Abstract
Background
Myocardial infarction with nonobstructive coronary artery disease (MINOCA) is defined as acute myocardial infarction (AMI) with angiographically nonobstructive coronary artery disease. MINOCA represents 6% of all AMI cases and is associated with increased mortality and morbidity. However, the wide array of pathophysiological factors and causes associated with MINOCA presents a diagnostic conundrum. Therefore, we conducted a contemporary systematic review of the pathophysiology of MINOCA.
Methods
A comprehensive systematic review of MINOCA was carried out through the utilization of the PubMed database. All systematic reviews, meta-analyses, randomized controlled trials, and cohort studies available in English or French that reported on the pathophysiology of MINOCA published after January 1, 2013 were retained.
Results
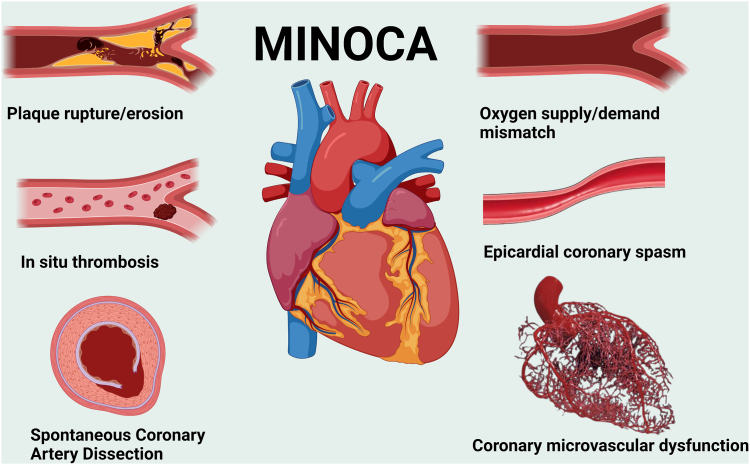
Of the 600 identified records, 80 records were retained. Central to the concept of MINOCA is the definition of AMI, characterized by the presence of myocardial damage reflected by elevated cardiac biomarkers in the setting of acute myocardial ischemia. As a result, a structured approach should be adopted to thoroughly assess and address clinically overlooked obstructive coronary artery disease, and cardiac and extracardiac mechanisms of myocyte injury. Once these options have been ruled out, a diagnosis of MINOCA can be established, and the appropriate multimodal assessment can be conducted to determine its specific underlying cause (plaque disruption, epicardial coronary vasospasm, coronary microvascular dysfunction, and coronary embolism and/or spontaneous coronary dissection or supply-demand mismatch).
Conclusions
Integrating a suitable definition of AMI and understanding the pathophysiological mechanisms of MINOCA are crucial to ensure an effective multimodal diagnostic evaluation and the provision of adequate tailored therapies.
RÉsumÉ
Contexte
L’infarctus du myocarde sans obstruction des artères coronaires (MINOCA) est défini comme un infarctus aigu du myocarde (IAM) en présence d’une coronaropathie non obstructive confirmée par angiographie. Le MINOCA représente 6 % de tous les cas d’IAM et est associé à une hausse des taux de mortalité et de morbidité. Cependant, le large éventail de facteurs physiopathologiques et de causes associés au MINOCA représente une énigme diagnostique. C’est pourquoi nous avons réalisé une analyse systématique des publications contemporaines sur la physiopathologie du MINOCA.
Méthodologie
Une analyse exhaustive des publications sur le MINOCA a été menée au moyen de la base de données PubMed. L’ensemble des analyses systématiques, des méta-analyses, des essais contrôlés randomisés et des études de cohorte publiés en anglais ou en français après le 1er janvier 2013 qui faisaient état de la physiopathologie du MINOCA ont été retenus.
Résultats
Parmi les 600 dossiers relevés, 80 ont été retenus. La définition de l’IAM était centrale au concept de MINOCA et était caractérisée par la présence d’une lésion myocardique attestée par des taux élevés de biomarqueurs cardiaques en contexte d’ischémie myocardique aiguë. Par conséquent, une approche structurée devrait être adoptée pour évaluer pleinement et traiter les coronaropathies obstructives qui passent inaperçues en clinique ainsi que les mécanismes cardiaques et extracardiaques des lésions aux myocytes. Une fois ces options exclues, un diagnostic de MINOCA peut être établi et l’évaluation multimodale appropriée peut être menée pour déterminer la cause sous-jacente précise (rupture de plaque, vasospasme d’une artère coronaire épicardique, dysfonction microvasculaire coronarienne et embolie coronarienne et/ou dissection spontanée d’une artère coronaire ou déséquilibre entre apports et besoins).
Conclusions
Il est crucial d’intégrer une définition convenable de l’IAM et de comprendre les mécanismes physiopathologiques du MINOCA pour assurer une évaluation diagnostique multimodale efficace et une prestation de traitements adaptés et adéquats.
Lay Summary
Myocardial infarction with nonobstructive coronary artery disease (MINOCA), or "heart attack with clear arteries," occurs when someone has the symptoms of a heart attack, but their coronary arteries are not significantly blocked. This condition occurs in a small number of heart attacks, but it is linked to a higher risk of complications and poor outcomes. Diagnosing MINOCA can be challenging. A review of published studies was done to improve our understanding of the causes of MINOCA, to thereby improve the diagnosis and treatment of this condition.
The contemporary diagnosis of acute myocardial infarction (AMI) is based on the demonstration of acute myocardial injury in the setting of myocardial ischemia.1 Most commonly, this condition occurs in the setting of underlying obstructive coronary artery disease (CAD), with therapy often consisting of timely revascularization.2 However, around 6% of AMI patients have no obstructive CAD and fit the criteria for the diagnosis of myocardial infarction with nonobstructive CAD (MINOCA).3 The prognosis of MINOCA is nevertheless not benign, as is commonly assumed based on early reports.4, 5, 6 In a large systematic review, MINOCA patients had unfavourable outcomes 12 months after their diagnosis, including 3.4% all-cause mortality, 9.6% major adverse cardiac events, 2.6% reinfarction, and 1.0% stroke.3 Both the prevalence and potential complications of MINOCA argue for prompt recognition of MINOCA, identification of its specific etiology, and its tailored treatment.
However, MINOCA patients represent a clinical and diagnostic challenge, given the multiplicity of etiologies and the pathophysiological mechanisms associated with this diagnosis. Given this context, an understanding of these underlying mechanisms is critical to ensuring that patients receive thorough and efficient diagnostic testing aimed at determining the precise cause of AMI. This testing, in turn, allows healthcare providers to offer specific therapies to treat the underlying condition. We therefore conducted a systematic review aimed at summarizing the contemporary understanding of the underlying causes and pathophysiological mechanisms of MINOCA.
