Summary
Background
Dengue shows high geographic heterogeneity within and across endemic countries. In the context of increasing burden and predicted outbreaks due to climate change, understanding the heterogeneity will enable us to develop region specific targeted interventions, including vaccination. World Health Organisation (WHO) suggests standard methodologies to study the burden and heterogeneity at national and subnational levels. Regional studies with robust and standard methodology to capture heterogeneity are scarce. We estimated the seroprevalence of dengue in children aged 9–12 years and the force of infection in Kerala, India, from where Zika cases also have been reported recently.
Methods
We conducted a school-based cross-sectional survey in 38 clusters; selected by stratified random sampling, representing rural, urban, high burden and low-burden administrative units. Validation of Indirect IgG ELISA was done by Plaque Reduction Neutralization Test (PRNT90) using the local isolates of all four serotypes. Force of infection (FOI) was estimated using the WHO-FOI calculator. We conducted a follow-up survey among a subsample of seronegative children, to estimate the rate of sero-conversion.
Results
Among 5236 children tested, 1521 were positive for anti-dengue IgG antibody. The overall seroprevalence in the state was 29% (95% CI 24.1–33.9). The validity corrected seroprevalence was 30.9% in the overall sample, 46.9% in Thiruvananthapuram, 26.9% in Kozhikkode and 24.9% in Kollam. Age-specific seroprevalence increased with age; 25.7% at 9 years, 29.5% at 10 years, 30.9% at 11 years and 33.9% at 12 years. Seroprevalence varied widely across clusters (16.1%–71.4%). The estimated force of infection was 3.3/100 person-years and the seroconversion rate was 4.8/100 person-years. 90% of children who tested positive were not aware of dengue infection. All the four serotypes were identified in PRNT and 40% of positive samples had antibodies against multiple serotypes.
Interpretation
The study validates the WHO methodology for dengue serosurveys and confirms its feasibility in a community setting. The overall seroprevalence in the 9–12 year age group is low to moderate in Kerala; there are regional variations; high burden and low burden clusters co-exist in the same districts. The actual burden of dengue exceeds the reported numbers. Heterogeneity in prevalence, the high proportion of inapparent dengue and the hyperendemic situation suggest the need for region-specific and targeted interventions, including vaccination.
Funding
World Health Organization.
Keywords: Burden of dengue: force of infection; Seroprevalence of dengue; Validation of IgG against PRNT, Plaque Reduction Neutralisation Test; Govt of Kerala-WHO Dengue study; Serprevalence of dengue in children; Seroconversion in dengue
Research in context.
Evidence before this study
We searched PubMed, for estimates of the seroprevalence of dengue infection among children. We identified 144 papers published between 2017 and 2022; of which 29 were from Southeast Asia and 15 from India. A national-level survey conducted in India in 2017 reported highly variable estimates, the lowest in the Northeastern region (1.6%) and the highest in the northern region (47%). It could not consider the cumulative nature of seroprevalence with age, as suggested in the methodology by WHO. This limits the validity of the estimates and the scope for comparison.
Added value of this study
‘Govt of Kerala-WHO Dengue study’ used one of the largest cohorts in India to precisely estimate the seroprevalence of dengue using methodology recommended by WHO (Indirect IgG ELISA validated with PRNT90 in children in the 9–12-year age group). The precise and narrow age band reflects the sero-conversions in the recent past and the data in this age group is critical for targeting vaccination policy. We conducted the study in the school setting in Kerala, where school enrolment is universal. We selected 5–7 administrative units (AUs) from each district, by stratified random sampling, representing rural and urban settings, with high and low prevalences, based on the surveillance data. Along with the estimates of seroprevalence and force of infection, the study generated serotype-specific data on dengue prevalence in subgroups. It has also built a large community-based cohort of children that can be used for follow-up evaluations. We found that 30% of the children are sero-converted and are at risk of antibody-mediated enhancement. The presence of antibodies against multiple serotypes was detected in 40.38% of positive samples (11.7% of the total). More importantly, 90% of children with serological evidence of dengue were unaware of the infection. It indicates the burden of inapparent dengue in a hyperendemic community and the need for urgent public health actions.
Implications of all the available evidence
This study estimated age-specific dengue seroprevalence and force of infection (FOI), calculated using the WHO-FOI calculator. This is the first field application of a validated WHO methodology. It reflects not only the rural–urban differences, but also the significant variability among high prevalent and low prevalent clusters within districts. Accurate burden estimates of dengue infection can help us plan targeted and region-specific interventions and guide us in formulating dengue vaccination and prevention policies. This paper provides a practical framework for replicating similar studies in different settings within and outside India.
Introduction
Dengue is the most prevalent mosquito-borne disease globally. There are around 100–400 million infections per year and nearly half the world’s population is at risk.1 The clinical spectrum ranges from asymptomatic infections to severe dengue. When multiple serotypes of Dengue Virus (DENV) circulate, rates of severe dengue are high.2,3 Traditional surveillance will not capture all infections4; WHO Global strategy highlights the urgent need to estimate the dengue burden.5 Cross-sectional, population-based, age-stratified seroprevalence studies can measure both the dengue burden and the historical disease transmission intensity in the region.6, 7, 8 Seroprevalence in children is a sensitive indicator and a more reliable measure of background incidence. Sero-prevalence in children of 9–12 years is critical to decide policies, while that in the 5–8 year group is desirable.6
In India, West Bengal, Uttar Pradesh, Punjab, Haryana, Delhi, Gujarat, Kerala, Karnataka and Tamil Nadu are the high dengue burden states.9 Kerala reports a high number of dengue cases; the highest from Thiruvananthapuram from where Zika cases were reported recently. During 2017, Kerala witnessed a large outbreak of dengue and Integrated Disease Surveillance Project (IDSP) reported around 20,000 confirmed cases.9 In this background the Government of Kerala conducted an in-depth epidemiological study on dengue, in collaboration with the World Health Organization. We estimated seroprevalence of dengue infection using indirect IgG Enzyme-linked Immunosorbent Assay (ELISA) in children, in the 9–12-year age group and force of infection, through follow-up of seronegative children. We also estimated seroprevalence in the 5–8 year age group in a hospital setting.
