Abstract
Background:
The Healthy Eating Index (HEI) and Alternate Healthy Eating Index (AHEI) are instruments developed by competing American research teams, aiming to assess the level of adherence to a dietary pattern, claimed to prevent chronic illness conditions such as dyslipidemia. This systematic review evaluated cross-sectional studies examining the association between HEI/AHEI score and the lipid profile in healthy participants.
Methods:
The systematic review was Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) compliant, and a search process was conducted through Scopus, Web of Knowledge, Google Scholar, Cochrane, PubMed, and ScienceDirect up to November 2022. Studies assessing the relationship between HEI/AHEI and lipid profile (low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and triglyceride (TG)) were eligible for inclusion. The statistical differences in outcomes, anthropometric indices, and demographic data were extracted from the selected studies. Also, the quality assessment of studies was performed using the Newcastle–Ottawa scale.
Results:
The systematic search presented 17 cross-sectional studies. Most of the studies revealed a significant correlation between HEI score and lipid profile (LDL-C, HDL-C, TG, and TC) (P < 0.05), while a few of them indicated a significant relationship between AHEI score and these factors. Overall, the elevation of HEI/AHEI score was associated with the improvement in lipid profile (P < 0.05), though this association was more obvious for HEI compared with AHEI.
Conclusions:
Overall, the results of the study indicated that an improved lipid profile in healthy individuals is associated with a higher score in either HEI or AHEI. Further research in the future is required to confirm the claim.
Keywords: Dyslipidemia, food quality, healthy eating index, lipid profile, systematic review
Introduction
Dyslipidemia is considered a modifiable risk factor for cardiovascular diseases. The control of this risk factor leads to reduced mortality caused by cardiovascular diseases.[1] Cardiovascular diseases remain the cause of 80% of deaths in developing countries.[2] The prevalence of dyslipidemia and thus cardiovascular diseases significantly changes with improvements in the economic status of societies and lifestyle modifications.[3] Although the prevalence of cardiovascular diseases has decreased over the past two decades in developed countries, recent findings suggest that 37% of Americans have a low high-density lipoprotein (HDL) cholesterol level, while this number in some Eastern countries such as Iran is 69%.[4,5] The results of a study in China indicated that the prevalence of dyslipidemia among women in 2012 was 34%.[3] Generally, the prevalence of dyslipidemia and cardiovascular diseases is higher among Eastern societies compared with their Western counterparts.[2,6]
The etiology of dyslipidemia and cardiovascular diseases indicates that lifestyle factors such as diet can play an important role in preventing these disorders.[7] A reduction in the consumption of saturated fatty acids, salt, and cholesterol can be a useful strategy in preventing dyslipidemia and cardiovascular disorders.[8] Most previous studies have dealt with examining individual food items. Nevertheless, assessing the quality of diet and its general components, because of the complexity of dietary patterns in different societies, can offer a better perspective for preventing dyslipidemia and cardiovascular diseases.[9] The American Healthy Eating Index (HEI) was designed to measure the general quality of an individual’s diet by assessing adherence to the Dietary Guidelines for Americans (DGA) and MyPyramid recommendations.[10] The HEI score is calculated by summing up the scores gained from assessing dietary diversity and the amount of sodium, cholesterol, saturated fatty acids, and total lipid consumed.[9] Obesity and lipid profile levels have an inverse relationship with HEI scores.[11] The Alternate Healthy Eating Index (AHEI) is a competing tool that assesses similar dietary parameters, while also taking the quality and source of nutrients consumed into account.[12] The AHEI is claimed to outperform HEI in predicting the risk of chronic disease.[13]
Adherence to special dietary patterns in different societies has increased considerably. For both HEI and AHEI, a high-scoring diet is claimed to help prevent chronic illness conditions, but previous studies have contradictory findings. Accordingly, this study aimed to systematically review cross-sectional studies conducted on evaluating the relationship between HEI/AHEI and lipid profile levels in healthy individuals.
Methods
Protocol
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Strategy was applied[14] with the registered code CRD42021287098. According to the research protocol and the local legislation, ethical approval was not needed.
Inclusion and exclusion criteria
Papers were included in this study according to the following inclusion criteria: i) studies that were issued as an original paper with full-text availability; ii) recruiting healthy individuals aged 18 years or older as study participants; iii) applying Food Frequency Questionnaire and 24-h recall as the dietary intake assessment tools; iv) those analyzing the association between HEI and AHEI with appropriate results; v) papers assessing at least one outcome from one of the following lipid profiles: total cholesterol (TC), TG, low-density lipoprotein cholesterol (LDL-C), or HDL-C; and vi) cross-sectional design. The exclusion criteria were as follows: i) studies documenting specific nutrients or other dietary patterns other than HEI and AHEI; ii) research conducted on nonhuman participants; iii) papers with unclear explained information about the research topic, study design, the data analysis method, and participants’ characteristics; and v) studies conducted on infants, children, adolescents, or pregnant or lactating women.
Data sources and search strategy
An online search was conducted for papers evaluating the relationship between HEI/AHEI score and lipid profiles (TG, LDL-C, HDL-C, and TC) using Scopus, Google Scholar, PubMed, Science Direct, Web of Knowledge, and Cochrane databases. Also, publisher databases including Springer Link, Wiley Online, and Elsevier were used up to the end of November 2022. No language limitations were considered in the literature search process, and supplementary data were gathered using reference lists of relevant publications. The keywords for searching in the PubMed database were the following: “Diet,” “Healthy,” “Food Quality,” “Diet Therapy,” “lipids,” “Lipid Metabolism,” “Triglycerides,” “High-Density Lipoproteins,” “Cholesterol, LDL,” “Cholesterol, HDL.” The keywords were searched as Medical Subject Headings (MeSH) terms and abstracts of documents. EndNote software (version 20, X9) was used to manage search results.
