Abstract
Using a strategy developed by R. Andino, D. Silvera, S. D. Suggett, P. I. Achacoso, C. J. Miller, D. Baltimore, and M. B. Feinberg (Science 265:1448–1451, 1994), we constructed recombinant polioviruses by fusing the open reading frame (ORF) of the green fluorescent protein gene (gfp) of Aequorea victoria or the gag gene (encoding p17-p24) of human immunodeficiency virus type 1 (HIV-1) to the N terminus of the poliovirus polyprotein. All poliovirus expression vectors constructed by us and those obtained from Andino et al. were found to be severely impaired in viral replication and genetically unstable. Upon replication, inserted sequences were rapidly deleted as early as the first growth cycle in HeLa cells. However, the vector viruses did not readily revert to the wild-type sequence but rather retained some of the insert plus the artificial 3Cpro/3CDpro cleavage site, engineered between the heterologous sequence and the poliovirus polyprotein, to give rise to genotypes reminiscent of cardioviruses. These virus variants that carry a small leader polypeptide were now relatively stable, and they grew better than their progenitor strains. Reverse transcription followed by PCR and sequence analysis of the genomic RNAs reproducibly revealed a few preferred genotypes among the isolated deletion variants. The remaining truncated inserts were retained through subsequent passages. In the immediate vicinity of the deletion borders, we observed short direct sequence repeats that we propose are involved in aligning RNA strands for illegitimate (nonhomologous) RNA recombination during minus-strand synthesis. On the basis of our results, which are at variance with published data, the utility of poliovirus vectors to express proteins >10 kDa in size through fusion with the polyprotein needs to be reevaluated.
It is an appealing idea to use RNA viruses as vectors for the delivery of immunogens. Most RNA viruses are rapidly replicating systems, and with the exception of retroviruses, their genomes are not integrated into the host cell’s genome. Infection with an RNA vector virus may lead to strong expression of exogenous immunogens over a limited time span, followed by clearance of the virus through the host’s immune system. Many attempts have been made to manipulate the genomes of RNA viruses so that they can function as expression vectors. Among these RNA viruses are Sindbis virus (8, 48, 51), Semliki Forest virus (31), influenza virus (19, 30), tobacco etch virus (16), mengovirus (2, 3), and poliovirus (PV) (1, 6, 9, 10, 12, 14, 22, 23, 32, 35, 36, 38, 44).
The genetic properties of RNA viruses, however, pose obstacles for the development of expression vectors. This problem relates to the rampant genetic variation of these viruses due to unedited misincorporation of nucleotides during RNA synthesis, which occurs at a rate of roughly 10−4 per base pair per genome replication (18). More formidable yet is the propensity of plus-strand RNA viruses to undergo genetic recombination (25, 29, 50). For PV, the frequency of homologous recombination is an astounding <10−3, depending on the extend of homology between the recombining RNAs (50). King (25) has estimated that 10 to 20% of progeny RNA in a single growth cycle may be products of recombination. The crossover events occur mostly between sibling RNA strands (11). Furthermore, illegitimate (nonhomologous) recombination can lead to the deletion of coding sequences of capsid protein, producing defective interfering particles (50), or to the rapid elimination of unwanted foreign sequences (1, 12, 32).
The genetic properties of RNA viruses have been held responsible for the generally small size of RNA genomes (50). Whereas the small size favors rapid genome replication and, combined with the frequencies of mutation and recombination, speedy viral adaptation to a new environment, RNA viruses must proliferate under conditions of genetic austerity with a minimum number of gene products, none of which can be spared. In the case of picornaviruses, the addition of extra genes is limited due to spatial restriction within the rigid viral capsid (1).
Our laboratory has generated dicistronic PV expression vectors by inserting the internal ribosomal entry site (IRES) of encephalomyocarditis virus (EMCV) and foreign ORFs into the PV genome (1, 12, 22, 32). These replication-competent viruses, although expressing the foreign gene, were genetically unstable. After a few passages in tissue culture, some or most of the inserts specifying the heterologous regulatory element or the foreign polypeptide were deleted. In view of these considerations, we were surprised that Andino et al. (6) reported genetic stability over many passages of a novel PV expression virus in which foreign ORFs were directly fused to the ORF of the PV polyprotein.
In constructing their vector viruses, Andino et al. (6) mimicked the genetic organization of cardioviruses such as mengovirus or EMCV. The coding region for the capsid proteins (P1) in cardioviruses is preceded by an ORF encoding a small leader polypeptide of unknown function. During polyprotein processing, the leader polypeptide is severed from P1 by the viral proteinase 3Cpro (17, 39). Indeed, Altmeyer et al. (3, 4) have used mengovirus to develop a novel RNA expression virus by inserting foreign ORFs in frame into the ORF of the leader polypeptide. These vectors were able to deliver foreign immunogens but proved unstable upon repeated passage in tissue culture cells (3, 4). Andino et al. (6) fused a foreign ORF in frame to the N terminus of the poliovirus ORF and cloned a cleavage site for the viral proteinase 3Cpro/3CDpro between the two ORFs. Thus, the foreign polypeptide, just like the leader polypeptide in cardiovirus polyproteins, was cleaved from the PV polyprotein during replication. Of particular interest to us was the viral vector moHgag, in which the human immunodeficiency virus type 1 (HIV-1) gag ORF (encoding p17-p24, or nearly 400 amino acids) was fused to the PV ORF (6) (see below).
We have made use of Andino’s strategy and fused the ORF of the green fluorescent protein (GFP) gene of Aequorea victoria (gfp) to the P1 region of the PV polyprotein (Fig. 1), with the intent to create a virus (PVMgfp) yielding an easily detectable marker for viral replication in tissue culture and for studies of the pathogenesis of PV in hPVR-transgenic mice (reference 21 and references therein). Unfortunately, the insertion of the gfp gene severely impaired viral replication, and the gene was deleted in the course of serial passage of PVMgfp2 in tissue culture. Even less stable than PVMgfp2 was an RNA expression virus, PVMgag2, in which the HIV-1 gag(p17-p24) ORF was fused to the PV ORF (Fig. 1), a virus almost identical to moHgag (6). Indeed, in parallel experiments, the two RNA expression vectors moHgag (generously provided by R. Andino) and PVMgag2 lost most of their HIV-1 gag(p17-p24) ORF during the first passage in HeLa cells. Moreover, these RNA virus vectors were severely impaired in viral replication. Currently, we cannot explain the discrepancy between the published data (6) and our results. Our data, however, must be taken into consideration when PV vectors are designed to deliver foreign genetic material.
FIG. 1.
(A) Genomic organization of expression constructs. Amino acids in capital letters are part of the PV genome; lowercase amino acids mark exogenous sequences, with boxes indicating the coding sequences of the inserted genes. The PV ORF initiating AUG is in its original context; the first 3 aa of VP4 (GAQ) are duplicated. The differences in pmogfp and pmoHgag are a more extensive multiple cloning site, six glycine residues upstream the cleavage site instead of four, and inclusion of the original P5 residue (glutamate) into the 3Cpro/3CDpro recognition site. (B) Schematic of the oligonucleotides used as RT-PCR primers and resulting fragment lengths.
A detailed study of the events leading to the elimination of the foreign ORF produced the surprising result that the initial deletion was incomplete. Recovery of wild-type (wt) PV genotypes was very rare. Instead, small fragments of the foreign ORF remained fused to the N terminus of the polyprotein, yielding PV variants with a gene organization just like that in cardioviruses. These PV variants, which carry a small leader polypeptide fused to the N terminus of the PV polyprotein, were now genetically relatively stable, and they grew better than their progenitor strains. Analysis of the sequences surrounding the deletion has allowed us to propose a mechanism of the deletion by illegitimate recombination leading to cardioviruslike genomes.
(This work was performed in part as a requirement for the Diplom in Biology bestowed to S.M. by the University of Cologne, Cologne, Germany.)
MATERIALS AND METHODS
Cells, viruses, plasmids, and bacteria.
HeLa R19 cell monolayers were maintained in Dulbecco’s modified eagle medium (DMEM) supplemented with 5% bovine calf serum (BCS) at 37°C. Plasmids pT7PVMgfp2 and pT7PVMgag2 are based on PV type 1 Mahoney [PV1(M)] cDNA clone pT7PVM (10, 49). Plasmids pmoHgag (6) and pmogfp (unpublished) were kindly provided by Raul Andino, University of California, San Francisco. Escherichia coli DH5α was used for plasmid transformation and propagation. Viruses were amplified by infection of HeLa R19 cell monolayers with 10 PFU per cell. Infected cells were incubated in DMEM (2% BCS) at 37°C until complete cytopathic effect (CPE) was apparent. After three rounds of freezing and thawing, the lysate was clarified of cell debris by low-speed centrifugation and the supernatant, containing the virus, was used for further passaging or reinfection followed by RNA isolation.
DNA manipulations.
