Abstract
The primary objective of this rapid review is to describe community-partner and patient-partner engagement in women’s cardiovascular disease (CVD) research. Secondary objectives are to: (i) describe the phase of the research in which community and patient partners were engaged; (ii) define the level of engagement at each research phase; and (iii) make recommendations for future engagement of community and/or patient partners in women’s CVD research. Rapid review guidelines recommended by the Cochrane Rapid Reviews Methods Group and Tricco et al. were used to search 5 databases using medical subject headings (MeSH) and/or keywords. Participants included women (cis and trans) aged > 18 years who had ischemic heart disease, heart failure, or stroke. A risk of bias assessment was not undertaken. Findings are summarized and/or clustered as community-based participatory research, or patient-oriented and/or patient-partner research. Our search yielded 39,998 titles and abstracts. Of these, 35 were included in a final narrative synthesis, comprising data from 474 community and/or patient partners, including 417 (88%) women. Over 85% of community partners collaborated in the design and/or planning and implementation of women’s CVD research; most originated in the US; only one originated in Canada. Most patient-oriented and patient-partner research originated in Canada. However, less than 50% of patient partners collaborated in any phase of research. Sex, gender, race, and ethnicity were rarely reported. Results suggest negligible community and inadequate patient-oriented and/or patient-partner engagement in women’s CVD research in Canada. Improved CVD outcomes for women may be achieved with better community- and patient-partner collaboration across all phases of research, genders, race, and ethnicities.
Graphical abstract
Résumé
L'objectif principal de cette brève revue de littérature est de décrire l'engagement des partenaires communautaires et des patients partenaires dans la recherche sur les maladies cardiovasculaires (MCV) chez les femmes. Les objectifs secondaires sont les suivants (i) décrire la phase de la recherche dans laquelle la communauté et les patients partenaires ont été impliqués; (ii) définir le niveau d'engagement à chaque phase de la recherche; et (iii) formuler des recommandations pour l'engagement futur des partenaires communautaires et/ou des patients partenaires dans la recherche sur les MCV chez les femmes. Les lignes directrices pour effectuer des revues de littérature rapides recommandées par le Cochrane Rapid Reviews Methods Group et Tricco et coll. ont été utilisées pour effectuer des recherches dans 5 bases de données à l'aide de rubriques médicales sous-jacentes (MeSH) et/ou de mots-clés. Les participants étaient des femmes (cis et trans) âgées de plus de 18 ans et ayant eu une cardiopathie ischémique, une insuffisance cardiaque ou un accident vasculaire cérébral. Aucune évaluation du risque de biais n'a été entreprise. Les résultats sont résumés et/ou regroupés en tant que recherche communautaire participative, ou recherche orientée vers le patient et/ou recherche avec partenariat patient. Notre recherche a collecté 39 998 titres et résumés. Parmi ceux-ci, 35 ont été inclus dans une synthèse narrative finale, comprenant des données provenant de 474 partenaires communautaires et/ou patients, dont 417 (88%) femmes. Plus de 85% des partenaires communautaires ont collaboré à la conception et/ou à la planification et à la mise en œuvre de la recherche sur les MCV chez les femmes; la plupart étaient originaires des États-Unis; une étude seulement était originaire du Canada. La plupart des recherches axées sur le patient et sur les patients partenaires ont été menées au Canada. Cependant, moins de 50 % des patients partenaires ont collaboré à l'une ou l'autre phase de la recherche. Le sexe, le genre, la race et l'origine ethnique étaient rarement rapportés. Les résultats suggèrent un engagement négligeable des communautés et un engagement insuffisant des patients et/ou des patients partenaires dans la recherche sur les MCV chez les femmes au Canada. Une meilleure collaboration entre la communauté et les patients partenaires à toutes les étapes de la recherche, quel que soit le sexe, la race ou l'origine ethnique, permettrait d'améliorer les résultats de la recherche sur les MCV chez les femmes.
Lay Summary
The aims of this review are to describe community- and patient-partner engagement in women’s heart and blood vessel (cardiovascular disease [CVD]) research and make recommendations for future research. Most patient-partnered research originated in Canada, but less than 50% of patient partners truly collaborated in the research. Only 1 community-partnered women’s CVD research study originated in Canada. Improved CVD outcomes may be achieved with better community- and patient-partner collaborations across all phases of research.
The leading cause of premature death for Canadian women is cardiovascular disease (CVD).1 In low-income countries, the highest rates of CVD mortality have shifted from men to women, and since 2017, CVD mortality has increased in 2 high-income countries—Canada and the US.2 Approximately 32,000 women die of CVD in Canada each year3; ischemic heart disease (IHD), stroke, and heart failure are the most common causes of mortality.1,2 CVD is also a leading cause of healthcare utilization for Canadian women, resulting in > 115,000 emergency department visits and > 132,000 inpatient hospitalizations each year.4 CVD in women is complex; it varies across the lifespan and is influenced by sex, gender, race, and ethnicity.5 The Lancet Women and Cardiovascular Disease Commission advocates for a global imperative to reduce the global burden of CVD in women by 2030.2
Myocardial infarction (MI) has increased in younger women, and recent data show an increase in its incidence and related deaths among women aged 45 to 54 years.6 Young Canadian women are more likely than men to die within 1 year of an MI,4,7 and up to 15% of women presenting with an MI have nonatherosclerotic (ie, nonobstructive) IHD (ie, MI with nonobstructive coronary arteries [MINOCA]).4 Twice as many women as men present with nonatherosclerotic IHD8; these women are usually younger, with few or no traditional risk factors, have coronary microvascular dysfunction, coronary vasospasm, and spontaneous coronary artery dissection (SCAD).9 Globally, ischemic stroke is the second most common cause of CVD mortality in women.10 Women have a higher lifetime stroke risk than men,2 and stroke affects women across their life course, with the risks being highest during pregnancy, menopause, and in later life.8 Women also have heart failure with preserved ejection fraction (HFpEF), peripartum cardiomyopathy, and Takotsubo syndrome,11,12 with few to no treatments available for specific heart failure phenotypes in women.2
Sex as a Biological Variable
Female hearts are biologically (sex) different than male hearts. Female hearts and coronary arteries are smaller, and atherosclerotic plaque builds up differently in female coronary arteries,13 causing atherosclerotic and nonatherosclerotic IHD. The difference in plaque formation, especially in younger female patients, partly explains why early signs of heart disease are missed in female patients. Nonatherosclerotic IHD, more prevalent in younger and middle-aged female patients, causes major adverse cardiac events (ie, MI) similar to those that occur in female patients with atherosclerotic IHD.14,15 Every year in Ontario, 45,000 people with cardiac pain and/or cardiac symptoms undergo coronary angiography; 10% to 30% are postmenopausal female patients with nonatherosclerotic IHD who suffer from persistent cardiac pain and/or cardiac symptoms and are frequent users of healthcare services.16 They are at risk for increased morbidity (ie, impaired function, depression, poor health-related quality of life) and mortality.17
Gender as a Sociocultural Factor
Results from the GENESIS-PRAXY (Gender and Sex Determinants of Cardiovascular Disease: From Bench to Beyond Premature Acute Coronary Syndrome) prospective cohort study suggest that gender is associated with higher rates of MI (hazard ratio [HR] 4.50, 95% confidence interval [CI], 1.05, 19.27)18 and poorer access to care.19 Gender refers to socially constructed roles, behaviours, and expressions,20 and it is described across the following 4 domains: gender identity, gender roles, gender relations, and institutionalized gender. Gender identity describes the way in which a female identifies (eg, personality traits, as a woman or man or nonbinary), with impacts on how they behave (eg, anxious, stressed, depressed) and how others in society treat them.21,22 Women describe signs of an MI differently than do men. This varied pattern and distribution of symptoms make interpretation of pain as cardiac-specific difficult for women and healthcare providers.14,23,24 Women minimize their symptoms, prefer to consult with family and friends (ie, gender relations), and have other gendered roles (ie, caring responsibilities and concerns for their family).25 As a result, women delay seeking appropriate care for cardiac pain and cardiac symptoms.26,27 Older age, race and ethnicity, lower socioeconomic status (ie, institutionalized gender), history of chronic disease, and symptom knowledge deficits also are associated with longer prehospital delay times.28
Race and Ethnicity
The incidence of risk factors for CVD is higher in South Asian, Chinese, and Black populations,29, 30, 31 and Indigenous women have a 53% higher CVD mortality risk than White women.32 Women from racial and ethnic minority groups in high-income countries, such as Canada and the US, live in poverty, which is associated with negative effects on access to healthcare.33 Low income, low levels of education, and living in disadvantaged areas increase CVD risk in women.34
System and Policy Mandates
International CVD priorities are led by the World Health Organization’s Global Action Plan for the Prevention and Control of Non-Communicable Diseases (2013-2020) and the United Nations Sustainable Development Goals (2015-2030).35 Sustainable development goal #17 advocates strengthening the means of implementation and revitalization of the global partnership for sustainable development. Targets and indicators include building capacity, enhancing regional and international cooperation and knowledge sharing, and encouraging multi-stakeholder partnerships. The World Heart Federation has been advocating globally for better CVD outcomes, suggesting advocacy tactics and strategies to reduce CVD by 25% by 2025.35 This approach includes addressing behavioural risk factors for better prevention and reducing IHD and stroke in women by identifying and engaging with national CVD priorities, strategic communications, media engagement, evidence-based research, partnerships, and collaborating with key decision-makers.35 National CVD priorities outlined by the Heart and Stroke Foundation of Canada (HSF) include promoting health, saving lives, and enhancing recovery.36 They advocate for heart-health equity to ensure that women are not underresearched, underdiagnosed, undertreated, under-supported, or under-aware.37 This goal aligns with those of the Canadian Institutes of Health Research (CIHR) and the Institute of Circulatory and Respiratory Health, which include partnering with individuals who have lived experience, collaborating with interdisciplinary teams, and developing capacity of the next generation of leaders.38
Patient Engagement and Patient-Oriented Research
