Abstract
In response to various environmental stresses, eukaryotic cells down-regulate protein synthesis by phosphorylation of the α subunit of eukaryotic translation initiation factor 2 (eIF-2α). In mammals, the phosphorylation was shown to be carried out by eIF-2α kinases PKR and HRI. We report the identification and characterization of a cDNA from rat pancreatic islet cells that encodes a new related kinase, which we term pancreatic eIF-2α kinase, or PEK. In addition to a catalytic domain with sequence and structural features conserved among eIF-2α kinases, PEK contains a distinctive amino-terminal region 550 residues in length. Using recombinant PEK produced in Escherichia coli or Sf-9 insect cells, we demonstrate that PEK is autophosphorylated on both serine and threonine residues and that the recombinant enzyme can specifically phosphorylate eIF-2α on serine-51. Northern blot analyses indicate that PEK mRNA is expressed in all tissues examined, with highest levels in pancreas cells. Consistent with our mRNA assays, PEK activity was predominantly detected in pancreas and pancreatic islet cells. The regulatory role of PEK in protein synthesis was demonstrated both in vitro and in vivo. The addition of recombinant PEK to reticulocyte lysates caused a dose-dependent inhibition of translation. In the Saccharomyces model system, PEK functionally substituted for the endogenous yeast eIF-2α kinase, GCN2, by a process requiring the serine-51 phosphorylation site in eIF-2α. We also identified PEK homologs from both Caenorhabditis elegans and the puffer fish Fugu rubripes, suggesting that this eIF-2α kinase plays an important role in translational control from nematodes to mammals.
Mammalian protein synthesis is promptly adjusted in response to variety of different cellular stresses including nutrient starvation, iron deficiency, heat shock, and viral infection (27). One of the best-studied mechanisms regulating translation involves phosphorylation of the α subunit of eukaryotic initiation factor 2 (eIF-2α) (6, 7, 18, 39, 45). eIF-2 associates with initiator Met-tRNA and GTP and participates in the ribosomal selection of the start codon. During the process of translation initiation, GTP complexed with eIF-2 is hydrolyzed to GDP and eIF-2–GDP is released from the ribosomal machinery (28). To facilitate subsequent rounds of translation initiation, the GDP-bound eIF-2 has to be converted to active eIF-2–GTP. This guanine exchange reaction is carried out by eIF-2B. Phosphorylation of the α subunit of eIF-2 at residue serine-51 leads to inhibition of eIF-2B activity, reducing guanine nucleotide exchange and thus the rate of translation.
A family of protein kinases phosphorylate eIF-2α in response to different cellular stress conditions. While each member of the eIF-2α kinase family shares sequence and structural features distinguishable from those of other families of serine/threonine kinases, there is little similarity in their flanking regulatory regions, which facilitate the different stress signals controlling each eIF-2α kinase. Included in this family are two mammalian eIF-2α kinases: the double-stranded RNA (ds-RNA)-dependent kinase (PKR) (34) and the heme-regulated inhibitor kinase (HRI) (5). PKR participates in an antiviral defense mechanism that is mediated by interferon. It has been proposed that when cells are infected by viruses, ds-RNA that is synthesized during viral replication stimulates PKR activity by interacting with two ds-RNA-binding domains located in the amino terminus of this kinase (6, 26, 34). Phosphorylation of eIF-2α by PKR blocks total protein synthesis, preventing viral replication and infection of neighboring cells. Interestingly, RNA and protein products encoded by viruses have been shown to thwart the antiviral pathway by binding to PKR and inhibiting its kinase activity. Recent studies indicate that PKR also plays an important role in the regulation of cell growth and division and in the control of apoptosis (2, 22, 24, 25, 29, 42).
The second well-characterized mammalian eIF-2α kinase, HRI, is expressed in erythroid tissues and couples the synthesis of globin, the predominant protein product in these cells, to hemin and iron availability (5). In addition to being modulated by hemin, the activity of HRI is proposed to be modulated by association with heat shock proteins (5, 49). Another eIF-2α kinase that is regulated by hemin, PfPK4, was recently identified from the malarial parasite Plasmodium falciparum (30). PfPK4 is expressed during each stage of parasite development, and it is proposed that PfPK4 allows the parasite to sense its environment during the invasion process. Although PfPK4 and HRI are both inhibited by hemin, these two kinases do not have similar sequences flanking their kinase catalytic domains.
In contrast to mammalian kinases PKR and HRI, which inhibit global protein synthesis in response to stress signals, the eIF-2α kinase in Saccharomyces cerevisiae, GCN2, controls translation of a single species of mRNA encoding GCN4 (19, 45). GCN4 is a transcriptional activator of over 30 genes involved in amino acid biosynthesis. Control of GCN4 translation is mediated by four short upstream open reading frames (ORFs) located in the 5′-untranslated portion of the GCN4 mRNA. When cells are grown under conditions limiting for amino acids, the ORFs inhibit translation of the GCN4 coding region. In response to amino acid limitation, phosphorylation of eIF-2α by GCN2 kinase leads to reduced eIF-2–GTP levels that overcome the inhibitory effects of the ORFs, allowing for increased translation of GCN4 (1, 9, 19, 45). Activation of GCN2 kinase during starvation conditions involves sequences homologous to those of histidyl-tRNA synthetases, which bind uncharged tRNAs that accumulate when amino acids are limiting (46–48, 52). Recently, GCN2 kinase was characterized from Drosophila melanogaster (33, 41). Expression of Drosophila GCN2 is developmentally regulated and at later stages becomes restricted to the central nervous system. The physiological role of GCN2 kinase in Drosophila is currently unclear. Furthermore, it is not certain whether Drosophila GCN2 mediates total protein synthesis or controls gene-specific translation.
In the present study, we identified and characterized a new eIF-2α kinase from a rat pancreatic islet. Like the members of the eIF-2α kinase family, this new kinase, which we refer to as PEK, pancreatic eIF-2α kinase, phosphorylates the α subunit of eIF-2 at residue serine-51. While the kinase domain of PEK is similar to those of eIF-2α kinases, including the characteristic large insert between subdomains IV and V, its flanking 550-residue amino-terminal sequences are distinct. Our Northern analysis indicates that PEK is expressed in many different rat tissues, with the greatest levels in the pancreas. In agreement with pancreatic expression for this kinase, PEK was predominantly detected by an immunoprecipitation kinase assay of pancreas and pancreatic islet cells. PEK was found to function in translation regulation in both the yeast and reticulocyte lysate model systems. Results from these studies indicate that PEK is a new mammalian eIF-2α kinase important for mediating translational control.
MATERIALS AND METHODS
Isolation of cDNA clones encoding PEK.
cDNAs encoding proteins immunoreactive with antiphosphothreonine antibodies were isolated from a lambda Zap-Express library generated from rat pancreatic islet poly(A)-selected RNA. The library was screened with a picoBlue immunoscreening kit from Stratagene according to the manufacturer’s instructions. A total of 5 × 105 plaques were screened by infecting the XL1-Blue MRF′ bacterial strain with the phage library. Following incubation at 42°C for 4 to 5 h, plates were overlaid with filters presoaked with 10 mM isopropylthio-β-d-galactoside (IPTG) and incubated for an additional 3.5 h. Upon removal of the first membrane, a duplicate nitrocellulose membrane presoaked with 10 mM IPTG was overlaid, and the plates were incubated overnight at 37°C. The membranes were incubated with blocking solutions containing rabbit antiphosphothreonine antibody (Zymed), rinsed three times with washing solution, and treated with alkaline phosphatase conjugated to goat anti-rabbit secondary antibody (Zymed). Positive plaques were detected with Nitro Blue Tetrazolium and 5-bromo-4-chloro-3-indolylphosphate (Sigma). Following purification by two subsequent rounds of screening, the cDNA inserts from positive plaques were subcloned into plasmid pBK-CMV by in vivo excision from the lambda phages as described by Stratagene. Additional rounds of screening were carried out to isolate full-length cDNA clones by using an [α-32P]dCTP-labeled DNA insert from the subcloned plasmid as a probe to rescreen the library, according to a protocol recommended by Stratagene for plaque hybridization and purification.
Bacterial and baculoviral expression of PEK and eIF-2α.
PEK was expressed in Escherichia coli by using the T7 promoter system. A 3.4-kb EcoRI DNA fragment encoding the entire PEK cDNA was inserted into the EcoRI site of pET28a. An EcoRI site was engineered immediately 5′ to the predicted start codon of the PEK gene to facilitate direct subcloning of the cDNA without the 5′ untranslated region (UTR). The resulting plasmid, p259, contained the PEK ORF fused to an amino-terminal sequence containing a polyhistidine tag. To express a mutant version of PEK with residues 785 to 1108 deleted, a 2.4-kb EcoRI-to-HindIII fragment was inserted into the EcoRI-to-HindIII sites of pET28a, generating p260. The encoded PEK-Δ785-1108 was fused to amino terminal polyhistidine sequences in pET28a. Expression plasmid p259 or p260 or vector pET28a was introduced into E. coli BL21 (DE3) (F− ompT rB− mB−; containing lysogen DE3), and the strain was grown at 30°C in Luria-Bertani medium supplemented with 100 μg of ampicillin per ml until mid-logarithmic phase. Then, 1 mM IPTG was added to the culture, and the culture was incubated overnight at room temperature. The cell pellets were collected by centrifugation, washed, and then resuspended in solution A (20 mM Tris-HCl [pH 7.9], 500 mM NaCl, 10% glycerol, 1 mM β-mercaptoethanol, 0.1% Triton X-100, 1 μM pepstatin A, 1 μM leupeptin, 0.15 μM aprotinin, 0.1 mM phenylmethylsulfonyl fluoride [PMSF]) with 5 mM imidazole and lysed with a French press. Lysates were clarified by centrifugation at 39,000 × g and subjected to an immunoblot assay using a polyclonal antibody against the polyhistidine tag (Pierce) or used in kinase reaction mixtures containing the eIF-2α substrate. No immunoreactive protein was detected in the lysate prepared from E. coli containing only vector pET28a. To purify the PEK fusion proteins, the clarified lysates were loaded onto a column containing nickel chelation resin (Qiagen) that binds to the polyhistidine tag of the fusion proteins, and PEK was partially purified by elution with solution A containing 200 mM imidazole.
