Abstract
Inflammatory bowel disease (IBD) commonly requires immunosuppressive treatments to induce and maintain durable remission. Janus kinase inhibitors (JAKis) are a novel group of orally administered, small molecule drugs that work by attenuating multiple cytokine signalling pathways to mediate dysregulated immune responses involved in the pathogenesis of IBD. Tofacitinib, filgotinib and upadacitinib have demonstrated efficacy against placebo and are licensed for the treatment of moderate to severe ulcerative colitis; upadacitinib is the only JAKi also currently approved for the treatment of Crohn’s disease. Safety concerns stratified by age have led to class-wide regulatory restrictions for JAKi use across all inflammatory diseases. It is important for gastroenterologists managing patients with IBD to be aware of the key pivotal trial outcomes, to identify appropriate patients in whom to commence a JAKi, and to understand the safety considerations and ways to mitigate these risks in the patients they treat. This review provides a contemporaneous overview of this emerging therapeutic class and provides a practical guide for healthcare practitioners for initiating and monitoring JAKi in IBD.
Keywords: IBD CLINICAL, INFLAMMATORY BOWEL DISEASE, ULCERATIVE COLITIS, CROHN'S DISEASE
Key points.
Janus kinase inhibitors (JAKis) modulate the effect of several key proinflammatory cytokines involved in the pathogenesis of IBD through inhibition of JAK—signal transducer and activator of transcription pathways.
JAKi offer a non-immunogenic, rapidly acting, oral option and should be considered for the treatment of moderate to severe IBD.
Three JAKis are currently approved in the UK; tofacitinib and filgotinib are licensed for ulcerative colitis (UC), while upadacitinib is licensed for both UC and Crohn’s disease.
For safety reasons, JAKi should be used at the lowest dose possible and avoided in individuals with risk factors if alternatives are available. These include; age ≥65 years, cigarette smokers or significant smoking history, risk factors for cancer, or risk factors for major adverse cardiovascular events.
JAKis are small molecules that cross the placenta and into breastmilk and preclinical studies have shown teratogenicity. JAKi should be avoided in pregnancy and breastfeeding.
Further research is required to evaluate the efficacy of JAKi in acute severe UC, how to use sequentially following prior JAKi failure, and their role in advanced combination therapy with a biologic to treat resistant disease/concomitant immune-mediated inflammatory diseases.
Janus kinase inhibitors: a novel class of orally administered drugs for the treatment of inflammatory bowel disease
Inflammatory bowel diseases (IBD), comprising ulcerative colitis (UC) and Crohn’s disease (CD), are immune-mediated inflammatory diseases (IMIDs) that typically require long-term immunosuppression. Broadly speaking, immunosuppressive therapies work in two main ways; to attenuate the signalling of one or more proinflammatory cytokines, or to prevent leucocyte migration to sites of inflammation. Treatment inefficacy and intolerance of existing therapies, however, has driven a proliferation of approved and investigational drugs over the past decade. Janus kinase inhibitors (JAKis) are a novel class of drugs in IBD and were first introduced in 2019 with the approval of tofacitinib, followed more recently by the JAK1-selective filgotinib and upadacitinib (table 1). The drug development rationale for selective JAK inhibition is to maximise therapeutic efficacy without compromising the homoeostatic functions of other JAK isoforms.
Table 1.
Currently licensed JAK inhibitors for inflammatory bowel disease
| Tofacitinib (Xeljanz) | Filgotinib (Jyseleca) | Upadacitinib (Rinvoq) | |
| Manufacturer | Pfizer | Gálapagos | AbbVie |
| Selectivity | JAK3>JAK2 >JAK1 | JAK1>JAK2 >JAK3 and TYK2 | JAK1>JAK2 and JAK3 |
| Ulcerative colitis | MHRA/FDA/EMA licensed NICE approved |
MHRA/EMA licensed NICE approved |
MHRA/FDA/EMA licensed NICE approved |
| Crohn’s disease | Unlicensed | Unlicensed | MHRA licensed NICE approved |
| Induction dose | 10 mg bd for 8–16 weeks | 200 mg od for 10–22 weeks | 45 mg od for 8–16 weeks for UC 45 mg od for 12 weeks for CD |
| Maintenance dose | 5 mg–10mg bd | 200 mg od | 15–30 mg od |
| Drug half life | 3 hours | 7 hours | 9–14 hours |
| Metabolism | 65% Hepati c(CYP3A4 and CYP2C19) | Intestinal (CES2 (primarily)] and Hepatic (CES1) | 34% Hepatic(CYP3A4 and CYP2D6) |
| Liver disease | Avoid Child-Pugh C | Avoid Child-Pugh C | Avoid Child-Pugh C |
| Renal disease | ↓ dose if CC<30 mL/min | ↓ dose if CC<60 mL/min | ↓ dose if CC<30 mL/min |
| Concomitant IMM studied | Not studied for IBD | Thiopurine and methotrexate | Methotrexate |
| Additional indications | RA, PsA, AS, JIA | RA | RA, PsA, AS, AD |
AD, atopic dermatitis; AS, ankylosing spondylitis; bd, two times per day; CC, creatinine clearance; CES, carboxylesterase; CYP, cytochrome P450; EMA, European Medicines Agency; FDA, Food and Drug Administration; IMM, immunomodulator; JAK, Janus kinase; JIA, juvenile idiopathic arthritis; MHRA, Medicines and Healthcare products Regulatory Agency; NICE, National Institute for Health and Care Excellence; od, once daily; PsA, psoriatic arthritis; RA, rheumatoid arthritis.
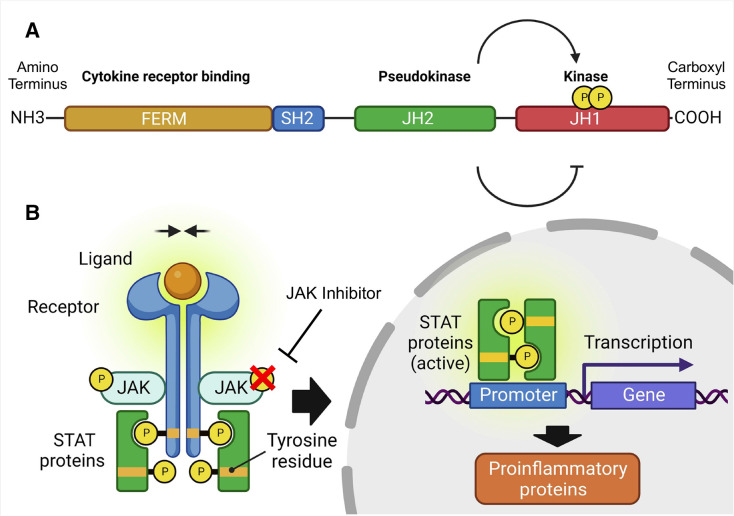
JAKs are non-receptor tyrosine kinase proteins constitutively linked with the cytoplasmic domains of type I and type II cytokine receptors.1 The four JAK isoforms mediate cytokine signalling via the signal transducer and activator of transcription (STAT) pathway for more than 50 factors, including interleukins, interferons, hormones and colony-stimulating factors that regulate a wide range of cellular processes.2 3 Inhibiting JAK phosphorylation and subsequent STAT activation leads to a broad disturbance of cytokine signalling, including pathways pivotal to intestinal homoeostasis and inflammation (figure 1). By corollary, this broad inhibitory effect can potentiate adverse events, and those seen with tofacitinib and baricitinib for the treatment of rheumatoid arthritis (RA) have led to regulatory restrictions for all JAKi across the spectrum of IMIDs.4 5
Figure 1.