Methods
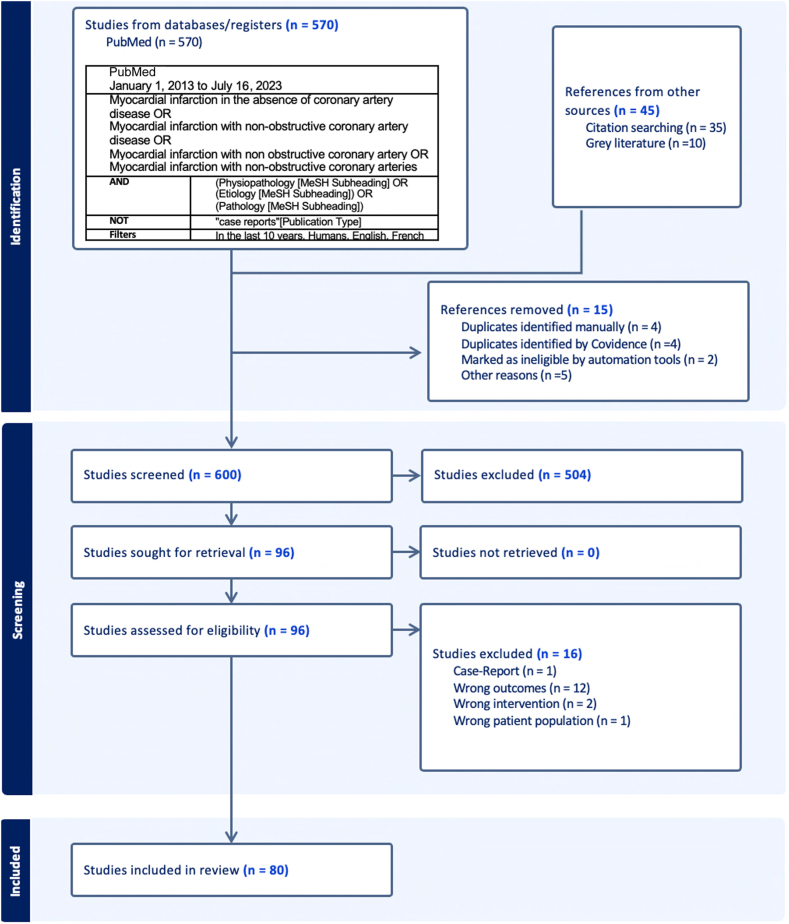
This systematic review was reported according to the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.7 PubMed was searched on July 16, 2023, using search terms for MINOCA as detailed in the example search algorithm presented in Figure 1.
Figure 1.
Search algorithm and flowchart of studies on the pathophysiology of myocardial infarction with no obstructive coronary artery disease.
We included systematic reviews, meta-analyses, randomized controlled trials, and cohort studies available in English or French that reported on the pathophysiology of MINOCA and were published after January 1, 2013. Case reports, case series, and abstracts that were not complete peer-reviewed articles were excluded.
We examined a wide range of potential etiologies contributing to MINOCA, and every etiology was searched for in the research database. Some key words were used, including “pathology,” “pathophysiology,” “cause,” and “diagnosis.”
One reviewer (K.H.) performed all database searches and imported the records into Covidence, a screening and data extraction tool for conducting systematic reviews. Using Covidence, 2 reviewers (L.-A.B.-P. and K.H.) independently screened article titles and abstracts and reviewed full-text articles for inclusion. In cases of disagreement regarding the inclusion of an article, a third researcher (C.P.) also reviewed the reference to determine its suitability for inclusion in this review.
Results
The database search returned 600 items. A primary screen excluded 504 unique references, and a comprehensive secondary screen excluded another 16 articles, leaving 80 references that informed this synthesis. The reasons for article exclusion are detailed in Figure 1.
Contemporary MINOCA definition
Central to defining MINOCA are the concepts of AMI and myocardial injury, which were thoroughly specified by the Joint European Society of Cardiology/American College of Cardiology/American Heart Association/World Heart Federation Task Force for the Universal Definition of Myocardial Infarction) 1 Myocardial injury is now defined by an elevated troponin level beyond the 99th percentile of the upper reference level.1 Although elevated troponin levels point to myocyte injury with the release of this intracellular protein into the systemic circulation, myocardial injury is nonspecific and can result from both ischemic and nonischemic mechanisms. AMI should be distinguished from myocardial injury, as the former is currently defined by the presence of myocardial damage reflected by elevated cardiac biomarkers in the setting of acute myocardial ischemia.1
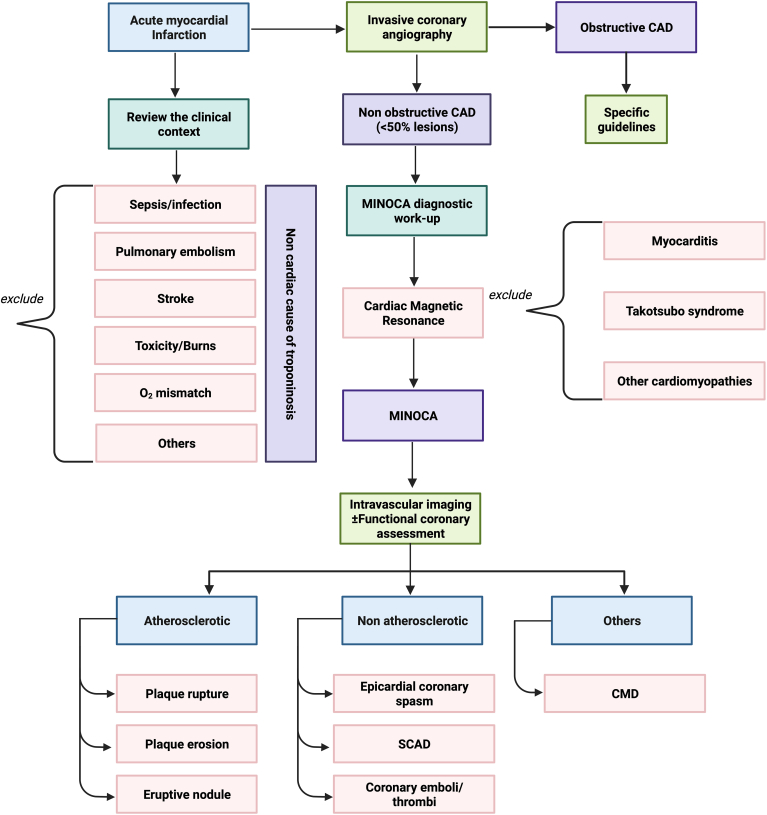
With this revision of the definition of AMI, the concept of MINOCA was updated also and should be reserved for patients who show evidence of ischemia based on their clinical presentation (Table 1).8 Consequently, when assessing patients with suspected AMI, clinically overlooked obstructive CAD, and cardiac (eg, myocarditis, Takotsubo syndrome [TTS], other cardiomyopathies) and extracardiac (eg pulmonary embolism, sepsis, cardiac contusion) mechanisms of myocyte injury should be considered and excluded (Fig. 2). Once these conditions have been excluded, a diagnosis of MINOCA can be made (Table 2). Although the mechanism of the TTS (ischemic vs nonischemic) is uncertain, the Fourth Universal Definition of Myocardial Infarction does not recognize this syndrome as a myocardial infarction.1 Therefore, this syndrome should be classified as separate from MINOCA.8
Table 1.
Myocardial infarction with no obstructive coronary artery disease (MINOCA) definition8
| The diagnosis of MINOCA is made in patients with acute myocardial infarction who fulfill the following criteria: |
|---|
|
|
|
Figure 2.
Clinical algorithm for the diagnosis of myocardial infarction with nonobstructive coronary artery disease (MINOCA; CAD). CMD, coronary microvascular dysfunction; SCAD, spontaneous coronary artery dissection. Created with Biorender.com.
Table 2.