Expression of PEK in TOP10 E. coli cells (Invitrogen) was carried out by using plasmid pBK-RK3, which was obtained by in vivo excision from the lambda phages from the antiphosphothreonine screen. Plasmid pBK-RK3 carries the full-length coding region for PEK along with 150 bp of 5′-untranslated sequence subcloned into the EcoRI and XhoI sites of expression vector pBK-CMV (Stratagene). Expression of PEK was driven by the lac promoter induced by IPTG. In addition to the coding region, both the 5′-untranslated sequences and part of the polylinker sequences upstream from the EcoRI site were included in the recombinant PEK gene. E. coli cells expressing PEK were collected by centrifugation, washed, and resuspended in a solution of 20 mM Tris-HCl (pH 7.9), 50 mM NaCl, and 10 mM MgCl2 and lysed with a French press. Lysates were clarified by centrifugation at 10,000 × g.
For baculoviral expression of PEK in Sf-9 cells, a 4.5-kb DNA fragment containing the entire coding region and a portion of the 5′-UTR of the PEK cDNA was subcloned from plasmid pBK-RK3 into the EcoRI and XhoI sites of the baculoviral expression vector pFastBac (Gibco-BRL). The selection of recombinant virus and the expression of PEK in Sf-9 cells were carried out according to a protocol provided by Gibco-BRL. Sf-9 cells expressing PEK were resuspended in cell lysis buffer (10 mM HEPES [pH 7.4], 1 mM EGTA, 1 mM MgCl2, 1 mM 2-aminoethylisothiouronium bromide, 1% Triton X-114, 1× Complete protease inhibitor cocktail [Boehringer Mannheim, Indianapolis, Ind.]), followed by centrifugation at 10,000 × g for 10 min to eliminate insoluble material. Human eIF-2α was similarly expressed in Sf-9 cells. The coding region of human eIF-2α was amplified by PCR with anchored primers and Marathon Ready human testis cDNAs (Clontech). Primers GCTAGAGCTCATGCCGGGTCTAAGTTGTAGATT and AGTCGAATTCAAATTGGACTCTGTTTCCCACAA contained a XhoI site or an EcoRI site to facilitate direct cloning into the respective sites in expression vector pTrcHis A (Invitrogen), generating plasmid pTrcHis-hIF2α. The human eIF-2α cDNA sequences were removed from plasmid pTrcHis-hIF2α and inserted between the BamHI and HindIII sites of the baculoviral expression vector pFastBacHTb (Gibco-BRL). The eIF-2α with six fused histidines at the N terminus was expressed in Sf-9 cells, and the recombinant protein was purified with a ProBond column (Invitrogen) containing nickel chelation resin. The column was washed, and human eIF-2α was eluted with native wash buffer containing 200 mM imidazole. Combined fractions containing the fusion protein were concentrated and desalted on a Centricon concentrator (Amicon) with 10,000-molecular-weight cutoff, washed once with kinase buffer (20 mM HEPES [pH 7.5], 50 mM NaCl, 10% [vol/vol] glycerol), and stored at −80°C. Protein concentrations were determined with bicinchoninic acid protein assay reagents from Pierce.
Yeast eIF-2α used in the in vitro kinase assays was a modified form lacking residues 200 to 304 and containing polyhistidine sequences for rapid purification (52). Deletion of the carboxy-terminal residues of eIF-2α removed phosphorylation sites for casein kinase II (12). Modified versions of yeast eIF-2α possessing or lacking the serine-51 phosphorylation site were expressed and purified from E. coli as previously described (52).
In vitro kinase assays.
The activity of recombinant rat PEK from Sf-9 cell lysate was assessed in immune-complex kinase assays using recombinant eIF-2α as a substrate. The supernatants were precleared with protein A-Sepharose, followed by immunoprecipitation with the polyclonal anti-PEK peptide antibody (PITK-289) at 4°C for 90 min. The PITK-289 antibody was developed by immunizing rabbits with synthetic peptides (ENAVFENLEFPGKTVLRQRS) derived from the C-terminal sequence of rat PEK. After incubation with protein A-Sepharose at 4°C for 1 h with rocking, the immune complexes were rinsed twice with wash buffer (10 mM HEPES [pH 7.4], 10 mM benzamidine, 150 mM NaCl, 0.5 mM methionine, 0.1 mg/ml bovine serum albumin [BSA], 5 mM EDTA) and twice with kinase buffer supplemented with 100 μM PMSF, 0.1 mM ATP, and 1 mM dithiothreitol. The kinase assay using human eIF-2α was carried out by the addition of 30 μl of reaction mixture containing 1, 2, or 4 μg of purified human eIF-2α and 20 μCi of [γ-32P]ATP in a final concentration of 0.1 mM ATP to the bead-bound PEK. After the reaction mixtures were incubated at 37°C for 30 min, the assays were terminated by boiling the mixtures with an equal volume of 2× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer for 3 min, followed by characterization by SDS-PAGE. The gels were dried and subjected to autoradiography at −70°C.
The in vitro kinase assays using recombinant yeast eIF-2α substrate were carried out as described previously (52). In a final reaction volume of 25 μl, 2 μg of recombinant PEK or PEK-Δ785-1108 was added, along with 1 μg of yeast eIF-2α and 10 μCi of [γ-32P]ATP in a final concentration of 60 μM ATP. After incubation for 6 min at 30°C, the phosphorylated proteins were analyzed by electrophoresis in an SDS–12.5% polyacrylamide gel, followed by autoradiography. Phosphoamino acid analysis of PEK radiolabeled by in vitro autophosphorylation was carried out by first transferring 32P-labeled PEK from the SDS-polyacrylamide gel to an Immobilon-P membrane (Millipore). The portion of the membrane containing PEK was excised, and the protein was hydrolyzed in 5.7 N HCl at 110°C for 60 min. Hydrolyzed samples were applied to cellulose-coated sheets (Kodak) and separated by one-dimensional thin-layer electrophoresis as described previously (53). Radiolabeled amino acids were detected by autoradiography.
Northern blot analysis.
A Northern blot containing 2 μg of poly(A)+ RNA from different rat tissues per lane was purchased from Clontech. To measure PEK mRNA levels in pancreas cells, which were not included in the multiple-tissue Northern blot, a separate blot was prepared with mRNA from pancreas, skeletal muscle, kidney, and testis cells. DNA probes for PEK and the internal controls, which include β-actin, α-tubulin, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), were radiolabeled with [α-32P]dCTP by random prime labeling with a kit from Gibco-BRL. Hybridization was carried out in 2× SSC (1× SSC is 0.15 M NaCl and 0.015 M sodium citrate) with 0.5% SDS, 0.1% BSA, 0.1% polyvinylpyrolidone, 0.1% Ficoll, 100 μg of heparin per ml, and 1 mM EDTA at 60°C overnight, followed by three washings at 60°C in 2× SSC buffer with 0.1% SDS. The relative levels of PEK and control mRNAs were measured with a Molecular Dynamics PhosphorImager.
Preparation of tissue lysates.
Tissues were freshly isolated from 8-week-old male Sprague-Dawley rats and were immediately frozen in liquid nitrogen. Frozen tissues were ground to a fine powder in liquid nitrogen and then resuspended in ice-cold lysis buffer containing 50 mM HEPES (pH 7.4), 2 mM EDTA, 1% Triton X-100, 10% glycerol, 10 mM NaF, 150 mM NaCl, and inhibitors (2 mM Na3VO4, 5 μg of leupeptin per ml, 1.5 mg of benzamidine per ml, 0.5 mg of pepstatin A per ml, 2 μg of aprotinin per ml, 1 mM PMSF, 10 μg of antipain per ml) at a final concentration of 100 mg of tissue/ml of buffer. The tissues were homogenized with a polytron for 30 s, and the homogenate was incubated on ice for 30 min, followed by centrifugation for 20 min at 10,000 × g. The supernatants were aliquotted and stored at −80°C. Isolated canine islets were lysed in cell lysis buffer (10 mM HEPES [pH 7.4], 1 mM EGTA, 1 mM MgCl2, 1 mM 2-aminoethylisothiouronium bromide, 1× Complete medium (Boehringer Mannheim) and precleared by centrifugation for 10 min at 10,000 × g. Immunoprecipitation kinase assays were carried out with human or yeast eIF-2α substrate as described above. As a control, similar assays were carried out with preimmune serum in the immunoprecipitations.
Expression of PEK in yeast.
