Abstract
Successful endovascular aneurysm repair can be achieved with favorable aortic and iliac arterial anatomies. However, patients with challenging iliac anatomy, such as stenotic, calcified, tortuous arteries, or concomitant iliac artery aneurysms, are commonly encountered. Such a hostile iliac anatomy increases the risk of intraprocedural complications and worsens long-term outcomes. This review addresses various technical options for treating patients with a hostile iliac anatomy, including innovative endovascular solutions, physician-modified endografts, and hybrid procedures. These considerations demonstrate the wide scope of therapies that may be offered to patients with an unfavorable iliac anatomy.
Keywords: Aortic aneurysm, Abdominal, Iliac artery, Endovascular, Stent graft
INTRODUCTION
Abdominal aortic aneurysm (AAA) treatment has significantly advanced over the last 20 years. With ongoing improvements in endograft technology and the growing experience of endovascular specialists, endovascular treatment has become more liberal. Consequently, endovascular aortic aneurysm repair (EVAR) has evolved as the first-line treatment for AAA. EVAR for both elective and ruptured AAAs has led to reduced perioperative morbidity and mortality, as well as shorter hospital stays [1].
The significance of the iliac artery during EVAR includes the delivery of a large and stiff device and proper sealing of aneurysms. Inappropriate distal landing zones remain a major limitation to achieving successful and durable EVAR results. Landing zone problems not only reduce primary technical success but also increase the risk of endoleaks. Up to 15%-40% of AAAs are accompanied by concomitant common iliac artery (CIA) aneurysms [2], and in these cases, endovascular treatment can be difficult because of the need for adequate distal landing zones to prevent type Ib and type II endoleaks [3].
Although the minimum requirement of commercially available devices was different, the clinical practice guidelines of the European Society for Vascular Surgery suggested that the ideal iliac anatomic characteristics for successful EVAR should be iliac luminal diameter >7 mm, angle between the long axis of the aneurysm and the iliac axis <60°, non-extensive circumferential iliac calcification, iliac neck diameter <22 mm, and iliac neck length >15 mm [4]. If preoperative imaging does not reveal these features, additional endovascular devices or techniques are required.
Hostile iliac anatomy consists of iliac tortuosity, stenotic iliac artery, severe calcification, short CIA, and combined iliac artery aneurysms [5]. We addressed various technical options for treating patients with hostile iliac anatomy. Novel endovascular solutions, physician-modified endografts, and hybrid procedures were included to demonstrate the wide scope of treatment that may be offered to patients with unfavorable iliac anatomy.
TORTUOUS ILIAC ARTERY
Tortuous iliac arteries can limit device delivery during EVAR. Previous studies have shown that up to 15% of patients are ineligible for EVAR because of excessive anatomical tortuosity [6]. The presence of tortuosity in the iliac anatomy has been shown to be associated with increased rates of complications, including arterial dissection, rupture, and failure to deploy a stent graft delivery system in patients undergoing EVAR [7,8]. In addition, iliac tortuosity increases the risk of reintervention during follow-up after a successful EVAR [9].
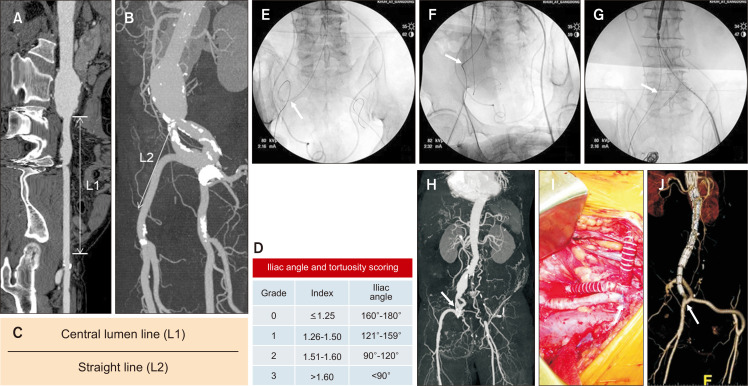
The tortuosity index (TI) was defined as TI=L1/L2 in accordance with the Society of Vascular Surgery Reporting Standards [10]. In the context of iliac tortuosity, L1 was defined as the distance along the central lumen line between the common femoral artery (CFA) and aortic bifurcation, while L2 represented the straight-line distance from the CFA to the aortic bifurcation (Fig. 1A-C) [11]. High iliac tortuosity was defined as TI>1.5, indicating that the true length of the CIA and external iliac artery (EIA) is more than 1.5 times longer than the imaginary straight line from the aortic bifurcation to the CFA owing to severe tortuosity. Fig. 1D demonstrated the iliac angle and tortuosity scoring according to the 4-tier iliac TI and iliac angle, consistent with previous studies demonstrating increased complications following EVAR [12].
Fig. 1.
Strategies for iliac artery tortuosity. (A-C) The iliac tortuosity index was calculated by dividing the centerline lumen length (A) by the straight-line length (B) from the aortic bifurcation to the common femoral artery. (D) Iliac tortuosity was classified with a 4-tier grade according to the iliac tortuosity index and iliac angle. (E-G) Strategy 1: (E) the severely tortuous right iliac artery (arrow) was nearly straightened with the insertion of a superstiff guidewire (F, arrow) and was completely straightened during endograft delivery (G, arrow). (H-J) Strategy 2: (H) the severely tortuous right external iliac artery (arrow) was resected segmentally and re-anastomosed (arrow). (J) Postoperative volume-rendering image showed a shortened right external iliac artery, an external-to-internal iliac bypass graft, and a right-to-left crossover iliofemoral bypass graft (arrow).
Techniques to straighten such arteries include buddy wires, use of large-bore intra-arterial sheaths, tensioning of the arterial tree via brachiofemoral pull-through wires, and manual iliac support via extraperitoneal access [13].
