Abstract
This paper aimed to find clues to treatment‐resistant depression (TRD) solutions. Depression comorbid with anxiety is often treatment‐resistant where anxious‐depressive attack (ADA) often lurks. ADA is a recently proposed clinical idea for just a psychological version of a panic attack. It mostly begins with an abrupt surge of intense anxiety followed by uninterrupted intrusive thoughts; lasting ruminations about regret or worry produced by violent anxiety, agitation, and loneliness. Acting‐out behaviors such as deliberate self‐injury and over‐dose may also be observed during the attack. As the basic psychopathology of ADA, rejection sensitivity (RS) was revealed by a structural equation model. It is said that the presence of RS in depressive disorders implies a poor prognosis. The following biological markers for RS were reviewed in the literature: first, the involvement of the μ‐opioid receptor function in RS and, secondly, hypersensitivity of the dopamine D4 receptor (DRD4) in the medial prefrontal cortex. The latter has been suggested in fear‐conditioned animal experiments. Manipulation of the μ‐opioid receptor function together with the DRD4 function may culminate in a treatment for RS, which could contribute to the development of a treatment for TRD via the improvement of ADA.
Keywords: anxious‐depressive attack, dopamine D4 receptor, rejection sensitivity, treatment‐resistant depression, μ‐opioid
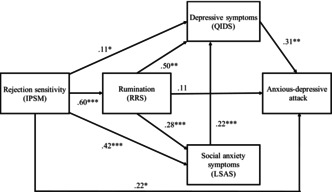
The structural equation model of the relationships between rejectionsensitivity (RS) and anxious‐depressive attack (ADA). RS contributes significantly to the onset of ADA.
1. INTRODUCTION
1.1. What is the anxious‐depressive attack?
Anxious‐depressive attack (ADA) was identified for the first time in patients who developed refractory depression during panic disorder, social anxiety disorder, or depression with atypical features. 1 , 2 ADA is thought of as just a psychological version of a panic attack. ADA begins with an abrupt surge of intense anxiety followed by uninterrupted intrusive thoughts. The eternal ruminations about regret or worry intrude one after another together with violent anxiety, agitation, or loneliness with durations ranging from several tens of minutes to half a day or an entire day. Some patients during ADA show acting‐out behaviors such as deliberate self‐injury, over‐dose, assaulting others, etc. ADA is rarely noticed by physicians or third parties, as it is almost impossible for the patient to objectively inform others of his or her own experiences during intense anxious seizures (Table 1). ADA is trans‐diagnostically observed in various disorders such as major depressive disorder with or without atypical features, bipolar disorder, social anxiety disorder, panic disorder with or without agoraphobia, specific phobia, obsessive‐compulsive disorder, and schizophrenia. 3 The proportion of patients having ADA was 43.2% among outpatients who presented regularly for treatment 2 but only 16.9% among first‐time visiting patients. 4 This discrepancy could be partially explained by the fact that patients with ADA were refractory.
TABLE 1.
Diagnostic criteria for anxious‐depressive attack. 3
| (A) Anxious‐depressive attack occurs suddenly and recurrently regardless of one's situation in various mental disorders |
|
(B) The following symptoms proceed in descending order; however, symptom no. 4 is elective: (1) Sudden instantaneous negative emotions occur. This is almost identical in nature to panic attacks. (2) Intrusive thoughts continue to arise immediately, becoming a rumination of past regrets or worries about the future, with a succession of changes in time and theme. The duration may range from a few minutes to more than 3 h, or sometimes an entire day. The intrusion may also appear as a flashback. (3) Prominent agitation, unrest, or loneliness occurs during rumination. The degree of anxiety is so strong that it does not match the content of the ruminating thoughts. (4) Various coping behaviors to manage intense discomfort, such as fugue, binge‐eating, smoking, shopping addiction, drinking, shouting, attacks on objects or people, over medication, sexual misconduct, self‐harm occasionally appear. |
| (C) Physical symptoms, if any, as seen in panic attacks, are extremely modest |
| (D) This symptom cluster is not attributable to the direct psychological effects of any stress, physiological effects of a substance, or a neurological or other medical condition |
| (E) The disturbance is not better explained by another neuropsychiatric disorder (e.g., panic disorder, posttraumatic stress disorder, non‐epileptic seizure, frontal epilepsy, intermittent explosive disorder, with anxious distress specifier for depressive disorder, sudden emotional excitement of schizophrenia, or ataque de nervios) |
1.2. Psychopathology of ADA
Considering the psychopathology of ADA, four components—rejection sensitivity (RS), rumination, social anxiety symptoms, and depressive symptoms—are thought to be associated with the development of ADA. To clarify the complex relationship between ADA and these four components, structural equation modeling was conducted. The structural equation model established depression (β = 0.31, p < 0.01) and RS (β = 0.22, p < 0.05) as factors contributing to ADA 3 (Figure 1). This could be interpreted clinically as meaning that if a depressed patient has rejection sensitivity, ADA is more likely to occur.
FIGURE 1.
The structural equation model of the relationships among RS, rumination, social anxiety symptoms, depressive symptoms, and anxious‐depressive symptoms. 3 Chi‐square value: χ 2 = 0.10, df = 1, p = 0.76; comparative fit index = 1.000; Tucker–Lewis Index = 1.000; root mean square error of approximation = 0.000, 90% confidence interval = 0.000–0.099; and standardized root mean square residual = 0.004. IPSM, interpersonal sensitivity measure; RRS, ruminative responses scale; LSAS, Liebowitz social anxiety scale; QIDS, quick inventory of depressive symptomatology. *p < 0.05; **p < 0.01; ***p < 0.001.
1.3. Aim of the present review
Given the above, this paper aims to clarify the true nature of RS, derive ADA treatments from it, and ultimately help to solve some of the treatment‐resistant depression (TRD). Thus, depression comorbid with anxiety disorders is intractable, 5 , 6 and the presence of RS in mood disorders implies a poor prognosis. 7 , 8 Furthermore, the depressive state of ADA is chronic and refractory.
2. REJECTION SENSITIVITY
2.1. History and definition of rejection sensitivity
Although RS is very important in psychology, it is rather thought to be an orphan word in psychiatry. 9 The term “RS” is also used for interpersonal rejection hypersensitivity, social pain, social RS, or ostracism.
The term “rejection‐sensitive” was first used in psychiatry by Klein & Davies. 10 They proposed the diagnostic term “rejection‐sensitive hysteroid dysphoria,” the original form of atypical depression. This term was changed afterward to hysteroid disphoria. 11 Klein and Davies described symptoms that were very likely ADA as “repeated episodes of abruptly depressed mood in response to feeling rejected.” Unfortunately, no detailed mention of this condition could be found in any of their papers afterward.
When Klein first became a physician, psychiatry in the USA was in its psychoanalytic heyday; therefore, it is assumed that Klein took the term “RS” from the vocabulary used in psychoanalysis. The psychoanalyst, Horney, 12 used the term “painful sensitivity to any rejection or rebuff” in psychology.
The term “RS” was found in only one place in the DSM‐5 diagnostic criteria. 13 It appears in the criteria of “with atypical features” of specifiers for bipolar and related disorders and depressive disorders. As per the criteria, RS is contained in two out of the four mandatory symptoms in section B. It is explained as a long‐standing pattern of interpersonal RS (not limited to episodes of mood disturbance) that results in significant social or occupational impairment. Furthermore, the note of the criteria describes that, unlike the other atypical features, pathological sensitivity to perceived interpersonal rejection is a trait that has an early onset and persists throughout most of adult life, and that RS occurs both when the person is and is not depressed, although it may be exacerbated during depressive episodes. In this context, RS is not a symptom but a disposition.
2.2. Measurement of rejection sensitivity
Stewart et al. 14 developed an atypical depression diagnostic scale in which they measured the extent to which sensitivity to interpersonal rejection interfered with functioning. The scale contains the following elements: (1) Interpersonal sensitivity–emotional overreaction to RS; (2) The quality of relationships–tumultuous or stormy relationships due to overreaction to rejection or criticism; (3) Functional impairment–work/school impairment due to overreaction to criticism or rejection; (4) Avoidance of relationships–lack of relationships due to fear or rejection; (5) Other rejection avoidance–avoidance of other important life tasks due to rejection avoidance.
Other measures of rejection hypersensitivity exist; Boyce and Parker 15 developed a scale to measure interpersonal sensitivity from clinical experience, defining high interpersonal sensitivity as “undue and excessive awareness of, and sensitivity to, the behaviors and feelings of others.” We have developed the Japanese version of the scale. 16 The interpersonal sensitivity measure (IPSM) consists of the following five subscales: interpersonal awareness, need for approval, separation anxiety, timidity, and a fragile inner‐self. Downey and Feldman 17 coined the RS questionnaire, operationalizing RS as “anxious or angry expectations of rejection in situations where rejection is possible.”
