Abstract
Distal femur fractures are challenging injuries to manage, and complication rates remain high. This article summarizes the international and basic science perspectives regarding distal femoral fractures that were presented at the 2022 Orthopaedic Trauma Association Annual Meeting. We review a number of critical concepts that can be considered to optimize the treatment of these difficult fractures. These include biomechanical considerations for distal femur fixation constructs, emerging treatments to prevent post-traumatic arthritis, both systemic and local biologic treatments to optimize nonunion management, the relative advantages and disadvantages of plate versus nail versus dual-implant constructs, and finally important factors which determine outcomes. A robust understanding of these principles can significantly improve success rates and minimize complications in the treatment of these challenging injuries.
Keywords: distal femur fractures, basic science, post-traumatic arthritis, biomechanics, nail–plate constructs, nonunion
1. Introduction
This article summarizes the international and basic science perspectives regarding distal femur fractures that were presented at the 2022 Orthopaedic Trauma Association Annual Meeting. A number of important concepts regarding the biomechanics, biology, and treatment of these difficult injuries are discussed.
2. Distal Femur Fracture Fixation: Biomechanical Considerations
The goals of operative treatment of distal femur fractures include fracture reduction, restoration of alignment, and provision of mechanical stability sufficient to maintain the reduction until the fracture heals. Recent developments have led to the introduction of advanced intramedullary and extramedullary fixation techniques for distal femur fractures. Despite the mechanical advantages of these modern nailing and plating techniques, healing complications in distal femur fractures remain relatively common. Rates of delayed union and nonunion have been as high as 15% and 19%, respectively.1 Ideally, early callus formation stabilizes the fracture and transfers load from the implant to the bone. Delays in callus formation can result in chronic implant overloading that challenges the fixation construct. Consequently, more than two-thirds of distal femur nonunions show mechanical hardware failure due to implant fatigue.2
Implant failure typically occurs greater than 6 months after treatment; however, implant failures can occur as early as 6 weeks after fracture fixation.1 Late implant failures together with fracture nonunion indicate fatigue failure. As osteosynthesis implants are designed for temporary mechanical support, late fatigue failures are inherent to their design. Early implant failures, on the other hand, are indicative of singular overloads, inappropriate implant application (Fig. 1), or material damage during implant insertion.3 Early failure is rarely due to intrinsic material defects in a modern-day implant. The mode and location of implant failure has been shown to be associated with the characteristics of the specific implant.2 In a retrospective review of cases, stainless steel locking plates most frequently bent or broke within the working length region. Plate breakage occurred after an average of 42 weeks. Titanium locking plates failed more frequently by loosening of the locking screws in the shaft region. If the screw design allows for variable angulation of the screws, fatigue can occur by plate and/or screw failure in the distal region of the locking plate.
Figure 1.
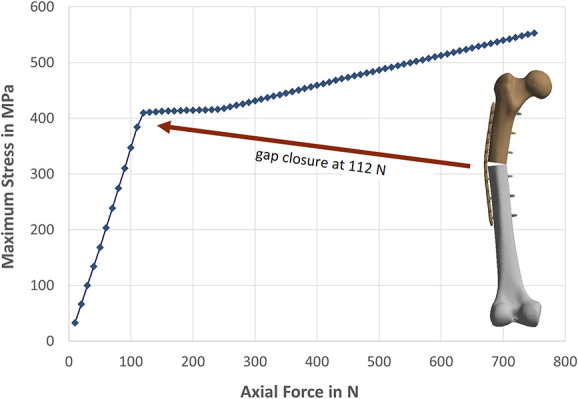
Computational analysis of mechanical stress within a locking plate shows the importance of fracture reduction. After closure of the 1.5-mm fracture gap at 112 N of load, stress in the plate increases much slower.
