Abstract
Background:
In 2019, South Africa, Nigeria, Tanzania, Democratic Republic of Congo, Uganda, Mozambique, Zambia, Angola, Cameroon, Zimbabwe, Ghana, Ethiopia, Malawi, Kenya, South Sudan and Côte d’Ivoire accounted for 80% of children living with HIV (CLHIV) not receiving HIV treatment. This manuscript describes pediatric HIV testing to inform case-finding strategies.
Methods:
We analyzed US President’s Emergency Plan for AIDS Relief monitoring, evaluation, and reporting data (October 1, 2018 to September 30, 2019) for these 16 countries. Number of HIV tests and positive results were reported by age band, country, treatment coverage and testing modality. The number needed to test (NNT) to identify 1 new CLHIV 1–14 years was measured by testing modality and country. The pediatric testing gap was estimated by multiplying the estimated number of CLHIV unaware of their status by NNT per country.
Results:
Among children, 6,961,225 HIV tests were conducted, and 101,762 CLHIV were identified (NNT 68), meeting 17.6% of the pediatric testing need. Index testing accounted for 13.0% of HIV tests (29.7% of positive results, NNT 30), provider-initiated testing and counseling 65.9% of tests (43.6% of positives, NNT 103), and universal testing at sick entry points 5.3% of tests (6.5% of positives, NNT 58).
Conclusions:
As countries near HIV epidemic control for adults, the need to increase pediatric testing continues. Each testing modality – PITC, universal testing at sick entry points, and index testing – offers unique benefits. These results illustrate the comparative advantages of including a strategic mix of testing modalities in national programs to increase pediatric HIV case finding.
Keywords: pediatric HIV, testing, risk screening, Africa
The United States President’s Emergency Plan for AIDS Relief (PEPFAR), launched in 2003, has supported notable achievements towards reaching HIV epidemic control in many countries. However, significant gaps in case identification remain, particularly among children, adolescent girls and young women (15–29 years), and men.1 Only 53% of the estimated 1.8 million children living with HIV (CLHIV) <15 years globally were receiving life-saving antiretroviral therapy (ART) in 2019.2 Sixteen sub-Saharan African countries accounted for 80% of this treatment gap, or approximately 668,500 CLHIV not yet on ART.3
One of the main challenges hindering pediatric treatment coverage is suboptimal early infant diagnosis (EID), which increases the need for HIV testing beyond infancy. In 2019, there were an estimated 150,000 new pediatric HIV infections.1 The United Nations Children’s Fund estimated only 58.8% of HIV-exposed infants had received virologic testing by 2 months1 due to numerous health systems challenges4 with poorer coverage historically affecting diagnoses among CLHIV now 1–14 years. PEPFAR-supported programs identified 17,080 infants living with HIV in 2019,5 accounting for approximately 11% of new pediatric infections. Recent trends indicate most new pediatric HIV infections occur during breastfeeding.6 The HIV risk among infants is particularly high following maternal acquisition of HIV during the breast-feeding period.7,8 However, many CLHIV remain undiagnosed due to poor implementation of maternal re-testing9–11 and misconceptions among frontline workers that older children are not at risk for HIV.12
The World Health Organization (WHO) and PEPFAR recommend a combination of index testing, provider-initiated testing and counseling (PITC), and universal testing of sick children to identify undiagnosed CLHIV.13,14 Index, or exposure-based, testing offered to the biological children of parents or siblings living with HIV facilitates the identification of well CLHIV before they develop symptoms.13,15,16 While PEPFAR supports exposure-based screening, testing for children is not dependent on maternal status alone, given challenges with stigma, disclosure, and mothers working in distant geographic locations, to avoid delays in pediatric HIV diagnoses. PEPFAR support for targeted PITC using HIV risk screening in outpatient and routine well-child health services, orphan and vulnerable children (OVC) programs, and voluntary medical male circumcision (VMMC) settings started in October 2019. Testing in VMMC programs confirms an individual’s status to provide relevant program interventions. While not considered a case-finding approach, many boys 10–14 were newly diagnosed through VMMC programs. PEPFAR guidance supported the use of screening tools in age groups with low HIV risk17 and recommended increasing the minimum age for VMMC to ≥15 years from ≥10 years,14 starting October 2020. Ideal screening tools would decrease the number needed to test (NNT) to identify 1 new CLHIV in these entry points while maintaining the overall number of CLHIV diagnosed.17,18 HIV risk screening tools assess signs, symptoms, or medical history to prioritize children for testing,14,17,19–21 yet risk missing some CLHIV depending on tool sensitivity.21 Although WHO recommends HIV testing among children in health facilities in inpatient and outpatient departments, PITC implementation has faced several challenges, especially in outpatient settings.12,22–26 Universal testing, or the testing of all children without a documented HIV status, is recommended for all children who are hospitalized, infected with tuberculosis (TB) or malnourished in generalized epidemics13,27 due to high HIV-positivity.28–32 While universal testing at sick entry points remains important, it typically identifies children late in disease progression when they are already symptomatic with advanced disease.33
PEPFAR-supported country programs report monitoring, evaluation, and reporting (MER) indicators quarterly to a central repository. These data facilitate close monitoring of pediatric HIV testing by age band and testing modality. This manuscript high-lights pediatric testing gaps in programs supported by PEPFAR and provides considerations to improve pediatric case findings.
MATERIALS AND METHODS
We analyzed routine program MER data from October 1, 2018 to September 30, 2019 (fiscal year, FY19) for children 1–14 years from 16 sub-Saharan African countries: South Africa, Nigeria, Tanzania, Democratic Republic of Congo (DRC), Uganda, Mozambique, Zambia, Angola, Cameroon, Zimbabwe, Ghana, Ethiopia, Malawi, Kenya, South Sudan, and Côte d’Ivoire. Infants <1 year were excluded because their virologic testing results are reported through a different EID MER indicator monitoring the prevention of mother-to-child transmission. Countries were purposively selected because they account for 80% of the pediatric treatment gap. MER data represent sub-national regions (eg, provinces, districts) receiving PEPFAR support, which varies by country. The Joint United Nations Programme on HIV/AIDS (UNAIDS) data were used to estimate the pediatric HIV treatment coverage by country and number of CLHIV, not on treatment (1 − % CLHIV on treatment = estimated % treatment gap; UNAIDS country CLHIV estimate × % on treatment = estimated number CLHIV on treatment).3 National HIV testing guidelines and the Barr-DiChiara updated review34 were used to classify countries into 2 categories according to the age of consent for HIV testing (<15 and ≥15 years). Countries were categorized by treatment coverage (% CLHIV receiving ART) into 3 categories (<50%, 50–69% and ≥70%).
Descriptive statistics were used to calculate the total number of HIV antibody tests conducted, positive test results, and newly diagnosed CLHIV initiated on ART. Proxy linkage was calculated, using (aggregate # CLHIV new on ART/aggregate # positive test results), due to the inability to measure ART initiation rates at the individual level among children newly diagnosed. NNT, which provides realistic expectations for frontline HIV testing counselors of the number of tests needed to diagnose a new CLHIV, was measured by testing modality, age band, and country; (# HIV tests/# positive results).35 Like testing yield, NNT is affected by serval factors, including the proportion of undiagnosed PHLIV in various settings, testing and ART coverage, and testing modality. The estimated pediatric testing gap (FY19 NNT × the estimated number of CLHIV unaware of their HIV status) and proportion of pediatric HIV testing need met (# tests conducted/# estimated tests needed) were calculated by country, region, and ART coverage. The proportion of HIV tests (# HIV number of tests conducted in a modality/total # of tests conducted) and positive test results (# positive results in a modality/total # of positive results across all modalities) were calculated by modality. Results were disaggregated by age band (1–4, 5–9, 10–14 years), testing approach (index testing in facility and community settings; PITC in outpatient, pediatric under-5 clinics, and VMMC settings; and universal testing in TB, inpatient and malnutrition entry points), geographic sub-region (Eastern and Southern Africa and Western and Central Africa), ART coverage, and age of consent for HIV testing.