To express PEK in yeast, a 3.5-kb SacI-to-XhoI DNA fragment containing the PEK cDNA was removed from pBK-RK3 and inserted between the SacI and SalI sites of yeast expression vector pEMBLyex4 (4), generating plasmid p504. p504 is a URA3-marked high-copy-number plasmid that contains the PEK cDNA downstream of the galactose-inducible GAL-CYC1 hybrid promoter. Plasmids p504, pEMBLyex4, and pC102-2 (47) encoding GCN2 were transformed into yeast strains H1894 (MATa ura3-52 leu2-3 leu 2-112 trp1 Δgcn2), H1816 (MATa ura3-52 leu2-3 leu2-112 Δgcn2 Δsui2 GCN4-lacZ p1097 [SUI2 LEU2]), and H1817 (MATa ura3-52 leu2-3 leu2-112 Δgcn2 Δsui2 GCN4-lacZ p1098 [SUI2S51A LEU2]) (19). Strains H1817 and H1816 are isogenic and differ only in their SUI2 alleles, which encode eIF-2α. Plasmid-containing strains were selected for by uracil prototrophy. Yeast transformants were grown in patches on agar plates containing synthetic medium supplemented with 10% galactose–2% raffinose (SGal) (21), 2 mM leucine, and 1 mM tryptophan. After the plates were incubated for 1 day at 30°C, cell patches were replica printed onto agar plates containing SGal medium supplemented with leucine, tryptophan, 0.5 μg of sulfometuron methyl (SM) per ml or 30 mM 3-aminotriazole (3-AT) (48), and all amino acids except histidine. Agar plates were incubated for the indicated times at 30°C and photographed.
Protein synthesis in rabbit reticulate lysate.
In vitro translation assays were carried out in a 20-μl reaction volume containing 50% untreated rabbit reticulocyte lysate (Promega), 20 mM HEPES-KOH (pH 6.8), 20 mM KCl, 1 mM Mg(OAc)2, 10 mM creatine phosphate, 50 μg of creatine phosphokinase per ml, 0.8 mM ATP, 0.2 mM GTP, a 25 μM concentration of each amino acid except methionine, and 1 μCi of [35S]methionine (1,200 Ci/mmol). Full-length PEK and PEK-Δ785-1008 were purified by using their amino-terminal polyhistidine sequences and nickel chelation resin. The partially purified PEK and the mutant were added to the in vitro translation reaction mixtures at the indicated concentrations, and the reaction mixtures were incubated at 30°C for 30 min. Proteins from 5-μl aliquots were precipitated with trichloroacetic acid, and the incorporation of 35S was quantified by scintillation counting.
Nucleotide sequence accession number.
The nucleotide sequences determined in this study have been submitted to the GenBank/EMBL data bank under accession no. AF096835.
RESULTS
Isolation of cDNA encoding new protein kinase from rat pancreatic islet cells.
In an effort to identify new threonine kinases in pancreatic tissue, we used antiphosphothreonine antibodies to screen a lambda Zap-Express cDNA library prepared from mRNA isolated from rat pancreatic islets. Since there was no detectable threonine kinase activity in the host strain, E. coli XL1-Blue MRF′, on the basis of the antiphosphothreonine antibodies, it was expected that any positive signal would come from the expression of introduced cDNA clones. After screening 5 × 105 recombinant plaques, we identified distinct clones encoding fusion proteins which reacted with the polyclonal antiphosphothreonine antibodies. The cDNA inserts were subcloned into plasmid pBK-CMV by in vivo excision from the lambda phages and characterized by sequence analysis. While the majority of the inserts encoded known threonine kinases, such as those encoded by lyn and pim-1, one cDNA clone with a 4.5-kb insert that did not match any previously characterized protein kinase coding sequence entered in the GenBank/EMBL database was identified. As will be described below, the corresponding new pancreatic kinase is related to the eIF-2α kinase family, and we will refer to it as pancreatic eIF-2α kinase, or PEK.
To verify that the cDNA contained the entire coding region for PEK, a probe derived from the cDNA insert was radiolabeled and used in a screen for additional clones from the same lambda library. A total of 2 × 106 recombinant plaques were screened, resulting in the identification of more than 20 positive clones. Sequence analyses confirmed that the majority of the clones carried the 4.5-kb insert. These clones differed in length by 10 to 20 bp at the 5′ end of the cDNA insert. Multiple rounds of rapid amplification of cDNA ends using cDNA prepared from rat pancreas, testis, and ovary cells yielded no additional sequences 5′ to the isolated PEK cDNA. The entire 4,526-nucleotide sequence of the PEK cDNA contains an ORF encoding a 1,108-residue polypeptide with a predicted molecular weight of 125,000 (Fig. 1). The sequences flanking the predicted start codon (underlined) (GCCGCTGATGG) match the consensus GCCA/GCCATGG described for translation initiation sites (23). The 5′-UTR contained in the PEK cDNA is 212 bp in length and contains termination codons in each of the three reading frames, indicating that the entire ORF was included in the isolated cDNA clones. The 3′-UTR spans 990 bp and includes a putative polyadenylation signal and a poly(A) tract.
FIG. 1.
The predicted sequence of pancreatic eIF-2α kinase, PEK. PEK is 1,108 residues in length. The kinase catalytic sequences are underlined. A predicted signal sequence and a hydrophobic region are boxed, and possible amino-terminal myristoylation sites are in boldface.
New pancreatic protein kinase is related to eIF-2α kinases.
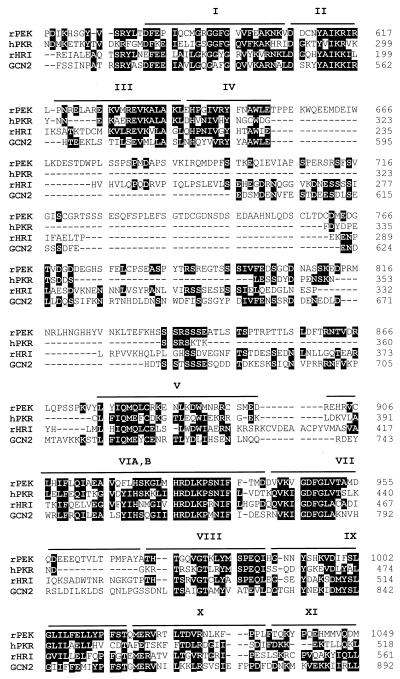
The catalytic domain of PEK spans 540 amino acid residues and contains sequences corresponding to those of the 12 subdomains described by Hanks and Hunter (17) (Fig. 1 and 2). By using the sequence of PEK as a query in a BLAST search of the GenBank/EMBL database, we found that it is most closely related to a family of protein kinases that regulate protein synthesis by phosphorylation of eIF-2α. The sum probability of random correspondence of the pairwise segments with the pancreatic kinase (i.e., the BLAST score) ranged from 6.0e−42 and 4.3e−40 with rat HRI and human PKR, respectively, to 2.4e−25 with yeast GCN2. A multiple alignment of the catalytic domain of PEK and the eIF-2α kinases was generated with the program Pileup and is illustrated in Fig. 2. In this alignment PEK is 31% identical to HRI, 31% identical to PKR, and 24% identical to GCN2. A distinguishing sequence proposed to be important for eIF-2α kinase function is LFIQME(Y/F)C(D/E), which is present in subdomain V of PEK (Fig. 2). Additionally, 11 residue positions dispersed among the catalytic domains of the eIF-2α kinases were observed to be conserved among the family members but absent in the majority of other protein kinases (36). PEK contains the same residues at 10 of these positions. The position that is the sole exception is also not conserved in the rat HRI kinase. A final feature shared among the eIF-2α kinases is an insert between subdomains IV and V (36, 45). The inserts of the different eIF-2α kinases are quite variable in sequence and in length, ranging from 35 residues in PKR to 550 residues in PfPK4 from P. falciparum. For PEK, this insert is 220 residues long and has only weak sequence identity to other members of the eIF-2α kinase family (Fig. 2). Together, the above-described sequence and structural similarities are highly suggestive that the pancreatic kinase is a new member of the eIF-2α kinase family.
FIG. 2.
The sequences of PEK are related to eIF-2α kinases. Shown is a sequence alignment of the kinase catalytic domains of rat PEK, human PKR, rat HRI, and yeast GCN2 generated by the program Pileup. Identical amino acid residues of the eIF-2α kinases are designated by black boxes, and gaps in the alignment are indicated by dashes. Kinase subdomains are highlighted by bars above the multisequence alignment.
While the sequences of the catalytic domain of PEK are similar to those of the eIF-2α kinases, the 550-residue amino-terminal segment of the new kinase is quite different, presumably reflecting the different physiological signals regulating its activity. Furthermore, there does not appear to be any significant similarity between the amino-terminal region of the new kinase and those of characterized proteins encoded by sequences in the GenBank/EMBL database. However, we did find uncharacterized sequences from other organisms that encode presumed PEK homologs. Multiple expressed sequence tags from mice and humans that closely matched portions of rat PEK were identified. The only gene that has significant homology to that encoding PEK was an uncharacterized gene identified recently from Caenorhabditis elegans. The C. elegans sequences were found in two overlapping cosmid inserts with accession no. Z66563 and Z68104. The predicted C. elegans polypeptide is 1,085 residues in length and is 29% identical over 965 residues to rat PEK. In contrast to the homology between PEK and the eIF-2α kinases, which is restricted to the catalytic domain, the homology with the C. elegans sequences extends over the entire length of these proteins. Additionally, there are genomic sequence entries from puffer fish Fugu rubripes encoding deduced polypeptide sequences that are over 80% identical to portions of rat PEK.
The properties of PEK were also assessed with the Wisconsin Genetics Computer Group software package. A hydropathy plot of the protein sequence indicates that PEK is composed of densely populated hydrophilic regions. Located at the amino terminus between residues 6 and 45 is a sequence predicted with 99.5% probability to be a transmembrane region or a leader sequence for secretion (Fig. 1). Two consensus N-myristoylation sites are localized within the signal sequences at residues 20 and 44. The pancreatic kinase also contains another highly hydrophobic region from residues 517 to 532 with the potential to function as a transmembrane region (Fig. 1).
PEK phosphorylates eIF-2α on residue serine-51.
