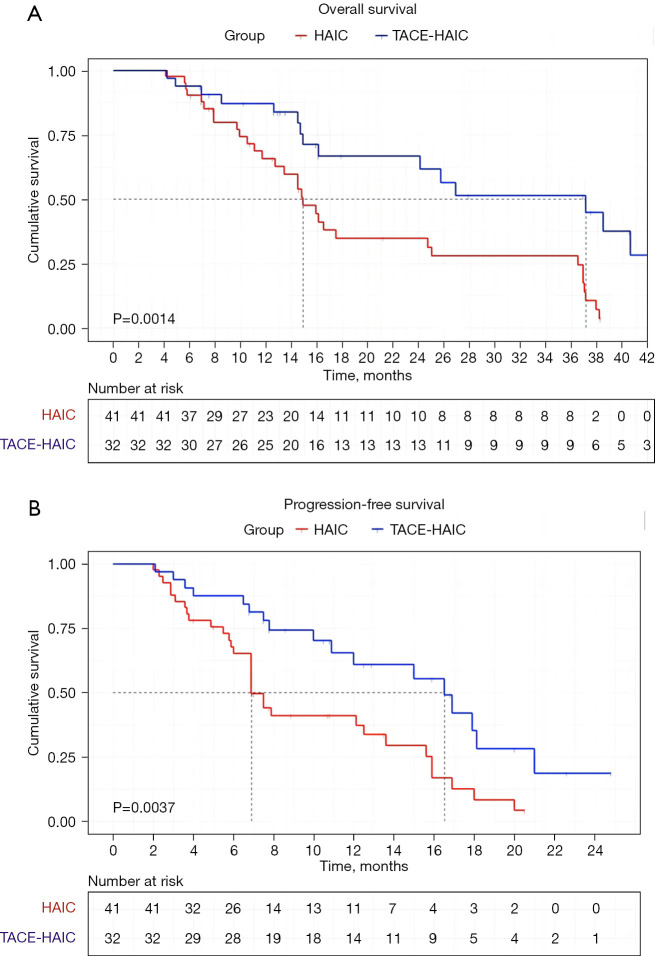
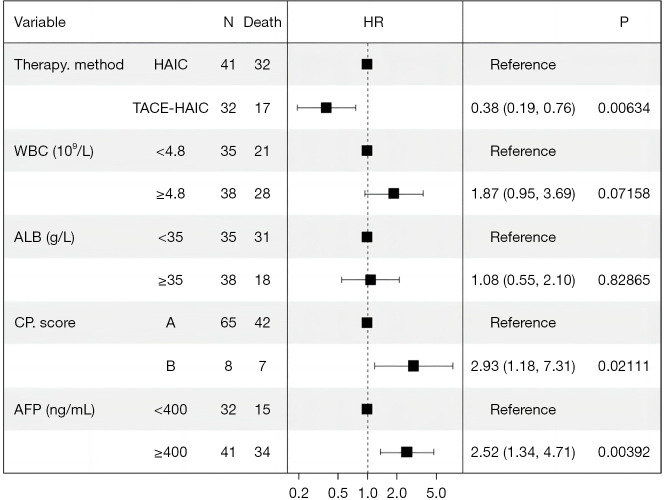
At the end of the follow-up period, 17 of 32 (53.1%) patients in the TACE-HAIC group and 32 of 41 (78.0%) patients in the HAIC group died. The PFS was longer in the TACE-HAIC group than in the HAIC group (median PFS: 16.5 vs. 6.9 months) (P=0.0037). Patients in the TACE-HAIC group had a median OS of 37.1 months (range, 4.2–45 months), which was significantly higher than that in the HAIC group (median OS: 14.9 months, range, 4.1–38.3 months) (P=0.0014) (Figure 2). The OS rates for 1-, 2-, and 3-year in the TACE-HAIC group were 78.1%, 40.6%, and 28.1%, respectively, whereas those in the HAIC group were 56.1%, 24.4%, and 19.5%, respectively. Univariate analysis showed that CP score [hazard ratio (HR) =4.346, 95% CI: 1.851–10.205, P=0.001], white blood cell (HR =2.197, 95% CI: 1.232–3.918, P=0.008), albumin (ALB) (HR =0.557, 95% CI: 0.309–1.004, P=0.052), alpha-fetoprotein (AFP) (HR =2.438, 95% CI: 1.317–4.516, P=0.005) and therapy method (HR =2.747, 95% CI: 1.433–5.266, P=0.002) were associated with OS in large unresectable HCC patients (Table 3). Multivariate Cox regression analyses revealed that CP score (HR =2.929, 95% CI: 1.175–7.314, P=0.021), AFP (HR =2.516, 95% CI: 1.336–4.708, P=0.004), and therapy method (HR =0.382, 95% CI: 0.185–0.763, P=0.006) were independent factors related to OS of patients with large unresectable HCC, among which, combined with TACE was a protective factor, as depicted in Figure 3.
