Abstract
Efficient splicing of the 5′-most intron of pre-mRNA requires a 5′ m7G(5′)ppp(5′)N cap, which has been implicated in U1 snRNP binding to 5′ splice sites. We demonstrate that the cap alters the kinetic profile of U1 snRNP binding, but its major effect is on U6 snRNA binding. With two alternative wild-type splice sites in an adenovirus pre-mRNA, the cap selectively alters U1 snRNA binding at the site to which cap-independent U1 snRNP binding is stronger and that is used predominantly in splicing; with two consensus sites, the cap acts on both, even though one is substantially preferred for splicing. However, the most striking quantitative effect of the 5′ cap is neither on U1 snRNP binding nor on the assembly of large complexes but on the replacement of U1 snRNP by U6 snRNA at the 5′ splice site. Inhibition of splicing by a cap analogue is correlated with the loss of U6 interactions at the 5′ splice site and not with any loss of U1 snRNP binding.
All precursor mRNAs transcribed by RNA polymerase II have a 5′ cap (m7G[5′]ppp[5′]N) added cotranscriptionally (63, 68) by enzymes associated with RNA polymerase II (8, 45). The 5′ cap protects pre-mRNA from 5′ exoribonucleases (17, 20, 70) and has been shown to have an important role in mRNA translation (62, 69). The cap also has roles in pre-mRNA processing; it is implicated in both polyadenylation and export from the nucleus, even if it is not essential for these processes (12, 15, 21, 28, 43, 51, 77, 78), and it has an important role in splicing.
A role for the 5′ cap structure in pre-mRNA splicing was shown by early studies in extracts. In HeLa whole-cell extracts, substrate pre-mRNA was spliced efficiently only when capped (32), and splicing reactions could be inhibited by adding low levels of m7GpppG or m7GTP cap analogues to the extract. In HeLa nuclear extracts, splicing was reduced, but only by one-third, in the absence of a cap (37, 52). Subsequent work showed that cap dependence was enhanced if nuclear extracts were preincubated in the presence of magnesium before the splicing reaction (13). The splicing of introns other than the cap-proximal intron is not affected much by the 5′ cap (25, 55) because of the presence of a polypyrimidine tract in an upstream intron (42). This is consistent with the suggestion that the cap functions in a manner analogous to that of an upstream polypyrimidine tract in the definition of the adjacent exon (1, 23, 24, 42, 58).
The effects of the cap on pre-mRNA processing and export are mediated by a cap-binding complex (CBC). In mammals, this comprises two proteins of 80 and 20 kDa (26, 30, 54). Immunodepletion of the CBC led to a loss of spliceosome assembly (26), including the loss of most U1 snRNP base pairing with the 5′ splice site (42). In Saccharomyces cerevisiae, loss of either capping enzyme activity or a CBC component affected splicing efficiency in vivo (9, 16, 66); in vitro, depletion of CBC reduced commitment complex formation and splicing (9, 41), but the absence of a 5′ cap on the pre-mRNA did not affect the efficiency of splicing (9, 66).
The finding that the CBC was required for efficient interactions of U1 snRNA with the 5′ splice site seemed to be inconsistent with other evidence. Not only are there reports that uncapped RNA can be spliced, but in some cases uncapped RNA has been used intentionally to detect complexes at 5′ splice sites (52, 53, 61). Furthermore, there are numerous reports of unaided interactions between pure U1 snRNPs and 5′ splice sites (5, 22, 27, 31, 49, 60, 75). These findings could be reconciled if the cap-CBC interaction was not required constitutively but only at specific sites where U1 snRNP binding is hindered by, for example, weak 5′ splice sites or sequestration of sites by proteins or RNA secondary structure. If this is true, then the exact mechanism of the cap effect on U1 snRNP binding would be expected to affect the selection of alternative 5′ splice sites in a 5′ exon. Thus, if binding of U1 snRNPs was enhanced by the cap, and the cap-proximal site was favored, then splicing would favor that site. If the cap enhanced binding at all sites, then weak sites would behave like strong sites and the presence of the cap would lead to a shift in splicing preferences towards the cap-distal (downstream) site, according to the model described in reference 14. If the cap enhanced binding only at specific sequestered sites, then the presence of the cap would lead to a shift towards those sites at the expense of the site favored in the absence of the cap. These schemes all suggest that the presence of the cap or the concentration of cap-binding proteins may have important effects on 5′ splice site selection. Previous studies have shown that the cap cannot just promote cap-proximal U1 snRNP binding with alternative strong 5′ splice sites, because initial U1 snRNP binding did not appear to depend on the proximity of the sites to the cap and the cap-distal site was spliced (14, 50), but discriminatory effects of the cap cannot be discounted for weaker sites, where the initial binding of U1 snRNPs is less well characterized (5, 74) and the cap-proximal site is sometimes preferred (57). The uncertainties about the generality of the cap requirement for U1 snRNP binding have such important implications for splice site selection that we sought to determine whether the cap is required for binding and splicing at all alternative splice sites, including strong consensus splice sites.
We investigated U1 snRNP binding to alternative 5′ splice sites on pre-mRNA capped with m7GpppG or ApppG. Splicing at all the sites was cap dependent. These experiments revealed that there were two kinetic modes for U1 snRNP binding, a rapid binding mode that depended on the 5′ cap and a slower mode that was seen in the absence of the correct cap or at sites that were not used for splicing. The cap itself did not appear to determine which site was bound rapidly and spliced. Strikingly, the m7GpppG cap was required for efficient interactions of U6 snRNA at active 5′ splice sites.
MATERIALS AND METHODS
Preparation of pre-mRNA.
Ad1 DNA constructs were derived from pBSAd1 (34). The Ad1 template used was mutated to introduce either a wild-type 5′ splice site (GGG/GTGAGT), a consensus 5′ splice site (CAG/GTAAGT), or a nonfunctional mutant sequence (GGCGAATTC) in place of either the natural 5′ splice site or a cryptic 5′ splice site in the intron. Mutagenesis of only the cryptic splice site produced templates named Ad1WW (wild type-wild type) and Ad1WM (wild type-mutant); mutations at both sites produced Ad1MW (mutant-wild type), Ad1CC (consensus-consensus), Ad1CM (consensus-mutant), Ad1MC (mutant-consensus), and Ad1MM (mutant-mutant). The templates were linearized with SauIIIa and transcribed with T3 RNA polymerase. The human β-globin IVS-1 substrate pSP64 5′Δ16 (37) was linearized with BamHI and transcribed with Sp6 RNA polymerase. Transcription reaction mixtures contained 0.5 μg of DNA template; 500 μM (each) ATP, CTP, and UTP; 100 μM GTP; 1 mM m7GpppG or ApppG cap analogues (New England Biolabs); 20 μCi [α-32P]GTP (NEN); and either T3 or SP6 RNA polymerase (Promega).
Splicing reactions.
HeLa nuclear extracts were obtained from the Computer Cell Culture Centre (Mons, Belgium). The extracts depleted of CBC were kindly given by J. Lewis and I. W. Mattaj (EMBL). Extracts were preincubated at 30°C for 15 min to deplete ATP. Splicing reactions were started by the addition of the pre-mRNA and splicing mix and then were incubated at 30°C for a maximum of 90 min. Reaction mixtures contained 40% (vol/vol) extract, ≈0.5 ng (10 to 20,000 cpm) of substrate per μl, and 1.6 mM MgCl2 (13, 26). Reactions were initiated such that different incubation times terminated together. The spliced products were resolved on 6% denaturing polyacrylamide gels.
Native gel analysis.
Splicing reactions were assembled as described previously. After incubation, 30 μl of buffer D supplemented with 0.4 mg of heparin per ml and an additional 150 mM KCl was added to each 7.5-μl reaction mixture, and the reaction mixtures were incubated at 30°C for an additional 10 min. Samples were then filtered through cellulose acetate filters (Costar) and loaded directly onto 0.5% agarose–3.0% polyacrylamide gels.
RNase H digestion.
Protection of consensus 5′splice sites in extracts was assayed by the addition of 100 pmol of the appropriate oligonucleotide in 2 μl of buffer D plus 2 mM MgCl2 and 4 U of RNase H (Pharmacia) to 10-μl splicing reactions either with the pre-mRNA for zero time points or 25 min later. Digestion was for 5 min. The oligonucleotides were 14-mers complementary to the consensus 5′ splice sites. For RNase H digestion of Ad1 pre-mRNA cross-linked to snRNA, splicing reaction products or gel-purified RNA was precipitated with ethanol and dissolved in Tris-EDTA; portions were incubated with 50 pmol of oligonucleotide in 3 μl at 80°C for 15 s and digested in 5 μl by the addition of buffer D, MgCl2 (to 3.2 mM), Nonidet P-40, (to 0.05% [vol/vol]), and RNase H. The products were analyzed on denaturing gels. Oligonucleotides used to cleave the snRNA were complementary to U1 snRNA nucleotides (nt) 123 to 142, U2 snRNA nt 1 to 15, U5 snRNA nt 68 to 88, or U6 snRNA nt 69 to 88. Oligonucleotides used to map the positions of the snRNA cross-links were complementary to the Ad1 pre-mRNA sequences centered at nt 40, 80, 120, 170, 210, 250, 290, and 330. The 5′ splice sites are at nt 92 and 93 and 185 and 186; the 3′ splice site is at position 326.