Efforts to improve the biomechanical performance of implants for distal femur fracture fixation aim to avoid implant failure and provide an adequate mechanical environment to enable uneventful fracture healing. Thus, implants must be strong enough to withstand early loads, yet flexible enough to stimulate secondary healing and callus formation. Implant strength of distal femur locking plates is predominantly determined by implant design and material.4 Secondary, but equally important considerations in implant design pertain to fatigue. Stainless steel plates consistently demonstrate superior failure strength and longer fatigue life compared with titanium plates. However, the disadvantage in material strength of titanium can partly be compensated for by appropriate implant design.5
Fixation construct stiffness is determined not only by implant material and implant design but it can also be directly modified by individually adjusting the screw configuration within a plate. Placing screws at different distances across the fracture site modifies the working length of the plate construct and constrains the amount of motion that occurs within the fracture gap. Increasing the working length reduces the stiffness of the construct. However, the effect of working length on the interfragmentary motion is complex because laterally placed locking plates are located at a distance from the primary mechanical axis. The resulting bending moment creates a nonuniform axial displacement and a shear movement. The axial displacement is large at the far cortex and small at the near cortex beneath the plate. Increasing the working length increases the axial motion at the far cortex and drastically amplifies the amount of shear motion within the fracture gap.6 The positive effect of stimulating axial motion at the fracture site is thus partly compensated by the simultaneous induction of shear motion, which is believed to be detrimental for fracture healing. The effect of working length on implant strength remains somewhat controversial. While larger working length results in larger strain on the plate surface, the fatigue life has not been strongly affected in stainless steel plates or has even slightly improved in titanium plates.7
Recent implant developments aim at providing stimulatory axial motion at the fracture site while maintaining implant strength and limiting shear motion. Modified designs such as dynamic locking screws, far cortical locking screws, or active plate inserts around the screw holes actively promote axial fracture gap motion. Their mechanical performance has been well documented and supported by preclinical studies showing accelerated fracture healing.8 Another solution for creating gap motion is the biphasic plating concept combining 2 different stiffness profiles within one locked bridging plate.9 Under low loads, the stiffness of the biphasic plate is low, assuring the desirable and advantageous mechanical fracture stimulation. Under large loads, the plate becomes stiff and leads to reduced plate strain for superior fatigue strength. Clinical studies to demonstrate the efficacy of these concepts are underway.
These biomechanical concepts represent important aspects to consider both in the preoperative plan and during the application of the fixation construct intraoperatively.
3. Preventing Post-Traumatic Arthritis in Articular Injuries: The Latest Basic Science Evidence
Post-traumatic osteoarthritis (PTOA) occurs after a joint injury, and it most predictably develops after intra-articular fracture (IAF). The exact mechanisms that lead to injury progression from the initial IAF to end-stage PTOA are unclear. PTOA development after intra-articular distal femur fracture is common. Rademakers et al10 reported that 36% of patients with an intra-articular distal femur fracture developed moderate/severe PTOA at a mean of 14 years from injury. A recent database review reported that 2.3% of distal femur fractures developed symptomatic PTOA and underwent total knee arthroplasty (TKA) within 5 years of injury.11 Patients undergoing TKA for distal femur fracture are at significantly greater risk of having a postoperative complication or requiring further revision surgery.12
Given the morbidity associated with PTOA in distal femur fractures, intra-articular injections have been investigated as early interventions to mitigate PTOA development. In a rabbit anterior cruciate ligament transection model, Heard et al13 reported mild improvement with hyaluronic acid injection and no improvement with hyaluronic acid and dexamethasone injection as compared with control limbs. In a similar model, Heard et al14 reported significantly better histologic scores in rabbits that received a single intra-articular injection of dexamethasone. Jayaram et al15 found no benefit in Osteoarthritis Research Society International (OARSI) or synovitis scores for either leukocyte-rich or leukocyte-poor platelet-rich plasma injections as compared with phosphate-buffered saline (PBS) in a mouse PTOA model. These results mirror the findings of a recent study investigating platelet-rich plasma injections in patients with early osteoarthritis.16 Amobarbital, a reversible inhibitor of the electron transport chain, has been demonstrated to improve histologic scores in a porcine IAF model and is currently undergoing trials in human patients with pilon fractures.17 Similarly, interleukin-1 receptor antagonist protein (IL-1Ra), an inhibitor of the potent cytokine interleukin-1β (IL-1β), has been demonstrated to improve both histologic and synovitis scores in a mouse IAF model.18 There are several injectable therapeutics that have demonstrated success in mitigating PTOA development in animal model studies and are primed for human investigation.