Figure 2.
Kaplan-Meier curves indicating OS (A) and PFS (B) of patients with large HCC who underwent TACE. OS, overall survival; PFS, progression-free survival; HCC, hepatocellular carcinoma; HAIC, hepatic arterial infusion chemotherapy; TACE, transarterial chemoembolization.
Table 3. Univariate analyses of features correlated with OS of HCC patients.
| Variables | HR | 95% CI | P |
|---|---|---|---|
| Age (years) | 0.976 | 0.940–1.014 | 0.213 |
| Sex (male/female) | 1.434 | 0.685–3.002 | 0.339 |
| HBsAg (presence/absence) | 0.684 | 0.378–1.239 | 0.210 |
| Cirrhosis (presence/absence) | 0.668 | 0.374–1.195 | 0.174 |
| Ascites (presence/absence) | 0.733 | 0.401–1.339 | 0.313 |
| Tumor size (cm) | 1.033 | 0.946–1.128 | 0.475 |
| Tumor number (single/multiple) | 0.858 | 0.480–1.533 | 0.605 |
| Extrahepatic metastasis (presence/absence) | 1.420 | 0.768–2.627 | 0.264 |
| Tumor thrombus (presence/absence) | 0.936 | 0.524–1.671 | 0.823 |
| ECOG (0/1) | 1.658 | 0.872–3.155 | 0.123 |
| CP score (A/B) | 4.346 | 1.851–10.205 | 0.001 |
| WBC (<4.8/≥4.8 109/L) | 2.197 | 1.232–3.918 | 0.008 |
| HGB (<150/≥150 g/L) | 0.916 | 0.512–1.638 | 0.768 |
| ALT (<69/≥69 U/L) | 1.637 | 0.903–2.968 | 0.104 |
| AST (<41/≥41 U/L) | 1.381 | 0.782–2.438 | 0.266 |
| ALP (<191/≥191 U/L) | 1.506 | 0.847–2.675 | 0.163 |
| GGT (<249/≥249 U/L) | 1.337 | 0.755–2.367 | 0.319 |
| ALB (<35/≥35 g/L) | 0.557 | 0.309–1.004 | 0.052 |
| TBIL (<25.5/≥25.5 μmol/L) | 1.238 | 0.687–2.231 | 0.478 |
| DBIL (<12.5/≥12.5 μmol/L) | 1.357 | 0.742–2.482 | 0.322 |
| PT (<12.8/≥12.8 s) | 1.574 | 0.876–2.828 | 0.129 |
| AFP (<400/≥400 ng/mL) | 2.438 | 1.317–4.516 | 0.005 |
| Therapy method (TACE-HAIC/HAIC) | 2.747 | 1.433–5.266 | 0.002 |
OS, overall survival; HCC, hepatocellular carcinoma; HR, hazard ratio; CI, confidence interval; HBsAg, hepatitis B surface antigen; ECOG, Eastern Cooperative Oncology Group; CP, Child-Pugh; WBC, white blood cell; HGB, hemoglobin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyl transpeptidase; ALB, albumin; TBIL, total bilirubin; DBIL, direct bilirubin; PT, prothrombin time; AFP, alpha-fetoprotein; TACE, transarterial chemoembolization; HAIC, hepatic arterial infusion chemotherapy.
Figure 3.