Methods
Study design and participants
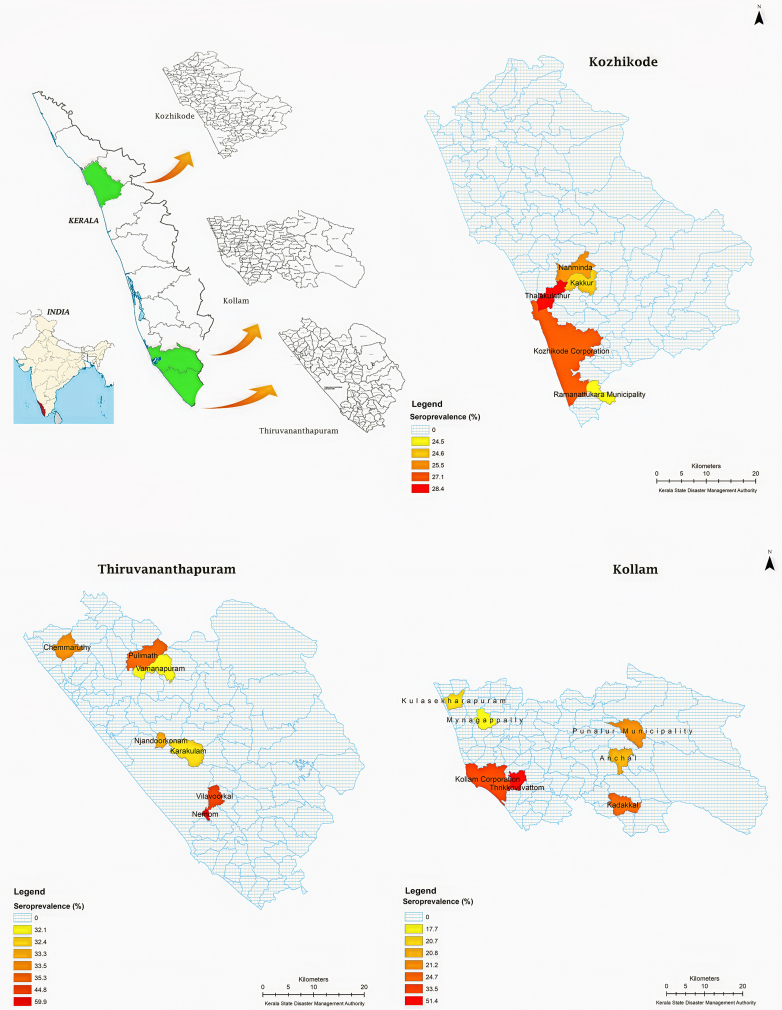
We conducted a school-based cross-sectional survey among 9–12-year-old children in 38 clusters of 19 Administrative Units (AU) selected from three districts of Kerala; Thiruvananthapuram (December 2017), Kollam (October 2018, January 2019) and Kozhikode (December 2018). Geospatial distribution of surveyed districts is given in Fig. 1. We estimated the sample size to be 1250 in each age stratum; assuming seroprevalence of 30%, absolute precision of 4%, design effect 2, and confidence interval of 95%; added 200 cases to account for potential missing information on the outcome status.
Fig. 1.
Administrative unit level dengue seroprevalence among 9–12 year old children.
Thiruvananthapuram district was included in the study because of epidemiological reasons; it reported the highest number of dengue cases. Kollam and Kozhikode were randomly selected from the list of southern and northern districts. From each district, we chose five to seven Administrative Units (AU), by stratified random sampling; two/three high prevalent rural (HR) units, two/three low prevalent rural (LR) units, one high prevalent urban (HU) unit and one low prevalent urban (LU) unit (list in the Supplementary File). An incidence proportion of 50 per 100,000, based on surveillance data was the cut-off point to decide whether the area is high or low prevalent. We randomly selected two schools from each AU and one classroom for each grade from 4 to 7; assuming that these grades in Indian schools represent age strata 9–12 years. We recruited all eligible children from the randomly selected classroom. This has resulted in less number of children in the lower age band (less number of 9-year old children in grade 4) and higher number of girls in the overall sample, since some of the selected classrooms had more girls. Moreover one of the randomly selected schools was a girls’ only school. All children from the selected classroom were invited to participate, due to practical reasons. We conducted class wise parent-teacher meetings to explain the objectives of the study, the data collection process and risk and benefits to the child and all eligible children in selected classrooms were invited to participate. Almost all parents were willing and gave consent and were eager to know the results of the testing. We obtained permission from the General Education and local self-government departments and interacted with people’s representatives, school management and teachers to appraise them about the objectives, process and expected outcomes of the study. The human ethics committee of Government Medical College, Thiruvananthapuram approved the study protocol (IEC No. 14/37/2017/MCT date 28/11/2017). We obtained written informed consent from parents, assent from children and subsequently informed their test results, along with appropriate risk communication.
After the initial survey of 5236 children, a follow-up survey was done in 386 children; 316 children from Kozhikode (2019) and 70 from Thiruvananthapuram (2020). We could not complete the follow-up survey because schools got closed due to COVID-19. Indirect IgG for dengue was done among 291 children who were seronegative in the initial survey. From a subsample of 92 children, who were seropositive in the initial survey, we collected data on fever and hospitalization during the one year follow up period. For the hospital-based survey, we recruited 153 children in the age group of 5–8 years, from Government Medical College, Thiruvananthapuram. Children suffering from acute febrile illness, chronic illnesses and long-term medications were excluded from both school-based and hospital-based surveys.
Procedures
Medical doctors from the Department of Community Medicine led the trained data collection teams. Socioeconomic status was assessed by the type of ration card10 of the household and by the educational and occupational status of family members. Trained staff nurses and qualified laboratory technicians collected blood samples (3 ml) under standard infection control precautions, in 5 ml screw-capped tubes and transported them to the State Public Health Laboratory in cold boxes on the collection day. The serum was separated from the whole blood and stored as aliquots at −20 °C.
