Abstract
The herpes simplex virus type 1 (HSV-1) gH-gL complex which is found in the virion envelope is essential for virus infectivity and is a major antigen for the host immune system. However, little is known about the precise role of gH-gL in virus entry, and attempts to demonstrate the immunologic or vaccine efficacy of gH and gL separately or as the gH-gL complex have not succeeded. We constructed a recombinant mammalian cell line (HL-7) which secretes a soluble gH-gL complex, consisting of gH truncated at amino acid 792 (gHt) and full-length gL. Purified gHt-gL reacted with gH- and gL-specific monoclonal antibodies, including LP11, which indicates that it retains its proper antigenic structure. Soluble forms of gD (gDt) block HSV infection by interacting with specific cellular receptors. Unlike soluble gD, gHt-gL did not block HSV-1 entry into cells, nor did it enhance the blocking capacity of gD. However, polyclonal antibodies to the complex did block entry even when added after virus attachment. In addition, these antibodies exhibited high titers of complement-independent neutralizing activity against HSV-1. These sera also cross-neutralized HSV-2, albeit at low titers, and cross-reacted with gH-2 present in extracts of HSV-2-infected cells. To test the potential for gHt-gL to function as a vaccine, BALB/c mice were immunized with the complex. As controls, other mice were immunized with gD purified from HSV-infected cells or were sham immunized. Sera from the gD- or gHt-gL-immunized mice exhibited high titers of virus neutralizing activity. Using a zosteriform model of infection, we challenged mice with HSV-1. All animals showed some evidence of infection at the site of virus challenge. Mice immunized with either gD or gHt-gL showed reduced primary lesions and exhibited no secondary zosteriform lesions. The sham-immunized control animals exhibited extensive secondary lesions. Furthermore, mice immunized with either gD or gHt-gL survived virus challenge, while many control animals died. These results suggest that gHt-gL is biologically active and may be a candidate for use as a subunit vaccine.
The virion glycoproteins gH and gL are among the few which have homologs in all three classes of herpesviruses (3, 24, 35). For many of these viruses, gH forms a hetero-oligomeric complex with gL (13, 29, 32, 33, 36, 55, 58). When herpes simplex virus type 1 (HSV-1) gH is expressed in the absence of gL, it is retained in the endoplasmic reticulum in an antigenically and structurally immature form (12, 25, 46, 48). The proper processing and transport of gH requires it to be coexpressed with gL as a hetero-oligomer (29). Thus, gL acts in part as a chaperone for gH. Interestingly, HSV gL contains an N-terminal signal peptide sequence but lacks a hydrophobic transmembrane region (TMR). When gL is expressed in the absence of gH, it is secreted from the cell (9); when gL is coexpressed in transfected cells, it is detected on the cell membrane (9). Likewise, both proteins require each other to be present in the viral envelope (48).
The conservation of the gH-gL complex among the herpesviruses suggests that it plays a central role in virus infection. In the case of HSV, gH and gL, along with gB and gD, are required for entry into susceptible cells and for cell-to-cell spread of HSV (54). Viruses lacking the gene for either gH or gL are noninfectious in cell culture (8, 14, 48). Also, certain monoclonal antibodies (MAbs) against HSV gH have high titers of complement-independent virus neutralizing activity (15, 49, 50), and some anti-gL MAbs can block virus spread, although they do not neutralize virus (44). These properties suggest that the gH-gL complex itself should stimulate neutralizing antibody responses in animals and that it might be a useful candidate for a subunit vaccine against HSV. However, the results to date in this regard have been disappointing. Immunization of animals with gH alone (15, 20, 21, 46), gL alone (3, 21), or gH-gL (3) induced little or no detectable virus neutralizing activity.
In this study, we decided to reexamine this issue by using a secreted form of the gH-gL complex. Previously, mammalian cells were cotransfected with plasmids which encode full-length gL and a truncated form of gH, gH(792t) (here referred to as gHt). The latter protein lacked the TMR and cytoplasmic tail. The transfected cells expressed and secreted the gHt-gL complex in a form which was recognized by conformation-dependent MAbs (9). To carry out more detailed studies, we cotransfected cells with these plasmids and selected a stable cell line, called HL-7, which constitutively expresses and secretes gHt-gL. The complex was purified in the absence of detergents by using immunoaffinity chromatography. Our results indicate that the complex can stimulate production of neutralizing antibody and affords good protection to mice challenged with HSV-1 in a zosteriform model.
MATERIALS AND METHODS
Cells and virus.
African green monkey kidney (Vero) and mouse L cells were grown in Dulbecco’s modified Eagle medium (DMEM) supplemented with 5% fetal bovine serum (FBS) at 37°C. D14 cells (Vero derived) which express HSV-1 ICP-6 (22) were grown in DMEM with 5% FBS and G418 (25 μg/ml) at 37°C. HL-7 cells which express gHt-gL were grown in DMEM supplemented with 10% FBS and hygromycin B (50 μg/ml). For protein production, hygromycin B was eliminated from the medium. HSV-1(hrR3) (22) was propagated on D14 cells and titered on Vero cells. The hrR3 strain of HSV-1 KOS and the D14 cells used to propagate the virus (22) were kindly provided by S. Weller. The propagation of HSV-1(NS) and HSV-2(333) stocks has been described previously (10).
Antibodies used.
The MAb-secreting cell lines 52S and 53S (recognizing gH) (49) were obtained from the American Type Culture Collection. Anti-gH MAb 37S was kindly provided by M. Zweig (49). Anti-gH MAb LP11 was the gift of A. Minson (4). MAb 8H4, which recognizes a linear epitope on gL, was described previously (9); rabbit antibody αUL1-2, which was prepared against a peptide sequence of gL, was kindly provided by D. Johnson (29). Rabbit antibody R83 (against gH) was described previously (46). Polyclonal antibodies R137, R138, R139, and R140 were prepared against purified gHt-gL (this study).
Construction of the HL-7 cell line and purification of the gHt-gL complex from the supernatant of HL-7 cells.
To construct a cell line which would secrete the gH-gL complex, L cells were cotransfected with three separate plasmids: (i) pCMV3gH(792) (9), which encodes gH truncated at amino acid 792 and lacks both the TMR and the cytoplasmic region; (ii) pCMV3gL-1 (9), which encodes the entire UL1(gL) open reading frame; and (iii) pX343, which confers resistance to hygromycin B (1). Transfected cells were grown in DMEM in the presence of hygromycin B, the surviving cells were expanded, and clones were obtained from single cells. The supernatant from each clone was screened by Western blot analysis using R83 (anti-gH) and αUL1-2 (anti-gL). In addition, cells which coexpressed the gHt-gL complex were detected by the ability of gH antisera to coimmunoprecipitate gL from the culture supernatant. Four different clones which express the gHt-gL complex were isolated. One cell line, designated HL-7, was selected for further study. HL-7 secreted significant amounts of gHt-gL, and the cells exhibited normal morphology and growth kinetics. For protein production, the cells were grown in roller bottles. The supernatant was obtained after 3 days and replaced with fresh medium. Two harvests of supernatant were obtained from each roller. The secreted gHt-gL complex was purified by chromatography on an immunoaffinity column of 53S, a gH-1 specific MAb, by a modification of a method used previously to purify gH from extracts of HSV-1-infected cells (46). Here, the clarified medium was passed over the column, and the bound protein was eluted with a low-pH buffer consisting of 50 mM glycine and 0.5 M NaCl (pH 2.5). The eluate was neutralized with 1 M Tris base (pH 9.0) and concentrated. Protein was quantitated by using a bicinchoninic acid kit (Pierce Chemical Co.). Approximately 400 μg of gH-gL complex was obtained per liter of HL-7 cell supernatant (∼10−4 ng/cell).
Purification of HSV-1.