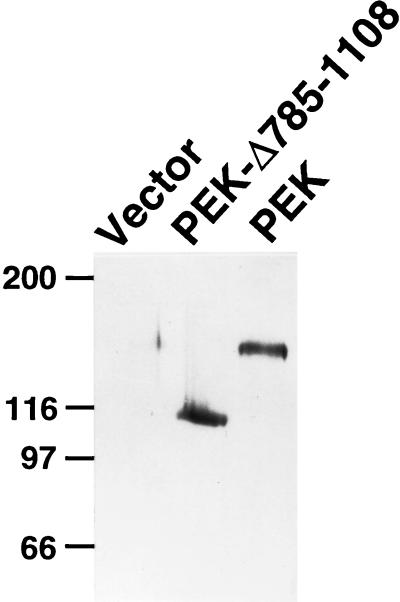
To verify the predicted size of PEK and address whether it phosphorylates eIF-2α, we expressed this protein kinase in E. coli and in Sf-9 cells. PEK sequences fused to an amino-terminal polyhistidine tag were expressed in E. coli by using the T7 promoter system. The recombinant protein was visualized by immunoblotting with a polyclonal antibody that recognizes the polyhistidine sequences (Fig. 3). This recombinant PEK had a molecular weight of 140,000, which is larger than the 128,000 molecular weight predicted for the fusion protein. This protein was absent in lysates prepared from E. coli containing only the parent vector, pET28a (Fig. 3). The reason why the recombinant PEK migrated more slowly by SDS-PAGE than the predicted molecular weight does not appear to be possible self-phosphorylation, because a similar preparation of a truncated version of PEK, lacking 324 residues in its carboxy catalytic domain, also revealed a higher molecular weight, calculated by SDS-PAGE, than that predicted on the basis of the deduced coding sequences (Fig. 3). Furthermore, we also expressed PEK in E. coli fused to a more-extended amino-terminal sequence and in Sf-9 cells by using the baculovirus expression system. In both cases, using immunoblot analysis and an antiphosphothreonine antibody, we detected recombinant PEK with a larger molecular weight than that predicted on the basis of the DNA sequence. No PEK protein was detected in lysates prepared from E. coli or Sf-9 cells containing the parent vector or expressing a heterologous control protein. It is noted that expression of PEK in the Sf-9 insect cells yielded very little protein, possibly due to the toxic effect of the expressed kinase or due to its instability.
FIG. 3.
Expression of PEK in E. coli as measured by immunoblotting. PEK or PEK-Δ785-1108 fused to amino-terminal polyhistidine tags was expressed in E. coli by using the T7 promoter system, as described in Materials and Methods. Equal amounts of total cell lysates were analyzed by SDS-PAGE, and PEK was detected by immunoblotting with a polyclonal antibody that recognizes the polyhistidine sequences. Vector, lysates prepared from E. coli containing only the parent vector, pET28a. Molecular weight markers, in kilodaltons, are on the left.
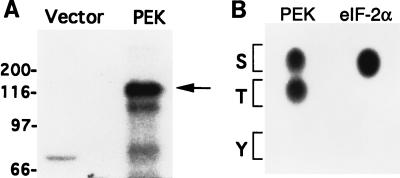
Our immunoblot analysis using the antiphosphothreonine antibodies suggested that PEK is autophosphorylated at a threonine residue(s) (data not shown). Previous studies indicate that autophosphorylation is essential for activation of the eIF-2α kinases (5, 34, 37, 39, 43). To directly test whether PEK is autophosphorylated on threonine residues, we incubated extracts from E. coli expressing PEK with [γ-32P]ATP and characterized the reaction mixture by SDS-PAGE. As shown in the autoradiogram in Fig. 4A, full-length PEK was radiolabeled and no phosphoprotein of this size was detected in a similar reaction mixture prepared from E. coli cells containing only the expression vector. Phosphoamino acid analysis of this radiolabeled PEK indicated that both threonine and serine residues were autophosphorylated (Fig. 4B).
FIG. 4.
PEK in an in vitro kinase assay is autophosphorylated on both serine and threonine residues. (A) Lysates prepared from E. coli expressing PEK or vector alone were incubated with [γ-32P]ATP and analyzed by SDS-PAGE, followed by autoradiography. The radiolabeled PEK is indicated by an arrow on the right. Sizes of protein standards in kilodaltons are on the left. (B) Phosphoamino acid analysis of 32P-labeled PEK from the in vitro assay. Radiolabeled PEK was hydrolyzed with HCl, applied to cellulose-coated sheets, and separated by one-dimensional thin-layer electrophoresis. In parallel, eIF-2α that was phosphorylated by PEK in an in vitro assay was similarly analyzed. The 32P-labeled amino acids were detected by autoradiography. The positions of serine, threonine, and tyrosine are indicated by one-letter codes.
Given the homology of PEK with members of the eIF-2α kinase family, we wished to determine whether the pancreatic kinase phosphorylates eIF-2α. The pancreatic kinase was immunoprecipitated from Sf-9 cell lysates with antiserum prepared against a synthetic peptide corresponding to the carboxy terminus of PEK, and the immunocomplex was incubated with [γ-32P]ATP and increasing concentrations of human eIF-2α. The eIF-2α substrate was phosphorylated in the reaction mixtures containing PEK but was not radiolabeled in reaction mixtures containing similarly prepared immunoprecipitates derived from Sf-9 cells expressing an unrelated bacterial protein (Fig. 5).
FIG. 5.
Immunoprecipitated PEK phosphorylates human eIF-2α. PEK or an unrelated bacterial protein was expressed in Sf-9 cells and the PEK was immunoprecipitated with polyclonal antisera prepared against a synthetic peptide derived from the carboxy terminus of the kinase. Immunocomplexes prepared from lysates expressing an unrelated bacterial protein (lanes 1 to 3) or PEK (lanes 4 to 6) were incubated with [γ-32P]ATP and increasing amounts of human eIF-2α as described in Materials and Methods. Radiolabeled proteins were separated by electrophoresis with an SDS-polyacrylamide gel and visualized by autoradiography. Kinase assay mixtures contained 1 (lane 1 and 4), 2 (lanes 2 and 5), or 4 (lanes 3 and 6) μg of purified human eIF-2α protein. The arrowhead indicates the position of the phosphorylated eIF-2α.
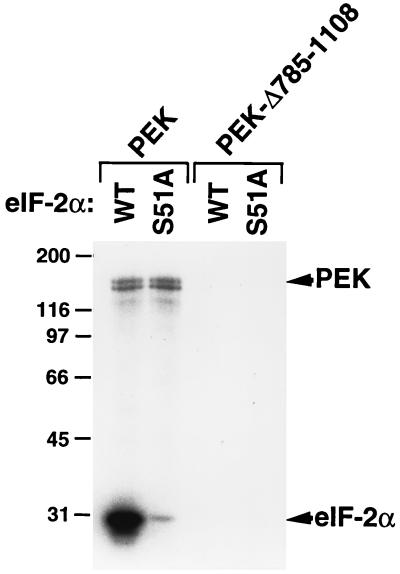
To address whether PEK phosphorylates eIF-2α on serine-51, we utilized modified yeast eIF-2α substrates. The eIF-2α is highly conserved from yeast to mammals, and yeast eIF-2α was previously shown to be a substrate for mammalian eIF-2α kinases in both in vitro and in vivo assays (8, 38, 51, 52). Yeast eIF-2α substrates containing wild-type serine-51 or alanine substituted for serine at this phosphorylation site (S51A) were included in kinase reaction mixtures containing PEK partially purified from E. coli (Fig. 6). We detected phosphorylation of both PEK and the recombinant eIF-2α substrate. As expected, only serine was found to be phosphorylated when we analyzed the radiolabeled eIF-2α by phosphoamino acid analysis (Fig. 4B). Phosphorylation was greatly reduced in the assay mixture containing the mutant substrate, eIF-2α–S51A (Fig. 6). As a control, we carried out kinase reactions with mixtures containing PEK-Δ785-1108 and found that the mutant PEK did not phosphorylate itself or eIF-2α. We conclude that PEK can specifically phosphorylate eIF-2α at residue serine-51. Furthermore, we added poly(I)·poly(C) or heparin, both of which are known in vitro activators of PKR, to the kinase reaction mixtures and found no changes in the levels of eIF-2α phosphorylation by PEK (data not shown). These activators are mediated through interaction with the amino-terminal sequences of PKR, and the inability of these ligands to alter PEK activity is consistent with there being no apparent homology between the regulatory region of PKR and that of PEK.
FIG. 6.
PEK phosphorylation of eIF-2α is dependent on residue serine-51. Full-length PEK and truncated PEK-Δ785-1108 were partially purified and added to kinase reaction mixtures containing [γ-32P]ATP and a modified form of yeast eIF-2 containing wild-type serine-51 (WT) or alanine substituted for serine at this phosphorylation site (S51A). Radiolabeled proteins were analyzed by SDS-PAGE, followed by autoradiography. The reason full-length PEK is a doublet appears to be in vitro proteolysis. In other preparations, as determined by autophosphorylation or immunoblotting, a single species of PEK was detected. Phosphorylated PEK and eIF-2α are indicated to the right. The sizes of protein standards in kilodaltons are on the left.
PEK is expressed in many different rat tissues, with the greatest level in the pancreas.
