Abstract
Cyclin E, a partner of the cyclin-dependent kinase Cdk2, has been implicated in positive control of the G1/S phase transition. Whereas degradation of cyclin E has been shown to be exquisitely regulated by ubiquitination and proteasomal action, little is known about posttranscriptional aspects of its biogenesis. In a yeast-based screen designed to identify human proteins that interact with human cyclin E, we identified components of the eukaryotic cytosolic chaperonin CCT. We found that the endogenous CCT complex in yeast was essential for the maturation of cyclin E in vivo. Under conditions of impaired CCT function, cyclin E failed to accumulate. Furthermore, newly translated cyclin E, both in vitro in reticulocyte lysate and in vivo in human cells in culture, is efficiently bound and processed by the CCT. In vitro, in the presence of ATP, the bound protein is folded and released in order to become associated with Cdk2. Thus, both the acquisition of the native state and turnover of cyclin E involve ATP-dependent processes mediated by large oligomeric assemblies.
Human cyclin E was initially identified in a genetic screen by virtue of its ability to rescue a deficiency of G1 cyclin function in the budding yeast Saccharomyces cerevisiae (10, 12). In mammalian cells, cyclin E, in association with its catalytic partner Cdk2 (22), is a positive regulator of the G1-to-S phase transition of the cell cycle (16–19, 30). Yet there is no consensus on the identity of the critical targets of cyclin E-Cdk2 phosphorylation involved in promoting the G1/S phase transition. More is understood concerning the regulation of cyclin E-Cdk2 activity in the cell cycle. Cyclin E-Cdk2 activity can be modulated by the phosphorylation and dephosphorylation of its catalytic partner, Cdk2 (15), as well as by association and dissociation of inhibitor proteins of the Cip-Kip family, which includes p21Cip1, p27Kip1, and p57Kip2 (23). In addition, regulated cyclin E proteolysis, which occurs through the ubiquitin-proteasome pathway, contributes to modulation of cyclin E-Cdk2 activity (2, 31). Specifically, autophosphorylation of cyclin E-Cdk2 complexes at threonine 380 of cyclin E, which occurs when cyclin E-Cdk2 complexes become active, targets cyclin E for ubiquitination and subsequent degradation by the 26S proteasome, a large protease-containing organelle. Thus, cyclin E remains stable as long as cyclin E-Cdk2 complexes are maintained in an inactive state, such as when inhibitor proteins are bound. However, cyclin E is rapidly turned over upon activation of such complexes, presumably allowing for dynamic regulation of the G1/S phase transition.
Here we show that a different large protein assembly also governs the biogenesis of cyclin E, the chaperonin complex known as CCT (chaperonin containing t-complex polypeptide 1) (5, 9, 29). CCT binds newly translated cyclin E and mediates its folding into a form that can associate with Cdk2. This provides another step at which cyclin E protein levels and activity may be regulated.
MATERIALS AND METHODS
Northern blot analysis, yeast cell extracts, and immunotechniques.
Northern blot analysis was performed as previously described (31) with the exception that after autoradiography for cyclin E, the filter was stripped and rehybridized with an actin probe. The preparation of yeast extracts by glass bead lysis, immunotechniques, and histone H1 kinase assays were essentially as previously described (31). Protein concentrations were determined using the Bio-Rad protein assay, and equal amounts of protein were used for Western blot analysis and immunoprecipitation. Proteins were separated by standard sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), blotted to an Immobilon-P membrane (Millipore), and incubated with specific antibodies as indicated. Immunoreactivity was visualized by using enhanced chemiluminescence (Pierce).
In vitro translation.
Cyclin E mRNA was translated in a reticulocyte lysate (Promega) for 6 min at 30°C in the presence of [35S]methionine. Cycloheximide and ribonuclease A were added, and 1 aliquot was chased for 30 min at 30°C with a mixture of 0.4 U of hexokinase/μl and 20 mM glucose (HK/glc) and the other was chased without HK/glc. Samples were then applied to a Superose 12 gel filtration column run in MES buffer (20 mM MES [morpholineethanesulfonic acid] [pH 6.8], 100 mM KCl, 2 mM MgCl2, 1 mM EGTA, and 5% glycerol), and fractions were analyzed by SDS-PAGE.
HeLa cell extracts and immunoprecipitations.