Psoralen cross-linking.
Splicing reaction mixtures were as normal but included 0.8% (vol/vol) of a 4′-aminomethyl-4,5′,8-trimethyl (AMT)-psoralen solution (2 mg of dimethylsulfoxide [DMSO]; HRI Associates). Reactions (2.5 μl each) were incubated in open wells of Thermowell C strips (Costar) in a 30°C water bath. Each strip was removed after a fixed time of incubation. When samples were to be irradiated, the strip was placed in a rigid holder that aligned the wands of a SpotCure (UVP) with the wells, and samples were irradiated in pairs with two wands that produced UV light of identical intensities. Irradiation was for 1 min at room temperature with long-wave UV filtered via a thin glass plate. Electrophoresis of the RNA was on 5% polyacrylamide gels containing 7 M urea and 30% (vol/vol) formamide. Unirradiated but parallel and otherwise identical reactions were run on the same gels and also on 8% gels. Dried gels were analyzed by PhosphorImager. The cross-links were quantified by the use of profiles derived from the summation of signals across the whole width of each lane; the intensity of each cross-linked band is given as a percentage of the sum of the intensities of the pre-mRNA, the major internal cross-links, and the identifiable snRNA cross-links. Where the cross-linked bands were very close (as in upstream U1, b, and U2), the baseline was set to the background outside the group and peaks were defined by vertical lines from the lowest point between the peaks. In some cases, the long exposures required for accurate measurement of faint signals led to saturation of the precursor, and the level of pre-mRNA was calculated from a scale factor derived from shorter exposures. The reactions shown in Fig. 3 differed in that the volumes were 5 μl, the psoralen was added at 1.6% (vol/vol), and where present, MgCl2 was at 2.7 rather than 1.6 mM.
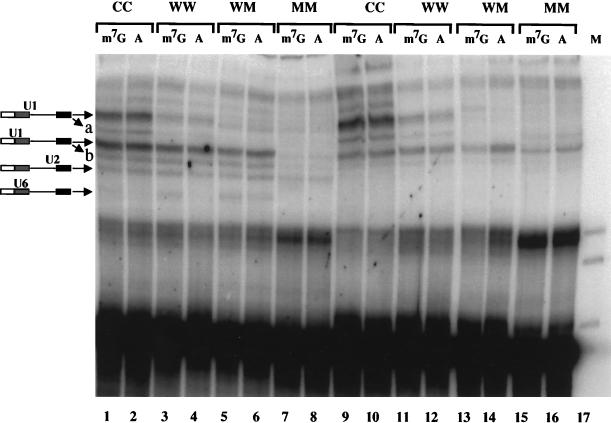
FIG. 3.
Psoralen cross-linking of U1 snRNA to m7GpppG- and ApppG-capped Ad1 substrates. The substrates used had two alternative 5′ splice sites, each of which was either consensus (C), wild-type (W), or mutant (M), as indicated above each lane. (A) Pre-mRNA capped with m7GpppG (m7G) or ApppG (A) was incubated for 20 min in splicing mixes containing AMT-psoralen in the presence (lanes 1 to 8) or absence (lanes 9 to 16) of magnesium, ATP, and phosphocreatine. Cross-linked products were resolved on a 5% polyacrylamide gel. The positions of the snRNA cross-links are shown diagramatically. Bands a and b are minor forms of the two U1 cross-links.
RESULTS
Dependence of alternative 5′ splice sites on the 5′ cap.
Adenovirus 1 and human β-globin IVS1 pre-mRNA transcripts primed with either m7GpppG or ApppG caps were spliced in vitro in HeLa nuclear extracts to confirm that splicing depended on the correct cap under the conditions used in this study (Fig. 1). The ApppG cap structure is unable to bind the CBC which mediates the effect of the m7GpppG (26) and therefore was used as a control. The ApppG-capped pre-mRNAs have been shown to behave as uncapped substrates in splicing, both in extracts and in microinjected Xenopus oocytes (25, 55). The substrates used all had alternative 5′ splice sites; the Ad1 template was mutated to create either an additional wild-type 5′ splice site in place of a cryptic 5′ splice site in the intron (Ad1WW) or a consensus sequence at both sites (Ad1CC).
FIG. 1.
Pre-mRNA substrates containing alternative 5′ splice sites require the m7GpppG 5′ cap structure for splicing to both sites. (A) Splicing of ApppG- and m7GpppG-capped human β-globin IVS-1 substrate psp64 5′Δ16, which contains alternative 5′ splice sites. ApppG-capped RNA is in lanes 1 to 10 and 21 to 23; m7GpppG-capped RNA is in lanes 11 to 20 and 24 to 26. Splicing reaction mixtures were incubated for the times indicated (in minutes) above each lane. Lanes 8 to 10 and 18 to 26 were incubated for 120 min. Lanes A, B, and C contain 0, 100, and 1 mM m7GpppG cap analogue as a competitor. Lanes D, E, and F contain increasing amounts of SR proteins: 0.75, 1.5, and 3 μg. Cap analogues and SR proteins were preincubated with the extract before the addition of the pre-mRNA and splicing mix. Lane 27 (M) contains size markers. Spliced products and intermediates were resolved on a 6% denaturing polyacrylamide gel. (B) Splicing of m7GpppG- and ApppG-capped adenovirus pre-mRNA with a duplicated wild-type 5′ splice site (Ad1WW). (C) Splicing of adenovirus pre-mRNA with duplicated consensus 5′ splice sites (Ad1CC). Splicing reactions were for 40 min (lanes 1 and 3) or 1.5 h (lanes 2 and 4).
Splicing to all 5′ splice sites was severely reduced with ApppG-capped substrates (Fig. 1A, lanes 1 to 7; 1B, lane 2; 1C, lanes 3 and 4). The inhibition of splicing of the β-globin substrate by exogenous m7GpppG (Fig. 1A, lanes 19 and 20) and the ability of SR proteins to compensate for an ApppG cap (Fig. 1A, lanes 21 to 23) show that the β-globin substrate behaves like the Ad1 pre-mRNA studied previously (42). We conclude that (i) the m7GpppG 5′ cap structure enhances splicing at all three 5′ splice site sequences (β-globin, Ad1, and consensus) and that (ii) both cap-proximal (β-globin and Ad1WW) and cap-distal (Ad1CC) sites are affected. The absence of the cap did not noticeably alter preferences in the residual splicing reactions. These results suggest that the role of the cap is not restricted to enhancing U1 snRNP binding at sites where it is hindered but that the cap affects a constitutive step in the reaction.
Protection of consensus 5′ splice sites in either m7GpppG- or ApppG-capped pre-mRNAs.
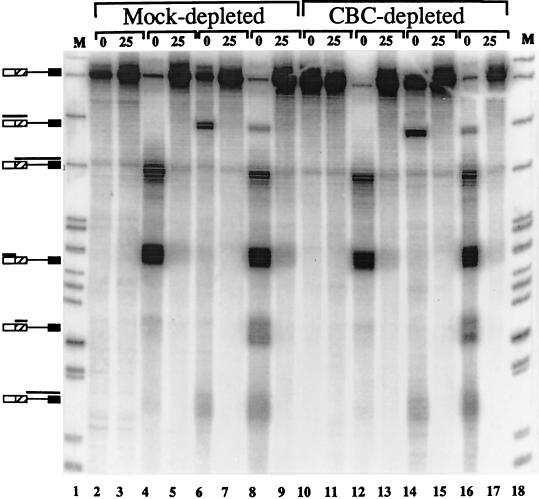
The effect of the 5′ cap on the interactions of U1 snRNPs was examined in extracts either depleted of CBC or mock depleted. The efficiency of the depleted extract in splicing was reduced compared to that of the mock-depleted extract by 50 to 90% in several trials (data not shown). U1 snRNP binding was measured by an incubation-dependent increase in the protection of consensus 5′ splice sites against ribonuclease H when RNA was added to extracts lacking MgCl2, ATP, and creatine phosphate (14). Under these conditions, splicing complex assembly proceeds only as far as the E complex, which contains U1 snRNP bound to the 5′ splice site (47, 48, 71, 79). The substrate used was a capped β-globin IVS-2 pre-mRNA (C174C) containing two consensus sites. When an oligonucleotide complementary to the upstream 5′ splice site was added to the reaction mixture before the pre-mRNA, incubation with RNase H produced almost complete cleavage (i.e., most of the pre-mRNA was cleaved into two fragments in Fig. 2, lanes 4, 8, 12, and 16), but if the oligonucleotide was added 25 min after the RNA, the site was almost completely protected (Fig. 2, lanes 5, 9, 13, and 17). A smaller proportion of the pre-mRNA was cleaved initially at the downstream site, probably because of interfering RNA secondary structure (Fig. 2, lanes 6, 8, 14, and 16), but this, too, became completely protected after 25 min (Fig. 2, lanes 7, 9, 15, and 17). If the reduction in splicing of 50% or more was caused by a corresponding loss of U1 snRNP binding in the depleted extract, then at least 50% of the RNase H product fragments in lanes 12, 14, and 16 should have remained in lanes 13, 15, and 17. In fact, lanes 13, 15, and 17 were identical to lanes 5, 7, and 9, from which we conclude that there was equivalent U1 snRNP association in the two extracts. This was also found to be the case for Ad1 substrates that contained duplicated consensus 5′ splice sites and for C174C capped with m7GpppG or ApppG (data not shown). Thus, the 5′ cap was required for splicing but not for U1 snRNP-dependent complex assembly at 5′ splice sites. This suggested that the cap, via CBC and interacting factors, may have a critical role in splicing beyond permitting U1 snRNP binding.