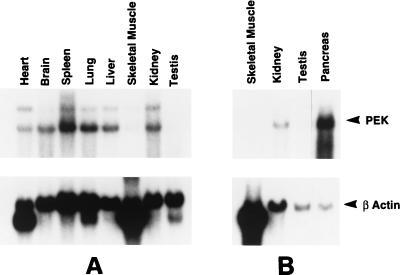
The expression of PEK mRNA in various rat tissues was examined by Northern blot analysis of poly(A)+ RNA using a cDNA probe containing the entire coding region of PEK. A major 5.2-kb transcript was detected in all tissues examined (Fig. 7). A higher-molecular-weight band of lower intensity was also detected in RNA samples from some of the tissues. This larger transcript was not reproducibly seen in our Northern blot analyses using different RNA preparations, suggesting an unspliced variant rather than different isoforms of PEK. PEK mRNA was readily detected in rat liver, kidney, spleen, brain, lung, and heart cells. Much lower levels of PEK mRNA were detected in testis and skeletal muscle cells. Given that the PEK cDNA was originally isolated from a pancreatic islet library, we carried out a separate Northern blot analysis using poly(A)+ RNA derived from rat pancreas cells. We found that the PEK transcript was greatly elevated in pancreas cells, with about 10 times the levels found in kidney cells. To ensure that similar amounts of RNA from each kind of tissue were used in the Northern analyses, we carried out a similar experiment using a DNA probe encoding β-actin. While β-actin mRNA levels were similar among most of the different tissue samples, the level was actually reduced in pancreas cells. As expected, the mRNA level of β-actin was elevated in skeletal muscle cells. We also carried out similar Northern analyses using DNA probes encoding frequently used standards α-tubulin and GAPDH and again detected lower mRNA levels in pancreas cells (data not shown). Based on these studies, we conclude that PEK mRNA is expressed at the highest level in the pancreas. The PEK mRNA level was 10-fold higher than those seen in other tissues, and, if the level was adjusted for differences in the amounts of RNA loaded between lanes by using the β-actin standard, this relative difference would be even greater.
FIG. 7.
Northern blot analysis of tissue distributions of the PEK mRNA. A Northern blot containing 2 μg of poly(A)+ RNA purified from the different rat tissues indicated (A) and a separate blot containing ∼2 μg of mRNA from rat skeletal muscle, kidney, testis, and pancreas tissue (B) were hybridized with an α-32P-labeled cDNA probe encoding PEK. Following autoradiography to visualize the ∼5.2-kb PEK mRNA (top panel), the membranes were rehybridized with a radiolabeled rat β-actin probe, and the resulting autoradiogram is shown in the bottom panel. The PEK and β-actin mRNAs are indicated by arrowheads.
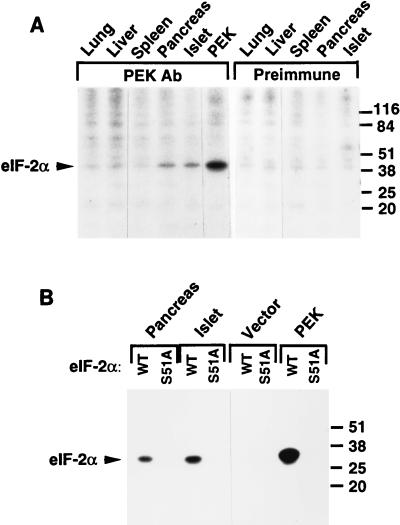
We next wished to identify PEK protein in rat tissues and correlate the levels of the kinase and PEK mRNA among the different cell types. Unfortunately, our polyclonal antibody prepared against the PEK carboxy terminus was not effective in an immunoblot assay, with minimal detection, even using recombinant PEK. As described earlier, the PEK antibody was effective in immunoprecipitation kinase reactions (Fig. 5). We identified eIF-2α kinase activity in PEK immunoprecipitates prepared from Sf-9 cells producing PEK that was absent in cells overexpressing an unrelated protein or vector alone (Fig. 5 and 8). We carried out similar PEK immunoprecipitation assays using cellular extracts prepared from different rat tissues, including those from the lung, liver, spleen, and pancreas, and canine pancreatic islets. Among all the tissues examined, eIF-2α kinase activities were reproducibly detected in lysates prepared from rat pancreas and canine islet cells (Fig. 8). By comparison, we did not detect human eIF-2α phosphorylation by using immunoprecipitates prepared from the tissue lysates with preimmune serum (Fig. 8A). Minimal eIF-2α kinase activity was detected in the immunoprecipitates from lung, liver, and spleen (Fig. 8A). To address the specificity for serine-51, we carried out the PEK immunoprecipitation kinase assays using yeast recombinant eIF-2α as the substrate. While immunoprecipitated PEK from pancreas cells or islets phosphorylated eIF-2α, no phosphorylation was detected with the eIF-2α–S51A mutant substrate (Fig. 8B). Together, these studies indicate that PEK is present in pancreas tissue, a tissue found to contain the highest levels of PEK mRNA, and in islets, the cells from which the cDNA encoding PEK was initially identified.
FIG. 8.
PEK immunoprecipitated from mammalian tissues phosphorylates eIF-2α at serine-51. (A) PEK was immunoprecipitated from the indicated tissues by using polyclonal PEK antibody and incubated with [γ-32P]ATP and human eIF-2α as described in Materials and Methods. As a control, a similar immunoprecipitation assay was carried out with preimmune serum. Radiolabeled proteins were separated by SDS-PAGE and were visualized by autoradiography. The arrowhead indicates the position of phosphorylated eIF-2α. All tissues used in this study are from rats, with the exception of islets, which were isolated from dogs. (B) PEK was immunoprecipitated from pancreas, islets, and Sf-9 cells. As a control, similar immunoprecipitations were carried out with lysates prepared from Sf-9 insect cells containing only the vector. Immunoprecipitates were added to kinase reaction mixtures containing [γ-32P]ATP and a modified form of yeast eIF-2 containing wild-type serine-51 (WT) or alanine substituted for serine at this phosphorylation site (S51A). Radiolabeled proteins were analyzed by SDS-PAGE, followed by autoradiography. Sizes of protein standards in kilodaltons on the right sides of both panels.
PEK mediates translational control in yeast and reticulocyte model system.
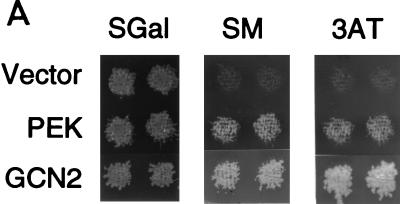
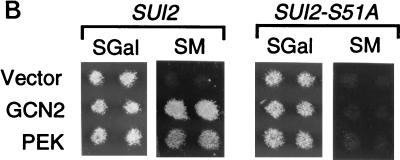
Yeast is a useful model system for studying the in vivo role of eIF-2α kinases in translational control. Several previous studies have shown that the expression of mammalian PKR or HRI or Drosophila GCN2 kinase in yeast can complement a deletion of the endogenous yeast eIF-2α kinase encoded by GCN2 (8, 33, 38, 51). GCN2 kinase in yeast participates in the general amino acid control response, and deletion of the GCN2 gene renders the cell hypersensitive to chemical inhibitors of amino acid biosynthesis, such as 3-AT and SM (19, 48). To address whether PEK can functionally substitute for the GCN2 kinase in strain H1894 (Δgcn2), we expressed PEK from a high-copy-number expression vector by using a galactose-inducible promoter. Yeast cells expressing PEK were compared to cells containing plasmid-encoded yeast GCN2 or only expression vector pEMBLyex4. While all three versions of H1894 grew on galactose-inducing medium, only the GCN2- and PEK-expressing cells were growth resistant to the same medium supplemented with 3-AT or SM (Fig. 9A). Minimal growth of cells containing only the expression vector was detected in the presence of either chemical inhibitor. This experiment indicates that PEK can function as an eIF-2α kinase in the yeast model system and replace GCN2 activity in this translational control pathway.
FIG. 9.
PEK functionally substitutes for the eIF-2α kinase GCN2 in a yeast model system. (A) Strain H1894 (Δgcn2) was transformed with plasmid pC102-2, encoding GCN2 kinase (GCN2), p504, expressing PEK from a galactose-inducible promoter (PEK), or only vector pEBMLyex4 (Vector). Patches of transformed cells were replica printed onto agar plates containing SGal, SGal supplemented with 3-AT, or SGal supplemented with SM. GCN2-deficient strains are hypersensitive to the amino acid inhibitors 3-AT and SM. Replicated plates were grown for 4 days at 30°C and photographed. (B) PEK was similarly expressed in strains H1816 (Δgcn2 SUI2) and H1817 (Δgcn2 SUI2-S51A), which are related to H1894, and cells were replica printed onto SGal or galactose-inducing medium containing SM. Strain H1817 contains a mutant version of eIF-2α that has an alanine substituted for the phosphorylated residue serine-51. While expression of either GCN2 or PEK in H1816 facilitates growth of this strain in SGal medium containing SM, neither eIF-2α kinase mediated growth when expressed in H1817 cells.
To determine whether the translational control mediated by PEK was dependent on the phosphorylation residue, serine-51, we expressed PEK by using the galactose-inducible promoter in two isogenic strains, H1816 (Δgcn2 SUI2) and H1817 (Δgcn2 SUI2-S51A). The SUI2 gene encodes eIF-2α, and strain H1817 contains an alanine residue substituted for serine-51. While yeast strain H1816 expressing either PEK or GCN2 grew in the galactose-containing medium supplemented with SM, the H1817 cells expressing either eIF-2α kinase failed to grow in the presence of this chemical inhibitor (Fig. 9B). We conclude that the translational control mediated by PEK in yeast cells depends upon the presence of serine-51 of eIF-2α.
