Abstract
Radioisotopes of metallic elements, or radiometals, are widely employed in both therapeutic and diagnostic nuclear medicine. For this application, chelators that efficiently bind the radiometal of interest and form a stable metal−ligand complex with it are required. Towards the development of new chelators for nuclear medicine, we recently reported a novel class of 18-membered macrocyclic chelators that is characterized by their ability to form stable complexes with both large and small rare-earth metals (Ln3+), a property referred to as dual size selectivity. A specific chelator in this class called py-macrodipa, which contains one pyridyl group within its macrocyclic core, was established as a promising candidate for 135La3+, 213Bi3+, and 44Sc3+ chelation. Building upon this prior work, here we report the synthesis and characterization of a new chelator called py2-macrodipa with two pyridyl units fused into the macrocyclic backbone. Its coordination chemistry with the Ln3+ series was investigated by NMR spectroscopy, X-ray crystallography, DFT calculations, analytical titrations, and transchelation assays. These studies reveal that py2-macrodipa retains the expected dual size selectivity and possesses an enhanced thermodynamic affinity for all Ln3+ compared to py-macrodipa. By contrast, the kinetic stability of Ln3+ complexes with py2-macrodipa is only improved for the light, large Ln3+ ions. Based upon these observations, we further assessed the suitability of py2-macrodipa for use with 225Ac3+, a large radiometal with valuable properties for targeted alpha therapy. Radiolabeling and stability studies revealed py2-macrodipa to efficiently incorporate 225Ac3+, and to form a complex that is inert in human serum over 3 weeks. Although py2-macrodipa does not surpass the state-of-the-art chelator macropa for 225Ac3+ chelation, it does provide another effective 225Ac3+ chelator. These studies shed light on the fundamental coordination chemistry of the Ln3+ and may inspire future chelator design efforts.
Graphical Abstract

INTRODUCTION
Radiopharmaceutical agents, which leverage radionuclides for medicinal therapy and diagnosis, have been receiving increasing attention as new modalities progress to the clinic.1–5 Across the entire periodic table, many metallic elements have been identified to possess radioisotopes suitable for this application.6–8 In order to harness these radiometals for clinical use, a chelator that can rapidly form thermodynamically and kinetically stable complexes is usually required. Facile complex formation minimizes radioactivity loss during handling before in vivo administration, and high complex stability prevents in vivo release of the potentially harmful radiometal. A significant body of research has been devoted to chelator design for radiometals by addressing their distinct chemical properties and coordination chemistry preferences.7–10
As a recent contribution to this area, we developed a class of ligands that exhibit “dual size selectivity”, a property characterized by their ability to preferentially bind both the large and small rare-earth metal ions (Ln3+). This dual-size-selective property is attributed to a significant conformational toggle that occurs in this ligand class as they bind metal ions of different sizes (Scheme 1) to optimize complex stability. For large Ln3+, a 10-coordinate, nearly C2-symmetric complex is formed (Conformation A), whereas for small Ln3+, an 8-coordinate asymmetric complex arises (Conformation B).10–12 The first member of this ligand class identified was the macrocyclic chelator macrodipa (Chart 1).11 Despite the unique selectivity profile of macrodipa, the kinetic lability of the corresponding Ln3+−macrodipa complexes limited its use for biomedical applications.12
Scheme 1.
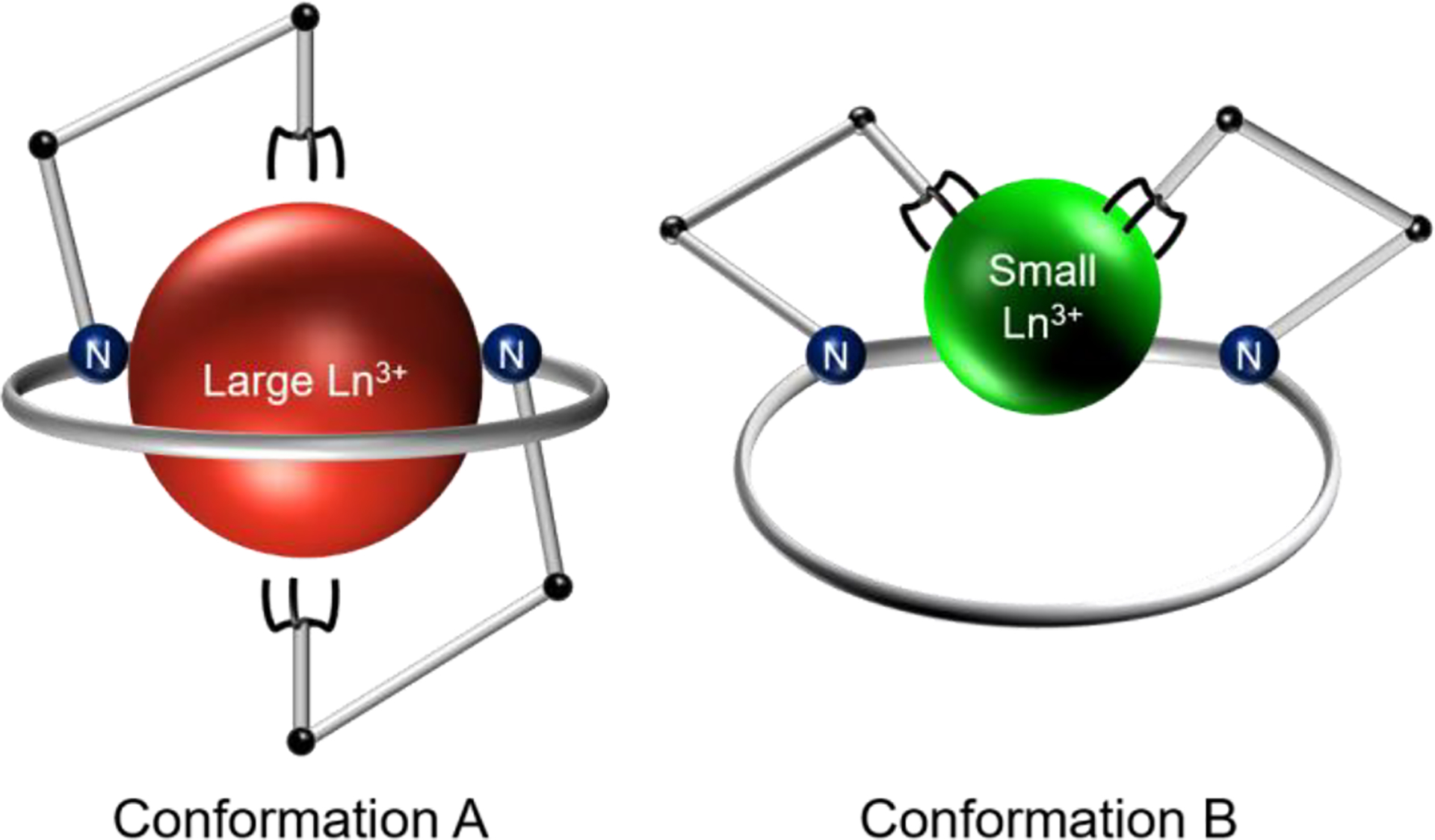
Depiction of the Conformational Toggle Present in Dual-Size-Selective Chelators when Binding Ln3+ Ions with Different Sizes.a
aReproduced from J. Am. Chem. Soc. 2020, 142, 13500. Copyright 2020 American Chemical Society.
Chart 1.
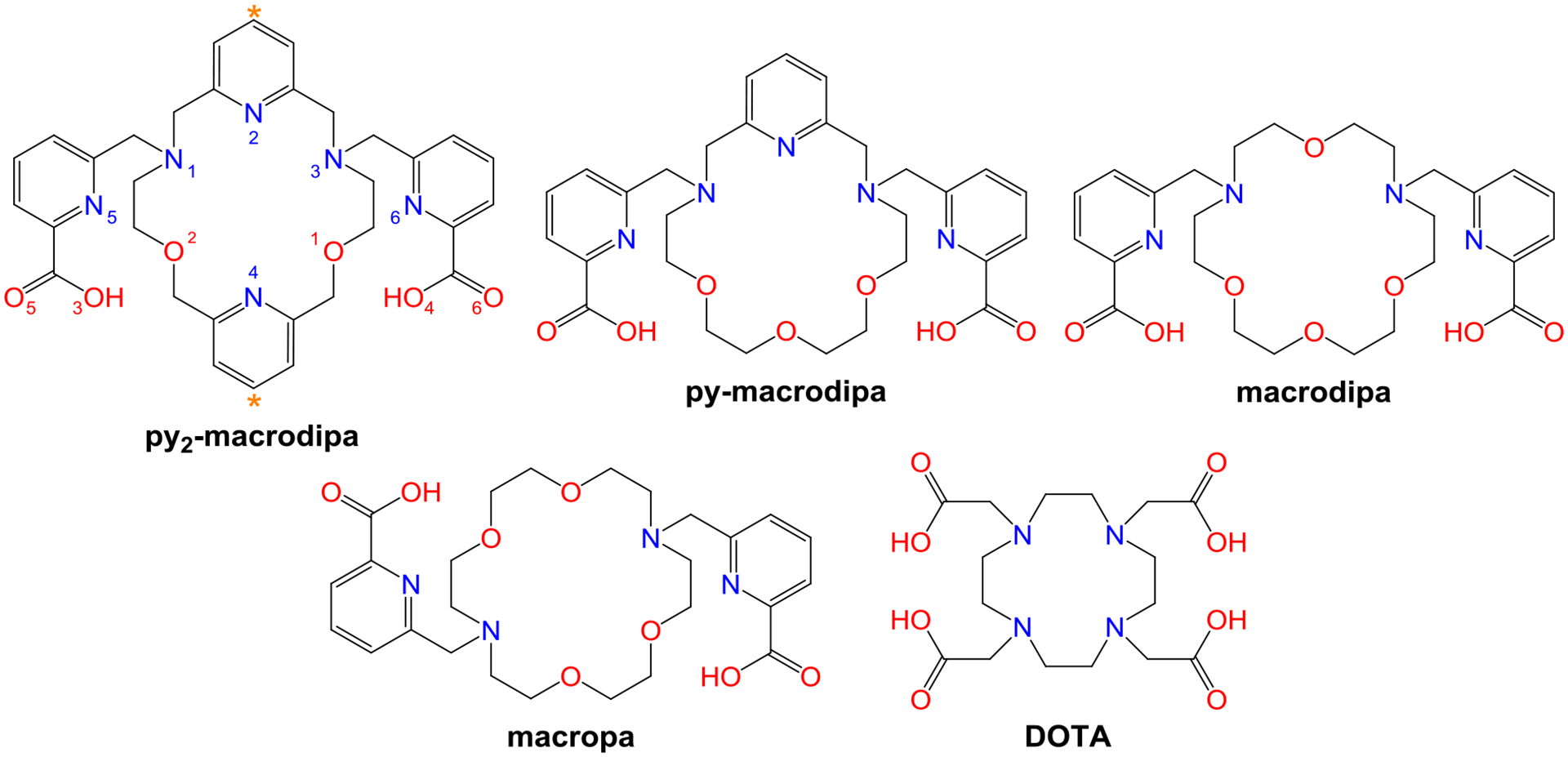
Structures of Ligands Discussed in This Work.