Underlying mechanisms and selective diagnostic investigations of myocardial infarction with no obstructive coronary artery disease (MINOCA)
| Underlying mechanism | Selective diagnostic investigations |
|---|---|
| Ischemic presentation (MINOCA) | |
| Atherosclerosis | |
| Plaque rupture | Angiographic review |
| Plaque erosion | Functional assessment |
| Calcified nodule | Intracoronary imaging |
| Epicardial coronary vasospasm | Angiographic review |
| Resolution with intracoronary | |
| vasodilators such as intracoronary | |
| nitroglycerin | |
| Spasm provocation testing | |
| Coronary microvascular dysfunction testing | Angiographic review |
| Coronary microvascular function | |
| testing | |
| Stress PET or CMRI with MBFR | |
| Coronary thrombosis or embolism | Angiographic review |
| Intracoronary imaging | |
| Blood tests and genetic studies | |
| Spontaneous coronary artery dissection | Angiographic review |
| Intracoronary imaging | |
| Fixed atherosclerosis + supply–demand mismatch OR Supply–demand mismatch alone | Review of potential stressors |
| Clinically overlooked nonischemic presentation (MINOCA mimickers) | |
| Cardiac causes | |
| Takotsubo | Left ventricular angiogram |
| Contrast CMRI | |
| Myocarditis | Contrast CMRI |
| Cardiomyopathy | Contrast CMRI |
| Extracardiac causes | |
| Pulmonary embolism | Review of clinical context |
| Sepsis | |
| Cardiac contusion | |
| Other noncardiac causes of elevation of CTn levels | |
CMRI, cardiac magnetic resonance imaging; CTn, cardiac troponins; MBFR; myocardial blood flow reserve: PET, positron emission tomography.
Atherosclerotic causes of myocardial necrosis
The angiographic 50% diameter stenosis threshold definition for obstructive CAD is consistent with the previous American Heart Association/American College of Cardiology coronary angiography guidelines, but it is somewhat arbitrary.9 Although an obstructive lesion is strictly a pathophysiological concept requiring functional or physiological evaluation, such assessment is not performed routinely in all patients undergoing coronary angiography, and the decision on whether to perform percutaneous coronary intervention is often based solely on a visual estimation of the angiographic diameter stenosis, in spite of good evidence that visual estimation of lesion severity is extremely subjective, with significant interobserver variability.10 The angiographic severity of a lesion also can vary between angiograms, because of fluctuation in the vasomotor tone. Consequently, careful review of angiographic findings should be performed in suspected MINOCA patients to exclude potentially overlooked obstructive CAD. Although the evidence is limited regarding the role of fractional flow reserve (FFR) testing in MINOCA patients with moderate stenosis, FFR can be considered when moderate coronary atherosclerotic lesions are present (> 30% but < 50%), based on extrapolation of data from stable patients.11 For instance, up to one quarter of patients labeled as having no significant CAD after angiography alone were found to have functionally significant lesions after FFR measurement.11
Coronary plaque disruption is common among MINOCA patients (Fig. 3). Plaque disruption includes plaque rupture, plaque erosion, and calcified nodules.12 Plaque disruption can trigger luminal thrombus formation or plaque hemorrhage superimposed on an atherosclerotic plaque with or without concomitant spasm.2 Plaque rupture is the most common cause of coronary thrombosis and is characterized by a structural flaw or opening in the fibrous cap that normally isolates the plaque's lipid-rich atheromatous core from the bloodstream, revealing the thrombogenic core of the plaque.13 Dislodged plaque material is sometimes found within the thrombus, indicating that rupture and thrombosis coincided.2 Plaque rupture is associated with thinning of the fibrous cap that usually involves the following 2 mechanisms: (i) the loss of vascular smooth muscle cells (VSMCs) from the fibrous cap; and (ii) the infiltration of macrophages that degrade the collagen-rich cap matrix.2 Plaque erosion is the second most common cause of coronary thrombosis. In this condition, a loss and/or dysfunction of endothelial cells occurs, but no structural gap or defect is present in the plaque.13 The mechanisms underlying plaque erosion are currently poorly understood, but they seem to be related to the apoptosis of endothelial cells and loss of endothelial contact with the underlying extracellular matrix.14 Vasospasm also has been suggested as a cause of endothelial damage and subsequent thrombosis.15 Consistent with this hypothesis, plaque erosion typically shows an intact internal and external elastic laminae and a well-developed media with contractile smooth muscle cells.16 Calcified nodules are a rare cause of coronary thrombosis. Overlying endothelial cells can be rarified or dysfunctional, creating a predisposition to thrombosis. Such nodules can be appreciated on optical coherence tomography (OCT) imaging as a signal-poor region with an irregular border that protrudes into the artery lumen.13
Figure 3.
Pathophysiological mechanisms and pathways underlying myocardial infarction with nonobstructive coronary artery disease. MINOCA, myocardial infarction with nonobstructive coronary artery disease. Created with Biorender.com.
Although the angiographic appearance of a lesion may suggest plaque disruption (eg, haziness or small filling defect), plaque disruption can be definitively diagnosed only by either intracoronary imaging, preferably by OCT with its higher near-field resolution, or high-definition intravascular ultrasound. The largest study on the use of OCT in MINOCA, conducted by Reynolds et al., identified a definite or culprit lesion, using OCT, in 46.2% of women (67 of 145) with MINOCA, most commonly plaque rupture, intraplaque cavitation, or layered plaque,17; smaller studies also have supported the utility of OCT in the diagnosis etiologic plaques in suspected MINOCA cases.18,19
Nonatherosclerotic causes of myocardial necrosis
Epicardial coronary vasospasm
Epicardial coronary spasm is defined as a transient total or subtotal coronary artery occlusion (≥ 90% constriction) with clinical angina and ischemic electrocardiogram changes either spontaneously or in response to a provocative stimulus. Vasospastic angina (VSA) in its turn is a clinical disorder characterised by the following: (i) classical clinical manifestations of VSA; (ii) the documentation of myocardial ischemia during spontaneous episodes; and (iii) the demonstration of coronary artery spasm (Supplemental Table S1).20 Although spontaneous episodes of epicardial vasospasms may occur, provocative spasm testing usually is required to demonstrate increased epicardial coronary artery spasm and is recommended in patients suspected to have VSA based on their symptoms (class I).20 Given its shorter effect duration, the preferred coronary spasm provocation test currently uses high-dose intracoronary acetylcholine boluses (20-200 mcg), with the epicardial coronary artery response assessed by invasive contrast angiography.20 Large spasm registries have reported that acetylcholine provocative testing of stable patients has an acceptable level of safety.21,22 Use of Intracoronary ergonovine boluses (20-60 mcg), with the epicardial coronary artery response assessed by invasive contrast angiography, is an alternative coronary provocation test that currently is regaining popularity.20 Some clinicians were reluctant to perform ergonovine provocation testing after deaths associated with its use were reported in the 1970s; however, these tests were performed bedside, often with intermittent electrocardiographic monitoring alone, with sublingual nitrates as the principal treatment for induced epicardial vasospasm.23 This method differs from contemporary catheterization laboratory-based provocative testing in which ergonovine is used, spasm is documented angiographically (ie, before significant ischemic electrocardiographic changes occur), and intracoronary nitrates are quickly administered. The safety of contemporary ergonovine testing has been demonstrated by a large spasm registry, in which the risk of major adverse reaction, including AMI and ventricular tachycardia, was 0.03%.24
Prolonged vasospastic episodes appear to be a common cause of MINOCA. In the study by Montone et al., epicardial vasospasm was diagnosed in 30% of MINOCA patients undergoing provocative testing.25 Another possibility is that epicardial vasospasm is more prevalent in Asian patients than it is in Caucasian patients. Postdischarge provocative testing in MINOCA patients induced epicardial vasospasm in 81% of Japanese patients and 61% of Korean patients, vs 15% of Caucasian patients.3
The 2 major mechanisms involved in epicardial spasm are endothelial dysfunction and VSMC hyperreactivity.26 On the one hand, vasoactive stimuli (eg, acetylcholine, ergonovine, histamine, and serotonin) normally mediate endothelium-dependent release of vasodilators, especially nitric oxide (NO).26 NO acts by suppressing vasoconstrictive metabolites (eg, angiotensin II and endothelium I).27 Lack of endogenous NO from endothelial dysfunction therefore causes an increase in vasoconstrictive metabolites.27 This mechanistic pathway explains why several endothelium-dependent vasoactive stimuli (eg, acetylcholine, ergonovine, histamine, and serotonin) provoke vasoconstriction in patients with VSA.28 On the other hand, VSMCs can become overreactive to vasoconstrictors, a condition known as VSCM hyperreactivity. VSMC contraction or relaxation is controlled by Rho-kinase myosin light-chain phosphorylation or dephosphorylation.29, 30, 31, 32 Increased Rho-kinase activity leads to an excessive myosin light-chain phosphorylation by inhibiting the myosin-binding subunit, leading to a state of hypercontractility.30 Additionally, several pathways involving NO, phospholipase C, and adenosince triphosphate–sensitive potassium (KATP) channels are also linked to VSCM hypercontractility.33, 34, 35 Chronic low-grade inflammation,36,37 magnesium deficiency, 38,39) overreactivity of the sympathetic or parasympathetic nervous system,40,41 and genetic polymorphisms (including genetic mutations in NO synthase, adrenergic receptors, serotonergic receptors, angiotensin-converting enzyme, and paroxonase I)42, 43, 44, 45 also have been described as potential additional contributors to epicardial coronary vasospasm.