Study selection
Initially, the publications extracted from the aforementioned databases were categorized based on the title and abstract, and duplicate papers were eliminated. Then, the authors separately evaluated the list of the specified references, and the remaining studies, which did not meet the inclusion criteria, were omitted. Next, study selection was done by assessing full texts based on the eligibility criteria from the remaining studies. The authors finally checked the reference lists of qualitative synthesis publications to discover other related studies. Discrepancies were resolved through consensus.
Data extraction and analysis
The data extraction process was performed using Microsoft Excel software. For data extraction in selected papers, the purpose and design of the studies were applied, along with study participants and sample size, research topic and location, measurement tools, the demographic information of individuals including age, body mass index (BMI), and gender, inclusion and exclusion criteria, time of the study, outcome data, result of analysis, food record template (e.g., 24-h recall or Food Frequency Questionnaire (FFQ)), and type of diet quality index. In addition, effect size correlation was extracted and calculated using the formula (rYl = d / √(d2 + 4) for each outcome. The authors solved any disagreement via consensus.
Method for quality assessment
The Newcastle–Ottawa scale was used to evaluate the quality of cross-sectional studies in systematic review research. This instrument uses a “star” system for quality assessment of non-randomized studies in three units: ascertainment of outcomes (maximum three stars), comparability of research groups (maximum two stars), and participant selection (maximum five stars).[15] Since the maximum score on the scale was 10, we considered studies with scores of 6 or higher to enter the qualitative synthesis phase.[16] The details of quality assessment via the Newcastle-Ottawa scale are presented in Table 1.
Table 1.
Quality assessment of studies based on the Newcastle–Ottawa Scale*
| Studies | Selection | Comparability Controlling for confounding factors | Outcome | Total | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Representative samples | Justice of sample size | Satisfactory response rate | Validated tool for exposure measurement | Outcome assessment | Appropriate statistical test | |||
| Kant et al. (2005)[17] | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆☆ | ☆ | 9 |
| Drewnowski et al. (2009)[18] | ☆ | ☆ | - | ☆ | ☆☆ | ☆ | ☆ | 7 |
| Shah et al. (2010)[10] | - | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | 7 |
| Tardivo et al. (2010)[19] | - | ☆ | ☆ | ☆ | - | ☆☆ | ☆ | 6 |
| Belin et al. (2011)[20] | ☆ | ☆ | ☆ | ☆ | - | ☆ | ☆ | 7 |
| Nicklas et al. (2012)[21] | ☆ | ☆ | - | ☆ | ☆☆ | ☆☆ | ☆ | 8 |
| Asghari et al. (2013)[22] | - | ☆ | - | ☆ | ☆☆ | ☆☆ | ☆ | 7 |
| Haghighatdoost et al. (2013)[9] | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆☆ | ☆ | 9 |
| De Almeida Ventura et al. (2014)[23] | - | ☆ | ☆ | ☆ | - | ☆☆ | ☆ | 6 |
| Saraf-Bank et al. (2017)[11] | ☆ | ☆ | - | ☆☆ | ☆☆ | ☆ | ☆ | 8 |
| Rashidipour-Fard et al. (2017)[24] | - | ☆ | - | ☆☆ | ☆ | ☆☆ | ☆ | 7 |
| AlEssa et al. (2017)[25] | ☆ | ☆ | - | ☆☆ | ☆☆ | ☆ | ☆ | 8 |
| Lavigne-Robichaud et al. (2018)[26] | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 |
| Fallaize et al. (2018)[27] | ☆ | ☆ | - | ☆☆ | ☆ | ☆ | ☆ | 7 |
| Whitton et al. (2018)[28] | ☆ | ☆ | ☆ | ☆☆ | ☆☆ | ☆☆ | ☆ | 10 |
| Khakpouri et al. (2019)[29] | ☆ | ☆ | ☆ | ☆☆ | - | ☆☆ | ☆ | 8 |
| Landry et al. (2019)[30] | - | ☆ | - | ☆ | ☆ | ☆☆ | ☆ | 6 |
*The Newcastle–Ottawa Scale included three sections: selection (representative samples: 0–1 star, justice of sample size: 0–1 star, satisfactory response rate: 0–1 star, validated tool for exposure measurement: 0–2 stars); comparability (controlling for confounding factors: 0–2 stars); outcome (appropriate statistical test: 0–1 star, outcome assessment: 0–2 star)
Results
Search results
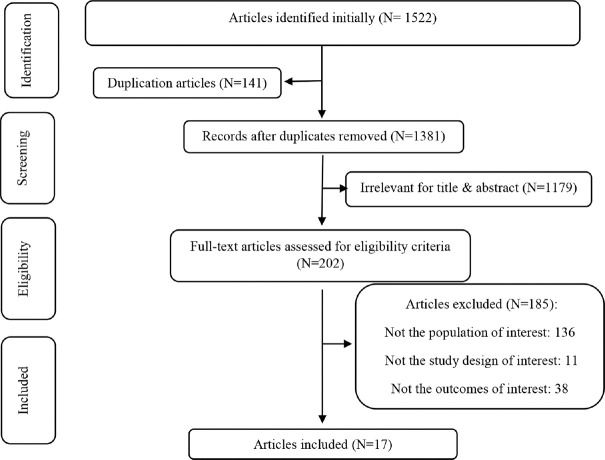
A primary database search resulted in the collection of 1522 articles, of which 141 articles were removed from the study due to duplication. Then, titles and abstracts were assessed and irrelevant studies were removed. Furthermore, 185 articles were removed from the study due to the following reasons: a) examining unrelated outcome variables, b) improper study design, and c) irrelevant target population. In the final step, 17 articles were included in the present systematic review. Figure 1 presents the search process.
