Abstract
Nucleotide sequences were determined for the complete M genome segments of two distinct hantavirus genetic lineages which were detected in hantavirus antibody- and PCR-positive white-footed mice (Peromyscus leucopus) from Indiana and Oklahoma. Phylogenetic analyses indicated that although divergent from each other, the virus lineages in Indiana and Oklahoma were monophyletic and formed a newly identified unique ancestral branch within the clade of Sin Nombre-like viruses found in Peromyscus mice. Interestingly, P. leucopus-borne New York virus was found to be most closely related to the P. maniculatus-borne viruses, Sin Nombre and Monongahela, and monophyletic with Monongahela virus. In parallel, intraspecific phylogenetic relationships of P. leucopus were also determined, based on the amplification, sequencing, and analysis of the DNA fragment representing the replication control region of the rodent mitochondrial genome. P. leucopus mitochondrial DNA haplotypes were found to form four separate genetic clades, referred to here as Eastern, Central, Northwestern, and Southwestern groups. The distinct Indiana and Oklahoma virus lineages were detected in P. leucopus of the Eastern and Southwestern mitochondrial DNA haplotypes, respectively. Taken together, our current data suggests that both cospeciation of Peromyscus-borne hantaviruses with their specific rodent hosts and biogeographic factors (such as allopatric migrations, geographic separation, and isolation) have played important roles in establishment of the current genetic diversity and geographic distribution of Sin Nombre-like hantaviruses. In particular, the unusual position of New York virus on the virus phylogenetic tree is most consistent with an historically recent host-switching event.
Hantaviruses are rodent-borne members of the family Bunyaviridae (34). They possess a single-stranded, negative-sense RNA genome contained within three separate segments referred to as the small, medium, and large (S, M, and L) segments. The S segment (1.7 to 2.0 kb) codes for the nucleocapsid (N) protein, the M segment (3.6 kb) codes for the glycoprotein precursor of two viral glycoproteins (G1 and G2), and the L segment (6.5 kb) codes for the viral RNA polymerase (14). The number of known distinct hantavirus serotypes and genotypes has grown significantly during the last 5 years and currently totals 16 (44).
Each hantavirus appears to be primarily associated with a specific rodent species, causing a persistent, asymptomatic, life-long infection in that species. They are thought to be primarily transmitted via infectious aerosol generated by contaminated urine and feces, and possibly via saliva during bites. Most of the current data appears consistent with cospeciation of hantaviruses and their rodent hosts being the predominant pattern in the long-term evolution of this group of viruses (2, 37, 43, 52, 62). This is certainly true at the higher levels, hantaviruses carried by species in the rodent subfamilies Murinae, Arvicolinae, and Sigmodontinae falling into three phylogenetically distinct groups, irrespective of their global geographic locations (22, 39, 44).
Many hantaviruses are pathogenic for humans. Well-characterized Old World hantaviruses which cause diseases collectively known as hemorrhagic fever with renal syndrome include Hantaan, Dobrava, Seoul, and Puumala viruses, associated with the striped field mouse, Apodemus agrarius, the yellow-necked field mouse, A. flavicollis, the Norway rat, Rattus norvegicus, and the bank vole, Clethrionomys glareolus, respectively (for reviews see references 22 and 44).
Sin Nombre (SN) virus was identified as the causative agent in an outbreak of severe pulmonary disease (hantavirus pulmonary syndrome) in the southwestern United States in 1993 (10, 12, 13, 40, 52). The deer mouse (Peromyscus maniculatus), an indigenous North American rodent of the subfamily Sigmodontinae, was shortly identified as the primary natural reservoir for SN virus (9). Subsequently, additional distinct hantaviruses associated with rodents of other sigmodontine genera were discovered in North and South America. Some, like Bayou (BAY), Black Creek Canal (BCC), Juquitiba, Andes, and Laguna Negra viruses (associated with Oryzomys palustris, Sigmodon hispidus, unknown, Oligoryzomys longicaudatus, and Calomys laucha rodents, respectively) have already proven to be pathogenic for humans (28, 31, 36, 39, 46, 47, 55, 59). Others, such as El Moro Canyon (ELMC) virus (from the harvest mouse, Reithrodontomys megalotis) (20, 21) and several hantaviruses from the Prospect Hill (PH) virus group (found in several different North American Microtus species) (42, 48, 51), have not been associated with human disease.
An extensive study of SN virus throughout North America revealed high genetic diversity as well as geographic clustering of genetic variants (18, 30, 35, 39, 40, 52), both of which may be connected with the genetic diversity of its natural rodent host, P. maniculatus (37). Adding further complexity to the picture, several other SN-like hantaviruses have been detected and genetically characterized. These include the P. leucopus-borne New York (NY) virus (23, 24) and Monongahela (MGL) virus (50), which was originally found in a morphologically distinct subspecies of P. maniculatus, P. maniculatus nubiterrae (cloudland deer mouse), in the Appalachians. These findings lead one to consider (i) how many hantaviruses are associated with Peromyscus rodents in North America, (ii) how many of them are capable of causing hantavirus pulmonary syndrome, and (iii) how hantavirus detection and differential diagnostics can potentially be improved. To begin to address such questions, a broad study of the genetic variability of different hantavirus strains, their geographic distribution, and their mode of maintenance in nature has been initiated. The pivotal component of such a study is investigation of the association and possible cospeciation of SN-like viruses with particular Peromyscus taxa.
To attempt to clarify these issues for P. leucopus-borne hantaviruses, the nucleotide sequences of the entire hantavirus M genome segments were determined for two previously unreported highly divergent SN-like virus lineages detected in P. leucopus from Indiana and Oklahoma. In parallel, intraspecific phylogenetic relationships of P. leucopus were also determined, based on the amplification, sequencing, and phylogenetic analysis of the mitochondrial DNA (mtDNA) fragment.
MATERIALS AND METHODS
Sample selection and origin.
Rodent blood and tissue samples were selected from an extensive collection at the Special Pathogens Branch, Centers for Disease Control and Prevention, Atlanta, Ga. This collection is supported with voucher specimens deposited at the Museum of Southwestern Biology, Albuquerque, N.Mex. Rodent samples from West Virginia were kindly provided by Richard Yanagihara.
P. leucopus samples used for amplification of the entire hantavirus M RNA genome segments were collected in La Porte County, Indiana (11), and Murray County, Oklahoma. DNA samples used for reconstructing rodent phylogeny included 49 P. leucopus samples from Arkansas, District of Columbia, Georgia, Illinois, Indiana, Iowa, Kansas, Kentucky, Maine, Maryland, Massachusetts, Missouri, New York, North Carolina, North Dakota, Ohio, Oklahoma, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, West Virginia, and Wisconsin.
RNA and DNA preparation.
Total RNA used for the recovery of the hantavirus genetic sequences was extracted from whole blood or lung tissue by using a standard guanidinium isothiocyanate-acid phenol extraction procedure as described previously (40). Total DNA from rodent blood or tissue samples was purified by proteinase K digestion followed by phenol-chloroform extraction and ethanol precipitation (7).
Oligonucleotide primer design.
A standard nested set of generic primers specific to the G2 region of the hantavirus M RNA genome segment (40) was used for the original recovery of the hantavirus genetic sequences (35). A variety of oligonucleotide primers were used to generate overlapping DNA fragments for the entire hantavirus M genome RNA segments. Nine sets of nested primer sequences were designed from conserved regions between SN (strains NMh10 and CC107), ELMC, BAY, BCC, and PH published sequences. These provided sufficient sequence for each virus that specific primers could be designed to fill gaps in the sequence. The virus sequences are reported in GenBank, and specific primer sequences are available from the authors upon request.
