Abstract
Parenteral administration of a mouse anti-human CD8 monoclonal antibody (MAb) to rhesus macaques resulted in a transient depletion of CD8+ cells in both the peripheral blood and lymphoid tissues. When administered during primary chimeric simian/human immunodeficiency virus infections, the CD8 MAb caused marked elevations of plasma and cell-associated virus levels in both the peripheral blood and lymphoid tissues and led to prolonged depletion of CD4 cells. Taken together, these results directly demonstrate that CD8+ T lymphocytes are actively involved in controlling the acute phase of primate lentivirus infections.
CD8 cytotoxic T-lymphocyte (CTL) responses are critical for the resolution of many viral infections (6, 21, 24, 29, 33). In human immunodeficiency virus type 1 (HIV-1) infections, a CTL response that occurs prior to seroconversion has been temporally linked with falling virus loads (3, 19, 27). A similar correlation has been reported for CTL suppression of acute simian immunodeficiency virus (SIV) infections (13, 34). Although it has been inferred that the CTL response mediates the resolution of the primary HIV-1 infection, direct proof that CD8+ T lymphocytes are responsible for the decline of viremia during acute lentivirus infections and/or the control of virus spread during chronic infection has not yet been obtained. Nonetheless, the presence of high levels of HIV-1-specific CD8+ CTL precursor cells in HIV-1-infected long-term nonprogressors and the loss of the lymphocyte subset in infected individuals with evidence of immunodeficiency suggest that these cells contribute to the control of virus replication in vivo (7, 15, 18, 26).
The development of knockout mice, in which genes specifying immunologically relevant proteins have been disrupted (35), has been useful in delineating the genes’ functions in suppressing viral infections (12, 16, 25, 34). Because this approach is not currently applicable to subhuman primate models of lentivirus infections, we turned instead to the parenteral administration of a CD8 monoclonal antibody (MAb) to specifically deplete this lymphocyte subset prior to or during acute infections of rhesus macaques (Macaca mulatta) with an SIV/HIV (SHIV) chimeric virus. The results presented in this report demonstrate that CD8 cells were transiently lost in both the peripheral blood and lymphoid tissue in animals receiving the CD8 MAb. When administered around the time of primary infection, the CD8 MAb caused greatly increased virus loads in the circulating blood and in lymph nodes and was associated with prolongation of the CD4 cell depletion attending acute infection. Unexpectedly, the administration of the anti-CD8 MAb selectively induced the depletion of the CD28− subset of CD8 cells in the peripheral blood.
MATERIALS AND METHODS
Animals.
Rhesus macaques and cynomolgus monkeys (Macaca fascicularis) tested negative for simian immunodeficiency and simian type D retroviruses before inoculation. The macaques used in this study were maintained in accordance with the Guide for the Care and Use of Laboratory Animals (22) and were housed in a biosafety level 2 facility; biosafety level 3 practices were followed. Physical examinations were performed under ketamine anesthesia by veterinarians at times of virus inoculation, phlebotomy, or lymph node biopsy.
Antibodies.
Antibody T87PT3F9, an immunoglobulin G2a (IgG2a) mouse anti-human CD8 MAb, was supplied by Gary Toedter (Coulter) and used to deplete macaque CD8 cells in vivo. This antibody was purified by ammonium sulfate precipitation and dialysis against phosphate-buffered saline as previously described (1). On the basis of preliminary dose-response experiments, two intravenous injections (2 mg/kg of body weight), 7 days apart, were routinely administered. For lymphocyte immunophenotyping, cells were triply or doubly stained with CD2-fluorescein isothiocyanate (FITC)–CD4-phosphatidylethanolamine (PE)–CD8-peridinin chlorophyll protein (PerCP), CD45RA–CD4-PE–CD8-PerCP, HLADR-FITC–CD4-PE–CD8-PerCP, and CD8-FITC–CD28-PE (Becton Dickinson) and analyzed by flow cytometry with FACSort (Becton Dickinson).
Viruses.
SHIVMD14YE is a molecularly cloned SIV/HIV-1 chimeric virus consisting of gag, pol, vif, vpx, nef, and vpr (approximately 20%) sequences from SIVmac239 linked to an HIV-1DH12 segment containing vpr (approximately 80%), tat, rev, vpu, and env genes (31). The animal challenge stock of SHIVMD14YE was prepared in cultured rhesus macaque peripheral blood mononuclear cells (PBMC) and contained approximately 105 50% tissue culture infective doses (TCID50)/ml (measured in human MT-4 cells) and 82 ng of p27 Gag antigen per ml.
