Abstract
According to WHO, “complementary feeding (CF) is the process starting when breast milk alone or infant formula alone is no longer sufficient to meet the nutritional requirements of infants, and therefore, other foods and liquids are needed, along with breast human milk or a breastmilk substitute”. CF is one of the most important “critical and sensitive periods” in human life: indeed, timing and approaches to solid foods introduction in an infant’s nutrition are of utmost importance as potential epigenetic factors from infancy to adulthood. CF is also deeply influenced by each country and single-family traditions, culture, and beliefs. The aim of our narrative review is to analyze traditional CF practices, including innovative and alternative ones that emerged in the last decades, such as baby-led weaning or plant-based weaning, and to evaluate their effects on the risk of developing non-communicable diseases. Moreover, we will discuss pitfalls and misunderstandings that pediatricians frequently have to face when dealing with complementary feeding. Health care professionals must not have prejudices against parents’ wishes or traditions about CF; rather, they should support and educate them in case of any alternative CF choice, always pursuing the infant’s adequate growth, neuro- and taste development, and the achievement of correct eating behavior as the primary goal.
Keywords: complementary feeding, infants, nutrition, baby-led weaning, food allergy
1. Introduction
Complementary feeding (CF) is defined by the World Health Organization (WHO) as “the process starting when breast milk alone or infant formula alone is no longer sufficient to meet the nutritional requirements of infants, and therefore, other foods and liquids are needed, along with breast milk or a breastmilk substitute” [1]. CF is one of the most important “critical periods” in human life; the timing and approaches of solid foods introduction in infant’s nutrition are a critical window in which, according to Barker’s hypothesis, a positive or negative insult can exert important epigenetic effects in terms of outcome, thus programming the individual’s future life [2]. This crucial process in each infant’s life is not always approached properly. Parents, caregivers, and pediatricians have to face and combine tradition, innovation, and, sometimes, misleading beliefs when approaching CF. Healthcare professionals’ beliefs can influence CF timing and characteristics. A recent Italian survey among pediatricians, aimed at assessing healthcare professionals’ beliefs on CF, showed that an earlier complementary feeding start, higher use of predefined schedules, and higher attention to meat and salt intake were recommended by professionals more focused on infants’ nutritional needs than on their neurodevelopmental performances [3]. Overall, health care professionals’ role in guiding parents and caregivers during CF is of utmost importance. Adequate timing and characteristics of CF are fundamental for infants worldwide. In low- and middle-income countries, correct CF is closely linked to parents’ and caregivers’ educational level, and it also represents a milestone in infants’ diarrhea and disease prevention [4]. Moreover, in a review analyzing CF practices in 80 low- and middle-income countries, the authors concluded that monitoring CF indicators across the world and implementing policies and programs to reduce wealth-related inequalities are essential to achieving children’s nutritional standards [5]. In our review, we report CF general recommendations worldwide, also focusing on the more relevant issues for clinicians both from middle- and high-income countries. The aim of our narrative review is to discuss traditional CF practices, as well as innovative and alternative ones that emerged in the last decades, such as baby-led weaning or plant-based weaning, and to evaluate their epigenetic effects on the development of non-communicable diseases (NCDs). Moreover, we will discuss pitfalls and misunderstandings that pediatricians frequently have to face when dealing with complementary feeding. The MEDLINE–PubMed database was searched to collect and select publications from 1990 to 2023. The search included randomized placebo-controlled trials, controlled clinical trials, double-blind, randomized controlled studies, and systematic reviews. The following combinations of keywords were used: “complementary feeding” AND “tradition” OR “ dietary patterns” OR “baby-led weaning” OR “plant-based diet” OR “ food allergy” OR “ preterm infants” OR “non-communicable diseases” OR “ type 1 diabetes mellitus” OR “ celiac disease” OR “ food allergy”. We also performed a manual search of the reference lists of the selected studies. The search was limited to English-language journals and full papers only.
2. Tradition
2.1. Human Milk and Infant Formula
Breastfeeding is the “ordinary” and optimal nourishment for newborns and infants, to achieve optimal growth, development, and health [1]. Indeed, human breast milk (HM) contains the most balanced nutrient concentrations and confers several benefits through its wide group of bioactive compounds, including proteins/peptides, indigestible oligosaccharides, cells, hormones, mRNAs, nucleotides, minerals, vitamins, and innate immune factors [6]. When breast milk is not available or it is not sufficient to cover an infant’s nutritional needs, infant formula must be introduced. Infant formula composition is aimed at reproducing HM’s nutritional and functional effects, even if the biochemical composition of HM is unique and cannot be entirely reproduced [7]. Many compounds can be added to infant formulas to reach this goal. Prebiotics and probiotics are mainly valued for their ability to modulate the intestinal flora and to regulate stool consistency, and frequency of evacuations. Prebiotics (e.g., fructo-oligosaccharides and galacto-oligosaccharides, and more recently other human milk oligosaccharides) are used for their ability to increase the proportion of Lactobacilli and Bifidobacteria gut colonization and decrease that of Escherichia and Clostridia species, without side effects [8]. Current evidence has linked long-chain polyunsaturated fatty acids (LCPUFAs) to the improvement in neurological development in breastfed (BF) infants compared to formula-fed (FF) infants [9]. HM nutritional composition changes dynamically over time, depending on the mammary gland physiology, maternal diet, maternal health, and many other environmental factors [10,11]. It can also vary according to prematurity, and whether hindmilk or foremilk, as well as mature, and transitional milk, or colostrum, are considered [12]. Nutritional characteristics of HM and starting infant formulas are summarized in Table 1.
Table 1.
Energy, macronutrient, and micronutrient composition of HM, and recommended composition in cow’s milk formula (adapted from Koletzko et al. [13]).
| Variable | Colostrum (1–5 Days) | Mature Milk (>14 Days) | Cows’ Milk–Based Starting Infant Formula (Min–Max) |
Cows’Milk-Based Starting Infant Formula (Min–Max) |
|---|---|---|---|---|
| Energy | 50–60 kcal/100 mL | 65–70 kcal/100 mL | 60–70 kcal/100 mL | 60–70 kcal/100 mL |
| Carbohydrate | 50–62 g/L | 60–70 g/L | 9.0–14.0 g/100 kcal | 54–98 g/L |
| Total protein | 14–16 g/L | 8–10 g/L | 1.8–3 g/100 kcal | 10.8–21 g/L |
| Total fat | 15–20 g/L | 35–40 g/L | 4.4–6.0 g/100 kcal | 26–42 g/L |
| Iron | 0.5–1.0 mg/L | 0.3–0.7 mg/L | 0.3–1.3 mg/100 kcal | 2–9 mg/L |
| Calcium | 250 mg/L | 200–250 mg/L | 50–140 mg/100 kcal | 300–980 mg/L |
| Phosphorus | 120–160 mg/L | 120–140 mg/L | 25–90 mg/100 kcal | 150–630 mg/L |
| Magnesium | 30–35 mg/L | 30–35 mg/L | 5–15 mg/100 kcal | 30–105 mg/L |
| Sodium | 300–400 mg/L | 150–250 mg/L | 20–60 mg/100 kcal | 120–420 mg/L |
| Chloride | 600–800 mg/L | 400–450 mg/L | 50–160 mg/100 kcal | 300–1120 mg/L |
| Potassium | 600–700 mg/L | 400–550 mg/L | 60–160 mg/100 kcal | 360–1120 mg/L |
| Manganese | 5–12 μg/L | 3–4 μg/L | 1–50 μg/100 kcal | 6–350 ug/L |
| Iodine | 40–50 μg/L | 140–150 μg/L | 10–50 μg/100 kcal | 60–350 μg/L |
| Selenium | 25–32 μg/L | 10–25 μg/L | 1–9 μg/100 kcal | 9–63 μg/L |
2.2. Traditional Complementary Feeding: Definition and Characteristics
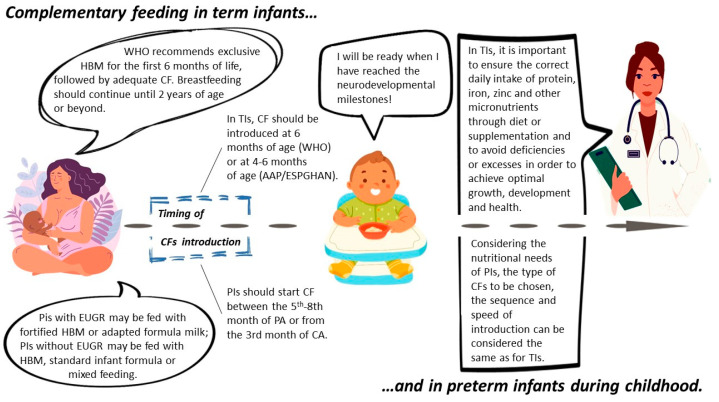
Complementary feeding (CF) is defined by WHO as “the process starting when breast milk alone or infant formula alone is no longer sufficient to meet the nutritional requirements of infants, and therefore, other foods and liquids are needed, along with breast milk or a breastmilk substitute”. According to WHO, complementary foods (CFs) are “any food or liquids, whether manufactured or locally prepared, suitable as a complement to breast milk or to a breast-milk substitute, fed to infants during the complementary feeding period” [1] and should not include low-nutrient beverages and drinks such as teas, coffee, and sugary drinks such as soda (e.g., tea and coffee contain compounds that can inhibit iron intestinal absorption) [14,15]. Thus, CFs may include finger foods, spoon-fed pureed foods, spoon-fed lumpy foods, or beverages, either prepared at home or commercially produced [16]. Traditionally, it was thought that CF should be started at 6 months of age for both BF [17] and FF infants. However, the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the American Academy of Pediatrics (AAP) both suggest that CFs may be introduced at 4–6 months, depending on the availability of safe CFs, the infant’s growth parameters, and the achievement of appropriate developmental milestones, thus widening the CF timeframe [18,19,20].
In the so-called “traditional complementary feeding”, CFs are first introduced in the form of purees, with gradual exposure to semisolid and finger foods (food that can be managed and eaten with hands), to finally acquire the dietary model of the entire family. Parents play a key role during this process, making decisions on the timing and content of the diet; indeed, they can adjust the pre-established amount, type, and consistency of foods usually given to infants [21] with the spoon. The foods, in the form of commercial or home-made baby foods, are specifically prepared for infants, and the correct proportions are recommended by pediatricians or other health professionals. As for this CF approach, the basic traditional meal consists of vegetable broth with semolina or rice/corn/tapioca flour, meat/fish purees, and grated fruit or fruit puree; the preparation of these meals is very quick and easy, and is thus usually preferred by parents.
CFs should be introduced no earlier than 4 months and no later than 7 months of age [14,18]. At around 6 months of age, infants should be ready to eat solid foods, due to the development of the appropriate renal, digestive, and oral motor skills (such as chewing and swallowing) [22]. A paper co-drafted by the Society for Preventive and Social Pediatrics (SIPPS), the Italian Federation of Pediatricians (FIMP), the Italian Society for Developmental Origins of Health and Disease (SIDOHaD), and the Italian Society of Pediatric Nutrition (SINUPE) reported the recommendations about the appropriate age and quantitative and qualitative modalities for the introduction of complementary foods into the diets of infants aged from 6 to 24 months [23]. The macronutrient-adequate intake for term infants aged 6 to 24 months is reported in Table 2.
Table 2.
Adequate intake of macronutrients in term infants from 6 to 36 months, expressed as percentage of total daily energy requirement, kcal/day or g/day (adapted from [23]).
| 6–12 Months | 12–24 Months | 24–36 Months | |
|---|---|---|---|
| Proteins | 14% | 14% | 14% |
| Carbohydrates | 45–55% | 45–60% | 45–60% |
| Fats | 40% | 35–40% | 35–40% |
| Fibers | 680–940 kcal/day | 10 g/day |
A month-by-month weaning schedule, which can provide useful guidance to facilitate the CF process, is summarized in Table 3 (to be considered as an example for illustrative purposes, and be adapted to local traditions, attitudes, and practices, and locally available food types).
Table 3.
Month-by-month weaning schedule (adapted from [23]).
| FOOD | AGE | |||
|---|---|---|---|---|
| 6–9 Months | 9–12 Months | 12–18 Months | 18–24 Months | |
| Cereals creams (rice, corn, and tapioca) | 25–30 g | |||
| Baby pasta and rice | 25–30 g | |||
| Bread | 5–10 g | |||
| Vegetable broth | 30–40 g | |||
| Fruits | 40 g (fresh fruit) 40 g (fruit puree) |
50 g (twice a day) 40 g (fruit puree) | 50 g three times a day | |
| Vegetables | 20 g | 30 g | ||
| Fish | 20 g (fresh fish), 40 g (fish puree) | 25 g (fresh fish) | ||
| Meat | 10 g (fresh meat), 40 g (meat puree) | 15–20 g (fresh meat) | ||
| Eggs | ¼ well cooked | ½ well cooked | ||
| Legumes | 25 g (Fresh peas) 10 g (Dried legumes) 40 g (legumes puree) |
30 g (Fresh peas) 30 g (Fresh green beans) 15–20 g (Dried legumes) |
||
| Extra virgin olive oil | 10 g | |||
Traditionally, CFs have been often referred to as “weaning foods”. Currently, the term “complementary foods” is preferred, as the term “weaning” implies the cessation of breastfeeding, while the goal of solid foods introduction is to supplement HM or infant formula without replacing it. HM alone is generally sufficient to meet nutritional infants’ needs for the first 6 months of life; thereafter, infants require additional sources of nutrients [24]. The period from conception to the beginning of the third year of life (the so-called “first thousand days of life”) is considered a “critical window” in which the foundations for appropriate neurological development and healthy growth throughout life are laid. This period is crucial, as the correct timing and choice of CF introduction may play a positive epigenetic influence on infants’ physical and cognitive development [25].
Early introduction of CFs may be considered for infants at high risk of iron deficiency (ID) or healthy BF infants if the mother is unable to breastfeed at 4–6 months of age [25]. However, Obbagy JE et al. conducted a systematic review on this topic, concluding that the introduction of CFs at 4 months instead of 6 months of age had no significant impact on iron status in healthy term infants (TIs) who were BF, fed with iron-fortified formula, or both. Iron-containing CFs (e.g., fortified cereals, meat), on the other hand, may help prevent ID and maintain adequate iron status in the first year of life among infants at risk of low iron intake or inadequate iron stores [26]. A similar result was found by Miniello VL et al.: no significant difference was detected between children (either BF or FF) who started CF at 4–6 months of age and those who started it at 6 months of age in terms of short-term (growth, iron status) and long-term outcomes (hypertension, overweight/obesity, type 2 diabetes mellitus) [27].
The main Italian and international dietary recommendations on complementary feeding practices are summarized in Table 4.
