Abstract
Sarcoma is a malignant tumor that originates from mesenchymal tissue. The common treatment for sarcoma is surgery supplemented with radiotherapy and chemotherapy. However, patients have a 5-year survival rate of only approximately 60%, and sarcoma cells are highly resistant to chemotherapy. Ferroptosis is an iron-dependent nonapoptotic type of regulated programmed cell death that is closely related to the pathophysiological processes underlying tumorigenesis, neurological diseases and other conditions. Moreover, ferroptosis is mediated via multiple regulatory pathways that may be targets for disease therapy. Recent studies have shown that the induction of ferroptosis is an effective way to kill sarcoma cells and reduce their resistance to chemotherapeutic drugs. Moreover, ferroptosis-related genes are related to the immune system, and their expression can be used to predict sarcoma prognosis. In this review, we describe the molecular mechanism underlying ferroptosis in detail, systematically summarize recent research progress with respect to ferroptosis application as a sarcoma treatment in various contexts, and point out gaps in the theoretical research on ferroptosis, challenges to its clinical application, potential resolutions of these challenges to promote ferroptosis as an efficient, reliable and novel method of clinical sarcoma treatment.
Keywords: Ferroptosis, Sarcoma, Mechanism, Prognosis prediction, Drug resistance
Introduction
Sarcoma is a type of malignant tumor originating from mesenchymal tissue and is broadly categorized into two primary groups: bone sarcomas and soft tissue sarcomas. Osteosarcoma, a bone sarcoma, is the most prevalent sarcoma among children and adolescents, with a 5-year survival rate ranging from 60 to 70%. Remarkably, this rate has seen little improvement over the past three decades [1, 2]. On the other hand, soft tissue sarcomas are complex malignancies that include at least 100 different histological and molecular subtypes [3]. The overall 5-year survival rate for soft tissue sarcomas stands at approximately 50% [4]. While local surgical resection coupled with chemotherapy and radiotherapy has demonstrated effectiveness in treating both types of sarcoma, its overall efficacy remains limited, and advancements in novel treatments have been sluggish [5].
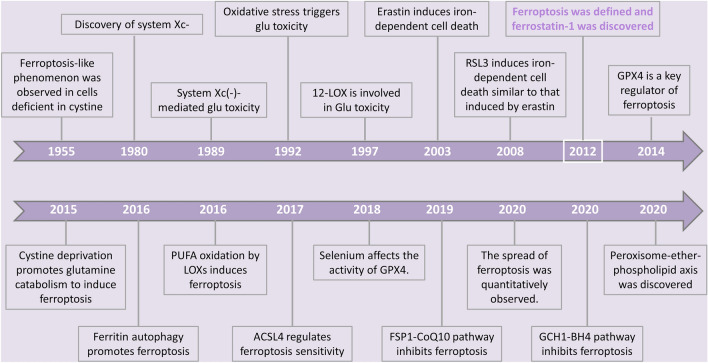
Ferroptosis represents a distinctive type of cell demise induced by erastin, which is a kind of oncogenic RAS-selective lethal small molecule (RSL). It is hallmarked by the intracellular accumulation of free iron and lipid peroxides and stands apart from apoptosis, necrosis, and autophagy in terms of morphology, biochemistry, and genetics [6]. Although the term "ferroptosis" was not formally introduced until 2012, with the discovery of the small-molecule inhibitor ferrostatin-1 by Dixon et al. [6], the characteristics of this mode of cell death had been described in earlier research and have continued to evolve since the coining of the term [7]. (Fig. 1). In normal conditions, cells manage stress associated with ferroptosis through the regulation of various antioxidant systems, which can, in turn, serve as targets for interventions aimed at inducing ferroptosis in cells.
Fig. 1.
Landmark events related to the development of ferroptosis
Ferroptosis has the potential to be a new and highly effective treatment for sarcoma. Recent research has unveiled a profound connection between ferroptosis and the pathophysiological mechanisms underlying a diverse spectrum of diseases, including cancer, neurological disorders, ischemia–reperfusion injury, kidney damage, and hematological disorders [8]. Notably, ferroptosis is emerging as a novel drug target, offering avenues to overcome chemotherapy resistance and predict disease prognosis in the context of sarcoma treatment [9–11]. Therefore, it is of great importance to study the mechanism underlying ferroptosis and its potential as a sarcoma treatment.
This comprehensive review meticulously delineates the molecular mechanisms that underlie ferroptosis. It distills and deliberates upon recent breakthroughs and advancements in ferroptosis research as a prospective strategy for the prevention and treatment of sarcoma. Furthermore, the review offers a visionary perspective on the clinical applications of ferroptosis, while candidly addressing the limitations and challenges inherent in these findings, providing a roadmap for future research directions.
In summary, ferroptosis stands at the forefront of innovative approaches to sarcoma therapy. Understanding the intricacies of this distinctive form of cell death not only holds the promise of more effective treatments but also the potential to prognosticate disease outcomes, thereby benefiting sarcoma patients and advancing the broader landscape of cancer research.
Molecular mechanisms underlying ferroptosis
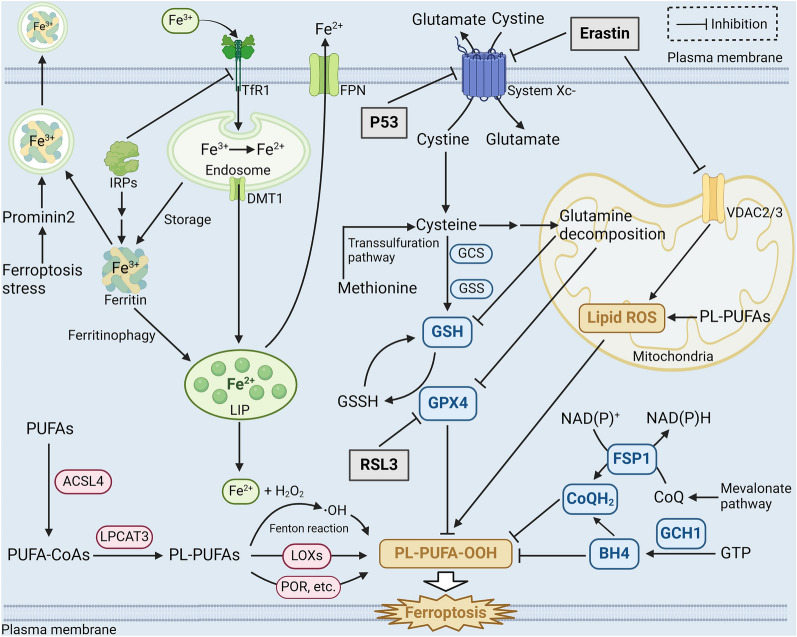
Ferroptosis, a relatively recent discovery in the realm of cell biology, represents a unique form of regulated cell death. This process hinges on a distinct chemical cascade ignited by the iron-dependent buildup of lipid peroxides. Ferroptosis, in essence, is orchestrated by an intricate interplay of various redox-active enzymes, each with roles in the generation or elimination of free radicals and lipid oxidation products. These molecular actors collectively orchestrate an intracellular redox imbalance, culminating in the cell’s demise. This tightly regulated cell death mechanism operates at multiple hierarchical levels, spanning epigenetic, transcriptional, posttranscriptional, and posttranslational tiers of control. In this orchestrated dance of molecular players, ferroptosis unveils itself as a multifaceted and carefully governed phenomenon in the intricate tapestry of cell biology [12]. (Fig. 2).
Fig. 2.
Molecular mechanism of the occurrence and regulation of ferroptosis. PUFAs are modified into PL-PUFAs, which produce lipid peroxides through the Fenton reaction involving Fe2+ or catalyzed by iron-dependent lipoxygenases (red), then attack cells to cause ferroptosis. The intracellular iron metabolism (green), three major regulatory pathways (system Xc(−)-GSH-GPX4 pathway, NADPH-FSP1-CoQ10 pathway and GCH1-BH4 pathway. blue) and mitochondrial involvement (orange) together regulate the process of ferroptosis. Three common ferroptosis inducers: erastin, RSL3, and p53 are exemplified (gray). (Created with BioRender.com.)
Abnormal iron metabolism initiates ferroptosis
Iron is an essential trace element for cell growth and metabolism and is a component of the catalytic site of many important redox enzymes. However, too much iron can be extremely hazardous to cells and cause oxidative DNA damage [13]. Intracellular iron is stored in a metabolically active pool called the “labile iron pool” (LIP). The LIP can store, export, or consume iron, and most of the iron (> 80%) in cells is available in ferrous form [14, 15].
When dysregulation of intracellular iron leads to abnormal accumulation of free iron, excessive amounts of lipid peroxide are produced, triggering ferroptosis. A study showed that iron chelation therapy attenuated ferroptosis in a rodent model of cerebral ischemia‒reperfusion injury [16]. Intracellular free iron is involved in two main pathways of ferroptosis, the nonenzymatic pathway and the enzymatic pathway. In the nonenzymatic pathway, excess iron generates hydroxyl radicals through the Fenton reaction, and these products enriches the intracellular reactive oxygen species (ROS) pool and promotes the oxidation of polyunsaturated fatty acids (PUFAs, such as arachidonic acid and linoleic acid) to generate lipid peroxides and hydroperoxides, which then attack adjacent PUFAs and trigger chain reactions [17, 18]. When the pathways that inhibit lipid peroxidation in a cell fail, the chain reaction eventually reaches cell membrane lipids, causing structural and functional damage and leading to ferroptosis [19].
Furthermore, iron-dependent lipoxygenase (LOX) serves as a catalyst in the enzymatic pathway, facilitating the production of lipid peroxides, thereby increasing the sensitivity of cells to ferroptosis. Notably, inhibition or knockdown of LOXs can inhibit ferroptosis in specific cell types, which further proves their importance to ferroptosis [20]. Remarkably, a specific lipoxygenase subtype, 12/15-LOX, has been identified as a key player in the oxidation of PUFAs linked to ferroptosis [21]. This observation hints at a potential pivotal role for 12/15-LOX in the regulation of ferroptosis, although further research is needed to solidify this connection [22]. In the context of iron overload, substances like hemoglobin and ferrous ammonium sulfate can also trigger ferroptosis. The mechanisms responsible for their effects involve the activation of distinct LOX protein subtypes, underscoring the diverse roles these enzymes play in driving ferroptotic cell death [23]. Additionally, other iron-dependent enzymes, notably cytochrome P450 oxidoreductase (POR), have been recognized as instigators of lipid peroxidation and ferroptosis under specific conditions [24], further illustrating the intricate network of molecular interactions governing this unique form of programmed cell death.
Hence, the development of ferroptosis can be influenced by interventions at various stages of cellular iron metabolism. Transferrin (TF) and transferrin receptor (TFRC) play crucial roles in this process. TF, binding to nearly all forms of circulating iron under physiological conditions, facilitates the entry of iron ions into cells by recognizing and binding to TFRC. In the SKBR3 and MDA-MB-231 cancer cell lines, ferroptosis induced by compounds like lapatinib and siramesine was observed to be mitigated when TF was knocked down [25]. Similarly, the deletion of TFRC prevented ferroptosis induced by erastin or cystine deficiency [26, 27]. These findings underscore the regulatory influence of TF and TFRC on iron uptake, subsequently affecting the sensitivity of cells to ferroptosis.
The mechanism governing the storage of iron within cells holds significant importance in the context of ferroptosis. Ferritin plays a key role in this process by storing intracellular iron in an inert form, primarily as Fe3+. Decreasing the LIP and elevating ferritin levels can be instrumental in preventing ferroptosis [28]. Moreover, in the cytoplasm, the overexpression of ferritin within mitochondria has been observed to thwart ferroptosis induced by compounds like erastin in neural cells [29]. Conversely, ferritin-targeted autophagy, also known as ferritin autophagy (chapter " The ferroptosis propagation”), has the opposite effect, increasing cell susceptibility to ferroptosis [30, 31]. Furthermore, research has revealed that prominin 2 mediates the release of ferritin and iron from cells via exosomes as a protective mechanism against ferroptosis [32]. This suggests that intracellular pathways governing iron degradation and secretion collectively regulate the LIP level, exerting a substantial influence on a cell’s susceptibility to ferroptosis.
Solute carrier family 40 member 1 (SLC40A1), also known as ferroportin (FPN), stands as the sole recognized iron transporter protein residing on mammalian cell membranes, primarily responsible for facilitating iron efflux. The modulation of SLC40A1 plays a pivotal role in the regulation of ferroptosis. Notably, when SLC40A1 is knocked down, it heightens the ferroptotic process. Conversely, the overexpression of SLC40A1 has been observed to decelerate the rate of ferroptosis [25, 33]. Thus, by regulating the outflow of cellular iron, SLC40A1 plays a crucial regulatory role in ferroptosis.
In conclusion, intracellular iron is involved in the formation of lipid peroxides and free radicals through the Fenton reaction or functions at the active sites of enzymes. Through these two functions, intracellular iron is related to ferroptosis. Moreover, modulation of multiple targets in the iron metabolism pathway can promote or prevent ferroptosis by affecting the amount and availability of intracellular iron, and many of the targets affected by iron metabolism have been demonstrated via experiments [34]. These discoveries open up numerous possibilities for leveraging ferroptosis as a therapeutic approach in the treatment of various diseases.
Lipid peroxidation provides materials essential for ferroptosis
Ferroptosis is characterized by the accumulation of intracellular lipid peroxides and their damaging effects on the cell membrane. Lipids, as the fundamental building blocks of cellular membranes, play a vital role in maintaining normal physiological processes. However, the excessive buildup of lipid peroxides can result in a range of structural and functional impairments within cells, a phenomenon frequently observed in the cells of diseased tissues [19].
When subjected to enzymatic processes or attacked by free radicals, PUFAs give rise to the production of lipid peroxides. For example, during a ROS assault, arachidonic and linoleic acids generate lipid peroxidation products, which trigger ferroptosis [35, 36]. Conversely, it was found that whether the fatty acid β-oxidation (FAO) in mitochondria consumes fatty acids, the formation of lipid droplets isolates PUFAs and protects them [34], or the competitive inhibition of PUFAs by monounsaturated fatty acids [37–39], they can all effectively prevent cells from ferroptosis by reducing the peroxidation of PUFAs. Moreover, PUFAs require modification before they can actively contribute to peroxidation reactions. Lysophosphatidylcholine acyltransferase-3 (LPCAT3) and acyl-CoA synthetase long-chain family member 4 (ACSL4) are recognized as significant regulators of ferroptosis [40–42]. They facilitate the conversion of PUFAs into phospholipid-bound polyunsaturated fatty acids (PL-PUFAs), which can directly participate in lipid peroxidation. Research indicates that phosphatidylcholine and phosphatidylethanolamine containing epinephrine or arachidonic acid (AA) are key phospholipids involved in the induction of ferroptosis [21]. Additionally, other members of the ACSL family might substitute for ACSL4, playing a role similar to that of ACSL4 in mediating ferroptosis [43].
In summary, lipid peroxides are generated from intracellular polyunsaturated fatty acids as the foundational substrates. This occurs through a combination of enzymatic modifications and iron-mediated mechanisms. These lipid peroxides subsequently attack the cell membrane, resulting in ferroptosis. Lipid peroxidation constitutes a fundamental process in ferroptosis and represents a potential target for clinical intervention.
Regulatory pathway of ferroptosis
In usual circumstances, cells possess intricate regulatory mechanisms to efficiently neutralize surplus peroxides and prevent ferroptosis-related reactions. Manipulation of these regulatory pathways can exert control over the onset of ferroptosis, holding promise for disease treatment. Consequently, ferroptosis regulation has emerged as a prominent research area in recent years. Among the identified ferroptosis-regulating mechanisms, the three primary pathways include the system Xc(−)-glutathione (GSH)-glutathione peroxidase 4 (GPX4) pathway, the nicotinamide adenine dinucleotide phosphate (NADPH)-ferroptosis suppressor protein 1 (FSP1)-coenzyme Q10 (CoQ10) pathway, and the GTP cyclohydrolase 1 (GCH1)-tetrahydrobiopterin (BH4) pathway. These pathways collectively orchestrate the cellular defense against ferroptosis [44].
The system Xc(−)-GSH-GPX4 pathway
The first regulatory pathway discovered and harnessed for inducing ferroptosis is the system Xc(−)-GSH-GPX4 pathway. This pathway relies on the catalytic activity of GPX4 and its cofactor GSH, which work in tandem to reduce lipid peroxides into harmless alcohols [45]. This enzymatic process is a crucial antioxidant mechanism within cells. Cysteine, the fundamental building block for GSH synthesis, is primarily supplied through the action of the system Xc- transporter protein on the cell membrane. System Xc- is a heterodimeric protein complex composed of solute carrier family 7 member 11 (SLC7A11/xCT) and solute carrier family 3 member 2 (SLC3A2). It facilitates the transport of cystine into the cell in a 1:1 ratio, which is then rapidly converted back into cysteine. Conducted by Dixon et al. in 2012, it was demonstrated that a small compound named erastin induced a unique form of cell death, subsequently termed ferroptosis [6]. Erastin’s mode of action centered on inhibiting the system Xc- transporter, thereby disrupting the cell’s ability to scavenge peroxides by reducing cellular cystine uptake. It was further observed that the tumor suppressor protein p53 downregulated the expression of SLC7A11, leading to ferroptosis via a similar mechanism [46, 47]. Additionally, the deletion of SLC7A11 selectively induced ferroptosis in pancreatic ductal adenocarcinoma cells driven by the KRAS proto-oncogene, effectively impeding tumor growth [48]. These findings illuminated the critical role of the system Xc- in ferroptosis regulation and its potential as a therapeutic target.
The synthesis of GSH plays a critical role in cellular ferroptosis. Activation of GSH synthase inhibitors can lead to ferroptosis [49–51]. Glutamate-cysteine ligase (GCL) is responsible for catalyzing the connection of cysteine to glutamate, a crucial step in the rate-limiting process of glutathione synthesis. The nuclear factor erythroid 2-related factor 2 (Nrf2) acts as a counterbalance to ferroptosis by promoting the expression of the GCL gene [52, 53]. Moreover, the expression of the multidrug resistance pump P-glycoprotein makes cells more susceptible to ferroptosis by pumping glutathione out of the cells [54]. This discovery reasonably explains why traditional drug resistance and ferroptosis sensitivity often appear at the same time and presents a novel approach for targeting drug-resistant bacteria or cancer cells. On the other hand, cells have alternative mechanisms to acquire cysteine, rendering them resistant to ferroptosis induced by compounds like erastin. Some cells can biosynthesize cysteine from methionine via the transsulfuration pathway. By blocking the transsulfuration pathway, cysteinyl-tRNA synthetase 1 (CARS1/CARS) facilitates erastin-induced ferroptosis [55]. In conclusion, reducing the cellular GSH concentration and diminishing the antioxidant capacity of cells, through means such as inhibiting system Xc- and other approaches, can induce ferroptosis.
