Abstract
Background
Many sports require maximal strength and endurance performance. Concurrent strength and endurance training can lead to suboptimal training adaptations. However, how adaptations differ between males and females is currently unknown. Additionally, current training status may affect training adaptations.
Objective
We aimed to assess sex-specific differences in adaptations in strength, power, muscle hypertrophy, and maximal oxygen consumption (O2max) to concurrent strength and endurance training in healthy adults. Second, we investigated how training adaptations are influenced by strength and endurance training status.
Methods
A systematic review and meta-analysis was conducted according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, and a Cochrane risk of bias was evaluated. ISI Web of science, PubMed/MEDLINE, and SPORTDiscus databases were searched using the following inclusion criteria: healthy adults aged 18–50 years, intervention period of ≥ 4 weeks, and outcome measures were defined as upper- and lower-body strength, power, hypertrophy, and/or O2max. A meta-analysis was performed using a random-effects model and reported in standardized mean differences.
Results
In total, 59 studies with 1346 participants were included. Concurrent training showed blunted lower-body strength adaptations in males, but not in females (male: − 0.43, 95% confidence interval [− 0.64 to − 0.22], female: 0.08 [− 0.34 to 0.49], group difference: P = 0.03). No sex differences were observed for changes in upper-body strength (P = 0.67), power (P = 0.37), or O2max (P = 0.13). Data on muscle hypertrophy were insufficient to draw any conclusions. For training status, untrained but not trained or highly trained endurance athletes displayed lower O2max gains with concurrent training (P = 0.04). For other outcomes, no differences were found between untrained and trained individuals, both for strength and endurance training status.
Conclusions
Concurrent training results in small interference for lower-body strength adaptations in males, but not in females. Untrained, but not trained or highly trained endurance athletes demonstrated impaired improvements in O2max following concurrent training. More studies on females and highly strength-trained and endurance-trained athletes are warranted.
Clinical Trial Registration
PROSPERO: CRD42022370894.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40279-023-01943-9.
Key Points
| Concurrent training resulted in blunted lower-body strength adaptations in males, but not in females. |
| For training status, untrained but not trained or highly trained endurance athletes displayed impaired improvements for maximal oxygen consumption with concurrent training. |
| Most concurrent training studies include relatively untrained participants, and highly trained athletes are under-represented in the literature. |
| Training status should be considered both in terms of strength and endurance training status and can be evaluated according to the mentioned classification framework. |
Introduction
When it comes to optimal physical performance, there are few sports that exclusively require a high maximal strength or a high endurance performance. Instead, many sports demand a combination of a high maximal strength and endurance performance [1, 2]. For this reason, athletes are encouraged to perform concurrent training, which combines both strength and endurance within their training program. Simultaneously training for strength and endurance is not without limitations when it comes to optimizing training adaptations. First, skeletal muscles of strength- and endurance-trained individuals are specifically trained, focused either on increasing muscle size and neuromuscular adaptations or on oxygen delivery and utilization. However, the extremes of these two traits are physiologically incompatible, as there is an inverse relationship between muscle fiber cross-sectional area and mitochondrial oxidative capacity [3–5]. Second, concurrent training potentially leads to blunted strength, power, or hypertrophic adaptations compared with strength training alone. This ‘interference effect’ was first reported in Hickson’s seminal publication on concurrent training in 1980 [6]. Since then, the literature on concurrent training has expanded. Recently, an updated meta-analysis [7] concluded that power gains were impaired with concurrent strength and endurance training, but that hypertrophy and maximal strength development were not compromised. Others [8] showed that maximal oxygen consumption (O2max) gains were also not compromised by concurrent training.
The magnitude of the interference effect with concurrent training may be influenced by inter-individual variations, differences in experimental design, training intervention, and the type of outcome measure. Many of these potentially contributing factors have been analyzed before [7–10]. One important inter-individual factor that has received very little attention in the literature is the differences in training responses between males and females. Sex-related adaptations with concurrent training may occur, owing to differences in endocrine and muscle physiology, or physical performance. On average, males have larger muscle fibers, more skeletal muscle mass, and greater strength [11–13], while females tend to have slower contractile properties [14]. Males tend to have greater improvements in absolute muscle size, strength, and power compared with females [15, 16], but females showed larger relative increases in upper-body strength than males [17]. Both sexes show similar relative increases in lower-body strength and hypertrophy after strength training. Grosso modo, males tend to have a higher O2max, even after normalization to body mass or fat-free mass [18–20], likely because females are generally smaller and have a lower cardiac output and stroke volume, hemoglobin levels, and blood volume [18–21]. Following endurance training, the absolute and relative increases in O2max were also larger in males compared with females [22]. It is therefore conceivable that there may be sex differences in the adaptations to concurrent training.
Another inter-individual variation includes the current training status of the participant, which may significantly impact the magnitude of the interference effect with concurrent training. Coffey and Hawley [23] hypothesized that adaptations are more compromised following concurrent training in subjects with a longer training history, i.e., will be more pronounced in highly trained compared with untrained participants. Accordingly, elite athletes of sports that require both high sprint/peak power and endurance (e.g., rowing and cycling) indeed seem to experience that optimizing both traits at the same time is more difficult, as highlighted by the inverse relationships between athletes’ sprint/peak power and endurance performance/O2max [3, 24]. However, previous meta-analyses on concurrent training did not find any differences in adaptations between different levels of training status for maximal strength, power, and hypertrophy [7, 9, 10], when there was sufficient time to recover between strength and endurance training sessions. In addition, for adaptations of O2max with concurrent training, the effect of training status has not yet been assessed. It should be noted that training status is generally considered to be one-dimensional: a participant is untrained, trained, or highly trained. However, this neglects the fact that athletes can optimize for both their strength and endurance capacities. For example, professional marathon runners are highly trained endurance athletes, but relatively untrained for maximal absolute strength. Importantly, prior meta-analyses on concurrent training did not distinguish between both endurance- and strength-trained status [7, 9, 10, 25].
The aim of this study was to assess sex-specific differences in adaptations to concurrent strength and endurance training for strength, power, muscle hypertrophy, and O2max in healthy adults. Second, we investigated how adaptations to concurrent training depend on strength and endurance training status. Such a systematic review and meta-analysis not only provides more insight into the concurrent training effects of various populations, but also highlights which populations may be under-represented in the concurrent training literature.
Methods
Systematic Literature Search
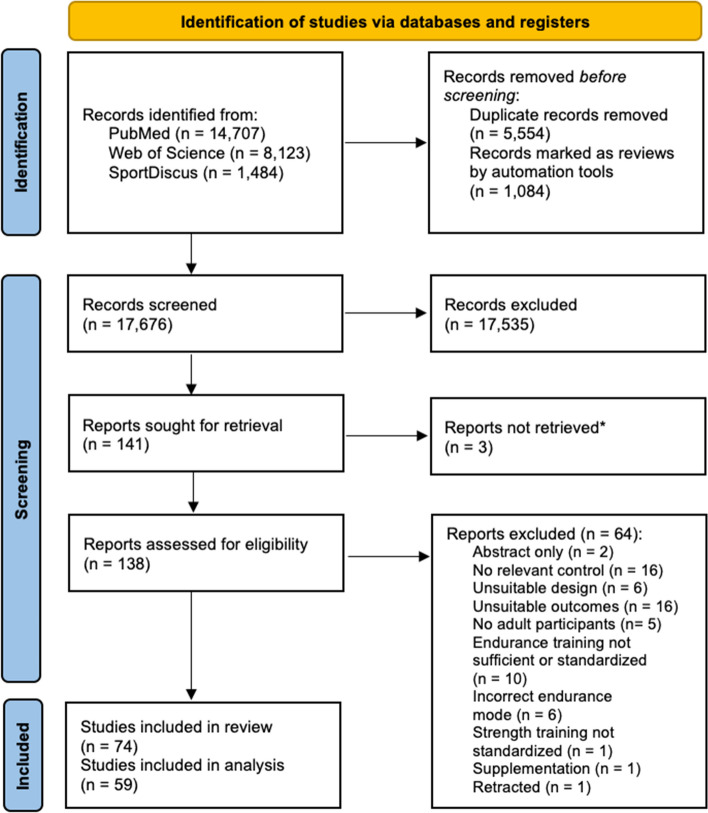
A systematic literature search was conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, and registered with the International Database of Prospectively Registered Systematic Reviews in Health and Social Care (PROSPERO, CRD42022370894). The ISI Web of science, PubMed/MEDLINE, and SPORTDiscus databases were searched on 14 November, 2022, using the following search string: ((“Concurrent training’’ OR “Combined training’’) OR ((“Endurance training’’ OR “Aerobic training’’) AND (“Resistance training” OR “Strength training’’ OR “Weight training’’)) AND (“Maximal strength’’ OR “1RM’’ OR “One repetition max’’ OR “Explosive strength’’ OR “Counter movement jump’’ OR “Squat jump’’ OR “Wingate test’’ OR “Peak power’’ OR “Hypertrophy’’ OR “Cross-sectional area’’ OR “Muscle thickness’’ OR “VO2max’’ OR “VO2peak’’ OR “Aerobic capacity’’ OR “Maximal oxygen uptake’’ OR “Maximal oxygen consumption’’)). Only studies from 1980 or later were included, which was after the seminal paper on concurrent training by Hickson [6]. Almost all eligible studies were identified by PubMed/MEDLINE (95%), and only a very small portion by WoS (4%) or SPORTDiscus (1%). Retrieved titles from the search were saved and both duplicates and review articles were removed using automated tools (i.e., custom-written R scripts, R version 4.2.2). Titles and abstracts of all remaining studies were screened individually by two reviewers (RH and SZ). If the reviewers could not reach consensus, a third reviewer was consulted (RW). The search process and selection of studies are summarized in the flowchart in Fig. 1.
Fig. 1.
Flowchart of data search and selection of studies. *From three studies, no full text could be obtained
Eligibility Criteria
Studies were included based on the PICO (Population, Intervention, Control and Outcomes) criteria [26]. The population included healthy male and female adults aged between 18 and 50 years. The intervention consisted of concurrent strength and endurance training for ≥ 4 weeks, where endurance training included cycling, cross-country skiing, swimming, rowing, or running and strength training was designed to improve strength, power, and/or hypertrophy, including concentric, eccentric, and plyometric training. Concurrent training groups were compared to an endurance training-only group (for O2max) or a strength training-only group (for other outcomes). Outcome measures related to maximal lower-body and upper-body strength, power, muscle hypertrophy, and O2max (Table 1). For maximal strength, one-repetition maximum (1-RM) and isometric measurements of lower and upper extremities were considered. For power, jump tests and dynamic power tests (e.g., Wingate test) were evaluated. For muscle hypertrophy, muscle thickness or whole-muscle cross-sectional area was measured objectively using ultrasound, magnetic resonance imaging, or computed tomography. Studies were excluded if participants experienced injuries or an illness or if they used ergogenic aids or other sport-enhancing supplements (except for proteins and vitamins).
Table 1.