All cloning enzymes and reaction buffers were obtained from New England Biolabs and Boehringer Mannheim, except Pfu polymerase (Stratagene). Plasmid DNA was purified according to the polyethylene glycol method (47).
In pT7PVM(E−), the BglII-PvuI fragment of pT7PVM was replaced by the respective BglII-PvuI fragment of pNENPO (1) to remove the EcoRI site at position 7525. At position 746 of pT7PVM(E−), a synthetic linker was inserted, introducing the first three codons of PV polyprotein, unique EcoRI and SacI sites, and a cleavage recognition site for PV proteinases 3Cpro and 3CDpro. This was done by means of PCR using primer pairs 01-05 and 04-02 to amplify portions of pT7PVM corresponding to nucleotides (nt) 487 to 748 and 746 to 1189, respectively. The two PCR fragments were ligated at their EcoRI sites, and the 750-bp fragment was amplified by using primers 01 and 02. After digestion with PflMI and NruI, the 750-bp fragment was ligated with the NruI-PflMI (position 3625) fragment and inserted between the PflMI and NruI sites of subclone ΔpT7PVM(P2−) to yield ΔpT7PVM(S+). From ΔpT7PVM(S+), the 2,033-bp PflMI (position 496)-NheI fragment, containing the manipulated region, was recovered to be ligated with the 1,115-bp fragment NheI-PflMI (position 3635) and vector PflMI (position 3625)-PflMI (position 496) of pT7PVM. The resulting plasmid from this three-fragment ligation was designated pT7PVM(S+E−).
The coding sequence of A. victoria gfp was PCR amplified from plasmid TU#65 (13) with primers 06 and 07. The PCR product was cut with EcoRI and SacI and inserted at the EcoRI-SacI cloning site of pT7PVM(S+E−) to produce plasmid pT7PVMgfp1. A later PV GFP expression vector, pT7PVMgfp2, features four consecutive glycine codons upstream of the 3CDpro cleavage site to improve cleavage (6). In this vector, the SacI-NruI fragment of pT7PVMgfp1 was replaced by the PCR product generated from pT7PVM with primers 02 and 4895. This adds 756 nt or 252 amino acids (aa) (approximately 10%) to the wt genome. The coding sequence for p17-p24 of HIV-1 gag was amplified from a subclone of the BH10 isolate of HIV-1 (45) by using primer pair 13-14. Replacing the gfp ORF of pT7PVMgfp2 with this PCR fragment generated pT7PVMgag2. This genome is 1,152 nt or 384 aa (about 15%) larger than PV1(M).
In vitro transcription of plasmid DNA and RNA transfection.
Driven by the T7 promoter, 5 μg of PvuI-linearized plasmids was transcribed by purified T7 RNA polymerase as described earlier (49) except that 20 mM dithiothreitol and 600 U of RNasin (Promega) per ml were used, and the reaction mixture was incubated for 60 min at 37°C. Ten microliters of fresh transcription reaction, containing between 1 and 10 μg of transcript RNA, was used to transfect 106 HeLa R19 cells on a 35-mm-diameter plate according to a modification of the DEAE-dextran method (27, 49). Following a 30-min incubation at room temperature, the supernatant was removed and cells were incubated at 37°C in 2 ml of DMEM containing 2% BCS until CPE appeared.
Metabolic labeling of infected cells.
Confluent 35-mm-diameter plates of HeLa R19 monolayers were infected with 20 PFU per cell. After the cells were rocked for 30 min, the supernatant was aspirated off, 1 ml of DMEM containing 5% BCS and 5 mg of actinomycin D per ml was added, and the cells were incubated at 37°C. After 4 h, the plates were washed twice with phosphate-buffered saline (PBS) and then incubated with 0.5 ml SMEM (5% BCS, 5 mg of actinomycin D per ml, Met−) for 30 min at 37°C. Then 20 μCi of Tran-(35S)-label (ICN Biomedicals) was added to the medium, followed by a 1-h incubation. The cells were washed twice with PBS, lysed in 0.1 ml of 0.5% Nonidet P-40 (NP-40 lysis buffer), and supplemented with 20 μl of 50% glycerol. Finally, 4 μl of lysate in 1× sodium dodecyl sulfate (SDS) gel-loading buffer per lane were run on an SDS–12.5% polyacrylamide gel (28).
RNA isolation from infected cells and reverse transcription (RT)-PCR.
Confluent HeLa R19 cell monolayers on 35-mm-diameter plates were infected with 10 PFU per cell. As soon as the first signs of CPE were observed, the cells were washed once with PBS and lysed in 0.2 ml of NP-40 lysis buffer. After 2 min of centrifugation at 14,000 rpm, the supernatant was recovered, supplemented with 2 μl of 10% SDS, and extracted twice with phenol-chloroform (1:1, vol/vol) and once with chloroform. The RNA was precipitated in 0.2 volume of 10 M ammonium acetate and 3 volumes of ethanol for 1 h at −70°C, washed with 70% ethanol, and resuspended in 20 μl of sterile filtered water.
First-strand cDNA synthesis was performed under the following conditions: 3 μl of total RNA from PV-infected cells, 0.5 mM each deoxynucleoside triphosphate, 0.1 mM random 9-mer oligonucleotides, 10 mM dithiothreitol, 8 U of avian myeloblastosis virus reverse transcriptase (Boehringer Mannheim), and 4 U of RNasin in 1× avian myeloblastosis virus reverse transcriptase transcription buffer at a total volume of 20 μl. The reaction mixture was incubated at 42°C for 1 h and then the enzyme was inactivated at 95°C for 5 min. The reaction mixture was then chilled on ice, supplemented with 1 μl of RNase A (0.5 mg/ml), and incubated for 30 min at room temperature. Two microliters of the RT reaction was used as the template for PCR as follows: 50 μM each deoxynucleoside triphosphate, 200 nM upstream primer, 200 nM downstream primer, 0.5 U of Taq polymerase (Boehringer Mannheim), 0.1 mg of bovine serum albumin per ml, and 1× PCR buffer in a total volume of 20 μl. The reaction mixture was overlaid with 50 μl of mineral oil, heated for 3 min at 95°C, and cycled 30 times for 30 s at 95°C and 90 s at 72°C (for primer pair 7215-6509). Figure 1 shows the primers used and the resulting fragment lengths. PCR products were analyzed on 1.2% agarose gels.
Sequencing of virus variants.
RNAs of plaque-purified virus variants were subjected to RT-PCR as outlined above, using primers 7215 and 6509. The PCR products were agarose gel purified and sequenced by closely following the protocol supplied with the SequiTherm cycle sequencing kit (Epicentre Technologies).
Oligonucleotide primers.
The following primers were used in this study: 01 (5′-GGT CAC AAA CCA GTG ATT GGC C-3′), 02 (5′-CCA CCA CCA CCC TCG CG-3′), 04 (5′-CAG GAA TTC GAA GAG CTC GCT TTG TTT CAA GGT GCT CAG GGT TCA TCA C-3′), 05 (5′-CCG AAT TCC TGA GCA CCC ATT ATG ATA CAA TTG TCT G-3′), 06 (5′-CAG GAA TTC AGT AAA GGA GAA GAA C-3′), 07 (5′-CTG GAG CTC TTT GTA TAG TTC ATC C-3′), 4895 (5′-GAA GAG CTC GGT GGT GGT GGT GCT TTG TTT CAA GGT GCT CAG GTT TCA TCA C-3′), 13 (5′-GCG AAT TCG GAG CGG CCG CTA TGG GTG CGA GAG CG-3′), 14 (5′-ACC GAG CTC GAG CGC CAA AAC TCT TGC CTT ATG-3′), 6509 (5′-GTC CTG TTT CGA AGC CGC GTT ACT AGC-3′) 7215 (5′-GCT GGA TCC GCT CCA TTG AGT GTG-3′), 7403 (5′-CTT GTC TAA AGC TTC CTT GGT GTC-3′), and 7404 (5′-CAG CAC GTG TCT TGT AGT TCC CG-3′).
RESULTS
Construction and growth characteristics of GFP- and Gag-expressing PVs.
PV-based RNA virus vectors PVMgfp2 and PVMgag2 were constructed as described in Materials and Methods (Fig. 1A). The original construct PVMgfp1 was modified to add four consecutive glycine residues just upstream of the favorable 3Cpro/3CDpro cleavage site ALFQ*G (11, 17) to possibly improve proteolytic processing (6). The difference in proteolytic cleavage between PVMgfp1 and PVMgfp2, however, was marginal (data not shown). The genotypes of constructs pmogfp and pmoHgag, two clones generously provided by R. Andino, are very similar to those of pT7PVMgfp2 and pT7PVMgag2. A difference relating to the genotype of the paternal PV cDNAs is a variation in the coding region for 3Dpol that leads to an altered mobility of 3Dpol-related polypeptides during SDS-polyacrylamide gel electrophoresis (see below).