Coronary microvascular dysfunction
Coronary microvascular dysfunction (CMD) is a clinical disorder defined by the presence of the following 4 factors: (i) symptoms of myocardial ischemia; (ii) the absence of obstructive CAD; (iii) evidence of myocardial ischemia; and (iv) signs of impaired coronary microvascular function (Supplemental Table S2). Impairment of coronary microcirculation is defined as one of the following: (i) impaired coronary flow reserve (CFR) < 2.0 in response to a vasodilator using a doppler or thermodilution technique; (ii) abnormal coronary microvascular resistance with an index of microvascular resistance (IMR) > 25; (iii) coronary slow-flow phenomenon, defined as a thrombolysis in myocardial infarction (TIMI) frame count > 25; or (iv) proof of microvascular spasm detected during provocative spasm testing.46 The definite diagnosis of CMD is made invasively using coronary functional testing and is recommended in patients with persistent angina and nonobstructive CAD (class 2a).47 However, noninvasive testing, such as positron emission tomography scan (class 2a), stress cardiac magnetic resonance (CMR) imaging (class 2a), and stress echocardiography with coronary flow velocity reserve measurement (class 2b) are alternative options.47,48 A positron emission tomography scan is now considered the gold-standard noninvasive tool for the assessment of myocardial blood flow, using dynamic rest and stress perfusion imaging with a vasodilator such as 13N ammonia or 82Rb rubidium to evaluate the vasodilatory response of the microcirculation.49
Among patients with stable CMD, 8% had magnetic resonance evidence of myocardial scar in the Women’s Ischemia Syndrome Evaluation (WISE) study ().50 However, CMD can be a cause of myocardial injury and can result from myocardial injury that has an either ischemic or nonischemic etiology.48 For example, among 40 female MINOCA patients who underwent stress CMR imaging, two-thirds of patients had an inducible perfusion abnormality, which implies the presence of CMD.51 Nonetheless, stress perfusion abnormalities were seen in patients with any-cause myocardial edema, including myocarditis. Consequently, a comprehensive approach always should be adopted when interpreting the causative link between CMD and MINOCA.
CMD arises as a result of functional or structural changes, or a mix of the 2.48 The functional alteration of the microcirculation could be due to microvascular spasm (enhanced vasoconstriction) or impaired vasodilation.52 Coronary microvascular spasm is characterized by the reproduction of chest pain and ischemic electrocardiographic alterations without any evidence of epicardial coronary spasm during intracoronary acetylcholine provocation testing.46 The major mechanism explaining enhanced microvascular vasoconstriction is Rho-kinase phosphorylation of myosin light-chain, inflammatory conditions in the coronary microvasculature resulting in increased coronary vasoconstrictive reactivity, and increased production of vasoconstrictive mediators, such as serotonin.53, 54, 55, 56 Vasodilation attenuation can be caused by either endothelium-dependent or endothelium-independent mechanisms.57 Endothelium-dependent impaired vasodilation is related to a lack of production or effect of endothelial-derived relaxing factors, such as prostaglandins, NO, and endothelium-dependent hyperpolarization factors.53 However, endothelium-dependent hyperpolarization factors (mainly hydrogen peroxide) seem to be the dominant modulators of resistance arteries, acting preferentially on prearterioles and arterioles instead of NO-mediated vasodilation of large conduit arteries.58 Endothelium-independent mechanisms of impaired vasodilation are debated, but an attenuated response to vasodilators (eg, adenosine, dipyridamole, and papaverine), and an increase in the sympathetic autonomic activity that impairs the microcirculation, have been suggested as potential mechanisms.48 Structural alterations (eg, luminal narrowing, vascular remodeling, vascular rarefication, or extramural compression) can also explain CMD via increased coronary microvascular resistance, particularly in the context of underlying cardiomyopathies and risk factors for CAD (eg, obesity, diabetes, hypertension, aging, dyslipidemia).59
Coronary embolism & thrombosis
Coronary embolism may be considered to be 1 of the following 3 types: direct, iatrogenic, and paradoxical.60 Direct coronary embolism is defined as that resulting from dislodged material arising from left-sided cardiac structures, such as mural thrombus, endocarditis, or intracardiac tumours, such as myxomas.60,61 Patients with an underlying cardiac condition, such as a cardiomyopathy with or without left ventricular dysfunction, atrial fibrillation, valvular disease, or coronary ectasia, are at higher risk of experiencing direct embolism due to blood stasis or slow flow.60,62 Infective endocarditis is a rare cause of coronary embolism. Endocarditis of the mitral valve, Staphylococcus aureus or fungal infection, and vegetation size > 10 mm are predictors of coronary embolism in patients with infective endocarditis.63 Marantic endocarditis also has been reported as an uncommon etiology of direct coronary embolism.64 Among myxomas, those with a villiform surface, as opposed to smooth-surface myxomas, are more likely to be associated with direct coronary embolism.61 Iatrogenic coronary embolism occurs mainly in the setting of coronary intervention, but also during cardiothoracic surgery and valvular procedures,60 whereas paradoxical embolism occurs as a result of embolization of material from the systemic venous circulation through a right-to-left shunt, such as a patent foramen ovale or an atrial septal defect.65
Inherited or acquired hypercoagulable states can also lead to a predisposition to in situ coronary thrombosis. The presence of a possible inherited disorder was detected in 14% of patients with MINOCA undergoing thrombophilia testing post-infarction, including factor V Leiden mutation, proteins C and S deficiency, and prothrombin gene mutation.3,66 Acquired hypercoagulable states include thrombocytopenic purpura, antiphospholipid syndrome, heparin-induced thrombocytopenia, and myeloproliferative neoplasms.67 Among a prospective cohort of 84 patients with MINOCA, 15.5% of patients were diagnosed with antiphospholipid syndrome.68 Therefore, in all patients with MINOCA, a reasonable consideration is inherited or acquired hypercoagulable states, and diagnostic testing should be performed preferably in consultation with a hematologist. Coagulation-factor levels should be measured after the acute-phase illness has resolved, and patients should be made aware of the pros and cons of genetic testing and understand the implications for family members.69
Spontaneous coronary artery dissection
Spontaneous coronary artery dissection (SCAD) is defined as a separation of the layers of the coronary artery wall with intramural hemorrhage, without evidence of either atherosclerosis, physical trauma, or iatrogenic injury.8 Although most cases of SCAD present with flow-obstructing lesions, the angiographic appearance can vary from being normal or near normal to being that of multiple radiolucent lumens or diffuse stenosis of varying severity. 70 SCAD increasingly is recognized as a cause of AMI that requires a high index of suspicion, especially in young women.70,71 Although the exact incidence of SCAD is controversial, because many events can be missed or misdiagnosed,40 SCAD-related AMI is estimated to represent 1%-5% of total cases of AMI,72 but the incidence may be as high as 35% among women aged < 50 years.50,73
