Abstract
Application of high-throughput sequencing and screening help to detect the transcriptional and metabolic discrepancies in organs provided with various levels of nutrients. The influences of individual essential amino acid (EAA) administration on transcriptomic and metabolomic profilings of bovine mammary epithelial cells (BMECs) were systematically investigated. A RNA sequencing and liquid chromatography-tandem mass spectrometry generated a comprehensive comparison of transcriptomics, non-targeted metabolomics and targeted amino acids profilings of BMECs with individual EAA stimulation by turn. The sequencing data and raw LC-MS/MS data of samples were presented in the databases of Gene Expression Omnibus, MetaboLights and Figshare for efficient reuse, including exploring the divergences in metabolisms between different EAAs and screening valuable genes and metabolites regulating casein synthesis.
Subject terms: Metabolomics, Transcriptomics
Background & Summary
Milk protein from mammary gland of dairy cows is a valuable protein source available for human consumption1. However, insufficient utilization of genetic potential restricts dairy’s competitiveness and weakens efforts to decline investments into food production2. Although it varies greatly, the efficiency of dietary nitrogen conversion into milk is only about 25% in lactating cows, which results in a huge pecuniary loss and a series of environmental pollutions3.
Given that, nitrogen intake has a significant correlation to nitrogen excretion4. Dairy cows are likely to be supplied protein that exceed their requirements resulting from unclear definitions of amino acid (AA) needs. The bovine mammary gland is a forceful milk protein synthesizing factory. Present diet formulation is according to the single limiting nutrient theory proposed by von Liebig, which suggested that AA transfer from lumen to milk follows a stationary efficiency until needs are satisfied5. However, the observations that several AAs regulated cell signaling pathways5,6 and the changeable AA transport activity of mammary gland indicate the mutable efficiency of AA utilization that violates the supposition raised by Mitchell and Block7. Therefore, more data about the individual AA metabolism in mammary gland is required to improve the predictions of AAs requirements for dairy cows.
When dairy cows were fed an average diet of 17.8% crude protein (CP), only 25% of dietary nitrogen is recovered in milk3, but this efficiency increased to 30% under a grass-based diet with a well-balanced supplement of histidine, lysine, methionine, and leucine to a 15% CP diet8. Similarly, Zhao et al. reported that lactating cows fed 12% CP diet supplemented with isoleucine, leucine, methionine and threonine had similar milk productions relative to those fed 16% CP diet9. These results indicated that a reasonable strategy to elevate postabsorptive nitrogen efficiency is to reduce present percentages of dietary nitrogen and add specific essential AAs (EAA), which requires a better understanding and comparison of different EAA conversions in mammary gland.
Similar to the low-protein diet supplemented with EAAs in dairy cows, single EAA addition to the standard medium devoid of total EAAs had been used to explore the influences of single EAA on milk protein synthesis in vitro1,5,10. Gao et al. found that cultivation with standard medium devoid of all AA and supplemented with histidine, lysine, methionine, and leucine for 6-h had different effects on β-casein synthesis11, respectively. Appuhamy et al. reported that media lack of total EAA and individually supplemented with arginine, isoleucine, leucine, methionine, or threonine exerted various influences on mTOR signaling pathways that regulate the rates of translation initiation and elongation5. These results indicated that different individual EAA addition under low total AA/EAA settings had diverse effects on casein synthesis. Besides, additions of several EAAs, such as arginine, leucine and branched-chain AAs were also used to improve the health status of humans with preeclampsia, sarcopenia and cirrhosis12–14, respectively. However, previous studies usually focused on few EAAs, and information about the systematic comparison of mammary metabolisms between all individual EAAs is relatively limited. Therefore, transcriptomic and metabolomic profilings as well as the AA concentrations of bovine mammary epithelial cells (BMECs) came from 72 samples with various single EAA availability (0 or standard concentration in medium for each EAA) were systematically compared in this study based on TruSeq Stranded mRNA LTSample Prep Kit (Illumina) and liquid chromatography-tandem mass spectrometry (LC-MS/MS). All of data were deposited in Gene Expression Omnibus15,16, MetaboLights17–19 and Figshare20,21 repositories.