The structure of a JAK protein and the mechanism of action of JAK inhibitors. (A) Functional domains of a JAK protein. This schematic displays the four functional domains common to all JAKs. The kinase domain is responsible for enzymatic activity and substrate phosphorylation while the pseudokinase domain lacks enzymatic activity but regulates the active catalytic kinase domain and is also associated with STAT recruitment. The Src-homology-2 (SH2) and FERM domains are involved in associating the JAK with the respective cell membrane receptors. Alternative nomenclature in the literature describes seven JAK homology domains (JH) numbered from the carboxyl terminus to the amino terminus. (B) JAK-STAT signal transduction and the mechanism of action of JAKi in attenuating cytokine signalling. The four members of the JAK family, JAK1, JAK2, JAK3 and Tyk2, are located on the intracellular domain of the cell surface receptor and are essential in mediating signalling downstream of cytokine receptors for a broad range of cellular processes. Numerous cytokines implicated in the pathogenesis of IBD, involved in both innate and adaptive immunity, signal through this pathway. Different cytokine-activated JAK-STAT combinations drive different cellular processes with a high degree of specificity. Extracellular cytokine binding result in conformational receptor changes, which brings the associated JAKs in close proximity and in turn, results in autophosphorylation and transphosphorylation of receptor chains. These forms docking sites for downstream STAT proteins, which after phosphorylation, receptor chains dissociation, and STAT homodimerisation or heterodimerisation, translocate to the nucleus to modulate gene transcription. JAKi reversibly bind onto the ATP site on the catalytic cleft of the kinase domain to prevent JAK phosphorylation and activation and inhibits the ensuing signalling cascade. IBD, inflammatory bowel disease; JAK, Janus kinase inhibitor. Adapted with permission using the Creative Commons Attribution 4.0 International License from Nielsen OH et al, Selective JAK1 inhibitors for the treatment of inflammatory bowel disease, Pharmacol Ther, 2023.
JAKis provide another treatment option for patients with moderate to severe IBD, particularly when failure and intolerance of existing therapies remains unacceptably high. JAKis are targeted, low molecular weight, synthetic drugs that hold a number of advantages over monoclonal antibodies (mAbs) including, oral administration, quick absorption and a rapid onset of action, short half-life and a lack of immunogenicity. This review provides a contemporary summary of the efficacy and safety data for the currently available JAKi to allow readers to take a balanced approach when considering this line of therapy for treating patients with IBD.
Tofacitinib: the first generation, non-selective JAKi licensed for UC
Tofacitinib (Xeljanz, Pfizer) is the first-in-class JAKi licensed for the treatment of moderate to severe UC. Originally developed as a JAK3 inhibitor to prevent solid organ transplant rejection, it is now considered to be a pan-JAK inhibitor with preferential selectively for JAK1 and JAK3.6 Tofacitinib gained regulatory approval for UC in 2018 and is indicated for a total of five diseases including, RA, psoriatic arthritis (PsA), juvenile idiopathic arthritis, and ankylosing spondylitis.7 Tofacitinib is not approved for CD after failing to meet the primary endpoint in the phase 2 trial.8 The OCTAVE UC phase III programme consisted of two identically designed 8-week induction studies (OCTAVE 1 and OCTAVE 2) and one maintenance 52-week study (OCTAVE SUSTAIN), which demonstrated superiority over placebo for clinical remission and several secondary endpoints.9 The more stringent definition of remission compared with earlier UC clinical trials had an additional requirement of a rectal bleeding sub score of 0. Table 2 summarises the registration trial data for approved JAKi in IBD.
Table 2.
Summary of pivotal trials for licensed JAK inhibitors in inflammatory bowel disease
| Trials | Treatment phase | n | Duration (weeks) | Primary outcome | Primary outcome findings | ||
| UC | Tofacitinib9 | OCTAVE 1 | Induction | 598 | 8 | Clinical remission* at week 8 | Clinical remission in 18.5% (tofacitinib 10 mg bd) vs 8.2% (placebo), p=0.007. |
| OCTAVE 2 | Induction | 541 | 8 | Clinical remission at week 8 | Clinical remission in 16.6% (tofacitinib 10 mg bd) vs 3.6% (placebo), p<0.001. | ||
| OCTAVE SUSTAIN | Maintenance | 593 | 52 | Clinical remission at week 52 | Clinical remission in 34.3% (tofacitinib 5 mg bd) vs 40.6% (tofacitinib 10 mg bd), vs 11.1% (placebo), p<0.001 for both comparisons with placebo. | ||
| Filgotinib23 | SELECTION STUDY A |
Induction | 659 | 10 | Clinical remission at week 10 | Clinical remission in 26.1% (filgotinib 200 mg od) vs 15.3% (placebo), p=0.0157. | |
| SELECTION STUDY B |
Induction | 689 | 10 | Clinical remission at week 10 | Clinical remission in 11.5% (filgotinib 200 mg od) vs 4.2% (placebo), p=0.0103. | ||
| SELECTION | Maintenance | 664 | 58 | Clinical remission at week 58 | Clinical remission in 37.2% (filgotinib 200 mg od) vs 11.2% (placebo), p<0.0001. Clinical remission was not significantly different between filgotinib 100 mg od and placebo at week 10 but was significant by week 58 (23.8% vs 13.5%, p=0.0420). | ||
| Upadacitinib62 | U-ACHIEVE | Induction | 474 | 8 | Clinical remission at week 8 | Clinical remission in 26% (upadacitinib 45 mg od) vs 5% (placebo), p<0.0001. | |
| U-ACCOMPLISH | Induction | 522 | 8 | Clinical remission at week 8 | Clinical remission in 34% (upadacitinib 45 mg od) vs 4% (placebo), p<0.0001. | ||
| U-ACHIEVE | Maintenance | 451 | 52 | Clinical remission at week 52 | Clinical remission in 52% (upadacitinib 30 mg od), 42% (upadacitinib 15 mg od), 12% (placebo), p<0.0001. | ||
| CD | Upadacitinib33 | U-EXCEL | Induction | 526 | 12 | Clinical remission and endoscopic response at week 12† | Clinical remission in 49.5% (upadacitinib 45 mg od) vs 29.1% (placebo) and endoscopic response in 45.5% (upadacitinib 45 mg od) vs 13.1% (placebo). p<0.001 for both comparisons. |
| U-EXCEED | Induction | 495 | 12 | Clinical remission and endoscopic response at week 12 | Clinical remission in 38.9% (upadacitinib 45 mg od) vs 21.1% (placebo) and endoscopic response in 34.6% (upadacitinib 45 mg od) vs 3.5% (placebo). p<0.001 for both comparisons. | ||
| U-ENDURE | Maintenance | 502 | 52 | Clinical remission and endoscopic response at week 52 | Clinical remission in 47.6% (upadacitinib 30 mg od) vs 37.3% (upadacitinib 15 mg od) vs 15.1% (placebo) and endoscopic response in 40.1% (upadacitinib 30 mg od) vs 27.6% (upadacitinib 15 mg od) vs 7.3% (placebo). p<0.001 for all comparisons. |
*Definition of clinical remission was similar across the UC trials and was defined as a total Mayo score of ≤2, with no subscore >1 and a rectal bleeding subscore of 0. For the filgotinib studies, a 1-point decrease in stool frequency from induction baseline for a subscore of 0 or 1 was required and for the upadacitinib studies, the Physician Global Assessment was removed due to subjectivity.