RESULTS
Ten of the 16 countries responsible for the largest pediatric treatment gaps were in Eastern and Southern Africa (Table 1). Four countries had a pediatric ART coverage of ≥70%, including Kenya (81%), Mozambique (72%), Zimbabwe (71%) and Malawi (70%). The gap in pediatric HIV treatment ranged from approximately 12,000 in Côte d’Ivoire to 175,000 in South Africa. South Africa, Nigeria, Tanzania and DRC had the largest numbers of CLHIV (<15 years) not on treatment. While countries in Eastern and Southern Africa met 23.8% of their estimated pediatric HIV testing need, Mozambique (45.6%), Kenya (41.4%) and Zambia (37.5%) exceeded the regional average. Countries in West and Central Africa met 7.0% of their estimated testing need, while Cote d’Ivoire (17.2%) and Nigeria (10.7%) exceeded the regional average. Age of consent for HIV testing ranged from 12 years in South Africa and Uganda to 18 years in Nigeria, DRC and South Sudan, and 72.2% of HIV tests among children 1–14 years were in countries where age of consent was ≥15 years.
TABLE 1.
Sixteen Countries With the Largest Pediatric HIV Testing and Treatment Gaps by Geographic Sub-region, 2019, and Age of Consent for HIV Testing
| Adult HIV Prevalence* (15–49 yr) (Uncertainty Bounds) | Estimated Number of CLHIV* (0–14 yr) (Uncertainty Bounds) | Estimated CLHIV Unaware of Their Status* | Estimated Testing Gap (NNT x # CLHIV Unaware) | Estimated Number of CLHIV on ART* | Pediatric ART Coverage* | Age of Consent for HIV Testing(yr)†,34 | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Eastern and Southern Africa | |||||||
| South Africa | 19.3% (12.2–25.0%) | 330,200 (205,700–547,800) | 86,600 | 4,850,400 | 156,200 | 47.3% | 12 |
| Tanzania | 4.8% (4.5–5.1%) | 121,100 (101,100–139,200) | 57,900 | 4,171,900 | 61,200 | 50.6% | 16 |
| Uganda | 5.7% (5.3–6.0%) | 106,300 (95,200–119,300) | 40,100 | 3,731,900 | 66,200 | 62.3% | 12 |
| Mozambique | 11.7% (9.4–14.6%) | 132,800 (104,400–179,800) | 37,800 | 2,302,800 | 95,100 | 71.6% | 15 |
| Zambia | 11.3% (10.8–11.8%) | 85,600 (76,700–95,100) | 35,300 | 2,190,800 | 50,200 | 58.7% | 16 |
| Zimbabwe | 12.4% (11.1–13.7%) | 84,300 (71,100–98,900) | 24,500 | 1,469,100 | 59,800 | 71.0% | 16 |
| Ethiopia | 0.9% (0.7–1.1%) | 47,900 (32,000–67,100) | 28,800 | 2,624,700 | 19,100 | 39.8% | 15 |
| Malawi | 8.5% (8.0–8.8%) | 68,600 (57,000–78,200) | 20,600 | 1,299,300 | 48,000 | 69.9% | 13 |
| Kenya | 4.4% (3.8–5.1%) | 90,000 (72,700–114,700) | 16,900 | 2,064,600 | 73,100 | 81.2% | 15 |
| South Sudan | 2.3% (1.8–2.9%) | 15,800 (11,500–20,700) | 13,900 | 833,000 | 1900 | 12.0% | 18 |
| Subtotal | 7.4% (4.7–9.5%) | 1,082,500 (674,400–1,796,200) | 362,400 | 25,014,700 | 630,700 | 58.3% | |
| Western and Central Africa | |||||||
| Nigeria | 1.3% (1.0–1.8%) | 128,500 (85,800 (194,300) | 75,000 | 4,123,000 | 53,500 | 41.7% | 18 |
| DRC | 0.8% (0.7–0.9%) | 74,200 (59,400–88,200) | 51,400 | 1,438,300 | 22,800 | 30.8% | 18 |
| Angola | 1.8% (1.6–2.2%) | 40,200 (32,700–50,000) | 35,100 | 702,500 | 5100 | 12.7% | 15 |
| Cameroon | 3.1% (2.8–3.3%) | 36,600 (29,200–42,500) | 26,200 | 3,356,200 | 10,400 | 28.4% | 14 |
| Ghana | 1.7% (1.5–2.0%) | 30,100 (23,900–36,300) | 23,400 | 398,300 | 6600 | 22.1% | 16 |
| Cote d’Ivoire | 2.2% (1.9–2.5%) | 23,500 (18,400–29,400) | 12,100 | 1,905,800 | 11,300 | 48.3% | 16 |
| Subtotal | 1.4% (1.1–1.9%) | 333,100 (222,300–503,700) | 223,200 | 14,510,700 | 109,800 | 33.0% | |
AIDSinfo | UNAIDS [Internet]. 2021 [cited 2021 Oct 5]. Available from: https://aidsinfo.unaids.org/ (data source for HIV prevalence, PLHIV and CLHIV estimates, and ART coverage and gap estimates by country). All figures are rounded to the nearest 100 to reflect level of precision.
Most countries allow emancipated minors, including adolescents that are sexually active, married or living with a partner, head of household, or pregnant, among other conditions, to consent to HIV testing.
In FY19, PEPFAR reported 6,961,225 HIV tests among children: 33.7% for children 1–4 years, 24.9% (5–9 years) and 41.4% (10–14 years) (Table 2) across supported sites. There were 101,762 positive HIV tests (63.0%) among school-aged children (5–14 years). Twenty fewer tests were needed to identify 1 new CLHIV 1–4 years (NNT 62) or 5–9 years (NNT 60) compared with a new CLHIV 10–14 years (NNT 82). The mean proxy linkage to ART rate for all newly diagnosed CLHIV 1–14 years was 80.5%, highest (89.6%) among younger children (1–4 years) and lowest (66.5%) among younger adolescents (10–14 years).
TABLE 2.
