Abstract
Cells initiate proliferation in response to growth factor stimulation, but the biochemical mechanisms linking signals received at the cell surface receptors to the cell cycle regulatory molecules are not yet clear. In this study, we show that the signaling molecule Raf-1 can physically interact with Rb and p130 proteins in vitro and in vivo and that this interaction can be detected in mammalian cells without overexpressing any component. The binding of Raf-1 to Rb occurs subsequent to mitogen stimulation, and this interaction can be detected only in proliferating cells. Raf-1 can inactivate Rb function and can reverse Rb-mediated repression of E2F1 transcription and cell proliferation efficiently. The region of Raf-1 involved in Rb binding spanned residues 1 to 28 at the N terminus, and functional inactivation of Rb required a direct interaction. Serum stimulation of quiescent human fibroblast HSF8 cells led to a partial translocation of Raf-1 into the nucleus, where it colocalized with Rb. Further, Raf-1 was able to phosphorylate Rb in vitro quite efficiently. We believe that the physical interaction of Raf-1 with Rb is a vital step in the growth factor-mediated induction of cell proliferation and that Raf-1 acts as a direct link between cell surface signaling cascades and the cell cycle machinery.
Various biological stimuli, such as growth factors, cytokines, and hormones, can modulate the cell cycle machinery leading to growth arrest, differentiation, or proliferation (63). Cell cycle progression is stringently restricted by a diverse array of regulatory molecules, which maintain cells in a quiescent state until an appropriate proliferative signal is received (61, 62). The retinoblastoma tumor suppressor protein (Rb) is one such molecule that constrains the progression of the cell cycle past the G1/S boundary, and successful passage of the cells from G1 to S phase requires the inactivation of Rb function (4, 31, 65, 74, 78). In normal cells, this is brought about by phosphorylation by cyclin D-cdk4/6 kinases, an event that occurs in mid- to late G1 (20, 38, 39, 58). Conditions that prevent the phosphorylation of Rb, including microinjection of cyclin D antibody or overexpression of the cyclin-dependent kinase (cdk) inhibitor p16INK4, can lead to a G1 arrest (48, 68, 70, 73). Such studies have established that Rb inactivation is vital for cell cycle progression and that Rb is inactivated at the appropriate point in the cell cycle upon mitogenic stimulation (4, 78).
It has been demonstrated that Rb and the related p107 and p130 proteins exert their growth inhibitory function to a great extent by targeting the E2F family of transcription factors (3, 5, 10, 15, 37, 75). The Rb-family tumor suppressors can bind to the transcriptional activation domain of E2F and repress its activity; this event is negated by the phosphorylation of Rb by the cyclin D-cdk4 complex (20). The expression of many vital genes involved in DNA synthesis is regulated by E2F (14, 41, 64), and it may be imagined that Rb-mediated repression of E2F ablates the expression of such genes, resulting in growth arrest. E2F by itself has been shown to have mitogenic potential, and microinjection or overexpression of E2F1 can induce S-phase entry (19, 35). Because of these results, it may be envisaged that E2F activity may be an appropriate downstream target of mitogenic signaling cascades, either directly or indirectly through cyclin-cdk’s and Rb-family proteins.
Even though the signal transduction pathways that are initiated upon growth factor stimulation have been well characterized (42, 43, 46), it is not yet clear how such pathways contact the cell cycle machinery. Since Rb has been well established to coordinate the cell cycle with transcription (30, 32), it is a logical candidate for being a target of signaling molecules. This hypothesis is based on the following reasons: (i) the phosphorylation and hence the activity of Rb changes in response to proliferative signals (7, 18); (ii) molecules involved in certain signaling pathways, such as c-Abl and protein phosphatase 1A, have been found to target Rb protein directly (78a, 78b); and (iii) since Rb is a conduit between cyclin-dependent kinases and the transcription machinery (29, 30, 32), it is ideally positioned to regulate gene expression in response to proliferative signals.
It has been demonstrated that mitogenic signaling from multiple classes of receptors converge on the Rb-controlled G1 checkpoint. In one study, it was found that mitogenic stimulation through membrane tyrosine kinase receptors, estrogen receptors, or G protein-coupled thryotropin receptors each required Rb inactivation and was sensitive to the cdk inhibitor p16 (47). Further, Ras-mediated stimulation of cell cycle progression required an ablation of Rb-mediated growth constraint (66). Though both of these studies demonstrated a requirement for cyclin D-cdk activity, it is not clear how a receptor activation leads to Rb inactivation. The studies we describe here add a new dimension to this scenario, in that the signaling molecule Raf-1 was found to directly interact with the Rb protein, contributing to its inactivation. Our results suggest that Raf-1 can overcome Rb-mediated regulation of cell proliferation and is probably one mechanism by which mitogenic signals received at the cell surface receptors are conveyed to the cell cycle machinery.
MATERIALS AND METHODS
In vitro binding assays.
Glutathione S-transferase (GST) fusion proteins of Rb and p107 containing pocket and C-terminal domains or the pocket domain of p130 were used in this study (12, 13, 52, 53). The mutant GST-Rb lacked exon 21 (37); the p130 mutant had a C894→F substitution. Full-length human Raf-1 was cloned into the pPCR-II vector and linearized with XbaI for in vitro transcription with SP6 RNA polymerase. Different Raf-1 mutants were generated by PCR and transcribed similarly. pSRαHAJNK1 and pSRαp38 were linearized with BglII and transcribed with SP6. pCDNA3E2F4 was digested with XhoI and pIBI31E7 with HindIII prior to transcription with T7 polymerase. The resulting mRNAs were translated in rabbit reticulocyte lysates (Promega) in the presence of [35S]methionine (New England Nuclear). Then, 8 μl of synthesized polypeptide was incubated with glutathione beads carrying equal amounts of GST fusion proteins in 200 μl of protein binding buffer (20 mM Tris, pH 7.5; 50 mM KCl; 0.5 mM EDTA; 1 mM dithiothreitol, 0.5% Nonidet P-40; 3 mg of bovine serum albumin [BSA] per ml) at 4°C for 2 h. The beads were washed six times with 1 ml of protein binding buffer and eluted with 10 mM glutathione. Eluates were separated in a sodium dodecyl sulfate (SDS)–10% polyacrylamide gel and visualized by autoradiography. The protein amounts in control input lanes were approximately one-fifth of the total used in the binding assay.
Yeast two-hybrid interaction assay.
Yeast strain L40 (His− Lys− Leu− Trp− Ura−) was used to assess the interaction between Raf-1 and Rb-family proteins by using previously described protocols (77). Human p107 cDNA containing the pocket domain and the C-terminal domain were cloned into pBTM116 vector as a LexA DNA binding domain fusion; pBTM116-Rb was similar and was a gift from Stephen Goff (36). Full-length human wild-type (WT) p130 or a point mutant with a C894→F substitution was cloned into pBTM116 as a BamHI-PstI fragment. Full-length human Raf-1 was cloned into pVP16 vector as a BamHI-EcoRI fragment. pBTM116 vectors carry selection markers for Ura and Trp, and pVP16 vector carries a selection marker for Leu-2. Transformation efficiency was assessed by selecting on Ura, Trp, and Leu, and protein-protein interaction was assessed by selecting on Trp−, His−, Ura−, Leu−, and Lys− plates. Positives for interaction were reconfirmed by transferring the colonies to a filter and performing an in situ β-galactosidase assay.
Immunoprecipitation and Western blot analysis.
Anti-Raf-1 mouse monoclonal antibody raised against residues 162 to 378 was obtained from Transduction Laboratories; rabbit polyclonal antibody specific to residues 253 to 269 was obtained from Upstate Biotechnologies, Inc., and an antibody specific to residues 637 to 648 came from Santa Cruz Biotechnology, Inc. Antibodies to Rb were obtained from Oncogene Science (mouse monoclonal) or Santa Cruz Biotechnology (rabbit polyclonal). Antibodies to JNK1, p38, ERK2, p107, and p130 were obtained from Santa Cruz Biotechnology.
Whole-cell extracts were prepared by hypotonic shock followed by salt extraction, as described previously (10). Then, 50 to 200 μg of whole-cell extracts were treated with 5 μl of the appropriate primary antibody in a volume of 100 μl at 4°C for 1 h. Next, 3 mg of protein A-Sepharose or protein G-Sepharose in a 100-μl volume was added and incubated for an additional 1 h. The binding was performed in a buffer containing 20 mM HEPES (pH 7.9), 40 mM KCl, 1 mM MgCl2, 0.1 mM EGTA, 0.1 mM EDTA, 0.1 mM dithiothreitol, 0.1 mM NaF, 0.1 mM Na3VO4, 0.5% Nonidet P-40, and 3 mg of BSA per ml. The beads were washed six times with 600 μl of the same buffer, boiled in 20 μl of SDS sample buffer, and separated on 8 or 10% polyacrylamide gels. After semidry transfer to a supported nitrocellulose membrane, the blots were probed with the appropriate antibody. The proteins were detected using an enhanced chemiluminescence system from Amersham.
EMSA.
Electrophoretic mobility shift assay (EMSA) following immunoprecipitation was performed as previously described (10). U937 whole-cell extracts (100 μg) were immunoprecipitated with 5 μl of c-myc antibody (Oncogene Science) or Raf-1 monoclonal antibody (Transduction Laboratories). The bound proteins were recovered on protein G-Sepharose beads and released by treatment with 0.8% deoxycholate, and the presence of E2F was determined by binding to a probe derived from adenovirus E2 promoter (2). Competition experiments were done by including 200 ng of oligonucleotides carrying a wild-type (TTTCGCGC) or a mutated (TTTATCGC) E2F site in the binding reaction.
In vitro kinase assays.