†Remission defined as CD activity index <150, and endoscopic response defined as a fall in Simple Endoscopic Score for CD score >50% from baseline.
bd, two times per day; CD, Crohn’s disease; JAK, Janus kinase; n, number of patients; od, once daily; UC, ulcerative colitis.;
Tofacitinib has been extensively studied since first entering the market for RA following Food and Drug Administration (FDA) approval in 2012.10 Databases of prescription and sales estimate that more than half a million patients have been treated globally across all indications.11 For UC, long-term clinical trial and real-world data demonstrate durable efficacy and highlight pertinent practice points, particularly with respect to dosing, which are not yet available for filgotinib and upadacitinib; safety outcomes are discussed later. In total, 1157 patients were studied through 5 randomised clinical trials, which include OCTAVE Open, a long-term extension (LTE) study of 944 patients evaluating the safety and efficacy with up to 7 years of treatment.12 First, the LTE supports long-term efficacy beyond 1 year. At 3 years, 59% and 34% of patients maintained or achieved clinical remission, with tofacitinib 5 mg two times per day and 10 mg two times per day, respectively.12 This correlates with the relatively high degree of persistence seen with a median of 5.6 years for tofacitinib responders. Second, OCTAVE Open suggests healthcare practitioners should consider an extended induction for cases of suboptimal response—in OCTAVE, response was defined as a decrease from induction-trial baseline in the total Mayo score of at least 3 points and at least 30%, with an accompanying decrease in the rectal bleeding subscore of at least 1 point or an absolute rectal bleeding subscore of 0 or 1. Here, 52% of initial non-responders after 8 weeks achieved a clinical response after an extended induction period totalling 16 weeks. At 36 months, 45% of delayed responders, that is, those who had a response at week 16 of therapy but not at week 8, maintained clinical remission. Finally, most patients in stable remission on 10 mg two times per day maintenance therapy maintained remission following dose reduction to 5 mg two times per day.13 Patients in deep endoscopic remission (endoscopic subscore 0) and those without prior anti-TNF-α failure were more likely to maintain remission on the lower dose. The wealth of real-world data that have been published are largely supportive of the efficacy data seen in the clinical trials. A recent meta-analysis of 17 studies and 1162 patients confirms tofacitinib effectiveness in a highly refractory patient population and showed pooled corticosteroid-free remission rates of 31% at 1 year.14
Filgotinib: a JAK1-selective drug approved for UC but not CD
Filgotinib (Jyseleca, Galapagos NV) received Medicines and Healthcare products Regulatory Agency (MHRA) licensing and National Institute for Health and Care Excellence (NICE) approval in 2022 for the treatment of moderate to severe UC after failure or intolerance of a biological or conventional therapy.15 16 While filgotinib is also approved for the treatment of RA in the UK, European Union and Japan, it is not available in the USA after a market authorisation application for RA was rejected by the FDA due to testicular toxicity concerns from preclinical studies. Gilead Sciences and Galapagos curtailed plans to pursue FDA authorisation despite subsequent safety trials showing no effect on semen parameters or sex hormones.17 Filgotinib is not approved for CD. Despite the favourable results of the phase II CD study (FITZROY), the induction cohorts of a large phase III study (DIVERSITY) of 1374 patients with moderate to severely active CD failed to meet the coprimary endpoints of endoscopic response and clinical remission at week 10.18 19 A phase II trial (DIVERGENCE 1) published in 2023 assessing the safety and efficacy of filgotinib in small bowel CD also did not show statistically significant differences against placebo.20
Filgotinib exhibits approximately 30-fold greater inhibition of JAK1 over JAK2-dependent and other isoform-dependent signalling, which mechanistically is thought to maximise efficacy and minimise side effects.21 22 Efficacy in UC was demonstrated in the pivotal phase IIb/III SELECTION studies for UC, which were published in the Lancet in 2021.23 Biological naïve and exposed patients were randomly assigned to receive once daily filgotinib at 100 mg or 200 mg or placebo in a 2:2:1 ratio across two 10-week induction studies; responders at the end of induction were then re-randomised into the 58-week maintenance study. The primary outcome was clinical remission as per the Mayo score defined by an endoscopic and stool frequency subscore of 0 or 1 and a rectal bleeding subscore of 0. The 200 mg but not the 100 mg once daily dose was efficacious at inducing and maintaining remission and met all prespecified primary and secondary endpoints. The SELECTION LTE study (NCT02914535) included 520 patients who completed the induction and maintenance SELECTION trial, and patients that either did not respond after the 10-week induction or patients with worsening disease during the maintenance trial.24 Interim analyses showed that 4 years of filgotinib treatment was efficacious at maintaining symptomatic remission and health-related quality of life (QoL) with mean reductions in partial Mayo clinical scores and increases in total IBDQ scores, respectively.24 Currently, there are no published real-world outcomes for the use of filgotinib in the treatment of UC but outcomes of the Galapagos-sponsored, prospective, observational study (GALOCEAN), are awaited.25
Upadacitinib: a promising JAK1-selective drug and the first to be approved for both UC and CD
Upadacitinib (Rinvoq, AbbVie) is a second-generation JAK1 selective inhibitor, and the only JAKi licensed for both moderate to severe UC and CD; MHRA licensing and NICE approval was received in 2023.26 27 Upadacitinib has six indications that also include RA, PsA, atopic dermatitis, axial spondyloarthritis.28 NICE recommends that upadacitinib is used for UC when conventional or biological therapy has been ineffective or intolerable. For CD, patients must have had prior biologic exposure or have a contraindication to anti-TNF-α therapy prior to upadacitinib use.
Progression to the phase III trial programmes was based on the success of the U-ACHIEVE and CELEST phase II dose-ranging studies for UC and CD, respectively.29 30 The phase III UC programme consisted of two replicate 8-week induction studies and a single 52-week maintenance study enrolling induction responders (table 2). The primary endpoint of clinical remission at both timepoints, based on an Adapted Mayo score that excluded Physician Global Assessment, was achieved with superiority above placebo in all three trials. For 125 patients not achieving a clinical response after 8 weeks, defined by an Adapted Mayo score decrease of ≥2 points and ≥30% from baseline, plus ≥1 point decrease in rectal bleeding score (RBS) or absolute RBS≤1, induction was extended at the same 45 mg once daily dose for another 8 weeks. Here, prolonged induction treatment for a total of 16 weeks was beneficial in almost half of UC patients (48.3%) who subsequently responded.31 All secondary endpoints in these trials were met, which included clinical, endoscopic, histological and QoL outcomes. Upadacitinib also improved faecal urgency, fatigue and abdominal pain, symptoms that impact patient QoL but are rarely assessed in UC trials.32
Marketing authorisation for upadacitinib in CD was supported by the results of the 12-week induction studies (U-EXCEL and U-EXCEED) including 1021 patients, and a 52-week maintenance study (U-ENDURE) of 502 patients.33 Three-quarters of patients had prior biological failure. The coprimary endpoints of clinical remission and endoscopic response were assessed at weeks 12 and 52. For induction, the proportion of patients in remission was nearly twice as high with upadacitinib compared with placebo and at least three times as high for endoscopic response. For maintenance, the delta above placebo was 2–3 fold higher for remission versus placebo and almost six times higher for endoscopic response (table 2). Differences of this magnitude have not been seen in prior registration CD trials.