Virus was purified as previously described (26). In brief, roller bottles (850 cm2) of D14 cells were infected with hrR3 at a multiplicity of infection (MOI) of 0.1. The growth medium was collected at 24 h postinfection, and extracellular virus was pelleted by centrifugation at 100,000 × g through a 5% sucrose–phosphate-buffered saline (PBS) cushion. Virus was further purified by first resuspending the pellet in PBS, followed by centrifugation at 30,000 × g for 5 h through a 10%-30%-60% sucrose-PBS step gradient. The virus band located at the 30%-60% sucrose interface was collected, titered, and stored at −80°C.
Soluble HSV glycoproteins and infected cell extracts used.
Soluble gD1(306t) was produced in baculovirus-infected Sf9 cells and was purified as previously described (52). Cytoplasmic extracts of HSV-1(NS) (16)- or HSV-2(333)-infected cells were prepared as previously described (10, 11). Full-length gD-1 was purified from cytoplasmic extracts of HSV-1-infected cells as previously described (10). Soluble gC-1(457t) was produced from baculovirus-infected insect cells and purified as previously described (57).
SDS-PAGE and Western blot analysis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under denaturing or native conditions was done as previously described (6), using Tris-glycine 10 or 4 to 12% gradient precast gels (Novex Experimental Technology). Silver staining was performed by using a silver staining kit (Pharmacia Biotech). For Western blot analysis, proteins were transferred to nitrocellulose and probed with antiserum R83 for gH or MAb 8H4 for gL. Goat anti-rabbit (for R83) or anti-mouse (for 8H4) immunoglobulin G-peroxidase (Boehringer) was then added as secondary antibody, and bands were visualized on X-ray film after addition of ECL chemiluminescent substrate (Amersham). To strip the blot, 50 mM glycine–0.5 M NaCl (pH 2.5) was added, and the mixture was incubated at room temperature (RT) for 15 min. The blot was then washed with PBS–0.2% Tween and reprobed.
Antigenic analysis of gHt-gL by ELISA.
Various concentrations of gHt-gL were coated onto enzyme-linked immunosorbent assay (ELISA) plates and incubated overnight at 4°C. The plates were blocked with PBS containing 1% bovine serum albumin and 1% ovalbumin. MAbs LP11, 53S, and 37S were each diluted in PBS with 0.05% bovine serum albumin and 0.05% ovalbumin and then added to the ELISA plate to detect gH. After 1 h at RT, the plate was washed three times with PBS–0.5% Tween 20. Goat anti-mouse IgG-horseradish peroxidase conjugate (Boehringer) was added, and the plate was incubated at RT for 30 min. After a rinse with citrate buffer (20 mM citrate acid, pH 4.5), ABTS substrate (2,2′-azino-di-3-ethylbenzthiozoline-6-sulfonic acid; Moss, Inc.) was added, and absorbance was read at 405 nm with a microtiter plate reader (BioTek Instruments).
HSV-1 entry assay.
Vero cells were seeded onto a 96-well plate and grown to confluence. The plate was cooled at 4°C for 10 min, and viral glycoproteins which had been serially diluted in 5% FBS–DMEM (with 0.03 M HEPES) were added. The medium was removed and replaced with 50 μl of a single purified virion glycoprotein and incubated at 4°C for 90 min. Purified HSV-1(hrR3) in 5% FBS–DMEM (2 × 104 PFU/ml) was added to each well (MOI was 0.5 PFU/cell) and incubated at 4°C for 90 min to allow for virus attachment. The cells were then incubated for 5 h at 37°C and lysed with 1% Nonidet P-40 in DMEM. Then 50 μl of lysate from each well was transferred to an ELISA plate and mixed with 50 μl of CPRG (chlorophenol red-β-d-galactopyranoside; 4.8 mg/ml; Boehringer), and β-galactosidase activity was measured by taking absorbance readings at 570 nm every 2 min for a total of 25 readings, using an ELISA plate reader (Bio-Tek). The slope of the line was used to calculate the amount of β-galactosidase activity as milli-optical density units/minute.
Immunization of rabbits with gHt-gL.
New Zealand rabbits were immunized with gHt-gL mixed with one of two adjuvants. Set I was immunized with gHt-gL (150 μg, total) mixed with Freund’s adjuvant. The first does was in Freund’s complete adjuvant (Sigma), and subsequent injections were given in Freund’s incomplete adjuvant. Set II was immunized with gHt-gL mixed with an equal volume of alum adjuvant (Pierce).
Virus neutralization assay.
Rabbit or mouse sera were treated at 56°C for 30 min to inactivate complement. Serial twofold dilutions of serum were prepared in DMEM containing 5% FBS and then mixed with an equal volume of HSV-1 or HSV-2 adjusted to give 100 plaques per well in the absence of neutralizing antibody. The virus cell mixture was incubated for 1 h at 37°C, overlaid with medium, and incubated at 37°C for 24 h. The medium was removed, the cells were fixed with a 2:1 mixture of methanol and acetone, and then dried. Plaques were visualized with a cocktail of polyclonal antibodies to gD, gB, and gC by black plaque assay (27, 57) using horseradish peroxidase-conjugated protein A, followed by addition of the substrate 4-chloro-1-naphthol. The neutralization titer was expressed as the dilution of serum that reduced the number of plaques by 50%.
Two assays were used to measure serum blocking (neutralization) of virus entry. In the first (antibody-plus-virus method), each antiserum was mixed with hrR3 (4 × 105 PFU/ml) in DMEM containing 5% FBS and 0.03 M HEPES, and the serum-virus mixture was incubated at 37°C for 90 min, cooled to 4°C, and added to Vero cells (96-well plates) (100 μl/well). Plates were rocked at 4°C for 90 min and then shifted to 37°C for 5 h. Cells were lysed, and β-galactosidase activity was measured on the cytoplasmic extract. In the second assay (antibody-after-virus method), hrR3 (4 × 105 PFU/ml) in DMEM containing 5% FBS and 0.03 M HEPES was added to Vero cells at 4°C for 90 min. The virus was removed and replaced by antiserum diluted in DMEM containing 5% FBS. Plates were rocked at 4°C for 90 min and then shifted to 37°C for 5 h. Cells were lysed, and β-galactosidase activity was measured.
Murine flank (zosteriform) model of HSV challenge.
A zosteriform model of HSV-1 infection (50, 51) was used to test the efficacy of gH-gL as a vaccine. Nine- to ten-week-old BALB/c (Charles River) mice were immunized intraperitoneally with 10 μg of antigen in complete Freund’s adjuvant, followed by three additional 10-μg doses of antigen given in incomplete Freund’s adjuvant at 2-week intervals. The antigens used were purified gHt-gL produced by HL-7 cells and purified full-length gD-1 from HSV-1-infected cells (10). Sham-immunized control animals received PBS emulsified with adjuvant at the same intervals. Mice were bled for sera between the third and fourth immunizations to test for virus neutralization. Two weeks after the last immunization, the right flank of each immunized or control animal was shaved and denuded by using a depilatory cream. Twenty-four hours later, 5 × 105 PFU of HSV-1 was applied to the depilated flank approximately 3 mm ventral to the spinal column, and the skin was scratched with a 27-gauge needle, using 20 horizontal strokes and 20 vertical strokes over an approximate area of 3 by 3 mm. The flank was observed daily for at least 10 days, and cumulative scores for primary and secondary areas were recorded from days 3 through 8. The period of recording lesions was limited to this period due to the deaths of unprotected animals beginning at day 8. Disease at the inoculation site was scored as follows: 0 points for no disease, 0.5 point for swelling without vesicles, and 1 point each for each vesicle or scab to a maximum score of 5. Swelling and lesions in locations separate from the inoculation site were considered to be secondary or zosteriform disease. Scoring of these lesions was the same as for the inoculation site except that a daily maximal score of 10 was used.
RESULTS
To construct the HL-7 cell line, mouse L cells were cotransfected with plasmids pCMV3gH(792) (9), which encodes gH truncated at amino acid 792 (Fig. 1); pCMV3gL-1 (9), which encodes the entire UL1(gL) open reading frame (Fig. 1); and pX343, which confers resistance to hygromycin B (1). Transfected cells were grown in the presence of hygromycin B, and clones were obtained from single surviving cells. One cell line, designated HL-7, was selected for further study. HL-7 cells exhibited normal morphology and growth kinetics (data not shown). These cells secreted significant amounts of gHt-gL which was detected on Western blots at the predicted sizes for gHt and gL (see below).