Plasmids pT7PVMgfp2, pmogfp, pT7PVMgag2, and pmoHgag were transcribed with T7 RNA polymerase, and the RNAs were used to transfect HeLa R19 cell monolayers. All constructs that contained foreign sequences showed significant delays in the development of CPE compared to PV wt transcripts. The delays were more pronounced with increased length of the insert (Table 1). Appearance of CPE with the different constructs followed the relationship pT7PVM < pT7PVMgfp2 = pmogfp < pmoHgag < pT7PVMgag2. Two independent transfections were performed, and viruses recovered from these experiments were labeled A and B and used for further study.
TABLE 1.
Correlation between retained insert length, plaque size, and virus titer
| Construct (nt insert)a | Time (h) when CPE was detected after transfection | 1st passage
|
6th passage
|
||||
|---|---|---|---|---|---|---|---|
| Retained insert (nt)b | Plaque phenotypec | Virus titer (PFU/ml) | Retained insert (nt) | Plaque phenotype | Virus titer (PFU/ml) | ||
| PVMgfp2-A (756) | 42 | Mostly undeleted | 90% <0.5 mm, 10% 1–1.5 mm | 2 × 107 | 300, 200 | 1–2 mm | 1.7 × 108 |
| PVMgfp2-B (756) | 42 | Undeleted | <0.5 mm | 1.5 × 107 | 400, 300 | 1 mm | 4 × 107 |
| mogfp-A (783) | 42 | Mostly undeleted | 95% <0.5 mm, 5% 1–1.5 mm | 4 × 107 | 400, 150 | 1–3 mm | 2.2 × 108 |
| mogfp-B (783) | 42 | Mostly undeleted | 80% <0.5 mm, 20% 1–2 mm | 4 × 107 | 300, 250 | 1–4 mm | 2.8 × 108 |
| PVMgag2-A (1152) | 68 | 90, 0 | 1 mm | 1.2 × 107 | 0 | 1–2 mm | 2.8 × 108 |
| PVMgag2-B (1152) | 68 | 100, 50 | 0.5 mm | 6 × 106 | 100, 50 | 0.5–1 mm | 5 × 107 |
| moHgag-A (1161) | 68 | 300, 250, 150, 70 | 1–3 mm | 7 × 107 | 150, 70, 0 | 2–4 mm | 8 × 108 |
| moHgag-B (1161) | 68 | 250, 150, 70 | 1–3 mm | 1.2 × 108 | 150, 70, 0 | 2–4 mm | 7 × 108 |
| PVM (0) | 24 | NA | 2–4 mm | 1.5 × 109 | |||
Two independent RNA transfections were performed for each construct (A and B) followed by passaging on HeLa R19 cells. The size of the inserted exogenous sequences is given as the number of nucleotides (in parentheses) after the construct.
Approximate number of nucleotides of the insert that were retained in the most predominant variant viruses, as determined by RT-PCR analysis (see Fig. 3). NA, not applicable.
Determined after 48-h plaque assay on HeLa R19 cells.
When the recombinant viruses isolated after transfections were used to metabolically label virus-specific proteins in infected HeLa cells [Tran-(35S)-label [ICN Biochemicals]; see Materials and Methods], unexpected results were obtained. Autoradiography of polyacrylamide gels prepared with lysates of metabolically labeled cells infected with PVMgag2 and moHgag, while producing nearly normal patterns of PV-specific polypeptides, did not reveal bands indicative of free Gag protein or its precursors (data not shown). This was observed in spite of the fact that Gag-related proteins contain 12 methionine and 4 cysteine residues that should allow ready detection of the polypeptide similar to the detection of PV-specific proteins. Moreover, Western blot analyses of the same lysates with anti-Gag monoclonal antibodies yielded a signal of the size expected for free Gag in only one experiment but failed to produce any signal in several other experiments (data not shown). In contrast to the PVMgag2 and moHgag isolates, first-passage PVMgfp2 and mogfp viruses revealed metabolically labeled GFP-containing polypeptides after metabolic labeling. However, these polypeptides were no longer detectable with sixth-passage PVMgfp2 and mogfp isolates (data not shown).
The failure to obtain Gag-related proteins in PVMgag2- and moHgag-infected cells, a result at variance with published data (6), prompted us to analyze viral properties such as plaque size and genotype of isolates obtained after various passages.
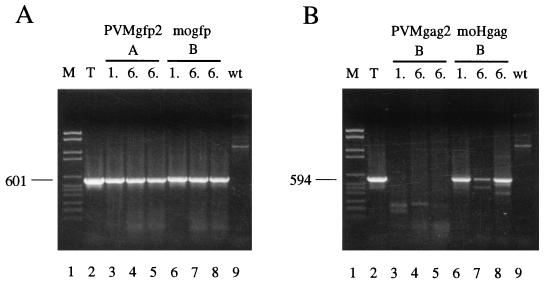
Plaque assays were carried out with stock virus recovered from RNA transfection and the fifth passage thereof. PVMgfp2 as well as mogfp showed mainly minute-plaque phenotypes after transfection (Fig. 2, first passage; similar plaque phenotypes were observed with either A or B isolates [not shown]). This result suggested that the proliferation of these viruses was severely impaired. Similar observations were also made by Andino for the construct mogfp (5). After five consecutive passages, a shift to medium plaque size for PVMgfp2, and to medium-large plaque size for mogfp, was observed (Fig. 2, sixth passage). In our experience, this is indicative of genomic rearrangements favorable to viral growth (Table 1; see also references 12 and 32).
FIG. 2.
Plaque phenotypes of GFP- and Gag-expressing constructs. Virus isolated from RNA transfections and after the fifth passage were plaque assayed on 35-mm-diameter six-well plates of HeLa R19 monolayers (considered first and sixth passages, respectively). After 48 h of incubation at 37°C and 5% CO2, the cells were stained with crystal violet.
First-passage PVMgag2 yielded a small-plaque phenotype, and this phenotype did not significantly change during passaging of the virus (Fig. 2). In stark contrast, first-passage moHgag yielded a medium- to large-plaque phenotype that changed after passaging to a large-plaque phenotype (Fig. 2). This observation may have led to the presumption that moHgag is a genetically stable virus vector with wt growth properties (6). An alternative explanation is that moHgag RNA is quasi-infectious (12, 20) and only progeny virus that lost detrimental elements appeared in the plaque assay, a scenario supported by the long time required to produce CPE upon transfection of the transcript RNAs (12, 32).
Genetic stability of the recombinant genomes.
We used RT-PCR to analyze the genotypes of viral RNAs that were extracted from infected cells.
Viruses isolated after transfection (A and B) and after five further passages (passaged in duplicates) were used to infect HeLa R19 monolayers (considered the sixth passage). After 7 to 10 h, total RNA was extracted as templates for RT-PCR. PVMgfp2 RNA isolated after one passage mostly carried the complete insert (Fig. 3A, lanes 4 and 7), although some products shorter than the complete insert were detectable in both isolates A and B. After six passages, full-length inserts could no longer be observed in our analyses and only shorter fragments were apparent (lanes 5, 6, 8, and 9). These results were strongly supported by analyses of PVMgfp2-infected HeLa cells for the expression of GFP by fluorescence microscopy. When cells were infected with first-passage PVMgfp2 at 1 PFU/cell, GFP-specific fluorescence could be detected in 50 to 80% of all cells, but only 2 to 5% of the cells were positive for GFP when sixth-passage PVMgfp2 was used (data not shown). This observation suggests loss of function of the gfp gene during passaging. Very similar results were obtained also with mogfp isolates A and B (Fig. 3B). Interestingly, the gfp gene truncations generated during the passages are different for each isolate (A or B) of either PVMgfp2 or mogfp (compare Fig. 3A, lanes 5, 6, 8, and 9, with Fig. 3B, lanes 5, 6, 8, and 9). On the other hand, independent passages of virus obtained from the same transfection yielded nearly identical deletion patterns after the sixth cycle (Fig. 3A and B; compare lanes 5 with lanes 6 and lanes 8 with lanes 9). This finding suggests that once novel, favored genotypes are produced during the first passage, they may prevail in subsequent passages (see also below).
FIG. 3.
RT-PCR analysis of PVMgfp2 (A), mogfp (B), PVMgag2 (C), and moHgag (D) virus variants with oligonucleotides flanking the exogenous sequences [7215, nt 667 to 690 of PV1(M); 6509, nt 851 to 877 of PV1(M)]. Two independent RNA transfections for each construct were done (A and B). Reinfection for RNA purification was considered first passage (1.). Each transfection was passaged five more times in duplicates (6.). Deletions within the foreign sequences result in shorter bands, accordingly. M, DNA molecular weight marker VI (Boehringer Mannheim); P, PCR product of the respective plasmid; T, RT-PCR of the respective transcript RNA. Products were analyzed on a 1.2% agarose gel. Sizes are indicated in nucleotides.