Engaging patients as partners in research implies that research will be conducted “with” rather than “to” or “about” patients; this means that patients are active participants throughout the research process and not simply study participants.39 Patient engagement in research originated in the late 1960s as participatory action research (PAR), to address issues related to unequal or harmful social systems,40,41 and when community members were actively engaged, this approach was more specifically described as community-based participatory research. Core principles of PAR included respect, humility, trust, patience, acceptance, flexibility, and openness to building relationships to define the issue and/or problem, gather and analyze data, and then plan and take action.40 An international movement to more formally engage patients and the public as active participants in research began in the United Kingdom (UK) with INVOLVE in 1996, funded by the National Institute for Health Research.42 Two incentives within this movement included improving trial efficiency (ie, recruitment) and ensuring trial outcomes were of value to patients.43 The Patient-Centered Outcomes Research Institute (PCORI) was created as part of the Patient Protection and Affordable Care Act in the US in 2010, which aimed to extend patient-centred research into healthcare in the US.44 Canada’s strategy for patient engagement or patient-oriented research (SPOR) began in 2011 with funding from CIHR.39 Patient engagement in research aims to enhance study recruitment and retention rates and increase relevance of outcomes to patients and the public; it is not intended to undermine the rigor of the research but to identify problems and outcomes that are important to patients and to inform study design, such as use of inclusion and exclusion criteria.44 Patient engagement is aligned with SPOR, which aims for patients, clinicians, researchers, and decision-makers to actively collaborate to build an equitable and sustainable healthcare system in Canada.39 Also aligned with SPOR are the SPOR Capacity Development Framework45 and the SPOR Patient Engagement Framework46; both provide definitions, values, principles, and strategies for engaging patients as equal and active members of investigative teams. The term ”patient” within these frameworks is broadly defined to include individuals who have personal experience with a health issue; this includes individuals with direct experience, but it is also inclusive of unpaid caregivers, family members, and friends.38 Patient engagement in research implies meaningful and active collaboration in setting priorities and conducting research, translating knowledge, and providing oversight and governance.38 Guidelines for establishing meaningful and active collaboration with patients require the following features: (i) adequate organizational policies; (ii) shared goals and strong communication practices between investigators and patient partners; (iii) principles of inclusiveness, support, mutual respect, flexibility, accountability, and co-learning; (iv) patient-oriented research training for all members of the research team; (v) tools and/or resources for successful patient engagement; and (vi) value for patient engagement across all phases of the research.47
Forms of patient-partner engagement
Patient partners can be engaged across a variety of health conditions utilizing a range of methodologies, including focus groups and/or interviews, surveys, workshops, and world cafés.48 Patient partners also can be engaged across different phases of the research process, including identifying problems and priorities for research, informing recruitment strategies, collecting and analyzing data, choosing outcome measures, and mobilizing or translating knowledge.48, 49, 50, 51 Polit and Beck52 describe 5 phases of the research process: conceptualization, design and planning, empirical, analytic, and dissemination. Recent systematic reviews suggest that patient engagement is most common at the conceptualization phase of study development.49,50
Levels of patient-partner engagement
The International Association for Public Participation (IAP2)53 defines a spectrum of 5 levels for patient and public participation in research, as follows: inform, consult, involve, collaborate, and empower. Community members (ie, public) and patient partners have increasing impact in research as they move along the spectrum from being informed, consulted, and involved to being truly collaborated with and empowered. When the public and patients are informed through pamphlets, brochures, and fact sheets, they are provided with information to assist them in understanding a problem, benefits, and risks; they are kept informed, and no advice or feedback is solicitated. Investigators consult patients for feedback using focus groups and/or interviews, informal dialogues, and surveys; through consultation, the concerns and aspirations of patients are acknowledged but are not necessarily considered when making research decisions. When patients are involved in research, their concerns and aspirations begin to influence research decisions through direct feedback in workshops, world cafés, and Delphi surveys. Patient partners have the greatest research impact when they collaborate and are empowered to make decisions through governance, as members of citizen and/or patient advisory committees.53 The IAP2 spectrum of engagement is similar to Health Canada’s public involvement continuum, which also defines 5 levels of engagement, as follows: communicate, listen, consult, engage, and partner.26 Partnerships include sharing values and acknowledging expertise, both scientific and experiential. Patient partners bring practical knowledge about living with a disease or condition, such as CVD.
Reporting patient-partner engagement
The Guidance for Reporting Involvement of Patients and the Public (GRIPP) checklist was developed and updated (GRIPP2—long form [LF]; GRIPP2—short form [SF]) to improve the quality, transparency, and consistency of reporting of patient and public involvement in health research.27 Adequate reporting helps to ensure that the evidence related to patient engagement can be synthesized in systematic reviews.54,55 Both the LF and SF of GRIPP2 are the first international informed guidelines for reporting patient engagement in research developed using the EQUATOR method (Enhancing the QUAlity and Transparency Of health Research) for guideline reporting.56 The GRIPP2-LF collects information about the abstract, background, aims, methods, and results, as well as the discussion and conclusions in a checklist format. Specific details related to patient engagement are included in the methods of the study—people involved, phases and/or level of involvement, and impact (if applicable)—and results should detail both the context and processes of patient engagement. The GRIPP2-SF presents similar patient engagement information in a table or narrative format.
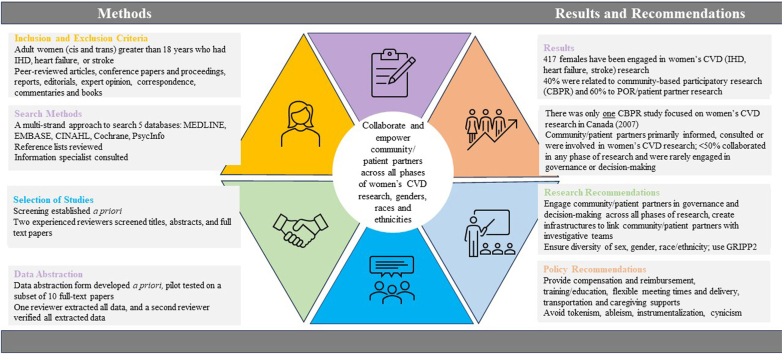
The primary objective of this rapid review is to describe community- and patient-partner engagement in women’s CVD (IHD, heart failure, stroke) research. Secondary objectives are as follows: (i) to describe the phase of the research in which community and patient partners were engaged; (ii) to define the level of engagement at each phase of research; and (iii) to make recommendations for future engagement of community and patient partners in women’s CVD research.
Material and Methods
This rapid review, utilizing accelerated forms of systematic review methodology, is reported using both PRISMA-S, an extension to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement28 (Supplemental Table S1), with rapid-review guidelines recommended by the Cochrane Rapid Reviews Methods Group57 and by Tricco et al.58 The protocol for this review has not been published. Two patient partners collaborated throughout the review process, from identifying priorities and search terms from a contemporaneous review through to reviewing the article prior to publication. Compensation was provided to patient partners using recommendations from the SPOR Chronic Disease Networks and the Primary and Integrated Health Care Innovations (PIHCI) Network.59 The GRIPP2-SF was used to report patient-partner engagement53 (Supplemental Table S2).
Inclusion and exclusion criteria
The inclusion criteria were kept broad rather than following the recommendation to optimize precision and sensitivity in a rapid review.57 The decision to maintain breadth and comprehensiveness to ensure that relevant studies were not missed was made in collaboration with our patient partners. This decision was based on the fact that a previous patient-engagement search60 identified a large number of irrelevant records that needed to be screened and manually removed because patients were participants and not partners, and many studies had a focus on study recruitment and retention of ethnically diverse populations and/or those with low literacy levels.60 The type of publications included peer-reviewed articles, conference papers and proceedings, reports, editorials, expert opinions, correspondence, commentaries, and books. Participants included adult women (cis and trans) aged > 18 years who had IHD, heart failure, or stroke. We searched broadly by “sex” and “gender,” and specifically by “females” and “women.” During title, abstract, and full-text screening, we ensured that women with IHD, heart failure, and stroke were included, although we did not define a specific minimum sample size of women, to avoid selection bias. Outcomes included patient-partner engagement, defined as the phase (ie, conceptual, design and/or planning, empirical, analytic, and dissemination)52 and level of engagement (ie, inform, consult, involve, collaborate, empower).53 According to the CIHR, patient engagement is defined as “meaningful and active collaboration in governance, priority setting, conducting research and knowledge translation” including “people who bring the collective voice of specific, affected communities.”38 “Patient” was considered to be “an overarching term used to include individuals with personal experience of a health issue and informal caregivers, including family and friends.”38 Patient partners who were consenting study participants were excluded.
Search methods for identification of relevant studies
A multi-strand approach was used to search the following: Ovid MEDLINE (1946 to June 19, 2023, including Epub Ahead of Print, and In-Process & Other Non-Indexed Citations); Ovid Embase (1947 to June 19, 2023); EBSCO CINAHL [Cumulative Index of Nursing and Allied Health] Plus with Full Text (1981 to June 19, 2023); Cochrane Central Register of Controlled Trials and Cochrane Database of Systematic Reviews; and American Psychological Association (APA) PsycINFO (1806 to June 2023) to identify peer-reviewed articles, conference papers and proceedings, reports, editorials, expert opinions, correspondence, commentaries, and books that described patient engagement in women’s CVD research. Reference lists of relevant studies also were reviewed.57 Search strategies were adapted by selecting relevant CVD terms (IHD, heart failure, stroke) from a previous search strategy61 that was drafted in MEDLINE using Ovid by an information specialist, was peer reviewed by a second information specialist, and then was translated using each database platform's command language, controlled vocabulary, and appropriate search fields. One review author (M.P.) checked the remaining primary search strategy for spelling errors, Boolean operator usage, and line number combinations. The strategy was then validated by confirming retrieval of 2 known relevant records62,63 and then was executed across the remaining databases. Medical subject headings (MeSH) and text words were used for the search concepts of patient partner, sex and gender, and CVD (IHD, heart failure, stroke). Search terms for patient partner were combined with the Boolean OR (strand 1). Search terms for sex and gender (strand 2) and search terms for CVD (IHD, heart failure, stroke) were combined with the Boolean OR (strand 3). Finally, this query was combined using search terms for patient partner, sex and gender, and CVD using the Boolean AND (strand 4). The search was conducted on June 20, 2023; no language or date limits were imposed in any database, grey literature searches were not completed, and no contact was made to authors or other experts to identify additional unpublished studies. EndNote (Clarivate, London, United Kingdom), a reference management software tool, was used to track search results. For full strategies, see Supplemental Table S3.