The public availability of this dataset helps to promote researches in biochemistry of EAA regulating milk protein synthesis, transcriptomic and metabolomic comparisons between different studies or explorations into repetitiveness of data analysis in multi-omics. Given that this study has a horizontal design, this dataset can be used in multiple perspectives. First, these data presented a landscape of transcriptomic and metabolomic profiles in BMECs with different individual AA supplies, which contributes to investigate the discrepancies between individual EAA metabolisms. Second, it helps researchers to study the pathways by which EAAs mediate BMECs function and milk protein synthesis. Third, data can be applied for optimizing mammary mathematical models to predict the responses of milk protein synthesis to EAA administrations and contributing to screening key genes and metabolites with potential breeding values.
Methods
Study design
Experimental protocols were ratified by Welfare and Health Committee of Qingdao Agricultural University. Primary BMECs were isolated as described previously22. Briefly, mammary gland tissues from healthy dairy cows were cut into 1mm3 pieces in D-Hanks solution (Solarbio, Beijing, China) containing 1% penicillin-streptomycin (Sigma-Aldrich, MO, USA). Following washing with PBS buffer three times, the pieces were incubated at 37 °C and 5% CO2. The tissues were removed when the cells isolated from tissues reached 80% confluence. BMECs and fibroblasts were divided according to their different sensitivity to 0.15% trypsin plus 0.02% EDTA (Sigma-Aldrich, MO, USA). BMECs (6 passages) were grown in DMEM-F12 medium (Gibco, NY, USA) including 1% L-glutamine and 10% (v/v) fetal bovine serum (Gibco, NY, USA) as well as 5 μg/mL insulin and 1% penicillin-streptomycin (both from Sigma-Aldrich, MO, USA) at 37 °C and 5% CO2.
The experimental design was shown in Fig. 1. For exploring the transcriptomic and metabolomic responses to diverse EAA stimuli, 0.5 × 107 BMECs were seeded in a 12-well plate. After nearly 48-h cultivation, the cells were serum-starved overnight when they reached a 90% confluence and had a aggregates-like formation, and then subsequently assigned to 1 of 12 treatment media (n = 6). Complete DMEM-F12 medium (Gibco, NY, USA) containing 0.70 L-arginine, 0.15 L-histidine, 0.42 L-isoleucine, 0.45 L-leucine, 0.5 L-lysine, 0.12 L-methionine, 0.22 L-phenylalanine, 0.45 L-threonine, 0.04 L-tryptophan, and 0.45 L-valine (all in mmol/L) serves as the positive control (POS) treatment, while DMEM-F12 medium without total EAA served as the negative control (NEG) treatment. Ten treatments were NEG individually supplemented with L-arginine, L-histidine, L-isoleucine, L-leucine, L-lysine, L-methionine, L-phenylalanine, L-threonine, L-tryptophan or L-valine (Sigma-Aldrich, MO, USA), respectively. Individual EAA was supplemented to achieve a concentration equal to that in the POS treatment. After 6-h treatment, cell samples were collected simultaneously and kept at −80 °C for subsequent analysis.
Fig. 1.
The experimental design and workflow to acquire data of transcriptomics, non-targeted metabolomics and targeted amino acids profilings in BMECs. Complete DMEM-F12 medium used for BMECs was the positive control (POS) treatment, while DMEM-F12 medium without total essential amino acid served as the negative control (NEG) treatment. Ten treatments were NEG individually supplemented with L-arginine, L-histidine, L-isoleucine, L-leucine, L-lysine, L-methionine, L-phenylalanine, L-threonine, L-tryptophan or L-valine, respectively (n = 6). Individual EAA was supplemented to achieve a concentration equal to that in POS.
Sample preparation
Total RNA of treated cells were collected with TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The quantity and quality (optical density at 260/280 nm = 1.8–2.0) of RNA were measured using a biophotometer (Eppendorf, Hamburg, Germany) and agarose gel electrophoresis for analyzing 28 S and 18 S rRNA subunits.
For non-targeted and targeted metabolomics, the metabolites of 1 × 106 cells were taken from each treatment using 1 mL of reagent including methanol, acetonitrile and water (2:2:1, v/v/v). The BMECs solutions were then vortexed for 1 min, following by ultrasonicating for 0.5-h at 4 °C and for 1-h at −20 °C to precipitate protein. A vacuum centrifuge was adopted to dry the supernatant of solution after a centrifugation with 14,000 rcf for 20 min, and then maintained at −80 °C until latter use. At last, the precipitation after drying was dissolved in 0.1 mL acetonitrile/water (1:1, v/v) and adequately vortexed, and then followed a 20 min centrifugation with 14,000 rcf to obtain supernatants for subsequent LC-MS/MS analysis.
