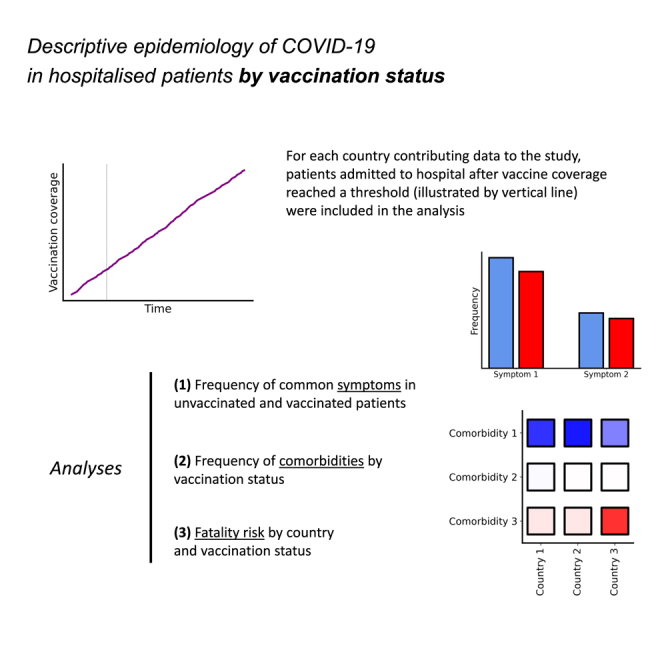
Summary
Background
Individuals vaccinated against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), when infected, can still develop disease that requires hospitalization. It remains unclear whether these patients differ from hospitalized unvaccinated patients with regard to presentation, coexisting comorbidities, and outcomes.
Methods
Here, we use data from an international consortium to study this question and assess whether differences between these groups are context specific. Data from 83,163 hospitalized COVID-19 patients (34,843 vaccinated, 48,320 unvaccinated) from 38 countries were analyzed.
Findings
While typical symptoms were more often reported in unvaccinated patients, comorbidities, including some associated with worse prognosis in previous studies, were more common in vaccinated patients. Considerable between-country variation in both in-hospital fatality risk and vaccinated-versus-unvaccinated difference in this outcome was observed.
Conclusions
These findings will inform allocation of healthcare resources in future surges as well as design of longer-term international studies to characterize changes in clinical profile of hospitalized COVID-19 patients related to vaccination history.
Funding
This work was made possible by the UK Foreign, Commonwealth and Development Office and Wellcome (215091/Z/18/Z, 222410/Z/21/Z, 225288/Z/22/Z, and 220757/Z/20/Z); the Bill & Melinda Gates Foundation (OPP1209135); and the philanthropic support of the donors to the University of Oxford’s COVID-19 Research Response Fund (0009109). Additional funders are listed in the “acknowledgments” section.
Keywords: COVID-19, descriptive epidemiology, vaccination, comorbidity, heterogeneity
Graphical abstract
Highlights
-
•
Vaccinated patients were on average older than unvaccinated individuals
-
•
Hospitalized COVID-19 patients with history of vaccination often had comorbidities
-
•
Fatality risk and vaccinated-versus-unvaccinated difference varied by country
Context and significance
Although SARS-CoV-2 vaccines are effective and protect against COVID-19, this protection is not perfect, and vaccinated individuals can still develop clinical disease that requires hospitalization. In this international clinical epidemiology study, Gonçalves et al. analyzed data collected in 38 countries to describe characteristics, including coexisting medical conditions, and outcomes of hospitalized COVID-19 patients by vaccination status. Hospitalized patients with history of SARS-CoV-2 vaccination were on average older than hospitalized unvaccinated individuals and more often had multiple comorbidities. There were differences in the risk of death between countries and by vaccination status. These observations will facilitate the design of longer-term studies to assess changes in the profile of COVID-19 patients linked to vaccination and inform better allocation of healthcare resources.
Gonçalves and colleagues analyzed a dataset from a large international clinical consortium to describe presentation, coexisting comorbidities, and outcomes of hospitalized COVID-19 patients by vaccination status. They observed that hospitalized vaccinated patients were on average older, and more often had comorbidities, compared with hospitalized unvaccinated individuals.
Introduction
The swiftness with which vaccines targeting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) were developed and tested1,2,3 in the first year of the pandemic allowed vaccination to be rolled out as early as December 2020 in some countries. Since then, coverage has increased in all regions, albeit to a variable extent, and millions of lives are estimated to have been saved by immunization programs.4 However, despite their effectiveness, current vaccines do not provide sterilizing immunity, and vaccinated individuals can still be infected and develop symptomatic disease. A consequence of this less-than-perfect vaccine-induced immunity, which has been shown to wane over time,5,6 and the increase in coverage is that coronavirus disease 2019 (COVID-19) cases, including those requiring hospital care, often occur in individuals with previous SARS-CoV-2 vaccination. Because disease presentation might differ between vaccinated and unvaccinated patients (for multiple reasons, including non-comparability in terms of prognostic factors linked to the prioritization of high-risk groups for immunization, and vaccine effects on disease progression after infection), the continuing change in the profile of COVID-19 patients with regard to vaccination history means that it is important to systematically describe symptoms, comorbidities, and severity of cases by vaccine status.
Although previous studies7,8,9,10,11 reported on characteristics and outcomes of hospitalized COVID-19 patients with history of SARS-CoV-2 vaccination, these studies often had limited sample sizes or did not allow for between-country comparisons. Here, we analyze data from an international consortium that collected detailed clinical information on hospitalized patients with COVID-19; our objective is to describe presentation, coexisting medical conditions, and outcome of patients admitted to hospital stratified by vaccination status. As this database includes hospital admission records from the beginning of the pandemic until the second half of 2022, to ensure that vaccinated and unvaccinated patients compared here were admitted during the same time period, we restricted analyses to individuals hospitalized after vaccine coverage reached 10% in the corresponding countries.
Results
Selection of analytic sample
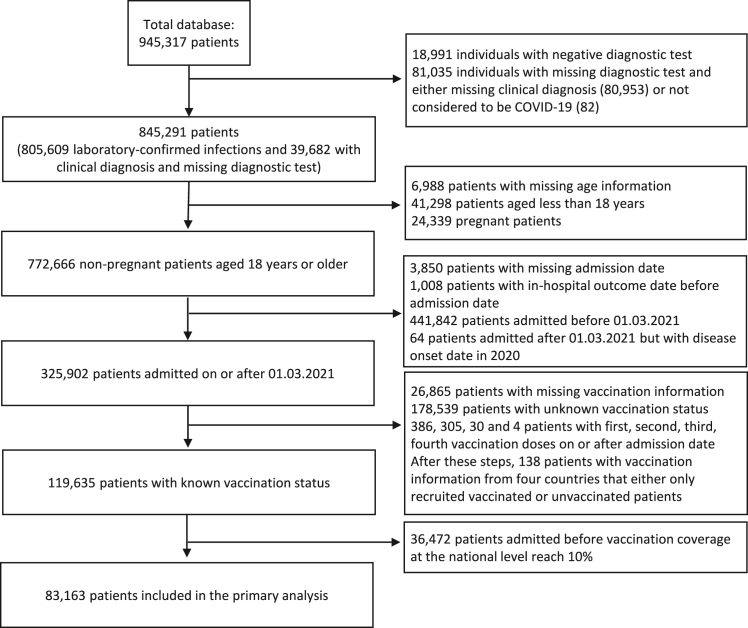
The clinical database used in this analysis includes information on 945,317 hospitalized patients from 76 countries; 845,291 either had laboratory-confirmed SARS-CoV-2 infection or were clinically diagnosed with COVID-19 in the absence of a diagnostic test. Data on vaccination were not collected by the consortium before March 2021; for this reason, analyses presented here exclude records of patients admitted to hospital before that month. Furthermore, since population-level vaccine coverage remained low for several months after March 2021 in many of the countries where participating hospitals are located, as mentioned above, only data from patients admitted after 10% of the corresponding country’s population had been vaccinated were included (Figure S1). Sensitivity analyses using a different coverage threshold are described in the Supplementary Appendix and Data S1. Because, in most settings, children and adolescents were offered vaccines after adult age groups, our analysis also excluded patients younger than 18 years of age. In Figure 1, the different steps of the selection of participants for the analysis are presented.
Figure 1.
Selection of the analytic sample
A database from the Our World in Data initiative with information on country-level vaccination coverage was used in the final step of selection. Pregnant women were excluded from the analysis because previous studies reported that delivery is a common reason for hospital admission in patients with incidental SARS-CoV-2 infections.12 See also Figure S1.
In the next subsections, data from 83,163 non-pregnant adult patients in 38 different countries with known vaccination status are presented. A comparison of these patients with those recruited during the same period but with unknown vaccination history is presented in Data S1. Note that, in some countries, patients requiring clinical management in intensive care units (ICUs) were preferentially enrolled; below, we refer to countries where more than 80% of study participants were admitted to an ICU during hospitalization as the ICU country group (18 countries; Table S1).
Vaccination data
Most hospital records in the analytic sample were contributed by South Africa and the United Kingdom (47,768 [57.4%] and 29,637 [35.6%]); 2,881 (3.5%) participants were from the ICU country group. Most (71.7%) patients in the analytic sample were admitted in 2021; 28.3% were admitted in 2022. A total of 37,147 (44.7%) patients were hospitalized during the period when the Delta variant was dominant; here, the period during which its frequency at the population level, relative to the other circulating variants, was above 90% (see STAR Methods and Figure S1); 36,976 (44.5%) participants were admitted when the Omicron variant was dominant. Other study participants were admitted either when the Alpha variant caused most infections locally (2,025, 2.4%) or during periods when relative frequencies of all circulating variants were below 90% (7,015, 8.4%).