Selection of studies
Screening criteria were established a priori. Two experienced reviewers (M.P., T.O.) screened all citations by title and abstract. Eligible full-text papers were retrieved (by T.O.) and screened (by M.P. and T.O.). Discrepancies during screening were resolved through discussion between M.P. and T.O. The final subset of full-text papers was reviewed (by M.P.) prior to data extraction. De-duplication and screening was done using Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia), a primary screening and data-extraction tool used by Cochrane authors.
Data extraction
A data abstraction form was developed a priori and pilot tested on a subset of 10 full-text papers. Minor revisions were made to the original data abstraction form. One review author (T.O.) extracted data, and a second review author (M.P.) verified all extracted data for accuracy. Data extraction was limited to key study characteristics and outcomes and included study details (ie, author, title, year of publication, study location, study aim, and sample size), patient-partner demographics (ie, biological sex, age, race and ethnicity, gender [identity, roles, relations, institutionalized], and primary cardiovascular diagnosis), and outcomes (ie, research phase and level of patient-partner engagement).
Risk-of-bias assessment
The primary objective of this review was to scope the available literature on patient engagement in women’s CVD research. As the intent was not to evaluate effect, a risk-of-bias assessment was not undertaken.
Knowledge synthesis
All studies meeting the inclusion criteria were summarized using a narrative synthesis process64 following guidance recommended by Popay et al.65 This summary incorporated a description of study details, patient-partner demographics, and research phase and level of engagement in cases in which patient partners had been engaged in women’s CVD (IHD, heart failure, stroke) research. Study findings were grouped or clustered into categories related to whether the focus was on participatory action research and/or community-based participatory research, or on patient-oriented and/or patient-partner research, distinguishing engagement at the level of either the community or the individual patient partner. Each individual study also was assessed according to both Polit and Beck’s52 5 phases of the research process—conceptual phase (ie, determining the research purpose, formulating the clinical problem); design and planning phase (ie, selecting the design and developing study procedures); empirical phase (ie, collecting data); analytic phase (ie, analyzing and interpreting results); and dissemination phase (ie, translating the results to an appropriate audience)—and the IAP253 level of engagement— inform (ie, provide the public with information); consult (ie, obtain public feedback); involve (ie, work with the public and consider their feedback); collaborate (ie, partner with the public in making decisions); and empower (ie, have public make the final decision).
Results
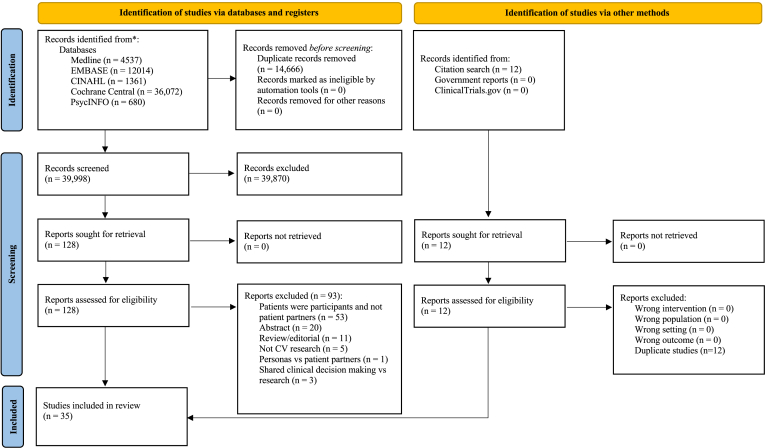
Search strategies of databases yielded 39,998 titles and abstracts and were reported using the PRISMA 2020 statement: an updated guideline for reporting systematic reviews66 (Fig. 1), from which 128 peer-reviewed articles, conference proceedings, and reports were deemed to meet the inclusion criteria and were assessed for full-text review. In addition, 12 published manuscripts were identified from a specific citation search; these were included as a compendium to the original record identified in the search (ie, full-text papers to support conference abstracts). In total, 35 records62,63,67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98 were included in the final review.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 flow diagram.66 ∗Consider, if feasible, reporting the number of records identified from each database or register searched (rather than the total number across all databases/registers). CINAHL, Cumulative Index of Nursing and Allied Health; CV, cardiovascular.
The 35 records represent data from 474 community and patient partners62,63,67,68,70,71,73, 74, 75, 76, 77, 78,80, 81, 82, 83, 84, 85, 86, 87,89, 90, 91,96, 97, 98, 99 who completed studies, including a total of 417 (88%) female partners. Of the 35 papers, the majority originated in the US (n = 16; 46%),68,71, 72, 73,76,77,79,81,83,84,87,90,93, 94, 95, 96 Canada (n = 7; 20%),62,63,69,70,74,75,98 and the UK (n = 6; 17%).67,80,82,85,92,97 The remaining papers originated in Ireland (n = 1; 2%),78 Sweden (n = 1; 2%),86 Turkey (n = 1; 2%),88 Germany (n = 1; 2%),89 Denmark (n = 1; 2%),91 and Greece (n = 1; 2%).99 Patient engagement focused on women with cerebrovascular disease and stroke (n = 19; 54.2%),63,67,68,76,78,79,82,83,85, 86, 87,89,90,93, 94, 95, 96, 97,99 heart disease and myocardial infarction (n = 10; 28.6%),62,63,73,74,76,80,84,91,94,98 and CVD and heart failure (n = 10; 28.6%).69, 70, 71, 72,75,77,81,83,88,92 Four were randomized controlled trials (RCTs),69,84.87,90 and one was a protocol for an RCT.72 Of the 5 RCTs, only 1 study90 reported patient-partner demographics, which included seven female patients who collaborated across all phases of research (Table 1).
Table 1.
Included studies, patient partner and/or community demographics and engagement details by region
| Author(s) (Year) Location |
Sample size Method |
Biological sex (female), n (%) | Age, y; mean (SD) | Race and/or ethnicity, n (%) | Gender, n (%) | Primary diagnosis | Research phase of engagement | Level of engagement |
|---|---|---|---|---|---|---|---|---|
| Canada | ||||||||
| Blumer et al.69 (2021) Ontario, Canada |
NR Stepped wedge cluster RCT |
NR | NR | NR | NR | HF | Design/Planning | Inform (PROMs) |
| Cho et al.70 (2019) British Columbia, Canada |
Patients, partners and family members formed the PPC (n = 6) Mixed methods |
2 (33) | NR | NR | NR | Premature atherosclerotic CVD | Conceptual Design/Planning Empirical Analytic Dissemination |
Collaborate Collaborate Collaborate Collaborate Collaborate |
| Etowa et al.74 (2007) Nova Scotia, Canada |
Black community leaders and community research facilitators (n = 3) Interviews |
3 (100) | NR | African Canadian | NR | Heart disease, cerebrovascular disease | Design/planning Empirical Analytic Dissemination |
Involve Collaborate Collaborate Empower |
| Ghisi et al.75 (2022) Canada, USA, Bangladesh |
Patient partners on writing panel (n = 2) Survey |
2 (100) | Patient partner ages: 74 and 82 y | NR | One patient partner had lower education level and one patient partner had higher education level | CVD | Analytic Dissemination |
Collaborate Involve |
| Parry et al.62 (2020) Ontario, Canada |
Patient Advisory Committee (n = 7) Protocol for a mixed methods study |
7 (100) | NR | NR | NR | Coronary artery disease | Conceptual Design/Planning Empirical Analytic Dissemination |
Collaborate Collaborate Collaborate Collaborate Collaborate |
| Teed et al.63 (2021) Canada |
Participants in cross-Canada tour (n = 204) Participants in collaboration sessions (n = 57) Focus groups, interviews, workshop |
261 (100) | NR | Efforts made to have representation from Indigenous women living in Northern communities and those of South and East Asian descent | NR | Heart disease and stroke | Conceptual Design/Planning Empirical Analytic Dissemination |
Collaborate Collaborate Involve Involve Involve |
| Wilson et al.98 (2023) Alberta, Canada |
Patient partners (n = 2) Survey |
2 | NR | NR | NR | Acute coronary syndrome | Design/Planning Empirical Dissemination |
Collaborate Collaborate Collaborate |
| United Kingdom | ||||||||
| Benn et al.67 (2022) Manchester, UK |
Stroke survivors (n = 2) Interviews |
NR | NR | NR | NR | Stroke | Conceptual Design/Planning Empirical Analytic Dissemination |
Involve Collaborate Collaborate Collaborate Collaborate |
| Jalal et al.80 (2020) UK |
n = 9 Focus groups |
5 (56) | NR | NR | Two of the patient partners were carers∗ | MI | Design/Planning Analytic Dissemination |
Collaborate Involve Unclear |
| Johnson et al.82 (2022) Cambridge, UK |
Three stroke survivor groups consisting of 4-8 participants per group (including carers) (n = 18) Workshops |
NR | NR | White | NR | Stroke | Conceptual Design/Planning |
Consult Consult |
| Kilbride et al.85 (2018) London, UK |
Stroke survivors (n = 20) Protocol for a nonrandomized intervention |
NR | NR | NR | NR | Stroke | Design/Planning Dissemination |
Involve Involve |
| Porter et al.92 (2016) UK |
A group of patients were recruited, actual number NR Survey, interviews |
NR | NR | NR | NR | Comorbidities include asthma, COPD, diabetes, HF, depression, and hip/knee osteoarthritis | Design/Planning | Involve |
| Williamson et al.97 (2015) Manchester, UK |
Lay advisors (n = 10) Interviews |
6 (60) | 40 – 75 y∗ | NR | NR | Stroke | Design/Planning Empirical Analytic Dissemination |
Involve Collaborate Involve Consult |
| USA | ||||||||
| Bess et al.68 (2019) North Carolina, USA |
Executive directors from community organizations (n = 2) Focus groups (n = 8) Semi-structured individual interviews (n = 48) Focus groups, interviews, surveys |