Measurement of virus loads.
Plasma antigenemia was measured by the SIV core antigen assay (Coulter), capable of detecting 50 pg of p27 Gag protein per ml. The number of proviral DNA copies in PBMC and lymph node cell lysates was measured by DNA PCR as previously described (31); the lower limit of detection in this assay is 0.3 copies/105 CD4+ cells.
RESULTS
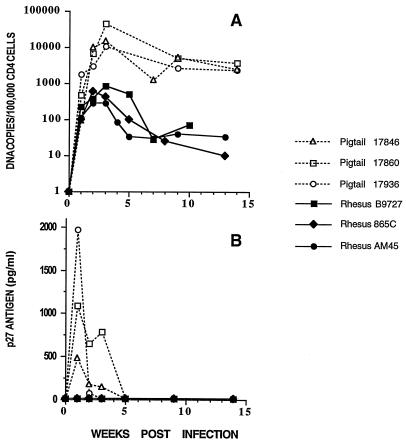
The SHIV used in these experiments, designated SHIVMD14YE, contains most of vpr and the complete tat, rev, vpu, and env genes from the dual-tropic HIV-1 isolate HIV-1DH12 (30) and the gag, pol, vif, vpx, and nef genes plus the 5′ 48 nucleotides of vpr and LTR sequences from SIVmac239 (17); codons 17 and 18 of the SIVmac239 nef gene present in SHIVMD14YE were changed from RQ to YE by site-specific mutagenesis (8). SHIVMD14YE induces an immunodeficiency in pig-tailed macaques characterized by a loss of CD4 cells, Pneumocystis carinii pneumonia, lymphoid tissue depletion, thymic atrophy, and disseminated fibromatosis 10 to 60 weeks following inoculation (31). However, in SHIVMD14YE-inoculated rhesus macaques, virus loads are usually 100-fold lower than those in pig-tailed macaques (Fig. 1A), p27 antigenemia cannot be detected (Fig. 1B), and immunodeficiency has not yet been observed.
FIG. 1.
Comparison of virus loads in pig-tailed and rhesus macaques infected with SHIVMD14YE. Animals were inoculated intravenously with 1.2 × 104 to 3.0 × 105 TCID50 of SHIVMD14YE. Proviral DNA copy numbers in PBMC (A) and the concentration of p27 Gag antigen in plasma (B) are shown.
CD8 MAb administration to naive macaques.
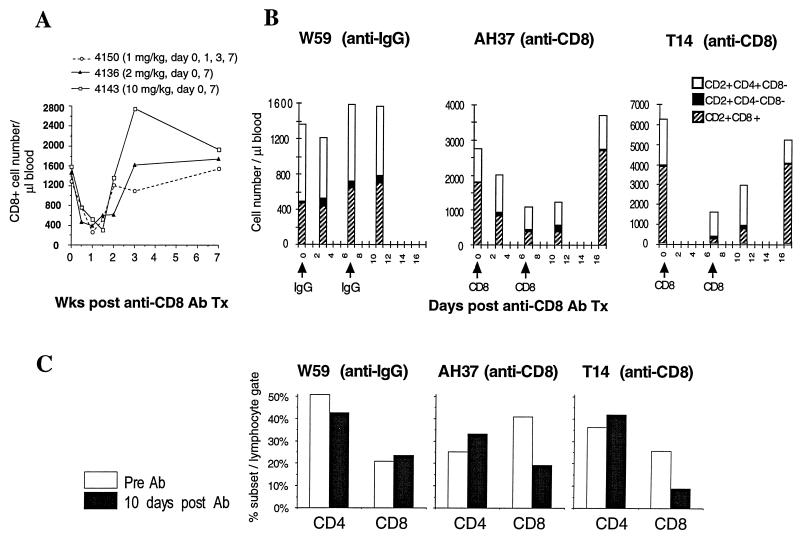
The anti-human CD8 mouse MAb (T87PT3F9 [Coulter]) administered to animals was shown to bind to a subset of PBMC from rhesus macaques in vitro, as determined by flow cytometry, following incubation with an FITC-conjugated secondary antibody (anti-mouse IgG rabbit serum). A second anti-human CD8 mouse MAb (PerCP-conjugated Leu-2A; Becton Dickinson) was used for lymphocyte immunophenotyping. Flow cytometry revealed that the staining of monkey PBMC with PerCP–Leu-2A was unaffected by prior incubation with the T87PT3F9 anti-human CD8 MAb (data not shown). Preliminary dose-response experiments indicated that animals inoculated intravenously with the T87PT3F9 anti-CD8 MAb experienced a consistent and transient reduction of circulating CD8 T lymphocytes lasting approximately 2 weeks (Fig. 2A). On the basis of these results, a regimen of two intravenous T87PT3F9 injections (2 mg/kg) given 7 days apart was used in the experiments to be described.