Total RNA sequencing
Poly-T oligo-attached magnetic beads were applied to purify mRNA. The synthesis of first-strand cDNA was conducted by random hexamer primer and M-MuLV Reverse Transcriptase(RNase H-), and the second-strand cDNA was generated with DNA Polymerase I and RNase H. Adaptor having hairpin loop structure were ligated after the adenylation of 3’ ends of DNA fragments. AMPure XP system was used to purify PCR products. The quality of library was assessed by the Bioanalyzer 2100 system (Agilent Technologies, CA, USA). The library establishments were then sequenced using an Illumina sequencing platform (HiSeqTM 2500) and 150 bp paired-end reads were producted.
Chromatography
BMECs samples were dissociated on an UHPLC (Vanquish UHPLC, Thermo) along with a Orbitrap. The mobile phase included A = ammonium acetate (25 mM) and ammonium hydroxide (25 mM) in water as well as B = acetonitrile. The process was 98% acetonitrile for 90 seconds and was decreased to 2% during 10.5 min linearly, and maintained for 2 min following by increasing to 98% in 6 seconds. Continuous analysis of samples was conducted arbitrarily to reduce the influences of instrument detection signal fluctuations. Quality control (QC) samples that were composed of aliquots from total samples were operated 3 times before the queue to monitor the column condition and every 6 inserts after that to evaluate discrepancies.
BMECs samples for targeted metabolomics of AA profiling were separated on an Agilent 1290 Infinity LC UHPLC System (Agilent Technologies, CA, USA) using a HILIC column. The mobile phase of composition A includes water, ammonium formate (25 mM) and formic acid (0.1%), and B was 0.1% formic acid in acetonitrile. The process of elution was: 85% B during 0-1 min; B reduced from 85% to 50% (1–11 min) in a linear manner, B was kept at 40% (11.1–12 min), B elevated from 40% to 75% (12–12.1 min), and finally B was kept at 75% (12.1–19 min). Continuous analysis of samples was conducted arbitrarily to reduce the influences of instrument detection measuring undulations. QC samples were operated 3 times before the queue to monitor the column condition and every 6 inserts after that to evaluate discrepancies. Chromatographic retention time was corrected by the blend of standard AA metabolites.
Mass spectrometry
ESI positive and negative ion modes were adopted in non-targeted metabolomics. BMECs samples were dissociated through UHPLC and underwent mass spectrometry using a Thermo Scientific Orbitrap Exploris 480 (Thermo Fisher Scientific). The ESI source settings were: source temperature, 600 °C; ion source gas 1 (nitrogen), 60; ion source gas 2 (nitrogen), 60; curtain gas, 30; ion spray voltage floating, ±5,500 V. In MS only acquisition, 80–1,200 Da m/z was got by the system coupled with the resolution of 60,000 and the accumulation time of 100 ms. In auto MS/MS acquisition, the system was adjusted to get over the m/z from 70 to 1,200 Da and the resolution was adjusted as 30,000. The accumulation time was adjusted as 50 ms, and exclude time within 4 s.
For targeted metabolomics of AA profilings, ESI positive ion mode was adopted for detection. Mass spectrometry of sampes adopted a 6500/5500 QTRAP mass spectrometer (AB SCIEX, Framingham, MA, USA). The ESI source setting was: ion source gas 1 (nitrogen): 40; ion source gas 2 (nitrogen): 40; curtain gas: 30; source temperature: 500 °C; ion spray voltage floating ±5,500 V. Ion pair was detected using the mode of multiple reaction monitoring.
Data analysis
The QC and reads statistics in transcriptomics were measured by Trimmomatic23. Subsequent analyses were conducted with high-quality clean data. Hisat2 was used for the mapping of Bos taurus clean reads to the corresponding reference genome24. StringTie (v1.3.3b) was adopted to assemble the mapped reads of each sample25. Each transcript’s expression was quantified using the amount of Fragments Per Kilobase of transcript sequence per Millions base pairs sequenced (FPKM) method26, and the read counts as well as FPKM value were computed by cufflinks and htseq-count27, respectively. The two expression profilings between each treatment and control group were quantified by DESeq R package using nbinom test28. Significant differentially expressed genes (DEG) were discriminated when the unigenes have P < 0.05 and |log2(fold-change)| > 1.
