Abstract
The prevalent use of opioids for pain management in patients with advanced cancer underscores the need for research on their neuropsychiatric impacts, particularly delirium. Therefore, we aimed to investigate the potential association between opioid use and the risk of delirium in patients with advanced cancer admitted to the acute palliative care unit. We conducted a retrospective observational study utilizing a multicenter, patient-based registry cohort by collecting the data from January 1, 2019, to December 31, 2020, in South Korea. All data regarding exposures, outcomes, and covariates were obtained through retrospective chart reviews by a team of specialized medical professionals with expertise in oncology. Full unmatched and 1:1 propensity-score matched cohorts were formed, and stratification analysis was conducted. The primary outcome, delirium, was defined and diagnosed by the DSM-IV. Of the 2,066 patients with advanced cancer, we identified 42.8% (mean [SD] age, 64.4 [13.3] years; 60.8% male) non-opioid users and 57.2% (62.8 [12.5] years; 55.9% male) opioid users, respectively. Opioid use was significantly associated with an increased occurrence of delirium in patients with advanced cancer (OR, 2.02 [95% CI 1.22–3.35]). The risk of delirium in patients with advanced cancer showed increasing trends in a dose-dependent manner. High-dose opioid users showed an increased risk of delirium in patients with advanced cancer compared to non-opioid users (low-dose user: OR, 2.21 [95% CI 1.27–3.84]; high-dose user: OR, 5.75 [95% CI 2.81–11.77]; ratio of OR, 2.60 [95% CI 1.05–6.44]). Patients with old age, male sex, absence of chemotherapy during hospitalization, and non-obese status were more susceptible to increased risk of delirium in patients with cancer. In this multicenter patient-based registry cohort study, we found a significant, dose-dependent association between opioid use and increased risk of delirium in patients with advanced cancer. We also identified specific patient groups more susceptible to delirium. These findings highlight the importance of opioid prescription in these patients with advanced cancer, balancing effective doses for pain management and adverse dose-inducing delirium.
Keywords: Opioid, Delirium, Cancer, Palliative care
Subject terms: Oncology, Risk factors
Introduction
Delirium, a common and acute neuropsychiatric complication in patients with advanced cancer1,2, remains significantly underrecognized and undetermined among patients with advanced cancer3. Characterized by a fluctuating disturbance in attention and awareness4, delirium adversely impacts the disease course by impairing communication and hindering the participation of patients in care, such as treatment decisions, counseling, and diagnosis5. Although frequently reported in advanced cancer, previous researches on delirium are predominantly limited to case reports and small patients cohorts6–8.
The etiology of delirium in these patients is typically multifactorial, and our prior research identified several risk factors for delirium in patients with cancer, including old age, absence of chemotherapy during hospitalization, hearing impairment, underweight status, current opioid use, and history of delirium and other psychiatric disorders9. Opioid prescriptions, in particular, have been identified in various studies as potential triggers of delirium, observed across different surgical and disease groups, including those with neurological injury, pain, infection, fever, and hypotension4,10,11. However, previous studies exploring the association between opioid use and delirium in patients with advanced cancer showed controversy and limited cohort size12–15.
The inevitability of using analgesics, particularly opioids, in managing chronic pain in patients with cancer, especially those with advanced stage, further complicates this issue16. In cancer pain management, approximately 50% of patients are estimated to use analgesics, with opioids being the choice for half of these individuals17. This significant reliance on opioids, despite their critical role in pain management, raises concerns about their neuropsychiatric side effects, particularly delirium, in patients with advanced cancer. Addressing this concern, we aimed to investigate the association between opioid use and the occurrence of delirium in patients with advanced cancer admitted to the acute palliative care unit (APCU), by utilizing a large-scale, multicenter, patient-based registry cohort.
Methods
Data source
In this retrospective observational study, we constructed a patient-based multicenter registry cohort by collecting data from four centers, including Seoul National University Hospital, Seoul National University Bundang Hospital, Yonsei University Severance Hospital, and CHA University Bundang Medical Center in South Korea. This patient-based registry cohort is distinguished by the following strengths9: (1) Four academic and accredited cancer centers compilated the data; (2) A team of specialized medical professionals with expertise in oncology was responsible for constructing and managing the dataset; (3) Patients who received supportive care during treatment and those who terminated their treatment were incorporated into the study; and (4) Since all of the analyzed data were anonymized, the requirement for prior consent was unnecessary. The Institutional Review Boards of the four centers (Seoul National University, H-2103-028-1201; Seoul National University Bundang Hospital, B-2104/681-405; Yonsei University, 4-2021-0323; and CHA University, CHAMC 2021-03-054-002) approved the protocol. The requirement for informed consent was waived by the four centers (Seoul National University; Seoul National University Bundang Hospital; Yonsei University; and CHA University) because only retrospective anonymized data were examined. This research adhered to the ethical guidelines established by relevant national and institutional review boards for human research and followed the 1975 Helsinki Declaration, as amended in 2008.
Study design and population
This study incorporated 2152 patients with advanced cancer admitted to the ACPU of four centers from January 1, 2019, to December 31, 2020, with follow-up until the date of death or March 31, 2022, based on the admission date. Exclusions were made based on: (1) hospital stay exceeding three months (excluded, n = 5); (2) transfers to other departments (excluded, n = 6); (3) observation of terminal delirium (excluded, n = 3); (4) patient with a history of delirium (excluded, n = 55); and (5) missing baseline characteristics of study subjects (excluded, n = 17). If delirium occurs within two weeks before death, we define it as terminal delirium. Following these criteria, 2066 individuals were included in the analysis. The assessment of opioid use, delirium, and other covariates was conducted through meticulous retrospective chart reviews, ensuring a thorough evaluation of patient histories and clinical outcomes.