32 (68.1)‡ | 21–30 y: n = 5 (10.6%) 31–45 y: n = 6 (12.8%) 46–60 y: n = 19 (40.4%) > 61 y: n = 17 (36.2%) |
African American: 47 (100) | Married / partnered: 19 (40) Not married / widowed: 28 (60) |
CVD | Conceptual Design/Planning Empirical Analytic Dissemination |
Collaborate Collaborate Collaborate Collaborate Collaborate |
| Crabbe et al.71 (2021) Philadelphia, USA |
Town hall #1 (n = 11) Town hall #2 (n = 41) 5 focus groups, 1 Twitter event, 2 Facebook live events, survey |
Survey: (100)† | Survey: 35–50 y (17%)† 51–60 y (33%)† 61–70 y (22%)† > 71 y (28%)† |
Survey: African American (100)† |
Survey: Income > $50,000 (82)† Income > $80,000 (41)† |
CVD | Conceptual Design/Planning Empirical Analytic Dissemination |
Collaborate Collaborate Collaborate Collaborate Collaborate |
| Dickson et al.72 (2015) USA |
NR Protocol for a staggered RCT |
NR | NR | NR | NR | HF | Conceptual Design/Planning Empirical |
Collaborate Collaborate Collaborate |
| Dodgen et al.73 (2020) Texas, US |
Community members (n = 6) Interviews |
6 (100) | 25– 60 y | African American, Hispanic, White† | Patient partners on the advisory committee consisted of women at various life stages (eg, young mothers, early to mid-career, professionals, and retirement) | Chronic diseases including heart disease, cancer, and diabetes | Conceptual Design/Planning Dissemination |
Inform Collaborate Collaborate |
| Gleason-Comstock et al.76 (2022) Michigan, USA |
No. of patient partners on the Community Advisory Committee (n = 12) Pre-post test |
6 (50) | NR | African American | Patient partners had LGBTQ+ representation, but this was not clearly defined | Heart disease | Design/planning Empirical Analytic Dissemination |
Collaborate Collaborate Collaborate Collaborate |
| Heo et al.77 (2015) USA |
n = 4 Interviews Pre-post test |
2 (50) | NR | White female: 1 (25) White male: 1 (25) African American female: 1 (25) African American male: 1 (25) |
NR | HF | Design/Planning | Consult |
| Inam et al.79 (2021) Texas, USA |
NR Survey |
NR | NR | NR | NR | Acute ischemic stroke | Design/Planning | Consult |
| Jankowska et al.81 (2023) Americas, Asia-Pacific, Europe, and Central Asia |
Patient survey (n = 98) 3 patient partners on the working group |
Survey: 60 (61.2) |
35–44 y: n = 13 (13.3%)∗ 45–54 y: n = 20 (20.4%)∗ 55–64 y: n = 30 (30.6%)∗ 65–74 y: n = 25 (25.5%)∗ ≥ 75 y: n = 10 (10.2%)∗ |
Americas: 25 (25.5)∗ Asia Pacific: 24 (24.5)∗ Europe and Central Asia: 49 (50)∗ |
NR | HF | Design/Planning Empirical Analytic Dissemination |
Involve Involve Involve |
| Jones et al.83 (2022) USA |
C-RAB working group members (n = 5) Health fairs, workshops |
5 (100) | > 40 y | Black: 5 (100) | Four primary sources of stress: workplace, parenting, finances, and social media. Gendered racism and discrimination and life imbalance emerged as underlying stressors. | CVD and stroke | Conceptual Design/Planning Empirical Analytic Dissemination |
Collaborate Collaborate Collaborate Unclear Collaborate |
| Kandula et al.84 (2015) Illinois, USA |
Community partner (Metropolitan Asian Family Services) (n = 1) RCT |
NR | NR | South Asian | NR | Heart disease | Conceptual Design/Planning Empirical Analytic Dissemination |
Collaborate Collaborate Collaborate Unclear Unclear |
| Kronish et al.87 (2014) New York, USA |
Stroke survivors (community-based PAR) (n = 39) RCT |
NR | NR | NR | NR | Stroke | Conceptual Design/Planning Empirical Analytic Dissemination |
Collaborate Collaborate Collaborate Collaborate Collaborate |
| Menkin et al.90 (2019) Los-Angeles, USA |
n = 8 Randomized wait-list controlled trial |
7 (88) | NR | Black: 2 (25) Latino: 1 (12.5) Chinese American: 1 (12.5) Korean American: 4 (50)∗ |
NR | Stroke | Conceptual Design/Planning Empirical Analytic Dissemination |
Collaborate Collaborate Collaborate Collaborate Collaborate |
| Prabhakaran, et al.93 (2020) Illinois, USA |
NR Community members and stroke survivors with CG on a CAB, stakeholders for recruitment Focus groups |
NR | NR | NR | NR | Ischemic stroke | Conceptual Design/Planning Empirical Dissemination |
Involve Involve Collaborate Involve |
| Robles et al.94 (2021) Michigan, USA |
Interviews (n = 15) Community members who engaged as partners (NR) Interviews |
NR | NR | NR | NR | Acute stroke and MI | Conceptual Design/Planning Empirical Analytic Dissemination |
Collaborate Collaborate Collaborate Collaborate Collaborate |
| Schwertfeger et al.95 (2024) USA |
Stroke camps led by PWS, CG, music therapist, and nurse but no. for PWS and CG (NR) Survey |
NR | NR | NR | NR | Stroke | Conceptual Design/Planning Empirical Dissemination |
Consult Consult Collaborate Collaborate |
| Skolarus et al.96 (2013) Michigan, USA |
One faith-based community organization was the community partner, incorporating 3 African American churches Focus groups |
NR | NR | African American: (100) | NR | Stroke | Conceptual Design/Planning Empirical Analytic |
Collaborate Empower Collaborate Collaborate |
| Other | ||||||||
| Horgan et al.78 (2022) Ireland |
PPI stakeholders (n = 34) PPI Advisory Committee (n = 20) Mixed methods |
NR | NR | NR | NR | Stroke | Conceptual Design/Planning Empirical Analytic Dissemination |
Collaborate Collaborate Collaborate Collaborate Involve |
| Kjörk et al.86 (2022) Sweden |
Expert panel (including 1 patient partner) (n = 11) Participants in usability testing (n = 22) Workshops |
Expert Panelists: 8 (73), patient partner was male Participants in usability testing: 9 (41) |
Expert Panel: median age 55 y (42–70 y)∗ Patient partner: NR Median age of participants in usability testing: 59 y (42–83 y)∗ |
NR | NR | Stroke | Design/Planning Analytic Dissemination |
Involve Involve Involve |
| Küçükkaya et al.88 (2023) Turkey |
NR Cross-sectional study |
NR | NR | NR | NR | HF | Design/Planning | Consult |
| Leifeld et al.89 (2009) North-Rhine Westphalia, Germany |
Hairdressers (n = 33) Surveys |
NR | NR | NR | NR | Stroke | Conceptual Design/Planning |
Collaborate Collaborate |
| Pedersen et al.91 (2022) Denmark |
PPI Board (n = 4) Single-arm feasibility study |
NR | NR | NR | NR | MI | Design/Planning | Consult |
| Proios & Tsakpounidou99 (2020) Northern Greece |
Patient partners (stroke survivors) (n = 2) Cross-sectional study |
2 (100) | 27 and 44 y | NR | NR | Stroke | Conceptual Design/Planning |
Involve Involve |
CAB, Community Advisory Board; CBPR, community-based participatory research; CG, caregivers; COPD, chronic obstructive pulmonary disease; C-RAB, Community Research Advisory Board; CVD, cardiovascular disease; HF, heart failure; LGBTQ+, lesbian, gay, bisexual, transgender, queer and/or questioning, plus others such as nonbinary, intersex, etc; MI, myocardial infarction; NR, no response; PPC, Patient-Partner Committee; PPI, patient and public involvement; PROMS, patient-reported outcome measures; PWS, persons with stroke; RCT, randomized controlled trial; SD, standard deviation; UK, United Kingdom.
Data not disaggregated by sex.
Total numbers not reported.
Missing data, n = 1.
Participatory action research and community-based participatory research
Fourteen studies utilized a participatory action research and/or community-based participatory research approach.68,71, 72, 73, 74,76,83,84,87,89,90,93,94,96 The majority of these were undertaken in the US from 2013 to 2022,68,71, 72, 73,76,83,84,87,90,93,94,96 one was conducted in Canada in 2007,74 and one was conducted in Germany in 2009.89 Race and ethnicity was primarily reported as African American,68,71,73,76,96, Black,83,90 African Canadian,74 South Asian,84 other non-White (Chinese American, Hispanic, Korean-American, Latino)73,90 and White73 populations. Five studies did not report race or ethnicity information,72,87,89,93,94 and data were not disaggregated by race or ethnicity.73 The number of African American, African Canadian, Black, Latino, Chinese American, and Korean American female community partners was reported to be 59, or 73% of the total patient partners reported across these studies.68,73,74,76,83,90 Some aspect of gender (identity, roles, relations, institutionalized) was reported in 5 of the 14 studies that utilized a participatory action research and/or community-based participatory research approach.68,71,73,76,83 Most community partners were not married or were widowed (n = 28),68 with incomes between US$50,000 to $80,000.71 Community partners on advisory committees represented African American, Hispanic, and White female partners across various life stages (ie, young mothers, early to mid-career, professionals, and retirement; n = 6)73 with LGBTQ+ (lesbian, gay, bisexual, transgender, queer and/or questioning, plus others such as nonbinary, intersex, etc.) African American representation (n = 6).76 Four primary sources of stress (ie, aspect of gender identity) were reported to be workplace, parenting, finances, and social media; gendered racism, discrimination, and life imbalance also emerged as underlying stressors in Black female partners (n = 5).83 Ages were primarily > 46 years (n = 36; 77%)68 or > 51 years (83%)71 (range: 25-60 years,73 > 21 years,68 > 35 years,71 or > 40 years83) and were reported in only 4 of the participatory action research and/or community-based participatory research studies.
