Abstract
Cancer, a complex and heterogeneous disease, arises from genomic instability. Currently, DNA damage-based cancer treatments, including radiotherapy and chemotherapy, are employed in clinical practice. However, the efficacy and safety of these therapies are constrained by various factors, limiting their ability to meet current clinical demands. Metal nanoparticles present promising avenues for enhancing each critical aspect of DNA damage-based cancer therapy. Their customizable physicochemical properties enable the development of targeted and personalized treatment platforms. In this review, we delve into the design principles and optimization strategies of metal nanoparticles. We shed light on the limitations of DNA damage-based therapy while highlighting the diverse strategies made possible by metal nanoparticles. These encompass targeted drug delivery, inhibition of DNA repair mechanisms, induction of cell death, and the cascading immune response. Moreover, we explore the pivotal role of physicochemical factors such as nanoparticle size, stimuli-responsiveness, and surface modification in shaping metal nanoparticle platforms. Finally, we present insights into the challenges and future directions of metal nanoparticles in advancing DNA damage-based cancer therapy, paving the way for novel treatment paradigms.
Key words: DNA damage, Metal nanoparticles, Nucleus-targeting, DNA repair inhibition, Immune response, Size optimization, Stimuli-responsiveness, Surface modification
Graphical abstract
Metal nanoparticles offer a versatile programmable platform for precise DNA damage induction and synergistic various tumor treatment strategies to effectively treat tumors.
1. Introduction
Cancer is a complex and heterogeneous disease that has been extensively studied and researched over the past few decades1. The hallmark of various cancers is genomic instability, characterized by the increasing accumulation of DNA damage2, which forms the basis for the application of radiotherapy and chemotherapy in cancer treatment3,4. The most common mechanism of both is to rapidly promote the accumulation of DNA damage within cancer cells through DNA crosslinkers or X-rays, thereby inducing cancer cell apoptosis5. However, the safety and efficacy of DNA damage-based cancer therapy are greatly limited by factors such as drug efflux, DNA repair mechanisms, and off-target toxicity6,7. Therefore, there is an urgent need to optimize DNA damage-based cancer therapy to combat tumor drug resistance.
Nanoparticles play a pivotal role in DNA damage-based tumor treatment approaches. These minute particles, typically ranging from 1 to 100 nm in size, possess unique properties that make them ideal candidates for targeted therapy8. By utilizing nanoparticle-based delivery systems, therapeutic agents can be precisely delivered to tumor cells, maximizing treatment efficacy while minimizing off-target effects9. Recently, metal nanoparticles have emerged as promising tools for cancer therapy due to their unique physicochemical properties and biocompatibility10. Unlike traditional cancer treatment strategies, metal nanoparticles can be considered as a personalized toolbox11, and a DNA damage-based therapy induced by well-designed metal nanoparticles can effectively avoid complex drug resistance factors in cancer cells. The capacity of metal nanoparticles to directly induce DNA damage and subsequently eliminate tumor cells has been extensively explored and refined. One example is the utilization of tumor pH-sensitive Pt nanoclusters, which can be engineered with targeting peptides and pH-responsive release mechanisms12. This design enables the efficient delivery of Pt to the nucleus of tumor cells, where it forms stable Pt-DNA complexes that are difficult to eliminate. Consequently, the induction of DNA damage occurs, leading to the demise of tumor cells. In combination with multiple potential treatment modalities, it can amplify the subsequent killing effects triggered by DNA damage. For example, Au nanoclusters are used to deliver platinum, effectively sensitizing cancer cells to platinum-based drugs by clearing intracellular GSH13. PEG-modified Gd-based nanosheets have a larger surface area for radiation deposition and stronger penetration ability in solid tumors, amplifying radiation-induced oxidative stress and DNA damage14. Copper sulfide nanoparticles, modified for nuclear targeting, can be delivered to the nucleus and efficiently damage DNA using photothermal therapy15. The ability of metal nanoparticles to induce DNA damage in cancer cells, either as a standalone therapy or in combination with other treatments, presents a new approach to combat drug resistance in cancer therapy.
In this review, we begin by providing a brief overview of the mechanisms and limitations of DNA damage-based cancer therapy strategies, including the challenges associated with resistance and toxicity. To address these limitations, we present a series of existing solutions based on metal nanoparticles, which offer personalized and precise approaches to cancer therapy. We then delve into a comprehensive summary of the adjustments of physical and chemical factors that affect the anti-tumor effect of metal nanoparticles. These factors include size, responsiveness, and surface modification, among others (Scheme 1). By examining the impact of these factors, we aim to provide insight into the optimal design of metal nanoparticles for inducing DNA damage and enhancing therapeutic efficacy. Finally, we discuss the current challenges and future prospects of using metal nanoparticles to induce DNA damage for cancer therapy, including the need for further research to optimize drug delivery and reduce toxicity, as well as the potential for combination therapies and personalized treatment strategies.
Scheme 1.
Metal nanoparticles-induced DNA damage for tumor treatment. Metal nanoparticles offer a powerful approach for inducing DNA damage to effectively treat tumors. Through size optimization and surface modifications, metal nanoparticles enable precise nucleus-targeted delivery and responsive release. This unique capability allows them to penetrate deep into the cellular nucleus, where they induce DNA damage. Additionally, metal nanoparticles can simultaneously interfere with normal cellular physiological functions and disrupt DNA damage repair mechanisms, leading to the accumulation of DNA damage load within cancer cells. The combined effect of these actions induces cancer cell death and triggers the release of a substantial amount of tumor antigens, including DNA fragments, which in turn activates immune responses. Overall, metal nanoparticles present a versatile and promising strategy for tumor treatment by exploiting its ability to induce DNA damage and engage immune-mediated anti-tumor responses.
2. Mechanisms of DNA damage-based cancer therapies
The integrity of DNA is crucial for normal cellular function and proliferation. DNA damage occurring in the S phase of the cell cycle can hinder the progression of replication forks and may result in replication-associated DNA double-strand breaks (DSBs), which is the most toxic form of all DNA damage16. The cell cycle checkpoint proteins detect high levels of DNA damage, and their activation induces cell cycle arrest to prevent the transfer of damaged DNA during mitosis17,18. If damaged DNA cannot be properly repaired, it may lead to cell death19. Cancer cells typically have relaxed DNA damage sensing/repair capabilities, and importantly, they can bypass cell cycle checkpoints, allowing them to achieve high proliferation rates, which makes them more susceptible to DNA damage, as replication of damaged DNA increases the likelihood of cell death20, 21, 22. The concept of using DNA as a target has spurred the development of numerous cancer therapies based on DNA damage strategies, such as platinum-based chemotherapy, radiotherapy, and photothermal therapy23, 24, 25.
Chemotherapeutic agents and other treatments commonly used for cancer therapy directly target cellular DNA molecules to combat tumors26. The major manifestations of this direct mechanism are damage to DNA chains, including intra-/inter-strand crosslinking, and single-/double-strand breaks27, 28, 29. Nitrogen mustard, cyclophosphamide, methotrexate, and platinum-based drugs, among others, can interfere with DNA replication and transcription by forming DNA adducts and inducing DNA strand crosslinking, leading to cell death30,31. Radiation therapy induces cell apoptosis directly by inducing DNA double-strand breaks and indirectly by generating reactive oxygen species32. Photothermal therapy utilizes photosensitizers to absorb light energy, generating heat and reactive oxygen species, which cause thermal damage and oxidative stress to cancer cells, resulting in double-strand breaks and other types of DNA damage33.
Moreover, certain chemotherapy drugs can also cause irreversible DNA damage indirectly, mainly by utilizing nucleotide analogs or nucleic acid synthesis inhibitors to block DNA synthesis, thereby interfering with the proliferation and division of cancer cells34,35. For example, drugs such as gemcitabine and methotrexate can block the synthesis of purines and pyrimidines during DNA synthesis, thereby preventing normal DNA replication36,37. Meanwhile, topoisomerase inhibitors, such as etoposide and doxorubicin, can interfere with DNA repair, thereby accelerating the accumulation of DNA damage and inducing cancer cell death38,39.
3. Metal nanoparticles for optimizing DNA damage-based cancer therapy
The conventional treatment strategies targeting DNA damage have inevitable limitations in practical applications. DNA is deeply embedded within the nucleus of cancer cells, and most DNA-damaging agents are required to traverse a sequence of barriers, including the cell membrane, lysosomes, and nuclear envelope, before gaining access to the nucleus40, 41, 42. Moreover, the efflux function of transport proteins on the cancer cell membrane leads to insufficient intracellular drug concentrations to induce DNA damage43, 44, 45. On the other hand, cancer cells have enhanced antioxidant capacity to protect DNA from oxidative stress induced by radiotherapy and chemotherapy or promote the efflux of Pt-GSH complexes formed by platinum-based drugs from the cells by overexpressing the glutathione (GSH) detoxification system46, 47, 48.
Even if DNA damage is eventually caused, cancer cells can evade cell death by enhancing DNA damage repair or increasing tolerance to genetoxicty49,50. For example, DNA damage caused by platinum-based chemotherapy drugs is typically repaired by nucleotide excision repair (NER), preventing the accumulation of DNA damage and subsequent cell death49,51,52. Meanwhile, mutations in the mismatch repair (MMR) pathway in cancer cells prevent the recognition and binding of MMR complexes with Pt-DNA adducts, silencing downstream signaling pathways that induce cell death and lead to tolerance to DNA damage49,51.
Fortunately, the use of metal nanoparticles has provided us with a personalized arsenal to design a diverse range of therapeutic strategies that target various mechanisms of resistance to DNA damage in tumors. These nanoparticles serve as a nexus that links DNA damage therapies with other treatment strategies, enabling the circumvention of complex resistance mechanisms in tumors while amplifying the cytotoxic effects of DNA damage and subsequent cytotoxicity. By incorporating various strategies such as targeted delivery, spatiotemporal release, and combination therapy, metal nanoparticles offer the potential for more effective cancer treatment options (Scheme 2). These strategies provide promising avenues to overcome resistance mechanisms in tumors and improve the therapeutic outcomes for cancer patients.
Scheme 2.
Metal nanoparticles offer a range of optimization strategies for DNA damage-based cancer treatment approaches. The unique properties of metal nanoparticles enable the precise targeting of cell nucleus, facilitating the specific delivery of DNA-damaging agents to cancer cells. Metal nanoparticles can act by inhibiting DNA damage repair mechanisms, leading to the accumulation of DNA damage load within cancer cells. Moreover, it employs various therapeutic strategies that disrupt normal physiological functions, inducing rapid cancer cell death. Additionally, metal nanoparticles can activate immune pathways, resulting in sustained anti-tumor responses. By addressing the limitations associated with DNA damage-based cancer treatment and enhancing the tumor-killing capacity linked to DNA damage, metal nanoparticles hold great promise for improved therapeutic outcomes.
Different metals exhibit distinct characteristics and mechanisms in DNA damage therapy (Table 1)12, 13, 14, 15,53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72. Gold (Au) is commonly employed in photothermal and photodynamic therapies, leveraging its ability to generate reactive oxygen species (ROS) to induce DNA damage. Platinum (Pt), on the other hand, exerts its DNA-damaging effects through the formation of DNA-Pt adducts. Additionally, high atomic number metals serve as potent radiosensitizers, enhancing the efficacy of radiation therapy by facilitating chromosome fragmentation or intensifying oxidative stress-induced DNA damage.
Table 1.