The experiments with PVMgag2 and moHgag (Fig. 3D and C) show results similar to those obtained with PVMgfp2 or mogfp. However, no full-length insert of gag(p17-p24) was detected by RT-PCR after the first passage (Fig. 3C and D, lanes 4 and 7). The rapid deletion of sequences of the gag insert may explain the relatively large plaque phenotypes seen with moHgag (Fig. 2). It should be noted that RT-PCR from purified virion RNA showed deletion patterns identical to those obtained with total RNA of infected cells (data not shown). This finding rules out the unlikely possibility that genome RNAs carrying the foreign sequences are preferentially encapsidated and carried through viral passages.
The deletion events indicate that the parental expression constructs are impaired in replication and that any of the deletions observed in Fig. 3 confers a highly selective advantage to the genomes. The shorter genotypes that, in the case of moHgag, retained between 250 and 70 nt of foreign sequence did not seem to readily undergo further deletions but served as founder genomes in subsequent bulk passages. To test the stability of these newly generated genotypes, viruses from four plaques of moHgag transfections A and B (Fig. 2) were purified and passaged four more times. As can be seen in Fig. 4, RT-PCR analyses of each isolated variant RNA at the beginning and end of passages revealed only one specific genotype (compare lanes 3 with 4, 5 with 6; 7 with 8, and 9 with 10). We conclude that the founder variants that arise by deletion events not only replicate well but also are genetically quite stable, thereby resisting elimination by competition with other variants or wt revertants.
FIG. 4.
Genetic stability of deletion variants of plaque-purified (p.p.) moHgag. Virus recovered from RNA transfections A and B was plaque assayed. Two small plaques from each plate were picked (A1, A2, B1, and B2), grown up (1.), and passaged four more times (5.). Total RNA was subjected to RT-PCR with primers 7215 and 6509. M, DNA molecular weight marker VI (Boehringer Mannheim); T, RT-PCR of pmoHgag transcript RNA; wt, RT-PCR of PV1(M) virion RNA. Products were analyzed on a 1.2% agarose gel. Sizes are indicated in nucleotides.
Competition in RT-PCR of mixed RNA populations.
The evidence presented so far indicates that rapid deletions of the inserted foreign sequences occur during the very first rounds of replication of the RNA virus vectors. However, the analysis of genotypes by RT-PCR may be flawed if the PCR discriminated against larger templates in the presence of smaller templates simply because the smaller DNA products multiply faster. Therefore, our analyses may not necessarily exclude the possibility that the population of progeny viruses of the expression RNA vectors, after transfection or passages, contains some genotypes that carry a large if not complete insert.
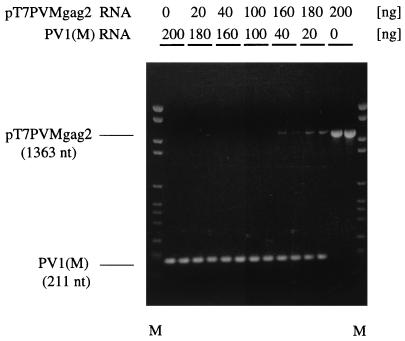
To test this possibility, we performed competition experiments in the polymerization reaction, using two templates of significantly different lengths. Specifically, the RT-PCR was carried out with a mixture of pT7PVMgag2 and pT7PVM (wt) transcript RNAs, using identical primers (Fig. 1B) but varying the concentration of the two RNA templates. The predicted sizes of the DNA products were 1,363 and 211 bp for the pT7PVMgag2 and pT7PVM RNAs, respectively (Fig. 1B). The results showed significant discrimination against the larger product even at a template ratio of pT7PVMgag2 to pT7PVM RNA of 9:1 (Fig. 5, lane 180 over 20). At a mass ration of 4:1, the 1,363-bp-long DNA product is barely visible, and at equal mass ratio, no pT7PVMgag2 product was seen (Fig. 5, lane 100 over 100).
FIG. 5.
Competition in RT-PCR. Transcript RNAs of pT7PVMgag2 and pT7PVM (wt) were mixed as indicated to a total of 200 ng and subjected to RT-PCR with primers 7215 and 6509. M, DNA molecular weight marker VI (Boehringer Mannheim). Products were run on a 1.2% agarose gel (ethidium bromide stained).
To investigate this phenomenon further, we carried out RT-PCR that, in the absence of competition, would exclusively yield products from viral RNAs that retained some or all of the insert. This was achieved by using primer 01, annealing to the PV 5′ nontranslated region (NTR), and the negative-sense primers 7403 and 7404, annealing to sites within the gag and gfp inserts, respectively (Fig. 1B). As shown in Fig. 6A for PVMgfp2 and mogfp, fragments of the size expected for undeleted parental virus could be detected in RNAs recovered from all passages, an observation suggesting that some progeny of the original PVMgfp2 may have retained the complete gfp gene (Fig. 6A, lanes 3 to 8). This agrees with our observation, mentioned above, that GFP fluorescence could still be detected in 2 to 5% of cells infected with virus stock after the sixth passage (data not shown). Of the Gag-expressing constructs, only moHgag could produce a signal, suggesting the possibility that some virus stock after the sixth passage retained some or all of the gag insert (Fig. 6B, lanes 6 to 8). This analysis, however, is also flawed in that it does not allow us to quantitate the fraction of recombinant virus in the total virus population that retained the insert, since deletion variants that lost most of the insert, including the annealing site of the internal primer, will not produce a signal. Furthermore, it is possible that deletions that would escape detection occurred within the foreign gene segments downstream of the primer annealing site.
FIG. 6.
Selective RT-PCR using one flanking primer [01, nt 487 to 508 of PV1(M)] and one primer mapping within the inserted sequence (7404, nt 309 to 331 of gfp10 ORF [A], or 7403, nt 286 to 309 of HIV-1 gag [B]). M, DNA molecular weight marker VI (Boehringer Mannheim); T, RT-PCR of the respective transcript RNA; 1., first passage; 6., sixth passage; wt, RT-PCR of PV1(M) virion RNA (negative control; neither 7404 nor 7403 can anneal). Products were analyzed on a 1.2% agarose gel (ethidium bromide stained). Sizes are indicated in nucleotides.
Ratios of different variant genotypes and deletion dynamics of an RNA virus vector.
It has been demonstrated previously that different genotypes in a swarm of viruses of an RNA quasispecies can be analyzed individually if competition is minimized and fitness to proliferate in a given environment no longer plays a major role (18, 50). We have shown above that a small fraction of RNA vector viruses carrying the complete foreign gene may survive the competition during proliferation in mixed populations, but that these parental genotypes are difficult to detect due to experimental limitations. We therefore turned to analyzing genotypes present in individual plaques, using moHgag as an example.
Following transfection with pmoHgag RNA, the cell monolayers were immediately overlaid with agar, thereby minimizing the mixing of parental RNA with RNAs of deletion variants. From the emerging plaques, 16 were further analyzed. Their genotypes should represent independent deletion events (Fig. 7A, lanes 3 to 18). Plaque assays were also done with bulk virus isolated from a parallel pmoHgag transfection (31 plaques purified) (Fig. 7A, lanes 22 to 28; Fig. 7B, lanes 3 to 26) and with bulk virus of the same transfection after five passages (Fig. 7C, lanes 3 to 17 and 19 to 27). Thus, RNAs in individual plaques were analyzed after (i) transfection, (ii) one passage, or (iii) six passages (71 RNA samples altogether). In none of the 47 viruses that were plaque purified after either transfection or first passage could the full-length gag insert be detected. We can therefore estimate that after the first passage, less than 2% of the bulk viruses carried the full-length insert.
FIG. 7.
Determination of the ratio of moHgag deletion variants during progressive passaging. Viruses were plaque purified from initial RNA transfection (A), first passages (A and B), and sixth passages (C). Viral RNAs were subjected to RT-PCR with flanking primers 7215 and 6509. M, DNA molecular weight marker VI (Boehringer Mannheim); T, RT-PCR of pmoHgag transcript RNA; wt, RT-PCR of PV1(M) virion RNA. Products were run on a 1.2% agarose gel (ethidium bromide stained).
In the course of these analyses, we detected six classes of fragments, labeled a to f, of different lengths. These fragments correspond to variants that had retained between 500 and 0 nt of the original gag-specific insert. A tendency to shorter insert lengths in later passages was observed. However, between the first and sixth passages, the average insert length decreased by only about 50 nt, whereas during the events occurring in the original transfection, a drastic reduction of the original insert (from 1,161 to an average of 233 nt) was noted (Table 2). These observations further support our hypothesis that during the early rounds of replication, the bulk of the deletion variants may arise from a single genetic event rather than from progressive shortening during passaging, and that these founder variants then persist during further passages and compete well with other genotypes. However, occasionally several genotypes were detected after plaque purification of a single plaque (Fig. 7A, lanes 14, 15, 16, and 23; Fig. 7C, lane 26). Whether these genotypes arose from independent genetic events or are the result of stepwise deletions remains unknown.
TABLE 2.
