Abstract
Vascular endothelial growth factor (VEGF) signaling is known to be involved in the antidepressant‐like effects of conventional antidepressants, such as desipramine (DMI), a tricyclic antidepressant, and fluoxetine (FLX), a selective serotonin reuptake inhibitor; however, the precise role of neuronal VEGF signaling in mediating these effects remains unclear. Using mice with excitatory neuron‐specific deletion of VEGF and its receptor, fetal liver kinase 1 (Flk‐1) in the forebrain, we examined the effects of forebrain excitatory neuron‐specific deletion of VEGF or Flk‐1 on the antidepressant‐like effects of repeated DMI and chronic FLX administration in the forced swim test (FST). Repeated intraperitoneal (i.p.) injections of DMI (10, 10, and 20 mg/kg at 24, 4, and 1 h before the FST, respectively) significantly decreased immobility in control mice; however, this effect was completely blocked in mice with neuron‐specific VEGF or Flk‐1 deletion. Although chronic treatment with FLX (18 mg/kg/day, i.p.) did not impact immobility in control mice 1 day after the 22nd injection, immobility was significantly reduced 1 day after the preswim and the 23rd FLX injection. However, in mice with neuron‐specific Flk‐1 deletion, chronic FLX treatment significantly increased immobility in the preswim and failed to produce antidepressant‐like effects. Collectively, these findings indicate that neuronal VEGF–Flk‐1 signaling contributes to the antidepressant‐like actions of conventional antidepressants.
Keywords: major depressive disorder, selective serotonin reuptake inhibitor, tricyclic antidepressant, vascular endothelial growth factor receptor 2, vascular endothelial growth factor‐a
This study demonstrates that the antidepressant‐like effects of desipramine and fluoxetine in the forced swim test were blocked in mice with neuron‐specific deletion of VEGF or its receptor Flk‐1. These results indicate that neuronal VEGF–Flk‐1 signaling contributes to the antidepressant‐like actions of conventional antidepressants.
1. INTRODUCTION
Vascular endothelial growth factor (VEGF, also known as VEGF‐A) is a pleiotropic growth factor expressed by endothelial cells, neurons, astrocytes, and perivascular macrophages in the brain. 1 , 2 VEGF levels were found to be reduced in the prefrontal cortex (PFC) and hippocampus of a rat model of depression, 3 and treatment with chronic fluoxetine (FLX), a selective serotonin reuptake inhibitor, enhanced VEGF expression in neurons and endothelial cells, but not in astrocytes, in the hippocampal dentate gyrus. 1 , 4 , 5 Moreover, pharmacological blockade of fetal liver kinase 1 (Flk‐1, also known as VEGF receptor 2) reportedly suppressed the neurogenic and behavioral effects of chronic treatment with FLX or desipramine (DMI), a tricyclic antidepressant. 1 , 6 Although these findings suggest the essential role of VEGF–Flk‐1 signaling in the antidepressant‐like effects of conventional antidepressants, the precise function of neuronal VEGF–Flk‐1 signaling in mediating these antidepressant‐like effects remain unclear. Thus, in the present study, we addressed this question using mice with forebrain excitatory neuron‐specific deletion of VEGF or Flk‐1, recently developed by our research group. 7 , 8 , 9
2. METHODS
2.1. Animals
Adult male Flk‐1 flox/flox , 10 Vegfa flox/flox , 11 Camk2a‐Cre;Flk‐1 flox/flox (hereafter, Flk‐1 NEURON−/− ), 8 , 9 and Camk2a‐Cre;Vegfa flox/flox (hereafter, Vegf NEURON−/− ) 7 , 8 , 9 mice, aged 2–6 months, were group‐housed and maintained under standard conditions with a 12‐h light/dark cycle (light on 07:00) and access to food and water ad libitum. All experiments were in accordance with the National Institutes of Health guidelines and were approved by the Yale University Animal Care and Use Committee.
2.2. Drug treatments and forced swim test (FST)
DMI (Sigma) and FLX (Spectrum Chemical) were dissolved in sterile saline prior to administration. DMI (10, 10, and 20 mg/kg, respectively) or saline was administered repeatedly by intraperitoneal (i.p.) injections 24, 4, and 1 h before the FST, as previously described. 12 FLX (18 mg/kg/day) or saline was i.p. administered for 23 days, as earlier described. 13 The preswim and FST were conducted 24 h after the 22nd and 23rd FLX/saline injections, respectively, as previously described. 7 , 8 , 13 , 14 , 15 The 23rd FLX/saline injection was performed 30–60 min after the preswim. Briefly, mice were subjected to a 10‐min swim in a 4‐L glass beaker (16 cm diameter, 24.5 cm height) containing water (24 ± 1°C, 15 cm depth) and the experimental session was recorded. The duration of immobility was measured between 2 and 6 min in a blinded manner.