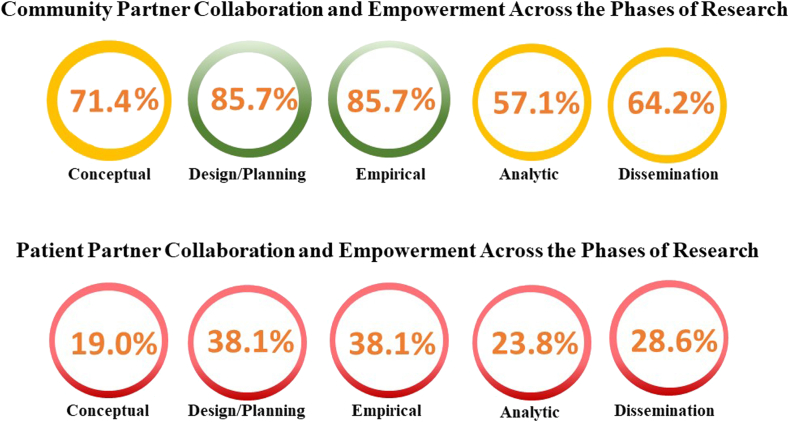
Community partners were engaged across all research phases—conceptual, design and planning, empirical, analytic, and dissemination. They were most engaged as collaborators in the design and/or planning (n = 11; 78.6%)68,71, 72, 73,76,83,84,87,89,90,94 and empirical (n = 12; 85.7%)68,71,72,74,76,83,84,87,90,93,94,96 phases of research. Community partners also were empowered to make final recruitment decisions in the design and/or planning of a stroke-related study in the US96 (n = 1; 7.1%). Community partners were less engaged in the conceptual (n = 10; 71.4%),68,71,72,83,84,87,89,90,94,96 analytic (n = 8; 57.1%),68,71,74,76,87,90,94,96 and dissemination (n = 8; 57.1%)68,71,73,76,83,87,90,94 phases of research. In 5 studies, community partners collaborated with investigators across all phases to make decisions focused on CVD,68,71 stroke,87,90,94 and MI94; all 5 studies were conducted in the US. Community partners were empowered to lead dissemination strategies through the creation of a travelling quilt to depict study findings, presentation at public events, and organization of media interviews in the only CVD-community-based participatory research study conducted in Canada.74 In 3 studies,71,83,87 community partners received training and either compensation for training,87 US$30 for meeting attendance,83 or a US$50 monthly stipend for their role as research advisory council members.71 A fourth study did not provide compensation to community partners who participated as equal members of the study team, but it did specify that community partners had already received funding through a local agency on aging.90 Dodgen et al.73 compensated community partners for their time during and outside meetings, and meetings were scheduled in the evening to accommodate work schedules, meals were provided at the start of each meeting, and community members were encouraged to bring children to the meetings, as childcare was not provided. The remaining studies68,72,74,76,84,89,93,94,96 did not acknowledge or provide details on compensation or reimbursement structures for community partners.
Patient-oriented and individual patient-partner research
The remaining 21 studies focused on engagement at the level of individual patient partners.62,63,67,69,70,75,77, 78, 79, 80, 81, 82,85,86,88,91,92,95,97, 98, 99 The majority of these were undertaken in Canada,62,63,69,70,75,98 the UK,67,80,82,85,92,97 and the US,77,79,81,95 from 2012 to 2023; 1 was conducted in Greece99 in 2020, 1 was conducted in each of Denmark,91 Ireland,78 and Sweden86 in 2022, and 1 was conducted in Turkey88 in 2023. Only 2 studies (10%)77,82 reported the race and ethnicity of patient-partner working group members. Investigators of 1 study indicated that efforts were made to have representation from those of South and East Asian descent, as well as Indigenous women living in Northern communities of Canada.63 Of the 2 studies that reported race and ethnicity, 1 study77 reported 1 White female partner, 1 African American female partner, 1 White male partner, and 1 African American male partner. The second study included all White patient partners (n = 18), but the results were not disaggregated by sex.82 One study mentioned institutionalized gender75 (ie, 1 patient partner had a lower education level, and the second patient partner had a higher education level) and 1 study referred to gender roles80 (ie, 2 patient partners were caregivers). However, gender was not reported in detail in any of the 21 studies focused on individual patient-partner engagement.62,63,67,69,70,75,77, 78, 79, 80, 81, 82,85,86,88,91,92,95,97, 98, 99 Age was reported in 3 studies, for a total of 14 patient partners; 2 patient partners were aged 74 and 82 years, respectively,75 2 were aged 27 and 44 years, respectively,99 and others had an age range of 40 to 75 years.97
Patient partners were engaged across all research phases—conceptual, design and planning, empirical, analytic, and dissemination; this engagement was defined primarily as being informed, consulted, or involved. Patients were engaged as partners or collaborators in the design and/or planning (n = 8; 38.1%)62,63,67,70,78,80,82,98 and empirical (n = 8; 38.1%)62,67,70,78,82,95,98,99 phases of research. Patient partners also collaborated in analyzing and/or interpreting the data (n = 5; 23.8%)62,67,70,75,78 and disseminating the results (n = 6; 28.6%).62,67,70,82,95,98 Patient partners were least engaged in identifying the problem (n = 4; 19.0%)62,63,70,78 to be researched. In 2 studies, patient partners collaborated with investigators across all research phases to make decisions focused on CVD70 and coronary artery disease,62 both studies were conducted in Canada, and 1 study utilized the GRIPP2 guidelines for reporting patient engagement.70 A second protocol paper also proposed use of the GRIPP2 guidelines.78 No studies reported patient-partner engagement at the level of empowerment (ie, making final decisions). Investigators in 2 studies reported using the definitions and guidelines of INVOLVE80,97 and compensated patient partners with grant funding,80 which included £50 for meeting attendance.97 Others also compensated patient partners for training91 and provided reimbursement for travel expenses63,91 and caregiving costs.63 Meetings were arranged with accessibility in mind, such as ensuring reasonable distances to parking, elevators, and washrooms.97 Consideration was also given to providing proper acoustic and/or visual resources, with suitable seating and adequate space for wheelchairs and other mobility aids.97 The remaining studies did not acknowledge or provide details on compensation or reimbursement structures for patient partners (Fig. 2).
Figure 2.
Community- and patient-partner collaboration and empowerment across the phases of research. Green = ≥ 75% community or patient-partner collaboration; yellow = 50%-75% community- or patient-partner collaboration; red = ≤ 50% community- or patient-partner collaboration.
Discussion
This review was conducted using rapid-review guidelines recommended by the Cochrane Rapid Reviews Methods Group57 and by Tricco et al.58 The inclusion criteria were kept broad to ensure that relevant studies were not missed; this decision was made in collaboration with 2 patient partners and was based on a previous patient-engagement search.60 PAR and community-based participatory research originated in the late 1960s, with a movement toward patient and public involvement in research in the UK (INVOLVE) in 1996,42 PCORI in the US in 2010,44 and SPOR in Canada in 2011.39 Findings from this rapid review suggest 417 female partners have been engaged in women’s CVD (IHD, heart failure, and stroke) research. Forty percent of studies were clustered as PAR and/or community-based participatory research that primarily originated in the US.68,71, 72, 73, 74,76,83,84,87,89,90,93,94,96 When reported, most patient partners identified as African American middle-class unmarried or widowed female partners across various life stages. Only 1 Canadian community-based participatory research study was identified in this rapid review; the study was undertaken because most of the health research on Black women in 2007 originated in the US. In the past 2 decades, little progress has been made in advancing community-based participatory research in Canada. The remaining 60% of patient-engagement studies focused on patient-oriented and/or patient-partner research; it was primarily conducted in Canada, the UK, and the US,62,63,67,69,70,75,77, 78, 79, 80, 81, 82,85,86,88,91,92,95,97, 98, 99 which is not surprising given the origin of INVOLVE, PCORI, and SPOR across these countries. Six studies were conducted in Canada, and demographic patient-partner details (ie, sex, age, race/ethnicity, gender) were rarely reported.
Community and patient partners were most engaged in the design and/or planning and implementation phases of research, in contrast to findings from previous systematic reviews that reported patient engagement to be most common at the conceptualization phase of study development.49,50 Community partners in community-based participatory research were more engaged than individual patient partners in patient-oriented research across all phases of research. In fact, less than 50% of patient partners collaborated or were empowered to make study decisions through governance, which is how patient partners can have the greatest impact in research.53 The results of our rapid review are similar to those of a review of 126 studies funded by PCORI, which found that patient-partner engagement ranged from passive input (ie, focus groups, 12%), to consultation (ie, working groups, 46%), to true collaboration and shared leadership (ie, being research team members, 37%).100
Implications for research
Community-based participatory research aims to reduce inequities and/or injustices identified by members of marginalized communities through community and researcher collaboration.38,101 True collaboration implies that community members are involved in every step of the research process—conceptualization, design and planning, empirical, analytic, and dissemination.52 Community-based participatory research is a partnership; it is iterative and often unpredictable, and community and researcher collaboration is imperative. The skills, time, and resources necessary for community-based participatory research can be counterintuitive to university researchers, who are driven by an academic infrastructure to generate rapid knowledge for scientific advancement—that is, research that perpetuates oppressive power relations. Most recently, Cornish et al.40 identified a call-to-action for academic researchers and institutions to be fully accountable to both academic and community audiences, and to support not only community collaboration but also its emancipation.
The impetus to engage patients as partners in research hinged on improving trial efficiency (ie, recruitment) and ensuring that trial outcomes were of value to patients.43 In a previous meta-analysis of 19 studies, engaging patient partners on research teams resulted in 3.14 times greater recruitment, compared to that for trials that did not engage patient partners (P = 0.02).43 In this rapid review, only 4 studies were RCTs, with one RCT protocol paper; only 1 study provided patient-partner demographics that included 7 female partners who collaborated across all phases of research.90 A total of 740 cardiovascular clinical trials have been completed between 2010 and 2017, with only 38.2% of participants being women, and with fewer women being recruited to procedural trials and to those trials focused on acute coronary syndrome.102 More recent data from the US Food and Drug Administration (2015 to 2019) suggest that, in general, clinical trials have a 51% representation of female participants , although the numbers appeared to be lower for trials that were focused on CVD.103 In addition, 76% of female participants in these trials were White, reflecting an urgent need to improve the level of diversity in regard to not only sex, but also gender, race, and ethnicity.103 Canada is a diverse nation with a population that represents broad social identities of race and ethnicity, gender, etc. Participation logistics and clinical trial processes affect recruitment102; these can be ameliorated by collaborating with communities and patient partners.