Suspension cultures of HeLa cells were synchronized at the G1/S phase boundary by 2 mM thymidine treatment for 16 h, collected in the presence of 0.02% sodium azide, and washed with ice-cold phosphate-buffered saline containing 15 mM EDTA. The cell pellets were snap-frozen and homogenized in lysis buffer (15 mM HEPES [pH 7.5], 0.1 M KCl, 0.5% Triton X-100, 1 mM EGTA, 15 mM EDTA, and protease inhibitors). Human CCTβ was immunoprecipitated by incubating extracts with anti-CCTβ antibodies (StressGen) cross-linked to protein A-Sepharose (7) in the modified lysis buffer adjusted to 50 mM HEPES on ice for 4.5 h with occasional tapping. The immune complexes were washed four times with ice-cold Nonidet P-40 (NP-40) buffer (0.5% NP-40, 150 mM NaCl, 10 mM MgCl2, and 15 mM HEPES [pH 7.5]) before being subjected to SDS-PAGE.
Pulse-chase analysis.
A549 cells (1 × 106 cells per dish) were transduced with either recombinant adenoviruses (Ad5) expressing cyclin E from the cytomegalovirus promoter or control viruses at a multiplicity of infection of 250. After 6 h, the cells were preincubated for 1 h in methionine-deficient Dulbecco’s modified Eagle’s medium (DMEM) plus 10% dialyzed serum and pulse-labeled for 1.5 min with [35S]methionine (500 μCi). The cells were then washed and chased for 10 min with DMEM plus 10% fetal bovine serum supplemented with 20 mM unlabeled methionine. Extracts were prepared by lysing the cells on ice using FB buffer with 15 mM HEPES (pH 7.5) as described previously (25). CCT immune complexes were dissociated by boiling in 1% SDS for 5 min, diluted 1:10 in FB buffer containing 0.5% Triton X-100 and 0.5% deoxycholate, and then immunoprecipitated with anti-cyclin E antibody overnight at 4°C. After incubation with protein A-Sepharose beads for 1 h, the cyclin E immune complexes were washed three times with RIPA buffer and once with buffer containing 50 mM Tris (pH 7.5), 0.5% NP-40 and 10 mM MgCl2. For the direct cyclin E immunoprecipitation, the cell extracts were boiled and diluted as described above before the immunoprecipitation with anti-cyclin E antibody.
Nucleotide sequence accession numbers.
The accession numbers (GenBank) for the human CCT sequences reported here are AF026291 for delta, AF026292 for eta, and AF026293 for beta.
RESULTS
Genetic interaction between human cyclin E and human CCT subunits in yeast.
Previously, we observed that overexpression of human cyclin E at high levels from a galactose-inducible promoter along with its catalytic partner Cdk2 is lethal to yeast cells, but expression at lower levels from a less active promoter is tolerated (31). The lethality associated with cyclin E overexpression, although associated with elevated kinase activity, is not understood, but based on this phenotype we performed a genetic screen to identify negative regulators of cyclin E function that could rescue the cyclin E-associated growth inhibition. Operationally, a yeast strain was constructed in which the endogenous cyclin-dependent kinase Cdc28p was replaced by the human homologue Cdk2 and human cyclin E was expressed from the conditional GAL1 promoter (31). We used the p21Cip1 cDNA as a positive control, since the encoded protein has been shown to function as a cyclin E-Cdk2 inhibitor (3, 8); when cyclin E was induced, yeast cells coexpressing p21Cip1 grew well, whereas cells containing the vector plasmid did not, confirming the feasibility of the screen (data not shown). Next, cells containing Cdk2 and cyclin E expression constructs were grown in glucose medium to repress cyclin E expression and transformed with a human cDNA library (21), and the transformants were plated on glucose medium. Colonies were then replica plated to galactose medium to induce expression of cyclin E protein, and those that were dependent on a human cDNA for survival were identified. We recovered these cDNAs, subjected them to sequence analysis, and found three of them to encode human homologues of the beta, delta, and eta subunits of the eukaryotic CCT or TCP1-ring complex (5, 6, 9, 13, 29). The CCT consists of two back-to-back rings, each with eight unique but homologous subunits (11). It assists the folding of newly translated polypeptide substrates through multiple rounds of ATP-driven release and rebinding of partially folded intermediate forms (4). Previously, the only known substrates of the CCT complex had been the cytoskeletal proteins tubulin and actin (25, 27, 28); more recently, α-transducin, the Gα protein associated with phototransduction, has also been identified as a substrate in vitro and in vivo (4). Although it remains unclear why expression of single CCT subunits should rescue cyclin E-associated lethality (but see Discussion), the fact that CCT subunits interacted with cyclin E genetically prompted us to ask whether the intact CCT complex is a molecular chaperone involved in cyclin E biogenesis.