The raw data files of non-targeted metabolomics were transformed to the format of mzML by ProteoWizard29. XCMS program was applied for peak alignment, retention time correction, and peak area extraction30. The following settings for peak picking were applied: centWave m/z = 25 ppm, peakwidth = c (10, 60), prefilter = c (10, 100). Bw = 5, mzwid = 0.025, minfrac = 0.5 were applied for peak grouping. Metabolite identification was conducted by MS/MS spectra using an in-house database. To make the metabolomics data reproducible, the relative standard derivation (RSD) of the peaks in the QC samples larger than 30% were filtered out. After normalized to total peak intensity, the processed data were uploaded into SIMCA-P (version 14.1, Umetrics, Umea, Sweden), where it was subjected to multivariate data analysis, including Pareto-scaled principal component analysis (PCA) and orthogonal partial least-squares discriminant analysis (OPLS-DA). Response permutation testing along with 7-fold cross-validation were used to assess the robustness of model. The variable importance in the projection (VIP) value of each variable in the OPLS-DA model was calculated to indicate its contribution to the classification. Significance was measured with an unpaired Student’s t test. Compounds with VIP value > 1 and P < 0.05 were considered as differentially expressed metabolites (DEM).
For targeted metabolomics of AA profiling in BMECs samples, multiQuant software was adopted to extract the chromatographic peak area and retention time. AA standards were used for retention time correction and metabolites identification31. Compounds having coefficient of variation under 30% cross samples were identified as reproducible measurements. For DEM identification, statistical analyses between two groups for each EAA administration (NEG vs. individual EAA treatment or POS vs. individual EAA treatment) were conducted with fold changes and P-values that were obtained according to a Student’s t test. Metabolites having P-values < 0.05 and VIP > 1 were considered as DEM.
Data Records
The sequencing data of BMECs with different EAA administrations were presented in Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo) with number of GSE23259116. Correlation coefficient matrix among the samples used for transcriptomics was uploaded to Figshare20. Raw LC-MS/MS data files for non-targeted and targeted metabolomics in BMECs were uploaded to the MetaboLights17 database (http://www.ebi.ac.uk/metabolights) under MTBLS778918 and MTBLS395619, respectively. Metabolic annotation and DEM analysis were present in Figshare21.
Technical Validation
Quality of the sequencing data was evaluated according to the sequence quality, GC content, presence of adaptors and overrepresented k-mers with FastQC. Samples subjected to routine data cleaning to guarantee that no base was called with a Phred quality below 20. Statistical robustness was supported by the 6 biological replicates of each treatment. A number of raw reads came from 12 treatments, ranging from 40,612,706 to 53,705,974 (Table 1). A total of 40,253,216 to 52,418,068 clean reads were kept after trimming and the overall mapping efficiency ranged from 95.92 to 97.71%. These results suggested that the sequencing data has a high quality for subsequent analysis. Correlation of gene expressions between samples is an important parameter to check the experimental reliability. Our data deposited at Figshare20 showed that the pearson correlation coefficient (r) with a square value between samples were all greater than 0.85, which was a prerequisite for subsequent differential expression analysis.
Table 1.
Statistics analysis of clean reads mapping onto reference genome.
| Sample | Raw reads | Clean reads | Mapping efficiency | Phred> 20 (%) | Sample | Raw reads | Clean reads | Mapping efficiency | Phred >20 (%) |
|---|---|---|---|---|---|---|---|---|---|
| NEG_1 | 48,526,890 | 47,549,694 | 92.61% | 96.85 | Met_1 | 46,384,336 | 45,506,434 | 93.78% | 96.19 |
| NEG_2 | 43,176,084 | 42,530,258 | 92.74% | 96.88 | Met_2 | 41,498,318 | 40,413,838 | 93.45% | 97.31 |