Although the obstruction to coronary blood flow in SCAD is commonly agreed to be due to a separation of the media and the adventitial vascular walls associated with intramural hematoma protrusion into the lumen, the precise pathophysiology remains a matter of discussion.74 The “outside-in” hypothesis proposes that the primary event is the development of an intramural hematoma due to the rupture of vasa vasorum in the arterial wall, which then leads to a secondary intimal tear in some patients, whereas the “inside-out” hypothesis states that the primary event is the intimal tear, leading to blood penetration into the vessel wall, forming a secondary intramural hematoma.74 Although the “outside-in” hypothesis has more support in the literature, another possibility is that both mechanisms operate simultaneously in some clinical scenarios.49
SCAD might be the cause via an underlying vasculopathy, precipitated by a stressor, such as a catecholamine surge, emotional stress, extreme physical activity, the use of sympathomimetic drugs, and hormonal changes. 49,75 Fibromuscular dysplasia was found in 72% of patients in a consecutive cohort of 168 patients with SCAD, by Saw et al., and approximately half reported the presence of a physical or emotional trigger.75 Female sex hormones and pregnancy also are associated with SCAD, with most of the pregnancy-associated myocardial infarction occurring within the first week postpartum.76
The presence of tortuosity (eg, corkscrew appearance, multivessel symmetrical tortuosity) should raise the suspicion for SCAD.77 Intravascular imaging techniques such as OCT and intravascular ultrasound may be necessary for confirmation of the diagnosis by demonstrating the lack of significant atherosclerotic plaque and the presence of dissection or intramural hematoma. OCT may be the preferred imaging modality in the case of suspected SCAD, owing to its superior spatial resolution, but it should be used judiciously to avoid propagation of the dissection plan as a result of high-pressure contrast injection.78
Supply–demand mismatch
MINOCA related to supply-demand mismatch, like other MINOCA etiologies, requires corroborative clinical evidence of infarction (Table 1). From one patient to the next, stressors of similar magnitude may not result in MINOCA, depending on the presence of noncardiac comorbidities, the extent of underlying CAD, and the presence of structural abnormalities.1 Stressors that reduce oxygen supply include severe brady-arrhythmia, respiratory failure with severe hypoxemia, and severe anemia, whereas stressors that increase oxygen demand include sustained tachy-arrhythmia, severe hypertension, and thyrotoxicosis.
In patients with stable CAD, an acute stressor with clinical manifestations of ischemia may result in a MINOCA, because of the insufficient blood flow to the myocardium to meet the increased myocardial oxygen demands, or further reduction in myocardial oxygen supply related to the stressor. Depending on the clinical situation, coronary angiography may be indicated to assess the likelihood of underlying CAD.1
MINOCA “mimics”
A number conditions leading to myocardial damage may be mistaken for MINOCA. In a study of patients with suspected MINOCA, Reynolds et al. found nonischemic patterns on CMR imaging (myocarditis, TTS, or nonischemic cardiomyopathy) in 20.7% of patients (24 of 116).17
Stress-induced cardiomyopathy, or TTS, is a condition that can lead to the release of cardiac troponins from cardiomyocytes, with a concentration rise and fall compatible with acute myocardial injury.79 However, the Fourth Universal Definition of Myocardial Infarction does not recognize TTS as a MINOCA etiology,1 owing to the current prevailing hypothesis that TTS is caused by cardiotoxicity triggered by a catecholamine surge secondary to severe physical or emotional stress,1,79, 80, 81 despite the fact that a clear trigger cannot be identified in up to one third of cases.79 Other theories have been proposed and deliberated upon to understand TTS, encompassing CMD, coronary epicardial vasospasm, inflammation and reperfusion injury, and disruptions in cardiac fatty acid metabolism with energetic alteration.79, 80, 81 TTS explains the symptoms of 2%-3% of patients presenting with suspected ACS, and most patients who develop TTS are women (80%-90%).79,81 TTS can be triggered by physical or emotional stress. Nonetheless, a crucial point to acknowledge is that a distinct trigger is not identified in one third of patients.79 Concomitant atherosclerotic coronary disease is reported in up to one third of patients with TTS,82 which can make accurate diagnosis challenging, particularly in the absence of left ventricular imaging.
Although electrocardiographic abnormalities frequently are impressive in TTS, the associated troponin level elevation can be comparatively modest.83 In TTS, left ventricular wall motion abnormalities that extend beyond a single epicardial coronary distribution are typical,79,84 with apical ballooning being the most common, followed by mid-cavitary dyskinesia. Additional patterns may be observed due to asymmetrical recuperation of left ventricular dysfunction, particularly if imaging is performed at a time point distant from symptom onset, as recovery may occur over a span of hours to several weeks. Signs of myocardial edema are frequently observed in CMR imaging during the acute phase, again in a distribution out of proportion to coronary anatomy, but late gadolinium enhancement is rare.84
In contrast, myocarditis is an inflammatory condition that impacts an estimated 4 to 14 individuals per 100,000 annually worldwide.85 Often, myocarditis is triggered by viral infection, although it can also result from other infectious conditions, autoimmune and systemic disorders, toxic substances, and drugs.86 The acute myocardial injury of myocarditis patients typically is associated with a rise in inflammatory markers. Electrocardiographic changes, including shifts in ST-T segments, and echocardiographic left ventricular dysfunction also may be observed. Myocarditis patients also may demonstrate increased ventricular wall thickness and pericardial effusion.85 CMR can provide support for a diagnosis of myocarditis based on the revised Lake Louise Criteria after integration of the T1 and T2 mapping techniques.85 If diagnostic uncertainty persists, the gold standard for diagnosing myocarditis remains endomyocardial biopsy, which shows lymphocytic infiltrates in association with myocyte necrosis (Dallas Criteria), and this may be required in some cases.87,88
Conclusion
Given the variety of etiologies of MINOCA, accurate diagnosis remains a challenge for clinicians. Combining an appropriate application of the Universal Definition of Myocardial Infarction and an understanding of the different pathophysiological mechanisms of MINOCA is paramount and will help ensure an efficient multimodal diagnostic workup, as well as appropriately tailored therapies for these patients.
Acknowledgments
Ethics Statement
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (ref: 10.1126/bmj.n71). Obtaining patient consent was waived by the institutional ethical board review (EBR), as no patient are directly involved in this article.
Patient Consent
This is a review article that does not involve original patient data or case reports; therefore, patient consent is not applicable.
Funding Sources
L.-A.B.-P. is supported by a grant from the University of Ottawa Heart Institute Foundation and the Whit & Heather Tucker Endowed Funds (Ottawa, Ontario, Canada). M.L. is supported by a grant from the Fondazione Enrio ed Enrica Sovena (Rome, Italy). C.P. has received research funding from Novartis and Fondation Caroline Durand, Fondation Jaccques et Michel Auger, and Fondation Tanguay. The other authors have no funding sources to declare.
Disclosures
C.P. has received honoraria or speaker fees from Novartis and KYE Pharmaceuticals. The other authors have no conflicts of interest to disclose.
Footnotes
See page 388 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2023.11.014.