1) Techniques for super-stiff wire advancement
After gaining femoral access, advancing the sheath through a tortuous iliac artery can be challenging, even for experienced interventionists. First, most interventionists might advance a relatively soft guidewire. The soft guidewire is then changed to a stiff guidewire after the advancement of the angiographic supporting catheter. Most of the iliac tortuosity can be overcome by gently pushing a stiff guidewire, as shown in Fig. 1E-G. Despite severe tortuosity in the right iliac artery, a soft guidewire can be passed (Fig. 1E). However, this soft guidewire must be changed to a stiff guidewire to facilitate the delivery of a large introducer sheath and endograft. The gentle push of the stiff guidewire can nearly straighten the iliac artery (Fig. 1F) and completely straighten the artery during endograft delivery (Fig. 1G). If a stiff guidewire cannot be passed with this maneuver, one useful option is the use of a large-bore, long, and flexible sheath, such as the Flexor Ansel sheath (Cook Medical). This approach facilitates the delivery of super-stiff wire because the sheath is typically stiffer than an angiographic catheter. If the previous two techniques fail, another endovascular option is the “body-flossing” technique after brachial artery catheterization [14].
The body-flossing technique can be achieved through the following steps: the guidewire is inserted into the thoracic aorta in an antegrade manner after brachial access. Then, the guidewire inserted from the femoral artery is grasped with a snare wire, which is introduced through the brachial access. The distal guidewire is pulled out through the brachial access site. This pull-through guidewire is straightened by pulling it proximally and distally. It can rescue situations in which a technically successful endograft delivery is impossible. This is particularly relevant for straightening tortuous iliac arteries, enabling retrograde transluminal delivery of the device using a brachiofemoral technique.
2) Hybrid procedure: segmental excision and end-to-end anastomosis
Open surgical procedures are inevitable if all endovascular options fail to advance the stiff guidewire. In addition, if surgical procedures such as bypass surgery are required, the severely tortuous iliac anatomy can be overcome with surgical options, such as resection of the tortuous segment and end-to-end anastomosis. Fig. 1H-J shows the typical surgical procedure. The patient presented with a right CIA aneurysm, a severely tortuous right EIA, and long-segment occlusion of the left common, internal, and external iliac arteries. Revascularization of the right internal iliac artery (IIA) was required to preserve pelvic flow. Therefore, we resected the tortuous segment of the right EIA and performed EVAR using a unibody endograft. Right external-to-internal iliac and right-to-left iliofemoral bypasses were also performed. The details of this procedure have been previously reported [15].
STENOTIC ILIAC ARTERY OR SEVERE CALCIFICATION
Due to the overlapping risk factors for AAA and atherosclerosis, iliac artery stenosis or occlusion is highly prevalent in patients with AAA. Up to 30% of patients with aortic aneurysms present with relevant stenosis of the iliac arteries [5,16]. An excessive plaque burden in the iliac arteries can make it difficult or even impossible to advance the delivery sheath or endograft, especially when combined with tortuosity. Occlusion of the residual lumen of a pre-existing stenotic lesion by the sheath during EVAR can cause temporary lower-limb malperfusion, posing a risk of post-procedural compartment syndrome. Regarding long-term complications, patients with calcified iliac artery lesions face a higher risk of limb occlusion after EVAR, and this risk is further heightened by tortuosity [17,18]. For patients with stenotic or severely calcified iliac arteries, the liberal use of a low-profile endograft or an aorto-uni-iliac device, along with femorofemoral bypass, is an excellent option. However, if these types of endografts are unavailable, the following endovascular options are valuable.
1) Balloon dilatation and stenting
Endovascular specialists attempt to implement endovascular solutions even in patients with heavily calcified and stenotic iliac arteries. This can include balloon dilatation with or without stent implantation, the use of a special dilator designed to dilate the iliac axis, or the use of a specially designed sheath. Stepwise dilatation of narrow iliac vessels can be carefully performed using endovascular dilatators [19]. In isolated short stenosis, transluminal angioplasty is sufficient for sheath delivery. If this approach fails, additional stenting should be considered.
2) Endoconduit technique
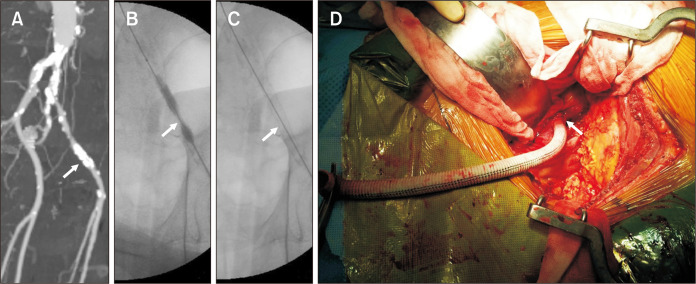
In cases with eccentric, heavily calcified stenosis with a high risk of rupture during forced angioplasty, the “paving and cracking” approach can be employed. After a covered stent is implanted over the total length of the stenosis (paving), it is maximally dilated to the necessary diameter (cracking). Even if the vessel ruptures, hemorrhage can be prevented by a previously implanted covered stent. Most endovascular specialists refer to this as the “endoconduit technique” [20]. In our center, we used the Viabahn-covered stent. Fig. 2A-C shows a severely calcified stenotic EIA in a patient with an AAA. After an 8×100 mm Viabahn stent graft (WL Gore and Associates) was placed in the calcified lesion, balloon dilatation was performed using a high-pressure balloon catheter. Despite an artificial rupture in the EIA, massive bleeding was avoided owing to the use of a covered stent. Fig. 2C shows the creation of a widely open lumen in the EIA. Asciutto et al. [20] reported 19 patients who underwent EVAR with an endoconduit. Fourteen (73.7%) had type D Trans-Atlantic Inter-Society Consensus lesions. Endoconduit creation was technically successful in all cases, and all endoconduits were patent after the completion of EVAR. One patient required two endovascular reinterventions at 1 and 5 years postoperatively to restore the patency of the endoconduit. No open reinterventions related to the endoconduit were necessary. After a median follow-up period of 17 months, the primary assisted patency rate for endoconduits was 88.9%.