| NEG_3 | 45,940,308 | 44,989,364 | 92.71% | 96.73 | Met_3 | 40,793,346 | 40,266,998 | 93.33% | 97.35 |
| NEG_4 | 45,406,952 | 44,616,416 | 92.71% | 96.66 | Met_4 | 43,268,450 | 427,36,254 | 93.03% | 97.33 |
| NEG_5 | 50,541,714 | 49,594,468 | 92.88% | 96.79 | Met_5 | 47,697,704 | 47,125,238 | 93.38% | 97.35 |
| NEG_6 | 46,829,238 | 46,111,516 | 92.94% | 97.00 | Met_6 | 45,551,840 | 44,703,604 | 93.52% | 96.90 |
| Arg_1 | 46,390,570 | 45,987,698 | 92.96% | 97.35 | Phe_1 | 47,526,272 | 46,582,198 | 93.02% | 96.84 |
| Arg_2 | 46,964,992 | 46,211,062 | 92.11% | 97.22 | Phe_2 | 47,428,866 | 46,399,916 | 93.30% | 96.81 |
| Arg_3 | 46,015,946 | 45,668,658 | 93.15% | 96.84 | Phe_3 | 53,705,974 | 52,418,068 | 92.90% | 96.87 |
| Arg_4 | 44,911,936 | 44,367,842 | 83.80% | 97.30 | Phe_4 | 46,669,402 | 45,601,374 | 92.69% | 96.50 |
| Arg_5 | 44,816,418 | 44,285,818 | 93.62% | 97.21 | Phe_5 | 50,288,164 | 49,017,216 | 92.47% | 96.61 |
| Arg_6 | 46,753,196 | 46,193,354 | 93.47% | 97.12 | Phe_6 | 51,493,910 | 49,931,934 | 92.55% | 96.77 |
| His_1 | 47,151,788 | 46,601,100 | 92.49% | 97.10 | Thr_1 | 46,907,428 | 45,578,642 | 93.25% | 96.67 |
| His_2 | 45,263,000 | 44,714,050 | 92.90% | 96.90 | Thr_2 | 53,469,642 | 52,135,856 | 93.44% | 96.78 |
| His_3 | 40,612,706 | 40,253,216 | 92.46% | 97.10 | Thr_3 | 45,859,072 | 44,557,912 | 92.85% | 96.94 |
| His_4 | 41,962,688 | 41,542,730 | 92.18% | 97.25 | Thr_4 | 50,051,988 | 48,869,674 | 93.29% | 96.82 |
| His_5 | 47,219,594 | 46,860,716 | 92.97% | 97.41 | Thr_5 | 47,181,948 | 46,294,180 | 93.25% | 96.79 |
| His_6 | 44,961,658 | 44,630,518 | 92.75% | 97.09 | Thr_6 | 45,299,044 | 44,021,930 | 92.32% | 96.67 |
| Ile_1 | 44,409,430 | 44,143,866 | 92.82% | 97.28 | Trp_1 | 45,961,922 | 44,819,548 | 93.18% | 96.79 |
| Ile_2 | 42,285,282 | 42,018,938 | 92.79% | 96.82 | Trp_2 | 45,661,406 | 43,839,576 | 92.11% | 97.01 |
| Ile_3 | 45,644,514 | 45,232,916 | 93.15% | 96.90 | Trp_3 | 45,596,684 | 44,770,264 | 93.58% | 97.03 |
| Ile_4 | 47,122,466 | 46,714,988 | 92.97% | 96.97 | Trp_4 | 46,588,266 | 45,742,108 | 92.20% | 97.23 |
| Ile_5 | 43,589,468 | 43,218,066 | 93.24% | 97.23 | Trp_5 | 45,209,728 | 43,980,576 | 92.38% | 97.07 |
| Ile_6 | 44,043,016 | 43,591,956 | 92.39% | 96.9 | Trp_6 | 45,515,404 | 44,238,466 | 91.39% | 97.24 |
| Leu_1 | 45,489,370 | 45,012,398 | 83.91% | 97.22 | Val_1 | 46,208,488 | 44,557,688 | 92.17% | 97.32 |
| Leu_2 | 46,056,674 | 45,504,358 | 93.09% | 97.17 | Val_2 | 45,995,318 | 44,540,748 | 91.72% | 97.19 |
| Leu_3 | 44,373,600 | 44,016,548 | 92.87% | 97.28 | Val_3 | 52,639,776 | 51,529,692 | 93.29% | 95.92 |
| Leu_4 | 44,005,676 | 43,663,748 | 92.79% | 97.22 | Val_4 | 44,879,374 | 43,045,218 | 93.40% | 97.47 |
| Leu_5 | 42,430,082 | 42,029,276 | 92.66% | 97.33 | Val_5 | 45,300,600 | 44,302,592 | 92.99% | 97.10 |
| Leu_6 | 44,428,110 | 43,925,098 | 93.46% | 97.47 | Val_6 | 47,799,064 | 46,034,646 | 93.95% | 97.40 |
| Lys_1 | 44,060,770 | 43,658,652 | 93.07% | 97.18 | POS_1 | 47,010,526 | 46,347,678 | 93.84% | 97.71 |
| Lys_2 | 45,720,748 | 45,363,288 | 93.50% | 96.93 | POS_2 | 43,969,636 | 42,918,656 | 92.93% | 96.97 |
| Lys_3 | 42,002,798 | 41,668,272 | 93.41% | 96.57 | POS_3 | 47,616,334 | 46,756,190 | 92.58% | 97.15 |
| Lys_4 | 41,221,456 | 40,887,146 | 93.18% | 96.61 | POS_4 | 47,351,276 | 45,510,318 | 91.82% | 97.39 |
| Lys_5 | 47,273,766 | 46,400,114 | 93.43% | 97.04 | POS_5 | 46,272,932 | 45,139,976 | 91.67% | 97.25 |
| Lys_6 | 46,279,044 | 45,825,968 | 93.05% | 96.95 | POS_6 | 47,241,486 | 46,172,198 | 91.83% | 97.15 |
Complete DMEM-F12 medium used for BMECs was the positive control (POS) treatment, while DMEM-F12 medium without total essential amino acid served as the negative control (NEG) treatment. Ten treatments were NEG individually supplemented with L-arginine, L-histidine, L-isoleucine, L-leucine, L-lysine, L-methionine, L-phenylalanine, L-threonine, L-tryptophan or L-valine, respectively (n = 6). Individual EAA was supplemented to achieve a concentration equal to that in POS. After 6-h treatment, total RNA was extracted for latter RNA-seq.