Exposure
Opioid exposure was considered for patients who received opioid medications during hospitalization. The morphine equivalent daily dose (MEDD) was used to assess exposed dose levels. We used a cutoff of the upper 25% of the MEDD (100 mg MEDD) threshold to categorize low-dose and high-dose users. Prescriptions of medications were conducted through medical specialists.
Outcome
The primary outcome, delirium in patients with advanced cancer, was identified via medical records and diagnosed based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5)18. At least two medical specialists performed a detailed review and recorded potentially related symptoms and associated medications. In cases of conflicting opinions, additional experts participated in the diagnosis and voted to reach a conclusion9.
Covariates
The study considered the following various patient related covariates: age (< 50, 50–59, 60–69, and ≥ 70 years), sex, the status of chemotherapy during hospitalization, living with family, eligibility for medical aid, education level (high school graduated or under, college graduated or higher, and unknown), visual impairment (wearing glasses), hearing impairment (using hearing aids), alcohol consumption (non-drinker, 1–3 times a week, and ≥ 4 times a week), smoking (non-smoker, ex-smoker, and current smoker), obesity (< 18.5 kg/m2 [underweight], 18.5–25 kg/m2 [normal], 25–30 kg/m2 [overweight], and ≥ 30 kg/m2 [obese])19–21, blood pressure (systolic blood pressure [SBP] ≥ 140 mmHg or diastolic blood pressure [DBP] ≥ 90 mmHg and SBP < 140 mmHg and DBP < 90 mmHg)22, body temperature (normal [< 38 ℃] and hyperthermia [≥ 38 ℃]), operation received, current cancer treatment status (cytotoxic chemotherapy, immunotherapy, targeted chemotherapy, radiation therapy, no further treatment, and other), use of concomitant medication (sedatives, antidepressant, antiepileptic, cholinergic, and anticholinergic), and history of diseases, such as cardiovascular disease, diabetes mellitus, respiratory disease, liver, mental illness, and head injury. These all variables were obtained through medical chart reviews and categorized according to the International Classification of Diseases, 10th edition (ICD-10)23,24.
Statistical analysis
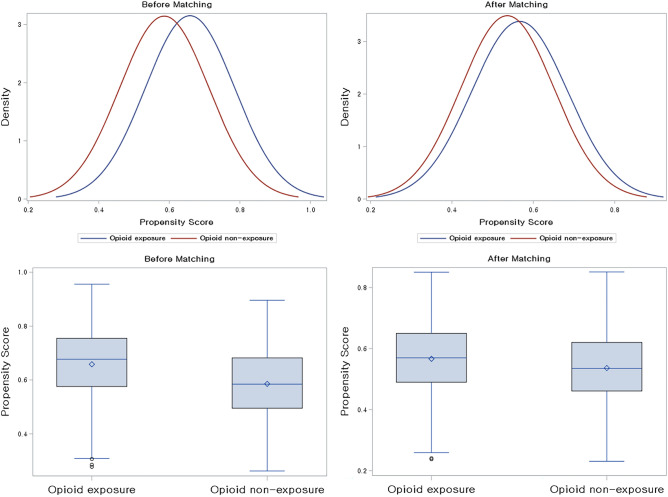
In this study, we aimed to investigate the association between opioid use and the development of delirium in patients with advanced cancer. To control for potential confounding variables and balance demographic characteristics between comparison groups, we constructed a propensity score (PS)-matching cohort (Fig. 1)21,25–27. All variables listed in Table 1 were used for matching, with PS calculated through a multivariate logistic regression model. Individuals with PS differences within the specified caliper (0.1) were matched in a 1:1 ratio using the greedy nearest-neighbor algorithm. Finally, 776 patients were allocated to each of the opioid-exposed and unexposed groups. The adequacy of PS matching was evaluated by standardized mean differences (SMD), with an SMD less than 0.1 indicating no significant imbalance25.
Figure 1.
Density plot and box plot of 1:1 propensity score matching cohort.
Table 1.
Baseline characteristics of study subjects.