Supplementary Material
References
- 1.Thygesen K., Alpert J.S., Jaffe A.S., et al. Fourth universal definition of myocardial infarction (2018) Circulation. 2018;138:e618–e651. doi: 10.1161/CIR.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 2.Michael J.D. The pathophysiology of acute coronary syndromes. Heart. 2000;83:361. doi: 10.1136/heart.83.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pasupathy S., Air T., Dreyer R.P., Tavella R., Beltrame J.F. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. 2015;131:861–870. doi: 10.1161/CIRCULATIONAHA.114.011201. [DOI] [PubMed] [Google Scholar]
- 4.Bugiardini R., Bairey Merz C.N. Angina with “normal” coronary arteries: a changing philosophy. JAMA. 2005;293:477–484. doi: 10.1001/jama.293.4.477. [DOI] [PubMed] [Google Scholar]
- 5.Kang W.Y., Jeong M.H., Ahn Y.K., et al. Are patients with angiographically near-normal coronary arteries who present as acute myocardial infarction actually safe? Int J Cardiol. 2011;146:207–212. doi: 10.1016/j.ijcard.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Larsen A.I., Nilsen D.W., Yu J., et al. Long-term prognosis of patients presenting with ST-segment elevation myocardial infarction with no significant coronary artery disease (from the HORIZONS-AMI trial) Am J Cardiol. 2013;111:643–648. doi: 10.1016/j.amjcard.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamis-Holland J.E., Jneid H., Reynolds H.R., et al. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American Heart Association. Circulation. 2019;139:e891–e908. doi: 10.1161/CIR.0000000000000670. [DOI] [PubMed] [Google Scholar]
- 9.Scanlon P.J., Faxon D.P., Audet A.M., et al. ACC/AHA guidelines for coronary angiography: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Coronary Angiography) Developed in collaboration with the Society for Cardiac Angiography and Interventions. J Am Coll Cardiol. 1999;33:1756–1824. doi: 10.1016/s0735-1097(99)00126-6. [DOI] [PubMed] [Google Scholar]
- 10.Zir L.M., Miller S.W., Dinsmore R.E., Gilbert J.P., Harthorne J.W. Interobserver variability in coronary angiography. Circulation. 1976;53:627–632. doi: 10.1161/01.cir.53.4.627. [DOI] [PubMed] [Google Scholar]
- 11.Curzen N., Rana O., Nicholas Z., et al. Does routine pressure wire assessment influence management strategy at coronary angiography for diagnosis of chest pain? Circ Cardiovasc Interv. 2014;7:248–255. doi: 10.1161/CIRCINTERVENTIONS.113.000978. [DOI] [PubMed] [Google Scholar]
- 12.Falk E., Shah P.K., Fuster V. Coronary plaque disruption. Circulation. 1995;92:657–671. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- 13.Schaar J.A., Muller J.E., Falk E., et al. Terminology for high-risk and vulnerable coronary artery plaques. Eur Heart J. 2004;25:1077–1082. doi: 10.1016/j.ehj.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 14.White S.J., Newby A.C., Johnson T.W. Endothelial erosion of plaques as a substrate for coronary thrombosis. Thromb Haemost. 2016;115:509–519. doi: 10.1160/TH15-09-0765. [DOI] [PubMed] [Google Scholar]
- 15.Virmani R., Burke A.P., Farb A., Kolodgie F.D. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47(8 suppl):C13–C18. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 16.Hao H., Gabbiani G., Camenzind E., et al. Phenotypic modulation of intima and media smooth muscle cells in fatal cases of coronary artery lesion. Arterioscler Thromb Vasc Biol. 2006;26:326–332. doi: 10.1161/01.ATV.0000199393.74656.4c. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds H.R., Maehara A., Kwong R.Y., et al. Coronary optical coherence tomography and cardiac magnetic resonance imaging to determine underlying causes of myocardial infarction with nonobstructive coronary arteries in women. Circulation. 2021;143:624–640. doi: 10.1161/CIRCULATIONAHA.120.052008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerbaud E., Arabucki F., Nivet H., et al. OCT and CMR for the diagnosis of patients presenting with MINOCA and suspected epicardial causes. JACC Cardiovasc Imaging. 2020;13:2619–2631. doi: 10.1016/j.jcmg.2020.05.045. [DOI] [PubMed] [Google Scholar]
- 19.Opolski M.P., Spiewak M., Marczak M., et al. Mechanisms of myocardial infarction in patients with nonobstructive coronary artery disease: results from the Optical Coherence Tomography Study. JACC Cardiovasc Imaging. 2019;12:2210–2221. doi: 10.1016/j.jcmg.2018.08.022. [DOI] [PubMed] [Google Scholar]
- 20.Beltrame J.F., Crea F., Kaski J.C., et al. International standardization of diagnostic criteria for vasospastic angina. Eur Heart J. 2017;38:2565–2568. doi: 10.1093/eurheartj/ehv351. [DOI] [PubMed] [Google Scholar]
- 21.Ong P., Athanasiadis A., Borgulya G., et al. Clinical usefulness, angiographic characteristics, and safety evaluation of intracoronary acetylcholine provocation testing among 921 consecutive White patients with unobstructed coronary arteries. Circulation. 2014;129:1723–1730. doi: 10.1161/CIRCULATIONAHA.113.004096. [DOI] [PubMed] [Google Scholar]
- 22.Takagi Y., Yasuda S., Takahashi J., et al. Clinical implications of provocation tests for coronary artery spasm: safety, arrhythmic complications, and prognostic impact: Multicentre Registry Study of the Japanese Coronary Spasm Association. Eur Heart J. 2013;34:258–267. doi: 10.1093/eurheartj/ehs199. [DOI] [PubMed] [Google Scholar]
- 23.Buxton A., Goldberg S., Hirshfeld J.W., et al. Refractory ergonovine-induced coronary vasospasm: importance of intracoronary nitroglycerin. Am J Cardiol. 1980;46:329–334. doi: 10.1016/0002-9149(80)90080-6. [DOI] [PubMed] [Google Scholar]
- 24.Harding M.B., Leithe M.E., Mark D.B., et al. Ergonovine maleate testing during cardiac catheterization: a 10-year perspective in 3447 patients without significant coronary artery disease or Prinzmetal's variant angina. J Am Coll Cardiol. 1992;20:107–111. doi: 10.1016/0735-1097(92)90145-d. [DOI] [PubMed] [Google Scholar]
- 25.Montone R.A., Niccoli G., Fracassi F., et al. Patients with acute myocardial infarction and non-obstructive coronary arteries: safety and prognostic relevance of invasive coronary provocative tests. Eur Heart J. 2018;39:91–98. doi: 10.1093/eurheartj/ehx667. [DOI] [PubMed] [Google Scholar]
- 26.Fu B., Wei X., Lin Y., Chen J., Yu D. Pathophysiologic basis and diagnostic approaches for ischemia with non-obstructive coronary arteries: a literature review. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.731059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandoo A., van Zanten J.J., Metsios G.S., Carroll D., Kitas G.D. The endothelium and its role in regulating vascular tone. Open Cardiovasc Med J. 2010;4:302–312. doi: 10.2174/1874192401004010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimokawa H. 2014 Williams Harvey Lecture: importance of coronary vasomotion abnormalities—from bench to bedside. Eur Heart J. 2014;35:3180–3193. doi: 10.1093/eurheartj/ehu427. [DOI] [PubMed] [Google Scholar]
- 29.Hirano K. Current topics in the regulatory mechanism underlying the Ca2+ sensitization of the contractile apparatus in vascular smooth muscle. J Pharmacol Sci. 2007;104:109–115. doi: 10.1254/jphs.cp0070027. [DOI] [PubMed] [Google Scholar]