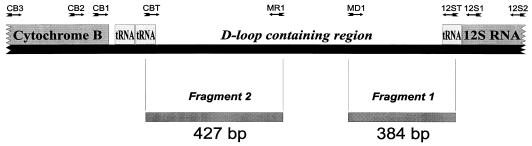
To identify highly variable genetic sequences suitable for the determination of the phylogenetic relationship among Peromyscus rodents, the GenBank sequence database and existing literature were examined, and the noncoding region of rodent mtDNA surrounding the origin of replication (D-loop-containing region) was chosen for study. Known mtDNA sequences of Norway rat (R. norvegicus) (15), house mouse (Mus domesticus) (6), and human (1) were aligned by using the LINEUP program of the Genetics Computer Group (Madison, Wis.) software package. Three pairs of generic primers (named CB1, CB2, CB3, 12S1, 12S2, and 12S3) were designed (Fig. 1; Table 1) and used in different combinations to amplify a replication control region of Peromyscus mtDNA. The mtDNA sequences obtained were aligned by using the LINEUP program. Then two P. leucopus/P. maniculatus-specific primer pairs were designed for amplification of the variable parts of the rodent mtDNA (Fig. 1; Table 1).
FIG. 1.
Linear map of the P. leucopus 1.8-kb mitochondrial genome fragment containing the replication control region. Directions of primers used are shown with arrows. Grey bars represent fragments selected for amplification, sequencing, and phylogenetic analyses.
TABLE 1.
Oligonucleotide primers used to amplify mtDNA sequences of P. leucopus
| Name | Sequence (5′→3′) |
|---|---|
| CB1 | GGCCAACCAGTAGAACACCCATTTAT |
| CB2 | CTTTACTGAATCCTAGTAGCCAACCT |
| CB3 | GA(T/C)GTTCT(C/A)GGAGACCCAGACAATTA |
| CBT | CCGCCATCAACACCCAAAGCTG |
| CBTR | CAGCTTTGGGTGTTGATGGTGG |
| MD1 | GTCTAGCTGGACTTTTCAATTCAAGC |
| MR1 | CCCTGAAGTAAGAACCAGATGCCTG |
| 12ST | GCATTTTCAGTGCTTTGCTTTATTG |
| 12S1 | TAATTATAAGGCCAGGACCAAACCTTT |
| 12S2 | CGTATGACCGCGGTGGCTGGCACGA |
| 12S3 | ACCGCCAAGTCCTTTGAGTTTTAAGC |
RT-PCR amplification and sequencing.
Extracted total RNA was assayed for the presence of hantavirus RNA by using nested reverse transcriptase PCR (RT-PCR) as described previously (40). Amplifications were performed on a TwinBlock system thermocycler (Ericomp, Inc., San Diego, Calif.). Peromyscus mtDNA fragments were amplified in a simple PCR using 40 cycles of 93°C for 30 s, 48°C for 30 s, and 72°C for 120 s. Synthesized DNA products were separated on agarose gels, purified by using a MERMaid kit (Bio 101, La Jolla, Calif.), and sequenced directly, using the DyeDeoxy cycle sequencing technique (Applied Biosystems, Foster City, Calif.). The exact sequences of 3′ and 5′ termini of the M genome segment of Indiana (IN) virus were determined by ligation of the individual RNA segment termini, followed by RT-PCR amplification and nucleotide sequence analysis through the ligation site (33).
Phylogenetic analyses.
Phylogenetic analyses of nucleotide sequence differences between different hantavirus strains, as well as between subspecies of P. leucopus, were performed by using both the distance-based neighbor-joining (NJ) method (MEGA software [29]) and the maximum-parsimony (MP) method (PAUP software [54]). Both analyses were carried out by using different algorithms and weighting schemes.
In the NJ method, different distance corrections including Jukes-Cantor and Tamura models, and gamma distance for the Tamura-Nei model, were used. Since the rate of transitional substitutions is known to be higher than the rate of transversional substitutions in the hantavirus genetic sequences (52), and frequencies of the four different nucleotides deviate from 25%, the Tamura model was selected for the final phylogenetic analysis of the hantavirus data set. This model includes estimations for the frequencies of different nucleotides in the analyzed sequences and for differences in the rate of transitional and transversional substitutions. For Peromyscus mtDNA sequences, the Jukes-Cantor model and gamma distances for the Tamura-Nei model were used in the analysis. The former algorithm is generally recommended when the Jukes-Cantor estimate of the number of nucleotide substitutions per site between different sequences is about 0.05 or less (29), as it was in our data set. However, since the variable region of the mammalian mtDNA is known to have extreme bias in the frequencies of the four nucleotides and in the rate of nucleotide substitutions among different sites, and a higher rate of transitional over transversional substitutions, the latter algorithm was also applied.
For the MP analysis, the transition/transversion ratio was estimated by using MacClade software (32) analyses of unweighted trees generated from nucleotide sequence differences detected among hantavirus strains (transition/transversion ratio of 4:1 [reference 52 and this report]) or among Peromyscus rodents (transition/transversion ratio of 7:1 [this report]). Such weighting is predicted to improve the effectiveness of the MP method for estimation of the correct phylogeny (19). Phylogenetic trees were obtained by using the branch-and-bound (hantavirus sequence data set) or heuristic (rodent mtDNA sequence data set) search method. Bootstrap confidence limits were calculated by 1,000 heuristic search repetitions of the analyses.
Nucleotide sequence accession numbers.
The nucleotide sequences reported in this article can be obtained from GenBank. The accession numbers for the IN and Oklahoma (OK) hantavirus lineages are AF030551 and AF030552, respectively. The accession numbers for P. leucopus mtDNA fragments are AF031710 to AF031807.
RESULTS
Genetic characterization of the M RNA segments of the virus lineages present in P. leucopus samples from Indiana and Oklahoma.
Earlier analysis of PCR fragments amplified from P. leucopus samples from Indiana and Oklahoma indicated the existence of two previously unrecognized hantavirus lineages (35). Two individual rodents containing the representative IN and OK virus lineages were chosen for further virus sequence analysis. The complete sequences of the M segments of the IN and OK virus lineages were determined to be 3,664 and 3,662 nucleotides in length, respectively. The terminal nucleotides (28 bases at the 3′ end and 31 bases at the 5′ end) of the OK virus lineage M genome segment were not determined directly but inferred from primers used for cDNA synthesis. The single open reading frame (ORF) (nucleotides 52 to 3472) for the glycoprotein precursor (GPC) was found to potentially encode an unprocessed GPC 1,140 amino acids in length. Percentage identity of both the nucleotide and amino acid sequences of the entire M segments and encoded proteins of IN and OK virus lineages compared with previously published sequences of other hantaviruses are shown in Table 2. The M RNA genome segments of IN and OK virus lineages show 84.3% nucleotide identity and 95.8% amino acid identity of encoded GPCs. As expected, they also display a high degree of nucleotide identity with SN (79.9 and 80.5% for IN and OK, respectively), MGL (81.2 and 80.6%), and NY (80.0 and 80.4%) viruses. The distribution of nucleic and amino acid substitutions found in IN and OK lineage M segments correlates well with the pattern of conserved and variable areas described for other hantaviruses (14, 42, 52).
TABLE 2.