Serological analysis and Plaque Reduction Neutralization (PRNT) assay
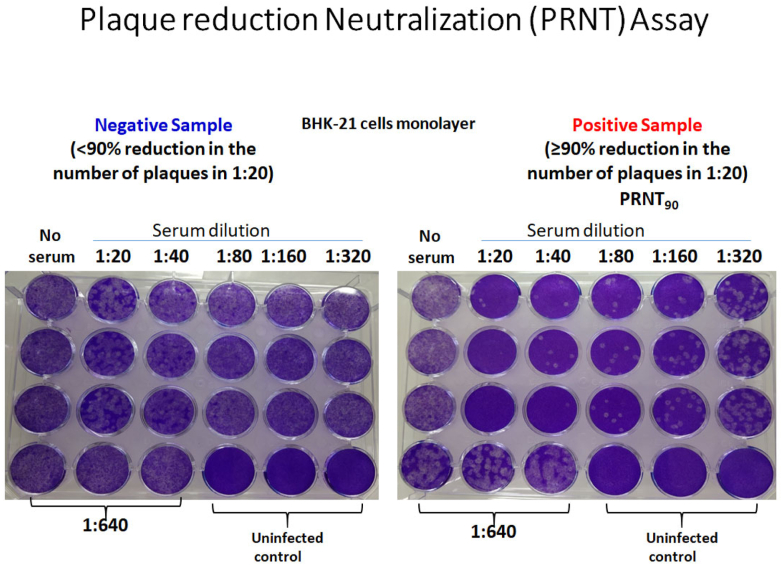
Indirect IgG Enzyme-linked Immunosorbent Assay (ELISA) (Panbio™) was used for the qualitative detection of anti-dengue IgG antibodies. An in-house PRNT assay was developed and standardized for the study in line with the approved protocols suggested by WHO.11 Kerala is hyperendemic for dengue; four viral strains were in circulation; we used the local isolates of all four serotypes. DENV-1 isolate RGCB419/2008 (GenBank JN903579); DENV-2 isolate RGCB880/2010 (GenBank KY427084); DENV-3 isolate RGCB1734/2017: and DENV-4 isolate RGCB1814/2019 were used. All the virus strains, originally isolated in C6/36 mosquito cells, were further passaged at least once in Vero cells. Briefly, 10-fold dilutions of heat-inactivated serum samples were incubated with 100 plaque-forming units (pfu) of the virus at 37 °C for 1 h for neutralization. The mixture was used for infecting confluent monolayers of Vero cells in 24-well culture plates in duplicate wells for each dilution. Serum-untreated, but virus-infected controls and uninfected controls were kept. After the initial incubation for virus adsorption, the monolayers were overlayed with carboxymethyl cellulose to a final concentration of 1.5% in 1X DMEM (Sigma) added with 2% Foetal Bovine serum (Gibco™) and 1X Antibiotic-antimycotic cocktail (Sigma). After six days of incubation at 37 °C in a 5% CO2 atmosphere, the cells were fixed overnight with 30% formaldehyde solution and stained with 0.1% crystal violet. The number of plaques in each well corresponding to each of the serum dilutions was counted and compared against the serum-untreated, but infected wells and the % reduction in plaques were calculated. A 90% or more reduction in plaque numbers (PRNT90) at the 1:10 dilution of patient serum against a DENV- serotype was taken as a positive result for the presence of antibodies against that serotype (Fig. 2).
Fig. 2.
Plaque Reduction Neutralization Assay (PRNT90) using the local DENV isolates.
We used 55 random samples from the IgG positive group and 50 random samples from IgG negative group for the PRNT assay; restricted to a subset since the assay is cumbersome and resource intensive. Even with 105 samples, the number of PRNT tests performed was 420 because each sample must be tested individually against all the four serotypes. We purposefully used more positive samples for PRNT to increase the yield of serotype-specific infections contributing to a positive PRNT. Since Kerala is a dengue-endemic area, we used high-stringency criteria for PRNT positivity; PRNT90. The strength of our PRNT assay is the use of locally circulating isolates.
Statistical analysis
We estimated seroprevalence using indirect IgG ELISA; validated and corrected it using the sensitivity and specificity estimates of the test against PRNT. Corrected prevalence was calculated using the formula; (apparent prevalence + specificity − 1)/(sensitivity + specificity − 1). We present the interval estimates for the clustered data.12 Force of Infection (FOI) was calculated from the increasing trend of seroprevalence with age, using the WHO-FOI calculator,4 which assumes a constant force of infection over time. The seroconversion rate was estimated from the follow-up data. Trend analysis was done using Extended Mantel–Haenszel Chi-square for linear trend.
Role of funding source
None.
Results
We enumerated 5566 children from 38 clusters; excluded 269 children, due to the non-availability of parental consent. We recruited 5297 children, from whom data and blood samples were collected; 61 samples were discarded due to lysis. Data from 5236 children are included in the analysis.
Our sample had more girls (3206/5236, 61.2%) and more children were in the 12 year age band (1740, 33.2%). Most (90%) parents had at least secondary schooling. More mothers were homemakers (64.3%), while fathers (98.6%) were employed (Table 1). Five percent of children gave a history of dengue infection during their lifetime (264), suspected or confirmed; while 16% of children reported that at least one family member was ever infected (845). Nearly half (2567, 48.5%) had a history of fever during the previous one-year; while 167 (6.5%) had clinical dengue, 83 (3.2%) had laboratory confirmation.
Table 1.
Socio-demographic characteristics of the study participants (n = 5236).