| Full unmatched cohort (n = 2066) | 1:1 PS-matched cohort (n = 1552) | ||||
|---|---|---|---|---|---|
| Non-opioid user | Opioid user | Non-opioid user | Opioid user | SMD* | |
| Total, n | 884 | 1,182 | 776 | 776 | |
| Age (years; mean, SD) | 64.4 (13.3) | 62.8 (12.5) | 63.5 (13.4) | 63.5 (12.5) | < 0.01 |
| Age group (years; n, %) | 0.05 | ||||
| < 50 | 111 (12.6) | 168 (14.2) | 109 (14.1) | 99 (12.8) | |
| 50–59 | 179 (20.3) | 256 (21.7) | 163 (21.0) | 161 (20.8) | |
| 60–69 | 255 (28.9) | 399 (33.8) | 239 (30.8) | 253 (32.6) | |
| ≥ 70 | 339 (38.4) | 359 (30.4) | 265 (34.2) | 263 (33.9) | |
| Sex (n, %) | 0.01 | ||||
| Male | 537 (60.8) | 661 (55.9) | 456 (58.8) | 454 (58.5) | |
| Female | 347 (39.3) | 521 (44.1) | 320 (41.2) | 322 (41.5) | |
| Chemotherapy during hospitalization (n, %) | 0.02 | ||||
| Yes | 309 (35.0) | 273 (23.1) | 254 (32.7) | 246 (31.7) | |
| No | 575 (65.1) | 909 (76.9) | 522 (67.3) | 530 (68.3) | |
| Living with family (n, %) | 0.05 | ||||
| Yes | 547 (61.9) | 833 (70.5) | 490 (63.1) | 507 (65.3) | |
| No | 337 (38.1) | 349 (29.5) | 286 (36.9) | 269 (34.7) | |
| Medical aid recipients (n, %) | 0.01 | ||||
| Yes | 33 (3.7) | 61 (5.2) | 27 (3.5) | 26 (3.4) | |
| No | 851 (96.3) | 1,121 (94.8) | 749 (96.5) | 750 (96.7) | |
| Education level (n, %) | 0.03 | ||||
| High school graduated or under | 357 (40.4) | 591 (50.0) | 328 (42.3) | 336 (43.3) | |
| College graduated or higher | 199 (22.5) | 316 (26.7) | 194 (25.0) | 188 (24.2) | |
| Unknown | 328 (37.1) | 275 (23.3) | 254 (32.7) | 252 (32.5) | |
| Visual impairment (wearing glasses; n, %) | 0.01 | ||||
| Yes | 51 (5.8) | 53 (4.5) | 44 (5.7) | 43 (5.5) | |
| No | 833 (94.2) | 1,129 (95.5) | 732 (94.3) | 733 (94.5) | |
| Hearing impairment (using hearing aids; n, %) | 0.03 | ||||
| Yes | 12 (1.4) | 9 (0.8) | 6 (0.8) | 8 (1.0) | |
| No | 872 (98.6) | 1,173 (99.2) | 770 (99.2) | 768 (99.0) | |
| Alcohol consumption (n, %) | < 0.01 | ||||
| Non-drinker | 751 (85.0) | 988 (83.6) | 667 (86.0) | 664 (85.6) | |
| 1–3 times a week | 82 (9.3) | 140 (11.8) | 72 (9.3) | 69 (8.9) | |
| ≥ 4 times a week | 51 (5.8) | 54 (4.6) | 37 (4.8) | 43 (5.5) | |
| Smoking (n, %) | 0.05 | ||||
| Non-smoker | 632 (71.5) | 791 (66.9) | 555 (71.5) | 544 (70.1) | |
| Ex-smoker | 27 (3.1) | 22 (1.9) | 19 (2.5) | 18 (2.3) | |
| Current smoker | 225 (25.5) | 369 (31.2) | 202 (26.0) | 214 (27.6) | |
| Obesity (n, %)† | 0.08 | ||||
| Underweight | 172 (19.5) | 274 (23.2) | 160 (20.6) | 165 (21.3) | |
| Normal weight | 546 (61.8) | 729 (61.7) | 476 (61.3) | 481 (62.0) | |
| Overweight | 139 (15.7) | 160 (13.5) | 122 (15.7) | 112 (14.4) | |
| Obese | 27 (3.1) | 19 (1.6) | 18 (2.3) | 18 (2.3) | |
| Blood pressure (n, %) | 0.02 | ||||
| SBP ≥ 140 mmHg or DBP ≥ 90 mmHg | 685 (77.5) | 877 (74.2) | 598 (77.1) | 605 (78.0) | |
| SBP < 140 mmHg and DBP < 90 mmHg | 199 (22.5) | 305 (25.8) | 178 (22.9) | 171 (22.0) | |
| Body temperature (n, %) | 0.01 | ||||
| Normal temperature (< 38 ℃) | 847 (95.8) | 1,131 (95.7) | 741 (95.5) | 742 (95.6) | |
| Hyperthermia (≥ 38 ℃) | 37 (4.2) | 51 (4.3) | 35 (4.5) | 34 (4.4) | |
| Operation (n, %) | 0.01 | ||||
| Yes | 484 (54.8) | 695 (58.8) | 435 (56.1) | 430 (55.4) | |
| No | 400 (45.3) | 487 (41.2) | 341 (43.9) | 346 (44.6) | |
| Cancer treatment (n, %) | 0.10 | ||||
| Cytotoxic chemotherapy | 425 (48.1) | 353 (29.9) | 350 (45.1) | 337 (43.4) | |
| Immunotherapy | 66 (7.5) | 84 (7.1) | 63 (8.1) | 61 (7.9) | |
| Targeted chemotherapy | 76 (8.6) | 64 (5.4) | 61 (7.9) | 58 (7.5) | |
| Radiation Therapy | 41 (4.6) | 47 (4.0) | 37 (4.8) | 37 (4.8) | |
| No further treatment | 257 (29.1) | 625 (52.9) | 254 (32.7) | 274 (35.3) | |
| Other | 19 (2.2) | 09 (0.8) | 11 (1.4) | 9 (1.2) | |
| Use of concomitant medication | |||||
| Sedatives (n, %) | 111 (12.6) | 230 (19.5) | 102 (13.1) | 123 (15.9) | 0.08 |
| Antidepressant (n, %) | 33 (3.7) | 91 (7.7) | 31 (4.0) | 35 (4.5) | 0.03 |
| Antiepileptic (n, %) | 54 (6.1) | 91 (7.7) | 50 (6.4) | 54 (7.0) | 0.02 |
| Cholinergic (n, %) | 21 (2.4) | 28 (2.4) | 19 (2.5) | 20 (2.6) | 0.01 |
| Anticholinergic (n, %) | 16 (1.8) | 18 (1.5) | 12 (1.6) | 16 (2.1) | 0.04 |
| History of disease | |||||
| Cardiovascular (n, %) | 338 (38.2) | 454 (38.4) | 290 (37.4) | 304 (39.2) | 0.04 |
| Diabetes mellitus (n, %) | 184 (20.8) | 268 (22.7) | 161 (20.8) | 172 (22.2) | 0.03 |
| Respiratory (n, %) | 83 (9.4) | 99 (8.4) | 71 (9.2) | 70 (9.0) | < 0.01 |
| Liver (n, %) | 57 (6.5) | 76 (6.4) | 52 (6.7) | 48 (6.2) | 0.02 |
| Mental illness (n, %) | 39 (4.4) | 84 (7.1) | 38 (4.9) | 43 (5.5) | 0.03 |
| Head injury (n, %) | 56 (6.3) | 82 (6.9) | 50 (6.4) | 51 (6.6) | 0.01 |
DBP diastolic blood pressure, PS propensity matching, SBP systolic blood pressure, SD standard deviation, SMD standardized mean difference.