To overcome this limitation, we modified the structure of macrodipa by incorporating a stronger pyridyl donor in place of an ethereal oxygen within the macrocycle to afford the second-generation ligand py-macrodipa (Chart 1). This change led to a significant enhancement of both the thermodynamic and kinetic stability of its Ln3+ complexes, while maintaining its dual size selectivity. Based on these improvements of macrodipa, we showed that py-macrodipa efficiently formed stable complexes with 135La3+ and 44Sc3+, two valuable radionuclides for nuclear medicine with significantly disparate ionic radii.12 To further validate the potential of py-macrodipa for nuclear medicine applications, we assessed its suitability for chelating 225Ac3+ and 213Bi3+, two therapeutically valuable α-emitters.13,14 These studies showed that py-macrodipa is a promising candidate for 213Bi3+ chelation, but does not form a complex of sufficient kinetic stability for 225Ac3+ nuclear medicine applications.15
The inefficacy of py-macrodipa for the stable chelation of 225Ac3+ motivated us to target a third-generation dual-size-selective chelator that forms complexes with even greater thermodynamic and kinetic stability. Based on the improvements that were observed upon the incorporation of a single pyridyl group into the macrocycle of macrodipa, we targeted the novel chelator py2-macrodipa (Chart 1), which contains an additional pyridyl donor. In this work, we discuss the synthesis of this new ligand, its coordination chemistry with Ln3+ ions, and its potential application for 225Ac3+ radiotherapy.
RESULTS AND DISCUSSION
The chelator py2-macrodipa was synthesized via the five-step procedure shown in Scheme 2, with an overall yield of 20%. The benzyl-protected macrocyclic backbone 2 was constructed in two steps commencing from commercially available 2,6-bis(bromomethyl)pyridine and 2-benzylaminoethanol. The benzyl groups were then removed by reduction with H2 over a Pd/C catalyst to yield the desired dipyridyl macrocycle 3. Subsequent installation of the two picolinate pendent arms via alkylation and acidic deprotection afforded py2-macrodipa. The identity and purity of the intermediates and final product were verified by NMR spectroscopy, mass spectrometry, and analytical HPLC (Figures S1−S13).
Scheme 2.
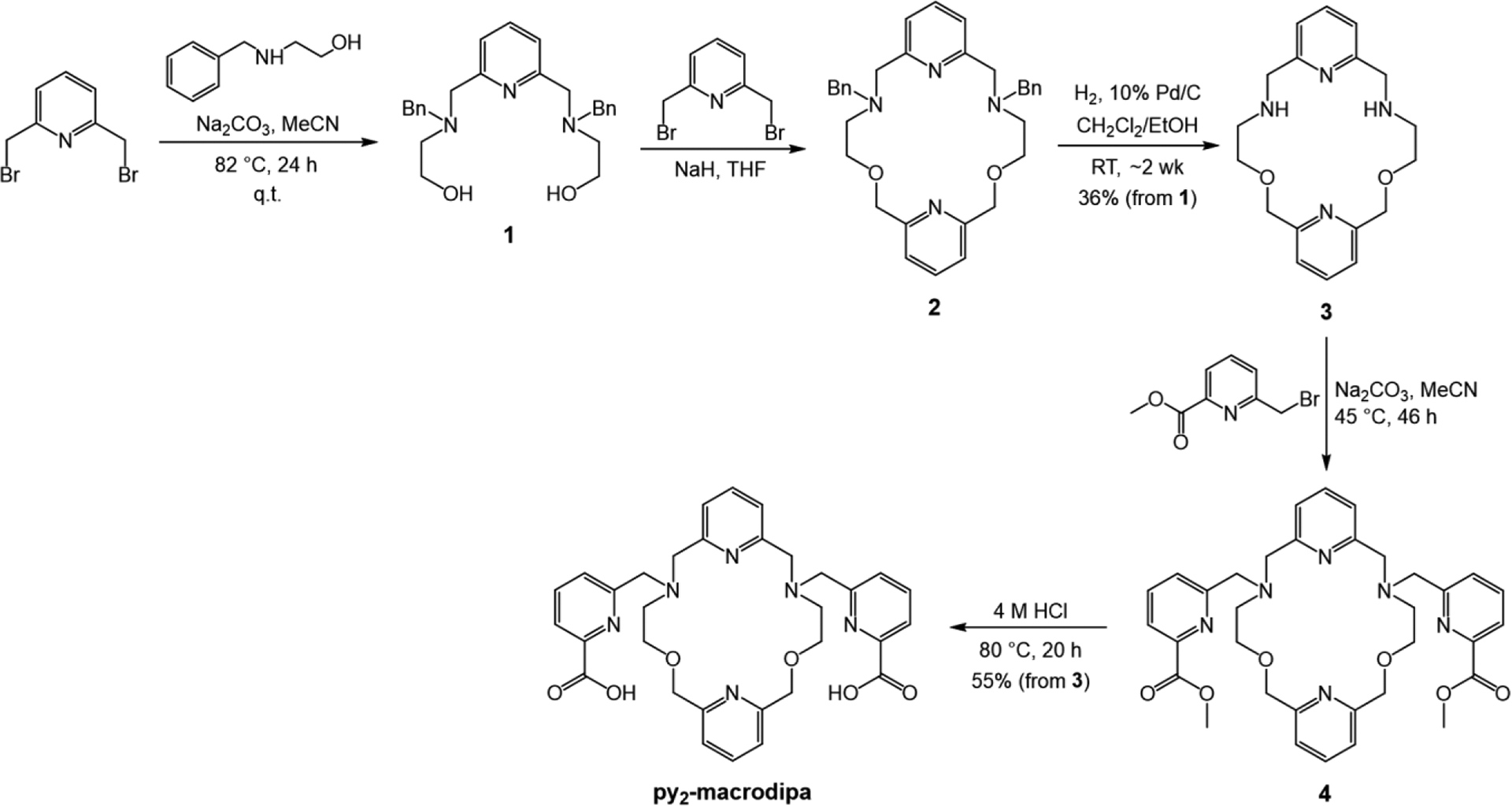
Synthetic Route to py2-macrodipa.
To understand the influence of the second pyridyl unit on the coordination chemistry of py2-macrodipa, the crystal structures of its complexes with two representative Ln3+ ions, La3+ and Sc3+, were determined by X-ray crystallography (Figure 1). La3+, the largest Ln3+, forms a complex with py2-macrodipa that attains the 10-coordinate, distorted C2-symmetric Conformation A, with all nitrogen and oxygen donor atoms on the ligand engaged in metal binding. By contrast, the py2-macrodipa complex of Sc3+, the smallest Ln3+, adopts the 8-coordinate, asymmetric Conformation B. In this crystal structure, three of the macrocycle-based ligand donor atoms (O1, O2, N4) do not directly interact with the Sc3+ center, whose coordination sphere is completed by an inner-sphere water molecule. The conformations observed for the La3+ and Sc3+ crystal structures are in line with our expectations, based on the well characterized conformational toggle (Scheme 1) observed for the earlier generation analogues macrodipa and py-macrodipa upon binding to Ln3+ of different sizes.
Figure 1.