Table 4.
| Dietary Recommendations for Term Infants (TIs) 0–6 Months | ||
|---|---|---|
| American Academy of Pediatrics (AAP) | ESPGHAN | Italian Intersociety Document |
|
|
|
| Dietary recommendations for TIs 7–12 months | ||
| AAP | ESPGHAN | Italian Intersociety Document |
|
|
|
WHO
| ||
Iron content in some CFs is shown in Table 5 [28].
Table 5.
Iron content of some foods used in complementary feeding (adapted from [28]).
| Food | Iron Content (mg/100 g of Food) |
|---|---|
| Dried borlotti beans | 9 |
| Whole chicken egg | 6.3 |
| Oatmeal | 5.2 |
| Dried peas | 4.5 |
| Seabass | 4.1 |
| Pork meat | 4 |
| Horse meat | 3.9 |
| Lamb meat | 3.2 |
| Anchovies | 3.2 |
| Guinea fowl | 2.8 |
| Beef meat | 2.3 |
| Veal | 2.3 |
| Mackerel | 2.1 |
| Trout | 2 |
| Chicken Meat | 0.23 |
| Cow’s milk | 0.1–0.2 |
Zinc may also be deficient during the CF period. Poor zinc status may affect cognitive and motor development, as well as immune functions [28]. Although its bioavailability is high, the HM zinc content is relatively low (1–3 μg/L) compared to that in infant formulas, [10]. However, HM zinc concentration is considered adequate for most healthy term BF infants up to 6 months of age and, therefore, it is not considered a critical factor in determining the timing of CF. In well-nourished populations, there are no reports of zinc deficiency in term BF infants up to 6 months of age [29]. Nevertheless, zinc supplementation from 1 to 9 months in developing countries has been shown to reduce mortality from infectious diseases among term and small-for-gestational-age infants [30], demonstrating that zinc intake in early childhood may be inadequate in some circumstances. According to the Italian Society of Human Nutrition, the appropriate daily intake of zinc for the Italian population is 3 mg/day from 6 to 12 months of age and 5 mg/day from 1 to 3 years of age.
Therefore, during the CF period, foods such as poultry, meat, fish, or eggs should be consumed daily, as they are rich in many essential nutrients, such as iron and zinc [17]. Adequate intake of these foods improves zinc status during the first year of life, particularly in BF infants who do not receive adequate zinc from other sources. Poorer evidence was found for infants who consumed zinc-fortified formulas [26].
Zinc content in some CFs is shown in Table 6 [28].
Table 6.
Zinc content in some complementary foods (CFs) (adapted from [28]).
| Food | Zinc Content (mg/100 g of Food) |
|---|---|
| Grana Padano (cheese) | 11 |
| Lamb meat | 5.8 |
| Sardines | 5.7 |
| Turkey meat | 5.1 |
| Beef | 5 |
| Anchovies | 4.2 |
| Parmigiano Reggiano (Parmesan cheese) | 4 |
| Rabbit meat | 3.9 |
| Sardines | 3.9 |
| Dried cannellini beans | 3.6 |
| Pork meat | 3.5 |
| Guinea fowl | 3.8 |
| Dried chickpeas | 3.2 |
| Died borlotti beans/dried lentils | 2.9 |
| Chicken meat | 2.8 |
| Hen eggs, yolk | 2.14 |
Other micronutrient deficiencies are not common in exclusively BF infants. However, if mothers’ diets are deficient or selective, their infants may have low intakes of specific vitamins and minerals [17]. Indeed, the HM concentrations of most group B vitamins, selenium, iodine, and LCPUFAs are directly influenced by dietary maternal intake [9]. Hence, exclusively BF infants born to mothers on a strict vegan diet and not receiving appropriate supplementation, or with unrecognized pernicious anemia, can present with clinical signs of vitamin B12 deficiency [29]. Vitamin D and phylloquinone are not decisive in choosing CF timing. HM is also low in phylloquinone (vitamin K) and vitamin D content, which may increase the risk of bleeding and rickets in exclusively BF infants, respectively. In any case, it is universally agreed to administer phylloquinone at pharmacological dose (0.5–1 mg i.m., once at birth) and vitamin D in the first year of life [31].
Vitamin D is a fundamental micronutrient related to early development, bone health, and the immune system [32]. HM has generally low vitamin D concentrations (from 10 to 80 UI/L in healthy lactating mothers); therefore, all newborns, infants, and toddlers must be granted adequate vitamin D levels. Most countries have elaborated health policies to prevent vitamin D deficiency in the first year of life, advocating vitamin D supplementation for the infant, the breastfeeding mother, or both [32]. According to most of the international recommendations and the global consensus on rickets prevention, all infants should receive 400 IU/day of vitamin D oral supplementation from birth to 12 months of age, regardless of feeding and nutrition types [33]. According to Italian guidelines, in the presence of risk factors for vitamin D deficiency (non-Caucasian ethnicity with dark skin pigmentation, vegan diet, chronic kidney disease, hepatic failure, cholestasis, malabsorption syndrome, chronic drug therapies such as steroids, and infants born from mothers with multiple risk factors for vitamin D deficiency) up to 1000 IU/day of vitamin D should be administered [34].
While protein concentration in each infant formula does not change, HM protein content ranges from 14 to 16 g/L at birth to 8–10 g/L at 3–4 months, and 7–8 g/L at 6 months [35], and then remains fairly stable until 12 months of age [29]. Protein intake in the first 6 months of life is up to 66–70% lower in BF infants than in FF infants. According to the “early protein hypothesis”, excessive protein intake in the first years of life results in increased concentrations of branched-chain amino acids, leading to increased insulin growth factor 1 and insulin secretion, which can lead to accelerated weight gain and fat storage, and thus early metabolic programming of adiposity [36].
2.3. Complementary Feeding Patterns Worldwide
CF is a universal practice involving the global population, but it is highly influenced by cultural, individual, and socioeconomic factors. Studies carried out in Ireland [36], Tanzania [37], and Bangladesh [38] have identified several factors that may influence CF practices: maternal age and educational level, caregivers’ socioeconomic status, the opinions of friends and relatives, traditional feeding practices, influence of social networks, father’s occupation, postnatal care, and lack of professional counseling. When HM alone is no longer sufficient to meet an infant’s nutritional needs, caregivers may be induced to early CF introduction, due to cultural or personal reasons [39]. As mentioned above, the timeframe from birth to the first 2 years of life is crucial for promoting development, health, and optimal growth [24]. Inadequate CF practices during this period, such as poor hygiene behaviors, a too early CF start, and inadequate CF nutritional content, have been identified as the main causes of diarrhea, increased infection rates, malnutrition, micronutrient deficiencies, growth retardation, poor cognitive development, and increased mortality among children worldwide [39]. Studies from USA [40] and Ireland [36] show that about 20% of mothers start CF below 4 months of age. Data collected in Europe show that most infants introduce CFs before 6 months of age [41]. Similar results have been collected in Indonesia and China, while in India, CF is started too late, if compared to WHO recommendations [42]. In a study conducted by Schiess S. et al. [43], data from 1678 European healthy TIs from Italy, Germany, Belgium, Spain, and Poland were evaluated between October 2002 and June 2004 to assess the timing of CFs introduction; 588 infants were BF for at least 4 months, while 1090 infants were FF. The results showed that the introduction of CFs was delayed in BF infants (median 21 weeks, interquartile range 19–24) compared with FF infants (median 19 weeks, interquartile range 17–21); 17.2% BF infants and 37.2% of FF infants received CFs at 4 completed months of age, earlier than recommended, whereas 97.7% of BF infants and 99.3% of FF infants had received CFs at 7 completed months. Multiple regression analysis revealed that low maternal age, low educational level, and maternal smoking were predictive of early introduction of CFs at 3 and 4 months. In Europe, it has also been reported that a considerable proportion of children consume cow’s milk before the age of 12 months. In a sample of 15,700 Italian children, 0.2% consumed cow’s milk before the age of 3 months, 1.5% between 5 and 6 months, 5.8% between 7 and 8 months, and 26.8% by the age of 1 year. On the other hand, in Sweden, it has been reported that 0.5% of infants received cow’s milk before 4 months, and 30.2% before 6 months of age [41]. Moreover, the first solid food offered during CF in Europe varies greatly in each country, due to different local traditions. For example, in Italy, the most frequently offered first solid foods are fruit (73.1%), cereals (63.9%), and vegetables (40.3%) [44]; in England, rice (74%) [45], while potatoes, carrots, sweet corn, and derivatives are the most common starting CFs in Sweden [46]. In China, India, and Indonesia, diets are characterized by a reduced variety of CFs, mostly of poor nutritional quality, such as rice, cereals, and noodles. On the other hand, nutrient-dense and protein-rich foods (e.g., animal foods) are poorly consumed, especially in rural areas of China and India [42]. In this context, a 2018 Cochrane meta-analysis reported that educational interventions have a significant impact on the timing of CF introduction and hygiene practices (moderate-quality evidence), but not on the duration of exclusive breastfeeding (very low-quality evidence). Overall, according to the authors, educational interventions lead to improved CF practices [39].
2.4. Complementary Feeding in Preterm Infants
Preterm infants (PIs) are defined as infants born before 37 weeks of gestational age (GA). PIs represent a particularly vulnerable population with specific nutritional needs, so the appropriate management of their early nutritional needs is of utmost importance [47]. However, the timing and methods of CF in PIs are extremely heterogeneous, both between and within different countries [48,49]. Traditionally, it was believed that several goals have to be met before starting CF in PIs, such as reaching 5 kg of body weight [49], 3–6 months or [50] 5–8 months of postnatal age (PA) [51], or 3 months of corrected age (CA) [52]. Surveys conducted among parents and caregivers show that the first solid food offered to PIs is often nutritionally inadequate (e.g., with low protein and energy content) [53], and it is usually offered before 4 months of PA, even earlier than the recommended timing for TIs [49,53,54,55]. In addition, there is wide variability in iron and vitamin supplementation patterns, and CF practices among primary care pediatricians [48]. Some studies have also shown that the degree of prematurity influences the CF; PIs born at 34–36 weeks of GA are weaned at a mean CA of 4.6 months and at a mean PA of 5.7 months [56], while those born at 22–32 weeks of GA have 9.90 higher probability of receiving CFs before 4 months of PA when compared to TIs [57]. In this population, the early introduction of CFs has been associated with a higher risk of anemia, allergy, and rapid weight gain, whereas a delayed introduction of CFs after 7–10 months of PA is likely to increase the risk of avoidant feeding disorders [52]. The Italian position paper (co-drafted by the Italian Society of Neonatology—SIN, Italian Society of Pediatrics—SIP and Italian Society of Pediatric Gastroenterology, Hepatology and Nutrition—SIGENP) recommended that CF should start for PIs between 5 and 8 months of PA, or from at least 3 months of CA [48]. In conclusion, it is not yet possible to establish a precise timing for CF initiation in these patients, as the evidence is insufficient. However, an individualized approach must be adopted, carefully analyzing the neurodevelopmental milestones reached by the infant, and his or her attitude towards CFs, using the above-reported timing as a plausible but non-mandatory guideline. Indeed, these timing criteria should correspond to the disappearance of the tongue protrusion reflex, the gradual appearance of the labial seal, the reduction in the sucking reflex in favor of lateral tongue movements, and good neck control achievement in most ex-PIs [48]. However, neurodevelopmental adequacy is not the only aspect to consider, as a difficult introduction of CFs may also be explained by possible comorbidities (e.g., bronchopulmonary dysplasia) or defensive behaviors at mealtimes [58,59,60]. Infants who have undergone neonatal surgery or who are born before 30 weeks of GA are considered at risk for oro-motor feeding problems at 12 months of CA [61]. Nutritional strategies for these patients should be reviewed and adjusted regularly by a multidisciplinary medical team, including a behavioral psychologist, speech therapist, and nutritionist [62]. Furthermore, energy requirements vary according to the degree of prematurity. It has been shown that PIs often do not meet their caloric and protein requirements from birth, and these deficits are not compensated for by the time of discharge [48]. It should also be considered that if catch-up growth has not been achieved by the time CFs are introduced, it is necessary to promote a high protein and energy intake, through an appropriate formula or specific foods [49]. However, given the specific nutritional needs of PIs, particular attention should also be paid to micronutrient intake, especially iron and vitamins. In this regard, iron and vitamin supplementation is useful to ensure adequate intake up to 6 months of age, after which iron-rich foods should be provided to ensure sufficient iron intake [48]. As a 2020 Cochrane review concluded that nutritional education for families may reduce the malnutrition risk in TIs [63], it is reasonable to assume that the same concept may be used for PIs. Based on the available evidence, there is no need to delay the onset of CF in PIs to prevent overweight and obesity later in life [48]. Regardless of the relative risk of allergy development, allergenic foods and gluten should be given at any time after 4 months of CA, limiting the amount of gluten during infancy [64].
National and international guidelines [18,23,48] promote a CF based on a wide variety of CFs. However, plant-based diets (e.g., vegetarian and vegan diets) are also gaining popularity [65] among parents, who often ask pediatricians to provide their children with CF based on such dietary regimes [66]. Due to the paucity of robust data supporting the feasibility and safety of these alternative CF regimens in the PI population, they should be carefully planned by a nutrition expert professional, who should recommend the consumption of foods rich in iodine, zinc, iron, calcium, and LCPUFAs, and low in fiber. Vitamin B12 (in the case of a vegan diet) and vitamin D supplementations are also recommended [67]. ESPGHAN made a statement on the type of milk that PIs should receive during CF. According to this scientific society, infants with extrauterine growth retardation (EUGR) or at high risk of long-term growth failure should be fed with fortified HM or formula milk adapted for PIs with LCPUFAs, zinc, phosphorus, calcium, and high protein content up to 40 (but preferably 52) weeks of postmenstrual age. Infants without EUGR should receive exclusively HM, standard formula enriched with LCPUFAs, or mixed feeding (in case of inadequate amounts of HM) [68]. Recommendations for CF in PIs, according to the SIN, SIP, and SIGENP position paper, are summarized in Table 7.
Table 7.