2.3. Statistical analysis
Data are presented as the mean ± standard error of the mean (SEM). Data were analyzed by two‐way analysis of variance (ANOVA), followed by the Tukey's post hoc test using the GraphPad Prism 6 software (GraphPad Software). Differences with P < 0.05 were considered statistically significant.
3. RESULTS
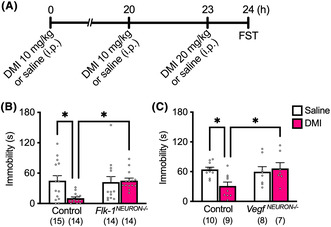
Repeated i.p. injections of DMI significantly reduced immobility in littermate controls (Flk‐1 flox/flox and Vegfa flox/flox , but Camk2a‐Cre negative), and this effect was completely blocked in Flk‐1 NEURON−/− (Figure 1A) and Vegf NEURON−/− mice (Figure 1B). In control Flk‐1 flox/flox mice, chronic FLX administration did not impact immobility in the preswim (Figure 2A) but significantly decreased immobility in the FST (Figure 2B). Conversely, Flk‐1 NEURON−/− mice chronically administered FLX exhibited a significant increase in immobility in the preswim when compared with chronic saline‐injected Flk‐1 NEURON−/− mice and littermate controls chronically administered saline or FLX (Figure 2A). In the FST, the antidepressant‐like effect of chronic FLX was completely blocked in Flk‐1 NEURON−/− mice (Figure 2B). The raw data are provided in Data S1.
FIGURE 1.
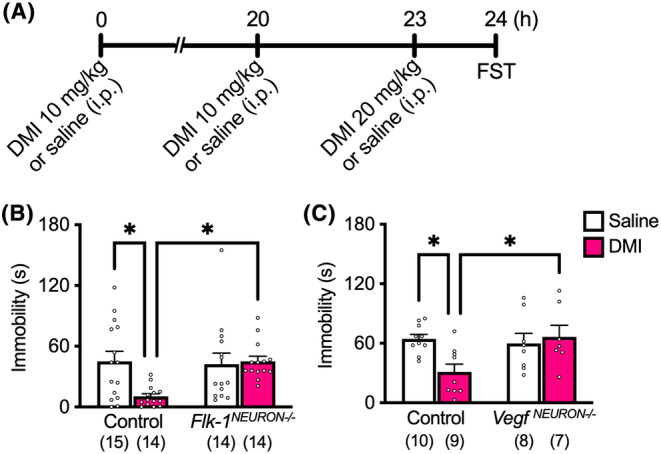
The antidepressant‐like effect of repeated DMI was blocked in Flk‐1 NEURON−/− and Vegf NEURON−/− mice. (A) Experimental timeline. The effects of forebrain excitatory neuron‐specific knockout of Flk‐1 (B; interaction, F 1, 53 = 5.59, P = 0.0218, two‐way ANOVA, n = 14 or 15) and VEGF (C; interaction, F 1, 30 = 5.62, P = 0.0244, two‐way ANOVA, n = 7–10) on the antidepressant‐like effect of repeated DMI in the FST. Data are expressed as means ± SEM. *P < 0.05 (Tukey's post hoc test).
FIGURE 2.
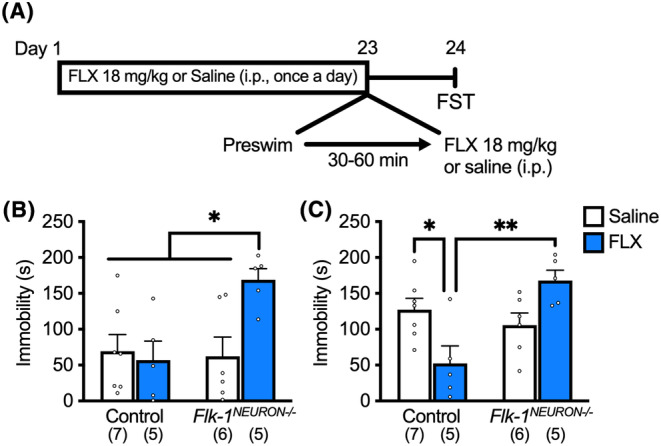
The antidepressant‐like effect of chronic FLX was blocked in Flk‐1 NEURON−/− mice. (A) Experimental timeline. The effect of forebrain excitatory neuron‐specific knockout of Flk‐1 on the antidepressant‐like effect of chronic FLX in the preswim (B; interaction, F 1, 19 = 5.97, P = 0.0245, two‐way ANOVA, n = 5–7) and FST (C; interaction, F 1, 19 = 14.6, P = 0.0012, two‐way ANOVA, n = 5–7). Data are expressed as means ± SEM. *P < 0.05, **P < 0.01 (Tukey's post hoc test).