Fig. 2.
Strategies for severely calcified stenotic iliac arteries. (A-C) Internal endoconduit: (A) a severely calcified stenotic external iliac artery (arrow) was seen in a patient with an abdominal aortic aneurysm. (B) After an 8×100 mm Viabahn stent graft (WL Gore and Associates) was placed in the calcified lesion (arrow), balloon dilatation was performed using a high-pressure balloon catheter. (C) The widely opened lumen (arrow) was created in the external iliac artery. (D) Temporary surgical conduit: a 10 mm Dacron graft (arrow) was attached to the calcium-free iliac artery using a retroperitoneal approach.
3) Surgical iliac conduit
Notwithstanding the “endovascular first” approach, hybrid procedures remain safe and effective for patients with excessive calcification of the iliac arteries, who are ineligible for standard endograft implantation. Via retroperitoneal access and iliac axis exposure, an iliac conduit using regular bypass grafts with a diameter of 8-10 mm is implanted through an end-to-side anastomosis to the distal common or the proximal EIA [21]. Using this conduit, the stent graft can be safely implanted without risking perforation or arterial thrombosis in the calcified lesions. At our center, we use a 10 mm Dacron graft to create a temporary iliac conduit (Fig. 2D). Preoperative evaluation should include the evaluation of the anastomotic region and the selection of the vessel segment with the least calcification. After excluding the aneurysm and removing the sheath, the conduit is transected and occluded using a continuous suture close to the initial anastomotic site. The conduit may serve as a graft for an iliofemoral bypass in patients with symptomatic claudication caused by stenosis or occlusion of the ipsilateral EIA. In this case, the proximal anastomosis is retained, and the bypass is tunneled to the groin and anastomosed with the CFA [21]. Rowse et al. [22] analyzed 137 patients who underwent surgical iliac conduit placement. Perioperative morbidities included a return to the operating room in 5 patients (5.6%) for bleeding (n=4) and graft thrombosis (n=1), 4 myocardial infarctions (2.8%), 5 episodes of respiratory failure (3.5%), 12 wound complications (8.3%), and 7 renal injuries (4.9%); of these, 3 patients progressed to dialysis (2.1%). They concluded that a surgical iliac conduit is a safe and viable option for EVAR in high-risk patients with challenging iliac anatomies.
Giannopoulos et al. [23] conducted a meta-analysis of iliac conduits, including 14 studies using random-effects modeling. The incidence of periprocedural adverse events was gauged based on iliac conduit versus non-conduit cases and planned versus unplanned iliac conduit placement. Outcomes of interest included the length of hospital stay, morbidity and mortality associated with conduits, and all-cause mortality. Iliac conduits, either surgical conduits or endoconduits, were used in 17% of the 16,855 cases, with a technical success rate of 94%. Periprocedural complications occurred in 32% of cases, with an overall bleeding complication rate of 10%. Females with a positive history of smoking, pulmonary disease, and peripheral artery disease at baseline showed a higher frequency of iliac conduit use. Conduit use was associated with longer hospitalization, higher periprocedural all-cause mortality (odds ratio [OR], 2.85; 95% confidence interval [CI], 1.75-4.64; P<0.001), and bleeding complication rate (OR, 2.38; 95% CI, 1.58-3.58; P<0.001).
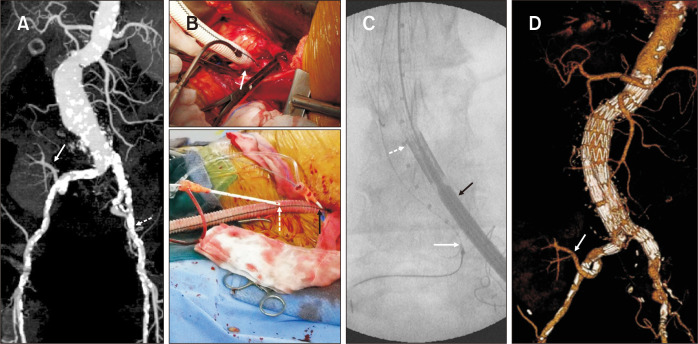
At our center, we encountered the most hostile iliac arteries in patients with AAA (Fig. 3). A 67-year-old female presented with an asymptomatic 6.5 cm-sized infrarenal AAA. Nineteen years previously, she underwent renal transplantation for renal failure secondary to diabetes and hypertension. The maximal intensity projection image of the preoperative computed tomographic angiography showed an AAA, the renal artery of the functioning transplanted kidney originating from the right IIA, and the heavily calcified left EIA (Fig. 3A). After the creation of the iliac conduit on the left iliac bifurcation using a 10 mm Dacron graft (Fig. 3B), the AAA was repaired with a Zenith bifurcated stent graft (Cook Medical). A 28×117 mm Zenith main body device was placed through the iliac conduit (Fig. 3C). After contralateral passage of the guidewire, we attempted to deliver the stiff wire. However, this approach failed due to previous surgical scarring and severe calcification. The guidewire was passed after snaring the wire, which was inserted through the left brachial artery (Fig. 3C). We were able to successfully complete the EVAR procedure using a surgical endoconduit and body flossing technique (Fig. 3D) [24].
Fig. 3.