Figure 1.
Flow chart of study selection
Quality assessment
The results of the quality assessment of studies using the Newcastle-Ottawa scale indicated that the studies by Tardivo et al. and Landry et al. had the lowest score (six stars), while the study by Whitton et al. obtained the highest score (ten stars) [Table 1]. Overall, the mean score on the scale was 7.47/10.
Study characteristics
The total number of participants in the 17 studies was 51510, with the age ranging from 18 to 90 years. The gender distribution was as follows: 17% male and 83% female. Also, the reported BMI values were within 18–43 kg/m2. Regarding the assessment of diet quality, four studies evaluated HEI, 11 studies assessed AHEI, and two studies examined HEI and AHEI simultaneously.[27,29] The average score of HEI and AHEI in the studies was within the range of 50 to 55. Assessment of the food intake of individuals was performed using FFQ (in eight studies) and 24-h recall (in nine studies) questionnaires [Table 1]. Studies had been carried out in the United States (n = 6), Brazil (n = 2), Iran (n = 5), France (n = 1), Canada (n = 1), and Singapore (n = 1). In addition, one study was conducted in seven countries of the European Union (EU).[27] Although the search strategy in this study included papers published by 2021, the publication date of studies was between 2005 and 2019 [Table 2].
Table 2.
Characteristics of the included studies investigating the association between diet quality score and lipid profile
| Author/year | Location | Sample size | Age range and BMI | Quality Index | Outcome | Food measurement tool | Adjustment | Effect size (r)* | Findings |
|---|---|---|---|---|---|---|---|---|---|
| Kant et al. (2005),[17] | USA | 8719 adults | <50 y: 67% ≥50 y: 33% BMI <25 kg/m2: 45% BMI ≥25 kg/m2: 55% | HEI | TC | 24-h recall | Age, race/ethnicity, education, smoking, alcohol use, BMI, recreational physical activity, hours of fasting, supplement use in the past 24 hours, supplement use in the past month | -0.57 | NS (P: 0.2) |
| HDL-C | -0.89 | S (P: 0.02) | |||||||
| LDL-C | -0.73 | S (P: 0.05) | |||||||
| TG | 0.70 | NS (P: 0.1) | |||||||
| Drewnowski et al. (2009),[18] | France | 5081 adults | 35–61 years | HEI | TC | 24-h recall | Age, energy intake, tobacco use, and alcohol consumption | NR | NS (P>0.05) |
| TG | NR | NS (P>0.05) | |||||||
| Shah et al. (2010),[10] | USA | 125 Women | 18–40 years; BMI >25 kg/m2 | HEI | TC | 24-hour recall | Energy intake and BMI | -0.75 | S (P: 0.04) |
| HDL-C | 0.78 | S (P: 0.03) | |||||||
| LDL-C | -0.82 | S (P: 0.033) | |||||||
| TG | -0.22 | NS (P: 0.333) | |||||||
| Tardivo et al. (2010),[19] | Brazil | 173 women | 45–75 years; BMI: 19.1–42.3 kg/m2 | HEI | TC | 24-h recall | NR | NR | NS (P>0.05) |
| HDL-C | NR | NS (P>0.05) | |||||||
| LDL-C | NR | NS (P>0.05) | |||||||
| TG | NR | NS (P>0.05) | |||||||
| Belin et al. (2011),[20] | USA | 1014 adults | 50–79 years; BMI: 24.6–27.8 kg/m2 | AHEI | HDL-C | FFQ | - | 0.39 | S (P<0.001) |
| LDL-C | -0.12 | NS (P: 0.279) | |||||||
| TG | -0.15 | S (P: 0.004) | |||||||
| Nicklas et al. (2012),[21] | USA | 18989 adults | ≥19 years; BMI: 27.4–29 kg/m2 | HEI | TC | 24-h recall | Ethnicity, gender, age, estimated energy ratio poverty–income ratio, BMI, physical activity, smoking, and alcohol | -0.92 | S (P: 0.001) |
| HDL-C | 0.86 | S (P: 0.005) | |||||||
| LDL-C | -0.91 | S (P: 0.004) | |||||||
| TG | 0.02 | NS (P: 0.70) | |||||||
| Asghari et al. (2013),[22] | Iran | 469 adults (Male: 33%) (Female: 67%) | 38.7±12.3 years; BMI <25: 43.03% BMI ≥25: 56.97% | HEI | TC | 24-h recall | Age, smoking status, waist circumference, body mass index, physical activity, and energy intake | NR | NS (P>0.05) |
| HDL-C | NR | NS (P>0.05) | |||||||
| LDL-C | NR | NS (P>0.05) | |||||||
| TG | NR | S (P: 0.038); just for male | |||||||
| Haghighatdoost et al. (2013),[9] | Iran | 9568 adults | 33–44 years; BMI: 25.6 kg/m2 | HEI | TC | FFQ | Age, smoking status, and BMI | 0.00 | NS (P: 0.4) |
| HDL-C | 0.00 | NS (P: 0.4) | |||||||
| LDL-C | 0.01 | NS (P: 0.05) | |||||||
| TG | 0.00 | NS (P: 0.3) | |||||||
| De Almeida Ventura et al. (2014),[23] | Brazil | 215 postmenopausal women | 44.5–90.1 years; BMI: 18.1–42.5 kg/m2 | HEI | TC | 24-hour recall | - | NR | NS (P: 0.27) |
| HDL-C | NR | NS (P: 0.37) | |||||||
| LDL-C | NR | NS (P: 0.75) | |||||||
| TG | NR | NS (P: 0.58) | |||||||
| Saraf-Bank et al. (2017),[11] | Iran | 1036 Iranian women | >30 years; BMI: 21.7–25.7 kg/m2 | HEI | HDL-C | FFQ | Age, BMI, cigarette smoking, physical activity, socioeconomic status, current estrogen use, menopausal status, and family history of diabetes and stroke | NR | S (P: 0.01) |