Next, 50-μg extracts from Sf9 cells producing FLAG-tagged ΔRaf-1YY (a kind gift of Martin McMahon and Elizabeth Bosch, DNAX) were immunoprecipitated with an anti-FLAG mouse monoclonal antibody, and the associated proteins were recovered on protein G-treated beads. The beads were washed four times with a buffer containing 20 mM Tris (pH 7.5), 1% Triton X-100, 0.1% SDS, 0.5% deoxycholate, and 0.5 M LiCl2 and twice with the kinase assay buffer (62.5 mM Tris, pH 7.5; 12.5 mM MnCl12; 6.25 mM MgCl12). The kinase reaction was performed in the same buffer containing 5 μCi of [γ-32P]ATP and 25 μM ATP, as well as 2 μg of GST-MEK1 or 5 μg of bacterially produced full-length human Rb (QED Bioscience, San Diego, Calif.) as substrates. Kinase assays were performed in a similar fashion on U937 whole-cell extracts immunoprecipitated with an anti-Raf-1 mouse monoclonal antibody.
Transient and stable transfections.
Saos-2 cells derived from human osteosarcoma cells were transfected by calcium phosphate precipitation by using standard protocols. Generally, 2 μg of the plasmids were used unless noted otherwise, and a pSVβgal vector was included in all transfections. Construct pCDNA3-Raf-1 contained a full-length human cRaf-1 cDNA cloned as a BamHI-XhoI fragment. A cDNA fragment coding for amino acids 29 to 648 of human Raf-1 was generated by PCR using the upstream primer 5′-TCAGTAGGATCCATGTCTCCTACAATAGTTC-3′ and cloned similarly to obtain pCDNA3-Raf-1Δ28. Constructs pDCE2F1, pE2CAT, and pSVRb have been described before. Transient transfections were performed in 100-mm dishes for 72 h; assays for chloramphenicol acetyltransferase (CAT) and β-galactosidase were performed according to standard protocols.
Stable transfections were performed on 35-mm dishes, each having approximately 10,000 cells. Selection in 1 μg of puromycin per ml, 40 μg of neomycin per ml, or a combination of both began 48 h after transfection. After 14 days of selection, cells were fixed in 3.7% formaldehyde and stained with 0.5% crystal violet, and colonies with 20 or more cells were counted. In all the transfections, Rb was cotransfected with a pBABE-Puro vector; Raf-1 constructs carried a neomycin resistance marker.
Immunofluorescence analysis.
The human primary fibroblast HSF-8 cells were plated on coverslips attached to 35-mm dishes. The cells were fixed in 3.7% paraformaldehyde at room temperature for 10 min. Nonspecific binding was blocked by incubating the cells for 15 min in 5% calf serum in M2A buffer (20 mM HEPES, pH 7.4; 50 mM NH4Cl; 150 mM NaCl; 1 mM CaCl2; 5 mM KCl; 1 mM MgCl2). After incubation in antibody buffer (M2A buffer containing 100 μg of saponin per ml and 2 mg of BSA per ml) for 15 min at 37°C, 5 to 10 μg of Raf-1 or Rb antibody in the same buffer was added and incubated for 1 h at 37°C. The cells were then washed three times with the antibody buffer and treated with rhodamine-conjugated goat anti-mouse immunoglobulin G (IgG), Fc fragment, or fluorescein-conjugated donkey anti-rabbit IgG (Pierce) for 1 h under the same conditions. The cells were washed three times with the antibody buffer and stored in 0.3% paraformaldehyde in M2 buffer at 4°C.
The cells were examined under a Leica DMIRB inverted microscope (Leica, Inc., Malvern, Pa.) using a ×63 (NA 1.32) Planapo objective. Rhodamine and fluorescein staining were visualized separately by the appropriate standard fluorescence filter sets. Images were recorded by using a Videoscope VS2000N camera and a Videoscope KS1380 image intensifier (Videoscope International, Washington, D.C.). The images were digitized and processed using the Metamorph image analysis program (Universal Imaging Corp., Westchester, Pa.).
RESULTS
Raf-1 physically interacts with Rb and p130 in vivo and in vitro.
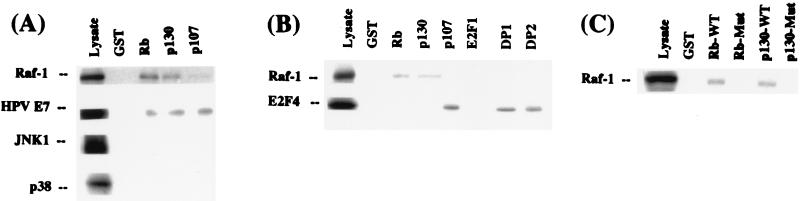
The ability of Raf-1 to interact with Rb, p130, and p107 in vitro was assessed by GST pulldown assays. 35S-labeled Raf-1 protein synthesized in rabbit reticulocyte lysates was tested for binding to glutathione-Sepharose beads primed with GST-Rb, GST-p107, and GST-p130. As can be seen from Fig. 1A, Raf-1 can be detected in the glutathione eluates of GST-Rb and GST-p130 beads but not in the control beads; the binding to GST-p107 was negligible or absent. In contrast, HPV16 E7 protein could bind equally well to all three beads. It was also observed that two other signaling kinases belonging to the MAP kinase family, JNK1 and p38, did not bind to any of the beads. Raf-1 did not bind to GST-E2F1 or its dimerization partners DP1 or DP2 (Fig. 1B), whereas 35S-labeled E2F4 protein could bind efficiently to GST-p107, DP1, and DP2 as expected (4). It may be concluded that Raf-1 can bind to Rb and p130 efficiently but not to p107, E2F1, or DP proteins. Raf-1 did not bind to Rb or p130, which harbored mutations in the pocket domain (Fig. 1C), indicating that a functional pocket domain is required for the binding.
FIG. 1.
Binding of 35S-labeled Raf-1 to GST fusions of Rb, p130, and p107 in vitro. (A) 35S-labeled Raf-1 (top) was incubated with the indicated beads, and binding was assessed by electrophoresis and autoradiography of the glutathione eluate. Lysate shows one-fifth of the loading, and GST indicates the control beads. HPV E7 protein could bind to Rb, p130, and p107 beads, but JNK1 and p38 proteins did not bind to any of the beads in parallel experiments. (B) Binding of Raf-1 to Rb and p130 but not to p107, E2F1, or DP proteins in a similar assay. E2F4 protein could effectively bind to p107, DP1, and DP2. (C) Binding of Raf-1 to wild-type and mutant GST-Rb and GST-p130 beads. The mutant Rb had a deletion of exon 21, and the p130 had a single C894→F mutation in the pocket domain.
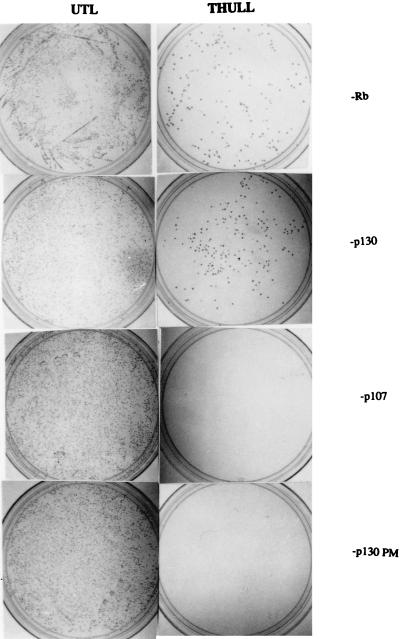
A yeast two-hybrid system was utilized to verify whether Raf-1 can interact with Rb family proteins in vivo (36, 77). Rb, p107, and p130 proteins were fused to the DNA binding domain of LexA, and their ability to interact with a Raf-1–VP16-AD fusion was determined. As shown in Fig. 2, Raf-1 could interact with wild-type Rb and p130 quite efficiently, whereas it was unable to bind to a C894→F pocket domain mutant of p130 (12, 13, 52, 53). There was no detectable interaction with p107 under the same conditions. The in vitro binding experiment, along with the yeast two-hybrid result, strongly suggests that the Raf-1 protein can directly interact with Rb and the Rb-related p130 protein and that this requires an intact pocket domain.
FIG. 2.
Interaction of Raf-1 with Rb and p130 in a yeast two-hybrid system. Yeast strain L40 was transformed with pVP16Raf-1 and also pBTM116-Rb, p130, or p107 as indicated on the right. The left panels show colonies obtained in UTL selection medium and serve as a control for transformation. The right panels depict colonies observed upon THULL selection after transformation with the same vectors, and the appearance of colonies suggests a physical interaction between Raf-1 and the indicated protein.
Raf-1 is associated with Rb and p130 in mammalian cells.
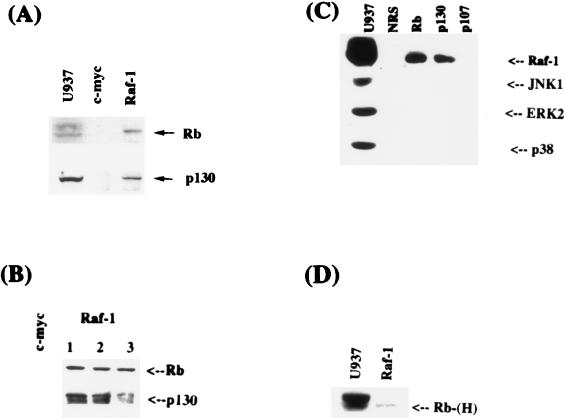
Since Raf-1 could physically interact with Rb and p130 in vitro and in a yeast system, we sought to determine whether such an interaction occurs in mammalian cells in vivo under normal circumstances. The approach was to perform coimmunoprecipitation experiments under native conditions by using whole-cell extracts from U937 cells. In the first set of experiments, extracts from U937 promonocytic cells were immunoprecipitated with a polyclonal antibody raised against residues 253 to 269 of Raf-1. The presence of Rb or p130 in the immunoprecipitates was determined by Western blotting. As can be seen from Fig. 3A, Rb and p130 could be detected in Raf-1 immunoprecipitates but not in control immunoprecipitates, where a c-myc antibody was used. To establish that the coimmunoprecipitation of Rb and p130 with Raf-1 is not due to a cross-reactivity of the antibody, three different Raf-1 antibodies (one monoclonal and two polyclonal) raised against different regions of Raf-1 were used for the immunoprecipitations (Fig. 3B). Western blot analysis as in the previous experiment showed that Rb and p130 can indeed be detected only in Raf-1 immunoprecipitates. This suggests that Raf-1 physically interacts with Rb and p130 in vivo. To further confirm this finding, the experiment was performed in the opposite fashion: i.e., immunoprecipitations were performed with anti-Rb, -p130, and -p107 antibodies, and the presence of Raf-1 was checked by Western blotting by using a Raf-1 monoclonal antibody. As can be seen in Fig. 3C, Raf-1 protein could be detected only in Rb and p130 immunoprecipitates. This result suggests that Raf-1 is associated with Rb and p130 in cells and that this interaction can be detected without overexpressing any component. Other signaling kinases, such as JNK1, p38, or ERK2, could not be detected in association with the Rb-family proteins in similar coimmunoprecipitation experiments. An analysis of the phosphorylation status of Rb associated with Raf-1 suggests that it is predominantly hypophosphorylated (Fig. 3D). Quantitation of the interaction suggests that approximately 5% of Rb is associated with Raf-1 in an asynchronously dividing population of U937 cells, whereas approximately 24% of Raf-1 could be detected in association with Rb.