2.3. Developmental correlates of rejection sensitivity
Maternal harshness, 18 parental coercion and psychological control, 19 and maltreatment 20 or emotional abuse and neglect 21 in the early years of life were all predictive of the occurrence of RS at a later date.
2.4. Psychological correlates of rejection sensitivity
RS is associated with aggression and victimization. 22 Low self‐esteem predicted later depression via RS and loneliness. 23 RS and submissive behavior (overly accommodating, non‐assertive, and self‐sacrificing) prospectively predicted increased rumination underlying depression. 24 , 25
2.5. Clinical correlates of rejection sensitivity
RS was related to suicide ideation, 26 depression, anxiety, borderline personality disorder, and body dysmorphic disorder. 27 State RS was significantly predicted by bipolar depression, which could be used for the differentiation of bipolar and unipolar depression. 28 This evidence could be closely related to the high prevalence of bipolar disorder in atypical depression, 29 where RS is an important symptom. Furthermore, it was reported that heightened RS in bipolar patients indicates a poor prognosis. 30 Depressive patients with high RS were associated with poor outcomes 1 year following the baseline assessment 7 or 6 months after the end of treatment. 8 Thus, the presence of RS in mood disorders implies a poor prognosis. 7 , 8
Parker et al. 31 statistically examined the symptoms of atypical depression and found that RS was the most major symptom of atypical depression. It is only natural that patients with atypical depression showed ADA at the highest frequency (78.3%). 2 Parker et al. 31 further showed lifetime panic disorder and social anxiety disorder as higher‐order determinants of atypical depression.
Posternak and Zimmerman 32 examined in detail atypical depressive symptoms in 1130 patients with anxiety disorders or depression or both (Figure 2). The frequency of the presence of RS was the highest in bipolar disorder among mood disorders (58.1%). In anxiety disorders without depression, RS was highest in social anxiety disorder (44.6%). In depression, the frequency of RS more than doubled when comorbid with anxiety disorders (21.4% vs. 53.7%). A notable finding of this study was that anxiety disorders alone were nearly twice as likely to have RS in comparison with depression alone (32.3% vs. 18.3%). In summary, when anxiety disorders occur alongside depression, the frequency of RS is higher, and more than half of the patients involved showed probable atypical depression (53.1%).
FIGURE 2.
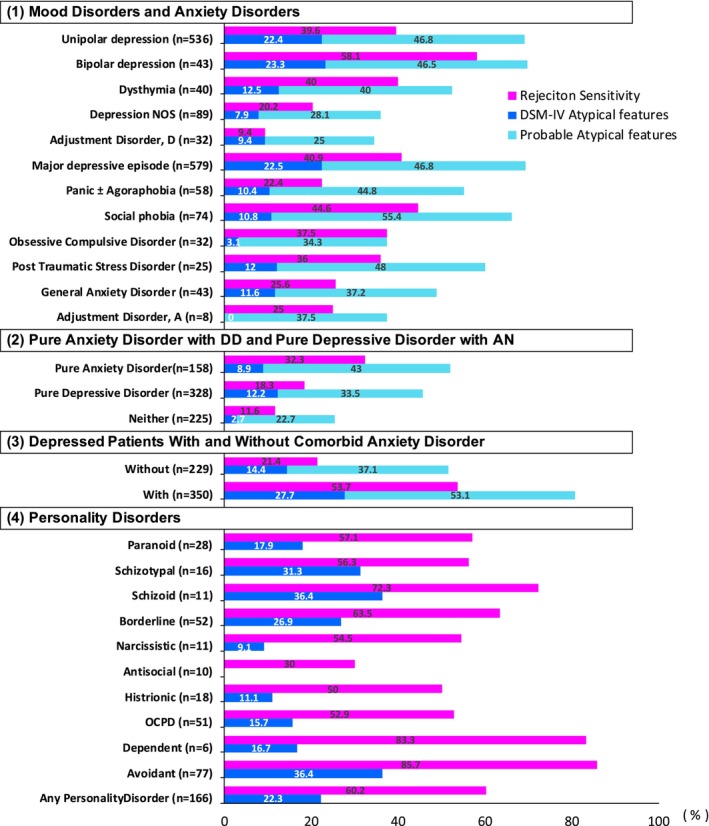
Prevalence of rejection sensitivity and atypical features in anxiety or mood disorders. The figure was prepared by the author from the data of Posternak and Zimmerman. 32 Probable atypical features mean consisting of mood reactivity plus only one of four of the atypical B symptoms (DSM‐IV). Depression NOS, depression not otherwise specified; adjustment disorder, D, adjustment disorder, depressed mood; panic ± agoraphobia, a panic disorder with and without agoraphobia; adjustment disorder, A, adjustment disorder, anxious features; OCPD, obsessive‐compulsive personality disorder.
3. OPIOID INVOLVEMENT IN REJECTION SENSITIVITY
3.1. The nature of the opioid system
The opioid system has various biological functions such as reward, analgesia, and stress responsivity. 33 Herman and Panksepp 34 were the first to report that the μ‐opioid receptor (MOR) agonist, morphine, relieved a sad squealing condition when guinea pig pups were separated from their mothers. Similar phenomena were subsequently reported in various animals. 35 , 36 , 37 , 38 Because the parent–child separation is an archetype of rejection, the opioid system is thought to be deeply involved in RS.
3.2. Brain imaging study of pain
RS induces social pain, while physical invasion causes physical pain. The brain imaging findings of patients experiencing psychological pain are very similar to those of patients experiencing physical pain. Eisenberger 39 reviewed the shared neural substrates of physical and social pain. She pointed out two regions mainly related to psychological pain, which are the dorsal anterior cingulate cortex and the anterior insula. The activity of these regions was correlated with psychological distress. Lesions in the insular region 40 or cingulate cortex 41 produce pain asymbolia, a condition in which pain is perceived but does not cause distress or suffering. Other brain regions relating mainly to physical pain were the primary and secondary somatosensory cortex and the posterior insula, in which the activity was observed also by psychological pain. This fact is particularly evident in patients with social anxiety disorder 42 and people having A118G, 43 a variation of the μ‐opioid receptor gene.
Positron emission tomography scans demonstrated that RS did not cause MOR baseline binding potential or activity during acceptance or rejection tasks in any region of the brain in healthy participants. 44 However, in patients with depression, rejection caused reduced endogenous opioid release in brain regions relating to stress, mood, and motivation. 45
There are clinical studies suggesting that psychological pain and physical pain are compatible. Patients having RS in major depression 46 or bipolar disorder 47 were reported to be more likely to experience physical pain during their illness. The experience of pain during depression was predicted by a major increase in the state of RS for both bipolar and unipolar depression. 28
3.3. Polymorphism of the morphine receptor gene
3.3.1. The nature of A118G
The MOR gene has a single nucleotide polymorphism, the usually studied A118G, in which adenine is replaced by guanine in exon 1. Asparagine is replaced by aspartic acid in the structural formula. 48 It has been reported that A118G carriers experience greater pain intensity 49 , 50 and need a high amount of morphine to achieve a certain effect. 48 This fact means that the MOR gene with A118G has a weaker effect on opioids.
3.3.2. Human findings of A118G
In human studies, subjects with the 118G allele were prone to fearful attachment, which is a state of refusing external communication for fear of hurting themselves in relation to others, regardless of the quality of maternal care. 51 A118G carriers reported higher NEO‐Neuroticism scores; a personality trait associated with increased pain and lower placebo responses. 52 Among G‐allele carriers, the effects of the negative impact of childhood adversity on personality were greater than among A‐allele homozygotes. 53 An insecure attachment style was more frequent in mothers and children carrying the G allele, and mothers with the G allele showed higher interpersonal sensitivity, depression, hostility, and paranoid ideation than A/A mothers. 54
An interesting clinical study of depression was conducted on G‐allele carriers. 55 found that G‐allele carriers were more severely depressed and twice as likely to meet the criteria for major depressive disorders following a recent targeted rejection major life event (e.g., being broken up with, getting fired) relative to A/A homozygotes who also experienced such stress.