- 30.Kandabashi T., Shimokawa H., Miyata K., et al. Inhibition of myosin phosphatase by upregulated Rho-kinase plays a key role for coronary artery spasm in a porcine model with interleukin-1β. Circulation. 2000;101:1319–1323. doi: 10.1161/01.cir.101.11.1319. [DOI] [PubMed] [Google Scholar]
- 31.Lanza G.A., Careri G., Crea F. Mechanisms of coronary artery spasm. Circulation. 2011;124:1774–1782. doi: 10.1161/CIRCULATIONAHA.111.037283. [DOI] [PubMed] [Google Scholar]
- 32.Somlyo A.P., Somlyo A.V. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 33.Büssemaker E., Pistrosch F., Förster S., et al. Rho kinase contributes to basal vascular tone in humans: role of endothelium-derived nitric oxide. Am J Physiol Heart Circ Physiol. 2007;293:H541–H547. doi: 10.1152/ajpheart.00770.2006. [DOI] [PubMed] [Google Scholar]
- 34.Chutkow W.A., Pu J., Wheeler M.T., et al. Episodic coronary artery vasospasm and hypertension develop in the absence of Sur2 KATP channels. J Clin Invest. 2002;110:203–208. doi: 10.1172/JCI15672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kakkar R., Ye B., Stoller D.A., et al. Spontaneous coronary vasospasm in KATP mutant mice arises from a smooth muscle-extrinsic process. Circ Res. 2006;98:682–689. doi: 10.1161/01.RES.0000207498.40005.e7. [DOI] [PubMed] [Google Scholar]
- 36.Ong P., Aziz A., Hansen H.S., et al. Structural and functional coronary artery abnormalities in patients with vasospastic angina pectoris. Circ J. 2015;79:1431–1438. doi: 10.1253/circj.CJ-15-0520. [DOI] [PubMed] [Google Scholar]
- 37.Yoo S.-Y., Kim J.-Y. Recent insights into the mechanisms of vasospastic angina. Korean Circ J. 2009;39:505–511. doi: 10.4070/kcj.2009.39.12.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goto K., Yasue H., Okumura K., et al. Magnesium deficiency detected by intravenous loading test in variant angina pectoris. Am J Cardiol. 1990;65:709–712. doi: 10.1016/0002-9149(90)91375-g. [DOI] [PubMed] [Google Scholar]
- 39.Miyagi H., Yasue H., Okumura K., et al. Effect of magnesium on anginal attack induced by hyperventilation in patients with variant angina. Circulation. 1989;79:597–602. doi: 10.1161/01.cir.79.3.597. [DOI] [PubMed] [Google Scholar]
- 40.Robertson R.M., Bernard Y., Robertson D. Arterial and coronary sinus catecholamines in the course of spontaneous coronary artery spasm. Am Heart J. 1983;105:901–906. doi: 10.1016/0002-8703(83)90387-3. [DOI] [PubMed] [Google Scholar]
- 41.Waters D.D., Miller D.D., Bouchard A., Bosch X., Theroux P. Circadian variation in variant angina. Am J Cardiol. 1984;54:61–64. doi: 10.1016/0002-9149(84)90304-7. [DOI] [PubMed] [Google Scholar]
- 42.Ito T., Yasue H., Yoshimura M., et al. Paraoxonase gene Gln192Arg (Q192R) polymorphism is associated with coronary artery spasm. Hum Genet. 2002;110:89–94. doi: 10.1007/s00439-001-0654-6. [DOI] [PubMed] [Google Scholar]
- 43.Murase Y., Yamada Y., Hirashiki A., et al. Genetic risk and gene-environment interaction in coronary artery spasm in Japanese men and women. Eur Heart J. 2004;25:970–977. doi: 10.1016/j.ehj.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 44.Oike Y., Hata A., Ogata Y., et al. Angiotensin converting enzyme as a genetic risk factor for coronary artery spasm. Implication in the pathogenesis of myocardial infarction. J Clin Invest. 1995;96:2975–2979. doi: 10.1172/JCI118369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park J.S., Zhang S.Y., Jo S.H., et al. Common adrenergic receptor polymorphisms as novel risk factors for vasospastic angina. Am Heart J. 2006;151:864–869. doi: 10.1016/j.ahj.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 46.Ong P., Camici P.G., Beltrame J.F., et al. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. 2018;250:16–20. doi: 10.1016/j.ijcard.2017.08.068. [DOI] [PubMed] [Google Scholar]
- 47.Gulati M., Levy P.D., Mukherjee D., et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;144:e368–e454. doi: 10.1161/CIR.0000000000001029. [DOI] [PubMed] [Google Scholar]
- 48.Del Buono M.G., Montone R.A., Camilli M., et al. Coronary microvascular dysfunction across the spectrum of cardiovascular diseases: JACC state-of-the-art review. J Am Coll Cardiol. 2021;78:1352–1371. doi: 10.1016/j.jacc.2021.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayes S.N., Tweet M.S., Adlam D., et al. Spontaneous coronary artery dissection: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76:961–984. doi: 10.1016/j.jacc.2020.05.084. [DOI] [PubMed] [Google Scholar]
- 50.Wei J., Bakir M., Darounian N., et al. Myocardial scar is prevalent and associated with subclinical myocardial dysfunction in women with suspected ischemia but no obstructive coronary artery disease: from the Women's Ischemia Syndrome Evaluation-Coronary Vascular Dysfunction Study. Circulation. 2018;137:874–876. doi: 10.1161/CIRCULATIONAHA.117.031999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mauricio R., Srichai M.B., Axel L., Hochman J.S., Reynolds H.R. Stress cardiac MRI in women with myocardial infarction and nonobstructive coronary artery disease. Clin Cardiol. 2016;39:596–602. doi: 10.1002/clc.22571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crea F., Camici P.G., Bairey Merz C.N. Coronary microvascular dysfunction: an update. Eur Heart J. 2014;35:1101–1111. doi: 10.1093/eurheartj/eht513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Godo S., Suda A., Takahashi J., Yasuda S., Shimokawa H. Coronary microvascular dysfunction. Arterioscler Thromb Vasc Biol. 2021;41:1625–1637. doi: 10.1161/ATVBAHA.121.316025. [DOI] [PubMed] [Google Scholar]
- 54.Godo S., Takahashi J., Yasuda S., Shimokawa H. Role of inflammation in coronary epicardial and microvascular dysfunction. Eur Cardiol. 2021;16:e13. doi: 10.15420/ecr.2020.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mohri M., Shimokawa H., Hirakawa Y., Masumoto A., Takeshita A. Rho-kinase inhibition with intracoronary fasudil prevents myocardial ischemia in patients with coronary microvascular spasm. J Am Coll Cardiol. 2003;41:15–19. doi: 10.1016/s0735-1097(02)02632-3. [DOI] [PubMed] [Google Scholar]
- 56.Odaka Y., Takahashi J., Tsuburaya R., et al. Plasma concentration of serotonin is a novel biomarker for coronary microvascular dysfunction in patients with suspected angina and unobstructive coronary arteries. Eur Heart J. 2017;38:489–496. doi: 10.1093/eurheartj/ehw448. [DOI] [PubMed] [Google Scholar]
- 57.Pries A.R., Badimon L., Bugiardini R., et al. Coronary vascular regulation, remodelling, and collateralization: mechanisms and clinical implications on behalf of the working group on coronary pathophysiology and microcirculation. Eur Heart J. 2015;36:3134–3146. doi: 10.1093/eurheartj/ehv100. [DOI] [PubMed] [Google Scholar]