FIG. 2.
Effect of CD8 MAb administration on macaque T-lymphocyte subsets. (A) The indicated amounts of the T87PT3F9 anti-CD8 MAb were injected intravenously into three cynomolgus macaques, and the number of CD8 cells in peripheral blood was determined. Three naive rhesus macaques were injected (2 mg/kg) with either the T87PT3F9 anti-CD8 MAb (animals T14 and AH37) or control IgG (animal W59). The number of cells present in different T-lymphocyte subsets in peripheral blood (B) and the percentage of CD4 or CD8 cells in unfractionated lymphocytes from inguinal lymph node samples (C) were measured by flow cytometry as described in Materials and Methods. Ab, antibody; Tx, transfusion.
The effect of CD8 MAb administration on lymphocyte subsets was initially evaluated in two uninfected rhesus monkeys (T14 and AH37), injected with CD8 MAb on days 0 and 7 (Fig. 2B). As a control, a third animal (W59) received the same amount of purified IgG. Animals T14 and AH37 lost substantial numbers of CD8+ (CD2+ CD8+) T cells (3,825/liter [day 0] to 192/liter [day 7] and 1,776/liter [day 0] to 375/liter [day 7], respectively) from their peripheral blood, whereas the CD4+ T-cell level (CD2+ CD4+ CD8−) changed only modestly. More importantly, the total number of CD2+ cells (the sum of CD2+ CD8+, CD2+ CD4+ CD8−, and CD2+ CD4− CD8−) also declined, paralleling the fall in CD8+ cells. This latter result is consistent with the earlier observation that preincubation with the T87PT3F9 anti-CD8 MAb does not block reactivity with PerCP-conjugated Leu-2A; if the observed fall in CD8+ T cells merely represented the masking of PerCP-conjugated Leu-2A staining, the decline in the number of CD2+ CD8+ cells would have been accompanied by an increase of CD2+ CD4− CD8− cells, thereby maintaining a constant number of CD2+ cells. As expected, no CD8 cell loss was observed in the control animal (W59), which received IgG. The levels of circulating HLADR (positive and negative) and CD45RA (positive and negative) CD8 subsets did not change in either of the macaques receiving the CD8 MAb (data not shown).
Lymph node biopsies were obtained from all three animals prior to and 10 days following CD8 MAb treatment (Fig. 2C). Substantial CD8 cell loss occurred in the lymph node specimens from both macaques receiving the anti-CD8 MAb, with little change in the numbers of their CD4 cells; the CD4 and CD8 profiles in the macaque given IgG were essentially unaltered. Taken together, these results indicate that administration of the CD8 MAb induces transient CD8 cell depletion in both circulating blood and peripheral lymphoid tissue.
CD8 MAb administration during acute virus infection.
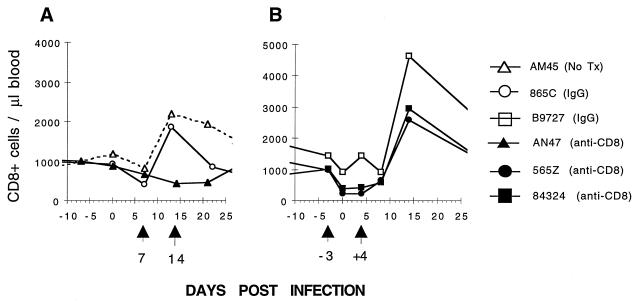
The effect of CD8 MAb administration on primary lentivirus infections was evaluated in an experiment in which six rhesus macaques were inoculated with 3 × 104 TCID50 of SHIVMD14YE. Three of the animals were treated with the T87PT3F9 CD8 MAb, two received control IgG, and one was untreated. Two different schedules of antibody administration were used as follows: (i) single injections 7 and 14 days after SHIV inoculation (Fig. 3A) or (ii) single injections 3 days prior to and 4 days following virus challenge (referred to hereafter as the −3/+4-day injection regimen) (Fig. 3B). Among the macaques on the +7/+14-day injection regimen, animal 865C (control IgG) exhibited an increase in the number of CD8 cells in response to the primary lentivirus infection comparable to that observed in macaque AM45, which received no antibody (Fig. 3A). This elevation of circulating CD8 cells was not observed in animal AN47 (Fig. 3A), presumably reflecting the opposing effects of CD8 cell depletion, induced by the administration of the anti-CD8 MAb on days 7 and 14 postinfection, and CD8 T-lymphocyte expansion in response to the primary virus infection. A more significant loss of CD8 cells was observed in the two macaques receiving the anti-CD8 MAb on the −3/+4-day injection schedule (animals 565Z and 84296 [Fig. 3B]). This fall in the number of CD8 cells was followed by robust elevations during the third week of SHIVMD14 infection. The control (IgG) animal for the second injection schedule (B9727) exhibited the expected CD8 cell response.