MOR gene polymorphism was studied by using Cyberball, a virtual ball‐tossing game, to induce experiences of non‐self‐dependent social rejection. And it was shown that the intensity of RS was in the order G/G, A/G, and A/A. 56 A similar, larger study using Cyberballs failed to prove the result that RS is significantly more common in people having the G allele. 57 Relative to A/A homozygotes, G‐allele carriers were more sensitive to their partners' self‐reported quarrelsome behaviors. 58 A study has submitted diametrically opposed results. The dispositional interpersonal sensitivity scores were higher in the A/A group than in the A/G (p = 0.023) and G/G (p = 0.009) group. 59 The results of this study seem contradictory to the author's view. Thus, because individuals with the A118G allele are hypersensitive to pain, 49 , 50 such individuals are more likely to show the social pain of rejection, given the homology between physical and psychological pain. There is more evidence to support this hypothesis. People with the A118G allele, with or without mental disorders, were found to be more likely to seek out emotional relationships and pursue enjoyment in social situations. 51 The author considers this to be the behavioral flip side of RS. Such behavior could be presumed as sociotropy—the tendency to place an inordinate value on relationships over personal independence (APA Dictionary of Psychology). There is a study showing that sociotropy was strongly correlated with RS. 60 This means that A118G, sociotropy, and RS are on the same line.
The close findings on opioids and RS discussed so far may provide clues for the treatment of TRD, as well as that of ADA.
3.3.3. Animal findings of μ‐opioid receptor gene polymorphisms relating attachment behavior
Barr et al. 61 reported that infants of monkeys with C77G spent more time fawning their mothers, had limited contact with other infants along with prolonged screams when separated from their mothers. In addition, rhesus monkey mothers with A118G did not attempt to separate from their offspring. 62 Mouse pups with genetic ablation of the opioid receptor gene did not scream when separated from their mothers and showed no interest in their mothers. 63 Taken together, these studies indicate that animals with μ‐opioid receptor gene polymorphisms are more prone to attachment behavior.
4. DOPAMINE'S INVOLVEMENT IN REJECTION SENSITIVITY
4.1. An animal model of rejection sensitivity
We found a study in the literature that could be considered an animal model of RS. 64 It is a fear conditioning experiment in rats using an odor (almond or pepper mint) as a conditioned stimulus and 0.8 mA electric shocks of the cage floor as an unconditioned stimulus. Before the fear conditioning experiment, it was confirmed that neurons in the medial prefrontal cortex (mPFC), which have been identified electrophysiologically as receiving monosynaptic orthodromic input from the basolateral nucleus of the amygdala (BLA), are neurons involved in the encoding, expression, and extinction of emotionally salient learned information (Figure 3). These neurons showed burst activity in response to the conditioned stimuli. In contrast, neurons in the mPFC without input from the BLA did not show this phenomenon. When animals received bilateral intra‐mPFC, the dopamine D4 receptor (DRD4) antagonist (L‐741741), the fear‐conditioned response did not occur and no more burst activity was seen in those neurons in the mPFC. 65 The research group then used the same experimental system to produce rats that did not show a fear‐conditioned response with unconditioned stimuli with an electric shock of 0.4 mA (subthreshold). In these animals, when a DRD4 agonist (PD 168077) was microinjected in the mPFC, a fear‐conditioned response was elicited. 67
FIGURE 3.
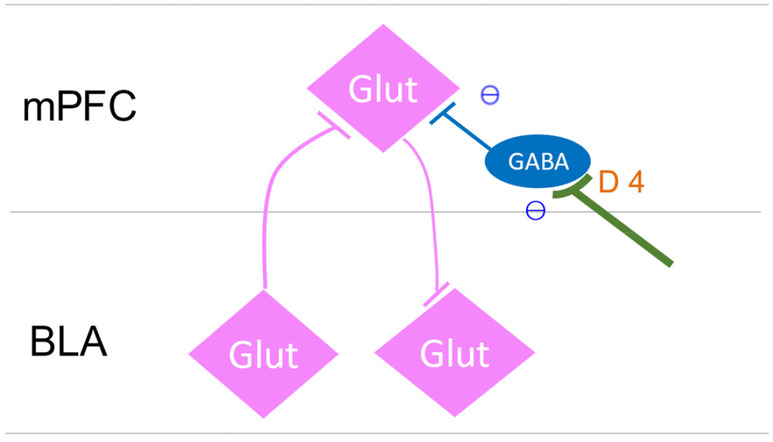
The schematic diagram of the author's estimation of the interrelationships between mPFC neurons and BLA neurons based on the literature of Laviolette et al. 64 ; Wang et al. 65 ; and Floresco and Maric. 66 Glutamate neurons in the mPFC are disinhibited and become more active when inhibitory GABA inter‐neurons are suppressed by the DRD4 effect. mPFC, the medial prefrontal cortex; BLA, basolateral nucleus of the amygdala.
Both these studies showed that the direct stimulation of DRD4 in neurons in the mPFC receiving monosynaptic input from the BLA produced fear‐conditioned responses, while the administration of a DRD4 blocker prevented the fear‐conditioned response.
Now, let us discuss how the animal experiment results can be used as an RS model for humans. The odor of a conditioned stimulus is clinically an insult in human relationships. This may be verbal or behaviorally demonstrated. The fear behaviors of animals induced by electric shock for an unconditioned stimulus are clinically ADA. The conditioned stimulus does not elicit a fear response in rats when the unconditioned stimulus is reduced from 0.8 to 0.4 mA. This may be comparable to a human situation where the accusation is less severe but still retains a subtle critical nuance. Therefore, a healthy person would not show any ADA at this level. However, people with RS do exhibit ADA. Animal experiments revealed that fear responses are produced even with weak unconditioned stimuli (0.4 mA) by giving DRD4 agonists, which increase the excitability of neurons in the prefrontal cortex that induce amygdala firing (Figure 3). The results of this animal study indicate that RS in humans is a situation in which the amygdala is easily excited by the hypersensitive state of the DRD4 in the prefrontal cortex. In other words, increased hypersensitivity to rejection can be believed to be a state of increased DRD4 function. Hence, the hypothesis arises that DRD4 antagonists may be effective for ADA by lowering RS.
4.2. Rationale for using the results of the animal studies as a model for RS in humans
The neuroscientific discussion of fear and anxiety by LeDoux and Pine 68 proposed neural circuits of two types of responses: (1) physiological (autonomic, immune, and endocrine) and behavioral responses (approach, avoidance, attack facial expressions, posture, etc.) and (2) subjective emotional states (orbital brain, ventral and dorsal prefrontal cortex, posterior parietal, and insula). These, in ADA, include physical symptoms of very mild panic attacks, acting‐out behaviors (wrist cutting, overeating, etc.), and severe anxiety and agitation.
The rationale for applying the experimental animal results to humans in these studies is as follows. First, the emotion of anxiety is the most primitive of all human mental states. Thus, animal experiment results could be applied to humans without much bias. Additionally, this study focused only on the intensity of fear and does not deal with more complex subjective emotions. Hence, applying the experimental animal results to humans in this study would be permissible.
4.3. Dopamine receptor D4 polymorphism
4.3.1. Nature the DRD4 polymorphism
The human DRD4 gene has many polymorphisms in its coding sequence. The third exon of this gene contains a 48‐base pair variable number tandem repeat with 2–11 repeats. 75 The 7R genotype is considered to be linked to the suppressed DRD4 expression in vitro. 76 The 2R is considered a “displacement” for the 7R in the Asian population; it is also assumed to function as the “risk” allele. 77 , 78 The “risk” allele might mean the poor expression of DRD4 effects.
4.3.2. Correlation of DRD4 polymorphism with human behavior
The DRD4 gene polymorphism is related to the diversity of temperaments, behaviors, and (eventually) psychiatric disorders. A meta‐analytic study identified a significant association of “longer” DRD4 variants with lower levels of executive function and social/emotional development. 79 No clear association between DRD4 polymorphism and novelty‐seeking was confirmed in two meta‐analyses. 80 , 81 In an international meta‐analysis, 82 the association between attention‐deficit hyperactivity disorder (ADHD) and DRD4 7R was confirmed in people of European–Caucasian (OR 1.64, p < 0.00001), South American (OR 2.41, p = 0.001), and Middle Eastern ancestry (OR 0.72, p = 0.014) but not confirmed in people of Asian ancestry (OR 1.65, p = 0.49). Many studies have suggested that the relationship between DRD4 polymorphism and the occurrence of ADHD might be affected by the environment in which childhood development took place and the presence/absence of adverse events.