4. DISCUSSION
Herein, our findings revealed that the antidepressant‐like effects of repeated DMI and chronic FLX were blocked by excitatory neuron‐specific deletion of VEGF or Flk‐1 in the forebrain, and extended our recently reported findings, which demonstrated the key role of neuronal VEGF–Flk‐1 signaling in the antidepressant‐like effects of ketamine, an N‐methyl‐D‐aspartate receptor antagonist. 8 Furthermore, the present results are consistent with previous findings that pharmacological blockade of VEGF–Flk‐1 signaling could attenuate the antidepressant‐like effects of conventional antidepressants, 1 , 6 as well as resolvin E1, an eicosapentaenoic acid metabolite, 16 and exercise. 17 Given that VEGF is not only expressed by neurons but also endothelial cells, astrocytes, and perivascular macrophages in the brain, 1 , 2 we cannot rule out the possibility that VEGF–Flk‐1 signaling in non‐neuronal cells could mediate the behavioral effects of these antidepressants. However, to our knowledge, the present results provide the first evidence that neuronal VEGF–Flk‐1 signaling plays an essential role in the antidepressant‐like effects of repeated DMI and chronic FLX.
Chronic treatment with FLX induced a prodepressant‐like effect in Flk‐1 NEURON−/− mice, consistent with our previous finding that a single dose of ketamine elicited prodepressant‐like effects in Flk‐1 NEURON−/− mice in the preswim. 8 Although mechanisms underlying these prodepressant‐like effects in Flk‐1 NEURON−/− mice remain unclear, these abnormal behavioral responses to FLX and ketamine may be related to neuronal Flk‐1 knockout across the forebrain, given that ketamine did not induce prodepressant‐like effects in mice with medial PFC‐specific Flk‐1 knockdown. 8 Further studies are required to identify brain regions responsible for mediating the abnormal behavioral responses to FLX and ketamine in Flk‐1 NEURON−/− mice. Moreover, it will be interesting in future studies to determine whether other antidepressants can also induce prodepressant‐like effects in Flk‐1 NEURON−/− mice, although three injections of DMI did not induce these effects (Figure 1B).
In conclusion, our findings highlight the relevance of neuronal VEGF–Flk‐1 signaling in mediating the antidepressant‐like effects of conventional antidepressants, as well as those of the rapid‐acting antidepressant ketamine. 4 , 5 , 8 , 18 Nevertheless, in‐depth investigations are needed to ascertain whether neuronal VEGF–Flk‐1 signaling mediates the behavioral effects of conventional antidepressants and ketamine in chronic stress models of depression, such as chronic unpredictable stress and chronic social defeat, and in females because females are twice as likely to develop depression than males. 19
AUTHOR CONTRIBUTIONS
SD and RSD designed the study, analyzed data, interpreted the results, and wrote the manuscript. SD and XYL conducted the experiments. All authors contributed to this study and approved to submit this manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Approval of the research protocol by an Institutional Reviewer Board: N/A.
Informed Consent: N/A.
Registry and the Registration No. of the study/trial: N/A.
Animal studies: All experiments were in accordance with the National Institutes of Health guidelines and were approved by the Yale University Animal Care and Use Committee.
Supporting information
Data S1.
ACKNOWLEDGMENTS
This study was supported by NIMH grants MH045481 and MH093897, the Uehara Memorial Foundation, and the State of Connecticut, Department of Mental Health and Addiction Services.
Deyama S, Li X‐Y, Duman RS. Neuron‐specific deletion of VEGF or its receptor Flk‐1 occludes the antidepressant‐like effects of desipramine and fluoxetine in mice. Neuropsychopharmacol Rep. 2024;44:246–249. 10.1002/npr2.12393
Dr. Ronald S. Duman passed away on February 1, 2020.