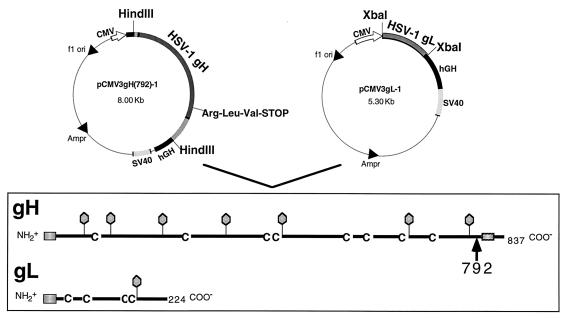
FIG. 1.
Plasmids used to construct the HL-7 cell line and diagrammatic representations of gHt and gL. HL-7 cells were obtained by cotransfecting mouse L cells with pCMV3gH(792)-1, pCMV3gL-1 (9), and pX343, which confers resistance to hygromycin B (1) (not shown). HL-7 was one of four separate clones which expressed and secreted gHt-gL as a complex. The stick diagrams illustrate major structural features of full-length gH-1 and gL-1. An arrow indicates the location of the truncation of gH at amino acid 792. Balloons indicate positions of predicted N-linked oligosaccharides, and C’s indicate positions of cysteine residues. The predicted signal peptide and transmembrane anchor regions are indicated with shaded boxes. SV40, simian virus 40; hGH, human growth hormone.
Purification and analysis of gHt-gL.
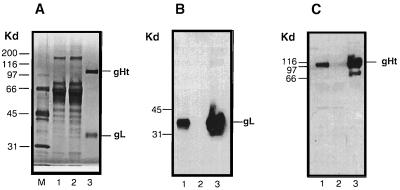
gHt-gL was purified from the growth medium of HL-7 cells by immunoaffinity chromatography on an anti-gH (53S) MAb column. Purification was monitored by SDS-PAGE followed by silver staining (Fig. 2A) as well as by Western blot analysis (Fig. 2B and C). The purified complex contained two silver-stained bands of 110 and 35 kDa (Fig. 2A, lane 3), although neither of these was prominent in the culture supernatant or column flowthrough (Fig. 2A, lanes 1 and 2). Both glycoproteins were readily detected in the culture supernatant by Western blot analysis (Fig. 2B and C, lanes 1). The absence of both gH and gL from the column flowthrough fraction (Fig. 2B and C, lanes 2) shows that all of the secreted gL was associated with gH and both proteins bound to MAb 53S as a stable complex. Both proteins were eluted by low pH (Fig. 2B and C, lanes 3). We estimate that the eluted complex was greater than 95% pure by silver staining (Fig. 2A, lane 3).
FIG. 2.
Extracellular expression and purification of gHt-gL complex from HL-7 cells. (A) Twenty microliters of HL-7 cell supernatant (lane 1), 20 μl of flowthrough (lane 2), and 1 μg of protein eluted from the 53S immunoabsorbent column (lane 3) were analyzed by electrophoresis on an SDS–10% polyacrylamide gel. The gel was stained for protein with silver stain. (B) The proteins were electrophoresed on an SDS–10% polyacrylamide gel, transferred to nitrocellulose, and probed with anti-gL ascites 8H4. (C) The proteins were electrophoresed on an SDS–10% polyacrylamide gel, transferred to nitrocellulose, and probed with anti-gH serum R83.
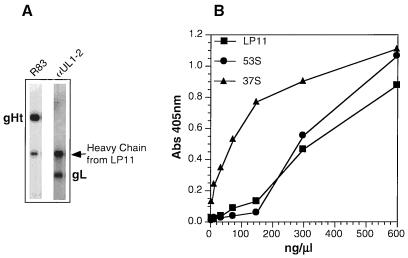
LP11 reactivity is considered to be a critical test of gH-gL conformation, since this MAb reacts with gH only when it is part of the native complex (29). Second, LP11 neutralizes virus infectivity at high titers and therefore recognizes an immunologically important epitope (4). Purified gHt-gL was immunoprecipitated with LP11, separated by SDS-PAGE, and analyzed by Western blotting, probing for gH (Fig. 3A, lane 1) and gL (Fig. 3A, lane 2) on individual nitrocellulose strips. Both proteins were detected, showing that the complex was reactive with LP11. Similar results were obtained in assays using MAb 52S (49) for the initial immunoprecipitation (results not shown). As a second method, we used ELISA to show that the purified complex reacts with MAbs LP11, 53S, and 37S (49). Previous studies had shown that MAbs 52S, 53S, and LP11 recognize different conformation-dependent epitopes (15, 18, 23, 46) and 37S recognizes a linear epitope (46). Thus, these two experiments indicate that gHt in the complex is antigenically correct. Similar studies were not done on gL, as no conformation-dependent MAbs are available. However, the complex does react by ELISA with gL MAbs which recognize linear epitopes (data not shown).
FIG. 3.
Reactivity of purified gHt-gL with gH-specific antibodies. (A) Purified gHt-gL was immunoprecipitated with MAb LP11 and then electrophoresed on an SDS–10% polyacrylamide gel. The proteins were transferred to nitrocellulose and probed with R83 (anti-gH serum) (lane 1) or with αUL1-2 (anti-gL serum) (lane 2) (B) Various concentrations of gHt-gL were coated onto an ELISA plate for 2 h at RT. Wells were reacted with anti-gH MAb LP11, 53S, or 37S. Binding of these antibodies was detected with horseradish peroxidase-labeled goat anti-mouse antibody and ABTS substrate. Abs, absorbance.
gHt-gL does not inhibit virus entry.
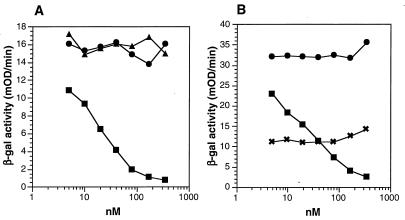
Both gH-1 and gL-1 are essential for HSV-1 penetration and cell-to-cell spread and are most likely involved in a cell fusion event (7, 8, 14, 44, 48). However, little is known about gH-gL function or whether the proteins work individually or together with other glycoproteins to effect virus entry. Soluble forms of gD (gDt) are able to block HSV infection (31, 42, 43). This is due to the interaction of gDt with cellular receptors such as herpesvirus entry mediator (HVEM) (40, 60), making them unavailable to bind to gD in the virion. In contrast, soluble forms of gC-1 (gC-1t) do not block plaque formation by HSV (57). We recently showed that gC-1t, gB-1t, and gHt-gL did not bind directly to HVEM (60). Here we asked whether soluble gHt-gL could block HSV-1 entry into cells, perhaps by binding to a different receptor than HVEM. We used an entry assay employing HSV-1(hrR3) which contains the lacZ gene under the control of the ICP6 promoter (22). Virus entry was measured as an increase in β-galactosidase activity at 5 h postinfection (Fig. 4A). As expected from previous studies (57), gC-1t did not block virus entry and served as a negative control for the assay. Fifty percent inhibition of virus entry was observed at 50 nM gD-1(306t), a result similar to that obtained in a 50% inhibition of plaque formation assay (43). In contrast, gHt-gL did not inhibit virus entry even at protein concentrations as high as 350 nM (50 ng/μl). We next asked whether gHt-gL could enhance the ability of soluble gD-1(306t) to block infection by enhancing its binding to HVEM or other gD receptors. Increasing amounts of gHt-gL were added to cells together with 40 nM gD (Fig. 4B). At this concentration, gD inhibited virus entry by 40 to 50%. gHt-gL did not enhance the inhibition achieved with gDt alone.
FIG. 4.