Different functions of metal nanoparticles for DNA damage.
| Formulation | Types of metal | Drug | Mechanism of inducing DNA damage | Optimization strategy | Ref. |
|---|---|---|---|---|---|
| Pt-NA | Pt | – | Formation of Pt-DNA adducts | pH-Responsive release | 12 |
| AuNCs–Pt | Au, Pt | – | Formation of Pt-DNA adducts | Depletion of GSH | 13 |
| Phy@PLGdH | Gd | Physcion | Radiation sensitization | Morphology control for deposition of X-rays | 14 |
| CuS@MSN-TAT-RGD | Cu | – | Localized high temperatures within the cell nucleus | Nuclear localization signals modified for nuclear targeting | 15 |
| PZGE | Zn | aPD-L1 | Generation of ROS | Size optimization for nuclear targeting | 53 |
| C-CD/TiO2 | Ti | – | Localized high temperatures within the cell nucleus | Surface hydrophilization for nuclear targeting | 54 |
| Pd-TAT | Pd | – | Localized high temperatures within the cell nucleus | Nuclear localization signals modified for nuclear targeting | 55 |
| FMSN@FITC-TAT | Au | – | Localized high temperatures within the cell nucleus | Nuclear localization signals modified for nuclear targeting | 56 |
| NMPN | Pt | – | Formation of Pt-DNA adducts | DNA repair inhibition | 57 |
| uPA-SP@CaP | Pt, Ca | BRCA1 siRNA | Formation of Pt-DNA adducts | DNA repair inhibition | 58 |
| BHT | Hf | 3-BrPA | Radiation sensitization | DNA repair inhibition | 59 |
| Bio-Pt-I | Pt | Iodine | Formation of Pt-DNA adducts | Downregulation of Bcl-2 to promote cell apoptosis | 60 |
| PWE | W | EGCG | Radiation sensitization | Demethylation of DNA to promote cell pyroptosis | 61 |
| CuCH–NCs | Cu | – | Generation of ROS | Activation of cytotoxic complexes | 62 |
| SR@PMOF | Zr | SR-717 | Generation of ROS | Activation of the STING pathway | 63 |
| aPD-L1@MnO2 | Mn | aPD-L1 | Radiation sensitization | Immune microenvironment reprogramming | 64 |
| Zn-Fu MNs | Zn | 5-Fu | Generation of ROS | DNA repair inhibition | 65 |
| PtNCs | Pt | – | Formation of Pt-DNA adducts | Light-triggered thermal-responsive release | 66 |
| Pt(IV) NPs | Pt | – | Formation of Pt-DNA adducts | GSH-responsive release | 67 |
| PEG-ZnO | Zn | – | Generation of ROS | PEG modified to promote cellular uptake | 68 |
| PEG-coated gold NRs | Au | – | Generation of ROS | PEG modified to achieve long-circulating | 69 |
| R-GAuNP | Au | – | Localized high temperatures within the cell nucleus | Glycosylation modification promotes tumor targeting and stability | 70 |
| PEG@Pt/Dox | Pt | Doxorubicin | Formation of Pt-DNA adducts | PEG modified to promote drug loading | 71 |
| LnPS@TiO2 | Ti,La,Ga,Ce | – | Radiation sensitization | Radiocatalysis based on core–shell structure | 72 |
‒, not applicable.
Metal nanoparticles harness the intrinsic DNA-damaging properties of metals while incorporating a range of optimization strategies. Through meticulous size control, surface modification, and stimulus-responsive release mechanisms, metal nanoparticles achieve enhanced DNA damage efficacy and subsequent anti-tumor effects, considering both the depth and intensity of the therapeutic intervention. By capitalizing on the unique characteristics of different metals and employing a diverse array of optimization techniques, metal nanoparticles hold immense promise for advancing DNA damage-based therapies and augmenting anti-cancer interventions.
3.1. Improving the nucleus-targeting ability
Whether the cancer therapeutic strategy targeting DNA damage can achieve the expected therapeutic effect largely depends on whether the drug can enter the nucleus smoothly73,74. Metal nanoparticles benefit from the customizable size and surface modification, becoming an excellent carrier for targeted drug delivery to the nucleus.
A series of metal nanoparticle-based DNA damage strategies focus on passive diffusion to achieve nuclear-targeted delivery of DNA-damaging agents. Zhang et al.53 loaded zinc phthalocyanine (ZnPc) and aPD-L1 onto the surface of GQD-PEG to obtain small-sized nanoparticles with a diameter of approximately 32 nm. Leveraging the size-dependent nuclear-targeting effect, the photosensitizer can be efficiently delivered to the chromosomal regions within the cell nucleus, facilitating the generation of light-induced DNA damage. Moreover, by fully utilizing the physical and chemical properties of metal nanoparticles, passive nuclear targeting can be effectively achieved75. Phuong et al.54 designed a TiO2 nanoparticle-embedded carbon dot system for nuclear targeting controllable photothermal therapy. After internalization into cells, the photocatalytic effect of TiO2 under visible light irradiation could oxidize the surface of carbon dots, resulting in a hydrophobic-to-hydrophilic transition of carbon dots, which could then cross the hydrophilic nuclear membrane and enter the cell nucleus76.
Most passive strategies for targeting cell nuclear rely on the passive diffusion of metal nanoparticles into the nucleus77. However, this results in low delivery efficiency and restricts the size design of metal nanoparticles78. To overcome this limitation, many efficient active targeting strategies have been developed for DNA damage-based cancer therapy.
The primary approach for delivering metal nanoparticles that exceeds the size of NPC to the cell nucleus is surface functionalization with nuclear localization signals (NLS)79. NLS enabled metal nanoparticles to overcome the size barrier for nuclear delivery, facilitating tumor therapy based on DNA damage80. With NLS's active targeting capability, metal nanoparticles induced rapid and effective accumulation of DNA damage in the nuclear area that exceeded the threshold for triggering apoptosis79. The trans-activator of transcription (TAT) derived from human immunodeficiency virus type 1 (HIV-1) is the most commonly used nuclear targeting peptide, which can mediate the nuclear transport of exogenous nanoparticles via the importin α/β pathway81,82. Li et al.83 conjugated TAT peptide to Fe3O4 nanoparticles that carried camptothecin (CPT), a DNA-damaging agent. The TAT peptide facilitated the recognition of the nanoparticles by NPC and guided the nanoparticles from cytosol to the nucleus, resulting in the accumulation of CPT inside the nucleus and efficient DNA damage. Photothermal therapy also faces the challenge of damaging DNA deep in the nucleus due to the short diffusion distance of ROS and the limited heat diffusion rate induced by the photothermal effect41. A possible solution is to employ nuclear targeting to enable in situ phototherapy that can directly destroy DNA. Gao et al.55 demonstrated an innovative approach to enhance the biocompatibility and stability of Pd nanosheets (PD-NSs) by surface coating them with a modified cell-penetrating peptide, TAT (Fig. 1A). This modification endowed PD-NSs with the ability to specifically target the cell nucleus, which serves as the central control center (Fig. 1B‒E). The enhanced nuclear targeting capability of PD-TAT greatly improved the photothermal therapeutic efficacy, as the nucleus is highly sensitive to thermal treatments (Fig. 1F). Multiple potential strategies for cell nucleus targeting can be integrated and applied on the platform of metal nanoparticles. By conjugating NLS (CGGGPKKKRKVGG) peptide and RGD peptides to AuNP, Mena et al.56 achieved cancer cell nuclear targeting and delivery of AuNP, which enabled in situ photothermal therapy to induce severe DNA damage.
Figure 1.
Enhancing the targeting efficiency of DNA damage drugs through metal nanoparticles platform. (A) Pd-TAT with perinuclear accumulation capability for cancer cell migration inhibition and photothermal therapy. (B) Confocal images of a representative MCF-7 cell after incubation with Pd-TAT-Cy7 for 12 h. (C) Confocal images of a representative MCF-7 cell after Pd-TAT treatment. (D) Line-scan fluorescence intensity of Hoechst and line-scan grayscale intensity of Pd-TAT along the arrowed region as shown in (C). (E) Confocal images of Pd-TAT-treated cells without or with mild NIR laser irradiation (808 nm, 0.3 W/cm2, 10 min). (F) Confocal fluorescence images of MCF-7 cells co-stained by calcein-AM and PI after different treatments as indicated. Reprinted with the permission from Ref. 55. Copyright © 2019 Elsevier.
3.2. Blocking DNA repair
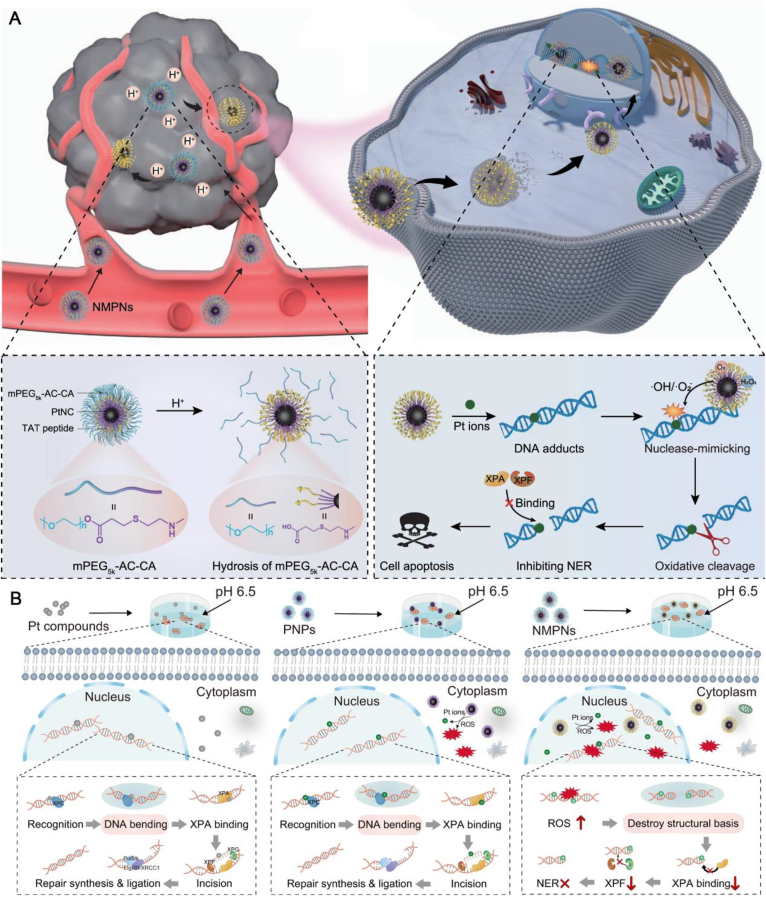
Tumor therapy strategies aimed at inducing DNA damage to kill cancer cells may encounter challenges due to the cellular DNA damage repair response, especially in platinum-based chemotherapy84, 85, 86. The development of metal nanoparticles that incorporate functionalized ligands to disrupt DNA damage repair responses may offer new insights into overcoming resistance in tumor therapies that target DNA damage. Our group57 has constructed a nuclease-mimetic platinum nanozyme (NMPN) that penetrates into the nucleus of cancer cells using surface-modified TAT peptides and releases Pt particles to promote the production of Pt-DNA adducts, thereby directly damaging the DNA of tumor cells. At the same time, NMPN with oxidase and peroxidase-like activities can produce active oxygen in situ to oxidatively cleave double-stranded DNA near the Pt–DNA binding site (Fig. 2A), interfere with the formation of DNA bending conformation required for NER (Fig. 2B)87,88, thus causing irreversible DNA damage and reversing tumor chemotherapy resistance89. Yang et al.58 considered a more direct strategy by loading DNA repair inhibitor BRCA1 small interfering RNA (siRNA) together with cisplatin into a pH-sensitive nano-platform, irreversible Pt-DNA adducts were formed by silencing DNA repair-related gene expression, ultimately leading to the fate of cancer cells towards apoptosis. Alternatively, Fu et al.59 sensitized radiotherapy using Hf-nMOFs loaded with 3-BrPA. While high-Z metal Hf deposited X-rays generate ROS sufficient to damage DNA90, 3-BrPA interferes with biosynthesis by inhibiting glycolysis, thus impeding the energy and material supply required for DNA repair91, ultimately inducing cancer cell apoptosis. Both direct interferences with DNA damage repair pathways and disruption of the supply of DNA repair materials can effectively enhance the efficacy of DNA damage therapies. The multifunctionality and customization of metal nanoparticles provide a variety of possibilities for their application.
