Abstract
Despite advances in understanding the development and progression of cancer in recent years, there remains a lack of comprehensive characterization of the cancer glycoproteome. Glycoproteins play an important role in medicine and are involved in various human disease conditions including cancer. Glycan-moieties participate in fundamental cancer processes like cell signaling, invasion, angiogenesis, and metastasis. Aberrant N-glycosylation significantly impacts cancer processes and targeted therapies in clinic. Therefore, understanding N-glycosylation in a tumor is essential for comprehending disease progression and discovering anti-cancer targets and biomarkers for therapy monitoring and diagnosis. This review presents the fundamental process of protein N-glycosylation and summarizes glycosylation changes in tumor cells, including increased terminal sialylation, N-glycan branching, and core-fucosylation. Also, the role of N-glycosylation in tumor signaling pathways, migration, and metabolism are discussed. Glycoproteins and glycopeptides as potential biomarkers for early detection of cancer based on site specificity have been introduced. Collectively, understanding and exploring the cancer glycoproteome, along with its role in medicine, implication in cancer and other human diseases, highlights the significance of N-glycosylation in tumor processes, necessitating further research for potential anti-cancer targets and biomarkers.
Key words: N-Glycosylated proteins, Cancer signaling, Cancer angiogenesis, Cancer metabolism, Cancer biomarkers
Graphical abstract
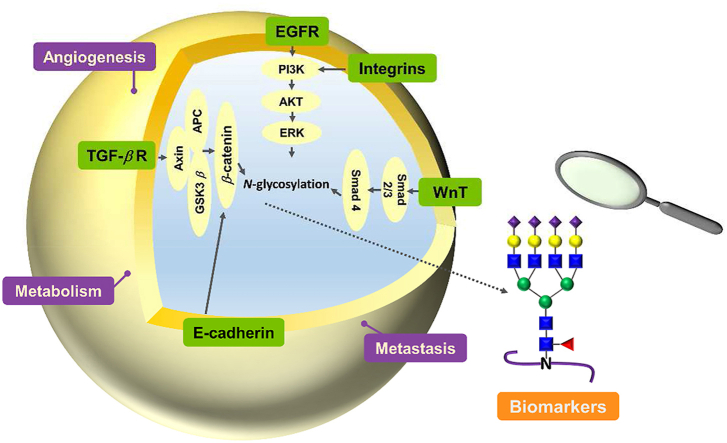
Glycosylation has a great impact on cancer biology involving signaling, tumor invasion, metastasis, metabolism and angiogenesis, as well as contributes to biomarker discovery.
1. Introduction
Cancer is widely recognized as one of the most life threatening diseases globally, with 10 million deaths in 2020, accounting for nearly one in six deaths1. Currently, therapeutic failures frequently arise from late-stage tumor detection, tumor recurrence, metastasis and drug resistance, all of which are associated with protein glycosylation. Thus, the discovery of early-stage cancer biomarkers and identification of specific targets for therapies based on protein glycosylation are crucial. Understanding and identifying the alterations in protein glycosylation in cancer cells could be the key to unraveling tumorigenesis, biomarker discovery and anti-cancer drug design.
Over the past two decades, there have been significant advances in the understanding of protein glycosylation, which involves peptides with diverse and heterogeneous glycan structures attached. Unlike protein and nucleic acid synthesis, glycan synthesis is a non-template catalytic process that requires coordinated involvement of multiple enzymes, leading to numerous significant discoveries in the field. Approximately 50% of proteins are estimated to be glycosylated2.
The biosynthetic capacity of glycans is believed to depend on the abundance of glycosylation enzymes and the sugar donors. Most glycoproteins are translated and pre-matured within the endoplasmic reticulum (ER) lumen, whereas part of the glycosylated proteins needs to be further trimmed in the Golgi apparatus. Matured glycoproteins are then assigned to different sites, such as the cell membrane or are secreted outside the cell. Protein glycosylation plays an important role in numerous intracellular and intercellular activities, such as protein folding, cell adhesion, cell–cell recognition, and host-pathogen recognition among others.
Accumulating evidence suggests that atypical glycosylation of glycoproteins, such as truncated glycan structures, multiple branching N-glycans, core fucosylation and sialylated termination increase are associated with the malignant cell transformation and metastasis. Differences in serum glycoproteins between tumor and normal patients hold promise for identifying tumor biomarkers. Certain atypical glycoproteins like core fucosylation of AFP and the sialyl Lewis A antigen (SLeA) of carbohydrate antigen 19-9 (CA19-9) are being used as serological cancer markers in clinical settings. However, their clinical application is limited, and many specific processes are still to be well-defined. This review aims to provide a clear understanding of the fundamental concepts of protein N-glycosylation in cancer and discuss its impact on cancer biology, as well as novel glycoproteins/glycopeptides as potential cancer biomarkers.
2. Glycosylation and glycan changes in cancer
It is common for cell membrane proteins and secretory proteins to be decorated by glycosylation. Protein glycosylation involves the attachment of glycans onto the immature proteins which occur in the ER and then they are trimmed in the Golgi apparatus, where the reactions are performed by a series of glycosyltransferase and glycosidase enzymes.
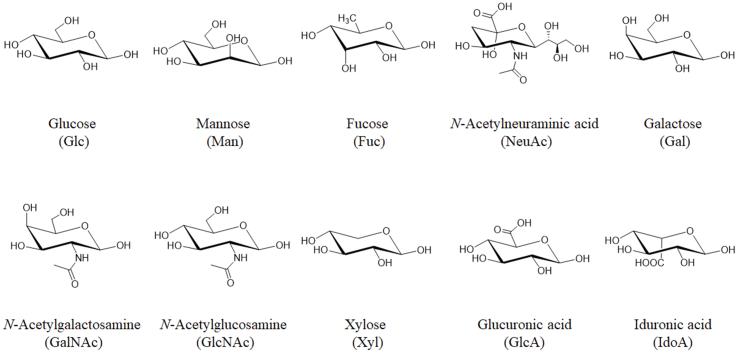
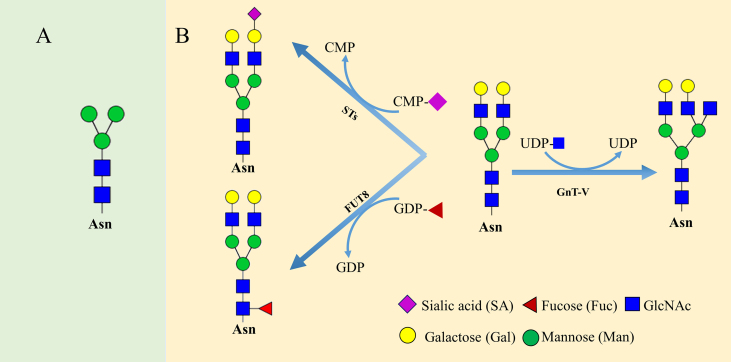
There are two main types of protein glycosylation according to the attached amino acid residues: (1) the glycan covalently attached to the Asn residue forms a typical consensus motif N-X-S/T (X≠P) termed N-glycan, and (2) O-glycosylation linked to the hydroxyl group of side chains from serine or threonine residues. Glycosylation occurs during the process where proteins go through the ER and Golgi compartments after transcription. In humans the glycan structures are mainly composed of the following 10 monosaccharides: (1) N-acetyl-d-glucosamine (GlcNAc), (2) d-mannose (Man), (3) L-fucose (Fuc), (4) d-glucose (Glc), (5) d-galactose (Gal), (6) N-acetylneuraminic acid sialic acid (Neu5Ac), (7) N-acetyl-d-galactosamine (GalNAc), (8) d-glucuronic acid (GlcA), (9) l-iduronic acid (IdoA) and (10) d-xylose (Xyl) (Fig. 1). N-Glycopeptides contain the glycan at the residue Asn in a conserved amino acid sequence N-X-S/T (X≠P), with a common but conserved five monosaccharides core structure GlcNAc2Man3, also called core glycans (Fig. 2A).
Figure 1.
Structures and names of 10 monosaccharides in humans which make up glycans.
Figure 2.
(A) Five monosaccharides core structure GlcNAc2Man3. (B) Procedure of sialylation, core-fucosylation and branching of glycan chain are enzymatically catalyzed by sialyltransferases, α1,6-fucosyltransferase (FUT8) and N-acetylglucosaminyltransferase-V (GnT-V) by transferring the monosugar moieties from activated CMP-SA, GDP-Fuc and UDP-GlcNAc, respectively.
Usually, both N- and O-glycans are terminated with sialic acids. Unlike DNA synthesis, the non-template driven glycan synthesis generates the glycans structural diversity and heterogeneity. The onset of disease may exhibit different forms of glycosylation. Therefore, uncovering the changes of disease-related glycosylation is essential for a deeper understanding of disease progression. Thus, we discuss N-glycosylation, fucosylation, sialylation and multiple-branching glycosylation as well as their roles in cancer progression.
2.1. N-Glycosylation
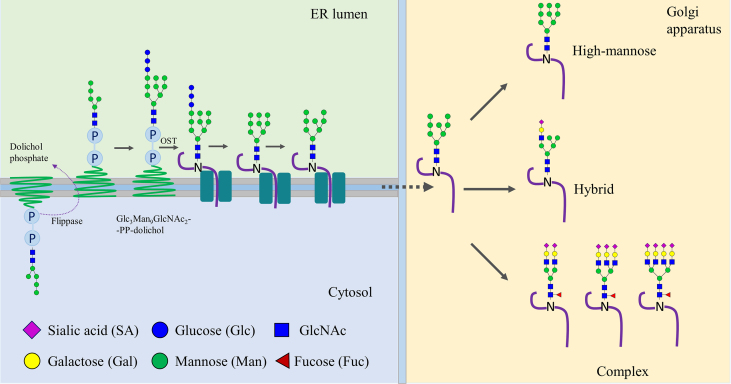
N-Linked glycosylation is a post-translational modification which plays an important role in determining the folding state and oligomerization of the protein. This process initiates in the ER where the GlcNAc2Man5-dolichol phosphate is flipped into the ER luminal side from cytosolic side by the flippase. After that, to the GlcNAc2Man5-dolichol phosphate is added four mannose and three glucose forming Glc3Man9GlcNAc2-PP-dolichol. The glycan moiety of this product is then transferred to a sequon N-X-S-T of a nascent peptide chain by oligosaccharyltransferase (OST) complex. Then three terminal glucose and one mannose are removed from the nascent glycopeptide, and the glycopeptide leaves the ER and enters to the Golgi apparatus for further trimming and branching, to produce the glycopeptide with diverse glycans (Fig. 3).