Effect of gHt-gL on HSV cell entry. Various concentrations of purified proteins gC1(457t) (gCt), gD-1(306t) (gDt), and gHt-gL were added to Vero cell monolayers in 96-well plates for 90 min at 4°C. HSV-1(hrR3) was added at an MOI of 0.5, and the plate was incubated for another 90 min at 4°C. Plates were then shifted to 37°C for 5 h. Cells were lysed, and β-galactosidase (β-gal) activity was measured on aliquots of the cytoplasmic extract using the substrate CPRG and measuring the increase in absorption at 570 nm (expressed as milli-optical density units [mOD]). (A) Blocking of virus entry with purified gCt (▴), gDt (▪), or gHt-gL (•); (B) blocking of virus entry with gDt alone (▪), gHt-gL (•), or a mixture of 40 nM gD (concentration which gave 50% inhibition of entry) with various concentrations of gHt-gL (×).
Antibodies to gHt-gL block virus entry and neutralize virus infectivity.
The previous experiments were inconclusive as to the role played by gH-gL in virus entry. It was previously shown that anti-gH neutralizing MAbs such as LP11 are able to block HSV infection even when added after virus attachment (18). We argued that if the conformation of gHt-gL is close to that of the functional form in the virus, then antibodies to the complex should be able to neutralize infection and block virus entry whether added before or after virus attachment.
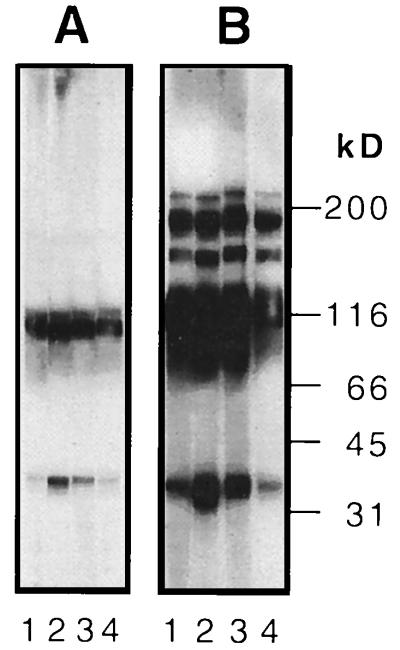
Rabbits were immunized with gHt-gL by using either Freund’s or alum adjuvant. All four animals produced antibodies which recognized gHt and gL on a Western blot of a denaturing gel (Fig. 5A). On a Western blot of a nondenaturing (native) gel (6), these antibodies also recognized higher-molecular-weight forms (Fig. 5B). The composition of these bands remains to be determined.
FIG. 5.
Immunoblot (Western blot) analysis of serum samples from rabbits immunized with gHt-gL. (A) Purified gHt-gL was electrophoresed on a denaturing SDS–10% polyacrylamide gel, transferred to nitrocellulose, and reacted with R136 (lane 1), R137 (lane 2), R138 (lane 3), or R139 (lane 4). (B) Purified gHt-gL was electrophoresed on a nondenaturing (native) SDS–10% polyacrylamide gel, transferred to nitrocellulose, and reacted with R136 (lane 1), R137 (lane 2), R138 (lane 3), or R139 (lane 4).
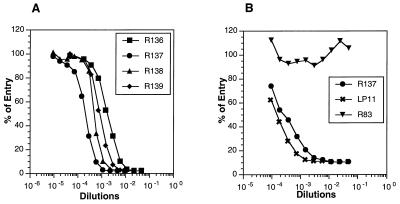
All four sera exhibited significant titers of complement-independent HSV-1 neutralizing activity (Table 1). In addition, these sera also neutralized HSV-2, albeit at a much reduced potency. These results indicated that the immunizing protein had biologic activity. In addition, each of the sera, when premixed with hrR3 virus, was able to block virus entry (Fig. 6A). As a second approach, we first adsorbed the virus to cells at 4°C and then added either R83 (anti-gH), R137, or MAb LP11 to the virus-cell mixture. As expected, LP11 blocked virus entry when added after virus adsorption (Fig. 6B). R137 had blocking activity similar to that observed for LP11, indicating that both antibodies recognized a site on gH-gL which was critical for postbinding steps in virus entry. This experiment suggests that the gHt-gL complex used to prepare R137 contains a functionally active conformation. In contrast, antibody R83 was unable to block virus entry. This was an important control for the present study because R83 had been prepared against gH purified from infected cells in such a way that it lacked gL and therefore lacked the proper biologically active conformation (46). Thus, although we were unable to directly demonstrate blocking activity by purified gHt-gL complex, studies with rabbit antisera to gHt-gL provide indirect evidence that the complex contains the conformation necessary for function in virus infection.
TABLE 1.
HSV neutralizing activity of sera from rabbits immunized with gHt-gL
| Adjuvant | Rabbit | Virus neutralization titer (50%)
|
||
|---|---|---|---|---|
| Entry assay,a HSV-1(hrR3) | Plaque assayb
|
|||
| HSV-1(KOS) | HSV-2(333) | |||
| Freund’s | R136 (prebleed) | <1:20 | <1:20 | <1:20 |
| R137 (prebleed) | <1:20 | <1:20 | <1:20 | |
| R136 (3rd bleed) | 1:640 | 1:640 | <1:20 | |
| R137 (3rd bleed) | 1:4,500 | 1:2,000 | 1:60 | |
| Alum | R138 (prebleed) | <1:20 | <1:20 | <1:20 |
| R139 (prebleed) | <1:20 | <1:20 | <1:20 | |
| R138 (3rd bleed) | 1:2,560 | 1:1,800 | 1:100 | |
| R139 (3rd bleed) | 1:1,280 | 1:640 | 1:100 | |
Virus entry was measured by infecting cells with HSV-1(hrR3) (22) and measuring β-galactosidase activity at 5 h postinfection. The neutralization titer represents the dilution of antiserum needed to reduce β-galactosidase activity to 50% of the maximum seen with no serum added.
Virus infection was measured by plaque formation by HSV-1(KOS) on Vero cells, using the black plaque assay. The neutralization titer represents the dilution of antiserum needed to reduce the number of plaques to 50% of the number found on control plates with no antiserum added.
FIG. 6.
Blocking of HSV entry by rabbit antibodies to gHt-gL. (A) HSV-1(hrR3) was incubated for 90 min at 37°C with various concentrations of rabbit anti-gHt-gL serum R136, R137, R138, or R139, and the serum-virus mixture was added to Vero cell monolayers in a 96-well plate, incubated at 4°C for 90 min, and then incubated at 37°C for 5 h. Virus entry was assayed as an increase in β-galactosidase activity in cytoplasmic extracts from each well and expressed as percentages of control values obtained with virus alone. (B) HSV-1(hrR3) was added to Vero cell monolayers at 4°C for 90 min. The medium was removed, various dilutions of either R83, R137, or LP11 were added, and monolayers were incubated at 4°C for 90 min and then at 37°C for 5 h. Virus entry was assayed as described for panel A.
Immunization of mice with gHt-gL.