Figure 2.
Metal nanoparticles enhance tumor DNA damage load by blocking DNA repair responses. (A) NMPNs can readily target cancer cell nuclei to efficiently induce Pt-DNA adducts, as well as change the NER-required DNA conformation for overcoming Pt-resistant tumors. (B) In comparison to Pt compounds and PNPs that cannot effectively generate ROS in the nucleus, NMPNs can readily accumulate in the nucleus by acidity-induced exposure of TAT peptides and induce in situ ROS generation to induce DNA oxidative cleavage, thus destroying the DNA conformation required for NER. Reprinted with the permission from Ref. 57. Copyright © 2023 Springer Nature Limited.
3.3. Transforming DNA damage into cellular death
An important factor that researchers tend to overlook is the inability to link DNA damage with eventual cell death. Cells have a certain tolerance for DNA damage, and the dysregulated expression of some apoptotic molecules may confer drug resistance to tumors92. By breaking the balance of apoptosis while inducing DNA damage, the effect of DNA damage therapy can be amplified93. The balance of Bcl-2/Bax apoptosis is disrupted in cancer cells, where the apoptotic inhibitor Bcl-2 has a unique carcinogenic effect, which can resist DNA damage by inhibiting multiple apoptotic deaths94, 95, 96. Yu et al.60constructed iodine-conjugated Pt4+ nanoparticles, which formed DNA damage while using iodine to downregulate Bcl-2 expression97, sensitizing cancer cells to apoptosis. By shifting our focus away from DNA damage-induced apoptosis, we can explore the multitude of possibilities that metal nanoparticles offer for tumor ablation.
As an example, Wang et al.61 developed a tungsten-based nanoparticles loaded with the epigenetic modulator EGCG. While inducing DNA damage with X-rays, EGCG can alleviate local hypermethylation of tumor DNA and upregulate the expression of the membrane pore-forming protein GSDME98,99. After tungsten ions are activated by X-rays and generate a significant amount of ROS, the potential Bax-cytochrome c-caspase-9-caspase-3 pathway is initiated, cleaving GSDME and forming gasdermin pores on the cell membrane, ultimately inducing pyroptosis100,101. The induction of DNA damage alone does not guarantee cell death in cancer therapy, as several barriers can hinder therapeutic efficacy.
Furthermore, metal nanoparticles can activate multiple pathways to synergistically trigger cell death mechanisms. For instance, Li et al.62 developed copper (II) carbonate hydroxide nanocrystals (CuCH–NCs) encapsulated within a single albumin nanocage, functioning as peroxidase-like biomineralization agents. These CuCH–NCs exhibit pH-responsive release of Cu2+. Upon reduction by glutathione (GSH), Cu2+ is converted to Cu+, catalyzing the generation of hydroxyl radicals (·OH) from H2O2. While damaging tumor cell DNA, Cu2+ also activates the transformation of a non-toxic disulfiram into a cytotoxic complex, thereby inducing selective chemotherapeutic damage through inhibition of cell proliferation and amplification of apoptosis. Metal nanoparticles have emerged as a promising class of agents that can serve both as inducers of DNA damage and as mediators of cell death, thereby providing new perspectives for cancer treatment.
3.4. Inducing anti-tumor immune response
In addition to their ability to induce DNA damage and subsequent cytotoxicity, metal nanoparticles also hold the potential to introduce immunotherapy by exploiting the neoantigens and immunological features generated by DNA damage and cancer cell death102, 103, 104, 105. Exposure of cancer cells to chemotherapy drugs, radiation, oxidative stress, and other factors can lead to the formation of lethal double-stranded DNA breaks (DSBs) and chromosomal fragments106,107. These fragments can be recognized by the cyclic GMP-AMP (cGAMP) synthase (cGAS), which induces the generation of cGAMP and activates the stimulator of interferon genes (STING) pathway108. This ultimately triggers a cascade of phosphorylation reactions (TBK1-IRF3-NF-κB) and upregulates the expression of type I interferons, thereby activating anti-tumor immunity109,110. Zhou et al.63 fabricated polymeric metal–organic framework nanoparticles (PMOF NPs) with a polyethylene glycol (PEG) shell by coordinating a segment copolymer of poly (propylene imine) dendrimer with 1,4-benzenedicarboxylic acid (PABDA), meso-tetrakis(4-carboxyphenyl) porphyrin (TCPP), disulfide succinic acid, and zirconium chloride. Subsequently, the researchers loaded the STING agonist SR-717 into the porous structure of PMOF to obtain SR@PMOF NPs. Under light irradiation, TCPP within the SR@PMOF NPs generated singlet oxygen (1O2), leading to DNA damage in tumor cells and subsequent release of fragmented DNA and tumor-associated antigens. Moreover, the disulfide bonds were cleaved by 1O2, causing the collapse of the PMOF structure and enabling the rapid release of SR-717. The combination of SR-717 and photodynamic therapy (PDT) resulted in the activation of the endogenous STING pathway. The simultaneous release of fragmented DNA and tumor antigens facilitated by metal nanoparticles further enhanced the treatment efficacy by inducing instantaneous tumor cell killing and long-term anti-tumor immune responses.
Also, Metal nanoparticles have the ability to reprogram the immune microenvironment, thus removing obstacles to subsequent immune responses triggered by DNA damage. Deng et al.64 have developed a biomineralized MnO2 nanoplatform to facilitate the delivery of immune checkpoint inhibitors. The manganese dioxide not only mitigates hypoxia within the tumor, thereby sensitizing it to radiotherapy but also activates the cGAS-STING pathway to initiate an anti-tumor immune response111,112. Moreover, the aPD-L1 carried by the manganese-based nanoparticles specifically blocks the binding of PD-L1 on cancer cells to PD-1 on cytotoxic T lymphocytes (CTLs)113,114, thereby reversing the immunosuppressive tumor microenvironment and synergizing with cancer immunotherapy. Chemotherapeutic agents, radiotherapy, or DDR inhibitors, among a series of therapeutic strategies that can induce DNA damage, can further increase the inherent genomic instability of tumors to elicit innate immune responses115.
In addition, various DNA damage strategies can be combined to induce immunogenic cell death (ICD), release tumor antigens, and activate immune responses. Lei et al.65 designed a zinc-fluorouracil metal drug network, where the dual damage caused by the 5-Fu-induced DNA transcriptional inhibition and the zinc particle-induced ferroptosis led to a violent outbreak of ICD in cancer cells (Fig. 3A‒D), resulting in a massive leakage of tumor neoantigens, inducing extensive infiltration of immune cells (Fig. 3E), and initiating subsequent anti-tumor immune responses (Fig. 3F and G). Metal nanoparticles offer a promising approach to increase chromosome fragmentation, which can activate subsequent anti-tumor immune responses or alleviate the immunosuppressive microenvironment, thus providing new avenues for tumor treatment strategies116,117. By selectively targeting cancer cells and inducing DNA damage, metal nanoparticles can trigger apoptosis or necrosis, leading to the release of tumor antigens and the subsequent activation of immune cells. Moreover, some metal nanoparticles possess intrinsic immunomodulatory properties, which can help to overcome the tumor-induced immunosuppressive microenvironment and enhance the anti-tumor immune response118. Therefore, the use of metal nanoparticles has the potential to revolutionize the field of cancer immunotherapy, and their development and optimization will undoubtedly lead to more effective and personalized cancer treatment options in the future.
Figure 3.
Harnessing anti-tumor immunity through metal nanoparticles in DNA damage-based cancer therapy. (A) Zinc-Fluorouracil metallodrug networks can remold immunosuppressive TME and induce immune activation through the synergistic effect of Fu-induced DNA damages and Zn-induced mitochondrial dysfunction. (B–D) Zn-Fu MNs mediated ICD and release of damage associated molecular patterns (DAMPs). (E, F) The tumor treated with Zn-Fu MNs exhibited higher T cell infiltration and secretion levels of IL-6 and TNF-α. Reprinted with the permission from Ref. 65. Copyright©2023 Ivyspring International Publisher.
4. Modulating the physicochemical properties of metal nanoparticles
The delivery and DNA-damaging abilities of metal nanoparticles are largely determined by their physical and chemical properties, including size, surface modification, and responsiveness. These properties directly affect the interaction between the nanoparticles and the biological system, which ultimately impacts therapeutic efficacy and safety. Therefore, a deep understanding of the structure-activity relationships of metal nanoparticles is crucial for the rational design of effective and safe nanoparticles.
In this chapter, we aim to provide a comprehensive review of the physical and chemical properties of metal nanoparticles and how they affect their DNA-damaging behavior (Scheme 3). We discuss the impact of nanoparticles size on cellular uptake, tumor penetration, and biodistribution, as well as the importance of surface modification to improve biocompatibility, stability, and targeting. Furthermore, we highlight the role of nanoparticle responsiveness in enhancing therapeutic efficacy through spatiotemporal control of drug release. We hope to provide insights into the design principles of metal nanoparticles for optimal DNA-damaging behavior and facilitate the development of safe and effective metal nanoparticles for further clinical use.
Scheme 3.
Influencing DNA damage potential of metal nanoparticles through physical and chemical factors. Metal nanoparticles serve as a versatile and customizable therapeutic platform. Size optimization plays a crucial role in controlling the pharmacokinetic properties and intracellular transport of metal nanoparticles. By precisely tuning the particle size, optimal cellular uptake and distribution can be achieved, enhancing the overall DNA damage potential. Additionally, metal nanoparticles can be engineered for responsive release, enabling the controlled and targeted delivery of DNA-damaging agents. This responsive release can be triggered by external stimuli or endogenous factors, ensuring precise drug release at the desired site. Furthermore, the surface structure of metal nanoparticles is highly tunable, allowing for the attachment of multiple functional modules. This enables the incorporation of tailored functionalities, enhancing the therapeutic efficacy and enabling personalized treatments. The comprehensive control over physical and chemical factors offers exciting opportunities to optimize the DNA damage potential of metal nanoparticles in cancer therapy.