†Obesity (body mass index, kg/m2) was categorized as < 18.5 kg/m2 (underweight), 18.5–25.0 kg/m2 (normal), 25.0–30.0 kg/m2 (overweight), and ≥ 30.0 kg/m2 (obese).
*SMD < 0.1 indicates no major imbalance.
Odds ratio (OR) with 95% confidence intervals (CIs) using binary logistic regression models were used for estimation27. In addition, an adjusted model was used to minimize the impact of potential confounders, incorporating the following variables: age, sex, chemotherapy during hospitalization, living with family, medical aid recipients, alcohol consumption, smoking, and obesity. Statistical significance was established at a two-sided P value < 0.05. All analyses and visualization were performed using SAS software (version 9.4; SAS Institute Inc., Cary, NC, USA) and R software (version 4.1.0; R Foundation for Statistical Computing, Vienna, Austria)28,29.
Ethical approval
The Korean government anonymized all patient-related data, including personal identification numbers, to enhance confidentiality. The protocol was approved by the institutional review boards of the four centers (CHA University, CHAMC 2021-03-054-002; Seoul National University, H-2103-028-1201; Seoul National University Bundang Hospital, B-2104/681-405; and Yonsei University, 4-2021-0323). We conducted this study using de-identified administrative data that were obtained without prior consent.
Results
Of the 2066 eligible patients with cancer admitted to the APCU, we identified 42.8% (884/2,066) non-opioid users (mean [standard deviation, SD] age, 64.4 [13.3] years; 60.8% male) and 57.2% (1182/2124) opioid users (62.8 [12.5] years; 55.9% male) in the full matched cohort (Table 1). Following the 1:1 PS matching, the SMD values were below 0.1, suggesting no major imbalances in the baseline characteristics. Table 1 details these baseline demographic characteristics.
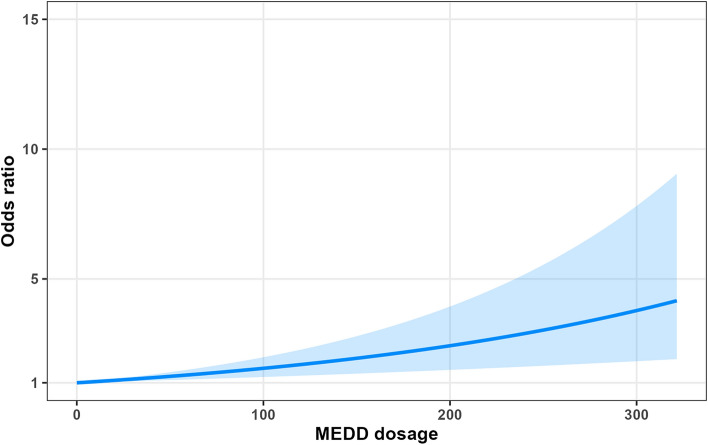
Opioid use was associated with an increased occurrence of delirium in patients with advanced cancer (OR, 2.02 [95% CI 1.22–3.35]) in Table 2. In particular, high-dose opioid users showed an increased risk of delirium in patients with advanced cancer compared to non-opioid users (low-dose user: OR, 2.21 [95% CI 1.27–3.84]; high-dose user: OR, 5.75 [95% CI 2.81–11.77]; ratio of OR, 2.60 [95% CI 1.05–6.44]). Furthermore, the risk of delirium in patients with advanced cancer showed increasing trends in a dose-dependent manner (Table 2 and Fig. 2).
Table 2.