FIG. 3.
(A) Interaction of Raf-1 with Rb and p130 in mammalian cells. U937 whole-cell extracts were immunoprecipitated with a control (c-myc) or a Raf-1 antibody. The immunoprecipitates were examined for the presence of Rb (top) or p130 (bottom) by Western blotting. The U937 lane shows an equivalent amount of U937 extracts used for immunoprecipitation. (B) Multiple anti-Raf-1 antibodies can coimmunoprecipitate Rb. U937 cell extracts were immunoprecipitated with a c-myc antibody as a control or with three different anti-Raf-1 antibodies. Lanes 1, 2, and 3 show immunoprecipitations performed with an anti-Raf-1 mouse monoclonal antibody to residues 162 to 378 and rabbit polyclonal antibodies specific to residues 253 to 269 or 637 to 648, respectively. Western blotting was performed with an anti-Rb mouse antibody (top) or an anti-p130 antibody (bottom). (C) Coimmunoprecipitation of Raf-1 with Rb and p130. U937 whole-cell extracts were immunoprecipitated with normal rabbit serum and with antibodies to Rb, p130, and p107. A Western blot analysis revealed the presence of Raf-1 in Rb and p130 immunoprecipitates but not in p107 immunoprecipitates. There were no detectable amounts of JNK1, ERK2, or p38 in the immunoprecipitates. (D) Raf-1 preferentially associates with the hypophosphorylated form of Rb. U937 whole-cell extracts were immunoprecipitated with an anti-Raf-1 antibody and subjected to prolonged electrophoresis, and the status of associated Rb was examined by Western blotting with an anti-Rb mouse monoclonal antibody.
The association of Raf-1 with Rb is serum inducible.
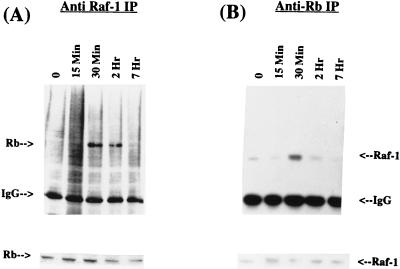
Since Raf-1 is activated in response to proliferative-stimulus-like growth factors, experiments were designed to determine whether Raf-1 binds to Rb in response to mitogen stimulation. The strategy was to examine the kinetics of the Rb–Raf-1 interaction by immunoprecipitation and Western blots in quiescent or serum-stimulated HSF8 (human fibroblast) cell extracts. First, an immunoprecipitation was performed with an anti-Raf-1 antibody, and the precipitates were blotted to detect the presence of Rb. As shown in Fig. 4A (upper panel), Rb can be detected in the Raf-1 immunoprecipitates of extracts prepared 30 min after serum stimulation; the association persists for up to 2 h. There is no significant amount of interaction in serum-starved cells (indicated by a “0” in Fig. 3) or in cells stimulated for 15 min; the interaction was maximal at the 30-min time point and persisted for 2 h before dissipating. There was no significant change in the levels of the Rb protein during this period (Fig. 4A, lower panel). This result was verified by reversing the antibodies, i.e., the immunoprecipitation was done with an anti-Rb antibody and the Western blot was done with an anti-Raf-1 monoclonal antibody (Fig. 4B, upper panel). It was found that here, too, the maximal interaction occurred at 30 min after serum stimulation. There was no marked change in the levels of the Raf-1 protein during this time period (lower panel).
FIG. 4.
(A) Induction of Rb–Raf-1 interaction by serum. Extracts from HSF8 human fibroblast cells stimulated with serum for the indicated periods of time were immunoprecipitated with a Raf-1 monoclonal antibody. The presence of Rb was detected by Western blot analysis. “0” indicates serum-starved cells. The lower panel shows a Western blot analysis of Rb protein in the same extracts. (B) The same extracts from serum-stimulated HSF8 cells were immunoprecipitated with an anti-Rb monoclonal antibody, and the presence of Raf-1 was detected by Western blotting. The lower panel shows the Raf-1 protein in the same extracts as detected by Western blotting.
These experiments demonstrate that Raf-1 interacts with Rb in response to mitogen stimulation and that this interaction is very precisely regulated. It is conceivable that the interaction of Raf-1 with Rb contributes to the functional inactivation of Rb, thus allowing the cells to progress through the cell cycle in response to such stimuli. The kinetics of the interaction confirms that the association of Raf-1 with Rb occurs when Rb is in the functional, hypophosphorylated state, prior to the cyclin D-cdk4-mediated phosphorylation; the binding of Raf-1 could be contributing to its phosphorylation and subsequent inactivation.
Raf-1 is present in E2F-containing complexes.
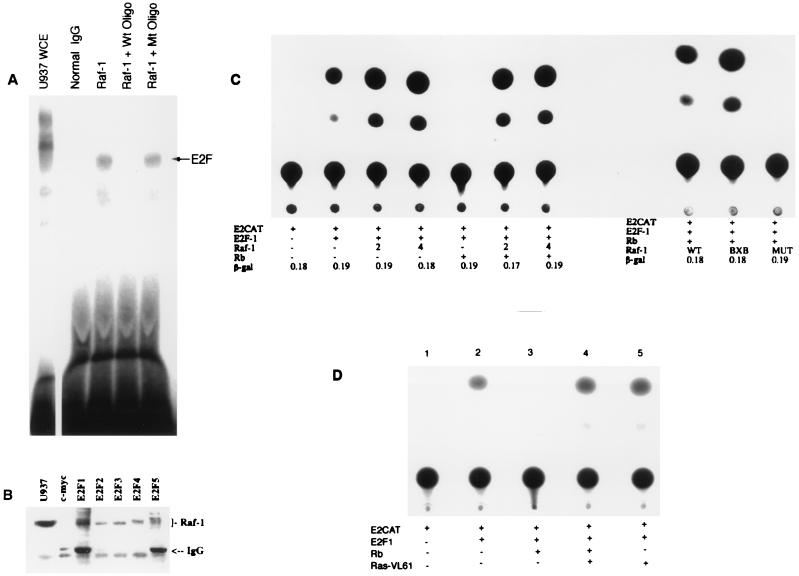
It is well established that Rb physically interacts with the transcription factor E2F and that viral oncoproteins such as adenovirus E1A, HPV-E7, and simian virus 40 (SV40) large T antigen can disrupt this interaction (3, 10, 11). We therefore examined whether Raf-1 can also execute similar effects in vitro, but Raf-1 produced in rabbit reticulocyte lysates could not dissociate E2F from Rb or other pocket proteins (data not shown). Experiments were designed to see whether Raf-1 is present in association with E2F as a part of a multiprotein complex. This was done by performing an immunoprecipitation on U937 extracts with a control antibody (c-myc) or an Raf-1 antibody. The bound proteins were recovered on protein G-Sepharose beads, dissociated by deoxycholate, and the presence of E2F was checked by a mobility shift assay as described in earlier studies (10). As shown in Fig. 5A, E2F could be detected in the Raf-1 immunoprecipitates, but there was no activity in the control (c-myc) immunoprecipitates. This result suggests that Raf-1 protein and E2F can coexist in multiprotein complexes in the cell.
FIG. 5.
Association of Raf-1 with E2F. (A) E2F can be detected in Raf-1 immunoprecipitates. Whole-cell extracts (WCE) from dividing U937 cells were immunoprecipitated with a Raf-1 antibody or a control (c-myc) antibody and collected on protein G-treated beads. The proteins associated with the beads were released by deoxycholate after an extensive washing, and the presence of E2F was assessed by EMSA. (B) All five E2Fs can be detected in association with Raf-1. U937 whole-cell extracts were immunoprecipitated with antibodies to c-myc or to E2Fs 1 through 5. The presence of Raf-1 in the immune complexes was assessed by Western blotting. (C) Raf-1 reverses Rb-mediated repression of E2F activity. Saos-2 cells were transiently transfected with an E2-CAT reporter plasmid activated by pDC-E2F-1. Then, 2 μg of E2F-1, pCMVRb, or pCDNA3-Raf-1 (wild type, constitutively active BXB, as well as a kinase-deficient mutant) were cotransfected as indicated. Next, 4 μg of wild-type Raf-1 was used in the two lanes so marked. A typical result obtained from four separate experiments is shown; the activity of a pSVβgal vector included in the transfections was comparable in all lanes. (D) A dominant-acting form of Ras can reverse Rb-mediated repression of E2F activity. A transient-transfection experiment was conducted as in panel C with 2 μg of Ras VL61 in the indicated lanes.
Attempts were then made to see which E2F protein associates with Raf-1. U937 extracts were therefore immunoprecipitated with antibodies to E2Fs 1 to 5; a c-myc antibody was used as a control. A Western blot analysis of the immunoprecipitates with a Raf-1 monoclonal antibody showed that Raf-1 is present in association with all five of the E2Fs (Fig. 5B). We believe that the association of Raf-1 with the different E2Fs is mediated by Rb and p130 and is not a direct interaction, since Raf-1 could not associate with E2F-1 in the in vitro GST pulldown assay (Fig. 1B).
Raf-1 can reverse Rb-mediated regulation of E2F activity.