4.1. Size optimization
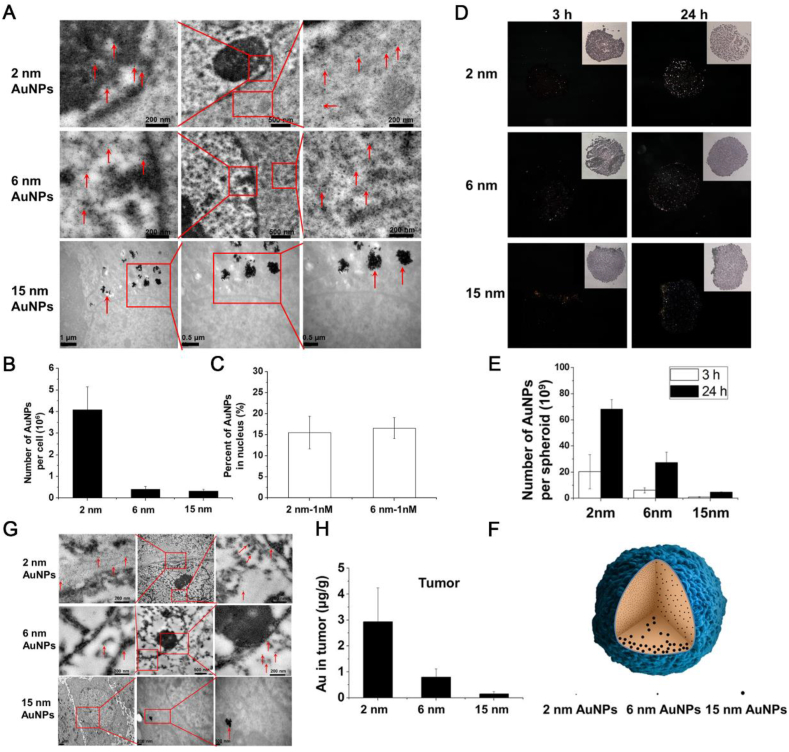
The size of metal nanoparticles is crucial for their ability to induce DNA damage61. In order to reach the vicinity of chromosomes and subsequently interact with biological systems, these nanoparticles need to penetrate multiple cellular barriers from the extracellular space to the nuclear membrane119, 120, 121. Therefore, the size of metal nanoparticles plays a critical role in determining their ability to penetrate these barriers and reach the desired target location. Specifically, the ability of metal nanoparticles to induce DNA damage is highly dependent on their sizes122. To deliver these nanoparticles from the extracellular space to the nuclear membrane, they must penetrate multiple cellular barriers, and their size largely determines whether they can be delivered to the vicinity of chromosomes and subsequently interact with biological processes. Huang et al.123 prepared a series of AuNPs with different sizes and found that AuNPs with ultra-small diameters can easily penetrate the nuclear pore complex to reach the periphery of chromosomes. In particular, 2 nm and 6 nm AuNPs were able to penetrate into the nucleus and disperse evenly (Fig. 4A and C), whereas 15 nm AuNPs were unable to enter the nucleus and instead aggregated in the cytoplasm due to nonspecific adsorption by serum proteins (Fig. 4A and B)124. In addition, the size of metal nanoparticles also affects their penetration and retention ability in solid tumors. Larger-sized AuNPs have difficulty penetrating into the interior of solid tumor tissues (Fig. 4D and E), only existing in the surface cell layer of a few tissues and in the cytoplasm of each cell (Fig. 4F). However, AuNPs with extremely small sizes can achieve better penetration efficiency (Fig. 4E and F) and longer retention time within the tumor tissue (Fig. 4G and H). In conclusion, the size of metal nanoparticles plays a critical role in their in vivo behavior, impacting their loading and delivery capabilities. Achieving an optimal balance between these factors is essential to create metal nanoparticles that exhibit high DNA cytotoxicity while maintaining biocompatibility. Therefore, careful size control is necessary for the design process of these drugs to achieve the desired therapeutic effects with minimal side effects. Further studies are required to fully elucidate the relationship between the size and in vivo behavior of metal nanoparticles, providing guidance for the development of more effective and safer therapeutic strategies.
Figure 4.
The effect of particle size on DNA damage induced by metal nanoparticles. (A) TEM images of cells treated with 1 nmol/L nanoparticles for 24 h. Red arrows indicate the gold nanoparticles. Boxed regions are enlarged in the adjacent panels. (B) Quantitative ICP-MS measurement of AuNP uptake by cells. (C) Percentage of gold nanoparticles localized in the nucleus compared to the whole cell after treatment with 2 and 6 nm Au nanoparticles at 1 nmol/L. (D) Dark field images of spheroids after culture with nanoparticles of different sizes. (E) ICP-MS analysis of the number of Au nanoparticles in each treated spheroid. (F) Schematic illustration of the penetration behavior of AuNPs of different sizes. (G) Representative TEM micrographs of tumor tissue taken 24 h after the administration of AuNPs. (h) Au content in tumor 24 h after iv injections of gold nanoparticles at 5 mg Au kg−1. Data represent mean ± SD (n = 3). Reprinted with the permission from Ref. 123. Copyright © 2012, American Chemical Society.
4.2. Stimuli-responsiveness
Some metal nanoparticles possess the direct ability to indiscriminately damage DNA, but their application may be limited by systemic toxicity generated during prolonged circulation. To optimize the direct DNA-damaging capacity of metal nanoparticles, it is crucial to maximize their intracellular accumulation while minimizing drug leakage during systemic circulation125. Achieving this goal requires the rational design of metal nanoparticles with precise spatiotemporal release capabilities, which can effectively deliver the drug payload to the targeted site and minimize off-target effects126. By harnessing advanced nanotechnology, metal nanoparticles can be engineered to exhibit tailored release kinetics, enabling controlled drug release in response to specific environmental stimuli or triggers, such as pH, temperature, enzymes, or light120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131. This approach can enhance therapeutic efficacy by increasing drug concentration at the desired site and minimizing systemic toxicity. Furthermore, the development of metal nanoparticles with enhanced release capabilities can facilitate the delivery of poorly soluble or highly toxic drugs, thereby expanding the range of therapeutic options available to patients132. Zhao and colleagues66 developed a Pt4+ PNA nanogel that retains the stability of its Pt shell during systemic circulation (Fig. 5A). Upon endocytosis by cancer cells, the nanogel responds to near-infrared (NIR) light as an external stimulus, generating a photothermal reaction (Fig. 5B) and triggering metal–metal interactions to release Pt2+ ions (Fig. 5C‒E). This results in synergistic damage to cancer cell DNA.
Figure 5.
Responsive release endows metal nanoparticles with spatiotemporal selectivity for activation. (A) In the absence of light, chemotherapy Pt2+ ions are tightly bound to the surface of PtNCs, effectively reducing adverse drug leakage and non-selective damage to normal tissues/cells. Upon exposure, PtNCs generate a large amount of heat through photothermal conversion of the Pt0 core, while triggering the rapid release of chemotherapy Pt2+ ions, resulting in a synergistic spatiotemporal photothermal-chemotherapy effect. (B) Infrared thermography of PtNCs with various Pt2+/Pt0 ratios after 10 min of 808 nm light exposure (1.0 W/cm2). (C) Hyperthermia-activated release profile of Pt2+ ions from PtNCs under five light exposures of 5 min at 1.0 W/cm2 at the interval of 1 h. (D) Long-term release profile of Pt2+ ions from PtNCs under six light exposures of 5 min at 1.0 W/cm2 at the interval of 2 days. (E) Release profile of Pt2+ ions from PtNCs under incubation at 37, 40, 60, and 80 °C in the dark. Reprinted with the permission from Ref. 66. Copyright © 2019 American Chemical Society.
Another strategy for responsive release is to utilize endogenous factors within the tumor to stimulate drug release. Our group12 designed tumor pH-sensitive Pt nanocluster assemblies (Pt–NAs) by immobilizing ultrasmall Pt nanoclusters (Pt–NCs) in a pH-sensitive polymer and derivatizing them with HCC-targeting peptides. After targeted entry into the acidic subcellular compartments of the tumor, they decompose into smaller-sized Pt nanoclusters, thereby accelerating the release and accumulation of toxic Pt ions intracellularly, achieving effective cancer treatment. The responsive release capability can also work in synergy with reversing tumor drug resistance133. Ling et al.67 designed a redox-responsive Pt prodrug nanoplatform capable of depleting GSH. The platinum prodrug remains stable during systemic circulation but it is decomposed by the GSH redox system in cancer cells, thereby promoting the massive release of Pt2+ for direct damage to tumor DNA while depleting GSH to alleviate drug resistance. This greatly enhances efficacy without weakening biological safety.
4.3. Surface modification
The surface modification of metal nanoparticles is indispensable for their multifunctionality, as unmodified highly reactive metal nanoparticles are prone to oxidation, deactivation, and agglomeration134, which can significantly reduce their biological activity and limit their therapeutic potential. By modifying the surface of metal nanoparticles, researchers can enhance their stability, solubility, and biocompatibility, while also introducing additional functional groups to enable targeted drug delivery and imaging capabilities135. Additionally, surface modification can help to control the release of drugs from metal nanoparticles, allowing for more precise spatiotemporal delivery and reducing the risk of off-target effects136.
Encapsulating metal nanoparticles with hydrophilic groups such as polyethylene glycol (PEG) is a common optimization method that can improve various shortcomings of metal nanoparticles, including dispersion, in vivo circulation, stability, and most importantly, toxicity reduction137, 138, 139. Chakraborti et al.68 used PEG to encapsulate ZnO nanoparticles, shielding the non-specific toxicity of ZnO to normal healthy cells and improving its solubility in biological media. This allowed the nanoparticles to exert their effect by inducing apoptosis in breast cancer cells through reactive oxygen-dependent damage to the DNA repair enzyme NEIL2140. Maltzahn and colleagues69 utilized PEG encapsulation of gold nanorods to enhance their in vivo circulation time and ability to passively accumulate in metastatic foci. This enabled the complete ablation of tumor tissue with only half the light intensity required for conventional photothermal therapy. Different surface modification techniques bring different functional optimizations to metal nanoparticles. Oyewumi et al.141 compared the pharmacokinetic characteristics of Gd-based nanoparticles coated with folic acid and PEG, respectively. Both folic acid-coated and PEG-coated nanoparticles exhibited considerable tumor accumulation. However, compared to PEG-coated nanoparticles, folic acid-coated nanoparticles showed significantly enhanced cellular uptake and tumor retention. This may be due to the high affinity binding of folic acid loaded on the surface of metal nanoparticles to the overexpressed folic acid receptors on the surface of cancer cells, and internalization through receptor-mediated endocytosis142.
Surface modification can also be used as a functional module loaded onto a metal nanoparticle platform to synergize with the DNA damage capability of the metal nanoparticles themselves. Zhao and colleagues70 constructed ribonuclease (RNase) conjugated glycol-gold nanoparticles (R-GAuNPs). The sugar polymer pMAG can act as a ligand binding to the cancer cell membrane and also increase the stability of RNase143. Under NIR, AuNPs produce photothermal effects, reaching a temperature of about 45 °C, damaging cancer cell DNA while approaching the optimal temperature for RNase and the melting temperature of RNA144, resulting in dual-mode damage to the genetic material of cancer cells and inhibiting the growth of cancer cells. The modular design of surface modification allows metal nanoparticles to integrate multiple functions, including imaging, photothermal conversion, and chemotherapy drug delivery. Fu et al.71 performed PEGylation on the surface of mesoporous platinum nanoparticles to ensure the long circulation ability of the nanoparticle platform. Doxorubicin was then loaded through electrostatic adsorption to prepare PEG@Pt/Dox. PEG@Pt/Dox can slowly release Dox in the acidic environment inside cancer cells while responding to 808 nm laser irradiation with mesoporous platinum nanoparticles to produce photothermal effects, synergistically damaging cancer cell DNA and inducing apoptosis. In addition, the Pt core can guide the progress of PTT through computed tomography (CT) imaging.