The study on DRD4 gene tandem polymorphisms by Tochigi et al. 83 contains the 2R (15.1%), 3R (0.5%), 4R (77.8%), 5R (4.3%), 6R (1.5%), and 7R (0.8%) and classified the genotypes into two groups: the short group, containing only alleles with two to four repeats (n = 170), and the long group, containing one or two alleles with five to seven repeats (n = 26). Because 83.3% of those in the genotypes of the short group are 4R, the most psychological nature of the short group could be regarded as resulting from the sufficient expression of the DRD4 effects. The authors demonstrated a higher score for neuroticism in the short group than in the long group in the examination by the revised NEO personality inventory. In the subscale of the NEO, the score of anxiety, anger‐hostility, and vulnerability in the short group was higher than that in the long group. These psychological characteristics could be thought to be related to those of RS and ADA. Considering the above findings on DRD4 polymorphism in the above‐mentioned discussion, it is plausible that ADA may have a high affinity for the 4R genotype with a high DRD4 effect.
At the moment, we could not find any direct relationship between RS and DRD4 polymorphisms.
5. NEURAL CIRCUIT OF A PANIC ATTACK AND ADA
The author sometimes encountered cases of clinically alternative appearances of panic attacks and ADA. This implies that primary anxiety underlies the two phenomena. Hence, when considering the neural circuit of the ADA, one must first consider that of the panic attack. Figure 4 shows the proposed neural circuit of a panic attack. In ADA, rumination is believed to be one of the primary symptoms. The currently proposed circulatory neural pathways of rumination in depression are ventromedial prefrontal cortex → posterior cingulate cortex (precuneus) → dorsomedial nucleus of the thalamus → subgenual prefrontal cortex → ventromedial prefrontal cortex, 72 which is a major part of the default mode network (Figure 5). In functional MRI studies of depression, the fiber connections between the amygdala and the two portions of the rumination pathway, including the amygdala–subgenual prefrontal cortex pathway 73 and the amygdala–precuneus pathway, 74 are hyperfunctional. As these two pathways are reciprocal, excitation of the amygdala may facilitate the rumination circuit and vice versa. This may explain why the duration of the ADA is longer than the duration of the panic attack. Alternatively, in the case of a panic attack, amygdala excitation moves downward to the brainstem and is eliminated by the outbreak of physical symptoms, resulting in an attack not lasting for a long time.
FIGURE 4.
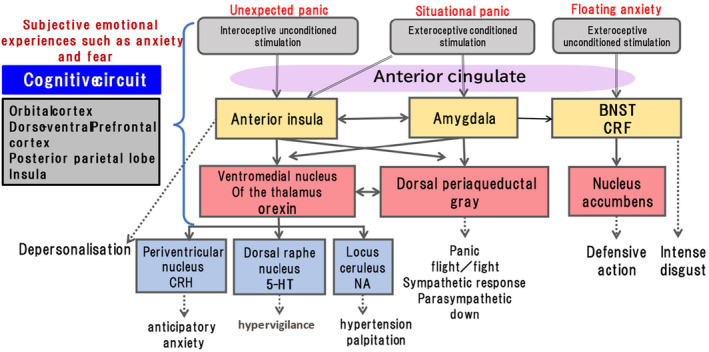
The schematic diagram of the neuronal circuit of panic attack prepared by the author based on literature of LeDoux and Pine, 68 ; Dreslerer al, 69 ; Johnson et al, 70 ; and Vollmer et al. 71 BNST, bed nucleus of the stria terminalis; CRF, corticotropin‐releasing factor; CRH, corticotropin releasing hormone; 5‐HT, 5‐hydroxytryptamine; NA, noradrenaline.
FIGURE 5.
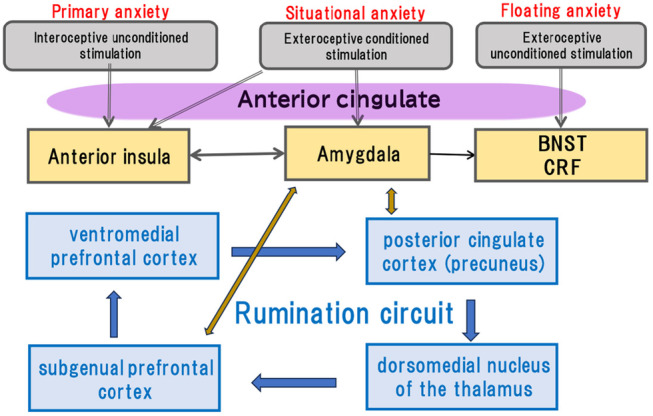
The tentative schematic diagram of the neuronal circuit of anxious depressive attack. See text for detailed explanation.
6. THERAPEUTIC ISSUES
6.1. Clinical application related to opioids
Clinically potent antidepressants, mirtazapine, fluoxetine, venlafaxine, 84 and imipramine, 85 were demonstrated to have analgesic effects at high doses, which were inhibited by naloxone, a non‐specific antagonist of the opioid receptor in animal experiments. Among them, fluoxetine, 86 , 87 venlafaxine, 88 and imipramine 89 , 90 were demonstrated to be effective for atypical depression.
Tramadol is a synthetic opioid with an analgesic effect, and its mechanism of action is the reuptake inhibition of norepinephrine and serotonin. 91 It has structural similarities with Venlafaxine. 92 Therefore, it has been suggested that tramadol could be used as an antidepressant with the benefits of an acute onset of action compared to modern antidepressants and the documentation of low abuse rates while maintaining an objective view of the risks. 93 Indeed, tramadol has been reported to be effective in augmenting treatment in 12 patients with a major depressive disorder who had a partial response to selective serotonin reuptake inhibitors. 94 A monotherapy with tramadol was also reported 95 : A 64‐year‐old male patient having long‐term intractable depression comorbid with obsessive‐compulsive disorder and panic disorder (in remission) described feeling the effect of tramadol within 30 min. Beforehand, the patient had failed 16 antidepressants (including monoamine oxidase inhibitors), three augmentation trials (including bupropion, lithium, and methylphenidate), and a combination of five anxiolytic drugs. Tramadol is marketed in Japan as a non‐narcotic analgesic in combination with Acetaminophen.
6.2. Clinical application related to the dopamine D4 receptor hypothesis
As shown in the previous chapter (4.1.), RS is considered to be related to the hypersensitivity of DRD4. Therefore, the treatment of RS requires drugs to decrease or block the effects of DRD4 actions. Many dopamine receptor blockers are used as antipsychotics and some block DRD4. The candidate drugs for anti‐RS among dopamine blockers are preferable to have a relatively higher blocking power for DRD4 and lower potential for DRD2 effects, which causes extrapyramidal symptoms. For patients having agitation or violent anxiety, α1 blocking effects might be necessary. Table 2 lists candidate drugs. Of these drugs, those likely to be available in clinical practice for the treatment of ADA would be pipamperone for regular treatment and asenapine for single use during highly excited attacks because of its fast action and sedative effect.
TABLE 2.
Ki values of the candidate drugs.
| DRD4 | DRD2 | D4/D2 | α1 | ||
|---|---|---|---|---|---|
| Pipamperone | 5.1 | 120.0 | 0.04 | 66.0 | Li et al. 96 |
| Clozapine | 40.0 | 152.0 | 0.26 | 123.0 | Schotte et al. 97 |
| 63.0 | 72.0 | 0.86 | 109.0 | Ishiyama et al. 98 | |
| 47.0 | 125.0 | 0.38 | – | Bymaster et al. 100 | |
| 13.0 | 74.0 | 0.17 | – | Patel et al. 102 | |
| Asenapine | 1.1 | 1.3 | 0.85 | 1.2 | Stoner et al. 99 |
| Olanzapine | 21.0 | 72.0 | 0.29 | 109.0 | Ishiyama et al. 98 |
| 26.0 | 11.0 | 2.36 | – | Bymaster et al. 100 | |
| 57.3 | 10.5 | 5.46 | – | Rasmussen et al. 101 | |
| Perospirone | 1.8 | 1.3 | 1.38 | 2.5 | Schotte et al. 97 |
| Mosapramine | 3.2 | 1.8 | 1.66 | 43.0 | Schotte et al. 97 |
| Risperidone | 16.0 | 3.3 | 4.84 | 2.3 | Schotte et al. 97 |
| 7.5 | 4.9 | 1.53 | 5.0 | Ishiyama et al. 98 | |
| Haloperidol | 15.0 | 2.0 | 7.50 | – | Li et al. 96 |
| 11.0 | 1.4 | 7.80 | 19.0 | Schotte et al. 97 | |
| 5.0 | 2.0 | 2.50 | 12.0 | Ishiyama et al. 98 | |
| 1.6 | 1.0 | 1.60 | – | Bymaster et al. 100 | |
| 2.3 | 1.4 | 1.64 | – | Patel et al. 102 | |
| Spiperone | 1.4 | 0.053 | 26.40 | 25.0 | Li et al. 96 |
In the practical clinical treatment of ADA, these laboratory data need to be used as a basis for selecting drugs, taking into account the patient's general condition (including agitation) and side effects.