Results from the upadacitinib trials have generated optimism in the IBD community for several reasons. First, the marked difference seen above placebo across the phase III IBD programme compared with previous advanced therapy trials cannot be discounted, even though between-trial efficacy comparisons are unwise. The differences are particularly notable given the evolution of clinical trials endpoints now incorporating the more objective endoscopic endpoints compared with the subjective symptom-based endpoints previously. Second, in the absence of head-to-head studies, network meta-analyses have repeatedly ranked upadacitinib highest for the induction of remission in UC, and the highest for maintenance of remission in CD.34–36 However, it is important to note that these meta-analyses also rank upadacitinib highest for adverse events, but not serious adverse events. Third, the rapidity of onset, as significant improvement in symptoms were seen as early as 24 hours for UC (stool frequency and rectal bleeding) and as early as 14 days (based on CD activity index) for CD.33 37 Finally, its efficacy in treating extraintestinal manifestations (EIMs) and coexisting IMIDs. In U-ENDURE, upadacitinib was more likely than placebo to resolve EIMs at the 30 mg dose. Among all JAKi, upadacitinib has the highest number of therapeutic indications for IMIDs. The U-ENDURE LTE and the prospective real-world studies PROFUNDUS (UC) and UPlift (CD) will determine the durability of efficacy and safety and its generalisation to everyday practice.38 39
Safety of JAK inhibitors
General safety considerations
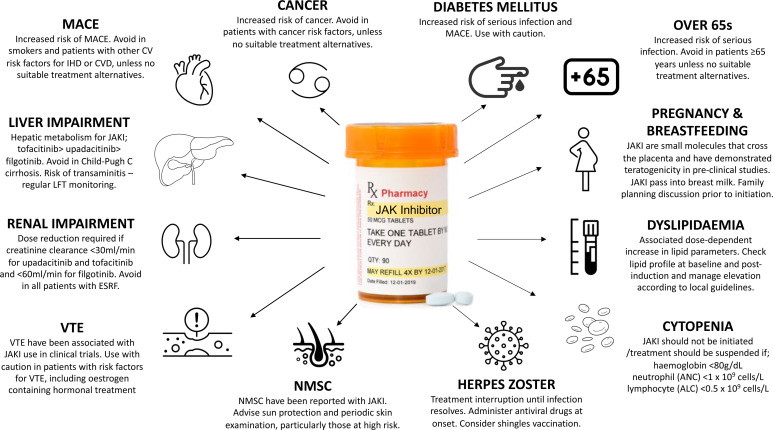
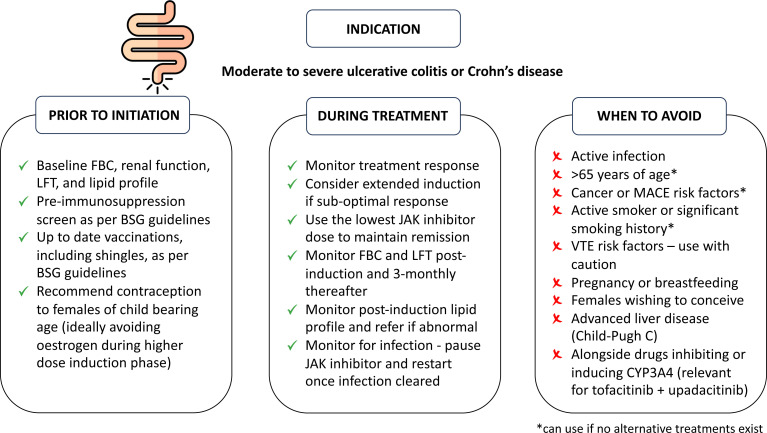
An important barrier to initiating any immunosuppressive therapy is the concern of adverse events from patients and clinicians. Most adverse events related to JAKi are mild to moderate, predictable and easy to manage. However, JAKis are also associated with more serious side effects. The randomised, open-label, noninferiority, safety study of tofacitinib (ORAL SURVEILLANCE) in 4362 patients aged ≥50 years with at least one cardiovascular disease risk factor, showed increased incident rates (IRs) of cancer and major adverse cardiovascular event (MACE) when compared with anti-TNF-α therapy.40 Similar FDA-mandated studies of baricitinib in RA are ongoing and are estimated to complete enrolment by 2025.41 It is difficult to ascertain with any certitude whether certain adverse events are class-specific, drug-specific or patient/population-specific. Figure 2 outlines how to mitigate and manage some of the potential adverse events from JAKi and figure 3 highlights important pre-initiation and postinitiation considerations of JAKi treatment and describes situations where JAKis are best avoided.42
Figure 2.
Managing potential adverse events of JAK inhibition. ALC, absolute lymphocyte count; ANC, absolute neutrophil count; CVS, cardiovascular; CVD, cerebrovascular disease; ESRF, end-stage renal failure; IHD, ischaemic heart disease; JAK, Janus kinase; LFTs, liver function tests; MACE, major adverse cardiovascular events; NMSC, non-melanoma skin cancer; VTE, venous thromboembolism.
Figure 3.
A checklist for initiating and monitoring therapy. *If no suitable alternative available. BSG, British Society of Gastroenterology; FBC, full blood count; JAKi, Janus kinase inhibitor; LFT, liver function tests; MACE, major adverse cardiovascular events; VTE, venous thromboembolism.
At baseline, all patients should have a preimmunosuppression screen for infection and be up to date with recommended vaccinations in accordance with BSG and ECCO guidelines.43 44 JAKi approved for IBD should be avoided in patients with advanced liver disease (Child-Pugh C), particularly as tofacitinib and upadacitinib are predominantly metabolised by hepatic CYP3A4. Filgotinib is metabolised by carboxylesterase-2 (CES2), is renally excreted and the creatinine clearance threshold for dose reduction is lower than other JAKi (table 1). Laboratory parameters should be monitored after induction and throughout maintenance therapy as these have been shown to be affected by JAKi. Abnormalities in blood counts and liver transaminases in the short and long-term were mild to moderate and included anaemia, leucopenia and elevations in aminotransferases (figure 2). Most patients experiencing these abnormalities were able to remain on the study drug and for patients where JAKi discontinuation was necessary, these changes resolved.12 45 46
Major adverse cardiovascular events
Chronic systemic inflammation is known to promote accelerated atherosclerosis and is a risk factor for MACE, defined by a fatal or non-fatal myocardial infarction or ischaemic stroke. In patients with IBD, this risk is modestly increased, particularly during active disease.47–49 The JAKi class is also associated with dose-dependent increases of high density lipoprotein (HDL) and low density lipoprotein (LDL) cholesterol without affecting the HDL:LDL ratio, changes that are reversible on treatment cessation.50 When compared with anti-TNF-α agents, tofacitinib has been shown to increase the incidence of MACE in RA patients, HR 1.33 (95% CI 0.91 to 1.94).40 It is important to note that the increased risk of MACE with JAKi has not been demonstrated in patients with IBD and the overall incidence is low.51 This includes interim outcomes from the LTE studies of filgotinib (SELECTION-LTE) and upadacitinib (U-ACTIVATE), which comprised 2055 and 2350 patient-years of exposure, respectively.24 52 No remarkable differences were observed in exposure-adjusted IRs of adjudicated MACE in these studies when compared with placebo. Nevertheless, steps should be taken to modify cardiovascular risk factors such as lipid-lowering and smoking cessation, and if no other treatments exist, JAKi should be avoided in high-risk populations.