FIG. 2.
RNase H protection of consensus 5′ splice sites. The substrate pre-mRNA was m7GpppG-capped C174C, derived from β-globin IVS-2, which contained two alternative consensus 5′ splice sites. Pre-mRNA was incubated in HeLa nuclear extract that had been depleted of CBC or had been mock depleted. Oligonucleotides that direct RNase H cleavage to the consensus 5′ splice sites were added either just before the pre-mRNA (0) or 25 min after the pre-mRNA (25). No MgCl2 or ATP was added to the reaction mixtures. No oligonucleotide was added to the reaction mixtures in lanes 2, 3, 10, and 11; an oligonucleotide directed against the upstream site was added in lanes 4, 5, 12, and 13; an oligonucleotide directed against the downstream site was added in lanes 6, 7, 14, and 15; both were added to the reaction mixtures in lanes 8, 9, 16, and 17. The reaction mixtures were resolved on a 6% polyacrylamide gel. The products of RNase H cleavage are indicated with bars above the diagrams of the pre-mRNA on the left-hand side.
Analysis of snRNA binding by cross-linking.
The lack of a requirement for the cap to mediate U1 snRNP binding appeared to be inconsistent with previous findings (42), but the inconsistency could be attributed to differences in the substrate or method of detection; Ad1 pre-mRNA and psoralen-mediated UV cross-linking were used in the earlier study (42). The effect of differences in the 5′ splice site sequence could not be tested by RNase H cleavage, which does not detect initial interactions at nonconsensus 5′ splice sites efficiently (14). Thus, we used psoralen-mediated UV cross-linking to examine Ad1 pre-mRNA. The presence of the psoralen did not affect splicing (see below). The Ad1 substrates were modified at the 5′ splice site and at a cryptic site 93 nt downstream such that they contained two wild-type sites (WW), two consensus sites (CC), two nonfunctional sites (MM), or mixtures (WM and MW). The sites of cross-linking by psoralen to Ad1 wild-type substrate (equivalent to Ad1WM) have been characterized previously (79).
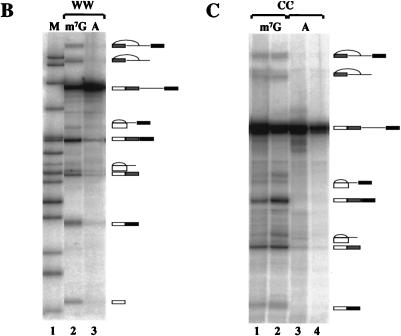
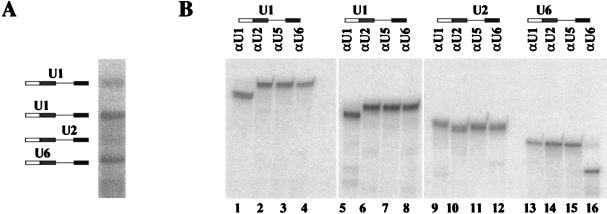
A number of UV-dependent bands were detected by electrophoresis that moved with lower mobility than the pre-mRNA (Fig. 3). These were characterized by oligonucleotide-directed RNase H cleavage of purified cross-linked molecules. A preparative cross-linking reaction was carried out with Ad1WW, and RNA was extracted from each band after electrophoresis. The RNA was treated with RNase H and oligonucleotides complementary to either U1, U2, U5, or U6 snRNA (Fig. 4). The results showed that there were two major cross-links to U1 snRNA and one to each of the U2 and U6 snRNAs. No cross-links to U5 snRNA were detected. The assignments of bands to the upstream and downstream 5′ splice sites were confirmed by separate RNase H digestions of each purified cross-linked product at eight sites in the pre-mRNA spaced approximately 40 nt apart (data not shown). The bands visible in Fig. 3 between the two major U1 snRNA-pre-mRNA cross-linked products (labelled band a) and between the upstream U1 cross-link and the U2-pre-mRNA cross-linked products (labelled band b) were also isolated and shown to comprise, respectively, wholly or largely U1 snRNA cross-linked to the downstream and upstream 5′ splice sites; their intensities changed in proportion to changes in the intensities of the corresponding major U1 snRNA cross-links. Double bands resulting from U1 snRNA cross-linking to the Ad1 5′ splice site have been observed previously (79, 80).
FIG. 4.
Identification of snRNA cross-linked to pre-mRNA. (A) Splicing reaction mixtures containing m7GpppG-capped Ad1WW were irradiated (as shown in Fig. 3, lane 3) and resolved on a 5% preparative polyacrylamide gel. (B) The cross-linked products shown in panel A were digested with RNase H and oligonucleotides complementary to either U1, U2, U5, or U6 snRNA and then fractionated onto a 5% polyacrylamide gel. The diagram above each gel indicates the identity of the snRNA shown to be cross-linked to the pre-mRNA.
The assignments made in Fig. 4 were shown to be correct for all the substrates shown in Fig. 3 by cleavage of parallel portions of each m7G-capped RNA reaction mixture with oligonucleotides complementary to U1 or U2 snRNA (data not shown). This showed that comigrating bands produced with different substrates contained the same snRNAs. In addition, the assignments fit the interactions expected with different substrates; Ad1WM has only the upstream U1 cross-links (Fig. 3, lanes 5 and 6), Ad1MM has no snRNA cross-links (Fig. 3, lanes 7 and 8), and neither U2 nor U6 cross-links are seen in reaction mixtures lacking ATP (Fig. 3, lanes 9 to 16).
With Ad1CC pre-mRNA, the cross-linking experiments confirmed the findings from RNase H protection (Fig. 2) that U1 snRNP interacted with both consensus 5′ splice sites and was not dependent on the m7GpppG or ApppG cap structure (Fig. 3, lanes 1 to 2 and 9 to 10). Similarly, U1 snRNA was cross-linked to wild-type 5′ splice sites whether the pre-mRNA had been primed with m7GpppG or ApppG (Fig. 3, lanes 3 to 4 and 11 to 12). This was true even when only E complex could be formed, in experiments with splicing reactions lacking magnesium and ATP (Fig. 3, lanes 11 to 14). Quantification showed that the absence of a m7GpppG cap caused no proportional reduction in the level of U1 cross-links in the latter experiments. In some other experiments, the level of U1 cross-linking in reactions lacking magnesium and ATP was reduced by one-third or a half with ApppG-capped RNA, but the extent was variable. We conclude that the association of U1 snRNPs with the pre-mRNA is not substantially dependent on the 5′ cap.
Effects of the 5′ cap on splicing complex assembly.
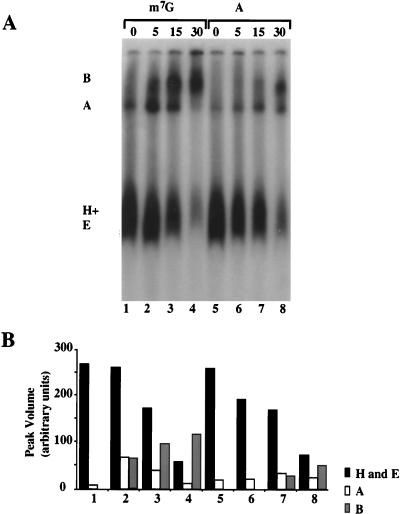
Comparisons on native gels of splicing complexes formed with m7GpppG- and ApppG-primed pre-mRNA indicated that both substrates can form A and B complexes (Fig. 5A, lanes 1 to 8; quantification in Fig. 5B). The A complex is the first ATP-dependent step in spliceosome assembly in which U2 snRNP enters the complex (2, 19, 33, 34, 47), and the ability of the ApppG-capped Ad1WW pre-mRNA to form this complex was expected from the psoralen cross-links to U2 snRNA described above. However, complex formation was less than might have been expected from the cross-linking. Thus, even though U1 snRNP binding to the substrate appeared to be normal, the ApppG cap appears to affect either the rate of the subsequent steps in complex assembly or the stability of the complexes on native gel electrophoresis (56). Furthermore, the B complexes formed do not splice in proportion to their abundance; even after prolonged incubation, the splicing efficiency of ApppG-capped RNA was usually extremely low (see below).
FIG. 5.
(A) Native gel analysis of splicing complexes formed with m7GpppG and ApppG primed Ad1 pre-mRNA. The incubation times for the splicing reactions are shown. The presumed identities of the bands are shown as complexes B, A, and H plus E. (B) Quantitative analysis of the data in panel A. Numbers correspond to the lanes in panel A.
Appearance of cap-dependent RNA cross-links.