Forest plot based on the results of the multivariate analysis of factors associated with overall survival. HR, hazard ratio; HAIC, hepatic arterial infusion chemotherapy; TACE, transarterial chemoembolization; WBC, white blood cell; ALB, albumin; CP, Child-Pugh; AFP, alpha-fetoprotein.
Safety
No TACE- or HAIC-related deaths occurred in this study. The adverse events caused by the treatment are shown in Table 4. The total incidence of AEs of any grade and grade 3/4 were not statistically significant (90.6% vs. 85.4% and 37.5% vs. 19.5% in the TACE-HAIC and HAIC groups, respectively). Fever and elevated alanine aminotransferase (ALT) levels were higher in the TACE-HAIC group than those in the HAIC group. However, there was no significant difference in the incidence of grade 3/4 AEs between the two groups. In the TACE-HAIC group, three patients discontinued treatment because they failed to tolerate AEs, whereas two patients in the HAIC group discontinued treatment (P=0.648).
Table 4. Adverse events related to therapy.
| Adverse events | Any grade | Grade 3–4 | |||||
|---|---|---|---|---|---|---|---|
| TACE-HAIC group (n=32) | HAIC group (n=41) | P value | TACE-HAIC group (n=32) | HAIC group (n=41) | P value | ||
| All | 29 (90.6%) | 35 (85.4%) | 0.722 | 12 (37.5%) | 8 (19.5%) | 0.115 | |
| General symptoms | |||||||
| Fever | 15 (46.9%) | 9 (22.0%) | 0.043 | 3 (9.4%) | 2 (4.9%) | 0.648 | |
| General fatigue | 10 (31.3%) | 9 (22.0%) | 0.427 | 2 (6.3%) | 1 (2.4%) | 0.578 | |
| Gastrointestinal symptoms | |||||||
| Nausea/vomit | 18 (56.3%) | 21 (51.2%) | 0.814 | 4 (12.5%) | 4 (9.8%) | 0.493 | |
| Abdomen pain | 16 (50.0%) | 19 (46.3%) | 0.816 | 5 (15.6%) | 3 (7.3%) | 0.287 | |
| Anorexia | 8 (25.0%) | 11 (26.8%) | >0.999 | 0 | 0 | – | |
| Ascites | 2 (6.3%) | 1 (2.4%) | 0.578 | 0 | 0 | – | |
| Laboratorial indexes abnormalities | |||||||
| Elevated ALT level | 11 (34.4%) | 5 (12.2%) | 0.044 | 4 (12.5%) | 3 (7.3%) | 0.692 | |
| Neutropenia | 7 (21.9%) | 7 (17.1%) | 0.766 | 0 | 0 | – | |
| Anemia | 6 (18.8%) | 4 (9.8%) | 0.317 | 0 | 0 | – | |
| Thrombocytopenia | 9 (28.1%) | 10 (24.4%) | 0.791 | 4 (12.5%) | 2 (4.9%) | 0.394 | |
| Hypoalbuminemia | 4 (12.5%) | 3 (7.3%) | 0.692 | 1 (3.1%) | 0 | 0.438 | |
| Others | |||||||
| Hypertension | 5 (15.6%) | 6 (14.6%) | >0.999 | 0 | 0 | – | |
| Sensory neuropathy | 1 (3.1%) | 0 | 0.438 | 0 | 0 | – | |
TACE, transarterial chemoembolization; HAIC, hepatic arterial infusion chemotherapy; ALT, alanine aminotransferase.
Discussion
In our study, we found that patients in TACE-HAIC group achieved longer OS and PFS in comparison with those in HAIC group (median OS, 37.1 vs. 14.9 months, median PFS, 16.5 vs. 6.9 months). The ORR and DCR in the TACE-HAIC group were higher than those in the HAIC group (65.6% vs. 53.7% and 90.6% vs. 78.0%, respectively), although the differences were not statistically significant. This may be explained as follows: TACE promotes tumor cell necrosis, intertissue ion transport, and interstitial hypertension changes, which together contribute to the distribution of chemotherapeutic drugs (16,17). TACE can block the tumor’s main blood vessels, whereas HAIC is more conducive to the control of tumors with deficient blood supply (17). The combination of TACE and HAIC integrates the advantages of both to achieve better tumor control.