Figure 3.
N-Glycosylation initiates in the ER and is elaborated in Golgi apparatus. The N-glycosylation is started by the transfer of GlcNAc2Man5 to dolichol phosphate on the cytosolic side of ER, and then flip to the luminal side by flippase. After adding four mannose and three glucose forming Glc3Man9GlcNAc2-PP-dolichol, the glycan moiety is then transferred to a sequon N-X-S-T of a nascent peptide chain by oligosaccharyltransferase (OST) complex. Next, three terminal glucose and one mannose are removed from the nascent glycopeptide, and the glycopeptide enters the Golgi apparatus to further trimming and branching, and thus produce the glycopeptide with diverse glycans.
According to the branching of side chains, the glycan types are divided into three main groups, such as high mannose N-glycans (elongated by mannose residues), complex N-glycans (further chain elongation by adding GlcNAc in the Golgi apparatus) and hybrid N-glycans (addition of galactose or fucose residues along with mannose in the Golgi apparatus). It has been believed that N-glycosylation is performed by a series of N-acetylglucosaminyltransferases called GnTs (encoded by MGAT genes), by which the glycan blocks are extended based on the conserved core glycan. The increase of branching, and the addition of fucosylation and sialylation have been believed to be associated with tumor progression. The FUT8 (α1,6-fucosyltransferase) adds a fucose into the innermost GlcNAc in the N-glycan core by formation of an α1,6-Fuc linkage, whereas terminal sialylation acted on by sialyltransferase forms an α2,6-linkage-specific bond, as shown in Fig. 2B.
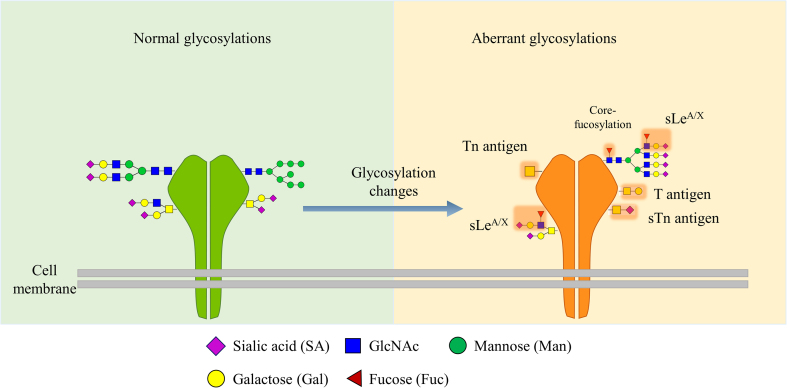
Aberrant protein glycosylation occurs in cancers involving core fucosylated, outer-arm fucosylated glycans, multiple branched N-glycans, truncated O-glycans, and multiple sialylated terminal glycans as shown in Fig. 4. The alterations in glycosylation arise from several mechanisms including the changes of expression levels of glycosyltransferases and glycosidases, and aberrant expression in glycosyltransferase localization within the secretory pathway involved in the ER and Golgi apparatus3. In addition, the changes of chaperone activity and donor substrates availability also result in the changes of glycosylation. Analysis of these changes has been used to discover biomarker candidates as the glycosylation changes of glycoproteins secreted from cancer cells into the serum. Some serum glycoproteins have been utilized for diagnosis and surveillance of cancers, for example, the CA19-9 bearing SLeA can be used for gastrointestinal tumors diagnosis, and the core-fucosylated form of AFP used for hepatocellular carcinoma diagnosis3.
Figure 4.
Changes of glycosylation during tumor progression. During malignancy, aberrant glycosylation displays abnormal expression of truncated glycans such as T antigen, Tn and their sialylated forms ST and STn, respectively, as well as the multiple branching N-glycans with terminal sialylation.
2.2. Sialylation by sialyltransferases
Sialic acid (N-acetylneuraminic acid, Neu5Ac), a nine-carbon sugar, typically occupying the terminal positions of the glycan chains of glycoproteins, is implicated in cellular interactions, and functions as a linkage molecule between the cell and its surrounding matrix, where aberrant sialylation has been shown to be involved in cancer cell adhesion and invasion. The terminal addition of sialic acid to the oligosaccharide is catalyzed by the sialyltransferases located in the Golgi apparatus, which allow glycans to have more diversity.
Accumulating evidence has shown that hypersialylation of glycoproteins is commonly found on the cancer cell surface. This increased sialylation on the cell surface glycoproteins is believed to be a signature of tumor transformation4. Increased sialylation in glycoproteins can be observed in tumor cells and is ascribed to enhanced sialyltransferase activity or reduced sialidase activity. Thus, detection and monitoring of glycoproteins from bio-fluids can be utilized for surveillance of cancer disease progression.
Although up to 20 sialyltransferases (STs) have been discovered, all involve alpha-linkage of sialic acid to N-glycan or O-glycans. It appears that each ST type can catalyze the formation of specific sialylated structures, such as sialylated Lewis antigen by ST3Gal III and ST3Gal IV. One example is CA19-9 bearing sialyl LewisA (sLeA) antigen, which can be attributed to ST3Gal III, and IV overexpression. Exploiting specific target inhibitors for STs would be a reasonable strategy for affecting tumor growth, invasion, and metastasis. For example, upregulation of STs has been shown to be associated with drug-resistant cancers treated with cisplatin or paclitaxel. Thus, these inhibitors can be used either alone or combined with current drugs to enhance their sensitivity to drug resistant cancers5.
Functionally, sialylated glycans have long been recognized to be implicated in diverse cellular interaction processes, such as cell–cell recognition and adhesion. Furthermore, they have also been reported as participants in tumor invasion and metastasis, as well as some immune responses. For example, hypersialylated integrins have been shown to take part in cancer cell migration in lung6,7, colon8 and ovarian cancers9.
2.3. Multiple-branching glycans regulated by GnT-V
Besides the sialylation by the sialyltranferases, multiple-branching glycans also contribute to cancer cells adhesion and metastasis. GnT-V (N-acetylglucosaminyltransferase-V), encoded by the MGAT5 gene in humans, extends the glycan chain branches by transfer of a GlcNAc residue from uridine diphosphate-GlcNAc (UDP-GlcNAc) into an α1,6-linked mannose in the conserved five monosaccharides core structure (GlcNac2Man3) forming a β1,6-linkage between them. However, galactose and sialic acid as well as bisected glycans from GnT-III activity, can inhibit the ability of the GnT-V to transfer the GlcNAc to the mannose of the conserved five monosaccharides core structure. Elevation of this glycan branch extension of glycoproteins on the cancer cell surface contributes to cancer invasion and metastasis, which makes GnT-V a promising anti-cancer drug target. Also, elevation of MGAT5 mRNA was observed in various cancer types, where MGAT5 gene knockout can reduce the tumor growth and metastasis10.
Dennis et al.11 in 1987 first reported the relationship between MGAT5 and cancer metastasis. Studies of cell lines and malignant human tissues demonstrated that the GnT-V increased the metastatic potential12. It was also observed that tumor cells deficient in GnT-V activity grew slowly and had lower metastatic ability than wild-type cells. Also, the mice lacking GnT-V could not add N-glycans into mannose forming β1,6-linkage so that tri-and tetra-antennary glycans were not formed. Further, the mice deficient in GnT-V could not develop normally and had autoimmune diseases, such as the enhancement of delayed-type hypersensitivity10.
The Dennis group also proposed that the glycosylation of the growth factor receptors makes them preferable for binding to lectins, such as insulin-like growth factor receptor (IGFR), epidermal growth factor receptor (EGFR), transforming growth factor β receptor (TGF-βR) and platelet-derived growth factors receptor (PDGFR). This leads to the formation of a molecular lattice composed of glycoproteins and galectins that resist glycoprotein endocytosis. Hence, the glycosylation of the growth factor receptors enhances cell signaling, migration and invasion13.
This group14 further demonstrated in epithelial cells that the number of N-glycans on proteins as well as the GlcNAc-branching activity contribute to galectin binding to N-glycans of cell membrane glycoproteins. This requires cells to have higher UDP-GlcNAc availability, the active form of the substrate for the GnT-V. Therefore, the glycoproteins on the cell membrane with a high number of N-glycan moieties, especially the growth factor receptors, such as fibroblast growth factor receptor (FGFR), IGFR, EGFR, and PDGFR, have higher galectin binding ability, and an increased expression of these glycoproteins on the cell surface would be a response to the increasing UDP-GlcNAc concentrations. Different from that, the cell surface glycoproteins bearing few N-glycan moieties, for example, TGF-β and glucose transporter4 (GLUT4), show delayed responses to the UDP-GlcNAc increase. The findings of this study demonstrated that branches of N-glycans can serve as metabolic sensors, regulating both cell growth and arrest signals. and thus inhibition of GnT-V might facilitate tumor treatment.
2.4. Core fucosylation regulated by α1,6-fucosyltransferase (FUT8)
Core fucosylation, also known as α1,6-fucosylation, is mediated by FUT8 (α1,6-fucosyltransferase), which transfers the fucose residue from GDP-fucose to the innermost GlcNAc residue of N-glycan on glycoproteins. This enzyme is the only core fucosyltransferase found in animals, and its activity is notably higher in the brain compared to other normal tissues.
Core-fucosylated glycoproteins are widely distributed in human tissues and involved in many diseases, such as hepatocellular carcinoma15,16. Studies have reported a direct association between the corresponding enzyme FUT8 expression with tumor size and lymph node metastasis. Moreover, in non-small cell lung cancer (NSCLC) cell lines, FUT8 upregulation is believed to contribute to cancer metastasis. In contrast, silencing FUT8 in CL1-5 and PC-5 which are known as aggressive lung cancer cell lines can significantly inhibit cell proliferation and invasion, as well as tumor growth and metastasis in mice17. Furthermore, microarray analyses and glycoproteomics have demonstrated that FUT8 can globally modify numerous glycoproteins involved in cancer progression, such as adhesion molecules, receptors, and surface antigens. These findings highlight the involvement of FUT8 in cancer progression through multiple mechanisms. In addition, core-fucosylated fetoprotein (AFP-L3) has been applied in the clinical diagnosis of liver cancer and several fucosylated glycopeptides show promise as potential cancer markers16. These advancements in core fucosylation research hold considerable potential for improvement in cancer diagnosis and treatment.