| Variable | Category | Number | Percentage (%) |
|---|---|---|---|
| Age (years) | 9 | 672 | 12.83 |
| 10 | 1344 | 25.67 | |
| 11 | 1480 | 28.27 | |
| 12 | 1740 | 33.23 | |
| Gender | Male | 2030 | 38.77 |
| Female | 3206 | 61.23 | |
| Socioeconomic status (SES) indicated by colour of ration card | Yellow (lowest SES) | 298 | 5.69 |
| Pink (low SES) | 1873 | 35.77 | |
| Blue (high SES) | 1639 | 31.30 | |
| White (highest SES) | 387 | 7.39 | |
| Data not available | 1039 | 19.84 | |
| Educational status of father (years of education) | No formal education | 11 | 0.21 |
| Primary (≤7 years) | 675 | 12.89 | |
| Secondary (8–10 years) | 2891 | 55.21 | |
| Higher secondary (11–12 years) | 827 | 15.79 | |
| Technical training | 94 | 1.79 | |
| Graduate/post graduate degree | 712 | 13.59 | |
| Professional degree | 26 | 0.49 | |
| Educational status of mother (years of education) | No formal education | 16 | 0.31 |
| Primary (≤7 years) | 413 | 7.88 | |
| Secondary (8–10 years) | 2625 | 50.13 | |
| Higher secondary (11–12 years) | 937 | 17.89 | |
| Technical training | 84 | 1.60 | |
| Graduate/post graduate degree | 1127 | 21.52 | |
| Professional degree | 34 | 0.65 | |
| Occupation of father | Unemployed | 71 | 1.36 |
| Unskilled worker | 1465 | 28.20 | |
| Semiskilled worker | 1155 | 22.23 | |
| Skilled worker | 1615 | 31.08 | |
| Clerical/shop owner/farmer | 620 | 11.93 | |
| Semi professional | 197 | 3.79 | |
| Professional | 72 | 1.38 | |
| Occupation of mother | Unemployed/home maker | 3369 | 64.34 |
| Unskilled worker | 661 | 12.62 | |
| Semiskilled worker | 331 | 6.32 | |
| Skilled worker | 304 | 5.81 | |
| Clerical/shop owner/farmer | 273 | 5.21 | |
| Semi professional | 199 | 3.80 | |
| Professional | 99 | 1.89 |
Of the 5236 sera tested, 1521 (29%) were positive, 3599 (68.8%) were negative and 116 (2.2%) were equivocal for IgG antibodies. We considered equivocal samples as negative. Overall seroprevalence for Kerala was 29% (95% CI 24.1%–33.9%); after validity correction based on validation against PRNT90, seroprevalence was 30.9%. The seroprevalence in Thiruvananthapuram was 41.2% (34.2%–49.5%), Kozhikode, 25.9% (24.0%–27.8%) and Kollam, 24.4% (19.3%–29.6%). Corrected seroprevalence was 46.9%, 24.9% and 26.9% in Thiruvananthapuram, Kollam and Kozhikkode, respectively (Table 2).
Table 2.
Seroprevalence of IgG antibodies against dengue virus in different districts, age groups and gender.
| Variable | Category | IgG positive | IgG negative | Seroprevalence (%) | Corrected seroprevalence (%) |
|---|---|---|---|---|---|
| District (5236, 100%) | Thiruvananthapuram (1261, 24%) | 520 (41.2%) | 741 (58.8%) | 41.2 | 46.9 |
| Kollam (1970, 37.6%) | 481 (24.4%) | 1489 (75.6%) | 24.4 | 24.9 | |
| Kozhikode (2005, 38.3%) | 520 (25.9%) | 1485 (74.1%) | 25.9 | 26.9 | |
| Age (5236, 100%) | 9 years (672, 12.9%) | 169 (25.1%) | 503 (74.9%) | 25.0 | 25.7 |
| 10 years (1344, 25.7%) | 376 (27.9%) | 968 (72.1%) | 27.9 | 29.5% | |
| 11 years (1480, 28.2%) | 431 (29.2%) | 1049 (70.8%) | 29 | 30.9 | |
| 12 years (1740, 33.2%) | 545 (31.3%) | 1195 (68.7%) | 31.3 | 33.9 | |
| Gender (5236, 100%) | Girls (3206, 61.2%) | 889 (27.7%) | 2317 (72.3%) | 27.7 | 29.2 |
| Boys (2030, 38.8%) | 631 (31.1%) | 1399 (68.9%) | 31.1 | 33.7 | |
| Overall | 1521 (29%) | 3715 (71%) | 29.0 | 30.9 | |
The heterogeneity in seroprevalence was evident across clusters in Thiruvanathapuram (35%–71.4%), Kollam (16.1%–60.3%) and Kozhikkode (25.0%–30.1%). The lowest seroprevalence was in a rural cluster (16.1%) and the highest was in urban cluster (71.4%). There was high variability in seroprevalence, even between AUs with geospatial proximity. The distance between a high prevalent urban cluster (60.3%) and a low prevalent (16.1%) rural cluster within the same district was hardly 20 miles (Table 3 and Fig. 1).
Table 3.
Seroprevalence of IgG antibodies against Dengue Virus in Administrative Units.
| District | Name of AU | Reported incidence of dengue | Rural/urban | Samples tested | Samples positive | Seroprevalence (%) | Corrected seroprevalence (%) |
|---|---|---|---|---|---|---|---|
| Thiruvananthapuram | Vilavoorkkal | High | Rural | 240 | 107 | 44.6 | 51.4 |
| Karakulam | High | Rural | 145 | 47 | 32.4 | 35.4 | |
| Vamanapuram | Low | Rural | 159 | 51 | 32.1 | 35.0 | |
| Pulimath | Low | Rural | 216 | 77 | 35.6 | 39.6 | |
| Chemmaruthy | Low | Rural | 185 | 62 | 33.5 | 36.8 | |
| Nemom | High | Urban | 267 | 160 | 59.9 | 71.4 | |
| Njandoorkonam | Low | Urban | 49 | 16 | 32.6 | 35.7 | |
| Total | 1261 | 520 | 41.2 | 46.9 | |||
| Kollam | Punalur | High | Rural | 149 | 32 | 21.5 | 21.1 |
| KS Puram | High | Rural | 343 | 71 | 20.7 | 20.1 | |
| Mynagapally | High | Rural | 220 | 39 | 17.7 | 16.1 | |
| Anchal | Low | Rural | 607 | 126 | 20.8 | 20.2 | |
| Kadakkal | Low | Rural | 283 | 70 | 24.7 | 25.3 | |
| Palathara | High | Urban | 111 | 57 | 51.4 | 60.3 | |
| Ramankulangara | Low | Urban | 257 | 86 | 33.5 | 36.8 | |
| Total | 1970 | 481 | 24.4 | 24.9 | |||
| Kozhikode | Thalakulathoor | High | Rural | 141 | 40 | 28.4 | 30.1 |
| Kakkoor | High | Rural | 349 | 86 | 24.6 | 25.3 | |
| Nanmanda | Low | Rural | 322 | 82 | 25.5 | 26.3 | |
| Ramanattukara | High | Urban | 425 | 104 | 24.5 | 25.0 | |
| Corporation | Low | Urban | 768 | 208 | 27.1 | 28.4 | |
| Total | 2005 | 520 | 25.9 | 26.9 | |||
“71.4” is the highest seroprevalence in the study population. “16.1” is the lowest seroprevaelnce in the study population.
The seroprevalence is higher among higher age groups and lower among girls. Hence the higher number of children in the higher age group in our sample might have inflated the seroprevalence while the higher number of girls might have deflated it. As a sensitivity analysis, we applied the stratum-specific seroprevalences to a standard population with equal proportions of the population in all age-gender strata. The adjusted seroprevalence obtained by the sensitivity analysis is 28.95%, while the unadjusted seroprevalence is 29.02% (Supplementary File).