Attempts were made to see whether Raf-1 can affect E2F-mediated transcription. Saos-2 cells, which lack a functional Rb, were transfected with an E2-CAT vector and an E2F-1 expression vector (Fig. 5C, lanes 1 and 2). Cotransfection of wild-type Raf-1 appeared to have little effect on E2F-mediated transcription (Fig. 5C, lanes 3 and 4). More significant effects were obtained when Rb was used to suppress the E2F activity (lane 5), as has been demonstrated before (28). Cotransfection of a wild-type Raf-1 along with Rb could completely reverse Rb-mediated transcriptional repression (lanes 6 and 7). The contribution of the Raf-1 kinase activity to this reversal was assessed with a kinase-deficient mutant of Raf-1 or a constitutively active form, Raf-1 BXB, which lacked the Ras binding domain (6). Raf-1 BXB was comparable to the wild type in reversing Rb function (lane 8); in contrast, the kinase-deficient mutant was totally unable to reverse Rb-mediated repression of E2F (lane 9). This result suggests that the kinase activity of Raf-1 is essential for abrogating Rb-mediated repression of E2F, and the mechanism of Rb inactivation is different from that used by viral oncoproteins. Despite the apparent difference in the mechanisms involved, it is significant that a transforming cellular protein can inactivate Rb function in a way similar to that of viral oncoproteins, which physically interact with Rb, further supporting the notion that the inactivation of Rb and the induction of E2F activity are an essential part of the oncogenic process.
Direct binding of Raf-1 is required to reverse Rb-mediated suppression of E2F activity.
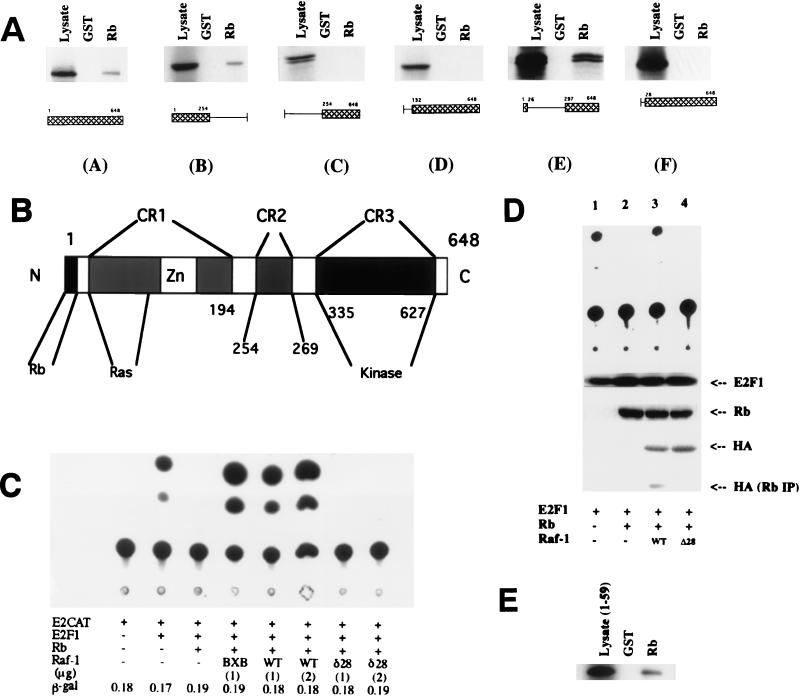
Since it was found that the kinase activity of Raf-1 is involved in inactivating Rb, attempts were made to check whether Raf-1 has to physically interact with Rb to reverse its function. The strategy used to address this issue was to identify the Rb-binding domain of Raf-1 and to check whether a Raf-1 molecule with a mutation in this domain is able to inactivate Rb. First, the region of Raf-1 that is involved in binding to Rb was identified by synthesizing deletion mutants of Raf-1 in vitro in rabbit reticulocyte lysates and by checking their ability to bind GST-Rb beads. As can be seen from Fig. 6A, the 5′ half of Raf-1 constituting the CR1 domain (see Fig. 6B) was sufficient to bind to Rb (Fig. 6A, panel B). Conversely, deletion of the entire CR1 abolished its binding; further, deletion of 131 amino-terminal residues, which eliminated the entire Ras-binding domain, impaired the ability of Raf-1 to bind to Rb (Fig. 6A, panels C and D). We next examined the ability of Raf-1 BXB to bind to Rb; Raf-1 BXB has amino-terminal residues 1 to 26 fused to the carboxy-terminal kinase domain spanning residues 297 to 648 (Fig. 6A, panel E). Raf-1 BXB was quite efficient in binding to Rb, suggesting that the amino-terminal 26 amino acids are essential for binding to Rb. This was confirmed by deleting the amino-terminal 28 amino acids from wild-type Raf-1; this Raf-1 mutant, Raf-1Δ28, was totally impaired in its ability to bind to Rb (Fig. 6A, panel F). This region is outside the CR1 motif and Ras binding domain and has no known function. There was no discernible LXCXE motif within this region, and it had no significant homology to any protein in the database.
FIG. 6.
Direct binding of Raf-1 is required for reversal of Rb function. (A) Mapping of the region of Raf-1 required for binding to Rb. Deletion mutants of Raf-1 were prepared by in vitro translation, and their binding to GST-Rb was evaluated as in Fig. 1. Mutant E is same as the constitutively active Raf-1 BXB. (B) Schematic of the Raf-1 structure. CR-1, CR-2, and CR-3 are domains conserved between different Raf-1 family members. The Rb-binding domain, the Ras-binding domain, and the kinase domain are also indicated. (C) Transient-transfection assay to examine the effect of Raf-1Δ28 on Rb-mediated repression of E2F. Saos-2 cells were transfected as described in the legend to Fig. 4C with 2 μg of Rb and the indicated amounts of wild type (WT) Raf-1, Raf-1 BXB, and Raf-1Δ28 (δ28). The Δ28 mutant had no effect on Rb-mediated repression of E2F. A typical result obtained from four separate experiments is shown here; the activity of a cotransfected pSVβgal vector was comparable in all lanes. (D) Correlation between association of Raf-1 and the functional inactivation of Rb. Saos-2 cells were transfected with E2-CAT, E2F1, and the vectors as indicated. A CAT assay revealed that HA-tagged wild-type Raf-1 (WT) can reverse Rb-mediated repression of E2F1 (lane 3) but that HA-Raf-1Δ28 cannot (lane 4). A Western blot analysis of the whole-cell extracts shows a comparable amount of Rb in lanes 2 to 4; similarly, there are comparable amounts of wild-type Raf-1 and Raf-1Δ28 expressed in the transfected cells as seen by an anti-HA Western blot. Western blot analysis of the Rb immunoprecipitates of the same extracts with an anti-HA antibody reveals the presence of HA–wild-type Raf-1 but not HA–Raf-1Δ28. (E) The amino-terminal sequences of Raf-1 are sufficient to bind to Rb. A peptide corresponding to residues 1 to 59 of Raf-1 was synthesized in vitro and its binding to GST-Rb was assayed as in Fig. 1.
The region of Raf-1 involved in binding to Rb is sufficiently removed from the kinase domain (Fig. 6B), and it has been shown that deletions of the entire CR1 and CR2 do not affect the kinase activity of Raf-1 (27, 49). Transient-transfection experiments were designed to examine whether the Raf-1Δ28 mutant can reverse Rb-mediated repression of E2F transcription. As can be seen from Fig. 6C, the constitutively active Raf-1 BXB and wild-type Raf-1 can reverse Rb-mediated repression of E2F activity, but the Raf-1Δ28 mutant was totally impaired in this aspect. It should be pointed out that Raf-1 BXB has the N-terminal 26 amino acids that are lacking in Raf-1Δ28 but that the entire Ras binding domain, as well as CR2, is missing. Since the N-terminal region has no other known function, it appears that a direct binding is required for Raf-1 to reverse Rb-mediated repression of E2F.
Attempts were made to test directly whether there is a correlation between the physical interaction of Raf-1 with Rb and its inactivation. Saos-2 cells were cotransfected with E2-CAT and E2F1 (Fig. 6D, top panel, lane 1); the E2F-mediated transcription was inhibited by Rb (lane 2), which could be reversed by cotransfecting a hemagglutinin (HA)-tagged full-length Raf-1 (lane 3). Cotransfection of an HA-tagged Raf-1Δ28 was unable to reverse the inhibition, as was seen in Fig. 6C (lane 4). A Western blot analysis revealed that there was a comparable amount of E2F1 in all of the transfected lanes; similarly, comparable amounts of Rb could be detected in lanes 2, 3, and 4. Western blotting with an anti-HA antibody showed comparable amounts of full-length Raf-1 and Raf-1Δ28 in lanes 3 and 4, respectively. An immunoprecipitation of all of the extracts with an anti-Rb antibody, followed by Western blot analysis with an anti-HA antibody, revealed the presence of Raf-1 only in lane 3, where full-length Raf-1 was cotransfected. Since there is no Raf-1Δ28 associated with Rb when cotransfected and since there was no reversal of Rb function in this lane, it appears that a physical interaction is indeed required to inactivate Rb. It was further tested whether only the amino-terminal region of Raf-1 was sufficient to interact with Rb by using an in vitro binding assay (Fig. 6D). It was observed that a 35S-labeled Raf-1 fragment spanning residues 1 to 59 could efficiently bind to GST-Rb. This result, in conjunction with the functional studies, strongly suggested that only the N-terminal region of Raf-1 is necessary and sufficient to interact with Rb but that the kinase domain is also needed for functional inactivation.
Raf-1 can reverse Rb-mediated growth suppression.
It had been demonstrated that viral oncoproteins such as adenovirus E1A and SV40 large T antigen, which bind to the pocket domain of Rb, can reverse Rb-mediated growth suppression (9, 18, 28, 45, 64). To assess whether Raf-1 has a similar effect on Rb-mediated growth control, we performed stable transfections on the human osteosarcoma cell line Saos-2 to evaluate the number of antibiotic-resistant colonies that grow on plastic (4). Saos-2 cells transfected with neomycin or puromycin control vectors, or a combination of both, gave rise to approximately 170 colonies after selection for 14 days (Table 1). But upon cotransfection with 2 μg of wild-type human Rb gene, the number of colonies was reduced about threefold; Raf-1 alone increased the number of colonies to about 300. Cotransfection of two different amounts of a neomycin control vector with Rb had no considerable effect on the number of colonies after double selection in the two antibiotics. In contrast, cotransfection of wild-type Raf-1 totally reversed the Rb-mediated repression of colony formation, suggesting that Raf-1 can indeed reverse Rb-mediated growth suppression.