This work was carried out at Dr. Ronald S. Duman lab in the Department of Psychiatry, Yale University School of Medicine, New Haven, CT 06519, USA.
DATA AVAILABILITY STATEMENT
The data that supports the present results are available in the supplementary material of this article.
REFERENCES
- 1. Greene J, Banasr M, Lee B, Warner‐Schmidt J, Duman RS. Vascular endothelial growth factor signaling is required for the behavioral actions of antidepressant treatment: pharmacological and cellular characterization. Neuropsychopharmacology. 2009;34(11):2459–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jais A, Solas M, Backes H, Chaurasia B, Kleinridders A, Theurich S, et al. Myeloid‐cell‐derived VEGF maintains brain glucose uptake and limits cognitive impairment in obesity. Cell. 2016;165(4):882–895. [DOI] [PubMed] [Google Scholar]
- 3. Elfving B, Plougmann PH, Wegener G. Differential brain, but not serum VEGF levels in a genetic rat model of depression. Neurosci Lett. 2010;474(1):13–16. [DOI] [PubMed] [Google Scholar]
- 4. Deyama S, Duman RS. Neurotrophic mechanisms underlying the rapid and sustained antidepressant actions of ketamine. Pharmacol Biochem Behav. 2020;188:172837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deyama S, Kaneda K. Role of neurotrophic and growth factors in the rapid and sustained antidepressant actions of ketamine. Neuropharmacology. 2023;224:109335. [DOI] [PubMed] [Google Scholar]
- 6. Warner‐Schmidt JL, Duman RS. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci U S A. 2007;104(11):4647–4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deyama S, Bang E, Kato T, Li XY, Duman RS. Neurotrophic and antidepressant actions of brain‐derived neurotrophic factor require vascular endothelial growth factor. Biol Psychiatry. 2019;86(2):143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deyama S, Bang E, Wohleb ES, Li XY, Kato T, Gerhard DM, et al. Role of neuronal VEGF signaling in the prefrontal cortex in the rapid antidepressant effects of ketamine. Am J Psychiatry. 2019;176(5):388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deyama S, Li XY, Duman RS. Neuron‐specific deletion of VEGF or its receptor Flk‐1 impairs recognition memory. Eur Neuropsychopharmacol. 2020;31:145–151. [DOI] [PubMed] [Google Scholar]
- 10. Albuquerque RJ, Hayashi T, Cho WG, Kleinman ME, Dridi S, Takeda A, et al. Alternatively spliced vascular endothelial growth factor receptor‐2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat Med. 2009;15(9):1023–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, et al. VEGF is required for growth and survival in neonatal mice. Development. 1999;126(6):1149–1159. [DOI] [PubMed] [Google Scholar]
- 12. Adachi M, Barrot M, Autry AE, Theobald D, Monteggia LM. Selective loss of brain‐derived neurotrophic factor in the dentate gyrus attenuates antidepressant efficacy. Biol Psychiatry. 2008;63(7):642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29(7):1321–1330. [DOI] [PubMed] [Google Scholar]
- 14. Deyama S, Kaneda K. The duration of the antidepressant‐like effects of a single infusion of brain‐derived neurotrophic factor into the medial prefrontal cortex in mice. Behav Brain Res. 2020;394:112844. [DOI] [PubMed] [Google Scholar]
- 15. Deyama S, Kondo M, Shimada S, Kaneda K. IGF‐1 release in the medial prefrontal cortex mediates the rapid and sustained antidepressant‐like actions of ketamine. Transl Psychiatry. 2022;12(1):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deyama S, Aoki S, Sugie R, Fukuda H, Shuto S, Minami M, et al. Intranasal administration of resolvin E1 produces antidepressant‐like effects via BDNF/VEGF‐mTORC1 signaling in the medial prefrontal cortex. Neurotherapeutics. 2023;20(2):484–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kiuchi T, Lee H, Mikami T. Regular exercise cures depression‐like behavior via VEGF‐Flk‐1 signaling in chronically stressed mice. Neuroscience. 2012;207:208–217. [DOI] [PubMed] [Google Scholar]
- 18. Duman RS, Deyama S, Fogaca MV. Role of BDNF in the pathophysiology and treatment of depression: activity‐dependent effects distinguish rapid‐acting antidepressants. Eur J Neurosci. 2021;53(1):126–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Health Organization . Depressive disorder (depression). 2023. https://www.who.int/news‐room/fact‐sheets/detail/depression/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Data Availability Statement
The data that supports the present results are available in the supplementary material of this article.