A randomized phase III trial (18) reported that HAIC achieved a higher tumor response in large HCC than TACE (DCR: 82% vs. 61%, ORR: 46% vs. 18%). HAIC group got better prognosis than TACE group, with the median OS reached 23.1 and 16.1 months, respectively. With the prevalence of combination therapy, the TACE plus HAIC regimen was used in large HCC and revealed more promising results than TACE alone, as reported by He et al. and Huang et al. (12,13). A retrospective study by Huang et al. (13) verified that compared with TACE, TACE combined with HAIC can improve the median OS (19 vs. 14 months, P=0.008) and PFS (9.3 vs. 6.3 months, P=0.005). Furthermore, no serious adverse events were observed in the combination therapy group. Within the scope of our study, we concluded that the combination of TACE and HAIC had a superior clinical effect to HAIC alone in patients with large HCC. To the best of our knowledge, this is the first study to compare the efficacy and safety of TACE combined with HAIC and HAIC alone in the treatment of large HCC.
In addition, multivariate analyses revealed that the CP score and AFP level were independent predictive factors for OS in patients with large HCC. A previous study has reported that the higher CP score, the worse liver function, and the worse patient prognosis (19). AFP, a serum biomarker sensitive to liver cancer, has been reported by several studies to be closely related to the prognosis of patients (20-22).
The challenge in the current study was the extent of embolism. On one hand, large tumors cannot be completely embolized during the first surgery. In the other hand, increased tumor necrosis may lead to more adverse events related to embolization. Therefore, we attempted to achieve embolization mostly or completely between 2–4 procedures, not at one time. Our study showed that the therapeutic schedule was safe and tolerable. Patients who underwent TACE had a higher frequency of fever and increased ALT levels; however, these were all manageable. Furthermore, combined TACE did not increase the occurrence of grade 3/4 AEs.
In our study, approximately 30% of patients have the feature of extrahepatic metastases. For these patients, subsequent therapy may be followed alone or in combination with other topical therapies, molecular-targeted agents, and/or immune checkpoint inhibitors after initial local therapy failure or progression.
This study had several limitations. First, this was a retrospective study with a small number of enrolled patients and inevitable selection bias in the two groups, which may have affected the research. Future prospective multi-center randomized controlled studies are warranted to conduct. Second, not all patients reached complete embolism, and some large tumors were treated with gelatin sponge particles, which may have effect on the study. Third, the follow-up period was limited, and long-term efficacy and safety are needed to further study between TACE-HAIC and HAIC groups. Fourth, in this study, the patients in HAIC group had more ascites, more multiple HCC, and even lower ALB and higher total bilirubin, although no significant differences were found in baseline characteristics between the two groups, this may lead OS lower than TACE-HAIC group. So the conclusion of this study needs to be verified by large samples.
Conclusions
TACE combined with HAIC yielded superior efficacy to HAIC alone in patients with large unresectable HCC, which dramatically prolonged the OS and PFS of the patients. Additionally, the combined therapeutic regimen was safe and tolerable.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (No. XYFY2023-KL116-01), and the procedures were performed in accordance with the principles of the Declaration of Helsinki (as revised in 2013). Informed consent was not required owing to the retrospective nature of the study.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-821/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-821/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-821/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-821/coif). The authors have no conflicts of interest to declare.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Singal AG, El-Serag HB. Hepatocellular Carcinoma From Epidemiology to Prevention: Translating Knowledge into Practice. Clin Gastroenterol Hepatol 2015;13:2140-51. 10.1016/j.cgh.2015.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang C, Zhang H, Zhang L, et al. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2023;20:203-22. 10.1038/s41575-022-00704-9 [DOI] [PubMed] [Google Scholar]