Venous thromboembolism
Patients with IBD with active disease have a twofold increased risk of venous thromboembolism (VTE).53 Cases of VTE have been observed with tofacitinib, filgotinib and upadacitinib in the trial programmes and although not powered to assess VTE, there was an increased number of VTE events in ORAL SURVEILLANCE, particularly with the 10 mg two times per day tofacitinib dose. However, two large meta-analyses of 5143 and 6542 JAKi-exposed patients across all IMIDs, did not find an increased risk.54 55 It remains unclear whether JAKi have a direct causal role in the development of VTEs or whether these VTEs occur in the context of a higher baseline risk in IMIDs. For now, the regulatory guidance is to use JAKi with caution in patients with VTE risk factors.4 5
Cancer including non-melanoma skin cancer
Uncontrolled inflammation is a critical component of the neoplastic process, driving cell proliferation, survival and migration.56 Compared with the general population, IBD may be associated with an increased risk of overall cancer and cancer-specific mortality.57
ORAL SURVEILLANCE did not meet its prespecified non-inferiority criteria and tofacitinib was associated with an increased cancer incidence (mainly lymphoma and lung cancer) compared with anti-TNF-α agents, HR 1.48 (95% CI 1.04 to 2.09).40 58 In a recent meta-analysis incorporating 62 randomised controlled trials, 16 LTE studies and 82 366 person-years of JAKi exposure, risk of malignancy did not differ significantly between JAKi and placebo but was associated with a higher incidence of malignancy when compared with anti-TNF-α agents, largely influenced by ORAL SURVEILLANCE.59 Interim findings of LTE studies for filgotinib and upadacitinib showed no remarkable differences in exposure-adjusted malignancies against placebo.24 52 JAKi-induced cancers remain a rare occurrence but until this risk is precisely elucidated, JAKi should be avoided in patients with an increased cancer risk, such as those with an active or past cancer.
Aggressive squamous cell carcinomas have been reported with JAKi; previous non-melanoma skin cancer (NMSC), prior anti-TNF failure and older age, are associated with increased NMSC risk.60 61 Manufacturer guidance for JAKi suggest periodic skin examination, particularly those at higher risk of skin cancer. Reassuringly, however, the IR of NMSC across the clinical trial programmes for JAKi were low at 0.51/100 patient-years; IR among patients exposed to comparator (mainly placebo) was 0.27/100 patient-years.
Infection
The most common infections affecting the IBD-approved JAKi in the trials were nasopharyngitis, upper respiratory tract infections and influenza.9 23 33 62 The IR of serious infections across all JAKi was 2.81/100 person-years and were mainly bacterial, including pneumonias, urinary tract and skin infections.55 Rates of tuberculosis were low in regions of low to medium incidence but should be screened for preinitiation.63 The most notable viral infection was a dose-dependent increased risk of herpes zoster infection, which has been associated across the JAKi class. This may be explained by the JAK1-mediated suppression of interferon, which has an antiviral role.64 Most cases are non-serious affecting a single dermatome and did not lead to treatment withdrawal. Using the lowest JAKi dose possible and vaccination against varicella zoster reactivation are the best ways of mitigating this risk. Live vaccines are contraindicated during JAKi therapy but the inactivated shingles vaccine (Shingrix) is to be made available in the UK later in 2023 for immunocompromised patients aged ≥50 where previously it was reserved for those aged ≥70 years.65
Pregnancy and breast feeding
JAKis are small molecule drugs that cross the placenta throughout pregnancy and have recently been shown to be excreted into human breast milk.66 Preclinical studies have shown that JAKi at doses much higher than those approved for humans were teratogenic and feticidal.7 16 67 Contraception is advised for all female patients during treatment JAKi and for 1 week after the last dose of filgotinib and 4 weeks for tofacitinib and upadacitinib, as per manufacturer instructions. Breast feeding is not recommended. Outcomes from maternal tofacitinib exposure of 62 patients across the UC, RA and psoriasis trials were similar to the general population, although this finding should be interpreted with caution given the small sample size.68 69 Pregnancy outcomes for filgotinib and upadacitinib are scarce but the same principles should apply.
Restrictions to JAKi use: update from the European Medicines Agency and MHRA
Following the publication of ORAL SURVEILLANCE in early 2022, the JAKi class of drugs was placed under safety review by the Pharmacovigilance Risk Assessment Committee (PRAC), a branch of the European Medicines Agency (EMA).4 PRAC recommendations were issued in October 2022 and were endorsed and approved by the EMA’s European Commission in March 2023.4 The MHRA followed suit and issued a statement in April 2023.5 Box 1 outlines these recommendations, which apply to all JAKi indicated for chronic inflammatory diseases; fedracitinib (Inrebic) and ruxolitinib (Jakavi) for myeloproliferative diseases, and baricitinib, when used short term for the treatment of COVID-19, are exempt. There is a degree of clinical judgement with the wording of the recommendations, for example, the degree of previous smoking exposure and risk factors for cancer are open to interpretation. This is unlikely to be the end of the turbulent JAKi story; while filgotinib and upadacitinib are exempt from postmarket FDA-required studies, results from the ongoing baricitinib studies may well lead to yet another change in the recommendations and guidance.
Box 1. European Medicines Agency and Medicines and Healthcare products Regulatory Agency recommendations on Janus kinase (JAK) inhibitors use.
JAKi only to be used in the following groups at the lowest effective dose if no suitable alternative agent available:
Aged ≥65 years.
At increased risk of major cardiovascular problems (such as heart attack or stroke).
Smokers or have smoked for a long time in the past.
Increased risk of cancer.
JAKis to be used with caution in those with venous thromboembolism risk factors and at the lowest effective dose.
Future perspectives and conclusions
There are several pan-JAK and selective JAKi, including the newer TYK2 inhibitors, in various stages of development for IBD.70–72 Even with the three available options, how do we select between therapies in this increasingly crowded space? While many unanswered questions remain, factors that will guide decision-making will likely centre on reimbursement cost, available direct and indirect safety and efficacy data, number of licensed indications to treat EIMs/coexisting IMIDs, clinician familiarity and dosing regimens; filgotinib and upadacitinib are dosed once daily as the prolonged release tofacitinib tablets are unavailable in the UK. The limelight on tofacitinib’s safety profile has led to the perception that JAK-1 selective inhibitors are safer. Whether selective inhibition of a JAK isoform confers a better safety and efficacy profile remains to be seen; filgotinib and upadacitinib will not be subjected to the same FDA-mandated testing as tofacitinib and baricitinib were so longer-term studies are essential.73 Theoretically, selective JAK isoform inhibition could limit side effects, although this selectivity is dose and tissue dependent, and may be lost with increasing doses.74 Key areas of further research with direct clinical relevance include, JAKi use in advanced combination therapy with a mAb, JAKi positioning in relation to other advanced therapies, sequencing after prior JAKi failure/intolerance, mechanisms of resistance and loss of response, and its role in the treatment of acute severe UC.