Seroprevalence among 5–8-year-old children (hospital-based)
Among 153 children in the 5–8-year age band, 37 were positive for IgG antibodies. The age-specific seroprevalences were 25% (11/25), 20% (9/45), 25.6% (10/39) and 28% (7/25) for 5–8-year age groups, respectively. The overall validity corrected seroprevalence in the age 5–8 year age group was 24.6% and in each age band 25.7%, 19.1%, 26.5% and 29.6%, respectively.
Validation of IgG against Plaque Reduction Neutralization Test (PRNT90)
Among the 55 samples that tested positive in indirect IgG ELISA, 48 (87.27%) were positive in PRNT assay and out of 50 negative samples, 46 (92%) were true negatives. So, the positive predictive value and negative predictive value of indirect IgG ELISA in our setting were 87.27% and 92%, respectively. Applying this data to the existing situation of 29.04% test positivity, the sensitivity and specificity were 81.7% and 94.6%, respectively. We used this sensitivity and specificity to estimate the corrected seroprevalence.
The pattern of the serotype-specific antibody as per PRNT assay
The predominant serotype in our study was DENV-2 (73%). The pattern of serotype-specific antibodies, observed in PRNT-positive samples (n = 52) was as follows; DENV-1, 24 (46.2%); DENV-2, 38 (73%); DENV-3, 14 (26.9%); and DENV-4, 2 (3.8%). All the four false negative samples (PRNT positive but ELISA negative) tested positive for DENV-2. The presence of antibodies against multiple serotypes was detected in 21 (40.38%) samples; of which the commonest combination was DENV-1 and DENV-2 detected in 11 (21.1%) samples (Table 4).
Table 4.
Serotype specific antibodies in PRNT positive samples.
| DENV serotype | Number N = 52 | Percentage (100%) |
|---|---|---|
| Monotypic | ||
| DENV-1 | 6 | 11.5 |
| DENV-2 | 20 | 38.5 |
| DENV-3 | 5 | 9.6 |
| DENV-4 | 0 | 0% |
| Multitypic | ||
| DENV-1 & DENV-2 | 11 | 21.1 |
| DENV-1 & DENV-3 | 1 | 1.9 |
| DENV-1 & DENV-4 | 0 | 0 |
| DENV-2 & DENV-3 | 1 | 1.9 |
| DENV-2 & DENV-4 | 0 | 0 |
| DENV-1, DENV-2 & DENV-3 | 6 | 11.6 |
| DENV-1, DENV-2 & DENV-4 | 1 | 1.9 |
| DENV-1, DENV-2, DENV-3 & DENV-4 | 1 | 1.9 |
Force of infection (FOI) and seroconversion rate
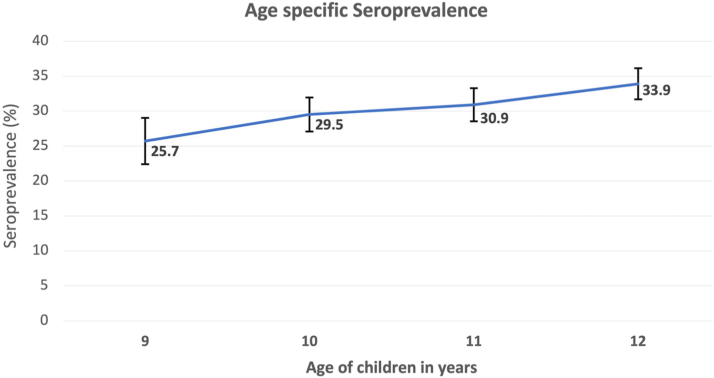
Seroprevalence increased with age; from 25.7% at age 9 to 33.9% at 12 years in the school-based survey (Tables 2 and 5 and Fig. 3) and from 25.7% at five years to 29.6% at the age of eight in the hospital-based survey. The estimated FOI in the 9–12 year band was 3.3 per 100 person-years and in 5–8 years, it was 4.3 per 100 person-years. In the follow up study, among the 291 seronegative children seroconversion was observed in thirteen children in Kozhikode (n = 246) and three in Thiruvananthapuram (n = 45). Seroconversion rate was 5.3 per 100 person-years in Kozhikode and 3.3 per 100 person-years in Thiruvananthapuram. The overall seroconversion rate was 4.8 per 100 person-years. On follow-up, two (2.17%) children from the seropositive cohort (92) developed fever and the diagnosis was dengue. Both were hospitalized and one child required ICU care and prolonged hospitalization (14 days).
Table 5.
Factors associated with dengue IgG seroprevalence.
| Variable | Category | Number of positives | Total | IgG seroprevalence (%) | p value |
|---|---|---|---|---|---|
| Age in years | 9 | 169 | 672 | 25.0 | 0.024 |
| 10 | 376 | 1345 | 27.9 | ||
| 11 | 431 | 1481 | 29.0 | ||
| 12 | 545 | 1741 | 31.3 | ||
| Gender | Boys | 631 | 2031 | 31.1 | 0.005 |
| Girls | 889 | 3206 | 27.7 | ||
| Colour of ration card | Yellow | 105 | 293 | 35.8 | <0.001 |
| Pink | 584 | 1858 | 31.4 | ||
| Blue | 424 | 1625 | 26.1 | ||
| White | 104 | 380 | 27.4 | ||
| NA | 268 | 995 | 26.9 | ||
| Reported history of confirmed or suspected dengue fever | Yes | 171 | 262 | 65.3 | <0.001 |
| No | 1335 | 4932 | 27.1 | ||
| Family member affected by dengue fever | Yes | 323 | 837 | 38.6 | <0.001 |
| No | 1180 | 4360 | 27.1 |
Fig. 3.
Age specific seroprevalence of IgG antibodies against dengue virus.
Among 1521 children who were IgG positive, only 171 reported history of confirmed or suspected dengue (11.2%). Seroprevalence was significantly higher among children with reported family history of dengue (38.6%) compared to those who did not report family history (27.1%).
Seroprevalence showed a positive trend with age (Extended Mantel–Haenszel Chi-square for linear trend, p value = 0.002). Boys had significantly higher seroprevalence (33.7%) compared to girls (29.2%) (p = 0.009). The lowest socioeconomic stratum reported the highest seroprevalence. Factors associated with seroprevalence are given in Table 5. High seroprevalence is observed in families adopting mosquito control measures like burning anti-mosquito incense inside the rooms, using mosquito coils and liquidator/vaporizer. However, closing windows and doors to prevent mosquito bites was protective.