TABLE 1.
Raf-1 can reverse Rb-mediated repression of colony formationa
| Vectors transfected (μg) | No. of colonies
|
|
|---|---|---|
| First expt | Second expt | |
| pSVNeo | 148 | 194 |
| pBABE-Puro | 153 | 185 |
| Puro+Neo | 164 | 176 |
| Raf-1-Neo, WT (2) | 315 | 287 |
| Raf-1-Neo, Δ28 (2) | 139 | 132 |
| Puro-Rb (2) | 78 | 59 |
| Rb+pSVNeo (1) | 83 | 62 |
| Rb+pSVNeo (2) | 63 | 54 |
| Rb+Raf-1, WT (1) | 325 | 296 |
| Rb+Raf-1, WT (2) | 343 | 318 |
| Rb+Raf-1, Δ28 (1) | 75 | 69 |
| Rb+Raf-1, Δ28 (2) | 62 | 67 |
Suppression of colony formation by Rb is reversed by Raf-1. Saos-2 osteosarcoma cells grown in 35-mm dishes were stably transfected with the indicated vectors and selected in antibiotic for 14 days. Colonies with 20 or more cells were counted. pCMV-Rb was cotransfected with pBABE-Puro in all cases to impart resistance; pCDNA3-Raf-1 vectors, wild type (WT) and Δ28, carried neomycin resistance markers. Selection was done in neomycin, puromycin, or a combination of both depending on the vectors transfected.
Unlike wild-type Raf-1, Raf-1Δ28 could not enhance colony formation in Saos-2 cells and was totally impaired in its ability to reverse Rb-mediated growth control. This result, in combination with the effects on E2F transcriptional activity, suggest that physical interaction with Rb is required for Raf-1 to affect its function. Since the kinase activity of Raf-1 is also required for such events to occur, it is possible that Raf-1 is phosphorylating Rb itself or other molecules which are bound to Rb, leading to an abrogation of Rb function.
Raf-1 protein can be detected in the nucleus of human fibroblasts.
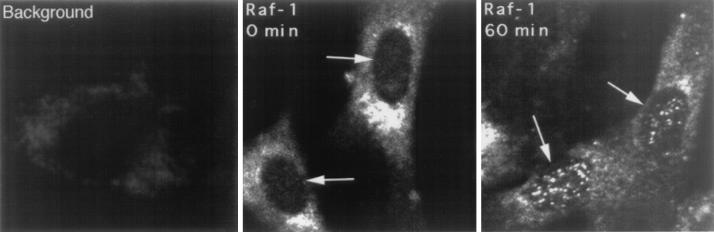
The observation that Raf-1 protein can modulate Rb function after direct binding suggested that at least a portion of the Raf-1 molecules should be entering the nucleus, since Rb is predominantly a nuclear protein. This notion is further supported by the effects of Raf-1 on E2F-mediated transcription. Though it has been suggested that Raf-1 is mostly cytoplasmic and gets activated at the membrane (54-56), it is not clear whether it gets translocated to other subcellular compartments, including the nucleus. It has recently been demonstrated that Raf-1 gets translocated to the mitochondria in association with Bcl-2 (49); in addition, there are earlier reports suggesting that Raf-1 enters the nucleus under certain conditions, such as hypothalamic ischemia (51). To examine whether Raf-1 indeed translocates to the nucleus in normal cells, we performed an immunofluorescence experiment with a Raf-1 monoclonal antibody on quiescent or serum-stimulated HSF8 cells. As shown in Fig. 7 (left panel), Raf-1 is exclusively in the cytoplasm in serum-starved cells; in contrast, significant amounts of Raf-1 can be detected in the nucleus after 30 min of serum stimulation. This result has been reproduced multiple times with two different Raf-1 antibodies, both monoclonal and polyclonal; in either case, there was no detectable immunofluorescence in the absence of a primary antibody, suggesting that the signal observed in the nucleus is indeed from Raf-1.
FIG. 7.
Raf-1 can localize to the nucleus. HSF8 cells serum starved for 48 h or stimulated for 30 min were fixed and stained with an anti-Raf-1 mouse monoclonal antibody. A rhodamine-conjugated anti-mouse secondary antibody was used to visualize the staining by immunofluorescence. The background panel represents the staining of a serum-stimulated cell in the absence of primary antibody.
Since Raf-1 and Rb have been found to interact physically and functionally in the cell and since Raf-1 was detected in the nucleus, attempts were made to assess whether these molecules colocalize in the cell. To test this, a Raf-1 polyclonal antibody and an Rb monoclonal antibody were used in a double immunofluorescence experiment (Rb polyclonal antibody was not very efficient in the immunofluorescence studies). The anti-mouse IgG secondary antibodies were labeled with rhodamine (for Rb) and the anti-rabbit antibodies were labeled with fluorescein (for Raf-1); the results are shown in Fig. 8. When the images are superimposed, it can be seen that Raf-1 and Rb colocalize in the nucleus considerably. The colocalization appears, reproducibly, in distinct spots in the nucleus. The nature of these structures is not known, but it has been suggested that Rb can form multimers and exist as large complexes in association with growth-promoting proteins in the nucleus (44). It may be that Raf-1 is localizing to such complexes. Since Raf-1 lacks an identifiable nuclear localization signal, its mechanism of translocation is not clear; it is conceivable that Raf-1 enters the nucleus in association with another protein chaperone.
FIG. 8.
Colocalization of Rb and Raf-1 in the nucleus. HSF8 cells were fixed after 60 min of serum stimulation and then immunostained with an anti-Raf-1 antibody (against residues 23 to 269) and an anti-Rb mouse monoclonal antibody. A fluorescein-conjugated anti-rabbit antibody or a rhodamine-conjugated anti-mouse antibody was used to visualize the staining. Arrows indicate the regions that were stained by both of the antibodies. Superimposition of the two images shows the regions of colocalization in yellow (bottom panel).
Raf-1 can phosphorylate Rb in vitro.
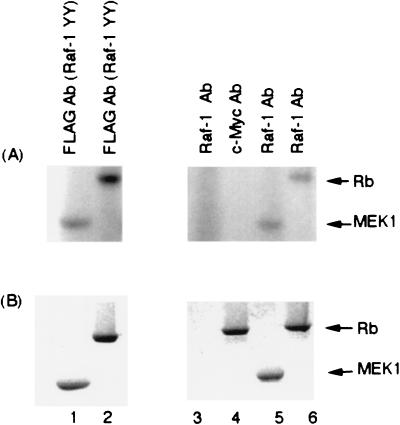
Attempts were made to assess the potential mechanisms by which Raf-1 may be regulating Rb function. Since it is well established that Rb function is regulated by phosphorylation and since Raf-1 is a kinase, experiments were designed to ascertain whether Raf-1 is capable of phosphorylating the Rb protein. Two separate types of experiments were conducted for this purpose. In the first set of experiments, Sf9 cell extracts expressing baculovirus-produced FLAG-tagged Raf-1 were used as a source for Raf-1 (55, 67, 71). Then, 50 μg of such an extract was immunoprecipitated with an anti-FLAG antibody, and the proteins were recovered on protein G-treated beads. The beads were washed thoroughly in M2 buffer or an immunoprecipitation buffer and used to conduct in vitro kinase reactions on 2 μg of GST-MEK1 or 5 μg of bacterially produced full-length Rb protein. Different amounts of these two substrates were used to ensure that they were in comparable molar amounts. In this experiment, it was found that immunoprecipitated FLAG-tagged Raf-1 could phosphorylate Rb quite efficiently (Fig. 9A, left panel). Experiments that were more direct were conducted by immunoprecipitating Raf-1 from U937 whole-cell extracts and examining its ability to phosphorylate these two substrates. As can be seen from Fig. 9A (right panel), Rb appeared to be phosphorylated as efficiently as MEK1 by the immunoprecipitated Raf-1. There was no phosphorylation when a control c-myc antibody was used in the immunoprecipitation. As expected, there were no bands visible when no substrate was added to the Raf-1 immunoprecipitation lane. From this experiment, it appears that Raf-1 can phosphorylate Rb in vitro quite efficiently and that such a direct phosphorylation probably contributes to its inactivation.
FIG. 9.
Raf-1 can phosphorylate Rb in vitro. (A) Baculovirus-produced FLAG-Raf-1 was immunoprecipitated with an anti-FLAG antibody and used for in vitro kinase assays on 2 μg of GST-MEK1 (lane 1) or 5 μg of full-length human Rb (lane 2). Similar assays were performed on a U937 whole-cell extract with Raf-1 antibody (lanes 3, 5, and 6) or a control antibody (lane 4). GST-MEK1 (lane 5) and Rb (lanes 4 and 6) were used as substrates. (B) The gel in panel A was rehydrated and stained with Coomassie blue to show the amounts of the substrates used.
DISCUSSION
Raf kinases are signal-integrating enzymes that have the ability to switch tyrosine kinase signaling to serine-threonine phosphorylation and to connect growth factor receptors to transcription factors (16, 24, 26). Tyrosine and/or serine-threonine phosphorylation of Raf-1 is required for its activation (8, 21, 33, 50, 59, 60, 81). Raf-1 has been shown to bind to Ras, as well as its substrate MEK (26, 77); in addition, it has been shown to bind to receptor tyrosine kinases and to Src in a mitogen-dependent manner (24). Recent studies have suggested that Raf-1 can interact with and regulate the function of cdc25A (22), a dual-specificity phosphatase involved in cell cycle regulation (23, 34). Further, it has been demonstrated that Raf-1 can physically interact with Bcl-2 and inhibit its antiapoptotic properties. These results suggest that Raf-1 can directly affect targets outside the MAP kinase cascade and modulate vital cellular events like apoptosis. The results we present here demonstrate that the cellular Raf-1 protein can physically interact with Rb and p130 proteins.