Pediatric HIV Testing, Linkage and Treatment Cascade by Age, Geographic Sub-region, National ART Coverage, and Age of Consent for Testing in 16 PEPFAR-funded Countries, October 1, 2018 to September 30, 2019
| Number of HIV Tests Conducted | % Pediatric HIV Testing Need Met | Number of Positive Test Results | NNT* | Number of PLHIV† New on HIV Treatment | Proxy Linkage Rate‡ | |
|---|---|---|---|---|---|---|
|
| ||||||
| Age (1–14 yr) (%) | 6,961,225 | 17.6% | 101,762 | 68 | 81,926 | 80.5% |
| 1–4 | 2,342,723 (33.7%) | — | 37,651 (37.0%) | 62 | 33,750 (41.2%) | 89.6% |
| 5–9 | 1,735,040 (24.9%) | — | 28,747 (28.2%) | 60 | 24,672 (30.1%) | 85.8% |
| 10–14 | 2,883,492 (41.4%) | — | 35,364 (34.8%) | 82 | 23,504 (28.7%) | 66.5% |
| Sub-region or country (%) | ||||||
| Eastern and Southern Africa | 5,945,318 (85.4%) | 23.8% | 86,101 (84.6%) | 69 | 68,027 (83.0%) | 79.0% |
| South Africa | 1,108,589 (15.9%) | 22.9% | 19,695 (19.4%) | 56 | 11,374 (13.9%) | 57.8% |
| Mozambique | 1,049,816 (15.1%) | 45.6% | 17,282 (17.0%) | 61 | 12,751 (15.6%) | 73.8% |
| Kenya | 854,915 (12.3%) | 41.4% | 7034 (6.9%) | 122 | 6158 (7.5%) | 87.5% |
| Uganda | 441,806 (6.3%) | 11.8% | 4726 (4.6%) | 93 | 6043 (7.4%) | 127.9% |
| Tanzania | 994,436 (14.3%) | 23.8% | 13,751 (13.5%) | 72 | 11,348 (13.9%) | 82.5% |
| Zimbabwe | 281,296 (4.0%) | 19.1% | 4697 (4.6%) | 60 | 3680 (4.5%) | 78.3% |
| Ethiopia§ | 103,840 (1.5%) | 4.0% | 1144 (1.1%) | 91 | 1670 (2.0%) | 146.0% |
| Malawi | 259,896 (3.7%) | 20.0% | 4138 (4.1%) | 63 | 4419 (5.4%) | 106.8% |
| Zambia | 822,166 (11.8%) | 37.5% | 13,156 (12.9%) | 62 | 10,155 (12.4%) | 77.2% |
| South Sudan | 28,558 (0.4%) | 3.4% | 478 (0.5%) | 60 | 429 (0.5%) | 89.7% |
| Western and Central Africa | 1,015,937 (14.6%) | 7.0% | 15,661 (15.4%) | 65 | 13,899 (17.0%) | 88.7% |
| Nigeria | 442,908 (6.4%) | 10.7% | 8079 (7.9%) | 55 | 7291 (8.9%) | 90.2% |
| DRC§ | 90,733 (1.3%) | 6.3% | 3265 (3.2%) | 28 | 2981 (3.6%) | 91.3% |
| Angola | 18,244 (0.3%) | 2.6% | 918 (0.9%) | 20 | 569 (0.7%) | 62.0% |
| Cameroon | 131,454 (1.9%) | 3.9% | 1024 (1.0%) | 128 | 906 (1.1%) | 88.5% |
| Ghana | 4833 (0.1%) | 1.2% | 287 (0.3%) | 17 | 248 (0.3%) | 86.4% |
| Cote d’Ivoire | 327,765 (4.7%) | 17.2% | 2088 (2.1%) | 157 | 1904 (2.3%) | 91.2% |
| ART coverage¶ | ||||||
| <50% | 2,256,924 (32.4%) | 11.2% | 36,978 (36.3%) | 61 | 27,372 (33.4%) | 74.0% |
| 50%–69% | 2,258,408 (32.4%) | 22.4% | 31,633 (31.1%) | 71 | 27,546 (33.4%) | 86.0% |
| ≥70% | 2,445,923 (35.1%) | 34.3% | 33,151 (32.6%) | 74 | 27,008 (33.0%) | 81.0% |
| Age of consent (yr)‖ | ||||||
| <15 | 1,941,745 (27.9%) | 14.7% | 29,583 (29.1%) | 66 | 22,742 (27.8%) | 76.9% |
| ≥15 | 5,019,510 (72.1%) | 19.1% | 72,179 (70.9%) | 70 | 59,184 (72.2%) | 82.0% |
NNT to identify 1 new CLHIV (eg, the number of tests divided by the number of positive test results).
PLHIV = people living with HIV.
Proxy linkage rate = percent of newly diagnosed children linked to HIV treatment divided by the total number of newly diagnosed children.
PEPFAR supports 3 of 26 provinces in DRC; PEPFAR Ethiopia noted the majority of tests and results were not reported for the outpatient modality in FY19, which contributed to the high proxy linkage.
ART coverage: <50%: South Sudan, Angola, Ghana, Cameroon, DRC, Ethiopia, Nigeria, South Africa, Cote d’Ivoire; 50%−69%: Tanzania, Uganda, Zambia; ≥70%: Malawi, Zimbabwe, Mozambique, Kenya.
Age of consent: <15: South Africa, Uganda, Cameroon, Malawi; ≥15: Ethiopia, Kenya, Angola, CDI, Nigeria, Mozambique, Zambia, Zimbabwe, DRC, Tanzania, Ghana, South Sudan.
The Western and Central Africa sub-region accounted for 14.6% of HIV tests (Table 2). Although HIV prevalence is generally lower in Western and Central Africa, NNT was similar in countries in Western and Central Africa (NNT 65, range NNT17–NNT157) and Eastern and Southern Africa (NNT 69, range NNT56–NNT122). Nine countries with <50% ART coverage—South Africa, South Sudan, Ethiopia, Nigeria, DRC (geographical coverage for PEP-FAR DRC includes support for 3 of 26 provinces). Angola, Ghana, Cameroon, and Cote d’Ivoire—accounted for 32.4% of HIV tests (2,256,924), 11.2% of their pediatric testing need met and 36,978 positive tests (36.3% of positive results). Four countries with ≥70% ART coverage accounted for 35.1% of HIV tests (2,445,923), 34.3% of their pediatric testing need met, and 33,151 positive tests (32.6% of positive results). While 68 tests were needed to identify 1 new CLHIV, the ratio of tests per CLHIV unaware of their status for the 9 countries with <50% ART coverage was much lower (4.6 tests per CLHIV unaware of their status) compared with the 4 countries with ≥70% ART coverage (16.4 tests per CLHIV unaware of their status). The proxy linkage rate, however, was higher for countries in Western and Central African (88.7%) than Eastern and Southern Africa (79.0%).
PITC accounted for 65.9% of tests and 43.6% of positive test results (Table 3). The PITC NNT (103) was higher than the overall NNT (68) to identify one new CLHIV. Nearly half of all pediatric HIV testing (46.6%) occurred in outpatient departments (OPD). Pediatric (<5 years) well-child clinics accounted for 6.4% of tests and 4.5% of positive results. VMMC programs accounted for 12.8% of tests and 6.1% of positive results with 99.5% of tests and 99.9% of positive results among boys 10–14 years. South Africa (20.6%), Mozambique (20.6%), and Uganda (20.7%) accounted for more than half of the testing conducted in VMMC, and South Africa (59.8%) and Mozambique (24.0%) accounted for over two- thirds of positive results.
TABLE 3.
Key Outcomes by HIV Case Finding Modality, October 1, 2018 to September 30, 2019
| Number of HIV Tests Conducted | Proportion of HIV Tests Conducted* | Number of Positive HIV Tests | Proportion of Positive HIV Tests† | NNT‡ | |
|---|---|---|---|---|---|
|
| |||||
| PITC | 4,589,157 | 65.9% | 44,375 | 43.6% | 103 |
| Outpatient (OPD) | 3,247,106 | 46.6% | 33,745 | 33.2% | 96 |
| Pediatric (<5 yr) Clinics | 447,931 | 6.4% | 4453 | 4.5% | 101 |
| VMMC | 894,120 | 12.8% | 6177 | 6.1% | 145 |
| Index | 906,847 | 13.0% | 30,199 | 29.7% | 30 |
| Index facility | 667,027 | 9.6% | 23,654 | 23.9% | 28 |
| Index community | 239,820 | 3.4% | 6545 | 6.5% | 37 |
| Universal | 366,778 | 5.3% | 6653 | 6.5% | 58 |
| TB | 45,109 | 0.6% | 2896 | 2.9% | 16 |
| Inpatient | 301,180 | 4.3% | 3482 | 3.6% | 86 |
| Malnutrition | 20,489 | 0.3% | 275 | 0.3% | 75 |
Proportion of HIV tests = number of tests conducted in that modality divided by the total number of tests conducted.
Proportion of positive tests = the number of positive results in that modality divided by the total number of positive results.
NNT to identify 1 child living with HIV (eg, the number of tests divided by the number of positive test results).