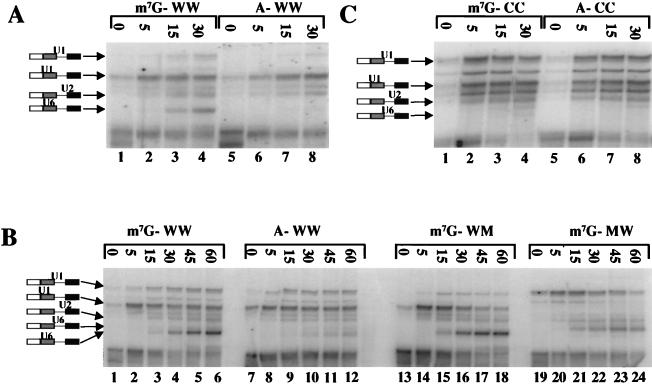
The native gels suggested that the 5′ cap affects both the rate of formation of complexes and steps after the assembly of complex B but prior to step 1. These effects were examined by psoralen cross-linking. Splicing reactions with the Ad1 substrate WW were incubated in the presence of psoralen at 30°C for the times shown. Samples were then irradiated in pairs at ambient temperature for 1 min each. Representative results are shown in Fig. 6A; Fig. 7A shows the results of quantitative analysis. Samples were removed also at 40 min and 1.5 h, without cross-linking, for analysis of splicing. The splicing efficiencies (calculated as mRNA/[mRNA + pre-mRNA]) after 1.5 h were 44 and 4% (for mRNA resulting from upstream and downstream 5′ splice site use, respectively) for m7GpppG-Ad1WW and 1.8 and 0% for the corresponding products from ApppG-Ad1WW. Thus, the difference in splicing efficiency was over 20-fold.
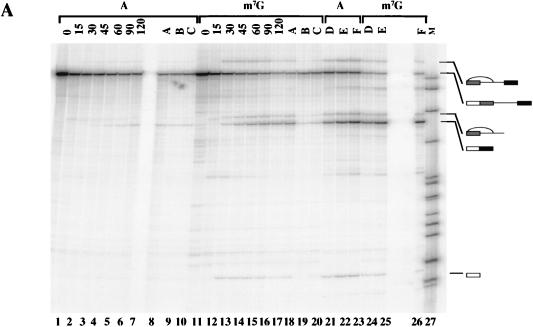
FIG. 6.
Effects of the 5′ cap and splice site sequence on snRNA interactions with pre-mRNA during time courses of splicing reactions. (A) Ad1WW pre-mRNA, capped with m7GpppG or ApppG, was incubated under splicing conditions in the presence of psoralen at 30°C. After the times shown above the lanes, reaction mixtures were removed and irradiated for 1 min each at ambient temperature. The results were analyzed by gel electrophoresis. The bands at the foot of the panel are intramolecular cross-links. Parallel reactions included (i) duplicates that contained an exogenous protein but showed the same features on quantitative analysis, (ii) reactions in the absence of MgCl2 and ATP, and (iii) reactions that were incubated for 40 and 90 min but not irradiated to allow splicing efficiency to be measured. (B) Ad1WW, Ad1WM, and Ad1MW pre-mRNAs were incubated under splicing conditions as in panel A, but for longer periods; irradiated; and analyzed as in panel A. The unlabelled lanes contained RNA from parallel reactions for 2.7 h that had not been irradiated so that the efficiency of splicing could be measured. The band shown as comprising U6 snRNA cross-linked to the downstream site, which is most obvious in the reaction of Ad1MW (lanes 21 to 24), has not been demonstrated directly to be this molecule, but its substrate specificity, kinetics, and mobility are consistent with this identification. (C) Identical to panel A in all respects, including the parallel reactions, but with Ad1CC pre-mRNA.
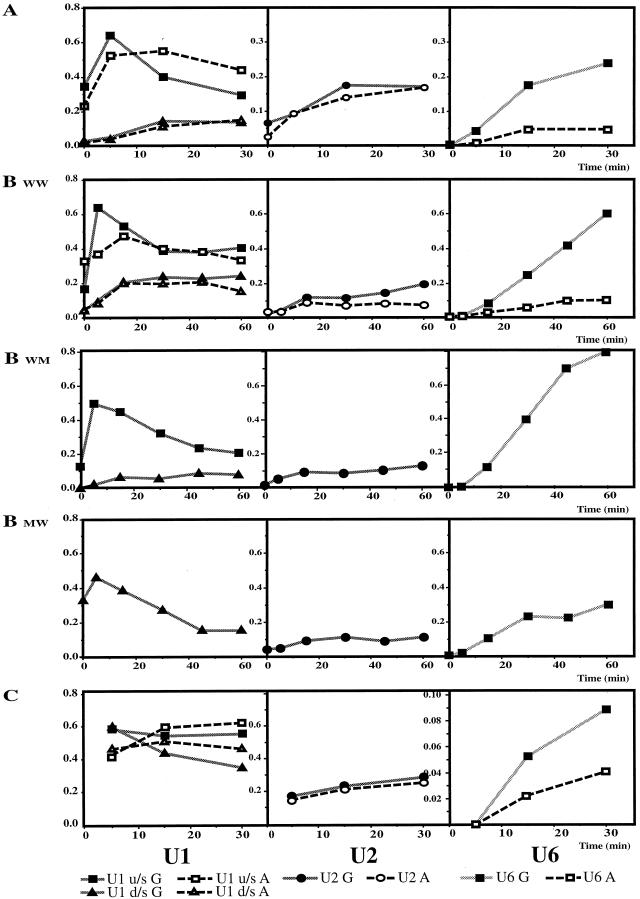
FIG. 7.
Quantitative analysis of the cross-links shown in Fig. 6A, B, and C refers to the three panels of Fig. 6, with the corresponding substrate designations for panel B. The y axis in every case shows the percentage of the cross-linked RNA molecule in the reaction mixture (see Materials and Methods); the x axis shows the time of incubation under splicing conditions (in minutes). The reactions of RNA capped with m7GpppG and ApppG are shown on the same graphs. The three graphs in each row show results for U1, U2 and U6 cross-links, respectively, with the identities of the lines shown below. u/s, upstream; d/s, downstream.
The cross-linking results quantified in Fig. 7A showed five main features: (i) the m7GpppG cap did not increase the final level of U1 binding to the upstream 5′ splice site, but it increased the rate of both the initial binding and subsequent dissociation; (ii) there was no significant effect of the cap on U1 snRNA cross-linking to the downstream 5′ splice site; (iii) U1 snRNA cross-linking to the upstream 5′ splice site predominated over the downstream 5′ splice site, regardless of the cap; (iv) the cap made little reproducible difference to the level of U2 snRNA cross-linking; (v) U6 snRNA cross-linking was virtually undetectable in the absence of the correct cap.
The steady increase in U6 cross-linking appeared to be the major quantitative difference between the reaction mixtures containing Ad1WW RNA capped with m7GpppG or ApppG. The sharp rise and fall in U1 cross-linking to the upstream 5′ splice site also appeared to depend on the cap, but it was not clear whether this property was associated with use of a site for splicing or with the upstream site in particular. Both of these aspects were followed during longer time courses (Fig. 6B; quantitative results shown in Fig. 7B). The results from the comparison of Ad1WW caps were very much the same as before, but it was noticeable that, whereas U6 snRNA cross-linking to the upstream 5′ splice site of m7GpppG pre-mRNA continued to accumulate, its cross-linking to the RNA capped with ApppG did not accelerate with time, indicating that this step was slower rather than delayed in its onset. The sharp rise and fall in U1 snRNA cross-linking, noted at the predominant (upstream) 5′ splice site of Ad1WW, was seen at the upstream site of Ad1WM and at the downstream site of Ad1MW. Thus, this pattern was seen with both splice sites when they were used significantly for splicing but neither at the less-used site in m7GpppG-capped Ad1WWn or in the absence of the m7GpppG cap. The samples incubated for 2.7 h without irradiation were also run on an 8% polyacrylamide gel. The percentages of RNA at that time that had undergone at least step 1 of splicing were as follows: Ad1WW G cap, 24 and 6.3% (upstream and downstream, respectively); Ad1WW A cap, 3.3 and 0.5%; Ad1 WM G cap, 49%; Ad1 MW G cap, 55%.
The cross-linking results with wild-type splice sites are consistent not with a role for the cap in determining whether U1 snRNPs will bind but, rather, in enabling binding to be productive. One possible mechanism might involve the recruitment of U1 snRNPs such that they bind rapidly to the site closest to the cap and permit subsequent interactions there. This possibility was tested with Ad1CC RNA which, as predicted for a pre-mRNA with two consensus 5′ splice sites (14), splices preferentially to the downstream (cap-distal) site. A time course of incubation, followed by cross-linking, is shown in Fig. 6C; quantitative data are given in Fig. 7C. After 1.5 h, 5% of the RNA had undergone at least step 1 at the upstream site and 22% at the downstream site (where total RNA at 1.5 h is defined as follows: pre-mRNA + both 5′ exon intermediates, corrected + both mRNA products, corrected). No splicing was detected with the ApppG-capped RNA. If the 5′ cap is responsible for determining the site of splicing, the cap effect on U1 snRNA cross-linking should be the converse of the findings with Ad1WW, i.e., the effect at the upstream site should be greatly reduced or absent. As with Ad1WW, U1 snRNP binding to ApppG-capped RNA peaked at 15 min, but the levels of binding to both sites were approximately equal. However, although the m7GpppG-capped RNA did show more rapid U1 snRNP binding, peaking at 5 min, binding was enhanced equally at both sites (Fig. 7C, U1). The subsequent decline in U1 snRNA cross-linking was more marked at the site used predominantly for splicing. We conclude that the 5′ cap is required for the productive binding of U1 snRNPs at 5′ splice sites but that it does not determine the site at which this happens. The twofold difference in U6 snRNA cross-linking between the two RNA samples is comparatively small, but the intensities were very low, so the measurements are not reliable.