| TG | NR | S (P: 0.001) | |||||||
| Rashidipour-Fard et al. (2017),[24] | Iran | 107 elderly | 60–65 years BMI: 25.9 kg/m2 | HEI | TC | FFQ | Age, sex, energy intake, BMI | 0.01 | NS (P: 0.985) |
| HDL-C | -0.02 | NS (P: 0.975) | |||||||
| LDL-C | -0.16 | NS (P: 0.816) | |||||||
| TG | 0.47 | NS (P: 0.452) | |||||||
| AlEssa et al. (2017),[25] | USA | 775 healthy women | 51–75 years; BMI: 26.7 kg/m2 | AHEI | TC | FFQ | Age, BMI, Caucasian race, postmenopausal status and postmenopausal hormone use, parity, and age at first birth, family history of myocardial infarction, family history of diabetes, diabetes, diabetes medication use, statin use, smoking status, moderate-to-vigorous physical activity, multivitamin use | 0.50 | NS (P: 0.15) |
| HDL-C | 0.18 | NS (P: 0.40) | |||||||
| TG | 0.00 | NS (P: 0.94) | |||||||
| Lavigne-Robichaud et al. (2018),[26] | Canada | 811 adults | 35–37 years BMI: 33.1 kg/m2 | AHEI | HDL-C | 24-h recall | Age, sex, area of residence, total daily dietary energy intake, smoking status | NR | NS (P: 0.88) |
| TG | NR | NS (P: 0.37) | |||||||
| Fallaize et al. (2018),[27] | Seven European Union (EU) countries | 1480 adults (Male: 41.6%) (Female: 58.4%) | 18–75 years; BMI: 25.4 kg/m2 | HEI | TC | FFQ | Sex, age, country, energy intake (kcal), objective PAL | 0.00 | NS (P: 0.20) |
| AHEI | 0.04 | NS (P: 0.39) | |||||||
| Whitton et al. (2018),[28] | Singapore | 2108 Singapore residents | 27–53 years BMI: NR | AHEI | TC | FFQ | BMI, age, sex, ethnic group, daily energy intake, physical activity, cigarette smoking, working status, and housing type | NR | S (P<0.05) |
| HDL-C | NR | S (P<0.05) | |||||||
| LDL-C | NR | S (P<0.05) | |||||||
| TG | NR | NS (P>0.05) | |||||||
| Khakpouri et al. (2019),[29] | Iran | 748 men | 43.50±8.88 years BMI: 27.16±3.76 kg/m2 | HEI | TC | FFQ | - | 0.07 | NS (P: 0.1) |
| HDL-C | - 0.03 | NS (P: 0.6) | |||||||
| LDL-C | 0.09 | NS (P: 0.08) | |||||||
| TG | - 0.03 | NS (P: 0.5) | |||||||
| AHEI | TC | 0.01 | NS (P: 0.7) | ||||||
| HDL-C | - 0.03 | NS (P: 0.5) | |||||||
| LDL-C | 0.00 | NS (P: 0.9) | |||||||
| TG | 0.00 | NS (P: 0.9) | |||||||
| Landry et al. (2019),[30] | USA | 92 Hispanic college freshmen | 18–19 years BMI: NR | HEI | TC | 24-h recall | Sex, BMI percentile, total moderate-to-vigorous physical activity | NR | NS (P: 0.653) |
| HDL-C | NR | NS (P: 0.244) | |||||||
| LDL-C | NR | NS (P: 0.898) | |||||||
| TG | NR | S (P: 0.037) |
AHEI=Alternative Healthy Eating Index, HEI=Healthy Eating Index, FFQ=Food Frequency Questionnaire, BMI=body mass index, LDL-C=low-density lipoprotein cholesterol, HDL-C=high-density lipoprotein cholesterol, TG=total cholesterol, TG=triglycerides, PAL=physical activity level, NR=not reported, S=significant, NS=not significant, NR=no reported suitable data. * Calculate the effect size correlation, rYl, using the means and standard deviations of two quartiles (the first quartile and the last quartile); effect size correlation: rYl=d/ √(d2+4)
Outcomes
Considering the lipid profile components in this study, one of the included studies assessed the relationship between cholesterol and HEI and AHEI. However, two studies evaluated only two components of lipid factors including TG/TC and TG/HDL-C [Table 2]. Also, two studies examined the association between the three components of the lipid profile (TG, TC, and HDL-C) and AHEI. Likewise, other studies have evaluated the association between each of the four components of the lipid profile and dietary quality indices, including HEI and AHEI [Table 2].
Regarding the relationship between lipid profile and HEI and AHEI, two studies showed that a reduction in TC is associated with an increase in HEI score (P = 0.001 and P < 0.001).[10,21] In addition, the results of Whitton et al. indicated an inverse association between AHEI score and TC (β (95% CI): - 0.05 (- 0.07, - 0.03), P < 0.05).[28] However, other studies did not show a significant relationship between TC and dietary quality score (P > 0.05).
The results of four studies revealed that a significant increase in HDL-C occurs following an increase in HEI score (P = 0.03, P = 0.01, P = 0.02, P = 0.005).[10,11,17,21] Also, the findings of Whitton et al. and Belin et al. indicated a direct association between AHEI and HDL-C in a significant manner (β (95% CI) = 0.02 (0.01, 0.02), P < 0.05), β (95% CI) = 57.0 (48.0, 71.0) (quintile 1) vs 66.0 (56.0, 77.0) (quintile 5), P < 0.001)[20,28] [Table 2].