FIG. 3.
The effect of CD8 MAb administration on the number of circulating CD8+ T lymphocytes during primary SHIV infections. Six rhesus macaques were inoculated intravenously with SHIVMD14YE (3 × 104 TCID50) on day 0. (A) On days 7 and 14 following infection, monkey AN47 received anti-CD8 MAb (2 mg/kg), monkey 865C was administered IgG (2 mg/kg), and monkey AM45 served as an untreated control. (B) Three days prior to and 4 days following SHIV infection, monkeys 565Z and 84324 received anti-CD8 MAb (2 mg/kg) and monkey B9727 was given IgG (2 mg/kg). Tx, transfusion.
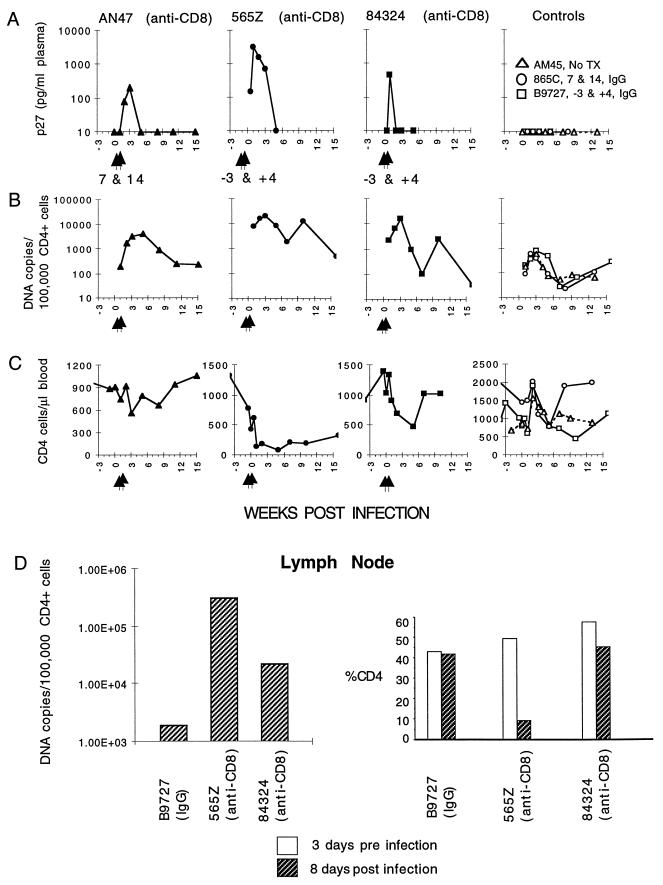
The administration of the anti-CD8 MAb prior to or during acute infection significantly increased virus loads; all three of the treated macaques developed p27 antigenemia, while the three control animals did not (Fig. 4A). The levels of PBMC-associated viral DNA in the animals receiving the anti-CD8 MAb were also substantially higher than that detected in the three control monkeys (Fig. 4B). At the peak of virus production during the primary infection, the mean PBMC-associated virus load in the three animals treated with the anti-CD8 MAb was 13,800 DNA copies/105 CD4+ cells compared with 660 copies/105 CD4+ cells in the control animals. During the first 30 weeks of virus infection, the average CD4 cell-associated virus loads (approximately 10 time points for each animal) were 4,200 and 190 copies/105 CD4+ cells for the treated and untreated groups, respectively. Both of the animals treated with the anti-CD8 MAb on the −3/+4-day schedule suffered significant CD4 cell depletion (Fig. 4C). In particular, the CD4 cell number in animal 565Z, which exhibited the highest plasma p27 and PBMC-associated viral DNA loads, fell to 88 cells/μl of blood at 5 weeks postinfection. Macaque 565Z continued to exhibit low numbers of CD4+ T lymphocytes in the peripheral blood (approximately 34% of the prechallenge cell count) 6 to 12 months following virus inoculation.
