Abstract
MacroH2A variants have been linked to inhibition of metastasis through incompletely understood mechanisms. Here, we reveal that solitary dormant disseminated cancer cells (DCCs) display increased levels of macroH2A variants in head and neck squamous cell carcinoma PDX in vivo models and patient samples compared to proliferating primary or metastatic lesions. We demonstrate that dormancy-inducing transforming growth factor–β2 and p38α/β pathways up-regulate macroH2A expression and that macroH2A variant overexpression is sufficient to induce DCC dormancy and suppress metastasis in vivo. Notably, inducible expression of the macroH2A2 variant in vivo suppresses metastasis via a reversible growth arrest of DCCs. This state does not require the dormancy-regulating transcription factors DEC2 and NR2F1; instead, transcriptomic analysis reveals that macroH2A2 overexpression inhibits cell cycle and oncogenic signaling programs, while up-regulating dormancy and senescence-associated inflammatory cytokines. We conclude that the macroH2A2-enforced dormant phenotype results from tapping into transcriptional programs of both quiescence and senescence to limit metastatic outgrowth.
A histone variant linked to repressive chromatin and senescence programs restricts carcinoma metastasis via dormancy induction.
INTRODUCTION
Microenvironmental and cellular intrinsic mechanisms control the dormancy state of disseminated cancer cells (DCCs), a stage of cancer cell quiescence (1–3) that can precede lethal metastasis of solid cancers by many years (4). However, less is known about the epigenetic mechanisms controlling dormancy in cancer cells with genetically altered genomes (5). In cancer models and human specimens, specific microenvironmental cues such as transforming growth factor–β2 (TGF-β2) (6), retinoic acid (7), bone morphogenetic protein 7 (BMP7), periostin, leukemia inhibitory factor, and growth arrest specific 6 (GAS6) can activate transcriptional programs of quiescence, pluripotency, and survival in dormant tumor cells (1). These programs are distinct from senescence and differentiation that generally result in irreversible growth arrest and lack expression of stem cell or diapause genes, as recently reviewed (1). We initially reported that in head and neck squamous cell carcinoma (HNSCC) and breast cancer models, a low ratio of the extracellular signal–regulated kinase to p38α/β kinase activities can predict a state of cancer cell dormancy in vivo (8) and regulates a specific dormancy gene expression signature (6, 9). One of the major transcription factors (TFs) of the dormancy program is nuclear receptor subfamily 2, group F, member 1 (NR2F1), a nuclear orphan receptor that contributes to a repressive chromatin state in dormant cancer cells characterized by high levels of H3K27me3 and H3K9me3 (7, 10). Combined treatment of 5-azacytidine and retinoic acid can induce dormancy in malignant cells and is also associated with the induction of a global chromatin repressive state (7). Similarly, senescent cells are associated with mechanisms of chromatin repression and induction of senescence-associated heterochromatin foci (SAHF) (11, 12). Thus, persistent growth arrest states such as dormancy (reversible) and senescence (irreversible) may exhibit similar chromatin alterations.
While epigenetic mechanisms control tumor onset, progression, and relapse (13, 14) and altered chromatin states can modulate the cell cycle and cellular responses to the microenvironment (15), our understanding of histone variants in the regulation of DCC fate remains limited. The H2A family of histones contains multiple variants, of which macroH2A is unique in having a large and evolutionarily conserved macro domain at the C terminus. In mammals, two different genes, MACROH2A1 and MACROH2A2, encode macroH2A1 and macroH2A2, respectively, and macroH2A1.1 and macroH2A1.2 are alternatively spliced variants encoded by MACROH2A1. MacroH2A variants are generally considered transcriptionally repressive histones that colocalize genome wide with histone modifications such as H3K27me3 and H3K9me3. This includes the inactive X chromosome in mammalian female cells (16) and broad repressive regions across autosomal chromatin (16), as well as SAHF (11). In somatic cells, these histone variants function as barriers in the reprogramming of differentiated cells toward pluripotency (17–19). Therefore, macroH2A variants may limit cellular plasticity in differentiated cells and cancer cells.
In keeping with this concept, macroH2A expression is lost or attenuated at different stages of cancer progression across multiple tumor types (13, 20, 21) and is largely considered tumor suppressive. For example, the abundance of macroH2A correlates inversely with proliferation in a lung cancer recurrence study (21). Moreover, the transcriptional loss of macroH2A correlates with melanoma metastasis in patients, while its overexpression substantially suppresses lung metastasis in mice (22). However, the mechanisms governed by macroH2A in inhibiting metastasis initiation remain unclear. Here, we show that the histone variant macroH2A plays a significant role in the induction of a unique program of DCC dormancy that suppresses metastasis. Using in vitro and in vivo models, as well as validation in human patient HNSCC DCCs, we show that macroH2A variants, and particularly macroH2A2, potently suppress metastasis. This occurs via a transcriptional program that involves inhibition of E2F and MYC signaling and induction of cytokines that are produced, albeit not exclusively, by senescent cells. Thus, macroH2A variants suppress metastasis via a dormancy program that contains components of both quiescence and senescence programs, the latter of which has not been detected in spontaneous dormancy models. This could be potentially exploited to force DCCs into a deep growth arrest and prevent metastasis initiation.
RESULTS
MacroH2A1 expression is enriched in dormant versus proliferative HNSCC cells and DCCs
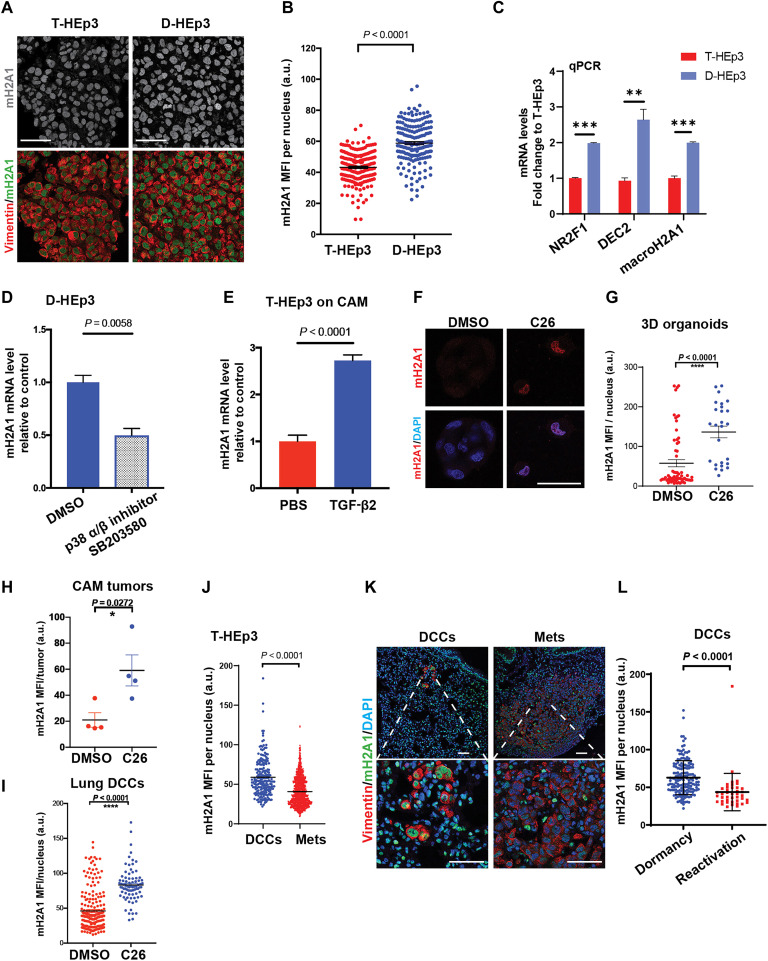
Taking advantage of HNSCC patient derived xenograft (PDX) models T-HEp3 (proliferative) and D-HEp3 (dormant) cells, which display these respective phenotypes when inoculated in vivo in the chick embryo chorioallantoic membrane (CAM) or nude mice (fig. S1A) (6, 23, 24) but proliferate similarly in two-dimensional (2D) culture, we examined expression of macroH2A1 and macroH2A2. By performing combined RNA sequencing (RNA-seq) and epigenomic analyses, we found that macroH2A1 levels are higher in D-HEp3 compared to T-HEp3 cells in vitro (fig. S1B), and while the macroH2A1 promoter is marked by H3K27ac in both, the D-HEp3 harbors putative enhancers as marked by both K3K27ac and H3K4me1. In contrast, macroH2A2 is not expressed in either proliferative or dormant HEp3 variants at the mRNA or protein levels, and its locus is coated by the repressive histone modification H3K27me3 (fig. S1, B and C). As reported (25), D-HEp3 (but not T-HEp3) cells inoculated on CAMs synchronously enter a sustained G0-G1 arrest within 48 hours, forming small fully quiescent tumor micromasses. Analysis of macroH2A1 protein levels via immunofluorescence (IF) directly from the CAM revealed that it was significantly up-regulated in D-HEp3 versus T-HEp3 cells (Fig. 1, A and B). The mRNA level of macroH2A1 is also enriched in D-HEp3 over T-HEp3 cells in CAMs via quantitative polymerase chain reaction (qPCR) analysis, along with dormancy-associated TFs, NR2F1 and DEC2/Bhlhe41 (basic helix-loop-helix family, member e41; Fig. 1C). Given that increased expression of NR2F1 and DEC2 in D-HEp3 cells is dependent on p38α/β signaling (6, 7), we treated D-HEp3 cells with a p38α/β inhibitor (SB203580) and observed an expected reduction in activating transcription factor 2 phosphorylation, a P-p38 substrate, and the cyclin-dependent kinase (CDK) inhibitor p27 (fig. S1D) (6, 7). We observed an ~50% reduction in macroH2A1 mRNA levels in D-HEp3 cells but not upon p38α/β inhibitor treatment of T-HEp3 cells (Fig. 1D and fig. S1E). We have also shown that DEC2 and p38α/β activation in D-HEp3 cells is dependent on TGF-β2 (6, 26). Upon daily treatments of TGF-β2 for 6 days, the growth of T-HEp3 cells in vivo was efficiently inhibited (fig. S1F) as reported (6) and significantly increased macroH2A1 mRNA levels (Fig. 1E). MacroH2A2 expression remained undetectable even after TGF-β2 treatment. Therefore, macroH2A1 is positively regulated by the TGF-β2 and p38α/β pathways in HEp3 cells and is associated with pathways that regulate dormancy.
Fig. 1. MacroH2A1 expression in dormant versus proliferative HNSCC cells and DCCs.