Novel strategy for the severely calcified bilateral iliac arteries with transplant kidney. (A) The preoperative maximal intensity projection image showed the abdominal aortic aneurysm and renal artery of a functioning transplanted kidney originating from the right internal iliac artery (arrow). Bilateral iliac arteries showed diffuse, severely calcified stenosis (dotted arrow). (B) (Upper) A 10 mm Dacron graft was anastomosed to the less calcified portion of the iliac artery (arrow). (Lower) Two 7F introducer sheaths (dotted and black arrows) were directly inserted into the graft for the insertion of a sizing pigtail catheter and endograft, respectively. (C) The endograft main body (black arrow) and a sizing pigtail catheter (dotted arrow) were inserted through the temporary conduit. Snaring was successfully used to cannulate the contralateral gate (white arrow). (D) The postoperative volume-rendering image showed no endoleak and a well-perfused transplanted kidney (arrow).
CONCOMITANT ILIAC ARTERY ANEURYSMS
In approximately 20%-30% of patients with AAA, at least one iliac artery is affected by aneurysmal dilatation (aortoiliac aneurysms) [25,26]. While these patients were traditionally considered contraindicated for EVAR in the past, there are now several technical approaches for the successful endovascular treatment of concomitant iliac artery aneurysms. Here, we address various endovascular options for these hostile conditions.
1) Extension of limb endograft after internal iliac artery embolization
CIA aneurysms can be treated endovascularly by shifting the distal landing zone towards the EIA. However, endovascular treatment requires sacrificing the IIA. It can be occluded by coiling or using an occlusion device, such as a vascular plug, or overstented without additional intervention. A disadvantage of this procedure is the possibility of ischemic symptoms occurring in the region supplied by the IIA. Occlusion of the IIA can have severe consequences, including colonic, spinal cord, and buttock ischemia. Minor complications, such as buttock claudication or sexual dysfunction, are more common [27,28]. Although severe complications are rare (approximately 3%), the incidence rate of buttock claudication is approximately 30% [29]. In approximately 50% of patients with buttock claudication, the symptoms resolve likely because of collateral formation in the pelvic plexus [29].
2) Bell-bottom technique
Ectatic CIAs can be treated using large-diameter flared endograft limbs or other large-diameter endografts positioned just before the iliac bifurcation. This allows the exclusion of aneurysms with sufficient distal sealing in anatomically suitable patients without disturbing the IIA perfusion. However, vigilant surveillance is required for the development of type Ib endoleaks [30].
3) Use of iliac-branched device
The development of iliac branch device (IBD) has allowed complete endovascular treatment of one or both IIAs in most cases, using an on-label option specifically designed for this purpose (Fig. 4A). Techniques for IBD implantation require a comprehensive inventory of endovascular tools, with a wide variety of guidewires, sheaths, catheters, balloons, and stents. While tools may vary depending on physician preference and local availability, a few are essential for procedural success. According to the instructions, through-and-through femoral access is required to snare the guidewire and guide the sheath through the device and into the side branch that exits to the IIA [31]. For successful IBD placement and to prevent late complications related to IBD, such as type Ic endoleak, it is vital to adhere to the instructions for use (IFU) of IBD [32]. However, IFU for IBD may not apply to a significant proportion of patients [33,34]. In addition, if the patient has a long CIA and large IIA diameter, a trifurcation technique, similar to IBD, can be a viable endovascular option [35].
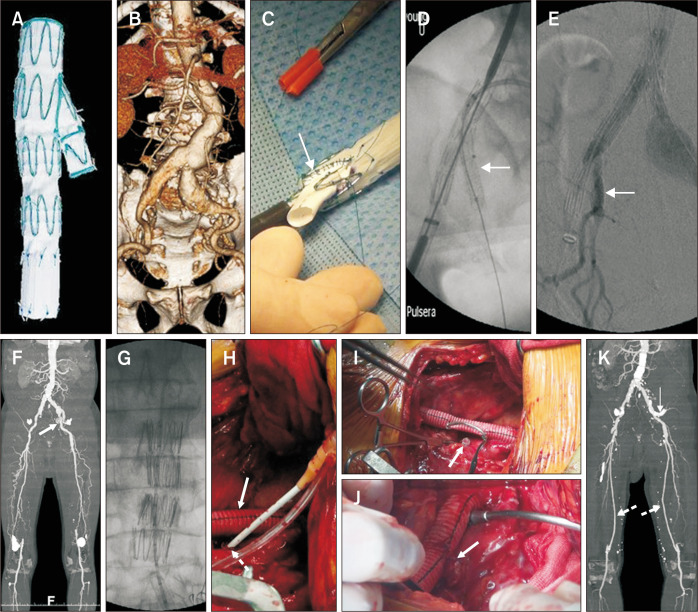
Fig. 4.
Strategies for the concomitant iliac aneurysms. (A) Commercially available iliac branch device (IBD). (B-E) A physician-modified IBD was used to treat a patient with an abdominal aortic aneurysm and concomitant bilateral common iliac artery aneurysms (B). (C) A polytetrafluoroethylene graft measuring 7×20 mm was attached to the deployed limb endograft at the back table (arrow). (D) After introducing the physician-modified IBD, an 8 mm covered stent was inserted into the internal iliac artery (arrow). (E) Completion angiography showed no type Ib or type II endoleak and a patent internal iliac stent (arrow). (F) A 70-year-old male presented with a saccular abdominal aortic aneurysm, bilateral external iliac artery stenosis, occluded right internal and bilateral superficial femoral arteries, and a concomitant left internal iliac artery aneurysm (arrow). (G) An aortic extender cuff was used to treat a saccular abdominal aortic aneurysm. (H) An 8 mm Dacron graft was anastomosed to the left common iliac artery in an end-to-end fashion. This temporary conduit was used to deliver the aortic extender cuff, which was anastomosed to the distal external iliac artery (arrow). A 7F introducer sheath was inserted in the left hypogastric aneurysm sac for inserting the covered stent (dotted arrow). (I) The Dacron bypass graft was partially clamped with a Satinsky clamp, and the inserted Viabahn covered stent was clamped (arrow). (J) The Dacron graft-Viabahn anastomosis was performed in an end-to-side manner (arrow). (K) Postoperative maximal intensity image demonstrated no endoleak, a patent hypogastric stent (arrow), and a patent bilateral femoropopliteal vein bypass graft (dotted arrows).