JAKis are an emerging group of highly efficacious drugs used to treat inflammatory, autoimmune and myeloproliferative diseases. For IBD, they represent another key line of therapy for patients with active disease and their advantages over mAbs, the crux of IBD pharmacological therapy over the past two decades, has attracted widespread attention. The safety profile of this drug class and the regulatory restrictions in place for all JAKi for inflammatory diseases require careful benefit–risk consideration when initiating and monitoring therapy. Although these risks in absolute terms are likely to be small for the typical IBD patient population, they are not inconsequential. Personalised stratification to appropriate JAKi use is an attractive future goal.
Footnotes
Contributors: SH and KP conceived the idea. SH wrote the original draft. SH, AA, MJC, SKM and FD were involved in the planning, conduct and reporting of the work. All authors critically reviewed and edited the manuscript before submission. SH is responsible for the overall content as guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: SH has served as a speaker, a consultant and/or advisory board for Pfizer, Janssen, Abbvie and Takeda, has received educational grants from Dr. Falk Pharma, Pharmacosmos and Ferring, and has had research supported by Pfizer. SKM has received speaker fees and/or advisory board fees from Dr. Falk Pharma. KP has received honoraria for educational meetings and speaker fees from Abbvie, Janssen, Takeda, Dr. Falk Pharma, PredictImmune, Pfizer and Ferring and has received advisory board fees from Abbvie, Galapagos, Pfizer, and Janssen. AA, MJC, FD, RP and AP report no conflicts.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Rane SG, Reddy EP. Janus Kinases: components of multiple signaling pathways. Oncogene 2000;19:5662–79. 10.1038/sj.onc.1203925 [DOI] [PubMed] [Google Scholar]
- 2. Garrido-Trigo A, Salas A. Molecular structure and function of Janus Kinases: implications for the development of inhibitors. J Crohns Colitis 2020;14:S713–24. 10.1093/ecco-jcc/jjz206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hu X, li J, Fu M, et al. The JAK/STAT signaling pathway: from bench to clinic. Sig Transduct Target Ther 2021;6:1–33. 10.1038/s41392-021-00791-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. EMA . Janus kinase inhibitors (JAKi) [European Medicines Agency]. 2022. Available: https://www.ema.europa.eu/en/medicines/human/referrals/janus-kinase-inhibitors-jaki [Accessed 06 Feb 2023].
- 5. Janus kinase (JAK) inhibitors: new measures to reduce risks of major cardiovascular events, malignancy, venous thromboembolism, serious infections and increased mortality. 2023. Available: https://www.gov.uk/drug-safety-update/janus-kinase-jak-inhibitors-new-measures-to-reduce-risks-of-major-cardiovascular-events-malignancy-venous-thromboembolism-serious-infections-and-increased-mortality
- 6. Flanagan ME, Blumenkopf TA, Brissette WH, et al. Discovery of CP-690,550: a potent and selective Janus kinase (JAK) inhibitor for the treatment of autoimmune diseases and organ transplant rejection. J Med Chem 2010;53:8468–84. 10.1021/jm1004286 [DOI] [PubMed] [Google Scholar]
- 7. XELJANZ 5 mg film-coated tablets - Summary of Product Characteristics (SmPC) - (emc), Available: https://www.medicines.org.uk/emc/product/2500/smpc [Accessed 09 Jul 2023].
- 8. Sandborn WJ, Ghosh S, Panes J, et al. A phase 2 study of tofacitinib, an oral Janus kinase inhibitor, in patients with Crohn's disease. Clin Gastroenterol Hepatol 2014;12:1485–93. 10.1016/j.cgh.2014.01.029 [DOI] [PubMed] [Google Scholar]
- 9. Sandborn WJ, Su C, Panes J. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;377:496–7. 10.1056/NEJMc1707500 [DOI] [PubMed] [Google Scholar]
- 10. Traynor K. FDA Approves tofacitinib for rheumatoid arthritis. Am J Health Syst Pharm 2012;69:2120. 10.2146/news120088 [DOI] [PubMed] [Google Scholar]
- 11. IQVIA . MIDAS® (sales) database. Available: https://www.iqvia.com/solutions/commercialization/brand-strategy-and-management/market-measurement/midas [Accessed 09 Jul 2023].
- 12. Sandborn WJ, Lawendy N, Danese S, et al. Safety and efficacy of tofacitinib for treatment of ulcerative colitis: final analysis of OCTAVE open, an open-label, long-term extension study with up to 7.0 years of treatment. Aliment Pharmacol Ther 2022;55:464–78. 10.1111/apt.16712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vermeire S, Su C, Lawendy N, et al. Outcomes of tofacitinib dose reduction in patients with ulcerative colitis in stable remission from the randomised RIVETING trial. J Crohns Colitis 2021;15:1130–41. 10.1093/ecco-jcc/jjaa249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taxonera C, Olivares D, Alba C. Real-world effectiveness and safety of tofacitinib in patients with ulcerative colitis: systematic review with meta-analysis. Inflamm Bowel Dis 2022;28:32–40. 10.1093/ibd/izab011 [DOI] [PubMed] [Google Scholar]
- 15. NICE . Overview | Filgotinib for treating moderately to severely active ulcerative colitis | Guidance; 2022. Available: https://www.nice.org.uk/guidance/ta792 [Accessed 13 Jul 2023].
- 16. Jyseleca 200 mg film-coated tablets - Summary of Product Characteristics (SmPC) - (emc), Available: https://www.medicines.org.uk/emc/product/11810/smpc [Accessed 13 Jul 2023].
- 17. Reinisch W, Hellstrom W, Dolhain R, et al. Effects of Filgotinib on semen parameters and sex hormones in male patients with inflammatory diseases: results from the phase 2, randomised, double-blind, placebo-controlled MANTA and MANTA-ray studies. Ann Rheum Dis 2023;82:1049–58. 10.1136/ard-2023-224017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vermeire S, Schreiber S, Petryka R, et al. Clinical remission in patients with moderate-to-severe Crohn's disease treated with Filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet 2017;389:266–75. 10.1016/S0140-6736(16)32537-5 [DOI] [PubMed] [Google Scholar]
- 19. Galapagos announces topline results from Phase 3 DIVERSITY trial of filgotinib in Crohn’s disease, Available: https://www.glpg.com/press-release/3766/galapagos-announces-topline-results-from-phase-3-diversity-trial-of-filgotinib-in-crohn-s-disease [Accessed 13 Jul 2023].