Different from the effects of erastin, RSL3-induced ferroptosis doesn’t significantly alter intracellular GSH levels. However, it leads to a significant production of intracellular lipid peroxides. This difference indicates that RSL3 targets a protein, distinct from erastin, to modulate the accumulation of peroxides. In a proteomic study based on an erastin experiment, it was discovered that RSL3 skips the upstream system Xc- and covalently binds to GPX4, reducing its functionality [56]. This effect was further confirmed when researchers overexpressed GPX4 in colorectal cancer cells, resulting in the inhibition of ferroptosis induced by RSL3 [57]. GPX4 emerges as a pivotal factor in the regulation of ferroptosis. It’s important to note that GPX4 is a selenoprotein, with selenocysteine serving as its active component. Selenium has been shown to decrease the rate of ferroptosis when added to cells or administered to animals, including mouse models of brain hemorrhage. Consequently, selenium may influence the sensitivity of cells to ferroptosis [58–60]. Furthermore, apart from GPX4, other selenoproteins might also play a role in ferroptosis [34]. In conclusion, in the system Xc(−)-GSH-GPX4 pathway, ferroptosis can be induced by inhibiting the system Xc- or GPX4. This dual mechanism provides a potential therapeutic approach for related diseases by targeting ferroptosis.
The NADPH-FSP1-CoQ10 pathway
In 2016, Shimada K et al. identified FIN56 during an investigation of fifty-six caspase-independent lethal compounds. Their experiments revealed that FIN56 induces ferroptosis through a dual mechanism involving the depletion of GPX4 and CoQ10. This discovery unveiled a novel pathway for regulating ferroptosis [61]. CoQ10 plays a vital role as an antioxidant in vivo, capturing free radical intermediates and preventing lipid peroxidation. Consequently, the depletion of CoQ10 heightens cellular susceptibility to ferroptosis. Experimental evidence has shown that statins, which inhibit 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase activity, can also increase the rate of ferroptosis by depleting cellular CoQ10 [61]. Researchers investigating how cells prevent ferroptosis in the absence of GPX4 discovered the flavoprotein apoptosis-inducing factor mitochondria-associated 2 (AIFM2), subsequently renamed FSP1. Further research revealed that FSP1 counteracts lipid peroxides by catalyzing the regeneration of reduced-state CoQ10 using NAD(P)H. This finding suggests that FSP1 regulates ferroptosis through a distinct pathway running parallel to the system Xc(−)-GSH-GPX4 pathway, known as the NADPH-FSP1-CoQ10 pathway [62–64].
The GCH1-BH4 pathway
GCH1 serves as the rate-limiting enzyme in the synthesis of BH4, a crucial coenzyme involved in phenylalanine metabolism. BH4 also contributes to the production of CoQ10 in its reduced state, bolstering the cell’s capacity to neutralize lipid peroxides and inhibit ferroptosis. Furthermore, BH4 directly reduces the ferroptosis rate by blocking the peroxidation of specific lipids [65]. Consequently, modulation of the GCH1-BH4 pathway can independently intervene in the process of ferroptosis.
The role of mitochondria in ferroptosis
A complex regulatory network involving multiple organelles collaborates to orchestrate ferroptosis, with mitochondria assuming particularly vital roles in this process. Experimental evidence has indicated a substantial increase in nonheme iron accumulation and lipid peroxidation specifically within mitochondria, rather than in the cytoplasm, during myocardial ferroptosis induced by Adriamycin [66]. While this outcome underscores the critical involvement of mitochondria in ferroptosis, the precise nature of their role remains unclear and warrants further investigation [51].
Research has shown that the depletion of mitochondria through parkin-mediated mitophagy effectively prevents ferroptosis induced by cysteine deprivation but doesn’t impact ferroptosis induced by GPX4 inhibition [67]. This observation suggests that mitochondria may promote ferroptosis when cysteine levels are deficient by influencing GSH metabolism. Further studies have highlighted the importance of mitochondrial glutamine degradation in initiating ferroptosis [26]. Two primary metabolic pathways of glutamine are the tricarboxylic acid (TCA) cycle and glutaminolysis [68]. The byproduct of glutamine catabolism, α-ketoglutarate (α-KG), and its downstream products within the TCA cycle are essential for the start of ferroptosis [26]. Moreover, in the absence of cysteine, glutamine catabolism enhances mitochondrial respiration and depletes GSH through GPX4, thereby promoting ferroptosis. Conversely, the prevention of ferroptosis induced by cysteine deficiency becomes possible when glutamine catabolism is suppressed [67]. This underscores the intricate relationship between mitochondrial function and ferroptosis regulation.
Mitochondrial lipids also play a significant role in inducing lipid peroxidation and subsequently ferroptosis. Knockdown of acyl CoA synthetase family member 2 (ACSF2) and citrate synthase (CS), both essential for mitochondrial lipid metabolism, has been shown to reverse erastin-induced ferroptosis [6]. Erastin targets the mitochondrial resident voltage-dependent anion channel 2/3 (VDAC2/3) located on the mitochondrial membrane. The interaction between VDAC2/3 and erastin impedes the entry of endogenous substrates and reduces the rate of NADH oxidation, leading to mitochondrial dysfunction and the release of oxidants, ultimately triggering cellular ferroptosis. Consequently, reducing the expression of VDAC2/3 can prevent ferroptosis induced by erastin [69, 70]. These findings underscore the close association between mitochondria and ferroptosis, making mitochondria a viable target for modulating ferroptosis.
The ferroptosis propagation
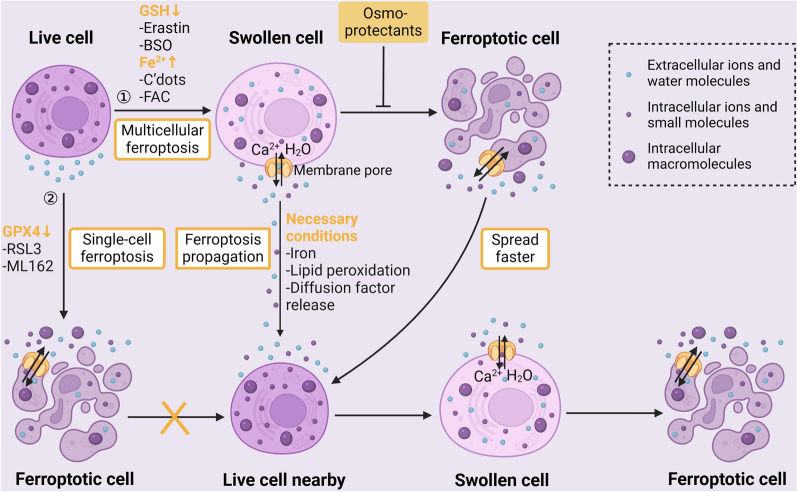
In addition to killing individual cells, it has been discovered that ferroptosis initiates a cascade of ferroptosis triggers among cell populations in a wave-like fashion. This results in a distinctive spatiotemporal pattern of cell death, which has been seen in cells exposed to C’dots nanoparticles, which induce ferroptosis, as well as in the kidney tubules of mice treated with erastin [71, 72]. However, this wave-like action is mediated by specific ferroptosis subtypes that depend on the continual presence of iron and lipid peroxides. In various circumstances, the existence of non-random spatiotemporal patterns of ferroptosis has been statistically demonstrated by Riegman M et al. [73]. Ferroptosis induced by GPX4 inhibition is described as "single cell ferroptosis" and does not trigger a propagation among neighboring cells. In contrast, ferroptosis caused by glutathione inhibition (such as BSO and erastin) or cellular iron overload (like C’dots and FAC) can initiate ferroptosis in multiple cells and is characterized as "multiple cell ferroptosis." [73]. The failure of ferroptosis propagation following GPX4 inhibition may be associated with factors related to glutathione function or iron activity rather than GPX4 itself [54]. This distinction highlights the complexity of ferroptosis regulation and propagation within cell populations.
The complete process of ferroptosis, including cell lysis, might be necessary for its propagation. But in recent years, it has been found that it can also propagate in the cell population without cell rupture [73]. Ferroptotic cells undergo swelling and rounding before eventually rupturing. This swelling and rounding are caused by the formation of pores in the plasma membrane, which allow the influx of water molecules and ions from the external environment [74, 75]. What’s intriguing is that lipid peroxidation, a key aspect of ferroptosis, can alter the shape of lipid domains and sections of the plasma membrane [76, 77]. This suggests the possibility that lipids themselves, rather than pore-forming proteins, mediate the formation of plasma membrane pores. Cells can propagate ferroptosis before they rupture by releasing factors through these plasma membrane pores, and this process can be effectively prevented by osmoprotectants [75]. However, research has shown that when cells undergo complete lysis (rupture), ferroptosis propagation is accelerated [73]. This acceleration is likely due to the release of more diffusible components from the ruptured cells. Furthermore, arachidonic acid, a ferroptosis inducer, has been shown to cause significant cellular deformation in zebrafish larvae, indicating that ferroptosis can also propagate in vivo and lead to substantial tissue damage [78]. Therefore, further investigation into the cascade of events in ferroptosis propagation could have significant clinical implications. The mechanism of intercellular ferroptosis propagation is shown by a picture (Fig. 3).
Fig. 3.
Mechanism of ferroptosis propagation between cells. This figure shows the inducers, conditions, processes, and changes in cell morphology and contents for the intercellular spread of ferroptosis, including single cell ferroptosis and multiple cell ferroptosis. (Created with BioRender.com.)
The role of ferroptosis in cancer
As a death mode first discovered in cancer [6], ferroptosis is closely related to the pathological process, metabolic state and microenvironment regulation of cancer. Therefore, we are here to elaborate the molecular mechanism related to ferroptosis in cancer.
Ferroptosis is involved in the formation and regulation of cancer
Studies have found that some classic cancer-related factors and pathways can affect the formation and regulation of cancer by inducing or inhibiting ferroptosis [79], indicating that ferroptosis is widely involved in the pathological process of cancer.
TP53
TP53 gene encodes an important tumor suppressor P53, which is mutated or inactivated in about 50% of cancers [80], leading to the development of cancer. The typical and well-known anticancer effect of P53 is achieved by inducing cell cycle arrest, senescence or apoptosis of tumor cells [81–83]. However, in recent years, it has been found that P53 can also affect tumor growth by inducing ferroptosis, which is called atypical effect. P53 3KR, a mutant product of TP53 gene, has lost the ability to induce cell cycle arrest, senescence and apoptosis, but it can enhance the susceptibility of cells to ferroptosis [47, 84]. The P53 3KR knockin mice will not form spontaneous tumors, which proves the existence of ferroptosis-induced anti-tumor pathway of p53 [47]. As mentioned above, under cellular stress, P53 affects the ability of cells to remove excess lipid peroxides and promotes ferroptosis of tumor cells mainly by mediating the transcriptional inhibition of SLC7A11. For example, the activation of p53 by nutlin-3 will trigger ferroptosis of osteosarcoma U2OS cells [47]. P53 R273H and P53 R175H, two mutants of P53, cannot bind to DNA, but can still inhibit the expression of SLC7A11 by inhibiting the activity of other transcription factors, which indicates that p53 participates in an integrated transcription factor network to regulate ferroptosis [85]. In addition, P53 can also indirectly mediate ferroptosis through metabolic target genes such as SAT1 [86], FDXR [87] and GLS2 [26].
However, under basal or low ROS stress, P53 may in turn inhibit ferroptosis, indicating its bidirectional effect [88]. For example, the complex formed by dipeptidyl peptidase-4 (DPP4) and NOX1 can mediate plasma membrane lipid peroxidation. By binding and blocking the activity of DPP4, P53 can inhibit ferroptosis induced by erastin in human colorectal cancer cells [89]. Moreover, in fibrosarcoma cells, P53 can limit ferroptosis by inducing CDKN1A expression [90].
To sum up, TP53, as an important regulatory gene of both ferroptosis and tumor, usually promotes ferroptosis and inhibits tumor growth, and may has the opposite effect in specific cases. Eprenetapopt and coti-2, both aimed at reactivating p53, are currently being tested in clinical trials involving patients with acute myeloid leukemia (AML; NCT03931291) and various solid malignant tumors (NCT04383938 and NCT02433626).
RAS
RAS genes, such as HRAS, NRAS and KRAS, are the most commonly mutated oncogenes in cancer [91], and closely related to ferroptosis. As mentioned above, erastin and RSL3 can significantly induce ferroptosis in RAS mutant cancer cells [6, 57, 92]. This is because the mutant RAS signal may increase the concentration of intracellular free iron by regulating the expression of iron metabolism-related genes (such as FTH1 and TFRC), which in turn increase the sensitivity of cells to ferroptosis [27, 93]. Therefore, in recent years, anti-tumor drugs targeting RAS to induce ferroptosis have been developed one after another. For example, sotorasib and adagrasib, the inhibitors against KRAS-G12C mutant protein have been proved to have good activity in patients with non-small-cell lung cancer (NSCLC) and other solid tumors [94, 95]. However, the RAS mutation of tumor may also inhibit ferroptosis under certain circumstances. Ectopic expression of oncogenic RAS mutants (NRAS12V, KRAS12V and HRAS12V) was found to enhance the resistance of rhabdomyosarcoma RMS13 cells to oxidative stress and ferroptosis [96]. In addition, a sensitivity analysis of 177 cancer cell lines to common ferroptosis-induced small molecules showed that the mechanism of ferroptosis can be RAS-dependent or independent, indicating that RAS mutation is not a necessary condition for ferroptosis in tumors [97]. In a word, RAS shows its potential as an ferroptosis inducing target to resist tumors. Its different mutation characteristics have different responses to ferroptosis, which needs further exploration.
Other tumor-related factors
The tumor suppressor BAP1 encodes a nuclear deubiquitinating enzyme, which is in the form of a polycomb-repressive deubiquitinase (PR-DUB) complex to reduce the ubiquitination of histone 2A (H2A) in nucleosomes, so as to perform epigenetic regulation gene expression [98, 99]. Studies have shown that the anti-tumor effect of BAP1 is partly due to the ubiquitination of H2A on the promoter of SLC7A11, thus inhibiting the expression of SLC7A11 and inducing ferroptosis [100]. However, the germline mutation of BAP1 is widely found in many cancers and makes tumor cells lose the vulnerability to ferroptosis, which is considered as an important susceptibility factor of hereditary cancers [101–103].
As an important regulator of oxidative stress signaling, NFE2L2 can promote the formation, progress and drug resistance of tumors [104, 105]. It has been found that NFE2L2 can help cells to resist oxidative stress of ferroptosis by activating protective genes involved in iron metabolism (including SLC40A1, MT1G, HMOX1 and FTH1), GSH metabolism (including SLC7A11, GCLM and CHAC1) and ROS detoxification (including TXNRD1, AKNRD1, AKR1C1, etc.) [106].
Hypoxia promotes tumor development and drug resistance [107]. The expression of HIF, the main regulator of hypoxia, can promote fatty acid uptake by increasing the expression of fatty acid binding proteins 3 and 7, so as to avoid ferroptosis caused by lipid peroxidation in HT-1080 fibrosarcoma cells [108]. On the contrary, the activation of HIF can also lead to ferroptosis vulnerability of clear-cell carcinomas [109]. This shows that HIF seems to have a dual role in the modulation of ferroptosis in cancer cells.
The epithelial-to-mesenchymal transition (EMT) is the process by which epithelial cells lose their junctions and apical-basal polarity, and then increase the mobility of single cells and make the development of invasive phenotype possible [110]. It can lead to cancer spread and drug resistance [110]. Studies have shown that the high mesenchymal-like cell state in human cancer cell lines and organs is related to the selective vulnerability to ferroptosis [111]. Moreover, metadherin, a positive regulator of EMT, can promote ferroptosis in many cancer cell lines by inhibiting the expression of GPX4 and SLC3A2 [112]. In addition, EMT also destroys cadherin 1-mediated cell–cell contact that can prevent ferroptosis [113–115]. Therefore, it can be seen that the tumor-promoting effect of EMT is accompanied by ferroptosis susceptibility, which is expected to become a breakthrough in the treatment of tumors with EMT phenomenon.
The role of ferroptosis in tumor microenvironment (TME)
The tumor microenvironment (TME) is composed of many different cellular and non-cellular components, which jointly drive tumor growth, invasion, metastasis and response to treatment. It makes cancer research change from a cancer-centered model to a model that regards TME as a whole [116, 117]. Immune cells, including T cells, macrophages, NK cells and so on, play a very important role in TME [118]. Moreover, a large number of studies have found that immune cells in TME have many overlaps with tumor cells in growth signals and metabolic characteristics, which further shows that they are closely interacted [119–122]. Therefore, we’ll ask two questions: does ferroptosis also occur in immune cells in TME when it’s induced in cancer cells and what is the interaction between immune cells and ferroptotic cancer cells.
Sensitivity of immune cells in TME to ferroptosis
T cells play an important role in anti-tumor immunity [123]. However, it was found that T cells lacking GPX4 will rapidly accumulate membrane lipid peroxides (LPO) after activation and lead to cell death, which will weaken their proliferation and anti-infection effects [124]. After that, this process was repeated in melanoma-related CD8+ T cells with the help of a high-throughput in vitro pharmacologic screening platform, and overexpression of GPX4 can effectively restore the anti-tumor immune effect of T cells [125]. Combined with the fact that cancer cells can promote the accumulation of reactive oxygen species in TME [126], it is reasonable to think that CD8+ T cells in TME are vulnerable to ferroptosis, and their susceptibility may be higher than that of T cells in physiological environment. This study also found that CD8+ T cells are even more sensitive to various ferroptosis inducers than some cancer cells (melanoma B16 cells) [125]. In contrast, Tregs in TME show a lower number of LPO, and are less prone to ferroptosis [125]. This might be because Tregs can rapidly induce the expression of GPX4 after being activated by TCR/CD28 co-stimulation [127]. However, targeted inhibition of GPX4 can still induce ferroptosis in Tregs to alleviate immunosuppression and exert antitumor effect [127].
In addition to T cells, other immune cells in TME will also respond to the induction of ferroptosis. It was found that M1 phenotype of tumor-associated macrophages (TAMs) is more resistant to ferroptosis caused by GPX4 deletion than M2 phenotype [128]. This might be because the high level of NO radical in M1 cells easily reacts with active intermediates produced by lipid free radicals and lipid peroxidation, and then replaces GPX4 to prevent ferroptosis [128, 129]. In addition, dendritic cells (DCs) have been proved to reduce the antigen processing ability due to ferroptosis when the lipid level increases [130–132]. Similarly, lipid peroxidation-associated oxidative stress caused by ferroptosis inhibits the glucose metabolism of NK cells to cause dysfunction, and the activation of Nrf2 antioxidant pathway may save them [133]. In short, not only tumor cells, but also immune cells in TME are susceptible to ferroptosis. The use of ferroptosis to treat tumors is a "double-edged sword", so efforts should be made to make the killing effect of tumors outweigh the damage to the immune system.
Interaction between ferroptotic cancer cells and immune cells in TME
Ferroptotic cancer cells and immune cells in TME can regulate each other. Firstly, ferroptosis can increase the immunogenicity of cancer cells. Studies have shown that cells undergoing early ferroptosis (within 1 h of treatment with the ferroptosis inducer RSL3) can induce dendritic cells to mature in vitro to kill fibrosarcoma cells by releasing damage-related molecular patterns (DAMPs) [134, 135]. Recently, a membrane oxidized phospholipid, 1-steaoryl-2–15-HpETE-sn-glycero-3-PE, has been found on the surface of ferroptotic cancer cells, which can guide macrophages to phagocytize [129]. But in some cases, ferroptotic cancer cells can also inhibit anti-tumor immunity. For example, the KRAS protein released from exosomes of ferroptotic tumor cells can be absorbed by TAMs. This, in turn, causes TAMs to switch to the M2 phenotype, thereby promoting the proliferation of pancreatic tumor [136].
Secondly, immune cells can also directly kill tumor cells through ferroptosis. Research shows that immunotherapy-activated CD8+ T cells can enhance ferroptosis-specific lipid peroxidation in tumor cells by downregulating the expression of SLC3A2 and SLC7A11, via the release of IFN-γ [137].