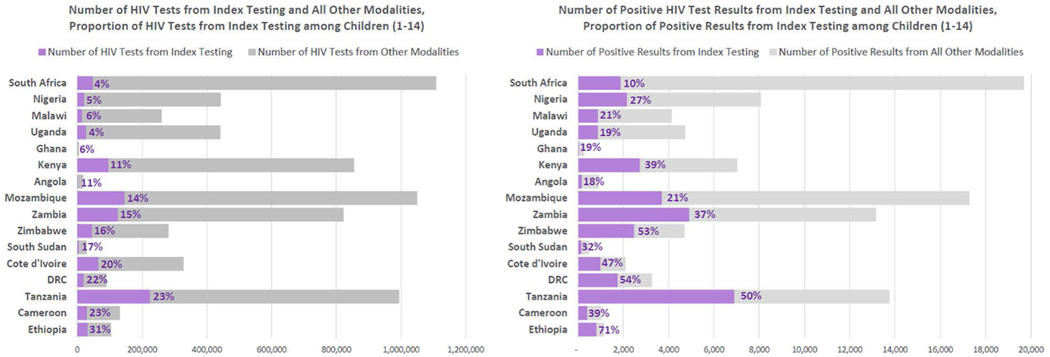
Index testing accounted for 13.0% of HIV tests and 29.7% of positive test results (NNT 30). The proportion of tests from facility-based index testing was nearly 3 times higher (667,027 tests) than community-based index testing (239,820 tests). The NNT for index testing was low in facility (28) and community (37) settings. Index testing accounted for ≥20% of positive test results for all countries except South Africa (10%), Angola (18%), Uganda (19%) and Ghana (19%) (Fig. 1). Index testing identified 32% less CLHIV (30,199) compared with PITC (44,375) due to the larger number of children tested through PITC. The proportion of tests conducted using index testing was lowest in South Africa (4%), Uganda (4%) and Nigeria (5%) (Fig. 1).
FIGURE 1.
Number of HIV tests and positive results from index testing and all other modalities and the proportion of HIV tests and positive results from index testing among children1–14 by country¥, October 1, 2018 to September 30, 2019. ¥Results for proportion of HIV tests and positive results through index testing for Ethiopia need to be cautiously interpreted because most HIV tests and results in outpatient entry points were not reported in FY19; Geographic coverage for PEPFAR DRC includes support for 3 of 26 provinces.
Universal testing at sick entry points accounted for 5.3% of tests and 6.5% of positive results (NNT 58). Nearly half of the CLHIV identified through universal testing were diagnosed in inpatient settings (3482 CLHIV), followed by TB (2896 CLHIV). The NNT was lowest for HIV testing overall in TB.16 Inpatient NNT was 86 and malnutrition NNT was 75.
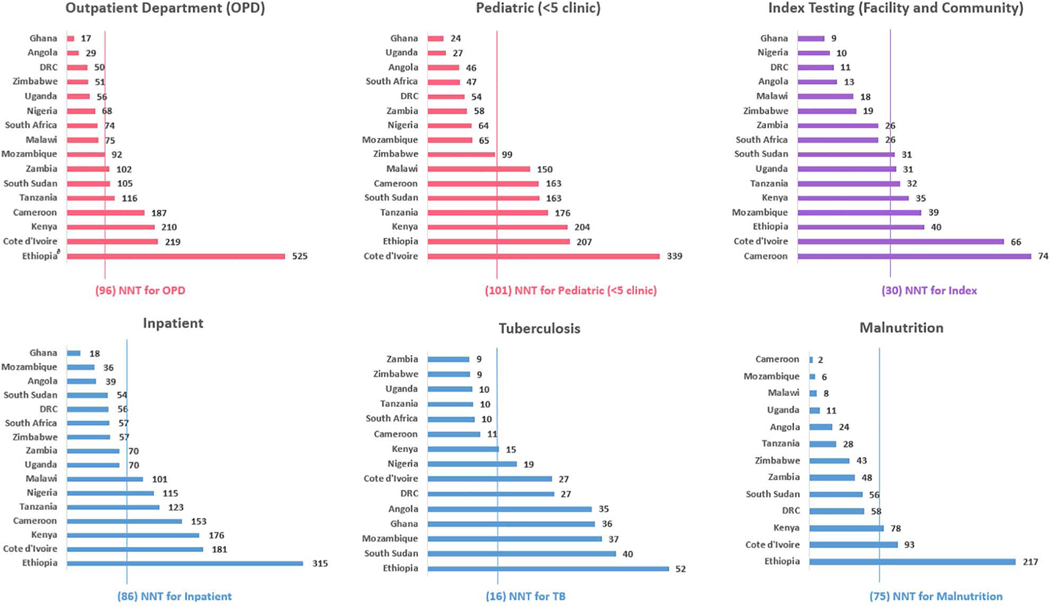
NNTs varied across HIV testing modalities and countries (Fig. 2). For PITC, the NNT was highest in OPD (NNT 96) and pediatric under-5 clinics (NNT 101). For OPD, the NNT was lower in countries implementing screening tools, including Ghana (NNT 17), DRC (NNT 50), Zimbabwe (NNT 51) and Uganda (NNT 56). These 4 countries implementing risk screening accounted for 6.6% of OPD tests and 12.4% of OPD positives (OPD NNT 52) compared with the other 12 countries (OPD NNT 102). Index testing required 50% less testing (NNT 30) to identify a CLHIV compared with pediatric HIV testing overall (NNT 68). The NNT was lowest for index testing in Ghana (NNT 9), Nigeria (NNT 10), DRC (NNT 11) and Angola (NNT 13)—countries with the lowest pediatric ART coverage.
FIGURE 2.
Number of children that programs needed to test (NNT) to identify 1 positive child by modality and country, October 1, 2018 to September 30, 2019∂. ∂Lines represent overall NNT by modality for pooled data from all countries. Results for outpatient department testing in Ethiopia need to be cautiously interpreted because most HIV tests and results in outpatient entry points were not reported in FY19; geographic coverage for PEPFAR DRC includes support for 3 of 26 provinces.
DISCUSSION
Key considerations for pediatric HIV testing programs are important across testing modalities (Figure, Supplemental Digital Content 1, http://links.lww.com/INF/E875). While countries determine what proportion of the estimated testing need is feasible to program annually, the ratio of HIV tests per CLHIV unaware of their status was low across countries, indicating the need for more pediatric testing overall. The testing ratio was lowest in countries with <50% pediatric ART coverage with the majority located in the West and Central Africa sub-region, highlighting the need to increase pediatric HIV testing in these settings. Low-prevalence countries, like Ethiopia, DRC, Nigeria, Ghana and Angola, can continue to strengthen the routine identification of pregnant women living with HIV to decrease maternal-to-child transmission of HIV, which ranges from 17% to 25% in these countries.1 Ethiopia’s extremely low HIV prevalence (0.9%) means pediatric programs test more children across modalities to identify 1 new CLHIV (Fig. 2).
Although several countries in Eastern and Southern Africa are close to achieving 90% of PLHIV overall knowing their status, awareness for CLHIV lags substantially. In Eastern and Southern Africa, 78% of adults were on treatment versus 57% of CLHIV with 95% of pregnant women living with HIV on ART. Disparities were even larger in Western and Central Africa where only 35% of CLHIV were on treatment versus 77% of adults with only 56% of pregnant women living with HIV on ART.3,36 Countries with low pediatric ART coverage (eg, South Africa, Nigeria, Ghana, South Sudan and Angola) or low ART coverage among pregnant women living with HIV and high rates of MTCT (eg, DRC, Nigeria, Angola, Ghana and Ethiopia, where MTCT ranges from 17% to 25%1) can scale up pediatric HIV testing, specifically index testing coverage for biological children of PLHIV and siblings of CLHIV to prevent delays in HIV diagnosis, which can lead to excess morbidity and mortality.37–40 It is important to ensure PEPFAR programs support host country governments and partners to prioritize a mix of facility and community testing approaches to address barriers to testing and allocate pediatric targets and budgets that adequately address the HIV testing and treatment gaps among children.