Maturation of human cyclin E requires CCT function in yeast.
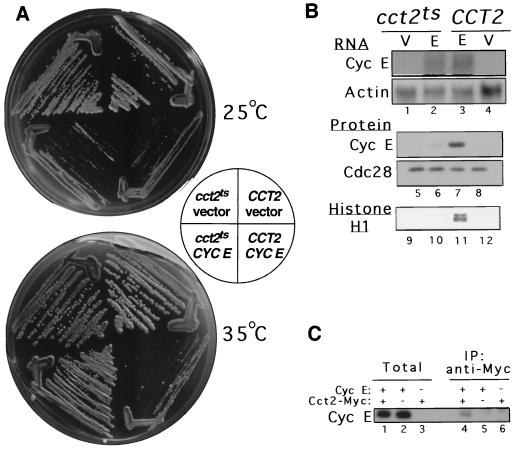
To determine whether CCT mediates folding of cyclin E in vivo, yeast strains expressing either the wild-type (CCT2) or a temperature-sensitive version of CCT containing a lesion in the β subunit (cct2ts) (14) were used. At the permissive temperature, both wild-type and cct2ts cells grew well in the absence of human cyclin E expression, but when cyclin E was induced, both strains exhibited severely reduced growth (Fig. 1A). When plated at 35°C, a semipermissive temperature for the cct2ts mutant, the wild-type cells still failed to grow when cyclin E was induced. However, the growth of cct2ts cells bearing a defective CCT complex was not affected by induction of cyclin E, and colonies were formed. Our interpretation of this result is that the CCT complex containing mutant Cct2p is unable to fold cyclin E efficiently at semipermissive temperature, thus protecting the cell from the toxicity associated with native cyclin E. We tested this idea by examining the steady-state levels of cyclin E in wild-type and cct2ts cells (Fig. 1B). Indeed, when mutant cells were grown at high temperature, cyclin E protein levels were greatly reduced, whereas the abundance of another protein, the Cdc28 kinase, was not affected (lanes 5 to 8). In contrast, cyclin E mRNA levels were similar in the wild-type and mutant cells (lanes 1 to 4), demonstrating that the reduction in cyclin E protein levels in the mutant cells is not due to effects on promoter activity or RNA stability. The differences in cyclin E protein levels were also reflected in the reduced activity of cyclin E-associated histone H1 kinase in the mutant cells compared to that in wild-type cells (lanes 9 to 12), showing that the overexpressed protein was biochemically active and, presumably, accounting for the toxicity. The foregoing results are thus consistent with the notion that impairment of CCT function protects the cell from toxicity of cyclin E overexpression by leaving cyclin E in a nonnative form that is subject to proteolysis. In order to confirm that the yeast CCT does, indeed, interact with human cyclin E, CCT was immunoprecipitated from yeast lysates after induction of cyclin E (Fig. 1C). A small portion of the steady-state pool of cyclin E coimmunoprecipitated with yeast Cct2, consistent with the idea that newly synthesized cyclin E interacts with the endogenous yeast CCT. Although these results do not directly address the question of whether cyclin E is a substrate of the CCT, and alternative explanations are possible, the data are certainly consistent with CCT-dependent folding of cyclin E.
FIG. 1.
A growth-defective phenotype of yeast cells overexpressing cyclin E is relieved by CCT deficiency, which is associated with reduced cyclin E protein levels and histone H1 kinase activity. (A) Growth on galactose plates at 25 or 35°C of wild-type (CCT2) and temperature-sensitive (cct2ts) yeast CCTβ cells transformed with either the cyclin E-expressing plasmid YCptG3(M)E or the insertless vector YCptG3(M) (31). (B) Effect of CCTβ mutation on cyclin E expression. CCT2 and cct2ts cells carrying either the cyclin E-expressing plasmid (E) or the vector (V) were grown at 35°C for 3 h in sucrose medium supplemented with 2% galactose and whole-cell RNA, and extracts were prepared. RNA was analyzed by Northern blotting with a cyclin E probe and an actin probe (lanes 1 to 4). Protein extracts were analyzed by Western blotting with an anti-cyclin E monoclonal antibody and a PSTAIRE monoclonal antibody for Cdc28p (lanes 5 to 8). Cyclin E-associated kinase activity was assayed by precipitation of extracts with the anti-cyclin E monoclonal antibody and kinase reactions with [γ-32P]ATP and histone H1 (lanes 9 to 12). (C) Cyclin E physically interacts with endogenous yeast CCT. Yeast cells expressing Cct2 bearing the Myc epitope (14) were induced for 2 h to express cyclin E (lanes 1 and 4). A cell extract was prepared, immunoprecipitated with anti-Myc antibody (Santa Cruz), and processed for Western blot analysis using anti-cyclin E monoclonal antibody. Protein extracts from cells expressing Cct1 bearing the influenza virus hemagglutinin epitope (14) after induction of cyclin E (lanes 2 and 5), and from cells expressing Cct2-Myc but lacking cyclin E (lanes 3 and 6), were also prepared and processed as negative controls.