To sum up, ferroptosis can affect the whole TME, not just tumor cells, and it can also serve as a bridge between tumor cells and related immune cells. Therefore, ferroptosis is both an internal factor and an external factor of TME regulation.
Progress of research on ferroptosis in sarcoma treatment
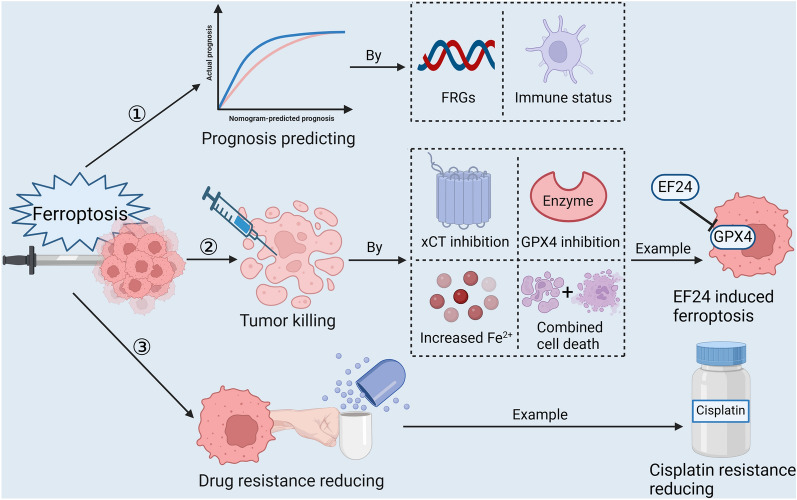
The importance of conducting further clinical research on sarcoma treatment cannot be overstated, primarily due to the high mortality rates among patients, the limited range of effective therapeutic options available, and the persistent challenge of drug resistance in treating this condition. Ferroptosis, a recently explored form of programmed cell death, has shown promise in effectively restraining tumor growth, invasion, and progression [28]. Consequently, there is a growing body of research focused on exploring the diverse strategies through which ferroptosis can be harnessed as a potential treatment approach for sarcoma. (Fig. 4).
Fig. 4.
Pathways of ferroptosis for sarcoma treatment. This figure shows three common ways in which ferroptosis is applied to sarcoma treatment, including predicting prognosis, killing sarcomas, and reducing drug resistance, with clarification of mechanisms and examples. (Created with BioRender.com.)
Research progress on ferroptosis applied to directly kill sarcoma cells
The current standard treatment for sarcoma, which involves surgery along with radiation and chemotherapy, has limitations in terms of its effectiveness, especially in cases of metastatic tumors, where the median survival rate is only 15–20 months [138, 139]. Consequently, there is a pressing need for novel and more potent systemic therapies for sarcoma patients [140]. Ferroptosis has emerged as a potentially transformative mechanism for targeting and eliminating sarcoma cells. What’s particularly promising is that there is evidence to suggest that tumor cells, including sarcoma cells, may be more vulnerable to ferroptosis than normal cells, thanks to the correlation between the expression of cancer-related genes and ferroptosis-related genes (FRGs) [114, 141–143]. As a result, ferroptosis has garnered significant attention as a research focal point in the pursuit of more effective sarcoma treatments. In Tables 1 and 2, we’ve summarized the mechanisms, effects, and the cell models used in some common ferroptosis inducers and inhibitors, both for sarcoma cell-targeted and non-sarcoma cell-targeted treatments.
Table 1.
Ferroptosis inducers targeting sarcoma cells and other cells
| Compound | Mechanism | Effect | Cell models | Refs. |
|---|---|---|---|---|
| ① Ferroptosis inducers in sarcoma | ||||
| 5-aminolevulinic acid (ALA) |
HMOX1 overexpression, iron and lipid peroxides overload |
Fe2+ ↑, GPX4 ↓, ROS ↑, MDA ↑ | SW872 (liposarcoma), MG63 (osteosarcoma) | [144] |
| ACXT-3102 | SLC7A11 inhibitor | GSH ↓ |
SK-LMS-1, MG-63, HTB-93, etc (synovial sarcoma) |
[145] |
| Bavachin |
Transferrin receptor ↑, divalent metal transporter-1 ↑, ferritin light chain ↓, ferritin heavy chain ↓, p53 ↑, p-STAT3 ↓, SLC7A11 ↓, GPX4 ↓ |
Fe2+ ↑, GSH ↓, GPX4 ↓, ROS ↑, Malondialdehyde ↑, mitochondrial morphology alteration |
MG63, HOS (osteosarcoma) | [146] |
| Buthionine-sulfoximine (BSO) | GCL inhibitor | GSH ↓ |
S4MH, F21 (rhabdomyosarcoma) |
[147, 148] |
|
β-Phenethyl isothiocyanate (PEITC) |
TfR1 ↑, FPN, FTH1, DMT1 and IRP2 ↓, GSH/GSSG and GPX4 ↓ |
Fe2+ ↑, GSH ↓, GPX4 ↓, ROS ↑ |
MNNG/HOS, U-2 OS, MG-63, 143B, K7M2 (osteosarcoma) |
[149, 150] |
| EF24 | HMOX1 overexpression | Fe2+ ↑, GPX4 ↓, ROS ↑, MDA ↑ | U2os, Saos-2 (osteosarcoma) | [11] |
| Erastin | SLC7A11 and VDAC2/3 inhibitor | GSH ↓, ROS ↑ |
HT1080; C2C12, RD, RH18, RH30, etc (various sarcomas) |
[6, 92, 151, 152] |
| Ferric ammonium citrate (FAC) | Iron supplement | Fe2+ ↑ |
K7M2 (murine osteosarcoma) |
[153] |
| Ferrous ammonium sulfate (FAS) | Iron supplement | Fe2+ ↑ |
K7M2 (murine osteosarcoma) |
[153] |
| KDM4A | H3K9me3 demethylation in the promoter region of SLC7A11 | GSH ↓ |
143 B, HOS (osteosarcoma) |
[154] |
| MicroRNA-1287-5p | Bound to the 3'-untranslated region of GPX4 | GPX4 ↓ | Human osteosarcoma cells | [155] |
| Pure Artemisinin/Artemisia annua L. hydroalcoholic extract | Ferritin autophagy | Fe2+ ↑ |
D-17, OSCA-8, OSCA-40 (canine osteosarcoma) |
[156, 157] |
| RSL3 | GPX4 inhibitor | GPX4 ↓ |
BJ-TERT; HT1080; C2C12, RD, RH18, RH30, etc (various sarcomas) |
[92, 97, 152] |
| Sorafenib | SLC7A11 inhibitor | GSH ↓ | RH30, RD, RMS, etc. (rhabdomyosarcoma) | [147, 158] |
|
Sulfasalazine (SAS) |
SLC7A11 inhibitor | GSH ↓ |
K7M2 (murine osteosarcoma) |
[153] |
| Theaflavin-3,3'-digallate (TF3) | Down-regulating FTH and GPX4, GSH consumption |
Fe2+ ↑, GSH ↓, GPX4 ↓ ROS ↑, MDA ↑ |
MG63. HOS, hFOB1.19 (osteosarcoma) | [159] |
|
Tirapazamine (under hypoxia) |
SLC7A11 and GPX4 inhibitor; up-regulating p53 |
Fe2+ ↑, GSH ↓, GPX4 ↓ | 143B, U2OS, MNNG/Hos (osteosarcoma) | [160] |
| Ursolic acid | Ferritin autophagy | Fe2+ ↑ | 143 B, HOS (osteosarcoma) | [161] |
| Zoledronic acid |
Up-regulating POR, down-regulating CoQ10, HMOX1 overexpression |
ROS ↑, lipid peroxides ↑ |
Human osteosarcoma cells | [162, 163] |
| ② Ferroptosis inducers in other cells | ||||
| BAY 11–7085 |
NFKBIA/IkBa inhibitor, HMOX1 overexpression |
Fe2+ ↑, GPX4 ↓, ROS ↑, MDA ↑ | MDA-MB-231, MCF-7, MDA-MB-468, SKBR3 | [164] |
| BAY 87–2243 | Mitochondrial complex I inhibitor | Mitochondrial membrane potential ↓, ROS ↑ | G361, SK-MEL-28 | [165] |
| Cyst(e)inase | Cyst(e)ine consumption | GSH ↓ |
AsPC-1, PANC-1, BxPC-3, S2-013; PCa cells, FVB/N mice |
[48, 166] |
| FeCl2 | Iron supplement | Fe2+ ↑ | OHSCs | [167] |
| FIN56 | GPX4 consumption and depleting CoQ10 via the mevalonate pathway |
GPX4 ↓, CoQ10 ↓, ROS ↑ |
BJeLR, HT-1080, MEFs, PACN1 | [61, 168] |
| FINO2 | Indirect inhibitor of GPX4 and direct oxidant of iron | Fe2+ ↑, GPX4 ↓ | HT-1080 | [169] |
| Glutamate | SLC7A11 inhibitor | GSH ↓ | HT1080, PC12 | [170] |
| Hemin | HMOX1 overexpression and iron supplement | Fe2+ ↑, GPX4 ↓, ROS ↑, MDA ↑ |
IMR-32, SK-N-SH; male Swiss albino mice |
[23, 171] |
| Hemoglobin |
Iron supplement and ROS production |
Fe2+ ↑, ROS ↑, MDA ↑ | OHSCs | [167] |
| (NH4)2Fe(SO4)2 | Iron supplement | Fe2+ ↑ | IMR-32 | [171] |
| Piperazine erastin | SLC7A11 inhibitor | GSH ↓ | HT-1080, BJeLR | [6, 97] |
| Statins (fluvastatin, lovastatin, simvastatin) | HMG-CoA reductase inhibitor and GPX4 biosynthesis suppression |
GPX4 ↓, CoQ10 ↓, ROS ↑ |
HT-1080, HCC4006 | [61, 111] |
Table 2.
Ferroptosis inhibitors targeting sarcoma cells and other cells
| Compound | Mechanism | Effect | Cell model | Refs. |
|---|---|---|---|---|
| ① Ferroptosis inhibitors in sarcoma | ||||
| Bathophenanthrolined-isulfonic acid (BPS) | Iron chelator | Fe2+ ↓ | C2C12, U57810 (rhabdomyosarcoma) | [92] |
| Bisindolylmaleimide I and Gö6976 | Protein kinase C inhibitor | Lipid peroxides ↓ | RD, RH18, RH30 | [151] |
|
Deferoxamine (DFO) |
Iron chelator | Fe2+ ↓ | MG63, HOS (Osteosarcoma); HT1080 (fibrosarcoma) | [6, 146] |
| Diphenyleneiodonium chloride (DPI) and GKT137831 | NOX inhibitor | NOX-mediated lipid peroxidation ↓, ROS ↓ | RD, RH18, RH30 | [151] |
| Fanconi anemia complementation group D2 (FANCD2) | JAK2/STAT3 pathway inhibitor, FTH1 ↑, GPX4 ↑, COX2 ↓, LIP ↓ | GPX4 ↑, Fe2+ ↓, ROS ↓ | MG-63, U2OS, hFOB1.19 (Osteosarcoma) | [172] |
| Ferrostatin-1 | Radical-trapping antioxidants |
ROS ↓, lipid peroxides ↓ |
HT1080, MG63, HOS, C2C12, RD, RH18, RH30, HEK-29, HT22, etc (various sarcomas) |
[6, 92, 146, 151, 173] |
| Liproxstatin-1 | Radical-trapping antioxidants |
ROS ↓, lipid peroxides ↓ |
HT1080, MG63, HOS, HEK-29, HT22 (various sarcomas) |
[6, 146, 173] |
| LncRNA- SNHG14 | Down-regulating miR-206 | GSH ↑ | NR-SJSA1 (nutlin3a-resistant osteosarcoma) | [174] |
| Mitochondrial NADP + -dependent isocitrate dehydrogenase (IDH2) | NADPH production | GSH ↑ | HT1080 (fibrosarcoma); Hepa1-6 (hepatoma) | [175] |
| N-acetyl cysteine (NAC), Glutathione (GSH) | Antioxidant, GSH supplement | GSH ↑, ROS ↓ | HT1080, C2C12, U57810 (rhabdomyosarcoma) | [6, 92, 97] |
| Pifithrin-α | p53 inhibitor | GSH ↑ |
MG63, HOS (Osteosarcoma) |
[146] |
| Vitamin E and tocopherols | Antioxidant, LOX inhibitor |
ROS ↓, lipid peroxides ↓ |
MG63, HOS (Osteosarcoma), Pfa1 | [21, 146] |
| ZnPPIX | Down-regulating HMOX1 | Fe2+ ↓, GPX4 ↑, ROS ↓, MDA ↓ |
SW872 (liposarcoma), MG63 (osteosarcoma) |
[144] |
| ② Ferroptosis inhibitors in other cells | ||||
| 1-methyl tryptophan |
Indoleamine 2, 3-dioxygenase (IDO) inhibitor, up-regulating SLC7A11, reduction of nitrative stress |
GSH ↑ | LO2 | [176] |
| AA-861 | 5-LOX inhibitor |
ROS ↓, lipid peroxides ↓ |
HEK-293 T, G401 | [177, 178] |
| Baicalein | 12/15-LOX inhibitor |
ROS ↓, lipid peroxides ↓ |
PANC1, BxPc3; Jurkat, Molt-4 |
[179, 180] |
| Butylated hydroxyanisole and butylated hydroxytoluene | Antioxidant |
ROS ↓, lipid peroxides ↓ |
HT1080; male C57BL/6 J mice |
[97, 181] |
| β-mercaptoethanol (2ME) | Reduction of cystine to cysteine | GSH ↑ |
BMDMΦ and OT-1 CD8+ T cells |
[6, 182] |
| CoQ10, idebenone | Antioxidant |
ROS ↓, lipid peroxides ↓ |
U-2 OS, NCI-H460, NCI-H2291, NCI-H1703, NCI-H446, HT1080 |
[62, 63] |
| Dopamine | Improvement of stability of GPX4 | GPX4 ↑ | HEK293, PANC1, HEY, MEF | [183] |
| Selenium | Active group of GPX4 | GPX4 ↑ | HT-1080, MEFs | [58] |
| Zileuton | 5-LOX inhibitor |
ROS ↓, lipid peroxides ↓ |
Pfa1, HT22 | [21, 184] |
Ferroptosis in the treatment of osteosarcoma
Certain drugs, such as bavachin [146], tirapazamine [160], and sulfasalazine [153, 185], have demonstrated the ability to induce ferroptosis in osteosarcoma cells by inhibiting the expression of the system Xc- component SLC7A11. In particular, bavachin has proven effective in inhibiting the growth of MG63 and HOS osteosarcoma cell lines. This inhibitory effect of bavachin on osteosarcoma cell growth can be reversed by ferroptosis inhibitors like ferrostatin-1 and liproxtin-1, iron chelators like desferrioxamine, and antioxidants like Vitamin E [146]. This suggests that bavachin induces cell death in osteosarcoma cells through ferroptosis. Further investigation into the mechanism revealed that bavachin increases the expression of transferrin receptor and divalent metal transporter-1 while decreasing the expression of ferritin light chain and ferritin heavy chain in osteosarcoma cells. This leads to an increase in intracellular ferrous iron content, making the cells more susceptible to ferroptosis. Additionally, bavachin upregulates the expression of p53 by downregulating phosphorylated signal transducer and activator of transcription 3 (p-STAT3). Then, p53 downregulates SLC7A11 and GPX4 expression, contributing to the accumulation of intracellular ROS and MDA. This finding highlights the importance of the STAT3/p53/SLC7A11 axis as a key pathway involved in ferroptosis induced by bavachin [146]. Conversely, the histone demethylase KDM4A has been found to increase SLC7A11 expression and inhibit ferroptosis in osteosarcoma cells by controlling the demethylation of H3K9me3 at the SLC7A11 promoter region [154]. Therefore, KDM4A is a potential therapeutic target for the treatment of osteosarcoma.
In addition to targeting the membrane receptor system Xc-, increasing the intracellular iron concentration has also been explored as a strategy to induce ferroptosis in sarcoma cells for potential treatment. Several studies have investigated the effects of iron supplementation on sarcoma cells. For instance, iron supplementation with compounds like ferric ammonium citrate (FAC) or ferrous ammonium sulfate (FAS) has been found to exacerbate ferroptosis induced by treatments in sarcoma cells, such as intensifying SAS-induced ferroptosis in K7M2 osteosarcoma cells [153]. This suggests that increasing intracellular iron levels can enhance ferroptosis in sarcoma cells. Another intriguing finding is related to ferritin autophagy, a process that involves the degradation of ferritin, which can increase the intracellular concentration of unstable iron. This mechanism appears to be critical for inducing ferroptosis in canine osteosarcoma cell lines when treated with a hydroalcoholic Artemisia annua extract [156, 157]. Furthermore, compounds like phenethyl isothiocyanate (PEITC), derived from cruciferous vegetables and available in plant extracts, have been shown to induce multiple forms of cell death, primarily ferroptosis, apoptosis, and autophagy, in osteosarcoma cells. This is achieved through mechanisms that include increasing active iron, depleting GSH, producing ROS, and activating the MAPK signaling pathway [149, 150]. However, it’s important to note that excessive PEITC intake can potentially affect normal cells due to expanded tissue distribution resulting from metabolic saturation [150, 186]. Additionally, EF24, a synthetic analog of curcumin, triggers ferroptosis by upregulating heme oxygenase 1 (HMOX1). This upregulation increases the Fe2+ concentration by breaking down heme and inhibiting GPX4 expression. EF24 is considered a promising candidate for treating HMOX1-positive osteosarcoma [11]. These studies demonstrate the potential of Chinese patent medicines in inducing sarcoma ferroptosis.
In vitro cell experiments showed that high concentrations of 5-aminolevulinic acid similarly induced ferroptosis in human sarcoma cells by overexpressing HMOX1 in the dark, suggesting new possibilities for the application of this drug [144]. Furthermore, zoledronic acid has exhibited multifaceted effects in promoting ferroptosis in osteosarcoma cells. It not only upregulates HMOX1 protein expression but also significantly reduces the levels of the antioxidant CoQ10. Additionally, zoledronic acid increases the expression of POR, an enzyme required for lipid peroxidation [162, 163]. These combined actions enhance the propensity for ferroptosis in osteosarcoma cells. Factors that can regulate ferroptosis in sarcoma cells by affecting both the Fe2+ concentration and GPX4 expression also include the fanconi anemia complementation group D2 (FANCD2) [172] and theaflavin-3,3′-digallate [159].
The latest approach to cancer treatment in recent years can be combined with ferroptosis to increase treatment efficacy. MicroRNAs (miRNAs) have emerged as promising candidates for personalized cancer treatment and appear to be involved in ferroptosis [187–190]. For instance, miR-1287-5p, which is downregulated in human osteosarcoma, exhibits upregulation in response to ferroptotic stimulation [155]. Elevated miR-1287-5p levels directly target the 3′-untranslated region of GPX4, inhibiting its activity and promoting ferroptosis in osteosarcoma cells. Additionally, miR-1287-5p mimics significantly heighten the sensitivity of human osteosarcoma cells to cisplatin chemotherapy [155]. Exosome-mediated miR-144-3p is another microRNA with a role in inhibiting osteosarcoma development. It regulates ZEB1 expression, thereby promoting ferroptosis [191]. Moreover, the long non-coding RNA (lncRNA) SNHG14 affects SLC7A11 activity and prevents ferroptosis by targeting and downregulating miR-206 expression in nutlin3a-resistant osteosarcoma cell lines [174]. Photodynamic therapy (PDT), which is a promising approach for various cancers, has been investigated in human osteosarcoma cells [192, 193]. Specifically, pyropheophorbide-α methyl ester-mediated PDT (MPPa-PDT) induces apoptosis while increasing unphosphorylated Yes-associated protein (YAP) levels, which in turn initiate Hippo pathway to inhibit apoptosis [194–196]. YAP knockdown enhances the sensitivity of human osteosarcoma cells to MPPa-PDT, increasing apoptosis rates and reducing drug resistance when administered in combination with erastin, an inducer of ferroptosis [196]. When utilized alongside homologous-sequence-targeting nanoparticles, PDT can further enhance apoptosis and ferroptosis rates in osteosarcoma cells [197].