Effects of concurrent training are evaluated for the following outcome measures. If multiple measurements exist for the same outcome measure, measurements are analyzed according to the presented hierarchy
| Outcome measures | Measurements |
|---|---|
| Maximal lower-body strength |
1-RM leg press (kg) 1-RM squat (kg) 1-RM half squat (kg) |
| Maximal upper-body strength | 1-RM bench press (kg) |
| Power |
Counter movement jump (in W or cm) Squat jump (in W or cm) Wingate peak power (W or W⋅kg−1) |
| Hypertrophy |
Cross-sectional area of the upper leg muscles (cm) Muscle thickness of upper leg muscles (cm) |
| Maximal oxygen consumption | O2max (mL⋅kg−1⋅min−1 or mL⋅min−1) |
1-RM one-repetition maximum, O2max maximal oxygen consumption
Classification of Training Status
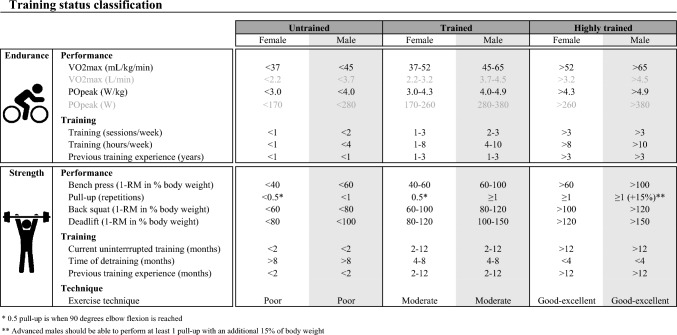
To classify training status within concurrent training studies, it is important to note that the training status of participants is not one-dimensional, but can be interpreted both in terms of strength and endurance training status. Unified classification systems have been introduced to categorize participants’ strength training status [27] and endurance training status [28, 29] based on multiple training and physiological performance indicators, such as training experience, attained O2max, and 1-RM. Training status is typically divided into three categories corresponding to untrained, trained, and highly trained athletes [30, 31]. In this meta-analysis, participants were characterized according to their combination of strength and endurance training status. Strength training status [27] was grouped into untrained, trained (i.e., intermediately trained athletes), and highly trained athletes (i.e., advanced and highly advanced athletes), and endurance training status [28, 29] into untrained, trained (i.e., recreationally trained and trained individuals), and highly trained athletes (i.e., well-trained and professional athletes). The classification framework to determine strength and endurance training status of participants is presented in Table 2 (adapted from [27–29]). To obtain the training status classification, scores were derived for each physiological performance, training, or technique indicator (if present) and were subsequently averaged and rounded. For strength training status, all strength performance indicators were grouped and counted as one score [27]. For endurance training status, the relative values of O2max and peak power (compared to body mass) were used by default and absolute values were only scored if relative values were not present. Using this unified classification system, participant groups can be classified for both their strength and endurance training status in order to determine how effects of concurrent training are dependent on training status.
Table 2.
Classification framework to determine strength and endurance training status of male and female participants. Guidelines are adapted from [27–29] and a full explanation of how training status is derived is provided in the description below
As an example of strength training status, a female individual is performing uninterrupted strength training for 6 months (score 2) with no break in her training routine (score 3) and therefore total training experience is also 6 months (score 2). She lifted < 40% of her body mass in the bench press and squatted 80% of her body mass in the back squat (average score 1.5), both with a good exercise technique (score 3). In this case, the average score is 2.3, meaning the individual is considered trained for her strength training status. The values for the deadlift and pull-up are not present and therefore not included in the calculation. Another example for endurance training status concerns a young male individual with a O2max) of 30 mL/kg/min (score 1) and peak power of 240 W and 2.8 W/kg (score 1; as relative peak power is used by default), who has been training for 6 months (score 1) with an average of 2 h per week (score 1) and 2 sessions per week (score 2). In this case, the average score is 1.2, meaning the individual is considered untrained for his endurance training status. 1-RM one repetition maximum
Data Extraction
Two reviewers independently evaluated the full-text articles using a standardized predefined form (RH and SZ). Articles were examined by a third reviewer if consensus was not reached (RW). Data extraction was performed by two reviewers (RH and SZ) and the following data were extracted from the articles: author(s), year of publication, age, sex, sample size, training status (for strength and endurance), training intervention (frequency, duration, intensity, modality), and outcome measures (maximal lower-body and upper-body strength, power, muscle hypertrophy, and O2max) before and after the intervention. If data on group mean and standard deviation were not presented in the text or tables, then baseline and post-intervention data were requested from the corresponding authors or obtained from figures (if present) using WebPlotDigitizer version 4.5 (Pacifica, CA, USA; [32]). If a particular study included more than one concurrent training group and if these groups were compared to the same control group, results for the intervention groups were combined based on guidelines from the Cochrane handbook [33], to avoid ‘double counts’ of participants in the control group.
Assessment of Methodological Quality
Methodological quality of identified studies was assessed independently by two reviewers (RH and RW) using the Cochrane risk-of-bias evaluation [33, 34], and consensus was reached after consultation. Reviewers assessed all studies for the risk of selection bias (sequence generation, allocation concealment), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting), and other biases. Biases were classified for each item as low, high, or unclear risk (i.e., when the risk is unknown or insufficient detail is reported). Relevant biases were assessed one by one according to the recommendation of the Cochrane Bias Methods Group and Statistical Methods Group [33].
Data Synthesis, Meta-Analysis, and Statistical Analyses
In this study, we compared the effects of (1) concurrent training versus endurance training only (for O2max) and (2) concurrent training versus strength training only (for other outcomes). Results for maximal strength were subdivided into lower-body and upper-body strength. In accordance with Cochrane recommendations [33], if studies presented multiple methods for the same outcome (e.g. a jump test and Wingate test for power), only one of these was included in the analysis according to the hierarchy presented in Table 1. This was to avoid inclusion of intervention effects that were statistically dependent in the analysis, as these were calculated from the same participants. For example, the hierarchy dictates evaluation of lower-body strength using 1-RM maximal strength during leg press (which is technically easier to execute) over the squat and evaluation of power using countermovement or squat jumps (which are more readily available) over Wingate peak power. Studies or intervention groups were excluded from the analysis if significant baseline differences were observed for the specific outcome measure between the concurrent training and control group or if only percentage changes were reported.
For the meta-analysis, effect sizes of each study were calculated based on standardized mean differences (SMDs) according to the Hedges’ g formula using the pooled standard deviation and incorporates an adjustment for small sample bias [35]. A random-effect model [36] was adopted and presented in forest plots, and records were weighted according to the inverse variance method for each outcome measure. Overall effects were evaluated using Z-tests and pooled effect sizes were presented with their 95% confidence interval (CI). Heterogeneity was calculated using the Q-test and expressed in terms of Chi2 and the I2 statistic. I2 describes the percentage of the variability that is attributable to heterogeneity rather than chance [37]. Heterogeneity was evaluated based on I2, with values of 25%, 50%, and 75% representing low, moderate, or high levels of heterogeneity, respectively [38]. Funnel plots with a 95% interval range were made in R for each outcome to quantify potential publication bias. Subgroup analyses were performed to compare concurrent training effects between (1) male and female participants; (2) untrained, trained, and highly trained levels of strength training status; and (3) untrained, trained, and highly trained levels of endurance training status. Last, sensitivity analyses were performed if one or more studies was considered to be an outlier and/or highly influential based on studentized residuals and Cook’s distances [39]. The meta-analysis was conducted using Review Manager (RevMan) software (Version 5.4.1, The Cochrane Collaboration, 2020) and R (Version 4.2.2, 2022) and the code is available in a GitHub repository (https://github.com/StephanvdZwaard/Concurrent-training-review). A significance level α of 0.05 was used to determine statistical differences.
Results
Study Characteristics
The database search resulted in a total of 24,314 articles. After automatic removal of the duplicates, removal of review articles, and screening of the remaining titles and abstracts, 141 articles remained. From these articles, 74 studies [6, 30, 40–111] met the inclusion criteria (see Fig. 1) of which 59 provided the necessary data to be included in the meta-analysis (see Tables S1–3 of the Electronic Supplementary Material [ESM]). Table S4 of the ESM summarizes all relevant characteristics of the studies, participants, and training interventions. The meta-analysis included 1346 participants, of whom 679 performed concurrent training, 270 performed strength training only, and 397 performed endurance training only. Training status of the participants (using Table 2) was determined for strength training status (38 studies), endurance training status (56 studies), and both strength and endurance training status (36 studies). Figure 2 displays the training status level of the participants within these studies, reflecting mostly untrained participants for strength (71%) and predominantly untrained or trained participants for endurance (34% and 46%, respectively). It should be noted that strength as well as endurance training status could only be determined in 61% of the studies included in the meta-analysis. From these studies, only two studies (5%) included participants with highly trained endurance status and no study included highly trained strength participants.
Fig. 2.
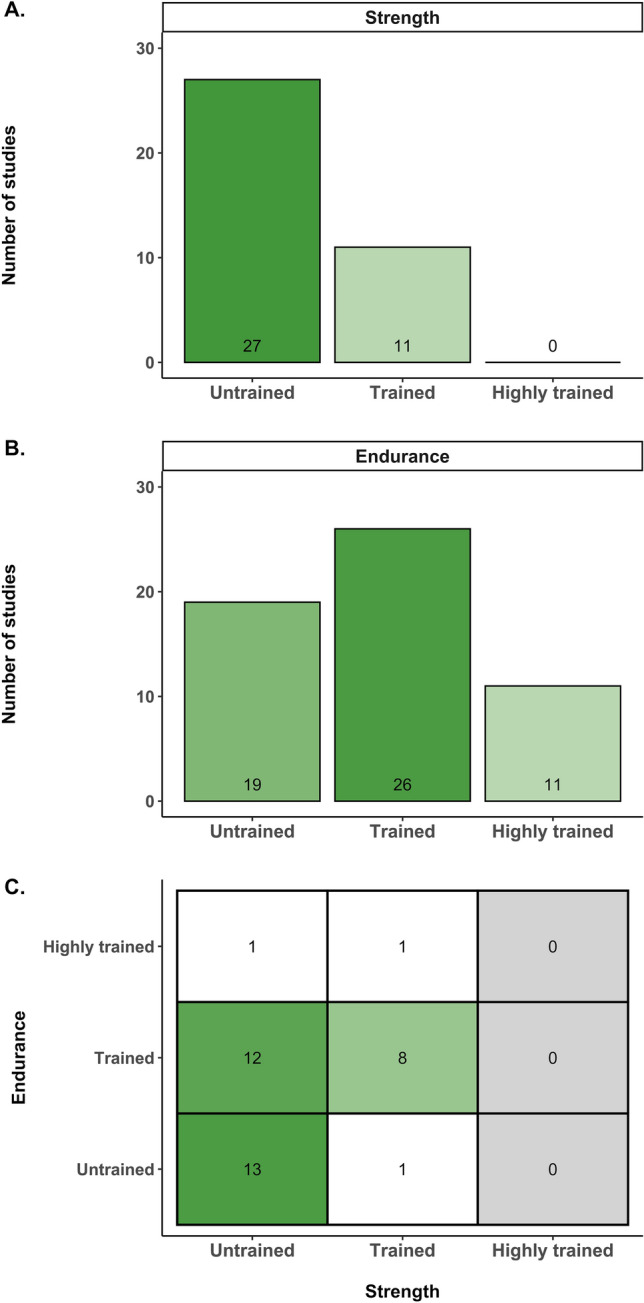
Overview of the training status of the participants in the included studies. Number of studies are reported in which levels of training status could be assessed for (A) strength, (B) endurance, and (C) both strength and endurance. Quantification of training status was performed according to the guidelines in Table 2
Sensitivity Analyses
Examination of the studentized residuals and Cook’s distance showed no indications of outliers or overly influential studies in all of the subgroup analyses.
Risk of Bias Analyses
The overall assessment for the risk of bias is presented in Fig. S17 of the ESM. For the selection bias, a random sequence generation had a low risk in ~ 75% of the studies and allocation concealment was mostly unclear. Detection bias was largely unclear (~ 95%). Attrition bias was low in ~ 80% and high in ~ 20% of the included studies. However, the bias for selective reporting was low in most studies (~ 95%).