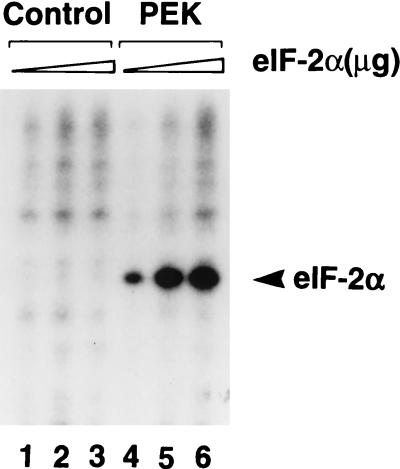
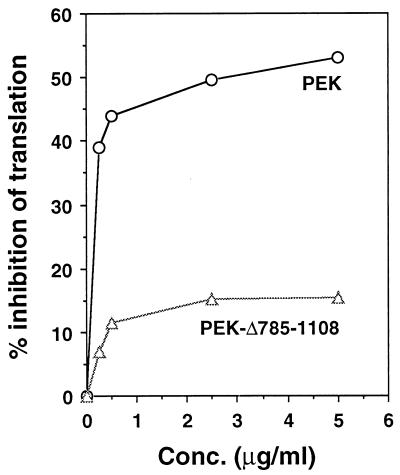
The eIF-2α kinases function to reduce general protein synthesis in mammalian cells in response to cellular stress. To address whether PEK can alter mammalian protein synthesis, we added partially purified recombinant PEK to rabbit reticulocyte lysate and measured [35S]methionine incorporation into proteins synthesized from endogenous mRNA templates. As a control we carried out similar measurements with purified recombinant PEK from which residues 785 to 1108 had been deleted. The deletions removed the carboxy-terminal portion of the catalytic domain and eliminated eIF-2α kinase activity in our in vitro assays (Fig. 6). The addition of the purified PEK to the cell-free system resulted in a dose-dependent reduction in protein synthesis, as measured by the incorporation of [35S]methionine into newly synthesized proteins. The inhibition was detected at low levels of added PEK, and with increasing concentrations of the kinase, there was more than a 50% reduction in protein synthesis (Fig. 10). We also carried out a similar experiment using an activated version of human PKR purified from yeast (51) and found a 60% reduction in the incorporation of [35S]methionine (data not shown). In contrast, the addition of the catalytically inactive PEK-Δ785-1108 caused only a modest reduction in protein synthesis in the reticulocyte lysates. Results from these studies suggest that PEK can function to reduce mammalian translation.
FIG. 10.
Addition of recombinant PEK to reticulocyte lysates reduced protein synthesis. Partially purified full-length PEK or truncated PEK-Δ785-1108 was added at the indicated concentrations to reticulocyte lysates. [35S]methionine was added to the cell-free translation system concomitant with recombinant PEK, and synthesized polypeptides were precipitated with tricarboxylic acid. The incorporation of [35S]methionine into the protein samples was measured by scintillation counting. The effect of PEK on protein synthesis in the cell-free system is expressed as a percentage of inhibition.
DISCUSSION
In this report, we describe the isolation and characterization of a new mammalian kinase, PEK, that phosphorylates the α subunit of eIF-2 at residue serine-51. Consistent with this observed kinase activity, the sequence of the catalytic domain of PEK is similar to those of members of the eIF-2α kinase family, including that of the characteristic large insert between subdomains IV and V. PEK contains a 550-residue flanking sequence that is quite divergent from those of previously characterized eIF-2α kinases, suggesting that PEK is regulated by different physiological conditions. Northern blot analysis suggests that while PEK is expressed in many different rat tissues, the highest levels are found in the pancreas. The eIF-2α kinase assays using PEK immunoprecipitated from different rat tissues further supported the idea that PEK is predominately expressed in pancreas and pancreatic islet tissues. PEK functionally substitutes for eIF-2α kinase GCN2, demonstrating that PEK can mediate translational control in the yeast model system. Furthermore, the addition of PEK to reticulocyte lysates reduced protein synthesis. Taken together, this study strongly suggests that PEK regulates translation initiation predominately in pancreatic cells by phosphorylation of eIF-2α.
Roles of eIF-2α kinases in cellular stress and proliferation.
A variety of cellular stresses have been observed to elicit the phosphorylation of mammalian eIF-2α, resulting in altered protein synthesis. In addition to viral infection and iron deficiency, known activators of PKR and HRI, respectively, identified stress conditions that increase eIF-2α phosphorylation include starvation for amino acids, glucose, or serum; growth factor deprivation; heat shock; ischemia; and altered calcium levels (6). Many of these conditions do not appear to be mediated by PKR and HRI. Additionally, as HRI is predominantly expressed in erythroid tissues, its role in translational control would appear to be restricted to certain cell types (5). This suggests that additional mammalian eIF-2α kinases, such as PEK, may mediate translation control in response to many of these stress conditions. Previous biochemical studies have partially characterized eIF-2α kinases, although it was unclear whether these enzymes were distinct from PKR and HRI and whether their activity was specific for serine-51 (7, 32, 35). Such new mammalian kinases may act individually or in combination with other eIF-2α kinase family members to increase the levels of eIF-2α phosphorylation. In support of a different physiological role for PEK, our preliminary studies show that PEK kinase activity in vitro was not affected by treatment with poly(I)·poly(C) or heparin, known activators of PKR.
In addition to antiviral activities, eIF-2α kinases are also proposed to have a role in the regulation of cell growth. Several reports observed that the expression of certain kinase-inactive mutant forms of PKR in NIH 3T3 cells confers a malignant-transformation phenotype and that subcutaneous injection of these transfected cells in nude mice gave rise to rapid tumor growth (2, 22, 29, 37). When similar experiments were carried out with wild-type PKR, no transformed phenotype was observed. These results suggest that the kinase may function as a tumor suppressor, since expression of a mutant PKR in NIH 3T3 cells reduced the activity of the endogenous wild-type PKR as measured by autophosphorylation (22). Furthermore, they support the idea that the level of eIF-2α phosphorylation in cells is important for the control of cell proliferation. Consistent with this premise, Donze et al. (10) reported that expression of the nonphosphorylatable eIF-2α mutant S51A in NIH 3T3 cells resulted in malignant transformation.
In contrast to these transfection studies, Yang et al. (50) bred mice in which the 5′ portion of the PKR gene was deleted and found these Pkro/o mice developed normally and showed no visible differences in appearance and behavior compared with their wild-type littermates. While the antiviral response induced by gamma interferon and ds-RNA was diminished in the Pkro/o mice, no spontaneous tumors were observed. Furthermore, no tumor formation was detected in nude mice injected with Pkro/o embryonic fibroblasts or in established NIH 3T3-like cell lines derived from the Pkro/o mice (50). Together, these results would appear to argue against PKR functioning as a direct tumor suppressor. A cautionary note to these studies is that the sequences encoding the kinase domain of PKR remained intact in these “knockout” mice and there may have been PKR activity that was not detectable in this study. An alternative explanation for the difference between the transfection and mouse studies is that an additional eIF-2α kinase, such as PEK, may carry out functions overlapping those of PKR. In this case, eIF-2α kinase activity important for controlling cellular proliferation would be sufficiently retained in mice in which PKR is deleted. It is possible that in the transfection studies overexpression of the dominant-negative form of PKR in NIH 3T3 cells interfered with the activity of an additional eIF-2α kinase by competing for regulatory factors or cellular targets, such as ribosomes (51).
Translational control by eIF-2α kinases in the yeast model system.
In mammalian cells, eIF-2α phosphorylation in response to viral infection or heme deprivation inhibits general protein synthesis. In contrast, GCN2 phosphorylation of eIF-2α in yeast does not reduce total protein synthesis but rather stimulates the translation of GCN4 mRNA. The GCN4 protein increases the transcription of over 30 genes involved in amino acid biosynthesis, including those that contribute to growth resistance in medium containing 3-AT or SM. It is thought that the level of eIF-2α phosphorylation during amino acid starvation in yeast leads to a modest decrease in the activity of this initiation factor that does not affect primary translation (45). By comparison, translation reinitiation that occurs during ribosome scanning of the 5′ leader of the GCN4 mRNA may be particularly sensitive to reductions in the levels of the eIF-2 ternary complex (19). It is this delay in reinitiation, which occurs during modest reductions in eIF-2-GTP levels, that is proposed to allow leaky scanning through the negative-acting ORFs, increasing the expression of the GCN4 coding sequence. Consistent with the idea that the levels of eIF-2α phosphorylation can be used to discriminate between general and gene-specific translation, cells expressing constitutively active mutants of GCN2 were found to suffer a slow-growth phenotype due to elevated eIF-2α phosphorylation that exceeded the levels measured in wild-type strains grown under amino acid starvation conditions (36, 44).
Rat PEK expressed in strain H1894 (Δgcn2) replaced GCN2 kinase function, allowing these yeast cells to grow in medium containing either 3-AT or SM (Fig. 9A). This function is dependent on the phosphorylated residue, serine-51, in eIF-2α (Fig. 9B). In previous studies, the expression of mammalian eIF-2α kinases PKR and HRI from a galactose-inducible promoter was also found to affect the translational control mechanism in yeast in a serine-51-dependent fashion (8, 38). The expression of PKR under galactose-inducing conditions was in fact found to lead to a severe slow-growth phenotype due to hyperphosphorylation of eIF-2α (8, 11, 38). Only when PKR was expressed at lower levels in glucose-containing medium or when partially defective PKR mutants were present was it found that GCN4 expression in cells was stimulated and that the cells grew in the presence of 3-AT (8, 38). In the example of PEK described in this report, no slow-growth phenotype was observed when PEK-expressing cells were grown in galactose medium (Fig. 9). A similar level of expression of HRI in Δgcn2 cells was also reported to allow growth under these galactose-inducing conditions (8). These results are consistent with the idea that the levels of eIF-2 phosphorylation in yeast cells expressing PEK or HRI were reduced compared to those in yeast cells expressing PKR. The basis for this activity difference would depend on the steady-state levels of these eIF-2α kinases expressed in yeast and on whether the activities of these kinases were impacted by regulators endogenous to yeast. In the PKR example, it is proposed that ds-RNA in yeast contributes to increased eIF-2α activity, as deletion of the RNA-binding domains of PKR led to loss of in vivo function (38). This interpretation, however, is complicated by the observation that the RNA-binding domains mediate the association of PKR with ribosomes, and this interaction is thought to facilitate in vivo phosphorylation of eIF-2α (51).