4) Physician-modified iliac-branched device
Unless a commercially available IBD is accessible, a physician-modified IBD can be created on the back table. In our center, we treated a patient with AAA and bilateral CIA aneurysms (Fig. 4B). Fig. 4C-E shows these details. A polytetrafluoroethylene graft measuring 7×20 mm was attached to the deployed limb endograft on the back table. After introducing the physician-modified IBD, an 8 mm covered stent was inserted into the IIA. Completion angiography revealed the absence of type Ib or II endoleaks and confirmed the patency of internal iliac stent.
5) Hybrid procedure: distal ‘sutureless’ anastomosis for IIA preservation
If a patient requires an open surgical procedure, a hybrid procedure can be used to preserve pelvic circulation and exclusion. At our center, we performed hybrid repair of the AAA and bilateral iliac artery aneurysms (Fig. 4F-K). A 70-year-old male presented with a saccular AAA, right internal and bilateral superficial femoral artery occlusion, and a concomitant left IIA aneurysm. An aortic extender cuff was used to treat the saccular AAA. An 8 mm Dacron graft was anastomosed end-to-end to the left CIA. This temporary conduit was used to deliver the aortic extender cuff, which was then anastomosed to the distal EIA. A 7F introducer sheath was inserted into the left IIA aneurysm sac to insert the covered stent. The Dacron bypass graft was partially clamped with a Satinsky clamp. The inserted Viabahn-covered stent is clamped (arrow). Dacron graft-Viabahn anastomosis was performed in an end-to-side fashion. Postoperative maximal intensity imaging revealed no endoleaks, patent hypogastric stents, and patent bilateral femoropopliteal vein bypass grafts.
6) Parallel graft technique
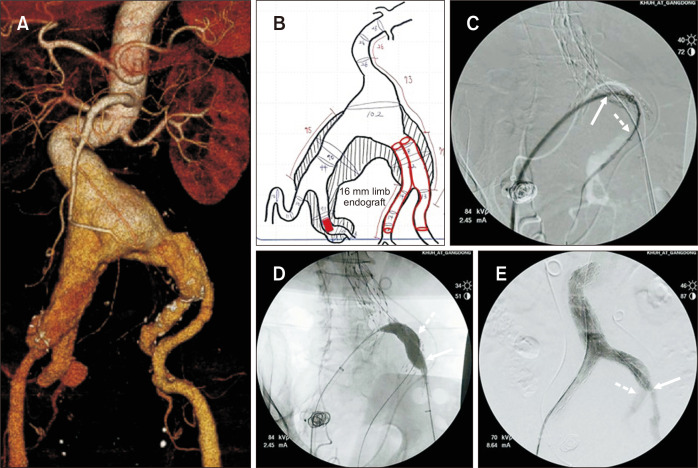
For short-necked aortic aneurysms, parallel graft technique such as chimneys has been established as a technical alternative to custom-made fenestrated grafts [36]. This technique, applied to iliac arteries (also called the double-barrel or sandwich technique), was first described by Lobato and Camacho-Lobato [37]. After implantation of the main EVAR graft, a covered stent graft is advanced into the IIA via a contralateral femoral or transbrachial approach. The iliac limb extension is advanced through an ipsilateral femoral approach, and both stent grafts are deployed. This technique allows the preservation of the IIA flow using standard endovascular grafts without the use of an IBD. For this procedure, the landing zone diameter of the IIA should ideally be less than 10 mm because of the availability of covered stents. In cases where the landing zone diameter of IIA exceeds 10 mm, the “parallel limb endograft technique” can be an alternative option. At our center, we use this technique for a very complicated hostile neck and iliac artery anatomy (Fig. 5). An 89-year-old male presented with a large AAA and bilateral CIA aneurysms. Preoperative measurements and the planning sheet showed that the diameter of the left IIA was 14 mm. To preserve unilateral pelvic flow, embolization of the right IIA and parallel limb endograft placement in the left external and internal iliac arteries were planned. A completion angiogram showed no endoleaks and patent endografts in the left external and internal iliac arteries. When the parallel graft technique is planned, post-deployment gutter leakage is a primary concern. To prevent gutter leakage, it is crucial to have a sufficient length of parallel graft within the iliac limb (>5 cm) [38]. Additionally, selection of the proper diameter of the covered stent and endograft limb is crucial and it is recommended to determine this diameter by calculating the arterial cross-sectional area [39].
Fig. 5.
Parallel limb endograft technique. (A) An 89-year-old male presented with a huge abdominal aortic aneurysm and bilateral common and internal iliac artery aneurysms. (B) The preoperative measuring and planning sheet demonstrated that the diameter of the left internal iliac artery was 14 mm. To preserve unilateral pelvic flow, embolization of the right internal iliac artery and parallel limb endograft placement in the left external and internal iliac arteries were planned. (C) After placement of the AFX (Endologix) main body (diameter=28 mm), a 14F introducer sheath was inserted with the up-and-over technique (arrow). Thereafter, a 16 mm limb endograft was inserted into the left internal iliac artery (dotted arrow). Subsequently, a 13 mm limb endograft was inserted into the left external iliac artery. (D) Kissing balloon angioplasty was performed to fix all endografts that were inserted into the left external (arrow) and internal (dotted arrow) iliac arteries. (E) Completion angiography showed no endoleak and patent endografts that were inserted into the left external (arrow) and internal (dotted arrow) iliac arteries.