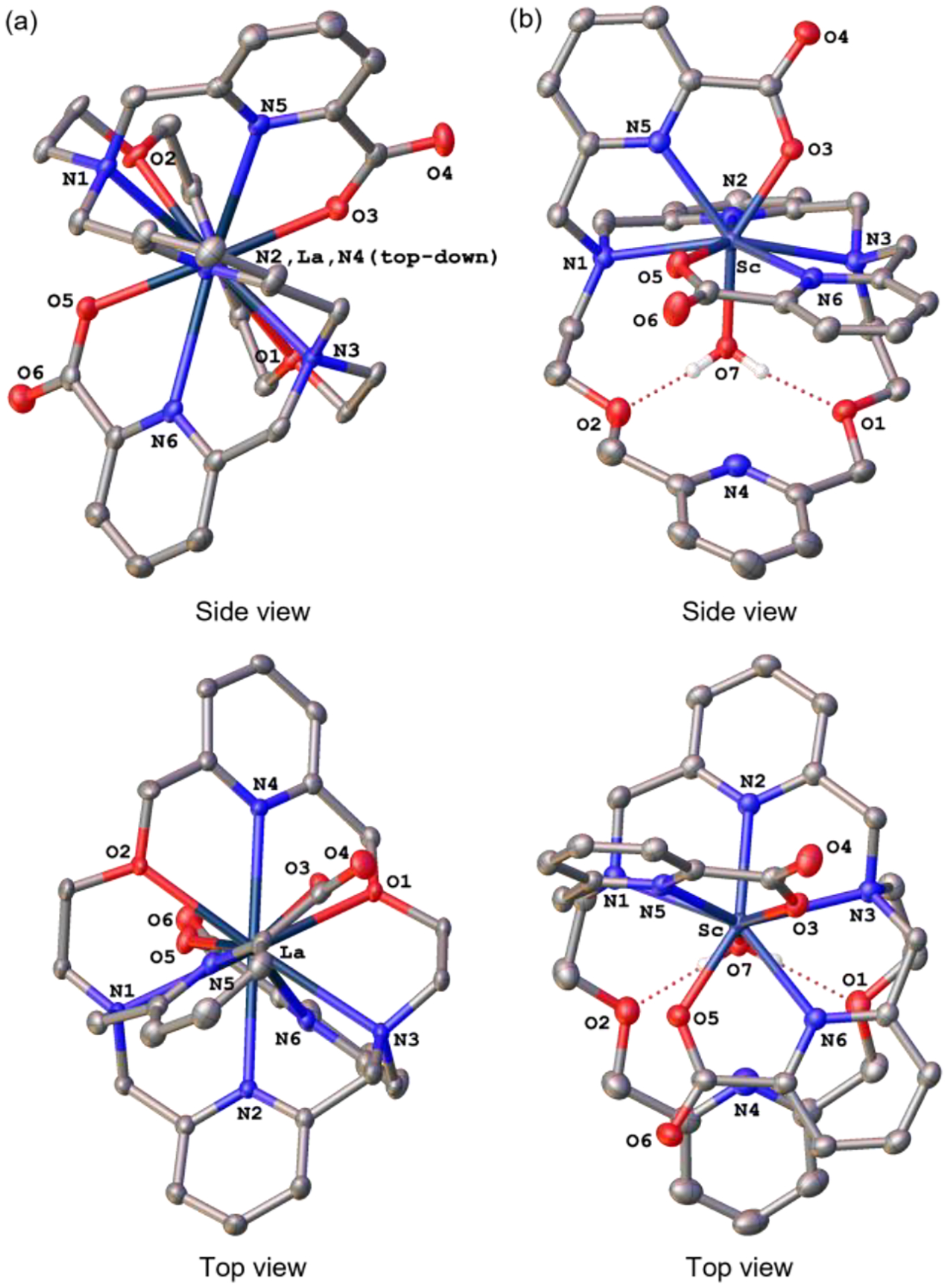
Crystal structures of (a) [La(py2-macrodipa)]+ and (b) [Sc(py2-macrodipa)(OH2)]+ complexes. Thermal ellipsoids are drawn at the 50% probability level. Solvent, counterions, and nonacidic hydrogen atoms are omitted for clarity.
Having verified that this conformational change occurs in the solid state, we next leveraged NMR spectroscopy to characterize the solution structures of Ln3+−py2-macrodipa complexes with the diamagnetic La3+, Y3+, Lu3+, and Sc3+ ions, for which the ionic radius decreases from 103.2 pm to 74.5 pm.16 The 1H and 13C{1H} NMR spectra of these four complexes were acquired in D2O at pD 7 (Figures 2 and S17−S24). Figure 2 shows a stacked arrangement of the 1H NMR spectra of these four complexes for comparison. The 1H NMR spectra of the complexes of La3+ and Y3+ show equivalency in their resonances, indicating that the symmetric Conformation A predominates for the py2-macrodipa complexes of these larger ions. By contrast, Sc3+, the smallest Ln3+, yields an NMR spectrum of lower symmetry, revealing it to attain the asymmetric Conformation B in aqueous solution. The 13C{1H} NMR spectra of these complexes are also consistent with these conformational assignments (Figures S18, S20, and S24). These NMR observations indicate that the solid-state crystallographic studies of the La3+ and Sc3+ complexes (Figure 1) are a good representation of their solution structures.
Figure 2.
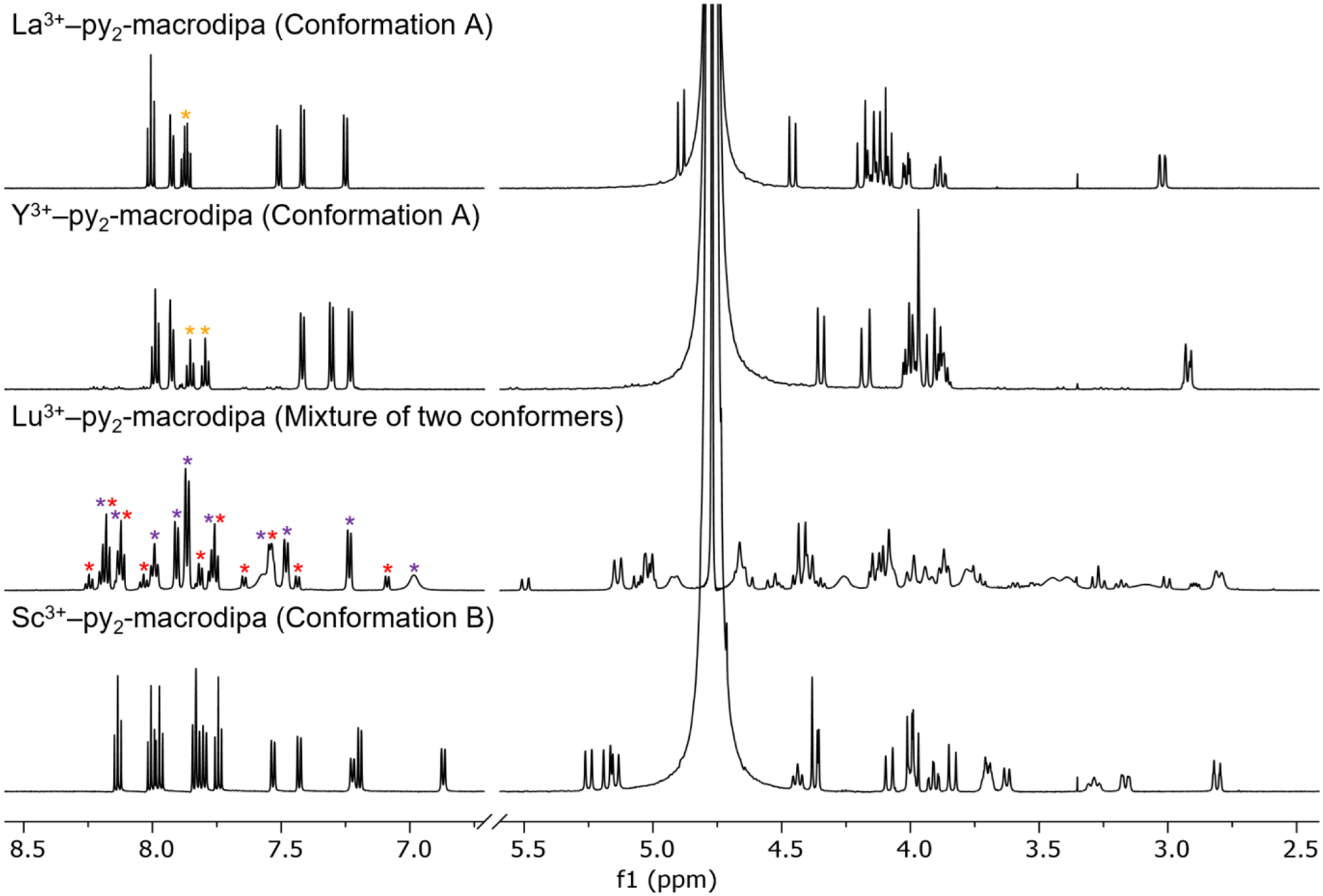
1H NMR spectra of La3+−, Y3+−, Lu3+−, and Sc3+−py2-macrodipa complexes (600 MHz, D2O, pD 7, 25 °C). The yellow asterisks in the spectra of the La3+ and Y3+ complexes highlight the decrease in symmetry of Conformation A in moving from larger to smaller ions. The purple (Conformation A) and red (Conformation B) asterisks in the spectrum of the Lu3+ complex indicate the two conformers present in solution.