Regulation of eIF-2α kinases by autophosphorylation and cellular targeting.
Autophosphorylation is an important step for the activation of the eIF-2α kinases (5, 34, 37, 39, 43). Romano et al. (37) presented evidence that autophosphorylation at two threonine residues in the predicted activation loops of PKR and GCN2 facilitates the function of these two eIF-2α kinases. Many protein kinases are known to be activated by the autophosphorylation of residues in this loop region (17). Such autophosphorylation may elicit a protein conformation that enhances the binding of ATP or peptide substrates or that facilitates the phosphoryl transfer reaction itself (17, 37). Interestingly, the autophosphorylated residues in subdomain VIII of PKR and GCN2 align with Thr-974 and Thr-979 of PEK. Given the observed PEK autophosphorylation at threonine residue(s) (Fig. 4B), it is inviting to speculate that a similar mode of PEK activation occurs by phosphorylation of these residues in its subdomain VIII.
Appropriate cellular localization of eIF-2α kinases also facilitates their in vivo function. The majority of PKR in mammalian cells was found to be associated with ribosomes (20, 39, 40), with the remaining portion, estimated at less than 20% of the total PKR, localized in the nucleus in close proximity to the nucleolus (20). Using the yeast model system, Zhu et al. (51) showed that the ds-RNA-binding domains mediate PKR targeting to ribosomes, and this interaction is proposed to facilitate kinase function in vivo by providing access to the eIF-2α substrate. Appropriate cellular localization appears to be important for the function of the eIF-2α kinase in P. falciparum. PfPK4 is proposed to be expressed in two major forms, 80 and 90 kDa in size (30). The larger form of PfPK4 is only found in mature parasites and is present in the membrane fraction of infected erythrocytes. On the basis of immunofluorescence studies, PfPK4 was localized to the apical complex that participates in the invasion of erythrocytes. Our analysis of the PEK sequences indicates that the enzyme does not possess the sequence motifs identified from PKR and GCN2 that are required for ribosomal association, suggesting that if PEK associates with the ribosome, it does so by a different mechanism. Furthermore, there is a putative signal sequence in the N terminus of PEK, indicating that membrane association may be important to facilitate its function.
New eIF-2α kinase predominately expressed in pancreas.
We have identified a new mammalian eIF-2α kinase that differs from PKR and HRI in tissue distribution. While our Northern analyses suggest that PEK is widely distributed among rat tissues, it is most highly expressed in the pancreas (Fig. 7 and 8). This was confirmed by our kinase assays that showed PEK activity in pancreas and islet cells (Fig. 8). The pancreas has both exocrine and endocrine functions that require regulated protein synthesis and secretion. The majority of the pancreas consists of exocrine acinar cells, which produce and secrete digestive enzymes. Scattered among the vast majority of exocrine tissue are the islets of Langerhans. Whereas these pancreatic islets occupy less than 5% of the total pancreatic mass, they play a pivotal role in regulating glucose homeostasis in mammals by secreting different hormones including insulin, glucagon, somatostatin, and pancreatic polypeptide (3). The synthesis and secretion of the hormones are coordinated and are regulated by changes in metabolic and nutritional conditions such as hyperglycemia or hypoglycemia. In contrast to transcriptional regulation over time intervals of hours, the glucose-stimulated biosynthesis of insulin occurs within minutes at the level of protein synthesis (13, 31). A number of other membrane and secretory proteins in the pancreas are also believed to be regulated at the translational level (14–16). Our identification and characterization of PEK may facilitate future studies on translational control of proteins secreted from pancreatic tissues.
ACKNOWLEDGMENTS
We thank James Miller and Bruce Konicek for assistance in establishing baculovirus expression systems, Bruce Glover for oligonucleotide synthesis, and members in the Lilly sequencing laboratory for DNA sequence analysis. We are especially grateful to Jose Caro, Armen Tashjian, and Amy Porter for valuable suggestions to the work presented here and to Julie Moyers and Scott Hayes for critically reading the manuscript. We also thank Santosh Mishra, Dennis Smith, and Rebecca Owens for help in the computer analysis and Wayne Wilson and Peter Roach for their assistance with phosphoamino acid analysis.
This work was supported in part by U.S. Public Health Service grant GM49164 from the National Institutes of Health and ACS grant RPG MBC-87806 (R.C.W.).
REFERENCES
- 1.Abastado J P, Miller P F, Jackson B M, Hinnebusch A G. Suppression of ribosomal reinitiation at upstream open reading frames in amino acid-starved cells forms the basis of GCN4 translational control. Mol Cell Biol. 1991;11:486–496. doi: 10.1128/mcb.11.1.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barber G N, Jagus R, Meurs E F, Hovanessian A G, Katze M G. Molecular mechanisms responsible for malignant transformation by regulatory and catalytic domain variants of the interferon-induced enzyme RNA-dependent protein kinase. J Biol Chem. 1995;270:17423–17428. doi: 10.1074/jbc.270.29.17423. [DOI] [PubMed] [Google Scholar]
- 3.Bonner-Weir S, Smith F E. Islets of langerhans: morphology and its implications. In: Kahn C R, Weir G C, editors. Joslin’s diabetes mellitus. 13th ed. Piladelphia, Pa: Lea & Febiger; 1994. pp. 15–28. [Google Scholar]
- 4.Cesareni G, Murray J. Plasmid vectors carrying the replication origin of filamentous single-stranded phages. In: Setlow J K, Hollaender A, editors. Genetic engineering: principles and methods. Vol. 9. New York, N.Y: Plenum Press; 1987. pp. 135–154. [Google Scholar]
- 5.Chen J J, London I M. Regulation of protein synthesis by heme-regulated eIF-2 alpha kinase. Trends Biochem Sci. 1995;20:105–108. doi: 10.1016/s0968-0004(00)88975-6. [DOI] [PubMed] [Google Scholar]
- 6.Clemens M J. Protein kinaes that phosphorylate eIF2 and eIF2β, and their role in eukaryotic cell translational control. In: Hershey J W B, Mathews M B, Sonenberg N, editors. Translational control. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 139–172. [Google Scholar]
- 7.de Haro C, Mendez R, Santoyo J. The eIF-2α kinases and the control of protein synthesis. FASEB J. 1996;10:1378–1388. doi: 10.1096/fasebj.10.12.8903508. [DOI] [PubMed] [Google Scholar]
- 8.Dever T E, Chen J J, Barber G N, Cigan A M, Feng L, Donahue T F, London I M, Katze M G, Hinnebusch A G. Mammalian eukaryotic initiation factor 2 alpha kinases functionally substitute for GCN2 protein kinase in the GCN4 translational control mechanism of yeast. Proc Natl Acad Sci USA. 1993;90:4616–4620. doi: 10.1073/pnas.90.10.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dever T E, Feng L, Wek R C, Cigan A M, Donahue T F, Hinnebusch A G. Phosphorylation of initiation factor 2 alpha by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell. 1992;68:585–596. doi: 10.1016/0092-8674(92)90193-g. [DOI] [PubMed] [Google Scholar]
- 10.Donze O, Jagus R, Koromilas A E, Hershey J W, Sonenberg N. Abrogation of translation initiation factor eIF-2 phosphorylation causes malignant transformation of NIH 3T3 cells. EMBO J. 1995;14:3828–3834. doi: 10.1002/j.1460-2075.1995.tb00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng G S, Chong K, Kumar A, Williams B R. Identification of double-stranded RNA-binding domains in the interferon-induced double-stranded RNA-activated p68 kinase. Proc Natl Acad Sci USA. 1992;89:5447–5451. doi: 10.1073/pnas.89.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng L, Yoon H, Donahue T F. Casein kinase II mediates multiple phosphorylation of Saccharomyces cerevisiae eIF-2α (encoded by SUI2), which is required for optimal function in S. cerevisiae. Mol Cell Biol. 1994;14:5139–5153. doi: 10.1128/mcb.14.8.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodison S, Kenna S, Ashcroft S J. Control of insulin gene expression by glucose. Biochem J. 1992;285:563–568. doi: 10.1042/bj2850563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grimaldi K A, Siddle K, Hutton J C. Biosynthesis of insulin secretory granule membrane proteins. Control by glucose. Biochem J. 1987;245:567–573. doi: 10.1042/bj2450567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guest P C, Bailyes E M, Rutherford N G, Hutton J C. Insulin secretory granule biogenesis. Co-ordinate regulation of the biosynthesis of the majority of constituent proteins. Biochem J. 1991;274:73–78. doi: 10.1042/bj2740073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guest P C, Rhodes C J, Hutton J C. Regulation of the biosynthesis of insulin-secretory-granule proteins. Co-ordinate translational control is exerted on some, but not all, granule matrix constituents. Biochem J. 1989;257:431–437. doi: 10.1042/bj2570431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanks S K, Hunter T. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 1995;9:576–596. [PubMed] [Google Scholar]
- 18.Hinnebusch A G. The eIF-2 alpha kinases: regulators of protein synthesis in starvation and stress. Semin Cell Biol. 1994;5:417–426. doi: 10.1006/scel.1994.1049. . (Review.) [DOI] [PubMed] [Google Scholar]