Another endovascular option for preserving pelvic flow is the “crossover chimney technique” [40]. Under bilateral CFA approach, a crossover covered stent was inserted from the contralateral CFA to the ipsilateral IIA involved in the CIA aneurysm. A covered stent was positioned 2 cm inside the IIA. The main body endograft was inserted through the ipsilateral CFA with distal sealing in the EIA. The gate was cannulated, and the limb extension was positioned in the contralateral CIA near the IIA orifice. Wu et al. [40] reported no acute branch occlusion or type I endoleak from the IIA or chimney graft gutters on imaging studies for up to 6 months. Although this technique is easy to use and avoids brachial access, as in the sandwich technique, it incurs additional costs.
7) Bilateral IIA occlusion
Exclusion of the IIA to prevent type Ib or II endoleaks is usually required when the aneurysm involves either the distal CIA or IIA itself. Although ipsilateral buttock claudication and erectile dysfunction have been reported in up to 40% of patients after unilateral IIA embolization, these symptoms tend to improve and abate over time. Bilateral IIA occlusion with endograft extension into both EIAs is occasionally required in high-risk patients when there is no distal fixation zone in either bilateral CIA or the aneurysm involves both common and internal iliac arteries.
CONCLUSION
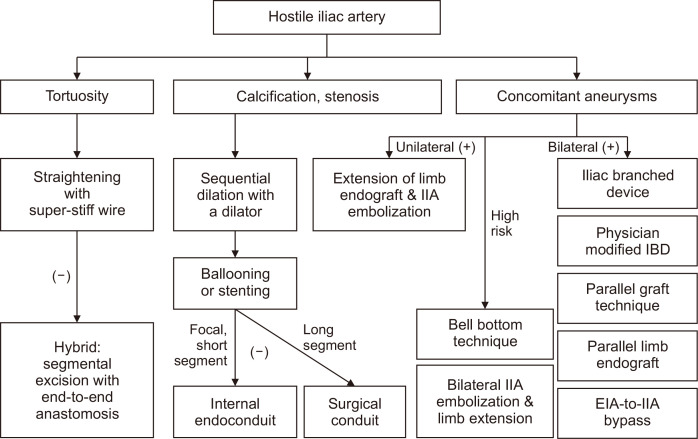
The versatility of current devices has allowed extended application to complex cases but must be considered carefully in difficult anatomies. Fig. 6 demonstrates the algorithm for applying various options for overcoming a hostile iliac anatomy. Adequate preoperative assessment and intraoperative manipulation are paramount for achieving success.
Fig. 6.
Algorithm to overcome hostile iliac arteries during endovascular aortic aneurysm repair. IIA, internal iliac artery; IBD, iliac branch device; EIA, external iliac artery.
Funding Statement
FUNDING None.
Footnotes
CONFLICTS OF INTEREST
The author has nothing to disclose.
REFERENCES
- 1.Dansey KD, Varkevisser RRB, Swerdlow NJ, Li C, de Guerre LEVM, Liang P, et al. Epidemiology of endovascular and open repair for abdominal aortic aneurysms in the United States from 2004 to 2015 and implications for screening. J Vasc Surg. 2021;74:414–424. doi: 10.1016/j.jvs.2021.01.044. https://doi.org/10.1016/j.jvs.2021.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naughton PA, Park MS, Kheirelseid EA, O'Neill SM, Rodriguez HE, Morasch MD, et al. A comparative study of the bell-bottom technique vs hypogastric exclusion for the treatment of aneurysmal extension to the iliac bifurcation. J Vasc Surg. 2012;55:956–962. doi: 10.1016/j.jvs.2011.10.121. https://doi.org/10.1016/j.jvs.2011.10.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hwang D, Kim HK, Huh S. Incidence and risk factors for sac expansion after endovascular aneurysm repair of abdominal aortic aneurysms. Vasc Spec Int. 2021;37:34. doi: 10.5758/vsi.210035. https://doi.org/10.5758/vsi.210035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moll FL, Powell JT, Fraedrich G, Verzini F, Haulon S, Waltham M, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg. 2011;41 Suppl 1:S1–S58. doi: 10.1016/j.ejvs.2010.09.011. https://doi.org/10.1016/j.ejvs.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Bischoff MS, Peters AS, Meisenbacher K, Böckler D. Challenging access in endovascular repair of infrarenal aortic aneurysms. J Cardiovasc Surg. 2014;55:75–83. [PubMed] [Google Scholar]
- 6.Gaudric J, Tresson P, Derycke L, Tezenas Du Montcel S, Couture T, Davaine JM, et al. Surgical internal iliac artery preservation associated with endovascular repair of infrarenal aortoiliac aneurysms to avoid buttock claudication and distal type I endoleaks. J Vasc Surg. 2018;68:1736–1743. doi: 10.1016/j.jvs.2018.03.416. https://doi.org/10.1016/j.jvs.2018.03.416. [DOI] [PubMed] [Google Scholar]
- 7.Coulston J, Baigent A, Selvachandran H, Jones S, Torella F, Fisher R. The impact of endovascular aneurysm repair on aortoiliac tortuosity and its use as a predictor of iliac limb complications. J Vasc Surg. 2014;60:585–589. doi: 10.1016/j.jvs.2014.03.279. https://doi.org/10.1016/j.jvs.2014.03.279. [DOI] [PubMed] [Google Scholar]