Though the focus of this investigation was the interaction of Raf-1 with Rb protein, it should be pointed out that Raf-1 can interact with p130 to a comparable extent. It is not yet known whether p130-E2F complexes, unlike Rb, play a dominant role in modulating the normal cell cycle or whether their disruption is essential for the progression of the cell cycle. Thus, we believe that the interaction of Raf-1 with Rb could be relatively more important than its interaction with p130. Binding of Raf-1 to p107 was considerably weaker or absent in the assays that were used here. p107 targets E2F family members only in the S phase (5, 25, 72, 75, 82) and thus it may not be necessary for Raf-1 to inactivate p107 to effect cell cycle progression if p107-E2F interactions exert their function past the G1/S transition point.
There are three Raf family genes present in vertebrates, and Raf-1 analogues have been studied in a Drosophila sp. and Caenorhabditis elegans (17). In humans, c-Raf-1 is ubiquitously expressed and is considered to be the main Raf-1 molecule; A-Raf-1 is a tissue-specific form of Raf-1, and B-Raf-1 is structurally similar to it (69). It is not yet clear whether Rb protein can interact with all three of the Raf-1 family members and whether all of the family members can inactivate Rb. Given the ubiquitous expression pattern of Rb and Raf-1, it would not be surprising if Rb shows preferential binding to Raf-1. Given the fact that there is only one Raf-1 and Rb homologue in organisms such as Drosophila and that they are functionally equivalent to the mammalian homologues, it is possible that they are interacting physically and/or functionally.
The Rb-Raf interaction appears to be stringently regulated following mitogenic stimulation. We found that the Raf-1 present in immunoprecipitates of Rb and p130 can cross-react with an anti-phosphotyrosine antibody (data not shown); and from the kinetics of the interaction, it appears that Raf-1 binds to Rb subsequent to its activation. Conversely, Rb is hypophosphorylated in the early G1 phase (18, 45), and it is a hypophosphorylated form of Rb that associates with Raf-1. This, along with the fact that Raf-1 does not associate with a pocket domain mutant of Rb, suggests that Raf-1 interacts with the functional form of Rb.
Data demonstrating the physical presence of Raf-1 in E2F-containing complexes raise some interesting possibilities. Raf-1 could be associating with E2F complexes at a step that precedes their dissolution by phosphorylation of Rb by cyclin-dependent kinases. It could be that the association of Raf-1 facilitates the subsequent inactivation of Rb-p130, leading to the release of transcriptionally active E2F. Functionally, the reversal of Rb-mediated repression of E2F activity by Raf-1 could have considerable repercussions on cell proliferation. We believe that Raf-1 is the first signaling molecule that can reverse Rb-mediated suppression of E2F activity in a manner analogous to viral oncoproteins such as E1A or SV40 large T antigen (9, 18, 28, 45, 64). Since transformation by viral oncoproteins correlates with a dissociation of E2F from Rb and a concomitant increase of E2F activity (64), it is possible that the activation of E2F by Raf-1 is an equivalent process inducing cell proliferation.
The kinase activity of Raf-1 was found to be necessary to reverse Rb-mediated repression of E2F activity, raising the possibility that Raf-1 can phosphorylate Rb directly or else proteins associated with Rb. We found that Raf-1 can phosphorylate Rb in in vitro assays. It is not yet clear whether such a phosphorylation of Rb by Raf-1 occurs in vivo, though it is conceivable that such a phosphorylation contributes to Rb inactivation. It should be pointed out that Rb harbors at least 16 phosphorylation sites (76) and has been shown to be a target of different kinases, making it plausible that direct phosphorylation by Raf-1 contributes to its inactivation. Rb inactivation by Raf-1 may involve additional mechanisms (40), and Rb-Raf-1 interaction could be one step in cyclin D-cdk4-mediated inactivation of Rb. For example, it has been observed that Raf-1 can associate with and activate cdc25A (22, 78), a phosphatase that is involved in the activation of cyclin-dependent kinases (23). It may be that Raf-1 activates cdc25A, allowing it to act on cyclin D-cdk4/6, leading to Rb phosphorylation, which may explain the temporal differences in the association of Raf-1 with Rb and the inactivation of Rb.
A dominant-negative Ras can block Ras transformation only in cells which have a functional Rb gene, suggesting a role for Rb inactivation in Ras-mediated transformation (66). Earlier studies showing that p16 can inhibit Ras-mediated transformation itself suggested a requirement for Rb inactivation, since the only known function of p16 is to maintain Rb in an active state (57). It has also been proposed that Ras inactivates Rb indirectly by inducing cyclin D expression, cdk activity, and downregulation of p27 (1, 40, 80). Our findings add a new dimension to this scenario, one in which a direct inactivation of Rb by Raf-1 contributes to G1 progression. A similar situation where Raf-1 is required is the v-Abl-mediated induction of the c-myc promoter through an E2F site, which requires the Ras signaling pathway (79). A functional Raf-1 protein was necessary for this induction (83). Though the c-Abl protein can modulate Rb function after direct binding, v-Abl has not been found to interact with Rb directly. Hence, it may be that v-Abl-mediated induction of E2F activity involves the inactivation of Rb by the pathways described above, as well as through a direct interaction of Raf-1 with Rb.
The finding that a portion of Raf-1 enters the nucleus following mitogen stimulation is intriguing, since it has been demonstrated that Raf-1 is activated by Ras in the cytoplasm (54-56). The finding that Raf-1 can affect the transcriptional activity of E2F, as well as its presence in E2F complexes, makes a strong argument that Raf-1 at some point enters the nucleus. It had been suggested previously that Raf-1 can be detected in the nucleus under certain specific circumstances, such as hypothalamic ischemia (65a). The presence of Raf-1 in the nucleus could raise the possibility that Raf-1 is targeting other nuclear proteins in addition to Rb and p130. Though it is not clear how Raf-1 enters the nucleus, perhaps it is chaperoned by another protein in a manner analogous to its localization to mitochondria in association with Bcl-2 (49). The colocalization of Rb and Raf-1 in the nucleus indeed strengthens this possibility. The kinetics of nuclear translocation, as well as the regions of Raf-1 required for this, is being examined now.
On the whole, we believe that the interaction of Raf-1 with Rb is a vital step in the way proliferative signals target the cell cycle and may thus contribute to oncogenic transformation. Perhaps agents that can disrupt Rb–Raf-1 interaction could have antiproliferative effects and could be important in designing novel strategies to combat cancer.
ACKNOWLEDGMENTS
We thank A. Giordano, N. Ahn, J. McClung, J. Padmanabhan, and J. Krolewski for different plasmid constructs and antibodies; R. Dalla-Favera, John Krolewski, Niharika Nath, and Mathew Adlam for critically reading the manuscript; Audrey Minden, Elizabeth Bosch, and Martin McMahon for reagents and helpful suggestions; and F. R. Maxfield for the use of microscope and imaging equipment.
This study was funded by NIH grant CA63136. S.P.C. is a recipient of the Irma-Hirschl Trust Research Award.
REFERENCES
- 1.Atkas H, Cai H, Cooper G M. Ras links growth factor signaling to the cell cycle machinery via regulation of cyclin D1 and the cdk inhibitor p27 Kip1. Mol Cell Biol. 1997;17:3850–3857. doi: 10.1128/mcb.17.7.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagchi S, Raychaudhuri P, Nevins J R. Adenovirus E1A proteins can dissociate heteromeric complexes involving the E2F transcription factor: a novel mechanism for E1A trans-activation. Cell. 1990;62:659–669. doi: 10.1016/0092-8674(90)90112-r. [DOI] [PubMed] [Google Scholar]
- 3.Bagchi S, Weinmann R, Raychaudhuri P. The retinoblastoma protein copurifies with E2F-I, an E1A-regulated inhibitor of the transcription factor E2F. Cell. 1991;65:1073–1082. doi: 10.1016/0092-8674(91)90558-g. [DOI] [PubMed] [Google Scholar]
- 4.Beijersbergen R L, Bernards R. Cell cycle regulation by the retinoblastoma family of growth inhibitory proteins. Biochim Biophys Acta. 1996;1287:103–120. doi: 10.1016/0304-419x(96)00002-9. [DOI] [PubMed] [Google Scholar]
- 5.Beijersbergen R L, Kerkhoven R M, Zhu L, Carlee L, Voorhoeve P M, Bernards R. E2F-4, a new member of the E2F gene family, has oncogenic activity and associates with p107 in vivo. Genes Dev. 1994;8:2680–2790. doi: 10.1101/gad.8.22.2680. [DOI] [PubMed] [Google Scholar]
- 6.Bruder J T, Heidecker G, Rapp U R. Serum-, TPA-, and Ras-induced expression from AP-1/Ets-driven promoters requires Raf-1 kinase. Genes Dev. 1992;6:545–556. doi: 10.1101/gad.6.4.545. [DOI] [PubMed] [Google Scholar]