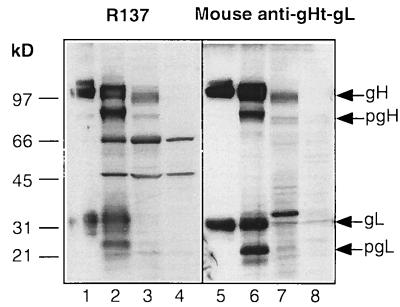
To further assess the ability of gHt-gL to elicit a humoral immune response, BALB/c mice were immunized with gHt-gL in two separate experiments (Table 2). Mice were separated into three groups in each experiment. Group 1 was sham immunized with PBS, as a negative control; group 2 was immunized with gD purified from HSV-infected cells, as a positive control; group 3 was immunized with gHt-gL. Prior to virus challenge, serum samples were obtained from each of the immunized animals. The reactivity of a pool of mouse anti-gHt-gL serum (from experiment I) was compared to that of R137 by immunoblotting. Both R137 and the mouse anti-gHt-gL reacted with gHt and gL on Western blots (Fig. 7, lanes 1 and 5). We also compared the reactivities of rabbit and mouse sera against cytoplasmic extracts of HSV-1- and HSV-2-infected cells. Both R137 and the pooled mouse serum reacted against bands migrating at the expected positions of gH-1 and gL-1 (Fig. 7, lanes 2 and 6). These sera also recognized the precursor forms of gH and gL. Both sera cross-reacted against bands we presume to be pgH-2 and gH-2 (Fig. 7, lanes 3 and 7). The mouse serum also reacted with a band at the presumed position of gL-2 (Fig. 7, lane 7). It should be noted that gL-2 is expected to be 500 Da larger than gL-1, based on predicted amino acid sequence, and gH-2 is predicted to be 700 Da smaller than gH-1. R137 reacted with two bands of 66 and 45 kDa in extracts from both infected and uninfected cells (Fig. 7, lanes 2 to 4). Therefore, these two bands are considered to react nonspecifically with the rabbit antibody.
TABLE 2.
Protection of mice from intradermal HSV-1 challenge following immunization with gD or gHt-gL
| Expa | Immunizing antigen | Avg primary scoreb | Avg secondary scorec | Group mortality |
|---|---|---|---|---|
| I | Mock (PBS) | 10.5 | 16.5 | 5/10 |
| gD | 5.5 | 0 | 0/10 | |
| gHt-gL | 2.8 | 0 | 0/9 | |
| II | Mock (PBS) | 22.3 | 19.6 | 5/5 |
| gD | 5.8 | 0 | 0/5 | |
| gHt-gL | 4.2 | 0 | 0/5 |
In experiment I, there were 10 mice in each group. One mouse in the gH-gL-immunized group of experiment I died prior to challenge. In experiment II, there were five mice in each group.
Primary lesions developed immediately around the infection site. The primary scores were cumulative from day 3 to day 8. Score range was 0 to 5.
Scored by the zosteriform lesions on the flank surrounding the primary infection site. The secondary scores were cumulative from days 3 to 8. Score range was 0 to 10.
FIG. 7.
Immunoblot (Western blot) analysis of cytoplasmic extracts of HSV-1 or HSV-2-infected Vero cells. Samples of purified gHt-gL or cytoplasmic extracts were electrophoresed on a denaturing SDS–10% polyacrylamide gel, transferred to nitrocellulose, and reacted with R137 (lanes 1 to 4) or mouse anti-gHt-gL (lanes 5 to 8). The mouse serum was pooled from nine animals immunized with gHt-gL (Table 2, experiment I). gHt-gL purified from HL-7 cells (lanes 1 and 5) was included on the gel as a control. Cytoplasmic extracts were prepared from cells infected with HSV-1(NS) (lanes 2 and 6) or HSV-2(333) (lanes 3 and 7) or from uninfected cells (lanes 4 and 8).
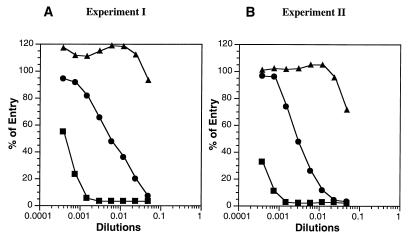
Sera obtained from each mouse immunized with either gD or gHt-gL exhibited high titers of virus neutralizing activity as measured by inhibition of virus entry (data for representative mice shown in Fig. 8). We observed that the titers were approximately 10-fold higher when animals were immunized with gD as opposed to gHt-gL.
FIG. 8.
Blocking of HSV entry by mouse antibodies to gD or to gHt-gL. (A) HSV-1(hrR3) was incubated for 90 min at 37°C with various concentrations of antisera from mice immunized either with full-length gD (from HSV-1-infected cells; ▪), with gHt-gL (from HL-7 cells; •), or with PBS (▴) according to experiment I (Table 2). The serum-virus mixture was added to Vero cell monolayers in a 96-well plate, incubated at 4°C for 90 min, and then incubated at 37°C for 5 h. Virus entry was assayed as an increase in β-galactosidase activity in cytoplasmic extracts from each well and expressed as percentages of control values obtained with virus alone. (B) Same as panel A except that the sera were from mice immunized as part of experiment II (Table 2). Each of the sera from both experiments was assayed, and only one representative curve for each experimental group is shown. All of the sera for each group gave similar curves.
gHt-gL protects mice from HSV-1 challenge.
A zosteriform model of HSV-1 infection was used to examine the ability of gHt-gL to act as a vaccine (50, 51). Following intraperitoneal immunization with either gD or gHt-gL, mice were challenged with HSV-1 by intradermal inoculation on the right flank (Table 2). In two separate experiments, some of the animals in each group showed some evidence of infection at the site of virus challenge (primary lesions). However, the primary lesion scores for mice immunized with either gD or gHt-gL were lower than those of sham-immunized mice. Of most significance was the finding that all of the sham-immunized mice that developed primary lesions went on to develop severe secondary zosteriform lesions. In contrast, mice immunized with either gD or with gHt-gL exhibited no secondary lesions, regardless of whether they developed any evidence of primary lesions. Furthermore, all of the immunized mice survived virus challenge, while many of the control animals (5 of 10 in experiment I and 5 of 5 in experiment II) died. These results suggest that gHt-gL purified from HL-7 cells is biologically active and should be considered as a candidate for use as a subunit vaccine against HSV-1 infection.
DISCUSSION
Structural, immunological, and functional studies of the HSV gH-gL complex have been hampered due to the lack of a suitable source of purified protein. Milligram amounts of a truncated mammalian form of HSV-1 gH-gL have now been obtained using a stably transfected L-cell line, HL-7, which secretes gHt and gL as a complex. In agreement with what we found for mammalian cells, Westra et al. (59) showed that an HSV-1 gHt-gL complex was secreted from insect cells coinfected with recombinant baculoviruses. Spaete et al. (53) showed that coexpression of a truncated form of cytomegalovirus (CMV) gH with the UL15 open reading frame gene product (the CMV homolog of HSV gL) results in association of the two proteins and secretion from a transfected cell. This issue may need to be revisited for CMV, since it was recently reported that a third protein, gp 145, coassociates with gH-gL in CMV-infected cells (28).
Purification of the HSV-1 gHt-gL complex produced by HL-7 cells was accomplished by immunosorbent chromatography, and the complex was obtained in reasonable quantities in the absence of detergents. However, the possibility that the presumed activity of the gHt-gL complex, i.e., membrane-membrane fusion, is compromised as a result of the truncation is a concern. Galdiero et al. (19) recently used site-directed mutagenesis to show that a region of gH just prior to the transmembrane region is critical for function. Several criteria suggest that the truncated soluble complex that we are studying reflects the actual structure of the complex in the virion envelope. First, the gHt-gL complex was antigenically intact, as judged by its capacity to bind to MAbs 52S and 53S, which recognize gH conformation (49), as well as to the “gold standard” MAb LP11 (4), which recognizes conformation of the gH-gL complex (23). The baculovirus-derived truncated form of HSV-1 gH-gL (59) also bound to these antibodies. gL conformation could not be determined since conformation-dependent MAbs to this protein are not available (9, 44, 48). Interestingly, all of the gL found in the supernatant was complexed to gHt, arguing for some type of regulation in the secretion process of these two proteins.
gHt-gL stimulates a protective immune response.
Previous attempts to immunize animals with gH alone (15, 20, 21, 46) or gL alone (3, 21) failed to induce virus neutralizing activity. These failures are now understood on the basis that an intact gH-gL complex is needed. Having demonstrated that gHt-gL can stimulate a robust humoral immune response and can protect mice from HSV-1 challenge, we are somewhat at a loss to explain the failure of other laboratories to achieve a similar result in assays using gH-gL expressed as a complex in a recombinant vaccinia virus (3). Those results are particularly puzzling since it was shown that the expressed gH-gL complex contained the LP11 epitope, considered to be an excellent prognosticator of proper gH-gL conformation (29). One possibility is that the level of gH-gL expression was too low to induce a sufficient immune response. Another possibility is that the assay used in this study has higher sensitivity. Such a possibility would also account for the lower neutralization titers reported for gD by those authors. However, the sera from the two systems should be tested in the same assay to verify this.