In the combined dataset, 41.9% (34,843/83,163) of hospitalized patients had been vaccinated. There was between-country variation (Table S2): in the United Kingdom, 64.1% of study participants reported vaccination before hospital admission, while in South Africa and the ICU country group, which includes settings with different population-level vaccination coverages, lower percentages of patients had received SARS-CoV-2 vaccine doses, 27.6% and 34.5% respectively. In some countries, e.g., Norway, there was an increase in the fraction of participants with history of vaccination during the study period (Figure S2), while in others this proportion remained relatively stable or fluctuated non-monotonously.
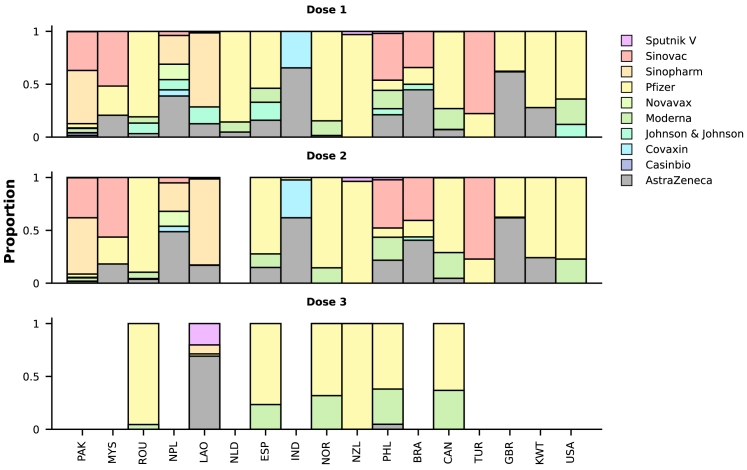
Our comparisons are based on a binary variable corresponding to vaccination history; however, information on the manufacturer of the vaccine used for the first four doses was also available for 78.5% (27,356 out of 34,843), 59.2% (20,618 out of 34,843), 1.6% (562 out of 34,843), and 0.1% (25 out of 34,843) of the vaccinated participants. Note that these numerators include patients who reported “Unknown vaccine type” (10,575, 4,512, 56, and 3, respectively, for the four doses), and the denominators are the same and correspond to the number of individuals with previous vaccination, rather than to those reporting different numbers of previous vaccine doses, as this information was not captured in the case report form. In the combined data, for first doses, the most frequently used vaccines were those produced by AstraZeneca (55.3%, 9,275 out of 16,781) and Pfizer (37.5%, 6,294 out of 16,781); distributions of vaccine manufacturers by country and dose are shown in Figure 2. Of patients with data on the first two doses, most received the same vaccine type in the first and second doses (16,048 out of 16,097).
Figure 2.
Manufacturers of vaccines administered to study participants
The y axes present the distributions of vaccine manufacturers, as proportions, by country (x axes; the same ordering applies to the three panels). For some participants, information on vaccine manufacturer was available for the second or third doses but not for the first dose (N = 25 and 15, respectively) or for the third dose but not the second dose (N = 18). Only countries with at least 20 participants for whom manufacturer information was available are included in this figure; this criterion was also used for each dose-specific panel. Information on vaccine manufacturer for the fourth dose was available for 22 patients and is not presented here. See also Figure S2 and Table S2.
Information on dates when patients received vaccine doses was available for 16,036, 13,312, and 500 participants for the first three doses. The median time interval between the first dose and hospital admission was 236 days (interquartile range [IQR] 157–306; N = 16,036), and between the most recent dose and admission was 172 days (IQR 104–237; N = 13,985). Of participants with data on vaccination dates, 2.6% (424 out of 16,036) were admitted in the 2 weeks following the first dose; this percentage was higher (3.4%) for patients admitted in 2021 compared with those admitted in 2022 (0.3%). For patients recruited in the ICU country group, median time intervals from the first and the most recent dose to hospital admission were, respectively, 165 (IQR 77–256) and 110 days (IQR 42–187). When calculating time from the most recent dose, patients for whom vaccination date was missing for a later vaccine dose with manufacturer information were not included.
Age and symptoms by vaccination status
In many settings, individuals at higher risk of severe disease were prioritized for vaccination. This initial susceptibility-based allocation of SARS-CoV-2 vaccines, aimed at maximizing public health impact, implies that vaccinated and unvaccinated patients are expected to differ in many clinically relevant characteristics. Consistent with this, although similar percentages of vaccinated and unvaccinated patients were male (50.0% and 48.0%, respectively), the median (IQR) age of those vaccinated was 65 (50–78) years, higher than the median age of unvaccinated patients (50 [35–65] years). In Table S3, median, and corresponding IQR, ages of vaccinated and unvaccinated participants are presented by country; in most countries, vaccinated patients were older than unvaccinated patients.
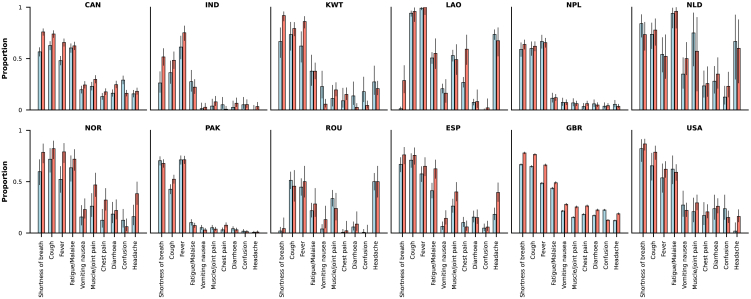
Data on symptoms were available for 30,341 out of 35,395 individuals; the denominator here does not include South African patients, as this information was not available for participants from that country. The analysis included variables on 24 different symptoms, and the median (IQR) number of symptoms with non-missing information per patient was 23 (19–24). Table S4 shows the frequencies of the different symptoms by vaccination status; some of the most common symptoms, e.g., fever, shortness of breath, and cough, were less frequent in the vaccinated group. Among patients with non-missing information on the five most common symptoms in the dataset (shortness of breath, cough, fever, fatigue/malaise, vomiting/nausea), 90.9% (12,041 out of 13,251) of those with history of vaccination had at least one of these symptoms; the percentage of unvaccinated patients with one or more of these typical symptoms was 95.6% (9,630 out of 10,070). Country-specific frequencies of symptoms by vaccination status are shown in Figure 3; data for most countries included in the figure suggest slightly higher frequencies in the unvaccinated compared with the vaccinated group. An exception to this pattern is the frequency of confusion or altered consciousness, which was more often seen in vaccinated patients. Table S4 presents the comparison by SARS-CoV-2 variant period.
Figure 3.
Country-specific frequencies of the 10 most common symptoms
Red bars correspond to data from unvaccinated patients and light blue bars to data from vaccinated patients. The ordering of symptoms (x axes) is the same in all panels; y axes present frequencies as proportions. The 95% confidence intervals are also shown. Only countries with at least 100 patients with data on one or more symptoms are included; the ISO3 code of each country included is presented as the corresponding panel title: CAN, Canada; IND, India; KWT, Kuwait; LAO, Laos; NPL, Nepal; NLD, the Netherlands; NOR, Norway; PAK, Pakistan; ROU, Romania; ESP, Spain; GBR, United Kingdom; USA, United States. See Table S4.
Comorbidities in vaccinated and unvaccinated patients
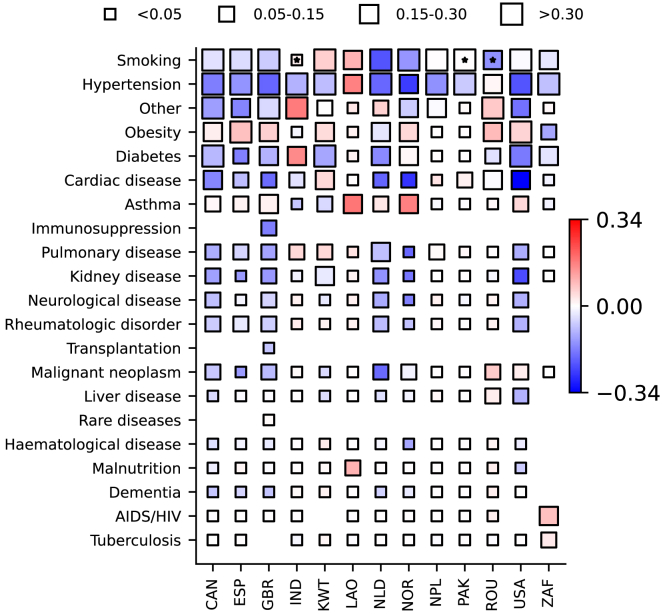
A total of 21 variables on specific comorbidities were analyzed; information on at least one of these variables was available for most participants (76,892 out of 83,163; 92.3% and 92.6% of vaccinated and unvaccinated individuals). The median number of comorbidities in patients with data on at least one comorbidity was two (IQR, 1–3) for the vaccinated group, and one (IQR, 0–2) for the unvaccinated group. Frequencies of the different medical conditions are presented in Table 1; several, e.g., chronic cardiac, pulmonary and kidney conditions, were more often reported in vaccinated compared with unvaccinated patients (respectively, 22.1% versus 7.1%, 13.7% versus 4.3%, 14.1% versus 3.7%). As both vaccination coverage and prevalence of comorbidities vary by country, we also analyzed country-specific data on coexisting conditions. In Figure 4, differences in frequencies of comorbidities between vaccinated and unvaccinated individuals are presented. Some conditions were more common in patients with a history of SARS-CoV-2 vaccination: for example, for several countries, hypertension and chronic cardiac disease were more frequent in the vaccinated group.
Table 1.