Ferroptosis applied to the treatment of other sarcomas
Rhabdomyosarcoma (RMS) cells have been reported to be susceptible to oxidative stress, and the mechanism of increasing GSH to increase antioxidant defense makes these cells more vulnerable to GSH depletion [198]. As a result, ferroptosis may be applied as a new RMS treatment, especially for refractory RMS. Recent studies have demonstrated that erastin and RSL3 induce ferroptosis in rapidly proliferating myogenic cells via the extracellular signal-regulated kinase (ERK) pathway. When combined with chemotherapeutic agents like adriamycin and actinomycin D, these compounds effectively inhibit all RMS cell lines [92]. Furthermore, sorafenib, which targets system Xc-, and buthionine-sulfonylimine, an inhibitor of GSH biosynthesis, have demonstrated the ability to hinder RMS cell line growth [147, 148, 158]. Protein Kinase C (PKC) and NADPH Oxidase (NOX) are also involved in the regulation of ferroptosis in RMS cells [151].
Ewing sarcoma (ES) is one of the most common malignant tumors in children, with a high degree of malignancy and limited treatment options [199]. Therefore, it is extremely urgent to identify novel potential therapeutic targets for ES and put them into use in clinical settings. Studies have shown that aurora kinase A (AURKA) is significantly up-regulated in ES, and its expression level is significantly related to the short overall survival and event-free survival of patients with ES [200, 201]. AURKA inhibition can trigger the apoptosis and ferroptosis of ES cells through the NPM1/Yes1 associated transcriptional regulator (YAP1) axis. Subsequently, this study identified an AURKA inhibitor TCS7010, which has the killing effect on ES cells, through the high-throughput screening of a small molecular pharmacy library [201]. In addition, cytosolic carbonic anhydrase (CA) may also be a potential target for ES therapy, and CA inhibitors can induce ferroptosis through Inhibition of AKT/FTH1 signaling in ES Cells [202].
Expanding on the potential of ferroptosis as a treatment strategy for various types of sarcomas, Kim H and colleagues found that the deletion of isocitrate dehydrogenase (IDH) increased the sensitivity of human HT1080 fibrosarcoma cells to ferroptosis induction when cultured in vitro [175]. In the context of synovial sarcoma, which is characterized by a deficiency in malic enzyme 1, these sarcoma cells exhibited heightened susceptibility to ferroptosis triggered by ACXT-3102 [145]. SHARPIN, an activator of NF-kappaB, can also induce the ferroptosis of synovial sarcoma cells, and the PGC1α/NRF2/SLC7A11 axis and BNIP3L/NIX-mediated mitophagy is involved in its downstream regulation [203]. Moreover, in the case of uterine carcinosarcoma, knocking out the ferroptosis-related gene named heat shock factor 1 (HSF1) increased the sensitivity of tumor cells to treatment with adriamycin or gemcitabine, suggesting a potential combination therapy approach [204]. These findings underscore the versatility of ferroptosis-based treatments across different types of sarcomas.
The utilization of ferroptosis as a means to eliminate tumor cells represents an innovative approach to sarcoma treatment. Within this domain, the objectives moving forward encompass the discovery of novel ferroptosis-inducing agents and intervention targets. Furthermore, efforts are aimed at enhancing the efficacy of tumor eradication and refining drug therapies through rigorous clinical trials. This promising avenue of research holds the potential to revolutionize the treatment landscape for sarcomas.
Targeting ferroptosis in immunotherapy to indirectly resist sarcoma
Immunotherapy for sarcoma has been studied for many years and achieved good results, which is an important supplement to chemotherapy [205, 206]. As mentioned above, ferroptosis can affect can affect tumor-related immune effects in TME. (chapter " The role of ferroptosis in TME "). Therefore, it is hopeful to enhance the immunotherapy effect of sarcoma by inducing ferroptosis, which can be achieved by two ways: inducing ferroptosis in sarcoma cells to enhance its immunogenicity or regulating ferroptosis in immune cells to enhance the anti-sarcoma immune effect. Efimova I et al. confirmed for the first time that ferroptosis is immunogenic in vivo and in vitro [135]. They found that early (rather than late) ferroptotic cells can promote the phenotypic maturation of bone marrow-derived dendritic cells (BMDCs) and elicit a vaccination-like effect in immune-competent mice but not in Rag-2-/-mice through the co-culture of ferroptotic mice fibrosarcoma MCA205 cells with immune cells in vitro and the preventive ferroptosis tumor vaccination inside the mice [135]. This indicates that the mechanism of ferroptosis-mediated immunogenicity is closely regulated by the adaptive immune system and is time-dependent. In theory, this method is expected to effectively reverse the treatment dilemma of patients with immune desert sarcoma. Some commonly used targeted therapies (such as sorafenib), chemotherapy (such as cisplatin) and radiotherapy for sarcoma are also ferroptosis inducers, which are helpful to enhance the immunogenicity of sarcoma cells [170, 207], reflecting the synergy between immunotherapy for sarcoma and other treatments.
Because immune cells are also vulnerable to ferroptosis (chapter “The role of ferroptosis in TME”), tumor immunotherapy can be carried out by regulating ferroptosis of immune cells. Common ideas include that ferroptotic stress inducing TAMs to repolarize from M2 type to M1 type [128, 208–210], inducing ferroptosis of Tregs to reduce negative immune effect [127], targeting system Xc- to alleviate cystine deprivation in TME mediated by myeloid-derived suppressor cells (MDSCs) to promote T cell survival [211] and so on. Recently, Yu K et al. designed a biomimetic hybrid cell membrane camouflaged by poly (lactic-co-glycolic) acid (PLGA)-loaded Fe3O4 and DHJS (a probe for ROS generation) to induce ferroptosis in osteosarcoma cells, and successfully mediated macrophage M1 polarization as well as the infiltration of CD8+ T cells and dendritic cells in tumors [212]. However, in sarcomas with different immunophenotypes, the effects of ferroptosis on tumor immunity may be different and different kinds of ferroptosis inducers will also have different effects on immune cells and tumor cells [121]. These factors determine the balance between tumor killing effect and immune system damage. Therefore, selecting the appropriate ferroptosis inducer for specific sarcoma is the most critical step for curative effect, and it is also a gap to be further explored.
Ferroptosis reduces drug resistance of sarcoma cells to chemotherapeutic agents
Despite the progress made in increasing the 5-year survival rate of sarcoma patients through conventional surgical treatment and postoperative neoadjuvant chemotherapy, chemotherapy resistance remains a significant obstacle to improving patient outcomes [213–215]. Cisplatin, a highly potent and commonly used chemotherapeutic agent for solid tumors, exerts its anti-tumor effects by triggering both apoptosis and ferroptosis [216, 217]. Nevertheless, tumor cells can develop resistance to cisplatin by engaging mechanisms that regulate autophagy and enhance the expression of antioxidant enzymes [218–220]. While previous studies have largely focused on reactivating proapoptotic pathways to enhance the sensitivity of sarcoma cells to cisplatin [221–223], this approach has not proven to be particularly effective.
Drug-resistant cancer cells, particularly those reliant on the GPX4 antioxidant system, are susceptible to ferroptosis induction [111, 224]. Consequently, combining ferroptosis inducers represents a novel approach to combat sarcoma resistance to chemotherapeutic agents like cisplatin. For instance, the combination of the ferroptosis agonist erastin with cisplatin has demonstrated a significant synergistic effect against A549/HCT116 tumor cells [10]. Additionally, both erastin and STAT3 inhibitors have been effective in reactivating ferroptosis in drug-resistant tumor cells, rendering them more susceptible to cisplatin [220]. Furthermore, research has highlighted the potential of the plant extract ursolic acid (UA) as an adjunct to cisplatin treatment for sarcoma [161]. UA promotes tumor cell apoptosis, inhibits metastasis [225], and, in the presence of cisplatin, activates ferritin autophagy and degradation. This leads to increased free iron levels, lipid peroxide accumulation, and ferroptosis induction, underlining the close relationship between autophagy and ferroptosis in cancer cell death [161, 226]. Moreover, with the assistance of nanomaterial technology, anti-Her2 affibody-decorated arsenene nanosheets have proven effective in depleting intracellular GSH and inhibiting GPX4 activity, thus inducing ferroptosis and overcoming cisplatin resistance [227].
In conclusion, ferroptosis holds significant promise for overcoming the resistance of sarcoma cells to chemotherapeutic agents, particularly cisplatin. The synergistic combination of ferroptosis inducers with conventional chemotherapeutic agents represents a potentially transformative approach in the treatment of sarcoma.
Predictive value of ferroptosis-related genes expression in sarcoma cells
Multiple molecular networks play critical roles in the regulation and understanding of ferroptosis. Exploring these molecular mechanisms can offer valuable insights into the potential clinical applications of ferroptosis in disease treatment. A growing body of research has identified and extensively studied ferroptosis-related genes (FRGs) across various fields [228, 229]. The expression of FRGs has been strongly associated with the development of several types of cancer, including hepatocellular carcinoma [230], glioma [231], esophageal adenocarcinoma [232], and lung adenocarcinoma [233]. Additionally, researchers have explored the use of FRGs in predicting the prognosis of sarcoma patients (Table 3).
Table 3.
Summary of prognostic models for sarcomas based on FRGs
| Time | Target | Screening method | Hub genes for Prognostic model | Efficiency verification | Refs. |
|---|---|---|---|---|---|
| 2021 | Soft tissue sarcoma | Univariate Cox → LASSO → Multivariate Cox | MUC1, GSS, HELLS, RPL8, ALOX15B, NOX5, CD44, ISCU, NCOA4, RGS4, SETD1B, GCLM |
① GEO database ② ROC curve ③ K-M survival analysis |
[234] |
| 2021 | Osteosarcoma |
Univariate Cox → LASSO → Multivariate Cox |
G6PD, PEBP1, PGD, DPP4, SLC39A8, SOCS1, ATG7, MYC, ALOX15B, CBS, EGLN1, MUC1 |
① GEO database ② Time-dependent ROC |
[9] |
| 2022 | Soft tissue sarcoma |
Log-rank → Wilcoxon rank sum → Univariate Cox → Multivariate Cox |
EPAS1, STMN1, CXCL2, NQO1, HELLS, IL6 |
① GEO database ② Time-dependent ROC |
[235] |
| 2022 | Uterine carcinosarcoma |
Univariate Cox → LASSO |
PGD, HSF1, ISCU, PLIN2, GPT2 |
① GEO database ② ROC curve |
[204] |
| 2022 | Sarcoma | Univariate Cox → LASSO | SLC7A11, FANCD2, CISD1, ATP3MC3 |
① ROC curve ② K-M survival analysis |
[236] |
| 2022 |
Osteosarcoma and Chemotherapy resistance |
Log-rank → Univariate Cox → Multivariate Cox | CBS, COCS1, EGFR |
① GEO database ② Time-dependent ROC |
[237] |
| 2022 | Osteosarcoma | Univariate Cox → LASSO → Multivariate Cox | PGD, G6PD, ACSF2, MT1G, FADS2, CBS |
① ROC curve ② K-M survival analysis |
[238] |
| 2022 | Ewing Sarcoma | Univariate Cox → Random survival forest algorithm → Multivariate Cox | AURKA, RGS4, RIPK1 |
① GEO database ② ROC curve ③ K-M survival analysis |
[239] |
| 2022 | Osteosarcoma | Univariate Cox → LASSO | TP53, HMOX1, SLC7A11, HRAS, VEGFA, TXNRD1, CBS, G6PD |
① GEO database ② ROC curve |
[240] |
| 2022 | Osteosarcoma | Univariate Cox → LASSO → Multivariate Cox |
LRRC1, ACO2, CTNNBIP1 (FRG subclusters) |
① ROC curve ② K-M survival analysis ③ Calibration curves |
[241] |
| 2022 | Osteosarcoma |
WGCNA → LASSO → Multivariate Cox |
COL5A2, HOXB4, UNC5B |
① GEO database ② ROC curve ③ K-M survival analysis |
[242] |
| 2022 | Osteosarcoma | Univariate Cox → LASSO → Multivariate Cox | ACSL4, HMOX1, GPX4, PRNP, ATG7 |
① TARGET and GEO database ② K-M survival analysis |
[243] |
| 2023 | Osteosarcoma | Univariate Cox → LASSO → Multivariate Cox | ACSL5, ATF4, CBS, CDO1, SCD, SLC3A2 |
① GEO database ② ROC curve ③ Subgroup analysis |
[244] |
| 2023 | Osteosarcoma | Univariate Cox → LASSO | MUC1, MAP3K5, LURAP1L, HMOX1, BNIP3 |
① GEO database ② ROC curve ③ Univariate and multivariate Cox |
[245] |
The first prognostic model for soft tissue sarcoma (STS) based on FRGs was developed by Huang W and colleagues [234]. They utilized RNA sequencing profile and employed various analytical techniques such as Cox regression analysis and LASSO analysis to identify 12 FRGs that are closely linked to the prognosis of STS. These FRGs were then integrated with clinical variables to construct a nomogram, which serves as a predictive tool for assessing the prognosis of STS patients. Furthermore, the independence and validity of these prognostic signals, along with the expression levels of key prognostic genes, were thoroughly validated in their study [234].
Subsequent to these developments, more researchers have endeavored to create prognostic models for sarcoma based on FRGs. For example, Lei T and his collaborators devised an innovative prognostic model specific to osteosarcoma [9]. Similarly, a prognostic model for Ewing sarcoma was successfully established, pinpointing three crucial genes, AURKA, RGS4, and RIPK1, that are intimately linked to disease prognosis [239]. As research efforts have expanded, an increasing number of key FRGs associated with sarcoma growth have been both identified and corroborated (Table 3 above). Furthermore, FRGs can serve as biomarkers to facilitate the screening of chemotherapeutic agents, thus aiding in the formulation of personalized chemotherapy strategies tailored to individual sarcoma patients [236, 239]. This same research strategy can also be applied to forecast tumor prognosis using ferroptosis-associated lncRNA genes [246].
GO and KEGG enrichment analyses have uncovered that FRGs are significantly enriched in pathways related to cancer and the immune system. Correlation analyses of key FRGs with immune checkpoint genes (ICGs) have revealed positive associations between the expression levels of CXCL2 and IL6, which are proteins encoded by FRGs, and the expression of immune factors [235]. RIPK1, a key FRG, has also been shown to be part of the same protein interaction network as immune checkpoints like PD-1 [239]. Furthermore, prognostic analyses based on FRGs have demonstrated that different risk groups exhibit varying patterns of immune cell infiltration, and individuals with more active immune cell involvement tend to have a better prognosis [9, 235, 237]. These findings suggest that FRG-encoded proteins can influence tumor prognosis by modulating the immune system, and further support the relationship between ferroptosis and tumor immune system.
Challenges and prospects
Indeed, while the potential of ferroptosis as a therapeutic approach in sarcoma treatment is promising, there are several critical issues that need to be addressed before its widespread clinical application.
Research perspectives on ferroptosis
Ferroptosis is a novel form of programmed cell death characterized by iron dependence and lipid peroxide accumulation, which differs from apoptosis, necrosis, and cellular autophagy. The activation of p53, a critical regulator of apoptosis, has been found to play a role in ferroptosis regulation. p53 can inhibit ferroptosis either by downregulating SLC7A11 expression or through the p53-p21 axis, indicating a connection between apoptosis and ferroptosis [46, 47, 90]. NCOA4-mediated ferritin autophagy can increase intracellular unstable iron levels, inducing cellular ferroptosis [30, 36, 247]. Interestingly, the ferroptosis inducer erastin can also promote ferritin autophagy, suggesting potential synergies between these two forms of cell death. Even whether ferroptosis is triggered by autophagy is a topic of debate in the scientific community, and further research is needed to clarify the relationship between these cell death modalities. Additionally, given the close ties between ferroptosis, the immune system, and lncRNA, there is potential for integrating these factors into a comprehensive regulatory network to better modulate cellular states and identify therapeutic targets.
Ferroptosis’s reliance on intracellular free iron is a defining feature. However, recent findings have revealed that copper, another crucial transition metal, can induce redox metabolism changes in cells similar to those seen with erastin-induced ferroptosis, by depleting GSH [248, 249]. Therefore, other metal ions, in addition to iron ions, have showed the potential to induce ferroptosis under specific conditions. This discovery raises questions about whether iron is necessary for ferroptosis and whether other metal ions or substances can disrupt intracellular redox balance and trigger ferroptosis. The emergence of cuproptosis underscores the potential for metal ions to influence cellular metabolism and cell death [250–253]. While cuproptosis and ferroptosis are currently understood to operate through distinct mechanisms, future research may uncover synergistic effects between them.
There is still much to explore in understanding the underlying mechanisms of ferroptosis, and further refinement of its description is necessary to support its clinical applications based on more comprehensive theories.
Clinical application and challenges of ferroptosis in the treatment of sarcoma
As previously mentioned, ferroptosis can currently be applied to sarcoma treatment through three primary approaches. The first and most common potential clinical application involves directly inducing ferroptosis in sarcoma cells, leading to their demise. Several ferroptosis inducers have been identified that function by depleting intracellular GSH, inactivating GPX4, activating the mevalonate pathway, and increasing intracellular lipid peroxidation and iron content [18, 171, 254]. In this context, FDA-approved drugs like sorafenib and octreotide have demonstrated their ability to induce ferroptosis in refractory cancers [18, 255]. Small-molecule drugs and proprietary Chinese medicines can also serve similar roles [11, 146, 160].The therapeutic significance of ferroptosis therapy is underscored by the observation that certain cancers inherently possess characteristics that make them sensitive to this form of cell death. For instance, rhabdomyosarcoma cells are susceptible to oxidative stress [151], while synovial sarcoma cells exhibit recurrent malic enzyme 1 expression deficiency [145], rendering them less tolerant to lipid peroxide accumulation and ferroptosis. Nevertheless, it’s important to note that not all cancer cells respond to ferroptosis inducers, and even well-characterized RAS-mutant cancer cell lines may not exhibit susceptibility [10, 45, 97]. Consequently, a key pending strategy is how to enhance tumor cell sensitivity to ferroptosis through epigenome editing, potentially paving the way for clinical applications of ferroptosis-based therapy. Alternatively, therapeutic effectiveness can be increased by exploiting tumor-specific characteristics. For instance, osteosarcoma cells display high expression of HMOX1, so EF24, which promotes HMOX1 expression, can be employed to expedite ferroptosis in these cells [11].