Surface modification of metal nanoparticles can also synergize with the metal core. Wang et al.72 developed a lanthanide scintillator (LnPS) coated with a TiO2 layer (Fig. 6A). LnPS can absorb radiation energy and convert it into UV–Visible light, but its weak catalytic ability is insufficient to support the generation of superoxide radicals required to induce DNA damage. The emission spectrum of LnPS precisely matches the absorption spectrum range of titanium dioxide, enabling efficient energy transfer (Fig. 6B). By modifying LnPS with a TiO2 coating, it is transformed into a TiO2 photocatalytic energy source, generating a large number of superoxide radicals to induce DNA damage (Fig. 6C and D), thereby killing cancer cells and inhibiting tumor proliferation (Fig. 6E).
Figure 6.
The synergistic interplay between the core and shell structure of LnPS@TiO2 maximizes the utilization of radiotherapy energy. (A) Lanthanide-doped scintillators absorb radiation and convert it into visible light, which in turn excites the surface TiO2 coating and generates a large amount of superoxide radicals. (B) Fluorescence spectra of LnPS (cyan) and LnPS@TiO2 (pink), compared with the absorption spectrum of TiO2 (blue). (C) DPBF was used as a probe to compare the production of reactive oxygen species in four groups under different radiation doses. (D) Quantification of fluorescence based on immunofluorescence assay of γ-H2AX of tumor tissues after different treatment. (E) Tumor growth curves of mice treated with different treatment. Reprinted with the permission from Ref. 72. Copyright © 2022 The Royal Society of Chemistry.
5. Summary and perspective
Metal nanoparticles have emerged as a versatile therapeutic platform that optimizes DNA damage-based cancer treatments through the integration of various components. In this review, we have provided a comprehensive summary of the key mechanisms and limitations of DNA damage-based cancer therapies. Metal nanoparticles provide a diverse range of optimization strategies to enhance biological processes beyond direct DNA damage induction. These strategies encompass targeted drug delivery to the nucleus, inhibition of DNA repair mechanisms, and induction of genotoxic cell death. By facilitating the precise delivery of therapeutic agents to the nucleus, metal nanoparticles can increase the intracellular concentration of DNA-damaging agents, maximizing their impact on tumor cells. Moreover, through the inhibition of DNA repair mechanisms, metal nanoparticles impede the repair of DNA damage, thereby enhancing the efficacy of conventional cancer treatments. Additionally, metal nanoparticles have the potential to synergize with tumor immunotherapy, harnessing the immune system to mount an effective response against cancer cells. By integrating these optimization strategies, metal nanoparticles offer promising prospects for refining cancer treatments and elevating overall therapeutic outcomes. We have explored the underlying physical and chemical principles governing these optimization strategies, including particle size, responsive release, and surface modification, thereby providing valuable insights for the future design of metal-based DNA damage agents.
However, the clinical translation of metal nanoparticles for DNA damage-based cancer therapy faces several challenges. Despite their unique physicochemical properties suitable for biomedical applications, concerns persist regarding the biotoxicity of metal nanoparticles79,143,144. For instance, gold nanoparticles tend to accumulate in the liver and spleen, leading to varying degrees of visceral inflammation145. Moreover, the biodistribution of gold nanoparticles is size-dependent, with smaller sizes offering excellent cellular nuclear penetration capability, essential for inducing DNA damage, but also resulting in extensive systemic distribution, including penetration across the blood–brain barrier146. Platinum nanoparticles face a delicate balance between safety and efficacy. While higher drug loading enables better DNA damage efficiency, it also carries the risk of off-target toxicity associated with platinum147. Therefore, achieving rational design and development of safer and more biocompatible metal nanoparticles through meticulous characterization and customization is crucial. Comprehensive toxicology studies are also necessary to thoroughly evaluate their biological safety. Furthermore, the design of targeted metal nanoparticles often overlooks the continuous biological barriers encountered by the vasculature system to deep-seated tumors78, 79, 80,82,143. To enhance drug accumulation at the tumor site and minimize off-target toxicity in normal tissues, exploring multiple targeting strategies within a single delivery system is recommended. This involves combining different targeting schemes to create a multi-faceted targeting strategy.
To mitigate the potential adverse effects associated with metals that exhibit potent DNA damaging properties, researchers often incorporate safety mechanisms into metal nanoparticle systems. These safety measures include the integration of responsive release switches or the application of surface coatings to enhance the stability and controlled release of the nanoparticles. However, overly complex designs of metal nanoparticles may result in unpredictable interactions among various factors and hinder their clinical translation72,148,149. Therefore, it is imperative to investigate simple and effective metal drug design strategies while simultaneously focusing on large-scale production and standardized preparation techniques for metal nanoparticles to ensure reproducibility and safety in clinical settings. Moreover, many metal nanoparticles used for DNA damage therapy typically rely on the introduction of external energy sources to activate their DNA-damaging capabilities, such as radiotherapy, photothermal therapy, and photodynamic therapy. Theoretically, the incorporation of multiple therapeutic strategies resembles an “AND gate” logic circuit, allowing for precise targeting of tumor DNA damage. However, in practice, tumors located deep within the body or non-solid tumors may not be efficiently targeted, leading to the indiscriminate or ineffective action of metal nanoparticles. Compared to the current mainstream organic nanocarriers, metal nanoparticles have certain limitations in addition to the aforementioned safety concerns. Their relatively smaller drug-loading capacity restricts their ability to carry a number of drugs, especially for large molecular or high-dose drugs, leading to a narrow therapeutic window150,151. Organic nanocarriers, on the other hand, typically have larger drug-loading space, higher drug-loading efficiency, and excellent biocompatibility, allowing for effective encapsulation and release of multiple drugs without compromising safety concerns associated with the carrier152,153. These limitations pose challenges in terms of achieving optimal targeting and therapeutic efficacy, highlighting the need for further research and development in the field of metal nanoparticles for DNA damage therapy. These limitations pose challenges in achieving optimal targeting and therapeutic efficacy, highlighting the need for further research and development in the field of metal nanoparticles for DNA damage therapy. Innovative strategies and advancements in metal nanoparticle technology are required to overcome these obstacles and fully harness their potential in precision cancer treatment.
We firmly believe that with a deeper understanding of the toxicology and pharmacology mechanisms associated with metal nanoparticles, a modular and customizable metal nanoparticles platform can be developed to fully optimize DNA damage-based cancer treatments and gradually transition towards clinical applications.
Acknowledgments
This work was funded by National Key Research and Development Program of China (2022YFB3203804, 2022YFB3203801, 2022YFB3203800), the Leading Talent of “Ten Thousand Plan”-National High-Level Talents Special Support Plan, National Natural Science Foundation of China (32071374, 32000985), Program of Shanghai Academic Research Leader under the Science and Technology Innovation Action Plan (21XD1422100, China), Program of Shanghai Science and Technology Development (22TS1400700, China), Zhejiang Provincial Natural Science Foundation of China (LR22C100001, LQ21H300003, China), Innovative Research Team of High-Level Local Universities in Shanghai (SHSMU-ZDCX20210900, China), and CAS Interdisciplinary Innovation Team (JCTD-2020-08, China).
Author contributions
Qian Chen, Chunyan Fang, and Fan Xia reviewed the literature, wrote the text, and created figures. Qian Chen, Chunyan Fang, Fan Xia and Qiyue Wang revised the manuscript. Fangyuan Li and Daishun Ling supervised Qian Chen, Chunyan Fang, and Fan Xia to revise the manuscript. All of the authors have read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Fangyuan Li, Email: lfy@zju.edu.cn.
Daishun Ling, Email: dsling@sjtu.edu.cn.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA-Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Basu A.K. DNA damage, mutagenesis and cancer. Int J Mol Sci. 2018;19:4–970. doi: 10.3390/ijms19040970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lord C.J., Ashworth A. The DNA damage response and cancer therapy. Nature. 2012;481:287–294. doi: 10.1038/nature10760. [DOI] [PubMed] [Google Scholar]
- 4.O'Connor M.J. Targeting the DNA damage response in cancer. Mol Cell. 2015;60:547–560. doi: 10.1016/j.molcel.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 5.Chatterjee N., Walker G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ Mol Mutagen. 2017;58:235–263. doi: 10.1002/em.22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearl L.H., Schierz A.C., Ward S.E., Al-Lazikani B., Pearl F.M. Therapeutic opportunities within the DNA damage response. Nat Rev Cancer. 2015;15:166–180. doi: 10.1038/nrc3891. [DOI] [PubMed] [Google Scholar]
- 7.Cheung-Ong K., Giaever G., Nislow C. DNA-damaging agents in cancer chemotherapy: serendipity and chemical biology. Chem Biol. 2013;20:648–659. doi: 10.1016/j.chembiol.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Shi J., Kantoff P.W., Wooster R., Farokhzad O.C. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17:20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu X., Li F.Y., Noor N., Ling D.S. Platinum drugs: from Pt(II) compounds, Pt(IV) prodrugs, to Pt nanocrystals/nanoclusters. Sci Bull. 2017;62:589–596. doi: 10.1016/j.scib.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Wang H., Mu X., He H., Zhang X.D. Cancer radiosensitizers. Trends Pharmacol Sci. 2018;39:24–48. doi: 10.1016/j.tips.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Huang J.L., Lin L.Q., Sun D.H., Chen H.M., Yang D.P., Li Q.B. Bio-inspired synthesis of metal nanomaterials and applications. Chem Soc Rev. 2015;44:6330–6374. doi: 10.1039/c5cs00133a. [DOI] [PubMed] [Google Scholar]