The 1H NMR spectrum of the py2-macrodipa complex of the intermediately-sized Lu3+ is more complicated than those of La3+, Y3+, and Sc3+. Two different species, in a molar ratio of 4:1, are observed. The 13C{1H} NMR spectrum of this complex likewise reveals the presence of two species (Figure S22). These two species are assigned to be a mixture of Conformation A and B in equilibrium. Close inspection of the aromatic region of the 1H NMR spectrum (Figures 2 and S21), reveals an asymmetry in both conformers, despite our prior observations that Conformation A typically gives rise to spectra with symmetry. Our tentative explanation for this observation is that the Conformation A gradually descends in symmetry as the Ln3+ gets smaller, and the size-matching becomes poorer. This argument is supported by comparing the 1H NMR spectra of the large La3+ and smaller Y3+ complexes (Figure 2), where a decrease in symmetry of Conformation A begins to appear. Most apparent are the two triplets in the aromatic region (labeled with orange asterisks), which can be assigned as the geminal hydrogens on the carbon atoms labeled with orange asterisks in Chart 1. For the large La3+, the chemical shifts of these two triplets are nearly identical, whereas for the smaller Y3+, their chemical shifts begin to diverge more substantially and indicate different chemical environments for these two H atoms, presumably due to a decrease in symmetry of Conformation A.
To further understand the conformational switch of py2-macrodipa across the Ln3+ series, we carried out density functional theory (DFT) calculations. All Ln3+−py2-macrodipa complexes in both Conformations A and B were optimized, using the same level of theory (ωB97XD/6–31G(d,p)/LCRECP) that we previously applied to study Ln3+−macrodipa and Ln3+−py-macrodipa.11,12,17–24 From these geometry optimizations and thermochemical calculations, the standard free energy difference (ΔG°) between Conformations A and B for each Ln3+ complex was determined (eq 1), as plotted in Figure 3.
Figure 3.
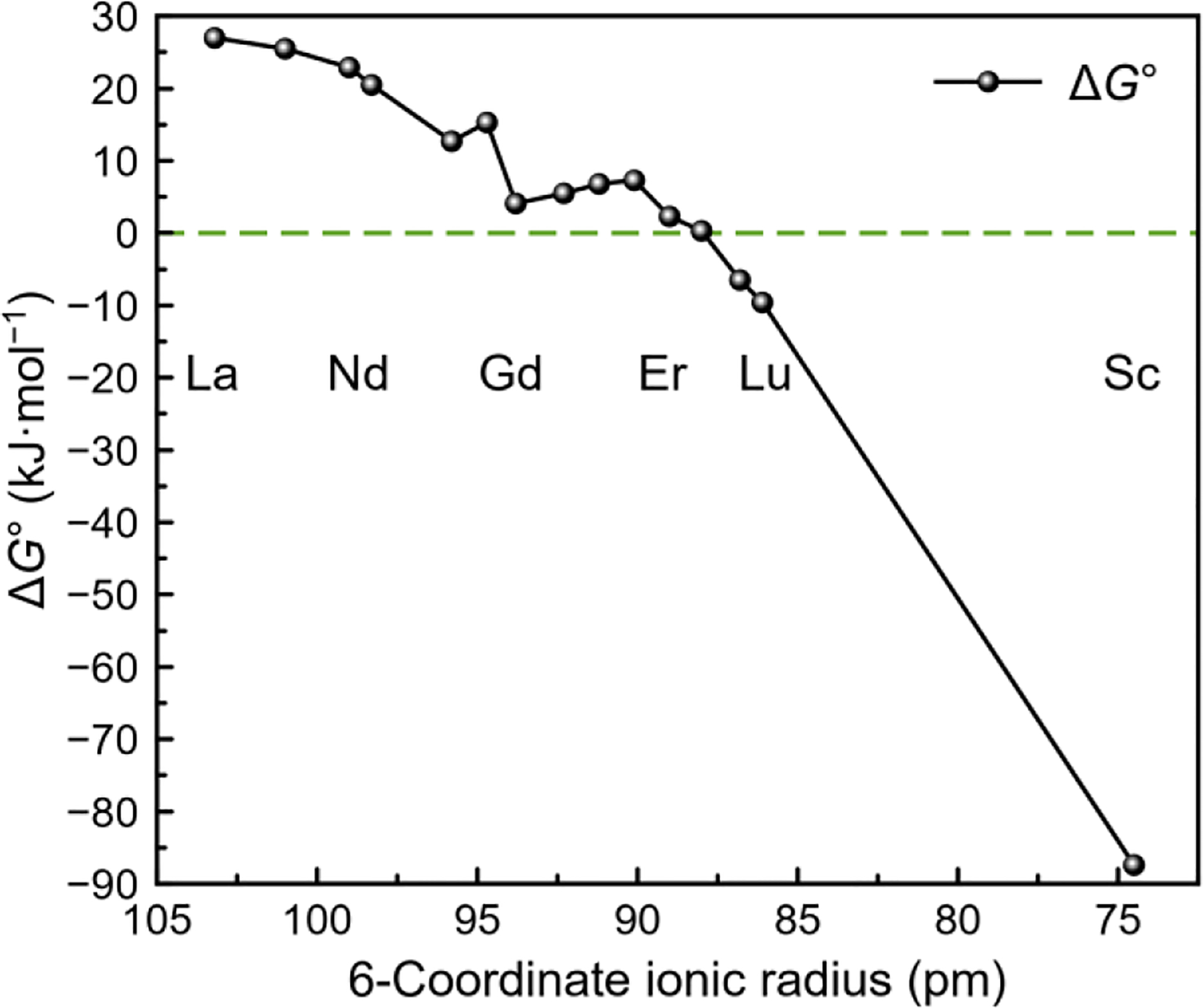
DFT-computed standard free energy differences (ΔG°) between Conformations A and B for Ln3+−py2-macrodipa complexes, plotted versus ionic radii.
| (1) |
As the Ln3+ series is traversed, ΔG° gradually shifts from positive to negative values, consistent with our experimental observations that Conformation A is favored for large Ln3+, whereas Conformation B is preferred for small Ln3+. A similar computational analysis was applied in our studies of macrodipa and py-macrodipa, which also showed that Conformation A was lower in free energy for large Ln3+ and vice versa. The crossover point where ΔG° switches its sign occurs roughly at Tm3+, Er3+, and Tb3+ for py2-macrodipa, py-macrodipa,12 and macrodipa11 systems, respectively. Thus, modifications of the macrocycle can alter the conformational dynamics and preferences of this ligand class.
Having confirmed by X-ray crystallography and NMR spectroscopy that Ln3+ complexes of py2-macrodipa undergo a Ln3+-size-dependent conformational toggle like its earlier analogues macrodipa and py-macrodipa, we next set out to verify our hypothesis that the additional macrocyclic pyridyl moiety would lead to enhanced complex stability. In this regard, we first evaluated the thermodynamic stability of Ln3+−py2-macrodipa complexes by determining their stability constants. The magnitude of stability constant measures the thermodynamic affinity of a ligand for a metal ion and is valuable for assessing its potential in different applications.10,25,26 The protonation constants (Ki) of py2-macrodipa, as well as the stability constants (KLnL) of its Ln3+ complexes, measured via either potentiometric titrations or UV−Vis spectrophotometric titrations,27–29 are collected in Table 1. These quantities are defined in eqs 2–3, where the concentration terms represent those at chemical equilibrium, and L signifies the uncomplexed ligand in its fully deprotonated state.
Table 1.
Protonation Constants of py2-macrodipa, py-macrodipa, macrodipa, macropa, and Stability Constants of Their Ln3+ Complexes.
| py2-macrodipaa | py-macrodipa | macrodipa | macropa | |
|---|---|---|---|---|
| log K1 | 7.58(4) | 7.20b | 7.79c | 7.41d, 7.41e |
| log K2 | 6.48(1) | 6.54b | 7.04c | 6.85d, 6.90e |
| log K3 | 3.52(3) | 3.17b | 3.18c | 3.32d, 3.23e |
| log K4 | 2.60(5) | 2.31b | 2.41c | 2.36d, 2.45e |
| log K5 | 2.10(11) | 1.69d | ||
| log KLaL | 16.68(8) | 14.31b | 12.19c | 14.99d |
| log KCeL | 17.13(7) | 14.65b | 12.50c | 15.11d |
| log KPrL | 17.28(6) | 14.81b | 12.41c | 14.70d |
| log KNdL | 17.11(3) | 14.51b | 12.25c | 14.36d |
| log KSmL | 16.56(4) | 13.66b | 11.52c | 13.80d |
| log KEuL | 15.93(4) | 13.29b | 10.93c | 13.01d |
| log KGdL | 15.25(7) | 12.63b | 10.23c | 13.02d |
| log KTbL | 14.76(6) | 11.95b | 9.68c | 11.79d |
| log KDyL | 14.04(2) | 11.47b | 9.36c | 11.72d |
| log KHoL | 12.68(5) | 10.69b | 9.36c | 10.59d |
| log KErL | 12.17(3) | 10.60b | 9.71c | 10.10d |
| log KTmL | 11.98(2) | 10.92b | 10.13c | 9.59d |
| log KYbL | 11.82(5) | 11.31b | 10.48c | 8.89d |
| log KLuL | 11.90(3) | 11.54b | 10.64c | 8.25c |
| log KScL | 16.28(4) | 15.83b | 14.37b |
| (2) |
| (3) |
The log KLnL values of py2-macrodipa, and those of the related structures macrodipa, py-macrodipa, and macropa, are plotted against Ln3+ 6-coordinate ionic radius16 in Figure 4. Like macrodipa and py-macrodipa, py2-macrodipa exhibits a dual-size-selective pattern that is not altered via the introduction of an additional pyridyl group in the macrocycle. Importantly, the log KLnL values of py2-macrodipa are systematically greater compared to those of py-macrodipa, indicating that the additional macrocyclic pyridyl group of py2-macrodipa has a pronounced effect on enhancing the overall Ln3+-binding affinity. The log KLnL values of py2-macrodipa are also significantly higher across the entire Ln3+ series than those of macropa, a chelator established as a promising candidate for several large radiometals including 225Ac3+, 132/135La3+, 131Ba2+, 223Ra2+, and 213Bi3+.30–34 This observation highlights the potential of py2-macrodipa for large radiometal chelation. Although these log KLnL values are smaller than those observed for the Ln3+ complexes of the commonly applied chelator DOTA (Chart 1), for which log KLnL spans 22.9−25.4 from La3+ to Lu3+, the established efficacy of macropa, which gives rise to substantially lower log KLnL values, for these radiometals demonstrate that such high KLnL values are not strictly needed for practical use in nuclear medicine. However, future ligand design efforts within this ligand class to further increase these stability constants may further improve value for use in nuclear medicine.