Teed et al.63 conducted a 3-staged quality improvement initiative on behalf of the Heart & Stroke Foundation of Canada to identify systemic healthcare gaps and the needs of women with heart disease and stroke. A total of 204 women informed a 5-year plan to address gaps. Patient engagement could be enhanced by utilizing the infrastructures of national health charities to connect patient partners with researchers, clinicians, and decision-makers. The GRIPP2 reporting guidelines can be used prospectively to plan patient-partner engagement in studies, and retrospectively, as a quality-assurance step in the documenting of patient-engagement processes in publications and reports.27
Implications for policy
An urgent need exists to engage community members and women as patient partners in CVD research focused on women. Community members and patient partners will have the greatest impact if they can collaborate and are empowered to make research decisions through governance, such as participating as members of citizen and/or patient advisory committees.53 To facilitate meaningful and active engagement of women on research teams, structures need to be in place for providing relationship building, financial compensation and reimbursement, flexibility in meeting times and delivery, support for transportation and caregiving, and attention to accessibility requirements. Currently, little community-based participatory CVD research is being conducted in Canada. Patient partners have rarely collaborated or been empowered in CVD research in Canada; they have been informed, consulted, or involved, but this approach risks tokenism, ableism, instrumentalization, and cynicism.104 CVD research focused on women should be undertaken with women, representing various gender identities, races, and ethnicities, across the lifespan. When reported, data were rarely disaggregated by sex, race, or ethnicity. The time has come to harness the innovative work of Teed et al.63 and the call-to-action proposed by Cornish et al.40 to engage communities and women as patient partners in setting research priorities to improve outcomes for women across race and ethnicities. Quality standards should mandate a minimal level of community and patient-partner engagement in women’s CVD research, to avoid perpetuating oppressive power relations and reducing the lived experience of women to that of a mere patient in the healthcare system.105 Women as patient partners and/or community members should identify research priorities; they can collaborate in designing trials and assisting in recruitment strategies, thereby improving the participation of women in clinical trials. This would ultimately better inform CVD guidelines and improve cardiovascular outcomes for women.106,107
Limitations
This review was conducted as a rapid review utilizing accelerated forms of systematic review methodologies to provide a timely and accurate summary of the literature on community and patient engagement in women’s CVD research. Evidence suggests that findings from rapid and full systematic reviews are similar, and conclusions from rapid reviews can therefore be used to make recommendations for clinical and policy decisions.108 However, this search did not include grey literature sources (eg, clinical trial registries), and therefore, some relevant information may have been missed.109 Moreover, a quality appraisal was not deemed necessary and was not undertaken, and results were presented as a narrative synthesis, which may provide less depth of information and detail in recommendation.110,111
Conclusions
Community-based participatory CVD research in Canada in virtually nonexistent. Patient-partner engagement in cardiovascular research has been reported to be only 5%,112 which continues to lag behind other specialty fields, such as oncology.43 The results of our rapid review suggest that female partners have been engaged in patient-oriented research in Canada, but their level of engagement is low. Improved CVD outcomes may be achieved with better community and patient-partner collaboration and empowerment across all phases of women’s CVD research, genders, races, and ethnicities. Including communities and patient partners as true collaborators in women’s CVD research could assist in defining unmet needs, ensuring that research priorities better align with patient priorities, and enhancing trial efficiency (eg, recruitment).
Acknowledgments
Ethics Statement
The research reported adhered to relevant ethical guidelines.
Patient Consent
The authors confirm that patient consent is not applicable to this article. This is a rapid review of the evidence and therefore, no patient-identified personal data were collected.
Funding Sources
The authors have no funding sources to declare.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
See page 499 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2023.12.016.
Supplementary Material
References
- 1.Norris C., Yip C., Nerenberg K., et al. Introducing the Canadian Women’s Heart Health Alliance ATLAS on Epidemiology, Diagnosis, and Management of Cardiovascular Disease in Women. CJC Open. 2020;2:145–150. doi: 10.1016/j.cjco.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogel B., Acevedo M., Appelman Y., et al. The Lancet Women and Cardiovascular Disease Commission: reducing the global burden by 2030. Lancet. 2021;397:2385–2438. doi: 10.1016/S0140-6736(21)00684-X. [DOI] [PubMed] [Google Scholar]
- 3.Statistics Canada Table 13-10-0394-01 Leading causes of death, total population, by age group. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310039401&pickMembers[0]=2.1&pickMembers[1]=3.3&cubeTimeFrame.startYear=2018&cubeTimeFrame.endYear=2022&referencePeriods=20180101%2C20220101 Available at:
- 4.Jaffer S., Foulds H.J.A., Parry M., et al. The Canadian Women's Heart Health Alliance ATLAS on the Epidemiology, Diagnosis, and Management of Cardiovascular Disease in Women; Chapter 2: scope of the problem. CJC Open. 2021;3:1–11. doi: 10.1016/j.cjco.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norris C., Yip C., Nerenberg K., et al. State of the science in women’s cardiovascular disease: a canadian perspective on the influence of sex and gender. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.015634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Center for Health Statistics . Health United States, 2011 With a Special Feature on Socioeconomic Status and Health. 2012. https://www.cdc.gov/nchs/data/hus/hus11.pdf Available at: [PubMed] [Google Scholar]
- 7.Izadegahdar M., Singer J., Lee M., et al. Do younger women fare worse? Sex differences in acute myocardial infarction hospitalization and early mortality rates over ten years. J Womens Health. 2014;23:10–17. doi: 10.1089/jwh.2013.4507. [DOI] [PubMed] [Google Scholar]
- 8.Pacheco C., Quesada O., Pepine C., Bairey Merz C. Why names matter for women: MINOCA/INOCA (myocardial infarction/ischemia and no obstructive coronary artery disease) Clin Cardiol. 2018;41:185–193. doi: 10.1002/clc.22894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bairey Merz C., Pepine C., Walsh M., Fleg J. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation. 2017;135:1075–1092. doi: 10.1161/CIRCULATIONAHA.116.024534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.GBD 2013 Mortality and Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benjamin E., Virani S., Callaway C., et al. Heart disease and stroke statistics—2018 update. a report from the American Heart Association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 12.Lam C., Arnott C., Beale A., et al. Sex differences in heart failure. Eur Heart J. 2019;40:3859–3868. doi: 10.1093/eurheartj/ehz835. [DOI] [PubMed] [Google Scholar]
- 13.Man J., Beckman J., Jaffe I. Sex as a biological variable in atherosclerosis. Circ Res. 2020;126:1297–1319. doi: 10.1161/CIRCRESAHA.120.315930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pepine C., Ferdinand K., Shaw L., et al. Emergence of nonobstructive coronary artery disease: a woman's problem and need for change in definition on angiography. J Am Coll Cardiol. 2015;66:1918–1933. doi: 10.1016/j.jacc.2015.08.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herscovici R., Sedlak T., Wei J., et al. Ischemia and no obstructive coronary artery disease (INOCA): What is the risk? J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.008868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arthur H., Campbell P., Harvey P., et al. Women, cardiac syndrome X, and microvascular heart disease. Can J Cardiol. 2012;28:S42–S49. doi: 10.1016/j.cjca.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Altintas E., Yigit F., Taskintuna N. The impact of psychiatric disorders with cardiac syndrome X on quality of life: 3 months prospective study. Int J Clin Exper Med. 2014;7:3520–3527. [PMC free article] [PubMed] [Google Scholar]
- 18.Pelletier R., Khan N., Cox J., et al. Sex versus gender-related characteristics: Which predicts outcome after acute coronary syndrome in the young? J Am Coll Cardiol. 2016;67:127–135. doi: 10.1016/j.jacc.2015.10.067. [DOI] [PubMed] [Google Scholar]
- 19.Pelletier R., Humphries K., Shimony A., et al. Sex-related differences in access to care among patients with premature acute coronary syndrome. CMAJ. 2014;186:497–504. doi: 10.1503/cmaj.131450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canadian Institutes of Health Research On-line training modules: integrating sex & gender in health research. http://www.cihr-irsc.gc.ca/e/49347.html Available at:
- 21.Canadian Institutes of Health Research Meet the Methods series: Methods for Prospectively and Retrospectively Incorporating Gender-Related Variables in Clinical Research. https://cihr-irsc.gc.ca/e/52608.html Available at:
- 22.Tadiri C., Raparelli V., Abrahamowicz M., et al. Methods for prospectively incorporating gender into health sciences research. J Clin Epidemiol. 2021;129:191–197. doi: 10.1016/j.jclinepi.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 23.Canto J., Canto E., Goldberg R. Time to standardize and broaden the criteria of acute coronary symptoms presentations in women. Can J Cardiol. 2014;30:721–728. doi: 10.1016/j.cjca.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 24.Kirchberger I., Heier M., Wende R., von Scheidt W., Meisinger C. The patient's interpretation of myocardial infarction symptoms and its role n the decision process to seek treatment: the MONICA/KORA Myocardial Infarction Registry. Clin Res Cardiol. 2012;101:909–916. doi: 10.1007/s00392-012-0475-8. [DOI] [PubMed] [Google Scholar]