3. N-Glycosylation in cancer signaling
3.1. Aberrant glycosylation in signal pathways
The presence of abnormal glycosylation can trigger detrimental metabolic and cellular signals that facilitate the progression of cancer. Nevertheless, the exact molecular process remains uncertain. Abnormal glycosylation has the potential to induce erroneous cellular signaling pathways, thereby promoting tumor growth. However, the specific mechanism underlying this phenomenon has not been comprehensively elucidated. It is widely believed that changes in glycosylation, gene mutations, and genomic instability collectively play a role in initiating cancer. All these irregularities result in the initiation of oncogenic signaling pathways, including Wnt/β-catenin, Hippo signaling, phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt), Janus kinase/signal transducer and activator of transcription (JAK/STAT), the transforming growth factor-β (TGF-β/Smad), and Notch signaling. Accumulating evidence indicates that the abnormal modification of cell surface proteins, such as transmembrane proteins and growth factors receptors, lead to tumor cell growth, invasion, and metastasis through activation of these signaling cascades.
3.2. EGFR
EGFR, a member of receptor tyrosine kinases (RTKs) which include many growth receptors such as VEGFR, IGFR, and FGFR, etc.18, is activated by growth factor ligands through binding the extracellular regions of receptors inducing dimerization or oligomerization. After that, receptors then phosphorylate each other triggering the downstream cascades of phosphorylation events, such as PI3K/Akt, JAK/STAT, and MAPK pathways. These chains of events are associated with several cancer cell processes, involving cellular growth, proliferation, migration, and metabolism. There are 13 N-glyco-sites in EGFR and previous studies indicated that aberrant glycosylation in EGFR can affect the conformation of the extracellular domain of the receptors, resulting in aberrant activation and cellular signaling transmission. Small molecules have been used to disrupt the glycosylation of the RTKs involving EGFR, ErbB2, ErbB3 and IGFR. These otherwise contribute to cancer cells proliferation and survival and have been regarded as potential therapeutic targets for treatment of malignancy19,20. Therefore, RTKs are therapeutic targets for the treatment of tumors18,19.
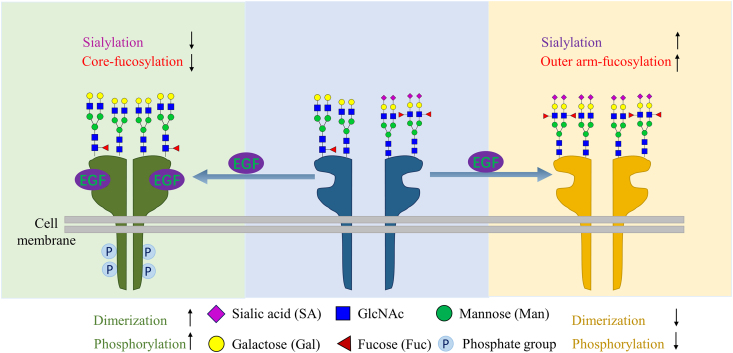
Accumulating evidence has shown that the changes in glycosylation in these RTKs, such as terminal sialylation, core or outer-arm fucosylation or oligosaccharides chain branching, can affect the dimerization or oligomerization and subsequently the RTKs activation. Sialylation in the branch terminal and fucosylation in the outer arm can suppress EGFR dimerization and subsequently inhibit the receptor activation in lung cancer cell lines, whereas an increase in core fucose by overexpression of FUT8 can promote EGFR activation as shown in Fig. 521. By comparing EGFR sialylation levels between cell lines CL1-0 and CL1-5, Liu et al.21 demonstrated that, of these two cell lines, the more invasive CL1-5 cell line has more sialylation and outer arm fucosylated N-glycans in EGFR. They found that the more terminal sialylation and/or outer arm fucosylation of EGFR suppressed the dimerization and the sequential phosphorylation even more, whereas these can be counteracted by adding sialidase and fucosidase, and treating sialidase can enhance EGF-mediated CL1-5 invasion.
Figure 5.
Core-fucosylation promotes EGFR dimerization, whereas sialylation and outer-arm fucosylation suppress the EGFR dimerization when the ligand EGF binds to the EGFR.
Different from the terminal sialylation and outer arm fucosylation, the core-fucosylation of the EGFR is required for EGFR dimerization and phosphorylation. Wang et al.22 also reported core fucosylation can regulate EGFR-mediated intracellular signaling. Their results demonstrated that the interactions between EGF and EGFR require core-fucosylation of EGFR, whereas the expression levels of EGFR did not affect this. They found that the phosphorylation of EGFR induced by EGF can be inhibited in FUT8−/− cells, whereas this blockade can be restored when the FUT8 gene contained plasmids were transfected into the FUT8−/− cells. These studies supported important roles of core-fucosylation in EGFR mediated biological functions.
However, in contrast to lung cell lines, Britain and colleagues23 reported that using 2 cell lines from ovarian cancer (OV4 and SKOV3) and BxPC3 from pancreatic cancer, the sialylation of EGFR by ST6Gal I sialyltransferase not only promotes EGFR activation but also has resistance to gefitinib-mediated cell death. At the same time, the gefitinib can induce the expression of ST6Gal I sialyltransferase. These results demonstrated that sialylation in the glycan branch terminals is associated with anti-cancer drug resistance.
The degree of branching of N-glycosylation in RTKs appears to contribute to their capability to induce or arrest cellular proliferation. For example, increasing hexosamine flux results in elevation of a number of N-glycans which can increase receptor association with galectin recognition and promotes the receptor to remain on the membrane of the cancer cell, and sequentially facilitates kinase activation14,24. Similarly, it has been documented that the MGAT5 expression product GnT-V, which contributes to the branching glycan, can promote receptor binding to galectins from the endosomes to the cell surface25. Furthermore, overexpression of MGAT5 can increase β 1,6-GlcNAc-branched N-glycans on EGFR and TGF-β receptor, thus enhancing sensitivities to their ligands, as well as promote cancer cell metastasis26. These β1,6-GlcNAc-branched N-glycans of glycoproteins can increase the binding ability to galectins, thus reducing endocytosis from the cell surface, whereas the RTKs tend to shift from the surface to the endosomes in MGAT5−/−cells27. Consistent with this, the MGAT−/− tumor cells show a non-migratory epithelial morphology, whereas MGAT+/+ display mesenchymal markers28. Beyond this, there are other factors modulating the activation of RTKs involved in the interaction with other glycosylated proteins on the cell surface29,30.
3.3. Wnt
It has been shown that Wnt signaling components are involved in cancer progression. It has been reported that the UDP-N-acetylglucosaminedolichylphosphate-N-acetylglucosamine phosphotransferase 1 (DPAGT1) is a target of the Wnt β–catenin signaling pathway, and β-catenin can bind to the promoter of DPAGT1 when Wnt is activated31. DPAGT1 is involved in the N-linked protein glycosylation pathway to catalyze the first step in dolichol-linked oligosaccharide biosynthesis, that is, transfer of GlcNAc-1-P from UDP-GlcNAc to the carrier lipid dolichyl phosphate (P-dolichol) to produce GlcNAc-P-P-dolichol. Furthermore, DPAGT1 is an upstream regulator of E-cadherin which is involved in the formation of intercellular adhesion complex and linked to cancer metastasis. Interestingly, the Wnts themselves are N-glycosylated proteins and the N-glycosylated form is required for the activation of Wnt signaling pathway32. Collectively, all of these suggest that the changes of glycosylation in Wnt signaling components affect cancer progression.
3.4. TGF-β
TGF-β is one of crucial cytokines in cellular communications and its signaling pathways are implicated in several cancer cellular functions such as cell proliferation, migration, invasion and metastasis through the Smad dependent pathway. The epithelial–mesenchymal transition (EMT) is a crucial step in cancer metastasis33, and the TGF-β is known to promote the EMT process and is tightly regulated by TGF-β receptors and co-receptors. After the TGF-β binding to the TGF-β receptor I (TβRI), TGF-β receptor II (TβRII) is recruited forming a complex. Then the serine residues in glycine–serine rich domain of TβRI are phosphorylated by TβRII resulting in the activation of TβRI kinase activity and trigger the intracellular signaling, which is regulated by Smad family proteins. Thus, TGF-β acts as an inducer of cancer progression by driving the EMT through the Smad pathway.
N-Glycosylation affects the TβRII interaction with TGF-β and controls the cell surface transport of TβRII. Kim et al.34 reported that inhibiting the N-linked glycosylation successfully blocks the binding of TGF-β to TβRII by preventing TβRII being transported to the cell surface and sequentially reducing the normal signaling transduction. Furthermore, the fully N-glycosylated form of TβRII in wild type (complex type) rather than the less processed N-glycosylation (high-mannose type or N70/94Q mutation) render TβRII more sensitive to its ligand binding on the cellular surface.
Core-fucosylation of TβRII is also required for its function whereas silencing the FUT8 by FUT8RNAi would decrease the TGF-β signaling35. Similarly, Tu et al.36 reported that FUT8 knockdown lead to invasive suppression in breast cancer cells, whereas upregulated expression of FUT8 by “gain of function” studies resulted in the EMT process, all of which are linked to the extent of TGF-β receptor. The activation of these receptors by core-fucosylation would further promote cancer cell migration and invasion.
Furthermore, the outer-arm fucosylated glycan sialyl-LewisX (sLeX) and sialyl-LewisA (sLeA) on the TGF-β receptor, regulated by FUT3 and FUT8, respectively, also affect the receptor activation. Using FUT3/FUT6 RNAi, the TβRI activation was suppressed, sequentially the phosphorylation of downstream components was also inhibited, thereby the invasion and migration of cancer cells were blocked37.
4. N-glycosylation in tumor metastasis
Metastasis is one of the major causes of death in patients with cancer, including EMT, migration, invasion, and extravasation, which finally form a metastatic tumor. In EMT the multi-step processes of tumor cell detachment from the primary place involve spreading to a secondary site, as the initial step in cancer metastasis. This process includes loss of epithelial markers and gain of mesenchymal markers, where EMT is associated with tumor invasion and poor prognosis, and EMT has been believed to be a crucial step of cancer metastasis38.