FIG. 4.
The effect of CD8 MAb administration on virus loads and CD4 cell levels in acutely infected animals. Arrows indicate the times of CD8 MAb administration. The three control macaques were inoculated with purified IgG or received no treatment. The concentration of p27 Gag antigen in plasma (A), the proviral DNA copy number in CD4+ PBMC (B), and the number of CD4 cells (C) are shown for the 15-week period following virus inoculation. The proviral DNA copy number and the percentage of CD4 in lymph node samples from three of the animals are also shown (D). Tx, transfusion.
Lymph node specimens were obtained from three of the animals (B9727, 565Z, and 84324) 3 days before and 8 days after inoculation with SHIV. The two anti-CD8 MAb-treated animals had striking elevations of SHIV DNA in their lymph nodes compared to the control animal B9727 (Fig. 4D). Monkey 565Z also suffered a marked loss of CD4 cells in the lymph node sample (from 50% of total lymph node cells prior to infection to 10% on day 8 postinfection).
CD8 MAb causes loss of circulating CD8+ CD28− cells.
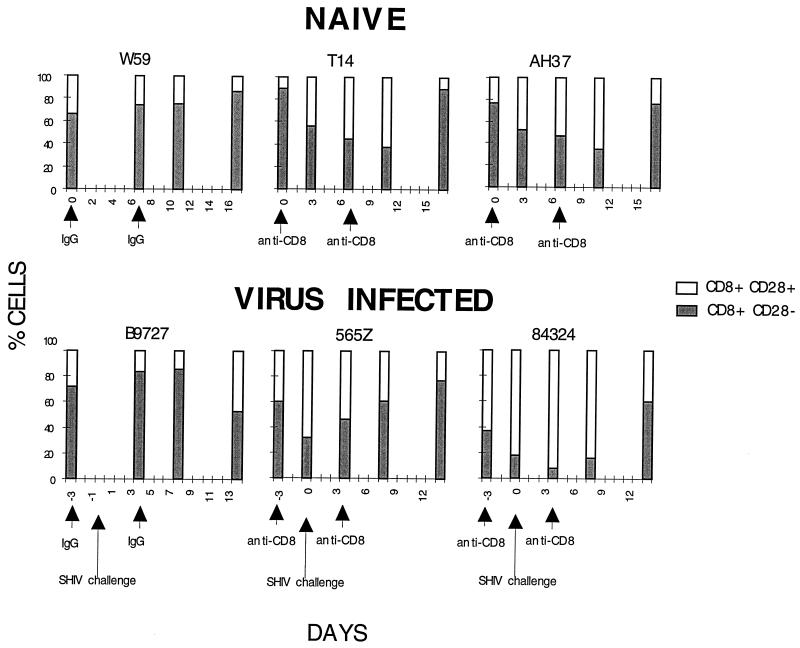
Although we had not intended to deplete a specific subset of CD8 cells, fluorescence-activated cell sorter analysis revealed the selective loss of CD8+ CD28− cells from the peripheral blood of two naive and two acutely infected animals following anti-CD8 MAb treatment (Fig. 5). In contrast to inbred mice, in which <5% of circulating CD8 T lymphocytes are CD28−, 30 to 40% of CD8 cells in human PBMC are CD28− (2, 4, 10, 14). In the macaques we have studied, the CD8+ CD28− subset comprises 30 to 80% of CD8 cells in the peripheral blood (data not shown). It should be noted that the CD8 T lymphocyte population in human lymphoid tissue is >95% CD28+ (4, 32), a value we have also observed in rhesus macaque lymph node specimens (data not shown).
FIG. 5.
The in vivo effect of T87PT3F9 anti-CD8 MAb administration on the CD28 subpopulation of CD8+ T lymphocytes in the peripheral blood. CD8+ PBMC from three naive (top) and three acutely infected (bottom) macaques were examined for surface expression of CD28. The total number of CD8+ cells is shown as 100%. The arrows indicate the times of antibody administration (IgG or anti-CD8 [2 mg/kg]) and SHIV challenge.