Recommendations on CF in PIs, according to the position paper by SIN, SIP, and SIGENP (modified from [48]).
| Item | Recommendation for Preterm Infants (PIs) | Evidence |
|---|---|---|
| Recommended time for initiation of complementary feeding | PIs should start complementary feeding (CF) between 5 and 8 months of postnatal age (PA) or from 3 months of correct age (CA), so that the neurodevelopmental milestones are attained. | Certainty of evidence: moderate; grade of recommendation: strong. |
| Management of PIs with comorbidities and/or oral dysfunction |
Preterm infants with comorbidities or oral dysfunctions may require a multidisciplinary assessment to evaluate when and how CF should be started. | Certainty of evidence: low; grade of recommendation: weak. |
| Complementary Foods (CFs) recommended during CF | Recommendations for PIs regarding type of foods to choose, sequence and speed of introduction may be considered the same as for term infants, currently. Consider starting CF encompassing sources of carbohydrates, proteins and vegetable fats (extra-virgin olive oil) and paying special attention to the intake of micronutrients (e.g., iron and vitamins). |
Certainty of evidence: low; grade of recommendation: weak. |
| Risk of developing overweight/obesity in relation to an early onset of CF | Timing of CF start in PIs is unlikely to influence the incidence of overweight and obesity in childhood and adulthood, so the onset of CF should not be delayed for this purpose. | Certainty of evidence: moderate; grade of recommendation: strong. |
| Risk of developing allergy in relation to an early onset of CF | The introduction of allergenic foods (e.g., eggs, fish, tomato, peanuts) may not be delayed in PIs. | Certainty of evidence: very low; grade of recommendation: weak. |
| Vegetarian and Vegan CF regimens in PIs | Vegetarian and vegan weaning may be carefully planned in PIs. | Certainty of evidence: very low; grade of recommendation: weak. |
| Recommended type of lactation during CF | Infants without Extra Uterine Growth Restriction (EUGR) may be fed with exclusive human breast milk (HM), standard infant formula enriched with long-chain polyunsaturated fatty acids (LCPUFAs), or mixed feeding (in case of inadequate amounts of HM). Infants with EUGR or at high risk of long-term growth failure may be fed with fortified HM or formula milk adapted for PIs as long as necessary to gain an optimal weight for CA. | Certainty of evidence: low; grade of recommendation: weak. |
Recommendations for CF in term and preterm infants are summarized in Figure 1.
Figure 1.
Recommendations for CF in term and preterm infants.
3. Baby-Led Weaning and On-Demand Complementary Feeding
CF occurs in a period of life, the so-called “first 1000 days”, that is well known as a crucial moment for children’s health and future development [69], as several epigenetic factors occurring in this timeframe may also affect later physical, cognitive, and socio-emotional health [70]. Hence, caregivers should receive adequate information about the CF process and the nutritional pattern to be adopted. Nowadays, several complementary feeding strategies are available. A correct CF approach may positively influence eating behaviors and other chronic diseases, such as overweight and obesity, allergic diseases, celiac disease, or diabetes. “Standard Weaning”, “Traditional Weaning”, “Traditional spoon feeding”, or “Parent-Led Weaning” (PLW) is widely supported by the consensus in the scientific literature. On the contrary, in the last 10–15 years, an alternative weaning approach, called “Baby-Led Weaning” (BLW) has grown up in popularity. In the UK, Rapley et al. introduced the definition of BLW as “the inclusion of the infant in family mealtimes, where food that is suitable for the infant to eat is made available to all” [71]. Through this weaning method, parents allow infants to choose where, how much, and what foods they eat, sitting at the table with the rest of the family [72]. The infant is offered the same foods as the family but as finger foods, large enough for them to be picked up by hand.
Baby-Led Introduction to SolidS (BLISS) is a modified form of BLW where caregivers are informed about choking, iron status, and failure to thrive. They are educated to cut foods into elongated formats, such as strips or sticks, and to offer children three types of food, sources of iron, energy, and fibers, at each meal [73]. BLW seems to be associated with better eating behaviors, with a lower incidence of fussiness, greater food enjoyment, and food responsiveness. In two cross-sectional studies, performed by Fu et al. and Komninou et al., infants who received BLW, followed up from 6 to 36 months, and from 12 to 36 months, respectively, had lower levels of food fussiness and major levels of food enjoyment when compared to those receiving traditional CF [74,75]. In a randomized clinical trial, Taylor et al. showed a lower satiety responsiveness in BLISS infants at 24 months (n = 166) compared to the spoon-weaning control group (adjusted difference, −0.24; 95% CI, −0.41 to −0.07). No body mass index (BMI) z-score differences were described between the two groups at 12 and 24 months [76]. Indeed, several studies have been carried out to investigate the growth and nutrient intake in BLW infants compared to traditionally weaned infants. An online questionnaire including sociodemographic and dietary questions has been administered to parents of 134 infants aged 6–12 months, among which 88 babies had followed Baby-Led Complementary Feeding (BLCF) and 44 babies following Standard Weaning (SW). In this cross-sectional study, the authors found no differences between the two groups in weight for age centiles, and energy, carbohydrate, protein, saturated fat, or Zn intake. However, BLCF infants received less iron from infant formulas (1.6 mg (SD 1.9) vs. 2.4 mg (SD 1.7); p = 0.012), and less fat and sodium from foods (p = 0.035 and p = 0.028, respectively) than SW babies [77]. In 2019, Rowan et al. conducted a study including 180 parents to compare the dietary composition of BLW to that of traditional spoon-fed children, aged 6–12 months. BLW infants were more prone to vegetables (p = < 0.0001) and proteins (p = 0.002) than traditionally weaned infants, whereas no differences were reported in exposure to iron-rich foods between the two groups [78]. In 2018, a randomized controlled trial carried out by Daniel et al. yielded the conclusion that a baby-led strategy is not correlated with the risk of iron deficiency. This trial included 206 participants assigned to control (n = 101) or BLISS (n = 105) groups [79].
Carruth and Skinner hypothesized that a baby-led approach may also permit a better development of motor ability, and both gross and fine movements, due to the repeated stimulations to use hands and fingers to handle and manage foods, as well as a concomitant better coordination of mouth and tongue movements [80].
BLW requires appropriate oral skills, including chewing and swallowing. Indeed, without proper parental control of food types and sizes, choking may become a serious risk factor for BLW. In a retrospective study, Ozyuksel et al. examined the clinical records of 75 infants aged from 5 to 12 months, who had undergone bronchoscopy due to foreign body aspiration, and they showed that 80% of aspiration occurred in BLW infants, compared to 14% during traditional CF [81]. Nevertheless, in a randomized controlled trial, Fangupo et al. showed no significant differences regarding choking episodes between the BLISS infants’ group (in which parents were educated about the relative risks) and infants following more traditional feeding practices; in the BLISS group, gagging episodes were more likely to occur at 6 months of age (relative risk (RR), 1.56; 95% confidence interval (CI), 1.13–2.17) than at 8 months (RR, 0.60; 95% CI, 0.42–0.87) [82]. The main limitation of the majority of these studies is the high prevalence of parent-reported information, which may bias the significance of the results [83].
Another alternative weaning approach, known as “self-weaning” or “on-demand complementary feeding”, emerged in Italy at the same time that BLW first arose in the UK. The main difference between the BLW strategy and the “on-demand complementary feeding”, described by the Italian pediatrician Lucio Piermarini, consists in the modality of feeding. In the BLW approach, the exclusive use of the hands is mandatory, whereas, according to on-demand complementary feeding, the use of a spoon is advisable. Indeed, times, manners, textures, and quantity of food offered during a “self-weaning” are based on the level of psycho-neuro-motor and physical development of the child (the food can be minced and mashed) [84]. This modality emphasizes the infant’s active behavior with food offers modulated as a parental response to the infant’s signs of request. Currently, there are no data available comparing BLW and on-demand CF efficacy [85].
WHO and AAP have also proposed Responsive Complementary Feeding (RCF), in which caregivers offer food only when the child is hungry and stop when the child stops demanding it. On the other hand, non-Responsive Complementary Feeding (NRCF) is characterized by a non-reciprocal relationship between caregiver and child, at least as far as mealtime is concerned. The caregiver is not involved in the child’s request or refusal of food and can force, insist, limit, or not limit food intake, or use food as a reward strategy [86].
However, should a family choose a non-traditional CF, the pediatrician’s role becomes of utmost importance, with the task of remembering the fundamental role of a healthy diet for all family members, to avoid the failure to thrive and micronutrient deficiency, and to provide proper advice to minimize choking and gabbing risks. Non-traditional CF characteristics and health implications are summarized in Table 8.
Table 8.
Main characteristics of different CF strategies.
| Traditional Weaning (TW) | Baby-Led Weaning (BLW) | BLISS (Baby-Led Introduction to SolidS) | On-Demand Complementary Feeding | |
|---|---|---|---|---|
| Parents involvement | Yes (spoon feeding) | No (use of hands) | No (use of hands), parents are instructed about relatives’ concerns | Yes (possibly use of spoon) |
| Food texture | Purees, semisolid, finger foods, solid | Finger foods | Finger foods | Based on the level of psycho-neuro-motor and physical development |
| Benefits |
|
|
|
|
| Risks |
|
|
|
4. Plant-Based Complementary Feeding
Over the last decades, the prevalence of people following vegan or vegetarian diets has dramatically increased [87]. According to the results of a survey conducted by Eurispes in 2023, people following a vegetarian or vegan diet are estimated to be 6.6% of the total population in Italy (4.2% vegetarian and 2.4% vegan) [88]. There are several types of plant-based diets, all characterized by an increased intake of plant foods and a reduced or absent intake of animal products: vegan diet (no animal products are permitted), lacto-ovo-vegetarian diet (only eggs and dairy products are allowed), and ovo-vegetarian or lacto-vegetarian diet (which excludes milk or eggs, respectively). Furthermore, some people follow a primary plant diet (similar to lacto-ovo-vegetarian but with small amounts of lean meat) or pescatarian (which excludes meat and poultry, while fish is permitted). There are also some very restrictive subtypes, such as raw vegan diet (all cooked foods and processed foods are excluded), fruitarian diet (only fruits, nuts, and seeds are permitted), and macrobiotic diet (based on Taoist “yin and yang” principles, which emphasize whole grains, beans, and vegetables) [65,89]. The Italian Position Paper on Vegetarian Diets in Pregnancy and Developmental Age states that there is yet not enough scientific evidence to determine at what age it is safe to start a vegetarian diet. On the other hand, there is strong evidence that excluding certain food groups can lead to nutritional deficiencies, which may require supplementation. The German Nutrition Society also recommends that people on a vegetarian diet supplement their diet and receive regular medical checkups. However, other organizations, such as the Academy of Nutrition and Dietetics, the Portuguese National Program for the Promotion of a Healthy Diet, the Canadian Pediatric Society, and the Australian National Health and Medical Research Council, state that a well-balanced vegetarian diet is appropriate for people of all ages, including infancy [90,91,92,93,94,95]. The joint position paper of the Italian Society of Preventive and Social Pediatrics (SIPPS) with the Italian Federation of Pediatricians (FIMP) and the Italian Society of Perinatal Medicine (SIMP) has also examined the appropriateness of a vegetarian diet during childhood. The authors conclude that a vegan diet should not be recommended for children below two years of age because it would lead to deficiencies in several important nutrients (vitamin B12, vitamin D, iron, docosahexaenoic acid—DHA, and calcium) [95]. According to the American Dietetic Association, the weaning guidelines and timing of solid food introduction are the same for vegetarian and non-vegetarian children. Mangels et al. proposed a schedule of solid food introduction for vegetarian and vegan infants. Until 6 months of age, both breast milk and infant formula provide adequate intake of macro- and micronutrients, except vitamin B12, which must be given as a supplement. Between 4 and 6 months, the first solid food to be introduced should be iron-fortified infant cereals, which provide adequate energy and iron intake in an easily digestible form. Rice cereals should be firstly preferred as they usually are hypoallergenic. In case iron-fortified infant cereals are not introduced, iron supplementation becomes essential. Once the child has well tolerated the cereals taken, vegetables and fruit can be introduced, without particular attention to the order of introduction. Between 7 and 8 months of age, protein sources should be introduced. Good sources of protein for vegetarian children include soy yogurt, tofu, and legume puree. Soy-based cheeses should be introduced later, and tempeh and soy burgers by 11–12 months of age. Once the infant has reached an adequate chewing ability, the sources of carbohydrates can be more varied. A proposal for different food introductions in plant-based CF is summarized in Table 9.
Table 9.
Food introduction timing in plant-based CF, modified from [96].
| Food | 4–6 Months | 6–8 Months | 9–10 Months | 11–12 Months |
|---|---|---|---|---|
| Milk | Human milk Soy formula |
Human milk Soy formula |
Human milk Soy formula |
Human milk Soy formula |
| Cereal and cereal-derived food | Iron fortified infant cereal (usually rice is the first introduced) | Infant cereal, crackers, unsweetened dry cereal for breakfast | Infant cereal, crackers, toast, unsweetened dry cereal for breakfast, soft bread | Infant cereal, crackers, toast, unsweetened dry cereal for breakfast, soft bread, rice pasta |
| Fruits and vegetables | - | Strained fruit or vegetables, fruit or vegetable juice | Soft or cooked fruit, fruit juice, cooked mashed vegetable, vegetable juice | Soft, canned or cooked fruit, peeled raw fruit, fruit juice, cooked pieces of vegetable, vegetables juice |
| Pulses | - | Pureed legumes | Pureed legumes, | Mashed legumes |
| Other food items with high protein content | Tofu, soy yogurt (after 8 months) | Soy cheese, soy yogurt (after 8 months) | Tofu, soy cheese or yogurt, tempeh | |
| Other food items with high fat content | Olive oil | Olive oil | Olive oil | Olive oil Small amount of light margarine |
Vitamin B12 is the only mandatory supplement in a plant-based diet, as even a varied and balanced diet does not guarantee sufficient intake of this micronutrient, due to the low vitamin B12 content and bioavailability in plant-based foods (algae, fungi). Thus, vitamin B12 supplementation in all people following a vegan diet, and especially in breastfeeding mothers and infants, is always mandatory. Infants’ vitamin B12 daily requirement ranges between 0.5 and 0.8 µg/day. Vitamin B12 deficiency is associated with neurological symptoms, anorexia, anemia, developmental delay, and palmar and plantar hyperpigmentation. Zinc is another micronutrient worth consideration, as its deficiency may be associated with increased susceptibility to infections, changes in taste, failure to thrive, and mucocutaneous alterations, such as dermatitis and alopecia. Fortified cereals are a good source of zinc. As for iodine, its daily requirement ranges from 50 to 80 µg/day in the first 12 months of life. Iodized salt is not recommended under 12 months of age. Fish and dairy products contain high levels of iodine, but 400 mL/day of breast milk or 900 mL/day of infant formula may also guarantee an adequate iodine intake [91]. DHA and eicosapentaenoic acid (EPA) have a significant role in retinal function, behavior, and brain development (both pre- and postnatal). Algae-derived DHA is a good option for vegan mothers’ supplementation, as the only very long-chain n-3 fatty acid precursor found in good amounts in plants is α-linolenic acid (ALA). ALA can be found in walnut, canola, soybean, linseed, echium seed oils, algae, paprika Capsicum annuum, and chia Salvia hispanica [9]. Indeed, breast milk and/or DHA-supplemented formula represents a good source of DHA in infants’ nutrition.