M segment nucleotide and amino acid sequence identitiesa
| Virus (strain) | Sequence identity (%)a
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SEO (SR-11) | THAI | HTN (76-118) | DOB | PUU (cg18-20) | TUL (Moravia) | PH (PHV-1) | ELMC (RM-97) | BAY | BCC | SN (NMh10) | NY (NY-1) | BR (IN) | BR (OK)b | |
| SEO (SR-11) | 73.5 | 71.7 | 70.1 | 58.1 | 58.7 | 58.4 | 58.6 | 59.0 | 59.4 | 58.1 | 57.7 | 57.8 | 58.1 | |
| THAI | 82.1 | 70.7 | 71.1 | 58.2 | 58.5 | 58.2 | 58.6 | 58.9 | 58.9 | 58.4 | 58.8 | 58.7 | 58.2 | |
| HTN (76-118) | 77.0 | 77.2 | 70.4 | 58.9 | 58.4 | 58.3 | 58.6 | 59.7 | 59.1 | 58.1 | 58.8 | 59.8 | 58.6 | |
| DOB | 77.6 | 77.0 | 77.4 | 58.1 | 57.8 | 57.7 | 58.3 | 58.8 | 58.8 | 58.8 | 58.8 | 59.3 | 58.0 | |
| PUU (cg18-20) | 53.9 | 54.1 | 54.1 | 53.7 | 71.9 | 70.2 | 63.5 | 64.0 | 65.0 | 66.2 | 65.3 | 65.9 | 65.6 | |
| TUL (Moravia) | 54.8 | 54.5 | 55.3 | 55.1 | 78.6 | 72.2 | 66.2 | 64.6 | 64.8 | 66.2 | 65.7 | 66.3 | 66.6 | |
| PH (PHV-1) | 54.5 | 54.2 | 54.6 | 53.8 | 75.7 | 80.4 | 64.6 | 64.5 | 64.1 | 65.2 | 66.2 | 65.5 | 65.8 | |
| ELMC (RM-97) | 54.2 | 55.8 | 56.4 | 55.0 | 66.3 | 68.6 | 66.5 | 69.2 | 69.7 | 71.2 | 70.8 | 70.8 | 70.4 | |
| BAY | 54.8 | 55.2 | 55.4 | 54.9 | 65.6 | 68.3 | 66.6 | 76.9 | 77.0 | 71.6 | 71.1 | 70.7 | 70.7 | |
| BCC | 53.7 | 53.7 | 54.7 | 54.2 | 65.9 | 68.4 | 65.4 | 76.7 | 88.5 | 72.1 | 71.8 | 70.8 | 72.1 | |
| SN (Nmh10) | 53.2 | 53.9 | 55.2 | 54.6 | 67.3 | 69.5 | 67.6 | 79.6 | 81.1 | 80.1 | 81.3 | 79.9 | 80.5 | |
| NY (NY-1) | 53.5 | 54.3 | 55.2 | 55.2 | 68.1 | 69.5 | 67.7 | 79.2 | 79.9 | 79.5 | 95.7 | 80.0 | 80.4 | |
| BR (Indiana) | 53.2 | 54.2 | 56.0 | 55.3 | 67.2 | 69.1 | 67.5 | 78.8 | 79.9 | 78.8 | 93.8 | 94.1 | 84.3 | |
| BR (Oklahoma) | 53.5 | 55.0 | 56.1 | 55.5 | 67.5 | 68.8 | 68.0 | 79.2 | 79.6 | 79.1 | 93.4 | 94.0 | 95.8 | |
Values above spaces are percent identical nucleotides, and those below spaces are percent identical amino acids. All values were calculated by using the GAP program of the Genetics Computer Group software. Abbreviations: SEO, Seoul virus; THAI, Thailand virus, HTN, Hantaan virus; DOB, Dobrava virus; PUU, Puumala virus; TUL, Tula virus, PH, Prospect Hill virus; ELMC, El Moro Canyon virus; BAY, Bayou virus; BCC, Black Creek Canal virus; SN, Sin Nombre virus; NY, New York virus; BR, Blue River virus.
Twenty-eight nucleotides from the 3′ end and 31 nucleotides from the 5′ end of the M segment genome of OK virus were not determined directly and therefore, were not included in comparisons.
The GPCs of IN and OK lineages possess a number of common structural features which are conserved among hantaviruses. These include conserved potential N-glycosylation sites, cysteine residues, locations of transmembrane regions of G1 and G2 (36, 49, 52), and the five amino acids preceding the proposed site for cotranslational cleavage of GPC (14, 36, 52). The major conserved type-specific linear epitope, reported to be located at amino acid positions 58 to 88 in the SN virus G1 protein (27), contains four amino acid substitutions in IN and OK virus lineages, with three of them being shared and one being unique for each virus. Whether these substitutions lead to the change of the immunological specificity of this epitope remains to be determined.
Phylogenetic relationship of the IN and OK viruses to other hantaviruses.
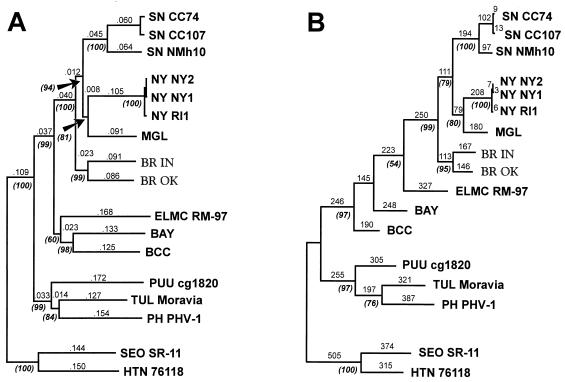
To clarify the phylogenetic relationship of IN and OK viruses to other SN-like hantaviruses, NJ and MP phylogenetic analyses of a 1,141-nucleotide fragment of the G2 ORF (positions from 2330 to 3470 of the IN sequence) were performed. The NJ tree and the single MP tree obtained display very similar topologies (Fig. 2). Although divergent from each other, IN and OK viruses group together and form a new, unique branch which diverges at a node that is ancestral to the node containing the clade including the other Peromyscus-borne hantaviruses. Interestingly, P. leucopus-borne NY virus appeared to be most closely related to the P. maniculatus-borne viruses, SN and MGL, and diverges from the same ancestral node as MGL virus. Bootstrap analysis of both NJ and MP trees provides good support for these clades, as well as for other major clades on the trees. The NJ and MP phylogenetic analyses of the complete virus M segments (data not shown) revealed a similar well-supported topology within the clade containing Peromyscus-borne SN-like viruses (except that the complete MGL virus M segment sequence was not available for inclusion in this analysis).
FIG. 2.
Phylogenetic relationships of the IN and OK hantavirus lineages to previously known hantaviruses, based on sequence differences of the 1,141-nucleotide fragment of the M genome RNAs. Sequences were aligned with BR IN and OK M genome RNA sequences and then edited to match the length of the shortest sequence which was the partial-length sequence of MGL. (A) NJ analysis was performed with MEGA, using the Tamura algorithm with options of using both transversional and transitional changes and complete deletion of gaps. Bootstrap values were calculated by 1,000 replicates of the analysis. (B) MP analysis was performed with PAUP, version 3.1.1, using the branch-and-bound search option and a 4:1 weighting of transversions over transitions. Bootstrap analysis was carried out by using 1,000 heuristic search replicates with the aforementioned weighting of transversions. Numbers above horizontal branches indicate distances (NJ) or nucleotide step differences (MP). The percentage of bootstrap support exceeding 50% is indicated by numbers in parentheses below corresponding branches near the appropriate branching point. Vertical branches are for visual clarity only. Previously published hantavirus sequences (for abbreviations not provided below, see the text and Table 2, footnote a) used in the analysis include the following: HTN virus strain 76118 (49), GenBank accession no. M14627; SEO virus strain SR-11 (3), M34882; PH virus strain PHV-1 (42), X55129; TUL virus strain Moravia (43), Z66538; PUU virus strain CG1820 (16), M29979; BAY virus (36), L36930; BCC virus (46), L39950; ELMC virus strain RM-97 (56), U26828; NY virus strains RI1, NY-1, and NY-2 (24), U36801, U36802, and U36803, respectively; SN virus strains New Mexico h10 (NMh10) (52), L25783; Convict Creek 74 (CC74) and Convict Creek 107 (CC107) (30), L33684 and L33474; and MGL virus strain Monongahela-2 (50), U32653.