This analysis shows many children (63%) are diagnosed later in childhood (school-aged, 5–14 years), reinforcing the need for programs, providers and parents to test older children.41–43 Countries can simultaneously work to strengthen EID and maternal retesting, while expanding testing options for school-age children 5–14 years. A pooled analysis from 12 studies showed children infected through breastfeeding have a longer net survival (25% alive at 16.9 years), even in the absence of HIV treatment, than those infected perinatally (25% alive at 10.6 years).44 Similarly, a study in Kenya found routine screening for children 10–14 years increased testing by 2.7 times and case identification by 2.4 times, indicating under-testing among eligible older children.20 Programmatically, countries may consider retraining frontline healthcare workers to consider HIV as an underlying diagnosis for older children 5–14 years. High-prevalence countries may consider policies to ensure all children entering school have a documented HIV status, or have received risk screening for underlying HIV infection, and offered HIV testing. This approach is currently being piloted in Zambia (Chalilwe Chungu, MD, email communication, June 2021). Countries can review their pediatric HIV estimates to better understand which age bands have the largest numbers of undiagnosed CLHIV (eg, Eswatini, Lesotho, Malawi among children 10–14 years, Zambia and Zimbabwe among children 5–9 years, and Tanzania among children 1–4 years).45 Programs may need to test more older children to identify those living with HIV, as reflected in a slightly higher NNT (82) for children 10–14 years, and identify strategies to improve linkage (66.5% among children 10–14 years). Potential interventions to improve linkage include enhanced case management,46 peer-navigated services,47 implementation of adolescent-friendly treatment services,48 disclosure counseling,49 and linkage and enrollment in OVC programs.50
CLHIV presenting to OPDs, well-child clinics, OVC programs, and VMMC services may appear healthy yet have an underlying HIV infection. Nearly half of the CLHIV identified (43.6%) were diagnosed through PITC in outpatient settings, which was the most common testing strategy, identified the largest number of CLHIV, and required more tests to identify 1 new CLHIV than index testing and universal testing of sick children. Programs may consider implementing a combination of screening for maternal HIV status, or exposure screening,51 screening using validated risk assessment tools,21,52 and eligibility screening among adolescents20 in OPD settings. Screening can address undertesting, especially when combined with a comprehensive intervention package (eg, clinical mentorship, monitoring and evaluation, extended clinic hours), as shown by 1 study in Kenya among children 10–18 years old across 139 healthcare facilities.20 Screening tools may offer more benefit in high-burden countries not currently implementing universal HIV testing for children and adolescents with an unknown HIV status, or with resource constraints limiting the implementation of universal PITC, given the 50% reduction in NNT. Screening tools, comprised of a mix of clinical, historical, exposure, and behavioral questions, offer the opportunity to accelerate HIV case identification20 by identifying children eligible for testing and linking them to testing services.21 Screening tools should be context-specific, validated to reduce missed diagnoses for CLHIV, and monitored closely to assess the impact on the absolute number of CLHIV identified.21,53 HIV risk screening tools validated in 3 countries had the following sensitivity and specificity, respectively: Uganda (87.8%, 62.6%), Tanzania (89.2%, 37.5%) and Zimbabwe (80.4%, 66.3%).19,52,53 As countries near HIV epidemic control for adults, it will be important to maintain access to HIV testing services for children in outpatient settings, as 37.7% of CLHIV in this analysis were identified in OPD and pediatric under 5 clinic settings.
While South Africa and Nigeria have the largest pediatric treatment gaps, accounting for 174,000 and 75,000 CLHIV not on treatment, respectively, they have the lowest proportion of pediatric HIV tests coming from index testing, ranging from 4% to 5%. Even small increases in index testing volumes for biological children of PLHIV and siblings of CLHIV, especially in countries with large gaps in pediatric treatment coverage and a low proportion of tests conducted in index testing, like Uganda, Ghana and Angola, could translate into more children identified (Fig. 1). Although index testing accounted for only 13% of all pediatric HIV tests, it identified 30% of all newly diagnosed CLHIV across these 16 countries. To increase access, countries can ensure all PLHIV accessing HIV health services in the facility and community are offered index testing at every encounter and implement systems to track the coverage of line-listed biological children, HIV testing of pediatric contacts, wraparound services to facilitate testing for children living apart from parents, and linkage to care for newly identified CLHIV.54 Countries may consider conducting file audits for adults newly diagnosed and already on treatment, especially clients with treatment interruptions or who are virally unsuppressed to ensure all biological children or siblings have a documented HIV status or are offered HIV testing. Index testing is proactive and can identify CLHIV earlier before they develop advanced HIV disease.15 Furthermore, it is important to integrate index testing into differentiated service delivery models as encounters with the health system may become less frequent for clients stable on treatment.
Finally, countries can improve monitoring to ensure 100% of children receiving inpatient, TB or malnutrition services are offered HIV testing (ie, universal testing), especially for countries with less effective prevention of mother-to-child transmission programs where children are more likely to be missed by EID or present later with advanced disease. WHO recommends HIV testing for all children in inpatient settings in high-prevalence countries,13 yet studies show testing coverage often remains below 100%.55,56 Pediatric HIV testing for hospitalized children has a lower NNT in Mozambique and South Africa, as well as many Western and Central African countries like Ghana, Angola and DRC (Fig. 2). PEPFAR guidance recommends household contact tracing for all PLHIV co-infected with TB as a highly effective case-finding strategy to identify other household members, including children, who may be infected with TB and/or HIV. Recommendations include screening all children in OPD settings for TB symptoms and linking children with presumptive TB to TB and HIV testing services.18 Furthermore, WHO recommends offering HIV testing to all children receiving TB or malnutrition services.13 Programs can conduct file audits in well-child clinics to identify children falling off the growth curve and refer them for HIV and TB testing, as studies show CLHIV are commonly stunted and underweight at diagnosis.42,57 Countries may consider strategies to routinely link CLHIV identified through inpatient, TB and malnutrition settings (which accounted for 6.5% of newly diagnosed CLHIV in this analysis), as well as for all CLHIV (<5) at diagnosis58 (representing 37.0% of newly diagnosed CLHIV in this analysis) to an appropriate evaluation for advanced disease and linkage to the required package of care.
This study was subject to 5 limitations. First, although countries follow PEPFAR monitoring and reporting guidance, data quality and reporting by testing strategy vary across countries. For example, Ethiopia noted underreporting in the outpatient modality, and there is the possibility that some children 12–17 months received antibody testing and were included in the testing calculations, even though guidelines recommend PCR testing. Second, treatment coverage and gaps are based on UNAIDS modeling data for entire countries and MER data (HIV testing, positive results, proxy linkage) reflect only provinces or districts receiving PEPFAR support, which varies by country. For example, PEPFAR only supports 3 of 26 provinces in DRC. Third, PEPFAR testing indicators track the number of tests conducted and not the number of individual children tested. The number of reported tests and positive results over-represents to an unknown extent the number of individual children who were tested, and individual CLHIV identified, overall and by country. Fourth, UNAIDS estimates for CLHIV unaware of their status is for children <15 years and MER data for HIV tests conducted are for children 1–14 years, causing the estimated tests needed to be higher and percent testing need met to be lower than expected, due to the inclusion of children <1 year in the aggregated Spectrum data. Fifth, ART initiation rates were not measured prospectively at the individual level among children who received a new HIV diagnosis. Thus, the extent to which proxy linkage rates represent the proportion of individual CLHIV newly initiated on ART is unknown overall and by country yet serves as a programmatic pulse check along the clinical cascade. Additional research is needed to calculate the cost to identify 1 new CLHIV using different testing modalities and inform the optimal mix of testing strategies to rapidly diagnose the largest number of CLHIV.