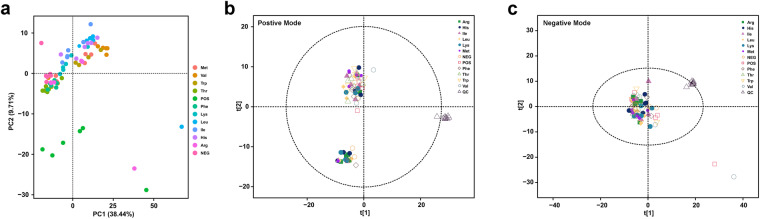
Various methods were used to improve the data quality in mass spectrometry experiments. First, sample extraction, LC-MS derivatization, and MS run followed the randomization sequences. Second, QCs that were made up of different samples were inserted during the measurement process to assess the reliability of data and system stability. Stability of measurement system was also examined using PCA analysis. PCA is a method that gives a overview of the data regarding questions about high variance as well as sample clusters and outliers32. QC samples cluster distinctively in Fig. 2, indicating that there was no significant variation induced by non-biology in this experiment.
Fig. 2.
Principal component analysis of the datasets obtained from transcriptomics (a) and metabolomics (b and c). Complete DMEM-F12 medium used for BMECs was the positive control (POS) treatment, while DMEM-F12 medium without total essential amino acid served as the negative control (NEG) treatment. Ten treatments were NEG individually supplemented with L-arginine, L-histidine, L-isoleucine, L-leucine, L-lysine, L-methionine, L-phenylalanine, L-threonine, L-tryptophan or L-valine, respectively (n = 6). Individual EAA was supplemented to achieve a concentration equal to that in POS. Quality control (QC) samples in metabolomics that were composed of aliquots from all samples were operated 3 times before the queue to monitor the column condition and every 6 inserts after that to evaluate discrepancies. The distinctive cluster of QC samples indicated that there was no significant variation induced by non-biology in this experiment.
Acknowledgements
The work is funded by National Natural Science Foundation of China (32102553, Beijing) and Key Research and Development Program of Ningxia Hui Autonomous Region (2023BCF01035, Ningxia).
Author contributions
L.B.X., X.W.W., X.L.L. and H.W.L. executed the experimental part, processed and performed data analysis, organized, structured and deposited the dataset into Gene Expression Omnibus, MetaboLights and Figshare. L.B.X. wrote the manuscript. L.B.X. and D.P.B. came up with the main research idea and designed the main study. L.B.X. and X.W.W. collected the samples. J.S.Z. and D.P.B. assisted in manuscript drafting. J.S.Z. and D.P.B. reviewed the manuscript. All authors read and approved the final documents.