Odds ratio models for the association between opioid use and delirium in patients with advanced cancer with the 1:1 propensity-score-matched cohort.
| Delirium event/exposed, n (%) | Crude OR (95% CI) | Adjusted OR (95% CI)† | |
|---|---|---|---|
| Opioid use | |||
| Non-user | 25/752 (3.32) | 1.00 (reference) | 1.00 (reference) |
| User | 46/752 (6.12) | 1.90 (1.15–3.12) | 2.02 (1.22–3.35) |
| Dose-dependent association (MEDD) | |||
| Non | 22/776 (2.84) | 1.00 (reference) | 1.00 (reference) |
| Low-dose user | 36/647 (5.56) | 2.02 (1.18–3.47) | 2.21 (1.27–3.84) |
| High-dose user | 15/129 (11.63) | 4.51 (2.27–8.95) | 5.75 (2.81–11.77) |
| Ratio of OR (High-dose vs. Low-dose [ref]) | – | 2.23 (0.93–5.35) | 2.60 (1.05–6.44) |
CI, confidence interval; MEDD, morphine equivalent daily dose; OR, odds ratio.
†The model was adjusted for age (< 50, 50–59, 60–69, and ≥ 70 years), sex, chemotherapy during hospitalization, living with family, medical aid recipients, alcohol consumption (0, 1–3, and ≥ 4), smoking (0, 1–3, and ≥ 4), and body mass index (< 18.5, 18.5–25.0, 25.0–30.0, ≥ 30.0 kg/m2).
Numbers in bold indicate significant differences (p < 0.05).
Figure 2.
Dose-dependent association between MEDD and incidence of delirium. Morphine equivalent daily dose.
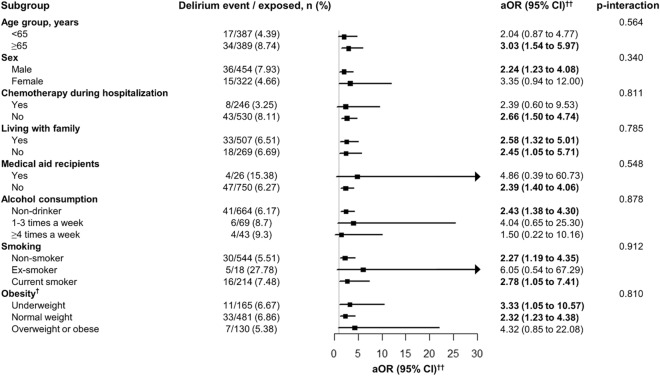
Stratification analysis was performed in 1:1 PS-matched cohorts (Fig. 3). Higher occurrences of delirium in patients with advanced cancer showed in individuals with old age (OR, 3.03 [95% CI 1.54–5.97]), male sex (2.24 [95% CI 1.23–4.08]), absence of chemotherapy during hospitalization (OR, 2.66 [95% CI 1.50–4.74]), and non-obese status (underweight status: OR, 3.33 [95% CI 1.05–10.57]; normal weight status: OR, 2.32 [95% CI 1.23–4.38]) in Fig. 3.
Figure 3.
Stratification analysis for odds ratio models of the association between opioid use and delirium in patients with advanced cancer with the 1:1 propensity-score-matched cohort. CI confidence interval, MEDD morphine equivalent daily dose, aOR adjusted odds ratio. †Obesity (body mass index, kg/m2) was categorized as < 18.5 kg/m2 (underweight), 18.5–25.0 kg/m2 (normal), 25.0–30.0 kg/m2 (overweight), and ≥ 30 kg/m2 (obese). ††The model was adjusted for age (< 50, 50–59, 60–69, and ≥ 70 years), sex, chemotherapy during hospitalization, living with family, medical aid recipients, alcohol consumption (0, 1–3, and ≥ 4), smoking (0, 1–3, and ≥ 4), and body mass index (< 18.5, 18.5–25.0, 25.0–30.0, ≥ 30.0 kg/m2). Numbers in bold indicate significant differences (p < 0.05).
Discussion
Findings and explanation
We investigated the impact of opioid use on the occurrence of delirium in patients with advanced cancer admitted to APCU. There are several key findings. First, in this large-scale multicenter patient-based registry cohort study that included 2124 eligible patients, opioid use was significantly associated with an increased occurrence of delirium in patients with advanced cancer. Second, the risk of delirium showed a dose-dependent relationship with opioid dose. High-dose opioid users showed higher odds of delirium in patients with advanced cancer. Third, patients with old age, male sex, absence of chemotherapy during hospitalization, and non-obese status showed a significant risk of delirium in stratification analysis.
Comparison with other studies
Previous studies explored the association between opioid use and delirium in individuals with various surgeries and diseases, including hip fractures, neurological injury, pain, infection, fever, and hypotension 4,10,11,30; however, investigations focusing on delirium in patients with advanced cancer are limited. A few studies suggested an association between opioid use and the occurrence of delirium in patients with cancer. However, these studies were limited to case-report studies or small cohort sizes to generalize the results (Table S1) 6–8,31. By contrast, our large-scale multicenter patient-based registry cohort, including 2124 eligible patients admitted to the APCU, highlighted the significant association between opioid use and delirium in patients with advanced cancer.
Possible mechanisms
Opioids are known to exert their effects primarily through the central nervous system by altering neurotransmitter release and neuronal activity32. This alternation can lead to neuropsychiatric outcomes, including cognitive impairment and delirium, particularly in patients with advanced cancer3,4. Moreover, opioids can disrupt the normal sleep–wake cycle, further exacerbating the risk of delirium33. In this cohort study, we found that the association between opioid use and the occurrence of delirium followed a dose-dependent nature. Higher doses of opioids are more likely to induce significant changes in the brain circuits by altering synaptic functions and neural pathways34, potentially leading to a higher risk of delirium.