Frequencies of comorbidities in vaccinated and unvaccinated patients in the combined (all-country) dataset
| Comorbidities | Vaccinated |
Unvaccinated |
||||
|---|---|---|---|---|---|---|
| % | Total (non-missing) | Missing data | % | Total (non-missing) | Missing data | |
| AIDS/HIV | 3.1 | 27,675 | 7,168 | 11.6 | 31,369 | 16,951 |
| Asthma | 11.9 | 28,421 | 6,422 | 7.4 | 32,532 | 15,788 |
| Cardiac disease | 22.1 | 28,480 | 6,363 | 7.1 | 32,412 | 15,908 |
| Hematological disease | 4.9 | 17,836 | 17,007 | 2.3 | 9,542 | 38,778 |
| Kidney disease | 14.1 | 28,280 | 6,563 | 3.7 | 32,067 | 16,253 |
| Neurological disease | 11.6 | 18,006 | 16,837 | 5.9 | 9,670 | 38,650 |
| Pulmonary disease | 13.7 | 28,386 | 6,457 | 4.3 | 32,102 | 16,218 |
| Dementia | 10.3 | 17,776 | 17,067 | 3.1 | 9,585 | 38,735 |
| Diabetes | 27.7 | 28,640 | 6,203 | 18.9 | 33,870 | 14,450 |
| Hypertension | 47.8 | 29,426 | 5,417 | 33.6 | 35,145 | 13,175 |
| Immunosuppression | 24.8 | 2,741 | 32,102 | 7.7 | 901 | 47,419 |
| Liver disease | 3.8 | 18,917 | 15,926 | 3.1 | 10,085 | 38,235 |
| Malignant neoplasm | 8.9 | 28,213 | 6,630 | 2.1 | 32,136 | 16,184 |
| Malnutrition | 1.7 | 16,732 | 18,111 | 1.4 | 9,285 | 39,035 |
| Obesity | 17.2 | 19,120 | 15,723 | 15.7 | 21,530 | 26,790 |
| Other | 28.7 | 30,251 | 4,592 | 10.8 | 43,344 | 4,976 |
| Rare diseases | 1.7 | 2,845 | 31,998 | 2.4 | 915 | 47,405 |
| Rheumatologic disorder | 12.0 | 17,801 | 17,042 | 5.6 | 9,524 | 38,796 |
| Smoking | 46.7 | 13,261 | 21,582 | 26.3 | 17,229 | 31,091 |
| Transplantation | 12.2 | 2,853 | 31,990 | 4.3 | 915 | 47,405 |
| Tuberculosis | 2.2 | 12,676 | 22,167 | 4.8 | 25,165 | 23,155 |
See also Table S5.
Figure 4.
Differences in frequencies of comorbidities between vaccinated and unvaccinated groups
Coordinates in the y axis correspond to comorbidities, ordered based on mean frequencies in all countries, and in the x axis, countries, represented by ISO3 codes, are ordered alphabetically. The four different sizes of the squares in the figure relate to the corresponding frequencies in the unvaccinated group (see top of the graph). Colors represent country- and comorbidity-specific numerical differences in frequencies between unvaccinated and vaccinated patients; red tones indicate that a comorbidity was more frequent in unvaccinated patients. Only countries with 100 or more patients with data on at least one comorbidity are presented. Stars indicate when the number of vaccinated or unvaccinated individuals was below 20. A different version of this figure is shown in the Supplementary Appendix (Figure S3) that accounts for frequencies of comorbidities in the unvaccinated group not only in the size of the squares but also in the color; i.e., the other version of this figure presents differences relative to the frequencies in the unvaccinated group rather than absolute differences.
The presence of multiple comorbidities was not infrequent in the analytic sample. Of the 58,330 participants with non-missing data on at least 10 comorbidities, 27.9% had three or more comorbidities. Of the vaccinated individuals, 42.0% (11,584 out of 27,571) had a medical history of three or more comorbidities, compared with 15.2% (4,692 out of 30,759) of the unvaccinated patients. A similar pattern was observed in each age group: for patients aged between 18 and 60 years, the frequency of three or more comorbidities was 20.4% (2,082 out of 10,198) and 10.4% (1,984 out of 19,105) for those with history of vaccination and those without previous vaccination, respectively; for patients older than 60 years, these percentages were respectively 54.7% (9,502 out of 17,373) and 23.2% (2,708 out of 11,654). For the ICU country group, the frequencies of multiple comorbidities in vaccinated and unvaccinated patients were 13.5% (132 out of 980) and 14.8% (274 out of 1,846), respectively. Table 2 presents country-specific data, and frequencies of multiple comorbidities for different variant periods are shown in Table S5.
Table 2.
Percentages of patients with three or more comorbidities by vaccination status and country
| Country | Vaccinated |
Unvaccinated |
||
|---|---|---|---|---|
| % with three or more comorbidities | Total | % with three or more comorbidities | Total | |
| Canada | 63.0 | 562 | 40.0 | 697 |
| India | 10.0 | 80 | 13.7 | 153 |
| Kuwait | 26.7 | 45 | 28.8 | 163 |
| Lao PDR | 0.6 | 344 | 14.3 | 49 |
| Nepal | 5.5 | 219 | 4.6 | 461 |
| The Netherlands | 64.7 | 68 | 30.3 | 66 |
| Norway | 58.6 | 70 | 23.3 | 86 |
| Pakistan | 1.0 | 483 | 1.3 | 603 |
| Romania | 12.5 | 152 | 15.2 | 46 |
| South Africa | 7.6 | 9,502 | 7.4 | 21,076 |
| Spain | 50.8 | 187 | 28.0 | 125 |
| United Kingdom | 65.3 | 15,450 | 37.1 | 6,642 |
| United States | 78.6 | 56 | 50.9 | 175 |
Only participants with non-missing information on 10 or more comorbidity-related variables were included; information is presented for countries with at least 100 patients meeting this criterion. See also Tables S7–S10.
In-hospital outcomes
We compared the risk of death during the first 28 days since admission or disease onset, whichever happened later, for vaccinated and unvaccinated patients (see STAR Methods for more details on the definition of the outcome). All data combined, there were 4,053 (12.4%) deaths in the vaccinated group, and, in the unvaccinated group, 7,832 out of 46,170 (17.0%) patients died. The fatality risk was higher in participants aged 60 years or older (17.1% [3,333 out of 19,432] and 28.8% [4,516 out of 15,698] in the vaccinated and unvaccinated groups, respectively) compared with younger patients, aged from 18 to 60 years (5.5% [720 out of 13,167] and 10.9% [3,316 out of 30,472] in the vaccinated and unvaccinated groups). Patients recruited in the ICU country group also had higher death risk, 38.9% (81 out of 208) and 33.1% (230 out of 694), respectively, for vaccinated and unvaccinated patients aged from 18 to 60 years, and 47.9% (185 out of 386) and 51.2% (353 out of 689) for vaccinated and unvaccinated patients aged 60 years or older. Country-specific data on the risk of death are presented in Table S5, and Figure 5 shows fatality risk by country, age, and vaccination group: while, in some countries, e.g., the United Kingdom and South Africa, unvaccinated patients had higher risk of death, in others, with more limited study sample size, a higher percentage of vaccinated patients died compared with unvaccinated patients. A multivariable analysis, adjusting for sex, age, and number of comorbidities, was also performed and the odds ratio estimated for the association between vaccination and death was 0.53 (95% confidence interval 0.50–0.56; Table S6). A post hoc regression analysis was also performed excluding data from the ICU country group; similar results were obtained (odds ratio 0.50, 0.47–0.53).
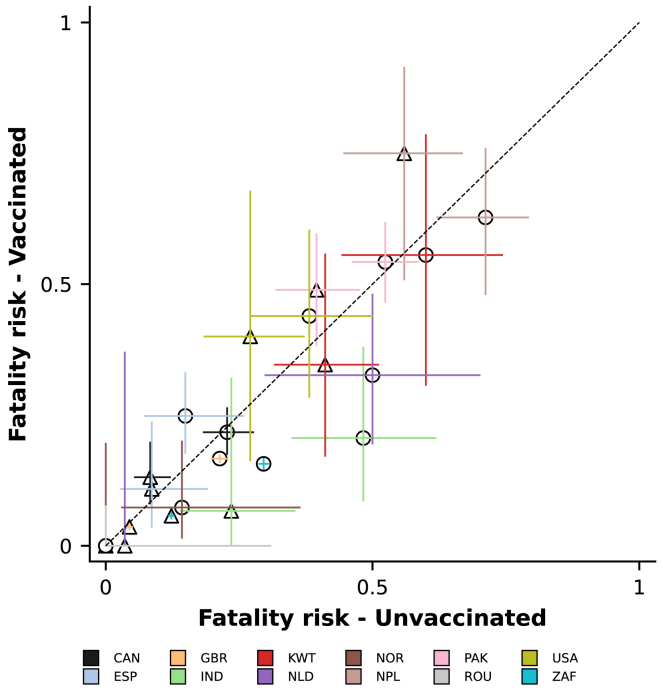
Figure 5.
Fatality risks in vaccinated (y axis) and unvaccinated (x axis) patients by country, represented by different colors, and age
Patients were grouped in two broad age categories for this figure: those aged between 18 and 60 years, represented by triangles, and patients older than 60 years, represented by circles. The 95% confidence intervals for both the vaccinated (vertical lines) and unvaccinated (horizontal lines) groups are shown. Figure S4 presents a version of this figure where the two axes range from 0 to 0.30, allowing better visualization of data from countries with lower fatality risks, including South Africa and the United Kingdom. See also Tables S7 and S8.
In addition to death, we also analyzed a composite outcome combining death and invasive mechanical ventilation. In analyses restricted to countries where less than 80% of the analytic sample required ICU admission, the composite outcome was more frequent in unvaccinated patients; in the ICU country group, the frequency of the composite outcome was high (>85%) regardless of vaccination status or age. Results of this analysis are detailed in the Supplementary Appendix and Data S1.