This investigation shows that active immunization of rabbits or mice with HSV-1 gHt-gL purified from the culture supernatant of mammalian cell line stimulates production of neutralizing antibodies to HSV-1. These antibodies were able to block HSV entry, though the entry blocking titers for gHt-gL were not as high as those seen with antibody to gD. Moreover, although there was some cross-reaction against gH and gL of HSV-2, seen by both Western blotting and virus neutralization assays, the cross-neutralization titers were lower than those achieved against HSV-1. One might have expected more cross-reactivity based on the 77% sequence homology between gH-1 and gH-2 (2, 38). However, the fact that we found any cross-reactivity is of interest because none of the MAbs available to gH-1 or gL-1 are cross-reactive and none of the polyclonal sera that we developed earlier to gH-1 cross-reacted with gH-2 (46). The cross-reactivity of the anti-gHt-gL serum was also lower than that of polyclonal anti-gD serum (11, 30). These results point out the need to develop reagents specific for HSV-2 gH-gL. However, it is also worth noting that both the rabbit and mouse sera can be utilized to visualize gH-2 and that the mouse serum appears to be a useful reagent for detecting gL-2.
Many previous studies using gD have shown that this protein can protect a variety of animals from HSV-1 or HSV-2 challenge (5, 20, 37, 41, 56). However, there have been no reports of studies using gD in the zosteriform model of HSV infection (50). According to Simmons and Nash, “infection of mice in the flank leads to ganglionic infection with subsequent delivery of the virus to the skin of the whole dermatome, by nerve fibers” (50). Mice infected by this method develop a band-like or zosteriform rash within a few days after virus inoculation. An interesting aspect of this model is that epidermal cells which are distant from where the virus is initially delivered become infected via nerve endings. Thus, although the secondary lesions that develop are not the result of reactivation from latency, they mimic events that occur after reactivation. Another very appealing aspect to this model is the ease with which one can visualize the results of infection. Moreover, the model allows one to assess the effect of immunological mechanisms on modulation of infection in the epidermis after the virus spreads centrifugally from the ganglion. It is of interest that in the study by Simmons and Nash (50), neutralizing antibodies against both gD and gH (i.e., LP2 and AP7 for gD and LP11 for gH, respectively) gave excellent passive protection against zosteriform infection, whereas nonneutralizing antibodies did not. Their findings suggested that this would be an excellent model in which to test the efficacy of purified gHt-gL.
Here, we found that neither gD nor gHt-gL was able to completely protect mice from developing lesions at the site of primary inoculation, although both protein preparations ameliorated the severity of the primary disease. Significantly, prior immunization with either gD or gHt-gL gave excellent protection against development of zosteriform lesions. These data, together with the neutralization titers of anti-gHt-gL sera, are the most encouraging results seen to date regarding the potential efficacy of gHt-gL as a vaccine. The weak cross-reactivity of the anti-gHt-gL sera makes it difficult to predict whether gHt-gL from HSV-1 will be cross-protective against HSV-2. Experiments to examine this possibility and to develop a truncated form of HSV-2 gH-gL are now in progress.
What is the role of gH-gL in virus entry?
At least one study shows that gH functions later in the infection process than gD (17), and several laboratories have speculated that gH-gL functions in the fusion step of several herpesviruses between the virus envelope and the plasma membrane of the cell (8, 14, 17, 18, 39, 45, 47, 48). The fact that gHt-gL stimulated a neutralizing antibody response is evidence that the purified complex is biologically active. How do we reconcile this result with the fact that the purified complex did not block HSV entry? The two assays are distinctly different and point out different features of gH-gL function. In order for R137 antibody to block entry, it must interact with and block a critical site on the ectodomain of virion associated gH-gL. In order for the soluble protein to block infection, it has to compete with virion associated gH-gL for a critical step, and this might not be seen if there is a marked difference in affinity between the soluble form and the virion-associated form of gH-gL for its functional partner(s) in the virus or the cell. It should be noted that the concentration of gDt needed to block infection is much higher than the concentration of neutralizing antibodies to accomplish the same goal. This is not surprising since soluble gDt must compete with virion gD for binding to receptor, whereas antibodies to gD can interfere with infection by reacting with the functional site on gD in the virus. There are several other ways to explain the failure of gHt-gL to enhance blocking by gD. One possibility is that gH-gL function requires that residues downstream of amino acid 792 be present even though these residues are not critical for LP11 conformation or for stimulating neutralizing antibody (61). A second possibility is that a functional association between gH-gL and gD requires the simultaneous presence of gB. Experiments to explore some of these possibilities are now in progress.
We cannot rule out the possibility that a third protein complexes with gH-gL during HSV infection, as has been found for CMV (28) and Epstein-Barr virus (34). Should such a protein exist, it might be needed for gHt-gL to block infection, but the present study predicts that should such a protein be identified, it would not be needed for proper folding and transport of gH-gL. Moreover, gHt-gL is sufficient to induce a protective immune response. Clearly, additional experiments will have to be done to further explore the role of gH-gL in HSV infection.
ACKNOWLEDGMENTS
We thank S. Weller for the hrR3 strain of HSV-1 KOS, D. Johnson for antibody αUL1-2, and A. Minson for MAb LP11.
This study was supported by Public Health Service grants NS-30606 from the National Institute of Neurological Diseases and Stroke, AI-18289 from the National Institute of Allergy and Infectious Diseases, and DE-08239 from the National Institute of Dental Research.
REFERENCES
- 1.Blochlinger K, Diggelmann H. Hygromycin B phosphotransferase as a selectable marker for DNA transfer experiments with higher eucaryotic cells. Mol Cell Biol. 1984;4:2929–2931. doi: 10.1128/mcb.4.12.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boursnell, M. E. Personal communication.