The patients with old age, male sex, absence of chemotherapy during hospitalization, and underweight status showed a significant risk of delirium in stratification analysis. These factors were aligned with identified risk factors for delirium in patients with cancer9. Older patients often have decreased physiological reserve and increased sensitivity to opioids, which can predispose them to delirium35. Moreover, the significant risk of delirium among male patients could be attributed to a higher incidence of hyperactive forms of delirium in males compared to females9. This discrepancy may also suggest potential underdiagnoses of delirium in female patients.
Furthermore, underweight individuals may exhibit different pharmacokinetics and pharmacodynamics, making them more susceptible to delirium36. This phenomenon aligns with the concept known as the "Obesity Paradox," where individuals with a higher body mass index (BMI) appear to possess protective factors against postoperative delirium9,36. This paradoxical relationship suggests that, similarly, in cancer patients, those with a higher BMI might exhibit a lower risk of delirium, highlighting the complex interplay between body weight and neuropsychiatric outcomes in medical conditions. The absence of chemotherapy during hospitalization could be indicative of a more advanced stage of cancer, where the physiological and psychological burden of the disease itself, coupled with opioid use, could heighten the risk of delirium.
Policy implications
Our findings not only highlight the need for cautious opioid use in patients with advanced cancer but also emphasize the broader impact of delirium37. Delirium poses a significant burden, not only affecting the patients but also placing a significant social burden on their families and healthcare providers9. In the context of advanced cancer, delirium adversely impacts the disease course by impairing communication and hindering the participation of patients in care, such as treatment decisions, counseling, and diagnosis5. Therefore, policy implications should extend beyond clinical management to include supportive measures for families and caregivers. Healthcare systems should implement policies promoting regular mental status assessments, individualized pain management strategies, and comprehensive support systems for patients with advanced cancer. These measures should be designed to minimize the occurrence of delirium and its associated burdens, thereby improving the overall conditions of patients and families in advanced cancer care contexts.
Strengths and limitations of the study
This study presents a novel association between opioid use and delirium among patients with advanced cancer by utilizing data from a large-scale, multicenter, patient-based registry cohort. However, several limitations must be acknowledged. First, we collected the information on opioid use relying on the medical records, but they do not necessarily equate to actual consumption of the medication, leading to potential exposure misclassification. Second, while we observed an association, the observational nature of our study precludes a definitive explanation of the causal relationship. It remains unclear whether the association is due to the chronic pain associated with cancer or the opioids themselves. This ambiguity underscores the need for future research to determine the appropriate dosage of opioids that balances analgesic effects and the risk of delirium, as well as to explore the underlying mechanisms. Third, our focus on patients with advanced cancer admitted to the APCU limits the generalizability of our findings to the general patients with cancer. Further studies are needed to assess whether these associations are consistent in patients with less advanced stages of cancer and in different care settings. Fourth, our study is subject to the inherent limitations of a retrospective observational design38. The reliance on a patient registry and retrospective chart reviews for evaluating exposures, outcomes, and covariates may introduce bias and affect the generalizability of our findings. To mitigate these limitations, we employed PS matching; however, we acknowledge that it does not fully address the problem38. Fifth, the prevalence of delirium observed in our study is lower than in previous studies. However, it is important to note that patients with advanced cancers often exhibit hypoactive delirium, which is more challenging to detect due to its less pronounced symptoms39. Thus, the nature of our retrospective chart review study may have resulted in the under-diagnosis of hypoactive delirium.
Conclusion
In this multicenter patient-based registry cohort study, opioid use was significantly associated with a substantial increase in the risk of delirium in patients with advanced cancer. This association was observed to be dose-dependent, with higher opioid dosages associated with an increased risk of delirium. In addition, we identified various vulnerable groups, including old age, male sex, absence of chemotherapy during hospitalization, and underweight status, for delirium among patients with advanced cancer. These findings highlight the critical need for healthcare providers to carefully prescribe opioids to manage pain in patients with advanced cancer; however, further studies are needed to focus on determining the optimal opioid dosages that minimize the risk of delirium and investigating underlying mechanisms of these associations.
Supplementary Information
Author contributions
Dr. DKY had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. All authors approved the final version of the manuscript before submission. Study concept and design: JK, HJK, and DKY; Acquisition, analysis, or interpretation of data: JK, HJK, and DKY; Drafting of the manuscript: JK, HJK, and DKY; Critical revision of the manuscript for important intellectual content: all authors; Statistical analysis: JK, HJK, and DKY; Study supervision: DKY. DKY and BK contributed equally as corresponding authors. SHY, JK, and HJK contributed equally to this work as first authors. The corresponding author attests that all listed authors meet the authorship criteria and that no one meeting the criteria has been omitted.
Funding
This study was supported by a Grant from the Ministry of Health and Welfare, Republic of Korea (Grant number: HC20C0040). The funders had no role in the study design, data collection, data analysis, data interpretation, or manuscript writing.
Data availability
Data are available on reasonable request. Study protocol, statistical code: available from DKY (email: yonkkang@gmail.com).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Shin Hye Yoo, Jiseung Kang and Hyeon Jin Kim.
Contributor Information
Dong Keon Yon, Email: yonkkang@gmail.com.
Beodeul Kang, Email: wb0707@cha.ac.kr.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-56675-1.