In the metastasis process, the epithelial cells undergo biological changes and attain a mesenchymal phenotype, then migrate to the secondary sites. EMT is characterized by a loss of cell adhesion ability and acquisition of cell mobility. In this context, it can be characterized by the decrease of cell adhesion molecules expression, such as the epithelial marker E-cadherin, and an increase of intermediate filament protein expression such as N-cadherin and α5β1 integrin. In this regard EMT can be believed to decrease cell adhesion and increase integrin-mediated cell-extracellular matrix adhesion. Therefore, EMT promotes metastasis of cancers and thus understanding the molecular mechanism implicated in EMT is critical for developing effective therapies for tumor growth and metastasis.
Accumulating evidence has shown that the aberrant expression of glycan epitopes is implicated in cancer metastasis. Some key glycoproteins are implicated in this complicated process, such as E-cadherin, N-cadherin, epithelial cell adhesion molecule (EpCAM), which are important in transition of normal liver cells to mesenchymal cell.
4.1. E-cadherin
Cadherins are calcium-dependent cell adhesion proteins. E-cadherin is a tumor suppressor glycoprotein with 13 glycosites and plays an inhibitory role in cancer metastasis by blocking cell migration or invasion. Loss of E-cadherin expression has been found in many human cancer cell types with increased invasiveness. It has been believed for a long time that low expression of E-cadherin is related to EMT in tumor progression, whereas E-cadherin activation inhibits tumor metastasis through reducing the number of circulating tumor cells arising from the primary tumor. Furthermore, E-cadherin at the cell surface activated by a monoclonal antibody can trigger apoptosis of tumor cells in the circulation and therefore reduce lung metastasis. This apoptosis was proposed as to be linked to the signaling pathways such as Wnt and PI3Kinase signaling pathways39.
The core-fucosylation might contribute to the conformation changes of E-cadherin which can destroy its asymmetry of dimers to suppress E-cadherin. In normal tissues, the core-fucosylated oligosaccharide is rather low. Geng et al.40 has reported that the core fucosylated E-cadherin was selectively expressed in highly metastatic lung cancer cells but less in lowly metastatic lung cancer cells. They found that α1,6-FUT (fucosyltransferase)-targeted RNAi could not affect the total expression of E-cadherin but can suppress core-fucosylation of E-cadherin. Furthermore, the RNAi of α1,6-FUT could also promote cell–cell aggregation, but it did not have impact on cells adhesion to human umbilical vein endothelial cells. The proliferation and migration of highly metastatic lung giant cancer cells (95D cells) also were inhibited by the RNAi of α1,6-FUT. These indicated that the core-fucosylation of E-cadherin plays an important role in cancer cells metastasis. Next, a molecular docking model was employed to demonstrate that the core-fucosylation of E-cadherin might destroy the 3-dimensional structure of the normal E-cadherin, and thus suppress the function of E-cadherin. Collectively, the levels of core-fucosylated E-cadherin are correlated positively with cancer metastasis, whereas this function of E-cadherin might be regulated by α1,6-FUT.
The biological functions of E-cadherin are also mediated by the cross talk of GnT-III and GnT-V enzymes. In normal epithelial cells, the E-cadherin mainly bear bisecting N-glycosylation attributed to the GnT-III enzyme, whereas its expression decreased by its promoter methylation, and the contributor of tri- and tetra-antennary glycosylation of E-cadherin and other proteins GnT-V enzyme is increased41. Bisected-glycosylation of E-cadherin helps to stabilize its position on the surface of the cell membrane preventing E-cadherin from being endocytosed, and thus promotes forming functional adherens-junctions, ultimately increased cell aggregation. In contrast, branched glycosylation enables E-cadherin dislocated from the cell surface to the cytoplasm, resulting in metastasis.
As mentioned above, sialyltransferases are implicated in E-cadherin expression. Wu et al.5 reported that activation of TGF-β in ovarian cancer cell SKOV-3 increased the ST3Gal I which subsequently reduced the E-cadherin expression. A reasonable observation is when the ST3Gal I was knocked down by RNAi, the TGF-β has no effect in the expression of E-cadherin4. This indicated that the sialyltransferases play an important role in the E-cadherin expression.
4.2. Selectins
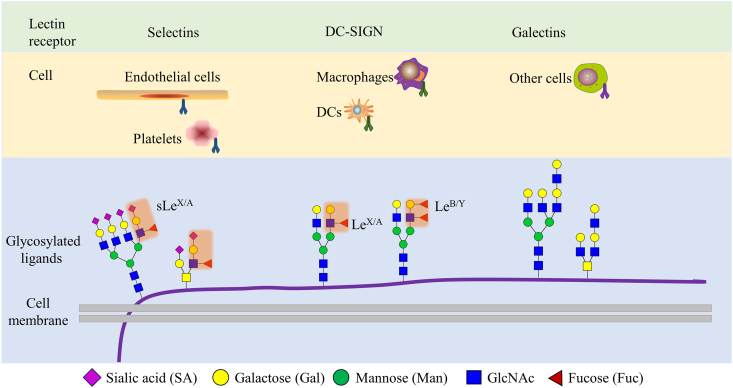
Selectins are a family of calcium-dependent transmembrane glycoproteins characterized as cell adhesion molecules which are mainly presented on the lymphocytes (L-selectin), activated platelets (P-selectin) and endothelial cells (E-selectin) surface42,43. Selectins binding to sialyl Lewis antigens, is important for endothelial cells recruiting of leukocytes and cancer cells. The sialyl Lewis antigens sLeA/X are tetra-saccharides at the end of the cell surface glycoproteins, and six fucosyltransferases (FUT3, 4, 5, 6, 7 and 9) are involved in the formation of sLeA/X 44,45.
In the case of inflammation, E- and P-selectin presented on the endothelial cell surface, can recruit the circulating immune cells bearing the sialyl Lewis antigens on the cell surface (Fig. 6), enabling immune cells to extravasate into the tissue. Similarly, it was also reported that the sialyl Lewis antigens SLeA/X on endothelial cells can help capture the circulating tumor cells and is highly linked to tumor metastasis44,45. The interaction of sialyl Lewis antigens on the cancer cell membrane and E-selectin on the endothelial cell membrane has been regarded as the first step of cancer cells extravasation entering the other tissue and forming a new metastatic lesion44. Considering these contexts, therapies targeting selectins and ligands interactions have been studied in cancer metastasis and bone marrow transplantation.
Figure 6.
Lectin recognition of glycosylation changes of tumor cells. Different lectin types expressed from cells are depicted, as well as the aberrant glycosylations in cancer cells. DC, dendritic cell; MGL, macrophage galactose-specific lectin; DC-SIGN, dendritic cell (DC)-specific ICAM-3-grabbing non-integrin.
4.3. Integrins
Integrins are a family of adhesion receptors ubiquitously expressed in almost all cell types with heterodimeric subunits where mediated cells attach to the extracellular matrix (ECM) and are involved in cell migration46, 47, 48, 49. The complete integrin receptor is composed of an α and a β subunit without a covalent bond, and there are 18 α and 8 β subunits that make up 25 combinations in humans48,50. Integrins are involved in cancer cellular signaling pathways regulation, which are associated with the receptor tyrosine kinase, such as EGFR mentioned above. It is believed that integrin binding to ECM is required for cell adhesion, migration, and proliferation. Accumulating evidence shows that N-glycosylation of integrins is implicated in its heterodimeric formation and thus has impact on its biological functions51.
A representative mesenchymal integrin is α5β1, which can modulate several cellular responses and may contribute to tumor phenotype. It has been reported that N-glycan of integrin α5 can bind to EGFR forming a stable complex, thus resulting in suppression of EGFR signaling. However, the N-glycosylation sites of the mutant integrin reversed the suppression of EGFR-mediated signaling. Thus, it further illustrates the involvement of integrin in the regulation of the EGFR signaling pathway52.
It also has been reported51,53 that the integrin functions can be modulated by sialylation of N-glycans through STs, which can enhance tumor progression as well as facilitate cancer cells to escape from apoptosis, promote invasion and metastasis, and form resistance to therapy. For example, α3β1 integrin bearing sialylated tetra-antennary glycans in metastatic A375 human melanoma cells might change cancer cell adhesive ability by reducing the ligand binding ability of α3β1 integrin54. Also, some cancer cell integrins, such as αVβ3 can be observed to interact with fibronectin, laminin and vitronectin, are associated with tumor cell invasion and metastasis.
It was thus considered that the integrin molecular species is implicated in the second stage of cancer cells adhesion to endothelial cells, when cancer cells already touch the vascular endothelial cells55. When the cancer cells adhere to endothelia, cancer cells are stimulated by cytokines from the endothelial cells, which lead to the activation of integrins on cancer cells. For example, in HepG2 cells, adding hepatocyte growth factor (HGF) into culture medium can activate α2β1 integrin leading to an increased adhesive activity to collagen-coated plates whereas this process can be blocked by anti-HGF antibody56. However, glycosylation can directly influence the interaction between integrins and ECM on the surface of cancer cells, then affecting biological functions of cancer cells, such as adhesion, migration and invasion57,58. For example, the number of N-glycans determined by GnT-V on α3β1 integrin can affect cell migration in MDA-MB-23151. Also, the increased expression of N-glycans of α5β1 integrin by GnT-V can result in cells with increased mobility, and promotion of tumor growth and invasion10,59. Therefore, it is believed that there are cross-talks among the N-glycosylated glycoproteins on the cell surface, which may control tumor cell behaviors.
5. Glycosylation for angiogenesis
The endothelial cells (ECs) cover the inner surfaces of blood vessels and release cytokines that control vascular functions. Cancer progress is closely associated with endothelial cells. To maintain rapid cell proliferation and high metabolic rates, solid tumors perform angiogenesis, which forms a network of blood vessels, to meet the tumor's needs for nutrients and to help remove metabolic waste. The angiogenic switch in growing tumor is usually triggered by hypoxia-induced expression of vascular endothelial growth factor (VEGF), leading to proliferation of normally quiescent endothelial cells. Some inducers of angiogenic signaling such as vascular endothelial growth factor receptor-2 (VEGFR-2) and Notch receptors are highly glycosylated. Aberrant glycosylation of these receptors may have wide effects on their biological activity.
The interaction of VEGF and VEGFR2 plays a central role in physiologic and pathologic angiogenesis60. VEGF can be divided into six main classes: VEGF-A, -B, -C, -D, -E and placental growth factor (PlGF), where VEGF-A is the most important member involved in angiogenesis. There are six different VEGF-A isoforms consisting of 121, 165, 189 and 206 amino acids, whereas the VEGF-A165 has the best activity of binding to the VEGFR. Once activated by VEGF, the VEGFR undergoes dimerization, phosphorylation of tyrosine residue and sequential signaling transduction. This process is quite like the EGFR activation for both of them are related to RTKs signaling.