Inhibition of the U6 but not U1 snRNA cross-links by the addition of m7GpppG cap analogue as a competitor.
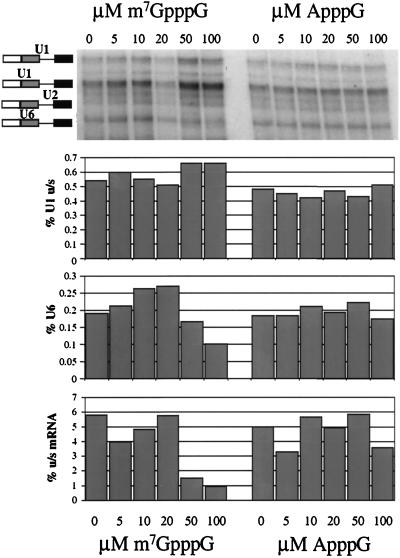
The cap dependence of splicing and cross-linking reported here could be attributed to inhibition by the ApppG cap rather than to dependence on an m7GpppG cap. This was tested by incubation of m7GpppG-capped Ad1WW RNA with increasing concentrations of free cap analogues; a requirement for recognition of the correct cap would be reflected in inhibition by m7GpppG but not by ApppG. The results showed that the U6 snRNA cross-link was reduced by 50 and 100 μM m7GpppG but that it was unaffected by equivalent concentrations of ApppG (Fig. 8). The efficiency of splicing was also reduced at these concentrations. In contrast, U1 snRNA cross-linking was not reduced. The drop in U6 cross-linking and splicing at 50 and 100 μM m7GpppG is highly reproducible. The apparent increase in U6 snRNA cross-linking at lower concentrations was also seen in other experiments, but it has not been investigated further. We conclude that m7GpppG is an effective competitor and that recognition of the m7GpppG 5′ cap is required for the replacement of U1 snRNA at a 5′ splice site by U6 snRNA.
FIG. 8.
Inhibition of U6 snRNA interactions with m7GpppG-capped Ad1WW pre-mRNA by the m7GpppG cap analogue but not the ApppG cap analogue. HeLa nuclear extract was preincubated with the m7GpppG or the ApppG cap analogue for 15 min prior to the addition of the pre-mRNA. The final concentrations of m7GpppG and ApppG were 0, 5, 10, 20, 50, and 100 μM, as marked above the gel. The cross-linked RNA was resolved on a 5% polyacrylamide gel. The bar charts aligned below the image show the relative intensities of specific products and the pre-mRNA (expressed as percentages) for each lane. The products measured are U1 cross-linked to the upstream 5′ splice site (the major site of splicing), U6 cross-linked, and the upstream (u/s) mRNA, as indicated by the y axis labels.
DISCUSSION
The purpose of these experiments was to investigate further the implications of previous evidence that the binding of U1 snRNPs to an adenovirus 5′ splice site depended on an interaction between the m7GpppG cap and the CBC and that this caused the observed dependence of splicing on the cap. Strikingly, our first results showed that U1 snRNP binding did not require the correct cap, even though splicing was all but eliminated in the absence of the cap. Thus, even though the low concentrations of pre-mRNA used may have precluded the detection of changes in the overall affinity of the U1 complex for the 5′ splice site in the presence of different caps, the fact that splicing was inhibited in these reactions allows us to conclude that the dependence of splicing on a 5′ cap is not a consequence of any effect of the cap on the overall level of U1 binding. Subsequent quantitative analysis of psoralen cross-linking during time courses of splicing showed that there are two different kinetic pathways for U1 snRNA to cross-link to the 5′ splice sites: wild-type splice sites that were not to undergo splicing (either because of their location or because of the lack of a cap) bound U1 snRNA more slowly, although the final level might be substantial, but sites that were to be spliced bound U1 snRNPs much more rapidly. The 5′ cap was required for splicing and thus for the productive mode of binding; binding to an unused alternative site was unaffected by the 5′ cap. Substrates that lacked the correct cap did form large complexes, albeit less efficiently, but these did not lead to proportional levels of splicing. Instead, there was a very marked effect of the cap on U6 snRNA interactions at the wild-type 5′ splice site.
Our finding that the correct cap was not required for U1 snRNP binding as such is consistent with early studies on complex formation (52, 61) and with the properties of purified U1 snRNPs (see the introduction) but in clear disagreement with the first report that the cap and CBC are required in extracts (42). However, most of the evidence in that report was based on psoralen cross-linking and gel filtration with a 5′ fragment of the Ad1 pre-mRNA, and it is possible that U1 snRNP binding to this portion is unstable in nuclear extracts in the absence of the cap. Thus, the kinetic effect on U1 snRNP binding that we have detected on intact pre-mRNA may reflect interactions that, in the absence of the 3′ splice site region, stabilize binding.
Does the 5′ cap act directly or via U1 to promote U6 snRNP interactions at the 5′ splice site?
The rapid rise and fall in U1 snRNP binding to active 5′ splice sites was seen in earlier studies with psoralen and site-specific 4-thiouridine cross-linking (71, 79, 80). The fall in U1 snRNP binding is likely to be associated with displacement by U6 snRNA, as it in turn base pairs with the 5′ splice site (24, 29, 35, 36, 40, 44, 64, 65, 73, 79). The effects of the cap on these two processes might be contingent or separate. Thus, it is possible that the slower binding of U1 snRNPs to ApppG-capped pre-mRNA results in a conformation that permits the association of other components to form large complexes (albeit at a slower rate) but that is not compatible with the interactions required to displace U1 snRNA and introduce U6 snRNA. It is notable that U1 snRNP binding to short uncapped 5′ splice site RNA oligonucleotides prevented the assembly of U2-U5-U6 complexes (36). The alternative possibility is that the CBC acts directly at a later stage in the spliceosome to facilitate U6 snRNA interactions.
Some support for the contingent effect on U6 snRNA comes from experiments in which m7GpppG was added as a competitor at various times before or after the addition of m7GpppG-capped Ad1WW RNA; splicing was inhibited if the competitor was added before or with the substrate but not if it was added even as early as 5 min afterwards (data not shown). The ability of exogenous SR proteins (members of the serine/arginine family of proteins) to circumvent cap dependence (reference 42 and Fig. 1) might also suggest that the determining effect of the cap is on U1 snRNP binding to 5′ splice sites, because some SR proteins enhance this (14, 27, 31, 42, 74, 81), but there is evidence that they affect splicing reactions at later stages as well (3, 46, 59, 74). A separate and direct effect of the cap on the replacement of U1 snRNP by U6 snRNA at the 5′ splice site might require the presence of the CBC in mature spliceosomes. The CBC appears to remain associated throughout splicing (42). Thus, neither mechanism can be ruled out yet. It should be possible to determine whether the effect on U6 snRNA cross-linking is an indirect consequence of the cap effect on U1 snRNP binding by testing pre-mRNA sequences that can splice in the absence of U1 snRNPs and without additional SR proteins (10).
The 5′ cap acts on U1 snRNPs that bind to the preferred 5′ splice sites.
The effects of the cap on the binding of U1 snRNPs to the wild-type splice sites in Ad1WW might be consistent with several possible functions. Given that Ad1WW spliced almost exclusively from the upstream 5′ splice sites, it might be argued that the rapid rise and fall in U1 cross-linking was a unique property of that site. However, when that site was deleted (Ad1MW), the downstream site of the cap-dependent substrate (data not shown) showed a similar profile (Fig. 6B and 7B). Thus, the cap seems to induce the profile associated with productive binding at whichever site is used. The function of the 5′ cap, therefore, might be to determine the site at which U1 snRNP binding would be rapid, or it might simply be required for rapid binding at sites specified previously. The first possibility can be excluded by the results with Ad1CC (Fig. 6B and 7B), which showed that the cap promoted rapid binding of U1 snRNPs at both alternative sites, even though the use of the upstream site was as small as that of the downstream site in Ad1WW. Instead, it appears that the stronger U1 snRNP binding to consensus sites is sufficient for rapid binding in the presence of the cap, whereas cap-dependent productive binding to Ad1WW was restricted to the upstream site. We conclude that the cap is required for rapid binding of U1 snRNPs but that this activity itself is not selective. We cannot exclude an additional earlier role in other substrates for the cap in the process that determines which of the two wild-type sites is to be used.
Potentially productive U1 snRNP binding at both consensus 5′ splice sites is associated with use of the downstream site.