Vitamin D supplementation and adequate calcium intake are always recommended for all infants. In the first year of life, the calcium daily requirement is 500 mg/day, whereas the vitamin D daily intake should be at least 10 µg/day (400 IU/day). Calcium is mainly provided by dairy products, and the levels of phytates and oxalates are inversely proportional to its bioavailability. Rice- or soy-based infant formula provides adequate calcium intake. As for vitamin D supplementation in vegan infants, the only vitamin D drop formulation is sheep’s wool lanoline-derived vitamin D3, so vegans often refuse it. In this case, vitamin D2 (derived from fungi) supplementation, at a dosage of 2000 IU/day or 60,000 IU/month for 3 months, combined with adequate sun exposure, may represent a valid alternative for breastfeeding mothers [97], see Table 10.
Table 10.
Micronutrient requirements and supplementation for plant-based complementary feeding.
| Daily Requirement 0–12 Months |
Plant-Based Food That Contains | Supplementation | |
|---|---|---|---|
| Vitamin B12 | 0.5–0.8 µg/day | Algae, fungi, tempeh | Necessary |
| Calcium | 500 mg/day | Dairy products, broccoli, kale, cabbage, soy drinks, tofu. Nuts, dried beans, spinach (low bioavailability) | Not necessary |
| Vitamin D | 10 µg/day (400 IU/day) |
Dairy products or cereals, fortified soy drink | Suggested |
| Iron | 6–8 mg/day | Soaking pulses, iron-rich vegetables, iron-fortified food (cereals) | Not necessary |
| Zinc | 5 mg/day | Zinc-fortified cereal, whole seeds, nuts, legumes, dairy products | Not necessary |
| Iodine | 50–80 µg/day | Dairy products, breast milk, infant formula | Not necessary |
Concerning protein intake, a well-balanced plant-based diet looks to be adequate. For formula-fed vegan infants, the use of rice-protein-based infant formulas, supplemented with lysine, tryptophan, and threonine, or soy-based infant formulas fortified with methionine, should be recommended.
In conclusion, the decision to start a vegetarian or vegan weaning should be made under close medical monitoring to avoid significant nutritional deficiencies. Vegetarian weaning under medical supervision is possible and should not be obstructed. Vegan weaning, on the other hand, should be carefully evaluated as related serious risks have been demonstrated (rickets, growth retardation, cognitive deficits), and it is not yet recommended by the main international scientific institutions. For ex-preterm infants, any form of alternative weaning should be discouraged. Infants following restrictive diets should be given the right vitamin supplementation throughout the weaning period, with periodic blood tests and clinical monitoring to document any micronutrient deficiencies.
5. Complementary Feeding Practices and Risk for Non-Communicable Diseases
NCDs are medical conditions associated with a long duration and slow progression of the illness. Most NCDs are the result of a combination of genetic, physiological, behavioral, and environmental factors. Multiple determinants interact to influence health and well-being throughout life [98]. Nutrition is a highly significant epigenetic factor in influencing health, either positively or negatively; nowadays, growing scientific evidence supports the statement that nutrition in early childhood may affect the risk of developing NCDs [24], although its epigenetic effect does not seem to be that strong, and further studies are needed in this field [99]. The CF timeframe is one of the so-called “susceptibility windows” where a positive or negative insult can have long-term effects on health outcomes in later childhood and even in adulthood.
5.1. Overweight and Obesity
Children with weight excess have an increased risk of becoming overweight and obese adults, and they may experience earlier onset of chronic diseases like hypertension, dyslipidemia, heart failure, and type 2 diabetes mellitus (T2DM). There are multiple risk factors for the development of overweight in infancy, both genetics and environmental; among environmental factors, dietary habits seem to act from the very early stages of life [100]. In this review, we focus on the possible correlation between the timing of CF and obesity risk, the role of the type of milk taken during CF, the type of weaning, and the quality of foods.
Data about the timing of CF and obesity risk are scarce. Sun et al. report that, in term infants, the introduction of solids at the age of 5–6 months decreases the risk of having a high BMI at 1 year of age, whereas infants weaned before 4 months have a higher risk of being overweight regardless of the duration of breastfeeding. Introduction of solid foods after 7 months is associated with increased BMI in infants breastfed for <4 months, but not in infants breastfed for ≥4 months. So, the authors concluded that longer duration of breastfeeding is associated with decreased risk of having above-normal BMI and CF should be introduced at 5–6 months of age [101].
Concerning the impact of the type of milk on weaning and weight gain, Jones et al. observed that spoon feeding was associated with increased infant weight only in formula-fed infants, while there was no significant weight difference in BLW infants who were breastfed or formula-fed. They also calculated gain velocity, as rapid weight gain during infancy may be a predictor of adiposity, but they did not find any significant differences between the two groups [102].
In a systematic review, Nazareth Martinón-Torres et al. analyzed the effect of BLW on the risk of obesity in late childhood, but their results were inconclusive [96]. In a Turkish randomized controlled study, Dogan et al. [103] showed that traditional spoon-fed infants gained more weight than the BLW group at 12 months of life. Similar results have been reported by Townsend and Pitchford [104].
Given that gut microbiota can have an impact on growth patterns in animal models and human studies [105,106,107,108], many studies have analyzed how CF and the composition of the gut microbiota may influence long-term body weight, body composition, and disease risk [109,110,111,112].
Tang et al. published a randomized controlled trial on infant growth and gut composition, in which almost 300 five-month-old infants were randomized to receive a meat-protein-predominant diet, dairy-protein-predominant diet, or plant-protein-predominant diet; they were compared to a reference infants group following traditional CFs. The authors reported an increased risk of overweight in the dairy group, even if the underlying mechanisms remain unclear. One possible cause is that they observed a higher serum Insulin-Like Growth Factor 1 (IGF-1) concentration in this group of infants, which is associated with a higher risk of obesity early in life [113]. Nevertheless, higher IGF-1 values were found from 6 to 12 months, but not at 12 or 24 months. They found that the meat and dairy groups had some specific differences in gut microbiota diversity and composition, but it is unclear how these observations could affect growth and risk of overweight. Therefore, although the plausibility of a direct protein effect on obesity during complementary feeding exists, evidence is still weak [114].
5.2. Type 1 Diabetes
As in other inflammatory disorders, it has been hypothesized that diet may also have epigenetic effects and affect immune dysregulation in type 1 diabetes mellitus (T1DM) pathogenesis. Breast milk seems to play a protective role against T1DM development. The MIDIA study investigated the association between breastfeeding duration and CF starting age, and the risk of islet autoimmunity and T1DM. The authors concluded that the duration of exclusive breastfeeding, solid food introduction timing, and breastfeeding at the time of introduction of any solid food cannot influence the risk of islet autoimmunity or type 1 diabetes. Breastfeeding, for 12 months or longer, is related to a lower risk of progression from islet autoimmunity to T1DM in genetically predisposed children [115]. One meta-analysis suggested an increased risk of T1DM in infants who had been early exposed to cow’s milk, but this result has not been further confirmed [116,117].
The Trial to Reduce T1DM in the Genetically at Risk (TRIGR) included 2159 newborn infants from 15 countries. In this study, CF with extensively hydrolyzed formula was not related to reduction in islet autoimmunity risk, if compared to conventional formula [118].
In 2003, the National Institutes of Health elaborated The Environmental Determinants of Diabetes in the Young (TEDDY), a multicenter prospective cohort study aimed at identifying environmental triggers of islet autoimmunity leading to T1DM; 8676 children with T1DM predisposing HLA-DR-DQ genotypes have been followed since birth in the USA, Finland, Germany, and Sweden, and environmental triggers, including infections, probiotics, micronutrients, and microbiome have been evaluated [118]. A possible protective role of breast milk was hypothesized. Breastfeeding duration was not associated with a lower risk of either islet or transglutaminase autoimmunity, while breastfeeding for more than 6 months of age, and exclusive breastfeeding for more than 3 months, were associated with decreased risk of obesity [119]. Conversely, accelerated weight gain may increase the risk for T1DM because of the establishment of insulin resistance and beta-cell overload, and consequent damage [49]. In the TEDDY study, the timing of solid food introduction was associated with islet autoimmunity in children with the HLA DR3/4 genotype not exposed to probiotics, even if the microbiome composition under these exposure combinations requires further studies [120]. In conclusion, most prospective cohort studies showed that early infant feeding practices, breastfeeding at gluten introduction, infant’s age at the time of gluten introduction, and type of milk cannot decrease the risk of developing T1DM [121].
The intake of soluble fibers has also been studied. Deficiency of soluble fiber intake has been suggested to dysregulate the local immune response, as soluble fibers are usually converted into short-chain fatty acids (SCFAs) by bacterial fermentation in the gut, with several anti-inflammatory properties. Moreover, fibers directly affect the gut microbiome, so a low fiber intake may lead to a status of dysbiosis, but no statistically significant associations between a high intake of soluble fiber and islet autoimmunity or T1DM have been found [122].
The associations between erythrocyte fatty acids and the risk of islet autoimmunity have been investigated in erythrocytes collected at the ages of 3, 6, and 12 months, and then annually up to 6 years of age. Higher EPA and DHA levels during infancy were associated with a lower risk of islet autoimmunity. Fatty acid status in early life may indicate the risk for islet autoimmunity, which may be preceded by increased levels of some short-chain and mono-unsaturated fatty acids [123].
5.3. Celiac Disease
Celiac disease (CD) is a disorder in which gluten intake, combined with genetic susceptibility, causes an autoimmune reaction affecting the gut and other organs. It is a permanent condition that affects approximately 1% to 3% of the general population almost worldwide, and the genetic predisposition is determined by the presence of HLA alleles DQ2 and/or DQ8 [124]. Identifying preventive strategies to reduce the prevalence of CD is one of the major targets of research in recent years, and many attempts and trials have been proposed with no univocal conclusions.
Increased serum antibody titers against cow’s milk proteins have been observed in subjects with CD [117] as well as in T1DM, and avoidance of cow’s milk-based formula has been tested [118].
In a randomized controlled trial [113], enrolling the same population of the above-mentioned TEDDY trial, the effect of extensively hydrolyzed formula on CD risk was assessed. The sample was composed of 230 infants with HLA predisposition to T1DM and at least one family member affected. Infants were divided into two groups, one fed a casein hydrolysate formula, and the other a conventional formula or breastmilk. Infants who later progressed to CD had casein antibody titers significantly higher than those of unaffected subjects. When diagnosed with CD, they also had IgG anti-beta lactoglobulin titers higher than those of non-affected infants. Nevertheless, they did not find evidence that extensively hydrolyzed formula would decrease the risk for CD later in life [118].
The timing of CF, in particular of gluten introduction, has been long debated. In 2008, ESPGHAN recommended avoiding both early (<4 months) and late (>7 months) gluten introduction, and to start it while continuing breastfeeding, to reduce CD risk [15]. The results of further observational studies showed no significant differences in CD risk in children exposed to gluten earlier than 4 months compared with first exposure at 4 to 6 months [124]. For this reason, in 2016, new evidence prompted ESPGHAN to revise recommendations, concluding that age and type of gluten introduction in infants do not seem to influence the absolute risk of developing CDA (celiac disease autoimmunity) or CD during childhood [124].
In conclusion, breastfeeding or not during gluten introduction does not reduce the risk for CD, and the avoidance of milk proteins is not protective. Hence, gluten should be introduced in a period between 4 and 8 months of age. In children at high genetic risk for CD, earlier introduction of gluten (around 4 months) is associated with earlier development of autoimmunity (defined as positive serology), but the cumulative incidence in later childhood is similar [125].
6. Complementary Feeding and Food Allergy
The risk of developing a food allergy (FA) is influenced by a combination of genetic and environmental factors. The recent and substantial rise in FA prevalence is primarily attributed to environmental factors. These factors could impact the food tolerance process, either directly or through epigenetic modifications [126,127]. Historically, the AAP discouraged the consumption of peanuts in children at an increased risk of atopy (i.e., those with ≥1 first-degree family relative with atopic diseases) before 3 years of age. AAP also advised against the consumption of cow’s milk throughout the entire first year of life, eggs during the second year of life, and fish and nuts during the third year of life [128]. Therefore, in the past, it was usually recommended to delay the introduction of foods with higher potential allergenic properties, as the immaturity of gut structure and function, coupled with its heightened permeability, was thought to provoke an elevated susceptibility to allergic sensitization [129]. Despite these strategies involving food avoidance, the prevalence of food allergies continued to increase in Western societies [130], suggesting that early allergen exposure might play a crucial role in attaining food tolerance, a process driven by antigens, as indicated by findings from animal models [18]. Considering this evidence, in 2008, the AAP revised recommendations on CF and FA, recognizing the uncertainty regarding the preventive aspects of allergen avoidance. They acknowledged that there was insufficient evidence to endorse maternal avoidance and delayed introduction of potential food allergens into infants’ diets as a primary tool for preventing food allergies [131]. Current guidelines suggest starting oral allergen exposure from the fourth month of age, although the most appropriate critical window for the introduction of CF for allergy prevention is still unknown [132]. Some data indicate that initiating CF before 3 or 4 months of age may increase the risk of developing allergic diseases during later infancy and childhood [133,134]. At that critical age, the intestinal barrier exhibits higher permeability, and the establishment of gastrointestinal colonization is not yet fully developed. These factors may contribute to the observed increase in the risk for allergies [135,136]. Therefore, several international guidelines aimed at allergy prevention recommend the introduction of solid foods, including egg and peanuts, after 4 months of age [135,136]. Several studies have demonstrated that delayed exposure to allergenic foods did not reduce the FA risk, both for infants with or without a positive family history of atopy [136,137]. A recent expert committee statement by the Section of Pediatrics of the European Academy of Allergology and Clinical Immunology is in close agreement with the revised AAP position [138]. In 2016, the Australasian Society of Clinical Immunology and Allergy (ASCIA) updated its guidelines for FA prevention. ASCIA recommended CF initiation “around 6 months, but not before 4 months of age,” irrespective of a family history of atopy, and preferably while continuing breastfeeding [139]. Similarly, the 2018 guidelines from the Asian Pacific Association of Pediatric Allergy, Respirology, and Immunology advised the introduction of solid foods, including those with allergenic potential, starting at six months of age, both for the general population and infants with a family history of atopic disorders [140]. The WHO strategy to prevent allergies is to promote exclusive breastfeeding during the first 6 months of the infant’s life, as a preventive strategy for the later development of allergies [141]. Current guidelines on the introduction of allergenic food during CF are summarized in Table 11.
Table 11.
Recommendations on allergenic foods introduction in complementary feeding.
| Guidelines | |
|---|---|
| American Academy of Pediatrics (AAP) 2019 |
|
| Asia Pacific Association of Pediatric Allergy, Respirology & Immunology (APAPARI); 2017 |
|
| National Institute of Allergy and Infectious Diseases (NIAID), 2017 |
|
| European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN), 2017 |
|
| Asian Pacific Association of Pediatric Allergy, Respirology and Immunology (APAPARI), 2018 |
|
| German Society for Allergology and Clinical Immunology (DGAKI) |
|
| European Academy of Allergy and Immunology (EAACI) |
|
Currently, there is insufficient conclusive evidence to support the hypothesis that early introduction of potentially allergenic foods may prevent the same food allergies, except for the introduction of peanuts between 4 and 11 months of age in infants at high risk of developing peanut allergies.