Among the VEGFR family, VEGFR2 is the main pro-angiogenic receptor expressed by endothelial cells potentially bearing 17 N-glycosylation sites on its extracellular region which is important for its stability and activation upon VEGF-A165 binding, and thus VEGFR2 is the most important mediator of VEGF-A angiogenic activity. However, the mechanisms underlying the angiogenic activity of the glycans of VEGFR2 have not been fully uncovered.
Chiodelli et al.61 identified the VEGFR2 bearing outer-arm fucose (α,1–3/4 fucose) and terminal α2,6-linked sialic acid by various types of lectins which can interact with specific glycan structure. whereas only a terminal α2,6-linked sialic acid is required for VEGF binding to VEGFR2. Based on this, they found that the α2,6-linked sialic acid binding lectin sambucus nigra (SNA) can inhibit VEGF-dependent VEGFR2 activation and EC motility, and therefore prevented angiogenesis. This indicated that the sialylation of VEGFR2 plays a crucial role in regulating VEGF and VEGFR2 interaction, EC pro-angiogenic activation and neovascularization. In this context, inhibition of interaction of VEGF and EGFR2 by disturbing VEGFR2 sialylation should be a reasonable strategy for treatment of angiogenesis associated cancer.
However, Chandler et al.62 reported that the N-glycosylation of VEGFR2 at site N247 hindered the VEGF ligand and receptor interaction and sequential activation and signaling in endothelial cells. By removal of sialic acid from VEGFR2, with a N247Q mutation and treating the cells with α2,3-neuraminidase, this group proved that the N247 terminal α2,6 rather than α2,3-sialylation of VEGF2 can oppose VEGF2 activation whereas the asialo-glycans favor activation of VEGF2.
The results of the above studies may appear to be contradictory but perhaps the glycosylation of different Asn residues of VEGFR affects the activity of VEGFR differently. Chandler et al.62 also pointed out that the Asn145, Asn160, and Asn320 has no structure-specific role in VEGFR2 activation.
These findings indicate that glycosylation of VEGFR2 has diverse and complex effects on its activity. However, regardless of the complexity, N-glycosylation of VEGFR2 can regulate ligand-mediated activation and signaling in ECs.
Accumulating evidence demonstrated that the secreted galectin-1 and galectin-3 are involved in angiogenesis and tumor progression. The animal lectins having a carbohydrate recognition domain (CRD) composed of about 130 consensus amino acids forming two antiparallel beta-sheets that can bind to β-galactoside containing glycans, which usually can be observed in extracellular glycoproteins, such as VEGFR2. Hsieh et al.63 demonstrated that galectin-1 can bind to the VEGFR co-receptor neuropilin-1 to activate the VEGFR2, and thus result in the increase of migration and adhesion of endothelial cells. Thijssen et al.64 reported the tumor cells that secrete galectin-1 can promote tumor angiogenesis in mice. Moreover, Croci et al.65 pointed out that the α2,6-linked sialic acid can prevent galectin-1 binding to VEGFR2, whereas elimination of α2,6-linked sialic acid from VEGFR2 offered resistance to anti-VEGF, and therefore they cause the disruption of galectin-1-N-glycan axis which should be efficient for anti-VEGF treatment.
Similarly, galectin-3 is also involved in angiogenesis through binding to VEGFR2 and inducing phosphorylation of VEGFR2 and thus activating its signaling in endothelial cells66. Furthermore, the interaction of galectin-3 and VEGFR2 on the cell membrane can be observed, which is dependent on the expression of GnT-V. Knockdown of galectin-3 and GnT-V can reduce the VEGF-A mediated angiogenesis in vitro. This is due to GnT-V which can provide high affinity glycans for galectin-3. Also, knockdown of both of these two genes lead to the increase of internalized VEGFR2, which suggested the galectin-3 contributed to the VEGFR2 delaying endocytosis from the cell membrane and therefore angiogenesis.
6. Glycosylation in cancer metabolism
The main characteristic of cancer cell metabolism is the requirement for a large amount of glucose uptake to meet the high energy demands of tumor growth. Acquiring a large amount of glucose in cancer cells increases glycolysis and other metabolic pathways, such as the hexosamine pathway67. The end product of the hexosamine pathway, UDP-GlcNAc is the essential substrate involved in the N-glycosylation process, which is sensitive to the amount of UDP-GlcNAc in cancer cells. Receptors have varied numbers of N-glycan sites and the type of N-glycan structure is ultrasensitive to the amount of hexosamine flux in the Golgi apparatus where N-glycosylation of proteins is produced.
It is generally accepted that the growth arrest receptors have few N-glycosylation sites, such as TGF-β receptors, whereas receptors with high numbers of N-glycans are associated with tumor growth such as the various “growth factor receptors”. A mechanism was proposed by Lau et al.14 for the metabolic regulation of cellular transition between arrest and growth coming from cooperation of the number of N-glycosylation and branching. Therefore, metabolic flux through the hexosamine pathway could affect the stability of the receptors on the cell surface by regulating the interaction of receptors with branched glycans and galectins as discussed above. Hence, more branched-glycosylated receptors could be bound with galectins preventing endocytosis and sequentially increasing signaling68. Furthermore, in MGAT5−/− cells cultured with GlcNAc, the TGF-β receptor complex was increased, and cell surface EGFR bound to galectins can be observed. Although galectin-3 was reduced in MGAT5−/− cells, it can be rescued by GlcNAc. This indicates that the remodeling of N-glycans on the surface of cancer cells is highly sensitive to metabolism14. In conclusion, nutrient fluxes regulating complex N-glycan biosynthesis orchestrate the cellular response of cancer cells, determining the cancer cell's behavior.
7. Glycosylation proteins as biomarkers
7.1. Rationale of glycopeptides as biomarkers
Serum proteins and peptides changes have been found to be associated with cancer processes69,70. A growing body of evidence suggests that aberrant glycosylation of proteins on the cancer cell surface or glycoproteins secreted into human fluids is related to cancer progression15,16,71,72. Therefore, monitoring alteration of glycosylation of markers for early cancer detection could be used for disease surveillance during cancer development and treatment15,16,72. For example, in hepatocellular carcinoma (HCC), the fucosylation level has been shown to be relatively low in normal people but may increase significantly as the disease develops73,74. AFP-L3, a serum glycoprotein which carries a core-fucosylated (α1,6-fucosylated) glycan structure has been used widely in the clinic to monitor HCC surveillance. Recently, the fucosylation level of other proteins were studied, including alpha-1-antitrypsin (A1AT) and Hp. The fucosylation levels of these proteins were enhanced in HCC patients, thus they might be promising liver cancer biomarker candidates. In addition, differentially expressed fucosylated glycoproteins in the serum of ovarian cancer and pancreatic cancer patients was uncovered by mass spectrometry, and these fucosylated glycoproteins/glycopeptides may also serve as potential cancer biomarkers for ovarian cancer and pancreatic cancer75,76. Sialylation usually occurs in the terminal of the glycan structure, where an increase has also been considered associated with HCC. In addition, several groups have reported that the branching of glycan structure is related to HCC progression72,77,78.
7.2. Current cancer biomarkers
Some current serological biomarkers are glycoproteins which are used in the clinic for cancer diagnosis and monitoring of disease progression and therapeutic treatment. These glycoproteins include widely used biomarkers in patients with HCC [AFP, AFP-L3 and Des-gamma-carboxy prothrombin (DCP)], prostate cancer (PSA), ovarian cancer (CA125), colon cancer [CA19-9 and carcinoembryonic antigen (CEA)], pancreatic cancer (CA19-9), and breast cancer (CA15-3). They are summarized in Supporting Information Table S1. Currently, although these serological biomarkers have been widely applied in the clinic, they have serious limitations in terms of their relatively low specificity for cancer screening and potential diagnosis.
α-Fetoprotein (AFP) from human serum is widely utilized as a surveillance biomarker for HCC. It has a high specificity (around 90%) for late-stage HCCs with around 60% sensitivity, whereas the sensitivity drops down to 35% in early HCC cases79. A lectin-bound AFP, AFP-L3, containing a core-fucosylated glycan structure had been reported as more specific to HCC than AFP. The ratio of AFP-L3 to total AFP was utilized as an indicator of HCC80, but the AUC value of this variant form of AFP cannot outperform that of AFP alone. Thus, although AFP has been widely utilized in the clinic, a limitation is that it can be elevated in patients with cirrhosis, resulting in an unreliable role of AFP in surveillance for HCC. AFP-L3 alone was not sufficient for HCC detection. Another glycoprotein DCP was used as a complement to AFP-L3 in east Asia81, but still does not meet the requirements of early detection. These limitations have motivated researchers to look forward for a new generation of biomarkers based on specific glycoforms of proteins with higher sensitivity and specificity for cancer detection15,16,82, 83, 84, 85, 86, 87, 88, 89, 90. The other biomarkers such as CA125, CEA et al. are summarized in Table S1.
7.3. Promising cancer biomarkers
Several glycoproteins or site-specific glycopeptides from serum have been shown to be promising cancer biomarker candidates. For example, Golgi protein-73 (GP-73) is a transmembrane glycoprotein expressed predominantly in the biliary epithelial cells, but it was rarely detectable in hepatocytes91. However, GP-73 was elevated in hepatocytes when the patients were infected with a virus or suffered from other non-viral liver diseases, which contributes to it being a promising biomarker for early HCC diagnosis92.
Haptoglobin (Hp) is an abundant serum protein secreted primarily by the liver, where it is involved in the metabolism of renal iron where it prevents kidney damage by releasing the iron component. It contains 4 glycosites N184, N207, N211 and N241. It has been reported that Hp can be a promising marker for early detection based on the aberrant glycosylation of these sites for several cancers, especially in HCC72. Interestingly, although it has been shown that the core-fucosylated glycan is associated with different cancers, the outer-arm fucosylated form sLe antigen is the main glycoform in Hp. Zhu and colleagues et al.71,72,78,93 reported that the multiple-branching fucosylated sialylated glycopeptides were associated with HCC development.