Newly translated cyclin E associates with the CCT in vitro.
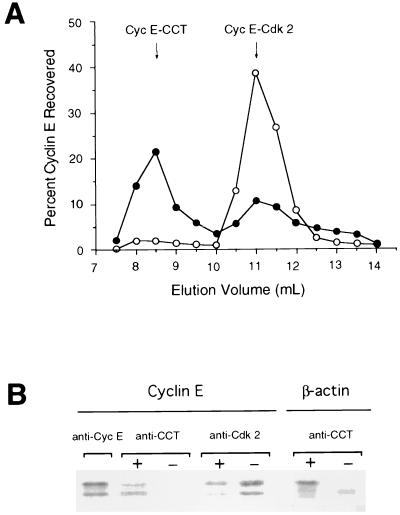
To directly address the issue of whether cyclin E is a substrate of CCT, the biogenesis of cyclin E upon translation in a reticulocyte lysate was analyzed. After a 6-min translation with [35S]methionine, further translation was halted by addition of cycloheximide. The mixture was split into two fractions. ATP, required for chaperone action, was depleted from one by addition of HK/glc, and both fractions were further incubated for 30 min at 30°C. The samples were then subjected to gel filtration chromatography (Fig. 2A). Most of the newly translated cyclin E from the ATP-depleted sample localized to a 900-kDa fraction containing CCT (4, 25, 27, 28). An additional, smaller portion of cyclin E was present in a 150-kDa fraction, where it was found by coimmunoprecipitation with anti-Cdk2 antibody to associate with Cdk2 (data not shown). This portion of cyclin E most likely represents native cyclin E that has already reached maturity during the 6-min translation reaction. When ATP was not depleted, only a small amount of cyclin E was detected in the 900-kDa fraction, whereas most of the cyclin E now localized to the 150-kDa fraction. This suggests that cyclin E is released from the CCT complex in an ATP-dependent manner, consistent with its being a CCT substrate. Physical association of cyclin E with CCT was demonstrated by coimmunoprecipitation of cyclin E with CCT, using anti-CCT antibodies (Fig. 2B). Here, since CCT requires both Mg and ATP to release a bound substrate, EDTA was used to quench CCT-mediated folding and release. When newly synthesized cyclin E was incubated in the presence of EDTA, cyclin E was coimmunoprecipitated with CCT (Fig. 2B). In contrast, cyclin E was not present in the CCT immunoprecipitate when the sample was incubated without any quenching reagent (Fig. 2B), confirming the ATP-dependent release of cyclin E from the CCT complex. Moreover, when incubated in the absence of EDTA, the released cyclin E associated with Cdk2, its natural partner, present in the lysate, as demonstrated by coimmunoprecipitation with anti-Cdk2 antibodies (Fig. 2B). Although the association of newly translated cyclin E with the CCT and its ATP-dependent release to complex with Cdk2 do not constitute proof of CCT-dependent folding of cyclin E, taken with the CCT-dependence of cyclin E accumulation in yeast and the known role of the CCT, this is the most likely explanation for the behavior of cyclin E in the experiments described above.
FIG. 2.