Discussion
Among the many important contributions of epidemiology to the pandemic response are studies on the effectiveness of SARS-CoV-2 vaccines.13,14,15,16 These have been instrumental to generate evidence on the effect of vaccines against different variants and in settings different from those where clinical trials were performed. These studies were often designed as test-negative studies or population-based studies that use electronic medical records. Our study has a different design; here, only patients who developed disease severe enough to require hospital admission were included, and non-COVID-19 patients were not recruited as part of this consortium. Hence, we did not aim to quantify vaccine effectiveness against infection or hospitalization; we rather aimed to describe the clinical profile of hospitalized COVID-19 patients by vaccination status. In the “results” section, we reported clinical data from 83,163 patients from 38 countries. Our analyses show that hospitalized vaccinated individuals were on average older than hospitalized unvaccinated individuals and more often had multiple comorbidities. Despite the variable fatality risk observed in different countries, descriptive analyses indicate that history of vaccination is associated with lower death risk in some settings despite initial prioritization of high-risk group for vaccination. Although these findings suggest differences between these groups, more consistently with regard to coexisting medical conditions, as vaccine coverage, including of booster doses, increases, a finer comparison based on time since most recent dose might be required to aid clinical practice and allocation of healthcare resources. The data presented here, in particular on the between-country heterogeneity in patient characteristics, will inform design of future international studies on this question.
The observation that, in the population of hospitalized COVID-19 patients, vaccinated individuals were on average older than unvaccinated individuals was expected given that, in many settings, age was used in prioritizing the initial allocation of vaccine doses. However, in some countries, this pattern persisted during the entire study period: for example, in the United Kingdom, participants aged 60 years or older represented 70.3% of the vaccinated patients and 29.2% of the unvaccinated patients in 2021 and 75.5% and 50.4%, respectively, in 2022; in South Africa, vaccinated patients were also more often older than 60 years compared with unvaccinated patients in both calendar years (see Table S3 for other similar country-specific comparisons). One possible explanation is that collider bias,17 due to selection of patients with severe disease, i.e., patients requiring hospital admission, might have contributed to this pattern. Collider bias18 can occur when study selection is affected by the two factors being studied or by their causes. Because both previous SARS-CoV-2 vaccination and age affect disease severity, and consequently risk of hospitalization, an association between these two factors is expected in the hospitalized population. Other explanations could relate to non-optimal immunological response to vaccination19,20,21 or higher immunization uptake in older age groups.
One of the effects of SARS-CoV-2 vaccines is on clinical progression after viral infection,22 and it seems reasonable to assume that this might also influence frequencies of symptoms, even among those individuals with severe disease. In our analysis, we observed that typical symptoms were more often present in unvaccinated than vaccinated individuals, although the difference was not substantial. The inclusion of patients with incidental infections might have contributed to this observation. In hospital-based studies, incidental infections complicate quantitative descriptions of clinical disease as, for patients with these infections, COVID-19 is not the primary cause of hospitalization, and COVID-19-related symptoms are thus not necessarily present, or rather, by definition, are less likely to be present. As previous studies suggest that delivery is a common cause of hospitalization in patients with incidental SARS-CoV-2 infections, pregnant women were excluded from this analysis (Figure 1). Relative frequencies of different variants should also be considered when interpreting these results, as a recent study provided evidence that infections caused by different variants could be associated with different symptoms.23 As mentioned in the “results” section, unlike most other common symptoms, confusion or altered consciousness was more often observed in vaccinated individuals; it is possible that this relates to differences in age distribution between the vaccinated and unvaccinated groups.24
The vaccinated and unvaccinated groups also differed in terms of comorbidities. In several countries, some of the most frequent comorbidities, e.g., hypertension and cardiac disease, were more prevalent in vaccinated patients. This pattern was also observed in analyses performed by SARS-CoV-2 variant periods. The two mechanisms described above, prioritization of high-risk groups for vaccination and potential collider bias, are likely to have contributed to this observation, which is consistent with a few previous hospital-based studies.25 For example, in a study from the United States,26 vaccinated patients admitted to hospital, in addition to being older than unvaccinated patients, more often had comorbidities; in an Israeli study,27 comorbidities were also more frequent in vaccinated versus unvaccinated patients. Epidemiological studies, including some performed before vaccine introduction,28,29 estimated that many of these conditions are associated with COVID-19-related death; this imbalance in coexisting comorbidities between vaccinated and unvaccinated patients might thus influence in-hospital prognosis for these patients. A limitation of this analysis is that only information on the presence or absence of specific comorbidities was collected, but not on their relative severities, which would have helped to understand their clinical significance for hospital admission. Note that, in South Africa, vaccinated and unvaccinated patients had similar frequencies of multiple comorbidities; it is unclear whether this relates to local epidemiology and transmission patterns, to a higher proportion of incidental infections, or to data missingness (for this country, only 12 out of 21 comorbidity-related variables were available for analysis).
Previous studies reported conflicting results regarding the risk of death in vaccinated and unvaccinated patients hospitalized with COVID-19. In the study conducted by Mielke and colleagues, fewer vaccinated individuals died (10.3%) compared with unvaccinated patients (12.8%).11 However, in other studies,26,27 similar fatality risks were observed in these groups. In our analysis, the odds ratio for the association between previous vaccination and death in the combined dataset, dominated by data from South Africa and the United Kingdom, suggests vaccinated patients had a lower risk of death. There was, however, considerable between-country variation both in the overall risk of death and in the differences between vaccinated and unvaccinated individuals (Figure 5). While for patients recruited in the United Kingdom, South Africa, and India, the risk of death was lower in the vaccinated group, for countries that might have primarily recruited more severely ill patients, e.g., Pakistan and the United States, the opposite pattern was observed. More generally, differences in fatality risk between vaccinated and unvaccinated hospitalized patients should not be interpreted as measures of vaccine effectiveness: both confounding due to vaccine prioritization and collider bias due to study sample selection can affect associations between vaccination and disease severity in these analyses, but, foremost, these estimates do not take into account the components of the vaccine-induced protection against infection and against progression to disease that requires hospital care conditional on infection.
Given the increasing coverage of vaccination, it is possible that the next surge of SARS-CoV-2 infections will affect primarily vaccinated individuals. Our analysis, which used data from one of the largest cohorts of hospitalized COVID-19 patients, suggests that, when these individuals are infected, develop disease, and are admitted to hospital, they might be more likely to have comorbidities and be older than patients with no previous vaccination. However, it does not necessarily follow that the same pattern will be observed in comparisons of vaccinated individuals with versus without booster doses, and international studies with design similar to ours and that are able to more comprehensively capture information on vaccination dates will be informative in the next waves of this pandemic.
Limitations of the study
The main strength of our study is the joint description of data from more than one country, which allowed assessment of the consistency of findings in countries with different SARS-CoV-2 epidemiological trajectories and vaccine coverage. On the other hand, the high proportion of hospitalizations with missing data on previous vaccination is an important weakness of our analysis. In Data S1, we present comparisons between participants for whom information on vaccination status was available versus those with missing vaccine data; we did not observe clear differences between these groups in aggregated analyses. Information on dates when vaccine doses were administered was also incomplete, only available for 46.0% of first doses in patients reporting previous vaccination. Another aspect of our analysis that should be addressed in future studies on similar questions relates to the identification of incidental SARS-CoV-2 infections as these might affect comparisons, especially if their frequency is influenced by vaccination. Finally, the inclusion of patients with more severe presentation in some countries is both a weakness and a strength; while it increases heterogeneity in the study population, complicating interpretation of aggregated summaries, we were able to describe data from this group of countries and compare with the combined dataset.