Concurrent Training Effects in Males and Females
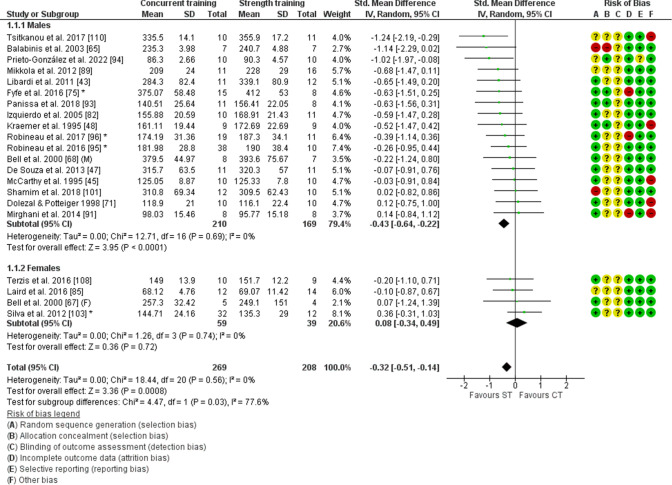
The analysis of lower-body strength included 20 studies [43, 45, 47, 48, 65, 68, 71, 75, 82, 85, 89, 91, 93–96, 101, 103, 108, 110], with 269 participants that performed concurrent training and 208 participants that performed strength training only. Males demonstrated blunted lower-body strength adaptations with concurrent training compared with strength training (− 0.43; 95% CI [− 0.64 to − 0.22], P < 0.001), whereas females did not (0.08, 95% CI [− 0.34 to 0.49], P = 0.72). These sex differences were significant (P = 0.03, Figs. 3 and 4). There was no significant heterogeneity within males (I2 = 0%, P = 0.69) or females (I2 = 0%, P = 0.74). These results highlight a small interference effect for lower-body strength with concurrent training in males, but not in females.
Fig. 3.
Forest plot of studies comparing differences in adaptations in lower-body strength with concurrent training between males and females. Risk of bias elements are highlighted in the legend below the forest plot and bias was indicated as low (green), unclear (yellow), or high (red). * reflects studies with multiple concurrent training groups that were combined. CI confidence interval, CT concurrent training group, SD standard deviation, ST strength training-only control group, Std. standard
Fig. 4.
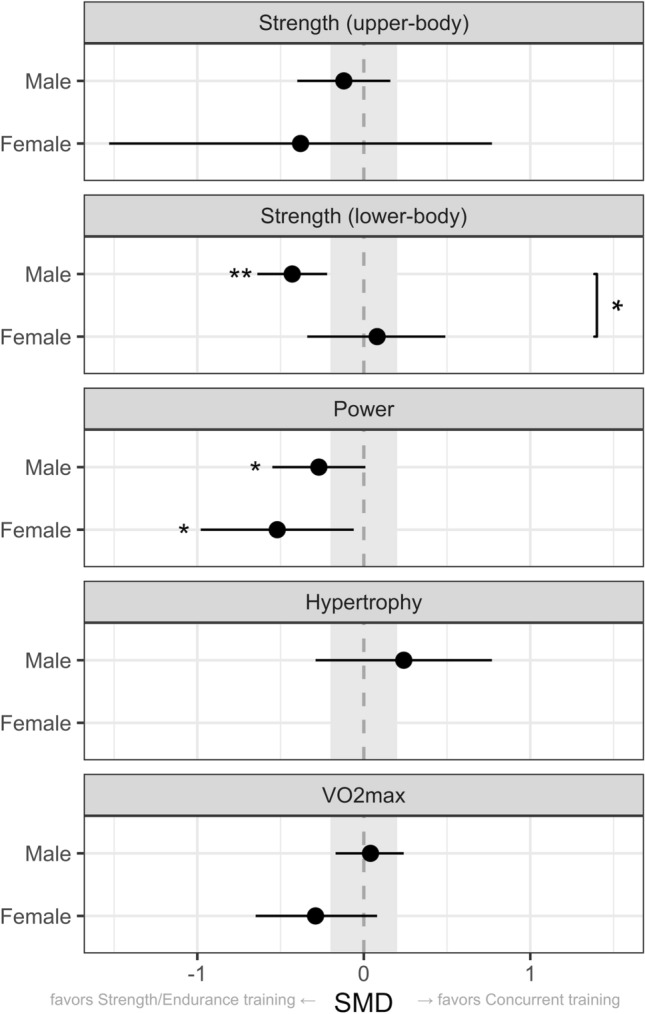
Point range plot demonstrating differences in the effects of concurrent training between males and females for each outcome measure. Effects are reported as the average standardized mean difference (SMD) and their 95% confidence intervals. The gray area highlights the smallest worthwhile change (SMD = 0.2), illustrating what effects can be considered to be non-trivial. Significant effects of concurrent training versus control are reported for males and females separately (next to point range). Significant sex differences were also reported (on the right). *P < 0.05. CI confidence interval; SMD standardized mean differences
Concurrent training resulted in impaired power gains compared with strength training only in both males (− 0.27, 95% CI [− 0.55 to 0.01], P = 0.05) and females (− 0.52, 95% CI [− 0.98 to − 0.06], P = 0.03), but without significant sex-differences (P = 0.37, based on 13 studies). No sex differences were observed for upper-body strength (P = 0.67, based on 13 studies) or O2max (P = 0.13, based on 32 studies). Data on muscle hypertrophy were available only in males, precluding us to draw any conclusions on sex differences. Forest plots for each outcome measure are also displayed in Figs. S1–5 of the ESM. Our results indicate a small interference with concurrent training for lower-body strength in males, but not in females, and a small-to-moderate interference for power in both sexes.
Training Status and Interference of Concurrent Training
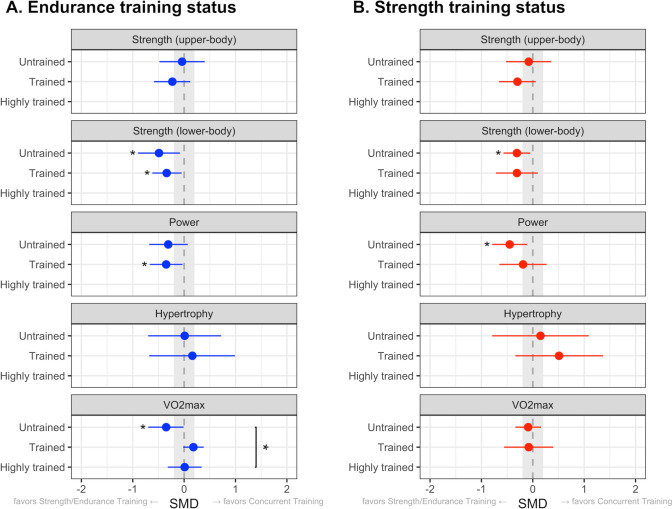
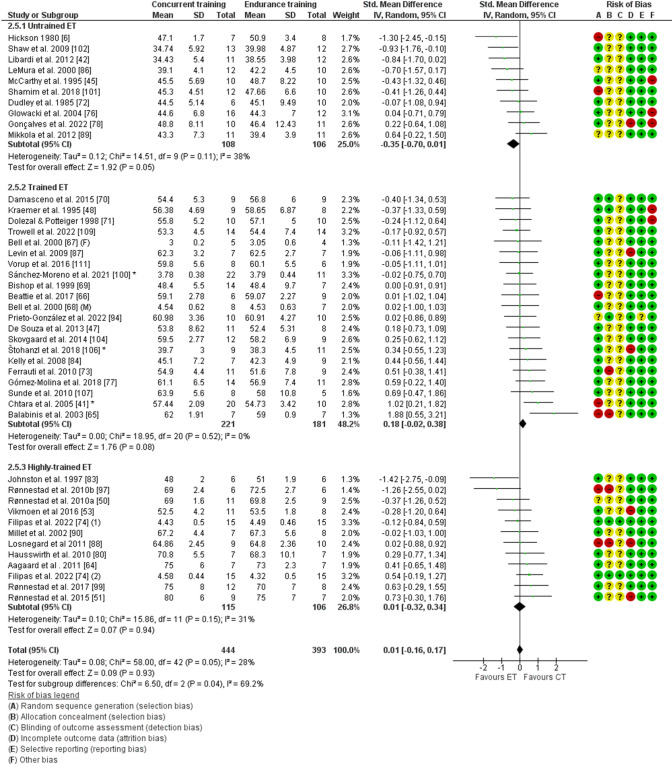
Figure 5 displays the effects of concurrent training related to the participants’ strength and endurance training status. Regarding strength training status, concurrent training resulted in similar adaptations for lower-body strength (P = 1.00, based on 16 studies), upper-body strength (P = 0.45, based on 14 studies), power (P = 0.38, based on 11 studies), and O2max (P = 0.96, based on 24 studies) between untrained and trained individuals. Similarly, for endurance training status, untrained and trained participants did not show different adaptations for lower-body strength (P = 0.55, based on 20 studies), upper-body strength (P = 0.50, based on 13 studies), and power (P = 0.86, based on 13 studies) with concurrent training. However, improvements in O2max with concurrent training were different for untrained, trained, and highly trained endurance athletes (P = 0.04, based on 42 studies, see Fig. 6). More specifically, adaptations in O2max were slightly blunted in untrained (− 0.35, CI95% [− 0.70 to − 0.01], P = 0.05) but not in trained (0.18, 95% CI [− 0.02 to 0.38], P = 0.08) and highly trained endurance athletes (0.01, 95% CI [− 0.32 to 0.34], P = 0.94). Only data from four studies were available on muscle hypertrophy, which precluded us to perform meaningful subgroup analyses. Limited data other than O2max in highly trained endurance athletes precluded us to draw any conclusions for other outcome measures in highly trained athletes.
Fig. 5.
Point range plot demonstrating differences in the effects of concurrent training between levels of training status for endurance (A) and strength (B) for each outcome measure. Effects are reported as the average standardized mean differences (SMDs) and their 95% confidence intervals. The gray area highlights the smallest worthwhile change (SMD = 0.2), illustrating what effects can be considered non-trivial. Significant effects of concurrent training versus control are reported for each level of training status (next to point range). Significant differences were also reported between training status levels (on the right). *P < 0.05. CI confidence interval; SMD standardized mean differences
Fig. 6.
Forest plot of studies comparing differences in adaptations in maximal oxygen consumption with concurrent training between untrained, trained, and highly trained endurance athletes. Risk of bias elements are highlighted in the legend below the forest plot and bias was indicated as low (green), unclear (yellow), or high (red). * reflects studies with multiple concurrent training groups that were combined. CI confidence interval, CT concurrent training group, ET endurance training-only control group, SD standard deviation, Std. standard
Lower-body strength adaptations were blunted with concurrent training in untrained and trained endurance athletes (P = 0.02 and P = 0.02), and untrained strength athletes (P = 0.02), but did not reach significance in trained strength athletes (P = 0.14). For gains in power, a small-to-moderate interference effect was observed with concurrent training in untrained strength and trained endurance athletes (P = 0.01 and P = 0.03), whereas trained strength athletes did not demonstrate impaired adaptations in power with concurrent training (P = 0.42; Figs. S6–15 of the ESM).
Discussion
The aim of this meta-analysis was to identify how sex or individual training status affect the adaptations in strength, power, muscle hypertrophy, and O2max with concurrent training in healthy adults. Concurrent training resulted in blunted lower-body strength adaptations in males, but not in females. No sex differences were observed for adaptations in upper-body strength, power, or O2max. There were insufficient studies to investigate muscle hypertrophy. We observed a blunted improvement in O2max in untrained, but not in trained or highly trained endurance athletes, and noted a small-to-moderate interference with concurrent training for lower-body strength and power gains in untrained, whereas this did not reach statistical significance in strength-trained athletes. With respect to strength and endurance training status, there were no differences in improvements in maximal lower- and upper-body strength and power between untrained and trained participants.
Sex Differences in Adaptations to Concurrent Training
Our meta-analysis shows that adaptations to concurrent training differ between sexes. We observed an impaired improvement in maximal lower-body strength in males (SMD: − 0.43), but not in females. There were no significant sex differences for improvements in power, upper-body strength, and O2max after concurrent training. When including both males and females, there was a small interference effect for power (SMD: − 0.35) but not for upper-body strength and O2max. This is in line with two recent meta-analyses that showed that concurrent training hampered improvements in power (SMD: − 0.28), but not in upper-body strength or O2max, when both sexes are combined [7, 8].
The underlying mechanism for this sex-specific interference remains unknown, but sex differences in muscle and training physiology likely contribute. While sex-related neuromuscular differences could contribute, no sex differences have been observed in the number of motor units or motor unit activation patterns for upper- and lower-body muscles [112, 113], nor in neuromuscular adaptations following exercise training [114].