While limiting the postinjury inflammatory cascade seems to be critical to lessening PTOA development, addressing osteochondral loss is another concern in managing intra-articular distal femur fractures. In a recent study of osteochondral defects in a porcine intra-articular pilon fracture model, DeKeyser et al reported significantly worse subchondral bone porosity, increased vascular invasion, and worse OARSI histologic scores in pigs with a defect as compared with anatomically reduced pigs.19 Tissue engineering may provide a substantial breakthrough in addressing osteochondral defects through 3D-printed scaffolds that can be successfully seeded with mesenchymal stem cells (MSCs) and attached to a bone plug for implantation. Three dimensional printing allows scaffolds to be printed in a variety of shapes to accommodate the bone of interest and minimize point loading at the transition from scaffold to native joint. These MSC-seeded scaffolds can be further modified with gene therapy using lentivirus to promote chondrocyte differentiation and produce IL-1Ra to limit the impacts of local inflammation on differentiation.20 In a recent canine model with a 10-mm osteochondral lesion of the femoral head, investigators demonstrated that a 3D-printed scaffold with implanted MSCs had successful incorporation with surrounding osteochondral tissue, similar mechanical properties, and successful return to function by 6 months.20
At present, the clinically available options for minimizing PTOA are limited to anatomic reduction and salvage/reconstruction of osteochondral fragments whenever possible. However, with progress in the above innovations, we are hopeful that other avenues for the treatment and prevention of PTOA will be available in the future.
4. Managing NonUnions in Distal Femur Fractures: Biological Options
The modern treatment of nonunions involves optimizing both the patient and maximizing anticipated fixation. This section will address the first issue, including both systemic biology and local biological options. A study by Niikura et al21 in 2014 reviewed the causative factors of nonunion in a large series, and the authors divided them into biological and mechanical factors. They found that in a series of 102 consecutive patients with nonunion, 24 cases were due to mechanical factors alone, 23 cases were due to biological factors, and 55 cases had a combination of both factors. Thus, almost 75% of nonunions had some biological deficit that could be optimized before revision fixation or surgery. At present, some of the more common factors that can be optimized in patients before surgery include low vitamin D levels, low calcium levels, low albumin levels, uncontrolled diabetes with high blood sugar, hyponatremia, hypothyroidism, and smoking. These are all biological factors that can be improved on in the reconstructive or semielective setting, before embarking on surgical intervention, and that will help to maximize the chance of success in these difficult and complex cases. The negative effects of most of these factors, if left uncorrected, are supported by evidence-based medicine and prospective studies. For example, series of patients undergoing limb reconstruction using controlled distraction osteogenesis with circular external fixation frames demonstrated higher rates of delayed union, nonunion, failure of regenerate bone formation, and amputation in patients who were active smokers.22 Similarly, a recent study by Bergin et al23 examined patients who underwent operative intervention for a nonunion at 2 Level I trauma centers and found that 42% of patients had an undiagnosed metabolic abnormality, particularly vitamin D deficiency. This study clearly supported Brinker's original 2007 article describing metabolic and endocrine abnormalities in patients with nonunion.24 It is important that everything feasible is done to improve the patient's systemic biology before the initiation of surgical intervention.
Once the patient's systemic biology had been optimized, distal femoral nonunion treatment also includes the use of osteobiologics locally, including autogenous bone grafting, allograft struts, and synthetic osteobiologics. A study by Wang and Weng25 described 13 patients with a distal femoral nonunion treated with open reduction and internal fixation in conjunction with autogenous bone graft and cortical allograft struts. They found that all nonunions healed at a mean of 5 months postoperatively, with significant improvements in the knee motion and functional outcome scores. In this study, autogenous bone graft was used for biological stimulation of healing and the allograft struts were used to augment mechanical fixation.
The use of osteobiologics in distal femoral nonunion has received limited attention in the medical literature. In general, morselized autograft, or allograft combined with an osteoinductive agent, such as a bone morphogenic protein (BMP) or demineralized bone matrix (DBM) are used to fill small defects in or around the nonunion site. Allograft struts are typically used to augment the strength of nonunion fixation, especially in the setting of a periprosthetic nonunion repair. In addition, as in the proximal humerus, the use of intramedullary fibular strut allografts in the distal femur has been successful.26
To summarize, the treatment of distal femoral nonunions should include a careful metabolic and nutritional workup to identify potentially correctable systemic factors that may have predisposed the patient to nonunion initially, with subsequent correction of any deficiencies. Allograft struts, either intramedullary or cortical onlay, play an important role in the augmentation of fixation around a distal femoral nonunion. Lastly, autograft, or allograft with or without osteoinductive agents such as BMP's or DBM's are useful in promoting local union. With thorough preoperative workup and proper operative techniques, including the use of osteobiologics, the majority of these patients can be treated successfully and relatively few will require conversion to a distal femoral replacement (Fig. 2). For example, in a study by Rajasekran et al,26 only 4 patients out of 58 with a distal femoral nonunion treated operatively following these principles required conversion to an arthroplasty.