Alpha-1-antitrypsin (A1AT) is another high abundance serum protein which is around 0.75–1.75 mg/mL in circulating blood and its concentration can reach up to 4-fold upon infection or inflammation94. By binding to elastase, A1AT's anti-trypsin activity can protect elastin in alveoli of the lung during infection where white blood cells produce the elastase which can attack the elastin. A1AT has three glycosites including, Asn70, Asn107 and Asn271, and alteration of glycosylation of A1AT is associated with NASH HCC95,96.
Vitronectin (VTNC), a 52.4 kDa glycoprotein with 459 amino acids, also called serum spreading factor belongs to the plexin family. Glycosylated VTNC has an apparent molecular mass of around 75 kDa. It is produced by hepatocytes and secreted into the blood and present in the extracellular matrix. VTNC has been regarded as a cell adhesion molecule that is involved in response to tissue injury and repair. There are three glycosites that have been found in VTNC at sites N86, N169 and N242. Lin et al.31 used the Stepped-HCD-PRM-MS/MS to evaluate the changes of glycosylation of these sites in HCC and showed that a biantennary sialylated glycopeptide involving site 169, N169_A2G2S2, and a fucosylated sialylated triantennary glycopeptide N242_A3G3F1S3 might be promising biomarkers for early-stage HCC diagnosis.
8. Conclusions
In conclusion, glycosylation of proteins plays an important role in the cancer development process. Understanding the aberrant alteration of glycosylation of tumor cells can provide a further understanding of the cancer mechanism and thus improve efficacy of targeted therapies. Thus, we presented a brief description of the abnormal N-glycosylation formation in tumor cells, then introduced the role of N-glycosylation in tumor cell signaling, metabolism and angiogenesis. This knowledge will provide promising new strategies for cancer disease in the future and thus improve the clinical outcome.
Several strategies related to clinical management appear to be particularly encouraging. Glycosylated proteins may provide novel biomarkers for therapies. The glycans in glycoproteins can provide more information than the protein alone, thus these epitopes can affect treatment responses of target proteins, such as EGFR20,97,98. Also, glycan epitopes from glycoproteins may serve for tumor subtype classification and provide a potential therapeutic for patients99.
Considering the diversity of glycoforms, targeting a specific glycoform protein on the cancer cell surface is an important aspect of glycosylation in therapeutics, which may serve as an alternative means to increase the anti-tumor specificity. Also, glycan–lectin interactions are implicated in tumor development involving cancer cell invasion, metastasis and angiogenesis. Thus, blockade of lectin-glycan recognition by specific chemicals is a promising approach where inhibitors are being developed. Finally, novel cancer biomarker candidates, such as Hp, VTNC, A1AT have shown encouraging results for early-stage cancer diagnosis. Collectively, the knowledge of glycosylation in a tumor is essential for the understanding of cancer disease progression and therefore it is also expected to serve for novel new biomarkers for cancer diagnosis.
Author contributions
Yu Lin wrote this manuscript and David M. Lubman revised this manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
We acknowledge the support of this work from the National Cancer Institute under Grants 1R01 CA160254 and U01 CA225753 (David M. Lubman, USA). David M. Lubman acknowledges support under the Maud T. Lane Professorship.
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2023.10.014.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Cancer . Feb 3, 2022. World health organization.https://www.who.int/news-room/fact-sheets/detail/cancer Available from: [Google Scholar]
- 2.Cho K.C., Chen L., Hu Y., Schnaubelt M., Zhang H. Developing workflow for simultaneous analyses of phosphopeptides and glycopeptides. ACS Chem Biol. 2019;14:58–66. doi: 10.1021/acschembio.8b00902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adamczyk B., Tharmalingam T., Rudd P.M. Glycans as cancer biomarkers. Biochim Biophys Acta Gen Subj. 2012;1820:1347–1353. doi: 10.1016/j.bbagen.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Büll C., den Brok M.H., Adema G.J. Sweet escape: sialic acids in tumor immune evasion. Biochim Biophys Acta Rev Cancer. 2014;1846:238–246. doi: 10.1016/j.bbcan.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Wu X., Zhao J., Ruan Y., Sun L., Xu C., Jiang H. Sialyltransferase ST3GAL1 promotes cell migration, invasion, and TGF-β1-induced EMT and confers paclitaxel resistance in ovarian cancer. Cell Death Dis. 2018;9:1102. doi: 10.1038/s41419-018-1101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J.Y., Tang Y.A., Huang S.M., Juan H.F., Wu L.W., Sun Y.C., et al. A novel sialyltransferase inhibitor suppresses FAK/paxillin signaling and cancer angiogenesis and metastasis pathways. Cancer Res. 2011;71:473–483. doi: 10.1158/0008-5472.CAN-10-1303. [DOI] [PubMed] [Google Scholar]
- 7.Chiang C.H., Wang C.H., Chang H.C., More S.V., Li W.S., Hung W.C. A novel sialyltransferase inhibitor AL10 suppresses invasion and metastasis of lung cancer cells by inhibiting integrin-mediated signaling. J Cell Physiol. 2010;223:492–499. doi: 10.1002/jcp.22068. [DOI] [PubMed] [Google Scholar]
- 8.Seales E.C., Jurado G.A., Brunson B.A., Wakefield J.K., Frost A.R., Bellis S.L. Hypersialylation of β1 integrins, observed in colon adenocarcinoma, may contribute to cancer progression by up-regulating cell motility. Cancer Res. 2005;65:4645–4652. doi: 10.1158/0008-5472.CAN-04-3117. [DOI] [PubMed] [Google Scholar]
- 9.Christie D.R., Shaikh F.M., Lucas JAt, Lucas J.A., 3rd, Bellis S.L. ST6Gal-I expression in ovarian cancer cells promotes an invasive phenotype by altering integrin glycosylation and function. J Ovarian Res. 2008;1:3. doi: 10.1186/1757-2215-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Granovsky M., Fata J., Pawling J., Muller W.J., Khokha R., Dennis J.W. Suppression of tumor growth and metastasis in Mgat5-deficient mice. Nat Med. 2000;6:306–312. doi: 10.1038/73163. [DOI] [PubMed] [Google Scholar]
- 11.Dennis J.W., Laferté S., Waghorne C., Breitman M.L., Kerbel R.S. β1-6 Branching of Asn-linked oligosaccharides is directly associated with metastasis. Science. 1987;236:582–585. doi: 10.1126/science.2953071. [DOI] [PubMed] [Google Scholar]
- 12.Seberger P.J., Chaney W.G. Control of metastasis by Asn-linked, β1–6 branched oligosaccharides in mouse mammary cancer cells. Glycobiology. 1999;9:235–241. doi: 10.1093/glycob/9.3.235. [DOI] [PubMed] [Google Scholar]
- 13.Partridge E.A., Le Roy C., Di Guglielmo G.M., Pawling J., Cheung P., Granovsky M., et al. Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science. 2004;306:120–124. doi: 10.1126/science.1102109. [DOI] [PubMed] [Google Scholar]
- 14.Lau K.S., Partridge E.A., Grigorian A., Silvescu C.I., Reinhold V.N., Demetriou M., et al. Complex N-glycan number and degree of branching cooperate to regulate cell proliferation and differentiation. Cell. 2007;129:123–134. doi: 10.1016/j.cell.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 15.Lin Y., Zhu J., Pan L., Zhang J., Tan Z., Olivares J., et al. A panel of glycopeptides as candidate biomarkers for early diagnosis of NASH hepatocellular carcinoma using a stepped HCD method and PRM evaluation. J Proteome Res. 2021;20:3278–3289. doi: 10.1021/acs.jproteome.1c00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin Y., Zhang J., Arroyo A., Singal A.G., Parikh N.D., Lubman D.M. A fucosylated glycopeptide as a candidate biomarker for early diagnosis of NASH hepatocellular carcinoma using a stepped HCD method and PRM evaluation. Front Oncol. 2022;12:818001. doi: 10.3389/fonc.2022.818001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen C.Y., Jan Y.H., Juan Y.H., Yang C.J., Huang M.S., Yu C.J., et al. Fucosyltransferase 8 as a functional regulator of nonsmall cell lung cancer. Proc Natl Acad Sci U S A. 2013;110:630–635. doi: 10.1073/pnas.1220425110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemmon M.A., Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Contessa J.N., Bhojani M.S., Freeze H.H., Rehemtulla A., Lawrence T.S. Inhibition of N-linked glycosylation disrupts receptor tyrosine kinase signaling in tumor cells. Cancer Res. 2008;68:3803–3809. doi: 10.1158/0008-5472.CAN-07-6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaszuba K., Grzybek M., Orłowski A., Danne R., Róg T., Simons K., et al. N-Glycosylation as determinant of epidermal growth actor receptor conformation in membranes. Proc Natl Acad Sci U S A. 2015;112:4334–4339. doi: 10.1073/pnas.1503262112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y.C., Yen H.Y., Chen C.Y., Chen C.H., Cheng P.F., Juan Y.H., et al. Sialylation and fucosylation of epidermal growth factor receptor suppress its dimerization and activation in lung cancer cells. Proc Natl Acad Sci U S A. 2011;108:11332–11337. doi: 10.1073/pnas.1107385108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X., Gu J., Ihara H., Miyoshi E., Honke K., Taniguchi N. Core fucosylation regulates epidermal growth factor receptor-mediated intracellular signaling. J Biol Chem. 2006;281:2572–2577. doi: 10.1074/jbc.M510893200. [DOI] [PubMed] [Google Scholar]
- 23.Britain C.M., Holdbrooks A.T., Anderson J.C., Willey C.D., Bellis S.L. Sialylation of EGFR by the ST6Gal-I sialyltransferase promotes EGFR activation and resistance to gefitinib-mediated cell death. J Ovarian Res. 2018;11:12. doi: 10.1186/s13048-018-0385-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanley P. A method to the madness of N-glycan complexity? Cell. 2007;129:27–29. doi: 10.1016/j.cell.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 25.Mendelsohn R., Cheung P., Berger L., Partridge E., Lau K., Datti A., et al. Complex N-glycan and metabolic control in tumor cells. Cancer Res. 2007;67:9771–9780. doi: 10.1158/0008-5472.CAN-06-4580. [DOI] [PubMed] [Google Scholar]