- 25.Sjostrom-Strand A., Fridlund B. Women's descriptions of symptoms and delay reasons in seeking medical care at the time of a first myocardial infarction: a qualitative study. Int J Nurs Stud. 2008;45:1003–1010. doi: 10.1016/j.ijnurstu.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Government of Canada The Health Canada Policy Toolkit for Public Involvement in Decision Making. https://www.canada.ca/en/health-canada/corporate/about-health-canada/reports-publications/health-canada-policy-toolkit-public-involvement-decision-making.html#a11 Available at:
- 27.Staniszewska S., Brett J., Simera I., et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ. 2017;358:1–7. doi: 10.1136/bmj.j3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rethlefsen M., Kirtley S., Waffenschmidt S., et al. PRISMA-S: an extension to the PRISMA statement for reporting literature searches in systematic reviews. Syst Rev. 2021;10:39. doi: 10.1186/s13643-020-01542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gasevic D., Ross E., Lear S. Ethnic differences in cardiovascular disease risk factors: a systematic review of North American evidence. Can J Cardiol. 2015;31:1169–1179. doi: 10.1016/j.cjca.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 30.Rana A., de Souza R., Kandasamy S., Lear S., Anand S. Cardiovascular risk among South Asians living in Canada: a systematic review and meta-analysis. CMAJ Open. 2014;2:E183–E191. doi: 10.9778/cmajo.20130064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anand S., Razak F., Davis A., et al. Social disadvantage and cardiovascular disese: development of an index and analysis of age, sex, and ethnicity effects. Int J Epidemiol. 2006;35:1239–1245. doi: 10.1093/ije/dyl163. [DOI] [PubMed] [Google Scholar]
- 32.Tjepkema M., Wilkins R., Goedhuis N., Pennock J. Cardiovascular disease mortality among First Nations people in Canada, 1991-2001. Chronic Dis Inj Can. 2012;32:200–207. [PubMed] [Google Scholar]
- 33.Schultz W., Kelli H., Lisko J., et al. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation. 2018;137:2166–2178. doi: 10.1161/CIRCULATIONAHA.117.029652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Backholer K., Peters S., Bots S., et al. Sex differences in the relationship between socioeconomic status and cardiovascular disease: a systematic review and meta-analysis. J Epidemiol Commun Health. 2017;71:550–557. doi: 10.1136/jech-2016-207890. [DOI] [PubMed] [Google Scholar]
- 35.Markbreiter J., Buckley P. CVD Advocacy Toolkit: The road to 2018. World Heart Federation; 2016. https://world-heart-federation.org/wp-content/uploads/2017/05/WHF9421_Advocacy_toolkit_PDF-1.pdf [Google Scholar]
- 36.Heart&Stroke. What we do/Strategy. https://www.heartandstroke.ca/what-we-do/strategy Available at:
- 37.Heart & Stroke Foundation of Canada Ms. Understood: Heart & Stroke 2018 Heart Report. https://www.heartandstroke.ca/-/media/pdf-files/canada/2018-heart-month/hs_2018-heart-report_en.ashx Available at:
- 38.Canadian Institutes of Health Research Strategy for patient-oriented research. https://cihr-irsc.gc.ca/e/41204.html Available at:
- 39.Street C., Twells L., Leamon T., Taylor L., Etchegary H. In: Clinical Epidemiology: Practice and Methods. 3rd ed. Parfrey P., Barrett B., editors. Springer; New York: 2021. Changing health behaviors 1: patient-oriented research and patient engagement in health research. [DOI] [PubMed] [Google Scholar]
- 40.Cornish F., Breton N., Moreno-Tabarez U., et al. Participatory action research. NatRev. 2023;3:1–14. [Google Scholar]
- 41.Jacobs S. The use of participatory action research within education—benefits to stakeholders. World J Educ. 2016;6:48–55. [Google Scholar]
- 42.INVOLVE. About INVOLVE. https://www.involve.org.uk/about/about-involve Available at:
- 43.Zannad F., Chauhan C., Gee P., et al. Patient partnership in cardiovascular clinical trials. Eur Heart J. 2022;43:1432–1437. doi: 10.1093/eurheartj/ehab835. [DOI] [PubMed] [Google Scholar]
- 44.Frank L., Basch E., Selby J. The PCORI perspective on patient-centred outcomes research. JAMA. 2014;312:1513–1514. doi: 10.1001/jama.2014.11100. [DOI] [PubMed] [Google Scholar]
- 45.Canadian Institutes of Health Research Strategy for patient-oriented research capacity development framework. http://www.cihr-irsc.gc.ca/e/documents/spor_capacity_development_framework-en.pdf Available at:
- 46.Canadian Institutes of Health Research Strategy for patient-oriented research patient engagement framework. http://www.cihr-irsc.gc.ca/e/48413.html Available at:
- 47.Kirwan J.R., de Wit M., Frank L., et al. Emerging guidelines for patient engagement in research. Value Health. 2017;20:481–486. doi: 10.1016/j.jval.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Manafo E., Petermann L., Mason-Lai P., Vandall-Walker V. Patient engagement in Canada: a scoping review of the ‘how’ and ‘what’ of patient engagement in health research. Health Res Policy Syst. 2018;16:5. doi: 10.1186/s12961-018-0282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Concannon T., Fuster M., Saunders T., et al. A systematic review of stakeholder engagement in comparative effectiveness and patient-centered outcomes research. J Gen Intern Med. 2014;29:1692–1701. doi: 10.1007/s11606-014-2878-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Domecq J., Prutsky G., Elraiyah T., et al. Patient engagement in research: a systematic review. Health Serv Res. 2014;14:1–9. doi: 10.1186/1472-6963-14-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shippee N., Domecq J., Prutsky G., et al. Patient and service user engagement in research: a systematic review and synthesized framework. Health Expect. 2015;18:1151–1166. doi: 10.1111/hex.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Polit D., Beck C. 7th ed. Lippincott Williams & Wilkins; Philadelphia: 2004. Nursing Research: Principles and Methods. [Google Scholar]
- 53.IAP2 International Association IAP2 spectrum of public participation. https://cdn.ymaws.com/www.iap2.org/resource/resmgr/pillars/Spectrum_8.5x11_Print.pdf Available at:
- 54.Mockford C., Staniszewska S., Griffiths F., Herron-Marx S. The impact of patient and public involvement on UK NHS health care: a systematic review. Int J Qual Health Care. 2012;24:28–38. doi: 10.1093/intqhc/mzr066. [DOI] [PubMed] [Google Scholar]
- 55.Brett J., Staniszewska S., Mockford C. A systematic review of the impact of patient and public involvement on service users, researchers and communities. Patient. 2014;7:387–395. doi: 10.1007/s40271-014-0065-0. [DOI] [PubMed] [Google Scholar]
- 56.Moher D., Schulz K., Simera I., Altman D. Guidance for developers of health research reporting guidelines. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klerings I., Robalino S., Booth A., et al. Rapid reviews methods series: guidance on literature search. BMJ Evid-Based Med. 2023;28:412–417. doi: 10.1136/bmjebm-2022-112079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tricco A., Langlois E., Straus S. World Health Organization; Geneva: 2017. Rapid Reviews to Strengthen Health Policy and Systems: A Practical Guide. [Google Scholar]
- 59.SPOR Recommendations on patient engagement compensation. https://diabetesaction.ca/wp-content/uploads/2018/07/TASK-FORCE-IN-PATIENT-ENGAGEMENT-COMPENSATION-REPORT_FINAL-1.pdf Available at:
- 60.Parry M., Bjørnnes A., Toupin April K., et al. Patient engagement partnerships in clinical trials: development of patient partner and investigator decision aids. Patient-Centered Outcomes Res. 2020;13:745–756. doi: 10.1007/s40271-020-00460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parry M., Visintini S., Johnston A., et al. Peer-support interventions for women with cardiovascular disease: protocol for synthesising the literature using an evidence map. BMJ Open. 2022;12 doi: 10.1136/bmjopen-2022-067812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parry M., Dhukai A., Clarke H., et al. Development and usability testing of HEARTPAIN: protocol for a mixed methods strategy to develop an integrated smartphone and web-based intervention for women with cardiac pain. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2019-033092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Teed M., Ianiro J., Culhane C., et al. Engaging women with lived experience: a novel cross-Canada approach. J Patient Exp. 2021;8 doi: 10.1177/23743735211008300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pope C., Mays N., Popay J. McGraw Open University Press; New York: 2007. Synthesizing Qualitative and Quantitative Health Evidence. [Google Scholar]
- 65.Popay J., Roberts H., Sowden A., et al. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews. A Product from the ESRC Methods Programme. 2006. https://www.lancaster.ac.uk/media/lancaster-university/content-assets/documents/fhm/dhr/chir//NSsynthesisguidanceVersion1-April2006.pdf Available at: [Google Scholar]
- 66.Page M., McKenzie J., Bossuyt P., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Benn Y., Jayes M., Casassus M., et al. A qualitative study into the experience of living with acalculia after stroke and other forms of acquired brain injury. Neuropsychol Rehabil. 2022;33:1512–1536. doi: 10.1080/09602011.2022.2108065. [DOI] [PubMed] [Google Scholar]
- 68.Bess K.D., Frerichs L., Young T., et al. Adaptation of an evidence-based cardiovascular health intervention for rural African Americans in the Southeast. Prog Commun Health Partner. 2019;13:385–396. doi: 10.1353/cpr.2019.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blumer V., Gayowsky A., Xie F., et al. Effect of patient-centered transitional care services on patient-reported outcomes in heart failure: sex-specific analysis of the PACT-HF randomized controlled trial. Eur J Heart Fail. 2021;23:1488–1498. doi: 10.1002/ejhf.2312. [DOI] [PubMed] [Google Scholar]
- 70.Cho R.Y., Weng J., Lynch K., et al. Priorities for services in young patients with atherosclerotic cardiovascular disease and their family members: an exploratory mixed-methods study. CJC Open. 2019;1:107–114. doi: 10.1016/j.cjco.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crabbe D., Gardiner H. Temple University; Philadelphia: 2021. Building Capacity for a Patient-Centered Outcomes Research Agenda on Racial and Sex-Specific Disparities in Cardiovascular Disease in COVID-19. [Google Scholar]