Figure 2.
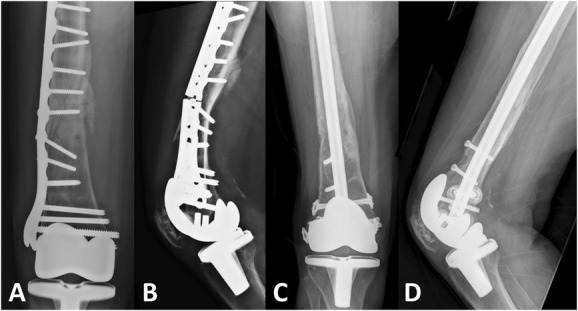
A and B, Preoperative radiographs of a 73-year-old woman with a distal femur nonunion 1 year after locking plate fixation of a periprosthetic distal femur fracture. Preoperative workup revealed the patient was a smoker with low vitamin D. Vitamin D supplementation and smoking cessation therapy were initiated. C and D, Radiographs 1 year postrevision fixation with an intramedullary nail and application of bone morphogenic protein-2 (BMP-2, Infuse, Medtronic, Memphis, TN) demonstrating solid union. This represents an off-label indication for the use of BMP-2.
5. Nailing, Plating, or Both: Optimizing Stability
There is controversy regarding the optimal fixation for distal femur fractures. Both locked lateral plating and retrograde intramedullary nailing have important roles. Each fixation strategy has relative strengths and weaknesses.
The principal advantage of locked plating is improved distal fixation. This is particularly true when there is intra-articular involvement because the fixation traverses the typical sagittal split between the 2 condyles. However, plates have notable disadvantages compared to intramedullary nails. Perhaps the most important relates to the position of the plate on the lateral surface of the femur, offset from the anatomic axis (Fig. 3). This leads to increased cantilever bending forces on the implant at the level of the fracture. To resist these forces, locked plates are commonly thick, large fragment implants. While this may be beneficial to prevent plate breakage, this added stiffness may negatively affect fracture healing.27 This may be further exacerbated by the surgical approach, which can cause additional soft tissue stripping, particularly with poor soft tissue handling. A number of techniques have been developed to mitigate these issues, including less-invasive surgical approaches and the use of longer constructs with lower screw density to reduce overall stiffness and promote healing.28,29 Titanium plates may also offer some advantages over stainless steel plates with regard to stiffness and risk of nonunion.28,30 Despite these efforts, failure rates for locked lateral plating in modern series range from 10% to 25%.28,29,31,32
Figure 3.
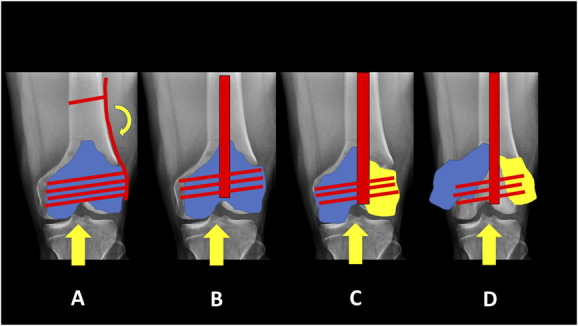
A, Laterally based plates are offset from the anatomic axis of the femur leading to cantilever bending forces on the implant. B, Intramedullary nails along the anatomic axis experience more axial loading. C/D, In the presence of intercondylar fracture extension, one or both condyles may “escape” from the limited fixation provided by interlocking screws in an intramedullary nail.