Timing of Solid Food Introduction and Risk of Food Allergy
Peanuts, eggs, cow milk, and fish are potentially allergenic foods whose timing of introduction in CF is often analyzed and debated.
Du Toit et al. showed a ten-fold higher prevalence of peanut hypersensitivity among Jewish children living in the United Kingdom in comparison to their counterparts in Israel. Notably, in Israel, there is an important early-life peanut consumption in comparison to the limited peanut intake observed in the United Kingdom [142]. The Learning Early About Peanut Allergy Study (LEAP study), a landmark randomized, open-label, controlled trial conducted in “high-risk” infants aged 4 to 11 months with severe eczema or egg allergy or both, and with a skin-prick test (SPT) for peanut allergy <4 mm, was aimed at assessing peanut allergy in this children population. A cohort of children was randomly allocated to either receive 6 g of peanut protein per week, provided as peanut snacks or peanut butter, or to abstain from peanut intake until the age of 5 years. The authors showed that the incidence of allergies decreases when peanuts are introduced around 4 to 6 months of age. After five years, children allocated to the intervention group had a significantly lower prevalence of peanut allergy (documented with an oral food challenge) compared with those of the avoidance group (3.2% vs. 17.2%, p < 0.001), corresponding to a 14% and 80% absolute and relative risk reduction, respectively. It is worth noting that this difference was observed in both groups of children who initially tested negative at peanut SPT (1.9% vs. 13.7%, p < 0.001), and in those with SPT ranging from 1 to 4 mm (10.6% vs. 35.3%, p = 0.004) [130]. The LEAP-On follow-up trial further demonstrated a significantly lower (74%) peanut allergy prevalence in infants who introduced peanuts early when compared to those who had avoided peanuts intake, suggesting a persistent tolerance in the early introduction infants’ group, even one year after ceasing peanut consumption, and in the absence of repeated exposures [143]. Furthermore, early peanut introduction was observed to be allergen-specific and did not influence the development or resolution of other allergic conditions, such as asthma and atopy [144]. Following these data, a consensus statement signed by the LEAP trial team recommended peanut introduction into the diet of “high-risk” (as defined by the LEAP study) infants’ diet between 4 and 11 months of age [145]. Based on this evidence, in 2017, the American National Institute of Allergy and Infectious Diseases issued supplementary guidelines for peanut allergies [146]. These recommendations suggest an early peanut introduction at around 4–6 months of age for infants with severe atopic dermatitis and/or egg allergy (EA) following SPT or specific IgE (sIgE) testing for peanuts. If the SPT is ≤2 mm, or sIgE < 0.35 kUA/L, parents can introduce peanuts at home. On the contrary, if SPT falls between 3 and 7 mm or sIgE ≥ 0.35 kUA/L, a supervised oral peanut challenge in a medical setting is advised. Finally, infants with an SPT ≥ 8 mm have a notably elevated risk of peanut allergy and should be followed up by a pediatric allergologist [147].
Several studies investigating the impact of introducing eggs on allergy risk have yielded conflicting findings. This disparity may be likely related to uncontrolled variables within diverse study populations, as well as to variations in the dosage and form of eggs administered, including whether they were raw or cooked. In the Hen’s Egg Allergy Prevention (HEAP) study [148], 383 infants, aged 4 to 6 months, with no prior egg sensitization, were randomly assigned to receive freeze-dried white egg or a placebo, three times a week for six months. At one year of age, only 12 infants had developed IgE antibodies in response to eggs, with eight babies (5.6%) in the group receiving the active intervention, and four babies (2.6%) in the placebo group. The incidence of egg allergy was found to be 2.1% in the active group and 0.6% in the placebo group. In summary, this study did not provide evidence supporting the hypothesis that early egg consumption prevents food allergies and egg sensitization. Similar findings were obtained in the Australian Study Starting Time of Egg Protein (STEP) trial [149]. The Prevention of Egg Allergy with Tiny Amount Intake (PETIT) trial [150] was aimed at assessing the effectiveness and safety of introducing heated eggs as a preventive measure against egg allergies in 147 high-risk infants with atopic eczema. These infants did not exhibit immediate allergic reactions to eggs, and showed no delayed reactions to any type of food; they were randomly divided into two groups: one received heated egg powder (50 mg/day from 6 to 9 months, and 250 mg/day from 9 to 12 months), while the other received a placebo (squash). The primary outcome, which included egg allergy confirmation through open egg challenges at 12 months of age, could not be established in 26 out of 147 (17%) infants. The primary analysis included 60 infants (50%) in the egg group and 61 infants (50%) in the placebo group. By the time the infants reached 12 months of age, clinical hypersensitivity reactions to eggs were significantly less frequent in the group receiving heated eggs compared to the control group (8% vs. 38%). Furthermore, at the 12-month test, levels of egg white and ovalbumin (OVA) sIgE were notably higher in the placebo group, while OVA IgG4 antibody concentration exhibited a significant increase in the group receiving heated eggs. It is worth noting that, at baseline, levels of egg-white sIgE were higher in the control group than in the group receiving heated eggs. Additionally, it is essential to approach these findings with caution since an intention-to-treat (ITT) analysis was not conducted. Furthermore, when addressing infants with severe eczema, it would be advisable [151] to conduct egg-specific SPT/sIgE testing before egg introduction into their diet. In case sIgE and/or SPT indicate a positive reaction, both egg and peanut should be administered under medical supervision, due to the potential for clinical hypersensitivity reactions upon exposure, whereas peanut introduction may occur at home if infants exhibit negative peanut sIgE and/or peanut SPT ≤ 2 mm. Additionally, it is recommended to offer cooked food to encourage tolerance and reduce the risk of FPIES [152].
In conclusion, current guidelines recommend peanut introduction during the first year of life at home for most infants. However, for infants with severe eczema, egg allergies, or both, a medical assessment, including sensitivity testing for peanuts, should be conducted before introducing peanuts at 4–6 months of age [153]. It remains uncertain whether other allergenic foods, such as eggs, should also be introduced to an infant’s diet between 4 and 6 months of age.
When breastfeeding is unfeasible or inadequate, cow’s milk proteins are typically introduced in the early days or weeks of infants’ life, via a cow’s milk-based formula, as also advised by the AAP [152]. AAP [153] and ESPGHAN recommend refraining from relying solely on whole cow’s milk for infants’ nutrition before the age of 12 months, citing its low iron content and the potential for causing intestinal microhemorrhages [154].
Many observational studies have evaluated the timing of fish introduction in infants’ diet, and whether it affects their chances of getting asthma and allergies. In the Enquiring About Tolerance (EAT) study [155], the introduction of six allergenic foods (cow’s milk, wheat, sesame, white fish, peanut, and egg) between three and six months of age did not lower the risk of developing food allergies to these specific foods, when compared to their standard introduction after six months of age, as commonly practiced in the general population.
7. Conclusions
CF is a fundamental milestone in infants’ nutrition. It is a critical period in which a positive or negative insult can have effects on long-term outcomes in later life, such as growth, non-communicable diseases, and food allergies. Solid food introduction is also deeply rooted in each country and each family’s tradition and culture, but it is also influenced by new modes and trends. In this context, pediatricians should be competent guides for children and their families, enabling adequate growth and neurodevelopment, while respecting each family’s beliefs and traditions. Healthcare professionals must not have prejudices against parents’ wishes or traditions about CF; rather, they should support and educate them in case of any alternative CF choice, always pursuing the infant’s adequate growth, neuro- and taste development, and the achievement of correct eating behavior as the primary goal.
Abbreviations
| AAP | American Academy of Pediatrics |
| AD | Atopic Dermatitis |
| ALA | Alfa-Linolenic Acid |
| BB | Breastfed |
| BLCF | Baby-Led Complementary Feeding |
| BLISS | Baby-Led Introduction to SolidS |
| BLW | Baby-Led Weaning |
| CD | Celiac Disease |
| CF | Complementary feeding |
| CFs | Complementary foods |
| CM | Cow Milk |
| DHA | Docosahexaenoic Acid |
| EA | Egg Allergy |
| EAT | Enquiring About Tolerance |
| EFSA | European Food Safety Authority |
| ESPGHAN | European Society for Paediatric Gastroenterology Hepatology and Nutrition |
| EUGR | Extra Uterine Growth Restriction |
| FA | Food Allergy |
| FIMP | Italian Federation of Pediatricians |
| FF | Formula-fed |
| HEAP | Hens Egg Allergy Prevention |
| HM | Human Breast Milk |
| ID | Iron Deficiency |
| IDA | Iron-Deficiency Anemia |
| IGF 1 | Insulin-Like Growth Factor 1 |
| LA | Linoleic Acid |
| LCPUFAs | Long-Chain Polyunsaturated Fatty Acids |
| LEAP | Learning Early About Peanut Allergy Study |
| NCDs | Non-Communicable Diseases |
| NRCF | Non-Responsive Complementary Feeding |
| PETIT | Prevention of Egg Allergy with Tiny Amount Intake |
| PLW | Parent-Led Weaning |
| PCPs | Primary Care Pediatricians |
| PIs | Preterm Infants |
| RCF | Responsive Complementary Feeding |
| SACN | UK Scientific Advisory Committee on Nutrition |
| SIDOAhD | Italian Society for Developmental Origins of Health and Disease |
| SIGENP | Italian Society of Pediatric Gastroenterology, Hepatology and Nutrition |
| SIMP | Italian Society of Perinatal Medicine |
| SIN | Italian Society of Neonatology |
| SIP | Italian Pediatric Society |
| SPT | Skin Prick Tests |
| STEP | Australian Study Starting Time of Egg Protein |
| SW | Standard Weaning |
| TEDDY | The Environmental Determinants of Diabetes in the Young |
| T1DM | Type 1 Diabetes Mellitus |
| T2DM | Type 2 Diabetes Mellitus |
| TRIGR | Trial to Reduce IDDM in the Genetically at-Risk |
| Tis | Term Infants |
| WHO | World Health Organization |
Author Contributions
M.E.C. wrote the first draft of the manuscript and supervised the literature review; B.S., A.G., D.M., S.R.L. and N.M.D. performed the literature review and co-wrote the manuscript; S.E. designed the project, revised the manuscript, and made a substantial scientific contribution; G.B. made a substantial scientific contribution and revised the first draft. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.World Health Organization. UNICEF Strengthening Action to Improve Feeding of Infants and Young Children 6–23 Months of Age in Nutrition and Child Health Programmes: Geneva, 6–9 October 2008. Report of Proceedings. [(accessed on 4 September 2023)]. Available online: https://iris.who.int/bitstream/handle/10665/44034/9789241597890%20eng.pdf;jsessionid=87A00C0BA248EC40EE35A22A2E9984DB?sequence=1.
- 2.Barker D.J.P. Editorial: The developmental origins of adult disease. Eur. J. Epidemiol. 2002;18:733–736. doi: 10.1023/A:1025388901248. [DOI] [PubMed] [Google Scholar]
- 3.Brambilla P., Giussani M., Picca M., Bottaro G., Buzzetti R., Milani G.P., Agostoni C., Becherucci P. Do the opinions of pediatricians influence their recommendations on complementary feeding? Preliminary results. Eur. J. Pediatr. 2020;179:627–634. doi: 10.1007/s00431-019-03548-9. [DOI] [PubMed] [Google Scholar]
- 4.Hamer D.H., Solomon H., Das G., Knabe T., Beard J., Simon J., Nisar Y.B., MacLeod W.B. Importance of breastfeeding and complementary feeding for management and prevention of childhood diarrhoea in low- and middle-income countries. J. Glob. Health. 2022;12:10011. doi: 10.7189/jogh.12.10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gatica-Domínguez G., Neves P.A., Barros A.J., Victora C.G. Complementary Feeding Practices in 80 Low- and Middle-Income Countries: Prevalence of and Socioeconomic Inequalities in Dietary Diversity, Meal Frequency, and Dietary Adequacy. J. Nutr. 2021;151:1956–1964. doi: 10.1093/jn/nxab088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Βasdeki A.M., Fatouros D.G., Βiliaderis C.G., Moschakis T. Physicochemical properties of human breast milk during the second year of lactation. Curr. Res. Food Sci. 2021;4:565–576. doi: 10.1016/j.crfs.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skórka A., Pieścik-Lech M., Kołodziej M., Szajewska H. Infant formulae supplemented with prebiotics: Are they better than unsupplemented formulae? An updated systematic review. Br. J. Nutr. 2018;119:810–825. doi: 10.1017/S0007114518000120. [DOI] [PubMed] [Google Scholar]
- 8.Riva E., Verduci E., Agostoni C., Giovannini M. Closer to the gold standard: An appraisal of formulae available in Italy for use in formula-fed infants. J. Int. Med. Res. 2005;33:595–611. doi: 10.1177/147323000503300601. [DOI] [PubMed] [Google Scholar]
- 9.Capra M.E., Stanyevic B., Giudice A., Monopoli D., Decarolis N.M., Esposito S., Biasucci G. Long-Chain Polyunsaturated Fatty Acids Effects on Cardiovascular Risk in Childhood: A Narrative Review. Nutrients. 2023;15:1661. doi: 10.3390/nu15071661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ballard O., Morrow A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013;60:49–74. doi: 10.1016/j.pcl.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin C.R., Ling P.R., Blackburn G.L. Review of Infant Feeding: Key Features of Breast Milk and Infant Formula. Nutrients. 2016;8:279. doi: 10.3390/nu8050279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Underwood M.A. Human milk for the premature infant. Pediatr. Clin. N. Am. 2013;60:189–207. doi: 10.1016/j.pcl.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koletzko B., Baker S., Cleghorn G., Neto U.F., Gopalan S., Hernell O., Hock Q.S., Jirapinyo P., Lonnerdal B., Pencharz P., et al. Global standard for the composition of infant formula: Recommendations of an ESPGHAN coordinated international expert group. J. Pediatr. Gastroenterol. Nutr. 2005;41:584–599. doi: 10.1097/01.mpg.0000187817.38836.42. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization Exclusive Breastfeeding for Optimal Growth, Development and Health of Infants. [(accessed on 2 September 2023)]. Available online: https://www.who.int/tools/elena/interventions/exclusive-breastfeeding.
- 15.Pan American Health Organization, World Health Organization Guiding principles for Complementary Feeding of the Breastfed Children. [(accessed on 4 September 2023)]. Available online: https://www.who.int/publications/i/item/9275124604.