In addition to enhancing sensitivity, the targeting and absorption rate by cancer cells are factors that limit the clinical efficacy of ferroptosis-inducing treatments. Erastin, as the first discovered ferroptosis inducer, exhibited limited efficacy in clinical trials, largely due to its inefficient uptake by cancer cells [145, 256]. Combining sigma-2 ligands with demethylated erastin has proven to be a promising strategy as it significantly enhances drug targeting and uptake. This approach takes the sigma-2 receptor, which is typically overexpressed in solid tumor cells, as the drug target [257–263]. Furthermore, the utilization of nanocarriers can also improve the targeting of ferroptosis inducers while often presenting fewer toxic side effects compared to other administration methods [18, 197]. Moreover, it is essential to explore specific genetic markers or biomarkers associated with ferroptosis induction in both preclinical and clinical cancer settings. This research can aid in the identification of potential side effects related to ferroptosis treatments and help develop strategies to manage and enhance treatment safety.
Secondly, a promising strategy is the combination of ferroptosis inducers with other chemotherapeutic drugs, particularly for overcoming chemotherapy resistance, which is frequently encountered in sarcoma treatment, notably resistance to cisplatin. Additionally, the concurrent use of inducers targeting different cell death modalities, such as ferroptosis and apoptosis, holds the potential for enhanced antitumor effects. Consequently, it is crucial to carefully select suitable ferroptosis-inducing drugs, explore their combination therapies to mediate better effects, and lay a solid theoretical foundation for their clinical application.
Lastly, it’s worth noting that FRGs hold promise in predicting sarcoma prognosis and refining clinical staging, providing a direction for the future research of molecular targeted therapy of sarcoma [9, 235]. However, the lack of research on FRGs, the data set from retrospective study and the limited clinical variable data have affected the accuracy and practicability of this technology. Further research and data collection are needed to enhance their precision and clinical relevance.
Conclusion
In summary, gaining a comprehensive understanding of the mechanisms underlying ferroptosis is crucial for its effective application in treating various diseases. Ferroptosis holds significant promise in sarcoma treatment by effectively targeting sarcoma cells, reducing chemotherapy resistance, and aiding in prognosis prediction. Future research directions include exploring additional drugs and targets for inducing ferroptosis and identifying novel strategies to enhance sarcoma treatment. However, the field is currently hindered by the lack of well-established theoretical foundations and clinical trials, presenting challenges to the clinical use of ferroptosis inducers. Despite these obstacles, the potential in this area is immense, and further exploration is warranted.
Acknowledgements
Not applicable.
Abbreviations
- RSL
RAS-selective lethal small molecule
- LIP
Labile iron pool
- ROS
Reactive oxygen species
- PUFAs
Polyunsaturated fatty acids
- LOX
Lipoxygenase
- POR
Cytochrome P450 oxidoreductase
- TF
Transferrin
- TFRC
Transferrin receptor
- SLC40A1
Solute carrier family 40 member 1
- FPN
Ferroportin
- FAO
Fatty acid β-oxidation
- LPCAT3
Lysophosphatidylcholine acyltransferase-3
- ACSL4
Acyl-CoA synthetase long-chain family member 4
- PL-PUFAs
Phospholipid-bound polyunsaturated fatty acids
- AA
Arachidonic acid
- GSH
Glutathione
- GPX4
Glutathione peroxidase 4
- NADPH
Nicotinamide adenine dinucleotide phosphate
- FSP1
Ferroptosis suppressor protein 1
- CoQ10
Coenzyme Q10
- GCH1
GTP cyclohydrolase 1
- BH4
Tetrahydrobiopterin
- SLC7A11
Solute carrier family 7 member 11
- SLC3A2
Solute carrier family 3 member 2
- GCL
Glutamate-cysteine ligase
- Nrf2
Nuclear factor erythroid 2-related factor 2
- CARS1
Cysteinyl-tRNA synthetase 1
- HMG-CoA
3-Hydroxy-3-methylglutaryl-coenzyme A
- AIFM2
Apoptosis-inducing factor mitochondria-associated 2
- TCA
Tricarboxylic acid
- α-KG
α-Ketoglutarate
- ACSF2
Acyl CoA synthetase family member 2
- CS
Citrate synthase
- VDAC2/3
Mitochondrial resident voltage-dependent anion channel 2/3
- DPP4
Dipeptidyl peptidase-4
- AML
Acute myeloid leukemia
- NSCLC
Non-small-cell lung cancer
- PR-DUB
Polycomb-repressive deubiquitinase
- H2A
Histone 2A
- EMT
Epithelial-to-mesenchymal transition
- TME
Tumor microenvironment
- LPO
Lipid peroxides
- TAMs
Tumor-associated macrophages
- DCs
Dendritic cells
- DAMPs
Damage-related molecular patterns
- FRGs
Ferroptosis-related genes
- IDO
Indoleamine 2, 3-dioxygenase
- p-STAT3
Phosphorylated signal transducer and activator of transcription 3
- FAC
Ferric ammonium citrate
- FAS
Ferrous ammonium sulfate
- PEITC
Phenethyl isothiocyanate
- HMOX1
Heme oxygenase 1
- FANCD2
Fanconi anemia complementation group D2
- miRNA
MicroRNA
- lncRNA
Long non-coding RNA
- PDT
Photodynamic therapy
- MPPa-PDT
Pyropheophorbide-α methyl ester-mediated PDT
- YAP
Yes-associated protein
- RMS
Rhabdomyosarcoma
- ERK
Extracellular signal-regulated kinase
- PKC
Protein Kinase C
- NOX
NADPH Oxidase
- ES
Ewing sarcoma
- AURKA
Aurora kinase A
- CA
Carbonic anhydrase
- IDH
Isocitrate dehydrogenase
- HSF1
Heat shock factor 1
- BMDCs
Bone marrow-derived dendritic cells
- MDSCs
Myeloid-derived suppressor cells
- PLGA
Poly (lactic-co-glycolic) acid
- UA
Ursolic acid
- STS
Soft tissue sarcoma
- ICGs
Immune checkpoint genes
Author contributions
JZ, ZJL and TL contributed to conceive, design and revision of the manuscript sections. JZ wrote the manuscript. JZ and YZ designed figures and created Tables. XHZ, ZJL, JY, PCD and TL supervised the manuscript by providing critical feedbacks and revisions. The authors read and approved the final manuscript.
Funding
This study was supported by grants from National Natural Science Foundation of China (No. 82072441), Hunan Outstanding Youth Fund (No. 2022JJ10095), Natural Science Foundation of Xinjiang Uygur Autonomous Region (No. 2022D01C565) and Natural Science Foundation of Changsha, China (No. kq2202402).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors consent to publication.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Beird HC, Bielack SS, Flanagan AM, Gill J, Heymann D, Janeway KA, Livingston JA, Roberts RD, Strauss SJ, Gorlick R. Osteosarcoma. Nat Rev Dis Primers. 2022;8:77. doi: 10.1038/s41572-022-00409-y. [DOI] [PubMed] [Google Scholar]
- 2.Fathizadeh H, Mirzaei H, Asemi Z. Melatonin: an anti-tumor agent for osteosarcoma. Cancer Cell Int. 2019;19:319. doi: 10.1186/s12935-019-1044-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gamboa AC, Gronchi A, Cardona K. Soft-tissue sarcoma in adults: an update on the current state of histiotype-specific management in an era of personalized medicine. CA Cancer J Clin. 2020;70:200–229. doi: 10.3322/caac.21605. [DOI] [PubMed] [Google Scholar]
- 4.Meyer M, Seetharam M. First-line therapy for metastatic soft tissue sarcoma. Curr Treat Options Oncol. 2019;20:6. doi: 10.1007/s11864-019-0606-9. [DOI] [PubMed] [Google Scholar]
- 5.Meltzer PS, Helman LJ. New Horizons in the Treatment of osteosarcoma. N Engl J Med. 2021;385:2066–2076. doi: 10.1056/NEJMra2103423. [DOI] [PubMed] [Google Scholar]
- 6.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirschhorn T, Stockwell BR. The development of the concept of ferroptosis. Free Radic Biol Med. 2019;133:130–143. doi: 10.1016/j.freeradbiomed.2018.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N, Sun B, Wang G. Ferroptosis: past, present and future. Cell Death Dis. 2020;11:88. doi: 10.1038/s41419-020-2298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lei T, Qian H, Lei P, Hu Y. Ferroptosis-related gene signature associates with immunity and predicts prognosis accurately in patients with osteosarcoma. Cancer Sci. 2021;112:4785–4798. doi: 10.1111/cas.15131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo J, Xu B, Han Q, Zhou H, Xia Y, Gong C, Dai X, Li Z, Wu G. Ferroptosis: A Novel Anti-tumor Action for Cisplatin. Cancer Res Treat. 2018;50:445–460. doi: 10.4143/crt.2016.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin H, Chen X, Zhang C, Yang T, Deng Z, Song Y, Huang L, Li F, Li Q, Lin S, Jin D. EF24 induces ferroptosis in osteosarcoma cells through HMOX1. Biomed Pharmacother. 2021;136:111202. doi: 10.1016/j.biopha.2020.111202. [DOI] [PubMed] [Google Scholar]
- 12.Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31:107–125. doi: 10.1038/s41422-020-00441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morales M, Xue X. Targeting iron metabolism in cancer therapy. Theranostics. 2021;11:8412–8429. doi: 10.7150/thno.59092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egyed A, Saltman P. Iron is maintained as Fe(II) under aerobic conditions in erythroid cells. Biol Trace Elem Res. 1984;6:357–364. doi: 10.1007/BF02989243. [DOI] [PubMed] [Google Scholar]
- 15.Breuer W, Epsztejn S, Cabantchik ZI. Iron acquired from transferrin by K562 cells is delivered into a cytoplasmic pool of chelatable iron(II) J Biol Chem. 1995;270:24209–24215. doi: 10.1074/jbc.270.41.24209. [DOI] [PubMed] [Google Scholar]
- 16.García-Yébenes I, Sobrado M, Moraga A, Zarruk JG, Romera VG, Pradillo JM. Perez de la Ossa N, Moro MA, Dávalos A, Lizasoain I: iron overload, measured as serum ferritin, increases brain damage induced by focal ischemia and early reperfusion. Neurochem Int. 2012;61:1364–1369. doi: 10.1016/j.neuint.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 17.Stoyanovsky DA, Tyurina YY, Shrivastava I, Bahar I, Tyurin VA, Protchenko O, Jadhav S, Bolevich SB, Kozlov AV, Vladimirov YA, et al. Iron catalysis of lipid peroxidation in ferroptosis: Regulated enzymatic or random free radical reaction? Free Radic Biol Med. 2019;133:153–161. doi: 10.1016/j.freeradbiomed.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hassannia B, Vandenabeele P, Vanden Berghe T. Targeting ferroptosis to iron out cancer. Cancer Cell. 2019;35:830–849. doi: 10.1016/j.ccell.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Gaschler MM, Stockwell BR. Lipid peroxidation in cell death. Biochem Biophys Res Commun. 2017;482:419–425. doi: 10.1016/j.bbrc.2016.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah R, Shchepinov MS, Pratt DA. Resolving the role of lipoxygenases in the initiation and execution of ferroptosis. ACS Cent Sci. 2018;4:387–396. doi: 10.1021/acscentsci.7b00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS, Dar HH, Liu B, Tyurin VA, Ritov VB, et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13:81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li QQ, Li Q, Jia JN, Liu ZQ, Zhou HH, Mao XY. 12/15 lipoxygenase: a crucial enzyme in diverse types of cell death. Neurochem Int. 2018;118:34–41. doi: 10.1016/j.neuint.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 23.NaveenKumar SK, Hemshekhar M, Kemparaju K, Girish KS. Hemin-induced platelet activation and ferroptosis is mediated through ROS-driven proteasomal activity and inflammasome activation: Protection by Melatonin. Biochim Biophys Acta Mol Basis Dis. 2019;1865:2303–2316. doi: 10.1016/j.bbadis.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Zou Y, Li H, Graham ET, Deik AA, Eaton JK, Wang W, Sandoval-Gomez G, Clish CB, Doench JG, Schreiber SL. Cytochrome P450 oxidoreductase contributes to phospholipid peroxidation in ferroptosis. Nat Chem Biol. 2020;16:302–309. doi: 10.1038/s41589-020-0472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma S, Henson ES, Chen Y, Gibson SB. Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells. Cell Death Dis. 2016;7:e2307. doi: 10.1038/cddis.2016.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and Transferrin Regulate Ferroptosis. Mol Cell. 2015;59:298–308. doi: 10.1016/j.molcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang WS, Stockwell BR. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol. 2008;15:234–245. doi: 10.1016/j.chembiol.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manz DH, Blanchette NL, Paul BT, Torti FM, Torti SV. Iron and cancer: recent insights. Ann N Y Acad Sci. 2016;1368:149–161. doi: 10.1111/nyas.13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang YQ, Chang SY, Wu Q, Gou YJ, Jia L, Cui YM, Yu P, Shi ZH, Wu WS, Gao G, Chang YZ. The protective role of mitochondrial ferritin on erastin-induced ferroptosis. Front Aging Neurosci. 2016;8:308. doi: 10.3389/fnagi.2016.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ, 3rd, Kang R, Tang D. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12:1425–1428. doi: 10.1080/15548627.2016.1187366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao M, Monian P, Pan Q, Zhang W, Xiang J, Jiang X. Ferroptosis is an autophagic cell death process. Cell Res. 2016;26:1021–1032. doi: 10.1038/cr.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown CW, Amante JJ, Chhoy P, Elaimy AL, Liu H, Zhu LJ, Baer CE, Dixon SJ, Mercurio AM. Prominin2 drives ferroptosis resistance by stimulating iron export. Dev Cell. 2019;51:575–586.e574. doi: 10.1016/j.devcel.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geng N, Shi BJ, Li SL, Zhong ZY, Li YC, Xua WL, Zhou H, Cai JH. Knockdown of ferroportin accelerates erastin-induced ferroptosis in neuroblastoma cells. Eur Rev Med Pharmacol Sci. 2018;22:3826–3836. doi: 10.26355/eurrev_201806_15267. [DOI] [PubMed] [Google Scholar]
- 34.Chen X, Li J, Kang R, Klionsky DJ, Tang D. Ferroptosis: machinery and regulation. Autophagy. 2021;17:2054–2081. doi: 10.1080/15548627.2020.1810918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seibt TM, Proneth B, Conrad M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic Biol Med. 2019;133:144–152. doi: 10.1016/j.freeradbiomed.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 36.Latunde-Dada GO. Ferroptosis: Role of lipid peroxidation, iron and ferritinophagy. Biochim Biophys Acta Gen Subj. 2017;1861:1893–1900. doi: 10.1016/j.bbagen.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 37.Tesfay L, Paul BT, Konstorum A, Deng Z, Cox AO, Lee J, Furdui CM, Hegde P, Torti FM, Torti SV. Stearoyl-CoA desaturase 1 protects ovarian cancer cells from ferroptotic cell death. Cancer Res. 2019;79:5355–5366. doi: 10.1158/0008-5472.CAN-19-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang Y, Mao C, Yang R, Yan B, Shi Y, Liu X, Lai W, Liu Y, Wang X, Xiao D, et al. EGLN1/c-myc induced lymphoid-specific helicase inhibits ferroptosis through lipid metabolic gene expression changes. Theranostics. 2017;7:3293–3305. doi: 10.7150/thno.19988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Magtanong L, Ko PJ, To M, Cao JY, Forcina GC, Tarangelo A, Ward CC, Cho K, Patti GJ, Nomura DK, et al. Exogenous monounsaturated fatty acids promote a ferroptosis-resistant cell state. Cell Chem Biol. 2019;26:420–432.e429. doi: 10.1016/j.chembiol.2018.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dixon SJ, Winter GE, Musavi LS, Lee ED, Snijder B, Rebsamen M, Superti-Furga G, Stockwell BR. Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chem Biol. 2015;10:1604–1609. doi: 10.1021/acschembio.5b00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13:91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan H, Li X, Zhang X, Kang R, Tang D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun. 2016;478:1338–1343. doi: 10.1016/j.bbrc.2016.08.124. [DOI] [PubMed] [Google Scholar]
- 43.Chu B, Kon N, Chen D, Li T, Liu T, Jiang L, Song S, Tavana O, Gu W. ALOX12 is required for p53-mediated tumour suppression through a distinct ferroptosis pathway. Nat Cell Biol. 2019;21:579–591. doi: 10.1038/s41556-019-0305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stockwell BR, Jiang X, Gu W. Emerging mechanisms and disease relevance of ferroptosis. Trends Cell Biol. 2020;30:478–490. doi: 10.1016/j.tcb.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao JY, Dixon SJ. Mechanisms of ferroptosis. Cell Mol Life Sci. 2016;73:2195–2209. doi: 10.1007/s00018-016-2194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang L, Hickman JH, Wang SJ, Gu W. Dynamic roles of p53-mediated metabolic activities in ROS-induced stress responses. Cell Cycle. 2015;14:2881–2885. doi: 10.1080/15384101.2015.1068479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, Baer R, Gu W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Badgley MA, Kremer DM, Maurer HC, DelGiorno KE, Lee HJ, Purohit V, Sagalovskiy IR, Ma A, Kapilian J, Firl CEM, et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science. 2020;368:85–89. doi: 10.1126/science.aaw9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang F, Lv H, Zhao B, Zhou L, Wang S, Luo J, Liu J, Shang P. Iron and leukemia: new insights for future treatments. J Exp Clin Cancer Res. 2019;38:406. doi: 10.1186/s13046-019-1397-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.El Hout M, Dos Santos L, Hamaï A, Mehrpour M. A promising new approach to cancer therapy: Targeting iron metabolism in cancer stem cells. Semin Cancer Biol. 2018;53:125–138. doi: 10.1016/j.semcancer.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 51.Xu T, Ding W, Ji X, Ao X, Liu Y, Yu W, Wang J. Molecular mechanisms of ferroptosis and its role in cancer therapy. J Cell Mol Med. 2019;23:4900–4912. doi: 10.1111/jcmm.14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Z, Lv X, Song E, Song Y. Fostered Nrf2 expression antagonizes iron overload and glutathione depletion to promote resistance of neuron-like cells to ferroptosis. Toxicol Appl Pharmacol. 2020;407:115241. doi: 10.1016/j.taap.2020.115241. [DOI] [PubMed] [Google Scholar]