Figure 4.
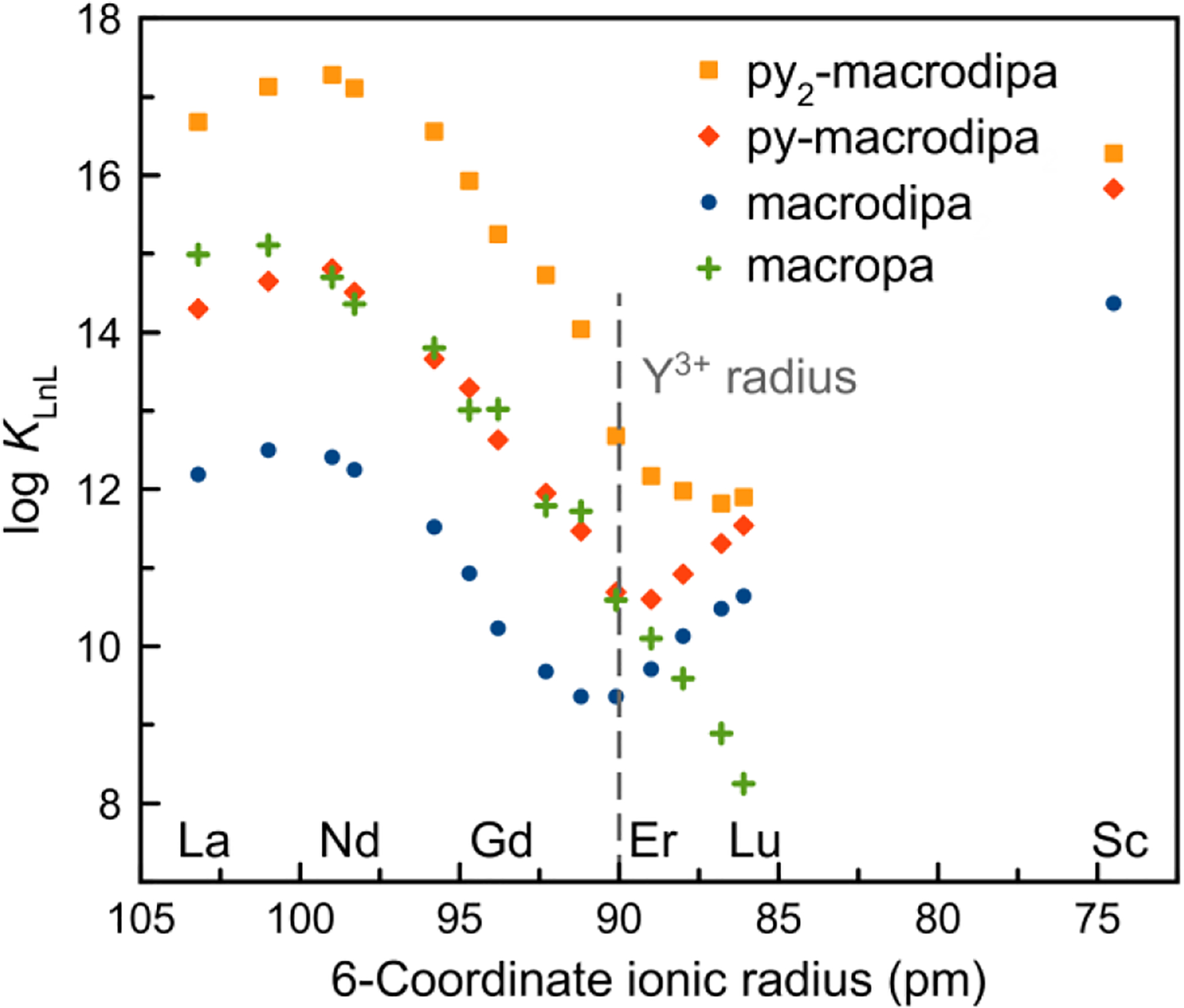
Stability constants of Ln3+ complexes formed with py2-macrodipa, py-macrodipa, macrodipa, and macropa plotted versus ionic radii.
Notably, the increase in log KLnL in moving from py-macrodipa to py2-macrodipa is much greater for the large Ln3+ than for the small Ln3+. This observation can be rationalized by the crystal structures. The newly introduced second pyridyl unit directly interacts and stabilizes the metal center in Conformation A (Figure 1a), whereas it is not bound to the metal in Conformation B (Figure 1b). As such, Conformation A benefits more significantly from the presence of the additional macrocyclic pyridyl donor present in py2-macrodipa. Thus, for large Ln3+ complexes, for which Conformation A prevails, a substantial log KLnL enhancement is observed, but diminished impact is found for small Ln3+ complexes, where Conformation B is dominant. Another noteworthy characteristic discerned from our potentiometric titrations is the identification of the protonated complex species (LnHL) for late Ln3+−py2-macrodipa systems (Figures S28−S32), which was not observed for any Ln3+ complexes of py-macrodipa.12 Based on this information, the most likely protonation site to form the LnHL species is the second pyridyl group (N4, Chart 1), which is substantially basic and not engaged with the Ln3+ center (Figure 1b) in Conformation B.
After demonstrating an enhancement of Ln3+ complex thermodynamic stability afforded by the second pyridyl group of py2-macrodipa, we next evaluated its impact on complex kinetic stability, a property of critical importance for chelators applied in radiopharmaceutical settings.1,9 For comparison to our previous studies, an identical DTPA transchelation challenge12,39 was applied to assess the Ln3+−py2-macrodipa complex kinetic stability. Specifically, the Ln3+−py2-macrodipa complexes were treated with 100 equiv of DTPA at pH 7.4 and RT (22 °C), a condition that thermodynamically favors the formation of the Ln3+–DTPA complexes.40–42 This transchelation process, which follows pseudo-first-order kinetics with the large excess of DTPA, was monitored by UV−Vis spectroscopy. The half-lives (t1/2) afforded from these pseudo-first-order processes (Table 2) provide a quantitative comparative measure of the complex kinetic stability.
Table 2.
Half-lives of Ln3+−py2-macrodipa, Ln3+−py-macrodipa, and Ln3+−macrodipa Complexes when Challenged with 100 Equivalents of DTPA.a
| Ln3+–py2-macrodipa | Ln3+–py-macrodipab | Ln3+–macrodipab | |
|---|---|---|---|
| La3+ | ≫ 5 weeks | 6.3 d | 1678 s |
| Gd3+ | 4.5 ± 0.2 d | 5524 s | 54 s |
| Lu3+ | 253 ± 6 s | 853 s | 65 s |
| Sc3+ | 5.9 ± 0.4 h | 16.6 h | 782 s |
[LnL] = 100 μM, pH 7.4 in MOPS, 22 °C.
Ref 12.
The trend in kinetic stability of the Ln3+−py2-macrodipa complexes follows closely with that observed for their thermodynamic stability, with the light and heavy Ln3+ complexes undergoing slower transchelation than those in the middle of the series. In comparison to those of macrodipa and py-macrodipa, the early Ln3+ complexes of py2-macrodipa exhibit a remarkable kinetic stability, with <10% dissociation of the La3+ complex observed after 5 weeks. However, for the smallest Sc3+, the kinetic stability of its py2-macrodipa complex is significantly lower than that of py-macrodipa, indicating that the second macrocyclic pyridyl is detrimental in this regard. The enhancement of kinetic stability of the La3+ complex is easily rationalized from the X-ray crystallographic data (Figure 1a), where both pyridyl groups strongly engage the La3+ center. By contrast, the non-coordinated pyridyl group present in the Sc3+−py2-macrodipa complex is positioned closely to the inner-sphere water molecule (N4–O7 distance of 2.91 Å), within a range that is consistent with hydrogen bonding, for which the N···O distances typically span from 2.8−3.0 Å.43 Thus, the pendent pyridyl group is oriented appropriately to facilitate proton-assisted metal-ion dissociation, which will substantially labilize the complex in aqueous solution. The increasing kinetic lability by protonation was also illustrated in other metal complex systems.44–46 Collectively, the inclusion of this second pyridyl unit of py2-macrodipa enhances the thermodynamic stability for all Ln3+ complexes, but only improves the kinetic stability of complexes with the large Ln3+. Thus, py2-macrodipa is a promising candidate for large Ln3+ chelation.