The statistical results of four studies showed that an increase in the score of HEI led to a significant decline in LDL-C (P = 0.033, P = 0.006, P = 0.02, P = 0.004).[9,10,17,21] A study by Whitton et al. reported that the AHEI score also has a significant inverse relationship with LDL-C (β (95% CI) = - 0.04 (- 0.06, - 0.02), P < 0.05).[28] The results of three studies indicated that elevation of HEI score led to a significant reduction in blood TG (P = 0.001, P = 0.005, and P = 0.037).[9,11,30] Also, the study of Asghari et al. revealed an opposite association between TG status and HEI score in men only (TG changes = - 8.8 vs 2.9; P = 0.038).[22] Furthermore, the results of Belin et al.’s study showed a significant reduction in blood TG following an increase in the AHEI score (β (95% CI) = 132.0 (95.0, 187.5) (quintile 1) vs 120.0 (94.0, 150.0) (quintile 5), P = 0.004).[20] Overall, the effect size of each of the outcomes was also extracted from papers included in the final phase, as shown in Table 2.
Discussion
The current systematic review evaluated 17 cross-sectional studies exploring the link between HEI/AHEI and lipid profile. Based on the studies reviewed here, four papers reported a negative significant association between HEI and TC, four between HEI and LDL-C, and four between HEI and TG, respectively, while two papers indicated a positive correlation between HEI and HDL-C. However, only one study revealed a negative relationship between AHEI and TC, one between AHEI and LDL-C, and one between AHEI and TG, respectively. In addition, two articles showed a positive association between AHEI and HDL-C.
Since diet and dietary patterns have a direct, pivotal effect on maintaining health, dietary quality indicators should be investigated thoroughly. To track dietary quality, HEI was created to improve health behaviors and prevent chronic complications, which is revised every 5 years.[31,32] The HEI scores up to 100 points to measure adherence to the US Dietary Guidelines, consumption of the five food groups, dietary variety, plus intakes of fat, cholesterol, and sodium.[19] Also, the most recent update of the HEI added an emphasis on healthy choices within groups, including whole grains, plant proteins, seafood, and an appropriate ratio of unsaturated to saturated fatty acids.[33] The AHEI was developed by making adjustments to the original HEI, with more focus on food sources and quality, with the particular target of mortality prediction.[12,34] Higher AHEI scores have been strongly related to a lower risk of chronic diseases such as heart failure and cardiovascular disease, diabetes, breast and colorectal cancer, and total mortality.[29] The key differences between AHEI and the original HEI include attention to cereal fiber, moderate alcohol intake, the red-to-white meat ratio, fat quality, and duration of multivitamin consumption.[35] However, overall both HEI and AHEI have been linked to significant risk reductions for all-cause mortality.[36,37]
The current study found that the studies by Saraf-Bank, Belin, Haghighatdoost, Kant, Whitton, and Nicklas et al. were powerful and well-designed with large sample sizes, which found an association between HEI/AHEI and different components of lipid profile improvements. In line with these findings, an updated meta-analysis indicated that diets with high scores of HEI and AHEI were associated with a remarkable decrease in the risk of neurodegenerative diseases, cardiovascular diseases (CVDs), type 2 diabetes, cancer, and all-cause mortality.[38] Also, in a cohort study with 12,413 participants, those in the highest quintile of HEI-2015, compared with those in the lowest quintile, had 16, 32, and 18% lower risk of CVD incident, CVD mortality, and all-cause mortality, respectively.[32] Nevertheless, the participants included in the study by Saraf-Bank et al.[11] were female nurses, which would make the results less generalizable to other women and men due to different socio-demographic situations, educational level, occupation, and income.
As Asghari et al.[22] revealed, HEI conformity has been linked to a lower level of TG, particularly in men. Also, Yu et al. demonstrated that a higher HEI score is associated with a lower mortality rate in men.[39] These results might be attributed to a higher intake of favorable dietary factors such as vegetables and fruits.[40] Although Fallaize et al.[27] did not find a significant link between either HEI or AHEI and TC, higher HEI/AHEI was associated with more advanced age, total carotenoids, and omega-3 index, as well as lower BMI, waist circumference (WC), and waist-to-height ratio (WHtR). The lack of significant results for TC could be due to the uncommon approach used, called dried blood spot (DBS), to assess cholesterol level, which differs from previous studies.[41,42]
However, there is a lack of information about other aspects of lipid profile including TG, LDL-C, and HDL-C. Another study by Drewnowski et al.[18] showed that HEI was a poor predictor of lipid indices. However, this result might reflect the fact that plasma TG and TC were normal in the participants. In this regard, Huffman et al. revealed that there was no association between HEI score and congenital heart defect (CHD) risk among Cuban American individuals. However, AHEI was a predictor of 10-year CHD risk in diabetic patients.[43] In addition, another cross-sectional study indicated no possible relationship between HEI and overweight/obesity in adolescents.[44] Note that HEI/AHEI does not involve functional foods and phytochemicals as a component, which is an important limitation of both indices.[9]
Shah et al.[10] reported that HEI scores were linked to improved TC, LDL-C, and HDL-C, but since the 24-hour recall was used to collect dietary information, measurement error related to within-person variability is possible. Furthermore, insignificant improvements in lipid indices in Ventura[23] and Landry et al.[30] studies might result from small sample sizes and one-day 24-hour recall to gather dietary information. Since dietary guideline recommendations are intended to be fulfilled over time,[12] a single day’s intake is inadequately representative of dietary pattern and seasonal variations.