- 58.Takahashi J., Suda A., Nishimiya K., et al. Pathophysiology and diagnosis of coronary functional abnormalities. Eur Cardiol. 2021;16:e30. doi: 10.15420/ecr.2021.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kunadian V., Chieffo A., Camici P.G., et al. An EAPCI expert consensus document on ischaemia with non-obstructive coronary arteries in collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation endorsed by Coronary Vasomotor Disorders International Study Group. EuroIntervention. 2021;16:1049–1069. doi: 10.4244/EIJY20M07_01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raphael C.E., Heit J.A., Reeder G.S., et al. Coronary embolus: an underappreciated cause of acute coronary syndromes. JACC Cardiovasc Interv. 2018;11:172–180. doi: 10.1016/j.jcin.2017.08.057. [DOI] [PubMed] [Google Scholar]
- 61.El Sabbagh A., Al-Hijji M.A., Thaden J.J., et al. Cardiac myxoma: the great mimicker. JACC Cardiovasc Imaging. 2017;10:203–206. doi: 10.1016/j.jcmg.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 62.Boles U., Rakhit R., Shiu M.F., Patel K., Henein M. Coronary artery ectasia as a culprit for acute myocardial infarction: review of pathophysiology and management. Anadolu Kardiyol Derg. 2013;13:695–701. doi: 10.5152/akd.2013.227. [DOI] [PubMed] [Google Scholar]
- 63.Zhao J., Yang J., Chen W., et al. Acute myocardial infarction as the first sign of infective endocarditis: a case report. J Int Med Res. 2020;48 doi: 10.1177/0300060520980598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karameh M., Golomb M., Arad A., Kalmnovich G., Herzog E. Multi-valvular non-bacterial thrombotic endocarditis causing sequential pulmonary embolism, myocardial infarction, and stroke: a case report and literature review. Cureus. 2022;14 doi: 10.7759/cureus.32261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Talebi S., Jadhav P., Tamis-Holland J.E. Myocardial infarction in the absence of obstructive coronary artery disease (MINOCA): a review of the present and preview of the future. Curr Atheroscler Rep. 2021;23:49. doi: 10.1007/s11883-021-00945-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pasupathy S., Rodgers S., Tavella R., McRae S., Beltrame J.F. Risk of thrombosis in patients presenting with myocardial infarction with nonobstructive coronary arteries (MINOCA) TH Open. 2018;2:e167–e172. doi: 10.1055/s-0038-1645875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shen Y.M., Nagalla S. Hypercoagulable workup in thrombotic cardiovascular diseases. Circulation. 2018;138:229–231. doi: 10.1161/CIRCULATIONAHA.117.031699. [DOI] [PubMed] [Google Scholar]
- 68.Stepien K., Nowak K., Wypasek E., Zalewski J., Undas A. High prevalence of inherited thrombophilia and antiphospholipid syndrome in myocardial infarction with non-obstructive coronary arteries: comparison with cryptogenic stroke. Int J Cardiol. 2019;290:1–6. doi: 10.1016/j.ijcard.2019.05.037. [DOI] [PubMed] [Google Scholar]
- 69.Cushman M. Thrombophilia testing in women with venous thrombosis: the 4 P's approach. Clin Chem. 2014;60:134–137. doi: 10.1373/clinchem.2013.202648. [DOI] [PubMed] [Google Scholar]
- 70.Saw J., Starovoytov A., Aymong E., et al. Canadian spontaneous coronary artery dissection cohort study: 3-year outcomes. J Am Coll Cardiol. 2022;80:1585–1597. doi: 10.1016/j.jacc.2022.08.759. [DOI] [PubMed] [Google Scholar]
- 71.García-Guimaraes M., Bastante T., Macaya F., et al. Spontaneous coronary artery dissection in Spain: clinical and angiographic characteristics, management, and in-hospital events. Rev Esp Cardiol (Engl Ed) 2021;74:15–23. doi: 10.1016/j.rec.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 72.Machanahalli Balakrishna A., Ismayl M., Thandra A., et al. Diagnostic value of cardiac magnetic resonance imaging and intracoronary optical coherence tomography in patients with a working diagnosis of myocardial infarction with non-obstructive coronary arteries—a systematic review and meta-analysis. Curr Probl Cardiol. 2023;48 doi: 10.1016/j.cpcardiol.2022.101126. [DOI] [PubMed] [Google Scholar]
- 73.Saw J., Humphries K., Aymong E., et al. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol. 2017;70:1148–1158. doi: 10.1016/j.jacc.2017.06.053. [DOI] [PubMed] [Google Scholar]
- 74.Waterbury T.M., Tweet M.S., Hayes S.N., et al. Early natural history of spontaneous coronary artery dissection. Circ Cardiovasc Interv. 2018;11 doi: 10.1161/CIRCINTERVENTIONS.118.006772. [DOI] [PubMed] [Google Scholar]
- 75.Saw J., Aymong E., Sedlak T., et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv. 2014;7:645–655. doi: 10.1161/CIRCINTERVENTIONS.114.001760. [DOI] [PubMed] [Google Scholar]
- 76.Tweet M.S., Hayes S.N., Codsi E., et al. Spontaneous coronary artery dissection associated with pregnancy. J Am Coll Cardiol. 2017;70:426–435. doi: 10.1016/j.jacc.2017.05.055. [DOI] [PubMed] [Google Scholar]
- 77.Eleid M.F., Guddeti R.R., Tweet M.S., et al. Coronary artery tortuosity in spontaneous coronary artery dissection: angiographic characteristics and clinical implications. Circ Cardiovasc Interv. 2014;7:656–662. doi: 10.1161/CIRCINTERVENTIONS.114.001676. [DOI] [PubMed] [Google Scholar]
- 78.Alfonso F., Paulo M., Gonzalo N., et al. Diagnosis of spontaneous coronary artery dissection by optical coherence tomography. J Am Coll Cardiol. 2012;59:1073–1079. doi: 10.1016/j.jacc.2011.08.082. [DOI] [PubMed] [Google Scholar]
- 79.Komamura K., Fukui M., Iwasaku T., Hirotani S., Masuyama T. Takotsubo cardiomyopathy: pathophysiology, diagnosis and treatment. World J Cardiol. 2014;6:602–609. doi: 10.4330/wjc.v6.i7.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lyon A.R., Citro R., Schneider B., et al. Pathophysiology of Takotsubo syndrome: JACC state-of-the-art review. J Am Coll Cardiol. 2021;77:902–921. doi: 10.1016/j.jacc.2020.10.060. [DOI] [PubMed] [Google Scholar]
- 81.Singh T., Khan H., Gamble D.T., et al. Takotsubo syndrome: pathophysiology, emerging concepts, and clinical implications. Circulation. 2022;145:1002–1019. doi: 10.1161/CIRCULATIONAHA.121.055854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ghadri J.R., Wittstein I.S., Prasad A., et al. International expert consensus document on Takotsubo syndrome (part i): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J. 2018;39:2032–2046. doi: 10.1093/eurheartj/ehy076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boyd B., Solh T. Takotsubo cardiomyopathy: review of broken heart syndrome. JAAPA. 2020;33:24–29. doi: 10.1097/01.JAA.0000654368.35241.fc. [DOI] [PubMed] [Google Scholar]
- 84.Jensch P.J., Stiermaier T., Eitel I. Takotsubo syndrome—Is there a need for CMR? Curr Heart Fail Rep. 2021;18:200–210. doi: 10.1007/s11897-021-00518-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ammirati E., Moslehi J.J. Diagnosis and treatment of acute myocarditis: a review. JAMA. 2023;329:1098–1113. doi: 10.1001/jama.2023.3371. [DOI] [PubMed] [Google Scholar]
- 86.Tschöpe C., Ammirati E., Bozkurt B., et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. 2021;18:169–193. doi: 10.1038/s41569-020-00435-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ferreira V.M., Schulz-Menger J., Holmvang G., et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 88.Aretz H.T. Myocarditis: the Dallas criteria. Hum Pathol. 1987;18:619–624. doi: 10.1016/s0046-8177(87)80363-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.