- 8.Taudorf M, Jensen LP, Vogt KC, Grønvall J, Schroeder TV, Lönn L. Endograft limb occlusion in EVAR: iliac tortuosity quantified by three different indices on the basis of preoperative CTA. Eur J Vasc Endovasc Surg. 2014;48:527–533. doi: 10.1016/j.ejvs.2014.04.018. https://doi.org/10.1016/j.ejvs.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 9.Saldana-Ruiz N, Tachida A, Mossman A, Cure R, Larimore A, Dansey K, et al. Iliac tortuosity increases reinterventions but not adverse outcomes following repair of juxtarenal aneurysms using physician-modified endografts. J Vasc Surg. 2023 doi: 10.1016/j.jvs.2023.10.053. https://doi.org/10.1016/j.jvs.2023.10.053. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Oderich GS, Forbes TL, Chaer R, Davies MG, Lindsay TF, Mastracci T, et al. Reporting standards for endovascular aortic repair of aneurysms involving the renal-mesenteric arteries. J Vasc Surg. 2021;73:4S–52S. doi: 10.1016/j.jvs.2020.06.011. https://doi.org/10.1016/j.jvs.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Chaikof EL, Fillinger MF, Matsumura JS, Rutherford RB, White GH, Blankensteijn JD, et al. Identifying and grading factors that modify the outcome of endovascular aortic aneurysm repair. J Vasc Surg. 2002;35:1061–1066. doi: 10.1067/mva.2002.123991. https://doi.org/10.1067/mva.2002.123991. [DOI] [PubMed] [Google Scholar]
- 12.Lee K, Leci E, Forbes T, Dubois L, DeRose G, Power A. Endograft conformability and aortoiliac tortuosity in endovascular abdominal aortic aneurysm repair. J Endovasc Ther. 2014;21:728–734. doi: 10.1583/14-4663MR.1. https://doi.org/10.1583/14-4663MR.1. [DOI] [PubMed] [Google Scholar]
- 13.Chaudhuri A. Using a large bore sheath to untwist tortuous iliac arteries at EVAR: a simple and effective technique. Eur J Vasc Endovasc Surg. 2019;57:433. doi: 10.1016/j.ejvs.2018.08.039. https://doi.org/10.1016/j.ejvs.2018.08.039. [DOI] [PubMed] [Google Scholar]
- 14.Criado FJ, Wilson EP, Abul-Khoudoud O, Barker C, Carpenter J, Fairman R. Brachial artery catheterization to facilitate endovascular grafting of abdominal aortic aneurysm: safety and rationale. J Vasc Surg. 2000;32:1137–1141. doi: 10.1067/mva.2000.109335. https://doi.org/10.1067/mva.2000.109335. [DOI] [PubMed] [Google Scholar]
- 15.Joh JH, Park HC. Reconstruction of the internal iliac artery in patients with aneurysmal disease: two case reports. Exp Ther Med. 2014;7:579–582. doi: 10.3892/etm.2013.1459. https://doi.org/10.3892/etm.2013.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henretta JP, Karch LA, Hodgson KJ, Mattos MA, Ramsey DE, McLafferty R, et al. Special iliac artery considerations during aneurysm endografting. Am J Surg. 1999;178:212–218. doi: 10.1016/S0002-9610(99)00156-7. https://doi.org/10.1016/s0002-9610(99)00156-7. [DOI] [PubMed] [Google Scholar]
- 17.Mantas GK, Antonopoulos CN, Sfyroeras GS, Moulakakis KG, Kakisis JD, Mylonas SN, et al. Factors predisposing to endograft limb occlusion after endovascular aortic repair. Eur J Vasc Endovasc Surg. 2015;49:39–44. doi: 10.1016/j.ejvs.2014.09.012. https://doi.org/10.1016/j.ejvs.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Moulakakis KG, Antonopoulos CN, Klonaris C, Kakisis J, Lazaris AM, Sfyroeras GS, et al. Bilateral endograft limb occlusion after endovascular aortic repair: predictive factors of occurrence. Ann Vasc Surg. 2018;46:299–306. doi: 10.1016/j.avsg.2017.07.019. https://doi.org/10.1016/j.avsg.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 19.Etkin Y, Baig A, Foley PJ, Wang GJ, Woo EY, Carpenter JP, et al. Management of difficult access during endovascular aneurysm repair. Ann Vasc Surg. 2017;44:77–82. doi: 10.1016/j.avsg.2017.03.190. https://doi.org/10.1016/j.avsg.2017.03.190. [DOI] [PubMed] [Google Scholar]
- 20.Asciutto G, Aronici M, Resch T, Sonesson B, Kristmundsson T, Dias NV. Endoconduits with "pave and crack" technique avoid open ilio-femoral conduits with sustainable mid-term results. Eur J Vasc Endovasc Surg. 2017;54:472–479. doi: 10.1016/j.ejvs.2017.07.010. https://doi.org/10.1016/j.ejvs.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Peterson BG, Matsumura JS. Tips and tricks for avoiding access problems when using large sheath endografts. J Vasc Surg. 2009;49:524–527. doi: 10.1016/j.jvs.2008.11.047. https://doi.org/10.1016/j.jvs.2008.11.047. [DOI] [PubMed] [Google Scholar]
- 22.Rowse JW, Morrow K, Bena JF, Eagleton MJ, Parodi FE, Smolock CJ. Iliac conduits remain safe in complex endovascular aortic repair. J Vasc Surg. 2019;70:424–431. doi: 10.1016/j.jvs.2018.10.099. https://doi.org/10.1016/j.jvs.2018.10.099. [DOI] [PubMed] [Google Scholar]
- 23.Giannopoulos S, Malgor RD, Sobreira ML, Siada SS, Rodrigues D, Al-Musawi M, et al. Iliac conduits for endovascular treatment of aortic pathologies: a systematic review and meta-analysis. J Endovasc Ther. 2021;28:499–509. doi: 10.1177/15266028211007468. https://doi.org/10.1177/15266028211007468. [DOI] [PubMed] [Google Scholar]
- 24.Joh JH, Nam DH, Park HC. Endovascular abdominal aortic aneurysm repair in patients with renal transplant. J Korean Surg Soc. 2013;84:189–193. doi: 10.4174/jkss.2013.84.3.189. https://doi.org/10.4174/jkss.2013.84.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffin CL, Scali ST, Feezor RJ, Chang CK, Giles KA, Fatima J, et al. Fate of aneurysmal common iliac artery landing zones used for endovascular aneurysm repair. J Endovasc Ther. 2015;22:748–759. doi: 10.1177/1526602815602121. https://doi.org/10.1177/1526602815602121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee WA, Nelson PR, Berceli SA, Seeger JM, Huber TS. Outcome after hypogastric artery bypass and embolization during endovascular aneurysm repair. J Vasc Surg. 2006;44:1162–1168. discussion 1168–1169. doi: 10.1016/j.jvs.2006.08.047. https://doi.org/10.1016/j.jvs.2006.08.047. [DOI] [PubMed] [Google Scholar]