7. DISCUSSION
In an immunohistochemical experiment in the rat striatum, the DRD4 agonist, PD168,870, reduced the immunoreactivity of μ‐opioids; conversely, the DRD4 antagonist, L745,870, enhanced the immunoreactivity of μ‐opioids. 103 This mechanism may also apply to the human frontal cortex because DRD4 gene expression 104 and MOR messenger RNA 105 are present at high concentrations in the human frontal cortex. If so, the treatment of ADA with underlying RS using DRD4 blockers may also have an additive effect on opioid systems.
The DRD4 polymorphism tandem repeat 4, which may be associated with ADA, has a high distribution rate in Asia, particularly in Japan. 106 Furthermore, the MOR polymorphism, A118G, has the highest distribution rate in Japan. 107 ADA cases are more common in Japan than in Europe and the USA due to these ethnic differences in gene polymorphisms. Therefore, there are still no studies on ADA in Western countries.
RS is associated with borderline personality disorder (BPD), 21 , 27 , 108 , 109 in which 91% of adolescents engaged in nonsuicidal self‐injury at least once. 110 Patients with ADA often attempt self‐mutilation during attacks. We treated ADA in a 30‐year female patient diagnosed with borderline personality disorder using haloperidol, a DRD4 blocker. After 10 months of treatment, this patient's BPD together with ADA completely disappeared. 111 Notably, not all patients with BPD have ADA. We believe that the symptoms described in section 6 of the DSM‐5 diagnostic criteria for BPD were evident in people with ADA, but this condition does not correspond to ADA. Thus, some patients diagnosed with BPD may have ADA, which can be treated with a DRD4 blocker.
In a recent study on borderline personality disorder, separation anxiety was highlighted as a cross‐diagnostic target symptom from a therapeutic perspective. 112 As biological markers of separation anxiety, variants in the oxytocin receptor, serotonin transporter, MOR, DRD4, and translocator protein were proposed. 113 Separation anxiety is a contributing factor to the emergence of rejection sensitivity. Separation anxiety was adopted as one of the five subscales of the IPSM. 15 Extensive twin studies have shown that separation anxiety, carbon dioxide sensitivity, and panic disorder are genetically closely related. Within these phenotypes, separation anxiety, carbon dioxide sensitivity, and early separation are all closely related to opioids. 34 , 114 Taken together, opioids may play an important role in the pathophysiology of both RS and separation anxiety, which are key symptoms of various internalized disorders.
Research domain criteria recommended a dimensional approach focusing on narrower domains of psychopathology, rather than the use of diagnostic criteria in clinical research, to characterize brain‐behavior relationships. 115 Especially in emotional disorders having complicated comorbidities, the dimensional approach seems more important than disorder‐specific criteria. 116 Such directivity could also look at developmental and environmental aspects that would help experts to treat the disorders from a more fundamental level. 117 The present paper has gone along with these directions, in which RS is thought to be a psychological marker of TRD, and MOR and DRD4 function are regarded as biological markers of RS. Furthermore, ADA mediates between RS and TRD.
8. CONCLUSION
Although the incidence of ADA is not low, very few physicians can diagnose ADA in clinical practice because patients rarely complain of ADA on their own. ADA is treatable, as shown in this study. Thus, if ADA were recognized by more physicians, the incidence of TRD would be reduced.
AUTHOR CONTRIBUTIONS
The author confirms sole responsibility for the following: Review of previous studies and preparation of manuscript.
FUNDING INFORMATION
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
CONFLICT OF INTEREST STATEMENT
Hisanobu Kaiya has received lecture fees from Meiji Seika Pharma and Mochida Pharmaceutical outside the submitted work.
ETHICS STATEMENT
Approval of the Research Protocol by an Institutional Reviewer Board: N/A.
Informed Consent: N/A.
Registry and the Registration No. of the Study/Trial: N/A.
Animal Studies: N/A.
DISCLAIMER
The effectiveness of the drugs described in this paper for refractory depression and ADA are only inferred based on a survey of the literature and a discussion of its contents. These drugs have not been subjected to formal clinical studies. Therefore, they have not been approved for use by the authorities.
ACKNOWLEDGMENTS
I would like to express my sincere thanks to Prof. Kenji Otowa and Prof Tsukasa Sasaki for reviewing this paper. I would like to thank Enago (www.enago.jp) for the English language editing.
Kaiya H. Anxious‐depressive attack and rejection sensitivity—Toward a new approach to treatment‐resistant depression. Neuropsychopharmacol Rep. 2024;44:17–28. 10.1002/npr2.12399
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Kaiya H. Anxious‐depressive attack: an overlooked condition a case report. Anxiety Disord Res. 2016;8(1):22–30. [Google Scholar]
- 2. Kaiya H. Distinctive clinical features of “anxious‐ depressive attack”. Anxiety Disord Res. 2017;9(1):2–16. [Google Scholar]
- 3. Noda S, Masaki M, Kishimoto T, Kaiya H. Effect of rejection sensitivity on the development of anxious‐depressive attack in Japanese outpatients: the mediating roles of rumination, social anxiety, and depressive symptoms. Front Psychol. 2022;13:1016879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Matsumoto S, Mina M, Komatsu C, Noguchi K, Kawasaki N, Kishino Y, et al. Examination of the prevalence of anxious‐depressive attacks in new outpatients. The 12th Japanese Society of Anxiety and Related Disorders Academic Disorders Academic Conference, Hyogo . 2020.
- 5. Fava M, Rush AJ, Alpert JE, Balasubramani GK, Wisniewski SR, Carmin CN, et al. Difference in treatment outcome in outpatients with anxious versus non‐anxious depression: a STAR*D report. Am J Psychiatry. 2008;165(3):342–351. [DOI] [PubMed] [Google Scholar]
- 6. Lundberg J, Cars T, Lööv SÅ, Söderling J, Sundström J, Tiihonen J, et al. Association of Treatment‐Resistant Depression with Patient Outcomes and Health Care Resource Utilization in a population‐wide study. JAMA Psychiatry. 2022;80(2):167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boyce P, Hickie I, Parker G, Mitchell P, Wilhelm K, Brodaty H. Interpersonal sensitivity and the one‐year outcome of a depressive episode. Aust N Z J Psychiatry. 1992;26(2):156–161. [DOI] [PubMed] [Google Scholar]
- 8. De Rubeis J, Lugo RG, Witthöft M, Sütterlin S, Pawelzik MR, Vögele C. Rejection sensitivity as a vulnerability marker for depressive symptom deterioration in men. PloS One. 2017;12(10):e0185802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hsu DT, Jarcho JM. Next up for psychiatry: rejection sensitivity and the social brain. Neuropsychopharmacology. 2021;46(1):239–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klein DF, Davis JM. Diagnosis and drug treatment of psychiatric disorders. Baltimore, Maryland: Williams & Wilkins Co.; 1969. [Google Scholar]
- 11. Liebowitz MR, Klein DF. Hysteroid dysphoria. Psychiat Clin North Am. 1979;2(3):555–575. [Google Scholar]
- 12. Horney K. The neurotic personality of our time. New York: Norton; 1937. [Google Scholar]
- 13. American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5th ed. Washington, D.C.: American Psychiatric Association; 2013. [Google Scholar]
- 14. Stewart JW, McGrath PJ, Rabkin JG, Quitkin FM. Atypical depression. A valid clinical entity? Psychiatr Clin North Am. 1993;16(3):479–495. [PubMed] [Google Scholar]
- 15. Boyce P, Parker G. Development of a scale to measure interpersonal sensitivity. Aust N Z J Psychiatry. 1989;23(3):341–351. [PubMed] [Google Scholar]