(A and B) Representative images and quantification of T-HEp3 or D-HEp3 CAM tumors stained for macroH2A1 (green), vimentin (red), and 4′,6-diamidino-2-phenylindole (DAPI; blue). n > 100 cells per group were assessed, Mann-Whitney test. Scale bars, 50 μm. a.u., arbitrary units. (C) qPCR-measured mRNA levels in both D-HEp3 cells (blue bars) and in T-HEp3 cells (red bars). (D) D-HEp3 cells were treated with dimethyl sulfoxide (DMSO) or SB 203580 (5 μM, 48 hours) in serum-free medium in vitro. See fig. S1D for the drug efficacy. qPCR measured the relative mRNA level of macroH2A1 to DMSO. (E) qPCR-measured macroH2A1 mRNA levels in T-HEp3 CAM tumors treated with or without TGF-β2 (10 ng/ml) for 6 days. n = 4 tumors per group. All qPCR assays were in triplicate, means + SEM, two-tailed Student’s t test. (F and G) Images and quantification of T-HEp3 cells grown in 3D organoids and cells were treated with 0.2 μM C26 (NR2F1 agonist) or DMSO as indicated for 4 days in vitro and stained for macroH2A1 (green) and DAPI (blue). Scale bars, 25 μm. (H and I) Bar graph quantification of macroH2A1 MFI in T-HEp3 cells grown in CAM model (H) or DCCs found in mouse lungs (I). Mean + SEM, two-tailed Student’s t test. (J to L) Experimental metastasis assay. See fig. S1G for the schematic. n = 10 animals were assessed. (J) Quantification of macroH2A1 MFI per nucleus in either DCCs or metastatic lesions. Single cells and small clusters (<20 cells) were considered as DCC events. Mann-Whitney test. (K) IF representative images show the stain for macroH2A1 (green), human vimentin (red), and DAPI (blue). Scale bars, 50 μm. (L) Quantification of macroH2A1 MFI in DCC events found in dormancy phase or reactivation phase. n = 165 cells pooled from five animals at dormancy phase, n = 41 cells pooled from five animals at reactivation phase. Mean + SD. Unpaired Student’s t test. m2HA1, macroH2A1.
We also explored whether NR2F1, which can be induced by morphogens like all-trans retinoic acid and BMP7 to induce dormancy, could up-regulate macroH2A1 expression. To this end, we made use of a recently identified NR2F1 agonist (C26) (27), which induces a dormancy-like program and suppresses metastasis in the HEp3 HNSCC model (27). T-HEp3 cells were treated with C26 in 3D organoid cultures for 4 days, which caused a robust up-regulation of macroH2A1 protein levels (Fig. 1, F and G). By treating T-HEp3 cells in vivo in the CAM, we also found that growth suppression induced by C26 was associated with a strong induction of macroH2A1 in these dormant-like lesions (Fig. 1H). Last, because C26 prevents solitary DCCs from forming proliferative metastasis (27), we tested whether this dormant solitary DCC state was associated with macroH2A induction. In accordance, C26-mediated suppression of metastasis in lungs (27) was associated with an up-regulation of macroH2A1 in DCCs (Fig. 1I), which also up-regulate NR2F1 and p27, while down-regulating H3S10 phosphorylation levels (27). We conclude that NR2F1 activity may directly or indirectly regulate macroH2A1 expression.
We next tested whether macroH2A expression is different between metastatic solitary HEp3 DCCs and proliferative lesions in secondary organs, as solitary DCCs can be found in a nonproliferative dormant state (10, 27–30). T-HEp3 cells, which can efficiently colonize distal organs in both spontaneous and experimental metastasis models (6, 31), were tail vein–injected in nude mice. DCCs that arrive in the lung in either of these assays can persist in a dormant state for at least 6 weeks, while a fraction reactivate and resume proliferation as early as 2 weeks after arrival to the lung (16, 23, 32, 33). We harvested lungs at 1 or 3 weeks after intravenous injection to detect dormant DCCs at high frequency at week 1 (dormancy) and reactivating DCCs at week 3 (reactivation; fig. S1G). To locate human solitary DCCs, DCC clusters, and metastasis in murine lungs, we used an antibody against the intermediate filament vimentin, which preferentially binds to the human over mouse antigen (6, 7, 31) and costained for macroH2A1. We considered single cells, doublets, and clusters of <20 cells (previously found to be devoid of proliferation markers (6, 7, 31)) as dormant DCC events; events with >20 cells were categorized as proliferative metastasis, as they commonly express proliferation markers (6, 7, 31). When comparing by cluster size, we found that macroH2A1 was significantly higher in solitary and small DCC cluster populations compared to metastases (Fig. 1, J and K). When focusing only on solitary and small DCC cluster events (<20 cells per cluster) during dormancy (week 1) and reactivation phases (week 3), we found that macroH2A1 signal was higher in dormant DCCs (Fig. 1L). Collectively, the above experiments support that signaling and transcriptional mechanisms that control cancer cell dormancy downstream of microenvironmental cues can promote macroH2A expression, which may enforce a dormancy-like state of DCCs.
MacroH2A variants are enriched in solitary DCCs versus primary tumor and metastatic lesions in patients with HNSCC
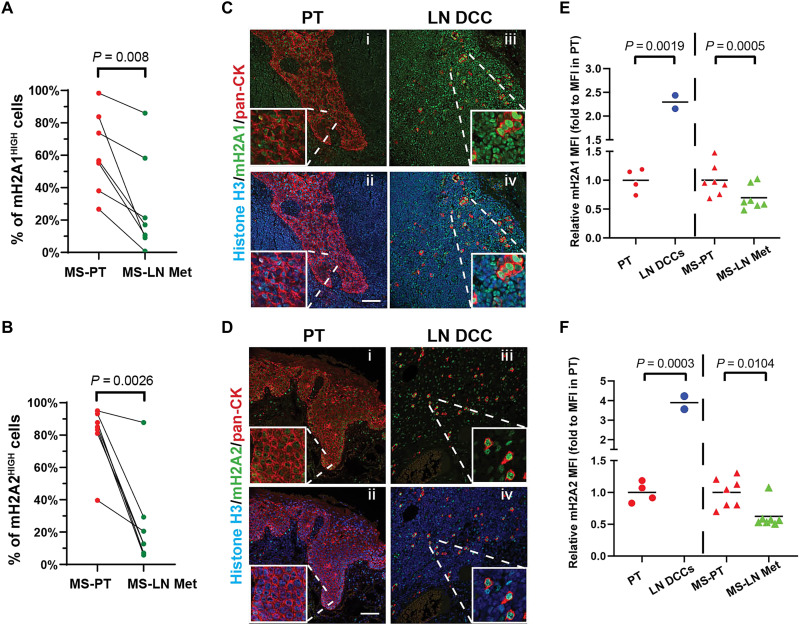
We next tested whether the association of macroH2A variant expression in DCCs held true in patients with HNSCC by quantifying their levels in formalin fixed paraffin embedded (FFPE) samples from poorly differentiated tumors. Examination of seven pairs of primary tumors (MS-PT) and matching lymph node metastases (MS-LN Met) via IF revealed that both macroH2A1 and macroH2A2 signals decrease in LN metastases versus primary tumors (Fig. 2, A and B, and fig. S2, A to D). While the T-HEp3 PDX model and SQ20B cells express no to little macroH2A2, patient samples, albeit with great heterogeneity, and the FaDu cell line does express macroH2A2 (fig. S1C). Thus, macroH2A2 silencing seems to occur heterogeneously. In addition, we observed heterogeneity in macroH2A expression patterns in both MS-PT and MS-LN Met samples across patients. Even within one patient, macroH2A expression is heterogenous in different regions of primary tumors and LN metastasis, suggesting dynamic regulation of its expression. Nevertheless, careful quantification of single-cell nuclear signal revealed that the proportion of macroH2A1High cells was significantly reduced in 57% (four of seven) of patient LN metastases compared to primary lesions (fig. S2A). Notably, the percentage of macroH2A2High cells significantly decreased in 86% (six of seven) of patient LN metastases compared to primary lesions (fig. S2B).
Fig. 2. MacroH2A variants are enriched in solitary DCCs versus primary tumor and metastatic lesions in patients with HNSCC.
(A and B) Quantification of the percentage of macroH2A1- or macroH2A2-high cells in either primary tumors or LN metastases. Each paired primary tumor (MS-PT) tissue section (red) and LN metastatic lesion (MS-LN Met, green) were from the same patient. See fig. S2 for the detailed quantification method and representative images. n = 7 patients; each data point represents the mean percentage of macroH2AHIGH cells pooled from five to six fields of view. Paired Student’s t test. (C and D) Representative images of IF staining in primary tumor tissues or clinically negative LN tissues. macroH2A1 (C) or macroH2A2 (D) are in green; pan-cytokeratin is in red, and Histone H3 is in blue. Scale bar, 75 μm. (E and F) Measurement of relative macroH2A1 or macroH2A2 intensity (normalized to histone H3 intensity) in HNSCC primary tumors (red dots) or DCCs (blue dots) found in clinically negative patient LN tissues after the primary tumor removal, as well as paired PT section (red triangles) and MS-LN Met (green triangles).
In the HEp3 model, macroH2A1 is expressed at higher levels in solitary DCCs and small clusters (Fig. 1). We next tested whether solitary DCCs present in the LN of patients with HNSCC also show increased levels of macroH2A compared to PT samples. While LN samples are not commonly processed for the detection of solitary DCCs, we were able to gain access to a small repository of samples from two patients available for analysis where clinically negative LN FFPE samples were collected during HNSCC primary tumor removal surgeries and confirmed to contain solitary cytokeratin positive DCCs (34, 35). IF staining revealed that both macroH2A1 and macroH2A2 signals were detectable in LN DCCs (Fig. 2, C and D) and at least two- to fivefold higher levels in solitary DCCs compared to nonpaired PT (Fig. 2, E and F). When normalizing for signal abundance in PTs across the patient cohort 1 (Fig. 2, A and B) and cohort 2 (Fig. 2, C and D), we took the PT in both cohorts as baseline expression (see Materials and Methods) and found that macroH2A signals were also up-regulated in solitary DCCs versus LN metastasis (Fig. 2, E and F). A similar pattern was reported recently for NR2F1 expression in these same samples, supporting that macroH2A up-regulation occurs in solitary DCCs that express a dormancy regulator (10, 27). A limitation of our study is that it is a small sample set and that we used normalization to account for intercohort differences. Nonetheless, this analysis suggests that solitary or small clusters of DCCs show increased macroH2A levels compared to PT and LN metastatic samples.
We also explored whether macroH2A1 was differentially expressed in DCCs isolated from the bone marrow of patients with prostate cancer with no evidence of disease (NED; n = 7) for up to 18 years or with advanced metastatic disease (ADV; n = 37) (7, 36). These NED DCCs were previously profiled and found to express dormancy signatures compared to the ADV DCCs, including up-regulation of the p38 signaling pathway, TGF-β2, and NR2F1 (7, 36). We found that macroH2A1, but not macroH2A2, mRNA levels were significantly higher in DCCs from the NED versus the ADV group of patients (fig. S2E). While also small sample–sized patient cohorts, these data support that macroH2A variant expression may be a feature of dormant disease across different human cancers.