- 12.Xia H.P., Li F.Y., Hu X., Wooram P., Wang S.F., Jang Y.J., et al. pH-Sensitive Pt nanocluster assembly overcomes cisplatin resistance and heterogeneous stemness of hepatocellular carcinoma. ACS Cent Sci. 2016;11:802–811. doi: 10.1021/acscentsci.6b00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Y.Y., Yu Y.J., Chen H., Meng X.X., Ma W., Yu M., et al. Illuminating platinum transportation while maximizing therapeutic efficacy by gold nanoclusters via simultaneous near-Infrared-I/II imaging and glutathione scavenging. ACS Nano. 2020;14:13536–13547. doi: 10.1021/acsnano.0c05541. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y.X., Chen J., Duan R.M., Gu R., Wang W.R., Wu J.H., et al. High-Z-sensitized radiotherapy synergizes with the intervention of the pentose phosphate pathway for in situ tumor vaccination. Adv Mater. 2022;34 doi: 10.1002/adma.202109726. [DOI] [PubMed] [Google Scholar]
- 15.Li N., Sun Q.Q., Yu Z.Z., Gao X.N., Pan W., Wan X.Y., et al. Nuclear-targeted photothermal therapy prevents cancer recurrence with near-infrared triggered copper sulfide nanoparticles. ACS Nano. 2018;12:5197–5206. doi: 10.1021/acsnano.7b06870. [DOI] [PubMed] [Google Scholar]
- 16.Hosoya N., Miyagawa K. Targeting DNA damage response in cancer therapy. Cancer Sci. 2014;105:370–388. doi: 10.1111/cas.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kloeber J.A., Lou Z. Critical DNA damaging pathways in tumorigenesis. Semin Cancer Biol. 2022;85:164–184. doi: 10.1016/j.semcancer.2021.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoeijmakers J.H. DNA damage, aging, and cancer. N Engl J Med. 2009;361:1475–1485. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- 19.Lodovichi S., Cervelli T., Pellicioli A., Galli A. Inhibition of DNA repair in cancer therapy: toward a multi-target approach. Int J Mol Sci. 2020;21:6684. doi: 10.3390/ijms21186684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson S.P., Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campos A., Clemente-Blanco A. Cell cycle and DNA repair regulation in the damage response: protein phosphatases take over the reins. Int J Mol Sci. 2020;21:446. doi: 10.3390/ijms21020446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthews H.K., Bertoli C., de Bruin R.A.M. Cell cycle control in cancer. Nat Rev Mol Cell Biol. 2022;23:74–88. doi: 10.1038/s41580-021-00404-3. [DOI] [PubMed] [Google Scholar]
- 23.Czajkowski D., Szmyd R., Gee H.E. Impact of DNA damage response defects in cancer cells on response to immunotherapy and radiotherapy. J Med Imaging Radiat Oncol. 2022;66:546–559. doi: 10.1111/1754-9485.13413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helleday T., Petermann E., Lundin C., Hodgson B., Sharma R.A. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer. 2008;8:193–204. doi: 10.1038/nrc2342. [DOI] [PubMed] [Google Scholar]
- 25.Chabanon R.M., Rouanne M., Lord C.J., Soria J.C., Pasero P., Postel-Vinay S. Targeting the DNA damage response in immuno-oncology: developments and opportunities. Nat Rev Cancer. 2021;21:701–717. doi: 10.1038/s41568-021-00386-6. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein M., Kastan M.B. The DNA damage response: implications for tumor responses to radiation and chemotherapy. Annu Rev Med. 2015;66:129–143. doi: 10.1146/annurev-med-081313-121208. [DOI] [PubMed] [Google Scholar]
- 27.Cromie G.A., Connelly J.C., Leach D.R. Recombination at double-strand breaks and DNA ends: conserved mechanisms from phage to humans. Mol Cell. 2001;8:1163–1174. doi: 10.1016/s1097-2765(01)00419-1. [DOI] [PubMed] [Google Scholar]
- 28.Nikjoo H., Uehara S., Wilson W.E., Hoshi M., Goodhead D.T. Track structure in radiation biology: theory and applications. Int J Radiat Biol. 1998;73:355–364. doi: 10.1080/095530098142176. [DOI] [PubMed] [Google Scholar]
- 29.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 30.Goldstein M., Roos W.P., Kaina B. Apoptotic death induced by the cyclophosphamide analogue mafosfamide in human lymphoblastoid cells: contribution of DNA replication, transcription inhibition and Chk/p53 signaling. Toxicol Appl Pharmacol. 2008;229:20–32. doi: 10.1016/j.taap.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Fichtinger-Schepman A.M., van der Veer J.L., den Hartog J.H., Lohman P.H., Reedijk J. Adducts of the antitumor drug cis-diamminedichloroplatinum (II) with DNA: formation, identification, and quantitation. Biochemistry. 1985;24:707–713. doi: 10.1021/bi00324a025. [DOI] [PubMed] [Google Scholar]
- 32.Lomax M.E., Folkes L.K., O'Neill P. Biological consequences of radiation-induced DNA damage: relevance to radiotherapy. Clin Oncol. 2013;25:578–585. doi: 10.1016/j.clon.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 33.Chen L., Huang Q., Zhao T.J., Sui L.H., Wang S.Y., Xiao Z.X., et al. Nanotherapies for sepsis by regulating inflammatory signals and reactive oxygen and nitrogen species: new insight for treating COVID-19. Redox Biol. 2021;45 doi: 10.1016/j.redox.2021.102046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tiwari M. Antimetabolites: established cancer therapy. J Cancer Res Ther. 2012;8:510–519. doi: 10.4103/0973-1482.106526. [DOI] [PubMed] [Google Scholar]
- 35.Delgado J.L., Hsieh C.M., Chan N.L., Hiasa H. Topoisomerases as anticancer targets. Biochem J. 2018;475:373–398. doi: 10.1042/BCJ20160583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mini E., Nobili S., Caciagli B., Landini I., Mazzei T. Cellular pharmacology of gemcitabine. Ann Oncol. 2006;175:7–12. doi: 10.1093/annonc/mdj941. [DOI] [PubMed] [Google Scholar]
- 37.Abolmaali S.S., Tamaddon A.M., Dinarvand R. A review of therapeutic challenges and achievements of methotrexate delivery systems for treatment of cancer and rheumatoid arthritis. Cancer Chemother Pharmacol. 2013;71:1115–1130. doi: 10.1007/s00280-012-2062-0. [DOI] [PubMed] [Google Scholar]
- 38.Baldwin E.L., Osheroff N. Etoposide, topoisomerase II and cancer. Curr Med Chem Anticancer Agents. 2005;5:363–372. doi: 10.2174/1568011054222364. [DOI] [PubMed] [Google Scholar]
- 39.Rivankar S. An overview of doxorubicin formulations in cancer therapy. J Cancer Res Ther. 2014;10:853–858. doi: 10.4103/0973-1482.139267. [DOI] [PubMed] [Google Scholar]
- 40.Lasorsa A., Nardella M.I., Rosato A., Mirabelli V., Caliandro R., Caliandro R., et al. Mechanistic and structural basis for inhibition of copper trafficking by platinum anticancer drugs. J Am Chem Soc. 2019;141:12109–12120. doi: 10.1021/jacs.9b05550. [DOI] [PubMed] [Google Scholar]
- 41.Long X.Y., Zhang X.J., Chen Q.H., Liu M., Xiang Y.T., Yang Y.Q., et al. Nucleus-targeting phototherapy nanodrugs for high-effective anti-cancer treatment. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.905375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao Y., Wu T.T., Zhang K., Meng X.D., Dai W.H., Wang D.D., et al. Engineered exosome-mediated near-infrared-II region V(2)C quantum dot delivery for nucleus-target low-temperature photothermal therapy. ACS Nano. 2019;13:1499–1510. doi: 10.1021/acsnano.8b07224. [DOI] [PubMed] [Google Scholar]
- 43.DeGorter M.K., Xia C.Q., Yang J.J., Kim R.B. Drug transporters in drug efficacy and toxicity. Annu Rev Pharmacol Toxicol. 2012;52:249–273. doi: 10.1146/annurev-pharmtox-010611-134529. [DOI] [PubMed] [Google Scholar]
- 44.Zhitomirsky B., Assaraf Y.G. Lysosomes as mediators of drug resistance in cancer. Drug Resist Updates. 2016;24:23–33. doi: 10.1016/j.drup.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 45.Szakács G., Hall M.D., Gottesman M.M., Boumendjel A., Kachadourian R., Day B.J., et al. Targeting the Achilles heel of multidrug-resistant cancer by exploiting the fitness cost of resistance. Chem Rev. 2014;114:5753–5774. doi: 10.1021/cr4006236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lai Y.-H., Kuo C., Kuo M.T., Chen H.H.W. Modulating chemosensitivity of tumors to platinum-based antitumor drugs by transcriptional regulation of copper homeostasis. Int J Mol Sci. 2018;19:1486. doi: 10.3390/ijms19051486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galadari S., Rahman A., Pallichankandy S., Thayyullathil F. Reactive oxygen species and cancer paradox: to promote or to suppress? Free Radical Biol Med. 2017;104:144–164. doi: 10.1016/j.freeradbiomed.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 48.Nunes J.H.B., Hager S., Mathuber M., Pósa V., Roller A., Enyedy E.A., et al. Cancer cell resistance against the clinically investigated thiosemicarbazone COTI-2 is based on formation of intracellular copper complex glutathione adducts and ABCC1-mediated efflux. J Med Chem. 2020;63:13719–13732. doi: 10.1021/acs.jmedchem.0c01277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riddell I.A. Cisplatin and oxaliplatin: our current understanding of their actions. Met Ions Life Sci. 2018;18:1–42. doi: 10.1515/9783110470734-007. [DOI] [PubMed] [Google Scholar]
- 50.Damia G., Broggini M. Platinum resistance in ovarian cancer: role of DNA repair. Cancers. 2019;11:119. doi: 10.3390/cancers11010119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martinez-Balibrea E., Martínez-Cardús A., Ginés A., Ruiz de Porras V., Moutinho C., Layos L., et al. Tumor-related molecular mechanisms of oxaliplatin resistance. Mol Cancer Therapeut. 2015;14:1767–1776. doi: 10.1158/1535-7163.MCT-14-0636. [DOI] [PubMed] [Google Scholar]
- 52.Brabec V., Kasparkova J. Modifications of DNA by platinum complexes: relation to resistance of tumors to platinum antitumor drugs. Drug Resist Updates. 2005;8:131–146. doi: 10.1016/j.drup.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 53.Zhang X.L., Yi C., Zhang L.J., Zhu X.Y., He Y., Lu H.Z., et al. Size-optimized nuclear-targeting phototherapy enhances the type I interferon response for “cold” tumor immunotherapy. Acta Biomater. 2023;159:338–352. doi: 10.1016/j.actbio.2023.01.023. [DOI] [PubMed] [Google Scholar]
- 54.Phuong P.T.M., Won H.J., Robby A.I., Kim S.G., Im G.B., Bhang S.H., et al. NIR-vis-induced pH-sensitive TiO2 immobilized carbon dot for controllable membrane-nuclei targeting and photothermal therapy of cancer cells. ACS Appl Mater Interfaces. 2020;12:37929–37942. doi: 10.1021/acsami.0c11979. [DOI] [PubMed] [Google Scholar]
- 55.Gao G., Jiang Y.W., Jia H.R., Sun W., Guo Y., Yu X.W., et al. From perinuclear to intranuclear localization: a cell-penetrating peptide modification strategy to modulate cancer cell migration under mild laser irradiation and improve photothermal therapeutic performance. Biomaterials. 2019;223 doi: 10.1016/j.biomaterials.2019.119443. [DOI] [PubMed] [Google Scholar]
- 56.Aioub M., El-Sayed M.A. A real-time surface enhanced Raman spectroscopy study of plasmonic photothermal cell death using targeted gold nanoparticles. J Am Chem Soc. 2016;138:1258–1264. doi: 10.1021/jacs.5b10997. [DOI] [PubMed] [Google Scholar]
- 57.Li F.Y., Sun H., Ren J.F., Zhang B., Hu X., Fang C.Y., et al. A nuclease-mimetic platinum nanozyme induces concurrent DNA platination and oxidative cleavage to overcome cancer drug resistance. Nat Commun. 2022;13:7361. doi: 10.1038/s41467-022-35022-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dong Y., Liao H.Z., Fu H., Yu J., Guo Q.Q., Wang Q., et al. pH-Sensitive shell–core platform block DNA repair pathway to amplify irreversible DNA damage of triple negative breast cancer. ACS Appl Mater Interfaces. 2019;11:38417–38428. doi: 10.1021/acsami.9b12140. [DOI] [PubMed] [Google Scholar]