Scoring of moHgag deletion variants according to the size of their retained insert after RNA transfection, first passage, and sixth passage
| Retained insert (nt)a | No. of variants detected for each size cluster afterb:
|
||
|---|---|---|---|
| Transfection | 1st passage | 6th passage | |
| 1,161 (undeleted) | 0 | 0 | 0 |
| 500 (cluster a) | 3 | 1 | 0 |
| 450 (cluster b) | 2 | 0 | 0 |
| 250 (cluster c) | 4 | 5 | 2 |
| 150 (cluster d) | 9 | 15 | 9 |
| 70 (cluster e) | 2 | 12 | 12 |
| 0 (cluster f, wt) | 1 | 0 | 4 |
| Avg insert (nt) retained | 233 | 147 | 100 |
Approximate size of retained inserts. Deletion clusters as defined in Fig. 7.
Sizes of the retained exogenous sequences of individual variants plaque purified after RNA transfection, first passage, and sixth passage were determined by RT-PCR and divided into clusters.
Sequence analysis of moHgag deletion variants.
The RT-PCRs with variant RNAs yielded fragments of distinct sizes rather than a collection of random deletion products. Indeed, independent transfection experiments with moHgag RNAs reproducibly yielded a set of common deletions, a finding indicating the existence of favored sites for a genetic event such as illegitimate recombination. We were interested whether this phenomenon could be linked to specific viral sequences. For this purpose, genomic RNAs of plaque purified variants (Fig. 7) were subjected to RT-PCR, and the agarose-gel-purified DNA fragments were sequenced by the dideoxy method, using a SequiTherm cycle sequencing kit (Epicentre Technologies). Representatives of size clusters a (Fig. 7A, lanes 10 and 18), b (Fig. 7A, lanes 4 and 8), c (Fig. 7B, lanes 6, 10, and 18), d (Fig. 7A, lanes 3, 7, 11, 12, and 13), e (Fig. 7C, lanes 8 to 10), and f (Fig. 7C, lanes 5, 6, 14, and 20) of moHgag variants were sequenced. In addition, four variant RNAs of PVMgag2 (Fig. 3C, lanes 6 and 9, and RNAs of two other independent transfection [data not shown]) were analyzed in a fashion similar to the analyses of moHgag RNAs.
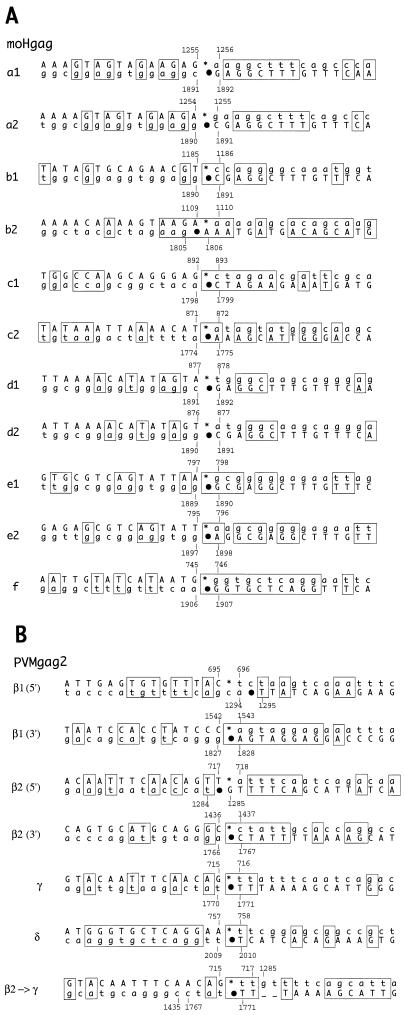
Variants within a size cluster were found to display heterogeneous genotypes. In most cases, two distinct genotypes (e.g., b1 and b2 [Fig. 8]) were found in the same size cluster. In all isolated moHgag variants, the deletions mapped to within the gag insert (Fig. 8). The largest deletion generated the exact PV wt genotype (cluster f). Interestingly, of 24 variants, only 4 had reverted to the PV wt sequence after six passages (Fig. 7C).
FIG. 8.
Sequence analysis of moHgag and PVMgag2 variants. Variant genotypes were divided into six and three categories based on the size of the retained foreign sequence as determined by RT-PCR (see text) (a, b, c, d, e, and f for moHgag; β, γ, and δ for PVMgag2). Several variants of each category were sequenced by cycle sequencing of their RT-PCR products. Black boxes represent gag-specific sequences. Dotted lines mark deleted portions (drawn to scale). Asterisks and dots indicate the 5′ and 3′ borders, respectively, of each deletion. Numerals above the deletions identify positions in the parental plasmid (pmoHgag or pT7PVMgag2) of the last upstream nucleotide before and the first downstream nucleotide after each deletion. The following deviations from the sequence shown were found in plasmid pmoHgag: position 1872, A→G (Glu→Gly); position 1875, G→T (Gly→Val) (both map within the linker/cleavage site and are also present in mogfp, which originates from the same vector plasmid); and position 1184 A→G (Ile→Val).
All variants other than wt revertants retained the artificial 3Cpro/3CDpro cleavage site necessary to remove the remainders of Gag protein fused to the viral polyprotein. This is vital to the variant’s replication, since the N terminus of the P1 capsid precursor must be posttranslationally myristoylated (15, 41).
PVMgag2 variants were much more complex than moHgag variants. In three of four variants, deletions included sequences of the PV 5′ NTR, including the original AUG initiation codon (Fig. 8, PVMgag2 β1, β2, and γ). Apparently, these variants use alternative in-frame AUG codons within the retained foreign sequences to initiate translation. Two of the PVMgag2 variants carried two deletions each (β1 and β2), an observation suggesting stepwise deletions. Particularly fascinating is variant PVMgag2 δ, which retained the first four amino acids (GAQE) of the inserted sequence and then deleted the entire gag insert plus the first four amino acids of VP4 (GAQV). The resulting PV variant has a wt sequence except for a Val4Glu mutation in capsid protein VP4. Interestingly, this variant displays a small-plaque phenotype (Fig. 2, PVMgag2 B) which may link this residue to inefficient myristoylation of the N terminus of the P1 polyprotein (41).
The sequences flanking the endpoints of each deletion were aligned (Fig. 9). In several variants, short direct sequence repeats could be identified in the sequences immediately surrounding the deletion endpoints. These repeats might have served as parting and anchoring sites for template switching as proposed by Pilipenko and coworkers (43).
FIG. 9.
Sequence alignments of the regions surrounding the deletion borders in moHgag (A) and PVMgag2 (B) variants. Thirty nucleotides around the 5′ and 3′ borders of each deletion (15 nt upstream and downstream) were aligned and analyzed for homologies. The upper sequence shows the 5′ border, and the lower sequence shows the 3′ border, with asterisks and dots indicating the respective deletion endpoints (see also Fig. 8 for positions of these marks). Note that both sequences are positive sense and can be located either on the same template strand or on two sibling strands. Numerals refer to positions within the full-length cDNA clones, pmoHgag and pT7PVMgag2. Capital letters represent the sequence of the variant (upper left to lower right) as determined by sequencing; lowercase letters show deleted parental sequences. Sequence homologies are boxed.
As shown in Fig. 8, the variants PVMgag2 β1 and β2 have probably arisen by multiple deletions. Therefore, stepwise deletion events are also likely to occur, in that a smaller, first deletion gives rise to a second, larger deletion. For example, PVMgag2 γ could have been formed by further extension of the deletion found in PVMgag2 β2. Indeed, sequence alignment of PVMgag2 γ revealed more significant sequence homologies, if alignment was done on the assumption that PVMgag2 β2 (and not the full-length PVMgag2 parent) served as an intermediate and direct predecessor of PVMgag2 γ (Fig. 9B, β2→γ). If template switching occurs during negative-strand synthesis (24, 26, 29), the sequence repeats should be located downstream of the deletion points on the positive-sense templates. This was the case for most of the variants except PVMgag2 δ (Fig. 9B), where a perfect sequence repeat of 10 nucleotides was found upstream of the deletion sites. If this repeat served as parting and anchoring site, template switching to generate this variant may have occurred during positive-strand synthesis. Clearly, more variant genotypes will have to be analyzed to obtain a more reliable picture about the genetic events leading to deletions in this experimental system.
DISCUSSION
Genetic stability of PV vectors in relation to the potential as live vaccines for foreign immunogens.
Numerous studies have attested to the genetic plasticity of the PV genome, a property common to all RNA viruses. Structural changes engineered into the viral RNA genome, whether as insertions, as deletions, or by a complete exchange of genetic elements (e.g., coding regions for a protein), generally lead to a rapid genetic response of the mutilated virus during replication. A common outcome is the selection of new genotypes that confer a replication advantage over the parental genotype. The selection of favored variants may be fast if the original genetic alteration still allowed a low level of translation and RNA synthesis. Emergence of variants may then occur within the first round of replication. Phenotypically, this is apparent by mixed-plaque or wt plaque sizes seen when the cell monolayers are overlaid with agar after transfection with the parental RNA (1, 32, 33). On the other hand, the genetic alteration may be so debilitating that the selection of viable variants is rare and may then take several days. In this case, the parental genotype may not even be detectable among progeny virions. Such parental viral genomes have been termed quasi-infectious (19; see also references 11 and 12).