Discussion
Our study reports a seroprevalence of 30.9% among 9–12 year old children and 24.6% among 5–8 year old children; this can be classified as low to moderate (<50%). The sample size for this study is 5236 which is the largest for a study done for the 9–12 year age band recommended by WHO till date. The reported seroprevalence in Kerala is higher than Singapore,13 but is lower than most endemic countries such as Cambodia, Mexico, Vietnam and Indonesia.14, 15, 16, 17, 18 Despite the high reported incidence in the surveillance data, the seroprevalence of dengue in Kerala is lower than that in many other regions in India. The age-specific seroprevalences reported from our study are closer to that of areas with lower seroprevalence in a nationally representative study done in 2017, which reported an overall seroprevalence of 48.7% (95% CI 43.5–54.0), 28.3% (21.5–36.2) among children aged 5–8 years and 41.0% (32.4–50.1) among children aged 9–17 years.19 Seroprevalence and force of infection are highly variable across countries and often underestimated.18,20 Areas with good surveillance, report more infections but the actual burden may be less than an area with poor surveillance. Seroprevalence among children in Singapore is just above 10%,13 whereas, in Indonesia it is more than 80%.14
Our study also affirms that the seroprevalence is more in urban areas and among the lower socio-economic group.19,21 Urban-to-rural spread is seen across India, in recent decades.22 The three principal drivers for the emergence of epidemic dengue are urbanization, globalization and lack of effective mosquito control.23 Thiruvananthapuram is the most urbanized among the regions studied and has the highest seroprevalence.
A Brazilian study reported that 60% of children in the deprived group are infected and the force of infection in that group is three times compared to the affluent group.24 Potential risk factors of dengue transmission at the household level are low socioeconomic status, littering in and around the home and poor sanitary and water storing facilities.17,25,26 Boys have significantly higher seroprevalence in our study; this may be due to difference in pattern of clothing and activities. Increasing seroprevalence with age is well documented and is attributed to the force of infection.25,27, 28, 29 We estimated the force of infection from the primary data on age-specific seroprevalence rates, using the WHO-FOI calculator. We estimated the seroconversion rate per 100 person-years as a measure of the force of infection in a subset of originally seronegative children. The triangulated data confirms that the force of infection in Kerala is three to five percent per annum, which can be considered as a low to moderate rate among the endemic regions of the world. The FOI reported in Indonesia and Vietnam is high (more than 10%).14,21 Another study from India estimated an annual force of infection of 11.9%.30 The incidence reported in our study is less than that from Maharashtra, another state in India; which reported an overall incidence rate of primary dengue as 54.2 infections/1000 children years (95% CI 43.0–67.3), in the 5–15 year age group.31
Apparent dengue represents the tip of the iceberg; only one in 10 children with serological evidence of dengue was aware of dengue infection. Most dengue infections result in either no perceptible symptoms or symptoms so mild that they go undetected by surveillance systems. The proportion of inapparent infections was earlier estimated to be 80%; in our study 89% of children with positive IgG did not know that they had dengue in the past.32 Individuals undetected by surveillance systems may be the primary reservoir of dengue virus transmission and that policy for dengue control and prevention must be revised accordingly.32
According to the PRNT data, the dengue infection is largely contributed by DENV2, which is the dominating strain elsewhere in India. However, there was a significant contribution by DENV1 also. The large outbreak of 2017 in Kerala was due to the emergence of DENV1 in an endemic situation dominated by DENV 2, while in subsequent years DENV 4 and more recently DENV 3 cases are on the increase.33 Our study affirms the hyperendemic situation in Kerala, where all the four serotypes circulate; with high incidence of multiple infections dominated by DENV2-DENV1 combination.32 The presence of antibodies against multiple serotypes was detected in 40.4% of positive samples (11.7% of total). In 2021, Zika cases were reported from Thiruvananthapuram, the first ever reports from Kerala and in 2022, from Kollam. Chikungunya cases are also reported from these districts.
Our data on anti-mosquito measures has the risk of reverse causality. Practices like the burning of anti-mosquito incense and the use of mosquito mats or coils may be a surrogate measure of mosquito menace itself. However, the seroprevalence of dengue was lower among children from houses which practised closure of windows and doors regularly to prevent the entry of mosquitoes.
We adopted stratified random sampling and in the last step all students in the randomly selected classrooms were recruited. This resulted in less number of children in the 9 year age band and more girls in the sample. As a sensitivity analysis, we applied the stratum-specific seroprevalences to a standard population with equal proportions of the population in all age-gender strata.
The overall seroprevalence and force of infection of dengue among children in the 9–12 year age group is low to moderate in Kerala; however there is high variability across clusters and there are high burden clusters even in the geographic proximity of low burden clusters. The overall seroprevalence is lower compared to reported number of cases and seroprevalence reported in this age group from many high-burden countries; but the high heterogeneity in the seroprevalence between clusters within the same districts has important implications, for the vaccination policy and clinical outcomes. Dengue infections in this population have been mostly asymptomatic and children are unaware of their positivity status. Multiple serotypes are in circulation in the same area and one in ten children was already infected with more than one serotype. Preventive measures need to be strengthened urgently to prevent high morbidity and mortality due to severe dengue. Heterogeneity across the country and even within the same state underscores the importance of conducting repeated cross-sectional studies following robust methodology to better inform dengue vaccination policies. In this context, the WHO methodology used here was found feasible and may be used for future comparison across different settings. FOI calculator and the burden estimation tool kit are now incorporated in the WHO website ‘A toolkit for national dengue burden estimation’.4
Contributors
IPS, ATS, CS, LGK, TL, RS, SS, RV, JMA, NB, SE, SA and SNS conceptualized the study; IPS, ATS, CS, LGK, TL, SNS, SE, SA, AR, SKL, JJ, AMG, TM, RKJ, MV, SS, NP, PKN, RK, JMA, NB, SS, RS and RV contributed to methodology and project administration; IPS, ATS, CS, LGK and TL had access to data and did the analysis; CS prepared the initial draft, further worked up on by IPS, ATS, CS, LGK and TL; SS, RV, RS, SE, NB, SA, AR, SKL, JJ, AMG, TM, RKJ, MV, SS, NP, PKN, RK, JMA and SNS contributed to the manuscript and all the authors approved the final manuscript.