- 59.Fu Z., Liu Z., Wang J.X., Deng L.F., Wang H., Tang W., et al. Interfering biosynthesis by nanoscale metal-organic frameworks for enhanced radiation therapy. Biomaterials. 2023;295 doi: 10.1016/j.biomaterials.2023.122035. [DOI] [PubMed] [Google Scholar]
- 60.Yu J., He X.D., Zhang Q.F., Zhou D.F., Wang Z.G., Huang Y.G. Iodine conjugated Pt(IV) nanoparticles for precise chemotherapy with iodine–Pt guided computed tomography imaging and biotin-mediated tumor-targeting. ACS Nano. 2022;16:6835–6846. doi: 10.1021/acsnano.2c01764. [DOI] [PubMed] [Google Scholar]
- 61.Wang G.H., Li B., Tian H., Xie L.S., Yan J., Sang W., et al. A metal–phenolic nanocoordinator launches radiotherapeutic cancer pyroptosis through an epigenetic mechanism. Adv Funct Mater. 2023;33 [Google Scholar]
- 62.Li T., Zhang Y., Zhu J., Zhang F.R., Xu A.A., Zhou T., et al. A pH-activatable copper-biomineralized proenzyme for synergistic chemodynamic/chemo-immunotherapy against aggressive cancers. Adv. Mater. 2023;35 doi: 10.1002/adma.202210201. [DOI] [PubMed] [Google Scholar]
- 63.Zhou Q.H., Dutta D., Cao Y.F., Ge Z.S. Oxidation-responsive polyMOF nanoparticles for combination photodynamic-immunotherapy with enhanced STING activation. ACS Nano. 2023;17:9374–9387. doi: 10.1021/acsnano.3c01333. [DOI] [PubMed] [Google Scholar]
- 64.Deng Z., Xi M., Zhang C., Wu X.R., Li Q.G., Wang C.J., et al. Biomineralized MnO2 nanoplatforms mediated delivery of immune checkpoint inhibitors with STING pathway activation to potentiate cancer radio-immunotherapy. ACS Nano. 2023;17:4495–4506. doi: 10.1021/acsnano.2c10352. [DOI] [PubMed] [Google Scholar]
- 65.Lei L.L., Dong Z., Xu L., Yang F.R., Yin B.L., Wang Y.J., et al. Metal-fluorouracil networks with disruption of mitochondrion enhanced ferroptosis for synergistic immune activation. Theranostics. 2022;12:6207–6222. doi: 10.7150/thno.75323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao H., Xu J.B., Huang W.J., Zhan G.T., Zhao Y.B., Chen H.B., et al. Spatiotemporally light-activatable platinum nanocomplexes for selective and cooperative cancer therapy. ACS Nano. 2019;13:6647–6661. doi: 10.1021/acsnano.9b00972. [DOI] [PubMed] [Google Scholar]
- 67.Ling X., Tu J., Wang J., Shajii A., Kong N., Feng C., et al. Glutathione-responsive prodrug nanoparticles for effective drug delivery and cancer therapy. ACS Nano. 2019;13:357–370. doi: 10.1021/acsnano.8b06400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chakraborti S., Chakraborty S., Saha S., Manna A., Banerjee S., Adhikary A., et al. PEG-functionalized zinc oxide nanoparticles induce apoptosis in breast cancer cells through reactive oxygen species-dependent impairment of DNA damage repair enzyme NEIL2. Free Radical Biol Med. 2017;103:35–47. doi: 10.1016/j.freeradbiomed.2016.11.048. [DOI] [PubMed] [Google Scholar]
- 69.von Maltzahn G., Park J.H., Agrawal A., Bandaru N.K., Das S.K., Sailor M.J., et al. Computationally guided photothermal tumor therapy using long-circulating gold nanorod antennas. Cancer Res. 2009;69:3892–3900. doi: 10.1158/0008-5472.CAN-08-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao Y.X., Jia R., Liu Y.P., Shen X., Wang H.W., Yuan L. Specific photothermal killing of cancer cells by RNase-conjugated glyco-gold nanoparticles. Mater Today Commun. 2021;28 [Google Scholar]
- 71.Fu B., Dang M., Tao J., Li Y.J., Tang Y.X. Mesoporous platinum nanoparticle-based nanoplatforms for combined chemo-photothermal breast cancer therapy. J Colloid Interface Sci. 2020;570:197–204. doi: 10.1016/j.jcis.2020.02.051. [DOI] [PubMed] [Google Scholar]
- 72.Wang C., Wang H.R., Luo L.F., Gan S.J., Yao Y.F., Wei Q.H., et al. Scintillator-based radiocatalytic superoxide radical production for long-term tumor DNA damage. Biomater Sci. 2022;10:3433–3440. doi: 10.1039/d2bm00101b. [DOI] [PubMed] [Google Scholar]
- 73.Song G.S., Cheng L., Chao Y., Yang K., Liu Z. Emerging nanotechnology and advanced materials for cancer radiation therapy. Adv Mater. 2017;29 doi: 10.1002/adma.201700996. [DOI] [PubMed] [Google Scholar]
- 74.Han K., Zhang W.Y., Zhang J., Lei Q., Wang S.B., Liu J.W., et al. Acidity-triggered tumor-targeted chimeric peptide for enhanced intra-nuclear photodynamic therapy. Adv Funct Mater. 2016;26:4351–4361. [Google Scholar]
- 75.Fu X., Shi Y., Qi T., Qiu S., Huang Y., Zhao X., et al. Precise design strategies of nanomedicine for improving cancer therapeutic efficacy using subcellular targeting. Signal Transduct Tar. 2020;5:262. doi: 10.1038/s41392-020-00342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim Y.K., Kang E.B., Kim S.H., Sharker S.M., Kong B.Y., In I., et al. Visible-light-driven photocatalysts of perfluorinated silica-based fluorescent carbon dot/TiO2 for tunable hydrophilic–hydrophobic surfaces. ACS Appl Mater Interfaces. 2016;8:29827–29834. doi: 10.1021/acsami.6b12618. [DOI] [PubMed] [Google Scholar]
- 77.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzym Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 78.Attia M.F., Anton N., Wallyn J., Omran Z., Vandamme T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J Pharm Pharmacol. 2019;71:1185–1198. doi: 10.1111/jphp.13098. [DOI] [PubMed] [Google Scholar]
- 79.Pan L.M., Liu J.N., Shi J.L. Cancer cell nucleus-targeting nanocomposites for advanced tumor therapeutics. Chem Soc Rev. 2018;47:6930–6946. doi: 10.1039/c8cs00081f. [DOI] [PubMed] [Google Scholar]
- 80.Tkachenko A.G., Xie H., Coleman D., Glomm W., Ryan J., Anderson M.F., et al. Multifunctional gold nanoparticle−peptide complexes for nuclear targeting. J Am Chem Soc. 2003;125:4700–4701. doi: 10.1021/ja0296935. [DOI] [PubMed] [Google Scholar]
- 81.Jeang K.T., Xiao H., Rich E.A. Multifaceted activities of the HIV-1 transactivator of transcription, Tat. J Biol Chem. 1999;274:28837–28840. doi: 10.1074/jbc.274.41.28837. [DOI] [PubMed] [Google Scholar]
- 82.Pan L.M., He Q.J., Liu J.N., Chen Y., Ma M., Zhang L.L., et al. Nuclear-targeted drug delivery of TAT peptide-conjugated monodisperse mesoporous silica nanoparticles. J Am Chem Soc. 2012;134:5722–5725. doi: 10.1021/ja211035w. [DOI] [PubMed] [Google Scholar]
- 83.Li Z.H., Dong K., Huang S., Ju E., Liu Z., Yin M.L., et al. A smart nanoassembly for multistage targeted drug delivery and magnetic resonance imaging. Adv Funct Mater. 2014;24:3612–3620. [Google Scholar]
- 84.Makovec T. Cisplatin and beyond: molecular mechanisms of action and drug resistance development in cancer chemotherapy. Radiol Oncol. 2019;53:148–158. doi: 10.2478/raon-2019-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Song X., Wang S., Hong X., Li X., Zhao X., Huai C., et al. Single nucleotide polymorphisms of nucleotide excision repair pathway are significantly associated with outcomes of platinum-based chemotherapy in lung cancer. Sci Rep. 2017;7 doi: 10.1038/s41598-017-08257-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jackson S.P., Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Janićijević A., Sugasawa K., Shimizu Y., Hanaoka F., Wijgers N., Djurica M., et al. DNA bending by the human damage recognition complex XPC-HR23B. DNA Repair. 2003;2:325–336. doi: 10.1016/s1568-7864(02)00222-7. [DOI] [PubMed] [Google Scholar]
- 88.Missura M., Buterin T., Hindges R., Hübscher U., Kaspárková J., Brabec V., et al. Double-check probing of DNA bending and unwinding by XPA-RPA: an architectural function in DNA repair. EMBO J. 2001;20:3554–3564. doi: 10.1093/emboj/20.13.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sears C.R., Turchi J.J. Complex cisplatin-double strand break (DSB) lesions directly impair cellular non-homologous end-joining (NHEJ) independent of downstream damage response (DDR) pathways. J Biol Chem. 2012;287:24263–24272. doi: 10.1074/jbc.M112.344911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Varzandeh M., Labbaf S., Varshosaz J., Laurent S. An overview of the intracellular localization of high-Z nanoradiosensitizers. Prog Biophys Mol Biol. 2022;175:14–30. doi: 10.1016/j.pbiomolbio.2022.08.006. [DOI] [PubMed] [Google Scholar]
- 91.Deng Y.Y., Song P.Y., Chen X.H., Huang Y., Hong L.J., Jin Q., et al. 3-Bromopyruvate-conjugated nanoplatform-induced pro-death autophagy for enhanced photodynamic therapy against hypoxic tumor. ACS Nano. 2020;14:9711–9727. doi: 10.1021/acsnano.0c01350. [DOI] [PubMed] [Google Scholar]
- 92.Evan G., Littlewood T. A matter of life and cell death. Science. 1998;281:1317–1322. doi: 10.1126/science.281.5381.1317. [DOI] [PubMed] [Google Scholar]
- 93.Kaufmann S.H., Earnshaw W.C. Induction of apoptosis by cancer chemotherapy. Exp Cell Res. 2000;256:42–49. doi: 10.1006/excr.2000.4838. [DOI] [PubMed] [Google Scholar]
- 94.Roos W.P., Thomas A.D., Kaina B. DNA damage and the balance between survival and death in cancer biology. Nat Rev Cancer. 2016;16:20–33. doi: 10.1038/nrc.2015.2. [DOI] [PubMed] [Google Scholar]
- 95.Knudson C.M., Korsmeyer S.J. Bcl-2 and Bax function independently to regulate cell death. Nat Genet. 1997;16:358–363. doi: 10.1038/ng0897-358. [DOI] [PubMed] [Google Scholar]
- 96.Yin X.-M., Oltvai Z.N., Korsmeyer S.J. BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature. 1994;369:321–323. doi: 10.1038/369321a0. [DOI] [PubMed] [Google Scholar]
- 97.Liu X.H., Chen G.G., Vlantis A.C., Tse G.M., van Hasselt C.A. Iodine induces apoptosis via regulating MAPKs-related p53, p21, and Bcl-xL in thyroid cancer cells. Mol Cell Endocrinol. 2010;320:128–135. doi: 10.1016/j.mce.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 98.Fang M.Z., Wang Y., Ai N., Hou Z., Sun Y., Lu H., et al. Tea polyphenol (‒)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003;63:7563–7570. [PubMed] [Google Scholar]
- 99.Gopal P., Yard B., Petty A., Castrillon J., Patel J.D., Abazeed M.E. The mutational landscape of the sensitivity of cancer to ionizing radiation. J Clin Oncol. 2021;39:15–3129. [Google Scholar]
- 100.Wang Y.P., Gao W.P., Shi X.Y., Ding J.J., Liu W., He H.B., et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547:99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 101.Hou J.W., Zhao R.C., Xia W.Y., Chang C.W., You Y., Hsu J.M., et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat Cell Biol. 2020;22:1264–1275. doi: 10.1038/s41556-020-0575-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Irvine D.J., Dane E.L. Enhancing cancer immunotherapy with nanomedicine. Nat Rev Immunol. 2020;20:321–334. doi: 10.1038/s41577-019-0269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gotwals P., Cameron S., Cipolletta D., Cremasco V., Crystal A., Hewes B., et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer. 2017;17:286–301. doi: 10.1038/nrc.2017.17. [DOI] [PubMed] [Google Scholar]