On account of the remarkable thermodynamic and kinetic stability of the La3+−py2-macrodipa complex, we next sought to investigate the capability of py2-macrodipa to chelate the largest trivalent cation Ac3+, as both ions possess similar coordination chemistry.47 Furthermore, the radioisotope 225Ac3+ (t1/2 = 9.9 d)48 emits four α particles through its decay chain, making it valuable for use in targeted internal radiotherapy.49–51 To assess the suitability of py2-macrodipa as a chelator for this radiometal, we carried out 225Ac3+ radiolabeling studies with py2-macrodipa and benchmarked the results to macropa and DOTA (Chart 1), two macrocyclic chelators that have established precedence for 225Ac3+ chelation.30,52
Concentration-dependent radiolabeling studies were carried out by incubating different concentrations of py2-macrodipa, macropa, and DOTA with 9.5–11.1 kBq of 225Ac3+ at pH 6.0 and 25 °C. The radiochemical yields (RCYs), determined by radio-TLC, are summarized in Figure 5 and Table S3. Notably, py2-macrodipa quantitatively incorporates 225Ac3+ at a low concentration of 10−5 M within 5 min, revealing it to be an efficient chelator for 225Ac3+. This property is nearly on par with that of macropa, the current gold standard, which quantitatively radiolabels 225Ac3+ at micromolar concentration, an observation consistent with previously reported results.15,30 By contrast, DOTA did not significantly undergo 225Ac3+ radiolabeling even at a millimolar concentration. Increasing the reaction time to 60 min had only a slight effect on the RCYs for py2-macrodipa and macropa, indicating that the radiolabeling process is nearly complete within 5 min under these mild conditions for both chelators. In addition, the radiolabeling efficiency of py2-macrodipa is comparable to that of py-macrodipa and significantly better than macrodipa.15
Figure 5.
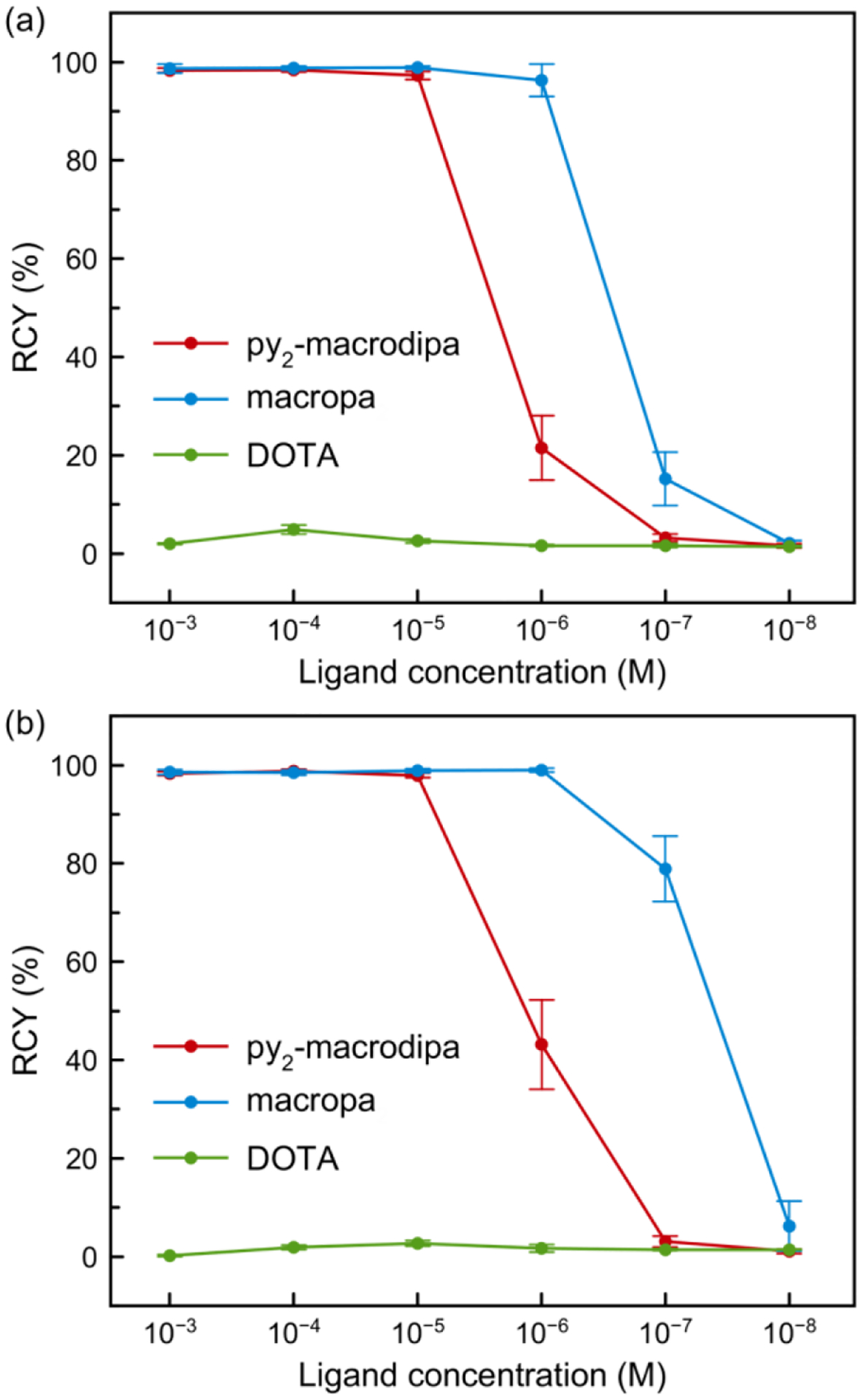
Radiochemical yields (RCYs) of 225Ac3+ radiolabeling with py2-macrodipa, macropa, and DOTA at different ligand concentrations (pH 6.0, 25 °C). (a) 5-min reaction time; (b) 60-min reaction time.
After establishing effective radiolabeling of py2-macrodipa with 225Ac3+, the kinetic stability of its 225Ac3+ complex was assessed. Specifically, the 225Ac3+ complexes of py2-macrodipa and macropa were incubated in human serum at 37 °C over 3 weeks to model the conditions that would be encountered when a radiopharmaceutical agent is administered in vivo. Figure 6 shows the percentage of intact complex remaining throughout the course of this experiment. Both radiocomplexes are sufficiently stable in human serum, as reflected by the fact that they remain >90% intact after 3 weeks. The resistance to human serum for [225Ac]Ac3+−macropa observed here is also consistent with prior studies.15,30,53 Although the radiolabeling efficiency of py2-macrodipa is comparable to that of py-macrodipa, the kinetic stability of [225Ac]Ac3+−py2-macrodipa is substantially enhanced relative to that of [225Ac]Ac3+−py-macrodipa, which showed a ~90% complex dissociation in human serum at 37 °C after 24 h.15 This observation is also consistent with the DTPA transchelation challenge results (Table 2), which also revealed an overall higher Ln3+ complex kinetic stability for py2-macrodipa. Collectively, py2-macrodipa shows promise for use with 225Ac3+ based on its efficient radiolabeling and high complex stability. It exhibits the best performance for 225Ac3+ chelation among the dual-size-selective chelators.
Figure 6.
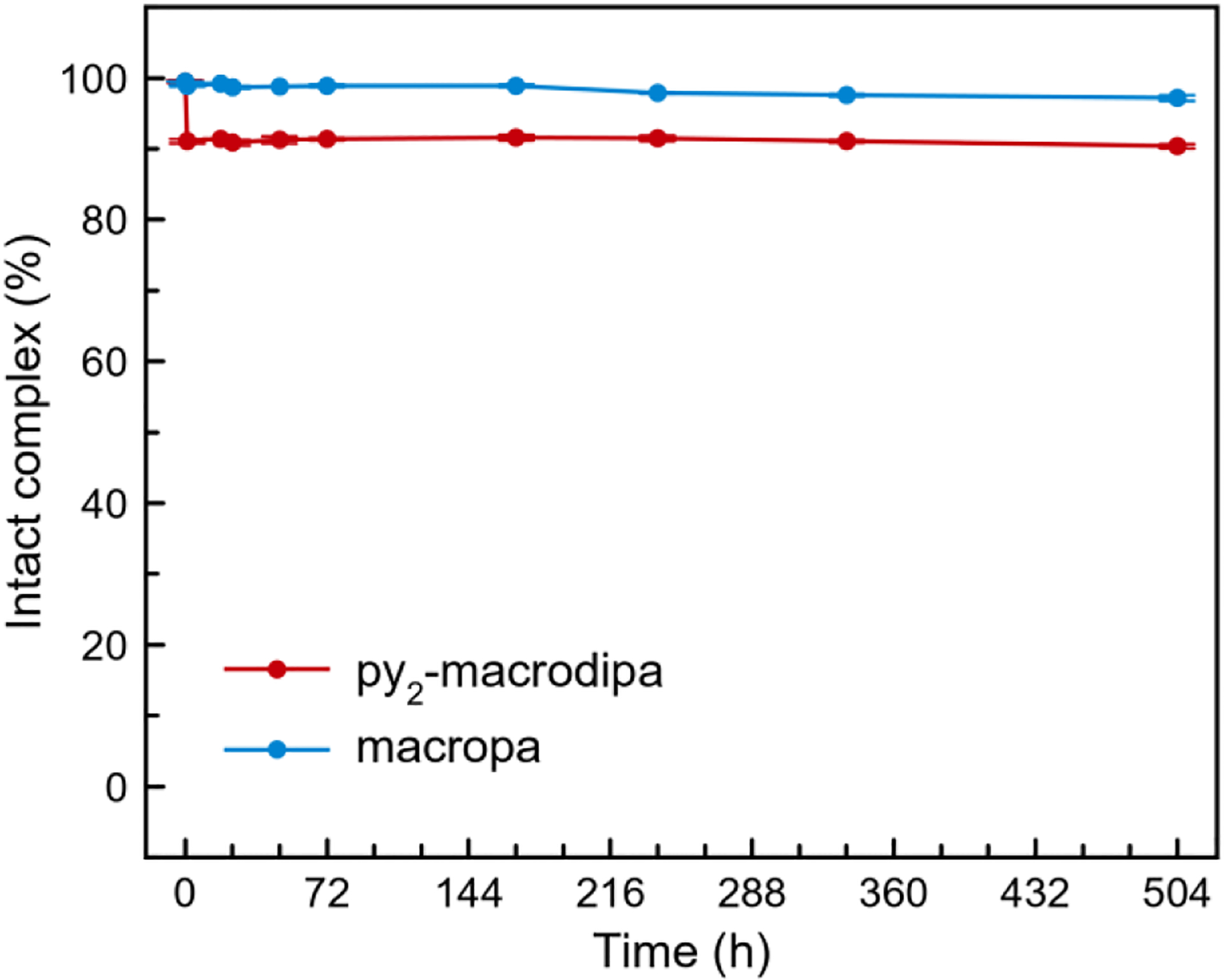
The stability of [225Ac]Ac3+−py2-macrodipa and [225Ac]Ac3+−macropa in human serum at 37 °C.