We have previously analyzed the potential of PV as a vector for the delivery of foreign antigens, either by exchanging the neutralization antigenic sites on the surface of PV (4, 37, 38) or by engineering the genome to express foreign proteins (1, 22, 32). The latter strategy involved the conversion of the PV genome to a dicistronic entity expressing a foreign gene (e.g., the chloramphenicol acetyltransferase [CAT] gene or a segment of the HIV env gene) independently from the PV polyprotein. Such a PV expression vector bearing an extra IRES and the CAT gene [PV(ECAT] whose genome was 17% larger than the wt genome efficiently expressed CAT activity (1). However, after six passages of PV(ECAT), expression of the foreign gene was weak due to deletions of the insert (1). Even less genetically stable than PV(ECAT) were our constructs designed to express the truncated gene of the HIV envelope protein (32). An additional restriction of the construction of dicistronic PV vectors was the observation that extending the length of the genome to more than 20% of the wt sequence interfered with packaging into the rigid PV capsid (1). Finally, we discovered that the presence of a signal sequence in a foreign protein encoded by the PV genome is lethal (32, 34). This observation further limits the utility of PV as an expression vector for neutralizing antigenic sites of infectious agents.
Based on these observation, we were not surprised that the PV vectors designed to express 252 amino acids of GFP or 384 amino acids of Gag(p17-p24) as polyprotein fusion proteins (6) were no more stable than the dicistronic expression vectors. Destabilization of the GFP or Gag fusion constructs may be due to one or all of the following. First, the new 3Cpro/3CDpro cleavage site between foreign protein and P1 may be suboptimal for proteolytic processing and myristoylation (36). Second, the enlarged genome may be suboptimal for synthesis and encapsidation. Third, fortuitous RNA structures formed in the coding sequence of the foreign ORF may interfere with the function of RNA signals (particularly the IRES) in the wt genome. The latter possibility is supported by our finding that translation of pT7PVMgfp2, pmogfp, pT7PVMgag2, and pmoHgag transcript RNAs is severely impaired in vitro (data not shown). Since the foreign gene product does not confer any advantage to the virus, any in-frame deletion within the foreign gene may relieve the virus from some restriction of replication. In a large population of viruses, this means greater fitness and rapid selection of the variants. Indeed, the deletions in the Gag(p17-p24)-expressing viruses occurred rapidly during the very first round of transfection such that the presence of the original genotype could be detected only by RT-PCR using internal primers.
Available evidence suggests that PV expression vectors based on polyprotein fusion are more stable if the foreign ORF is small (<400 nt) (35, 36, 52), a phenomenon seen also with dicistronic viruses (32). Although the fusion of small neutralization epitopes of rotavirus, herpes simplex virus type 2, and hepatitis B virus to PV polyprotein yielded genotypes of increased genetic stability, the resulting viral expression vectors were impaired in growth at 37°C, either because they expressed a temperature-sensitive phenotype or because they were retarded at the level of encapsidation (35, 36, 52). A detailed analysis of the genetic properties of these viruses after multiple rounds of replication has not been carried out. However, the vectors with short ORFs may reflect the rather stable genetic organization of the variants of PVMgag2 and moHgag that we have observed (see also below). In any event, the limited capacity of these PV expression vectors for foreign genes and the poor growth properties are likely to restrict the use of these agents as delivery vehicles for foreign antigens.
A special case of recombinant PV carrying a fusion with the polyprotein is PV1/HCV(701), a chimeric virus whose translation is controlled by genetic elements (IRES and adjacent fragment of the core polypeptide) of hepatitis C virus (HCV) (34). In PV1/HCV(701), the foreign gene product (the HCV core fragment) conferred a replication advantage to the replication of the virus; hence, the coding sequence of this polypeptide was retained in its entirety over many passages (34). Similarly, Hahm et al. (23) reported a stable chimeric PV expressing HCV protease NS3. In this vector, proliferation of the virus is dependent on the proteolytic activity of the exogenous HCV protein (23).
Another strategy to use PV as an expression vector is the replacement of the coding region for the capsid protein (P1) with a foreign ORF. Cleavage of the foreign polypeptide from the P2-P3 polyprotein is then carried out by viral proteinase 2Apro at an endogenous cleavage site (7, 42, 44). The corresponding replicons can be encapsidated by PV coat proteins provided in trans. The genomes of these replicons appear to be relatively stable (reference 42 and references therein) presumably because of their small genome size, the use of a natural processing site, and their limitation in proliferation. However, just as the P1 region is dispensable for PV replication (formation of defective interfering particles [50]), these vectors, too, may lose their inserts and replicate without genetic information in the P1 region. In any event, the replication-defective expression vectors, although suitable for expression of relatively large foreign proteins, have the disadvantage that the delivery of immunogens is restricted to a single round of replication.
In antigenic hybrid viruses mentioned above, the bulging loops connecting the beta strands of the beta barrels that serve as PV neutralization antigenic sites have been exchanged with heterologous sequences known to elicit a neutralization response (4, 9, 37, 38). Unfortunately, the coding capacity for expression of foreign antigenic determinants is highly restricted to 10 to 30 aa and the immunologic presentation of the antigenic sites may be very poor. Moreover, these chimeric viruses generally grow very poorly.
Dynamics of the deletions in the fusion protein expression vectors.
RT-PCR as sole method for the analysis of progeny virus derived from PV expression vectors can be misleading, as the result may vary depending on the nature of the primers used for RT-PCR. We have solved this dilemma by analyzing the nucleotide sequences of viral genomes that emerged from the first transfection. These RNAs were recovered under conditions that would minimize the mixing of genotypes. We then compared these earliest genotypes by RT-PCR with genotypes recovered after one and after five bulk passages (Fig. 7). Finally, 19 representative moHgag-derived variant genomes were subjected to sequence analyses. To our surprise, no complete insert was detectable in genomes of early-progeny virus harvests. However, complete deletion of the foreign gene insert in moHgag to yield wt poliovirus was relatively rare. Rather, deletion events yielding different size clusters (a to f) of residual inserts occurred during the first round of replication. The resulting founder genotypes were relatively stable upon further bulk passage.
The most surprising result of this study was the rapid emergence of distinct deletion variants that persisted in subsequent bulk passages. This result indicates that (i) an efficient and specific mechanism leading to different deletion clusters exists and (ii) the variants replicate well to resist elimination by variants with shorter inserts or with a wt genotype that may have arisen during later passages.
The growth and genetic properties of PVMgag2 were, unexpectedly, quite different from those of moHgag. In comparison to moHgag, PVMgag2 transcript RNA was less infectious, produced smaller plaques after transfection (Fig. 2), and yielded different deletion patterns of the gag insert. Moreover, in contrast to moHgag variants, PVMgag2 variants carried deletions in the parental viral genome. Particularly striking are variants with deletions reaching into the PV 5′ NTR, eliminating up to 50 PV genomic nucleotides including the original initiating AUG, and another deletion including the first four amino acids of VP4 (Fig. 8). Apart from possible differences in the parental poliovirus cDNA clone, a significant difference may be the P5 position of the engineered 3Cpro/3CDpro cleavage signal, which is Gly in PVMgag2 and Glu in moHgag (Fig. 1).
Relationship of the moHgag and PVMgag2 variants to cardioviruses.
The genetic organization of the newly generated moHgag variants (ORFs preceding the PV polyprotein) reveal striking similarities to the genetic organization of cardiovirus genomes. These viruses encode a small leader protein that precedes the viral capsid region. As in the PVMgag2 and moHgag variants, the leader protein in cardioviruses is released from the capsid precursor by 3Cpro cleavage. In the two most prevalent moHgag variant types, size clusters d and e, the Gag-derived leaders are approximately 50 and 20 aa, respectively, compared to 67 aa in EMCV and mengovirus (39, 40, 53). The function of the cardiovirus leader protein is unknown. Increasing the size of the leader by artificially engineering a foreign ORF into it has generated a mengovirus-based expression vector (2, 3). Immunization of mice with such a recombinant virus protected the animals against infection with lymphocytic choriomeningitis virus, the source of the inserted antigen (3). However, the insert was deleted upon repeated passage of the recombinant virus, an observation suggesting that even cardioviruses do not tolerate extended leader proteins. It is intriguing to speculate that the generation of the PV-related variants of PVMgag2 and moHgag portrays the evolution of a cardiovirus in a tissue culture system.
It is noteworthy that in 6 of 10 of the moHgag variants (Fig. 8, a1, a2, b1, d1, d2, and e1), the downstream border of the deletion is located exactly at, or within one nucleotide upstream of, the P5 position (Glu) of the cleavage recognition site. We therefore suggest that retaining the proper P5 position might be a result of selection for optimal proteolytic cleavage at this Gln*Gly cleavage site. We note that the two Gln*Gly bonds within the PV polyprotein known to be cleaved most rapidly in trans (at the 2A-2B and 2C-3A junctions) both carry a glutamate in P5.