On average, males tend to have larger muscle fibers and a larger percentage of their cross-sectional area occupied by type II fibers [13, 112, 113, 115, 116]. With these larger fibers, males produce higher absolute forces than females [112]. For strength training adaptations, however, relative improvements in lower-body strength and hypertrophy are similar between males and females [17, 113, 117–119]. Men show larger absolute gains in fiber size [120, 121], which may relate to the observation that glycolytic type II fibers have the larger potential to hypertrophy than type I fibers [122].
Endocrine differences between males and females may contribute to the muscular adaptations with strength training. Circulating testosterone levels in males acutely increase following strength exercise [123, 124], which activates muscle protein synthesis via the anabolic Akt/mTOR pathway and androgen receptors [125, 126]. Despite the lower testosterone levels in females [127], strength exercise-induced muscle protein synthesis seems to be similar between sexes [128–130], suggesting that other factors, such as mechanical signals contribute to the activation of muscle protein synthesis.
The impaired lower-body strength gains in males could also relate to skeletal muscle metabolism and fatigability [131–134], associated with their lower percentage type I fibers. Following concurrent training, this difference could translate into larger residual fatigue from endurance training in men, which may compromise the quality of strength training sessions and subsequent strength gains [135]. Men potentially experience a larger interference effect when combining strength and endurance training because of the tight negative correlation between muscle fiber size and oxidative capacity within skeletal muscle [3, 4] and their larger fiber size. As such, sex-related differences in fiber size, endocrine physiology, and muscle fatigability could help to explain why we observed blunted lower-body strength adaptations in males, but not in females.
Current Training Status and Interference with Concurrent Training
The adaptations with concurrent training compared to single-mode training may be compromised more in highly trained athletes compared with trained and untrained individuals (known as the ceiling effect) [23]. In contrast, we observed the opposite for adaptations in O2max in this meta-analysis, demonstrating blunted adaptations in untrained (SMD: − 0.35), but not in trained and highly trained endurance athletes.
It should be noted that the participant training status classification framework depends on selection criteria for what is regarded ‘untrained,’ ‘trained,’ and ‘highly trained’. A recent meta-analysis [7] distinguished between untrained and trained based on the subgroup description in prior studies (e.g., sedentary, recreationally active). Another meta-analysis [10] distinguished training status based on the World Health Organization physical activity guidelines. Recently, a new classification framework was proposed for determining training status in sport science studies [136]. This framework discriminates very well between world-class, elite, highly trained athletes, and other lower levels of training status, which is very important for avoiding the misuse of the term elite or world-class when addressing participant groups. However, to discriminate between untrained, trained, and highly trained athletes, this framework uses physical activity guidelines and competitive performance characteristics, which are often not reported in the included studies. Based on such criteria, a participant could have a very low endurance capacity (O2max of 30 mL/kg/min), but also perform individual competitive exercise, which classify this individual as a “trained participant”, despite being physically unfit. Additionally, such training status classification does not distinguish between endurance and strength-related training status.
Because of these considerations, we decided to apply a training classification framework that (1) is based on physical capacities reflecting the physiology of participants, (2) is based on variables that are commonly reported in previous studies, and (3) that clearly distinguishes between training status in terms of strength and endurance capabilities, which is a necessity for the present meta-analysis. Cut-off values used to distinguish untrained, trained, and highly trained athletes were based on previous classification frameworks that were established based on measurement data from 130 studies in males [28] and 82 studies in females [29] for endurance training status. Although these cut-off points for O2max (< 45 mL/kg/min for untrained and > 65 mL/kg/min for highly trained males) may seem like quite the range, we have previously shown that O2max can range from ~ 25 to 80 mL/kg/min in healthy adults [137]. Therefore, this large range indeed seems to give a broad indication of untrained, trained, or highly trained individuals. The same goes for strength measurements. Therefore, we are confident that our classification framework based on the physical strength and endurance capacities of the participant gives an accurate representation of the untrained, trained, and highly trained athletes.
Untrained participants tend to have less type I fibers with fewer mitochondria and capillaries, a lower stroke volume and cardiac output, and a lower O2max compared with endurance-trained athletes [5, 19, 138–141]. Whole-body O2max is considered to be limited by oxygen supply to the mitochondria, although O2max is well aligned with a small overcapacity of mitochondrial oxidative phosphorylation [137]. Therefore, improvements in O2max with training are expected to be accompanied by improvements in both oxygen supply to the mitochondria and mitochondrial oxidative capacity. In response to endurance training, increases in O2max are mostly explained by improvements in cardiac output [142–144], although early increases in O2max seem to relate to greater maximal arterial-venous oxygen differences [142]. This is in line with the time courses of adaptations to endurance training, showing very rapid increases in mitochondrial oxidative capacity [139, 145, 146] and improvements in capillarization [139, 147] and blood volume [148] with ~ 2 to 4 weeks of training, while central cardiovascular adaptations in maximal stroke volume and cardiac output occur only after month(s) of training [142, 145, 149, 150].
Differences in O2max between untrained and trained endurance athletes are largely explained by differences in cardiac output, stroke volume, and mitochondrial oxidative capacity [19, 137]. With concurrent training, untrained individuals are expected to elicit generic adaptations to both strength and endurance training with additive effects [23], that is, untrained individuals may even increase their muscle size after endurance training [151] or their oxidative capacity after strength training [152]. Together with the greater muscle hypertrophy in untrained participants after strength training [153], one may expect untrained participants to show larger increases in the fiber cross-sectional area following concurrent training. As muscle fiber size inverse relates to oxidative capacity [3, 4], this may explain why untrained individuals have more compromised O2max adaptations when combining strength and endurance training compared with trained or highly trained endurance athletes.
For maximal lower-body and upper-body strength and power, no differences in adaptations to concurrent training were observed between untrained and trained participants. Not enough studies have been performed to draw conclusions about highly trained athletes. Prior observations in highly trained powerlifters revealed that stronger males, but not females, gain less strength over time, indicative of a ceiling effect [154]. During the general and competitive preparation phase, elite female rowers showed large gains in their muscle physiological cross-sectional area and/or fascicle length [155], indicating that such a ceiling effect was absent during this phase of the season in females. In brief, more studies on highly trained (female) athletes are necessary to evaluate potential interference effects with concurrent training and its underlying mechanisms. Similar to our findings, the meta-analysis of Schumann et al. [7] reported no differences in adaptations with concurrent training between untrained and trained participants for maximal strength, power, and muscle hypertrophy. In contrast, the meta-analysis of Petré et al. [10] observed that maximal lower-body strength gains after concurrent training were impaired in trained, but not in untrained participants. However, they included 27 studies in the meta-analysis (vs 59 studies in our study) and applied a different classification for training status (i.e., several participant groups that were considered moderately trained in [10] were classified as untrained in the our study). The present meta-analysis provides a new perspective on the influence of training status by assessing training status both in terms of strength and endurance capacities of the participants.
Future Perspectives and Limitations
Perspectives and limitations are considered first regarding sex differences and second related to the influence of training status for adaptations with concurrent training. In general, female participation in research tends to be under-represented compared with male participants in sport science and sports medicine studies (~ 70% of studies included only male participants, often college students [156, 157]). Similarly, we observed more concurrent studies with male participants compared with female participants in this meta-analysis, and our sex-specific conclusions are therefore based on fewer studies. Only four studies on concurrent training effects for muscle hypertrophy were included, all in males, which precluded us to draw any conclusions on the influence of sex. In addition, phases of the menstrual cycle as well as the use of the oral conceptive pill could have an influence on training adaptations and exercise metabolism [117, 158–160], which have not always been controlled for or monitored in training studies with females. Last, highly trained endurance female athletes can suffer from female athlete triad or relative energy deficiency in sport, affecting their reproductive endocrinology and training adaptability [161], which might provide an additional complicating factor in understanding the interference effect of concurrent training in female athletes.
Differences in training volume or design of training interventions could also potentially contribute to observed sex differences (see Table S5 of the ESM). Training adaptations are intrinsically heterogenous, even when performed at the same intensity and load, and therefore, such training studies should include a large enough sample size to account for this intrinsic heterogeneity in training adaptations, independent of an interference effect. Future research could focus on studying the gain and interference effects at different training intensities and volumes, taking into account female sex and pre-training status.
Regarding the assessment of risk of bias, the majority of included studies had some (unclear) risk of bias. Part of this may be owing to the fact that blinding of participants/researchers is not always possible with exercise training interventions or because the required information was not reported in the studies. However, our sensitivity analyses did not indicate the presence of outliers or overly influential studies.
To avoid statistical dependency between analyzed data points, we selected only one measurement method per study if multiple methods were reported for an outcome according to the hierarchy in Table 1. Alternatively, a three-level multivariate meta-analysis could be used to assess a third level of variance within studies, which would allow for the analysis of multiple methods per outcome [162]. Such a statistical analysis could provide additional insights into how adaptations with concurrent training may differ between measurement methods, such as between 1-RM squat and 1-RM leg press for lower-body strength. In the present meta-analysis, however, the number of analyzed studies was too small to perform a three-level meta-analysis [163, 164] and could result in biased estimates of the variance and standard error at the third level (i.e., within studies) [163]. Consequently, we performed a traditional two-level meta-analysis based on random effects [165] and selected one measurement method for each outcome instead. For future systematic reviews and meta-analyses that include more concurrent training studies, preferably 50 or more [164], we encourage the use of three-level meta-analyses to also assess how adaptations with concurrent training may differ between measurement methods.
It should be noted that not all studies reported the required descriptors to determine the training status of participants according to the framework in Table 2. Only ~ 60% of the studies reported sufficient information to determine both strength and endurance training status, and therefore not all studies could be included in this meta-analysis. This complicates meta-analyses, and the general applicability of the study findings. Future concurrent training studies should provide detailed descriptions of their participants’ strength and endurance training status, to allow better assessment of potential differences in the interference effect with concurrent training between untrained, trained, and highly trained individuals.
Furthermore, it should be noted that the framework to determine training status did not account for age. However, this effect is likely marginal for this study, as the average age was 27 ± 7 years (see Table S4 of the ESM). Reductions in strength and O2max are expected only with advancing age [166–168], but we do not know how our results extrapolate to older age.
It has been suggested that instead of O2max, other physiological performance-based parameters such as critical power, could be used to classify endurance training status [169, 170]. O2max is appropriate to discriminate between individuals that largely differ in their endurance training status, such as untrained, trained, and highly trained endurance athletes. However, a measure like critical power may be more valuable to discriminate between more homogeneous groups of athletes with rather similar training status, such as well-trained amateurs, sub-elite athletes, and elite athletes [170]. Therefore, adding critical power as a criterion to the framework to classify training status could be worthwhile to describe more specific subgroups, such as highly trained athletes.
In terms of practical advice, individuals and coaches who incorporate concurrent strength and endurance training within their training should consider the potential small-to-moderate interference effects resulting in blunted training adaptations. Not only highly trained athletes, but also untrained individuals should be aware of these effects. Male athletes should be extra cautious when aiming to improve lower-body strength, whereas this seems to be less of an issue in female athletes. In addition, contrary to prior beliefs, these blunted adaptations may also occur for endurance-related outcomes (i.e., O2max). Therefore, when optimizing an individual prescription of concurrent training in athletes, inter-individual differences in sex and training status should be considered when evaluating magnitudes of the observed adaptations. Furthermore, we encouraged scientists to provide a detailed description of their participants’ strength and endurance training status.
Conclusions
This meta-analysis shows that concurrent strength and endurance training resulted in blunted lower-body strength adaptations in males, but not in females. Concurrent training also resulted in impaired improvements in O2max in untrained, but not in trained or highly trained athletes. Our meta-analysis indicated that highly strength- or endurance-trained athletes are under-represented in the concurrent training literature. In summary, inter-individual differences in sex and training status should be considered when optimizing concurrent training prescription in individual athletes.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
No financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
Raven O. Huiberts, Rob C.I. Wüst, and Stephan van der Zwaard have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The datasets analyzed during the current study are available in a GitHub repository (https://github.com/StephanvdZwaard/Concurrent-training-review).
Code availability
The code is available in a GitHub repository (https://github.com/StephanvdZwaard/Concurrent-training-review).