Data sharing statement
The data collected for the study is not available in the public domain. The de-identified data can made available from the corresponding author based on reasonable request.
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
None.
Acknowledgements
We acknowledge Mr. Mohammed Haneesh IAS and Dr. Rajan Khobragade IAS, Pricipal Secretaries, Health and Family Welfare, Govt of Kerala, Mr. A. Shajahan IAS, Secretary, General Education, Govt of Kerala, Dr. Remla Beevi, Director of Medical Education, Dr. Ajayakumar M.K., Dr. Sara Varghese, Dr. Kala Kesavan and Dr. Linette Morris, Principals of Govt Medical College, Thiruvananthapuram and Dr. Raveendra Mohan Pandey, Professor and head, Biostatistics, All India Institute of Medical Sciences, New Delhi, for their support and guidance. We acknowledge Dr. Oliver Brady, Department of Infectious Disease Epidemiology and Dynamics, London School of Hygiene and Tropical Medicine for development of the WHO dengue burden estimation toolkit and its associated analysis tools and training materials. We specially acknowledge our core team from the Department of Community Medicine, Government Medical College, Thiruvananthapuram who were closely involved with the project at different stages of implementation; Dr. Mathew J. Valamparambil, Mr. Akhil T., Dr. Ananth M., Dr. Sreekanth K.B., Dr. Ajitha V., Dr. Jose Vincent, Dr. Ameena S.R., Dr. Deepthi K. Satheesan, Dr. Indu R.M., Dr. Vipin K. Ravi, Dr. Merin Sara Jose, Dr. Reshma R.S., Dr. Greeshma R.L., Dr. Betsy A. Jose, Dr. Rehna Rahman, Dr. Praseeda Chandran, Dr. Abin S. Sebastian, Dr. Princy S., Dr. Mariyam Alex, Dr. Prajitha K.C., Dr. Meenu M. Suresh, Dr. Sruthi C.M., Dr. Lekshmi Krishnan, Dr. Arya R., Dr. Anjana N.K., Dr. Vincy S., Mr. Prasanth F., Mrs. Usha P. and Mrs. Jayasree G. We acknowledge the District Surveillance Officers (DSO); Dr. Neena Rani, DSO of Thiruvananthapuram, Dr. Sandhya Raveendran, DSO of Kollam and Dr. Ashadevi, DSO of Kozhikode, for their leadership and facilitation; all the three District Vector Control teams, Dr. Jyothi R., Associate Professor, Microbiology, faculty and staff of Paediatrics department, Dr. Sekhar Lukose Kuriakose, Member Secretary and Chief Scientist, Kerala Disaster Management Authority (SDMA), and Sreeja S., Molecular Virology Laboratory, RGCB for their technical support. We thank the Primary Health Centre Medical Officers, field staff and district and state officials of Health department, Education Department and Local Self Government Departments of Government of Kerala for facilitating field operations. We thank all our study participants, teachers, parents and management of schools for their co-operation and support. This study was funded by the World Health Organization through a grant from Bill and Melinda Gates Foundation.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lansea.2023.100337.
Appendix A. Supplementary data
References
- 1.Dengue and severe dengue. https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue [cited 2022 January 21]. Available from:
- 2.Soo K.M., Khalid B., Ching S.M., Chee H.Y. Meta-analysis of dengue severity during infection by different dengue virus serotypes in primary and secondary infections. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0154760. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0154760 [cited 2022 January 21]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fried J.R., Gibbons R.V., Kalayanarooj S., et al. Serotype-specific differences in the risk of dengue hemorrhagic fever: an analysis of data collected in Bangkok, Thailand from 1994 to 2006. PLoS Negl Trop Dis. 2010;4(3) doi: 10.1371/journal.pntd.0000617. https://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0000617 [cited 2022 January 21]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.A toolkit for national dengue burden estimation. World Health Organization; Geneva: 2018. https://www.who.int/publications/i/item/WHO-CDS-NTD-VEM-2018.05 Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 5.Global Strategy for dengue prevention and control, 2012–2020. https://www.who.int/publications-detail-redirect/9789241504034 [cited 2022 January 21]. Available from:
- 6.Dengue_Serosurveys_020617.pdf. https://www.who.int/immunization/research/development/Dengue_Serosurveys_020617.pdf [cited 2020 February 24]. Available from:
- 7.Farrington C.P., Kanaan M.N., Gay N.J. Estimation of the basic reproduction number for infectious diseases from age-stratified serological survey data. J R Stat Soc Ser C Appl Stat. 2001;50(3):251–292. http://oro.open.ac.uk/2266/ [cited 2020 September 4]; Available from: [Google Scholar]
- 8.Ferguson N.M., Donnelly C.A., Anderson R.M. Transmission dynamics and epidemiology of dengue: insights from age-stratified sero-prevalence surveys. Philos Trans R Soc Lond B Biol Sci. 1999;354(1384):757–768. doi: 10.1098/rstb.1999.0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dengue/DHF situation in India: National Center for Vector Borne Diseases Control (NCVBDC) https://nvbdcp.gov.in/index4.php?lang=1&level=0&linkid=431&lid=3715 [cited 2022 December 8]. Available from:
- 10.Kerala ration card - types, eligibility and application procedure. IndiaFilings - Learning Centre; 2018. https://www.indiafilings.com/learn/kerala-ration-card/ [cited 2020 September 4]. Available from: [Google Scholar]
- 11.Roehrig J.T., Hombach J., Barrett A.D.T. Guidelines for plaque-reduction neutralization testing of human antibodies to dengue viruses. Viral Immunol. 2008;21(2):123–132. doi: 10.1089/vim.2008.0007. https://www.liebertpub.com/doi/10.1089/vim.2008.0007 [cited 2022 February 13]; Available from: [DOI] [PubMed] [Google Scholar]
- 12.Abramson J.H. WINPEPI updated: computer programs for epidemiologists, and their teaching potential. Epidemiol Perspect Innov EPI. 2011;8(1):1. doi: 10.1186/1742-5573-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ang L.W., Cutter J., James L., Goh K.T. Seroprevalence of past dengue virus infection among children and adolescents in Singapore. J Med Virol. 2015;87(12):2159–2162. doi: 10.1002/jmv.24287. https://onlinelibrary.wiley.com/doi/abs/10.1002/jmv.24287 [cited 2022 January 23]; Available from: [DOI] [PubMed] [Google Scholar]