- 7.Buchkovich K, Duffy L A, Harlow E. The retinoblastoma protein is phosphorylated during specific phases of the cell cycle. Cell. 1989;58:1097–1105. doi: 10.1016/0092-8674(89)90508-4. [DOI] [PubMed] [Google Scholar]
- 8.Burgering B M, Bos J L. Regulation of Ras-mediated signalling: more than one way to skin a cat. Trends Biochem Sci. 1995;20:18–22. doi: 10.1016/s0968-0004(00)88944-6. [DOI] [PubMed] [Google Scholar]
- 9.Chellappan S, Kraus V B, Kroger B, Munger K, Howley P M, Phelps W C, Nevins J R. Adenovirus E1A, simian virus 40 tumor antigen, and human papillomavirus E7 protein share the capacity to disrupt the interaction between transcription factor E2F and the retinoblastoma. Proc Nat Acad Sci USA. 1992;89:4549–4553. doi: 10.1073/pnas.89.10.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chellappan S P, Hiebert S, Mudryj M, Horowitz J M, Nevins J R. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991;65:1053–1061. doi: 10.1016/0092-8674(91)90557-f. [DOI] [PubMed] [Google Scholar]
- 11.Chittenden T, Livingston D M, Kaelin W G., Jr RB associates with an E2F-like, sequence-specific DNA-binding protein. Cold Spring Harbor Symp Quant Biol. 1991;56:187–195. doi: 10.1101/sqb.1991.056.01.024. [DOI] [PubMed] [Google Scholar]
- 12.Claudio P P, De L A, Howard C M, Baldi A, Firpo E J, Koff A, Paggi M G, Giordano A. Functional analysis of pRb2/p130 interaction with cyclins. Cancer Res. 1996;56:2003–2008. [PubMed] [Google Scholar]
- 13.Claudio P P, Howard C M, Baldi A, De L A, Fu Y, Condorelli G, Sun Y, Colburn N, Calabretta B, Giordano A. p130/pRb2 has growth suppressive properties similar to yet distinctive from those of retinoblastoma family members pRb and p107. Cancer Res. 1994;54:5556–5560. [PubMed] [Google Scholar]
- 14.Cobrinik D. Regulatory interactions among E2Fs and cell cycle proteins. Curr Top Microbiol Immunol. 1996;208:32–63. doi: 10.1007/978-3-642-79910-5_2. [DOI] [PubMed] [Google Scholar]
- 15.Cobrinik D, Whyte P, Peeper D S, Jacks T, Weinberg R A. Cell cycle-specific association of E2F with the p130 E1A-binding protein. Genes Dev. 1993;7:2392–2404. doi: 10.1101/gad.7.12a.2392. [DOI] [PubMed] [Google Scholar]
- 16.Cox L A, Chen G, Lee E Y. Tumor suppressor genes and their roles in breast cancer. Breast Cancer Res Treat. 1994;32:19–38. doi: 10.1007/BF00666203. [DOI] [PubMed] [Google Scholar]
- 17.Daum G, Eisenmann-Tappe I, Fries H W, Troppmair J, Rapp U R. The ins and outs of Raf kinases. Trends Biochem Sci. 1994;19:474–479. doi: 10.1016/0968-0004(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 18.DeCaprio J A, Ludlow J W, Lynch D, Furukawa Y, Griffin J, Piwnica-Worms H, Huang C M, Livingston D M. The product of the retinoblastoma susceptibility gene has properties of a cell cycle regulatory element. Cell. 1989;58:1085–1095. doi: 10.1016/0092-8674(89)90507-2. [DOI] [PubMed] [Google Scholar]
- 19.DeGregori J, Leone G, Ohtani K, Miron A, Nevins J R. E2F-1 accumulation bypasses a G1 arrest resulting from the inhibition of G1 cyclin-dependent kinase activity. Genes Dev. 1995;9:2873–2887. doi: 10.1101/gad.9.23.2873. [DOI] [PubMed] [Google Scholar]
- 20.Ewen M E, Sluss H K, Sherr C J, Matsushime H, Kato J, Livingston D M. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993;73:487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- 21.Fabian J R, Daar I O, Morrison D K. Critical tyrosine residues regulate the enzymatic and biological activity of Raf-1 kinase. Mol Cell Biol. 1993;13:7170–7179. doi: 10.1128/mcb.13.11.7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galaktionov K, Jessus C, Beach D. Raf-1 interaction with Cdc25 phosphatase ties mitogenic signal transduction to cell cycle activation. Genes Dev. 1995;9:1046–1058. doi: 10.1101/gad.9.9.1046. [DOI] [PubMed] [Google Scholar]
- 23.Gautier J, Solomon M, Booher R, Bazan J, Kirshner M. Cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell. 1991;67:67–72. doi: 10.1016/0092-8674(91)90583-k. [DOI] [PubMed] [Google Scholar]
- 24.Gerwins P, Blank J L, Johnson G L. Cloning of a novel mitogen-activated protein kinase kinase kinase, MEKK4, that selectively regulates the c-Jun amino terminal kinase pathway. J Biol Chem. 1997;272:8288–8295. doi: 10.1074/jbc.272.13.8288. [DOI] [PubMed] [Google Scholar]
- 25.Ginsberg D, Vairo G, Chittenden T, Xiao Z X, Xu G, Wydner K L, DeCaprio J A, Lawrence J B, Livingston D M. E2F-4, a new member of the E2F transcription factor family, interacts with p107. Genes Dev. 1994;8:2665–2679. doi: 10.1101/gad.8.22.2665. [DOI] [PubMed] [Google Scholar]
- 26.Glazer R I, Rohlff C. Transcriptional regulation of multidrug resistance in breast cancer. Breast Cancer Res Treat. 1994;31:263–271. doi: 10.1007/BF00666159. [DOI] [PubMed] [Google Scholar]
- 27.Heidecker G, Huleihel M, Cleveland J L, Kolch W, Beck W, Lloyd P, Pawson T, Rapp U R. Mutational activation of c-Raf-1 and the definition of the minimal transforming sequence. Mol Cell Biol. 1990;10:2503–2512. doi: 10.1128/mcb.10.6.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hiebert S W, Chellappan S P, Horowitz J M, Nevins J R. The interaction of RB with E2F coincides with an inhibition of the transcriptional activity of E2F. Genes Dev. 1992;6:177–185. doi: 10.1101/gad.6.2.177. [DOI] [PubMed] [Google Scholar]
- 29.Hinds P W, Mittnacht S, Dulic V, Arnold A, Reed S I, Weinberg R A. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell. 1992;70:993–1006. doi: 10.1016/0092-8674(92)90249-c. [DOI] [PubMed] [Google Scholar]
- 30.Hollingsworth R J, Chen P L, Lee W H. Integration of cell cycle control with transcriptional regulation by the retinoblastoma protein. Curr Opin Cell Biol. 1993;5:194–200. doi: 10.1016/0955-0674(93)90102-v. [DOI] [PubMed] [Google Scholar]
- 31.Hollingsworth R J, Hensey C E, Lee W H. Retinoblastoma protein and the cell cycle. Curr Opin Genet Dev. 1993;3:55–62. doi: 10.1016/s0959-437x(05)80341-7. [DOI] [PubMed] [Google Scholar]
- 32.Horowitz J M. Regulation of transcription by the retinoblastoma protein. Genes Chromosomes Cancer. 1993;6:124–131. doi: 10.1002/gcc.2870060211. [DOI] [PubMed] [Google Scholar]
- 33.Jelinek T, Dent P, Sturgill T W, Weber M J. Ras-induced activation of Raf-1 is dependent on tyrosine phosphorylation. Mol Cell Biol. 1996;16:1027–1034. doi: 10.1128/mcb.16.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jinno S, Suto K, Nagata A, Igarashi M, Kanaoka Y, Nojima H, Okayama H. Cdc25A is a novel phosphatase functioning early in the cell cycle. EMBO J. 1994;13:1549–1556. doi: 10.1002/j.1460-2075.1994.tb06417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson D G, Schwarz J K, Cress W D, Nevins J R. Expression of transcription factor E2F1 induces quiescent cells to enter S phase. Nature. 1993;365:349–352. doi: 10.1038/365349a0. [DOI] [PubMed] [Google Scholar]
- 36.Junaief J L, Strober B E, Guha S, Khavari P A, Alin K, Luban J, Begenman M, Goff S P. The retinoblastoma protein and Brg1 form a complex and co-operate to induce cell cycle arrest. Cell. 1994;79:119–130. doi: 10.1016/0092-8674(94)90405-7. [DOI] [PubMed] [Google Scholar]
- 37.Kaelin W G, Jr, Pallas D C, DeCaprio J A, Kaye F J, Livingston D M. Identification of cellular proteins that can interact specifically with the T/E1A-binding region of the retinoblastoma gene product. Cell. 1991;64:521–532. doi: 10.1016/0092-8674(91)90236-r. [DOI] [PubMed] [Google Scholar]
- 38.Kato J, Matsushime H, Hiebert S W, Ewen M E, Sherr C J. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993;7:331–342. doi: 10.1101/gad.7.3.331. [DOI] [PubMed] [Google Scholar]
- 39.Kato J Y, Matsuoka M, Strom D K, Sherr C J. Regulation of cyclin D-dependent kinase 4 (cdk4) by cdk4-activating kinase. Mol Cell Biol. 1994;14:2713–2721. doi: 10.1128/mcb.14.4.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kerkhoff E, Rapp U R. Induction of cell proliferation in quiescent NIH 3T3 cells by oncogenic c-Raf-1. Mol Cell Biol. 1997;17:2576–2586. doi: 10.1128/mcb.17.5.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.La Thangue N. DRTF1/E2F: an expanding family of heterodimeric transcription factors implicated in cell-cycle control. Trends Biochem Sci. 1994;19:108–114. doi: 10.1016/0968-0004(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 42.Lee C S, deFazio A, Ormandy C J, Sutherland R L. Inverse regulation of oestrogen receptor and epidermal growth factor receptor gene expression in MCF-7 breast cancer cells treated with phorbol ester. J Steroid Biochem Mol Biol. 1996;58:267–275. doi: 10.1016/0960-0760(96)00039-8. [DOI] [PubMed] [Google Scholar]
- 43.Lee E Y, To H, Shew J Y, Bookstein R, Scully P, Lee W H. Inactivation of the retinoblastoma susceptibility gene in human breast cancers. Science. 1988;241:218–221. doi: 10.1126/science.3388033. [DOI] [PubMed] [Google Scholar]
- 44.Lee W H, Hollingsworth R E, Jr, Qian Y W, Chen P L, Hong F, Lee E Y. RB protein as a cellular “corral” for growth-promoting proteins. Cold Spring Harbor Symp Quant Biol. 1991;56:211–217. doi: 10.1101/sqb.1991.056.01.026. [DOI] [PubMed] [Google Scholar]
- 45.Lees J A, Buchkovich K J, Marshak D R, Anderson C W, Harlow E. The retinoblastoma protein is phosphorylated on multiple sites by human cdc2. EMBO J. 1991;10:4279–4290. doi: 10.1002/j.1460-2075.1991.tb05006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lehman T A, Greenblatt M, Bennett W P, Harris C C. Mutational spectrum of the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Drug Metab Rev. 1994;26:221–235. doi: 10.3109/03602539409029793. [DOI] [PubMed] [Google Scholar]