Code availability
FastQC (version 0.11.3, https://www.bioinformatics.babraham.ac.uk/projects/fastqc/) was adopted to check the quality of raw FASTQ sequencing files. Metabolite profiling was analysed with ProteoWizard package (http://proteowizard.sourceforge.net), XCMS Online software (https://xcmsonline.scripps.edu/), SIMCA 13.0 (Umetrics AB, Umea, Sweden) software, MultiQuant software (https://sciex.com/products/software/multiquant-software) and MetaboAnalyst plotform (https://www.metaboanalyst.ca), respectively.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dai WT, White RR, Liu JX, Liu HY. Seryl-tRNA synthetase-mediated essential amino acids regulate β-casein synthesis via cell proliferation and mammalian target of rapamycin (mTOR) signaling pathway in bovine mammary epithelial cells. Journal of Dairy Science. 2018;101:10456–10468. doi: 10.3168/jds.2018-14568. [DOI] [PubMed] [Google Scholar]
- 2.Baumgard LH, Rhoads RP., Jr Effects of heat stress on postabsorptive metabolism and energetics. Annual Review of Animal Biosciences. 2013;1:311–337. doi: 10.1146/annurev-animal-031412-103644. [DOI] [PubMed] [Google Scholar]
- 3.Hristov AN, Price WJ, Shafii B. A meta-analysis examining the relationship among dietary factors, dry matter intake, and milk and milk protein yield in dairy cows. Journal of Dairy Science. 2004;87:2184–2196. doi: 10.3168/jds.S0022-0302(04)70039-9. [DOI] [PubMed] [Google Scholar]
- 4.Kebreab E, France J, Beever DE, Castillo AR. Nitrogen pollution by dairy cows and its mitigation by dietary manipulation. Nutrient Cycling in Agroecosystems. 2001;60:275–285. doi: 10.1023/A:1012668109662. [DOI] [Google Scholar]
- 5.Appuhamy JA, Knoebel NA, Nayananjalie WA, Escobar J, Hanigan MD. Isoleucine and leucine independently regulate mTOR signaling and protein synthesis in MAC-T cells and bovine mammary tissue slices. The Journal of Nutrition. 2012;142:484–491. doi: 10.3945/jn.111.152595. [DOI] [PubMed] [Google Scholar]
- 6.Appuhamy JA, Bell AL, Nayananjalie WA, Escobar J, Hanigan MD. Essential amino acids regulate both initiation and elongation of mRNA translation independent of insulin in MAC-T cells and bovine mammary tissue slices. The Journal of Nutrition. 2011;141:1209–1215. doi: 10.3945/jn.110.136143. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell HH, Block RJ. Some relationships between the amino acid contents of proteins and their nutritive values for the rat. Journal of Biological Chemistry. 1946;163:599–620. doi: 10.1016/S0021-9258(17)41289-0. [DOI] [PubMed] [Google Scholar]
- 8.Haque MN, et al. Milk protein synthesis in response to the provision of an “ideal” amino acid profile at 2 levels of metabolizable protein supply in dairy cows. Journal of Dairy Science. 2012;95:5876–5887. doi: 10.3168/jds.2011-5230. [DOI] [PubMed] [Google Scholar]
- 9.Zhao K, et al. Effects of rumen-protected methionine and other essential amino acid supplementation on milk and milk component yields in lactating Holstein cows. Journal of Dairy Science. 2019;102:7936–7947. doi: 10.3168/jds.2018-15703. [DOI] [PubMed] [Google Scholar]
- 10.Arriola Apelo SI, et al. Isoleucine, leucine, methionine, and threonine effects on mammalian target of rapamycin signaling in mammary tissue. Journal of Dairy Science. 2014;97:1047–1056. doi: 10.3168/jds.2013-7348. [DOI] [PubMed] [Google Scholar]
- 11.Gao HN, et al. Combination of histidine, lysine, methionine, and leucine promotes β-casein synthesis via the mechanistic target of rapamycin signaling pathway in bovine mammary epithelial cells. Journal of Dairy Science. 2017;9:7696–7709. doi: 10.3168/jds.2015-10729. [DOI] [PubMed] [Google Scholar]
- 12.Camarena Pulido EE, et al. Efficacy of L-arginine for preventing preeclampsia in high-risk pregnancies: A double-blind, randomized, clinical trial. Hypertension in Pregnancy. 2016;35:217–225. doi: 10.3109/10641955.2015.1137586. [DOI] [PubMed] [Google Scholar]
- 13.Chang MC, Choo YJ. Effects of whey protein, leucine, and vitamin D supplementation in patients with sarcopenia: A systematic review and meta-analysis. Nutrients. 2023;15:521. doi: 10.3390/nu15030521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruiz-Margáin A, et al. Effect of a high-protein, high-fiber diet plus supplementation with branched-chain amino acids on the nutritional status of patients with cirrhosis. Revista de Gastroenterología de México (English Edition) 2018;83:9–15. doi: 10.1016/j.rgmx.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Research. 2002;1:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu LB, Bu DP, Li XL. 2024. HydroShare. //identifiers.org/geo/GSE232591