CONCLUSIONS
As countries design pediatric HIV testing programs, no 1 approach will close the pediatric testing and treatment gap. Each testing modality offers unique benefits, when it comes to early identification (ie, index testing), cost (ie, OPD testing), identifying a high absolute number of children (ie, OPD testing), high yield (ie, TB and index), increasing the rate of HIV case identification (ie, exposure screening, risk screening, and eligibility screening), and identifying children most at risk for advanced disease (eg, TB, malnutrition, inpatient, and pediatric <5 years clinics) and linking them to the appropriate package of care. Programs can evaluate their current composition of testing modalities and re-design, where needed, to promote a more optimal and effective mix of testing modalities.
Supplementary Material
ACKNOWLEDGMENTS
The authors recognize frontline health workers, Ministries of Health, PEPFAR implementing partners, CDC, USAID and DOD country office colleagues advancing pediatric and adolescent HIV case-finding approaches and reporting HIV testing data to facilitate program improvements and targeted pediatric HIV testing strategies to identify C/ALHIV earlier and link them to life-saving treatment.
National HIV Testing Workgroup: Geoffrey Taasi, MPH (Uganda Ministry of Health, Kampala, Uganda), Esther Nazziwa, MMed (DGHT, CDC, Kampala, Uganda), Madina Apolot, MSc (DGHT, CDC, Kampala, Uganda), Esther Nkolo, PhD (PMCB, USAID, Kampala, Uganda), Peris Urasa, MPH (National AIDS Control Programme, Ministry of Health, Community Development, Gender, Elderly and Children, Dodoma, Tanzania), Margreth Kagashe, MPH (National AIDS Control Programme, Ministry of Health, Community Development, Gender, Elderly and Children, Dodoma, Tanzania), Edward Machege, MD (DGHT, CDC, Dar es Salaam, Tanzania), Oscar Rwabiyago, MD (DGHT, CDC, Dar es Salaam, Tanzania), Fredrick Rwegerera, MD (PMCB, USAID, Dar es Salaam, Tanzania), Zoraima Neto, PhD (DGHT, CDC, Luanda, Angola), E. Amaka Nwankwo-Igomu, DrPH (U.S. Embassy, Luanda, Angola), Magdalene Mayer, MD (DGHT, CDC, Yaoundé, Cameroon), Valery Nzima, MD (PMCB, USAID, Yaoundé, Cameroon), Aka Kouame Herve Prao, MD (DGHT, CDC, Abidjan, Cote d’Ivoire), Lucie Dagri, MS (PMCB, USAID, Abidjan, Cote d’Ivoire), Henri Longuma, MPH (DGHT, CDC, Kinshasa, Democratic Republic of Congo), Wondimu Teferi, MD (DGHT, CDC, Addis Ababa, Ethiopia), Chanie Temesgen, MD (DGHT, CDC, Addis Ababa, Ethiopia), Tsegaye Tilahun, MPH (PMCB, USAID, Addis Ababa, Ethiopia), Silas Quaye, MPH (DGHT, CDC, Accra, Ghana), Ekua Houphouet, MPH (PMCB, USAID, Accra, Ghana), Lennah Nyabiage, MMed (DGHT, CDC, Nairobi, Kenya), Immaculate Mutisya, MMed (PMCB, USAID, Nairobi, Kenya), Teresa Simiyu, MSc (PMCB, USAID, Nairobi, Kenya), Samson Anangwe, MScPH (Defense Health Agency, Nairobi, Kenya), Dumbani Kayira, MBBS (DGHT, CDC, Lilongwe, Malawi), Gerald Zomba, MPH (PMCB, USAID, Lilongwe, Malawi), Owen Kumwenda, MPH (PMCB, USAID, Lilongwe, Malawi), Maria Ines de Deus, MD (DGHT, CDC, Maputo, Mozambique), Mercia Matsinhe, MD (PMCB, USAID, Maputo, Mozambique), Bilkisu Ibrahim Jibrin (Federal Ministry of Health, Abuja, Nigeria), Omodele Johnson Fagbamigbe, MBCHB (DGHT, CDC, Abuja, Nigeria), Onyeka Igboelina (PMCB, USAID, Abuja, Nigeria), Dolapo Ogundehin (PMCB, USAID, Abuja, Nigeria), Gurpreet Kindra, PhD (DGHT, CDC Pretoria, South Africa), Hlamalani Mabasa (PCMB, USAID, Pretoria, South Africa), John Mondi, MD (DGHT, CDC), Sudhir Bunga, MD (DGHT, CDC), Nicholas Baabe, MD (Juba, South Sudan; PCMB, USAID, Juba, South Sudan), Kebby Musokotwane, MSc (DGHT, CDC, Lusaka, Zambia), Megumi Itoh, MD (DGHT, CDC, Lusaka, Zambia), Godfrey Lingenda, MD (PCMB, USAID, Lusaka, Zambia), Talent Maphosa, MD (DGHT, CDC, Harare, Zimbabwe), Solomon Mukungunugwa, MD (PCMB, USAID, Harare, Zimbabwe).
The findings and conclusions are those of the author(s) and do not necessarily represent the official position of the US Centers for Disease Control and Prevention or the US Agency for International Development.
This study was supported by the US President’s Emergency Plan for AIDS Relief through the U.S. Centers for Disease Control and Prevention and the U.S. Agency for International Development.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
The authors have no conflicts of interest to disclose.
J.G., M.P., E.R. and A.M. led the study design. K.B., M.H. and J.G. extracted the data. J.G. and M.H. conducted data analysis and developed visualizations. J.G., A.M. and P.S. drafted the manuscript. All co-authors reviewed the manuscript and approved the final version.
REFERENCES
- 1.Progress towards the Start Free, Stay Free, AIDS Free targets. 2020. Report [Internet]. [cited Jan 13, 2021]. Available at: https://www.unaids.org/sites/default/files/media_asset/start-free-stay-free-aids-free-2020-progress-report_en.pdf.