- 16.EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens) Castenmiller J., de Henauw S., Hirsch-Ernst K.-I., Kearney J., Knutsen H.K., Maciuk A., Mangelsdorf I., McArdle H.J., Naska A., et al. Scientific Opinion on the appropriate age range for introduction of complementary feeding into an infant’s diet. EFSA J. 2019;17:5780. doi: 10.2903/j.efsa.2019.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dewey K. Guiding Principles for Feeding Non-Breastfed Children 6–24 Months of Age. World Health Organization; Geneva, Switzerland: 2005. [Google Scholar]
- 18.Fewtrell M., Bronsky J., Campoy C., Domellöf M., Embleton N., Fidler Mis N., Hojsak I., Hulst J.M., Indrio F., Lapillonne A., et al. Complementary Feeding: A Position Paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017;64:119–132. doi: 10.1097/MPG.0000000000001454. [DOI] [PubMed] [Google Scholar]
- 19.Gartner L.M., Morton J., Lawrence R.A., Naylor A.J., O’Hare D., Schanler R.J., Eidelman A.I. Breastfeeding and the use of human milk. Pediatrics. 2005;115:496–506. doi: 10.1542/peds.2004-2491. [DOI] [PubMed] [Google Scholar]
- 20.Section on Breastfeeding. Eidelman A.I., Schanler R.J., Johnston M., Landers S., Noble L., Szucs K., Viehmann L. Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827–e841. doi: 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- 21.Fernandes C., Martins F., Santos A.F., Fernandes M., Veríssimo M. Complementary Feeding Methods: Associations with Feeding and Emotional Responsiveness. Children. 2023;10:464. doi: 10.3390/children10030464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naylor A.J., Morrow A.L. Developmental Readiness of Normal Full Term Infants to Progress from Exclusive Breastfeeding to the Introduction of Complementary Foods: Reviews of the Relevant Literature Concerning Infant Immunologic, Gastrointestinal, Oral Motor and Maternal Reproductive and Lactational Development. The Linkages Project and Wellstart International; Washington, DC, USA: 2001. [Google Scholar]
- 23.Caroli M., Vania A., Verga M.C., Di Mauro G., Bergamini M., Cuomo B., D’Anna R., D’Antonio G., Dello Iacono I., Dessì A., et al. Recommendations on Complementary Feeding as a Tool for Prevention of Non-Communicable Diseases (NCDs)-Paper Co-Drafted by the SIPPS, FIMP, SIDOHaD, and SINUPE Joint Working Group. Nutrients. 2022;14:257. doi: 10.3390/nu14020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Acevedo N., Alhamwe B.A., Caraballo L., Ding M., Ferrante A., Garn H., Garssen J., Hii C.S., Irvine J., Llinás-Caballero K., et al. Perinatal and early-life nutrition, epigenetics, and allergy. Nutrients. 2021;13:724. doi: 10.3390/nu13030724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Domellöf M., Braegger C., Campoy C., Colomb V., Decsi T., Fewtrell M., Hojsak I., Mihatsch W., Molgaard C., Shamir R., et al. Iron requirements of infants and toddlers. J. Pediatr. Gastroenterol. Nutr. 2014;58:119–129. doi: 10.1097/MPG.0000000000000206. [DOI] [PubMed] [Google Scholar]
- 26.Obbagy J.E., English L.K., Psota T.L., Wong Y.P., Butte N.F., Dewey K.G., Fox M.K., Greer F.R., Krebs N.F., Scanlon K.S., et al. Complementary feeding and micronutrient status: A systematic review. Am. J. Clin. Nutr. 2019;109((Suppl. S7)):852S–871S. doi: 10.1093/ajcn/nqy266. [DOI] [PubMed] [Google Scholar]
- 27.Miniello V.L., Verga M.C., Miniello A., Di Mauro C., Diaferio L., Francavilla R. Complementary feeding and iron status: “the unbearable lightness of being” infants. Nutrients. 2021;13:4201. doi: 10.3390/nu13124201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daniels L., Heath A.L., Williams S.M., Cameron S.L., Fleming E.A., Taylor B.J., Wheeler B.J., Gibson R.S., Taylor R.W. Baby-Led Introduction to SolidS (BLISS) study: A randomised controlled trial of a baby-led approach to complementary feeding. BMC Pediatr. 2015;15:179. doi: 10.1186/s12887-015-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies) Scientific Opinion on the essential composition of infant and follow-on formulae. EFSA J. 2014;12:3760. doi: 10.2903/j.efsa.2014.3760. [DOI] [Google Scholar]
- 30.Sazawal S., Black R.E., Menon V.P., Dinghra P., Caulfield L.E., Dhingra U., Bagati A. Zinc supplementation in infants born small for gestational age reduces mortality: A prospective, randomized, controlled trial. Pediatrics. 2001;108:1280–1286. doi: 10.1542/peds.108.6.1280. [DOI] [PubMed] [Google Scholar]
- 31.Braegger C., Campoy C., Colomb V., Decsi T., Domellof M., Fewtrell M., Hojsak I., Mihatsch W., Molgaard C., Shamir R., et al. Vitamin D in the healthy European paediatric population. J. Pediatr. Gastroenterol. Nutr. 2013;56:692–701. doi: 10.1097/MPG.0b013e31828f3c05. [DOI] [PubMed] [Google Scholar]
- 32.Corsello A., Milani G.P., Giannì M.L., Dipasquale V., Romano C., Agostoni C. Different Vitamin D Supplementation Strategies in the First Years of Life: A Systematic Review. Healthcare. 2022;10:1023. doi: 10.3390/healthcare10061023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munns C.F., Shaw N., Kiely M., Specker B.L., Thacher T.D., Ozono K., Michigami T., Tiosano D., Mughal M.Z., Mäkitie O., et al. Global Consensus Recommendations on Prevention and Management of Nutritional Rickets. J. Clin. Endocrinol. Metab. 2016;101:394–415. doi: 10.1210/jc.2015-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saggese G., Vierucci F., Prodam F., Cardinale F., Cetin I., Chiappini E., De’ Angelis G.L., Massari M., Miraglia Del Giudice E., Miraglia Del Giudice M., et al. Vitamin D in pediatric age: Consensus of the Italian Pediatric Society and the Italian Society of Preventive and Social Pediatrics, jointly with the Italian Federation of Pediatricians. Ital. J. Pediatr. 2018;44:51. doi: 10.1186/s13052-018-0488-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor S.N., Fenton T.R., Groh-Wargo S., Gura K., Martin C.R., Griffin I.J., Rozga M., Moloney L. Exclusive Maternal Milk Compared with Exclusive Formula on Growth and Health Outcomes in Very-Low-Birthweight Preterm Infants: Phase II of the Pre-B Project and an Evidence Analysis Center Systematic Review. Front. Pediatr. 2022;9:793311. doi: 10.3389/fped.2021.793311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koletzko B., Dodds P.F., Akerblom H., Ashwell M. Early Nutrition and Its Later Consequences: New Opportunities. Springer Publishers; New York, NY, USA: 2005. [Google Scholar]
- 37.Victor R., Baines S.K., Agho K.E., Dibley M.J. Factors associated with inappropriate complementary feeding practices among children aged 6-23 months in Tanzania. Matern. Child Nutr. 2014;10:545–561. doi: 10.1111/j.1740-8709.2012.00435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kabir I., Khanam M., Agho K.E., Mihrshahi S., Dibley M.J., Roy S.K. Determinants of inappropriate complementary feeding practices in infant and young children in Bangladesh: Secondary data analysis of Demographic Health Survey 2007. Matern. Child Nutr. 2012;8((Suppl. S1)):11–27. doi: 10.1111/j.1740-8709.2011.00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arikpo D., Edet E.S., Chibuzor M.T., Odey F., Caldwell D.M. Educational interventions for improving primary caregiver complementary feeding practices for children aged 24 months and under. Cochrane Database Syst. Rev. 2018;5:CD011768. doi: 10.1002/14651858.CD011768.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fein S.B., Labiner-Wolfe J., Scanlon K.S., Grummer-Strawn L.M. Selected complementary feeding practices and their association with maternal education. Pediatrics. 2008;122((Suppl. S2)):S91–S97. doi: 10.1542/peds.2008-1315l. [DOI] [PubMed] [Google Scholar]
- 41.Caroli M., Mele R.M., Tomaselli M.A., Cammisa M., Longo F., Attolini E. Complementary feeding patterns in Europe with a special focus on Italy. Nutr. Metab. Cardiovasc. Dis. 2012;22:813–818. doi: 10.1016/j.numecd.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Sirkka O., Abrahamse-Berkeveld M., van der Beek E.M. Complementary Feeding Practices among Young Children in China, India, and Indonesia: A Narrative Review. Curr. Dev. Nutr. 2022;6:nzac092. doi: 10.1093/cdn/nzac092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schiess S., Grote V., Scaglioni S., Luque V., Martin F., Stolarczyk A., Vecchi F., Koletzko B., European Childhood Obesity Project Introduction of complementary feeding in 5 European countries. J. Pediatr. Gastroenterol. Nutr. 2010;50:92–98. doi: 10.1097/MPG.0b013e31819f1ddc. [DOI] [PubMed] [Google Scholar]
- 44.Giovannini M., Riva E., Banderali G., Scaglioni S., Veehof S.H., Sala M., Radaelli G., Agostoni C. Feeding practices of infants through the first year of life in Italy. Acta Paediatr. 2004;93:492–497. doi: 10.1080/08035250410025591. [DOI] [PubMed] [Google Scholar]
- 45.Wright C.M., Parkinson K.N., Drewett R.F. Why are babies weaned early? Data from a prospective population based cohort study. Arch. Dis. Child. 2004;89:813–816. doi: 10.1136/adc.2003.038448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brekke H.K., Ludvigsson J.F., van Odijk J., Ludvigsson J. Breastfeeding and introduction of solid foods in Swedish infants: The All Babies in Southeast Sweden study. Br. J. Nutr. 2005;94:377–382. doi: 10.1079/BJN20051499. [DOI] [PubMed] [Google Scholar]
- 47.Riskin A. Meeting the nutritional needs of premature babies: Their future is in our hands. Br. J. Hosp. Med. 2017;78:690–694. doi: 10.12968/hmed.2017.78.12.690. [DOI] [PubMed] [Google Scholar]
- 48.Baldassarre M.E., Panza R., Cresi F., Salvatori G., Corvaglia L., Aceti A., Giannì M.L., Liotto N., Ilardi L., Laforgia N., et al. Complementary feeding in preterm infants: A position paper by Italian neonatal, paediatric and paediatric gastroenterology joint societies. Ital. J. Pediatr. 2022;48:143. doi: 10.1186/s13052-022-01275-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liotto N., Cresi F., Beghetti I., Roggero P., Menis C., Corvaglia L., Mosca F., Aceti A., on Behalf of the Study Group on Neonatal Nutrition and Gastroenterology-Italian Society of Neonatology Complementary Feeding in Preterm Infants: A Systematic Review. Nutrients. 2020;12:1843. doi: 10.3390/nu12061843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DHSS (Department of Health and Social Security) Present day practice in infant feeding. Lancet. 1988;331:975–976. doi: 10.1016/S0140-6736(88)91786-2. [DOI] [PubMed] [Google Scholar]
- 51.King C. An evidence based guide to weaning preterm infants. Paediatr. Child Health. 2009;19:405–414. doi: 10.1016/j.paed.2009.06.005. [DOI] [Google Scholar]
- 52.Palmer D.J., Makrides M. Introducing solid foods to preterm infants in developed countries. Ann. Nutr. Metab. 2012;60((Suppl. S2)):31–38. doi: 10.1159/000335336. [DOI] [PubMed] [Google Scholar]
- 53.Fanaro S., Borsari G., Vigi V. Complementary feeding practices in preterm infants: An observational study in a cohort of Italian infants. J. Pediatr. Gastroenterol. Nutr. 2007;45((Suppl. S3)):S210–S214. doi: 10.1097/01.mpg.0000302974.90867.f1. [DOI] [PubMed] [Google Scholar]
- 54.Zielinska M.A., Rust P., Masztalerz-Kozubek D., Bichler J., Hamułka J. Factors Influencing the Age of Complementary Feeding-A Cross-Sectional Study from Two European Countries. Int. J. Environ. Res. Public Health. 2019;16:3799. doi: 10.3390/ijerph16203799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cleary J., Dalton S.M., Harman A., Wright I.M. Current practice in the introduction of solid foods for preterm infants. Public Health Nutr. 2020;23:94–101. doi: 10.1017/S1368980019002337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giannì M.L., Bezze E., Colombo L., Rossetti C., Pesenti N., Roggero P., Sannino P., Muscolo S., Plevani L., Mosca F. Complementary Feeding Practices in a Cohort of Italian Late Preterm Infants. Nutrients. 2018;10:1861. doi: 10.3390/nu10121861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Braid S., Harvey E.M., Bernstein J., Matoba N. Early introduction of complementary foods in preterm infants. J. Pediatr. Gastroenterol. Nutr. 2015;60:811–818. doi: 10.1097/MPG.0000000000000695. [DOI] [PubMed] [Google Scholar]
- 58.Menezes L.V.P., Steinberg C., Nóbrega A.C. Complementary feeding in infants born prematurely. Codas. 2018;30:e20170157. doi: 10.1590/2317-1782/20182017157. [DOI] [PubMed] [Google Scholar]
- 59.Crapnell T.L., Rogers C.E., Neil J.J., Inder T.E., Woodward L.J., Pineda R.G. Factors associated with feeding difficulties in the very preterm infant. Acta Paediatr. 2013;102:e539–e545. doi: 10.1111/apa.12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lau C. Development of infant oral feeding skills: What do we know? Am. J. Clin. Nutr. 2016;103:616S–621S. doi: 10.3945/ajcn.115.109603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanchez K., Spittle A.J., Slattery J.M., Morgan A.T. Oromotor Feeding in Children Born Before 30 Weeks’ Gestation and Term-Born Peers at 12 Months’ Corrected Age. J. Pediatr. 2016;178:113–118.e1. doi: 10.1016/j.jpeds.2016.07.044. [DOI] [PubMed] [Google Scholar]