Cyclin E newly translated in a reticulocyte lysate associates with the CCT complex. (A) Newly translated cyclin E fractionates at 900 kDa and in the presence of ATP chases to a 150-kDa fraction. Cyclin E mRNA was translated in a reticulocyte lysate in the presence of [35S]methionine. One aliquot was chased with HK/glc (closed circle) and the other was chased without HK/glc (open circle). Samples were then applied to a Superose 12 gel filtration column run, and fractions were analyzed by SDS-PAGE. Radioactivity incorporated into cyclin E was quantitated with a PhosphorImager. The amount of cyclin E recovered in each fraction is shown as a percentage of the total recovered from the column. (B) Newly translated cyclin E coimmunoprecipitates with CCT. Cyclin E was translated as described for panel A and was chased at 30°C in the presence (+) or absence (−) of 5 mM EDTA. The lysates were then immunoprecipitated by using anti-cyclin E monoclonal antibody, polyclonal anti-CCTβ serum, or polyclonal anti-Cdk2 antibody. A similar experiment performed with β-actin is shown for comparison.
Association of newly translated cyclin E with the CCT in vivo.
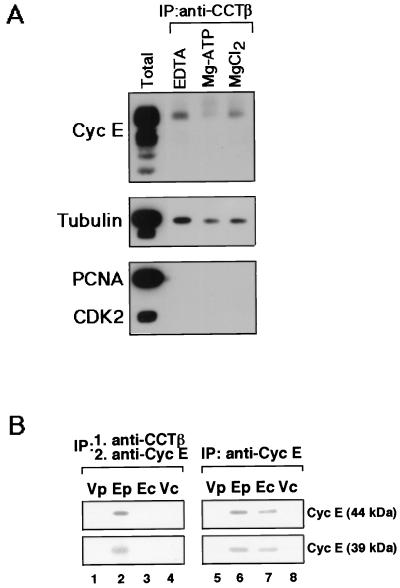
To determine whether cyclin E is also a substrate of CCT in vivo, HeLa cells were arrested by thymidine treatment in early S phase, which corresponds to the peak of cyclin E synthesis. Cell extracts were prepared and fractionated on a size exclusion column, and the fractions containing CCTβ and cyclin E protein were determined by Western blot analysis. A portion of the cyclin E cofractionated with the high-molecular-mass complex containing CCTβ (data not shown), suggesting that cyclin E associates with CCT in human cells. Cyclin E was present in an anti-CCTβ immunoprecipitate from an extract incubated with EDTA to quench ATP-dependent reactions, confirming a physical interaction between cyclin E and CCT (Fig. 3A). A significant decrease in the amount of cyclin E bound to CCT in a sample incubated with Mg-ATP compared to samples incubated with either EDTA or Mg alone was observed, demonstrating that here, as with cyclin E translated in vitro, cyclin E is dissociated from the CCT in an ATP-dependent manner. The same coimmunoprecipitates showed that the amount of the previously determined CCT substrate, β-tubulin, was also decreased in the sample incubated with Mg-ATP. In contrast, neither the DNA polymerase processivity factor PCNA nor Cdk2, the kinase partner of cyclin E, coimmunoprecipitated with CCTβ, indicating that neither of these proteins is a substrate of the CCT and that cyclin E associated with CCT is not already bound to Cdk2.
FIG. 3.
Interaction of newly translated cyclin E with CCT in human cells. (A) Cyclin E is a substrate of the CCT complex. Extracts from thymidine-blocked HeLa cells were aliquoted into three parts and incubated with either 15 mM EDTA, 5 mM MgCl2, or 5 mM Mg plus ATP at room temperature for 20 min. Human CCTβ was immunoprecipitated from these samples, and the immune complexes (IP) along with whole-cell extract (Total) were separated by SDS-PAGE and processed for Western blot analysis. The upper half of the blot was probed sequentially with anti-cyclin E antibody and anti-β-tubulin antibody (Boehringer Mannheim). The lower half was probed with anti-PCNA antibody (Santa Cruz) and anti-Cdk2 antibody (Transduction Laboratories). (B) Newly synthesized cyclin E in vivo associates with the CCT complex. A549 cells transduced with either an adenovirus expressing cyclin E (E) or a control virus (V) were pulse-labeled with [35S]methionine for 1.5 min (p), chased for 10 min (c), extracted and immunoprecipitated with anti-CCTβ antibody (lanes 1 to 4) or anti-cyclin E antibody (lanes 5 to 8). The CCT immune complexes were dissociated and reimmunoprecipitated with anti-cyclin E antibody (lanes 1 to 4), and cyclin E immune complexes were subjected to SDS-PAGE analysis.