MacroH2A overexpression induces a quiescent phenotype in malignant HNSCC cells
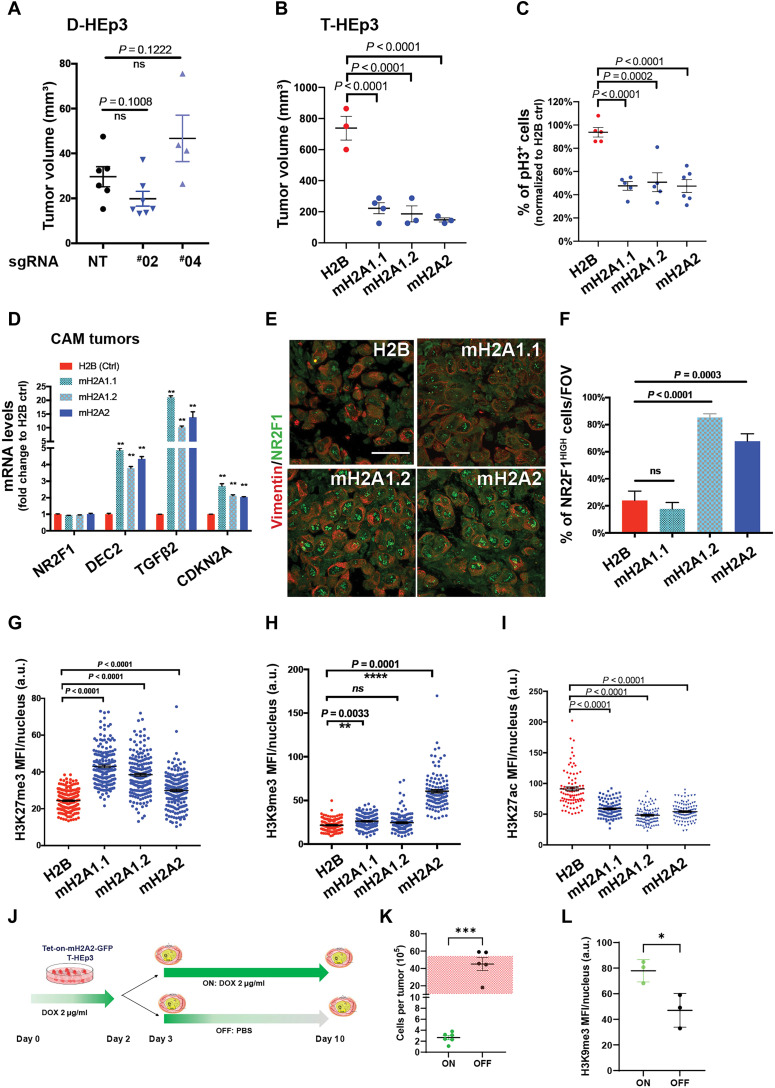
We next tested whether macroH2A1 is essential for the cellular dormancy in D-HEp3 cell variants. We applied CRIPSR-Cas9 knockout or short hairpin RNA (shRNA) methods to deplete macroH2A1 in spontaneously dormant D-HEp3 cells (macroH2A1 is the only variant expressed in D-HEp3 and T-HEp3 cells). D-HEp3 cells proliferate indistinguishably from T-HEp3 cells in vitro but, when inoculated in vivo, enter a growth arrest with <10% of the cells showing any proliferation within 48 hours, with less than one population doubling in the first week and no population doublings after 2 weeks (fig. S3, A to C) (3, 8, 37). We inoculated the engineered D-HEp3 cells in vivo on CAM for 7-day incubation, where, within 48 hours, they enter a long-lasting G0-G1 arrest that can last for at least 2 months (3, 23, 37). These experiments revealed that the dormant phenotype of D-HEp3 cells was not interrupted by macroH2A1 knockout or knockdown as tumor nodules remained small (20 to 50 mm3; Fig. 3A) compared to proliferative T-HEp3 cells that can reach >10 times that volume at the same time (23, 37). This suggests that macroH2A1 alone is not essential for the dormant phenotype of D-HEp3 cells.
Fig. 3. MacroH2A overexpression induces a quiescent phenotype in malignant HNSCC cells.
(A) D-HEp3 tumor growth on CAM with nontargeting guide RNA (NT) and two different guide RNAs targeting macroH2A1 (#02 and #04). n = 6 (NT), 7 (#02), and 4 (#04). Unpaired Student’s t test. (B) Tumor growth on CAM of T-HEp3 cells expressing H2B-GFP (Ctrl) or macroH2A variants. T-HEp3 cells were inoculated onto CAM (1.5 × 105 cells per animal) and transplanted into a new chicken embryo CAM for the second week (n = 3 to 4 tumors). (C) Quantification of percentage of histone H3–S10phos–positive cells on CAM tumors, normalized to H2B control tumors. n = 200 to 300 cells per tumor; n = 5 tumors per condition. One-way ANOVA test. (D) mRNA levels in the indicated 1-week CAM tumors measured by qPCR in triplicate; means + SEM. Multiple t tests with false discovery rate used. (E and F) Images and quantification of the indicated T-HEp3 CAM tumor sections stained for NR2F1 (green) and vimentin (red). Scale bar, 50 μm. (n > 100 cells assessed per tumor, n = 3 tumors per condition); One-way ANOVA test. (G to I) Quantification of IF MFI signal from repressive (H3K27me3 and H3K9me3) and active (H3K27ac) histone modification in the indicated T-HEp3 CAM tumors, n ≈ 200 cells assessed from three tumors per condition. (J) Cartoon of the experiment in (K) and (L). MacroH2A2-GFP expression was induced with DOX 48-hour treatment in vitro. T-HEp3 cells (2 × 105) were inoculated on CAMs. Some CAM tumors were treated with DOX, while the others were treated with PBS for 7 days. (K) Quantification of cell number per tumor in tumors with (ON) or without (OFF) DOX as in (J). n = 5 to 6 tumors. Two-tailed unpaired t test. Red grid box indicates the range of tumor cell numbers detected per T-HEp3 tumor after 7-day incubation (3, 8). (L) Quantification of H3K9me3 MFI via IF in the indicated CAM tumors, n ≈ 200 cells assessed from three tumors per condition.
Thus, we tested whether inducible overexpression of macroH2A might be sufficient to initiate a cellular dormancy mechanism. To test this possibility, we generated T-HEp3 cells overexpressing each individual isoform of macroH2A or the histone H2B as a control [as green fluorescent protein (GFP) fusions; fig. S3D] and tested their growth in vivo on the CAM and followed serial passages of tumors for 2 weeks, which is sufficient to confirm a dormancy-like phenotype (6, 7). Overexpression of all individual macroH2A variants was sufficient to strongly suppress tumor growth in vivo (Fig. 3B). This phenotype was due to reduced proliferation, similar to levels reported in control quiescent D-HEp3 cells (3), as observed by significantly reduced H3S10 phosphorylation and Ki67 (Fig. 3C and fig. S3E). We did not observe increased apoptosis (which was very low at basal levels) nor changes in cleaved caspase-3 abundance in macroH2A overexpressing cells (fig. S3, F and G).
MacroH2A-induced growth arrest in vivo was associated with up-regulation of TGF-β2 and DEC2, as well as the CDK inhibitor CDKN2A/p16 (Fig. 3D). While TGF-β2 can induce macroH2A1 expression in T-HEp3 cells (Fig. 1E), macroH2A variants also induced TGF-β2, suggesting that these genes may be part of an autocrine feedforward loop that initiates and maintains dormancy reprogramming of tumorigenic cancer cells. In contrast, the transcript level of NR2F1 was not affected by ectopic macroH2A variant expression (Fig. 3D). However, IF analysis revealed that macroH2A overexpression triggered a robust accumulation of NR2F1 signal in the nucleus that was observed as a punctate nuclear pattern (Fig. 3, E and F), as we previously reported (27). This is similar to that observed in D-HEp3 cells (fig. S3H) (7), spontaneously dormant DCCs in mice and humans (7, 10), or cells treated with an NR2F1 agonist (27). This change was significant when macroH2A1.2 or macroH2A2 but not macroH2A1.1 was overexpressed (Fig. 3, E and F), suggesting different dormancy mechanisms driven by the variants. NR2F1 and some macroH2A variants may be part of a self-reinforcing mechanisms because an NR2F1 agonist can also induce macroH2A1 expression (Fig. 1, F to I).
Previous studies demonstrated that dormant tumor cells with NR2F1high signal display a repressive chromatin state, with increased H3K9me3 and H3K27me3 nuclear signal (7). Accordingly, we detected an enrichment of repressive chromatin modifications by overexpressing macroH2A variants. These includes an increased H3K27me3 nuclear signal and decreased H3K27ac nuclear signal when overexpressing all individual macroH2A variants, and specifically, macroH2A1.1 overexpression increases the H3K27me3 nuclear signal the most, while macroH2A2 overexpression is much more robust for H3K9me3 (Fig. 3, G to I, and fig. S3, I to K).
Next, we tested the reversibility of the tumor growth arrest phenotype induced by macroH2A2 constitutive overexpression in vitro, which is manifested when the T-HEp3 cells are injected in vivo. To this end, we performed a tumor growth assay on CAM using engineered tetracycline-ON (Tet-ON)–inducible macroH2A2-GFP T-HEp3 cells. Doxycycline (DOX) treatment strongly induced the expression of macroH2A2-GFP 48 hours in vitro before inoculation in CAMs (fig. S3L) but did not affect proliferation in vitro (fig. S3M). Half of the CAM samples were then treated with DOX (to maintain the dormant phenotype (as in the constitutive overexpression; Fig. 3A), while the other half were treated with phosphate-buffered saline (PBS) during the 1-week incubation in CAMs (Fig. 3J). Seven days later, individual tumors were harvested and the number of cells per tumor was counted. This quantification showed that tumors without continuous macroH2A2 expression were able to proliferate, suggesting that the changes that occur during strong overexpression of macroH2A2 are reversible if the expression is not maintained (Fig. 3K and fig. S3N). This reversibility was accompanied by a reduction of H3K9me3 IF signal in growing tumors (Fig. 3L), arguing that this is a reversible growth arrest compatible with quiescence. These data support that macroH2A expression in malignant cancer cells can induce a reversible growth arrest program displaying hallmarks of repressive chromatin akin to spontaneous dormant tumor cells.
MacroH2A2-induced quiescence displays gene expression signatures of dormancy and senescence
While macroH2A2 expression is undetectable in T-HEp3 and D-HEp3 HNSCC cells at the mRNA and protein levels (fig. S1, B and C), its overexpression was sufficient to induce a dormancy-like program. These data led us to focus our attention on this macroH2A variant, as its down-regulation is also associated with melanoma metastasis in patients and acts as the strongest barrier to somatic cell reprogramming of all three macroH2A isoforms (17, 22). To decipher how macroH2A2 is driving cells into a dormancy-like state, we tested whether NR2F1 or DEC2 were required for macroH2A2-induced growth arrest in vivo. Knockdown of DEC2 or NR2F1 in macroH2A2-overexpressing cells did not reverse macroH2A2-induced growth arrest, (NR2F1 KD did partially rescue the increased H3K9me3 IF signals; Fig. 3G), indicating that mRNA up-regulation of DEC2 and nuclear accumulation of NR2F1 were not individually required for the full macroH2A2-induced dormancy phenotype (fig. S4, A to F).