Consortia
The members of the ISARIC Clinical Characterisation Group include Sheryl Ann Abdukahil, Kamal Abu Jabal, Nashat Abu Salah, Eka Airlangga, Ali Ait Hssain, Chika Akwani, Eman Al Qasim, Angela Alberti, Osama Aldabbourosama, Marta Alessi, Beatrice Alex, Abdulrahman Al-Fares, Jeffrey Aliudin, Mohammed Alkahlout, Lana Almasri, Yousef Al-Saba’a, Rita Alves, Joana Alves Cabrita, Maria Amaral, Phoebe Ampaw, Aditya John Anchan, Andrea Angheben, Yaseen Arabi, Antonio Arcadipane, Patrick Archambault, Lukas Arenz, Rakesh Arora, Elizabeth A. Ashley, Anika Atique, Moad Atlowly, Benjamin Bach, John Kenneth Baillie, J. Kevin Baird, Valeria Balan, Renata Barbalho, Nicholas Yuri Barbosa, Wendy S. Barclay, Michaela Barnikel, Netta Beer, Husna Begum, David Bellemare, Anna Beltrame, Giulia Bertoli, Claudia Bianco, Felwa Bin Humaid, Jonathan Bitton, Catherine Blier, Debby Bogaert, Diogo Borges, Dounia Bouhmani, Thipsavanh Bounphiengsy, Latsaniphone Bountthasavong, Bianca Boxma-de Klerk, Filipa Brás Monteiro, Luca Brazzi, Nina Buchtele, Danilo Buonsenso, Aidan Burrell, Ingrid G. Bustos, Joana Cabrita, Eder Caceres, Rui Caetano Garcês, Josie Campisi, Cecilia Canepa, Janice Caoili, Chiara Simona Cardellino, Filipa Cardoso, Filipe Cardoso, Sofia Cardoso, Gayle Carney, François Martin Carrier, Gail Carson, Mariana Cascão, José Casimiro, Silvia Castañeda, Nidyanara Castanheira, Paolo Cattaneo, Roberta Cavalin, Alexandros Cavayas, Muge Cevik, Bounthavy Chaleunphon, Adrienne Chan, Meera Chand, Anjellica Chen, Matthew Pellan Cheng, Danoy Chommanam, Yock Ping Chow, Nathaniel Christy, Rolando Claure-Del Granado, Sara Clohisey, Cassidy Codan, Marie Connor, Graham S. Cooke, Mary Copland, Amanda Corley, Andrea Cortegiani, Gloria Crowl, Claudina Cruz, Marc Csete, Paula Custodio, Ana da Silva Filipe, Andrew Dagens, Peter Daley, Zaina Dalloul, Heidi Dalton, Jo Dalton, Juliana Damas, Nick Daneman, Emmanuelle A. Dankwa, Jorge Dantas, Frédérick D'Aragon, Cristina De Rose, Thushan de Silva, William Dechert, Emmanuelle Denis, Yael Dishon, k Dhangar, Annemarie B. Docherty, Christl A. Donnelly, Chloe Donohue, Phouvieng Douangdala, James Joshua Douglas, Triona Downer, Mark Downing, Thomas Drake, Murray Dryden, Audrey Dubot-Pérès, Susanne Dudman, Jake Dunning, Mathilde Duplaix, Lucian Durham III, Anne Margarita Dyrhol-Riise, Michael Edelstein, Martina Escher, Mariano Esperatti, Catarina Espírito Santo, João Estevão, Amna Faheem, Cameron J. Fairfield, Pedro Faria, Nataly Farshait, Jorge Fernandes, Marília Andreia Fernandes, Joana Ferrão, Mário Ferraz, Bernardo Ferreira, Claudia Figueiredo-Mello, Tom Fletcher, Brigid Flynn, Patricia Fontela, Simon Forsyth, Giuseppe Foti, Robert A. Fowler, Diego Franch-Llasat, Christophe Fraser, John F. Fraser, Ana Freitas Ribeiro, Caren Friedrich, Nora Fuentes, Argin G, Linda Gail Skeie, Carrol Gamble, Rui Garcês, Julia Garcia-Diaz, Esteban Garcia-Gallo, Aisling Gavin, Nuno Germano, Moji Ghadimi, Praveen Kumar Ghisulal, Marco Giani, Jess Gibson, Michelle Girvan, Geraldine Goco, Joan Gómez-Junyent, Margarite Grable, Christopher A. Green, William Greenhalf, Fiona Griffiths, Heidi Gruner, Yusing Gu, Anne-Marie Guerguerian, Daniela Guerreiro, Daniel Haber, Hannah Habraken, Wael Hafez, Matthew Hall, Sophie Halpin, Shaher Hamdan, Raph L. Hamers, Summer Hamza, Hayley Hardwick, Janet Harrison, Alan Hartman, Lars Heggelund, Ross Hendry, Martina Hennessy, Liv Hesstvedt, Dawn Higgins, Rupert Higgins, Samuel Hinton, Antonia Ho, Jan Cato Holter, Juan Pablo Horcajada, Jimmy Ming-Yang Hsu, Abby Hurd, Samreen Ijaz, Carlos Cañada Illana, Hugo Inácio, Mariachiara Ippolito, Tiago Isidoro, Hamza Jaber, Clare Jackson, Denise Jaworsky, Synne Jenum, Philippe Jouvet, Alina Kalicinska, Chris Kandel, Kevin Katz, Aasmine Kaur, Seán Keating, Andrea Kelly, Sadie Kelly, Kalynn Kennon, Sommay Keomany, Imrana Khalid, Michelle E. Kho, Saye Khoo, Peter Kiiza, Beathe Kiland Granerud, Anders Benjamin Kildal, Paul Klenerman, Gry Kloumann Bekken, Stephen R. Knight, Volkan Korten, Caroline Kosgei, Deepali Kumar, Demetrios Kutsogiannis, François Lamontagne, Marina Lanza, Andrew Law, Andy Law, Teresa Lawrence, James Lee, Jennifer Lee, Todd C. Lee, Gary Leeming, Andrew Letizia, Gianluigi Li Bassi, Janet Liang, Wei Shen Lim, Andreas Lind, Samantha Lissauer, Diogo Lopes, Ruth Lyons, Sara Machado, Nimisha Abdul Majeed, Frank Manetta, Ceila Maria Sant`Ana Malaque, Catherine Marquis, Laura Marsh, John Marshall, Alejandro Martín-Quiros, Ana Martins, Caroline Martins Rego, Gennaro Martucci, David Maslove, Christina Matthew, Mayfong Mayxay, Colin McArthur, Anne McCarthy, Rachael McConnochie, Sarah E. McDonald, Allison McGeer, Chris McKay, Kenneth A. McLean, Kusum Menon, Alexander J. Mentzer, António Mesquita, Dan Meyer, Alison M. Meynert, Efstathia Mihelis, Agostinho Monteiro, Giorgia Montrucchio, Sarah Moore, Shona C. Moore, Lina Morales Cely, Lucia Moro, Ben Morton, Caroline Mudara, Mo’nes Muhaisen, Fredrik Müller, Karl Erik Müller, Laveena Munshi, Srinivas Murthy, Dana Mustafa, Dave Nagpal, Mangala Narasimhan, Prashant Nasa, Matthew Nelder, Emily Neumann, Pauline Yeung Ng, Alistair D. Nichol, Lisa Norman, Alessandra Notari, Mahdad Noursadeghi, Dwi Utomo Nusantara, Giovanna Occhipinti, Katie O'Hearn, Larissa Oliveira, David S.Y. Ong, Wilna Oosthuyzen, Peter Openshaw, Massimo Palmarini, Giovanna Panarello, Prasan Kumar Panda, Rachael Parke, Patricia Patricio, Lisa Patterson, Mical Paul, Jorge Paulos, William A. Paxton, Mare Pejkovska, Rui Pereira, Michele Petrovic, Frank Olav Pettersen, Scott Pharand, Ooyanong Phonemixay, Soulichanya Phoutthavong, Maria de Piero, Carlos Pimentel, Catarina Pires, Ayodhia Pitaloka, Riinu Pius, Sergio Poli, Georgios Pollakis, Andra-Maris Post, Diana Póvoas, Jeff Powis, Viladeth Praphasiri, Mark G. Pritchard, Bambang Pujo Semedi, Gregory Purcell, Luisa Quesada, Else Quist-Paulsen, Aldo Rafael, Mutia Rahardjani, José Ramalho, Rajavardhan Rangappa, Indrek Rätsep, Brenda Reeve, Dag Henrik Reikvam, Hongru Ren, Oleksa Rewa, Antonia Ricchiuto, Asgar Rishu, Maria Angelica Rivera Nuñez, Stephanie Roberts, David L. Robertson, Ferran Roche-Campo, Paola Rodari, Bernhard Roessler, Andrea Rossanese, Matteo Rossetti, Clark D. Russell, Aleksander Rygh Holten, Isabela Saba, Musharaf Sadat, Valla Sahraei, Leonardo Salazar, Gabriele Sales, Emely Sanchez, Vanessa Sancho-Shimizu, Gyan Sandhu, Oana Sandulescu, Marlene Santos, Shirley Sarfo-Mensah, Iam Claire E. Sarmiento, Egle Saviciute, Justin Schaffer, Michael Schwameis, Gary Schwartz, Janet T. Scott, James Scott-Brown, Malcolm G. Semple, Tânia Sequeira, Ellen Shadowitz, Anuraj Shankar, Catherine A. Shaw, Victoria Shaw, Dr. Rajesh Mohan Shetty, Bountoy Sibounheuang, Louise Sigfrid, Piret Sillaots, Wai Ching Sin, Dario Sinatti, Mahendra Singh, Vegard Skogen, Sue Smith, Joshua Solomon, Tom Solomon, Rima Song, Elisabetta Spinuzza, Shiranee Sriskandan, Thomas Staudinger, Stephanie-Susanne Stecher, Trude Steinsvik, Birgitte Stiksrud, Adrian Streinu-Cercel, Anca Streinu-Cercel, David Stuart, Decy Subekti, Jacky Y. Suen, Asfia Sultana, Charlotte Summers, Atie Suwarti, Jaques Sztajnbok, Shirin Tabrizi, Sara Taleb, Richard S. Tedder, João Teixeira, Hubert Tessier-Grenier, Shaun Thompson, Emma C. Thomson, Mathew Thorpe, Ryan S. Thwaites, Kristian Tonby, Marta Torre, Rosario Maria Torres Santos-Olmo, Alexis F. Turgeon, Lance C.W. Turtle, Anders Tveita, Pawel Twardowski, Roman Ullrich, Timothy M. Uyeki, Piero Valentini, Luís Val-Flores, Michael Varrone, José Ernesto Vidal, César Vieira, Joy Ann Villanueva, Judit Villar, Andrea Villoldo, Chiara Vitiello, Manivanh Vongsouvath, Marina Wainstein, Steve Webb, Jia Wei, Sanne Wesselius, Murray Wham, Nicole White, Sue Willems, Bailey Williams, Virginie Williams, Evert-Jan Wils, Jessica Wittman, Stephanie Yerkovich, Touxiong Yiaye, Maram Zahran, and Maria Zambon.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER | |
|---|---|---|---|
| Deposited data | |||
| Code – descriptive analyses in text | https://doi.org/10.5281/zenodo.8252909; https://github.com/BronnerG/Hosp_Vacc_analysis | ||
| Software and algorithms | |||
| Stata version 17 | Stata Corp, Texas, TX, USA | https://www.stata.com/ | |
| Python version 3.7 | Python Software Foundation | https://www.python.org/ | |
Resource availability
Lead contact
Further information should be directed to and will be fulfilled by the lead contact, Bronner P. Gonçalves (bronnergoncalves@gmail.com).
Materials availability
The study did not generate any new reagents or materials.
Experimental model and study participant details
Data on clinical presentation and outcomes of hospitalised COVID-19 patients were analyzed in this study. Information on the patients’ age and sex is reported in the results section. These data are part of the ISARIC (International Severe Acute Respiratory and Emerging Infections Consortium) COVID-19 database, and were collected using standardised case report forms, and are stored at a central repository at the University of Oxford. Additional information on ISARIC can be found here https://isaric.org/about-us/membership/, and on data collection and curation can be found in other publications.30,31
Method details
Population-level data on vaccination and SARS-CoV-2 variants
We used data available in the website https://ourworldindata.org/on country-level vaccination coverage32 to restrict analyses to patients hospitalised after 10% of a country population had been vaccinated; data were downloaded on April 7, 2023. The objective was to report on a more clinically relevant comparison, i.e., of vaccinated and unvaccinated patients admitted to hospital during the same period, rather than on a comparison that would reflect mostly vaccine prioritisation of high-risk groups and temporal changes in clinical prognosis independent of vaccination.