To determine whether the CCT complex interacts specifically with newly synthesized cyclin E in mammalian cells, as would be expected for an essential folding function, in vivo pulse-chase experiments were performed on human cells in culture (Fig. 3B). A549 human lung carcinoma-derived cells transduced with a recombinant adenovirus programmed to express high levels of cyclin E were pulse-labeled with [35S]methionine for 1.5 min and then subjected to a 10 min chase in the presence of excess nonradioactive methionine. Cell extracts were prepared and the CCT complex and associated proteins were then immunoprecipitated with anti-CCTβ antibody. Subsequently, CCT immune complexes were dissociated in SDS and diluted, and a second immunoprecipitation with anti-cyclin E antibody was performed to assess the association of radiolabeled cyclin E with the CCT complex (Fig. 3B). After the short interval of pulse-labeling, both the 44-kDa and 39-kDa species resulting from expression of recombinant cyclin E coimmunoprecipitated with CCTβ (lane 2), demonstrating an association of newly translated cyclin E with CCT in vivo. In contrast, cells transduced with control virus did not produce any detectable signal (lane 1) due to the much lower level of endogenous cyclin E synthesis that falls below the level of detection under these short-pulse conditions. The level of both species of cyclin E associated with CCT decreased dramatically during the 10-min chase (lane 3), confirming that the newly synthesized cyclin E is indeed transiently associated with the CCT complex in vivo. Tubulin and actin were also coimmunoprecipitated with CCT from the sample pulse-labeled extract, and the level of both of them likewise decreased during the chase (data not shown). However, whereas actin and tubulin are stable proteins with half-lives measured in mammalian cells of 65 and 48 h, respectively (20, 24), cyclin E is an unstable protein with a half-life of 30 min (31). Therefore, to control for the natural turnover of labeled cyclin E in vivo during the chase, cyclin E was directly immunoprecipitated from the same pulse-labeled and -chased extracts used for CCT coimmunoprecipitation (lanes 5 to 8). Both the 44- and 39-kDa species of labeled cyclin E showed a slight decrease during the chase (lane 7) consistent with the reported 30-min half-life, but this was only a small change compared with complete disappearance from the cyclin E-CCT complex (lane 3). These data indicate that the decrease in CCT-associated cyclin E during the chase is due not to turnover of cyclin E but to release from chaperonin. Alternatively, the pool of cyclin E associated with the CCT may have an uncharacteristically short half-life. However, taken with the essentiality of CCT function for cyclin E biogenesis in yeast and the demonstration that cyclin E translated in vitro associates with the CCT prior to association with Cdk2, these results are more consistent with the idea that newly translated cyclin E in vivo associates transiently with the CCT for folding and is then released in a mature active form.
DISCUSSION
To date, the CCT complex has been shown to mediate the ATP-dependent folding of only a limited set of proteins in vivo—tubulin, actin, and α-transducin. These proteins do not show any significant amino acid sequence similarity, nor do they share a common fold. Cyclin E, likewise, does not bear any recognizable primary structural similarity to the other known CCT substrates. Notably, however, all four of these proteins are known to be aggregation prone when expressed in bacterial systems or when subjected to refolding from denaturant in vitro. Thus, exposure of hydrophobic surfaces in the nonnative state may be a significant shared feature of recognition of these proteins by CCT, as it is for the bacterial chaperonin, GroEL (1). On the other hand, CCT in general does not efficiently bind GroEL substrates following their dilution from denaturant, for example (26). Thus, there must be additional features of recognition in addition to, or other than, exposed hydrophobic surfaces, which are operative in recognition by this machinery. Perhaps the heterologous nature of the eight different CCT apical domains, corresponding to eight different subunits, contributes to such specificity. In any case, here, as with other heterooligomeric proteins whose subunit folding is assisted by chaperonin, CCT-mediated folding of cyclin E precedes the step of oligomerization with its partner protein, Cdk2. In the present case, in contrast to α and β tubulins, both of which require CCT action, Cdk2 does not appear to require the action of CCT for acquisition of its native state. However, folding of cyclin E by the CCT appears to be a prerequisite for Cdk2 binding, as cyclin E associated with the CCT is not associated with Cdk2.
The findings reported here of involvement of the CCT in biogenesis of cyclin E complement previous studies showing the involvement of another complex, the proteasome, in the ubiquitin-mediated turnover of cyclin E. It remains to be understood exactly what conformational features of the same primary cyclin E sequence are recognized by CCT early after translation, leading to production of the native state and assembly with Cdk2, as opposed to those recognized by the ubiquitin conjugation machinery after the G1/S transition, leading to proteasome-mediated proteolysis.