- 20. D’Haens GR, Lee S, Taylor SA, et al. Filgotinib for the treatment of small bowel Crohn's disease: the DIVERGENCE 1 trial. Gastroenterology 2023;165:289–92. 10.1053/j.gastro.2023.03.234 [DOI] [PubMed] [Google Scholar]
- 21. Van Rompaey L, Galien R, van der Aar EM, et al. Preclinical characterization of GLPG0634, a selective inhibitor of JAK1, for the treatment of inflammatory diseases. J Immunol 2013;191:3568–77. 10.4049/jimmunol.1201348 [DOI] [PubMed] [Google Scholar]
- 22. Traves PG, Murray B, Campigotto F, et al. JAK selectivity and the implications for clinical inhibition of pharmacodynamic cytokine signalling by Filgotinib, Upadacitinib, tofacitinib and Baricitinib. Ann Rheum Dis 2021;80:865–75. 10.1136/annrheumdis-2020-219012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Feagan BG, Danese S, Loftus EV, et al. Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): a phase 2B/3 double-blind, randomised, placebo-controlled trial. Lancet 2021;397:2372–84. 10.1016/S0140-6736(21)00666-8 [DOI] [PubMed] [Google Scholar]
- 24. Feagan BG, Matsuoka K, Rogler G, et al. OP35 efficacy and safety outcomes up to ~4 years of treatment with Filgotinib 200 mg among patients with ulcerative colitis: results from the SELECTIONLTE study. J Crohns Colitis 2023;17:i47–50. 10.1093/ecco-jcc/jjac190.0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Galapagos NV. A prospective, non-Interventional, multi-country cohort study of the effectiveness and safety of Filgotinib in adult patients with moderately to severely active ulcerative colitis. Report no.: NCT05817942 [clinicaltrials.gov]. 2023. Available: https://clinicaltrials.gov/study/NCT05817942 [Accessed 19 Jul 2023].
- 26. NICE . Overview | Upadacitinib for treating moderately to severely active ulcerative colitis | Guidance; 2023. Available: https://www.nice.org.uk/guidance/ta856 [Accessed 09 Jul 2023].
- 27. NICE . Overview | Upadacitinib for previously treated moderately to severely active Crohn’s disease | guidance; 2023. Available: https://www.nice.org.uk/guidance/ta905 [Accessed 09 Jul 2023].
- 28. RINVOQ 15 mg prolonged-release tablets - Summary of Product Characteristics (SmPC) - (emc), Available: https://www.medicines.org.uk/emc/product/10972/smpc [Accessed 10 Jul 2023].
- 29. Sandborn WJ, Ghosh S, Panes J, et al. Efficacy of upadacitinib in a randomized trial of patients with active ulcerative colitis. Gastroenterology 2020;158:2139–49. 10.1053/j.gastro.2020.02.030 [DOI] [PubMed] [Google Scholar]
- 30. Sandborn WJ, Feagan BG, Loftus EV, et al. Efficacy and safety of upadacitinib in a randomized trial of patients with Crohn’s disease. Gastroenterology 2020;158:2123–38. 10.1053/j.gastro.2020.01.047 [DOI] [PubMed] [Google Scholar]
- 31. Vermeire S, Danese S, Zhou W, et al. DOP41 efficacy and safety of extended induction treatment with Upadacitinib 45 mg once daily followed by maintenance Upadacitinib 15 or 30 mg once daily in patients with moderately to severely active ulcerative colitis. J Crohns Colitis 2022;16:i090–1. 10.1093/ecco-jcc/jjab232.080 [DOI] [Google Scholar]
- 32. Ghosh S, Sanchez Gonzalez Y, Zhou W, et al. Upadacitinib treatment improves symptoms of bowel urgency and abdominal pain, and correlates with quality of life improvements in patients with moderate to severe ulcerative colitis. J Crohns Colitis 2021;15:2022–30. 10.1093/ecco-jcc/jjab099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Loftus EV, Panés J, Lacerda AP, et al. Upadacitinib induction and maintenance therapy for Crohn’s disease. N Engl J Med 2023;388:1966–80. 10.1056/NEJMoa2212728 [DOI] [PubMed] [Google Scholar]
- 34. Lasa JS, Olivera PA, Danese S, et al. Efficacy and safety of Biologics and small molecule drugs for patients with moderate-to-severe ulcerative colitis: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol 2022;7:161–70. 10.1016/S2468-1253(21)00377-0 [DOI] [PubMed] [Google Scholar]
- 35. Burr NE, Gracie DJ, Black CJ, et al. Efficacy of biological therapies and small molecules in moderate to severe ulcerative colitis: systematic review and network meta-analysis. Gut 2022;71:1976–87. 10.1136/gutjnl-2021-326390 [DOI] [PubMed] [Google Scholar]
- 36. Barberio B, Gracie DJ, Black CJ, et al. Efficacy of biological therapies and small molecules in induction and maintenance of remission in Luminal Crohn’s disease: systematic review and network meta-analysis. Gut 2023;72:264–74. 10.1136/gutjnl-2022-328052 [DOI] [PubMed] [Google Scholar]
- 37. Loftus EV, Colombel J-F, Takeuchi K, et al. Upadacitinib therapy reduces ulcerative colitis symptoms as early as day 1 of induction treatment. Clin Gastroenterol Hepatol 2023;21:2347–58. 10.1016/j.cgh.2022.11.029 [DOI] [PubMed] [Google Scholar]
- 38. ClinicalTrials.gov . An Observational Study to Assess Change in Disease Activity and Adverse Events of Upadacitinib in Adult Participants With Moderate to Severe Ulcerative Colitis (UC) in Real-World Practice - Full Text View, Available: https://clinicaltrials.gov/ct2/show/NCT05494606 [Accessed 12 Jul 2023].
- 39. ClinicalTrials.gov . Study to Assess Speed of Onset and Durability of Effectiveness of Upadacitinib in Adult Participants With Moderate to Severe Crohn’s Disease (CD) in Real World Clinical Practice. - Full Text View, Available: https://clinicaltrials.gov/ct2/show/NCT05930275 [Accessed 12 Jul 2023].
- 40. Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med 2022;386:316–26. 10.1056/NEJMoa2109927 [DOI] [PubMed] [Google Scholar]
- 41. Eli Lilly and Company . A randomized, active-controlled, parallel-group, phase 3B/4 study of Baricitinib in patients with rheumatoid arthritis. Report no.: NCT03915964. Clinicaltrials.gov; 2023. Available: https://clinicaltrials.gov/study/NCT03915964 [Accessed 17 Jul 2023].