In a study by AlEssa et al., almost all participants were nurses and they were healthy with normal lipid profiles, which makes the results of the study debatable.[25] Also, Lavigne-Robichaud et al.[45] revealed that the use of 24-hour recall would increase the risk of omitting or forgetting some foods. Although Khakpouri et al.[29] was a well-designed study, it was conducted only on Iranian male employees with normal health status, which made the result less representative of the entire population of Iran. Note that the sample sizes of Rashidipour[24] and Tardivo[19] studies were small, due to the nature of cross-sectional studies. Also, in Tardivo and colleagues’ research, all individuals came from low socioeconomic groups and the results may not reflect the general population. Some evidence indicated that consumption of whole grains, fish, lean meats, low-fat dairy, vegetables, and fruits is more likely in higher socioeconomic groups. In contrast, due to the association between food costs and food selection, people with a low-level income have weakened health, nutritional status, and diet quality.[46,47] Of note, due to different cultures, dietary behaviors, and habits, HEI and AHEI may not be valid in all populations.[48] Since the etiology of obesity and its related complications is multifactorial and lifestyle parameters such as socioeconomic status play an important role in its development, it may explain the different findings in the included studies, which had been conducted in developed and developing countries.[49] Developing countries experience several challenges related to corruption and political instability, while in developed countries various forms of food insecurity and urban poverty can occur such as establishment of more fast-food restaurants and few grocery stores.[50]
Our study had certain limitations based on the studies included in the review. First, FFQ was employed as a dietary assessment tool in some studies, while some others employed 24-h recall questionnaire. FFQ shows the dietary intake of participants in the long term, while 24-h recall reveals the dietary intake of individuals in the short term. This can make bias and affect our conclusion to some extent. Furthermore, according to the search strategy and published articles, the main limitation has been the exclusive inclusion of cross-sectional studies. In these studies, no causal associations can be established and individuals with higher serum lipids may have altered their nutrition because of their lipid profile. Moreover, studies have large variations in the determination of HEI, AHEI, blood parameters, and dietary evaluation. Also, considering the reported results of included studies and heterogeneity assessment, it was not possible to conduct a meta-analysis. Finally, although biological age is a pronounced risk factor for dyslipidemia, the eligible studies in our systematic review had been conducted on different group ages.
Conclusions
In conclusion, HEI/AHEI might have a positive correlation with lipid profile improvement, and healthcare professionals should be aware of the potential use of these indices for characterizing the diet-related risk of chronic disease conditions. Further studies are required to confirm this conclusion.
Conflict of Interest
The authors declare no conflict of interest.
Funding
This research has not received any funding from institutions.
Ethical Consideration
Not applicable.
Code of Ethics
Not applicable; Prisma Code: CRD42021287098.
Authors’ Contributions
1) H.Faraji and S.Ferrie: Paper searching, study designing, writing the manuscript, and final checking 2) S.Jamshidi and P.S.Azar: Manuscript editing, writing the manuscript, paper reading and outcomes assessing.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Not applicable.
References
- 1.Stein R, Ferrari F, Scolari F. Genetics, dyslipidemia, and cardiovascular disease: New insights. Curr Cardiol Rep. 2019;21:68. doi: 10.1007/s11886-019-1161-5. [DOI] [PubMed] [Google Scholar]
- 2.Zaribaf F, Mohammadifard N, Sarrafzadegan N, Karimi G, Gholampour A, Azadbakht L. Dietary patterns in relation to lipid profiles among Iranian adults. J Cardiovasc Thorac Res. 2019;11:19–27. doi: 10.15171/jcvtr.2019.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J, Wang Z, Wang H, Du W, Su C, Zhang J, et al. Association between dietary patterns and blood lipid profiles among Chinese women. Public Health Nutr. 2016;19:3361–8. doi: 10.1017/S136898001600197X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: Findings from the third national health and nutrition examination survey. JAMA. 2002;287:356–9. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 5.Sarrafzadegan N, Kelishadi R, Sadri G, Malekafzali H, Pourmoghaddas M, Heidari K, et al. Outcomes of a comprehensive healthy lifestyle program on cardiometabolic risk factors in a developing country: The isfahan healthy heart program. Arch Iran Med. 2013;16:4–11. [PubMed] [Google Scholar]
- 6.Schober A, Nazari-Jahantigh M, Wei Y, Bidzhekov K, Gremse F, Grommes J, et al. MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat Med. 2014;20:368–76. doi: 10.1038/nm.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez-Delgado F, Katsiki N, Lopez-Miranda J, Perez-Martinez P. Dietary habits, lipoprotein metabolism and cardiovascular disease: From individual foods to dietary patterns. Crit Rev Food Sci Nutr. 2021;61:1651–69. doi: 10.1080/10408398.2020.1764487. [DOI] [PubMed] [Google Scholar]
- 8.Liu C, Xue Y, Wang Y, Zhang Y, Qiao D, Wang B, et al. Association between dietary patterns and dyslipidemia in adults from the henan rural cohort study. Asia Pac J Clin Nutr. 2020;29:299–308. doi: 10.6133/apjcn.202007_29(2).0013. [DOI] [PubMed] [Google Scholar]
- 9.Haghighatdoost F, Sarrafzadegan N, Mohammadifard N, Sajjadi F, Maghroon M, Boshtam M, et al. Healthy eating index and cardiovascular risk factors among Iranians. J Am Coll Nutr. 2013;32:111–21. doi: 10.1080/07315724.2013.767590. [DOI] [PubMed] [Google Scholar]