DISCUSSION
These results represent the first direct demonstration that CD8 T lymphocytes play a critical role in resolving primary lentiviral infections; administration of the anti-CD8 MAb caused transient yet consistent losses of CD8 cells in both the peripheral blood as well as lymphoid tissue, currently thought to be the primary site of virus replication (9, 23). Greatly increased virus loads in both of these compartments were observed in macaques receiving the anti-CD8 MAb during the early phase of acute infection. In one animal (565Z), the CD8 cell deficit delayed resolution of the primary infection, and the resulting high levels of circulating virus were associated with marked CD4 cell loss in both PBMC and lymph nodes.
The effects of MAb-mediated CD8 depletion on virus loads appeared to be considerably greater in animals receiving the CD8 MAb prior to and shortly after SHIV inoculation compared to those in animals receiving antibody beginning 1 week postinfection. This result is consistent with a previous study in which CTL precursors directed against Gag peptides were reported to be present as early as 4 to 6 days following SIV infection of rhesus monkeys (34). Since the CD8 MAb administered to the macaques depletes CD8 cells only transiently, its effect will probably be greatest when it is given prior to the expansion of the virus-specific CTL population (viz. within the first days of the acute infection). Conversely, if it is administered too late, perhaps even 1 week postinfection, the capacity of the MAb to deplete CD8 cells may be severely compromised by the CD8 T-lymphocyte response normally elicited by the virus.
An unexpected result of this study was the selective depletion of macaque CD8+ CD28− T lymphocytes in animals receiving the anti-CD8 MAb. In humans, this CD8 cell subset comprises more than one-third of circulating CD8 T cells and is thought to represent terminally differentiated, nonproliferating effector cells that require no costimulation for immediate cytotoxic activity (2). Compared to CD8+ CD28+ lymphocytes, which produce soluble factors that suppress HIV-1 replication (20), CD8+ CD28− cells are enriched for perforin and granule-associated TIA-1 molecules (4) and have potent CTL activity against major histocompatibility complex-matched lymphocytes expressing HIV-1 Gag and Pol proteins (11). It is also worth noting that the CD8+ CD28− lymphocyte subset in peripheral blood has been reported to expand during the course of HIV-1 infections (4, 5, 28). Although researchers cannot presently explain the selective depletion of CD8+ CD28− cells following MAb treatment, one could speculate that the binding of antibody to these terminally differentiated, nonproliferating cells might trigger apoptosis and their disappearance from the PBMC compartment.
ACKNOWLEDGMENTS
We are indebted to Ali A. Azadegan and Randy Elkins for assisting in animal procurement and procedures and to George Coleman and Charles W. Thornton for animal care.
REFERENCES
- 1.Andrew S M, Titus J A. Purification of immunoglobulin G. In: Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. Vol. 1. New York, N.Y: John Wiley & Sons, Inc.; 1997. pp. 2.7.2–2.7.4. [Google Scholar]
- 2.Azuma M, Phillips J H, Lanier L L. CD28− T lymphocytes. J Immunol. 1993;150:1147–1159. [PubMed] [Google Scholar]
- 3.Borrow P, Lewicki H, Hahn B H, Shaw G M, Oldstone M B A. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borthwick N J, Bofill M, Gombert W M, Akbar A N, Medina E, Sagawa K, Lipman M C, Johnson M A, Janossy G. Lymphocyte activation in HIV-1 infection. II. Functional defects of CD28− T cells. AIDS. 1994;8:431–441. doi: 10.1097/00002030-199404000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Brinchmann J E, Dobloug J H, Heger B H, Haaheim L L, Sannes M, Egeland T. Expression of costimulatory molecule CD28 on T cells in human immunodeficiency virus type 1 infection: functional and clinical correlations. J Infect Dis. 1994;169:730–738. doi: 10.1093/infdis/169.4.730. [DOI] [PubMed] [Google Scholar]