The genetic interaction between cyclin E and components of the CCT was discovered because expression of individual human CCT subunits was able to rescue the lethality associated with cyclin E overexpression in yeast, presumably by interfering with some aspect of cyclin E biogenesis or function. This result would seem to be at odds with the notion that CCT function is associated with folding and maturation of cyclin E into an active form. However, we propose two models that might provide resolution to this apparent paradox. Firstly, human CCT components, when expressed in yeast might be coassembled into the endogenous CCT, acting as poison or dominant negative subunits. Reducing the efficiency of the endogenous CCT in this manner might interfere with the maturation of cyclin E, accounting for the rescue of cyclin E-associated toxicity. Consistent with this interpretation, human CCT subunits coeluted with endogenous high-molecular-weight CCT complexes when yeast extracts expressing the recombinant human protein were subjected to gel filtration chromatography (data not shown). Secondly, excess monomeric human CCT subunits might have the capacity to bind and sequester cyclin E in a nonfunctional state, thereby reducing the functional level of cyclin E and accounting for the rescue of cyclin E-associated toxicity. Although gel filtration profiles revealed elution patterns consistent with CCT subunit-cyclin E heterodimer formation, the steady-state levels of such complexes were relatively low (data not shown). However, we cannot exclude the possibility that such complexes exist at much high levels in vivo and dissociate upon lysis and preparation of extracts. Therefore, further investigation is required to choose from between these models or additional models in order to explain our initial genetic observations linking cyclin E to the CCT.
ACKNOWLEDGMENTS
We thank John Cogswell and Sue Neill of Glaxo Wellcome for providing the recombinant adenoviruses and Suwon Kim for the CCT mutant yeast strains. We thank members of the Reed laboratory and the Horwich laboratory for valuable discussions.
This work was supported by U.S. Army grant DAMD 17-94-J4208 to S.I.R. and National Institutes of Health Postdoctoral Fellowship CA09292 to K.-A.W. A.L.H. is an investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Bukau B, Horwich A L. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- 2.Clurman B E, Sheaff R J, Thress K, Groudine M, Roberts J M. Turnover of cyclin E by the ubiquitin-proteasome pathway is regulated by cdk2 binding and cyclin phosphorylation. Genes Dev. 1996;10:1979–1990. doi: 10.1101/gad.10.16.1979. [DOI] [PubMed] [Google Scholar]
- 3.El-Deiry W S, Tokino T, Velculescu V E, Levy D B, Parsons R, Trent J M, Lin D, Mercer W E, Kinzler K W, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 4.Farr G W, Scharl E C, Schumacher R J, Sondek S, Horwich A L. Chaperonin-mediated folding in the eukaryotic cytosol proceeds through rounds of release of native and nonnative forms. Cell. 1997;89:927–937. doi: 10.1016/s0092-8674(00)80278-0. [DOI] [PubMed] [Google Scholar]
- 5.Frydman J, Nimmesgern E, Erdjument-Bromage H, Wall J S, Tempst P, Hartl F U. Function in protein folding of TRiC, a cytosolic ring complex containing TCP-1 and structurally related subunits. EMBO J. 1992;11:4767–4778. doi: 10.1002/j.1460-2075.1992.tb05582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao Y, Thomas J O, Chow R L, Lee G H, Cowan N J. A cytoplasmic chaperonin that catalyzes beta-actin folding. Cell. 1992;69:1043–1050. doi: 10.1016/0092-8674(92)90622-j. [DOI] [PubMed] [Google Scholar]
- 7.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 8.Harper J W, Adami G R, Wei N, Keyomarsi K, Elledge S J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 9.Hartl F U. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 10.Koff A, Cross F, Fisher A, Schumacher J, Leguellec K, Philippe M, Roberts J M. Human cyclin E, a new cyclin that interacts with two members of the CDC2 gene family. Cell. 1991;66:1217–1228. doi: 10.1016/0092-8674(91)90044-y. [DOI] [PubMed] [Google Scholar]