The role of U1 snRNP binding in 5′ splice site selection is still unclear (reviewed in reference 4). Despite genetic evidence showing that U1 snRNA base pairing can affect preferences between alternative sites (67, 82), at least when they are well separated (24), and that this is in turn can determine U6 recruitment (24), the molecular mechanisms have not been established. We have shown previously that both of two alternative consensus 5′ splice sites can be occupied initially simultaneously by U1 snRNPs (14), and we proposed that this would be normal with strong sites; the marked preference among tandem strong 5′ splice sites for the downstream site, both in vitro and in vivo (11, 14), could be accounted for by interactions of 3′ components with the nearest occupied site (14). Interestingly, the same preference for a downstream site was elicited upon transfection of U1 and U6 snRNA genes that complemented tandem weak 5′ splice sites perfectly, suggesting that interactions with these two components were sufficient for a site to behave as a strong site (24). The cross-linking results shown here (Fig. 7C) with Ad1CC are also consistent with this proposal; the cap-dependent rapid binding to both sites, followed by splicing primarily to the downstream site, suggests that potentially productive or competent U1 snRNP binding has taken place on both sites simultaneously and that selection of the downstream site depends neither on the 5′ cap nor on any selectivity in the U1 snRNP interaction. Genetic evidence (24) suggests that U6 snRNA is likely to mediate the selection.
Mechanisms that restrict the cap effect on U1 snRNP binding to one alternative wild-type site.
For weaker sites, we suggested previously (14) that the probability of simultaneous binding was lower and that the outcome would depend on the relative levels of U1 snRNP binding; if the use of a site depended on its being occupied at some critical point in the reaction, the relative probabilities of the sites being occupied at that point would determine the ratio of use. The psoralen cross-linking data from Ad1WW show that both sites can be bound by U1 snRNPs, but the levels are quite unequal. In the absence of the m7GpppG cap, the level of binding to the downstream site is considerably lower, especially at early times. Thus, cap proximity does not appear to determine the preferences. Instead, the exclusive effect of the cap on binding to the upstream site may merely reflect the existence of a limited period of time, up to the critical point described above, during which the cap accelerates binding; afterwards, when one U1 snRNP has occupied 100% of the upstream sites and is interacting with 3′ components, the 5′ cap may not be able to facilitate U1 snRNP binding elsewhere (it might, for example, be sequestered within the nascent spliceosome). This interpretation is consistent with the evidence from Ad1CC, in which the levels of U1 snRNP binding to the two sites in ApppG-capped RNA are more equal and the m7GpppG 5′ cap accelerates both.
The identities of the proteins that mediate the effects of the cap on U1 snRNA interactions at the 5′ splice site are not known. Yeast two-hybrid screens have shown that CBP80 can interact with several proteins (30), including hnRNP F (18), but none of these has been shown to affect U1 snRNP binding directly. Some of the factors that might influence cap-independent U1 snRNP binding rates can be surmised. In the present case, for example, the sequences of the two 5′ splice sites to which U1 snRNA could base pair in Ad1WW are identical, but the rates of U1 snRNP binding might be determined by either RNA secondary structure or the proximity of proteins that impede or hasten binding. U1 snRNP binding is enhanced by several SR proteins (14, 27, 31, 81), which in Ad1 pre-mRNA are known to cross-link in the E complex to sequences in the 5′ exon 26 to 31 nt 5′ of the 5′ splice site (6); at limiting concentrations, these may favor U1 snRNP binding at the closest 5′ splice site. Cross-linking does not support the proposal (83) that SR proteins bind to or select the 5′ splice site directly. Several other unidentified proteins have been shown by cross-linking to contact the 5′ splice site with or before U1 snRNA (80). These might affect U1 snRNP preferences for the upstream 5′ splice site, or they might mediate the effects of the 5′ cap.
The 5′ cap had relatively little effect on the interaction of U2 snRNA at the branch site. Given that U2-containing complexes can assemble in the absence of a functional 5′ splice site (7, 33, 36, 38, 39, 48, 61), this result suggests that the primary effect of the cap is at the 5′ splice site and that it does not have a more general role in facilitating the assembly of complexes.
Correlations between the 5′ cap, splicing efficiency, and U6 cross-links.
The effect of the cap on U6 snRNA interactions at the 5′ splice site was substantial. Interestingly, the rate of the linear increase in U6 snRNA cross-linking to the upstream 5′ splice site between 5 and 45 min for three substrates (m7GpppG-capped Ad1WW, m7GpppG-Ad1WM, and ApppG-Ad1WW) in Fig. 6B was proportional to the level of splicing (step 1) after 2.7 h (data not shown), perhaps implying that no subsequent step is affected grossly by the cap. In contrast, the m7GpppG-capped substrates Ad1WM and Ad1MW spliced with similar efficiencies, even though the level of cross-linking to U6 snRNA was quite different (Fig. 6B and 7B). This result might imply that U6 snRNA interactions are not rate limiting at the downstream splice site because of its distance from the 5′ cap or for other reasons, but it is also possible that the site itself affects the cross-linking efficiency and that comparisons should not be made between sites.
A candidate protein for mediating the effects of the cap directly on the replacement of U1 snRNP at the 5′ splice site by U6 snRNP is the U5 100-kDa component of the U5 and [U4-U6-U5] tri-snRNPs, which contains an N-terminal RS domain and a C-terminal domain that has sequences suggestive of RNA unwinding activity (76). The C-terminal domain is similar in sequence to the yeast Prp28p, which has been proposed to facilitate the U1-to-U6 transition (72). It would be most interesting to determine whether these proteins interact with the CBC.
Whether the 5′ cap participates directly in snRNA rearrangements in the spliceosome or whether it only ensures that U1 snRNP is bound in a manner that is sterically or kinetically compatible with such rearrangements, its role seems to be more subtle and more complex than the mere recruitment of U1 snRNPs to the 5′ splice site.
ACKNOWLEDGMENTS
We are grateful to J. Lewis and I. W. Mattaj for hospitality, advice, and materials during a visit by L.O. to EMBL.
This work was supported by a Human Capital and Mobility grant from the Commission of the European Communities and by the Medical Research Council, with an equipment grant from the Wellcome Trust.
REFERENCES
- 1.Berget S M. Exon recognition in vertebrate splicing. J Biol Chem. 1995;270:2411–2414. doi: 10.1074/jbc.270.6.2411. [DOI] [PubMed] [Google Scholar]
- 2.Bindereif A, Green M R. An ordered pathway of snRNP binding during mammalian pre-messenger RNA splicing complex assembly. EMBO J. 1987;6:2415–2424. doi: 10.1002/j.1460-2075.1987.tb02520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao W H, Jamison S F, Garcia-Blanco M A. Both phosphorylation and dephosphorylation of ASF/SF2 are required for pre-mRNA splicing in vitro. RNA. 1997;3:1456–1467. [PMC free article] [PubMed] [Google Scholar]
- 4.Chabot B. Directing alternative splicing: cast and scenarios. Trends Genet. 1996;12:472–477. doi: 10.1016/0168-9525(96)10037-8. [DOI] [PubMed] [Google Scholar]
- 5.Chabot B, Steitz J A. Recognition of mutant and cryptic 5′ splice sites by the U1 small nuclear ribonucleoprotein in vitro. Mol Cell Biol. 1987;7:698–707. doi: 10.1128/mcb.7.2.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiara M D, Gozani O, Bennett M, Champion-Arnaud P, Palandjian L, Reed R. Identification of proteins that interact with exon sequences, splice sites, and the branchpoint sequence during each stage of spliceosome assembly. Mol Cell Biol. 1996;16:3317–3326. doi: 10.1128/mcb.16.7.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiara M D, Reed R. A 2-step mechanism for 5′ and 3′ splice site pairing. Nature. 1995;375:510–513. doi: 10.1038/375510a0. [DOI] [PubMed] [Google Scholar]