- 6.Byrne J A, Oldstone M B A. Biology of cloned cytotoxic T lymphocytes specific for lymphocytic choriomeningitis virus: clearance of virus in vivo. J Virol. 1984;51:682–686. doi: 10.1128/jvi.51.3.682-686.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carmichael A, Jin X, Sissons P, Borysiewicz L. Quantitative analysis of the human immunodeficiency virus type 1 (HIV-1)-specific cytotoxic T lymphocyte (CTL) response at different stages of HIV-1 infection: differential CTL responses to HIV-1 and Epstein-Barr virus in late disease. J Exp Med. 1993;177:249–256. doi: 10.1084/jem.177.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du Z, Lang S M, Sasseville V G, Lackner A A, Ilyinskll P O, Daniel M D, Jung J U, Desrosiers R C. Identification of a nef allele that causes lymphocyte activation and acute disease in macaque monkeys. Cell. 1995;82:665–674. doi: 10.1016/0092-8674(95)90038-1. [DOI] [PubMed] [Google Scholar]
- 9.Embretson J, Zupancic M, Ribas J L, Burke A, Racz P, Tenner-Racz K, Haase A T. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993;362:359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- 10.Fagnoni F F, Vescovini R, Mazzola M, Bologna G, Nigro E, Lavagetto G, Franceschi C, Passeri M, Sansoni P. Expansion of cytotoxic CD8+ CD28− T cells in healthy ageing people, including centenarians. Immunology. 1996;88:501–507. doi: 10.1046/j.1365-2567.1996.d01-689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiorentino S, Dalod M, Olive D, Guillet J-G, Gomard E. Predominant involvement of CD8+ CD28− lymphocytes in human immunodeficiency virus-specific cytotoxic activity. J Virol. 1996;70:2022–2026. doi: 10.1128/jvi.70.3.2022-2026.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fung-Leung W P, Schilham M W, Rahemtulla A, Kundig T M, Vollenweider M, Potter J, van Ewijk W, Mak T W. CD8 is needed for development of cytotoxic T cells but not helper T cells. Cell. 1991;65:443–449. doi: 10.1016/0092-8674(91)90462-8. [DOI] [PubMed] [Google Scholar]
- 13.Gallimore A, Cranage M, Cook N, Almond N, Bootman J, Rud E, Silvera P, Dennis M, Corcoran T, Stott J, McMichael A, Gotch F. Early suppression of SIV replication by CD8+nef-specific cytotoxic T cells in vaccinated macaques. Nat Med. 1995;1:1167–1173. doi: 10.1038/nm1195-1167. [DOI] [PubMed] [Google Scholar]
- 14.Giorgi J V, Boumsell L, Autran B. Reactivity of workshop T-cell section mAb with circulating CD4+ and CD8+ T cells in HIV disease and following in vitro activation. In: Schlossman S F, Boumsell L, Gilks W, et al., editors. Leucocyte typing V: white cell differentiation antigens. Oxford, England: Oxford University Press; 1995. pp. 446–461. [Google Scholar]
- 15.Hoffenbach A, Langlade-Demoyen P, Dadaglio G, Vilmer E, Michel F, Mayaud C, Autran B, Plata F. Usually high frequencies of HIV-1 specific cytotoxic T lymphocytes in humans. J Immunol. 1989;142:452–462. [PubMed] [Google Scholar]
- 16.Kägi D, Ledermann B, Bürki K, Seiler P, Odermatt B, Olsen K J, Podack E R, Zinkernagel R M, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 17.Kestler H, Kodama T, Ringler D, Marthas M, Pederson N, Lackner A, Regier D, Sehgal P, Daniel M, King N, Desrosiers R C. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science. 1990;248:1109–1112. doi: 10.1126/science.2160735. [DOI] [PubMed] [Google Scholar]
- 18.Klein M R, van Baalen C A, Holwerda A M, Kerkhof-Garde S R, Bende R J, Keet I P, Eeftinck-Schattenkerk J K, Osterhaus A D, Schuitemaker H, Miedema F. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landay A L, Mackewicz C E, Levy J A. An activated CD8+ T cell phenotype correlates with anti-HIV activity and asymptomatic clinical status. Clin Immunol Immunopathol. 1993;69:106–116. doi: 10.1006/clin.1993.1157. [DOI] [PubMed] [Google Scholar]
- 21.McMichael A J, Gotch F M, Noble G R, Beare P A S. Cytotoxic T-cell immunity to influenza. N Engl J Med. 1983;309:13–17. doi: 10.1056/NEJM198307073090103. [DOI] [PubMed] [Google Scholar]