- 11.Kubota H, Hynes G, Carn A, Ashworth A, Willison K. Identification of six Tcp-1-related genes encoding divergent subunits of the TCP-1-containing chaperonin. Curr Biol. 1994;4:89–99. doi: 10.1016/s0960-9822(94)00024-2. [DOI] [PubMed] [Google Scholar]
- 12.Lew D J, Dulic V, Reed S I. Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell. 1991;66:1197–1206. doi: 10.1016/0092-8674(91)90042-w. [DOI] [PubMed] [Google Scholar]
- 13.Lewis V A, Hynes G M, Zheng D, Saibil H, Willison K. T-complex polypeptide-1 is a subunit of a heteromeric particle in the eukaryotic cytosol. Nature. 1992;358:249–252. doi: 10.1038/358249a0. [DOI] [PubMed] [Google Scholar]
- 14.Miklos D, Caplan S, Mertens D, Hynes G, Pitluk Z, Kashi Y, Harrison-Lavoie K, Stevenson S, Brown C, Barrell B, Horwich A L, Willison K. Primary structure and function of a second essential member of the heterooligomeric TCP1 chaperonin complex of yeast, TCP1 beta. Proc Natl Acad Sci USA. 1994;91:2743–2747. doi: 10.1073/pnas.91.7.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan D O. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 16.Ohtsubo M, Roberts J M. Cyclin-dependent regulation of G1 in mammalian fibroblasts. Science. 1993;259:1908–1912. doi: 10.1126/science.8384376. [DOI] [PubMed] [Google Scholar]
- 17.Ohtsubo M, Theodoras A M, Schumacher J, Roberts J M, Pagano M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol. 1995;15:2612–2624. doi: 10.1128/mcb.15.5.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quelle D E, Ashmun R A, Shurtleff S A, Kato J Y, Bar S D, Roussell M F, Sherr C J. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 1993;7:1559–1571. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- 19.Resnitzky D, Gossen M, Bujard H, Reed S I. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol. 1994;14:1669–1679. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubinstein N, Chi J, Holtzer H. Coordinated synthesis and degradation of actin and myosin in a variety of myogenic and non-myogenic cells. Exp Cell Res. 1976;97:387–393. doi: 10.1016/0014-4827(76)90630-3. [DOI] [PubMed] [Google Scholar]
- 21.Schild D, Brake A J, Kiefer M C, Young D, Barr P J. Cloning of three human multifunctional de novo purine biosynthetic genes by functional complementation of yeast mutations. Proc Natl Acad Sci USA. 1990;87:2916–2920. doi: 10.1073/pnas.87.8.2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherr C J. G1 phase progression cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 23.Sherr C J, Roberts J M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 24.Spiegelman B M, Penningroth S M, Kirschner M W. Turnover of tubulin and the N site GTP in Chinese hamster ovary cells. Cell. 1977;12:587–600. doi: 10.1016/0092-8674(77)90259-8. [DOI] [PubMed] [Google Scholar]
- 25.Sternlicht H, Farr G W, Sternlicht M L, Driscoll J K, Willison K, Yaffe M B. The t-complex polypeptide 1 complex is a chaperonin for tubulin and actin in vivo. Proc Natl Acad Sci USA. 1993;90:9422–9426. doi: 10.1073/pnas.90.20.9422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian G, Vainberg I E, Tap W D, Lewis S A, Cowan N J. Specificity in chaperonin-mediated protein folding. Nature. 1995;375:250–253. doi: 10.1038/375250a0. [DOI] [PubMed] [Google Scholar]
- 27.Ursic D, Culbertson M R. The yeast homolog to mouse Tcp-1 affects microtubule-mediated processes. Mol Cell Biol. 1991;11:2629–2640. doi: 10.1128/mcb.11.5.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vinh D B, Drubin D G. A yeast TCP-1-like protein is required for actin function in vivo. Proc Natl Acad Sci USA. 1994;91:9116–9120. doi: 10.1073/pnas.91.19.9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willison K R, Horwich A L. Structure and function of chaperonins in Archaebacteria and eukaryotic cytosol. In: Ellis R J, editor. The chaperonins. San Diego, Calif: Academic Press; 1996. pp. 107–136. [Google Scholar]
- 30.Wimmel A, Lucibelio F C, Sewing A, Adolph S, Muller R. Inducible acceleration of G1 progression through tetracycline-regulated expression of human cyclin E. Oncogene. 1994;9:995–997. [PubMed] [Google Scholar]
- 31.Won K-A, Reed S I. Activation of cyclin E/CDK2 is coupled to site-specific autophosphorylation and ubiquitin-dependent degradation of cyclin E. EMBO J. 1996;15:4182–4193. [PMC free article] [PubMed] [Google Scholar]