- 8.Cho E J, Takagi T, Moore C R, Buratowski S. Messenger RNA capping enzyme is recruited to the transcription complex by phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev. 1997;11:3319–3326. doi: 10.1101/gad.11.24.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colot H V, Stutz F, Rosbash M. The yeast splicing factor mud13p is a commitment complex component and corresponds to CBP20 the small subunit of the nuclear cap-binding complex. Genes Dev. 1996;10:1699–1708. doi: 10.1101/gad.10.13.1699. [DOI] [PubMed] [Google Scholar]
- 10.Crispino J D, Mermoud J E, Lamond A I, Sharp P A. Cis-acting elements distinct from the 5′ splice site promote U1-independent pre-messenger RNA splicing. RNA. 1996;2:664–673. [PMC free article] [PubMed] [Google Scholar]
- 11.Cunningham S A, Else A J, Potter B, Eperon I C. Influences of separation and adjacent sequences on the use of alternative 5′ splice sites. J Mol Biol. 1991;217:265–281. doi: 10.1016/0022-2836(91)90541-d. [DOI] [PubMed] [Google Scholar]
- 12.Daneholt B. A look at messenger RNP moving through the nuclear pore. Cell. 1997;88:585–588. doi: 10.1016/s0092-8674(00)81900-5. [DOI] [PubMed] [Google Scholar]
- 13.Edery I, Sonenberg N. Cap-dependent RNA splicing in a HeLa nuclear extract. Proc Natl Acad Sci USA. 1985;82:7590–7594. doi: 10.1073/pnas.82.22.7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eperon I C, Ireland D C, Smith R A, Mayeda A, Krainer A R. Pathways for selection of 5′ splice sites by U1 snRNPs and SF2/ASF. EMBO J. 1993;12:3607–3617. doi: 10.1002/j.1460-2075.1993.tb06034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flaherty S M, Fortes P, Izaurralde E, Mattaj I W, Gilmartin G M. Participation of the nuclear cap binding complex in pre-MRNA 3′ processing. Proc Natl Acad Sci USA. 1997;94:11893–11898. doi: 10.1073/pnas.94.22.11893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fresco L D, Buratowski S. Conditional mutants of the yeast messenger RNA capping enzyme show that the cap enhances, but is not required for, messenger RNA splicing. RNA. 1996;2:584–596. [PMC free article] [PubMed] [Google Scholar]
- 17.Furuichi Y, La Fiandra A, Shatkin A J. 5′ Terminal structure and mRNA stability. Nature. 1977;266:235–239. doi: 10.1038/266235a0. [DOI] [PubMed] [Google Scholar]
- 18.Gamberi C, Izaurralde E, Beisel C, Mattaj I W. Interaction between the human nuclear cap-binding protein complex and hnRNP F. Mol Cell Biol. 1997;17:2587–2597. doi: 10.1128/mcb.17.5.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grabowski P J, Sharp P A. Affinity chromatography of splicing complexes: U2, U5, and U4+U6 small nuclear ribonucleoprotein particles in the spliceosome. Science. 1986;233:1294–1299. doi: 10.1126/science.3638792. [DOI] [PubMed] [Google Scholar]
- 20.Green M R, Maniatis T, Melton D A. Human beta-globin pre-mRNA synthesized in vitro is accurately spliced in Xenopus oocyte nuclei. Cell. 1983;32:681–694. doi: 10.1016/0092-8674(83)90054-5. [DOI] [PubMed] [Google Scholar]
- 21.Grummt I, Skinner J A. Efficient transcription of a protein-coding gene from the RNA polymerase-I promoter in transfected cells. Proc Natl Acad Sci USA. 1985;82:722–726. doi: 10.1073/pnas.82.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heinrichs V, Bach M, Winkelmann G, Lührmann R. U1-specific protein C needed for efficient complex formation of U1 snRNP with a 5′ splice site. Science. 1990;247:69–72. doi: 10.1126/science.2136774. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman B E, Grabowski P J. U1 snRNP targets an essential splicing factor, U2AF65, to the 3′ splice site by a network of interactions spanning the exon. Genes Dev. 1992;6:2554–2568. doi: 10.1101/gad.6.12b.2554. [DOI] [PubMed] [Google Scholar]
- 24.Hwang D Y, Cohen J B. U1 snRNA promotes the selection of nearby 5′ splice sites by U6 snRNA in mammalian cells. Genes Dev. 1996;10:338–350. doi: 10.1101/gad.10.3.338. [DOI] [PubMed] [Google Scholar]
- 25.Inoue K, Ohno M, Sakamoto H, Shimura Y. Effect of the cap structure on pre-mRNA splicing in Xenopus oocyte nuclei. Genes Dev. 1989;3:1472–1479. doi: 10.1101/gad.3.9.1472. [DOI] [PubMed] [Google Scholar]
- 26.Izaurralde E, Lewis J, McGuigan C, Jankowska M, Darzynkiewicz E, Mattaj I W. A nuclear cap-binding protein complex involved in pre-mRNA splicing. Cell. 1994;78:657–668. doi: 10.1016/0092-8674(94)90530-4. [DOI] [PubMed] [Google Scholar]
- 27.Jamison S F, Pasman Z, Wang J, Will C, Lührmann R, Manley J L, Garcia-Blanco M A. U1 snRNP-ASF/SF2 interaction and 5′ splice site recognition: characterization of required elements. Nucleic Acids Res. 1995;23:3260–3267. doi: 10.1093/nar/23.16.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jarmolowski A, Boelens W C, Izaurralde E, Mattaj I W. Nuclear export of different classes of RNA is mediated by specific factors. J Cell Biol. 1994;124:627–635. doi: 10.1083/jcb.124.5.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kandels-Lewis S, Séraphin B. Role of U6 snRNA in 5′ splice site selection. Science. 1993;262:2035–2039. doi: 10.1126/science.8266100. [DOI] [PubMed] [Google Scholar]
- 30.Kataoka N, Ohno M, Moda I, Shimura Y. Identification of the factors that interact with nCBP, an 80 kDa nuclear cap-binding protein. Nucleic Acids Res. 1995;23:3638–3641. doi: 10.1093/nar/23.18.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohtz J D, Jamison S F, Will C L, Zuo P, Lührmann R, Garcia-Blanco M A, Manley J L. Protein-protein interactions and 5′ splice site recognition in mammalian messenger RNA precursors. Nature. 1994;368:119–124. doi: 10.1038/368119a0. [DOI] [PubMed] [Google Scholar]
- 32.Konarska M M, Padgett R A, Sharp P A. Recognition of cap structure in splicing in vitro of mRNA precursors. Cell. 1984;38:731–736. doi: 10.1016/0092-8674(84)90268-x. [DOI] [PubMed] [Google Scholar]
- 33.Konarska M M, Sharp P A. Electrophoretic separation of complexes involved in the splicing of precursors to messenger RNAs. Cell. 1986;46:845–855. doi: 10.1016/0092-8674(86)90066-8. [DOI] [PubMed] [Google Scholar]
- 34.Konarska M M, Sharp P A. Interactions between small nuclear ribonucleoprotein particles in formation of spliceosomes. Cell. 1987;49:763–774. doi: 10.1016/0092-8674(87)90614-3. [DOI] [PubMed] [Google Scholar]
- 35.Konforti B B, Konarska M M. U4/U5/U6 snRNP recognizes the 5′ splice site in the absence of U2 snRNP. Genes Dev. 1994;8:1962–1973. doi: 10.1101/gad.8.16.1962. [DOI] [PubMed] [Google Scholar]
- 36.Konforti B B, Koziolkiewicz M J, Konarska M M. Disruption of base-pairing between the 5′ splice site and the 5′ end of U1 snRNA is required for spliceosome assembly. Cell. 1993;75:863–873. doi: 10.1016/0092-8674(93)90531-t. [DOI] [PubMed] [Google Scholar]
- 37.Krainer A R, Maniatis T, Ruskin B, Green M R. Normal and mutant human beta-globin pre-mRNAs are faithfully and efficiently spliced in vitro. Cell. 1984;36:993–1005. doi: 10.1016/0092-8674(84)90049-7. [DOI] [PubMed] [Google Scholar]
- 38.Krämer A. Pre-splicing complex formation requires 2 proteins and U2 snRNP. Genes Dev. 1988;2:1155–1167. doi: 10.1101/gad.2.9.1155. [DOI] [PubMed] [Google Scholar]
- 39.Lamond A I, Konarska M M, Sharp P A. A mutational analysis of spliceosome assembly—evidence for splice site collaboration during spliceosome formation. Genes Dev. 1987;1:532–543. doi: 10.1101/gad.1.6.532. [DOI] [PubMed] [Google Scholar]
- 40.Lesser C F, Guthrie C. Mutations in U6 snRNA that alter splice site specificity: implications for the active site. Science. 1993;262:1982–1988. doi: 10.1126/science.8266093. [DOI] [PubMed] [Google Scholar]
- 41.Lewis J D, Görlich D, Mattaj I W. A yeast cap binding protein complex (yCBC) acts at an early step in pre-mRNA splicing. Nucleic Acids Res. 1996;24:3332–3336. doi: 10.1093/nar/24.17.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewis J D, Izaurralde E, Jarmolowski A, McGuigan C, Mattaj I W. A nuclear cap-binding complex facilitates association of U1-snRNP with the cap-proximal 5′ splice site. Genes Dev. 1996;10:1683–1698. doi: 10.1101/gad.10.13.1683. [DOI] [PubMed] [Google Scholar]
- 43.Lo H J, Huang H K, Donahue T F. RNA polymerase I-promoted HIS4 expression yields uncapped, polyadenylated mRNA that is unstable and inefficiently translated in Saccharomyces cerevisiae. Mol Cell Biol. 1998;18:665–675. doi: 10.1128/mcb.18.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luukkonen B, Séraphin B. Genetic interaction between U6 snRNA and the first intron nucleotide in Saccharomyces cerevisiae. RNA. 1998;4:167–180. [PMC free article] [PubMed] [Google Scholar]