- 2.UNAIDS_FactSheet_en.pdf [Internet]. [cited Jan 8, 2021]. Available at: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf. [Google Scholar]
- 3.AIDSinfo | UNAIDS [Internet]. 2021. [cited Oct 5, 2021]. Available at: https://aidsinfo.unaids.org/. [Google Scholar]
- 4.Mofenson LM, Cohn J, Sacks E. Challenges in the early infant HIV diagnosis and treatment cascade. JAIDS J Acquir Immune Defic Syndr. 2020;84:S1–S4. Available at: https://journals.lww.com/jaids/Fulltext/2020/07011/Challenges_in_the_Early_Infant_HIV_Diagnosis_and.1.aspx. [Internet] Jul1citedJun92021. [DOI] [PubMed] [Google Scholar]
- 5.PEPFAR Monitoring and Evaluation Reporting (MER): Annual Program Review (APR) 2019 data for Early Infant Diagnosis (EID)-positive. PEPFAR; 2019. [Internet]. Cited February 3, 2022. [Google Scholar]
- 6.Elimination of mother-to-child transmission [Internet]. UNICEF DATA. [cited Feb 19, 2020]. Available at: https://data.unicef.org/topic/hivaids/emtct/. [Google Scholar]
- 7.Molès JP, Méda N, Kankasa C, et al. A new plan for extended paediatric HIV testing is needed in Africa. Lancet Glob Health. 2019;7:e1603–e1604. Available at: https://www.sciencedirect.com/science/article/pii/S2214109X19304085. [Internet]Dec1citedJun92021. [DOI] [PubMed] [Google Scholar]
- 8.Bhardwaj S, Carter B, Aarons GA, et al. Implementation research for the prevention of mother-to-child HIV transmission in Sub-Saharan Africa: existing evidence, current gaps, and new opportunities. Curr HIV/AIDS Rep. 2015;12:246–255. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4430362/. [Internet]JuncitedJan132021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Start Free. Stay Free. AIDS Free. [Internet]. [cited Feb 19, 2020]. Available at: https://free.unaids.org/. [Google Scholar]
- 10.Drake AL, Thomson KA, Quinn C, et al. Retest and treat: a review of national HIV retesting guidelines to inform elimination of mother-to-child HIV transmission (EMTCT) efforts. J Int AIDS Soc. 2019;22:e25271. Available at: https://onlinelibrary.wiley.com/doi/abs/10.1002/jia2.25271. [Internet]citedFeb192020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mandala J, Kasonde P, Badru T, et al. HIV retesting of HIV-negative pregnant women in the context of prevention of mother-to-child transmission of HIV in primary health centers in rural Zambia: what did we learn? J Int Assoc Provid AIDS Care. 2019;18:2325958218823530. [Internet]. Cited February 3, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kranzer K, Meghji J, Bandason T, et al. Barriers to provider-initiated testing and counselling for children in a high HIV prevalence setting: a mixed methods study. PLoS Med. 2014;11:e1001649. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4035250/. [Internet]citedFeb62020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Consolidated Guidelines on HIV Testing Services: 5Cs: Consent, Confidentiality, Counselling, Correct Results and Connection 2015 [Internet]. Geneva: World Health Organization; 2015. [cited Apr 23, 2020]. (WHO Guidelines Approved by the Guidelines Review Committee). Available at: http://www.ncbi.nlm.nih.gov/books/NBK316021/. [PubMed] [Google Scholar]
- 14.COP20-Guidance_Final-1–15-2020.pdf [Internet]. [cited Mar 10, 2020]. Available at: https://www.state.gov/wp-content/uploads/2020/01/COP20-Guidance_Final-1-15-2020.pdf. [Google Scholar]
- 15.Simon KR, Flick RJ, Kim MH, et al. Family testing: an index case finding strategy to close the gaps in pediatric HIV diagnosis. J Acquir Immune Defic Syndr 1999. 2018;78(Suppl 2):S88–S97. [Internet]. Cited February 11, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lasry A, Medley A, Behel S, et al. Scaling up testing for human immunodeficiency virus infection among contacts of index patients — 20 countries, 2016–2018. Morb Mortal Wkly Rep. 2019;68:474–477. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6542477/. [Internet]cited-Jan302020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.PEPFAR 2019 Country Operational Plan Guidance for all PEPFAR Countries. U.S. President’s Emergency Plan for AIDS Relief (PEPFAR); 2019. Available at: https://www.state.gov/wp-content/uploads/2019/08/PEPFAR-Fiscal-Year-2019-Country-Operational-Plan-Guidance.pdf. [Internet]. Cited February 3, 2022. [Google Scholar]
- 18.PEPFAR-COP21-Guidance-Final.pdf [Internet]. [cited Jan 22, 2021]. Available at: https://www.state.gov/wp-content/uploads/2020/12/PEPFAR-COP21-Guidance-Final.pdf. [Google Scholar]
- 19.Bandason T, McHugh G, Dauya E, et al. Validation of a screening tool to identify older children living with HIV in primary care facilities in high HIV prevalence settings. AIDS Lond Engl. 2016;30:779–785. [Internet]. Cited February 3, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kose J, Tiam A, Ochuka B, et al. Impact of a comprehensive adolescent-focused case finding intervention on uptake of HIV testing and linkage to care among adolescents in Western Kenya. J Acquir Immune Defic Syndr 1999. 2018;79:367–374. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6203422/. [Internet]citedFeb122020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clemens SL, Macneal KD, Alons CL, et al. Screening algorithms to reduce burden of pediatric HIV testing: a systematic review and meta-analysis. Pediatr Infect Dis J. 2020;39:e303–e309. [Internet]. Cited February 3, 2022. [DOI] [PubMed] [Google Scholar]
- 22.Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: Recommendations for a public health approach. WHO; 2021. Report No.: ISBN: 978–92-4–003159-3. Available at: https://www.who.int/publications/i/item/9789240031593. [Internet]. Cited February 3, 2022. [PubMed] [Google Scholar]
- 23.Marwa R, Anaeli A. Perceived barriers toward provider-initiated HIV testing and counseling (PITC) in pediatric clinics: a qualitative study involving two regional hospitals in Dar-Es-Salaam, Tanzania. HIVAIDS Auckl NZ. 2020;12:141–150. [Internet]. Cited February 3, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohn J, Whitehouse K, Tuttle J, et al. Paediatric HIV testing beyond the context of prevention of mother-to-child transmission: a systematic review and meta-analysis. Lancet HIV. 2016;3:e473–e481. Available at: https://www.sciencedirect.com/science/article/pii/S2352301816300509. [Internet] Oct1citedJun92021. [DOI] [PubMed] [Google Scholar]
- 25.Evans C, Nalubega S, McLuskey J, et al. The views and experiences of nurses and midwives in the provision and management of provider-initiated HIV testing and counseling: a systematic review of qualitative evidence. JBI Database Syst Rev Implement Rep. 2016;13:130–286. [Internet]. Cited February 3, 2022. [DOI] [PubMed] [Google Scholar]
- 26.Roura M, Watson-Jones D, Kahawita TM, et al. Provider-initiated testing and counselling programmes in sub-Saharan Africa: a systematic review of their operational implementation. AIDS Lond Engl. 2013;27:617–626. [Internet]. Cited February 3, 2022. [DOI] [PubMed] [Google Scholar]
- 27.Tanser F, de Oliveira T, Maheu-Giroux M, et al. Concentrated HIV sub-epidemics in generalized epidemic settings. Curr Opin HIV AIDS. 2014;9:115–125. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4228373/. [Internet]MarcitedJan132021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siberry G, Roffenbender J Volume and yield of pediatric HIV testing by modality in 21 PEPFAR-supported African programs. 2018. [cited Feb 5, 2020]. Available at: http://www.abstract-archive.org/Abstract/Share/78261. [Google Scholar]
- 29.Nasuuna E, Babirye L, Namimbi F, et al. Where are the HIV Positive Children? A Comparison of Facility and Community Testing Approaches in 14 Public Health Facilities in Five Ugandan Districts. 2019. [Internet]. Cited February 3, 2022. [Google Scholar]
- 30.Kisanga R Provider-initiated testing and counseling for children under 15 yrs at inpatient ward, quality improvement experience from Morogoro Regional Hospital, Tanzania (Jan-Dec 2015). 2016. [cited Feb 10, 2020]. Available at: http://www.abstract-archive.org/Abstract/Share/71083. [Google Scholar]
- 31.Bitimwine H, Kisitu GP, Ssebunya RN, et al. High HIV testing yield found in children attending or accompanying those attending TB, malnutrition, and HIV clinics in Uganda, 2017. 2017 [cited Feb 5, 2020]. Available at: http://www.abstract-archive.org/Abstract/Share/77729. [Google Scholar]