Author contributions
RH and SZ conceived and designed the work. RH and SZ performed the literature search and the data screening and extraction. RH and RW evaluated the risk of bias. RH and SZ performed the statistical analyses. SZ was responsible for the visualizations and RH, RW, and SZ interpreted the data. SZ wrote the manuscript. All authors read and revised the manuscript and approved the final version of the manuscript for publication.
References
- 1.Nader GA. Concurrent strength and endurance training: from molecules to man. Med Sci Sports Exerc. 2006;38:1965–1970. doi: 10.1249/01.mss.0000233795.39282.33. [DOI] [PubMed] [Google Scholar]
- 2.Egan B, Sharples AP. Molecular responses to acute exercise and their relevance for adaptations in skeletal muscle to exercise training. Physiol Rev. 2022;103:2057–2070. doi: 10.1152/physrev.00054.2021. [DOI] [PubMed] [Google Scholar]
- 3.van der Zwaard S, van der Laarse WJ, Weide G, Bloemers FW, Hofmijster MJ, Levels K, et al. Critical determinants of combined sprint and endurance performance: an integrative analysis from muscle fiber to the human body. FASEB J. 2018;32:2110–2123. doi: 10.1096/fj.201700827R. [DOI] [PubMed] [Google Scholar]
- 4.van Wessel T, de Haan A, van der Laarse WJ, Jaspers RT. The muscle fiber type–fiber size paradox: hypertrophy or oxidative metabolism? Eur J Appl Physiol. 2010;110:665–694. doi: 10.1007/s00421-010-1545-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Zwaard S, Brocherie F, Jaspers RT. Under the hood: skeletal muscle determinants of endurance performance. Front Sports Act Living. 2021;3:719434. doi: 10.3389/fspor.2021.719434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hickson RC. Interference of strength development by simultaneously training for strength and endurance. Eur J Appl Physiol Occup Physiol. 1980;45:255–263. doi: 10.1007/BF00421333. [DOI] [PubMed] [Google Scholar]
- 7.Schumann M, Feuerbacher JF, Sünkeler M, Freitag N, Rønnestad BR, Doma K, et al. Compatibility of concurrent aerobic and strength training for skeletal muscle size and function: an updated systematic review and meta-analysis. Sports Med. 2022;52:601–612. doi: 10.1007/s40279-021-01587-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pito PG, Cardoso JR, Tufano J, Guariglia D. Effects of concurrent training on 1RM and VO2 in adults: systematic review with meta-analysis. Int J Sports Med. 2021;43:297–304. doi: 10.1055/a-1506-3007. [DOI] [PubMed] [Google Scholar]
- 9.Wilson JM, Marin PJ, Rhea MR, Wilson SMC, Loenneke JP, Anderson JC. Concurrent training: a meta-analysis examining interference of aerobic and resistance exercises. J Strength Cond Res. 2012;26:2293–2307. doi: 10.1519/JSC.0b013e31823a3e2d. [DOI] [PubMed] [Google Scholar]
- 10.Petré H, Hemmingsson E, Rosdahl H, Psilander N. Development of maximal dynamic strength during concurrent resistance and endurance training in untrained, moderately trained, and trained individuals: a systematic review and meta-analysis. Sports Med. 2021;51:991–1010. doi: 10.1007/s40279-021-01426-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol. 2000;89:81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 12.Schantz P, Randall-Fox E, Hutchison W, Tydén A, Astrand PO. Muscle fibre type distribution, muscle cross-sectional area and maximal voluntary strength in humans. Acta Physiol Scand. 1983;117:219–226. doi: 10.1111/j.1748-1716.1983.tb07200.x. [DOI] [PubMed] [Google Scholar]
- 13.Toft I, Lindal S, Bønaa KH, Jenssen T. Quantitative measurement of muscle fiber composition in a normal population. Muscle Nerve. 2003;28:101–108. doi: 10.1002/mus.10373. [DOI] [PubMed] [Google Scholar]
- 14.Wüst R, Morse CI, de Haan A, Jones D, Degens H. Sex differences in contractile properties and fatigue resistance of human skeletal muscle. Exp Physiol. 2008;93:843–850. doi: 10.1113/expphysiol.2007.041764. [DOI] [PubMed] [Google Scholar]
- 15.Cureton KJ, Collins MA, Hill DW, McElhannon FM. Muscle hypertrophy in men and women. Med Sci Sports Exerc. 1988;20:338–344. doi: 10.1249/00005768-198808000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Jozsi AC, Campbell WW, Joseph L, Davey SL, Evans WJ. Changes in power with resistance training in older and younger men and women. J Gerontol A Biol Sci Med Sci. 1999;54:M591–M596. doi: 10.1093/gerona/54.11.m591. [DOI] [PubMed] [Google Scholar]
- 17.Roberts BM, Nuckols G, Krieger JW. Sex differences in resistance training: a systematic review and meta-analysis. J Strength Cond Res. 2020;34:1448–1460. doi: 10.1519/JSC.0000000000003521. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell JH, Tate C, Raven P, Cobb F, Kraus W, Moreadith R, et al. Acute response and chronic adaptation to exercise in women. Med Sci Sports Exerc. 1992;24:S258–S265. [PubMed] [Google Scholar]
- 19.Ogawa T, Spina RJ, Martin WH, Kohrt WM, Schechtman KB, Holloszy JO, et al. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation. 1992;86:494–503. doi: 10.1161/01.cir.86.2.494. [DOI] [PubMed] [Google Scholar]
- 20.Peltonen JE, Hägglund H, Koskela-Koivisto T, Koponen AS, Aho JM, Rissanen A-PE, et al. Alveolar gas exchange, oxygen delivery and tissue deoxygenation in men and women during incremental exercise. Respir Physiol Neurobiol. 2013;188:102–112. doi: 10.1016/j.resp.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 21.Lundsgaard A-M, Fritzen AM, Kiens B. Chapter 36. Exercise physiology in men and women. In: Legato MJ, editor. Principles of gender-specific medicine (Third Edition). San Diego: Academic Press; 2017. p. 525–42.
- 22.Diaz-Canestro C, Montero D. Sex dimorphism of VO2max trainability: a systematic review and meta-analysis. Sports Med. 2019;49:1949–1956. doi: 10.1007/s40279-019-01180-z. [DOI] [PubMed] [Google Scholar]
- 23.Coffey VG, Hawley JA. Concurrent exercise training: do opposites distract? J Physiol. 2017;595:2883–2896. doi: 10.1113/JP272270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Zwaard S, Weide G, Levels K, Eikelboom MRI, Noordhof DA, Hofmijster MJ, et al. Muscle morphology of the vastus lateralis is strongly related to ergometer performance, sprint capacity and endurance capacity in Olympic rowers. J Sports Sci. 2018;36:2111–2120. doi: 10.1080/02640414.2018.1439434. [DOI] [PubMed] [Google Scholar]
- 25.Lundberg TR, Feuerbacher JF, Sünkeler M, Schumann M. The effects of concurrent aerobic and strength training on muscle fiber hypertrophy: a systematic review and meta-analysis. Sports Med. 2022;52:2391–2403. doi: 10.1007/s40279-022-01688-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang X, Lin J, Demner-Fushman D. Evaluation of PICO as a knowledge representation for clinical questions. AMIA Annu Symp Proc. 2006;2006:359–363. [PMC free article] [PubMed] [Google Scholar]
- 27.Santos Junior ERT, de Salles BF, Dias I, Ribeiro AS, Simão R, Willardson JM. Classification and determination model of resistance training status. Strength Cond J. 2021;43:77–86. [Google Scholar]
- 28.Pauw KD, Roelands B, Cheung SS, de Geus B, Rietjens G, Meeusen R. Guidelines to classify subject groups in sport-science research. Int J Sports Physiol Perform. 2013;8:111–122. doi: 10.1123/ijspp.8.2.111. [DOI] [PubMed] [Google Scholar]
- 29.Decroix L, Pauw KD, Foster C, Meeusen R. Guidelines to classify female subject groups in sport-science research. Int J Sports Physiol Perform. 2016;11:204–213. doi: 10.1123/ijspp.2015-0153. [DOI] [PubMed] [Google Scholar]
- 30.Kraemer WJ, Ratamess NA. Fundamentals of resistance training: progression and exercise prescription. Med Sci Sports Exerc. 2004;36:674–688. doi: 10.1249/01.mss.0000121945.36635.61. [DOI] [PubMed] [Google Scholar]
- 31.American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41. [DOI] [PubMed]
- 32.Drevon D, Fursa SR, Malcolm AL. Intercoder reliability and validity of WebPlotDigitizer in extracting graphed data. Behav Modif. 2017;41:323–339. doi: 10.1177/0145445516673998. [DOI] [PubMed] [Google Scholar]
- 33.Higgins JPT, Green S, Cochrane Collaboration, editors. Cochrane handbook for systematic reviews of interventions. Chichester; Hoboken: Wiley-Blackwell; 2008.
- 34.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deeks J, Higgins J. Statistical algorithms in Review Manager 5. 2010.