- 62.Lipner H.S., Huron R.F. Developmental and Interprofessional Care of the Preterm Infant: Neonatal Intensive Care Unit through High-Risk Infant Follow-up. Pediatr. Clin. N. Am. 2018;65:135–141. doi: 10.1016/j.pcl.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 63.Ojha S., Elfzzani Z., Kwok T.C., Dorling J. Education of family members to support weaning to solids and nutrition in later infancy in term-born infants. Cochrane Database Syst. Rev. 2020;7:CD012241. doi: 10.1002/14651858.CD012241.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chiale F., Maggiora E., Aceti A., Liotto N., Coscia A., Peila C., Baldassarre M.E., Bertino E., Cresi F. Complementary Feeding: Recommendations for the Introduction of Allergenic Foods and Gluten in the Preterm Infant. Nutrients. 2021;13:2477. doi: 10.3390/nu13072477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Capra M.E., Monopoli D., Decarolis N.M., Giudice A., Stanyevic B., Esposito S., Biasucci G. Dietary Models and Cardiovascular Risk Prevention in Pediatric Patients. Nutrients. 2023;15:3664. doi: 10.3390/nu15163664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bivi D., Di Chio T., Geri F., Morganti R., Goggi S., Baroni L., Mumolo M.G., de Bortoli N., Peroni D.G., Marchi S., et al. Raising Children on a Vegan Diet: Parents’ Opinion on Problems in Everyday Life. Nutrients. 2021;13:1796. doi: 10.3390/nu13061796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baldassarre M.E., Panza R., Farella I., Posa D., Capozza M., Mauro A.D., Laforgia N. Vegetarian and Vegan Weaning of the Infant: How Common and How Evidence-Based? A Population-Based Survey and Narrative Review. Int. J. Environ. Res. Public Health. 2020;17:4835. doi: 10.3390/ijerph17134835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.ESPGHAN Committee on Nutrition. Aggett P.J., Agostoni C., Axelsson I., De Curtis M., Goulet O., Hernell O., Koletzko B., Lafeber H.N., Michaelsen K.F., et al. Feeding preterm infants after hospital discharge: A commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2006;42:596–603. doi: 10.1097/01.mpg.0000221915.73264.c7. [DOI] [PubMed] [Google Scholar]
- 69.Mameli C., Mazzantini S., Zuccotti G.V. Nutrition in the First 1000 Days: The Origin of Childhood Obesity. Int. J. Environ. Res. Public Health. 2016;13:838. doi: 10.3390/ijerph13090838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Agostoni C., Decsi T., Fewtrell M., Goulet O., Kolacek S., Koletzko B., Michaelsen K.F., Moreno L., Puntis J., Rigo J., et al. Complementary feeding: A commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2008;46:99–110. doi: 10.1097/01.mpg.0000304464.60788.bd. [DOI] [PubMed] [Google Scholar]
- 71.Rapley G., Forste R., Cameron S., Brown A., Wright C. Baby-Led Weaning: A New Frontier? ICAN Infant Child Adolesc. Nutr. 2015;7:77–85. doi: 10.1177/1941406415575931. [DOI] [Google Scholar]
- 72.Brown A., Jones S.W., Rowan H. Baby-Led Weaning: The Evidence to Date. Curr. Nutr. Rep. 2017;6:148–156. doi: 10.1007/s13668-017-0201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taylor R.W., Williams S.M., Fangupo L.J., Wheeler B.J., Taylor B.J., Daniels L., Fleming E.A., McArthur J., Morison B., Erickson L.W., et al. Effect of a Baby-Led Approach to Complementary Feeding on Infant Growth and Overweight: A Randomized Clinical Trial. JAMA Pediatr. 2017;171:838–846. doi: 10.1001/jamapediatrics.2017.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fu X., Conlon C.A., Haszard J.J., Beck K.L., von Hurst P.R., Taylor R.W., Heath A.M. Food fussiness and early feeding characteristics of infants following Baby-Led Weaning and traditional spoon-feeding in New Zealand: An internet survey. Appetite. 2018;130:110–116. doi: 10.1016/j.appet.2018.07.033. [DOI] [PubMed] [Google Scholar]
- 75.Komninou S., Halford J.C.G., Harrold J.A. Differences in parental feeding styles and practices and toddler eating behaviour across complementary feeding methods: Managing expectations through consideration of effect size. Appetite. 2019;137:198–206. doi: 10.1016/j.appet.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 76.Cameron S.L., Heath A.L., Taylor R.W. How feasible is Baby-led Weaning as an approach to infant feeding? A review of the evidence. Nutrients. 2012;4:1575–1609. doi: 10.3390/nu4111575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alpers B., Blackwell V., Clegg M.E. Standard v. baby-led complementary feeding: A comparison of food and nutrient intakes in 6-12-month-old infants in the UK. Public Health Nutr. 2019;22:2813–2822. doi: 10.1017/S136898001900082X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rowan H., Lee M., Brown A. Differences in dietary composition between infants introduced to complementary foods using Baby-led weaning and traditional spoon feeding. J. Hum. Nutr. Diet. 2019;32:11–20. doi: 10.1111/jhn.12616. [DOI] [PubMed] [Google Scholar]
- 79.Daniels L., Taylor R.W., Williams S.M., Gibson R.S., Fleming E.A., Wheeler B.J., Taylor B.J., Haszard J.J., Heath A.M. Impact of a modified version of baby-led weaning on iron intake and status: A randomised controlled trial. BMJ Open. 2018;8:e019036. doi: 10.1136/bmjopen-2017-019036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carruth B.R., Skinner J.D. Feeding behaviors and other motor development in healthy children (2–24 months) J. Am. Coll. Nutr. 2002;21:88–96. doi: 10.1080/07315724.2002.10719199. [DOI] [PubMed] [Google Scholar]
- 81.Özyüksel G., Soyer T., Üzümcügil F., Yalçın Ş., Ekinci S., Karnak İ., Çiftçi A.Ö., Tanyel F.C. Foreign Body Aspiration in Infants: Role of Self-Feeding. Pediatr. Allergy Immunol. Pulmonol. 2019;32:52–55. doi: 10.1089/ped.2019.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fangupo L.J., Heath A.M., Williams S.M., Erickson Williams L.W., Morison B.J., Fleming E.A., Taylor B.J., Wheeler B.J., Taylor R.W. A Baby-Led Approach to Eating Solids and Risk of Choking. Pediatrics. 2016;138:e20160772. doi: 10.1542/peds.2016-0772. [DOI] [PubMed] [Google Scholar]
- 83.Boswell N. Complementary Feeding Methods-A Review of the Benefits and Risks. Int. J. Environ. Res. Public Health. 2021;18:7165. doi: 10.3390/ijerph18137165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alvisi P., Congiu M., Ficara M., De Gregorio P., Ghio R., Spisni E., Di Saverio P., Labriola F., Lacorte D., Lionetti P. Complementary Feeding in Italy: From Tradition to Innovation. Children. 2021;8:638. doi: 10.3390/children8080638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Addessi E., Galloway A.T., Wingrove T., Brochu H., Pierantozzi A., Bellagamba F., Farrow C.V. Baby-led weaning in Italy and potential implications for infant development. Appetite. 2021;164:105286. doi: 10.1016/j.appet.2021.105286. [DOI] [PubMed] [Google Scholar]
- 86.Bergamini M., Simeone G., Verga M.C., Doria M., Cuomo B., D’Antonio G., Dello Iacono I., Di Mauro G., Leonardi L., Miniello V.L., et al. Complementary Feeding Caregivers’ Practices and Growth, Risk of Overweight/Obesity, and Other Non-Communicable Diseases: A Systematic Review and Meta-Analysis. Nutrients. 2022;14:2646. doi: 10.3390/nu14132646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ferrara P., Corsello G., Quattrocchi E., Dell’Aquila L., Ehrich J., Giardino I., Pettoello-Mantovani M. Caring for Infants and Children Following Alternative Dietary Patterns. J. Pediatr. 2017;187:339–340.e1. doi: 10.1016/j.jpeds.2017.04.053. [DOI] [PubMed] [Google Scholar]
- 88.Eurispes Rapporto Italia 2023. [(accessed on 1 October 2023)]. Available online: https://eurispes.eu/news/risultati-del-rapporto-italia-2023/
- 89.Simeone G., Bergamini M., Verga M.C., Cuomo B., D’Antonio G., Iacono I.D., Mauro D.D., Mauro F.D., Mau-ro G.D., Leonardi L., et al. Do Vegetarian Diets Provide Adequate Nutrient Intake during Complementary Feeding? A Systematic Review. Nutrients. 2022;14:3591. doi: 10.3390/nu14173591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Melina V., Craig W., Levin S. Position of the Academy of Nutrition and Dietetics: Vegetarian Diets. J. Acad. Nutr. Diet. 2016;116:1970–1980. doi: 10.1016/j.jand.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 91.National Health and Medical Research Council (Hg) Eat for Health. Australian Dietary Guidelines. National Health and Medical Research Council; Canberra, Australia: 2013. [Google Scholar]
- 92.Direção-Geral da Saúde (Hg) National Programme for the Promotion of a Healthy Diet, Guidelines for a Healthy Vegetarian Diet. Direção-Geral da Saúde; Lissabon, Portugal: 2015. [Google Scholar]
- 93.Amit M. Vegetarian diets in children and adolescents. Paediatr. Child Health. 2010;15:303–314. [PMC free article] [PubMed] [Google Scholar]
- 94.Richter M., Boeing H., Grünewald-Funk D., Heseker H., Kroke A., Leschik-Bonnet E., Oberritter H., Strohm D., Watzl B. Vegan Diet Position of the German Nutrition Society (DGE) Ernaehrungs Umsch. Int. 2016;63:92–102. [Google Scholar]
- 95.SIPPS. FIMP. SIMA. SIMP Position Paper—Diete vegetariane in gravidanza ed età evolutiva. Riv. Ital. Pediatr. Prev. Soc. 2017;12((Suppl. S3)):119–193. [Google Scholar]
- 96.Mangels A.R., Messina V. Considerations in planning vegan diets: Infants. J. Am. Diet. Assoc. 2001;101:670–677. doi: 10.1016/S0002-8223(01)00169-9. [DOI] [PubMed] [Google Scholar]
- 97.Farella I., Panza R., Baldassarre M.E. The Difficult Alliance between Vegan Parents and Pediatrician: A Case Report. Int. J. Environ. Res. Public Health. 2020;17:6380. doi: 10.3390/ijerph17176380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Budreviciute A., Damiati S., Sabir D.K., Onder K., Schuller-Goetzburg P., Plakys G., Katileviciute A., Khoja S., Kodzius R. Management and Prevention Strategies for Non-communicable Diseases (NCDs) and Their Risk Factors. Front. Public Health. 2020;8:788. doi: 10.3389/fpubh.2020.574111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mallisetty Y., Mukherjee N., Jiang Y., Chen S., Ewart S., Arshad S.H., Holloway J.W., Zhang H., Karmaus W. Epigenome-Wide Association of Infant Feeding and Changes in DNA Methylation from Birth to 10 Years. Nutrients. 2021;13:99. doi: 10.3390/nu13010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Latorre-Millán M., Rupérez A.I., González-Gil E.M., Santaliestra-Pasías A., Vázquez-Cobela R., Gil-Campos M., Aguilera C.M., Gil Á., Moreno L.A., Leis R., et al. Dietary patterns and their association with body composition and cardiometabolic markers in children and adolescents: Genobox cohort. Nutrients. 2020;12:3424. doi: 10.3390/nu12113424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sun C., Foskey R.J., Allen K.J., Dharmage S.C., Koplin J.J., Ponsonby A.L., Lowe A.J., Matheson M.C., Tang M.L., Gurrin L., et al. The Impact of Timing of Introduction of Solids on Infant Body Mass Index. J. Pediatr. 2016;179:104–110.e1. doi: 10.1016/j.jpeds.2016.08.064. [DOI] [PubMed] [Google Scholar]
- 102.Jones S.W., Lee M., Brown A. Spoonfeeding is associated with increased infant weight but only amongst formula-fed infants. Matern. Child Nutr. 2020;16:1–8. doi: 10.1111/mcn.12941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Martinón-Torres N., Carreira N., Picáns-Leis R., Pérez-Ferreirós A., Kalén A., Leis R. Baby-led weaning: What role does it play in obesity risk during the first years? A systematic review. Nutrients. 2021;13:1009. doi: 10.3390/nu13031009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dogan E., Yilmaz G., Caylan N., Turgut M., Gokcay G., Oguz M.M. Baby-led complementary feeding: Randomized controlled study. Pediatr. Int. 2018;60:1073–1080. doi: 10.1111/ped.13671. [DOI] [PubMed] [Google Scholar]
- 105.Schwarzer M., Makki K., Storelli G., Machuca-Gayet I., Srutkova D., Hermanova P., Martino M.E., Balmand S., Hudcovic T., Heddi A., et al. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science. 2016;351:854–857. doi: 10.1126/science.aad8588. [DOI] [PubMed] [Google Scholar]
- 106.Blanton L.V., Charbonneau M.R., Salih T., Barratt M.J., Venkatesh S., Ilkaveya O., Subramanian S., Manary M.J., Trehan I., Jorgensen J.M., et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science. 2016;351:aad3311. doi: 10.1126/science.aad3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kamng’ona A.W., Young R., Arnold C.D., Kortekangas E., Patson N., Jorgensen J.M., Prado E.L., Chaima D., Malamba C., Ashorn U., et al. The association of gut microbiota characteristics in Malawian infants with growth and inflammation. Sci. Rep. 2019;9:12893. doi: 10.1038/s41598-019-49274-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Forbes J.D., Azad M.B., Vehling L., Tun H.M., Konya T.B., Guttman D.S., Field C.J., Lefebvre D., Sears M.R., Becker A.B., et al. Association of exposure to formula in the hospital and subsequent infant feeding practices with gut microbiota and risk of overweight in the first year of life. JAMA Pediatr. 2018;172:e181161. doi: 10.1001/jamapediatrics.2018.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Townsend E., Pitchford N.J. Baby knows best? The impact of weaning style on food preferences and body mass index in early childhood in a case-controlled sample. BMJ Open. 2012;2:e000298. doi: 10.1136/bmjopen-2011-000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tang M., Matz K.L., Berman L.M., Davis K.N., Melanson E.L., Frank D.N., Hendricks A.E., Krebs N.F. Effects of Complementary Feeding with Different Protein-Rich Foods on Infant Growth and Gut Health: Study Protocol. Front. Pediatr. 2022;9:793215. doi: 10.3389/fped.2021.793215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tang M., Ma C., Weinheimer-Haus E.M., Robertson C.E., Kofonow J.M., Berman L.M., Waljee A., Zhu J., Frank D.N., Krebs N.F. Different gut microbiota in U.S. formula-fed infants consuming a meat vs. dairy-based complementary foods: A randomized controlled trial. Front. Nutr. 2023;9:1063518. doi: 10.3389/fnut.2022.1063518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Magne F., Hachelaf W., Suau A., Boudraa G., Mangin I., Touhami M., Bouziane-Nedjadi K., Pochart P. A longitudinal study of infant faecal microbiota during weaning. FEMS Microbiol. Ecol. 2006;58:563–571. doi: 10.1111/j.1574-6941.2006.00182.x. [DOI] [PubMed] [Google Scholar]
- 113.Koletzko B., Broekaert I., Demmelmair H., Franke J., Hannibal I., Oberle D., Schiess S., Baumann B.T., Verwied-Jorky S. Protein intake in the first year of life: A risk factor for later obesity? The E.U. childhood obesity project. Adv. Exp. Med. Biol. 2005;569:69–79. doi: 10.1007/1-4020-3535-7_12. [DOI] [PubMed] [Google Scholar]