Amplification and sequencing of the mtDNA replication control region of P. leucopus and P. maniculatus.
All three pairs of generic primers used (Fig. 1) produced amplicons of rodent mtDNA. However, the most efficient amplifications were obtained with primers CB3 and 12S2. A 1.8-kb fragment of mtDNA containing the replication control region was generated for P. leucopus, P. gossypinus, P. maniculatus, and P. polionotus (species identification supported by analysis of respective voucher specimens). Two variable fragments of mtDNA surrounding the replication control region were identified (fragments 1 and 2 in Fig. 1). Changes among the rodent species mentioned above included numerous nucleotide substitutions, a 75-nucleotide duplication in fragment 2 in all P. polionotus samples compared with P. leucopus and P. maniculatus samples, and a 9-nucleotide duplication in some P. gossypinus samples. Intraspecies variability appeared to be lower (up to 4.3% in P. leucopus), with most changes being transitional nucleotide substitutions.
Based on these data, P. leucopus/P. maniculatus-specific primers were designed and used to amplify variable 384- and 427-nucleotide mtDNA fragments (333 and 380 nucleotides in length, respectively, after exclusion of primer sequences) surrounding the replication control region (using primer pairs MD1-12ST and CBT-MR1, respectively [Fig. 1]). These fragments were successfully amplified for 49 P. leucopus samples from different regions of its geographic distribution (see Materials and Methods).
Phylogenetic relationship of white-footed mice from different geographic areas.
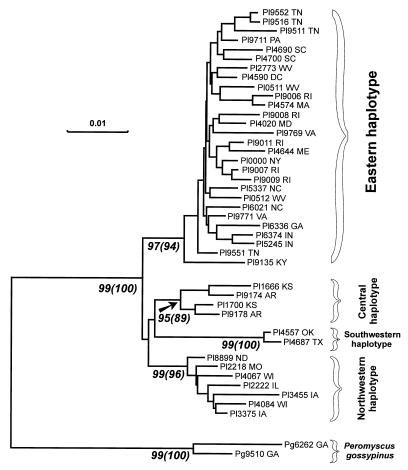
To determine the phylogenetic relationship of geographically distant populations of P. leucopus, data obtained by sequencing two variable mtDNA fragments were combined and aligned. The original data set was 717 nucleotides long and contained 49 sequences of P. leucopus, with two sequences of P. gossypinus used as the outgroup. Redundant taxa were omitted in the final phylogenetic analyses, which were performed by NJ and MP methods. Despite extreme bias in nucleotide composition and a high transition/transversion ratio in the mtDNA data set, the two models used in the NJ analysis produced very similar phylogenetic trees. The phylogenetic tree based on the Jukes-Cantor model is shown in Fig. 3. All MP trees obtained in both unweighted and weighted (7:1) analyses also displayed the same major clades of P. leucopus mtDNA sequences (data not shown). In both NJ and MP analyses, the bootstrap estimates provide good support for all these major clades.
FIG. 3.
Phylogeny of P. leucopus inferred from NJ analysis of combined 333- and 380-nucleotide fragments of the mtDNA replication control region. NJ analysis was performed with MEGA, using the Jukes-Cantor model. Bootstrap values were obtained by 1,000 repetitions of the analysis. The standard error test support numbers and bootstrap support numbers (in parentheses) for major clades are indicated below the corresponding branches near the appropriate branching point. The horizontal scale bar indicates a distance of 0.01. Vertical branches are for visual clarity only. Prefixes: Pl, P. leucopus; Pg, P. gossypinus. States of origin are indicated by two-letter postal code. Four clades or haplotypes are indicated. The first clade contains mtDNA sequences from the eastern United States including East Coast (Maine, Massachusetts, New York, Pennsylvania, Rhode Island, Maryland, and District of Columbia), Appalachian Mountain region (West Virginia, Virginia, Tennessee, Kentucky, South Carolina, and Georgia), and areas between the Appalachians and Great Lakes (Indiana and Ohio). The second clade is comprised of mtDNA sequences from the northern region of the Central Plains (Illinois, Wisconsin, Iowa, Missouri, and North Dakota). The third clade contains mtDNA sequences from Kansas and Arkansas, while the fourth clade is comprised of mtDNA sequences from Texas and Oklahoma.
P. leucopus mtDNA haplotypes appear to form four genetically distinct clades, referred to here for simplicity as the Eastern, Northwestern, Central, and Southwestern groups of haplotypes (Fig. 3 and 4). It is noteworthy that the P. leucopus from Indiana, from which IN virus lineage sequence was obtained, belongs to the Eastern group, while the P. leucopus from Oklahoma, which carries OK virus lineage, belongs to the Southwestern group.
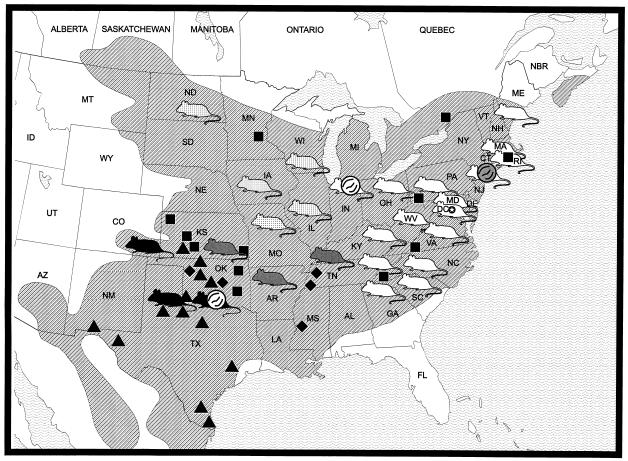
FIG. 4.
Geographic distribution of mtDNA haplotypes of P. leucopus and P. leucopus-borne hantaviruses. The shaded area represents the geographic distribution of P. leucopus according to Hall (17). White, dotted, grey, and black mouse figures designate localities containing Eastern, Northwestern, Central, and Southwestern mtDNA haplotypes, respectively. Black squares, triangles, and diamonds indicate localities containing northeastern, southwestern, and “hybrid” karyotypes, respectively, according to Baker et al. (5). White virion figures indicate localities where IN and OK hantavirus lineages were recovered from P. leucopus. The grey virion figure shows NY hantavirus. Mice from northern Ohio, southwestern Kentucky, and Colorado were assigned to mtDNA haplotype groups based on phylogenetic analysis of the fragment 1 only and therefore were not included in Fig. 3.
DISCUSSION
To gain a better understanding of the genetic variability, geographic distribution, and association (and potential cospeciation) of SN-like viruses with particular species and subspecies of P. leucopus, the complete M RNA genome segment sequences were determined for two divergent hantavirus lineages detected in P. leucopus from distant geographic areas (35). As expected, nucleotide sequences of the entire M RNA genome segments of IN and OK virus lineages possess all of the specific features (i.e., overall structure, imperfectly complementary ends of the genome, length of the GPC ORF and nontranslated regions, features of encoded proteins, etc.) known for other hantaviruses (24, 36, 46, 49, 52).