CONCLUSION
Building upon macrodipa and py-macrodipa, we designed and prepared the third-generation dual-size-selective chelator py2-macrodipa. Its coordination chemistry with Ln3+ ions was characterized by multiple techniques including X-ray crystallography, NMR spectroscopy, DFT calculations, analytical titrations, and transchelation assays. These three chelators all exhibit dual-size selective properties and are based on the same 18-membered macrocyclic core structures. They differ by the presence of 0, 1, or 2 pyridyl groups within the macrocyclic core. Without an integrated pyridyl group, macrodipa is a relatively poor chelator with respect to both the thermodynamic and kinetic stability of its Ln3+ complexes. The single pyridyl group in py-macrodipa serves to enhance both properties. In this study, we showed that the introduction of a second pyridyl group in py2-macrodipa further enhanced the thermodynamic stability for all Ln3+ complexes, but the kinetic stability was only improved for the large Ln3+ complexes. As a general conclusion, it appears that the inclusion of pyridyl groups in place of ethereal donors does benefit complex stability, but the conformations of the resulting complexes also need to be considered when trying to anticipate the magnitudes of these effects. Despite the weaker performance of py2-macrodipa for small Ln3+, its promising chelation properties for large Ln3+ prompted us to investigate its use with the therapeutic radiometal 225Ac3+. Radiolabeling and serum stability studies with 225Ac3+ revealed py2-macrodipa to perform comparably to the state-of-the-art chelator macropa. Although the dual-size-selective properties were demonstrated both thermodynamically and kinetically, efforts need to be undertaken to improve the stability of its complexes with small metal ions for practical nuclear medicine applications. Additionally, ongoing work is also directed toward preparation of a bifunctional analogue of py2-macrodipa that can be used to conjugate with biomolecules. Both the macrocyclic backbone and the picolinate pendant arms provide potential opportunities for this functionalization. In any case, this work affords a new potential candidate for 225Ac3+ chelation as well as provides new insights on the fundamental Ln3+ coordination chemistry of and 225Ac3+ radiochemistry for future chelator development endeavors.
Supplementary Material
Synopsis.
A novel macrocyclic chelator called py2-macrodipa is reported, and its coordination chemistry with the rare-earth metals is described. These studies show it to have promising properties for the chelation of large radiometal ions for nuclear medicine. Thus, its ability to chelate the therapeutic radionuclide 225Ac3+ was assessed, demonstrating efficient formation of inert 225Ac3+ complexes.
ACKNOWLEDGEMENTS
This research was supported by the U.S. Department of Energy Isotope Program, managed by the Office of Science for Isotope R&D and Production, and by the Laboratory Directed Research and Development Program of Oak Ridge National Laboratory (ORNL). The authors would like to thank the staff of the Technical Services, Finishing, and Dispensing Group at ORNL for their isotope production and purification contributions. The manuscript was produced by UT-Battelle, LLC under Contract No. DE-AC05-00OR22725 with the U.S. Department of Energy. The publisher acknowledges the U.S. Government license to provide public access under the DOE Public Access Plan (http://energy.gov/downloads/doe-public-access-plan). This research was also supported by the National Institutes of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Numbers R21EB027282 and R01EB029259, as well as by the U.S. Department of Energy Office of Science under Award Number DE-SC0021662. J.J.W. thanks the Research Corporation for Science Advancement for a Cottrell Scholar Award. A.H. was funded by a summer research fellowship at ORNL via the ORISE/ORAU (GSO program) to work on this project. V. K. was supported by the U. S. Department of Energy Office of Biological and Environmental Research. We thank Dr. Samantha MacMillan for data collection on the X-ray diffractometer.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/xxxxxxx.
Experimental procedures and supplementary data for ligand synthesis, X-ray crystallography, NMR spectroscopy, DFT calculations, potentiometric titrations, UV−Vis spectrophotometric titrations, DTPA transchelation studies, radiolabeling studies, and serum challenge assays (PDF)
Crystallographic data for La3+− and Sc3+−py2-macrodipa complexes (CIF)
Geometry outputs for all DFT-optimized structures (ZIP)
Accession Codes
CCDC 2178140–2178141 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
A.H, J.J.W., and N.A.T. are co-inventors on a patent application filed on the use of py2-macrodipa and related analogues for nuclear medicine applications.
REFERENCES
- (1).Radiopharmaceutical Chemistry; Lewis JS, Windhorst AD, Zeglis BM, Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2019. [Google Scholar]
- (2).Dondi M; Kashyap R; Paez D; Pascual T; Zaknun J; Mut Bastos F; Pynda Y Trends in Nuclear Medicine in Developing Countries. J. Nucl. Med 2011, 52, 16S–23S. [DOI] [PubMed] [Google Scholar]
- (3).Delbeke D; Segall GM Status of and Trends in Nuclear Medicine in the United States. J. Nucl. Med 2011, 52, 24S–28S. [DOI] [PubMed] [Google Scholar]
- (4).Kramer-Marek G; Capala J The Role of Nuclear Medicine in Modern Therapy of Cancer. Tumor Biol. 2012, 33, 629–640. [DOI] [PubMed] [Google Scholar]
- (5).Sgouros G; Bodei L; McDevitt MR; Nedrow JR Radiopharmaceutical Therapy in Cancer: Clinical Advances and Challenges. Nat. Rev. Drug Discovery 2020, 19, 589–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Blower PJ A Nuclear Chocolate Box: the Periodic Table of Nuclear Medicine. Dalton Trans. 2015, 44, 4819–4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Kostelnik TI; Orvig C Radioactive Main Group and Rare Earth Metals for Imaging and Therapy. Chem. Rev 2019, 119, 902–956. [DOI] [PubMed] [Google Scholar]
- (8).Boros E; Packard AB Radioactive Transition Metals for Imaging and Therapy. Chem. Rev 2019, 119, 870–901. [DOI] [PubMed] [Google Scholar]
- (9).Price EW; Orvig C Matching Chelators to Radiometals for Radiopharmaceuticals. Chem. Soc. Rev 2014, 43, 260–290. [DOI] [PubMed] [Google Scholar]
- (10).Hu A; Wilson JJ Advancing Chelation Strategies for Large Metal Ions for Nuclear Medicine Applications. Acc. Chem. Res 2022, 55, 904–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Hu A; MacMillan SN; Wilson JJ Macrocyclic Ligands with an Unprecedented Size-Selectivity Pattern for the Lanthanide Ions. J. Am. Chem. Soc 2020, 142, 13500–13506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Hu A; Aluicio-Sarduy E; Brown V; MacMillan SN; Becker KV; Barnhart TE; Radchenko V; Ramogida CF; Engle JW; Wilson JJ Py-Macrodipa: A Janus Chelator Capable of Binding Medicinally Relevant Rare-Earth Radiometals of Disparate Sizes. J. Am. Chem. Soc 2021, 143, 10429–10440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Morgenstern A; Apostolidis C; Kratochwil C; Sathekge M; Krolicki L; Bruchertseifer F An Overview of Targeted Alpha Therapy with 225Actinium and 213Bismuth. Curr. Radiopharm 2018, 11, 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Bruchertseifer F; Kellerbauer A; Malmbeck R; Morgenstern A Targeted Alpha Therapy with Bismuth-213 and Actinium-225: Meeting Future Demand. J. Label. Compd. Radiopharm 2019, 62, 794–802. [DOI] [PubMed] [Google Scholar]
- (15).Hu A; Brown V; MacMillan SN; Radchenko V; Yang H; Wharton L; Ramogida CF; Wilson JJ Chelating the Alpha Therapy Radionuclides 225Ac3+ and 213Bi3+ with 18-Membered Macrocyclic Ligands Macrodipa and Py-Macrodipa. Inorg. Chem 2022, 61, 801–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Shannon RD Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr., Sect. A: Found. Adv 1976, 32, 751–767. [Google Scholar]