- 26.Cheung P., Pawling J., Partridge E.A., Sukhu B., Grynpas M., Dennis J.W. Metabolic homeostasis and tissue renewal are dependent on β1,6GlcNAc-branched N-glycans. Glycobiology. 2007;17:828–837. doi: 10.1093/glycob/cwm048. [DOI] [PubMed] [Google Scholar]
- 27.Dennis J.W., Lau K.S., Demetriou M., Nabi I.R. Adaptive regulation at the cell surface by N-glycosylation. Traffic. 2009;10:1569–1578. doi: 10.1111/j.1600-0854.2009.00981.x. [DOI] [PubMed] [Google Scholar]
- 28.Taniguchi N., Ohkawa Y., Maeda K., Harada Y., Nagae M., Kizuka Y., et al. True significance of N-acetylglucosaminyltransferases GnT-III, V and α1,6 fucosyltransferase in epithelial-mesenchymal transition and cancer. Mol Aspect Med. 2021;79 doi: 10.1016/j.mam.2020.100905. [DOI] [PubMed] [Google Scholar]
- 29.Shintani Y., Takashima S., Asano Y., Kato H., Liao Y., Yamazaki S., et al. Glycosaminoglycan modification of neuropilin-1 modulates VEGFR2 signaling. EMBO J. 2006;25:3045–3055. doi: 10.1038/sj.emboj.7601188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Julien S., Bobowski M., Steenackers A., Le Bourhis X., Delannoy P. How do gangliosides regulate RTKs signaling? Cells. 2013;2:751–767. doi: 10.3390/cells2040751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sengupta P.K., Bouchie M.P., Kukuruzinska M.A. N-Glycosylation gene DPAGT1 is a target of the Wnt/β-catenin signaling pathway. J Biol Chem. 2010;285:31164–31173. doi: 10.1074/jbc.M110.149195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Komekado H., Yamamoto H., Chiba T., Kikuchi A. Glycosylation and palmitoylation of Wnt-3a are coupled to produce an active form of Wnt-3a. Gene Cell. 2007;12:521–534. doi: 10.1111/j.1365-2443.2007.01068.x. [DOI] [PubMed] [Google Scholar]
- 33.Liu J., Ren L., Li S., Li W., Zheng X., Yang Y., et al. The biology, function, and applications of exosomes in cancer. Acta Pharm Sin B. 2021;11:2783–2797. doi: 10.1016/j.apsb.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim Y.W., Park J., Lee H.J., Lee S.Y., Kim S.J. TGF-β sensitivity is determined by N-linked glycosylation of the type II TGF-β receptor. Biochem J. 2012;445:403–411. doi: 10.1042/BJ20111923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venkatachalam M.A., Weinberg J.M. New wrinkles in old receptors: core fucosylation is yet another target to inhibit TGF-β signaling. Kidney Int. 2013;84:11–14. doi: 10.1038/ki.2013.95. [DOI] [PubMed] [Google Scholar]
- 36.Tu C.F., Wu M.Y., Lin Y.C., Kannagi R., Yang R.B. FUT8 promotes breast cancer cell invasiveness by remodeling TGF-β receptor core fucosylation. Breast Cancer Res. 2017;19:111. doi: 10.1186/s13058-017-0904-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirakawa M., Takimoto R., Tamura F., Yoshida M., Ono M., Murase K., et al. Fucosylated TGF-β receptors transduces a signal for epithelial–mesenchymal transition in colorectal cancer cells. Br J Cancer. 2014;110:156–163. doi: 10.1038/bjc.2013.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Padmanaban V., Krol I., Suhail Y., Szczerba B.M., Aceto N., Bader J.S., et al. E-cadherin is required for metastasis in multiple models of breast cancer. Nature. 2019;573:439–444. doi: 10.1038/s41586-019-1526-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Na T.-Y., Schecterson L., Mendonsa A.M., Gumbiner B.M. The functional activity of E-cadherin controls tumor cell metastasis at multiple steps. Proc Natl Acad Sci U S A. 2020;117:5931–5937. doi: 10.1073/pnas.1918167117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geng F., Shi B.Z., Yuan Y.F., Wu X.Z. The expression of core fucosylated E-cadherin in cancer cells and lung cancer patients: prognostic implications. Cell Res. 2004;14:423–433. doi: 10.1038/sj.cr.7290243. [DOI] [PubMed] [Google Scholar]
- 41.Pinho S.S., Figueiredo J., Cabral J., Carvalho S., Dourado J., Magalhães A., et al. E-cadherin and adherens-junctions stability in gastric carcinoma: functional implications of glycosyltransferases involving N-glycan branching biosynthesis, N-acetylglucosaminyltransferases III and V. Biochim Biophys Acta Gen Subj. 2013;1830:2690–2700. doi: 10.1016/j.bbagen.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 42.McEver R.P. Selectins: initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc Res. 2015;107:331–339. doi: 10.1093/cvr/cvv154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guan X. Cancer metastases: challenges and opportunities. Acta Pharm Sin B. 2015;5:402–418. doi: 10.1016/j.apsb.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li J., Guillebon A.D., Hsu Jw, Barthel S.R., Dimitroff C.J., Lee Y.F., et al. Human fucosyltransferase 6 enables prostate cancer metastasis to bone. Br J Cancer. 2013;109:3014–3022. doi: 10.1038/bjc.2013.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Esposito M., Mondal N., Greco T.M., Wei Y., Spadazzi C., Lin S.C., et al. Bone vascular niche E-selectin induces mesenchymal–epithelial transition and Wnt activation in cancer cells to promote bone metastasis. Nat Cell Biol. 2019;21:627–639. doi: 10.1038/s41556-019-0309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Desgrosellier J.S., Cheresh D.A. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campbell I.D., Humphries M.J. Integrin structure, activation, and interactions. Cold Spring Harbor Perspect Biol. 2011;3:a004994. doi: 10.1101/cshperspect.a004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ganguly K.K., Pal S., Moulik S., Chatterjee A. Integrins and metastasis. Cell Adhes Migrat. 2013;7:251–261. doi: 10.4161/cam.23840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li M., Wang Y., Li M., Wu X., Setrerrahmane S., Xu H. Integrins as attractive targets for cancer therapeutics. Acta Pharm Sin B. 2021;11:2726–2737. doi: 10.1016/j.apsb.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caswell P.T., Vadrevu S., Norman J.C. Integrins: masters and slaves of endocytic transport. Nat Rev Mol Cell Biol. 2009;10:843–853. doi: 10.1038/nrm2799. [DOI] [PubMed] [Google Scholar]
- 51.Isaji T., Im S., Kameyama A., Wang Y., Fukuda T., Gu J. A complex between phosphatidylinositol 4-kinase IIα and integrin α3β1 is required for N-glycan sialylation in cancer cells. J Biol Chem. 2019;294:4425–4436. doi: 10.1074/jbc.RA118.005208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hang Q., Isaji T., Hou S., Im S., Fukuda T., Gu J. Integrin α5 suppresses the phosphorylation of epidermal growth factor receptor and its cellular signaling of cell proliferation via N-glycosylation. J Biol Chem. 2015;290:29345–29360. doi: 10.1074/jbc.M115.682229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Büll C., Stoel M.A., den Brok M.H., Adema G.J. Sialic acids sweeten a tumor's life. Cancer Res. 2014;74:3199–3204. doi: 10.1158/0008-5472.CAN-14-0728. [DOI] [PubMed] [Google Scholar]
- 54.Pocheć E., Lityńska A., Amoresano A., Casbarra A. Glycosylation profile of integrin α3β1 changes with melanoma progression. Biochim Biophys Acta Gen Subj. 2003;1643:113–123. doi: 10.1016/j.bbamcr.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 55.Hood J.D., Cheresh D.A. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 56.Kawakami-Kimura N., Narita T., Ohmori K., Yoneda T., Matsumoto K., Nakamura T., et al. Involvement of hepatocyte growth factor in increased integrin expression on HepG2 cells triggered by adhesion to endothelial cells. Br J Cancer. 1997;75:47–53. doi: 10.1038/bjc.1997.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paszek M.J., DuFort C.C., Rossier O., Bainer R., Mouw J.K., Godula K., et al. The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature. 2014;511:319–325. doi: 10.1038/nature13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pinho S.S., Reis C.A. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015;15:540–555. doi: 10.1038/nrc3982. [DOI] [PubMed] [Google Scholar]
- 59.Guo H.B., Lee I., Kamar M., Akiyama S.K., Pierce M. Aberrant N-glycosylation of β1 integrin causes reduced α5β1 integrin clustering and stimulates cell migration. Cancer Res. 2002;62:6837–6845. [PubMed] [Google Scholar]
- 60.Claesson-Welsh L., Welsh M. VEGFA and tumour angiogenesis. J Intern Med. 2013;273:114–127. doi: 10.1111/joim.12019. [DOI] [PubMed] [Google Scholar]
- 61.Chiodelli P., Rezzola S., Urbinati C., Federici Signori F., Monti E., Ronca R., et al. Contribution of vascular endothelial growth factor receptor-2 sialylation to the process of angiogenesis. Oncogene. 2017;36:6531–6541. doi: 10.1038/onc.2017.243. [DOI] [PubMed] [Google Scholar]
- 62.Chandler K.B., Leon D.R., Kuang J., Meyer R.D., Rahimi N., Costello C.E. N-Glycosylation regulates ligand-dependent activation and signaling of vascular endothelial growth factor receptor 2 (VEGFR2) J Biol Chem. 2019;294:13117–13130. doi: 10.1074/jbc.RA119.008643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hsieh S.H., Ying N.W., Wu M.H., Chiang W.F., Hsu C.L., Wong T.Y., et al. Galectin-1, a novel ligand of neuropilin-1, activates VEGFR-2 signaling and modulates the migration of vascular endothelial cells. Oncogene. 2008;27:3746–3753. doi: 10.1038/sj.onc.1211029. [DOI] [PubMed] [Google Scholar]
- 64.Thijssen V.L., Barkan B., Shoji H., Aries I.M., Mathieu V., Deltour L., et al. Tumor cells secrete galectin-1 to enhance endothelial cell activity. Cancer Res. 2010;70:6216–6224. doi: 10.1158/0008-5472.CAN-09-4150. [DOI] [PubMed] [Google Scholar]