- 10.Shah BS, Freeland-Graves JH, Cahill JM, Lu H, Graves GR. Diet quality as measured by the healthy eating index and the association with lipid profile in low-income women in early postpartum. J Am Diet Assoc. 2010;110:274–9. doi: 10.1016/j.jada.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 11.Saraf-Bank S, Haghighatdoost F, Esmaillzadeh A, Larijani B, Azadbakht L. Adherence to healthy eating index-2010 is inversely associated with metabolic syndrome and its features among Iranian adult women. Eur J Clin Nutr. 2017;71:425–30. doi: 10.1038/ejcn.2016.173. [DOI] [PubMed] [Google Scholar]
- 12.McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, et al. Diet quality and major chronic disease risk in men and women: Moving toward improved dietary guidance. Am J Clin Nutr. 2002;76:1261–71. doi: 10.1093/ajcn/76.6.1261. [DOI] [PubMed] [Google Scholar]
- 13.McCullough ML, Willett WC. Evaluating adherence to recommended diets in adults: The Alternate Healthy Eating Index. Public Health Nutr. 2006;9:152–7. doi: 10.1079/phn2005938. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Modesti PA, Reboldi G, Cappuccio FP, Agyemang C, Remuzzi G, Rapi S, et al. Panethnic differences in blood pressure in Europe: A systematic review and meta-analysis. PLoS One. 2016;11:e0147601. doi: 10.1371/journal.pone.0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J, Hoang T, Bu SY, Kim JM, Choi JH, Park E, et al. Associations of dietary intake with cardiovascular disease, blood pressure, and lipid profile in the Korean Population: A systematic review and meta-analysis. J Lipid Atheroscler. 2020;9:205–29. doi: 10.12997/jla.2020.9.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kant AK, Graubard BI. A comparison of three dietary pattern indexes for predicting biomarkers of diet and disease. J Am Coll Nutr. 2005;24:294–303. doi: 10.1080/07315724.2005.10719477. [DOI] [PubMed] [Google Scholar]
- 18.Drewnowski A, Fiddler EC, Dauchet L, Galan P, Hercberg S. Diet quality measures and cardiovascular risk factors in france: Applying the Healthy Eating Index to the SU. VI. MAX study. J Am Coll Nutr. 2009;28:22–9. doi: 10.1080/07315724.2009.10719757. [DOI] [PubMed] [Google Scholar]
- 19.Tardivo AP, Nahas-Neto J, Nahas EA, Maesta N, Rodrigues MA, Orsatti FL. Associations between healthy eating patterns and indicators of metabolic risk in postmenopausal women. Nutr J. 2010;9:64. doi: 10.1186/1475-2891-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belin RJ, Greenland P, Allison M, Martin L, Shikany JM, Larson J, et al. Diet quality and the risk of cardiovascular disease: The Women's Health Initiative (WHI) Am J Clin Nutr. 2011;94:49–57. doi: 10.3945/ajcn.110.011221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicklas TA, O'Neil CE, Fulgoni VL., 3rd Diet quality is inversely related to cardiovascular risk factors in adults. J Nutr. 2012;142:2112–8. doi: 10.3945/jn.112.164889. [DOI] [PubMed] [Google Scholar]
- 22.Asghari G, Mirmiran P, Hosseni-Esfahani F, Nazeri P, Mehran M, Azizi F. Dietary quality among Tehranian adults in relation to lipid profile: Findings from the Tehran Lipid and Glucose Study. J Health Popul Nutr. 2013;31:37–48. doi: 10.3329/jhpn.v31i1.14747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Almeida Ventura D, Vânia de Matos F, Ramos EG, Marinheiro LP, Souza RA, Chaves CR, et al. Association between quality of the diet and cardiometabolic risk factors in postmenopausal women. Nutr J. 2014;13:121. doi: 10.1186/1475-2891-13-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rashidipour-Fard N, Karimi M, Saraf-Bank S, Baghaei MH, Haghighatdoost F, Azadbakht L. Healthy eating index and cardiovascular risk factors among iranian elderly individuals. ARYA Atheroscler. 2017;13:56–65. [PMC free article] [PubMed] [Google Scholar]
- 25.AlEssa HB, Malik VS, Yuan C, Willett WC, Huang T, Hu FB, et al. Dietary patterns and cardiometabolic and endocrine plasma biomarkers in US women. Am J Clin Nutr. 2017;105:432–41. doi: 10.3945/ajcn.116.143016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavigne-Robichaud M, Moubarac JC, Lantagne-Lopez S, Johnson-Down L, Batal M, Laouan Sidi EA, et al. Diet quality indices in relation to metabolic syndrome in an Indigenous Cree (Eeyouch) population in northern Québec, Canada. Public Health Nutr. 2018;21:172–80. doi: 10.1017/S136898001700115X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fallaize R, Livingstone KM, Celis-Morales C, Macready AL, San-Cristobal R, Navas-Carretero S, et al. Association between diet-quality scores, adiposity, total cholesterol and markers of nutritional status in european adults: Findings from the Food4Me study. Nutrients. 2018;10:49. doi: 10.3390/nu10010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitton C, Rebello SA, Lee J, Tai ES, van Dam RM. A healthy asian a posteriori dietary pattern correlates with a priori dietary patterns and is associated with cardiovascular disease risk factors in a multiethnic Asian population. J Nutr. 2018;148:616–23. doi: 10.1093/jn/nxy016. [DOI] [PubMed] [Google Scholar]
- 29.Khakpouri S, Safari M, Ghazizadeh H, Parizadeh SMR, Nematy M, Tayefi M, et al. The relationship between the healthy eating index and an alternate healthy eating index with the risk factors for cardiovascular disease in a population from northeastern Iran. Translational Metabolic Syndrome Research. 2019;2:1–6. [Google Scholar]