- 114.Grote V., Theurich M. Complementary feeding and obesity risk. Curr. Opin. Clin. Nutr. Metab. Care. 2014;17:273–277. doi: 10.1097/MCO.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 115.Lund-Blix N.A., Stene L.C., Rasmussen T., Torjesen P.A., Andersen L.F., Rønningen K.S. Infant feeding in relation to islet autoimmunity and type 1 diabetes in genetically susceptible children: The MIDIA study. Diabetes Care. 2015;38:257–263. doi: 10.2337/dc14-1130. [DOI] [PubMed] [Google Scholar]
- 116.Knip M., Virtanen S.M., Seppä K., Ilonen J., Savilahti E., Vaarala O., Reunanen A., Teramo K., Hämäläinen A.M., Paronen J., et al. Dietary Intervention in Infancy and Later Signs of Beta-Cell Autoimmunity. N. Engl. J. Med. 2010;363:1900–1908. doi: 10.1056/NEJMoa1004809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hyytinen M., Savilahti E., Virtanen S.M., Härkönen T., Ilonen J., Luopajärvi K., Uibo R., Vaarala O., Åkerblom H.K., Knip M., et al. Avoidance of Cow’s Milk–Based Formula for At-Risk Infants Does Not Reduce Development of Celiac Disease: A Randomized Controlled Trial. Gastroenterology. 2017;153:961–970.e3. doi: 10.1053/j.gastro.2017.06.049. [DOI] [PubMed] [Google Scholar]
- 118.Rewers M., Hyöty H., Lernmark Å., Hagopian W., She J.X., Schatz D., Ziegler A.G., Toppari J., Akolkar B., Krischer J. The environmental determinants of diabetes in the young (TEDDY) study. Ann. N. Y. Acad. Sci. 2008;1150:1–13. doi: 10.1196/annals.1447.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hummel S., Weiß A., Bonifacio E., Agardh D., Akolkar B., Aronsson C.A., Hagopian W.A., Koletzko S., Krischer J.P., Lernmark Å., et al. Associations of breastfeeding with childhood autoimmunity, allergies, and overweight: The Environmental Determinants of Diabetes in the Young (TEDDY) study. Am. J. Clin. Nutr. 2021;114:134–142. doi: 10.1093/ajcn/nqab065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Uusitalo U., Mramba L.K., Aronsson C.A., Vehik K., Yang J., Hummel S., Lernmark Å., Rewers M., Hagopian W., McIndoe R., et al. HLA Genotype and Probiotics Modify the Association Between Timing of Solid Food Introduction and Islet Autoimmunity in the TEDDY Study. Diabetes Care. 2023;46:1839–1847. doi: 10.2337/dc23-0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pieścik-Lech M., Chmielewska A., Shamir R., Szajewska H. Systematic review: Early infant feeding and the risk of type 1 Diabetes. J. Pediatr. Gastroenterol. Nutr. 2017;64:454–459. doi: 10.1097/MPG.0000000000001293. [DOI] [PubMed] [Google Scholar]
- 122.Beyerlein A., Liu X., Uusitalo U.M., Harsunen M., Norris J.M., Foterek K., Virtanen S.M., Rewers M.J., She J.X., Simell O., et al. Dietary intake of soluble fiber and risk of islet autoimmunity by 5 y of age: Results from the TEDDY study. Am. J. Clin. Nutr. 2015;102:345–352. doi: 10.3945/ajcn.115.108159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Niinistö S., Erlund I., Lee H.S., Uusitalo U., Salminen I., Aronsson C.A., Parikh H.M., Liu X., Hummel S., Toppari J., et al. Children’s erythrocyte fatty acids are associated with the risk of islet autoimmunity. Sci. Rep. 2021;11:3627. doi: 10.1038/s41598-021-82200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Szajewska H., Shamir R., Mearin L., Ribes-Koninckx C., Catassi C., Domellöf M., Fewtrell M.S., Husby S., Papadopoulou A., Vandenplas Y., et al. Gluten introduction and the risk of coeliac disease: A position paper by the european society for pediatric gastroenterology, hepatology, and nutrition. J. Pediatr. Gastroenterol. Nutr. 2016;62:507–513. doi: 10.1097/MPG.0000000000001105. [DOI] [PubMed] [Google Scholar]
- 125.Knip M., Åkerblom H.K., Becker D., Dosch H.M., Dupre J., Fraser W., Howard N., Ilonen J., Krischer J.P., Kordonouri O., et al. Hydrolyzed infant formula and early β-cell autoimmunity: A randomized clinical trial. JAMA. 2014;311:2279–2287. doi: 10.1001/jama.2014.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.du Toit G., Tsakok T., Lack S., Lack G. Prevention of food allergy. J. Allergy Clin. Immunol. 2016;137:998–1010. doi: 10.1016/j.jaci.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 127.Hong X., Ladd-Acosta C., Hao K., Sherwood B., Ji H., Keet C.A., Kumar R., Caruso D., Liu X., Wang G., et al. Epigenome-wide association study links site-specific DNA methylation changes with cow’s milk allergy. J. Allergy Clin. Immunol. 2016;138:908–911.e9. doi: 10.1016/j.jaci.2016.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.American Academy of Pediatrics Committee on Nutrition. Hypoallergenic infant formulas. Pt 1Pediatrics. 2000;106:346–349. doi: 10.1542/peds.106.2.346. [DOI] [PubMed] [Google Scholar]
- 129.Devonshire A.L., Robison R.G. Prevention of food allergy. Allergy Asthma Proc. 2019;40:450–452. doi: 10.2500/aap.2019.40.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Prescott S.L., Pawankar R., Allen K.J., Campbell D.E., Sinn J.K., Fiocchi A., Ebisawa M., Sampson H.A., Beyer K., Lee B.W. A global survey of changing patterns of food allergy burden in children. World Allergy Organ. J. 2013;6:21. doi: 10.1186/1939-4551-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Greer F.R., Sicherer S.H., Burks A.W., American Academy of Pediatrics Committee on Nutrition. American Academy of Pediatrics Section on Allergy and Immunology Effects of early nutritional interventions on the development of atopic disease in infants and children: The role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics. 2008;121:183–191. doi: 10.1542/peds.2007-3022. [DOI] [PubMed] [Google Scholar]
- 132.Prescott S.L., Smith P., Tang M., Palmer D.J., Sinn J., Huntley S.J., Cormack B., Heine R.G., Gibson R.A., Makrides M. The importance of early complementary feeding in the development of oral tolerance: Concerns and controversies. Pediatr. Allergy Immunol. 2008;19:375–380. doi: 10.1111/j.1399-3038.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- 133.Forsyth J.S., Ogston S.A., Clark A., Florey C.D., Howie P.W. Relation between early intro-duction of solid food to infants and their weight and illnesses during the first two years of life. BMJ. 1993;306:1572–1576. doi: 10.1136/bmj.306.6892.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zutavern A., Brockow I., Schaaf B., Bolte G., von Berg A., Diez U., Borte M., Herbarth O., Wichmann H.E., Heinrich J., et al. Timing of solid food introduction in relation to atopic dermatitis and atopic sensitization: Results from a prospective birth cohort study. Pediatrics. 2006;117:401–411. doi: 10.1542/peds.2004-2521. [DOI] [PubMed] [Google Scholar]
- 135.West C.E., D’Vaz N., Prescott S.L. Dietary immunomodulatory factors in the development of immune tolerance. Curr. Allergy Asthma Rep. 2011;11:325–333. doi: 10.1007/s11882-011-0200-0. [DOI] [PubMed] [Google Scholar]
- 136.Zutavern A., Brockow I., Schaaf B., von Berg A., Diez U., Borte M., Kraemer U., Herbarth O., Behrendt H., Wichmann H.E., et al. Timing of solid food intro-duction in relation to eczema, asthma, allergic rhinitis, and food and inhalant sensiti-zation at the age of 6 years: Results from the prospective birth cohort study LISA. Pediatrics. 2008;121:e44–e52. doi: 10.1542/peds.2006-3553. [DOI] [PubMed] [Google Scholar]
- 137.Du Toit G., Katz Y., Sasieni P., Mesher D., Maleki S.J., Fisher H.R., Fox A.T., Turcanu V., Amir T., Zadik-Mnuhin G., et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J. Allergy Clin. Immunol. 2008;122:984–991. doi: 10.1016/j.jaci.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 138.Høst A., Halken S., Muraro A., Dreborg S., Niggemann B., Aalberse R., Arshad S.H., von Berg A., Carlsen K.H., Duschén K., et al. Dietary prevention of allergic diseases in infants and small children. Pediatr. Allergy Immunol. 2008;19:1–4. doi: 10.1111/j.1399-3038.2007.00680.x. [DOI] [PubMed] [Google Scholar]
- 139.Australasian Society of Clinical Immunology and Allergy Infant Feeding and Allergy Prevention. [(accessed on 28 April 2019)]. Available online: http://www.allergy.org.au/images/pcc/ASCIA_guidelines_infant_feeding_and_allergy_prevention.pdf. [DOI] [PubMed]
- 140.Tham E.H., Shek L.P., Van Bever H.P., Vichyanond P., Ebisawa M., Wong G.W., Lee B.W., Asia Pacific Association of Pediatric Allergy, Respirology & Immunology (APAPARI) Early introduction of allergenic foods for the prevention of food allergy from an Asian perspective-An Asia Pacific Association of Pediatric Allergy, Respirology & Immunology (APAPARI) consensus statement. Pediatr. Allergy Immunol. 2018;29:18–27. doi: 10.1111/pai.12820. [DOI] [PubMed] [Google Scholar]
- 141.Fifty-Fourth World Health Assembly . Provisional Agenda Item 13.1.1. Global Strategy for Infant and Young Child Feeding: The Optimal Duration of Exclusive Breastfeeding. World Health Organization; Geneva, Switzerland: 2001. [Google Scholar]
- 142.Du Toit G., Roberts G., Sayre P.H., Bahnson H.T., Radulovic S., Santos A.F., Brough H.A., Phippard D., Basting M., Feeney M., et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N. Engl. J. Med. 2015;372:803–813. doi: 10.1056/NEJMoa1414850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Du Toit G., Sayre P.H., Roberts G., Sever M.L., Lawson K., Bahnson H.T., Brough H.A., Santos A.F., Harris K.M., Radulovic S., et al. Effect of Avoidance on Peanut Allergy after Early Peanut Consumption. N. Engl. J. Med. 2016;374:1435–1443. doi: 10.1056/NEJMoa1514209. [DOI] [PubMed] [Google Scholar]
- 144.du Toit G., Sayre P.H., Roberts G., Lawson K., Sever M.L., Bahnson H.T., Fisher H.R., Feeney M., Radulovic S., Basting M., et al. Allergen specificity of early peanut consumption and effect on development of allergic disease in the Learning Early About Peanut Allergy study cohort. J. Allergy Clin. Immunol. 2018;141:1343–1353. doi: 10.1016/j.jaci.2017.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fleischer D.M., Sicherer S., Greenhawt M., Campbell D., Chan E.S., Muraro A., Halken S., Katz Y., Ebisawa M., Eichenfield L., et al. Consensus communication on early peanut introduction and the prevention of peanut allergy in high-risk infants. Allergy Asthma Clin. Immunol. 2015;11:23. doi: 10.1186/s13223-015-0087-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Togias A., Cooper S.F., Acebal M.L., Assa’ad A., Baker J.R., Jr., Beck L.A., Block J., Byrd-Bredbenner C., Chan E.S., Eichenfield L.F., et al. Addendum guidelines for the prevention of peanut allergy in the United States: Report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. J. Allergy Clin. Immunol. 2017;139:29–44. doi: 10.1016/j.jaci.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gupta R.S., Bilaver L.A., Johnson J.L., Hu J.W., Jiang J., Bozen A., Martin J., Reese J., Cooper S.F., Davis M.M., et al. Assessment of Pediatrician Awareness and Implementation of the Addendum Guidelines for the Prevention of Peanut Allergy in the United States. JAMA Netw. Open. 2020;3:e2010511. doi: 10.1001/jamanetworkopen.2020.10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bellach J., Schwarz V., Ahrens B., Trendelenburg V., Aksünger Ö., Kalb B., Niggemann B., Keil T., Beyer K. Randomized placebo-controlled trial of hen’s egg consumption for primary prevention in infants. J. Allergy Clin. Immunol. 2017;139:1591–1599.e2. doi: 10.1016/j.jaci.2016.06.045. [DOI] [PubMed] [Google Scholar]
- 149.Palmer D.J., Sullivan T.R., Gold M.S., Prescott S.L., Makrides M. Randomized controlled trial of early regular egg intake to prevent egg allergy. J. Allergy Clin. Immunol. 2017;139:1600–1607.e2. doi: 10.1016/j.jaci.2016.06.052. [DOI] [PubMed] [Google Scholar]
- 150.Natsume O., Kabashima S., Nakazato J., Yamamoto-Hanada K., Narita M., Kondo M., Saito M., Kishino A., Takimoto T., Inoue E., et al. Two-step egg introduction for prevention of egg allergy in high-risk infants with eczema (PETIT): A randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:276–286. doi: 10.1016/S0140-6736(16)31418-0. [DOI] [PubMed] [Google Scholar]
- 151.Tham E.H., Lee B.W., Chan Y.H., Loo E.X.L., Toh J.Y., Goh A., Teoh O.H., Yap F., Tan K.H., Godfrey K.M., et al. Low Food Allergy Prevalence Despite Delayed Introduction of Allergenic Foods-Data from the GUSTO Cohort. J. Allergy Clin. Immunol. Pract. 2018;6:466–475.e1. doi: 10.1016/j.jaip.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Institute of Medicine (US) Committee on the Evaluation of the Addition of Ingredients New to Infant Formula . National Academies Press (US); Washington, DC, USA: 2004. [(accessed on 6 February 2019)]. Comparing Infant Formulas with Human Milk. Available online: https://www.ncbi.nlm.nih.gov/books/NBK215837/ [Google Scholar]
- 153.Greer F.R., Sicherer S.H., Burks A.W., Committee on Nutrition. Section on Allergy and Immunology The Effects of Early Nutritional Interventions on the Development of Atopic Disease in Infants and Children: The Role of Maternal Dietary Restriction, Breastfeeding, Hydrolyzed Formulas, and Timing of Introduction of Allergenic Complementary Foods. Pediatrics. 2019;143:e20190281. doi: 10.1542/peds.2019-0281. [DOI] [PubMed] [Google Scholar]
- 154.Alvisi P., Brusa S., Alboresi S., Amarri S., Bottau P., Cavagni G., Corradini B., Landi L., Loroni L., Marani M., et al. Recommendations on complementary feeding for healthy, full-term infants. Ital. J. Pediatr. 2015;41:36. doi: 10.1186/s13052-015-0143-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Perkin M.R., Logan K., Marrs T., Radulovic S., Craven J., Flohr C., Lack G., EAT Study Team Enquiring About Tolerance (EAT) study: Feasibility of an early allergenic food introduction regimen. J. Allergy Clin. Immunol. 2016;137:1477–1486.e8. doi: 10.1016/j.jaci.2015.12.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]