- 16. Suyama H, Kaneko Y, Ito R, Yokoyama S, Ito D, Kunisato Y, et al. Influences of interpersonal rejection sensitivity and depressive symptoms on social anxiety symptoms in severe social anxiety disorder patients. Anxiety Disord Res. 2014;6(1):7–16. [Google Scholar]
- 17. Downey G, Feldman SI. Implications of rejection sensitivity for intimate relationships. J Pers Soc Psychol. 1996;70(6):1327–1343. [DOI] [PubMed] [Google Scholar]
- 18. Godleski SA, Eiden RD, Kachadourian L, Lucke JF. Etiological pathways to rejection sensitivity in a high‐risk sample. Pers Soc Psychol Bull. 2019;45(5):715–727. [DOI] [PubMed] [Google Scholar]
- 19. Rowe SL, Gembeck MJZ, Rudolph J, Nesdale D. A longitudinal study of rejecting and autonomy‐restrictive parenting, rejection sensitivity, and socioemotional symptoms in early adolescents. J Abnorm Child Psychol. 2015;43:1107–1118. [DOI] [PubMed] [Google Scholar]
- 20. Nenov‐Matt T, Barton BB, Dewald‐Kaufmann J, Goerigk S, Rek S, Zentz K, et al. Loneliness, social isolation and their difference: a cross‐diagnostic study in persistent depressive disorder and borderline personality disorder. Front Psych. 2020;17(11):608476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Foxhall M, Hamilton‐Giachritsis C, Button K. The link between rejection sensitivity and borderline personality disorder: a systematic review and meta‐analysis. Br J Clin Psychol. 2019;58(3):289–326. [DOI] [PubMed] [Google Scholar]
- 22. Gao S, Assink M, Liu T, Chan KL, Ip P. Associations between rejection sensitivity, aggression, and victimization: a meta‐analytic review. Trauma Violence Abuse. 2021;22(1):125–135. [DOI] [PubMed] [Google Scholar]
- 23. Zhou J, Li X, Tian L, Huebner ES. Longitudinal association between low self‐esteem and depression in early adolescents: the role of rejection sensitivity and loneliness. Psychol Psychother. 2020;93(1):54–71. [DOI] [PubMed] [Google Scholar]
- 24. Pearson KA, Watkins ER, Mullan EG, Moberly NJ. Psychosocial correlates of depressive rumination. Behav Res Ther. 2010;48(8):784–791. [DOI] [PubMed] [Google Scholar]
- 25. Pearson KA, Watkins ER, Mullan EG. Rejection sensitivity prospectively predicts increased rumination. Behav Res Ther. 2011;49(10):597–605. [DOI] [PubMed] [Google Scholar]
- 26. Brown SL, Mitchell SM, Roush JF, La Rosa NL, Cukrowicz KC. Rejection sensitivity and suicide ideation among psychiatric inpatients: an integration of two theoretical models. Psychiatry Res. 2019;272:54–60. [DOI] [PubMed] [Google Scholar]
- 27. Gao S, Assink M, Cipriani A, Lin K. Associations between rejection sensitivity and mental health outcomes: a meta‐analytic review. Clin Psychol Rev. 2017;57:59–74. [DOI] [PubMed] [Google Scholar]
- 28. Ehnvall A, Mitchell PB, Hadzi‐Pavlovic D, Parker G, Frankland A, Loo C, et al. Rejection sensitivity and pain in bipolar versus unipolar depression. Bipolar Disord. 2014;16(2):190–198. [DOI] [PubMed] [Google Scholar]
- 29. Akiskal HS, Akiskal KK, Perugi G, Toni C, Ruffolo G, Tusini G. Bipolar II and anxious reactive "comorbidity": toward better phenotypic characterization suitable for genotyping. J Affect Disord. 2006;96(3):239–247. [DOI] [PubMed] [Google Scholar]
- 30. Ng TH, Johnson SL. Rejection sensitivity is associated with quality of life, psychosocial outcome, and the course of depression in euthymic patients with bipolar I disorder. Cog Ther Res. 2013;37:1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Parker G, Roy K, Mitchell P, Wilhelm K, Malhi G, Hadzi‐Pavlovic D. Atypical depression: a reappraisal. Am J Psychiatry. 2002;159(9):1470–1479. [DOI] [PubMed] [Google Scholar]
- 32. Posternak MA, Zimmerman M. The prevalence of atypical features across mood, anxiety, and personality disorders. Compr Psychiatry. 2002;43(4):253–262. [DOI] [PubMed] [Google Scholar]
- 33. Vaccarino AL, Kastin AJ. Endogenous opiates: 1999. Peptides. 2000;21(12):1975–2034. [DOI] [PubMed] [Google Scholar]
- 34. Herman BH, Panksepp J. Effects of morphine and naloxon on separation distress and approach attachment: evidence for opiate mediation of social affect. Pharmacol Biochem Behav. 1978;9(2):213–220. [DOI] [PubMed] [Google Scholar]
- 35. Panksepp J, Herman B, Conner R, Bishop P, Scott JP. The biology of social attachments: opiates alleviate separation distress. Biol Psychiatry. 1978;13(5):607–618. [PubMed] [Google Scholar]
- 36. Kalin NH, Shelton SE, Barksdale CM. Opiate modulation of separation‐induced distress in non‐human primates. Brain Res. 1988;440(2):285–292. [DOI] [PubMed] [Google Scholar]
- 37. Carden SE, Barr GA, Hofer MA. Differential effects of specific opioid receptor agonists on rat pup isolation calls. Brain Res Dev Brain Res. 1991;62(1):17–22. [DOI] [PubMed] [Google Scholar]
- 38. Wamick E, McCyrdy CR, Sufka KJ. Opioid receptor function in social attachment in young domestic fowl. Behav Brain Res. 2005;160(2):277–285. [DOI] [PubMed] [Google Scholar]
- 39. Eisenberger NI. The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nat Rev Neurosci. 2012;13(6):421–434. [DOI] [PubMed] [Google Scholar]
- 40. Berthier M, Starkstein MD, Leiguarda R. Asymbolia for pain: a sensory‐limbic disconnection syndrome. Ann Neurol. 1988;24:41–49. [DOI] [PubMed] [Google Scholar]
- 41. Foltz EL, White LE. Pain “relief” by frontal cingulumotomy. J Neurosurg. 1962;19:89–100. [DOI] [PubMed] [Google Scholar]
- 42. Burklund LJ, Torre JB, Lieberman MD, Taylor SH, Craske MG. Neural responses to social threat and predictors of cognitive behavioral therapy and acceptance and commitment therapy in social anxiety disorder. Psychiatry Res Neuroimaging. 2017;30:52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bonenberger M, Plener PL, Groschwitz RC, Grön G, Abler B. Polymorphism in the μ‐opioid receptor gene (OPRM1) modulates neural processing of physical pain, social rejection and error processing. Exp Brain Res. 2015;233(9):2517–2526. [DOI] [PubMed] [Google Scholar]
- 44. Hill KR, Hsu DT, Taylor SF, Ogden RT, DeLorenzo C, Parsey RV. Rejection sensitivity and mu opioid receptor dynamics associated with mood alterations in response to social feedback. Psychiatry Res Neuroimaging. 2022;324:111505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hsu DT, Sanford BJ, Meyers KK, Love TM, Hazlett KE, Walker SJ, et al. It still hurts: altered endogenous opioid activity in the brain during social rejection and acceptance in major depressive disorder. Mol Psychiatry. 2015;20(2):193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ehnvall A, Mitchell PB, Hadzi‐Pavlovic D, Malhi GS, Parker G. Pain during depression and relationship to rejection sensitivity. Acta Psychiatr Scand. 2009;119(5):375–382. [DOI] [PubMed] [Google Scholar]
- 47. Ehnvall A, Mitchell PB, Hadzi‐Pavlovic D, Loo C, Breakspear M, Wright A, et al. Pain and rejection sensitivity in bipolar depression. Bipolar Disord. 2011;13(1):59–66. [DOI] [PubMed] [Google Scholar]
- 48. Mague SD, Blendy JA. OPRM1 SNP (A118G): involvement in disease development, treatment response, and animal models. Drug Alcohol Depend. 2010;108(3):172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sia AT, Lim Y, Lim EC, Goh RW, Law HY, Landau R, et al. A118G single nucleotide polymorphism of human μ‐opioid receptor gene influences pain perception and patient‐controlled intravenous morphine consumption after intrathecal morphine for postcesarean analgesia. Anesthesiologists. 2008;109(3):520–526. [DOI] [PubMed] [Google Scholar]
- 50. Tan EC, Lim EC, Teo YY, Lim Y, Law HY, Sia AT. Ethnicity and OPRM variant independently predict pain perception and patient‐controlled analgesia usage for post‐operative pain. Mol Pain. 2009;5:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Troisi A, Frazzetto G, Carola V, Di Lorenzo G, Coviello M, D'Amato FR, et al. Social hedonic capacity is associated with the A118G polymorphism of the mu‐opioid receptor gene (OPRM1) in adult healthy volunteers and psychiatric patients. Soc Neurosci. 2011;6(1):88–97. [DOI] [PubMed] [Google Scholar]