- 32.Rurangwa A, Kouaasi E, N’Dabian D. Increasing HIV case identification and linkage to antiretroviral therapy though nutrition screening: lessons learned from the Cote d’Iviore nutrition and community-facility linkages activity. 2018. [cited Feb 11, 2020]. Available at: http://www.abstract-archive.org/Abstract/Share/78118. [Google Scholar]
- 33.Preidis GA, McCollum ED, Kamiyango W, et al. Routine inpatient provider-initiated HIV testing in Malawi, compared to client-initiated community-based testing, identifies younger children at higher risk of early mortality. J Acquir Immune Defic Syndr 1999. 2013;63:e16–e22. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4364280/. [Internet]citedFeb132020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barr-DiChiara M, Tembo M, Harrison L, et al. Adolescents and age of consent to HIV testing: an updated review of national policies in sub-Saharan Africa. BMJ Open. 2021;11:e049673. [Internet]. Cited February 3, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edwards JK, Arimi P, Ssengooba F, et al. Improving HIV outreach testing yield at cross-border venues in East Africa. AIDS Lond Engl. 2020;34:923–930. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7178496/. [Internet]May1citedJun92021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.2020 World AIDS Day Report: Reimagining a resilient HIV response for children, adolescents and pregnant women living with HIV [Internet]. UNICEF; 2020. Available at: https://data.unicef.org/resources/world-aids-day-report-2020/. [Internet]. Cited February 3, 2022. [Google Scholar]
- 37.Becquet R, Marston M, Dabis F, et al. Children who acquire HIV infection perinatally are at higher risk of early death than those acquiring infection through breastmilk: a meta-analysis. PLoS One. 2012;7:e28510. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3285615/. [Internet] Feb23citedJun92021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davies MA, Gibb D, Turkova A. Survival of HIV-1 vertically infected children. Curr Opin HIV AIDS. 2016;11:455–464. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5384722/. [Internet]SepcitedJun92021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walenda C, Kouakoussui A, Rouet F, et al. Morbidity in HIV-1-infected children treated or not treated with highly active antiretroviral therapy (HAART), Abidjan, Côte d’Ivoire, 2000–04*. J Trop Pediatr. 2009;55:170–176. Available at: 10.1093/tropej/fmn106. [Internet] Jun1citedJun92021. [DOI] [PubMed] [Google Scholar]
- 40.Knox J, Arpadi SM, Kauchali S, et al. Screening for developmental disabilities in HIV positive and HIV negative children in South Africa: results from the Asenze Study. PLoS One. 2018;13:e0199860. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6029795/. [Internet]Jul3citedJun92021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeap AD, Hamilton R, Charalambous S, et al. Factors influencing uptake of HIV care and treatment among children in South Africa - a qualitative study of caregivers and clinic staff. AIDS Care. 2010;22:1101–1107. [Internet]. Cited February 3, 2022. [DOI] [PubMed] [Google Scholar]
- 42.Ferrand RA, Munaiwa L, Matsekete J, et al. Undiagnosed HIV infection among adolescents seeking primary health care in Zimbabwe. Clin Infect Dis Off Publ Infect Dis Soc Am. 2010;51:844–851. [Internet]. Cited February 3, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davies MA, Kalk E. Provider-initiated HIV testing and counselling for children. PLoS Med. 2014;11:e1001650. [Internet]. Cited February 3, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marston M, Becquet R, Zaba B, et al. Net survival of perinatally and postnatally HIV-infected children: a pooled analysis of individual data from sub-Saharan Africa. Int J Epidemiol. 2011;40:385–396. [Internet]. Cited February 3, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teasdale CA, Zimba R, Abrams EJ, et al. Estimates of the prevalence of undiagnosed HIV among children living with HIV in Eswatini, Lesotho, Malawi, Namibia, Tanzania, Zambia, and Zimbabwe from 2015 to 2017: an analysis of data from the cross-sectional Population-based HIV Impact Assessment surveys. Lancet HIV. 2022;9:e91–e101. Available at: https://www.thelancet.com/journals/lanhiv/article/PIIS2352-3018(21)00291-5/fulltext. [Internet]Feb1citedFeb32022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fortenberry JD, Martinez J, Rudy BJ, et al. Linkage to care for HIV-positive adolescents: a multi-site study of the adolescent medicine trials units of the adolescent trials network. J Adolesc Health Off Publ Soc Adolesc Med. 2012;51:551–556. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3505853/. [Internet]DeccitedJun92021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruria EC, Masaba R, Kose J, et al. Optimizing linkage to care and initiation and retention on treatment of adolescents with newly diagnosed HIV infection. AIDS Lond Engl. 2017;31(Suppl 3):S253–S260. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5497791/. [Internet]Jul1citedJun92021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee L, Yehia BR, Gaur AH, et al. ; HIV Research Network. The impact of youth-friendly structures of care on retention among HIV-infected youth. AIDS Patient Care STDS. 2016;30:170–177. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4827281/.[Internet]Apr1citedJun92021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rakhmanina N, Kose J, Wallner K, et al. Disclosure of HIV status toolkit for pediatric and adolescent populations [Internet]. Elizabeth Glaser Pediatric AIDS Foundation; 2018. Available at: https://www.pedaids.org/wp-content/uploads/2019/01/NewHorizonsDisclosureToolkit_FINAL.pdf. [Internet]. Cited February 3, 2022. [Google Scholar]
- 50.Katbi M, Magaji D, Philips-Ononye T, et al. Closing the gap in pediatric HIV case finding: a review of the PASS strategy in Southern Nigeria. Int J Virol AIDS. 2020;7:1–6. Available at: https://www.clinmedjournals.org/articles/ijva/international-journal-of-virology-and-aids-ijva-7-068.php?jid=ijva. [Internet]Aug24citedMar52021. [Google Scholar]
- 51.Ahmed S, Cox C, Abrams E. Commentary on “symptom-based screening is not the solution to improve pediatric HIV testing”. Pediatr Infect Dis J. 2020;39:1101–1102. [Internet]. Cited February 3, 2022. [DOI] [PubMed] [Google Scholar]
- 52.Katureebe C, Ashburn K, Machekano R, et al. Developing and validating an effective pediatric and adolescent HIV testing eligibility screening tool for high-volume entry points in Uganda. J Acquir Immune Defic Syndr 1999. 2021;88:290–298. [Internet]. Cited February 3, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Antelman G, Gill MM, Jahanpour O, et al. Balancing HIV testing efficiency with HIV case-identification among children and adolescents (2–19 years) using an HIV risk screening approach in Tanzania. PLoS One. 2021;16:e0251247. [Internet]. Cited February 3, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Index testing for biological children and adolescents (<19y/o) of PLHIV: clinical and OVC partner collaboration to expand testing services [Internet]. PEPFAR Solutions Platform. 2021. Available at: https://www.pepfarsolutions.org/resourcesandtools-2/2021/10/5/index-testing-for-biological-children-and-adolescents-lt19yo-of-plhiv-clinical-and-ovc-partner-collaboration-to-expand-testing-services. [Internet]. Cited February 3, 2022. [Google Scholar]
- 55.Dougherty G, Panya M, Madevu-Matson C, et al. Reaching the first 90: improving inpatient pediatric provider-initiated HIV testing and counseling using a quality improvement collaborative strategy in Tanzania. J Assoc Nurses AIDS Care. 2019;30:682–690. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6698429/. [Internet]citedJan302020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nhabomba C, Chicumbe S, Muquingue H, et al. Clinical and operational factors associated with low pediatric inpatient HIV testing coverage in Mozambique. Public Health Action. 2019;9:113–119. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6827495/ [Internet]cited-Jan142020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Masi-Leone M, Arpadi S, Teasdale C, et al. growth and metabolic changes after antiretroviral initiation in South African Children. Pediatr Infect Dis J. 2021;40:1004–1010. [Internet]. Cited February 3, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Package of care for children and adolescents with advanced HIV disease: stop AIDS [Internet]. [cited 2021 Jan 22]. Available at: https://www.who.int/publications-detail-redirect/9789240008045. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.