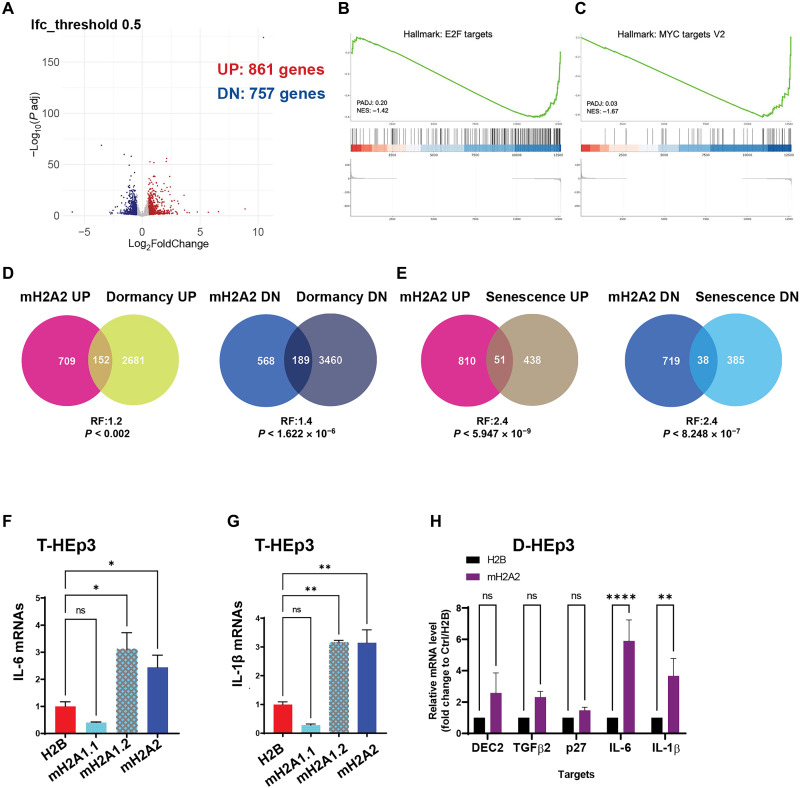
This suggested that macroH2A2 may activate a unique gene expression program distinct from D-HEp3 dormancy. Transcriptomic profiling by RNA-seq of T-HEp3 cells either overexpressing macroH2A2 or H2B control in vitro (2D culture) identified 1618 differentially expressed genes (DEGs), when using a log2 fold change cutoff of 0.5 with 757 genes down-regulated and 861 up-regulated (Fig. 4A; fig. S4, G and H; and data file S1). Pathway analysis using gene set enrichment analysis (GSEA; data files S2 and S3) or Enrichr, a comprehensive resource for curated gene sets dataset (data file S4) (38), supported that macroH2A2 overexpressing cells are poised to promote a tumor growth arrest phenotype when inoculated in vivo. For example, GSEA analysis revealed E2F targets and MYC targets as top negatively regulated hallmark gene sets regulated by macroH2A2 (Fig. 4, B and C). In addition, DNA replication, DNA repair, DNA strand elongation, and other S phase–associated terms were enriched with the down-regulated genes via Enrichr (data file S4) upon macroH2A2 overexpression, consistent with the potent growth arrest in vivo. Enrichr analysis with Molecular Signatures Database (MSigDB) Hallmark Gene Set also revealed that genes associated with cholesterol metabolism were suppressed by macroH2A2 overexpression. Our global DEG analysis supports the fact that lack of macroH2A2 in malignant cells allows for active proliferation and, potentially, cholesterol or other lipids metabolism changes, which may be linked to metabolic reprogramming that happens in the transition from quiescence to proliferation (39, 40). In contrast, the pathway enrichment analysis of up-regulated genes showed epithelial-to-mesenchymal transition seen in melanoma cells undergoing phenotype switching that also can enter dormancy (41), inflammatory response, and circadian rhythm regulation (data file S4).
Fig. 4. MacroH2A2-induced dormancy displays features of spontaneous dormancy and senescence.
(A) Volcano plot displays the DEGs detected by RNA-seq from T-HEp3 cells with mH2A2 versus H2B overexpression. Log2 fold change cutoff is 0.5; 861 up-regulated genes, 757 down-regulated genes. (B and C) Top negatively correlated Hallmark gene sets using RNA-seq data via GSEA. (D) Venn diagrams show the comparison of up-regulated and down-regulated genes in D-HEp3 with up- and down-regulated genes in macroH2A2 overexpressing cells. (E) Venn diagrams show the comparison of up- and down-regulated genes in oncogene-induced senescent fibroblasts (IMR90) with up- and down-regulated genes in macroH2A2 overexpressing T-HEp3 cells. (F and G) qPCR analysis of IL-6 and IL-1β transcript levels in T-HEp3 cells overexpressing individual macroH2A variants. PCR in triplicate, mean + SEM. P value is analyzed via one-way ANOVA. (H) qPCR analysis of dormancy and senescence gene mRNA levels in the indicated D-HEp3 cells with mH2A2 overexpression or H2B as control. PCR in triplicate, mean + SEM.
Enrichr analysis with MSigDB Hallmark Gene Set also (data file S4) revealed that genes commonly associated with inflammatory response or tumor necrosis factor–α signaling via nuclear factor κB programs are up-regulated by macroH2A2 overexpression. A similar trend was detected when using GSEA analysis (data file S2). This suggested that, potentially, cytokines, which are commonly associated with inflammation, might be up-regulated by macroH2A2. Some of these cytokines such as interleukin-6 (IL-6) and IL-1β have been linked to the senescence-associated secretory phenotype (SASP) (42). These associations and the fact that macroH2A is enriched in SAHF (11), led us to compare the macroH2A2 gene expression profile to those of dormant D-HEp3 versus isogenic proliferative T-HEp3 cells (GSE172115) and IMR90 fibroblasts undergoing oncogene-induced senescence versus proliferative fibroblasts (Fig. 4, D and E) (43), allowing us to obtain a comparison of macroH2A2-induced programs and those in dormant and senescent cells. These comparisons revealed that only a small fraction of genes regulated in dormant D-HEp3 cells is also regulated by macroH2A2 (Fig. 4D and data file S5). When we performed the same comparative analysis with senescent cells, we found that macroH2A2 overexpression resulted in an overlapping program although for a limited number of genes (Fig. 4E and data file S6). GSEA analysis of the macroH2A2-regulated genes compared to various senescence-associated signatures also confirmed an overlap with senescence signatures including DNA damage and telomere stress-induced senescence, SASP, and oxidative stress–induced senescence (data file S3). Thus, macroH2A2 induced a strong proliferative arrest, suggesting that a combination of dormancy and senescence subprograms regulated by this unique histone variant may be sufficient to induce a long-term arrest in malignant cells.
When considering the common genes up-regulated by macroH2A2 and senescent cells (data file S3), we found that several cytokines induced by macroH2A2 associated with SASP, or other molecules associated with senescence and aging, were not up-regulated in dormant D-HEp3 versus T-HEp3 cells (data files S5 and S6). These included IL-1β, IL-6, IL-11, growth differentiation factor 15 (GDF15), glial cell line derived neurotrophic factor (GDNF), tenascin C (TNC), laminin subunit alpha 4 (LAMA4), CDKN2A, CDKN1B, high mobility group AT-hook 1 (HMGA1), and HMGA2. We found that overexpression of macroH2A1.2 and macroH2A2, but not macroH2A1.1, increased IL-6 and IL-1β transcript levels via qPCR (Fig. 4, F and G). These data suggest that macroH2A2 may be inducing a specific subset of senescence-associated cytokines. These same genes were induced >5-fold upon macroH2A2 overexpression in D-HEp3 cells (Fig. 4H), suggesting that even in cells carrying a quiescence program of dormancy, macroH2A2 expression could induce senescence-associated genes. We conclude that macroH2A2 induces a dormancy-like program with a combination of quiescence and senescence genes that together may explain the strong induction of quiescence in malignant T-HEp3 cells.
Since primary lesions can still express macroH2A variants (Fig. 2), we wondered whether the expression levels of macroH2A and the enrichment of the macroH2A-regulated gene set described above would inform on overall and relapse-free survival in patients with HNSCC (44). Analysis of these parameters revealed that over a 4-year follow-up, patients carrying primary tumors with a high macroH2A2 gene signature (top 52 genes up-regulated log2 fold change > 1.9 by macroH2A2; see data file S1 for the genes used to define the signature) displayed statistically significantly (log rank P = 0.03) longer relapse-free periods (fig. S4, I and J). We selected up-regulated genes as we hypothesized that these may play an active role in the macroH2A2-induced dormancy and because the major pathways down-regulated were cell cycle programs that are likely less informative. These data suggest that tumors or cells within a tumor carrying a high macroH2A2 expression level may spawn DCCs with a higher propensity to enter dormancy and relapse later than those derived from macroH2A2-low tumors.
MacroH2A2 promotes the accumulation of long-lived dormant DCCs restricting their ability to initiate proliferative metastasis
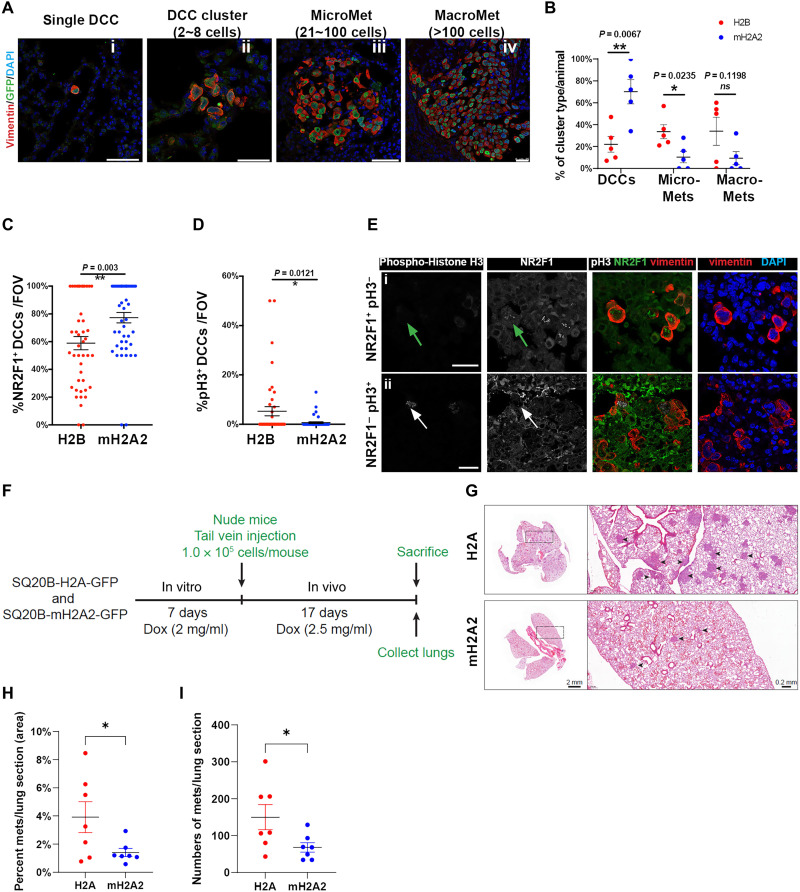
HEp3 cells can form overt metastases in lungs and LNs in either experimental or spontaneous metastasis models (6, 31). We next studied whether macroH2A2 could influence DCC fate in vivo. First, we performed an experimental metastasis assay with T-HEp3 cells expressing either H2B- or macroH2A2-GFP fusions. Three weeks after tail vein injection in nude mice, the lungs were harvested and processed for IF analysis. Using vimentin antibody (6, 7), we scored the frequency of solitary DCCs (including single cells and less than eight-cell clusters), micrometastasis, and large metastatic events in each animal (Fig. 5A). MacroH2A2 overexpression led to a higher frequency of solitary DCCs and fewer micro- and macrometastases in the lungs, compared to the control group (Fig. 5B). DCCs overexpressing macroH2A2 (confirmed by GFP expression) displayed a higher proportion of NR2F1-positive cells (also observed in Fig. 3E), which was inversely correlated with the frequency of H3S10phos-positive DCCs, compared to the H2B-GFP DCCs (Fig. 5, C to E). We also engineered the SQ20B HNSCC cell line that expresses low levels of macroH2A2 (fig. S1C) to carry the inducible Tet-On-macroH2A2-GFP or Tet-On-H2A-GFP (as control) constructs (fig. S5, A and B). These cells were pretreated with DOX to induce the transgene expressions for a week and then inoculated in nude mice. Animals were drinking water with DOX (2.5 mg/ml) for 17 days. Overexpression of macroH2A2 in SQ20B cells also reduced the frequency of metastasis and those that grew were smaller than the control SQ20B cells (Fig. 5, F to I, and fig. S5, C and D). This argues that more cells may be restrained from initiating and/or sustaining metastatic growth in the lung upon macroH2A2 expression. A similar result was observed for SQ20B cells grown in 3D Matrigel, where induction of macroH2A2 retained SQ20B cells as solitary single cells and significantly reduced the number of larger proliferative clusters in this 3D growth assay (fig. S5E). Thus, macroH2A2 can induce DCC growth arrest in different HNSCC models, and in T-HEp3 cells, this was associated with accumulation of the dormancy marker NR2F1. These data argue that macroH2A2 promotes the accumulation of dormant DCCs at the expense of proliferative metastases.