- 17.Haug K, et al. MetaboLights: a resource evolving in response to the needs of its scientific community. Nucleic Acids Research. 2020;48:D440–D444. doi: 10.1093/nar/gkz1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu LB, Bu DP, Li XL. 2024. MTBLS7789: Non-targeted metabolomics data in bovine mammary epithelial cells stimulated by ten different essential amino acids. MetaboLights. MTBLS7789 [DOI] [PubMed]
- 19.Xu LB, Bu DP. 2024. MTBLS3956: Targeted metabolomics of amino acid profilings of bovine mammary epithelial cells stimulated by ten different essential amino acids. MetaboLights MTBLS3956. MTBLS3956 [DOI] [PubMed]
- 20.Xu LB, Bu DP, Li XL. 2024. Coefficient matrix among the samples in RNA-Seq from bovine mammary epithelial cells stimulated by ten different essential amino acids. Figshare. [DOI] [PubMed]
- 21.Xu LB, Bu DP, Li XL. 2024. Non-targeted metabolomics data from bovine mammary epithelial cells stimulated by ten different essential amino acids. Figshare. [DOI] [PubMed]
- 22.Zhao K, Liu HY, Zhou MM, Liu JX. Establishment and characterization of a lactating bovine mammary epithelial cell model for the study of milk synthesis. Cell Biology International. 2010;34:717–721. doi: 10.1042/CBI20100023. [DOI] [PubMed] [Google Scholar]
- 23.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nature Methods. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shumate A, Wong B, Pertea G, Pertea M. Improved transcriptome assembly using a hybrid of long and short reads with StringTie. PLoS Computational Biology. 2022;18:e1009730. doi: 10.1371/journal.pcbi.1009730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trapnell C, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nature Biotechnology. 2010;28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anders S, Pyl PT, Huber W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anders, S. & Huber, W. Differential expression of RNA-seq data at the gene level -the DESeq package. EMBL (2012).
- 29.Kessner D, Chambers M, Burke R, Agus D, Mallick P. ProteoWizard: open source software for rapid proteomics tools development. Bioinformatics. 2008;24:2534–2536. doi: 10.1093/bioinformatics/btn323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith CA, Want EJ, O’Maille G, Abagyan R, Siuzdak G. XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Analytical Chemistry. 2006;78:779–787. doi: 10.1021/ac051437y. [DOI] [PubMed] [Google Scholar]
- 31.Cai Y, Weng K, Guo Y, Zhu ZJ. An integrated targeted metabolomic platform for high-throughput metabolite profiling and automated data processing. Metabolomics. 2015;11:1575–1586. doi: 10.1007/s11306-015-0809-4. [DOI] [Google Scholar]
- 32.Beisken S, et al. Metabolic differences in ripening of Solanum lycopersicum ‘Ailsa Craig’ and three monogenic mutants. Scientific Data. 2014;1:140029. doi: 10.1038/sdata.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Xu LB, Bu DP, Li XL. 2024. HydroShare. //identifiers.org/geo/GSE232591
- Xu LB, Bu DP, Li XL. 2024. MTBLS7789: Non-targeted metabolomics data in bovine mammary epithelial cells stimulated by ten different essential amino acids. MetaboLights. MTBLS7789 [DOI] [PubMed]
- Xu LB, Bu DP. 2024. MTBLS3956: Targeted metabolomics of amino acid profilings of bovine mammary epithelial cells stimulated by ten different essential amino acids. MetaboLights MTBLS3956. MTBLS3956 [DOI] [PubMed]
- Xu LB, Bu DP, Li XL. 2024. Coefficient matrix among the samples in RNA-Seq from bovine mammary epithelial cells stimulated by ten different essential amino acids. Figshare. [DOI] [PubMed]
- Xu LB, Bu DP, Li XL. 2024. Non-targeted metabolomics data from bovine mammary epithelial cells stimulated by ten different essential amino acids. Figshare. [DOI] [PubMed]
Data Availability Statement
FastQC (version 0.11.3, https://www.bioinformatics.babraham.ac.uk/projects/fastqc/) was adopted to check the quality of raw FASTQ sequencing files. Metabolite profiling was analysed with ProteoWizard package (http://proteowizard.sourceforge.net), XCMS Online software (https://xcmsonline.scripps.edu/), SIMCA 13.0 (Umetrics AB, Umea, Sweden) software, MultiQuant software (https://sciex.com/products/software/multiquant-software) and MetaboAnalyst plotform (https://www.metaboanalyst.ca), respectively.