Short direct repeats may facilitate illegitimate RNA recombination.
Monitoring the fate of the parental moHgag and PVMgag2 genomes in transfected cells facilitated an investigation of rare illegitimate RNA recombination events. Since the growth of the parental moHgag and PVMgag2 viruses is severely impaired (see Table 1 for the emergence of CPE), any in-frame deletion within the gag insert is likely to result in the generation of faster-replicating variants that quickly outgrow their parent. The generation of distinct size clusters strongly suggested that the deletion events were directed by specific sequence signals. Sequence analyses revealed short direct sequence repeats, downstream of the borders of most deletions, that we suggest may serve as parting and anchoring sites during template switching, as has been proposed by Pilipenko and coworkers (43). Although it is desirable to gather sequence information of more deletion mutants, we consider it possible that the excision of the foreign sequence could occur through structural intermediates depicted in Fig. 10. These structural arrangements would involve either a loop-out mechanism (Fig. 10A) or a copy choice mechanism by strand switching, during minus-strand synthesis (Fig. 10B). The latter is reminiscent of the events occurring during genetic recombination of poliovirus (50). Considering the astoundingly high frequency by which genetic recombination between sibling strands is scored during a single growth cycle (11, 50), the copy choice mechanism may be currently favored in the rapid generation of the PV deletion variants carrying a fusion protein at the amino terminus of their polyprotein.
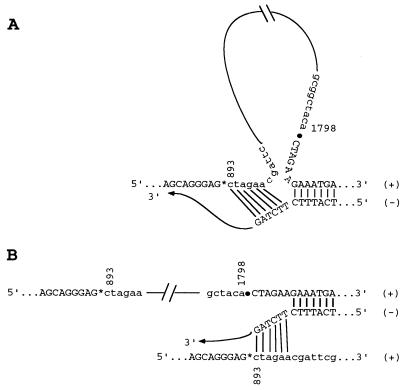
FIG. 10.
Two possible models of illegitimate recombination during minus-strand synthesis as exemplified for variant moHgag c1. Both models require a partial dissociation of the nascent minus [(−)] strand from the template plus [(+)] strand, caused by pausing of the polymerase. The free 3′ end of the nascent strand can reanneal to a short complementary sequence further upstream on the same template strand, thereby looping out the intervening sequences (A), or can reanneal to the same complementary sequence, but on a sibling plus strand, and complete synthesis on this second template (strand switching [B]). In both cases, the resulting minus strands would have excised the sequence between nt 893 and 1798 and can now, in turn, give rise to truncated positive-sense RNA genomes.
Explanation for the discrepancy between our data and data published by others.
It was reported previously that PV1(M) could stably express fusions to the N terminus of the polyprotein as large as 363 aa and replicate with near-wild-type characteristics (6). However, our data show that growth of the constructs with large inserts is severely impaired (as judged by delayed CPE) and that the block of replication is overcome by rapid deletion of different portions of the inserted foreign sequences. The genetic complexity of the genotypes of deleted variants, combined with the choice of probes to test for the inserts after several rounds of replication, may have given Andino et al. (5) an erroneous result of having retained the complete gag(p17-p24) in moHgag.
Interestingly, most of the isolated moHgag variants characterized here retained enough of the Gag coding sequence to contain at least one of the antigenic sites of HIV-1 Gag, located between aa 11 and 25 of matrix protein p17 (46). This may have led to the detection of Gag-specific neutralizing antibodies in test animals infected with such a variant (6). With this in mind, it might be possible to stably express and deliver small immunogenic peptides with polyprotein fusion vectors of the kind discussed here. However, genetic stability of the construct may vary from case to case and will have to be determined empirically.
Conclusion.
Four strategies to engineer PV vaccines for the delivery of foreign antigens (antigenic hybrid virions, dicistronic expression vectors, polyprotein fusion vectors, and P1 region replacement replicons) have failed so far to produce a vaccine that can be seriously considered for application in the human population. This is mainly due to the genetic and biochemical properties of these RNA viruses that defy prediction of the behaviors of altered genomes during proliferation. This pessimistic note does not mean that no picornavirus construct that is effective to deliver foreign antigens and serve as an efficacious vaccine will ever be found. However, given our current state of knowledge, such a vaccine will have to be found by trial and error rather than by design.
ACKNOWLEDGMENTS
We thank Raul Andino for the generous gift of plasmids pmogfp (prior to publication) and pmoHgag, and we thank Aniko Paul and Michael P. Shepley for suggestions and editing the manuscript. We are indebted to Walter Doerfler for encouragement.
This work was supported in part by NIH grants 5R01 AI32100-04 and 5R37 AI15122-24.
REFERENCES
- 1.Alexander L, Lu H H, Wimmer E. Polioviruses containing picornavirus type 1 and/or type 2 internal ribosomal entry site elements: genetic hybrids and the expression of a foreign gene. Proc Natl Acad Sci USA. 1994;91:1406–1410. doi: 10.1073/pnas.91.4.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altmeyer R, Escriou N, Girad M, Palmenberg A, van der Werf S. Attenuated Mengo virus as a vector for immunogenic human immunodeficiency virus type 1 glycoprotein 120. Proc Natl Acad Sci USA. 1994;91:9775–9779. doi: 10.1073/pnas.91.21.9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altmeyer R, Girad M, van der Werf S, Mimic V, Seigneur L, Saron M-F. Attenuated mengovirus: a new vector for live recombinant vaccines. J Virol. 1995;69:3193–3196. doi: 10.1093/benz/9780199773787.article.b00034516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altmeyer R, Murdin A D, Harber J J, Wimmer E. Construction and characterization of a poliovirus/rhinovirus antigenic hybrid. Virology. 1991;184:636–644. doi: 10.1016/0042-6822(91)90433-c. [DOI] [PubMed] [Google Scholar]
- 5.Andino, R. Personal communication.
- 6.Andino R, Silvera D, Suggett S D, Achacoso P L, Miller C J, Baltimore D, Feinberg M B. Engineering poliovirus as a vaccine vector for the expression of diverse antigens. Science. 1994;265:1448–1451. doi: 10.1126/science.8073288. [DOI] [PubMed] [Google Scholar]
- 7.Ansardi D C, Moldoveanu Z, Porter D C, Walker D E, Conry R M, LoBuglio A F, McPherson S, Morrow C D. Characterization of poliovirus replicons encoding carcinoembryonic antigen. Cancer Res. 1994;54:6359–6364. [PubMed] [Google Scholar]
- 8.Bredenbeek P J, Frolov I, Rice C M, Schlesinger S. Sindbis virus expression vectors: packaging of RNA replicons by using defective helper RNAs. J Virol. 1993;67:6439–6446. doi: 10.1128/jvi.67.11.6439-6446.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke K L, Almond J W, Evans D J. Antigen chimeras of poliovirus. Prog Med Virol. 1991;38:56–68. [PubMed] [Google Scholar]
- 10.Cao X, Kuhn R J, Wimmer E. Replication of poliovirus RNA containing two VPg coding sequences leads to a specific deletion event. J Virol. 1993;67:5572–5578. doi: 10.1128/jvi.67.9.5572-5578.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao X, Wimmer E. Genetic variation of the poliovirus genome with two VPg coding units. EMBO J. 1996;15:23–33. [PMC free article] [PubMed] [Google Scholar]
- 12.Cao X, Wimmer E. Intragenomic complementation of a 3AB mutant in dicistronic polioviruses. Virology. 1995;209:315–326. doi: 10.1006/viro.1995.1263. [DOI] [PubMed] [Google Scholar]
- 13.Chalfie M, Tu Y, Euskirchen G, Ward W W, Prasher D C. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 14.Choi W S, Pal-Gosh R S, Morrow C D. Expression of human immunodeficiency virus type 1 (HIV-1) Gag, Pol, and Env proteins from chimeric HIV-1 poliovirus minireplicons. J Virol. 1991;65:2875–2883. doi: 10.1128/jvi.65.6.2875-2883.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chow M, Newman J F E, Filman D, Hogle J M, Rowlands D J, Brown F. Myristylation of picornavirus capsid protein VP4 and its structural significance. Nature. 1987;327:482–486. doi: 10.1038/327482a0. [DOI] [PubMed] [Google Scholar]
- 16.Dolja V V, Herndon K L, Pirone T P, Carrington J C. Spontaneous mutagenesis of a plant potyvirus genome after insertion of a foreign gene. J Virol. 1993;67:5968–5975. doi: 10.1128/jvi.67.10.5968-5975.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dougherty W G, Semler B L. Expression of virus-encoded proteinases: functional and structural similarities with cellular enzymes. Microbiol Rev. 1993;57:781–822. doi: 10.1128/mr.57.4.781-822.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duarte E A, Novella I S, Weaver S C, Domingo E, Wain-Hobson S, Clarke D K, Moya A, Elena S F, de la Torre J C, Holland J J. RNA virus quasispecies: significance for viral disease and epidemiology. Infect Agents Dis. 1994;3:201–214. [PubMed] [Google Scholar]