- 3.Browne H, Baxter V, Minson T. Analysis of protective immune responses to the glycoprotein H-glycoprotein L complex of herpes simplex virus type 1. J Gen Virol. 1993;74:2813–2817. doi: 10.1099/0022-1317-74-12-2813. [DOI] [PubMed] [Google Scholar]
- 4.Buckmaster E A, Gompels U, Minson A. Characterisation and physical mapping of an HSV-1 glycoprotein of approximately 115 × 103 molecular weight. Virology. 1984;139:408–413. doi: 10.1016/0042-6822(84)90387-8. [DOI] [PubMed] [Google Scholar]
- 5.Burke R L. HSV vaccine development. In: Roizman B, Lopez C, editors. The herpesviruses. Vol. 5. New York, N.Y: Raven Press; 1993. pp. 367–380. [Google Scholar]
- 6.Cohen G H, Isola V J, Kuhns J, Berman P W, Eisenberg R J. Localization of discontinuous epitopes of herpes simplex virus glycoprotein D: use of a nondenaturing (“native” gel) system of polyacrylamide gel electrophoresis coupled with Western blotting. J Virol. 1986;60:157–166. doi: 10.1128/jvi.60.1.157-166.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis-Poynter N, Bell S, Minson T, Browne H. Analysis of the contribution of herpes simplex virus type 1 membrane proteins to the induction of cell-cell fusion. J Virol. 1994;68:7586–7590. doi: 10.1128/jvi.68.11.7586-7590.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desai P J, Schaffer P A, Minson A C. Excretion of non-infectious virus particles lacking glycoprotein H by a temperature-sensitive mutant of herpes simplex virus type 1: evidence that gH is essential for virion infectivity. J Gen Virol. 1988;69:1147–56. doi: 10.1099/0022-1317-69-6-1147. [DOI] [PubMed] [Google Scholar]
- 9.Dubin G, Jiang H. Expression of herpes simplex virus type 1 glycoprotein L (gL) in transfected mammalian cells: evidence that gL is not independently anchored to cell membranes. J Virol. 1995;69:4564–4568. doi: 10.1128/jvi.69.7.4564-4568.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisenberg R J, Ponce de Leon M, Friedman H M, Fries L F, Frank M M, Hastings J C, Cohen G H. Complement component C3b binds directly to purified glycoprotein C of herpes simplex virus types 1 and 2. Microb Pathog. 1987;3:423–435. doi: 10.1016/0882-4010(87)90012-x. [DOI] [PubMed] [Google Scholar]
- 11.Eisenberg R J, Ponce de Leon M, Pereira L, Long D, Cohen G H. Purification of glycoprotein gD of herpes simplex virus types 1 and 2 by use of monoclonal antibody. J Virol. 1982;41:1099–1104. doi: 10.1128/jvi.41.3.1099-1104.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foa-Tomasi L, Avitabile E, Boscaro A, Brandimarti R, Gualandri R, Manservigi R, Dall’Olio F, Serafini-Cessi F, Fiume G C. Herpes simplex virus (HSV) glycoprotein H is partially processed in a cell line that expresses the glycoprotein and fully processed in cells infected with deletion or ts mutants in the known HSV glycoproteins. Virology. 1991;180:474–482. doi: 10.1016/0042-6822(91)90061-f. [DOI] [PubMed] [Google Scholar]
- 13.Forghani B, Ni L, Grose C. Neutralization epitope of the varicella-zoster virus gH:gL glycoprotein complex. Virology. 1994;199:458–462. doi: 10.1006/viro.1994.1145. [DOI] [PubMed] [Google Scholar]
- 14.Forrester A, Farrell H, Wilkinson G, Kaye J, Davis-Poynter N, Minson T. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J Virol. 1992;66:341–348. doi: 10.1128/jvi.66.1.341-348.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forrester A J, Sullivan V, Simmons A, Blacklaws B A, Smith G L, Nash A A, Minson A C. Induction of protective immunity with antibody to herpes simplex virus type 1 glycoprotein H (gH) and analysis of the immune response to gH expressed in recombinant vaccinia virus. J Gen Virol. 1991;72:369–375. doi: 10.1099/0022-1317-72-2-369. [DOI] [PubMed] [Google Scholar]
- 16.Friedman H M, Cohen G H, Eisenberg R J, Seidel C A, Cines D B. Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature (London) 1984;309:633–635. doi: 10.1038/309633a0. [DOI] [PubMed] [Google Scholar]
- 17.Fuller A O, Lee W-C. Herpes simplex virus type 1 entry through a cascade of virus-cell interactions requires different roles of gD and gH in penetration. J Virol. 1992;66:5002–5012. doi: 10.1128/jvi.66.8.5002-5012.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuller A O, Santos R E, Spear P G. Neutralizing antibodies specific for glycoprotein H of herpes simplex virus permit viral attachment to cells but prevent penetration. J Virol. 1989;63:3435–3443. doi: 10.1128/jvi.63.8.3435-3443.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galdiero M, Whiteley A, Bruun B, Bell S, Minson T, Browne H. Site-directed and linker insertion mutangenesis of herpes simplex virus type 1 glycoprotein H. J Virol. 1997;71:2163–2170. doi: 10.1128/jvi.71.3.2163-2170.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghiasi H, Kaiwar R, Nesburn A B, Slanina S, Wechsler S L. Expression of seven herpes simplex virus type 1 glycoproteins (gB, gC, gD, gE, gG, gH, and gI): comparative protection against lethal challenge in mice. J Virol. 1994;68:2118–2126. doi: 10.1128/jvi.68.4.2118-2126.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghiasi H, Kaiwar R, Slanina S, Nesburn A B, Wechsler S L. Expression and characterization of baculovirus expressed herpes simplex virus type 1 glycoprotein L. Arch Virol. 1994;138:199–212. doi: 10.1007/BF01379126. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein D J, Weller S K. An ICP6::LacZ insertional mutagen is used to demonstrate that the UL52 gene of herpes simplex virus type 1 is required for virus growth and DNA synthesis. J Virol. 1988;62:2970–2977. doi: 10.1128/jvi.62.8.2970-2977.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gompels U A, Carss A L, Saxby C, Hancock D C, Forrester A, Minson A C. Characterization and sequence analyses of antibody-selected antigenic variants of herpes simplex virus show a conformationally complex epitope on glycoprotein H. J Virol. 1991;65:2393–2401. doi: 10.1128/jvi.65.5.2393-2401.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gompels U A, Craxton M A, Honess R W. Conservation of glycoprotein H (gH) in herpesviruses: nucleotide sequence of the gH gene from herpesvirus saimiri. J Gen Virol. 1988;69:2819–2829. doi: 10.1099/0022-1317-69-11-2819. [DOI] [PubMed] [Google Scholar]
- 25.Gompels U A, Minson A C. Antigenic properties and cellular localization of herpes simplex virus glycoprotein H synthesized in a mammalian cell expression system. J Virol. 1989;63:4744–4755. doi: 10.1128/jvi.63.11.4744-4755.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Handler C G, Eisenberg R J, Cohen G H. Oligomeric structure of glycoproteins in herpes simplex virus type 1. J Virol. 1996;70:6067–6075. doi: 10.1128/jvi.70.9.6067-6070.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Highlander S L, Sutherland S L, Gage P J, Johnson D C, Levine M, Glorioso J C. Neutralizing monoclonal antibodies specific for herpes simplex virus glycoprotein D inhibit virus penetration. J Virol. 1987;61:3356–3364. doi: 10.1128/jvi.61.11.3356-3364.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huber M T, Compton T. Characterization of a novel third member of the human cytomegalovirus glycoprotein H-glycoprotein L complex. J Virol. 1997;71:5391–5398. doi: 10.1128/jvi.71.7.5391-5398.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hutchinson L, Browne H, Wargent V, Davis-Poynter N, Primorac S, Goldsmith K, Minson A C, Johnson D C. A novel herpes simplex virus glycoprotein, gL, forms a complex with glycoprotein H (gH) and affects normal folding and surface expression of gH. J Virol. 1992;66:2240–2250. doi: 10.1128/jvi.66.4.2240-2250.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isola V J, Eisenberg R J, Siebert G R, Heilman C J, Wilcox W C, Cohen G H. Fine mapping of antigenic site II of herpes simplex virus glycoprotein D. J Virol. 1989;63:2325–2334. doi: 10.1128/jvi.63.5.2325-2334.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson D C, Burke R L, Gregory T. Soluble forms of herpes simplex virus glycoprotein D bind to a limited number of cell surface receptors and inhibit virus entry into cells. J Virol. 1990;64:2569–2576. doi: 10.1128/jvi.64.6.2569-2576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaye J F, Gompels U A, Minson A C. Glycoprotein H of human cytomegalovirus (HCMV) forms a stable complex with the HCMV UL115 gene product. J Gen Virol. 1992;73:2693–2698. doi: 10.1099/0022-1317-73-10-2693. [DOI] [PubMed] [Google Scholar]