We also stratified key analyses by periods defined based on the relative frequencies of SARS-CoV-2 variants. Data from the Global Initiative on Sharing All Influenza Data (GISAID) on each of the main SARS-CoV-2 variants were aggregated by sample collection date (epidemiologic week) using a bespoke pipeline available from here https://github.com/globaldothealth/covid19-variants-summary. GISAID data were downloaded on October 10, 2022. Country- and variant-specific periods were defined based on the dates when the relative frequency of the temporarily dominant variant was first above and subsequently below 90%; only epidemiological weeks with 10 or more samples in GISAID were analyzed, and after the start of each period, drops to frequencies between 80 and 90% were not considered in defining the end of the period. Note that information on country-level frequencies of variants was not available for the entire study period for some of the countries contributing data to the analytic sample.
Quantification and statistical analysis
Our aim was to compare clinical presentation, existing comorbidities, and disease progression after admission of vaccinated and unvaccinated patients hospitalised with COVID-19. Count and discretised continuous variables such as age were summarised with medians and interquartile ranges; categorical variables were presented using frequencies, proportions or percentages, of observations in each stratum.
In addition to frequencies of symptoms and comorbidities, we also analyzed risk of in-hospital death. For this, only data from patients still in hospital 28 days after admission or disease onset, regardless of their subsequent outcome, those discharged, and patients who died in the first four weeks of hospitalisation were analyzed; a total of 4,394 patients in the analytic sample were excluded from this analysis. We assessed the association between history of vaccination and in-hospital death using mixed effects logistic regression, including as covariates age, sex and presence of comorbidities, and random effects for different countries; age was included as a binary variable (age below versus above 60 years). Qualitatively similar results, not presented here, were obtained when using Cox proportional hazards models stratified by country.
R and Python were used for data processing and descriptive analyses, and Stata 17 was used to fit mixed effects logistic regression models.
Acknowledgments
This work was also funded by grants from the National Institute for Health Research (NIHR; award CO-CIN-01), the Medical Research Council (MRC; grant MC_PC_19059), and the NIHR Health Protection Research Unit (HPRU) in Emerging and Zoonotic Infections at the University of Liverpool in partnership with Public Health England (PHE) (award 200907), NIHR HPRU in Respiratory Infections at Imperial College London with PHE (award 200927), Liverpool Experimental Cancer Medicine Centre (grant C18616/A25153), NIHR Biomedical Research Centre at Imperial College London (award ISBRC-1215-20013), and NIHR Clinical Research Network providing infrastructure support; CIHR Coronavirus Rapid Research Funding Opportunity OV2170359 and the coordination in Canada by Sunnybrook Research Institute; funding by the Health Research Board of Ireland (CTN-2014-12); the Rapid European COVID-19 Emergency Response research (RECOVER) (H2020 project 101003589) and European Clinical Research Alliance on Infectious Diseases (ECRAID) (965313); Cambridge NIHR Biomedical Research Centre (award NIHR203312); a Research Council of Norway grant no. 312780 and a philanthropic donation from Vivaldi Invest A/S owned by Jon Stephenson von Tetzchner; the Comprehensive Local Research Networks (CLRNs), of which P.J.M.O. is an NIHR Senior Investigator (NIHR201385); Stiftungsfonds zur Förderung der Bekämpfung der Tuberkulose und anderer Lungenkrankheiten of the City of Vienna, project number APCOV22BGM; funding from Medical University of Vienna, Department of Anaesthesia, Intensive Care Medicine and Pain Medicine; Italian Ministry of Health “Fondi Ricerca corrente–L1P6” to IRCCS Ospedale Sacro Cuore–Don Calabria; Australian Department of Health grant (3273191); Gender Equity Strategic Fund at University of Queensland, Artificial Intelligence for Pandemics (A14PAN) at University of Queensland, the Australian Research Council Centre of Excellence for Engineered Quantum Systems (EQUS, CE170100009), the Prince Charles Hospital Foundation, Australia; a grant from foundation Bevordering Onderzoek Franciscus; the South Eastern Norway Health Authority and the Research Council of Norway; Institute of Cancer Research (ICR), National Institutes of Health (NIH) supported by the Ministry of Health Malaysia; and the US DoD Armed Forces Health Surveillance Division, Global Emerging Infectious Diseases Branch to the US Naval Medical ResearchUnit no. TWO (NAMRU-2) (Work Unit #: P0153_21_N2). These authors would like to thank Vysnova Partners, Inc., for the management of this research project. The Lao-Oxford-Mahosot Hospital-Wellcome Trust Research Unit is funded by the Wellcome Trust; M.U.G.K. was supported by the Branco Weiss Fellowship, Google.org, the Oxford Martin School, the Rockefeller Foundation, and the European Union Horizon 2020 project MOOD (#874850). The contents of this publication are the sole responsibility of the authors and do not necessarily reflect the views of the European Commission.
The investigators acknowledge the support of the COVID clinical management team, AIIMS, Rishikesh, India; the COVID-19 Clinical Management team, Manipal Hospital Whitefield, Bengaluru, India; the Liverpool School of Tropical Medicine and the University of Oxford; Imperial NIHR Biomedical Research Centre; the dedication and hard work of the Norwegian SARS-CoV-2 study team; endorsement of the Irish Critical Care Clinical Trials Group, co-ordination in Ireland by the Irish Critical Care Clinical Trials Network at University College Dublin; and preparedness work conducted by the Short Period Incidence Study of Severe Acute Respiratory Infection.
This work uses data provided by patients and collected by the NHS as part of their care and support #DataSavesLives. The data used for this research were obtained from ISARIC4C. We are extremely grateful to the 2,648 frontline NHS clinical and research staff and volunteer medical students who collected these data in challenging circumstances and for the generosity of the patients and their families for their individual contributions in these difficult times. The COVID-19 Clinical Information Network (CO-CIN) data were collated by ISARIC4C Investigators. We also acknowledge the support of Jeremy J. Farrar and Nahoko Shindo.
Author contributions
Conceptualization, B.P.G., J.B., A.R., I.M.-L., L.F.R., C.K., P.H., P.L.O., and L.M.; methodology and investigation, B.P.G., W.J., J.B., A.R., I.M.-L., L.F.R., B.W.C., C.K., S.S., P.L.O., and L.M.; clinical data curation, B.W.C., M.L., and L.M.; population-level variant data curation, A.D. and M.U.G.K.; statistical analysis, B.P.G. with inputs from J.B., C.K., A.R., I.M.-L., L.F.R., P.L.O., and L.M. B.P.G., B.W.C., M.L., and L.M. had unrestricted access to all data. B.P.G. wrote the original draft with inputs from P.L.O. and L.M. All authors reviewed and edited the manuscript. All authors agreed to submit the manuscript, read and approved the final draft, and take full responsibility for its content, including the accuracy of the data.
Declaration of interests
I.M.-L. declares lectures for Gilead, Thermo Fisher, and MSD; advisory board participation for Fresenius Kabi, Advanz Pharma, Gilead, Accelerate, and Merck; and consulting fees for Gilead outside of the submitted work. S.S. participated as an investigator for an observational study analyzing ICU patients with COVID-19 (for the Critical Care Consortium, including ECMOCARD) funded by The Prince Charles Hospital Foundation during the conduct of this study. B.L. declares travel/accommodation/meeting expenses from Mylan and Gilead, all outside the submitted work.
Published: September 21, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.medj.2023.08.005.
Contributor Information
Bronner P. Gonçalves, Email: bronnergoncalves@gmail.com.