- 19.Garcia-Sastre A, Palese P. Influenza virus vectors. Biologicals. 1995;23:171–178. doi: 10.1006/biol.1995.0028. [DOI] [PubMed] [Google Scholar]
- 20.Gmyl A P, Pilipenko E V, Maslova S V, Belov G A, Agol V I. Functional and genetic plasticities of the poliovirus genome: quasi-infectious RNAs modified in the 5′-untranslated region yield a variety of pseudorevertants. J Virol. 1993;67:6309–6316. doi: 10.1128/jvi.67.10.6309-6316.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gromeier M, Alexander L, Wimmer E. Internal ribosomal entry site substitution eliminates neurovirulence in intergeneric poliovirus recombinants. Proc Natl Acad Sci USA. 1996;93:2370–2375. doi: 10.1073/pnas.93.6.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gromeier M, Lu H H, Mueller S, Alexander L, Wimmer E. Attenuated poliovirus as live vaccine. In: Levine M, editor. New generation vaccines, in press. New York, N.Y: Marcel Dekker Inc.; 1997. pp. 315–330. [Google Scholar]
- 23.Hahm B, Back S H, Lee T G, Wimmer E, Jang S K. Generation of a novel poliovirus with a requirement of hepatitis C virus protease NS3 activity. Virology. 1996;226:318–326. doi: 10.1006/viro.1996.0659. [DOI] [PubMed] [Google Scholar]
- 24.Jarvis T C, Kirkegaard K. The polymerase in its labyrinth: mechanisms and implications of RNA recombination. Trends Genet. 1991;7:186–191. doi: 10.1016/0168-9525(91)90434-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King A M. Preferred sites of recombination in poliovirus RNA: an analysis of 40 intertypic cross-over sequences. Nucleic Acids Res. 1988;16:11705–11723. doi: 10.1093/nar/16.24.11705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirkegaard K, Baltimore D. The mechanism of RNA recombination in poliovirus. Cell. 1986;47:433–443. doi: 10.1016/0092-8674(86)90600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koch G. Interaction of poliovirus-specific RNAs with HeLa cells and E. coli. Curr Top Microbiol Immunol. 1973;62:89–138. doi: 10.1007/978-3-642-65772-6_4. [DOI] [PubMed] [Google Scholar]
- 28.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacterial T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 29.Lai M M C. RNA recombination in animal and plant viruses. Annu Rev Microbiol. 1992;56:61–79. doi: 10.1128/mr.56.1.61-79.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li S, Polonis V, Isobe H, Zaghouani H, Guinea R, Moran T, Bona C, Palese P. Chimeric influenza virus induces neutralizing antibodies and cytotoxic T cells against human immunodeficiency virus type 1. J Virol. 1993;67:6659–6666. doi: 10.1128/jvi.67.11.6659-6666.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liljestrom P, Garoff H. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Biotechnology (New York) 1991;9:1356–1361. doi: 10.1038/nbt1291-1356. [DOI] [PubMed] [Google Scholar]
- 32.Lu H H, Alexander L, Wimmer E. Construction and genetic analysis of dicistronic polioviruses containing open reading frames for epitopes of human immunodeficiency virus type 1 gp120. J Virol. 1995;69:4797–4806. doi: 10.1128/jvi.69.8.4797-4806.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu H H, Li X, Cuconati A, Wimmer E. Analysis of picornavirus 2Apro proteins: separation of proteinase from translation and replication functions. J Virol. 1995;69:7445–7452. doi: 10.1128/jvi.69.12.7445-7452.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu H H, Wimmer E. Poliovirus chimeras replicating under the translational control of genetic elements of hepatitis C virus reveal unusual properties of the internal ribosomal entry site of hepatitis C virus. Proc Natl Acad Sci USA. 1996;93:1412–1417. doi: 10.1073/pnas.93.4.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mattion N M, Reilly P A, Camposano E, Wu S L, DiMichele S J, Ishizaka S T, Fantini S E, Crowley J C, Weeks-Levy C. Characterization of recombinant polioviruses expressing regions of rotavirus VP4, hepatitis B surface antigen, and herpes simplex virus type 2 glycoprotein D. J Virol. 1995;69:5132–5137. doi: 10.1128/jvi.69.8.5132-5137.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mattion N M, Reilly P A, DiMichele S J, Crowley J C, Weeks-Levy C. Attenuated poliovirus strain as a live vector: expression of regions of rotavirus outer capsid protein VP7 by using recombinant Sabin 3 viruses. J Virol. 1994;68:3925–3933. doi: 10.1128/jvi.68.6.3925-3933.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murdin A D, Lu H H, Murray M G, Wimmer E. Poliovirus antigenic hybrids simultaneously expressing antigenic determinants from all three serotypes. J Gen Virol. 1992;73:607–611. doi: 10.1099/0022-1317-73-3-607. [DOI] [PubMed] [Google Scholar]
- 38.Murray M G, Kuhn R J, Arita M, Kawamura N, Nomoto A, Wimmer E. Poliovirus type 1/type 3 antigenic hybrid virus constructed in vitro elicits type 1 and type 3 neutralizing antibodies in rabbits and monkeys. Proc Natl Acad Sci USA. 1988;85:3203–3207. doi: 10.1073/pnas.85.9.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palmenberg A C. Proteolytic processing of picornaviral polyprotein. Annu Rev Microbiol. 1990;44:603–623. doi: 10.1146/annurev.mi.44.100190.003131. [DOI] [PubMed] [Google Scholar]
- 40.Palmenberg, A. C., and G. M. Duke. 1993. Mengo virus polyprotein genome. GenBank accession no. L22089.
- 41.Paul A V, Schultz A, Pincus S E, Oroszlan S, Wimmer E. Capsid protein VP4 of poliovirus is N-myristoylated. Proc Natl Acad Sci USA. 1987;84:7827–7831. doi: 10.1073/pnas.84.22.7827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Percy N, Barclay W S, Sullivan M, Almond J W. A poliovirus replicon containing the chloramphenicol acetyltransferase gene can be used to study the replication and encapsidation of poliovirus RNA. J Virol. 1992;66:5040–5046. doi: 10.1128/jvi.66.8.5040-5046.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pilipenko E V, Gmyl A P, Agol V I. A model for rearrangements in RNA genomes. Nucleic Acids Res. 1995;23:1870–1875. doi: 10.1093/nar/23.11.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Porter D C, Ansardi D C, Morrow C D. Encapsidation of poliovirus replicons encoding the complete human immunodeficiency virus type 1 gag gene by using a complementation system which provides the P1 capsid protein in trans. J Virol. 1995;69:1548–1555. doi: 10.1128/jvi.69.3.1548-1555.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ratner L, Haseltine W, Patarca R, Livak K J, Starcich B, Josephs S F, Doran E R, Rafalski J A, Whitehorn E A, Baumeister K, et al. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985;313:277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- 46.Robert-Hebmann V, Emiliani S, Resnicoff M, Jean F, Devaux C. Subtyping of human immunodeficiency virus isolates with a panel of monoclonal antibodies: identification of conserved and divergent epitopes on p17 and p25 core proteins. Mol Immunol. 1992;29:1175–1183. doi: 10.1016/0161-5890(92)90053-z. [DOI] [PubMed] [Google Scholar]
- 47.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 48.Schlesinger S. RNA viruses as vectors for the expression of heterologous proteins. Mol Biotechnol. 1995;3:155–165. doi: 10.1007/BF02789111. [DOI] [PubMed] [Google Scholar]
- 49.van der Werf S, Bradley J, Wimmer E, Studier F W, Dunn J J. Synthesis of infectious poliovirus RNA by purified T7 RNA polymerase. Proc Natl Acad Sci USA. 1986;83:2330–2334. doi: 10.1073/pnas.83.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wimmer E, Hellen C U, Cao X. Genetics of poliovirus. Annu Rev Genet. 1993;27:353–436. doi: 10.1146/annurev.ge.27.120193.002033. [DOI] [PubMed] [Google Scholar]
- 51.Xiong C, Levis R, Shen P, Schlesinger S, Rice C M, Huang H V. Sindbis virus: an efficient, broad host range vector for gene expression in animal cells. Science. 1989;243:1188–1191. doi: 10.1126/science.2922607. [DOI] [PubMed] [Google Scholar]
- 52.Yim T J, Tang S, Andino R. Poliovirus recombinants expressing hepatitis B virus antigens elicited a humoral immune response in susceptible mice. Virology. 1996;218:61–70. doi: 10.1006/viro.1996.0166. [DOI] [PubMed] [Google Scholar]
- 53.Zimmermann A, Nelsen-Salz B, Kruppenbacher J P, Eggers H J. The complete nucleotide sequence and construction of an infectious cDNA clone of a highly virulent encephalomyocarditis virus. Virology. 1994;203:366–372. doi: 10.1006/viro.1994.1495. [DOI] [PubMed] [Google Scholar]