- 36.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 37.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 38.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Viechtbauer W, Cheung MW-L. Outlier and influence diagnostics for meta-analysis. Res Synth Methods. 2010;1:112–125. doi: 10.1002/jrsm.11. [DOI] [PubMed] [Google Scholar]
- 40.Chtara M, Chaouachi A, Levin GT, Chaouachi M, Chamari K, Amri M, et al. Effect of concurrent endurance and circuit resistance training sequence on muscular strength and power development. J Strength Cond Res. 2008;22:1037–1045. doi: 10.1519/JSC.0b013e31816a4419. [DOI] [PubMed] [Google Scholar]
- 41.Chtara M, Chamari K, Chaouachi M, Chaouachi A, Koubaa D, Feki Y, et al. Effects of intra-session concurrent endurance and strength training sequence on aerobic performance and capacity. Br J Sports Med. 2005;39:555–560. doi: 10.1136/bjsm.2004.015248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Libardi CA, De Souza GV, Cavaglieri CR, Madruga VA, Chacon-Mikahil MPT. Effect of resistance, endurance, and concurrent training on TNF-α, IL-6, and CRP. Med Sci Sports Exerc. 2012;44:50–56. doi: 10.1249/MSS.0b013e318229d2e9. [DOI] [PubMed] [Google Scholar]
- 43.Libardi CA, Souza GV, Gáspari AF, Dos Santos CF, Leite ST, Dias R, et al. Effects of concurrent training on interleukin-6, tumour necrosis factor-alpha and C-reactive protein in middle-aged men. J Sports Sci. 2011;29:1573–1581. doi: 10.1080/02640414.2011.609896. [DOI] [PubMed] [Google Scholar]
- 44.McCarthy JP, Pozniak MA, Agre JC. Neuromuscular adaptations to concurrent strength and endurance training. Med Sci Sports Exerc. 2002;34:511–519. doi: 10.1097/00005768-200203000-00019. [DOI] [PubMed] [Google Scholar]
- 45.McCarthy JP, Agre JC, Graf BK, Pozniak MA, Vailas AC. Compatibility of adaptive responses with combining strength and endurance training. Med Sci Sports Exerc. 1995;27:429–436. [PubMed] [Google Scholar]
- 46.de Souza EO, Tricoli V, Aoki MS, Roschel H, Brum PC, Bacurau AVN, et al. Effects of concurrent strength and endurance training on genes related to myostatin signaling pathway and muscle fiber responses. J Strength Cond Res. 2014;28:3215–3223. doi: 10.1519/JSC.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 47.de Souza EO, Tricoli V, Roschel H, Brum PC, Bacurau AVN, Ferreira JCB, et al. Molecular adaptations to concurrent training. Int J Sports Med. 2013;34:207–213. doi: 10.1055/s-0032-1312627. [DOI] [PubMed] [Google Scholar]
- 48.Kraemer WJ, Patton JF, Gordon SE, Harman EA, Deschenes MR, Reynolds K, et al. Compatibility of high-intensity strength and endurance training on hormonal and skeletal muscle adaptations. J Appl Physiol. 1995;78:976–989. doi: 10.1152/jappl.1995.78.3.976. [DOI] [PubMed] [Google Scholar]
- 49.Rønnestad BR, Hansen EA, Raastad T. Effect of heavy strength training on thigh muscle cross-sectional area, performance determinants, and performance in well-trained cyclists. Eur J Appl Physiol. 2010;108:965–975. doi: 10.1007/s00421-009-1307-z. [DOI] [PubMed] [Google Scholar]
- 50.Rønnestad BR, Hansen EA, Raastad T. Strength training improves 5-min all-out performance following 185 min of cycling. Scand J Med Sci Sports. 2011;21:250–259. doi: 10.1111/j.1600-0838.2009.01035.x. [DOI] [PubMed] [Google Scholar]
- 51.Rønnestad BR, Hansen J, Hollan I, Ellefsen S. Strength training improves performance and pedaling characteristics in elite cyclists. Scand J Med Sci Sports. 2015;25:e89–98. doi: 10.1111/sms.12257. [DOI] [PubMed] [Google Scholar]
- 52.Vikmoen O, Raastad T, Seynnes O, Bergstrøm K, Ellefsen S, Rønnestad BR. Effects of heavy strength training on running performance and determinants of running performance in female endurance athletes. PLoS ONE. 2016;11:e0150799. doi: 10.1371/journal.pone.0150799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vikmoen O, Ellefsen S, Trøen Ø, Hollan I, Hanestadhaugen M, Raastad T, et al. Strength training improves cycling performance, fractional utilization of VO2max and cycling economy in female cyclists. Scand J Med Sci Sports. 2016;26:384–396. doi: 10.1111/sms.12468. [DOI] [PubMed] [Google Scholar]
- 54.Beattie K, Carson BP, Lyons M, Rossiter A, Kenny IC. The effect of strength training on performance indicators in distance runners. J Strength Cond Res. 2017;31:9–23. doi: 10.1519/JSC.0000000000001464. [DOI] [PubMed] [Google Scholar]
- 55.Gergley JC. Comparison of two lower-body modes of endurance training on lower-body strength development while concurrently training. J Strength Cond Res. 2009;23:979–987. doi: 10.1519/JSC.0b013e3181a0629d. [DOI] [PubMed] [Google Scholar]
- 56.Gravelle BL, Blessing DL. Physiological adaptation in women concurrently training for strength and endurance. J Strength Cond Res. 2000;14:5. [Google Scholar]
- 57.Jones TW, Howatson G, Russell M, French DN. Performance and endocrine responses to differing ratios of concurrent strength and endurance training. J Strength Cond Res. 2016;30:693–702. doi: 10.1519/JSC.0000000000001135. [DOI] [PubMed] [Google Scholar]
- 58.Kawano H, Fujimoto K, Higuchi M, Miyachi M. Effect of combined resistance and aerobic training on reactive hyperemia in men. J Physiol Sci. 2009;59:457–464. doi: 10.1007/s12576-009-0057-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee MJ-C, Ballantyne JK, Chagolla J, Hopkins WG, Fyfe JJ, Phillips SM, et al. Order of same-day concurrent training influences some indices of power development, but not strength, lean mass, or aerobic fitness in healthy, moderately-active men after 9 weeks of training. PLoS ONE. 2020;15:e0233134. doi: 10.1371/journal.pone.0233134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leveritt M, Abernethy PJ, Barry B, Logan PA. Concurrent strength and endurance training: the influence of dependent variable selection. J Strength Cond Res. 2003;17:503–508. doi: 10.1519/1533-4287(2003)017<0503:csaett>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 61.Psilander N, Frank P, Flockhart M, Sahlin K. Adding strength to endurance training does not enhance aerobic capacity in cyclists. Scand J Med Sci Sports. 2015;25:e353–e359. doi: 10.1111/sms.12338. [DOI] [PubMed] [Google Scholar]
- 62.Støren O, Helgerud J, Støa EM, Hoff J. Maximal strength training improves running economy in distance runners. Med Sci Sports Exerc. 2008;40:1087–1092. doi: 10.1249/MSS.0b013e318168da2f. [DOI] [PubMed] [Google Scholar]
- 63.Timmins RG, Shamim B, Tofari PJ, Hickey JT, Camera DM. Differences in lower limb strength and structure after 12 weeks of resistance, endurance, and concurrent training. Int J Sports Physiol Perform. 2020;15(9):1223–1230. doi: 10.1123/ijspp.2019-0788. [DOI] [PubMed] [Google Scholar]
- 64.Aagaard P, Andersen JL, Bennekou M, Larsson B, Olesen JL, Crameri R, et al. Effects of resistance training on endurance capacity and muscle fiber composition in young top-level cyclists. Scand J Med Sci Sports. 2011;21:e298–307. doi: 10.1111/j.1600-0838.2010.01283.x. [DOI] [PubMed] [Google Scholar]
- 65.Balabinis CP, Psarakis CH, Moukas M, Vassiliou MP, Behrakis PK. Early phase changes by concurrent endurance and strength training. J Strength Cond Res. 2003;17:393–401. doi: 10.1519/1533-4287(2003)017<0393:epcbce>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 66.Beattie K, Carson BP, Lyons M, Kenny IC. The effect of maximal- and explosive-strength training on performance indicators in cyclists. Int J Sports Physiol Perform. 2017;12:470–480. doi: 10.1123/ijspp.2016-0015. [DOI] [PubMed] [Google Scholar]
- 67.Bell G, Syrotuik D, Socha T, Maclean I, Quinney HA. Effect of strength training and concurrent strength and endurance training on strength, testosterone, and cortisol. J Strength Cond Res. 1997;11:57. [Google Scholar]
- 68.Bell GJ, Syrotuik D, Martin TP, Burnham R, Quinney HA. Effect of concurrent strength and endurance training on skeletal muscle properties and hormone concentrations in humans. Eur J Appl Physiol. 2000;81:418–427. doi: 10.1007/s004210050063. [DOI] [PubMed] [Google Scholar]
- 69.Bishop D, Jenkins DG, Mackinnon LT, McEniery M, Carey MF. The effects of strength training on endurance performance and muscle characteristics. Med Sci Sports Exerc. 1999;31:886–891. doi: 10.1097/00005768-199906000-00018. [DOI] [PubMed] [Google Scholar]
- 70.Damasceno MV, Lima-Silva AE, Pasqua LA, Tricoli V, Duarte M, Bishop DJ, et al. Effects of resistance training on neuromuscular characteristics and pacing during 10-km running time trial. Eur J Appl Physiol. 2015;115:1513–1522. doi: 10.1007/s00421-015-3130-z. [DOI] [PubMed] [Google Scholar]
- 71.Dolezal BA, Potteiger JA. Concurrent resistance and endurance training influence basal metabolic rate in nondieting individuals. J Appl Physiol. 1998;85:695–700. doi: 10.1152/jappl.1998.85.2.695. [DOI] [PubMed] [Google Scholar]
- 72.Dudley GA, Djamil R. Incompatibility of endurance- and strength-training modes of exercise. J Appl Physiol. 1985;59:1446–1451. doi: 10.1152/jappl.1985.59.5.1446. [DOI] [PubMed] [Google Scholar]
- 73.Ferrauti A, Bergermann M, Fernandez-Fernandez J. Effects of a concurrent strength and endurance training on running performance and running economy in recreational marathon runners. J Strength Cond Res. 2010;24:2770–2778. doi: 10.1519/JSC.0b013e3181d64e9c. [DOI] [PubMed] [Google Scholar]
- 74.Filipas L, Bonato M, Maggio A, Gallo G, Codella R. Effects of plyometric training on different 8-week training intensity distributions in well-trained endurance runners. Scand J Med Sci Sports. 2023;33:200–212. doi: 10.1111/sms.14257. [DOI] [PubMed] [Google Scholar]
- 75.Fyfe JJ, Bartlett JD, Hanson ED, Stepto NK, Bishop DJ. Endurance training intensity does not mediate interference to maximal lower-body strength gain during short-term concurrent training. Front Physiol. 2016;7:487. doi: 10.3389/fphys.2016.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Glowacki SP, Martin SE, Maurer A, Baek W, Green JS, Crouse SF. Effects of resistance, endurance, and concurrent exercise on training outcomes in men. Med Sci Sports Exerc. 2004;36:2119–2127. doi: 10.1249/01.mss.0000147629.74832.52. [DOI] [PubMed] [Google Scholar]
- 77.Gómez-Molina J, Ogueta-Alday A, Camara J, Stickley C, García-López J. Effect of 8 weeks of concurrent plyometric and running training on spatiotemporal and physiological variables of novice runners. Eur J Sport Sci. 2018;18:162–169. doi: 10.1080/17461391.2017.1404133. [DOI] [PubMed] [Google Scholar]
- 78.Gonçalves R, Motta-Santos D, Szmuchrowski L, Couto B, Soares YM, Damasceno VO, et al. Combined training is not superior to strength and aerobic training to mitigate cardiovascular risk in adult healthy men. Biol Sport. 2022;39:727–734. doi: 10.5114/biolsport.2022.107483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Häkkinen K, Alen M, Kraemer WJ, Gorostiaga E, Izquierdo M, Rusko H, et al. Neuromuscular adaptations during concurrent strength and endurance training versus strength training. Eur J Appl Physiol. 2003;89:42–52. doi: 10.1007/s00421-002-0751-9. [DOI] [PubMed] [Google Scholar]
- 80.Hausswirth C, Argentin S, Bieuzen F, Le Meur Y, Couturier A, Brisswalter J. Endurance and strength training effects on physiological and muscular parameters during prolonged cycling. J Electromyogr Kinesiol. 2010;20:330–339. doi: 10.1016/j.jelekin.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 81.Hendrickson NR, Sharp MA, Alemany JA, Walker LA, Harman EA, Spiering BA, et al. Combined resistance and endurance training improves physical capacity and performance on tactical occupational tasks. Eur J Appl Physiol. 2010;109:1197–1208. doi: 10.1007/s00421-010-1462-2. [DOI] [PubMed] [Google Scholar]
- 82.Izquierdo M, Häkkinen K, Ibáñez J, Kraemer WJ, Gorostiaga EM. Effects of combined resistance and cardiovascular training on strength, power, muscle cross-sectional area, and endurance markers in middle-aged men. Eur J Appl Physiol. 2005;94:70–75. doi: 10.1007/s00421-004-1280-5. [DOI] [PubMed] [Google Scholar]
- 83.Johnston RE, Quinn TJ, Kertzer R, Vroman NB. Strength training in female distance runners: impact on running economy. J Strength Cond Res. 1997;11:224. [Google Scholar]
- 84.Kelly CM, Burnett AF, Newton MJ. The effect of strength training on three-kilometer performance in recreational women endurance runners. J Strength Cond Res. 2008;22:396–403. doi: 10.1519/JSC.0b013e318163534a. [DOI] [PubMed] [Google Scholar]
- 85.Laird RH, Elmer DJ, Barberio MD, Salom LP, Lee KA, Pascoe DD. Evaluation of performance improvements after either resistance training or sprint interval-based concurrent training. J Strength Cond Res. 2016;30:3057–3065. doi: 10.1519/JSC.0000000000001412. [DOI] [PubMed] [Google Scholar]
- 86.LeMura LM, von Duvillard SP, Andreacci J, Klebez JM, Chelland SA, Russo J. Lipid and lipoprotein profiles, cardiovascular fitness, body composition, and diet during and after resistance, aerobic and combination training in young women. Eur J Appl Physiol. 2000;82:451–458. doi: 10.1007/s004210000234. [DOI] [PubMed] [Google Scholar]
- 87.Levin GT, Mcguigan MR, Laursen PB. Effect of concurrent resistance and endurance training on physiologic and performance parameters of well-trained endurance cyclists. J Strength Cond Res. 2009;23:2280–2286. doi: 10.1519/JSC.0b013e3181b990c2. [DOI] [PubMed] [Google Scholar]
- 88.Losnegard T, Mikkelsen K, Rønnestad BR, Hallén J, Rud B, Raastad T. The effect of heavy strength training on muscle mass and physical performance in elite cross country skiers. Scand J Med Sci Sports. 2011;21:389–401. doi: 10.1111/j.1600-0838.2009.01074.x. [DOI] [PubMed] [Google Scholar]
- 89.Mikkola J, Rusko H, Izquierdo M, Gorostiaga EM, Häkkinen K. Neuromuscular and cardiovascular adaptations during concurrent strength and endurance training in untrained men. Int J Sports Med. 2012;33:702–710. doi: 10.1055/s-0031-1295475. [DOI] [PubMed] [Google Scholar]
- 90.Millet GP, Jaouen B, Borrani F, Candau R. Effects of concurrent endurance and strength training on running economy and VO(2) kinetics. Med Sci Sports Exerc. 2002;34:1351–1359. doi: 10.1097/00005768-200208000-00018. [DOI] [PubMed] [Google Scholar]
- 91.Mirghani SJ, Alinejad HA, Azarbayjani MA, Mazidi A, Mirghani SA. Influence of strength, endurance and concurrent training on the lipid profile and blood testosterone and cortisol response in young male wrestlers. Baltic J Health Phys Activity. 2014;6.