- 19.Hinnebusch A G. Translational regulation of yeast GCN4. A window on factors that control initiator-tRNA binding to the ribosome. J Biol Chem. 1997;272:21661–21664. doi: 10.1074/jbc.272.35.21661. [DOI] [PubMed] [Google Scholar]
- 20.Jeffrey I W, Kadereit S, Meurs E F, Metzger T, Bachmann M, Schwemmle M, Hovanessian A G, Clemens M J. Nuclear localization of the interferon-inducible protein kinase PKR in human cells and transfected mouse cells. Exp Cell Res. 1995;218:17–27. doi: 10.1006/excr.1995.1126. [DOI] [PubMed] [Google Scholar]
- 21.Kaiser C, Michaelis S, Mitchell A. Methods in yeast genetics. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1994. pp. 207–217. [Google Scholar]
- 22.Koromilas A E, Roy S, Barber G N, Katze M G, Sonenberg N. Malignant transformation by a mutant of the IFN-inducible dsRNA-dependent protein kinase. Science. 1992;257:1685–1689. doi: 10.1126/science.1382315. [DOI] [PubMed] [Google Scholar]
- 23.Kozak M. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J Biol Chem. 1991;263:19867–19870. [PubMed] [Google Scholar]
- 24.Lee S B, Esteban M. The interferon-induced double-stranded RNA-activated protein kinase induces apoptosis. Virology. 1994;199:491–496. doi: 10.1006/viro.1994.1151. [DOI] [PubMed] [Google Scholar]
- 25.Lee S B, Rodriguez D, Rodriguez J R, Esteban M. The apoptosis pathway triggered by the interferon-induced protein kinase PKR requires the third basic domain, initiates upstream of Bcl-2,. and involves ICE-like proteases. Virology. 1997;231:81–88. doi: 10.1006/viro.1997.8494. [DOI] [PubMed] [Google Scholar]
- 26.Mathews M B. Interactions between viruses and the cellular machinery for protein synthesis. In: Hershey J W B, Mathews M B, Sonenberg N, editors. Translational control. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1996. pp. 505–548. [Google Scholar]
- 27.Mathews M B, Sonenberg N, Hershey J W B. Origins and targets of translational control. In: Hershey J W B, Mathews M B, Sonenberg N, editors. Translational control. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 1–30. [Google Scholar]
- 28.Merrick W C, Hershey J W B. The pathway and mechanism of eukaryotic protein synthesis. In: Hershey J W B, Mathews M B, Sonenberg N, editors. Translational control. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 31–70. [Google Scholar]
- 29.Meurs E F, Galabru J, Barber G N, Katze M G, Hovanessian A G. Tumor suppressor function of the interferon-induced double-stranded RNA-activated protein kinase. Proc Natl Acad Sci USA. 1993;90:232–236. doi: 10.1073/pnas.90.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohrle J J, Zhao Y, Wernli B, Franklin R M, Kappes B. Molecular cloning, characterization and localization of PfPk4, an eIF-2α kinase-related enzyme from the malarial parasite Plasmodium falciparum. Biochem J. 1997;328:677–687. doi: 10.1042/bj3280677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nielsen D A, Welsh M, Casadaban M J, Steiner D F. Control of insulin gene expression in pancreatic beta-cells and in an insulin-producing cell line, RIN-5F cells. I. Effects of glucose and cyclic AMP on the transcription of insulin mRNA. J Biol Chem. 1985;260:13585–13589. [PubMed] [Google Scholar]
- 32.Olmsted E A, O’Brien L, Henshaw E C, Panniers R. Purification and characterization of eukaryotic initiation factor (eIF)-2 alpha kinases from Ehrlich ascites tumor cells. J Biol Chem. 1993;268:12552–12559. [PubMed] [Google Scholar]
- 33.Olsen D S, Jordan B, Chen D, Wek R C, Cavener D R. Isolation of the gene encoding the Drosophila melanogaster homolog of the Saccharomyces cerevisiae GCN2 eIF-2 alpha kinase. Genetics. 1998;149:1495–1509. doi: 10.1093/genetics/149.3.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Proud C G. PKR: a new name and new roles. Trends Biochem Sci. 1995;20:241–246. doi: 10.1016/s0968-0004(00)89025-8. [DOI] [PubMed] [Google Scholar]
- 35.Proud C G. Protein phosphorylation in translational control. Curr Top Cell Regul. 1992;32:243–369. doi: 10.1016/b978-0-12-152832-4.50008-2. [DOI] [PubMed] [Google Scholar]
- 36.Ramirez M, Wek R C, Vazquez de Aldana C R, Jackson B M, Freeman B, Hinnebusch A G. Mutations activating the yeast eIF-2α kinase GCN2: isolation of alleles altering the domain related to histidyl-tRNA synthetases. Mol Cell Biol. 1992;12:5801–5815. doi: 10.1128/mcb.12.12.5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romano P R, Garcia-Barrio M T, Zhang X, Wang Q, Taylor D R, Zhang F, Herring C, Mathews M B, Qin J, Hinnebusch A G. Autophosphorylation in the activation loop is required for full kinase activity in vivo of human and yeast eukaryotic initiation factor 2α kinases PKR and GCN2. Mol Cell Biol. 1998;18:2282–2297. doi: 10.1128/mcb.18.4.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romano P R, Green S R, Barber G N, Mathews M B, Hinnebusch A G. Structural requirements for double-stranded RNA binding, dimerization, and activation of the human eIF-2α kinase DAI in Saccharomyces cerevisiae. Mol Cell Biol. 1995;15:365–378. doi: 10.1128/mcb.15.1.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samuel C E. The eIF-2 alpha protein kinases, regulators of translation in eukaryotes from yeasts to humans. J Biol Chem. 1993;268:7603–7606. . (Review.) [PubMed] [Google Scholar]
- 40.Samuel C E, Knutson G S, Berry M J, Atwater J A, Lasky S R. Purification of double-stranded RNA-dependent protein kinase from mouse fibroblasts. Methods Enzymol. 1986;119:499–516. doi: 10.1016/0076-6879(86)19070-7. [DOI] [PubMed] [Google Scholar]
- 41.Santoyo J, Alcalde J, Mendez R, Pulido D, de Haro C. Cloning and characterization of a cDNA encoding a protein synthesis initiation factor-2alpha (eIF-2alpha) kinase from Drosophila melanogaster. Homology to yeast GCN2 protein kinase. J Biol Chem. 1997;272:12544–12550. doi: 10.1074/jbc.272.19.12544. [DOI] [PubMed] [Google Scholar]
- 42.Srivastava S P, Kumar K U, Kaufman R J. Phosphorylation of eukaryotic initiation factor 2 mediates apoptosis in response to activation of the double-stranded RNA-dependent protein kinase. J Biol Chem. 1998;273:2416–2423. doi: 10.1074/jbc.273.4.2416. [DOI] [PubMed] [Google Scholar]
- 43.Taylor D R, Lee S B, Romano P R, Marshak D R, Hinnebusch A G, Esteban M, Mathews M B. Autophosphorylation sites participate in the activation of the double-stranded-RNA-activated protein kinase PKR. Mol Cell Biol. 1996;16:6295–6302. doi: 10.1128/mcb.16.11.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vazquez de Aldana C R, Dever T E, Hinnebusch A G. Mutations in the alpha subunit of eukaryotic translation initiation factor 2 (eIF-2 alpha) that overcome the inhibitory effect of eIF-2 alpha phosphorylation on translation initiation. Proc Natl Acad Sci USA. 1993;90:7215–7219. doi: 10.1073/pnas.90.15.7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wek R C. eIF-2 kinases: regulators of general and gene-specific translation initiation. Trends Biochem Sci. 1994;19:491–496. doi: 10.1016/0968-0004(94)90136-8. . (Review.) [DOI] [PubMed] [Google Scholar]
- 46.Wek R C, Jackson B M, Hinnebusch A G. Juxtaposition of domains homologous to protein kinases and histidyl-tRNA synthetases in GCN2 protein suggests a mechanism for coupling GCN4 expression to amino acid availability. Proc Natl Acad Sci USA. 1989;86:4579–4583. doi: 10.1073/pnas.86.12.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wek R C, Ramirez M, Jackson B M, Hinnebusch A G. Identification of positive-acting domains in GCN2 protein kinase required for translational activation of GCN4 expression. Mol Cell Biol. 1990;10:2820–2831. doi: 10.1128/mcb.10.6.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wek S A, Zhu S, Wek R C. The histidyl-tRNA synthetase-related sequence in the eIF-2α protein kinase GCN2 interacts with tRNA and is required for activation in response to starvation for different amino acids. Mol Cell Biol. 1995;15:4497–4506. doi: 10.1128/mcb.15.8.4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu Z, Pal J K, Thulasiraman V, Hahn H P, Chen C J J, Matts R L. The role of the 90-kDa heat-shock protein and its associated cohorts in stabilizing the heme-regulated eIF-2alpha kinase in reticulocyte lysates during heat stress. Eur J Biochem. 1997;246:461–470. doi: 10.1111/j.1432-1033.1997.t01-1-00461.x. [DOI] [PubMed] [Google Scholar]
- 50.Yang Y-L, Reis Y F L, Pavlovic J, Aguzzi A, Schafer R, Kumar A, Williams B R G, Aguet M, Weissman C. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 1995;14:6095–6106. doi: 10.1002/j.1460-2075.1995.tb00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu S, Romano P R, Wek R C. Ribosome targeting of PKR is mediated by two double-stranded RNA-binding domains and facilitates in vivo phosphorylation of eukaryotic initiation factor-2. J Biol Chem. 1997;272:14434–14441. doi: 10.1074/jbc.272.22.14434. [DOI] [PubMed] [Google Scholar]
- 52.Zhu S, Sobolev A Y, Wek R C. Histidyl-tRNA synthetase-related sequences in GCN2 protein kinase regulate in vitro phosphorylation of eIF-2. J Biol Chem. 1996;271:24989–24994. doi: 10.1074/jbc.271.40.24989. [DOI] [PubMed] [Google Scholar]
- 53.Zioncheck T F, Harrison M L, Geahlen R L. Purification and characterization of a protein-tyrosine kinase from bovine thymus. J Biol Chem. 1986;261:15637–15643. [PubMed] [Google Scholar]