Fig. 5. MacroH2A2 overexpression promotes the accumulation of quiescent DCCs to prevent metastatic growth.
(A) Experimental metastasis assay. T-HEp3 cells with mH2A2-GFP or H2B-GFP expression were tail vein–injected in nude mice. Three weeks later, the lungs were retrieved and processed for FFPE sections. n = 5 animals per condition. Representative IF images of single DCC, DCC cluster (<8 cells), micro-Met, and macro-Met were costained with GFP, human vimentin (red), and DAPI (blue). Scale bar, 50 μm. (B) Quantification of DCC, micro-Met, and large-Met event frequency in the freshly resected lungs in each animal. Single cells and small clusters (<8 cells) are all included as DCC events. Red dots annotate control animals with H2B expression; blue dots annotate experimental animals with mH2A2 overexpression. Mean ± SEM. Two-tailed unpaired t test. (C and D) Quantification of IF staining for NR2F1, H3S10phos phorylation (pH3+), and human vimentin in DCCs found in the lungs per condition. n = 80 fields of view pooled from five animals per condition. (E) Representative IF images of NR2F1 (green), human vimentin (red), and H3S10phos (gray) in lung DCCs. Green arrow, NR2F1-positive cell; white arrow, pH3-positive cell. Scale bar, 25 μm. (F) Schematic depicting the in vivo experimental metastasis assay timeline. (G) Representative images of each group showing metastatic lesions (arrowheads) in lungs stained with H&E. (H and I) Quantification of the percentage of metastatic area (H) and number of metastatic lesions per animal (I). Mann-Whitney test was used for statistical test. n = 7 per group.
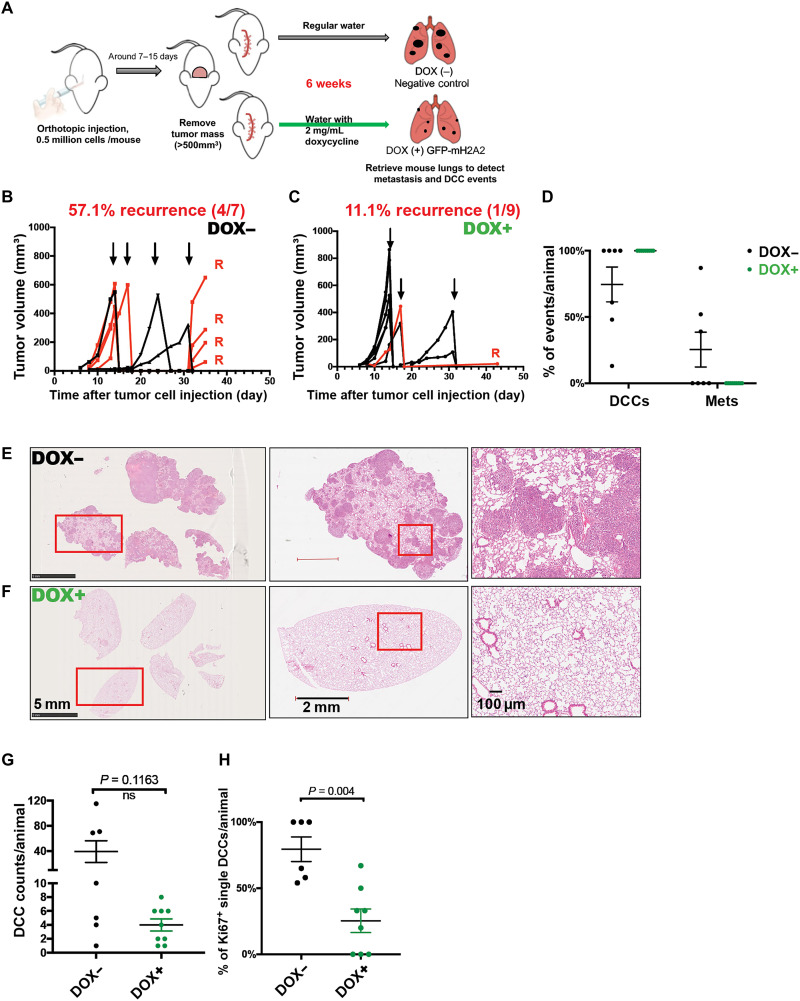
To further test the dormancy-inducing function of macroH2A2 in DCCs, we next performed a spontaneous metastasis assay (Fig. 6A) using engineered Tet-ON–inducible macroH2A2-GFP T-HEp3 cells (fig. S3, L and M). This assay allows the examination of whether an adjuvant “therapeutic” induction of macroH2A2, as it has been tested for oncogene deinduction in inducible mouse models (45, 46), could induce and/or maintain dormancy after cancer cells have completed all steps of dissemination with no macroH2A2 expression. To this end, 5 × 105 T-HEp3 Tet-ON-macroH2A2-GFP cells were injected subcutaneously into nude mice and when primary tumors reached ~500 mm3 they were surgically removed. Animals were then provided with oral treatment (therapeutic phase) of DOX (2 mg/ml) (47). Six weeks after surgery, we found that ~57% of animals in the no DOX treatment control group developed local recurrences in the surgery margins where dormant residual cells persist and fuel recurrences (7). In contrast, animals with inducible expression of macroH2A2 displayed greatly reduced local recurrences, with only 11% of animals relapsing (Fig. 6, B and C). Furthermore, the local recurring lesion expressing macroH2A2 grew at a much slower rate than those developed in the negative control group (Fig. 6, B and C). This indicates that macroH2A2 prevents local residual tumor cells in surgery margins of the primary tumor from reactivating.
Fig. 6. Induction of macroH2A2 after tumor cell dissemination restricts proliferative metastasis.
(A) Scheme of spontaneous metastasis assay. Tet-ON–inducible macroH2A2-GFP T-HEp3 cells were subcutaneously injected in nude mice. Tumors were surgically removed when the size reached ~500 mm3; then, the mice were provided with oral treatment [DOX water (2 mg ml−1), replenished every 48 hours] to induce the expression of macroH2A2-GFP or regular water without DOX. Six weeks later, the lungs were retrieved and processed for FFPE sections. (B and C) Measurement of the primary tumor volumes after the orthotopic injection. Arrows indicate the primary tumor removal times. Red lines annotate animals with local tumor recurrence after the surgery. (D) Frequency measurement of lung DCC and metastasis events in each animal. Single cells and doublets are both considered as DCC events. Black dots represent animals without DOX treatment (DOX−; n = 7), while green dots represent animals with oral DOX (DOX+; n = 9). (E and F) Representative H&E images of animal lungs with metastasis growth in DOX− group and those with no evidence of metastasis in DOX+ group. Red rectangular areas are zoomed in. Scale bars, 5 mm, 2 mm, and 100 μm. (G) Counts of DCC events in each animal, 12 tissue sections pooled from each animal. (H) Quantification of IF staining for Ki67-positive solitary DCCs found in the lungs. Mann-Whitney test.
At the same end point, we additionally quantified the frequency of lung DCC and metastasis in animals with or without DOX treatment using IF with vimentin and hematoxylin and eosin (H&E) staining (Fig. 6, D to F). We found that inducible expression of macroH2A2 suppressed overt metastatic growth (Fig. 6, D to F) that was present in three of seven control group animals (fig. S6, A and B). This was evidenced by inducible expression of macroH2A2 resulting in lungs only carrying solitary and small clusters of DCCs, with not even micrometastases (20 to 100 cells per lesion) being detectable (Fig. 6D). No statistically significant difference in the number of solitary DCCs detected in two groups was detected, suggesting that seeding was similar. However, the proportion of proliferative DCCs (Ki67 positive) is significantly higher in the negative control group (Fig. 6, G and H), arguing that macroH2A2 holds DCCs in a dormant state associated with a NR2F1hi/p-H3lo/Ki67lo profile. Our results indicate that restored macroH2A2 expression is sufficient to keep DCCs in a state of dormancy in lungs.
DISCUSSION
Clinical dormancy can be a long-lived event lasting many years (2). This led us to hypothesize that if DCCs persist in a long-term growth arrest with perhaps punctual divisions, like quiescent adult stem cells (48), convergence of both microenvironmental and epigenetic mechanisms might be necessary to maintain this phenotype. Previous work from our laboratories revealed that a repressive chromatin state found in HNSCC-dormant cancer cells was linked to the TF NR2F1 (7), and in melanoma cancer cells, metastasis-initiating capacity was suppressed by overexpression of macroH2A variants (22). However, whether metastasis suppression by macroH2A variants and dormancy induction were functionally linked was unknown.
Our analysis of the above question revealed that TGF-β2, a prodormancy microenvironmental cue found in the extracellular matrix and in soluble form (6, 26, 31), was able to induce macroH2A1 expression and a dormant phenotype. The p38α/β pathway, which also responds to other microenvironmental signals such as TGF-β1/2 (6, 26), BMP7 (49), and retinoic acid (7, 10), also induces and/or maintains expression of macroH2A1. Activation via an agonist of the orphan nuclear receptor NR2F1, also regulated by BMP7, Wnt5a (41), and retinoic acid (7), again resulted in macroH2A1 variant up-regulation. Thus, it is possible that niche-derived cues that control growth arrest programs of quiescence or differentiation can up-regulate macroH2A1 to induce cancer cell growth suppression. However, in the D-HEp3 dormancy model (23), macroH2A deficiency was insufficient to reverse dormancy. We reasoned that other transcriptional and signaling nodes active in these cells and known to induce dormancy (7, 9) still exert a growth-suppressive effect even in the absence of macroH2A. Nevertheless, stable or inducible overexpression of macroH2A1 or macroH2A2 in malignant HEp3 and SQ20B cells was sufficient to induce a dormancy-like program. MacroH2A variant up-regulation strongly up-regulated TGF-β2, DEC2, and p16, but this induced dormancy program did not depend on the known dormancy TFs, DEC2, and NR2F1 for reasons that remain unknown. Notably, NR2F1 was not induced at the mRNA level but accumulated robustly along with histone H3 repressive marks in the nucleus upon macroH2A variant expression, supporting that it might recruit NR2F1 and possibly other nuclear factors to induce a repressive chromatin state and enforce the growth arrest program.
The above results argued that an additional mechanism was operating downstream of macroH2A to induce growth suppression. RNA-seq analysis revealed that the growth arrest induced by macroH2A2 only partially overlapped with quiescence and senescence gene expression programs found in dormant D-HEp3 cells (7) or senescent fibroblasts (43), respectively. While macroH2A2 induced only a subset of quiescence- and senescence-associated genes, their combination was associated with a strong suppression of metastasis and local recurrence initiation by holding solitary DCCs in a dormant-like state. This program involves cytokines and other genes found in senescent cells, albeit not exclusively and in our system, uniquely regulated by macroH2A2 such as ILs (e.g., IL6), cell cycle inhibitors (e.g., CDKN2A), and chromatin structural proteins (e.g., HMGA1/2). Although unknown, we hypothesize that these may cooperate with TGF-β2 signaling induced by macroH2A2 to induce dormancy. The transcriptional regulators executing these programs downstream of macroH2A2 remain to be identified.