- 22.National Institutes of Health. Guide for the care and use of laboratory animals, rev. ed. Department of Health and Human Services publication no. (NIH)85-23. Bethesda, Md: National Institutes of Health; 1985. [Google Scholar]
- 23.Pantaleo G, Graziosi C, Demarest J F, Butini L, Montroni M, Fox C H, Orenstein J M, Kotler D P, Fauci A S. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993;362:355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 24.Quinnan G V, Kirmani N, Rook A H, Manishevitz J V, Jackson L, Moreschi G, Santos G W, Saral R, Burns W H. Cytotoxic T cells in cytomegalovirus infection. N Engl J Med. 1982;307:7–13. doi: 10.1056/NEJM198207013070102. [DOI] [PubMed] [Google Scholar]
- 25.Rahemtulla A, Fung-Leung W P, Schilham M W, Kundig T M, Sambhara S R, Narendran A, Arabian A, Wakeham A, Paige C J, Zinkernagel R M, Miller R G, Mak T W. Normal development and function of CD8+ cells but markedly decreased helper cell activity in mice lacking CD4. Nature. 1991;353:180–184. doi: 10.1038/353180a0. [DOI] [PubMed] [Google Scholar]
- 26.Rinaldo C, Huang X-L, Fan Z, Ding M, Beltz L, Logar A, Panicali D, Mazzara G, Liebmann J, Cottrill M, Gupta P. High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory cytotoxic T-lymphocyte activity and low viral load are associated with lack of disease in HIV-1-infected long-term nonprogressors. J Virol. 1995;69:5838–5842. doi: 10.1128/jvi.69.9.5838-5842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Safrit J T, Andrews C A, Zhu T, Ho D D, Koup R A. Characterization of human immunodeficiency virus type 1-specific cytotoxic T lymphocyte clones isolated during acute seroconversion: recognition of autologous virus sequences within a conserved immunodominant epitope. J Exp Med. 1994;179:463–472. doi: 10.1084/jem.179.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saukkonen J J, Kornfeld H, Berman J S. Expansion of a CD8+ CD28− cell population in the blood and lung of HIV-positive patients. J Acquired Immune Defic Syndr. 1993;6:1194–1204. [PubMed] [Google Scholar]
- 29.Sethi K K, Omata Y, Schnewer K E. Protection of mice from fatal herpes simplex virus type 1 infection by adoptive transfer of virus-specific and H-2 restricted cytotoxic T lymphocytes. J Gen Virol. 1983;64:443–447. doi: 10.1099/0022-1317-64-2-443. [DOI] [PubMed] [Google Scholar]
- 30.Shibata R, Hoggan M D, Broscius C, Englund G, Theodore T S, Buckler-White A, Arthur L O, Israel Z, Schultz A, Lane H C, Martin M A. Isolation and characterization of a syncytium-inducing, macrophage/T-cell line-tropic human immunodeficiency virus type 1 isolate that readily infects chimpanzee cells in vitro and in vivo. J Virol. 1995;69:4453–4462. doi: 10.1128/jvi.69.7.4453-4462.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shibata R, Maldarelli F, Siemon C, Matano T, Parta M, Miller G, Fredrickson T, Martin M A. Infection and pathogenicity of chimeric simian-human immunodeficiency viruses in macaques: determinants of high virus loads and CD4 cell killing. J Infect Dis. 1997;176:362–373. doi: 10.1086/514053. [DOI] [PubMed] [Google Scholar]
- 32.Turka L A, Ledbetter J A, Lee K, June C H, Thompson C B. CD28 is an inducible T cell surface antigen that transduces a proliferative signal in CD3+ mature thymocytes. J Immunol. 1990;144:1646–1653. [PubMed] [Google Scholar]
- 33.Yap K L, Ada G L, McKenzie I F C. Transfer of specific cytotoxic T lymphocytes protects mice inoculated with influenza virus. Nature. 1978;273:238–239. doi: 10.1038/273238a0. [DOI] [PubMed] [Google Scholar]
- 34.Yasutomi Y, Reimann K A, Lord C I, Miller M D, Letvin N L. Simian immunodeficiency virus-specific CD8+ lymphocyte response in acutely infected rhesus monkeys. J Virol. 1993;67:1707–1711. doi: 10.1128/jvi.67.3.1707-1711.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zijlstra M, Li E, Sajjadi F, Subramani S, Jaenisch R. Germ-line transmission of a disrupted β2-microglobulin gene produced by homologous recombination in embryonic stem cells. Nature. 1989;342:435–438. doi: 10.1038/342435a0. [DOI] [PubMed] [Google Scholar]