- 4.Biolato M, Marrone G, Racco S, et al. Transarterial chemoembolization (TACE) for unresectable HCC: a new life begins? Eur Rev Med Pharmacol Sci 2010;14:356-62. [PubMed] [Google Scholar]
- 5.Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol 2022;76:681-93. 10.1016/j.jhep.2021.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int 2015;35:2155-66. 10.1111/liv.12818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins JM. Pharmacologic rationale for regional drug delivery. J Clin Oncol 1984;2:498-504. 10.1200/JCO.1984.2.5.498 [DOI] [PubMed] [Google Scholar]
- 8.Lewandowski RJ, Geschwind JF, Liapi E, et al. Transcatheter intraarterial therapies: rationale and overview. Radiology 2011;259:641-57. 10.1148/radiol.11081489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikeda M, Morizane C, Ueno M, et al. Chemotherapy for hepatocellular carcinoma: current status and future perspectives. Jpn J Clin Oncol 2018;48:103-14. 10.1093/jjco/hyx180 [DOI] [PubMed] [Google Scholar]
- 10.Kudo M. Surveillance, Diagnosis, and Treatment Outcomes of Hepatocellular Carcinoma in Japan: 2021 Update. Liver Cancer 2021;10:167-80. 10.1159/000516491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyu N, Kong Y, Mu L, et al. Hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin vs. sorafenib for advanced hepatocellular carcinoma. J Hepatol 2018;69:60-9. 10.1016/j.jhep.2018.02.008 [DOI] [PubMed] [Google Scholar]
- 12.He MK, Le Y, Li QJ, et al. Hepatic artery infusion chemotherapy using mFOLFOX versus transarterial chemoembolization for massive unresectable hepatocellular carcinoma: a prospective non-randomized study. Chin J Cancer 2017;36:83. 10.1186/s40880-017-0251-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang J, Huang W, Zhan M, et al. Drug-Eluting Bead Transarterial Chemoembolization Combined with FOLFOX-Based Hepatic Arterial Infusion Chemotherapy for Large or Huge Hepatocellular Carcinoma. J Hepatocell Carcinoma 2021;8:1445-58. 10.2147/JHC.S339379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358-80. 10.1002/hep.29086 [DOI] [PubMed] [Google Scholar]
- 15.European Association for the Study of the Liver . EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. Erratum in: J Hepatol 2019;70:817. 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 16.Zhu D, Ma K, Yang W, et al. Transarterial chemoembolization plus apatinib with or without camrelizumab for unresected hepatocellular carcinoma: A two-center propensity score matching study. Front Oncol 2022;12:1057560. 10.3389/fonc.2022.1057560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen B, Dai H, Yang J, et al. Transarterial Chemoembolization Followed by Hepatic Arterial Infusion Chemotherapy Combined a Tyrosine Kinase Inhibitor for Treatment of Large Hepatocellular Carcinoma. Curr Cancer Drug Targets 2023;23:564-71. 10.2174/1568009623666230215142941 [DOI] [PubMed] [Google Scholar]
- 18.Li QJ, He MK, Chen HW, et al. Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin Versus Transarterial Chemoembolization for Large Hepatocellular Carcinoma: A Randomized Phase III Trial. J Clin Oncol 2022;40:150-60. 10.1200/JCO.21.00608 [DOI] [PubMed] [Google Scholar]
- 19.Ma D, Liu M, Zhai X, et al. Development and validation of prognostic risk prediction models for hepatocellular carcinoma patients treated with immune checkpoint inhibitors based on a systematic review and meta-analysis of 47 cohorts. Front Immunol 2023;14:1215745. 10.3389/fimmu.2023.1215745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ridder DA, Weinmann A, Schindeldecker M, et al. Comprehensive clinicopathologic study of alpha fetoprotein-expression in a large cohort of patients with hepatocellular carcinoma. Int J Cancer 2022;150:1053-66. 10.1002/ijc.33898 [DOI] [PubMed] [Google Scholar]
- 21.Bruix J, Cheng AL, Meinhardt G, Nakajima K, De Sanctis Y, Llovet J. Corrigendum to "Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: Analysis of two phase III studies" [J hepatol 67 (2017) 999-1008]. J Hepatol 2018;69:990-1. [DOI] [PubMed] [Google Scholar]
- 22.Kaewdech A, Sripongpun P, Assawasuwannakit S, et al. FAIL-T (AFP, AST, tumor sIze, ALT, and Tumor number): a model to predict intermediate-stage HCC patients who are not good candidates for TACE. Front Med (Lausanne) 2023;10:1077842. 10.3389/fmed.2023.1077842 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as