Analysis of virus sequence difference by both NJ and MP phylogenetic methods showed that (i) the IN and OK virus lineages form a new, unique ancestral branch within the clade of SN-like viruses and (ii) although placed together, IN and OK viruses are still relatively divergent, with the evolutionary distance being almost as great as that between two SN-like viruses currently recognized by others as distinct, i.e., NY and MGL (Fig. 2). Surprisingly, another P. leucopus-borne hantavirus, NY virus, is phylogenetically placed within the clade containing the P. maniculatus-borne hantaviruses and appears to be most closely related to the MGL virus. Considering that all well-known hantaviruses are each primarily associated with a single rodent species, a possible explanation for these results might lie in the phylogenetic relationship and history of the rodent hosts of these SN-like hantaviruses.
The two closely related species of Peromyscus rodents, P. maniculatus (about 60 named subspecies) and P. leucopus (17 subspecies), are widespread and abundant on the North American continent (4, 17, 25, 26, 41) (Fig. 4). Despite considerable efforts, intraspecific phylogenetic histories of both species remain unclear (reviewed in reference 8). In the case of P. leucopus, the existence of two distinct chromosomal races has been well documented during the last two decades (5, 38, 53, 57) (Fig. 4), supporting the earlier morphological subdivision of the P. leucopus species (41). White-footed mice belonging to both races were shown to hybridize easily along the contact line in Oklahoma, forming a geographically stable narrow hybrid zone (38, 53, 57). Chromosomal, allozymic, and mtDNA restriction pattern data suggest different phylogenetic histories for the two chromosomal races and an allopatric origin of the contact zone (38, 53). Presumed allopatric subdivision of the parental types took place between 20,000 and 250,000 years ago (53). Secondary contact took place approximately 9,000 generations ago (i.e., at less than 9,000 years, and, probably, less than half that time) (53). These estimates are in agreement with the hypothesis that the Wisconsin glaciation, which eliminated plant and animal life from a large section of North America for about 30,000 years, was responsible for splitting the range of this species (53). It is likely that during the Wisconsinian time the eastern population survived glaciation in the southern Appalachians (58), while the second (southwestern) population was probably located somewhere in the region of Texas, New Mexico, or Mexico.
The data presented here identify not two but four phylogenetically distinct mtDNA haplotypes of P. leucopus (Fig. 3 and 4). Since the geographic distribution of the previously defined Northeastern chromosomal race of P. leucopus (5) covers areas shown in this study to be inhabited by P. leucopus possessing the Eastern, Northwestern, and Central mtDNA haplotypes (Fig. 4), these mtDNA haplotypes probably represent P. leucopus populations which survived the Wisconsin glacial period in the southern Appalachians, while the Southwestern haplotype originated from another glacial age refuge (or, alternatively, represents an earlier division of P. leucopus). Unfortunately, the current analysis, which allowed us to clearly identify four distinct mtDNA haplotypes, cannot unambiguously resolve the phylogenetic relationships between them.
The data discussed above lead us to the conclusion that the two divergent SN-like hantavirus lineages from Indiana and Oklahoma are associated with two distinct contemporary chromosomal races and phylogenetically distinct mtDNA haplotypes of the white-footed mouse. The OK virus lineage is clearly associated with the Southwestern chromosomal race (and the Southwestern mtDNA haplotype) of P. leucopus. The IN virus lineage is associated with the Northeastern race but may not truly belong to P. leucopus of the Eastern mtDNA haplotype. Based on absence of IN hantavirus lineage in samples from the rest of the geographic range of P. leucopus of the Eastern mtDNA haplotype (Appalachians and coastal areas [Fig. 4]) (24, 35, 50), it seems likely that this virus lineage is predominantly associated with P. leucopus of the Northwestern mtDNA haplotype (which inhabits areas west from Indiana) and invaded border populations of P. leucopus of the Eastern mtDNA haplotype.
When discussing the association and possible cospeciation of closely related SN-like hantaviruses with their closely related Peromyscus rodent hosts, one could consider three possible hypotheses. (i) Cospeciation of hantaviruses with their specific rodent hosts remains a critical factor in the fine scale, short-term evolution (i.e., closely related Peromyscus-borne SN-like hantaviruses undergo cospeciation with their specific rodent hosts, genetically distinct forms of P. maniculatus and P. leucopus). (ii) At such a fine temporal scale, there is no cospeciation of closely related Peromyscus-borne SN-like hantaviruses with their rodent hosts: SN-like hantaviruses are able to be maintained in both P. maniculatus and P. leucopus, and only biogeographic factors (such as allopatric migrations, geographic separation, and isolation) and founder events in natural infections of rodents have led to the current divergence and geographic distribution of SN-like hantaviruses. (iii) Both factors mentioned above play an important role: cospeciation of Peromyscus-borne SN-like hantaviruses with their specific rodent hosts remains an important factor, although biogeographic factors are equally important, and host-switching events can occur when closely related rodent species are sympatric.
Our current data taken together with those from previously published studies are most consistent with the last hypothesis. There is ample evidence of cospeciation occurring at the rodent and virus species level. For instance, although P. maniculatus and P. leucopus are sympatric almost everywhere in the Central Plains (including Indiana and Oklahoma), the IN and OK virus lineages have not been detected in P. maniculatus captured in this region despite analysis of numerous specimens (35). At the subspecies level, the association of genetically distinct IN and OK virus genotypes with two genetically distinct chromosomal forms of P. leucopus, and the association of SN and MGL viruses with two genetically distinct forms of P. maniculatus (37, 50), are consistent with cospeciation.
However, the position of the P. leucopus-borne NY virus on the virus phylogenetic tree inside the clade of the P. maniculatus-borne SN-like hantaviruses (Fig. 2) suggests that one or more host-switching events may have occurred in the past. There are several possible scenarios which include multiple host-switching events. Nevertheless, from both historical and phylogenetic perspectives, the most likely and parsimonious explanation for the unusual position of NY virus on the hantavirus phylogenetic tree is a single historically recent host-switching event. Both P. maniculatus nubiterrae and P. leucopus are known to inhabit the Appalachian Mountain region, often sharing common habitats (4, 17, 60, 61). This situation is thought to have existed since the Wisconsin glacial period, when this region served as an ice age refuge for both species (58). Most likely an ancient MGL-like virus originally was associated with P. maniculatus, based on virus position on the phylogenetic tree and its current association solely with P. maniculatus in Quebec and Ontario, outside the geographic range of P. leucopus (35). At present, this virus can be found in the southern Appalachians predominantly in P. maniculatus nubiterrae but also in P. leucopus (35), suggesting that MGL virus may have become well adapted to both closely related rodent species during the ice age. After the glacial ice sheet retreated from New England more than 10,000 years ago, ancient populations of both species expanded northward along the Appalachian Mountain chain (P. maniculatus, which generally follows higher elevations) and coastal plains (P. leucopus, which generally follows lower elevations) (58). It seems likely that cospeciation of the ancient MGL-like virus strain with such coastal populations of P. leucopus resulted in the constitution of the SN-like hantavirus, now known as NY virus.