- 30.Landry MJ, Asigbee FM, Vandyousefi S, Khazaee E, Ghaddar R, Boisseau JB, et al. Diet quality is an indicator of disease risk factors in hispanic college freshmen. J Acad Nutr Diet. 2019;119:760–8. doi: 10.1016/j.jand.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Guenther PM, Casavale KO, Reedy J, Kirkpatrick SI, Hiza HA, Kuczynski KJ, et al. Update of the healthy eating index: HEI-2010. J Acad Nutr Diet. 2013;113:569–80. doi: 10.1016/j.jand.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu EA, Steffen LM, Coresh J, Appel LJ, Rebholz CM. Adherence to the healthy eating index-2015 and other dietary patterns may reduce risk of cardiovascular disease, cardiovascular mortality, and all-cause mortality. J Nutr. 2020;150:312–21. doi: 10.1093/jn/nxz218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Onvani S, Haghighatdoost F, Surkan PJ, Larijani B, Azadbakht L. Adherence to the healthy eating index and alternative healthy eating index dietary patterns and mortality from all causes, cardiovascular disease and cancer: A meta-analysis of observational studies. J Hum Nutr Diet. 2017;30:216–26. doi: 10.1111/jhn.12415. [DOI] [PubMed] [Google Scholar]
- 34.Al-Ibrahim AA, Jackson RT. Healthy eating index versus alternate healthy index in relation to diabetes status and health markers in U. S. adults: NHANES 2007-2010. Nutr J. 2019;18:26. doi: 10.1186/s12937-019-0450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huffman FG, De La Cera M, Vaccaro JA, Zarini GG, Exebio J, Gundupalli D, et al. Healthy eating index and alternate healthy eating index among Haitian Americans and African Americans with and without type 2 diabetes. J Nutr Metab 2011. 2011 doi: 10.1155/2011/398324. 398324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwingshackl L, Hoffmann G. Diet quality as assessed by the Healthy Eating Index, the Alternate Healthy Eating Index, the Dietary Approaches to Stop Hypertension score, and health outcomes: A systematic review and meta-analysis of cohort studies. J Acad Nutr Diet. 2015;115:780–800. doi: 10.1016/j.jand.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 37.Arem H, Reedy J, Sampson J, Jiao L, Hollenbeck AR, Risch H, et al. The healthy eating index 2005 and risk for pancreatic cancer in the NIH-AARP study. J Natl Cancer Inst. 2013;105:1298–305. doi: 10.1093/jnci/djt185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwingshackl L, Bogensberger B, Hoffmann G. Diet quality as assessed by the healthy eating index, alternate healthy eating index, dietary approaches to stop hypertension score, and health outcomes: An updated systematic review and meta-analysis of cohort studies. J Acad Nutr Diet. 2018;118:74–100. doi: 10.1016/j.jand.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 39.Yu D, Sonderman J, Buchowski MS, McLaughlin JK, Shu XO, Steinwandel M, et al. Healthy eating and risks of total and cause-specific death among low-income populations of african-americans and other adults in the southeastern United States: A prospective cohort study. PLoS Med. 2015;12:e1001830. doi: 10.1371/journal.pmed.1001830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drewnowski A, Monsivais P, Maillot M, Darmon N. Low-energy-density diets are associated with higher diet quality and higher diet costs in French adults. J Am Diet Assoc. 2007;107:1028–32. doi: 10.1016/j.jada.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 41.Rothman RL, Malone R, Bryant B, Shintani AK, Crigler B, Dewalt DA, et al. Arandomized trial of a primary care-based disease management program to improve cardiovascular risk factors and glycated hemoglobin levels in patients with diabetes. Am J Med. 2005;118:276–84. doi: 10.1016/j.amjmed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 42.Tantikosoom W, Thinkhamrop B, Kiatchusakul S, Jarernsiripornkul N, Srinakarin J, Ojongpian S. Randomized trial of atorvastatin in improving endothelial function in diabetics without prior coronary disease and having average cholesterol level. J Med Assoc Thai. 2005;88:399–406. [PubMed] [Google Scholar]
- 43.Huffman FG, Zarini GG, McNamara E, Nagarajan A. The healthy eating index and the alternate healthy eating index as predictors of 10-year chd risk in cuban americans with and without type 2 diabetes. Public Health Nutr. 2011;14:2006–14. doi: 10.1017/S1368980011001054. [DOI] [PubMed] [Google Scholar]
- 44.Moraeus L, Lindroos AK, WarensjöLemming E, Mattisson I. Diet diversity score and healthy eating index in relation to diet quality and socio-demographic factors: Results from a cross-sectional national dietary survey of swedish adolescents. Public Health Nutr. 2020;23:1754–65. doi: 10.1017/S1368980019004671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lavigne-Robichaud M, Moubarac JC, Lantagne-Lopez S, Johnson-Down L, Batal M, Laouan Sidi EA, et al. Diet quality indices in relation to metabolic syndrome in an indigenous cree (eeyouch) population in northern québec, canada. Public Health Nutr. 2018;21:172–80. doi: 10.1017/S136898001700115X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lo YT, Chang YH, Lee MS, Wahlqvist ML. Health and nutrition economics: Diet costs are associated with diet quality. Asia Pac J Clin Nutr. 2009;18:598–604. [PubMed] [Google Scholar]
- 47.Darmon N, Drewnowski A. Does social class predict diet quality? Am J Clin Nutr. 2008;87:1107–17. doi: 10.1093/ajcn/87.5.1107. [DOI] [PubMed] [Google Scholar]
- 48.Levine CS, Miyamoto Y, Markus HR, Rigotti A, Boylan JM, Park J, et al. Culture and healthy eating: The role of independence and interdependence in the United States and Japan. Pers Soc Psychol Bull. 2016;42:1335–48. doi: 10.1177/0146167216658645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khodarahmi M, Asghari-Jafarabadi M, Abbasalizad Farhangi M. A structural equation modeling approach for the association of a healthy eating index with metabolic syndrome and cardio-metabolic risk factors among obese individuals. PLoS One. 2019;14:e0219193. doi: 10.1371/journal.pone.0219193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Filippini R, Mazzocchi C, Corsi S. The contribution of Urban Food Policies toward food security in developing and developed countries: A network analysis approach. Sustainable Cities and Society. 2019;47:101506. [Google Scholar]