- 92.Nelson AG, Arnall DA, Loy SF, Silvester LJ, Conlee RK. Consequences of combining strength and endurance training regimens. Phys Ther. 1990;70:287–294. doi: 10.1093/ptj/70.5.287. [DOI] [PubMed] [Google Scholar]
- 93.Panissa VLG, Fukuda DH, de Oliveira FP, Parmezzani SS, Campos EZ, Rossi FE, et al. Maximum strength development and volume-load during concurrent high intensity intermittent training plus strength or strength-only training. J Sports Sci Med. 2018;17:623–632. [PMC free article] [PubMed] [Google Scholar]
- 94.Prieto-González P, Sedlacek J. Effects of running-specific strength training, endurance training, and concurrent training on recreational endurance athletes’ performance and selected anthropometric parameters. Int J Environ Res Public Health. 2022;19:10773. doi: 10.3390/ijerph191710773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Robineau J, Babault N, Piscione J, Lacome M, Bigard AX. Specific training effects of concurrent aerobic and strength exercises depend on recovery duration. J Strength Cond Res. 2016;30:672–683. doi: 10.1519/JSC.0000000000000798. [DOI] [PubMed] [Google Scholar]
- 96.Robineau J, Lacome M, Piscione J, Bigard X, Babault N. Concurrent training in Rugby Sevens: effects of high-intensity interval exercises. Int J Sports Physiol Perform. 2017;12:336–344. doi: 10.1123/ijspp.2015-0370. [DOI] [PubMed] [Google Scholar]
- 97.Rønnestad BR, Hansen EA, Raastad T. In-season strength maintenance training increases well-trained cyclists’ performance. Eur J Appl Physiol. 2010;110:1269–1282. doi: 10.1007/s00421-010-1622-4. [DOI] [PubMed] [Google Scholar]
- 98.Rønnestad BR, Hansen J, Hollan I, Spencer M, Ellefsen S. Impairment of performance variables after in-season strength-training cessation in elite cyclists. Int J Sports Physiol Perform. 2016;11:727–735. doi: 10.1123/ijspp.2015-0372. [DOI] [PubMed] [Google Scholar]
- 99.Rønnestad BR, Hansen J, Nygaard H. 10 weeks of heavy strength training improves performance-related measurements in elite cyclists. J Sports Sci. 2017;35:1435–1441. doi: 10.1080/02640414.2016.1215499. [DOI] [PubMed] [Google Scholar]
- 100.Sánchez-Moreno M, Rodríguez-Rosell D, Díaz-Cueli D, Pareja-Blanco F, González-Badillo JJ. Effects of velocity loss threshold within resistance training during concurrent training on endurance and strength performance. Int J Sports Physiol Perform. 2021;16:849–857. doi: 10.1123/ijspp.2020-0349. [DOI] [PubMed] [Google Scholar]
- 101.Shamim B, Devlin BL, Timmins RG, Tofari P, Lee Dow C, Coffey VG, et al. Adaptations to concurrent training in combination with high protein availability: a comparative trial in healthy, recreationally active men. Sports Med. 2018;48:2869–2883. doi: 10.1007/s40279-018-0999-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shaw BS, Shaw I. Compatibility of concurrent aerobic and resistance training on maximal aerobic capacity in sedentary males. Cardiovasc J Afr. 2009;20:104–106. [PMC free article] [PubMed] [Google Scholar]
- 103.Silva RF, Cadore EL, Kothe G, Guedes M, Alberton CL, Pinto SS, et al. Concurrent training with different aerobic exercises. Int J Sports Med. 2012;33:627–634. doi: 10.1055/s-0031-1299698. [DOI] [PubMed] [Google Scholar]
- 104.Skovgaard C, Christensen PM, Larsen S, Andersen TR, Thomassen M, Bangsbo J. Concurrent speed endurance and resistance training improves performance, running economy, and muscle NHE1 in moderately trained runners. J Appl Physiol. 2014;117:1097–1109. doi: 10.1152/japplphysiol.01226.2013. [DOI] [PubMed] [Google Scholar]
- 105.Spiliopoulou P, Zaras N, Methenitis S, Papadimas G, Papadopoulos C, Bogdanis GC, et al. Effect of concurrent power training and high-intensity interval cycling on muscle morphology and performance. J Strength Cond Res. 2021;35:2464–2471. doi: 10.1519/JSC.0000000000003172. [DOI] [PubMed] [Google Scholar]
- 106.Štohanzl M, Baláš J, Draper N. Effects of minimal dose of strength training on running performance in female recreational runners. J Sports Med Phys Fit. 2018;58:1211–1217. doi: 10.23736/S0022-4707.17.07124-9. [DOI] [PubMed] [Google Scholar]
- 107.Sunde A, Støren O, Bjerkaas M, Larsen MH, Hoff J, Helgerud J. Maximal strength training improves cycling economy in competitive cyclists. J Strength Cond Res. 2010;24:2157–2165. doi: 10.1519/JSC.0b013e3181aeb16a. [DOI] [PubMed] [Google Scholar]
- 108.Terzis G, Spengos K, Methenitis S, Aagaard P, Karandreas N, Bogdanis G. Early phase interference between low-intensity running and power training in moderately trained females. Eur J Appl Physiol. 2016;116:1063–1073. doi: 10.1007/s00421-016-3369-z. [DOI] [PubMed] [Google Scholar]
- 109.Trowell D, Fox A, Saunders N, Vicenzino B, Bonacci J. Effect of concurrent strength and endurance training on run performance and biomechanics: a randomized controlled trial. Scand J Med Sci Sports. 2022;32:543–558. doi: 10.1111/sms.14092. [DOI] [PubMed] [Google Scholar]
- 110.Tsitkanou S, Spengos K, Stasinaki A-N, Zaras N, Bogdanis G, Papadimas G, et al. Effects of high-intensity interval cycling performed after resistance training on muscle strength and hypertrophy. Scand J Med Sci Sports. 2017;27:1317–1327. doi: 10.1111/sms.12751. [DOI] [PubMed] [Google Scholar]
- 111.Vorup J, Tybirk J, Gunnarsson TP, Ravnholt T, Dalsgaard S, Bangsbo J. Effect of speed endurance and strength training on performance, running economy and muscular adaptations in endurance-trained runners. Eur J Appl Physiol. 2016;116:1331–1341. doi: 10.1007/s00421-016-3356-4. [DOI] [PubMed] [Google Scholar]
- 112.Miller AE, MacDougall JD, Tarnopolsky MA, Sale DG. Gender differences in strength and muscle fiber characteristics. Eur J Appl Physiol Occup Physiol. 1993;66:254–262. doi: 10.1007/BF00235103. [DOI] [PubMed] [Google Scholar]
- 113.Nuzzo JL. Narrative review of sex differences in muscle strength, endurance, activation, size, fiber type, and strength training participation rates, preferences, motivations, injuries, and neuromuscular adaptations. J Strength Cond Res. 2023;37:494. doi: 10.1519/JSC.0000000000004329. [DOI] [PubMed] [Google Scholar]
- 114.Sontag SA, Trevino MA, Herda TJ, Sterczala AJ, Miller JD, Parra ME, et al. Endurance training alters motor unit activation strategies for the vastus lateralis, yet sex-related differences and relationships with muscle size remain. Eur J Appl Physiol. 2021;121:1367–1377. doi: 10.1007/s00421-021-04622-7. [DOI] [PubMed] [Google Scholar]
- 115.Simoneau JA, Bouchard C. Human variation in skeletal muscle fiber-type proportion and enzyme activities. Am J Physiol. 1989;257:E567–E572. doi: 10.1152/ajpendo.1989.257.4.E567. [DOI] [PubMed] [Google Scholar]
- 116.Staron RS, Hagerman FC, Hikida RS, Murray TF, Hostler DP, Crill MT, et al. Fiber type composition of the vastus lateralis muscle of young men and women. J Histochem Cytochem. 2000;48:623–629. doi: 10.1177/002215540004800506. [DOI] [PubMed] [Google Scholar]
- 117.Knowles OE, Aisbett B, Main LC, Drinkwater EJ, Orellana L, Lamon S. Resistance training and skeletal muscle protein metabolism in eumenorrheic females: implications for researchers and practitioners. Sports Med. 2019;49:1637–1650. doi: 10.1007/s40279-019-01132-7. [DOI] [PubMed] [Google Scholar]
- 118.Abe T, DeHoyos DV, Pollock ML, Garzarella L. Time course for strength and muscle thickness changes following upper and lower body resistance training in men and women. Eur J Appl Physiol. 2000;81:174–180. doi: 10.1007/s004210050027. [DOI] [PubMed] [Google Scholar]
- 119.Staron RS, Karapondo DL, Kraemer WJ, Fry AC, Gordon SE, Falkel JE, et al. Skeletal muscle adaptations during early phase of heavy-resistance training in men and women. J Appl Physiol. 1994;76:1247–1255. doi: 10.1152/jappl.1994.76.3.1247. [DOI] [PubMed] [Google Scholar]
- 120.Folland JP, Williams AG. Morphological and neurological contributions to increased strength. Sports Med. 2007;37:145–168. doi: 10.2165/00007256-200737020-00004. [DOI] [PubMed] [Google Scholar]
- 121.Alway SE, Grumbt WH, Gonyea WJ, Stray-Gundersen J. Contrasts in muscle and myofibers of elite male and female bodybuilders. J Appl Physiol. 1985;1989(67):24–31. doi: 10.1152/jappl.1989.67.1.24. [DOI] [PubMed] [Google Scholar]
- 122.Fry AC. The role of resistance exercise intensity on muscle fibre adaptations. Sports Med. 2004;34:663–679. doi: 10.2165/00007256-200434100-00004. [DOI] [PubMed] [Google Scholar]
- 123.Kraemer WJ, Häkkinen K, Newton RU, McCormick M, Nindl BC, Volek JS, et al. Acute hormonal responses to heavy resistance exercise in younger and older men. Eur J Appl Physiol Occup Physiol. 1998;77:206–211. doi: 10.1007/s004210050323. [DOI] [PubMed] [Google Scholar]
- 124.Häkkinen K, Pakarinen A. Acute hormonal responses to heavy resistance exercise in men and women at different ages. Int J Sports Med. 1995;16:507–513. doi: 10.1055/s-2007-973045. [DOI] [PubMed] [Google Scholar]
- 125.Spiering BA, Kraemer WJ, Vingren JL, Ratamess NA, Anderson JM, Armstrong LE, et al. Elevated endogenous testosterone concentrations potentiate muscle androgen receptor responses to resistance exercise. J Steroid Biochem Mol Biol. 2009;114:195–199. doi: 10.1016/j.jsbmb.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 126.Basualto-Alarcón C, Jorquera G, Altamirano F, Jaimovich E, Estrada M. Testosterone signals through mTOR and androgen receptor to induce muscle hypertrophy. Med Sci Sports Exerc. 2013;45:1712–1720. doi: 10.1249/MSS.0b013e31828cf5f3. [DOI] [PubMed] [Google Scholar]
- 127.Roberts BM, Lavin KM, Many GM, Thalacker-Mercer A, Merritt EK, Bickel CS, et al. Human neuromuscular aging: Sex differences revealed at the myocellular level. Exp Gerontol. 2018;106:116–124. doi: 10.1016/j.exger.2018.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.West DWD, Burd NA, Churchward-Venne TA, Camera DM, Mitchell CJ, Baker SK, et al. Sex-based comparisons of myofibrillar protein synthesis after resistance exercise in the fed state. J Appl Physiol. 1985;2012(112):1805–1813. doi: 10.1152/japplphysiol.00170.2012. [DOI] [PubMed] [Google Scholar]