Data presented here once again demonstrate the great genetic complexity of the group of SN-like hantaviruses in North America. Considering the significant geographic variation among hantaviruses of a single species, it is not easy to determine whether the IN and OK virus lineages described here constitute true hantavirus species or whether they should be considered divergent strains of a single novel virus. It has been proposed that combination of three major pieces of evidence must be considered when one evaluates new hantavirus species (20). (i) The first is immunologic evidence, with a four-fold or greater two-way difference between viruses in plaque reduction neutralization assays. This is a problem in the case of hantaviruses, which are known to be generally difficult to adapt to cell culture. (ii) The second is ecological/physiological evidence, with a clear association of a new hantavirus with a different rodent host. “Different rodent host” usually means different rodent species. However, analysis of the mammalogical literature (for instance, reference 25; for a review, see reference 8) shows that in some cases such a definition is limited due to uncertainties in rodent taxonomy. We propose to adopt the definition of a different rodent host as a reproductively and/or ecologically and/or geographically separated rodent species or subspecies, which carries a phylogenetically distinct hantavirus and which is capable of being the sole carrier of this virus in a rodent community. (iii) Finally, one must consider molecular evidence, with significant nucleotide and amino acid differences from previously characterized hantaviruses. Commonly, a hantavirus is considered to be a new species if it has more than a 25% nucleotide difference in the GPC ORF. Nucleotide differences of 15 to 25% usually are considered a “grey area” where the significance of sequence differences cannot be properly evaluated without phylogenetic analysis. At the amino acid level, a threshold of 5 to 6% difference in the nucleocapsid protein sequence has been proposed to distinguish new hantavirus species (45).
Viruses of the IN and OK lineages have not been isolated in cell culture, and so immunological criteria cannot be used. They are associated with two phylogenetically distinct chromosomal races (and mtDNA haplotypes) of their rodent host. However, since these two groups of P. leucopus are in contact, and hybridize easily, it is hard to characterize this situation as a true separation. Finally, 16% nucleotide difference of the virus M genome segments and 4.2% amino acid difference of the corresponding GPC proteins are insufficient to constitute the IN and OK lineages as separate virus species. Based on these criteria, we consider these virus lineages to be two divergent strains of a new SN-like hantavirus for which we propose the name Blue River (BR) virus, after a geographic feature near the location of rodent capture in Oklahoma.
The average nucleotide and amino acid sequence differences between SN, NY, MGL viruses, which are currently recognized by others as distinct virus species (23, 24, 50, 52), and BR virus are still in the same grey area (Table 1). However, the genetic distance between these SN-like viruses is greater than that between the IN and OK lineages of BR virus (Fig. 2), and most of them are associated with reproductively isolated, phylogenetically distinct and/or geographically separated rodent hosts (9, 24, 50). Immunological comparisons for SN-like viruses have not yet been done, since only SN and NY viruses so far have been adapted to cell culture. Therefore, further studies are needed to determine whether SN, NY, MGL, and BR viruses are taxonomically distinct entities or divergent strains of SN virus and whether we should adopt the group name “Sin Nombre-like viruses” or “Sin Nombre group.”
ACKNOWLEDGMENTS
We thank Terry Yates, Jim Mills, and Richard Yanagihara for providing rodent blood and tissue samples; Wallace Dawson, Michael Bowen, Vladimir Chizhikov, and Michael Kosoy for fruitful discussions; Elmer Otteson for synthesizing oligonucleotide primers for this study; and Brian Mahy for support and encouragement. We are grateful to Marty Monroe, Mary Lane Martin, George Gallucci, Vera Semenova, Brad Hotard, and Lora Morgan for excellent technical assistance.
This work was supported in part by National Institutes of Health grants 5RO1AI36418-04 and 1PO1AI39808-01 through the University of Nevada—Reno.
REFERENCES
- 1.Anderson S, Bankier A T, Barrell B G, de Bruijn M H L, Coulson A R, Drouin J, Eperon I C, Nierlich D P, Roe B A, Sanger F, Schreier P H, Smith A J H, Staden R, Young I G. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 2.Antic D, Yong Kang C, Spik K, Schmaljohn C S, Vapalahti O, Vaheri A. Comparison of the deduced gene products of the L, M and S genome segments of hantaviruses. Virus Res. 1992;24:35–46. doi: 10.1016/0168-1702(92)90029-9. [DOI] [PubMed] [Google Scholar]
- 3.Arikawa J, Lapenotiere H F, Iacono-Connors L, Wang M, Schmaljohn C S. Coding properties of the S and M genome segments of Sapporo rat virus: comparison to other causative agents of hemorrhagic fever with renal syndrome. Virology. 1990;176:114–125. doi: 10.1016/0042-6822(90)90236-k. [DOI] [PubMed] [Google Scholar]
- 4.Baker R H. Habitats and distribution. In: King J A, editor. Biology of Peromyscus (Rodentia). Special publication. Lawrence, Kans: American Society of Mammalogists; 1968. pp. 98–126. [Google Scholar]
- 5.Baker R J, Robbins L W, Stangl F B, Jr, Birney E C. Chromosomal evidence for a major subdivision in Peromyscus leucopus. J Mammal. 1983;64:356–359. [Google Scholar]
- 6.Bibb M J, van Etten R A, Wright C T, Walberg M W, Clayton D A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981;26:167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- 7.Blin N, Stafford D W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976;3:2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carleton M D. Systematics and evolution. In: Kirkland G L Jr, Layne J N, editors. Advances in the study of Peromyscus (Rodentia). Lubbock, Tex: Texas Tech University Press; 1989. pp. 7–141. [Google Scholar]
- 9.Childs J E, Ksiazek T G, Spiropoulou C F, Krebs J W, Morzunov S, Maupin G O, Gage K L, Rollin P, Sarisky J, Enscore R, Peters C J, Nichol S T. Serologic and genetic identification of Peromyscus maniculatus as the primary rodent reservoir for a new hantavirus in the southwestern United States. J Infect Dis. 1994;169:1271–1280. doi: 10.1093/infdis/169.6.1271. [DOI] [PubMed] [Google Scholar]