A thought-provoking finding was that overexpression of macroH2A1.2 and macroH2A2, but not macroH2A1.1, was sufficient to induce the expression of the above-mentioned mRNAs commonly found to be produced in senescent cells, but this subset of genes limits the notion that these are fully senescent cells, supported by the reversibility experiments (Fig. 3J). Only the macroH2A1.1 macro domain is described to bind adenosine diphosphate (ADP)–ribose metabolites and ADP-ribosylated proteins, which is not present in the other variants (50) and may have a transcriptionally independent metabolism-regulating function (51). Thus, the selective function of macroH2A1.2 and macroH2A2 found in this model may be attributed to transcriptional regulation functions of these two variants. For example, our data suggest that only macroH2A1.2 and macroH2A2 induce NR2F1 nuclear accumulation. We believe, however, that the induction of SASP genes is likely an indirect effect of the macroH2A-induced growth arrest, rather than direct macroH2A binding at these loci and inducing their expression. MacroH2A1 has been shown to be specifically removed from SASP gene chromatin during oncogene-induced senescence (OIS) (52).
The literature has implicated loss of macroH2A2 as a common event in cancer (20–22), further supporting a restrictive role for cancer progression and metastasis initiation in our studies. Notably, however, the contribution of each variant to tumorigenesis appears highly cell type and context dependent (53), and an intriguing finding along these lines is that while the T-HEp3 cells express some macroH2A1, they were highly proliferative and metastatic at baseline. Nevertheless, overexpression of the same variants was able to reprogram the cells into growth arrest. This suggests that malignant cells may restrict the access of macroH2A variants to loci that control growth suppression programs but that these loci can be re-engaged by available macroH2A molecules. Finding ways to “reposition” macroH2A in malignant cells with basal level expression may activate partial quiescence and senescence programs.
The fact that macroH2A histone variant expression can persist to a certain level without causing growth suppression was also observed, as both macroH2A1 and macroH2A2 are present in aggressively proliferative lesions in head and neck primary tumor patient samples. However, an important distinction was that the solitary and potentially dormant cells robustly up-regulate the levels of macroH2A variants, and the expression of all variants significantly decreases in proliferative metastatic lesions. These data suggest that during progression, cancer cells can spontaneously up-regulate macroH2A variants to adapt to the switch from proliferation to quiescence but that metastatic growth is associated with a down-regulation of all variants consistent with our findings in melanoma (22).
We reveal that macroH2A histone variants can suppress metastasis by tapping into unique and a limited set of genes found in senescent and dormant D-HEp3 cells. These results suggest that to induce and maintain dormancy, activating a subset of components of distinct growth arrest programs may be sufficient. We further reveal that macroH2A1/2 variant function could be exploited for such a purpose and that their detection (or a macroH2A-regulated signature) in primary lesions or in DCCs might be useful biomarkers of patients with better prognosis and/or dormant DCCs and could be used in clinical trials to phenotypically profile DCCs (10, 26).
MATERIALS AND METHODS
Cell lines and tumor growth studies
Tumorigenic (T-HEp3) HEp3 cells were derived from a LN metastasis from a patient with HNSCC as described previously (37). D-HEp3 cells were obtained by passing T-HEp3 cells for more than 40 generations in vitro (37). When cultured in vitro, all these cells were passaged in DMEM cell growth medium [Dulbecco’s minimum essential medium with 10% of fetal bovine serum (FBS) and penicillin (100 U)/streptomycin (0.1 mg ml−1)]. MacroH2A-overexpressing cells were generated by plKO-GFP-macroH2A–encoding lentivirus infection of T-HEp3 cells then selected with puromycin (2.0 mg ml−1). Cells were routinely tested for mycoplasma (PCR Mycoplasma test kit PK-CA91-1096, PromoCell).
Tumor growth on chicken embryo CAMs (3) or Balb/c nude mice (Charles River Laboratories, #490) was performed as described previously (23). All animal studies were approved by Institutional Animal Care and Use Committees (IACUC) at Mount Sinai School of Medicine (protocol ID: 08-0366). Briefly, 150 × 103 to 200 × 103 T-HEp3 cells were inoculated on the CAM and allowed to grow for the indicated times in each experiment (from 4 to 7 days). Dormant D-HEp3 cells were inoculated onto CAMs at 500 × 103 cells per egg and allowed to grow for the indicated times in experiments (from 5 to 7 days). For TGF-β2 (R&D Systems, 302-B2) experiments on CAM, HEp3 cells were pretreated in vitro for 24 hours and then inoculated on CAMs for 6 days. CAM tumors were treated every day with TGF-β2 (10 ng ml−1). For small interfering RNA (siRNA) experiments on CAM, HEp3 cells were pretreated in vitro with scramble RNA or siRNA for 48 hours and then inoculated on CAMs for 5 days.
For HEp3 experimental metastasis assay, 3 × 105 cells were tail vein injected into BALB/c nu/nu female mice (4 to 6 weeks). Mice were inspected every 48 hours and were kept for 1 or 3 weeks. At the end of week 1 or week 3, mice were euthanized, and lungs were collected.
For SQ20B experimental metastasis assay, inducible H2A-GFP (as a control) and mH2A2-GFP SQ20B cell lines were treated with DOX (2 μg/ml) in culture for 7 days. Cells were harvested, and 1 × 105 cells were injected intravenously to each animal (n = 7 per group). Eight-week-old female mice were used for this experiment. After injection, mice were treated with DOX (2.5 mg/ml) in water with 1% sucrose for 17 days. At the end of the experiment, animals were euthanized, lungs were collected and fixed in 4% formaldehyde, and tissues were used for H&E staining. H&E images were scanned using Fast Scanner at Imaging Facility at the Albert Einstein College of Medicine. Scanned images were further analyzed using CaseViewer v2.4. This animal study was approved by the IACUC of the Albert Einstein College of Medicine.
For HEp3 spontaneous metastasis studies, 5 × 105 cells were injected subcutaneously into BALB/c nu/nu female mice (4 to 6 weeks) in the interscapular region. Mice were inspected every 48 hours and were kept (∼14 days) until primary tumors developed and grew up to >500 mm3. After surgical removal of the primary tumors, animals were randomly assigned to two groups. One as a negative control group was provided with regular drinking water, while the other group was provided with drinking water containing DOX (2 mg/ml). Drinking water was replenished every 48 hours. Animals were monitored for local tumor recurrence every 48 hours for 6 weeks. At the end, mice were euthanized and lungs were collected.
For NR2F1 agonist (C26 compound treatment), 3D organoid assay, CAM tumor assay, and spontaneous metastasis assay with C26 compound treatment were described previously (27).
3D Matrigel colony assay
SQ20B cells were treated with DOX (2 μg/ml) for 72 hours in culture to induce the expression of H2A-GFP or mH2A2-GFP. Then, 1000 cells were seeded in 40 μl of growth factor–reduced Matrigel (Corning, #356231), covered with the growth medium with reduced FBS content (5%) in eight-well chamber slides (Falcon). Cultures were treated with DOX (2 μg/ml). and 3 days later, single cells and clusters (more than four-cell colonies) were counted using a light microscope.
Human specimens
Paraffin-embedded sections from HNSCC primary tumors and matched LN metastasis lesions were obtained from the Cancer Biorepository at Icahn School of Medicine at Mount Sinai, New York, NY, USA. Paraffin-embedded tissue sections from LNs biopsied from clinically diagnosed patients with metastasis-free HNSCC were obtained from C. Sproll at the Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany (35). These patients presented small primary tumors, had no previously diagnosed malignancy in the head and neck region, and had not been subject to previous treatment. Samples were deidentified and obtained with institutional review board approval. FFPE samples were stained with pan-cytokeratin in red macroH2A1 or macroH2A2 in green and histone H3 in blue. Fields of view (n = 6) were assessed in each condition. Mean fluorescent intensity (MFI) of macroH2A1, macroH2A2, and histone H3 was quantitatively measured in ImageJ. Cells with macroH2A1/H3 MFI ratio higher than 0.75 were considered as macroH2A1HIGH. Cells with macroH2A2/H3 MFI ratio higher than 0.6 were considered as macroH2A2 HIGH. To normalize the signal abundance in PTs across the patient cohort 1 (U.S. patients in Fig. 2, A and B) and cohort 2 (German patients in Fig. 2, C and D), the mean macroH2A1 or macroH2A2 MFI of PT in both cohorts was used as baseline expression, and relative macroH2A1 or macroH2A2 MFI was calculated via the fold change to the corresponding PT baselines.
Plasmids
Constitutively expressed human H2B-GFP and macroH2A2-GFP fusions were previously described (17). Human macroH2A1.1 and macroH2A1.2-GFP fusions (transcript IDs XM_005272132.2 and NM_004893.3, respectively) were cloned into the pLKO.1 vector. Inducible H2A-GFP, and macroH2A2-GFP constructs were generated as described (54).
Quantitative reverse transcriptome PCR
Total RNA was isolated from HEp3 cells using TRIzol reagent (Invitrogen), and 2 μg of RNA was reverse-transcribed using M-MuLV reverse transcriptase enzyme (New England Biolabs, #M0253S) following the manufacturer’s instructions. PCR was performed using PowerUp SYBR Green Master Mix (Applied Biosystems, #A25741). Primers were purchased from IDT. Human tubulin was used as housekeeping gene control for all experiments. See table S1 for primer sequences.
Immunofluorescence
Paraffin-embedded tissue sections were incubated in xylene (5 min twice), followed by graded ethanol rehydration (3 min each). Antigen retrieval for human primary tumors, LN metastasis tissues, mouse lung tissues, and T-HEp3 and D-HEp3 tumors was performed in 10 mM citrate buffer (pH 6) for 40 min using a steamer. Tissues were permeabilized in 0.1% Triton X-100 + PBS for 10 min. Tissue sections were blocked with 3% bovine serum albumin (Fisher Bioreagents) and 5% normal goat serum (Gibco, #PCN5000) in PBS for 1 hour at room temperature. Primary antibodies (listed in table S2) in blocking buffer were incubated overnight at 4°C followed by washing and incubation with Alexa Fluor–conjugated secondary antibodies (1:1000 dilution; Invitrogen) at room temperature for 1 to 2 hours in the dark. Hoechst 33432 was used to stain nucleus (10 min) at room temperature. Slides were mounted with ProLong Gold Antifade reagent (Invitrogen, no. P36930). Images were obtained using Leica Software on a Leica SPE confocal microscope and analyzed using ImageJ software.
RNA interference
Gene-specific siRNAs (listed in table S3) were transfected to HEp3 cells at a final concentration of 40 nM using siPORT NeoFX Transfection Agent (Thermo Fisher Scientific, #AM4510). Lentiviral vectors of shRNAs were produced in human embryonic kidney (HEK) 293T cells, and viral supernatants were used to infect D-HEp3 cells. Detailed information of shRNA generation was described previously (22).
Generation of macroH2A1 knockout cell lines
macroH2A1-knockout cell lines were generated using CRISPR-Cas9–targeted genome editing. Briefly, two separate macroH2A1 guide RNAs were designed and cloned into pLentiCRISPRv2 (Addgene, #52961) (55). A nontargeting guide RNA was used as a control. Lentiviral vectors were produced in HEK293T cells, and viral supernatants were used to infect D-HEp3 cells. Cells were then selected using puromycin (Sigma-Aldrich, no. P8833). macroH2A1 knockout was confirmed by Western blot. See table S3 for guide RNA sequences.