- 72.Dickson V.V., Melkus G.D., Dorsen C., Katz S., Riegel B. Improving heart failure self-care through a community-based skill-building intervention: a study protocol. J Cardiovasc Nurs. 2015;30(4 Suppl 1):S14–S24. doi: 10.1097/JCN.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 73.Dodgen L., Spence-Almaguer E., Cantu Anguiano K., Hooker A., White S. Partnership processes to develop SHE tribe: a healthy lifestyle intervention. Health Promot Pract. 2020;21:591–600. doi: 10.1177/1524839918812428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Etowa J.B., Bernard W.T., Oyinsan B., Clow B. Participatory action research (PAR): an approach for improving black women's health in rural and remote communities. J Transcult Nurs. 2007;18:349–357. doi: 10.1177/1043659607305195. [DOI] [PubMed] [Google Scholar]
- 75.Ghisi G.L.M., Kin S.M.R., Price J., et al. Women-focused cardiovascular rehabilitation: an international council of cardiovascular prevention and rehabilitation clinical practice guideline. Can J Cardiol. 2022;38:1786–1798. doi: 10.1016/j.cjca.2022.06.021. [DOI] [PubMed] [Google Scholar]
- 76.Gleason-Comstock J., Calhoun C.B., Mozeb G., et al. Recruitment, retention, and future direction for a heart health education and risk reduction intervention led by community health workers in an African American majority city. J Racial Ethn Health Disparities. 2022;10:1432–1440. doi: 10.1007/s40615-022-01329-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Heo S., Moser D.K., Lennie T.A., et al. Development and testing of the feasibility and acceptability of a tailored dietary intervention in patients with heart failure. J Cardiovasc Nurs. 2015;30:213–221. doi: 10.1097/JCN.0000000000000148. [DOI] [PubMed] [Google Scholar]
- 78.Horgan F., Lennon O., Hickey A., et al. A protocol to evaluate the impact of embedding public and patient involvement in a structured PhD program for stroke care. Front Rehabil Sci. 2022;3 doi: 10.3389/fresc.2022.877598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Inam M.E., Sanzgiri A., Lekka E., et al. Exception from informed consent in the era of social media: the SEGA stroke trial experience. Brain Circ. 2021;7:253–258. doi: 10.4103/bc.bc_44_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jalal Z., Paudyal V., Al-Arkee S., Dyson G., Marriott J. Designing a clinical pharmacy primary care intervention for myocardial infarction patients using a patient and public involvement discussion. Pharmacy (Basel) 2020;8:13. doi: 10.3390/pharmacy8010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jankowska E.A., Liu P.P., Cowie M.R., et al. Personalized care of patients with heart failure: are we ready for a REWOLUTION? Insights from two international surveys on healthcare professionals' needs and patients' perceptions. Eur J Heart Fail. 2023;25:364–372. doi: 10.1002/ejhf.2798. [DOI] [PubMed] [Google Scholar]
- 82.Johnson V.L., Apps L., Hadjiconstantinou M., et al. The development of a self-management intervention for stroke survivors—my life after stroke (MLAS) Disabil Rehabil. 2022;45:226–234. doi: 10.1080/09638288.2022.2029959. [DOI] [PubMed] [Google Scholar]
- 83.Jones H.J., Bakas T., Nared S., et al. Co-designing a program to lower cardiovascular disease risk in midlife Black women. Int J Environ Res Public Health. 2022;19:1356. doi: 10.3390/ijerph19031356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kandula N.R., Dave S., De Chavez P.J., et al. Translating a heart disease lifestyle intervention into the community: the South Asian Heart Lifestyle Intervention (SAHELI) study; a randomized control trial. BMC Public Health. 2015;15:1064. doi: 10.1186/s12889-015-2401-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kilbride C., Scott D.J.M., Butcher T., et al. Rehabilitation via home based gaming exercise for the upper-limb post stroke (RHOMBUS): protocol of an intervention feasibility trial. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2018-026620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kjörk E.K., Sunnerhagen K.S., Lundgren-Nilsson A., Andersson A.K., Carlsson G. Development of a digital tool for people with a long-term condition using stroke as a case example: participatory design approach. JMIR Hum Factors. 2022;9 doi: 10.2196/35478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kronish I.M., Goldfinger J.Z., Negron R., et al. Effect of peer education on stroke prevention: the prevent recurrence of all inner-city strokes through education randomized controlled trial. Stroke. 2014;45:3330–3336. doi: 10.1161/STROKEAHA.114.006623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kücükkaya H., Gonenc I.M. An evaluation of the prevalence and predictive factors of sexual dysfunction in women with heart failure: a cross-sectional survey. J Clin Nurs. 2023;32:3929–3942. doi: 10.1111/jocn.16578. [DOI] [PubMed] [Google Scholar]
- 89.Leifeld T., Rau R., Mensing M. Community stroke knowledge: a new information strategy using a joint project of the Public Health Service and the Hairdressers’ Guild of the Wesel District. J Public Health. 2009;17:371–376. [Google Scholar]
- 90.Menkin J.A., McCreath H.E., Song S.Y., et al. "Worth the walk": culturally tailored stroke risk factor reduction intervention in community senior centers. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.011088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pedersen M., Bennich B., Boateng T., et al. Peer-mentor support for older vulnerable myocardial infarction patients referred to cardiac rehabilitation: single-arm feasibility study. Pilot Feasibility Stud. 2022;8:172. doi: 10.1186/s40814-022-01141-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.2016. Integrating patient reported outcome measures (PROMs) into routine primary care for patients with multimorbidity: a feasibility study. Paper presented at: Patient Reported Outcome Measures (PROMs), Sheffield, UK. vol. 14; p. 1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Prabhakaran S., Richards C.T., Kwon S., et al. A community-engaged stroke preparedness intervention in Chicago. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Robles M.C., Corches C., Bradford M., et al. Community and academic partner creation of a community-informed, behavior theory-based music video to encourage stroke preparedness during the Covid-19 pandemic. Stroke. 2021;52(Suppl 1) 871. [Google Scholar]
- 95.Schwertfeger J.L., Miller S.A., Jordan M., Jordan D., Schneider K.L. A 3-day 'stroke camp' addressed chronic disease self-management elements and perceived stress of survivors of stroke and their caregivers reduced: survey results from the 14 US camps. Top Stroke Rehabil. 2024;31:1–10. doi: 10.1080/10749357.2023.2196468. [DOI] [PubMed] [Google Scholar]
- 96.Skolarus L.E., Murphy J.B., Zimmerman M.A., et al. Individual and community determinants of calling 911 for stroke among African Americans in an urban community. Circ Cardiovasc Qual Outcomes. 2013;6:278–283. doi: 10.1161/CIRCOUTCOMES.111.000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Williamson T., Kenney L., Barker A.T., et al. Enhancing public involvement in assistive technology design research. Disabil Rehabil Assist Technol. 2015;10:258–265. doi: 10.3109/17483107.2014.908247. [DOI] [PubMed] [Google Scholar]
- 98.Wilson T.A., Hazlewood G.S., Sajobi T.T., et al. Preferences of patients with chronic kidney disease for invasive versus conservative treatment of acute coronary syndrome: a discrete choice experiment. J Am Heart Assoc. 2023;12 doi: 10.1161/JAHA.122.028492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Proios H., Tsakpounidou K. FAST 112 HEROES: patient engagement in an educational stroke awareness program for kindergarten. Arch Phys Med Rehabil. 2020;101 [Google Scholar]
- 100.Forsythe L., Carman K., Szydlowski V., et al. Patient engagement in research: early findings from the Patient-Centered Outcomes Research Institute. Health Aff (Millwood) 2019;38:359–367. doi: 10.1377/hlthaff.2018.05067. [DOI] [PubMed] [Google Scholar]
- 101.Jull J., Giles A., Graham I. Community-based participatory research and integrated knowledge translaation: advancing the co-creation of knowledge. ImplementSci. 2017;12:150. doi: 10.1186/s13012-017-0696-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jin X., Chandramouli C., Allocco B., et al. Women’s participation in cardiovascular clinical trials from 2010 to 2017. Circulation. 2020;141:540–548. doi: 10.1161/CIRCULATIONAHA.119.043594. [DOI] [PubMed] [Google Scholar]
- 103.Vasisht K., Nugent B., Woodcock J. Progress and opportunities for women in clinical trials: a look at recent data and initiatives from the U.S. FDA. Medicine. 2021;2:456–459. doi: 10.1016/j.medj.2021.04.010. [DOI] [PubMed] [Google Scholar]
- 104.Bombak A., Hanson H. A critical discussion of patient engagement in research. J Patient Cent Res Rev. 2017;4:39–41. doi: 10.17294/2330-0698.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Glasdam S., Oeye C., Thrysoee L. Patient’s participation in decision-making in the medical field—‘projectification’ of patients in a neoliberal framed healthcare system: patient’s participation in decision-making. Nurs Philos. 2015;16:226–238. doi: 10.1111/nup.12092. [DOI] [PubMed] [Google Scholar]
- 106.Norris C., Tannenbaum C., Pilote L., et al. Systematic incorporation of sex-specific information into clinical practice guidelines for the management of ST-segment-elevation myocardial infarction: feasibility and outcomes. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.011597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tannenbaum C., Norris C., McMurtry M. Sex-specific considerations in guidelines generation and application. Can J Cardiol. 2019;35:598–605. doi: 10.1016/j.cjca.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 108.Watt A., Cameron A., Sturm L., et al. Rapid verus full systematic reviews: validity in clinical practice. ANZ J Surg. 2008;78:1037–1040. doi: 10.1111/j.1445-2197.2008.04730.x. [DOI] [PubMed] [Google Scholar]
- 109.Moons P., Goossens E., Thompson D. Rapid reviews: the pros and cons of an accelerated review process. Eur J Cardiovasc Nurs. 2021;20:515–519. doi: 10.1093/eurjcn/zvab041. [DOI] [PubMed] [Google Scholar]
- 110.Tricco A., Antony J., Zarin W., et al. A scoping review of rapid review methods. BMC Med. 2015;13:224. doi: 10.1186/s12916-015-0465-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ganann R., Ciliska D., Thomas H. Expediting systematic reviews: methods and implications of rapid reviews. Implement Sci. 2010;5:56. doi: 10.1186/1748-5908-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McCoy M., Carniol M., Chockley K., et al. Conflicts of interest for patient-advocacy organizations. N Engl J Med. 2017;376:880–885. doi: 10.1056/NEJMsr1610625. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.