- 52. Peciña M, Love T, Stohler CS, Goldman D, Zubieta JK. Effects of the mu opioid receptor polymorphism (OPRM1 A118G) on pain regulation, placebo effects and associated personality trait measures. Neuropsychopharmacology. 2015;40(4):957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Carver CS, Johnson SL, Kim Y. Mu opioid receptor polymorphism, early social adversity, and social traits. Soc Neurosci. 2016;11(5):515–524. [DOI] [PubMed] [Google Scholar]
- 54. Cimino S, Carola V, Cerniglia L, Bussone S, Bevilacqua A, Tambelli R. The μ‐opioid receptor gene A118G polymorphism is associated with insecure attachment in children with disruptive mood regulation disorder and their mothers. Brain Behav. 2020;10(7):e01659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Slavich GM, Tartter MA, Brennan PA, Hammen C. Endogenous opioid system influences depressive reactions to socially painful targeted rejection life events. Psychoneuroendocrinology. 2014;49:141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Way BM, Taylor SE, Eisenberger NI. Variation in the mu‐opioid receptor gene (OPRM1) is associated with dispositional and neural sensitivity to social rejection. Proc Natl Acad Sci U S A. 2009;106(35):15079–15084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Persson E, Asutay E, Heilig M, Löfberg A, Pedersen N, Västfjäll D, et al. Variation in the μ‐opioid receptor gene (OPRM1) does not moderate social‐rejection sensitivity in humans. Psychol Sci. 2019;30(7):1050–1062. [DOI] [PubMed] [Google Scholar]
- 58. Tchalova K, Sadikaj G, Moskowitz DS, Zuroff DC, Bartz JA. Variation in the μ‐opioid receptor gene (OPRM1) and experiences of felt security in response to a romantic partner's quarrelsome behavior. Mol Psychiatry. 2021;26(8):3847–3857. [DOI] [PubMed] [Google Scholar]
- 59. Suzuki A, Shirata T, Noto K, Matsumoto Y, Muraosa H, Abe M, et al. Associations of the A118G OPRM1 polymorphism with sociotropy and interpersonal sensitivity. Brain Behav. 2022;12(7):e2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Otani K, Suzuki A, Kamata M, Matsumoto Y, Shibuya N, Sadahiro R. Interpersonal sensitivity is correlated with sociotropy but not with autonomy in healthy subjects. J Nerv Ment Dis. 2012;200(2):153–155. [DOI] [PubMed] [Google Scholar]
- 61. Barr CS, Schwandt ML, Lindell SG, Higley JD, Maestripieri D, Goldman D, et al. Variation at the mu‐opioid receptor gene (OPRM1) influences attachment behavior in infant primates. Proc Natl Acad Sci U S A. 2008;105(13):5277–5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Higham JP, Barr CS, Hoffman CL, Mandalaywala TM, Parker KJ, Maestripieri D. Mu‐opioid receptor (OPRM1) variation, oxytocin levels and maternal attachment in free‐ranging rhesus macaques Macaca mulatta. Behav Neurosci. 2011;125(2):131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Moles A, Kieffer BL, D' Amato FR. Deficit in attachment behavior in mice lacking the mu‐opioid receptor gene. Science. 2004;304:1983–1986. [DOI] [PubMed] [Google Scholar]
- 64. Laviolette SR, Lipski WJ, Grace AA. A subpopulation of neurons in the medial prefrontal cortex encodes emotional learning with burst and frequency codes through a dopamine D4 receptor‐dependent basolateral amygdala input. J Neurosci. 2005;25(26):6066–6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wang X, Zhong P, Yan Z. Dopamine D4 receptors modulate GABAergic signaling in pyramidal neurons of prefrontal cortex. J Neurosci. 2002;22:9185–9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Floresco SB, Maric TT. Dopaminergic regulation of inhibitory and excitatory transmission in the basolateral amygdala–prefrontal cortical pathway. J Neurosci. 2007;27(8):2045–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lauzon NM, Bishop SF, Laviolette SR. Dopamine D1 versus D4 receptors differentially modulate the encoding of salient versus nonsalient emotional information in the medial prefrontal cortex. J Neurosci. 2009;29(15):4836–4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. LeDoux JE, Pine DS. Using neuroscience to help understand fear and anxiety: a two‐system Framwork. Am J Psychiatry. 2016;173(11):1083–1093. [DOI] [PubMed] [Google Scholar]
- 69. Dresler T, Guhn A, Tupak SV, Ehlis AC, Herrmann MJ, Fallgatter AJ, et al. Revise the revised? New dimensions of the neuroanatomical hypothesis of panic disorder. J Neural Transm. 2013;120:3–29. [DOI] [PubMed] [Google Scholar]
- 70. Johnson PL, Federici LM, Shekhar A. Etiology, triggers and neurochemical circuits associated with unexpected, expected, and laboratory‐induced panic attacks. Neurosci Biobehav Rev. 2014;46(3):429–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Vollmer LL, Strawn JR, Sah R. Acid‐base dysregulation and chemosensory mechanisms in panic disorder: a translational update. Transl Psychiatry. 2015;5(5):e572. 10.1038/tp.2015.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hamilton JP, Farmer M, Fogelman P, Gotlib IH. Depressive rumination, the default‐mode network, and the dark matter of clinical neuroscience. Biol Psychiatry. 2015;78(4):224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jalbrzikowski M, Larsen B, Hallquist MN, Foran W, Calabro F, Luna B. Development of White matter microstructure and intrinsic functional connectivity between the amygdala and ventromedial prefrontal cortex: associations with anxiety and depression. Biol Psychiatry. 2017;82(7):511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cullen KR, Westlund MK, Klimes‐Dougan B, Mueller BA, Houri A, Eberly LE, et al. Abnormal amygdala resting‐state functional connectivity in adolescent depression. JAMA Psychiatry. 2014;71(10):1138–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Oak JN, Oldenhof J, Van Tol HH. The dopamine D4 receptor: one decade of research. Eur J Pharmacol. 2000;405(1–3):303–327. [DOI] [PubMed] [Google Scholar]
- 76. Schoots O, Van Tol HH. The human dopamine D4 receptor repeat sequences modulate expression. Pharmacogenomics J. 2003;3:343–348. [DOI] [PubMed] [Google Scholar]
- 77. Leung PW, Lee CC, Hung SF, Ho TP, Tang CP, Kwong SL, et al. Dopamine receptor D4 (DRD4) gene in Han Chinese children with attention‐deficit/hyperactivity disorder (ADHD): increased prevalence of the 2‐repeat allele. Am J Med Genet B Neuropsychiatr Genet. 2005;133B:54–56. [DOI] [PubMed] [Google Scholar]
- 78. Reist C, Ozdemir V, Wang E, Hashemzadeh M, Mee S, Moyzis R. Novelty seeking and the dopamine D4 receptor gene (DRD4) revisited in Asians: haplotype characterization and relevance of the 2‐repeat allele. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:453–457. [DOI] [PubMed] [Google Scholar]
- 79. Pappa I, Mileva‐Seitz VR, Bakermans‐Kranenburg MJ, Tiemeier H, van IJzendoorn MH. The magnificent seven: a quantitative review of dopamine receptor d4 and its association with child behavior. Neurosci Biobehav Rev. 2015;57:175–186. [DOI] [PubMed] [Google Scholar]
- 80. Kluger AN, Siegfried Z, Ebstein RP. A meta‐analysis of the association between DRD4 polymorphism and novelty seeking. Mol Psychiatry. 2002;7(7):712–717. [DOI] [PubMed] [Google Scholar]
- 81. Munafò MR, Yalcin B, Willis‐Owen SA, Flint J. Association of the dopamine D4 receptor (DRD4) gene and approach‐related personality traits: meta‐analysis and new data. Biol Psychiatry. 2008;63(2):197–206. [DOI] [PubMed] [Google Scholar]
- 82. Nikolaidis A, Gray JR. ADHD and the DRD4 exon III 7‐repeat polymorphism: an international meta‐analysis. Soc Cogn Affect Neurosci. 2010;5(2–3):188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tochigi M, Hibino H, Otowa T, Kato C, Marui T, Ohtani T, et al. Association between dopamine D4 receptor (DRD4) exon III polymorphism and neuroticism in the Japanese population. Neurosci Lett. 2006;398(3):333–336. [DOI] [PubMed] [Google Scholar]