- 47.Lukas J, Bartkova J, Bartek J. Convergence of mitogenic signaling cascades from diverse classes of receptors at the cyclin D-cyclin-dependent-kinase-pRb-controlled G1 checkpoint. Mol Cell Biol. 1996;16:6917–6925. doi: 10.1128/mcb.16.12.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lukas J, Bartkova J, Rohde M, Strauss M, Bartek J. Cyclin D1 is dispensable for G1 control in retinoblastoma gene-deficient cells independently of cdk4 activity. Mol Cell Biol. 1995;15:2600–2611. doi: 10.1128/mcb.15.5.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lumpkin C J, Moore T L, Tarpley M D, Taylor J M, Badger T M, McClung J K. Acute ethanol and selected growth suppressor transcripts in regenerating rat liver. Alcohol. 1995;12:357–362. doi: 10.1016/0741-8329(95)00018-m. [DOI] [PubMed] [Google Scholar]
- 50.Marais R, Light Y, Paterson H F, Marshall C J. Ras recruits Raf-1 to the membrane for activation by tyrosine phosphorylation. EMBO J. 1995;14:3136–3145. doi: 10.1002/j.1460-2075.1995.tb07316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mark H F, Annas G, Ricker R, Weitzel J. Clinical and research issues in breast cancer genetics. Ann Clin Lab Sci. 1996;26:396–408. [PubMed] [Google Scholar]
- 52.Mayol S, Garriga J, Grana X. G1 cyclin/CDK-independent phosphorylation and accumulation of p130 during the transition from G1 to G0 lead to its association with E2F-4. Oncogene. 1996;13:237–246. [PubMed] [Google Scholar]
- 53.Mayol S, Grana X, Baldi A, Sang N, Hu Q, Giordano A. Cloning of a new member of the retinoblastoma gene family (pRb2) which binds to the E1A transforming domain. Oncogene. 1993;8:2561–2566. [PubMed] [Google Scholar]
- 54.Mazzarelli J M, Atkins G B, Geisberg J V, Ricciardi R P. The viral oncoproteins Ad5 E1A, HPV16 E7 and SV40 TAg bind a common region of the TBP-associated factor-110. Oncogene. 1995;11:1859–1864. [PubMed] [Google Scholar]
- 55.McCarthy S A, Samuels M L, Pritchard C A, Abraham J A, McMahon M. Rapid induction of heparin-binding epidermal growth factor/diphtheria toxin receptor expression by Raf and Ras oncogenes. Genes Dev. 1995;9:1953–1964. doi: 10.1101/gad.9.16.1953. [DOI] [PubMed] [Google Scholar]
- 56.McClung J K, Danner D B, Stewart D A, Smith J R, Schneider E L, Lumpkin C K, Dell’Orco R T, Nuell M J. Isolation of a cDNA hybrid that selects anti-proliferative mRNA from rat liver. Biochem Biophys Res Commun. 1989;164:1316–1322. doi: 10.1016/0006-291x(89)91813-5. [DOI] [PubMed] [Google Scholar]
- 57.McClung J K, King R L, Walker L S, Danner D B, Nuell M J, Stewart C A, Dell’Orco R T. Expression of prohibitin, an antiproliferative protein. Exp Gerontol. 1992;27:413–417. doi: 10.1016/0531-5565(92)90074-a. [DOI] [PubMed] [Google Scholar]
- 58.Meyerson M, Harlow E. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol Cell Biol. 1994;14:2077–2086. doi: 10.1128/mcb.14.3.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mischak H, Seitz T, Janosch P, Eulitz M, Steen H, Schellerer M, Philipp A, Koldh W. Negative regulation of Raf-1 by phosphorylation of serine 621. Mol Cell Biol. 1996;16:5409–5418. doi: 10.1128/mcb.16.10.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morrison D K, Kaplan D R, Escobedo J A, Rapp U R, Roberts T M, Williams L T. Direct activation of the serine/threonine kinase activity of Raf-1 through tyrosine phosphorylation by the PDGF beta-receptor. Cell. 1989;58:649–657. doi: 10.1016/0092-8674(89)90100-1. [DOI] [PubMed] [Google Scholar]
- 61.Muller R, Mumberg D, Lucibello F C. Signals and genes in the control of cell cycle progression. Biochem Biophys Acta. 1993;1155:151–179. doi: 10.1016/0304-419x(93)90003-u. [DOI] [PubMed] [Google Scholar]
- 62.Murray A W. Creative blocks: cell cycle checkpoints and feedback controls. Nature. 1992;359:599–604. doi: 10.1038/359599a0. [DOI] [PubMed] [Google Scholar]
- 63.Murray A W, Hunt T. The cell cycle: an introduction. Oxford, United Kingdom: Oxford University Press; 1993. [Google Scholar]
- 64.Nevins J R. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science. 1992;258:424–429. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- 65.Norbury C, Nurse P. Animal cell cycles and their control. Annu Rev Biochem. 1992;61:441–470. doi: 10.1146/annurev.bi.61.070192.002301. [DOI] [PubMed] [Google Scholar]
- 65a.Olah Z, Komoly S, Nagashima N, Joo F, Rappa U R, Anderson W B. Cerebral ischemia induces transient intracellular redistribution and intranuclear translocation of the Raf proto-oncogene product in hippocampal pyramidal cells. Exp Brain Res. 1991;84:403–410. doi: 10.1007/BF00231462. [DOI] [PubMed] [Google Scholar]
- 66.Peeper D S, Upton T M, Ladha M H, Neuman E, Zalvide J, Bernards R, DeCaprio J, Ewen M E. Ras signaling linked to the cell-cycle machinery by the retinoblastoma protein. Nature. 1997;386:177–181. doi: 10.1038/386177a0. [DOI] [PubMed] [Google Scholar]
- 67.Pritchard C A, Samuels M L, Bosch E, McMahon M. Conditionally oncogenic form of the A-Raf and B-Raf protein kinases display different biological and biochemical properties in NIH 3T3 cells. Mol Cell Biol. 1995;15:6430–6442. doi: 10.1128/mcb.15.11.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Quelle D E, Ashmun R A, Shurtleff S A, Kato J-Y, Bar-Sagi D, Roussel M F, Sherr C J. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 1993;7:1559–1571. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- 69.Rapp U R. Role of Raf-1 serine/threonine protein kinase in growth factor signal transduction. Oncogene. 1991;6:495–500. [PubMed] [Google Scholar]
- 70.Resnitzky D, Gossen M, Bujard H, Reed S I. Acceleration of G1/S transition by expression of cyclins D and E with an inducible system. Mol Cell Biol. 1994;14:1669–1679. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Samuels M L, McMahon M. Inhibition of platelet-derived growth factor and epidermal growth factor-mediated mitogenesis and signaling in 3T3 cells expressing DRaf-1:ER, an estradiol-regulated form of Raf-1. Mol Cell Biol. 1994;14:7855–7866. doi: 10.1128/mcb.14.12.7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sardet C, Vidal M, Cobrinik D, Geng Y, Onufryk C, Chen A, Weinberg R A. E2F-4 and E2F-5, two members of the E2F family, are expressed in the early phases of the cell cycle. Proc Natl Acad Sci USA. 1995;92:2403–2407. doi: 10.1073/pnas.92.6.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Serrano M, Hannon G J, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- 74.Sherr C J. The ins and outs of RB: coupling gene expression to the cell cycle clock. Trends Cell Biol. 1994;4:15–18. doi: 10.1016/0962-8924(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 75.Shirodkar S, Ewen M, DeCaprio J A, Morgan J, Livingston D M, Chittenden T. The transcription factor E2F interacts with the retinoblastoma product and a p107-cyclin A complex in a cell cycle-regulated manner. Cell. 1992;68:157–166. doi: 10.1016/0092-8674(92)90214-w. [DOI] [PubMed] [Google Scholar]
- 76.Taya Y. Rb kinases and RB-binding proteins: new points of view. Trends Biochem Sci. 1997;22:14–17. doi: 10.1016/s0968-0004(96)10070-0. [DOI] [PubMed] [Google Scholar]
- 77.Vojtek A B, Hollenberg S M, Cooper J A. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993;74:205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- 78.Weinberg R A. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 78a.Welch P J, Wang J Y J. A C-terminal protein-binding domain in the retinoblastoma protein regulates nuclear c-Abl tyrosine kinase in the cell cycle. Cell. 1993;75:779–790. doi: 10.1016/0092-8674(93)90497-e. [DOI] [PubMed] [Google Scholar]
- 78b.Welch P J, Wang J Y J. Disruption of the retinoblastoma protein function by co-expression of its C pocket fragment. Genes Dev. 1995;9:31–46. doi: 10.1101/gad.9.1.31. [DOI] [PubMed] [Google Scholar]
- 79.Wong K K, Zou X, Merrell K T, Patel A J, Marcu K B, Chellappan S, Calame K. v-Abl activates c-myc transcription through the E2F site. Mol Cell Biol. 1995;15:6535–6544. doi: 10.1128/mcb.15.12.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Woods D, Parry D, Cherwinski H, Bosch E, Lees E, McMahon M. Raf-induced proliferation or cell cycle arrest is determined by the level of Raf activity with arrest mediated by p21Cip1. Mol Cell Biol. 1997;17:5598–5611. doi: 10.1128/mcb.17.9.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yao B, Zhang Y, Delikat S, Mathias S, Basu S, Kolesnick R. Phosphorylation of Raf by ceramide-activated protein kinase. Nature. 1995;378:307–310. doi: 10.1038/378307a0. [DOI] [PubMed] [Google Scholar]
- 82.Zhu L, van-den-Heuvel S, Helin K, Fattaey A, Ewen M, Livingston D M, Dyson N, Harlow E. Inhibition of cell proliferation by p107, a relative of the retinoblastoma protein. Genes Dev. 1993;7:1111–1125. doi: 10.1101/gad.7.7a.1111. [DOI] [PubMed] [Google Scholar]
- 83.Zou X, Rudchenko S, Wong K-K, Calame K. Induction of c-myc transcription by the v-Abl tyrosine kinase requires Ras, Raf-1 and cyclin-dependent kinases. Genes Dev. 1997;11:654–662. doi: 10.1101/gad.11.5.654. [DOI] [PubMed] [Google Scholar]