References
- 1.Hui D, Frisbee-Hume S, Wilson A, et al. Effect of Lorazepam with haloperidol vs haloperidol alone on agitated delirium in patients with advanced cancer receiving palliative care: A randomized clinical trial. JAMA. 2017;318(11):1047–1056. doi: 10.1001/jama.2017.11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawlor PG, Bush SH. Delirium in patients with cancer: Assessment, impact, mechanisms and management. Nat. Rev. Clin. Oncol. 2015;12(2):77–92. doi: 10.1038/nrclinonc.2014.147. [DOI] [PubMed] [Google Scholar]
- 3.Lawlor PG, Gagnon B, Mancini IL, et al. Occurrence, causes, and outcome of delirium in patients with advanced cancer: A prospective study. Arch. Intern. Med. 2000;160(6):786–794. doi: 10.1001/archinte.160.6.786. [DOI] [PubMed] [Google Scholar]
- 4.Ormseth CH, LaHue SC, Oldham MA, Josephson SA, Whitaker E, Douglas VC. Predisposing and precipitating factors associated with delirium: A systematic review. JAMA Netw. Open. 2023;6(1):e2249950. doi: 10.1001/jamanetworkopen.2022.49950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hui D, Dev R, Bruera E. Neuroleptics in the management of delirium in patients with advanced cancer. Curr. Opin. Support Palliat. Care. 2016;10(4):316–323. doi: 10.1097/SPC.0000000000000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiiba M, Takei M, Nakatsuru M, et al. Clinical observations of postoperative delirium after surgery for oral carcinoma. Int. J. Oral. Maxillofac. Surg. 2009;38(6):661–665. doi: 10.1016/j.ijom.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Lawlor P, Walker P, Bruera E, Mitchell S. Severe opioid toxicity and somatization of psychosocial distress in a cancer patient with a background of chemical dependence. J. Pain Symptom Manage. 1997;13(6):356–361. doi: 10.1016/s0885-3924(97)00081-x. [DOI] [PubMed] [Google Scholar]
- 8.Mihara N, Yazawa Y, Imanishi J, Torigoe T, Onishi H, Ishida M. Delirium in patients with musculoskeletal tumours: Incidence and risk factors—single-centre prospective study. BMJ Support Palliat Care. 2023 doi: 10.1136/spcare-2023-004544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SW, Jung EH, Kim HJ, et al. Risk factors for delirium among patients with advanced cancer in palliative care: A multicenter, patient-based registry cohort in South Korea. Eur. Rev. Med. Pharmacol. Sci. 2023;27(5):2068–2076. doi: 10.26355/eurrev_202303_31578. [DOI] [PubMed] [Google Scholar]
- 10.Sadeghirad B, Dodsworth BT, Schmutz Gelsomino N, et al. Perioperative factors associated with postoperative delirium in patients undergoing noncardiac surgery: An individual patient data meta-analysis. JAMA Netw. Open. 2023;6(10):e2337239. doi: 10.1001/jamanetworkopen.2023.37239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.SantaCruzMercado LA, Liu R, Bharadwaj KM, et al. Association of intraoperative opioid administration with postoperative pain and opioid use. JAMA Surg. 2023;158(8):854–864. doi: 10.1001/jamasurg.2023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moryl N, Kogan M, Comfort C, Obbens E. Methadone in the treatment of pain and terminal delirum in advanced cancer patients. Palliat. Support Care. 2005;3(4):311–317. doi: 10.1017/s1478951505050479. [DOI] [PubMed] [Google Scholar]
- 13.Hasuo H, Ishihara T, Kanbara K, Fukunaga M. Myofacial trigger points in advanced cancer patients. Indian J. Palliat. Care. 2016;22(1):80–84. doi: 10.4103/0973-1075.173956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mercadante S, Adile C, Ferrera P, et al. Methadone as first-line opioid for the management of cancer pain. Oncologist. 2022;27(4):323–327. doi: 10.1093/oncolo/oyab081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiratsuka Y, Tagami K, Inoue A, et al. Prevalence of opioid-induced adverse events across opioids commonly used for analgesic treatment in Japan: A multicenter prospective longitudinal study. Support Care Cancer. 2023;31(12):632. doi: 10.1007/s00520-023-08099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fitzgerald Jones K, Khodyakov D, Arnold R, et al. Consensus-based guidance on opioid management in individuals with advanced cancer-related pain and opioid misuse or use disorder. JAMA Oncol. 2022;8(8):1107–1114. doi: 10.1001/jamaoncol.2022.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu TC, Hsu CH, Sun WZ, Chen HM, Lin CP, Shao YY. Impact of expanded strong opioid availability on opioid prescription patterns in patients with cancer: A population-wide cohort study in Taiwan. Lancet Reg. Health West Pac. 2021;16:100255. doi: 10.1016/j.lanwpc.2021.100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European Delirium A, American DS. The DSM-5 criteria, level of arousal and delirium diagnosis: Inclusiveness is safer. BMC Med. 2014;12:141. doi: 10.1186/s12916-014-0141-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akyea RK, Doehner W, Iyen B, Weng SF, Qureshi N, Ntaios G. Obesity and long-term outcomes after incident stroke: A prospective population-based cohort study. J. Cachexia Sarcopenia Muscle. 2021;12(6):2111–2121. doi: 10.1002/jcsm.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eum S, Rhee SY. Age, ethnic, and sex disparity in body mass index and waist circumference: A bi-national large-scale study in South Korea and the United States. Life Cycle. 2023;3:e4. doi: 10.54724/lc.2023.e4. [DOI] [PubMed] [Google Scholar]