- 10.Chizhikov V E, Spiropoulou C F, Morzunov S P, Monroe M C, Peters C J, Nichol S T. Complete genetic characterization and analysis of isolation of Sin Nombre virus. J Virol. 1995;69:8132–8136. doi: 10.1128/jvi.69.12.8132-8136.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dietrich N, Pruden S, Ksiazek T G, Morzunov S P, Camp J W. A small-scale survey of hantavirus in mammals from Indiana. J Wildl Dis. 1997;33:818–822. doi: 10.7589/0090-3558-33.4.818. [DOI] [PubMed] [Google Scholar]
- 12.Duchin J S, Koster F T, Peters C J, Simpson G L, Tempest B, Zaki S R, Ksiazek T G, Rollin P E, Nichol S T, Umland E T, Moolenaar R L, Reef S E, Nolte K B, Gallaher M M, Butler J C, Breiman R F the Hantavirus Study Group. Hantavirus pulmonary syndrome: a clinical description of 17 patients with a newly recognized disease. N Engl J Med. 1994;330:949–955. doi: 10.1056/NEJM199404073301401. [DOI] [PubMed] [Google Scholar]
- 13.Elliott L H, Ksiazek T G, Rollin P E, Spiropoulou C F, Morzunov S P, Monroe M, Goldsmith C S, Humphrey C D, Zaki S R, Krebs J W, Maupin G, Gage K, Childs J E, Nichol S T, Peters C J. Isolation of the causative agent of hantavirus pulmonary syndrome. Am J Trop Med Hyg. 1994;51:102–108. doi: 10.4269/ajtmh.1994.51.102. [DOI] [PubMed] [Google Scholar]
- 14.Elliott R M, Schmaljohn C S, Collet M S. Bunyaviridae genome structure and gene expression. Curr Top Microbiol Immunol. 1991;169:91–141. doi: 10.1007/978-3-642-76018-1_4. [DOI] [PubMed] [Google Scholar]
- 15.Gadaleta G, Pepe G, De Candia G, Quagliariello C, Sbisa E, Saccone C. The complete nucleotide sequence of the Rattus norvegicus mitochondrial genome: cryptic signals revealed by comparative analysis between vertebrates. J Mol Evol. 1989;28:497–516. doi: 10.1007/BF02602930. [DOI] [PubMed] [Google Scholar]
- 16.Giebel L B, Stohwasser R, Zoeller L, Bautz E K, Darai G. Determination of the coding capacity of the M genome segment of Nephropathia epidemica virus strain Hallnas B1 by molecular cloning and nucleotide sequence analysis. Virology. 1989;172:498–505. doi: 10.1016/0042-6822(89)90192-x. [DOI] [PubMed] [Google Scholar]
- 17.Hall E R. Mammals of North America. 2nd ed. New York, N.Y: John Wiley and Sons; 1981. [Google Scholar]
- 18.Henderson W W, Monroe M C, St. Jeor S C, Thayer W P, Rowe J E, Peters C J, Nichol S T. Naturally occurring Sin Nombre virus genetic reassortants. Virology. 1996;214:602–610. doi: 10.1006/viro.1995.0071. [DOI] [PubMed] [Google Scholar]
- 19.Hillis D M, Huelsenbeck J P, Cunningham C W. Application and accuracy of molecular phylogenies. Science. 1994;264:671–677. doi: 10.1126/science.8171318. [DOI] [PubMed] [Google Scholar]
- 20.Hjelle B, Anderson B, Torrez-Martinez N, Song W, Gannon W L, Yates T L. Prevalence and geographic genetic variation of hantaviruses of New World harvest mice (Reithrodontomys): identification of a divergent genotype from a Costa Rican Reithrodontomys mexicanus. Virology. 1995;207:452–459. doi: 10.1006/viro.1995.1104. [DOI] [PubMed] [Google Scholar]
- 21.Hjelle B, Chavez-Giles F, Torrez-Martinez N, Yates T, Sarisky J, Webb J, Ascher M. Genetic identification of a novel hantavirus of the harvest mouse Reithrodontomys megalotis. J Virol. 1994;68:6751–6754. doi: 10.1128/jvi.68.10.6751-6754.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hjelle B, Jenison S A, Goade D E, Green W B, Feddersen R M, Scott A A. Hantaviruses: clinical, microbiologic, and epidemiologic aspects. Crit Rev Clin Lab Sci. 1995;32:469–508. doi: 10.3109/10408369509082592. [DOI] [PubMed] [Google Scholar]
- 23.Hjelle B, Krolikowski J, Torrez-Martinez N, Chavez-Giles F, Vanner C, Laposata E. Phylogenetically distinct hantavirus implicated in a case of hantavirus pulmonary syndrome in the Northeastern United States. J Med Virol. 1995;46:21–27. doi: 10.1002/jmv.1890460106. [DOI] [PubMed] [Google Scholar]
- 24.Hjelle B, Lee S-W, Song W, Torrez-Martinez N, Song J-W, Yanagihara R, Gavrilovskaya I, Mackow E R. Molecular linkage of hantavirus pulmonary syndrome to the white-footed mouse, Peromyscus leucopus: genetic characterization of the M genome of New York virus. J Virol. 1995;69:8137–8141. doi: 10.1128/jvi.69.12.8137-8141.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hogan K M, Hedin M C, Koh H S, Davis S K, Greenbaum I F. Systematic and taxonomic implications of karyotypic, electrophoretic, and mitochondrial-DNA variation in Peromyscus from the Pacific Northwest. J Mammal. 1993;74:819–831. [Google Scholar]
- 26.Hooper E T. Classification. In: King J A, editor. Biology of Peromyscus. Special publication. Lawrence, Kans: American Society of Mammalogists; 1968. pp. 27–74. [Google Scholar]
- 27.Jenison S, Yamada T, Morris C, Anderson B, Torrez-Martinez N, Keller N, Hjelle B. Characterization of human antibody responses to Four Corners hantavirus infections among patients with hantavirus pulmonary syndrome. J Virol. 1994;68:3000–3006. doi: 10.1128/jvi.68.5.3000-3006.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan A S, Spiropoulou C F, Morzunov S, Zaki S R, Kohn M A, Nawas S R, McFarland L, Nichol S T. Fatal illness associated with a new hantavirus in Louisiana. J Med Virol. 1995;46:281–286. doi: 10.1002/jmv.1890460320. [DOI] [PubMed] [Google Scholar]
- 29.Kumar S, Tamura K, Nei M. MEGA: molecular evolutionary genetics analysis, version 1.01. University Park, Pa: The Pennsylvania State University; 1993. . (Computer program.) [Google Scholar]
- 30.Li D, Schmaljohn A L, Anderson J, Schmaljohn C S. Complete nucleotide sequences of the M and S segments of two hantavirus isolates from California: evidence for reassortment in nature among viruses related to hantavirus pulmonary syndrome. Virology. 1995;206:973–983. doi: 10.1006/viro.1995.1020. [DOI] [PubMed] [Google Scholar]
- 31.Lopez N, Padula P, Rossi C, Lazaro M E, Franze-Fernandez M T. Genetic identification of a new hantavirus causing severe pulmonary syndrome in Argentina. Virology. 1996;220:223–226. doi: 10.1006/viro.1996.0305. [DOI] [PubMed] [Google Scholar]
- 32.Maddison W P, Maddison D R. MacClade: analysis of phylogeny and character evolution, version 3.05. Sunderland, Mass: Sinauer Associates; 1992. . (Computer program.) [Google Scholar]
- 33.Mandl C W, Heinz F X, Puchhammer-Stockl E, Kunz C. Sequencing the termini of capped viral RNA by 5′-3′ ligation and PCR. BioTechniques. 1991;10:485–486. [PubMed] [Google Scholar]
- 34.McKee K T, Jr, LeDuc J W, Peters C J. Hantaviruses. In: Belshe R B, editor. Textbook of human virology. 2nd ed. St. Louis, Mo: Mosby Year Book; 1991. pp. 615–632. [Google Scholar]
- 35.Monroe, M. C., S. P. Morzunov, A. Johnson, M. Bowen, H. Artsob, T. Yates, T. G. Ksiazek, and S. T. Nichol. 1997. Unpublished results.