- 84. Sikka P, Kaushik S, Kumar G, Kapoor S, Bindra VK, Saxena KK. Study of antinociceptive activity of SSRI (fluoxetine and escitalopram) and atypical antidepressants (venlafaxine and mirtazepine) and their interaction with morphine and naloxone in mice. J Pharm Bioallied Sci. 2011;3(3):412–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Takahashi RN, Paz MM. Influence of naloxone on analgesic effects of antidepressants in mice. Braz J Med Biol Res. 1987;20(5):607–610. [PubMed] [Google Scholar]
- 86. Joyce PR, Mulder RT, McKenzie JM, Luty SE, Cloninger CR. Atypical depression, atypical temperament and a differential antidepressant response to fluoxetine and nortriptyline. Depress Anxiety. 2004;19(3):180–186. [DOI] [PubMed] [Google Scholar]
- 87. Pande AC, Birkett M, Fechner‐Bates S, Haskett RF, Greden JF. Fluoxetine versus phenelzine in atypical depression. Biol Psychiatry. 1996;40(10):1017–1020. [DOI] [PubMed] [Google Scholar]
- 88. Roose SP, Miyazaki M, Devanand D, Seidman S, Fitzsimmons L, Turret N, et al. An open trial of venlafaxine for the treatment of late‐life atypical depression. Int J Geriatr Psychiatry. 2004;19(10):989–994. [DOI] [PubMed] [Google Scholar]
- 89. Quitkin FM, Stewart JW, McGrath PJ, Liebowitz MR, Harrison WM, Tricamo E, et al. Phenelzine versus imipramine in the treatment of probable atypical depression: defining syndrome boundaries of selective MAOI responders. Am J Psychiatry. 1988;145(3):306–311. [DOI] [PubMed] [Google Scholar]
- 90. McGrath PJ, Stewart JW, Janal MN, Petkova E, Quitkin FM, Klein DF. A placebo‐controlled study of fluoxetine versus imipramine in the acute treatment of atypical depression. Am J Psychiatry. 2000;157(3):344–350. [DOI] [PubMed] [Google Scholar]
- 91. Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43(13):879–923. [DOI] [PubMed] [Google Scholar]
- 92. Markowitz JS, Patrick KS. Venlafaxine‐tramadol similarities. Med Hypotheses. 1998;51(2):167–168. [DOI] [PubMed] [Google Scholar]
- 93. Barber J. Examining the use of tramadol hydrochloride as an antidepressant. Exp Clin Psychopharmacol. 2011;19(2):123–130. [DOI] [PubMed] [Google Scholar]
- 94. Fanelli J, Montgomery C. Use of the analgesic tramadol in antidepressant potentiation. Psychopharmacol Bull. 1996;32:442. [Google Scholar]
- 95. Shapiro DA, Renock S, Arrington E, Chiodo LA, Liu L‐X, Sibley DR, et al. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology. 2003;28(8):1400–1411. [DOI] [PubMed] [Google Scholar]
- 96. Li P, Snyder GL, Vanover KE. Dopamine targeting drugs for the treatment of schizophrenia: past, present and future. Curr Top Med Chem. 2016;16:3385–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Schotte A, Bonaventure P, Janssen PF, Leysen JE. In vitro receptor binding and in vivo receptor occupancy in rat and Guinea pig brain: risperidone compared with antipsychotics hitherto used. Jpn J Pharmacol. 1995;69(4):399–412. [DOI] [PubMed] [Google Scholar]
- 98. Ishiyama T, Loebel A, Cucchiaro J, Horisawa T, Tokuda K, Ogasa M, et al. Comparative receptor binding profile of lurasidone and other first and second generation antipsychotics. Poster Sessions #038 XXVII CINP Congress, Hong Kong. 2010.
- 99. Stoner SC, Pace HA. Asenapine: a clinical review of a second‐generation antipsychotic. Clin Ther. 2012;34(5):1023–1040. [DOI] [PubMed] [Google Scholar]
- 100. Bymaster FP, Rasmussen K, Calligaro DO, Nelson DL, DeLapp NW, Wong DT, et al. In vitro and in vivo biochemistry of olanzapine: a novel, atypical antipsychotic drug. J Clin Psychiatry. 1997;58(Suppl 10):28–36. [PubMed] [Google Scholar]
- 101. Rasmussen K, Benvenga MJ, Bymaster FP, Calligaro DO, Cohen IR, Falcone JF, et al. Preclinical pharmacology of FMPD [6‐fluoro‐10‐[3‐(2‐methoxyethyl)‐4‐methyl‐piperazin‐1‐yl]‐2‐methyl‐4H‐3‐thia‐4,9‐diaza‐benzo[f]azulene]: a potential novel antipsychotic with lower histamine H1 receptor affinity than olanzapine. J Pharmacol Exp Ther. 2005;315(3):1265–1277. [DOI] [PubMed] [Google Scholar]
- 102. Patel S, Patel S, Marwood R, Emms F, Marston D, Leeson PD, et al. Identification and pharmacological characterization of [125I] L‐750,667, a novel radioligand for the dopamine D4 receptor. Mol Pharmacol. 1996;50(6):1658–1664. [PubMed] [Google Scholar]
- 103. Gago B, Fuxe K, Agnati L, Peñafiel A, De La Calle A, Rivera A. Dopamine D4 receptor activation decreases the expression of μ‐opioid receptors in the rat striatum. J Comp Neurol. 2007;502(3):358–366. [DOI] [PubMed] [Google Scholar]
- 104. Mulcrone J, Kerwin RW. The regional pattern of D4 gene expression in human brain. Neurosci Lett. 1997;234(2–3):147–150. [DOI] [PubMed] [Google Scholar]
- 105. Peckys D, Landwehrmeyer GB. Expression of mu, kappa, and delta opioid receptor messenger RNA in the human CNS: a 33P in situ hybridization study. Neuroscience. 1999;88(4):1093–1135. [DOI] [PubMed] [Google Scholar]
- 106. Chang FM, Kidd JR, Livak KJ, Pakstis AJ, Kidd KK. The world‐wide distribution of allele frequencies at the human dopamine D4 receptor locus. Hum Genet. 1996;98:91–101. [DOI] [PubMed] [Google Scholar]
- 107. Way BM, Lieberman MD. Is there a genetic contribution to cultural differences? Collectivism, individualism and genetic markers of social sensitivity. Soc Cogn Affect Neurosci. 2010;5(2–3):203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Seidl E, Padberg F, Bauriedl‐Schmidt C, Albert A, Daltrozzo T, Hall J, et al. Response to ostracism in patients with chronic depression, episodic depression and borderline personality disorder a study using Cyberball. J Affect Disord. 2020;260:254–262. [DOI] [PubMed] [Google Scholar]
- 109. Sato M, Fonagy P, Luyten P. Rejection sensitivity and borderline personality disorder features: the mediating roles of attachment anxiety, need to belong, and self‐criticism. J Pers Disord. 2020;34(2):273–288. [DOI] [PubMed] [Google Scholar]
- 110. Zanarini MC, Frankenburg FR, Ridolfi ME, Jager‐Hyman S, Hennen J, Gunderson JG. Reported childhood onset of self‐mutilation among borderline patients. J Pers Disord. 2006;20(1):9–15. [DOI] [PubMed] [Google Scholar]
- 111. Kaiya H. The ravings of an old neuropsychiatrist: 18. History of ADA discovery. Borderline personality disorder. J Journal of Psychotherapy. 2017;43(6):865–870. [Google Scholar]
- 112. Matthies S, Schiele MA, Koentges C, Pini S, Schmahl C, Domschke K. Please don't leave me – separation anxiety and related traits in borderline personality disorder. Curr Psychiatry Rep. 2018;20:1–10. [DOI] [PubMed] [Google Scholar]
- 113. Schiele MA, Bandelow B, Baldwin DS, Pini S, Domschke K. A neurobiological framework of separation anxiety and related phenotypes. Eur Neuropsychopharmacol. 2020;33:45–57. [DOI] [PubMed] [Google Scholar]
- 114. Preter M, Klein DF. Panic, suffocation false alarms, separation anxiety and endogenous opioids. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(3):603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Insel T, Cuthbert B. Research domain criteria (RDoC): commentary. Toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748–751. [DOI] [PubMed] [Google Scholar]
- 116. Brown TA, Barlow DH. A proposal for a dimensional classification system based on the shared features of the DSM‐IV anxiety and mood disorders: implications for assessment and treatment. Psychol Assess. 2009;21(3):256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Clark LA, Cuthbert B, Lewis‐Fernández R, Narrow WE, Reed GM. Three approaches to understanding and classifying mental disorder: ICD‐11, DSM‐5, and the National Institute of Mental Health's research domain criteria (RDoC). Psychol Sci Public Interest. 2017;18(2):72–145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.