- 53.Kang YP, Mockabee-Macias A, Jiang C, Falzone A, Prieto-Farigua N, Stone E, Harris IS, DeNicola GM. Non-canonical Glutamate-Cysteine Ligase Activity Protects against Ferroptosis. Cell Metab. 2021;33:174–189.e177. doi: 10.1016/j.cmet.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cao JY, Poddar A, Magtanong L, Lumb JH, Mileur TR, Reid MA, Dovey CM, Wang J, Locasale JW, Stone E, et al. A Genome-wide Haploid Genetic Screen Identifies Regulators of Glutathione Abundance and Ferroptosis Sensitivity. Cell Rep. 2019;26:1544–1556.e1548. doi: 10.1016/j.celrep.2019.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hayano M, Yang WS, Corn CK, Pagano NC, Stockwell BR. Loss of cysteinyl-tRNA synthetase (CARS) induces the transsulfuration pathway and inhibits ferroptosis induced by cystine deprivation. Cell Death Differ. 2016;23:270–278. doi: 10.1038/cdd.2015.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang WS, Stockwell BR. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016;26:165–176. doi: 10.1016/j.tcb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sui X, Zhang R, Liu S, Duan T, Zhai L, Zhang M, Han X, Xiang Y, Huang X, Lin H, Xie T. RSL3 Drives Ferroptosis Through GPX4 Inactivation and ROS Production in Colorectal Cancer. Front Pharmacol. 2018;9:1371. doi: 10.3389/fphar.2018.01371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alim I, Caulfield JT, Chen Y, Swarup V, Geschwind DH, Ivanova E, Seravalli J, Ai Y, Sansing LH, Ste Marie EJ, et al. Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell. 2019;177:1262–1279.e1225. doi: 10.1016/j.cell.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 59.Ingold I, Berndt C, Schmitt S, Doll S, Poschmann G, Buday K, Roveri A, Peng X, Porto Freitas F, Seibt T, et al. Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell. 2018;172:409–422.e421. doi: 10.1016/j.cell.2017.11.048. [DOI] [PubMed] [Google Scholar]
- 60.Friedmann Angeli JP, Conrad M. Selenium and GPX4, a vital symbiosis. Free Radic Biol Med. 2018;127:153–159. doi: 10.1016/j.freeradbiomed.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 61.Shimada K, Skouta R, Kaplan A, Yang WS, Hayano M, Dixon SJ, Brown LM, Valenzuela CA, Wolpaw AJ, Stockwell BR. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat Chem Biol. 2016;12:497–503. doi: 10.1038/nchembio.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I, Goya Grocin A, Xavier da Silva TN, Panzilius E, Scheel CH, et al: FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019, 575:693–698. [DOI] [PubMed]
- 63.Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nakamura T, Hipp C, Santos Dias Mourão A, Borggräfe J, Aldrovandi M, Henkelmann B, Wanninger J, Mishima E, Lytton E, Emler D, et al: Phase separation of FSP1 promotes ferroptosis. Nature 2023, 619:371–377. [DOI] [PMC free article] [PubMed]
- 65.Kraft VAN, Bezjian CT, Pfeiffer S, Ringelstetter L, Müller C, Zandkarimi F, Merl-Pham J, Bao X, Anastasov N, Kössl J, et al. GTP cyclohydrolase 1/tetrahydrobiopterin counteract ferroptosis through lipid remodeling. ACS Cent Sci. 2020;6:41–53. doi: 10.1021/acscentsci.9b01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fang X, Wang H, Han D, Xie E, Yang X, Wei J, Gu S, Gao F, Zhu N, Yin X, et al. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci U S A. 2019;116:2672–2680. doi: 10.1073/pnas.1821022116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gao M, Yi J, Zhu J, Minikes AM, Monian P, Thompson CB, Jiang X. Role of mitochondria in ferroptosis. Mol Cell. 2019;73:354–363.e353. doi: 10.1016/j.molcel.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang C, Ko B, Hensley CT, Jiang L, Wasti AT, Kim J, Sudderth J, Calvaruso MA, Lumata L, Mitsche M, et al. Glutamine oxidation maintains the TCA cycle and cell survival during impaired mitochondrial pyruvate transport. Mol Cell. 2014;56:414–424. doi: 10.1016/j.molcel.2014.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yagoda N, von Rechenberg M, Zaganjor E, Bauer AJ, Yang WS, Fridman DJ, Wolpaw AJ, Smukste I, Peltier JM, Boniface JJ, et al. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. 2007;447:864–868. doi: 10.1038/nature05859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang Y, Luo M, Zhang K, Zhang J, Gao T, Connell DO, Yao F, Mu C, Cai B, Shang Y, Chen W. Nedd4 ubiquitylates VDAC2/3 to suppress erastin-induced ferroptosis in melanoma. Nat Commun. 2020;11:433. doi: 10.1038/s41467-020-14324-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Linkermann A, Skouta R, Himmerkus N, Mulay SR, Dewitz C, De Zen F, Prokai A, Zuchtriegel G, Krombach F, Welz PS, et al. Synchronized renal tubular cell death involves ferroptosis. Proc Natl Acad Sci U S A. 2014;111:16836–16841. doi: 10.1073/pnas.1415518111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim SE, Zhang L, Ma K, Riegman M, Chen F, Ingold I, Conrad M, Turker MZ, Gao M, Jiang X, et al. Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nat Nanotechnol. 2016;11:977–985. doi: 10.1038/nnano.2016.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Riegman M, Sagie L, Galed C, Levin T, Steinberg N, Dixon SJ, Wiesner U, Bradbury MS, Niethammer P, Zaritsky A, Overholtzer M. Ferroptosis occurs through an osmotic mechanism and propagates independently of cell rupture. Nat Cell Biol. 2020;22:1042–1048. doi: 10.1038/s41556-020-0565-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ros U, Peña-Blanco A, Hänggi K, Kunzendorf U, Krautwald S, Wong WW, García-Sáez AJ. Necroptosis execution is mediated by plasma membrane nanopores independent of calcium. Cell Rep. 2017;19:175–187. doi: 10.1016/j.celrep.2017.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8:1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 76.Agmon E, Solon J, Bassereau P, Stockwell BR. Modeling the effects of lipid peroxidation during ferroptosis on membrane properties. Sci Rep. 2018;8:5155. doi: 10.1038/s41598-018-23408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Runas KA, Acharya SJ, Schmidt JJ, Malmstadt N. Addition of cleaved tail fragments during lipid oxidation stabilizes membrane permeability behavior. Langmuir. 2016;32:779–786. doi: 10.1021/acs.langmuir.5b02980. [DOI] [PubMed] [Google Scholar]
- 78.Katikaneni A, Jelcic M, Gerlach GF, Ma Y, Overholtzer M, Niethammer P. Lipid peroxidation regulates long-range wound detection through 5-lipoxygenase in zebrafish. Nat Cell Biol. 2020;22:1049–1055. doi: 10.1038/s41556-020-0564-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen X, Kang R, Kroemer G, Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol. 2021;18:280–296. doi: 10.1038/s41571-020-00462-0. [DOI] [PubMed] [Google Scholar]
- 80.Bykov VJN, Eriksson SE, Bianchi J, Wiman KG. Targeting mutant p53 for efficient cancer therapy. Nat Rev Cancer. 2018;18:89–102. doi: 10.1038/nrc.2017.109. [DOI] [PubMed] [Google Scholar]
- 81.Hassin O, Oren M. Drugging p53 in cancer: one protein, many targets. Nat Rev Drug Discov. 2023;22:127–144. doi: 10.1038/s41573-022-00571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 83.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/S0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 84.Li T, Kon N, Jiang L, Tan M, Ludwig T, Zhao Y, Baer R, Gu W. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell. 2012;149:1269–1283. doi: 10.1016/j.cell.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu DS, Duong CP, Haupt S, Montgomery KG, House CM, Azar WJ, Pearson HB, Fisher OM, Read M, Guerra GR, et al. Inhibiting the system x(C)(−)/glutathione axis selectively targets cancers with mutant-p53 accumulation. Nat Commun. 2017;8:14844. doi: 10.1038/ncomms14844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ou Y, Wang SJ, Li D, Chu B, Gu W. Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc Natl Acad Sci U S A. 2016;113:E6806–e6812. doi: 10.1073/pnas.1607152113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang Y, Qian Y, Zhang J, Yan W, Jung YS, Chen M, Huang E, Lloyd K, Duan Y, Wang J, et al. Ferredoxin reductase is critical for p53-dependent tumor suppression via iron regulatory protein 2. Genes Dev. 2017;31:1243–1256. doi: 10.1101/gad.299388.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kruiswijk F, Labuschagne CF, Vousden KH. p53 in survival, death and metabolic health: a lifeguard with a licence to kill. Nat Rev Mol Cell Biol. 2015;16:393–405. doi: 10.1038/nrm4007. [DOI] [PubMed] [Google Scholar]
- 89.Xie Y, Zhu S, Song X, Sun X, Fan Y, Liu J, Zhong M, Yuan H, Zhang L, Billiar TR, et al. The tumor suppressor p53 limits ferroptosis by blocking DPP4 activity. Cell Rep. 2017;20:1692–1704. doi: 10.1016/j.celrep.2017.07.055. [DOI] [PubMed] [Google Scholar]
- 90.Tarangelo A, Magtanong L, Bieging-Rolett KT, Li Y, Ye J, Attardi LD, Dixon SJ. p53 suppresses metabolic stress-induced ferroptosis in cancer cells. Cell Rep. 2018;22:569–575. doi: 10.1016/j.celrep.2017.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ryan MB, Corcoran RB. Therapeutic strategies to target RAS-mutant cancers. Nat Rev Clin Oncol. 2018;15:709–720. doi: 10.1038/s41571-018-0105-0. [DOI] [PubMed] [Google Scholar]
- 92.Codenotti S, Poli M, Asperti M, Zizioli D, Marampon F, Fanzani A. Cell growth potential drives ferroptosis susceptibility in rhabdomyosarcoma and myoblast cell lines. J Cancer Res Clin Oncol. 2018;144:1717–1730. doi: 10.1007/s00432-018-2699-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lu R, Jiang Y, Lai X, Liu S, Sun L, Zhou ZW. A shortage of FTH induces ROS and sensitizes RAS-proficient neuroblastoma N2A cells to ferroptosis. Int J Mol Sci. 2021;22:8898. doi: 10.3390/ijms22168898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI, Falchook GS, Price TJ, Sacher A, Denlinger CS, et al. KRAS(G12C) inhibition with sotorasib in advanced solid tumors. N Engl J Med. 2020;383:1207–1217. doi: 10.1056/NEJMoa1917239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hallin J, Engstrom LD, Hargis L, Calinisan A, Aranda R, Briere DM, Sudhakar N, Bowcut V, Baer BR, Ballard JA, et al. The KRAS(G12C) inhibitor MRTX849 provides insight toward therapeutic susceptibility of kras-mutant cancers in mouse models and patients. Cancer Discov. 2020;10:54–71. doi: 10.1158/2159-8290.CD-19-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schott C, Graab U, Cuvelier N, Hahn H, Fulda S. Oncogenic RAS mutants confer resistance of RMS13 rhabdomyosarcoma cells to oxidative stress-induced ferroptotic cell death. Front Oncol. 2015;5:131. doi: 10.3389/fonc.2015.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Carbone M, Yang H, Pass HI, Krausz T, Testa JR, Gaudino G. BAP1 and cancer. Nat Rev Cancer. 2013;13:153–159. doi: 10.1038/nrc3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ventii KH, Devi NS, Friedrich KL, Chernova TA, Tighiouart M, Van Meir EG, Wilkinson KD. BRCA1-associated protein-1 is a tumor suppressor that requires deubiquitinating activity and nuclear localization. Cancer Res. 2008;68:6953–6962. doi: 10.1158/0008-5472.CAN-08-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang Y, Shi J, Liu X, Feng L, Gong Z, Koppula P, Sirohi K, Li X, Wei Y, Lee H, et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat Cell Biol. 2018;20:1181–1192. doi: 10.1038/s41556-018-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jiao Y, Pawlik TM, Anders RA, Selaru FM, Streppel MM, Lucas DJ, Niknafs N, Guthrie VB, Maitra A, Argani P, et al. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat Genet. 2013;45:1470–1473. doi: 10.1038/ng.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wiesner T, Obenauf AC, Murali R, Fried I, Griewank KG, Ulz P, Windpassinger C, Wackernagel W, Loy S, Wolf I, et al. Germline mutations in BAP1 predispose to melanocytic tumors. Nat Genet. 2011;43:1018–1021. doi: 10.1038/ng.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Abdel-Rahman MH, Pilarski R, Cebulla CM, Massengill JB, Christopher BN, Boru G, Hovland P, Davidorf FH. Germline BAP1 mutation predisposes to uveal melanoma, lung adenocarcinoma, meningioma, and other cancers. J Med Genet. 2011;48:856–859. doi: 10.1136/jmedgenet-2011-100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rojo de la Vega M, Chapman E, Zhang DD: NRF2 and the Hallmarks of Cancer. Cancer Cell 2018, 34:21–43. [DOI] [PMC free article] [PubMed]
- 105.Lignitto L, LeBoeuf SE, Homer H, Jiang S, Askenazi M, Karakousi TR, Pass HI, Bhutkar AJ, Tsirigos A, Ueberheide B, et al. Nrf2 activation promotes lung cancer metastasis by inhibiting the degradation of Bach1. Cell. 2019;178:316–329.e318. doi: 10.1016/j.cell.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Anandhan A, Dodson M, Schmidlin CJ, Liu P, Zhang DD. Breakdown of an ironclad defense system: the critical role of NRF2 in mediating ferroptosis. Cell Chem Biol. 2020;27:436–447. doi: 10.1016/j.chembiol.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jing X, Yang F, Shao C, Wei K, Xie M, Shen H, Shu Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol Cancer. 2019;18:157. doi: 10.1186/s12943-019-1089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang M, Chen P, Liu J, Zhu S, Kroemer G, Klionsky DJ, Lotze MT, Zeh HJ, Kang R, Tang D: Clockophagy is a novel selective autophagy process favoring ferroptosis. Sci Adv 2019, 5:eaaw2238. [DOI] [PMC free article] [PubMed]
- 109.Zou Y, Palte MJ, Deik AA, Li H, Eaton JK, Wang W, Tseng YY, Deasy R, Kost-Alimova M, Dančík V, et al. A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nat Commun. 2019;10:1617. doi: 10.1038/s41467-019-09277-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Viswanathan VS, Ryan MJ, Dhruv HD, Gill S, Eichhoff OM, Seashore-Ludlow B, Kaffenberger SD, Eaton JK, Shimada K, Aguirre AJ, et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature. 2017;547:453–457. doi: 10.1038/nature23007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bi J, Yang S, Li L, Dai Q, Borcherding N, Wagner BA, Buettner GR, Spitz DR, Leslie KK, Zhang J, Meng X. Metadherin enhances vulnerability of cancer cells to ferroptosis. Cell Death Dis. 2019;10:682. doi: 10.1038/s41419-019-1897-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang WH, Ding CC, Sun T, Rupprecht G, Lin CC, Hsu D, Chi JT. The Hippo Pathway Effector TAZ Regulates Ferroptosis in Renal Cell Carcinoma. Cell Rep. 2019;28:2501–2508.e2504. doi: 10.1016/j.celrep.2019.07.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu J, Minikes AM, Gao M, Bian H, Li Y, Stockwell BR, Chen ZN, Jiang X. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature. 2019;572:402–406. doi: 10.1038/s41586-019-1426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wenz C, Faust D, Linz B, Turmann C, Nikolova T, Dietrich C. Cell-cell contacts protect against t-BuOOH-induced cellular damage and ferroptosis in vitro. Arch Toxicol. 2019;93:1265–1279. doi: 10.1007/s00204-019-02413-w. [DOI] [PubMed] [Google Scholar]
- 116.Pitt JM, Marabelle A, Eggermont A, Soria JC, Kroemer G, Zitvogel L. Targeting the tumor microenvironment: removing obstruction to anticancer immune responses and immunotherapy. Ann Oncol. 2016;27:1482–1492. doi: 10.1093/annonc/mdw168. [DOI] [PubMed] [Google Scholar]
- 117.Elhanani O, Ben-Uri R, Keren L. Spatial profiling technologies illuminate the tumor microenvironment. Cancer Cell. 2023;41:404–420. doi: 10.1016/j.ccell.2023.01.010. [DOI] [PubMed] [Google Scholar]
- 118.Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Hedrick CC, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kishton RJ, Sukumar M, Restifo NP. Metabolic Regulation of T Cell Longevity and Function in Tumor Immunotherapy. Cell Metab. 2017;26:94–109. doi: 10.1016/j.cmet.2017.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Muri J, Kopf M. Redox regulation of immunometabolism. Nat Rev Immunol. 2021;21:363–381. doi: 10.1038/s41577-020-00478-8. [DOI] [PubMed] [Google Scholar]
- 121.Xu H, Ye D, Ren M, Zhang H, Bi F. Ferroptosis in the tumor microenvironment: perspectives for immunotherapy. Trends Mol Med. 2021;27:856–867. doi: 10.1016/j.molmed.2021.06.014. [DOI] [PubMed] [Google Scholar]
- 122.Kim R, Taylor D, Vonderheide RH, Gabrilovich DI. Ferroptosis of immune cells in the tumor microenvironment. Trends Pharmacol Sci. 2023;44:542–552. doi: 10.1016/j.tips.2023.06.005. [DOI] [PubMed] [Google Scholar]
- 123.Madden MZ, Rathmell JC. The Complex Integration of T-cell Metabolism and Immunotherapy. Cancer Discov. 2021;11:1636–1643. doi: 10.1158/2159-8290.CD-20-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Matsushita M, Freigang S, Schneider C, Conrad M, Bornkamm GW, Kopf M. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J Exp Med. 2015;212:555–568. doi: 10.1084/jem.20140857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Drijvers JM, Gillis JE, Muijlwijk T, Nguyen TH, Gaudiano EF, Harris IS, LaFleur MW, Ringel AE, Yao CH, Kurmi K, et al. Pharmacologic Screening Identifies Metabolic Vulnerabilities of CD8(+) T Cells. Cancer Immunol Res. 2021;9:184–199. doi: 10.1158/2326-6066.CIR-20-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Costa A, Scholer-Dahirel A, Mechta-Grigoriou F. The role of reactive oxygen species and metabolism on cancer cells and their microenvironment. Semin Cancer Biol. 2014;25:23–32. doi: 10.1016/j.semcancer.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 127.Xu C, Sun S, Johnson T, Qi R, Zhang S, Zhang J, Yang K. The glutathione peroxidase Gpx4 prevents lipid peroxidation and ferroptosis to sustain Treg cell activation and suppression of antitumor immunity. Cell Rep. 2021;35:109235. doi: 10.1016/j.celrep.2021.109235. [DOI] [PubMed] [Google Scholar]