- 21.Kim Y, Kim S, Kim MS. Obesity and COVID-19 pandemic in South Korea: Rapid review and a post-hoc analysis. Life Cycle. 2023;3:e15. doi: 10.54724/lc.2023.e15. [DOI] [Google Scholar]
- 22.Douros A, Tolle M, Ebert N, et al. Control of blood pressure and risk of mortality in a cohort of older adults: The Berlin Initiative Study. Eur. Heart J. 2019;40(25):2021–2028. doi: 10.1093/eurheartj/ehz071. [DOI] [PubMed] [Google Scholar]
- 23.Lee SW, Yang JM, Moon SY, et al. Association between mental illness and COVID-19 in South Korea: A post-hoc analysis. Lancet Psychiatry. 2021;8(4):271–272. doi: 10.1016/s2215-0366(21)00043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SW, Kim SY, Moon SY, et al. Estimating COVID-19 infection and severity risks in patients with chronic rhinosinusitis: A Korean Nationwide Cohort Study. J. Allergy Clin. Immunol. Pract. 2021;9(6):2262–2271.e2. doi: 10.1016/j.jaip.2021.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woo A, Lee SW, Koh HY, Kim MA, Han MY, Yon DK. Incidence of cancer after asthma development: 2 independent population-based cohort studies. J. Allergy Clin. Immunol. 2021;147(1):135–143. doi: 10.1016/j.jaci.2020.04.041. [DOI] [PubMed] [Google Scholar]
- 26.Lee SW, Ha EK, Yeniova AO, et al. Severe clinical outcomes of COVID-19 associated with proton pump inhibitors: A nationwide cohort study with propensity score matching. Gut. 2021;70(1):76–84. doi: 10.1136/gutjnl-2020-322248. [DOI] [PubMed] [Google Scholar]
- 27.Kim MJ, Lee KH, Lee JS, et al. Trends in body mass index changes among Korean adolescents between 2005–2020, including the COVID-19 pandemic period: A national representative survey of one million adolescents. Eur. Rev. Med. Pharmacol. Sci. 2022;26(11):4082–4091. doi: 10.26355/eurrev_202206_28978. [DOI] [PubMed] [Google Scholar]
- 28.Yon DK, Hwang S, Lee SW, et al. Indoor exposure and sensitization to formaldehyde among inner-city children with increased risk for asthma and rhinitis. Am. J. Respir. Crit. Care Med. 2019;200(3):388–393. doi: 10.1164/rccm.201810-1980LE. [DOI] [PubMed] [Google Scholar]
- 29.Yoo HW, Jin HY, Yon DK, et al. Non-alcoholic fatty liver disease and COVID-19 susceptibility and outcomes: A Korean Nationwide Cohort. J. Korean Med. Sci. 2021;36(41):e291. doi: 10.3346/jkms.2021.36.e291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sieber FE, Mears S, Lee H, Gottschalk A. Postoperative opioid consumption and its relationship to cognitive function in older adults with hip fracture. J. Am. Geriatr. Soc. 2011;59(12):2256–2262. doi: 10.1111/j.1532-5415.2011.03729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slatkin N, Rhiner M. Treatment of opioid-induced delirium with acetylcholinesterase inhibitors: A case report. J. Pain Symptom Manage. 2004;27(3):268–273. doi: 10.1016/j.jpainsymman.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Kibaly C, Xu C, Cahill CM, Evans CJ, Law PY. Non-nociceptive roles of opioids in the CNS: Opioids' effects on neurogenesis, learning, memory and affect. Nat. Rev. Neurosci. 2019;20(1):5–18. doi: 10.1038/s41583-018-0092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aizawa K, Kanai T, Saikawa Y, et al. A novel approach to the prevention of postoperative delirium in the elderly after gastrointestinal surgery. Surg. Today. 2002;32(4):310–314. doi: 10.1007/s005950200044. [DOI] [PubMed] [Google Scholar]
- 34.Quinn PD, Fine KL, Rickert ME, et al. Association of opioid prescription initiation during adolescence and young adulthood with subsequent substance-related morbidity. JAMA Pediatr. 2020;174(11):1048–1055. doi: 10.1001/jamapediatrics.2020.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLachlan AJ, Bath S, Naganathan V, et al. Clinical pharmacology of analgesic medicines in older people: Impact of frailty and cognitive impairment. Br. J. Clin. Pharmacol. 2011;71(3):351–364. doi: 10.1111/j.1365-2125.2010.03847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng X, Qin P, Lin Y, et al. The relationship between body mass index and postoperative delirium. Brain Behav. 2022;12(4):e2534. doi: 10.1002/brb3.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park S, Yon H, Ban CY, et al. National trends in alcohol and substance use among adolescents from 2005 to 2021: A Korean serial cross-sectional study of one million adolescents. World J. Pediatr. 2023;19(11):1071–1081. doi: 10.1007/s12519-023-00715-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gbolahan OB, Zhi X, Liu Y, Shah MM, Kooby DA, Alese OB. Adjuvant chemotherapy and outcomes in older adult patients with biliary tract cancer. JAMA Netw. Open. 2024;7(1):e2351502. doi: 10.1001/jamanetworkopen.2023.51502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang JH, Shin SH, Bruera E. Comprehensive approaches to managing delirium in patients with advanced cancer. Cancer Treat. Rev. 2013;39(1):105–112. doi: 10.1016/j.ctrv.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request. Study protocol, statistical code: available from DKY (email: yonkkang@gmail.com).