RNA-sequencing
RNA extraction, library preparations, and sequencing were conducted as described (27). Sequencing on Illumina NextSeq 500 was performed at the ISMMS Sinai Genomics Core Facility, using the standard manufacture procedures. A detailed method of RNA-seq and analysis was described previously (27).
RNA-seq alignment and quality control
A total of five RNA-seq library (three replicates for overexpression and two for control) systems were processed using the same pipeline for compatibility. Quality control was performed using FastQC (v0.11.8) (56). Trim Galore! (version 0.6.5) was used to trim the adapter sequences with a quality threshold of 20 (57). The human genome reference used was GRCh38.p13, and GENCODE release 36 was used as the transcriptome reference. The alignment is performed by using STAR aligner (v2.7.5b) (58). Gene level read counts were obtained by using Salmon (v1.2.1) for all libraries (59). All samples passed the quality control requirements with >85% of reads uniquely mapping (>14 million uniquely mapped reads for each library) using STAR aligner.
Differential gene expression and functional analysis
Differential expression analysis was performed using the gene level read counts and the DESeq2 (v1.28.1) R package (60). Genes with less than five reads in total across all samples are filtered as inactive genes. A gene is considered differentially expressed if the adjusted P value is less than 0.05 and the absolute fold change is greater than 0.5.
GSEA analysis with RNA-seq data of T-HEp3 cells either overexpressing macroH2A2 or H2B tested the enrichment of ranked genes against molecular Signature Database Hallmark gene sets (see data files S2 and S3). Enrichr analysis of differentially expressed up- or down-regulated genes by macroH2A2 overexpression was performed. The enrichment analysis is performed with MSigDB Hallmark gene sets, Kyoto Encyclopedia of Genes and Genomes Pathway Dataset, and NCATS BioPlanet resource (61) separately (see data file S4).
Principal components analysis and heatmaps
We performed the between sample normalization using the variance stabilizing transformation of the DESeq2 package. The 500 most variable genes are used to perform principal components analysis and calculate the Euclidean distances between each sample. Gene expression heatmaps show the z scores of DESeq2 VST normalized gene level read counts. The heatmaps were generated using all the DEGs identified to give an overview of the transcriptomic changes. All visualizations are generated using plotly R package (v4.9.2.1) except for heatmaps and volcano plots (62). The heatmaps and volcano plots are generated using heatmaply (v1.1.0) and Glimma (v1.16.0) R packages, respectively (63, 64). R (v.4.0.3) is used to perform all bioinformatics analysis.
Statistical analysis
All in vitro experiments were repeated at least three times unless indicated otherwise. For CAM tumor growth analysis, a minimum of four tumors were analyzed per group/experiment. For mouse experiments, a minimum of five mice per group were used for tumor growth, DCC, and metastatic burden measurement and immunostaining analysis. Statistical analysis was performed on Prism software using unpaired t test, Mann-Whitney test, and two-way analysis of variance (ANOVA) with multiple comparison test. A P value ≤0.05 was considered significant.
Acknowledgments
We thank the Aguirre-Ghiso, Sosa, and Bernstein laboratories for helpful discussions. J.A.A.-G. is a Samuel Waxman Cancer Research Foundation Investigator. We thank the Oncological Sciences Sequencing Core supported by Tisch Cancer Institute (TCI) of the Icahn School of Medicine at Mount Sinai (ISMMS) and the Mount Sinai Genomics Technology Facility and D. Demircioglu of the Bioinformatics for Next Generation Sequencing (BiNGS) shared resource facility within the TCI at ISMMS.
Funding: This work was supported by grants from NIH/National Cancer Institute (NCI) (CA109182, CA216248, CA218024, and CA196521 to J.A.A.-G. and R01CA154683 and CA218024 to E.B.). RNA-seq was supported, in part, through the Oncological Sciences Sequencing Core supported by TCI of the ISMMS Cancer Center Support Grant P30 (CA196521), Scientific Computing supported by the Office of Research Infrastructure of the NIH under award number S10OD026880 to ISMMS, and the BiNGS shared resource facility within the TCI at ISMMS, partially supported by P30 (CA196521).
Author contributions: D.S. designed, planned, and conducted experiments; analyzed data; and wrote the manuscript. D.S., D.K.S., D.F., and X.H. performed in vivo mouse experiments, and D.S. and D.K.S. further processed in vivo material. D.S., S.C., D.H., and D.K.S. performed RNA-seq experiments and analyzed data. B.A.M., W.W., and K.C.S. provided human tissue samples and assisted tissue anatomical analysis. B.K. performed the experiments in Fig. 1, F to I. J.A.A.-G. conceived the project, designed experiments, analyzed data, and wrote the manuscript. E.B. analyzed data, secured funding, and edited the manuscript.
Competing interests: J.A.A.-G. is a scientific cofounder, scientific advisory board member, and equity owner in HiberCell and receives financial compensation as a consultant for HiberCell, a Mount Sinai spin-off company focused on therapeutics that prevent or delay cancer recurrence. The other authors declare that they have no competing interests.
Data and materials availability: All datasets from this study have been uploaded to the Gene Expression Omnibus (GEO). RNA-seq dataset of T-HEp3 cells with macroH2A2 overexpression is accessible with the code of GSE182459. Previously published RNA-seq dataset of T-HEp3 versus D-HEp3 cells and H3K27ac, H3K27me3, and H3K4me1 ChIP-seq in T-HEp3 and D-HEp3 cell lines are available under the accession codes GSE172115 and GSE181838. Previously published RNA-seq dataset of oncogene-induced senescent human fibroblasts is available under the accession code GSE55949. All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Figs. S1 to S6
Tables S1 to S3
Other Supplementary Material for this manuscript includes the following:
Data files S1 to S6
REFERENCES AND NOTES
- 1.Risson E., Nobre A. R., Maguer-Satta V., Aguirre-Ghiso J. A., The current paradigm and challenges ahead for the dormancy of disseminated tumor cells. Nat. Cancer 1, 672–680 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phan T. G., Croucher P. I., The dormant cancer cell life cycle. Nat. Rev. Cancer 20, 398–411 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Aguirre Ghiso J. A., Kovalski K., Ossowski L., Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. J. Cell Biol. 147, 89–104 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crist S. B., Ghajar C. M., When a house is not a home: A survey of antimetastatic niches and potential mechanisms of disseminated tumor cell suppression. Annu. Rev. Pathol. 16, 409–432 (2021). [DOI] [PubMed] [Google Scholar]
- 5.M. S. Sosa, E. Bernstein, J. A. Aguirre-Ghiso, Epigenetic regulation of cancer dormancy as a plasticity mechanism for metastasis initiation, in Tumor Dormancy and Recurrence. Y. Wang, F. Crea, Eds. (Springer International Publishing, 2017), pp. 1–16. [Google Scholar]
- 6.Bragado P., Estrada Y., Parikh F., Krause S., Capobianco C., Farina H. G., Schewe D. M., Aguirre-Ghiso J. A., TGF-β2 dictates disseminated tumour cell fate in target organs through TGF-β-RIII and p38α/β signalling. Nat. Cell Biol. 15, 1351–1361 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sosa M. S., Parikh F., Maia A. G., Estrada Y., Bosch A., Bragado P., Ekpin E., George A., Zheng Y., Lam H.-M., Morrissey C., Chung C.-Y., Farias E. F., Bernstein E., Aguirre-Ghiso J. A., NR2F1 controls tumour cell dormancy via SOX9- and RARβ-driven quiescence programmes. Nat. Commun. 6, 6170 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aguirre-Ghiso J. A., Liu D., Mignatti A., Kovalski K., Ossowski L., Urokinase receptor and fibronectin regulate the ERK(MAPK) to p38(MAPK) activity ratios that determine carcinoma cell proliferation or dormancy in vivo. Mol. Biol. Cell 12, 863–879 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adam A. P., George A., Schewe D., Bragado P., Iglesias B. V., Ranganathan A. C., Kourtidis A., Conklin D. S., Aguirre-Ghiso J. A., Computational identification of a p38SAPK-regulated transcription factor network required for tumor cell quiescence. Cancer Res. 69, 5664–5672 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borgen E., Rypdal M. C., Sosa M. S., Renolen A., Schlichting E., Lønning P. E., Synnestvedt M., Aguirre-Ghiso J. A., Naume B., NR2F1 stratifies dormant disseminated tumor cells in breast cancer patients. Breast Cancer Res. 20, 120 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang R., Poustovoitov M. V., Ye X., Santos H. A., Chen W., Daganzo S. M., Erzberger J. P., Serebriiskii I. G., Canutescu A. A., Dunbrack R. L., Pehrson J. R., Berger J. M., Kaufman P. D., Adams P. D., Formation of macroH2A-containing senescence-associated heterochromatin foci and senescence driven by ASF1a and HIRA. Dev. Cell 8, 19–30 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Salama R., Sadaie M., Hoare M., Narita M., Cellular senescence and its effector programs. Genes Dev. 28, 99–114 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghiraldini F. G., Filipescu D., Bernstein E., Solid tumours hijack the histone variant network. Nat. Rev. Cancer 21, 257–275 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao S., Allis C. D., Wang G. G., The language of chromatin modification in human cancers. Nat. Rev. Cancer 21, 413–430 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sogabe Y., Seno H., Yamamoto T., Yamada Y., Unveiling epigenetic regulation in cancer, aging, and rejuvenation with in vivo reprogramming technology. Cancer Sci. 109, 2641–2650 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costanzi C., Pehrson J. R., Histone macroH2A1 is concentrated in the inactive X chromosome of female mammals. Nature 393, 599–601 (1998). [DOI] [PubMed] [Google Scholar]
- 17.Gaspar-Maia A., Qadeer Z. A., Hasson D., Ratnakumar K., Leu N. A., Leroy G., Liu S., Costanzi C., Valle-Garcia D., Schaniel C., Lemischka I., Garcia B., Pehrson J. R., Bernstein E., MacroH2A histone variants act as a barrier upon reprogramming towards pluripotency. Nat. Commun. 4, 1565 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrero M. J., Sese B., Kuebler B., Bilic J., Boue S., Martí M., Belmonte J. C. I., Macrohistone variants preserve cell identity by preventing the gain of H3K4me2 during reprogramming to pluripotency. Cell Rep. 3, 1005–1011 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Pasque V., Gillich A., Garrett N., Gurdon J. B., Histone variant macroH2A confers resistance to nuclear reprogramming. EMBO J. 30, 2373–2387 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu W.-H., Miyai K., Sporn J. C., Luo L., Wang J. Y. J., Cosman B., Ramamoorthy S., Loss of histone variant macroH2A2 expression associates with progression of anal neoplasm. J. Clin. Pathol. 69, 627–631 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sporn J. C., Kustatscher G., Hothorn T., Collado M., Serrano M., Muley T., Schnabel P., Ladurner A. G., Histone macroH2A isoforms predict the risk of lung cancer recurrence. Oncogene 28, 3423–3428 (2009). [DOI] [PubMed] [Google Scholar]