- 128.Kapralov AA, Yang Q, Dar HH, Tyurina YY, Anthonymuthu TS, Kim R, St Croix CM, Mikulska-Ruminska K, Liu B, Shrivastava IH, et al. Redox lipid reprogramming commands susceptibility of macrophages and microglia to ferroptotic death. Nat Chem Biol. 2020;16:278–290. doi: 10.1038/s41589-019-0462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Luo X, Gong HB, Gao HY, Wu YP, Sun WY, Li ZQ, Wang G, Liu B, Liang L, Kurihara H, et al. Oxygenated phosphatidylethanolamine navigates phagocytosis of ferroptotic cells by interacting with TLR2. Cell Death Differ. 2021;28:1971–1989. doi: 10.1038/s41418-020-00719-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Herber DL, Cao W, Nefedova Y, Novitskiy SV, Nagaraj S, Tyurin VA, Corzo A, Cho HI, Celis E, Lennox B, et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med. 2010;16:880–886. doi: 10.1038/nm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Veglia F, Tyurin VA, Mohammadyani D, Blasi M, Duperret EK, Donthireddy L, Hashimoto A, Kapralov A, Amoscato A, Angelini R, et al. Lipid bodies containing oxidatively truncated lipids block antigen cross-presentation by dendritic cells in cancer. Nat Commun. 2017;8:2122. doi: 10.1038/s41467-017-02186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ramakrishnan R, Tyurin VA, Veglia F, Condamine T, Amoscato A, Mohammadyani D, Johnson JJ, Zhang LM, Klein-Seetharaman J, Celis E, et al. Oxidized lipids block antigen cross-presentation by dendritic cells in cancer. J Immunol. 2014;192:2920–2931. doi: 10.4049/jimmunol.1302801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Poznanski SM, Singh K, Ritchie TM, Aguiar JA, Fan IY, Portillo AL, Rojas EA, Vahedi F, El-Sayes A, Xing S, et al. Metabolic flexibility determines human NK cell functional fate in the tumor microenvironment. Cell Metab. 2021;33:1205–1220.e1205. doi: 10.1016/j.cmet.2021.03.023. [DOI] [PubMed] [Google Scholar]
- 134.Tang D, Kepp O, Kroemer G. Ferroptosis becomes immunogenic: implications for anticancer treatments. Oncoimmunology. 2020;10:1862949. doi: 10.1080/2162402X.2020.1862949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Efimova I, Catanzaro E, Van der Meeren L, Turubanova VD, Hammad H, Mishchenko TA, Vedunova MV, Fimognari C, Bachert C, Coppieters F, et al. Vaccination with early ferroptotic cancer cells induces efficient antitumor immunity. J Immunother Cancer. 2020;8:e001369. doi: 10.1136/jitc-2020-001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dai E, Han L, Liu J, Xie Y, Kroemer G, Klionsky DJ, Zeh HJ, Kang R, Wang J, Tang D. Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy. 2020;16:2069–2083. doi: 10.1080/15548627.2020.1714209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang W, Green M, Choi JE, Gijón M, Kennedy PD, Johnson JK, Liao P, Lang X, Kryczek I, Sell A, et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569:270–274. doi: 10.1038/s41586-019-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tap WD, Wagner AJ, Schöffski P, Martin-Broto J, Krarup-Hansen A, Ganjoo KN, Yen CC, Abdul Razak AR, Spira A, Kawai A, et al. Effect of doxorubicin plus olaratumab vs doxorubicin plus placebo on survival in patients with advanced soft tissue sarcomas: the ANNOUNCE randomized clinical trial. JAMA. 2020;323:1266–1276. doi: 10.1001/jama.2020.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Seddon B, Strauss SJ, Whelan J, Leahy M, Woll PJ, Cowie F, Rothermundt C, Wood Z, Benson C, Ali N, et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): a randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1397–1410. doi: 10.1016/S1470-2045(17)30622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Klemen ND, Kelly CM, Bartlett EK. The emerging role of immunotherapy for the treatment of sarcoma. J Surg Oncol. 2021;123:730–738. doi: 10.1002/jso.26306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, Kang R, Tang D. Ferroptosis: process and function. Cell Death Differ. 2016;23:369–379. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kang R, Kroemer G, Tang D. The tumor suppressor protein p53 and the ferroptosis network. Free Radic Biol Med. 2019;133:162–168. doi: 10.1016/j.freeradbiomed.2018.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Moloney JN, Cotter TG. ROS signalling in the biology of cancer. Semin Cell Dev Biol. 2018;80:50–64. doi: 10.1016/j.semcdb.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 144.Horii S, Mori S, Ogata R, Nukaga S, Nishida R, Kishi S, Sasaki R, Ikemoto A, Owari T, Maesaka F, et al. 5-Aminolevrinic Acid Exhibits Dual Effects on Stemness in Human Sarcoma Cell Lines under Dark Conditions. Int J Mol Sci. 2023;24:6189. doi: 10.3390/ijms24076189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Brashears CB, Prudner BC, Rathore R, Caldwell KE, Dehner CA, Buchanan JL, Lange SES, Poulin N, Sehn JK, Roszik J, et al. Malic enzyme 1 absence in synovial sarcoma shifts antioxidant system dependence and increases sensitivity to ferroptosis induction with ACXT-3102. Clin Cancer Res. 2022;28:3573–3589. doi: 10.1158/1078-0432.CCR-22-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Luo Y, Gao X, Zou L, Lei M, Feng J, Hu Z. Bavachin induces ferroptosis through the STAT3/P53/SLC7A11 axis in osteosarcoma Cells. Oxid Med Cell Longev. 2021;2021:1783485. doi: 10.1155/2021/1783485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fanzani A, Poli M. Iron, oxidative damage and ferroptosis in rhabdomyosarcoma. Int J Mol Sci. 2017;18:1718. doi: 10.3390/ijms18081718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Castro B, Alonso-Varona A, del Olmo M, Bilbao P, Palomares T. Role of gamma-glutamyltranspeptidase on the response of poorly and moderately differentiated rhabdomyosarcoma cell lines to buthionine sulfoximine-induced inhibition of glutathione synthesis. Anticancer Drugs. 2002;13:281–291. doi: 10.1097/00001813-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 149.Lv H, Zhen C, Liu J, Shang P. β-phenethyl isothiocyanate induces cell death in human osteosarcoma through altering iron metabolism, disturbing the redox balance, and activating the mapk signaling pathway. Oxid Med Cell Longev. 2020;2020:5021983. doi: 10.1155/2020/5021983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lv HH, Zhen CX, Liu JY, Shang P. PEITC triggers multiple forms of cell death by GSH-iron-ROS regulation in K7M2 murine osteosarcoma cells. Acta Pharmacol Sin. 2020;41:1119–1132. doi: 10.1038/s41401-020-0376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dächert J, Ehrenfeld V, Habermann K, Dolgikh N, Fulda S. Targeting ferroptosis in rhabdomyosarcoma cells. Int J Cancer. 2020;146:510–520. doi: 10.1002/ijc.32496. [DOI] [PubMed] [Google Scholar]
- 152.Shintoku R, Takigawa Y, Yamada K, Kubota C, Yoshimoto Y, Takeuchi T, Koshiishi I, Torii S. Lipoxygenase-mediated generation of lipid peroxides enhances ferroptosis induced by erastin and RSL3. Cancer Sci. 2017;108:2187–2194. doi: 10.1111/cas.13380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu J, Lou C, Zhen C, Wang Y, Shang P, Lv H: Iron plays a role in sulfasalazine-induced ferroptosis with autophagic flux blockage in K7M2 osteosarcoma cells. Metallomics 2022, 14. [DOI] [PubMed]
- 154.Chen M, Jiang Y, Sun Y. KDM4A-mediated histone demethylation of SLC7A11 inhibits cell ferroptosis in osteosarcoma. Biochem Biophys Res Commun. 2021;550:77–83. doi: 10.1016/j.bbrc.2021.02.137. [DOI] [PubMed] [Google Scholar]
- 155.Xu Z, Chen L, Wang C, Zhang L, Xu W. MicroRNA-1287-5p promotes ferroptosis of osteosarcoma cells through inhibiting GPX4. Free Radic Res. 2021;55:1119–1129. doi: 10.1080/10715762.2021.2024816. [DOI] [PubMed] [Google Scholar]
- 156.Isani G, Bertocchi M, Andreani G, Farruggia G, Cappadone C, Salaroli R, Forni M, Bernardini C. Cytotoxic Effects of Artemisia annua L. and Pure Artemisinin on the D-17 Canine Osteosarcoma Cell Line. Oxid Med Cell Longev. 2019;2019:1615758. doi: 10.1155/2019/1615758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Salaroli R, Andreani G, Bernardini C, Zannoni A, La Mantia D, Protti M, Forni M, Mercolini L, Isani G. Anticancer activity of an Artemisia annua L. hydroalcoholic extract on canine osteosarcoma cell lines. Res Vet Sci. 2022;152:476–484. doi: 10.1016/j.rvsc.2022.09.012. [DOI] [PubMed] [Google Scholar]
- 158.Maruwge W, D'Arcy P, Folin A, Brnjic S, Wejde J, Davis A, Erlandsson F, Bergh J, Brodin B. Sorafenib inhibits tumor growth and vascularization of rhabdomyosarcoma cells by blocking IGF-1R-mediated signaling. Onco Targets Ther. 2008;1:67–78. doi: 10.2147/OTT.S3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.He T, Lin X, Yang C, Chen Z, Wang L, Li Q, Ma J, Zhan F, Wang Y, Yan J, Quan Z. Theaflavin-3,3′-digallate plays a ros-mediated dual role in ferroptosis and apoptosis via the MAPK pathway in human osteosarcoma cell lines and xenografts. Oxid Med Cell Longev. 2022;2022:8966368. doi: 10.1155/2022/8966368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shi Y, Gong M, Deng Z, Liu H, Chang Y, Yang Z, Cai L. Tirapazamine suppress osteosarcoma cells in part through SLC7A11 mediated ferroptosis. Biochem Biophys Res Commun. 2021;567:118–124. doi: 10.1016/j.bbrc.2021.06.036. [DOI] [PubMed] [Google Scholar]
- 161.Tang Z, Dong H, Li T, Wang N, Wei X, Wu H, Liu Y, Wang W, Guo Z, Xiao X. The synergistic reducing drug resistance effect of cisplatin and ursolic acid on osteosarcoma through a multistep mechanism involving ferritinophagy. Oxid Med Cell Longev. 2021;2021:5192271. doi: 10.1155/2021/5192271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jiacong H, Qirui Y, Haonan L, Yichang S, Yan C, Keng C. Zoledronic acid induces ferroptosis by upregulating POR in osteosarcoma. Med Oncol. 2023;40:141. doi: 10.1007/s12032-023-01988-w. [DOI] [PubMed] [Google Scholar]
- 163.Ren T, Huang J, Sun W, Wang G, Wu Y, Jiang Z, Lv Y, Wu G, Cao J, Liu M, Gu H. Zoledronic acid induces ferroptosis by reducing ubiquinone and promoting HMOX1 expression in osteosarcoma cells. Front Pharmacol. 2022;13:1071946. doi: 10.3389/fphar.2022.1071946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chang LC, Chiang SK, Chen SE, Yu YL, Chou RH, Chang WC. Heme oxygenase-1 mediates BAY 11–7085 induced ferroptosis. Cancer Lett. 2018;416:124–137. doi: 10.1016/j.canlet.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 165.Basit F, van Oppen LM, Schöckel L, Bossenbroek HM, van Emst-de Vries SE, Hermeling JC, Grefte S, Kopitz C, Heroult M, Hgm Willems P, Koopman WJ. Mitochondrial complex I inhibition triggers a mitophagy-dependent ROS increase leading to necroptosis and ferroptosis in melanoma cells. Cell Death Dis. 2017;8:e2716. doi: 10.1038/cddis.2017.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cramer SL, Saha A, Liu J, Tadi S, Tiziani S, Yan W, Triplett K, Lamb C, Alters SE, Rowlinson S, et al. Systemic depletion of L-cyst(e)ine with cyst(e)inase increases reactive oxygen species and suppresses tumor growth. Nat Med. 2017;23:120–127. doi: 10.1038/nm.4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li Q, Han X, Lan X, Gao Y, Wan J, Durham F, Cheng T, Yang J, Wang Z, Jiang C, et al. Inhibition of neuronal ferroptosis protects hemorrhagic brain. JCI Insight. 2017;2:e90777. doi: 10.1172/jci.insight.90777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wen Q, Liu J, Kang R, Zhou B, Tang D. The release and activity of HMGB1 in ferroptosis. Biochem Biophys Res Commun. 2019;510:278–283. doi: 10.1016/j.bbrc.2019.01.090. [DOI] [PubMed] [Google Scholar]
- 169.Gaschler MM, Andia AA, Liu H, Csuka JM, Hurlocker B, Vaiana CA, Heindel DW, Zuckerman DS, Bos PH, Reznik E, et al. FINO(2) initiates ferroptosis through GPX4 inactivation and iron oxidation. Nat Chem Biol. 2018;14:507–515. doi: 10.1038/s41589-018-0031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED, Hayano M, Thomas AG, Gleason CE, Tatonetti NP, Slusher BS, Stockwell BR. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife. 2014;3:e02523. doi: 10.7554/eLife.02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hassannia B, Wiernicki B, Ingold I, Qu F, Van Herck S, Tyurina YY, Bayır H, Abhari BA, Angeli JPF, Choi SM, et al. Nano-targeted induction of dual ferroptotic mechanisms eradicates high-risk neuroblastoma. J Clin Invest. 2018;128:3341–3355. doi: 10.1172/JCI99032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Li X, Liu J. FANCD2 inhibits ferroptosis by regulating the JAK2/STAT3 pathway in osteosarcoma. BMC Cancer. 2023;23:179. doi: 10.1186/s12885-023-10626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zilka O, Shah R, Li B, Friedmann Angeli JP, Griesser M, Conrad M, Pratt DA. On the mechanism of cytoprotection by ferrostatin-1 and liproxstatin-1 and the role of lipid peroxidation in ferroptotic cell death. ACS Cent Sci. 2017;3:232–243. doi: 10.1021/acscentsci.7b00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Li L, Zhang Y, Gao Y, Hu Y, Wang R, Wang S, Li Y, He Y, Yuan C. LncSNHG14 promotes nutlin3a resistance by inhibiting ferroptosis via the miR-206 /SLC7A11 axis in osteosarcoma cells. Cancer Gene Ther. 2023;30:704–715. doi: 10.1038/s41417-022-00581-z. [DOI] [PubMed] [Google Scholar]
- 175.Kim H, Lee JH, Park JW. Down-regulation of IDH2 sensitizes cancer cells to erastin-induced ferroptosis. Biochem Biophys Res Commun. 2020;525:366–371. doi: 10.1016/j.bbrc.2020.02.093. [DOI] [PubMed] [Google Scholar]
- 176.Zeng T, Deng G, Zhong W, Gao Z, Ma S, Mo C, Li Y, Huang S, Zhou C, Lai Y, et al. Indoleamine 2, 3-dioxygenase 1enhanceshepatocytes ferroptosis in acute immune hepatitis associated with excess nitrative stress. Free Radic Biol Med. 2020;152:668–679. doi: 10.1016/j.freeradbiomed.2020.01.009. [DOI] [PubMed] [Google Scholar]
- 177.Gregus AM, Dumlao DS, Wei SC, Norris PC, Catella LC, Meyerstein FG, Buczynski MW, Steinauer JJ, Fitzsimmons BL, Yaksh TL, Dennis EA. Systematic analysis of rat 12/15-lipoxygenase enzymes reveals critical role for spinal eLOX3 hepoxilin synthase activity in inflammatory hyperalgesia. Faseb j. 2013;27:1939–1949. doi: 10.1096/fj.12-217414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A. 2016;113:E4966–4975. doi: 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Xie Y, Song X, Sun X, Huang J, Zhong M, Lotze MT, Zeh HJR, Kang R, Tang D. Identification of baicalein as a ferroptosis inhibitor by natural product library screening. Biochem Biophys Res Commun. 2016;473:775–780. doi: 10.1016/j.bbrc.2016.03.052. [DOI] [PubMed] [Google Scholar]
- 180.Probst L, Dächert J, Schenk B, Fulda S. Lipoxygenase inhibitors protect acute lymphoblastic leukemia cells from ferroptotic cell death. Biochem Pharmacol. 2017;140:41–52. doi: 10.1016/j.bcp.2017.06.112. [DOI] [PubMed] [Google Scholar]
- 181.Sun Z, Tang Z, Yang X, Liu QS, Liang Y, Fiedler H, Zhang J, Zhou Q, Jiang G. Perturbation of 3-tert-butyl-4-hydroxyanisole in adipogenesis of male mice with normal and high fat diets. Sci Total Environ. 2020;703:135608. doi: 10.1016/j.scitotenv.2019.135608. [DOI] [PubMed] [Google Scholar]
- 182.Sha LK, Sha W, Kuchler L, Daiber A, Giegerich AK, Weigert A, Knape T, Snodgrass R, Schröder K, Brandes RP, et al. Loss of Nrf2 in bone marrow-derived macrophages impairs antigen-driven CD8(+) T cell function by limiting GSH and Cys availability. Free Radic Biol Med. 2015;83:77–88. doi: 10.1016/j.freeradbiomed.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 183.Wang D, Peng Y, Xie Y, Zhou B, Sun X, Kang R, Tang D. Antiferroptotic activity of non-oxidative dopamine. Biochem Biophys Res Commun. 2016;480:602–607. doi: 10.1016/j.bbrc.2016.10.099. [DOI] [PubMed] [Google Scholar]
- 184.Liu Y, Wang W, Li Y, Xiao Y, Cheng J, Jia J. The 5-lipoxygenase inhibitor zileuton confers neuroprotection against glutamate oxidative damage by inhibiting ferroptosis. Biol Pharm Bull. 2015;38:1234–1239. doi: 10.1248/bpb.b15-00048. [DOI] [PubMed] [Google Scholar]
- 185.Su Y, Zhao B, Zhou L, Zhang Z, Shen Y, Lv H, AlQudsy LHH, Shang P. Ferroptosis, a novel pharmacological mechanism of anti-cancer drugs. Cancer Lett. 2020;483:127–136. doi: 10.1016/j.canlet.2020.02.015. [DOI] [PubMed] [Google Scholar]
- 186.Ioannides C, Konsue N. A principal mechanism for the cancer chemopreventive activity of phenethyl isothiocyanate is modulation of carcinogen metabolism. Drug Metab Rev. 2015;47:356–373. doi: 10.3109/03602532.2015.1058819. [DOI] [PubMed] [Google Scholar]
- 187.Ali Syeda Z, Langden SSS, Munkhzul C, Song S, Lee M. Regulatory mechanism of MicroRNA expression in cancer. Int J Mol Sci. 2020;21:1723. doi: 10.3390/ijms21051723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wang JK, Wang Z, Li G. MicroRNA-125 in immunity and cancer. Cancer Lett. 2019;454:134–145. doi: 10.1016/j.canlet.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 189.Hussen BM, Hidayat HJ, Salihi A, Sabir DK, Taheri M, Ghafouri-Fard S. MicroRNA: a signature for cancer progression. Biomed Pharmacother. 2021;138:111528. doi: 10.1016/j.biopha.2021.111528. [DOI] [PubMed] [Google Scholar]
- 190.Qiu Z, Wang L, Liu H. Hsa_circ_0001982 promotes the progression of breast cancer through miR-1287-5p/MUC19 axis under hypoxia. World J Surg Oncol. 2021;19:161. doi: 10.1186/s12957-021-02273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jiang M, Jike Y, Liu K, Gan F, Zhang K, Xie M, Zhang J, Chen C, Zou X, Jiang X, et al. Exosome-mediated miR-144-3p promotes ferroptosis to inhibit osteosarcoma proliferation, migration, and invasion through regulating ZEB1. Mol Cancer. 2023;22:113. doi: 10.1186/s12943-023-01804-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Correia JH, Rodrigues JA, Pimenta S, Dong T, Yang Z. Photodynamic therapy review: principles, photosensitizers, applications, and future directions. Pharmaceutics. 2021;13:1332. doi: 10.3390/pharmaceutics13091332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang X, Liu T, Li Z, Zhang X. Progress of photodynamic therapy applications in the treatment of musculoskeletal sarcoma (Review) Oncol Lett. 2014;8:1403–1408. doi: 10.3892/ol.2014.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tao Y, Ou Y, Yin H, Chen Y, Zhong S, Gao Y, Zhao Z, He B, Huang Q, Deng Q. Establishment and characterization of human osteosarcoma cells resistant to pyropheophorbide-α methyl ester-mediated photodynamic therapy. Int J Oncol. 2017;51:1427–1438. doi: 10.3892/ijo.2017.4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Huang Q, Ou YS, Tao Y, Yin H, Tu PH. Apoptosis and autophagy induced by pyropheophorbide-α methyl ester-mediated photodynamic therapy in human osteosarcoma MG-63 cells. Apoptosis. 2016;21:749–760. doi: 10.1007/s10495-016-1243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhan F, Zhang Y, Zuo Q, Xie C, Li H, Tian L, Wu C, Chen Z, Yang C, Wang Y, et al. YAP knockdown in combination with ferroptosis induction increases the sensitivity of HOS human osteosarcoma cells to pyropheophorbide-α methyl ester-mediated photodynamic therapy. Photodiagnosis Photodyn Ther. 2022;39:102964. doi: 10.1016/j.pdpdt.2022.102964. [DOI] [PubMed] [Google Scholar]