- 104.Chen D.S., Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 105.Ngwa W., Irabor O.C., Schoenfeld J.D., Hesser J., Demaria S., Formenti S.C. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer. 2018;18:313–322. doi: 10.1038/nrc.2018.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roos W.P., Thomas A.D., Kaina B. DNA damage and the balance between survival and death in cancer biology. Nat Rev Cancer. 2016;16:20–33. doi: 10.1038/nrc.2015.2. [DOI] [PubMed] [Google Scholar]
- 107.Santivasi W.L., Xia F. Ionizing radiation-induced DNA damage, response, and repair. Antioxidants Redox Signal. 2014;21:251–259. doi: 10.1089/ars.2013.5668. [DOI] [PubMed] [Google Scholar]
- 108.Xu Y. DNA damage: a trigger of innate immunity but a requirement for adaptive immune homeostasis. Nat Rev Immunol. 2006;6:261–270. doi: 10.1038/nri1804. [DOI] [PubMed] [Google Scholar]
- 109.Schoggins J.W., Wilson S.J., Panis M., Murphy M.Y., Jones C.T., Bieniasz P., et al. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472:481–485. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dunphy G., Flannery S.M., Almine J.F., Connolly D.J., Paulus C., Jønsson K.L., et al. Non-canonical activation of the DNA sensing adaptor STING by ATM and IFI16 mediates NF-κB signaling after nuclear DNA damage. Mol Cell. 2018;71:745–760. doi: 10.1016/j.molcel.2018.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lv M.Z., Chen M.X., Zhang R., Zhang W., Wang C.G., Zhang Y., et al. Manganese is critical for antitumor immune responses via cGAS-STING and improves the efficacy of clinical immunotherapy. Cell Res. 2020;30:966–979. doi: 10.1038/s41422-020-00395-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jiang W., Chan C.K., Weissman I.L., Kim B.Y.S., Hahn S.M. Immune priming of the tumor microenvironment by radiation. Trends Cancer. 2016;2:638–645. doi: 10.1016/j.trecan.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 113.Melero I., Hervas-Stubbs S., Glennie M., Pardoll D.M., Chen L.P. Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer. 2007;7:95–106. doi: 10.1038/nrc2051. [DOI] [PubMed] [Google Scholar]
- 114.Mellman I., Coukos G., Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Reisländer T., Groelly F.J., Tarsounas M. DNA damage and cancer immunotherapy: a STING in the tale. Mol Cell. 2020;80:21–28. doi: 10.1016/j.molcel.2020.07.026. [DOI] [PubMed] [Google Scholar]
- 116.Dreaden E.C., Alkilany A.M., Huang X.H., Murphy C.J., El-Sayed M.A. The golden age: gold nanoparticles for biomedicine. Chem Soc Rev. 2012;41:2740–2779. doi: 10.1039/c1cs15237h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Çeşmeli S., Biray Avci C. Application of titanium dioxide (TiO2) nanoparticles in cancer therapies. J Drug Target. 2019;27:762–766. doi: 10.1080/1061186X.2018.1527338. [DOI] [PubMed] [Google Scholar]
- 118.Luo Y.H., Chang L.W., Lin P. Metal-based nanoparticles and the immune system: activation, inflammation, and potential applications. BioMed Res Int. 2015;2015 doi: 10.1155/2015/143720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hülsmann B.B., Labokha A.A., Görlich D. The permeability of reconstituted nuclear pores provides direct evidence for the selective phase model. Cell. 2012;150:738–751. doi: 10.1016/j.cell.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 120.Frey S., Görlich D. A saturated FG-repeat hydrogel can reproduce the permeability properties of nuclear pore complexes. Cell. 2007;130:512–523. doi: 10.1016/j.cell.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 121.Sakiyama Y., Mazur A., Kapinos L.E., Lim R.Y.H. Spatiotemporal dynamics of the nuclear pore complex transport barrier resolved by high-speed atomic force microscopy. Nat Nanotechnol. 2016;11:719–723. doi: 10.1038/nnano.2016.62. [DOI] [PubMed] [Google Scholar]
- 122.Jiang W., Kim B.Y.S., Rutka J.T., Chan W.C.W. Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol. 2008;3:145–150. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 123.Huang K.Y., Ma H.L., Liu J., Huo S.D., Kumar A., Wei T., et al. Size-dependent localization and penetration of ultrasmall gold nanoparticles in cancer cells, multicellular spheroids, and tumors in vivo. ACS Nano. 2012;6:4483–4493. doi: 10.1021/nn301282m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chithrani B.D., Ghazani A.A., Chan W.C.W. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6:662–668. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 125.Li H.J., Du J.Z., Du X.J., Xu C.F., Sun C.Y., Wang H.X., et al. Stimuli-responsive clustered nanoparticles for improved tumor penetration and therapeutic efficacy. Proc Natl Acad Sci U S A. 2016;113:4164–4169. doi: 10.1073/pnas.1522080113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Godin B., Tasciotti E., Liu X.W., Serda R.E., Ferrari M. Multistage nanovectors: from concept to novel imaging contrast agents and therapeutics. Acc Chem Res. 2011;44:979–989. doi: 10.1021/ar200077p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ling D.S., Park W., Park Sj, Lu Y., Kim K.S., Hackett M.J., et al. Multifunctional tumor pH-sensitive self-assembled nanoparticles for bimodal imaging and treatment of resistant heterogeneous tumors. J Am Chem Soc. 2014;136:5647–5655. doi: 10.1021/ja4108287. [DOI] [PubMed] [Google Scholar]
- 128.Yatvin M.B., Weinstein J.N., Dennis W.H., Blumenthal R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science. 1978;202:1290–1293. doi: 10.1126/science.364652. [DOI] [PubMed] [Google Scholar]
- 129.Agasti S.S., Chompoosor A., You C.C., Ghosh P., Kim C.K., Rotello V.M., et al. Photoregulated release of caged anticancer drugs from gold nanoparticles. J Am Chem Soc. 2009;131:5728–5729. doi: 10.1021/ja900591t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang Y.C., Wang F., Sun T.M., Wang J. Redox-responsive nanoparticles from the single disulfide bond-bridged block copolymer as drug carriers for overcoming multidrug resistance in cancer cells. Bioconjugate Chem. 2011;22:1939–1945. doi: 10.1021/bc200139n. [DOI] [PubMed] [Google Scholar]
- 131.Mura S., Nicolas J., Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12:991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 132.Qiu M., Wang D., Liang W.Y., Liu L.P., Zhang Y., Chen X., et al. Novel concept of the smart NIR-light-controlled drug release of black phosphorus nanostructure for cancer therapy. Proc Natl Acad Sci USA. 2018;115:501–506. doi: 10.1073/pnas.1714421115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Valente A., Podolski-Renić A., Poetsch I., Filipović N., López Ó., Turel I., et al. Metal- and metalloid-based compounds to target and reverse cancer multidrug resistance. Drug Resist Updates. 2021;58 doi: 10.1016/j.drup.2021.100778. [DOI] [PubMed] [Google Scholar]
- 134.Kumar Y., Sinha A.S.K., Nigam K.D.P., Dwivedi D., Sangwai J.S. Functionalized nanoparticles: tailoring properties through surface energetics and coordination chemistry for advanced biomedical applications. Nanoscale. 2023;15:6075–6104. doi: 10.1039/d2nr07163k. [DOI] [PubMed] [Google Scholar]
- 135.Harris J.M., Chess R.B. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2:214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 136.Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 137.Jokerst J.V., Lobovkina T., Zare R.N., Gambhir S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine. 2011;6:715–728. doi: 10.2217/nnm.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Alkilany A.M., Thompson L.B., Boulos S.P., Sisco P.N., Murphy C.J. Gold nanorods: their potential for photothermal therapeutics and drug delivery, tempered by the complexity of their biological interactions. Adv Drug Deliv Rev. 2012;64:190–199. doi: 10.1016/j.addr.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 139.van Vlerken L.E., Vyas T.K., Amiji M.M. Poly(ethylene glycol)-modified nanocarriers for tumor-targeted and intracellular delivery. Pharm Res (N Y) 2007;24:1405–1414. doi: 10.1007/s11095-007-9284-6. [DOI] [PubMed] [Google Scholar]
- 140.Zhang H.J., Chen B.A., Jiang H., Wang C.L., Wang H.P., Wang X.M. A strategy for ZnO nanorod mediated multi-mode cancer treatment. Biomaterials. 2011;32:1906–1914. doi: 10.1016/j.biomaterials.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 141.Xie Z.J., Fan T.J., An J.S., Choi W., Duo Y.H., Ge Y.Q., et al. Emerging combination strategies with phototherapy in cancer nanomedicine. Chem Soc Rev. 2020;49:8065–8087. doi: 10.1039/d0cs00215a. [DOI] [PubMed] [Google Scholar]
- 142.Kunitz M. Crystalline ribonuclease. J Gen Physiol. 1940;24:15. doi: 10.1085/jgp.24.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xu J.J., Zhang W.C., Guo Y.W., Chen X.Y., Zhang Y.N. Metal nanoparticles as a promising technology in targeted cancer treatment. Drug Deliv. 2022;29:664–678. doi: 10.1080/10717544.2022.2039804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nel A.E., Mädler L., Velegol D., Xia T., Hoek E.M.V., Somasundaran P., et al. Understanding biophysicochemical interactions at the nano–bio interface. Nat Mater. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 145.Li T., Albee B., Alemayehu M., Diaz R., Ingham L., Kamal S., et al. Comparative toxicity study of Ag, Au, and Ag–Au bimetallic nanoparticles on Daphnia magna. Anal Bioanal Chem. 2010;398:689–700. doi: 10.1007/s00216-010-3915-1. [DOI] [PubMed] [Google Scholar]
- 146.De Jong W.H., Hagens W.I., Krystek P., Burger M.C., Sips A.J., Geertsma R.E. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials. 2008;12:1912–1919. doi: 10.1016/j.biomaterials.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 147.Apps M.G., Choi E.H., Wheate N.J. The state-of-play and future of platinum drugs. Endocr Relat Cancer. 2015;22:219–233. doi: 10.1530/ERC-15-0237. [DOI] [PubMed] [Google Scholar]
- 148.Tarkistani M.A.M., Komalla V., Kayser V. Recent advances in the use of iron–gold hybrid nanoparticles for biomedical applications. Nanomaterials. 2021;11:1227. doi: 10.3390/nano11051227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rabaan A.A., Bukhamsin R., AlSaihati H., Alshamrani S.A., AlSihati J., Al-Afghani H.M., et al. Recent trends and developments in multifunctional nanoparticles for cancer theranostics. Molecules. 2022;27:8659. doi: 10.3390/molecules27248659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zheng X.J., Wu Y., Zuo H.L., Chen W.Y., Wang K. Metal nanoparticles as novel agents for lung cancer diagnosis and therapy. Small. 2023;18 doi: 10.1002/smll.202206624. [DOI] [PubMed] [Google Scholar]
- 151.Gutiérrez de la R.S.Y., Muñiz D.R., Villalobos G.P.T., Patakfalvi R., Gutiérrez C.Ó. Functionalized platinum nanoparticles with biomedical applications. Int J Mol Sci. 2022;16:9404. doi: 10.3390/ijms23169404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.López-Dávila V., Seifalian A.M., Loizidou M. Organic nanocarriers for cancer drug delivery. Curr Opin Pharmacol. 2012;4:414–419. doi: 10.1016/j.coph.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 153.Liu Y.M., Wang Q.Y., Li F.Y., Ling D.S. Nature-inspired supramolecular assemblies for precise biomedical imaging and therapy. Acta Pharm Sin B. 2022;10:4008–4010. doi: 10.1016/j.apsb.2022.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]