- 36.Morzunov S P, Feldmann H, Spiropoulou C F, Semenova V A, Rollin P E, Ksiazek T G, Peters C J, Nichol S T. A newly recognized virus associated with a fatal case of hantavirus pulmonary syndrome in Louisiana. J Virol. 1995;69:1980–1983. doi: 10.1128/jvi.69.3.1980-1983.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morzunov S P, St. Jeor S C, Yates T L, Rowe J, Ksiazek T G, Peters C J, Nichol S T. Abstracts of the Xth International Congress of Virology 1996. Jerusalem, Israel: Virology Division, International Union of Microbiological Societies; 1996. Co-evolution of North American Peromyscus-borne hantaviruses with specific rodent hosts, W 11-5; p. 23. [Google Scholar]
- 38.Nelson K, Baker R J, Honeycutt R L. Mitochondrial DNA and protein differentiation between hybridizing cytotypes of the white-footed mouse, Peromyscus leucopus. Evolution. 1987;4:864–872. doi: 10.1111/j.1558-5646.1987.tb05859.x. [DOI] [PubMed] [Google Scholar]
- 39.Nichol S T, Ksiazek T G, Rollin P E, Peters C J. Hantavirus pulmonary syndrome and newly described hantaviruses in the United States. In: Elliott R M, editor. The Bunyaviridae. New York, N.Y: Plenum Press; 1996. pp. 269–280. [Google Scholar]
- 40.Nichol S T, Spiropoulou C, Morzunov S, Rollin P E, Ksiazek T G, Feldmann H, Sanchez A, Childs J, Zaki S, Peters C J. Genetic identification of a hantavirus associated with an outbreak of acute respiratory illness. Science. 1993;262:914–917. doi: 10.1126/science.8235615. [DOI] [PubMed] [Google Scholar]
- 41.Osgood W H. Revision of the mice of the American genus Peromyscus. N Am Fauna. 1909;28:1–285. [Google Scholar]
- 42.Parrington M A, Lee P W, Kang C Y. Molecular characterization of the Prospect Hill virus M RNA segment: comparison with the M RNA segments of other hantaviruses. J Gen Virol. 1991;72:1845–1854. doi: 10.1099/0022-1317-72-8-1845. [DOI] [PubMed] [Google Scholar]
- 43.Plyusnin A, Vapalahti O, Lankinen H, Lehvaeslaiho H, Apekina N, Myasnikov Y, Kallio-Kokko H, Henttonen H, Lundkvist Å, Brummer-Korvenkontio M, Gavrilovskaya I, Vaheri A. Tula virus: a newly detected hantavirus carried by European common voles. J Virol. 1994;68:7833–7838. doi: 10.1128/jvi.68.12.7833-7839.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Plyusnin A, Vapalahti O, Vaheri A. Hantaviruses: genome structure, expression and evolution. J Virol. 1996;77:2677–2687. doi: 10.1099/0022-1317-77-11-2677. [DOI] [PubMed] [Google Scholar]
- 45.Puthavathana P, Dobbs M, Baek L-J, Chu Y-K, Lee H W, Kang C Y. Comparison of nucleotide sequences among hantaviruses belonging to the same serotype: an analysis of amplified DNA by thermal cycle sequencing. Virus Res. 1993;30:161–169. doi: 10.1016/0168-1702(93)90004-7. [DOI] [PubMed] [Google Scholar]
- 46.Ravkov E V, Rollin P E, Ksiazek T G, Peters C J, Nichol S T. Genetic and serologic analysis of the Black Creek Canal virus and its association with human disease and Sigmodon hispidus infection. Virology. 1995;210:482–489. doi: 10.1006/viro.1995.1366. [DOI] [PubMed] [Google Scholar]
- 47.Rollin P E, Ksiazek T G, Elliott L H, Ravkov E V, Martin M L, Morzunov S, Livingstone W, Monroe M, Glass G, Ruo S, Khan A S, Childs J E, Nichol S T, Peters C J. Isolation of Black Creek Canal virus, a new hantavirus from Sigmodon hispidus in Florida. J Med Virol. 1995;46:35–39. doi: 10.1002/jmv.1890460108. [DOI] [PubMed] [Google Scholar]
- 48.Rowe J E, St. Jeor S C, Riolo J, Otteson E, Monroe M C, Henderson W W, Ksiazek T G, Rollin P E, Nichol S T. Coexistence of several novel hantaviruses in rodents indigenous to North America. Virology. 1995;213:122–130. doi: 10.1006/viro.1995.1552. [DOI] [PubMed] [Google Scholar]
- 49.Schmaljohn C S, Schmaljohn A L, Dalrymple J M. Hantaan virus M RNA: coding strategy, nucleotide sequence, and gene order. Virology. 1987;157:31–39. doi: 10.1016/0042-6822(87)90310-2. [DOI] [PubMed] [Google Scholar]
- 50.Song J W, Baek L J, Nagle J W, Schlitter D, Yanagihara R. Genetic and phylogenetic analyses of hantaviral sequences amplified from archival tissues of deer mouse (Peromyscus maniculatus nubiterrae) captured in the eastern United States. Arch Virol. 1996;141:959–967. doi: 10.1007/BF01718170. [DOI] [PubMed] [Google Scholar]
- 51.Song W, Torrez-Martinez N, Irwin W, Harrison F J, Davis R, Ascher M, Jay M, Hjelle B. Isla Vista virus: a genetically novel hantavirus of the California vole Microtus californicus. J Gen Virol. 1995;76:3195–3199. doi: 10.1099/0022-1317-76-12-3195. [DOI] [PubMed] [Google Scholar]
- 52.Spiropoulou C F, Morzunov S, Feldmann H, Sanchez A, Peters C J, Nichol S T. Genome structure and variability of a virus causing hantavirus pulmonary syndrome. Virology. 1994;200:715–723. doi: 10.1006/viro.1994.1235. [DOI] [PubMed] [Google Scholar]
- 53.Stangl F B., Jr Aspects of a contact zone between two chromosomal races of Peromyscus leucopus (Rodentia: Cricetidae) J Mammal. 1986;67:465–473. [Google Scholar]
- 54.Swofford D L. PAUP: phylogenetic analysis using parsimony, version 3.1.1. Champaign, Ill: Illinois Natural History Survey; 1991. . (Computer program.) [Google Scholar]
- 55.Torrez-Martinez N, Hjelle B. Enzootic of Bayou hantavirus in rice rats (Oryzomys palustris) in 1983. Lancet. 1995;346:780–781. doi: 10.1016/s0140-6736(95)91541-9. [DOI] [PubMed] [Google Scholar]
- 56.Torrez-Martinez N, Song W, Hjelle B. Nucleotide sequence analysis of the M genomic segment of El Moro Canyon hantavirus: antigenic distinction from Four Corners hantavirus. Virology. 1995;211:336–338. doi: 10.1006/viro.1995.1413. [DOI] [PubMed] [Google Scholar]
- 57.Van Den Bussche R A, Chesser R A, Hamilton M J, Bradley R D, Porter C A, Baker R J. Maintenance of a narrow hybrid zone in Peromyscus leucopus: a test of alternative models. J Mammal. 1993;74:832–845. [Google Scholar]
- 58.Waters J H. Biochemical relationships of the mouse Peromyscus in New England. Syst Zool. 1963;12:122–132. [Google Scholar]
- 59.Williams R J, Bryan R T, Mills J N, Palma E, Vera I, de Velasquez F, Baez E, Schmidt W, Figueroa R, Mexa A E, Peters C J, Zaki S R, Khan A S, Ksiazek T G. An outbreak of hantavirus pulmonary syndrome among Mennonite colonies in western Paraguay. Am J Trop Med Hyg. 1997;57:274–282. doi: 10.4269/ajtmh.1997.57.274. [DOI] [PubMed] [Google Scholar]
- 60.Wolff J O. Getting along in Appalachia. Nat History. 1986;9:45–48. [Google Scholar]
- 61.Wolff J O. Social behavior. In: Kirkland G L Jr, Layne J N, editors. Advances in the study of Peromyscus (Rodentia). Lubbock, Tex: Texas Tech University Press; 1989. pp. 271–291. [Google Scholar]
- 62.Xiao S-Y, LeDuc J M, Chu Y K, Schmaljohn C S. Phylogenetic analysis of virus isolates in genus Hantavirus, family Bunyaviridae. Virology. 1994;198:205–217. doi: 10.1006/viro.1994.1023. [DOI] [PubMed] [Google Scholar]