- 197.Wang Y, Zhang L, Zhao G, Zhang Y, Zhan F, Chen Z, He T, Cao Y, Hao L, Wang Z, et al. Homologous targeting nanoparticles for enhanced PDT against osteosarcoma HOS cells and the related molecular mechanisms. J Nanobiotechnology. 2022;20:83. doi: 10.1186/s12951-021-01201-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Chen X, Stewart E, Shelat AA, Qu C, Bahrami A, Hatley M, Wu G, Bradley C, McEvoy J, Pappo A, et al. Targeting oxidative stress in embryonal rhabdomyosarcoma. Cancer Cell. 2013;24:710–724. doi: 10.1016/j.ccr.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ludwig JA, Meyers PA, Dirksen U. Ewing's Sarcoma. N Engl J Med. 2021;384:1476. doi: 10.1056/NEJMc2102423. [DOI] [PubMed] [Google Scholar]
- 200.Du R, Huang C, Liu K, Li X, Dong Z. Targeting AURKA in Cancer: molecular mechanisms and opportunities for cancer therapy. Mol Cancer. 2021;20:15. doi: 10.1186/s12943-020-01305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Chen H, Hu J, Xiong X, Chen H, Lin B, Chen Y, Li Y, Cheng D, Li Z. AURKA inhibition induces Ewing's sarcoma apoptosis and ferroptosis through NPM1/YAP1 axis. Cell Death Dis. 2024;15:99. doi: 10.1038/s41419-024-06485-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Fayzullina D, Yakushov S, Kantserova K, Belyaeva E, Aniskin D, Tsibulnikov S, Fayzullina N, Kalinin S, Romantsova O, Timashev PS, et al. Carbonic anhydrase inhibitors induce ferroptosis through inhibition of AKT/FTH1 signaling in ewing sarcoma tumor cells. Cancers. 2023;15:5225. doi: 10.3390/cancers15215225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Tamiya H, Urushihara N, Shizuma K, Ogawa H, Nakai S, Wakamatsu T, Takenaka S, Kakunaga S. SHARPIN enhances ferroptosis in Synovial Sarcoma Cells via NF-κB- and PRMT5-mediated PGC1α reduction. Cancers (Basel) 2023;15:3484. doi: 10.3390/cancers15133484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Han S, Liu Q, Yang Z, Ma J, Liu D, Yan C, Liang D. Identification of ferroptosis-related gene prognostic signature and HSF1 for reversing doxorubicin and gemcitabine resistance in uterine carcinosarcoma. Dis Markers. 2022;2022:6400227. doi: 10.1155/2022/6400227. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 205.Meyer CF. Immunotherapy for Sarcoma: a Work in Progress. J Clin Oncol. 2022;40:1267–1270. doi: 10.1200/JCO.21.01338. [DOI] [PubMed] [Google Scholar]
- 206.Panagi M, Pilavaki P, Constantinidou A, Stylianopoulos T. Immunotherapy in soft tissue and bone sarcoma: unraveling the barriers to effectiveness. Theranostics. 2022;12:6106–6129. doi: 10.7150/thno.72800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lei G, Zhang Y, Koppula P, Liu X, Zhang J, Lin SH, Ajani JA, Xiao Q, Liao Z, Wang H, Gan B. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 2020;30:146–162. doi: 10.1038/s41422-019-0263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zhu S, Luo Z, Li X, Han X, Shi S, Zhang T. Tumor-associated macrophages: role in tumorigenesis and immunotherapy implications. J Cancer. 2021;12:54–64. doi: 10.7150/jca.49692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Guo P, Wang L, Shang W, Chen J, Chen Z, Xiong F, Wang Z, Tong Z, Wang K, Yang L, et al. Intravesical in situ immunostimulatory gel for triple therapy of bladder cancer. ACS Appl Mater Interfaces. 2020;12:54367–54377. doi: 10.1021/acsami.0c15176. [DOI] [PubMed] [Google Scholar]
- 210.Jiang Q, Wang K, Zhang X, Ouyang B, Liu H, Pang Z, Yang W. Platelet membrane-camouflaged magnetic nanoparticles for ferroptosis-enhanced cancer immunotherapy. Small. 2020;16:e2001704. doi: 10.1002/smll.202001704. [DOI] [PubMed] [Google Scholar]
- 211.Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Yu K, Chen Y, Zhang L, Zheng Y, Chen J, Wang Z, Yu X, Song K, Dong Y, Xiong F, et al. Cancer-Erythrocyte Membrane-Mimicking Fe(3)O(4) Nanoparticles and DHJS for Ferroptosis/Immunotherapy Synergism in Tumors. ACS Appl Mater Interfaces. 2023;15:44689–44710. doi: 10.1021/acsami.3c07379. [DOI] [PubMed] [Google Scholar]
- 213.Smeland S, Bielack SS, Whelan J, Bernstein M, Hogendoorn P, Krailo MD, Gorlick R, Janeway KA, Ingleby FC, Anninga J, et al. Survival and prognosis with osteosarcoma: outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur J Cancer. 2019;109:36–50. doi: 10.1016/j.ejca.2018.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.O'Kane GM, Cadoo KA, Walsh EM, Emerson R, Dervan P, O'Keane C, Hurson B, O'Toole G, Dudeney S, Kavanagh E, et al. Perioperative chemotherapy in the treatment of osteosarcoma: a 26-year single institution review. Clin Sarcoma Res. 2015;5:17. doi: 10.1186/s13569-015-0032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Farfalli GL, Albergo JI, Lobos PA, Smith DE, Streitenberger PD, Pallotta Rodríguez MG, Aponte-Tinao LA. Osteosarcoma lung metastases Survival after chemotherapy and surgery. Medicina. 2015;75:87–90. [PubMed] [Google Scholar]
- 216.Zhang C, Xu C, Gao X, Yao Q. Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics. 2022;12:2115–2132. doi: 10.7150/thno.69424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 218.Kim M, Jung JY, Choi S, Lee H, Morales LD, Koh JT, Kim SH, Choi YD, Choi C, Slaga TJ, et al. GFRA1 promotes cisplatin-induced chemoresistance in osteosarcoma by inducing autophagy. Autophagy. 2017;13:149–168. doi: 10.1080/15548627.2016.1239676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Meng Y, Gao R, Ma J, Zhao J, Xu E, Wang C, Zhou X. MicroRNA-140-5p regulates osteosarcoma chemoresistance by targeting HMGN5 and autophagy. Sci Rep. 2017;7:416. doi: 10.1038/s41598-017-00405-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Liu Q, Wang K. The induction of ferroptosis by impairing STAT3/Nrf2/GPx4 signaling enhances the sensitivity of osteosarcoma cells to cisplatin. Cell Biol Int. 2019;43:1245–1256. doi: 10.1002/cbin.11121. [DOI] [PubMed] [Google Scholar]
- 221.Sun CY, Zhu Y, Li XF, Wang XQ, Tang LP, Su ZQ, Li CY, Zheng GJ, Feng B. Scutellarin increases cisplatin-induced apoptosis and autophagy to overcome cisplatin resistance in non-small cell lung cancer via ERK/p53 and c-met/AKT signaling pathways. Front Pharmacol. 2018;9:92. doi: 10.3389/fphar.2018.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Rudin CM, Yang Z, Schumaker LM, VanderWeele DJ, Newkirk K, Egorin MJ, Zuhowski EG, Cullen KJ. Inhibition of glutathione synthesis reverses Bcl-2-mediated cisplatin resistance. Cancer Res. 2003;63:312–318. [PubMed] [Google Scholar]
- 223.Weir NM, Selvendiran K, Kutala VK, Tong L, Vishwanath S, Rajaram M, Tridandapani S, Anant S, Kuppusamy P. Curcumin induces G2/M arrest and apoptosis in cisplatin-resistant human ovarian cancer cells by modulating Akt and p38 MAPK. Cancer Biol Ther. 2007;6:178–184. doi: 10.4161/cbt.6.2.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hangauer MJ, Viswanathan VS, Ryan MJ, Bole D, Eaton JK, Matov A, Galeas J, Dhruv HD, Berens ME, Schreiber SL, et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature. 2017;551:247–250. doi: 10.1038/nature24297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Sommerwerk S, Heller L, Kuhfs J, Csuk R. Urea derivates of ursolic, oleanolic and maslinic acid induce apoptosis and are selective cytotoxic for several human tumor cell lines. Eur J Med Chem. 2016;119:1–16. doi: 10.1016/j.ejmech.2016.04.051. [DOI] [PubMed] [Google Scholar]
- 226.Tang M, Chen Z, Wu D, Chen L. Ferritinophagy/ferroptosis: iron-related newcomers in human diseases. J Cell Physiol. 2018;233:9179–9190. doi: 10.1002/jcp.26954. [DOI] [PubMed] [Google Scholar]
- 227.He P, Xu S, Miao Z, Que Y, Chen Y, Li S, Ma Q, Yang R, Wei W, Zha Z, Hu Y. Anti-Her2 affibody-decorated arsenene nanosheets induce ferroptosis through depleting intracellular GSH to overcome cisplatin resistance. J Nanobiotechnology. 2023;21:203. doi: 10.1186/s12951-023-01963-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kim SW, Kim Y, Kim SE, An JY. Ferroptosis-related genes in neurodevelopment and central nervous system. Biology. 2021;10:35. doi: 10.3390/biology10010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Cui DJ, Chen C, Yuan WQ, Yang YH, Han L. Integrative analysis of ferroptosis-related genes in ulcerative colitis. J Int Med Res. 2021;49:3000605211042975. doi: 10.1177/03000605211042975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Du X, Zhang Y. Integrated analysis of immunity- and ferroptosis-related biomarker signatures to improve the prognosis prediction of hepatocellular carcinoma. Front Genet. 2020;11:614888. doi: 10.3389/fgene.2020.614888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zhuo S, Chen Z, Yang Y, Zhang J, Tang J, Yang K. Clinical and biological significances of a ferroptosis-related gene signature in glioma. Front Oncol. 2020;10:590861. doi: 10.3389/fonc.2020.590861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zhu L, Yang F, Wang L, Dong L, Huang Z, Wang G, Chen G, Li Q. Identification the ferroptosis-related gene signature in patients with esophageal adenocarcinoma. Cancer Cell Int. 2021;21:124. doi: 10.1186/s12935-021-01821-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Gao X, Tang M, Tian S, Li J, Liu W. A ferroptosis-related gene signature predicts overall survival in patients with lung adenocarcinoma. Future Oncol. 2021;17:1533–1544. doi: 10.2217/fon-2020-1113. [DOI] [PubMed] [Google Scholar]
- 234.Huang W, Duan Y, Yang X, Shang C, Chen X, Zhang H, Li F. Identification of novel prognostic risk signatures of soft tissue sarcoma based on ferroptosis-related genes. Front Oncol. 2021;11:629868. doi: 10.3389/fonc.2021.629868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zhao J, Zhao Y, Ma X, Feng H, Cui R. Immunological and prognostic significance of novel ferroptosis-related genes in soft tissue sarcoma. PLoS ONE. 2022;17:e0262234. doi: 10.1371/journal.pone.0262234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Guan Z, Liu S, Luo L, Wu Z, Lu S, Guan Z, Tao K. Identification of Ferroptosis-Related Genes as Biomarkers for Sarcoma. Front Cell Dev Biol. 2022;10:847513. doi: 10.3389/fcell.2022.847513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zhao J, Zhao Y, Ma X, Feng H, Jia L. Outstanding prognostic value of novel ferroptosis-related genes in chemoresistance osteosarcoma patients. Sci Rep. 2022;12:5029. doi: 10.1038/s41598-022-09080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Jiang M, Wang Z, He X, Hu Y, Xie M, Jike Y, Bo Z, Qin W. A risk-scoring model based on evaluation of ferroptosis-related genes in osteosarcoma. J Oncol. 2022;2022:4221756. doi: 10.1155/2022/4221756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zhao R, Li Z, Huang Y, Xiong C, Zhang C, Liang H, Xu J, Luo X. A novel ferroptosis-related gene signature for prognosis prediction in ewing sarcoma. Anal Cell Pathol. 2022;2022:6711629. doi: 10.1155/2022/6711629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Li J, Wu F, Xiao X, Su L, Guo X, Yao J, Zhu H. A novel ferroptosis-related gene signature to predict overall survival in patients with osteosarcoma. Am J Transl Res. 2022;14:6082–6094. [PMC free article] [PubMed] [Google Scholar]
- 241.Jiang M, Jike Y, Gan F, Li J, Hu Y, Xie M, Liu K, Qin W, Bo Z. Verification of ferroptosis subcluster-associated genes related to osteosarcoma and exploration of immune targeted therapy. Oxid Med Cell Longev. 2022;2022:9942014. doi: 10.1155/2022/9942014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 242.Zheng D, Xia K, Wei Z, Wei Z, Guo W. Identification of a novel gene signature with regard to ferroptosis, prognosis prediction, and immune microenvironment in osteosarcoma. Front Genet. 2022;13:944978. doi: 10.3389/fgene.2022.944978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ge Z, Song D. A five ferroptosis-related genes risk score for prognostic prediction of osteosarcoma. Medicine (Baltimore) 2022;101:e32083. doi: 10.1097/MD.0000000000032083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Huang H, Ye Z, Li Z, Wang B, Li K, Zhou K, Cao H, Zheng J, Wang G. Employing machine learning using ferroptosis-related genes to construct a prognosis model for patients with osteosarcoma. Front Genet. 2023;14:1099272. doi: 10.3389/fgene.2023.1099272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Yang L, Liu J, Liu S. Clinical significance and immune landscape of a novel ferroptosis-related prognosis signature in osteosarcoma. BMC Cancer. 2023;23:229. doi: 10.1186/s12885-023-10688-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Hou J, Lu Z, Cheng X, Dong R, Jiang Y, Wu G, Qu G, Xu Y. Ferroptosis-related long non-coding RNA signature predicts the prognosis of bladder cancer. BMC Cancer. 2022;22:719. doi: 10.1186/s12885-022-09805-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kang R, Tang D. Autophagy and ferroptosis—what's the connection? Curr Pathobiol Rep. 2017;5:153–159. doi: 10.1007/s40139-017-0139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Maher P. Potentiation of glutathione loss and nerve cell death by the transition metals iron and copper: Implications for age-related neurodegenerative diseases. Free Radic Biol Med. 2018;115:92–104. doi: 10.1016/j.freeradbiomed.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 249.Lewerenz J, Ates G, Methner A, Conrad M, Maher P. Oxytosis/ferroptosis-(Re-) emerging roles for oxidative stress-dependent non-apoptotic cell death in diseases of the central nervous system. Front Neurosci. 2018;12:214. doi: 10.3389/fnins.2018.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Tsvetkov P, Coy S, Petrova B, Dreishpoon M, Verma A, Abdusamad M, Rossen J, Joesch-Cohen L, Humeidi R, Spangler RD, et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science. 2022;375:1254–1261. doi: 10.1126/science.abf0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Song Q, Zhou R, Shu F, Fu W. Cuproptosis scoring system to predict the clinical outcome and immune response in bladder cancer. Front Immunol. 2022;13:958368. doi: 10.3389/fimmu.2022.958368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Tang D, Chen X, Kroemer G. Cuproptosis: a copper-triggered modality of mitochondrial cell death. Cell Res. 2022;32:417–418. doi: 10.1038/s41422-022-00653-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Wang Y, Zhang L, Zhou F. Cuproptosis: a new form of programmed cell death. Cell Mol Immunol. 2022;19:867–868. doi: 10.1038/s41423-022-00866-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Zhang Y, Tan H, Daniels JD, Zandkarimi F, Liu H, Brown LM, Uchida K, O'Connor OA, Stockwell BR. Imidazole ketone erastin induces ferroptosis and slows tumor growth in a mouse lymphoma model. Cell Chem Biol. 2019;26:623–633.e629. doi: 10.1016/j.chembiol.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Schut AW, Vriends ALM, Sacchetti A, Timbergen MJM, Alman BA, Al-Jazrawe M, Grünhagen DJ, Verhoef C, Sleijfer S, Wiemer EAC. In desmoid-type fibromatosis cells sorafenib induces ferroptosis and apoptosis, which are enhanced by autophagy inhibition. Eur J Surg Oncol. 2022;48:1527–1535. doi: 10.1016/j.ejso.2022.02.020. [DOI] [PubMed] [Google Scholar]
- 256.Su Y, Tatzel K, Wang X, Belt B, Binder P, Kuroki L, Powell MA, Mutch DG, Hawkins WG, Spitzer D. Mesothelin's minimal MUC16 binding moiety converts TR3 into a potent cancer therapeutic via hierarchical binding events at the plasma membrane. Oncotarget. 2016;7:31534–31549. doi: 10.18632/oncotarget.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Ohman KA, Hashim YM, Vangveravong S, Nywening TM, Cullinan DR, Goedegebuure SP, Liu J, Van Tine BA, Tiriac H, Tuveson DA, et al. Conjugation to the sigma-2 ligand SV119 overcomes uptake blockade and converts dm-Erastin into a potent pancreatic cancer therapeutic. Oncotarget. 2016;7:33529–33541. doi: 10.18632/oncotarget.9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Hashim YM, Spitzer D, Vangveravong S, Hornick MC, Garg G, Hornick JR, Goedegebuure P, Mach RH, Hawkins WG. Targeted pancreatic cancer therapy with the small molecule drug conjugate SW IV-134. Mol Oncol. 2014;8:956–967. doi: 10.1016/j.molonc.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Garg G, Vangveravong S, Zeng C, Collins L, Hornick M, Hashim Y, Piwnica-Worms D, Powell MA, Mutch DG, Mach RH, et al. Conjugation to a SMAC mimetic potentiates sigma-2 ligand induced tumor cell death in ovarian cancer. Mol Cancer. 2014;13:50. doi: 10.1186/1476-4598-13-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Hornick JR, Vangveravong S, Spitzer D, Abate C, Berardi F, Goedegebuure P, Mach RH, Hawkins WG. Lysosomal membrane permeabilization is an early event in Sigma-2 receptor ligand mediated cell death in pancreatic cancer. J Exp Clin Cancer Res. 2012;31:41. doi: 10.1186/1756-9966-31-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Spitzer D, Simon PO, Jr, Kashiwagi H, Xu J, Zeng C, Vangveravong S, Zhou D, Chang K, McDunn JE, Hornick JR, et al. Use of multifunctional sigma-2 receptor ligand conjugates to trigger cancer-selective cell death signaling. Cancer Res. 2012;72:201–209. doi: 10.1158/0008-5472.CAN-11-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Kashiwagi H, McDunn JE, Simon PO, Jr, Goedegebuure PS, Vangveravong S, Chang K, Hotchkiss RS, Mach RH, Hawkins WG. Sigma-2 receptor ligands potentiate conventional chemotherapies and improve survival in models of pancreatic adenocarcinoma. J Transl Med. 2009;7:24. doi: 10.1186/1479-5876-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Kashiwagi H, McDunn JE, Simon PO, Jr, Goedegebuure PS, Xu J, Jones L, Chang K, Johnston F, Trinkaus K, Hotchkiss RS, et al. Selective sigma-2 ligands preferentially bind to pancreatic adenocarcinomas: applications in diagnostic imaging and therapy. Mol Cancer. 2007;6:48. doi: 10.1186/1476-4598-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.