- (17).Frisch MJ; Trucks GW; Schlegel HB; Scuseria GE; Robb MA; Cheeseman JR; Scalmani G; Barone V; Petersson GA; Nakatsuji H; Li X; Caricato M; Marenich AV; Bloino J; Janesko BG; Gomperts R; Mennucci B; … Fox DJ Gaussian 09, Revision D. 01 Gaussian Inc.: Wallingford, CT: 2009. [Google Scholar]
- (18).Chai J-D; Head-Gordon M Systematic Optimization of Long-Range Corrected Hybrid Density Functionals. J. Chem. Phys 2008, 128, 084106. [DOI] [PubMed] [Google Scholar]
- (19).Chai J-D; Head-Gordon M Long-Range Corrected Hybrid Density Functionals with Damped Atom–Atom Dispersion Corrections. Phys. Chem. Chem. Phys 2008, 10, 6615–6620. [DOI] [PubMed] [Google Scholar]
- (20).Hehre WJ; Ditchfield R; Pople JA Self-Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian-Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys 1972, 56, 2257–2261. [Google Scholar]
- (21).Hariharan PC; Pople JA The Influence of Polarization Functions on Molecular Orbital Hydrogenation Energies. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar]
- (22).Dolg M; Stoll H; Savin A; Preuss H Energy-Adjusted Pseudopotentials for the Rare Earth Elements. Theor. Chim. Acta 1989, 75, 173–194. [Google Scholar]
- (23).Marenich AV; Cramer CJ; Truhlar DG Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [DOI] [PubMed] [Google Scholar]
- (24).Regueiro-Figueroa M; Esteban-Gómez D; de Blas A; Rodríguez-Blas T; Platas-Iglesias C Understanding Stability Trends along the Lanthanide Series. Chem. – A Eur. J 2014, 20, 3974–3981. [DOI] [PubMed] [Google Scholar]
- (25).Martell AE; Hancock RD Metal Complexes in Aqueous Solutions; Plenum Press: New York, 1996. [Google Scholar]
- (26).Peters JA; Djanashvili K; Geraldes CFGC; Platas-Iglesias C The Chemical Consequences of the Gradual Decrease of the Ionic Radius along the Ln-Series. Coord. Chem. Rev 2020, 406, 213146. [Google Scholar]
- (27).Gans P; O’Sullivan B GLEE, a New Computer Program for Glass Electrode Calibration. Talanta 2000, 51, 33–37. [DOI] [PubMed] [Google Scholar]
- (28).Gans P; Sabatini A; Vacca A Investigation of Equilibria in Solution. Determination of Equilibrium Constants with the HYPERQUAD Suite of Programs. Talanta 1996, 43, 1739–1753. [DOI] [PubMed] [Google Scholar]
- (29).Gans P; Sabatini A; Vacca A Determination of Equilibrium Constants from Spectrophometric Data Obtained from Solutions of Known pH: the Program pHab. Ann. Chim 1999, 89, 45–49. [Google Scholar]
- (30).Thiele NA; Brown V; Kelly JM; Amor-Coarasa A; Jermilova U; MacMillan SN; Nikolopoulou A; Ponnala S; Ramogida CF; Robertson AKH; Rodríguez-Rodríguez C; Schaffer P; Williams C; Babich JW; Radchenko V; Wilson JJ An Eighteen-Membered Macrocyclic Ligand for Actinium-225 Targeted Alpha Therapy. Angew. Chem., Int. Ed 2017, 56, 14712–14717. [DOI] [PubMed] [Google Scholar]
- (31).Aluicio-Sarduy E; Thiele NA; Martin KE; Vaughn BA; Devaraj J; Olson AP; Barnhart TE; Wilson JJ; Boros E; Engle JW Establishing Radiolanthanum Chemistry for Targeted Nuclear Medicine Applications. Chem. – A Eur. J 2020, 26, 1238–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Reissig F; Bauer D; Ullrich M; Kreller M; Pietzsch J; Mamat C; Kopka K; Pietzsch H-J; Walther M Recent Insights in Barium-131 as a Diagnostic Match for Radium-223: Cyclotron Production, Separation, Radiolabeling, and Imaging. Pharmaceuticals 2020, 13, 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Abou DS; Thiele NA; Gutsche NT; Villmer A; Zhang H; Woods JJ; Baidoo KE; Escorcia FE; Wilson JJ; Thorek DLJ Towards the Stable Chelation of Radium for Biomedical Applications with an 18-Membered Macrocyclic Ligand. Chem. Sci 2021, 12, 3733–3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Fiszbein DJ; Brown V; Thiele NA; Woods JJ; Wharton L; MacMillan SN; Radchenko V; Ramogida CF; Wilson JJ Tuning the Kinetic Inertness of Bi3+ Complexes: The Impact of Donor Atoms on Diaza-18-Crown-6 Ligands as Chelators for 213Bi Targeted Alpha Therapy. Inorg. Chem 2021, 60, 9199–9211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Stasiuk GJ; Long NJ The Ubiquitous DOTA and its Derivatives: the Impact of 1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic Acid on Biomedical Imaging. Chem. Commun 2013, 49, 2732–2746. [DOI] [PubMed] [Google Scholar]
- (36).Cacheris WP; Nickle SK; Sherry AD Thermodynamic study of Lanthanide Complexes of 1,4,7-Triazacyclononane-N,N′,N″-triacetic Acid and 1,4,7,10-Tetraazacyclododecane-N,N′,N″,N‴-tetraacetic Acid. Inorg. Chem 1987, 26, 958–960. [Google Scholar]
- (37).Roca-Sabio A; Mato-Iglesias M; Esteban-Gómez D; Tóth É; de Blas A; Platas-Iglesias C; Rodríguez-Blas T Macrocyclic Receptor Exhibiting Unprecedented Selectivity for Light Lanthanides. J. Am. Chem. Soc 2009, 131, 3331–3341. [DOI] [PubMed] [Google Scholar]
- (38).Thiele NA; MacMillan SN; Wilson JJ Rapid Dissolution of BaSO4 by Macropa, an 18-Membered Macrocycle with High Affinity for Ba2+. J. Am. Chem. Soc 2018, 140, 17071–17078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Hu A; Keresztes I; MacMillan SN; Yang Y; Ding E; Zipfel WR; DiStasio RA; Babich JW; Wilson JJ Oxyaapa: A Picolinate-Based Ligand with Five Oxygen Donors that Strongly Chelates Lanthanides. Inorg. Chem 2020, 59, 5116–5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Grimes TS; Nash KL Acid Dissociation Constants and Rare Earth Stability Constants for DTPA. J. Solution Chem 2014, 43, 298–313. [Google Scholar]
- (41).Moeller T; Thompson LC Observations on the Rare Earths—LXXV: The Stabilities of Diethylenetriaminepentaacetic Acid Chelates. J. Inorg. Nucl. Chem 1962, 24, 499–510. [Google Scholar]
- (42).Pniok M; Kubíček V; Havlíčková J; Kotek J; Sabatie-Gogová A; Plutnar J; Huclier-Markai S; Hermann P Thermodynamic and Kinetic Study of Scandium(III) Complexes of DTPA and DOTA: A Step Toward Scandium Radiopharmaceuticals. Chem. – A Eur. J 2014, 20, 7944–7955. [DOI] [PubMed] [Google Scholar]
- (43).Karle IL Hydrogen Bonds in Molecular Assemblies of Natural, Synthetic and ‘Designer’ Peptides. J. Mol. Struct 1999, 474, 103–112. [Google Scholar]
- (44).Tóth É; Brücher E; Lázár I; Tóth I Kinetics of Formation and Dissociation of Lanthanide(III)–DOTA Complexes. Inorg. Chem 1994, 33, 4070–4076. [Google Scholar]
- (45).van Leeuwen HP; Town RM; Buffle J Impact of Ligand Protonation on Eigen-Type Metal Complexation Kinetics in Aqueous Systems. J. Phys. Chem. A 2007, 111, 2115–2121. [DOI] [PubMed] [Google Scholar]
- (46).van Leeuwen HP; Town RM Outer-Sphere and Inner-Sphere Ligand Protonation in Metal Complexation Kinetics: The Lability of EDTA Complexes. Environ. Sci. Technol 2009, 43, 88–93. [DOI] [PubMed] [Google Scholar]
- (47).Deblonde GJ-P; Zavarin M; Kersting AB The Coordination Properties and Ionic Radius of Actinium: A 120-Year-Old Enigma. Coord. Chem. Rev 2021, 446, 214130. [Google Scholar]
- (48).Pommé S; Marouli M; Suliman G; Dikmen H; Van Ammel R; Jobbágy V; Dirican A; Stroh H; Paepen J; Bruchertseifer F; Apostolidis C; Morgenstern A Measurement of the 225Ac Half-Life. Appl. Radiat. Isot 2012, 70, 2608–2614. [DOI] [PubMed] [Google Scholar]
- (49).Geerlings MW; Kaspersen FM; Apostolidis C; van der Hout R The Feasibility of 225Ac as a Source of α-Particles in Radioimmunotherapy. Nucl. Med. Commun 1993, 14, 121–125. [DOI] [PubMed] [Google Scholar]
- (50).Thiele NA; Wilson JJ Actinium-225 for Targeted α Therapy: Coordination Chemistry and Current Chelation Approaches. Cancer Biother. Radiopharm 2018, 33, 336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Eychenne R; Chérel M; Haddad F; Guérard F; Gestin J-F Overview of the Most Promising Radionuclides for Targeted Alpha Therapy: The “Hopeful Eight”. Pharmaceutics 2021, 13, 906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).McDevitt MR; Ma D; Simon J; Frank RK; Scheinberg DA Design and Synthesis of 225Ac Radioimmunopharmaceuticals. Appl. Radiat. Isot 2002, 57, 841–847. [DOI] [PubMed] [Google Scholar]
- (53).Kadassery KJ; King AP; Fayn S; Baidoo KE; MacMillan SN; Escorcia FE; Wilson JJ H2BZmacropa-NCS: A Bifunctional Chelator for Actinium-225 Targeted Alpha Therapy. Bioconjugate Chem. 2022, 33, 1222–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