- 33.Klupp B G, Baumeister J, Karger A, Visser N, Mettenleiter T C. Identification and characterization of a novel structural glycoprotein in pseudorabies virus, gL. J Virol. 1994;68:3868–3878. doi: 10.1128/jvi.68.6.3868-3878.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Q, Turk S M, Hutt-Fletcher L M. The Epstein-Barr virus (EBV) BZLF2 gene product associates with the gH and gL homologs of EBV and carries an epitope critical to infection of B cells but not of epithelial cells. J Virol. 1995;69:3987–3994. doi: 10.1128/jvi.69.7.3987-3994.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu D X, Gompels U A, Foa-Tomasi L, Campadelli-Fiume G. Human herpesvirus-6 glycoprotein H and L homologs are components of the gp100 complex and the gH external domain is the target for neutralizing monoclonal antibodies. Virology. 1993;197:12–22. doi: 10.1006/viro.1993.1562. [DOI] [PubMed] [Google Scholar]
- 36.Liu D X, Gompels U A, Nicholas J, Lelliott C. Identification and expression of the human herpesvirus 6 glycoprotein H and interaction with an accessory 40K glycoprotein. J Gen Virol. 1993;74:1847–1857. doi: 10.1099/0022-1317-74-9-1847. [DOI] [PubMed] [Google Scholar]
- 37.Long D, Madara T J, Ponce de Leon M, Cohen G H, Montgomery P C, Eisenberg R J. Glycoprotein D protects mice against lethal challenge with herpes simplex virus types 1 and 2. Infect Immun. 1984;37:761–764. doi: 10.1128/iai.43.2.761-764.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGeoch D J, Davison A J. DNA sequence of the herpes simplex virus type 1 gene encoding glycoprotein gH, and identification of homologues in the genomes of varicella-zoster virus and Epstein-Barr virus. Nucleic Acids Res. 1986;14:4281–4292. doi: 10.1093/nar/14.10.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller N, Hutt-Fletcher L M. A monoclonal antibody to glycoprotein gp85 inhibits fusion but not attachment of Epstein-Barr virus. J Virol. 1988;62:2366–2372. doi: 10.1128/jvi.62.7.2366-2372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montgomery R I, Warner M S, Lum B J, Spear P G. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 41.Muggeridge M I, Roberts S R, Isola V J, Cohen G H, Eisenberg R J. Herpes simplex virus. In: Van Regenmortel M H V, Neurath A R, editors. Immunochemistry of viruses. II. The basis for serodiagnosis and vaccines. Amsterdam, The Netherlands: Elsevier Biochemical Press; 1990. pp. 459–481. [Google Scholar]
- 42.Nicola A V, Peng C, Lou H, Cohen G H, Eisenberg R J. Antigenic structure of soluble herpes simplex virus (HSV) glycoprotein D correlates with inhibition of HSV infection. J Virol. 1997;71:2940–2946. doi: 10.1128/jvi.71.4.2940-2946.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nicola A V, Willis S H, Naidoo N N, Eisenberg R J, Cohen G H. Structure-function analysis of soluble forms of herpes simplex virus glycoprotein D. J Virol. 1996;70:3815–3822. doi: 10.1128/jvi.70.6.3815-3822.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Novotny M J, Parish M L, Spear P G. Variability of herpes simplex virus 1 gL and anti-gL antibodies that inhibit cell fusion but not viral infectivity. Virology. 1996;221:1–13. doi: 10.1006/viro.1996.0347. [DOI] [PubMed] [Google Scholar]
- 45.Peeters B, de Wind N, Broer R, Gielkens A, Moormann R. Glycoprotein H of pseudorabies virus is essential for entry and cell-to-cell spread of the virus. J Virol. 1992;66:3888–3892. doi: 10.1128/jvi.66.6.3888-3892.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roberts S R, Ponce de Leon M, Cohen G H, Eisenberg R J. Analysis of the intracellular maturation of the herpes simplex virus type 1 glycoprotein gH in infected and transfected cells. Virology. 1991;184:609–624. doi: 10.1016/0042-6822(91)90431-a. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez J E, Moninger T, Grose C. Entry and egress of varicella virus blocked by same anti-gH monoclonal antibody. Virology. 1993;196:840–844. doi: 10.1006/viro.1993.1543. [DOI] [PubMed] [Google Scholar]
- 48.Roop C, Hutchinson L, Johnson D C. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J Virol. 1993;67:2285–2297. doi: 10.1128/jvi.67.4.2285-2297.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Showalter S D, Zweig M, Hampar B. Monoclonal antibodies to herpes simplex virus type 1 proteins, including the immediate-early protein ICP 4. Infect Immun. 1981;34:684–692. doi: 10.1128/iai.34.3.684-692.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simmons A, Nash A A. Role of antibody in primary and recurrent herpes simplex virus infection. J Virol. 1985;53:944–948. doi: 10.1128/jvi.53.3.944-948.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simmons A, Nash A A. Zosterform spread of herpes simplex as a model of recrudescence and its use to investigate the role of immune cells in prevention of recurrent disease. J Virol. 1984;52:816–821. doi: 10.1128/jvi.52.3.816-821.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sisk W P, Bradley J D, Leipold R J, Stoltzfus A M, Ponce de Leon M, Hilf M, Peng C, Cohen G H, Eisenberg R J. High-level expression and purification of secreted forms of herpes simplex virus type 1 glycoprotein gD synthesized by baculovirus-infected insect cells. J Virol. 1994;68:766–775. doi: 10.1128/jvi.68.2.766-775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spaete R R, Perot K, Scott P I, Nelson J A, Stinski M F, Pachl C. Coexpression of truncated human cytomegalovirus gH with the UL115 gene product or the truncated human fibroblast growth factor receptor results in transport of gH to the cell surface. Virology. 1993;193:853–861. doi: 10.1006/viro.1993.1194. [DOI] [PubMed] [Google Scholar]
- 54.Spear P G. Membrane fusion induced by herpes simplex virus. In: Bentz J, editor. Viral fusion mechanisms. Boca Raton, Fla: CRC Press, Inc.; 1993. pp. 201–232. [Google Scholar]
- 55.Stokes A, Alber D G, Greensill J, Amellal B, Carvalho R, Taylor L A, Doel T R, Killington R A, Halliburton I W, Meredith D W. The expression of the proteins of equine herpesvirus 1 which share homology with herpes simplex virus 1 glycoproteins H and L. Virus Res. 1996;40:91–107. doi: 10.1016/0168-1702(95)01256-7. [DOI] [PubMed] [Google Scholar]
- 56.Straus S E, Savarese B, Tigges M, Freifeld A G, Krause P R, Margolis D M, Meier J L, Paar D P, Adair S F, Dina D, Dekker C, Burke R L. Induction and enhancement of immune responses to herpes simplex virus type 2 in humans by use of a recombinant glycoprotein D vaccine. J Infect Dis. 1993;167:1045–1052. doi: 10.1093/infdis/167.5.1045. [DOI] [PubMed] [Google Scholar]
- 57.Tal-Singer R, Peng C, Ponce de Leon M, Abrams W R, Banfield B W, Tufaro F, Cohen G H, Eisenberg R J. The interaction of herpes simplex virus glycoprotein gC with mammalian cell surface molecules. J Virol. 1995;69:4471–4483. doi: 10.1128/jvi.69.7.4471-4483.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Drunen Lillel-van den Hurk S, Khattar S, Tikoo S K, Babiuk A, Baranowski E, Plainchamp D, Thiry E. Glycoprotein H (gII/gp108) and glycoprotein L form a functional complex which plays a role in penetration, but not in attachment, of bovine herpesvirus1. J Gen Virol. 1996;77:1515–1520. doi: 10.1099/0022-1317-77-7-1515. [DOI] [PubMed] [Google Scholar]
- 59.Westra D F, Glazenburg K L, Harmsen M C, Tiran A, Scheffer A J, Welling G W, The T H, Welling-Wester S. Glycoprotein H of herpes simplex virus type 1 requires glycoprotein L for transport to the surfaces of insect cells. J Virol. 1997;71:2285–2291. doi: 10.1128/jvi.71.3.2285-2291.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Whitbeck J C, Peng C, Lou H, Xu R, Willis S H, Ponce de Leon M, Peng T, Nicola A V, Montgomery R I, Warner M S, Soulika A M, Spruce L A, Moore W T, Lambris J D, Spear P G, Cohen G H, Eisenberg R J. Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of the TNFR superfamily and a mediator of HSV entry. J Virol. 1997;71:6083–6093. doi: 10.1128/jvi.71.8.6083-6093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilson D W, Davis-Poynter N, Minson A C. Mutations in the cytoplasmic tail of herpes simplex virus glycoprotein H suppress cell fusion by a syncytial strain. J Virol. 1994;68:6985–6993. doi: 10.1128/jvi.68.11.6985-6993.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]