ISARIC Clinical Characterisation Group:
Sheryl Ann Abdukahil, Kamal Abu Jabal, Nashat Abu Salah, Eka Airlangga, Ali Ait Hssain, Chika Akwani, Eman Al Qasim, Angela Alberti, Osama Aldabbourosama, Marta Alessi, Beatrice Alex, Abdulrahman Al-Fares, Jeffrey Aliudin, Mohammed Alkahlout, Lana Almasri, Yousef Al-Saba’a, Rita Alves, Joana Alves Cabrita, Maria Amaral, Phoebe Ampaw, Aditya John Anchan, Andrea Angheben, Yaseen Arabi, Antonio Arcadipane, Patrick Archambault, Lukas Arenz, Rakesh Arora, Elizabeth A. Ashley, Anika Atique, Moad Atlowly, Benjamin Bach, John Kenneth Baillie, J. Kevin Baird, Valeria Balan, Renata Barbalho, Nicholas Yuri Barbosa, Wendy S. Barclay, Michaela Barnikel, Netta Beer, Husna Begum, David Bellemare, Anna Beltrame, Giulia Bertoli, Claudia Bianco, Felwa Bin Humaid, Jonathan Bitton, Catherine Blier, Debby Bogaert, Diogo Borges, Dounia Bouhmani, Thipsavanh Bounphiengsy, Latsaniphone Bountthasavong, Bianca Boxma-de Klerk, Filipa Brás Monteiro, Luca Brazzi, Nina Buchtele, Danilo Buonsenso, Aidan Burrell, Ingrid G. Bustos, Joana Cabrita, Eder Caceres, Rui Caetano Garcês, Josie Campisi, Cecilia Canepa, Janice Caoili, Chiara Simona Cardellino, Filipa Cardoso, Filipe Cardoso, Sofia Cardoso, Gayle Carney, François Martin Carrier, Gail Carson, Mariana Cascão, José Casimiro, Silvia Castañeda, Nidyanara Castanheira, Paolo Cattaneo, Roberta Cavalin, Alexandros Cavayas, Muge Cevik, Bounthavy Chaleunphon, Adrienne Chan, Meera Chand, Anjellica Chen, Matthew Pellan Cheng, Danoy Chommanam, Yock Ping Chow, Nathaniel Christy, Rolando Claure-Del Granado, Sara Clohisey, Cassidy Codan, Marie Connor, Graham S. Cooke, Mary Copland, Amanda Corley, Andrea Cortegiani, Gloria Crowl, Claudina Cruz, Marc Csete, Paula Custodio, Ana da Silva Filipe, Andrew Dagens, Peter Daley, Zaina Dalloul, Heidi Dalton, Jo Dalton, Juliana Damas, Nick Daneman, Emmanuelle A. Dankwa, Jorge Dantas, Frédérick D'Aragon, Cristina De Rose, Thushan de Silva, William Dechert, Emmanuelle Denis, Yael Dishon, k Dhangar, Annemarie B. Docherty, Christl A. Donnelly, Chloe Donohue, Phouvieng Douangdala, James Joshua Douglas, Triona Downer, Mark Downing, Thomas Drake, Murray Dryden, Audrey Dubot-Pérès, Susanne Dudman, Jake Dunning, Mathilde Duplaix, Lucian Durham, III, Anne Margarita Dyrhol-Riise, Michael Edelstein, Martina Escher, Mariano Esperatti, Catarina Espírito Santo, João Estevão, Amna Faheem, Cameron J. Fairfield, Pedro Faria, Nataly Farshait, Jorge Fernandes, Marília Andreia Fernandes, Joana Ferrão, Mário Ferraz, Bernardo Ferreira, Claudia Figueiredo-Mello, Tom Fletcher, Brigid Flynn, Patricia Fontela, Simon Forsyth, Giuseppe Foti, Robert A. Fowler, Diego Franch-Llasat, Christophe Fraser, John F. Fraser, Ana Freitas Ribeiro, Caren Friedrich, Nora Fuentes, Argin G, Linda Gail Skeie, Carrol Gamble, Rui Garcês, Julia Garcia-Diaz, Esteban Garcia-Gallo, Aisling Gavin, Nuno Germano, Moji Ghadimi, Praveen Kumar Ghisulal, Marco Giani, Jess Gibson, Michelle Girvan, Geraldine Goco, Joan Gómez-Junyent, Margarite Grable, Christopher A. Green, William Greenhalf, Fiona Griffiths, Heidi Gruner, Yusing Gu, Anne-Marie Guerguerian, Daniela Guerreiro, Daniel Haber, Hannah Habraken, Wael Hafez, Matthew Hall, Sophie Halpin, Shaher Hamdan, Raph L. Hamers, Summer Hamza, Hayley Hardwick, Janet Harrison, Alan Hartman, Lars Heggelund, Ross Hendry, Martina Hennessy, Liv Hesstvedt, Dawn Higgins, Rupert Higgins, Samuel Hinton, Antonia Ho, Jan Cato Holter, Juan Pablo Horcajada, Jimmy Ming-Yang Hsu, Abby Hurd, Samreen Ijaz, Carlos Cañada Illana, Hugo Inácio, Mariachiara Ippolito, Tiago Isidoro, Hamza Jaber, Clare Jackson, Denise Jaworsky, Synne Jenum, Philippe Jouvet, Alina Kalicinska, Chris Kandel, Kevin Katz, Aasmine Kaur, Seán Keating, Andrea Kelly, Sadie Kelly, Kalynn Kennon, Sommay Keomany, Imrana Khalid, Michelle E. Kho, Saye Khoo, Peter Kiiza, Beathe Kiland Granerud, Anders Benjamin Kildal, Paul Klenerman, Gry Kloumann Bekken, Stephen R. Knight, Volkan Korten, Caroline Kosgei, Deepali Kumar, Demetrios Kutsogiannis, François Lamontagne, Marina Lanza, Andrew Law, Andy Law, Teresa Lawrence, James Lee, Jennifer Lee, Todd C. Lee, Gary Leeming, Andrew Letizia, Gianluigi Li Bassi, Janet Liang, Wei Shen Lim, Andreas Lind, Samantha Lissauer, Diogo Lopes, Ruth Lyons, Sara Machado, Nimisha Abdul Majeed, Frank Manetta, Ceila Maria Sant`Ana Malaque, Catherine Marquis, Laura Marsh, John Marshall, Alejandro Martín-Quiros, Ana Martins, Caroline Martins Rego, Gennaro Martucci, David Maslove, Christina Matthew, Mayfong Mayxay, Colin McArthur, Anne McCarthy, Rachael McConnochie, Sarah E. McDonald, Allison McGeer, Chris McKay, Kenneth A. McLean, Kusum Menon, Alexander J. Mentzer, António Mesquita, Dan Meyer, Alison M. Meynert, Efstathia Mihelis, Agostinho Monteiro, Giorgia Montrucchio, Sarah Moore, Shona C. Moore, Lina Morales Cely, Lucia Moro, Ben Morton, Caroline Mudara, Mo’nes Muhaisen, Fredrik Müller, Karl Erik Müller, Laveena Munshi, Srinivas Murthy, Dana Mustafa, Dave Nagpal, Mangala Narasimhan, Prashant Nasa, Matthew Nelder, Emily Neumann, Pauline Yeung Ng, Alistair D. Nichol, Lisa Norman, Alessandra Notari, Mahdad Noursadeghi, Dwi Utomo Nusantara, Giovanna Occhipinti, Katie O'Hearn, Larissa Oliveira, David S.Y. Ong, Wilna Oosthuyzen, Peter Openshaw, Massimo Palmarini, Giovanna Panarello, Prasan Kumar Panda, Rachael Parke, Patricia Patricio, Lisa Patterson, Mical Paul, Jorge Paulos, William A. Paxton, Mare Pejkovska, Rui Pereira, Michele Petrovic, Frank Olav Pettersen, Scott Pharand, Ooyanong Phonemixay, Soulichanya Phoutthavong, Maria de Piero, Carlos Pimentel, Catarina Pires, Ayodhia Pitaloka, Riinu Pius, Sergio Poli, Georgios Pollakis, Andra-Maris Post, Diana Póvoas, Jeff Powis, Viladeth Praphasiri, Mark G. Pritchard, Bambang Pujo Semedi, Gregory Purcell, Luisa Quesada, Else Quist-Paulsen, Aldo Rafael, Mutia Rahardjani, José Ramalho, Rajavardhan Rangappa, Indrek Rätsep, Brenda Reeve, Dag Henrik Reikvam, Hongru Ren, Oleksa Rewa, Antonia Ricchiuto, Asgar Rishu, Maria Angelica Rivera Nuñez, Stephanie Roberts, David L. Robertson, Ferran Roche-Campo, Paola Rodari, Bernhard Roessler, Andrea Rossanese, Matteo Rossetti, Clark D. Russell, Aleksander Rygh Holten, Isabela Saba, Musharaf Sadat, Valla Sahraei, Leonardo Salazar, Gabriele Sales, Emely Sanchez, Vanessa Sancho-Shimizu, Gyan Sandhu, Oana Sandulescu, Marlene Santos, Shirley Sarfo-Mensah, Iam Claire E. Sarmiento, Egle Saviciute, Justin Schaffer, Michael Schwameis, Gary Schwartz, Janet T. Scott, James Scott-Brown, Malcolm G. Semple, Tânia Sequeira, Ellen Shadowitz, Anuraj Shankar, Catherine A. Shaw, Victoria Shaw, Dr. Rajesh Mohan Shetty, Bountoy Sibounheuang, Louise Sigfrid, Piret Sillaots, Wai Ching Sin, Dario Sinatti, Mahendra Singh, Vegard Skogen, Sue Smith, Joshua Solomon, Tom Solomon, Rima Song, Elisabetta Spinuzza, Shiranee Sriskandan, Thomas Staudinger, Stephanie-Susanne Stecher, Trude Steinsvik, Birgitte Stiksrud, Adrian Streinu-Cercel, Anca Streinu-Cercel, David Stuart, Decy Subekti, Jacky Y. Suen, Asfia Sultana, Charlotte Summers, Atie Suwarti, Jaques Sztajnbok, Shirin Tabrizi, Sara Taleb, Richard S. Tedder, João Teixeira, Hubert Tessier-Grenier, Shaun Thompson, Emma C. Thomson, Mathew Thorpe, Ryan S. Thwaites, Kristian Tonby, Marta Torre, Rosario Maria Torres Santos-Olmo, Alexis F. Turgeon, Lance C.W. Turtle, Anders Tveita, Pawel Twardowski, Roman Ullrich, Timothy M. Uyeki, Piero Valentini, Luís Val-Flores, Michael Varrone, José Ernesto Vidal, César Vieira, Joy Ann Villanueva, Judit Villar, Andrea Villoldo, Chiara Vitiello, Manivanh Vongsouvath, Marina Wainstein, Steve Webb, Jia Wei, Sanne Wesselius, Murray Wham, Nicole White, Sue Willems, Bailey Williams, Virginie Williams, Evert-Jan Wils, Jessica Wittman, Stephanie Yerkovich, Touxiong Yiaye, Maram Zahran, and Maria Zambon
Supplemental information
Data and code availability
-
•
The data that underpin this analysis are highly detailed clinical data on individuals hospitalised with COVID-19. Due to the sensitive nature of these data and the associated privacy concerns, they are available via a governed data access mechanism following review of a data access committee. Data can be requested via the IDDO COVID-19 Data Sharing Platform (http://www.iddo.org/covid-19). The Data Access Application, Terms of Access and details of the Data Access Committee are available on the website. Briefly, the requirements for access are a request from a qualified researcher working with a legal entity who have a health and/or research remit; a scientifically valid reason for data access which adheres to appropriate ethical principles. The full terms are at https://www.iddo.org/document/covid-19-data-access-guidelines. A small subset of sites who contributed data to this analysis have not agreed to pooled data sharing as above. In the case of requiring access to these data, please contact the corresponding author in the first instance who will look to facilitate access. Code used in the descriptive analysis is available.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watson O.J., Barnsley G., Toor J., Hogan A.B., Winskill P., Ghani A.C. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect. Dis. 2022;22:1293–1302. doi: 10.1016/S1473-3099(22)00320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg Y., Mandel M., Bar-On Y.M., Bodenheimer O., Freedman L.S., Ash N., Alroy-Preis S., Huppert A., Milo R. Protection and Waning of Natural and Hybrid Immunity to SARS-CoV-2. N. Engl. J. Med. 2022;386:2201–2212. doi: 10.1056/NEJMoa2118946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberg Y., Mandel M., Bar-On Y.M., Bodenheimer O., Freedman L., Haas E.J., Milo R., Alroy-Preis S., Ash N., Huppert A. Waning Immunity after the BNT162b2 Vaccine in Israel. N. Engl. J. Med. 2021;385:e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruni A., Longhini F., Macheda S., Biamonte E., Pasqua P., Neri G., Guzzo M.L., Garofalo E., Calabria COVID-ICU Network authors Characteristics of unvaccinated and vaccinated critically ill COVID-19 patients in calabria region (Italy): A retrospective study. Front. Med. 2022;9 doi: 10.3389/fmed.2022.1042411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chanda D., Hines J.Z., Itoh M., Fwoloshi S., Minchella P.A., Zyambo K.D., Sivile S., Kampamba D., Chirwa B., Chanda R., et al. COVID-19 Vaccine Effectiveness Against Progression to In-Hospital Mortality in Zambia, 2021-2022. Open Forum Infect. Dis. 2022;9:ofac469. doi: 10.1093/ofid/ofac469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Modes M.E., Directo M.P., Melgar M., Johnson L.R., Yang H., Chaudhary P., Bartolini S., Kho N., Noble P.W., Isonaka S., Chen P. Clinical Characteristics and Outcomes Among Adults Hospitalized with Laboratory-Confirmed SARS-CoV-2 Infection During Periods of B.1.617.2 (Delta) and B.1.1.529 (Omicron) Variant Predominance - One Hospital, California, July 15-September 23, 2021, and December 21, 2021-January 27, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022;71:217–223. doi: 10.15585/mmwr.mm7106e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whittaker R., Bråthen Kristofferson A., Valcarcel Salamanca B., Seppälä E., Golestani K., Kvåle R., Watle S.V., Buanes E.A. Length of hospital stay and risk of intensive care admission and in-hospital death among COVID-19 patients in Norway: a register-based cohort study comparing patients fully vaccinated with an mRNA vaccine to unvaccinated patients. Clin. Microbiol. Infect. 2022;28:871–878. doi: 10.1016/j.cmi.2022.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mielke N., Johnson S., Bahl A. Boosters reduce in-hospital mortality in patients with COVID-19: An observational cohort analysis. Lancet Reg. Health. Am. 2022;8 doi: 10.1016/j.lana.2022.100227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai J., Traub E., Aoki K., Oyong K., Sato H., Rizik-Baer D., Gounder P. Incidentally Detected SARS-COV-2 Among Hospitalized Patients in Los Angeles County, August to October 2020. J. Hosp. Med. 2021;16:480–483. doi: 10.12788/jhm.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferdinands J.M., Rao S., Dixon B.E., Mitchell P.K., DeSilva M.B., Irving S.A., Lewis N., Natarajan K., Stenehjem E., Grannis S.J., et al. Waning of vaccine effectiveness against moderate and severe covid-19 among adults in the US from the VISION network: test negative, case-control study. BMJ. 2022;379 doi: 10.1136/bmj-2022-072141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., Hernán M.A., Lipsitch M., Reis B., Balicer R.D. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams K., Rhoads J.P., Surie D., Gaglani M., Ginde A.A., McNeal T., Talbot H.K., Casey J.D., Zepeski A., Shapiro N.I., et al. Vaccine effectiveness of primary series and booster doses against covid-19 associated hospital admissions in the United States: living test negative design study. BMJ. 2022;379 doi: 10.1136/bmj-2022-072065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lau J.J., Cheng S.M.S., Leung K., Lee C.K., Hachim A., Tsang L.C.H., Yam K.W.H., Chaothai S., Kwan K.K.H., Chai Z.Y.H., et al. Real-world COVID-19 vaccine effectiveness against the Omicron BA.2 variant in a SARS-CoV-2 infection-naive population. Nat. Med. 2023;29:348–357. doi: 10.1038/s41591-023-02219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu H., Cole S.R., Howe C.J., Westreich D. Toward a Clearer Definition of Selection Bias When Estimating Causal Effects. Epidemiology. 2022;33:699–706. doi: 10.1097/EDE.0000000000001516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernán M.A., Hernández-Díaz S., Robins J.M. A structural approach to selection bias. Epidemiology. 2004;15:615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 19.Romero-Olmedo A.J., Schulz A.R., Hochstätter S., Das Gupta D., Virta I., Hirseland H., Staudenraus D., Camara B., Münch C., Hefter V., et al. Induction of robust cellular and humoral immunity against SARS-CoV-2 after a third dose of BNT162b2 vaccine in previously unresponsive older adults. Nat. Microbiol. 2022;7:195–199. doi: 10.1038/s41564-021-01046-z. [DOI] [PubMed] [Google Scholar]
- 20.Newman J., Thakur N., Peacock T.P., Bialy D., Elrefaey A.M.E., Bogaardt C., Horton D.L., Ho S., Kankeyan T., Carr C., et al. Neutralizing antibody activity against 21 SARS-CoV-2 variants in older adults vaccinated with BNT162b2. Nat. Microbiol. 2022;7:1180–1188. doi: 10.1038/s41564-022-01163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collier D.A., Ferreira I.A.T.M., Kotagiri P., Datir R.P., Lim E.Y., Touizer E., Meng B., Abdullahi A., CITIID-NIHR BioResource COVID-19 Collaboration. Elmer A., et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021;596:417–422. doi: 10.1038/s41586-021-03739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams L.R., Ferguson N.M., Donnelly C.A., Grassly N.C. Measuring Vaccine Efficacy Against Infection and Disease in Clinical Trials: Sources and Magnitude of Bias in Coronavirus Disease 2019 (COVID-19) Vaccine Efficacy Estimates. Clin. Infect. Dis. 2022;75:e764–e773. doi: 10.1093/cid/ciab914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitaker M., Elliott J., Bodinier B., Barclay W., Ward H., Cooke G., Donnelly C.A., Chadeau-Hyam M., Elliott P. Variant-specific symptoms of COVID-19 in a study of 1,542,510 adults in England. Nat. Commun. 2022;13:6856. doi: 10.1038/s41467-022-34244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldberg E.M., Southerland L.T., Meltzer A.C., Pagenhardt J., Hoopes R., Camargo C.A., Jr., Kline J.A. Age-related differences in symptoms in older emergency department patients with COVID-19: Prevalence and outcomes in a multicenter cohort. J. Am. Geriatr. Soc. 2022;70:1918–1930. doi: 10.1111/jgs.17816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motos A., López-Gavín A., Riera J., Ceccato A., Fernández-Barat L., Bermejo-Martin J.F., Ferrer R., de Gonzalo-Calvo D., Menéndez R., Pérez-Arnal R., et al. Higher frequency of comorbidities in fully vaccinated patients admitted to the ICU due to severe COVID-19: a prospective, multicentre, observational study. Eur. Respir. J. 2022;59 doi: 10.1183/13993003.02275-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Havers F.P., Pham H., Taylor C.A., Whitaker M., Patel K., Anglin O., Kambhampati A.K., Milucky J., Zell E., Moline H.L., et al. COVID-19-Associated Hospitalizations Among Vaccinated and Unvaccinated Adults 18 Years or Older in 13 US States, January 2021 to April 2022. JAMA Intern. Med. 2022;182:1071–1081. doi: 10.1001/jamainternmed.2022.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freund O., Tau L., Weiss T.E., Zornitzki L., Frydman S., Jacob G., Bornstein G. Associations of vaccine status with characteristics and outcomes of hospitalized severe COVID-19 patients in the booster era. PLoS One. 2022;17 doi: 10.1371/journal.pone.0268050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L., Holden K.A., Read J.M., Dondelinger F., Carson G., et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., Curtis H.J., Mehrkar A., Evans D., Inglesby P., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kartsonaki C., Baillie J.K., Barrio N.G., Baruch J., Beane A., Blumberg L., Bozza F., Broadley T., Burrell A., Carson G., et al. Characteristics and outcomes of an international cohort of 600 000 hospitalized patients with COVID-19. Int. J. Epidemiol. 2023;52:355–376. doi: 10.1093/ije/dyad012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.ISARIC Clinical Characterization Group. Garcia-Gallo E., Merson L., Kennon K., Kelly S., Citarella B.W., Fryer D.V., Shrapnel S., Lee J., Duque S., et al. ISARIC-COVID-19 dataset: A Prospective, Standardized, Global Dataset of Patients Hospitalized with COVID-19. Sci. Data. 2022;9:454. doi: 10.1038/s41597-022-01534-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathieu E., Ritchie H., Ortiz-Ospina E., Roser M., Hasell J., Appel C., Giattino C., Rodés-Guirao L. A global database of COVID-19 vaccinations. Nat. Human Behav. 2021;5:947–953. doi: 10.1038/s41562-021-01122-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The data that underpin this analysis are highly detailed clinical data on individuals hospitalised with COVID-19. Due to the sensitive nature of these data and the associated privacy concerns, they are available via a governed data access mechanism following review of a data access committee. Data can be requested via the IDDO COVID-19 Data Sharing Platform (http://www.iddo.org/covid-19). The Data Access Application, Terms of Access and details of the Data Access Committee are available on the website. Briefly, the requirements for access are a request from a qualified researcher working with a legal entity who have a health and/or research remit; a scientifically valid reason for data access which adheres to appropriate ethical principles. The full terms are at https://www.iddo.org/document/covid-19-data-access-guidelines. A small subset of sites who contributed data to this analysis have not agreed to pooled data sharing as above. In the case of requiring access to these data, please contact the corresponding author in the first instance who will look to facilitate access. Code used in the descriptive analysis is available.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.