- 14.Prayitno A., Taurel A.F., Nealon J., et al. Dengue seroprevalence and force of primary infection in a representative population of urban dwelling Indonesian children. PLoS Negl Trop Dis. 2017;11(6) doi: 10.1371/journal.pntd.0005621. https://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0005621 [cited 2022 January 23]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox-Lewis A., Hopkins J., Sar P., et al. Seroprevalence of dengue virus and rickettsial infections in Cambodian children. Am J Trop Med Hyg. 2019;100(3):635–638. doi: 10.4269/ajtmh.18-0865. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6402902/ [cited 2022 January 23]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pavía-Ruz N., Barrera-Fuentes G.A., Villanueva-Jorge S., et al. Dengue seroprevalence in a cohort of schoolchildren and their siblings in Yucatan, Mexico (2015-2016) PLoS Negl Trop Dis. 2018;12(11) doi: 10.1371/journal.pntd.0006748. https://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0006748 [cited 2022 January 23]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thai K.T.D., Binh T.Q., Giao P.T., et al. Seroprevalence of dengue antibodies, annual incidence and risk factors among children in southern Vietnam. Trop Med Int Health. 2005;10(4):379–386. doi: 10.1111/j.1365-3156.2005.01388.x. [DOI] [PubMed] [Google Scholar]
- 18.Luna E., Figueiredo G., Levi J., et al. Data on dengue incidence in South-eastern Brazil, 2014–2018. Data Brief. 2020;29 doi: 10.1016/j.dib.2020.105266. https://www.sciencedirect.com/science/article/pii/S2352340920301608 [cited 2022 January 23]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amaya-Larios I.Y., Rojas-Russell M., López-Cervantes M., et al. Seroprevalence of dengue in school children in Mexico ages 6–17 years, 2016. Trans R Soc Trop Med Hyg. 2018;112(5):223–229. doi: 10.1093/trstmh/try046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Das S., Sarfraz A., Jaiswal N., Das P. Impediments of reporting dengue cases in India. J Infect Public Health. 2017;10(5):494–498. doi: 10.1016/j.jiph.2017.02.004. https://www.sciencedirect.com/science/article/pii/S1876034117300618 [cited 2022 January 23]; Available from: [DOI] [PubMed] [Google Scholar]
- 21.Thai K.T.D., Binh T.Q., Giao P.T., et al. Seroprevalence of dengue antibodies, annual incidence and risk factors among children in southern Vietnam. Trop Med Int Health. 2005;10(4):379–386. doi: 10.1111/j.1365-3156.2005.01388.x. https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-3156.2005.01388.x [cited 2022 January 23]; Available from: [DOI] [PubMed] [Google Scholar]
- 22.Chakravarti A., Arora R., Luxemburger C. Fifty years of dengue in India. Trans R Soc Trop Med Hyg. 2012;106(5):273–282. doi: 10.1016/j.trstmh.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Gubler D.J. Dengue, urbanization and globalization: the unholy trinity of the 21st century. Trop Med Health. 2011;39(4 Suppl):3–11. doi: 10.2149/tmh.2011-S05. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3317603/ [cited 2020 September 4]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braga C., Luna C.F., Martelli C.M., et al. Seroprevalence and risk factors for dengue infection in socio-economically distinct areas of Recife, Brazil. Acta Trop. 2010;113(3):234–240. doi: 10.1016/j.actatropica.2009.10.021. https://www.sciencedirect.com/science/article/pii/S0001706X09003556 [cited 2022 January 23]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garg S., Chakravarti A., Singh R., et al. Dengue serotype-specific seroprevalence among 5- to 10-year-old children in India: a community-based cross-sectional study. Int J Infect Dis. 2017;54:25–30. doi: 10.1016/j.ijid.2016.10.030. https://www.ijidonline.com/article/S1201-9712(16)31214-0/abstract [cited 2020 February 24]; Available from: [DOI] [PubMed] [Google Scholar]
- 26.Velasco-Salas Z.I., Sierra G.M., Guzmán D.M., et al. Dengue seroprevalence and risk factors for past and recent viral transmission in Venezuela: a comprehensive community-based study. Am J Trop Med Hyg. 2014;91(5):1039–1048. doi: 10.4269/ajtmh.14-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murhekar M.V., Kamaraj P., Kumar M.S., et al. Burden of dengue infection in India, 2017: a cross-sectional population based serosurvey. Lancet Glob Health. 2019;7(8):e1065–e1073. doi: 10.1016/S2214-109X(19)30250-5. https://www.thelancet.com/journals/langlo/article/PIIS2214-109X(19)30250-5/abstract [cited 2020 August 15]; Available from: [DOI] [PubMed] [Google Scholar]
- 28.Ganeshkumar P., Murhekar M.V., Poornima V., et al. Dengue infection in India: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12(7) doi: 10.1371/journal.pntd.0006618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tam C.C., Tissera H., de Silva A.M., De Silva A.D., Margolis H.S., Amarasinge A. Estimates of dengue force of infection in children in Colombo, Sri Lanka. PLoS Negl Trop Dis. 2013;7(6) doi: 10.1371/journal.pntd.0002259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhavsar A., Tam C.C., Garg S., et al. Estimated dengue force of infection and burden of primary infections among Indian children. BMC Public Health. 2019;19(1):1116. doi: 10.1186/s12889-019-7432-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah P.S., Alagarasu K., Karad S., et al. Seroprevalence and incidence of primary dengue infections among children in a rural region of Maharashtra, Western India. BMC Infect Dis. 2019;19(1):296. doi: 10.1186/s12879-019-3937-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ten Bosch Q.A., Clapham H.E., Lambrechts L., et al. Contributions from the silent majority dominate dengue virus transmission. PLoS Pathog. 2018;14(5) doi: 10.1371/journal.ppat.1006965. https://dx.plos.org/10.1371/journal.ppat.1006965 Ferguson NM, editor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahul A., Saini P., Valamparampil M.J., et al. Epidemiological and clinical characterization of dengue virus serotypes during 2017-2019 in southern Kerala, India. Trans R Soc Trop Med Hyg. 2022;116(10):900–909. doi: 10.1093/trstmh/trac001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.