- 27.Bosanquet DC, Wilcox C, Whitehurst L, Cox A, Williams IM, Twine CP British Society of Endovascular therapy (BSET), author Systematic review and meta-analysis of the effect of internal iliac artery exclusion for patients undergoing EVAR. Eur J Vasc Endovasc Surg. 2017;53:534–548. doi: 10.1016/j.ejvs.2017.01.009. https://doi.org/10.1016/j.ejvs.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Rayt HS, Bown MJ, Lambert KV, Fishwick NG, McCarthy MJ, London NJ, et al. Buttock claudication and erectile dysfunction after internal iliac artery embolization in patients prior to endovascular aortic aneurysm repair. Cardiovasc Interv Radiol. 2008;31:728–734. doi: 10.1007/s00270-008-9319-3. https://doi.org/10.1007/s00270-008-9319-3. [DOI] [PubMed] [Google Scholar]
- 29.Bratby MJ, Munneke GM, Belli AM, Loosemore TM, Loftus I, Thompson MM, et al. How safe is bilateral internal iliac artery embolization prior to EVAR? Cardiovasc Interv Radiol. 2008;31:246–253. doi: 10.1007/s00270-007-9203-6. https://doi.org/10.1007/s00270-007-9203-6. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Liang S, Xu X, Chen B, Jiang J, Shi Z, et al. A comparative study of the efficacy by using different stent grafts in bell-bottom technique for the treatment of abdominal aortic aneurysm concomitant with iliac artery aneurysm. Ann Vasc Surg. 2018;52:41–48. doi: 10.1016/j.avsg.2018.05.030. https://doi.org/10.1016/j.avsg.2018.05.030. [DOI] [PubMed] [Google Scholar]
- 31.D'Oria M, Mastrorilli D, DeMartino R, Lepidi S. Current status of endovascular preservation of the internal iliac artery with iliac branch devices (IBD) Cardiovasc Interv Radiol. 2019;42:935–948. doi: 10.1007/s00270-019-02199-5. https://doi.org/10.1007/s00270-019-02199-5. [DOI] [PubMed] [Google Scholar]
- 32.Kim HJ, Yun WS, Kim HK. Type ic endoleak after lifestream balloon-expandable stent graft and zenith iliac branch device placement. Vasc Spec Int. 2023;39:2. doi: 10.5758/vsi.230002. https://doi.org/10.5758/vsi.230002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muzepper M, Zhou M. Anatomic suitability of iliac branched devices for Chinese patients with abdominal-iliac aortic aneurysm. Ann Vasc Surg. 2020;67:178–184. doi: 10.1016/j.avsg.2020.03.010. https://doi.org/10.1016/j.avsg.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Itoga NK, Fujimura N, Hayashi K, Obara H, Shimizu H, Lee JT. Outcomes of endovascular repair of aortoiliac aneurysms and analyses of anatomic suitability for internal iliac artery preserving devices in Japanese patients. Circ J. 2017;81:682–688. doi: 10.1253/circj.CJ-16-1109. https://doi.org/10.1253/circj.CJ-16-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casey K, Al-Khatib WK, Zhou W. Hypogastric artery preservation during aortoiliac aneurysm repair. Ann Vasc Surg. 2011;25:133.e1–8. doi: 10.1016/j.avsg.2010.06.008. https://doi.org/10.1016/j.avsg.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 36.Donas KP, Lee JT, Lachat M, Torsello G, Veith FJ PERICLES investigators, author. Collected world experience about the performance of the snorkel/chimney endovascular technique in the treatment of complex aortic pathologies: the PERICLES registry. Ann Surg. 2015;262:546–553. discussion 552–553. doi: 10.1097/SLA.0000000000001405. https://doi.org/10.1097/SLA.0000000000001405. [DOI] [PubMed] [Google Scholar]
- 37.Lobato AC, Camacho-Lobato L. The sandwich technique to treat complex aortoiliac or isolated iliac aneurysms: results of midterm follow-up. J Vasc Surg. 2013;57:26S–34S. doi: 10.1016/j.jvs.2012.09.081. https://doi.org/10.1016/j.jvs.2012.09.081. [DOI] [PubMed] [Google Scholar]
- 38.Kim D, Chung JK, Park HS, Jung IM, Lee T. Early experiences of sandwich technique to preserve pelvic circulation during endovascular aneurysm repair. Vasc Spec Int. 2017;33:72–80. doi: 10.5758/vsi.2017.33.2.72. https://doi.org/10.5758/vsi.2017.33.2.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pang H, Chen Y, He X, Zeng Q, Ye P. Selection of stents by calculation of arterial cross-sectional area in modified sandwich technique for complex aortoiliac arterial lesions. Ann Vasc Surg. 2019;58:108–114. doi: 10.1016/j.avsg.2018.10.039. https://doi.org/10.1016/j.avsg.2018.10.039. [DOI] [PubMed] [Google Scholar]
- 40.Wu IH, Chan CY, Chen YS, Huang SC, Wang SS, Chi NH. Crossover chimney technique to preserve the internal iliac artery in abdominal aortic aneurysm with common iliac artery aneurysms. J Endovasc Ther. 2013;20:298–302. doi: 10.1583/13-4219R.1. https://doi.org/10.1583/13-4219R.1. [DOI] [PubMed] [Google Scholar]