- 45.McCracken S, Fong N, Rosonina E, Yankulov K, Brothers G, Siderovski D, Hessel A, Poster S, Shuman S, Bentley D L. 5′-capping enzymes are targeted to pre-MRNA by binding to the phosphorylated carboxy-terminal domain of RNA polymerase II. Genes Dev. 1997;11:3306–3318. doi: 10.1101/gad.11.24.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mermoud J E, Cohen P, Lamond A I. Regulation of mammalian spliceosome assembly by a protein: phosphorylation mechanism. EMBO J. 1994;13:5679–5688. doi: 10.1002/j.1460-2075.1994.tb06906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Michaud S, Reed R. An ATP-independent complex commits pre-mRNA to the mammalian spliceosome assembly pathway. Genes Dev. 1991;5:2534–2546. doi: 10.1101/gad.5.12b.2534. [DOI] [PubMed] [Google Scholar]
- 48.Michaud S, Reed R. A functional association between the 5′ and 3′ splice sites is established in the earliest prespliceosome complex (E) in mammals. Genes Dev. 1993;7:1008–1020. doi: 10.1101/gad.7.6.1008. [DOI] [PubMed] [Google Scholar]
- 49.Mount S M, Pettersson I, Hinterberger M, Karmas A, Steitz J A. The U1 small nuclear RNA-protein complex selectively binds a 5′ splice site in vitro. Cell. 1983;33:509–518. doi: 10.1016/0092-8674(83)90432-4. [DOI] [PubMed] [Google Scholar]
- 50.Nelson K K, Green M R. Splice site selection and ribonucleoprotein complex assembly during in vitro pre-mRNA splicing. Genes Dev. 1988;2:319–329. doi: 10.1101/gad.2.3.319. [DOI] [PubMed] [Google Scholar]
- 51.Nigg E A. Nucleocytoplasmic transport: signals, mechanisms and regulation. Nature. 1997;386:779–787. doi: 10.1038/386779a0. [DOI] [PubMed] [Google Scholar]
- 52.Noble J, Prives C, Manley J L. In vitro splicing of simian virus-40 early pre-messenger RNA. Nucleic Acids Res. 1986;14:1219–1235. doi: 10.1093/nar/14.3.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Noble J, Prives C, Manley J L. Alternative splicing of SV40 early pre-mRNA is determined by branch site selection. Genes Dev. 1988;2:1460–1475. doi: 10.1101/gad.2.11.1460. [DOI] [PubMed] [Google Scholar]
- 54.Ohno M, Kataoka N, Shimura Y. A nuclear cap binding protein from HeLa cells. Nucleic Acids Res. 1990;18:6989–6995. doi: 10.1093/nar/18.23.6989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ohno M, Sakamoto H, Shimura Y. Preferential excision of the 5′ proximal intron from messenger RNA precursors with 2 introns as mediated by the cap structure. Proc Natl Acad Sci USA. 1987;84:5187–5191. doi: 10.1073/pnas.84.15.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patzelt E, Thalmann E, Hartmuth K, Blaas D, Kuechler E. Assembly of pre-mRNA splicing complex is cap dependent. Nucleic Acids Res. 1987;15:1387–1399. doi: 10.1093/nar/15.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reed R, Maniatis T. A role for exon sequences and splice-site proximity in splice-site selection. Cell. 1986;46:681–690. doi: 10.1016/0092-8674(86)90343-0. [DOI] [PubMed] [Google Scholar]
- 58.Robberson B L, Cote G J, Berget S M. Exon definition may facilitate splice site selection in RNAs with multiple exons. Mol Cell Biol. 1990;10:84–94. doi: 10.1128/mcb.10.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roscigno R F, Garcia-Blanco M A. SR proteins escort the U4/U6.U5 tri-snRNP to the spliceosome. RNA. 1995;1:692–706. [PMC free article] [PubMed] [Google Scholar]
- 60.Rossi F, Forne T, Antoine E, Tazi J, Brunel C, Cathala G. Involvement of U1 small nuclear ribonucleoproteins (snRNP) in 5′ splice site-U1 snRNP interaction. J Biol Chem. 1996;271:23985–23991. doi: 10.1074/jbc.271.39.23985. [DOI] [PubMed] [Google Scholar]
- 61.Ruskin B, Green M R. Specific and stable intron-factor interactions are established early during in vitro pre-mRNA splicing. Cell. 1985;43:131–142. doi: 10.1016/0092-8674(85)90018-2. [DOI] [PubMed] [Google Scholar]
- 62.Sachs A B, Sarnow P, Hentze M W. Starting at the beginning, middle, and end: translation initiation in eukaryotes. Cell. 1997;89:831–838. doi: 10.1016/s0092-8674(00)80268-8. [DOI] [PubMed] [Google Scholar]
- 63.Salditt-Georgieff M, Harpold M, Chen-Kiang S, Darnell J E. The addition of 5′ cap structures occurs early in hnRNA synthesis and prematurely terminated molecules are capped. Cell. 1980;19:69–78. doi: 10.1016/0092-8674(80)90389-x. [DOI] [PubMed] [Google Scholar]
- 64.Sawa H, Abelson J. Evidence for a base-pairing interaction between U6 small nuclear RNA and the 5′ splice site during the splicing reaction in yeast. Proc Natl Acad Sci USA. 1992;89:11269–11273. doi: 10.1073/pnas.89.23.11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sawa H, Shimura Y. Association of U6 snRNA with the 5′-splice site region of pre-mRNA in the spliceosome. Genes Dev. 1992;6:244–254. doi: 10.1101/gad.6.2.244. [DOI] [PubMed] [Google Scholar]
- 66.Schwer B, Shuman S. Conditional inactivation of messenger-RNA capping enzyme affects yeast pre-messenger RNA splicing in vivo. RNA. 1996;2:574–583. [PMC free article] [PubMed] [Google Scholar]
- 67.Séraphin B, Kandels-Lewis S. 3′ splice site recognition in Saccharomyces cerevisiae does not require base-pairing with U1 snRNA. Cell. 1993;73:803–812. doi: 10.1016/0092-8674(93)90258-r. [DOI] [PubMed] [Google Scholar]
- 68.Shatkin A J. Capping of eukaryotic mRNAs. Cell. 1976;9:645–653. doi: 10.1016/0092-8674(76)90128-8. [DOI] [PubMed] [Google Scholar]
- 69.Shatkin A J. Messenger RNA cap binding proteins: essential factors for initiating translation. Cell. 1985;40:223–224. doi: 10.1016/0092-8674(85)90132-1. [DOI] [PubMed] [Google Scholar]
- 70.Shimotohno K, Kodama Y, Hashimoto J, Miura K I. Importance of 5′-terminal blocking structure to stabilize mRNA in eukaryotic protein synthesis. Proc Natl Acad Sci USA. 1977;74:2734–2738. doi: 10.1073/pnas.74.7.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sontheimer E J, Steitz J A. The U5 and U6 small nuclear RNAs as active-site components of the spliceosome. Science. 1993;262:1989–1996. doi: 10.1126/science.8266094. [DOI] [PubMed] [Google Scholar]
- 72.Staley J P, Guthrie C. Mechanical devices of the spliceosome: motors, clocks, springs, and things. Cell. 1998;92:315–326. doi: 10.1016/s0092-8674(00)80925-3. [DOI] [PubMed] [Google Scholar]
- 73.Sun J S, Manley J L. A novel U2-U6 snRNA structure is necessary for mammalian messenger-RNA splicing. Genes Dev. 1995;9:843–854. doi: 10.1101/gad.9.7.843. [DOI] [PubMed] [Google Scholar]
- 74.Tarn W Y, Steitz J A. Modulation of 5′ splice site choice in pre-messenger RNA by 2 distinct steps. Proc Natl Acad Sci USA. 1995;92:2504–2508. doi: 10.1073/pnas.92.7.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tatei K, Takemura K, Tanaka H, Masaki T, Ohshima Y. Recognition of 5′ and 3′ splice site sequences in pre-messenger RNA studied with a filter binding technique. J Biol Chem. 1987;262:11667–11674. [PubMed] [Google Scholar]
- 76.Teigelkamp S, Mundt C, Aschel T, Will C L, Lührmann R. The human U5 snRNP-specific 100-kD protein is an RS domain-containing, putative RNA helicase with significant homology to the yeast splicing factor Prp28p. RNA. 1997;3:1313–1326. [PMC free article] [PubMed] [Google Scholar]
- 77.Ullman K S, Powers M A, Forbes D J. Nuclear export receptors: from importin to exportin. Cell. 1997;90:967–970. doi: 10.1016/s0092-8674(00)80361-x. [DOI] [PubMed] [Google Scholar]
- 78.Visa N, Izaurralde E, Ferreira J, Daneholt B, Mattaj I W. A nuclear cap-binding complex binds Balbiani Ring pre-mRNA cotranscriptionally and accompanies the ribonucleoprotein particle during nuclear export. J Cell Biol. 1996;133:5–14. doi: 10.1083/jcb.133.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wassarman D A, Steitz J A. Interactions of small nuclear RNAs with precursor messenger RNA during in vitro splicing. Science. 1992;257:1918–1925. doi: 10.1126/science.1411506. [DOI] [PubMed] [Google Scholar]
- 80.Wyatt J R, Sontheimer E J, Steitz J A. Site-specific cross-linking of mammalian U5 snRNP to the 5′ splice site before the 1st step of pre-mRNA splicing. Genes Dev. 1992;6:2542–2553. doi: 10.1101/gad.6.12b.2542. [DOI] [PubMed] [Google Scholar]
- 81.Zahler A M, Roth M B. Distinct functions of SR proteins in recruitment of U1 small nuclear ribonucleoprotein to alternative 5′ splice sites. Proc Natl Acad Sci USA. 1995;92:2642–2646. doi: 10.1073/pnas.92.7.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhuang Y, Weiner A M. A compensatory base change in U1 snRNA suppresses a 5′ splice site mutation. Cell. 1986;46:827–835. doi: 10.1016/0092-8674(86)90064-4. [DOI] [PubMed] [Google Scholar]
- 83.Zuo P, Manley J L. The human splicing factor ASF/SF2 can specifically recognize pre-mRNA 5′ splice sites. Proc Natl Acad Sci USA. 1994;91:3363–3367. doi: 10.1073/pnas.91.8.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]