- 22.Kapoor A., Goldberg M. S., Cumberland L. K., Ratnakumar K., Segura M. F., Emanuel P. O., Menendez S., Vardabasso C., Roy G. L., Vidal C. I., Polsky D., Osman I., Garcia B. A., Hernando E., Bernstein E., The histone variant macroH2A suppresses melanoma progression through regulation of CDK8. Nature 468, 1105–1109 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aguirre-Ghiso J. A., Ossowski L., Rosenbaum S. K., Green fluorescent protein tagging of extracellular signal-regulated kinase and p38 pathways reveals novel dynamics of pathway activation during primary and metastatic growth. Cancer Res. 64, 7336–7345 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Aguirre-Ghiso J. A., Estrada Y., Liu D., Ossowski L., ERK(MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38(SAPK). Cancer Res. 63, 1684–1695 (2003). [PubMed] [Google Scholar]
- 25.Ossowski L., Reich E., Loss of malignancy during serial passage of human carcinoma in culture and discordance between malignancy and transformation parameters. Cancer Res. 40, 2310–2315 (1980). [PubMed] [Google Scholar]
- 26.Nobre A. R., Risson E., Singh D. K., Di Martino J. S., Cheung J. F., Wang J., Johnson J., Russnes H. G., Bravo-Cordero J. J., Birbrair A., Naume B., Azhar M., Frenette P. S., Aguirre-Ghiso J. A., Bone marrow NG2+/Nestin+ mesenchymal stem cells drive DTC dormancy via TGF-β2. Nat. Cancer 2, 327–339 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khalil B. D., Sanchez R., Rahman T., Rodriguez-Tirado C., Moritsch S., Martinez A. R., Miles B., Farias E., Mezei M., Nobre A. R., Singh D., Kale N., Sproll K. C., Sosa M. S., Aguirre-Ghiso J. A., An NR2F1-specific agonist suppresses metastasis by inducing cancer cell dormancy. J. Exp. Med. 219, e20210836 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goddard E. T., Bozic I., Riddell S. R., Ghajar C. M., Dormant tumour cells, their niches and the influence of immunity. Nat. Cell Biol. 20, 1240–1249 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Dasgupta A., Lim A. R., Ghajar C. M., Circulating and disseminated tumor cells: Harbingers or initiators of metastasis? Mol. Oncol. 11, 40–61 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naume B., Synnestvedt M., Falk R. S., Wiedswang G., Weyde K., Risberg T., Kersten C., Mjaaland I., Vindi L., Sommer H. H., Sætersdal A. B., Rypdal M. C., Schirmer C. B., Wist E. A., Borgen E., Clinical outcome with correlation to disseminated tumor cell (DTC) status after DTC-guided secondary adjuvant treatment with docetaxel in early breast cancer. J. Clin. Oncol. 32, 3848–3857 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Fluegen G., Avivar-Valderas A., Wang Y., Padgen M. R., Williams J. K., Nobre A. R., Calvo V., Cheung J. F., Bravo-Cordero J. J., Entenberg D., Castracane J., Verkhusha V., Keely P. J., Condeelis J., Aguirre-Ghiso J. A., Phenotypic heterogeneity of disseminated tumour cells is preset by primary tumour hypoxic microenvironments. Nat. Cell Biol. 19, 120–132 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ossowski L., Russo-Payne H., Wilson E. L., Inhibition of urokinase-type plasminogen activator by antibodies: The effect on dissemination of a human tumor in the nude mouse. Cancer Res. 51, 274–281 (1991). [PubMed] [Google Scholar]
- 33.Ossowski L., Russo H., Gartner M., Wilson E. L., Growth of a human carcinoma (HEp3) in nude mice: Rapid and efficient metastasis. J. Cell. Physiol. 133, 288–296 (1987). [DOI] [PubMed] [Google Scholar]
- 34.Sproll C., Fluegen G., Stoecklein N. H., Minimal residual disease in head and neck cancer and esophageal cancer. Adv. Exp. Med. Biol. 1100, 55–82 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Sproll C., Freund A. K., Hassel A., Hölbling M., Aust V., Storb S. H., Handschel J., Teichmann C., Depprich R., Behrens B., Neves R. P. L., Kübler N. R., Kaiser P., Baldus S. E., Tóth C., Kaisers W., Stoecklein N. H., Immunohistochemical detection of lymph node-DTCs in patients with node-negative HNSCC. Int. J. Cancer 140, 2112–2124 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Chéry L., Lam H.-M., Coleman I., Lakely B., Coleman R., Larson S., Aguirre-Ghiso J. A., Xia J., Gulati R., Nelson P. S., Montgomery B., Lange P., Snyder L. A., Vessella R. L., Morrissey C., Characterization of single disseminated prostate cancer cells reveals tumor cell heterogeneity and identifies dormancy associated pathways. Oncotarget 5, 9939–9951 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ossowski L., Reich E., Changes in malignant phenotype of a human carcinoma conditioned by growth environment. Cell 33, 323–333 (1983). [DOI] [PubMed] [Google Scholar]
- 38.Kuleshov M. V., Jones M. R., Rouillard A. D., Fernandez N. F., Duan Q., Wang Z., Koplev S., Jenkins S. L., Jagodnik K. M., Lachmann A., McDermott M. G., Monteiro C. D., Gundersen G. W., Ma’ayan A., Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 44, W90–W97 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee H.-J., Jedrychowski M. P., Vinayagam A., Wu N., Shyh-Chang N., Hu Y., Min-Wen C., Moore J. K., Asara J. M., Lyssiotis C. A., Perrimon N., Gygi S. P., Cantley L. C., Kirschner M. W., Proteomic and metabolomic characterization of a mammalian cellular transition from quiescence to proliferation. Cell Rep. 20, 721–736 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Angius F., Spolitu S., Uda S., Deligia S., Frau A., Banni S., Collu M., Accossu S., Madeddu C., Serpe R., Batetta B., High-density lipoprotein contribute to G0-G1/S transition in Swiss NIH/3T3 fibroblasts. Sci. Rep. 5, 17812 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fane M. E., Chhabra Y., Alicea G. M., Maranto D. A., Douglass S. M., Webster M. R., Rebecca V. W., Marino G. E., Almeida F., Ecker B. L., Zabransky D. J., Hüser L., Beer T., Tang H.-Y., Kossenkov A., Herlyn M., Speicher D. W., Xu W., Xu X., Jaffee E. M., Aguirre-Ghiso J. A., Weeraratna A. T., Stromal changes in the aged lung induce an emergence from melanoma dormancy. Nature 606, 396–405 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Acosta J. C., Banito A., Wuestefeld T., Georgilis A., Janich P., Morton J. P., Athineos D., Kang T.-W., Lasitschka F., Andrulis M., Pascual G., Morris K. J., Khan S., Jin H., Dharmalingam G., Snijders A. P., Carroll T., Capper D., Pritchard C., Inman G. J., Longerich T., Sansom O. J., Benitah S. A., Zender L., Gil J., A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 15, 978–990 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duarte L. F., Young A. R. J., Wang Z., Wu H.-A., Panda T., Kou Y., Kapoor A., Hasson D., Mills N. R., Ma’ayan A., Narita M., Bernstein E., Histone H3.3 and its proteolytically processed form drive a cellular senescence programme. Nat. Commun. 5, 5210 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagy A., Munkacsy G., Gyorffy B., Pancancer survival analysis of cancer hallmark genes. Sci. Rep. 11, 6047 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruth J. R., Pant D. K., Pan T.-C., Seidel H. E., Baksh S. C., Keister B. A., Singh R., Sterner C. J., Bakewell S. J., Moody S. E., Belka G. K., Chodosh L. A., Cellular dormancy in minimal residual disease following targeted therapy. Breast Cancer Res. 23, 63 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shachaf C. M., Kopelman A. M., Arvanitis C., Karlsson A., Beer S., Mandl S., Bachmann M. H., Borowsky A. D., Ruebner B., Cardiff R. D., Yang Q., Bishop J. M., Contag C. H., Felsher D. W., MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer. Nature 431, 1112–1117 (2004). [DOI] [PubMed] [Google Scholar]
- 47.Redelsperger I. M., Taldone T., Riedel E. R., Lepherd M. L., Lipman N. S., Wolf F. R., Stability of doxycycline in feed and water and minimal effective doses in tetracycline-inducible systems. J. Am. Assoc. Lab. Anim. Sci. 55, 467–474 (2016). [PMC free article] [PubMed] [Google Scholar]
- 48.Wilson A., Laurenti E., Oser G., van der Wath R. C., Blanco-Bose W., Jaworski M., Offner S., Dunant C. F., Eshkind L., Bockamp E., Lió P., Macdonald H. R., Trumpp A., Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135, 1118–1129 (2008). [DOI] [PubMed] [Google Scholar]
- 49.Kobayashi A., Okuda H., Xing F., Pandey P. R., Watabe M., Hirota S., Pai S. K., Liu W., Fukuda K., Chambers C., Wilber A., Watabe K., Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J. Exp. Med. 208, 2641–2655 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Timinszky G., Till S., Hassa P. O., Hothorn M., Kustatscher G., Nijmeijer B., Colombelli J., Altmeyer M., Stelzer E. H. K., Scheffzek K., Hottiger M. O., Ladurner A. G., A macrodomain-containing histone rearranges chromatin upon sensing PARP1 activation. Nat. Struct. Mol. Biol. 16, 923–929 (2009). [DOI] [PubMed] [Google Scholar]
- 51.Marjanović M. P., Hurtado-Bagès S., Lassi M., Valero V., Malinverni R., Delage H., Navarro M., Corujo D., Guberovic I., Douet J., Gama-Perez P., Garcia-Roves P. M., Ahel I., Ladurner A. G., Yanes O., Bouvet P., Suelves M., Teperino R., Pospisilik J. A., Buschbeck M., MacroH2A1.1 regulates mitochondrial respiration by limiting nuclear NAD+ consumption. Nat. Struct. Mol. Biol. 24, 902–910 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen H., Ruiz P. D., McKimpson W. M., Novikov L., Kitsis R. N., Gamble M. J., MacroH2A1 and ATM play opposing roles in paracrine senescence and the senescence-associated secretory phenotype. Mol. Cell 59, 719–731 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hsu C.-J., Meers O., Buschbeck M., Heidel F. H., The role of macroH2A histone variants in cancer. Cancers (Basel) 13, 3003 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun Z., Filipescu D., Andrade J., Gaspar-Maia A., Ueberheide B., Bernstein E., Transcription-associated histone pruning demarcates macroH2A chromatin domains. Nat. Struct. Mol. Biol. 25, 958–970 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sanjana N. E., Shalem O., Zhang F., Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods 11, 783–784 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.S. Andrews, (Babraham Bioinformatics, 2010).
- 57.F. Krueger (Babraham Bioinformatics, Babraham Institute, 2012).
- 58.Dobin A., Davis C. A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T. R., STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patro R., Duggal G., Love M. I., Irizarry R. A., Kingsford C., Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14, 417–419 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Love M. I., Huber W., Anders S., Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang R., Grishagin I., Wang Y., Zhao T., Greene J., Obenauer J. C., Ngan D., Nguyen D.-T., Guha R., Jadhav A., Southall N., Simeonov A., Austin C. P., The NCATS bioplanet—An integrated platform for exploring the universe of cellular signaling pathways for toxicology, systems biology, and chemical genomics. Front. Pharmacol. 10, 445 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.C, S. (Chapman and Hall, 2020).
- 63.Galili T., O’Callaghan A., Sidi J., Sievert C., heatmaply: An R package for creating interactive cluster heatmaps for online publishing. Bioinformatics 34, 1600–1602 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Su S., Law C. W., Ah-Cann C., Asselin-Labat M.-L., Blewitt M. E., Ritchie M. E., Glimma: Interactive graphics for gene expression analysis. Bioinformatics 33, 2050–2052 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S6
Tables S1 to S3
Data files S1 to S6