- 42. Namour F, Anderson K, Nelson C, et al. Filgotinib: a clinical pharmacology review. Clin Pharmacokinet 2022;61:819–32. 10.1007/s40262-022-01129-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lamb CA, Kennedy NA, Raine T, et al. British society of gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019;68:s1–106. 10.1136/gutjnl-2019-318484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kucharzik T, Ellul P, Greuter T, et al. ECCO guidelines on the prevention, diagnosis, and management of infections in inflammatory bowel disease. J Crohns Colitis 2021;15:879–913. 10.1093/ecco-jcc/jjab052 [DOI] [PubMed] [Google Scholar]
- 45. Kavanaugh A, Westhovens RR, Winthrop KL, et al. Safety and efficacy of Filgotinib: up to 4-year results from an open-label extension study of phase II rheumatoid arthritis programs. J Rheumatol 2021;48:1230–8. 10.3899/jrheum.201183 [DOI] [PubMed] [Google Scholar]
- 46. Burmester GR, Cohen SB, Winthrop KL, et al. Safety profile of upadacitinib over 15 000 patient-years across rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and atopic dermatitis. RMD Open 2023;9:e002735. 10.1136/rmdopen-2022-002735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Singh S, Singh H, Loftus EV, et al. Risk of cerebrovascular accidents and ischemic heart disease in patients with inflammatory bowel disease: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2014;12:382–93. 10.1016/j.cgh.2013.08.023 [DOI] [PubMed] [Google Scholar]
- 48. Kirchgesner J, Beaugerie L, Carrat F, et al. Increased risk of acute arterial events in young patients and severely active IBD: a nationwide French cohort study. Gut 2018;67:1261–8. 10.1136/gutjnl-2017-314015 [DOI] [PubMed] [Google Scholar]
- 49. Card TR, Zittan E, Nguyen GC, et al. Disease activity in inflammatory bowel disease is associated with arterial vascular disease. Inflamm Bowel Dis 2021;27:629–38. 10.1093/ibd/izaa156 [DOI] [PubMed] [Google Scholar]
- 50. Li N, Gou Z-P, Du S-Q, et al. Effect of JAK inhibitors on high- and low-density lipoprotein in patients with rheumatoid arthritis: a systematic review and network meta-analysis. Clin Rheumatol 2022;41:689–93. 10.1007/s10067-022-06064-8 [DOI] [PubMed] [Google Scholar]
- 51. Olivera PA, Lasa JS, Peretto G, et al. Review article: risk of cardiovascular events in patients with inflammatory bowel disease receiving small molecule drugs. Aliment Pharmacol Ther 2023;57:1231–48. 10.1111/apt.17509 [DOI] [PubMed] [Google Scholar]
- 52. Panaccione R, Lichtenstein G, Nakase H, et al. P518 safety of upadacitinib in ulcerative colitis: long-term data from the phase 3 open-label extension study (U-ACTIVATE). J Crohns Colitis 2023;17:i644–6. 10.1093/ecco-jcc/jjac190.0648 [DOI] [Google Scholar]
- 53. Yuhara H, Steinmaus C, Corley D, et al. Meta-analysis: the risk of venous thromboembolism in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2013;37:953–62. 10.1111/apt.12294 [DOI] [PubMed] [Google Scholar]
- 54. Yates M, Mootoo A, Adas M, et al. Venous thromboembolism risk with JAK inhibitors: a meta-analysis. Arthritis Rheumatol 2021;73:779–88. 10.1002/art.41580 [DOI] [PubMed] [Google Scholar]
- 55. Olivera PA, Lasa JS, Bonovas S, et al. Safety of Janus kinase inhibitors in patients with inflammatory bowel diseases or other immune-mediated diseases: a systematic review and meta-analysis. Gastroenterology 2020;158:1554–73. 10.1053/j.gastro.2020.01.001 [DOI] [PubMed] [Google Scholar]
- 56. Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–7. 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wu S, Xie S, Yuan C, et al. Inflammatory bowel disease and long-term risk of cancer: a prospective cohort study among half a million adults in UK Biobank. Inflamm Bowel Dis 2023;29:384–95. 10.1093/ibd/izac096 [DOI] [PubMed] [Google Scholar]
- 58. Curtis JR, Yamaoka K, Chen Y-H, et al. Malignancy risk with tofacitinib versus TNF inhibitors in rheumatoid arthritis: results from the open-label, randomised controlled ORAL surveillance trial. Ann Rheum Dis 2023;82:331–43. 10.1136/ard-2022-222543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Russell MD, Stovin C, Alveyn E, et al. JAK inhibitors and the risk of malignancy: a meta-analysis across disease indications. Ann Rheum Dis 2023;82:1059–67. 10.1136/ard-2023-224049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Greif CS, Srivastava D, Nijhawan RI. Janus kinase inhibitors and non-melanoma skin cancer. Curr Treat Options Oncol 2021;22:11. 10.1007/s11864-020-00815-y [DOI] [PubMed] [Google Scholar]
- 61. Sands BE, Long MD, Reinisch W, et al. Tofacitinib for the treatment of ulcerative colitis: analysis of nonmelanoma skin cancer rates from the ulcerative colitis clinical program. Inflamm Bowel Dis 2022;28:234–45. 10.1093/ibd/izab056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Danese S, Vermeire S, Zhou W, et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet 2022;399:2113–28. 10.1016/S0140-6736(22)00581-5 [DOI] [PubMed] [Google Scholar]
- 63. Winthrop KL, Park S-H, Gul A, et al. Tuberculosis and other opportunistic infections in tofacitinib-treated patients with rheumatoid arthritis. Ann Rheum Dis 2016;75:1133–8. 10.1136/annrheumdis-2015-207319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Colombel JF. Herpes Zoster in patients receiving JAK inhibitors for ulcerative colitis: mechanism, epidemiology, management, and prevention. Inflamm Bowel Dis 2018;24:2173–82. 10.1093/ibd/izy150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. GOV.UK . Introduction of Shingrix® vaccine for the whole programme and expansion of eligible cohorts letter. Available: https://www.gov.uk/government/publications/shingles-vaccination-programme-changes-from-september-2023-letter/introduction-of-shingrix-vaccine-for-the-whole-programme-and-expansion-of-eligible-cohorts-letter [Accessed 18 Jul 2023].
- 66. Julsgaard M, Mahadevan U, Vestergaard T, et al. Tofacitinib concentrations in plasma and breastmilk of a lactating woman with ulcerative colitis. Lancet Gastroenterol Hepatol 2023;8:695–7. 10.1016/S2468-1253(23)00158-9 [DOI] [PubMed] [Google Scholar]
- 67. AbbVie News Center . Upadacitinib (RINVOQ®) Achieved Clinical Remission and Endoscopic Response at One Year in Phase 3 Maintenance Study in Patients with Crohn’s Disease, Available: https://news.abbvie.com/news/press-releases/upadacitinib-rinvoq-achieved-clinical-remission-and-endoscopic-response-at-one-year-in-phase-3-maintenance-study-in-patients-with-crohns-disease.htm [Accessed 06 Feb 2023].
- 68. Mahadevan U, Baumgart DC, Dubinsky MC, et al. S0847 pregnancy outcomes in the tofacitinib ulcerative colitis OCTAVE studies: an update as of February 2020. Am J Gastroenterol 2020;115:S437–8. 10.14309/01.ajg.0000705436.64452.7d [DOI] [Google Scholar]
- 69. Clowse MEB, Feldman SR, Isaacs JD, et al. Pregnancy outcomes in the tofacitinib safety databases for rheumatoid arthritis and psoriasis. Drug Saf 2016;39:755–62. 10.1007/s40264-016-0431-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Herrera-deGuise C, Serra-Ruiz X, Lastiri E, et al. JAK inhibitors: a new dawn for oral therapies in inflammatory bowel diseases. Front Med (Lausanne) 2023;10:1089099. 10.3389/fmed.2023.1089099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Danese S, Peyrin-Biroulet L. Selective tyrosine kinase 2 inhibition for treatment of inflammatory bowel disease: new hope on the rise. Inflamm Bowel Dis 2021;27:2023–30. 10.1093/ibd/izab135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nielsen OH, Boye TL, Chakravarti D, et al. Selective tyrosine kinase 2 inhibitors in inflammatory bowel disease. Trends Pharmacol Sci 2022;43:424–36. 10.1016/j.tips.2022.02.008 [DOI] [PubMed] [Google Scholar]
- 73. Danese S, Argollo M, Le Berre C, et al. JAK selectivity for inflammatory bowel disease treatment: does it clinically matter Gut 2019;68:1893–9. 10.1136/gutjnl-2019-318448 [DOI] [PubMed] [Google Scholar]
- 74. Choy EH. Clinical significance of Janus kinase inhibitor selectivity. Rheumatology (Oxford) 2019;58:953–62. 10.1093/rheumatology/key339 [DOI] [PMC free article] [PubMed] [Google Scholar]