- 129.Fujita S, Rasmussen BB, Bell JA, Cadenas JG, Volpi E. Basal muscle intracellular amino acid kinetics in women and men. Am J Physiol Endocrinol Metab. 2007;292:E77–83. doi: 10.1152/ajpendo.00173.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Markofski MM, Volpi E. Protein metabolism in women and men: similarities and disparities. Curr Opin Clin Nutr Metab Care. 2011;14:93–97. doi: 10.1097/MCO.0b013e3283412343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Häkkinen K. Neuromuscular fatigue and recovery in male and female athletes during heavy resistance exercise. Int J Sports Med. 1993;14:53–59. doi: 10.1055/s-2007-1021146. [DOI] [PubMed] [Google Scholar]
- 132.Hunter SK. Sex differences in human fatigability: mechanisms and insight to physiological responses. Acta Physiol. 2014;210:768–789. doi: 10.1111/apha.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hunter SK. The relevance of sex differences in performance fatigability. Med Sci Sports Exerc. 2016;48:2247–2256. doi: 10.1249/MSS.0000000000000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ansdell P, Brownstein CG, Škarabot J, Hicks KM, Howatson G, Thomas K, et al. Sex differences in fatigability and recovery relative to the intensity-duration relationship. J Physiol. 2019;597:5577–5595. doi: 10.1113/JP278699. [DOI] [PubMed] [Google Scholar]
- 135.Leveritt M, Abernethy PJ, Barry BK, Logan PA. Concurrent strength and endurance training. A review. Sports Med. 1999;28(6):413–27. 10.2165/00007256-199928060-00004 [DOI] [PubMed]
- 136.McKay AKA, Stellingwerff T, Smith ES, Martin DT, Mujika I, Goosey-Tolfrey VL, et al. Defining training and performance caliber: a participant classification framework. Int J Sports Physiol Perform. 2022;17:317–331. doi: 10.1123/ijspp.2021-0451. [DOI] [PubMed] [Google Scholar]
- 137.van der Zwaard S, de Ruiter CJ, Noordhof DA, Sterrenburg R, Bloemers FW, de Koning JJ, et al. Maximal oxygen uptake is proportional to muscle fiber oxidative capacity, from chronic heart failure patients to professional cyclists. J Appl Physiol. 1985;2016(121):636–645. doi: 10.1152/japplphysiol.00355.2016. [DOI] [PubMed] [Google Scholar]
- 138.Gollnick PD, Armstrong RB, Saubert CW, Piehl K, Saltin B. Enzyme activity and fiber composition in skeletal muscle of untrained and trained men. J Appl Physiol. 1972;33:312–319. doi: 10.1152/jappl.1972.33.3.312. [DOI] [PubMed] [Google Scholar]
- 139.Andersen P, Henriksson J. Capillary supply of the quadriceps femoris muscle of man: adaptive response to exercise. J Physiol. 1977;270:677–690. doi: 10.1113/jphysiol.1977.sp011975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ingjer F. Capillary supply and mitochondrial content of different skeletal muscle fiber types in untrained and endurance-trained men: a histochemical and ultrastructural study. Eur J Appl Physiol. 1979;40:197–209. doi: 10.1007/BF00426942. [DOI] [PubMed] [Google Scholar]
- 141.Klausen K, Andersen LB, Pelle I. Adaptive changes in work capacity, skeletal muscle capillarization and enzyme levels during training and detraining. Acta Physiol Scand. 1981;113:9–16. doi: 10.1111/j.1748-1716.1981.tb06854.x. [DOI] [PubMed] [Google Scholar]
- 142.Murias JM, Kowalchuk JM, Paterson DH. Time course and mechanisms of adaptations in cardiorespiratory fitness with endurance training in older and young men. J Appl Physiol. 2010;108:621–627. doi: 10.1152/japplphysiol.01152.2009. [DOI] [PubMed] [Google Scholar]
- 143.Holloszy JO, Booth FW. Biochemical adaptations to endurance exercise in muscle. Annu Rev Physiol. 1976;38:273–291. doi: 10.1146/annurev.ph.38.030176.001421. [DOI] [PubMed] [Google Scholar]
- 144.Montero D, Diaz-Cañestro C, Lundby C. Endurance training and VO2max: role of maximal cardiac output and oxygen extraction. Med Sci Sports Exerc. 2015;47:2024–2033. doi: 10.1249/MSS.0000000000000640. [DOI] [PubMed] [Google Scholar]
- 145.MacInnis MJ, Gibala MJ. Physiological adaptations to interval training and the role of exercise intensity. J Physiol. 2017;595:2915–2930. doi: 10.1113/JP273196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.van der Zwaard S, Brocherie F, Kom BLG, Millet GP, Deldicque L, van der Laarse WJ, et al. Adaptations in muscle oxidative capacity, fiber size, and oxygen supply capacity after repeated-sprint training in hypoxia combined with chronic hypoxic exposure. J Appl Physiol. 2018;124:1403–1412. doi: 10.1152/japplphysiol.00946.2017. [DOI] [PubMed] [Google Scholar]
- 147.Liu Y, Christensen PM, Hellsten Y, Gliemann L. Effects of exercise training intensity and duration on skeletal muscle capillarization in healthy subjects: a meta-analysis. Med Sci Sports Exerc. 2022;54:1714–1728. doi: 10.1249/MSS.0000000000002955. [DOI] [PubMed] [Google Scholar]
- 148.Convertino VA. Blood volume: its adaptation to endurance training. Med Sci Sports Exerc. 1991;23:1338–1348. [PubMed] [Google Scholar]
- 149.Arbab-Zadeh A, Perhonen M, Howden E, Peshock RM, Zhang R, Adams-Huet B, et al. Cardiac remodeling in response to 1 year of intensive endurance training. Circulation. 2014;130:2152–2161. doi: 10.1161/CIRCULATIONAHA.114.010775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Howden EJ, Perhonen M, Peshock RM, Zhang R, Arbab-Zadeh A, Adams-Huet B, et al. Females have a blunted cardiovascular response to one year of intensive supervised endurance training. J Appl Physiol. 2015;119:37–46. doi: 10.1152/japplphysiol.00092.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Konopka AR, Harber MP. Skeletal muscle hypertrophy after aerobic exercise training. Exerc Sport Sci Rev. 2014;42:53–61. doi: 10.1249/JES.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tang JE, Hartman JW, Phillips SM. Increased muscle oxidative potential following resistance training induced fibre hypertrophy in young men. Appl Physiol Nutr Metab. 2006;31:495–501. doi: 10.1139/h06-026. [DOI] [PubMed] [Google Scholar]
- 153.Lopez P, Radaelli R, Taaffe DR, Newton RU, Galvão DA, Trajano GS, et al. Resistance training load effects on muscle hypertrophy and strength gain: systematic review and network meta-analysis. Med Sci Sports Exerc. 2021;53:1206–1216. doi: 10.1249/MSS.0000000000002585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Latella C, Teo W-P, Spathis J, van den Hoek D. Long-term strength adaptation: a 15-year analysis of powerlifting athletes. J Strength Cond Res. 2020;34:2412–2418. doi: 10.1519/JSC.0000000000003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.van der Zwaard S, Koppens TFP, Weide G, Levels K, Hofmijster MJ, de Koning JJ, et al. Training-induced muscle adaptations during competitive preparation in elite female rowers. Front Sports Act Living. 2021;3:344. doi: 10.3389/fspor.2021.781942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mujika I, Taipale RS. Sport science on women, women in sport science. Int J Sports Physiol Perform. 2019;14:1013–1014. doi: 10.1123/ijspp.2019-0514. [DOI] [PubMed] [Google Scholar]
- 157.Paul RW, Sonnier JH, Johnson EE, Hall AT, Osman A, Connors GM, et al. Inequalities in the evaluation of male versus female athletes in sports medicine research: a systematic review. Am J Sports Med. 2022;03635465221131281. [DOI] [PubMed]
- 158.Oosthuyse T, Strauss JA, Hackney AC. Understanding the female athlete: molecular mechanisms underpinning menstrual phase differences in exercise metabolism. Eur J Appl Physiol. 2023;123:423–450. doi: 10.1007/s00421-022-05090-3. [DOI] [PubMed] [Google Scholar]
- 159.Landen S, Hiam D, Voisin S, Jacques M, Lamon S, Eynon N. Physiological and molecular sex differences in human skeletal muscle in response to exercise training. J Physiol. 2023;601:419–434. doi: 10.1113/JP279499. [DOI] [PubMed] [Google Scholar]
- 160.Ansdell P, Thomas K, Hicks KM, Hunter SK, Howatson G, Goodall S. Physiological sex differences affect the integrative response to exercise: acute and chronic implications. Exp Physiol. 2020;105:2007–2021. doi: 10.1113/EP088548. [DOI] [PubMed] [Google Scholar]
- 161.Dipla K, Kraemer RR, Constantini NW, Hackney AC. Relative energy deficiency in sports (RED-S): elucidation of endocrine changes affecting the health of males and females. Hormones (Athens) 2021;20:35–47. doi: 10.1007/s42000-020-00214-w. [DOI] [PubMed] [Google Scholar]
- 162.Van den Noortgate W, López-López JA, Marín-Martínez F, Sánchez-Meca J. Meta-analysis of multiple outcomes: a multilevel approach. Behav Res. 2015;47:1274–1294. doi: 10.3758/s13428-014-0527-2. [DOI] [PubMed] [Google Scholar]
- 163.Van den Noortgate W, López-López JA, Marín-Martínez F, Sánchez-Meca J. Three-level meta-analysis of dependent effect sizes. Behav Res. 2013;45:576–594. doi: 10.3758/s13428-012-0261-6. [DOI] [PubMed] [Google Scholar]
- 164.Moeyaert M, Ugille M, Natasha Beretvas S, Ferron J, Bunuan R, Van den Noortgate W. Methods for dealing with multiple outcomes in meta-analysis: a comparison between averaging effect sizes, robust variance estimation and multilevel meta-analysis. Int J Soc Res Methodol. 2017;20:559–572. [Google Scholar]
- 165.Pastor DA, Lazowski RA. On the multilevel nature of meta-analysis: a tutorial, comparison of software programs, and discussion of analytic choices. Multivar Behav Res. 2018;53:74–89. doi: 10.1080/00273171.2017.1365684. [DOI] [PubMed] [Google Scholar]
- 166.Fleg JL, Lakatta EG. Role of muscle loss in the age-associated reduction in VO2 max. J Appl Physiol. 1988;65:1147–1151. doi: 10.1152/jappl.1988.65.3.1147. [DOI] [PubMed] [Google Scholar]
- 167.Keller K, Engelhardt M. Strength and muscle mass loss with aging process: age and strength loss. Muscles Ligaments Tendons J. 2014;3:346–350. [PMC free article] [PubMed] [Google Scholar]
- 168.Strait JB, Lakatta EG. Aging-associated cardiovascular changes and their relationship to heart failure. Heart Fail Clin. 2012;8:143–164. doi: 10.1016/j.hfc.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Podlogar T, Leo P, Spragg J. Using V̇O2max as a marker of training status in athletes: can we do better? J ApplPhysiol. 2022;133:144–147. doi: 10.1152/japplphysiol.00723.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Valenzuela PL, Mateo-March M, Muriel X, Zabala M, Lucia A, Barranco-Gil D, et al. Commentaries on viewpoint: using V̇O2max as a marker of training status in athletes: can we do better? J Appl Physiol. 1985;2022(133):148–164. doi: 10.1152/japplphysiol.00224.2022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.