- 65.Croci Diego O., Cerliani Juan P., Dalotto-Moreno T., Méndez-Huergo Santiago P., Mascanfroni Ivan D., Dergan-Dylon S., et al. Glycosylation-dependent lectin-receptor interactions preserve angiogenesis in anti-VEGF refractory tumors. Cell. 2014;156:744–758. doi: 10.1016/j.cell.2014.01.043. [DOI] [PubMed] [Google Scholar]
- 66.Markowska A.I., Jefferies K.C., Panjwani N. Galectin-3 protein modulates cell surface expression and activation of vascular endothelial growth factor receptor 2 in human endothelial cells. J Biol Chem. 2011;286:29913–29921. doi: 10.1074/jbc.M111.226423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lam C., Low J.Y., Tran P.T., Wang H. The hexosamine biosynthetic pathway and cancer: current knowledge and future therapeutic strategies. Cancer Lett. 2021;503:11–18. doi: 10.1016/j.canlet.2021.01.010. [DOI] [PubMed] [Google Scholar]
- 68.Taniguchi N. A sugar-coated switch for cellular growth and arrest. Nat Chem Biol. 2007;3:307–309. doi: 10.1038/nchembio0607-307. [DOI] [PubMed] [Google Scholar]
- 69.Fan J., Huang Y., Finoulst I., Wu H.J., Deng Z., Xu R., et al. Serum peptidomic biomarkers for pulmonary metastatic melanoma identified by means of a nanopore-based assay. Cancer Lett. 2013;334:202–210. doi: 10.1016/j.canlet.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deng Z., Li Y., Fan J., Wang G., Li Y., Zhang Y., et al. Circulating peptidome to indicate the tumor-resident proteolysis. Sci Rep. 2015;5:9327. doi: 10.1038/srep09327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu J., Warner E., Parikh N.D., Lubman D.M. Glycoproteomic markers of hepatocellular carcinoma–mass spectrometry based approaches. Mass Spectrom Rev. 2019;38:265–290. doi: 10.1002/mas.21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin Y., Zhu J., Zhang J., Dai J., Liu S., Arroyo A., et al. Glycopeptides with sialyl lewis antigen in serum haptoglobin as candidate biomarkers for nonalcoholic steatohepatitis hepatocellular carcinoma using a higher-energy collision-induced dissociation parallel reaction monitoring-mass spectrometry method. ACS Omega. 2022;7:22850–22860. doi: 10.1021/acsomega.2c02600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miyoshi E., Moriwaki K., Nakagawa T. Biological function of fucosylation in cancer biology. J Biochem. 2008;143:725–729. doi: 10.1093/jb/mvn011. [DOI] [PubMed] [Google Scholar]
- 74.Tan Y., Zhu J., Gutierrez Reyes C.D., Lin Y., Tan Z., Wu Z., et al. Discovery of core-fucosylated glycopeptides as diagnostic biomarkers for early HCC in patients with NASH cirrhosis using LC-HCD-PRM-MS/MS. ACS Omega. 2023;8:12467–12480. doi: 10.1021/acsomega.3c00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tan Z., Yin H., Nie S., Lin Z., Zhu J., Ruffin M.T., et al. Large-scale identification of core-fucosylated glycopeptide sites in pancreatic cancer serum using mass spectrometry. J Proteome Res. 2015;14:1968–1978. doi: 10.1021/acs.jproteome.5b00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu J., Xie X., Liu Y., He J., Benitez R., Buckanovich R.J., et al. Identification and confirmation of differentially expressed fucosylated glycoproteins in the serum of ovarian cancer patients using a lectin array and LC‒MS/MS. J Proteome Res. 2012;11:4541–4552. doi: 10.1021/pr300330z. [DOI] [PubMed] [Google Scholar]
- 77.Yin H., An M., So Pk, Wong M.Y.M., Lubman D.M., Yao Z. The analysis of alpha-1-antitrypsin glycosylation with direct LC‒MS/MS. Electrophoresis. 2018;39:2351–2361. doi: 10.1002/elps.201700426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhu J., Huang J., Zhang J., Chen Z., Lin Y., Grigorean G., et al. Glycopeptide biomarkers in serum haptoglobin for hepatocellular carcinoma detection in patients with non-alcoholic steatohepatitis. J Proteome Res. 2020;19:3452–3466. doi: 10.1021/acs.jproteome.0c00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mechref Y., Peng W., Gautam S., Ahmadi P., Lin Y., Zhu J., et al. Mass spectrometry based biomarkers for early detection of HCC using a glycoproteomic approach. Adv Cancer Res. 2023;157:23–56. doi: 10.1016/bs.acr.2022.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aoyagi Y., Isemura M., Suzuki Y., Sekine C., Soga K., Ozaki T., et al. Fucosylated alpha-fetoprotein as marker of early hepatocellular carcinoma. Lancet. 1985;326:1353–1354. doi: 10.1016/s0140-6736(85)92643-1. [DOI] [PubMed] [Google Scholar]
- 81.Liebman H.A., Furie B.C., Tong M.J., Blanchard R.A., Lo K.J., Lee S.D., et al. Des-gamma-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N Engl J Med. 1984;310:1427–1431. doi: 10.1056/NEJM198405313102204. [DOI] [PubMed] [Google Scholar]
- 82.Sanda M., Pompach P., Brnakova Z., Wu J., Makambi K., Goldman R. Quantitative liquid chromatography-mass spectrometry-multiple reaction monitoring (LC‒MS-MRM) analysis of site-specific glycoforms of haptoglobin in liver disease. Mol Cell Proteomics. 2013;12:1294–1305. doi: 10.1074/mcp.M112.023325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pompach P., Ashline D.J., Brnakova Z., Benicky J., Sanda M., Goldman R. Protein and site specificity of fucosylation in liver-secreted glycoproteins. J Proteome Res. 2014;13:5561–5569. doi: 10.1021/pr5005482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yin H., Tan Z., Wu J., Zhu J., Shedden K.A., Marrero J., et al. Mass-selected site-specific core-fucosylation of serum proteins in hepatocellular carcinoma. J Proteome Res. 2015;14:4876–4884. doi: 10.1021/acs.jproteome.5b00718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang Y., Zhu J., Yin H., Marrero J., Zhang X., Lubman D.M. ESI–LC–MS method for haptoglobin fucosylation analysis in hepatocellular carcinoma and liver cirrhosis. J Proteome Res. 2015;14:5388–5395. doi: 10.1021/acs.jproteome.5b00792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zacharias L.G., Hartmann A.K., Song E., Zhao J., Zhu R., Mirzaei P., et al. HILIC and ERLIC enrichment of glycopeptides derived from breast and brain cancer cells. J Proteome Res. 2016;15:3624–3634. doi: 10.1021/acs.jproteome.6b00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee J., Hua S., Lee S.H., Oh M.J., Yun J., Kim J.Y., et al. Designation of fingerprint glycopeptides for targeted glycoproteomic analysis of serum haptoglobin: insights into gastric cancer biomarker discovery. Anal Bioanal Chem. 2018;410:1617–1629. doi: 10.1007/s00216-017-0811-y. [DOI] [PubMed] [Google Scholar]
- 88.Ma J., Sanda M., Wei R., Zhang L., Goldman R. Quantitative analysis of core fucosylation of serum proteins in liver diseases by LC‒MS-MRM. J Proteonomics. 2018;189:67–74. doi: 10.1016/j.jprot.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gutierrez Reyes C.D., Huang Y., Atashi M., Zhang J., Zhu J., Liu S., et al. PRM-MS quantitative analysis of isomeric N-glycopeptides derived from human serum haptoglobin of patients with cirrhosis and hepatocellular carcinoma. Metabolites. 2021;11:563. doi: 10.3390/metabo11080563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Oh M.J., Lee S.H., Kim U., An H.J. In-depth investigation of altered glycosylation in human haptoglobin associated cancer by mass spectrometry. Mass Spectrom Rev. 2021;42:496–518. doi: 10.1002/mas.21707. [DOI] [PubMed] [Google Scholar]
- 91.Marrero J.A., Romano P.R., Nikolaeva O., Steel L., Mehta A., Fimmel C.J., et al. GP73, a resident Golgi glycoprotein, is a novel serum marker for hepatocellular carcinoma. J Hepatol. 2005;43:1007–1012. doi: 10.1016/j.jhep.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 92.Mao Y., Yang H., Xu H., Lu X., Sang X., Du S., et al. Golgi protein 73 (GOLPH2) is a valuable serum marker for hepatocellular carcinoma. Gut. 2010;59:1687–1693. doi: 10.1136/gut.2010.214916. [DOI] [PubMed] [Google Scholar]
- 93.Zhu J., Chen Z., Zhang J., An M., Wu J., Yu Q., et al. Differential quantitative determination of site-specific intact N-glycopeptides in serum haptoglobin between hepatocellular carcinoma and cirrhosis using LC‒EThcD-MS/MS. J Proteome Res. 2019;18:359–371. doi: 10.1021/acs.jproteome.8b00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yin H., Lin Z., Nie S., Wu J., Tan Z., Zhu J., et al. Mass-selected site-specific core-fucosylation of ceruloplasmin in alcohol-related hepatocellular carcinoma. J Proteome Res. 2014;13:2887–2896. doi: 10.1021/pr500043k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang M., Zhu J., Lubman D.M., Gao C. Aberrant glycosylation and cancer biomarker discovery: a promising and thorny journey. Clin Chem Lab Med. 2019;57:407–416. doi: 10.1515/cclm-2018-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ogawa K., Kobayashi T., Furukawa J-i, Hanamatsu H., Nakamura A., Suzuki K., et al. Tri-antennary tri-sialylated mono-fucosylated glycan of alpha-1 antitrypsin as a non-invasive biomarker for non-alcoholic steatohepatitis: a novel glycobiomarker for non-alcoholic steatohepatitis. Sci Rep. 2020;10:321. doi: 10.1038/s41598-019-56947-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chugh S., Meza J., Sheinin Y.M., Ponnusamy M.P., Batra S.K. Loss of N-acetylgalactosaminyltransferase 3 in poorly differentiated pancreatic cancer: augmented aggressiveness and aberrant ErbB family glycosylation. Br J Cancer. 2016;114:1376–1386. doi: 10.1038/bjc.2016.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mereiter S., Magalhães A., Adamczyk B., Jin C., Almeida A., Drici L., et al. Glycomic analysis of gastric carcinoma cells discloses glycans as modulators of RON receptor tyrosine kinase activation in cancer. Biochim Biophys Acta Gen Subj. 2016;1860:1795–1808. doi: 10.1016/j.bbagen.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 99.Mereiter S., Polom K., Williams C., Polonia A., Guergova-Kuras M., Karlsson N.G., et al. The Thomsen-Friedenreich antigen: a highly sensitive and specific predictor of microsatellite instability in gastric cancer. J Clin Med. 2018;7:256. doi: 10.3390/jcm7090256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.