In contrast to plates, nails are typically inserted without any exposure or soft tissue stripping of the metaphysis. In addition, nails are in line with the anatomic axis of the femur resulting in more axial loading rather than the cantilever bending experienced by a laterally based plate (Fig. 3). Hence, titanium intramedullary nails may result in a more favorable healing environment with a lower likelihood of implant failure due to metaphyseal nonunion.31 However, nails may compromise the amount of distal fixation compared with plates, particularly in extreme distal or intra-articular fractures.33 Modern nails now allow for 3 to 4 multiplanar interlocking screws within 3 to 4 cm of the intercondylar notch, which allows for improved fixation even in short segments. This is particularly true in extra-articular fractures where the nail itself contributes to fixation. However, this does not hold true when there is intra-articular extension of the fracture.33 In these cases, the nail does not contribute to fixation of the individual condyles and may act as a wedge driving the condyles apart. In these cases, the fixation is dependent on the interlocking screws, which are generally fewer in number than what would be achieved with a locking plate (Fig. 3). For that reason, plates are preferred for most of the displaced intra-articular distal femur fractures.
Other strong indications to choose a plate over nail include B-type partial articular fractures and some periprosthetic fractures. B-type fractures are best treated with a buttress plate applied at the apex of the fracture, whether that is medial or lateral.34 Use of a nail in these cases offers little advantage and may displace the fracture during nail insertion.
The optimal choice for periprosthetic fractures is dependent on the type of prosthesis. In the presence of an ipsilateral total hip, a short nail may be considered but carries a risk of interprosthetic fracture.35 In these cases, a plate may be a better alternative to protect the whole femur, or this may be an indication to combine a plate and nail together. For fractures about a total knee, only specific primary knee femoral components are compatible with a nail. For total knees with a narrow or closed box, plating is generally the only option assuming the fracture is too distal for antegrade nail placement. Gerow et al36 recently published an updated guide with tables listing total knee designs, along with the size of the notch and compatibility with a retrograde nail, which is an important reference if considering retrograde nailing in these scenarios.
Given the limitations of both plates and nails as a single implant, a number of techniques for combining implants have been described (Fig. 4). The use of a second plate to augment lateral fixation has been described for decades.37 Broadly there are 2 distinct techniques. The first is application of a medial plate through a separate approach, most commonly a medial subvastus approach. Although this is a very effective method for increasing stability, it may increase periosteal stripping and further disrupt vascular supply at the fracture site.37,38 Percutaneous medial approaches are limited by the crossing of the femoral artery, which is typically 16 to 18 cm proximal to the adductor tubercle.38 These downsides of the medial plate may be overcome using the endosteal plating technique described by Mast et al.39 In this technique, a plate is inserted through the fracture site into the intramedullary canal using the same lateral approach. The 2 implants can then be locked together by passing the screws from the lateral plate through the endosteal plate. While this technique has the advantage of increasing stability without the biologic cost of a medial approach, it is technically demanding and has not gained widespread popularity.39 Dual plating using a medial plate, however, remains a useful alternative in scenarios where a single plate may be at high risk of failure and a nail is not feasible, such as a periprosthetic fracture with poor bone quality, comminution, or bone loss. A recent systemic review demonstrated acceptable results of dual plating with nonunion rates of less than 5% despite a selection bias for complex, high-risk cases.37
Figure 4.
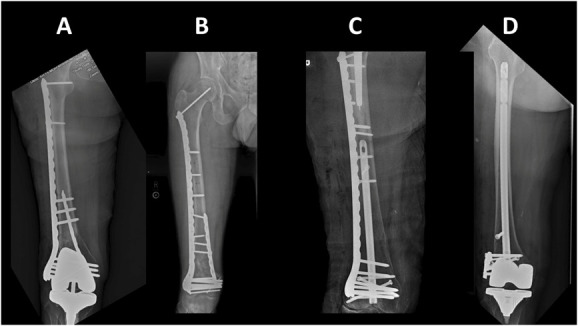
Numerous approaches for dual-implant fixation have been described, including endosteal substitution (A), dual plating (B), nail–plate combination (C), and nail-washer (D) constructs.
More recently there has been a trend toward the use of nail–plate combined constructs.40,41 This technique has been shown to improve biomechanical stability without the need for a second medial approach or additional metaphyseal soft tissue stripping.42 Furthermore, while it is technically more difficult than a single implant construct, it does not present the same technical challenges of endosteal plating.41 Several small case series have shown promising results with this technique.43
Unfortunately, the indications for dual-implant constructs have yet to be fully determined. Common indications described include acute fractures at high risk for nonunion, such as fractures with extensive comminution, bone loss, or open fractures.32,37 A further argument for dual implant constructs is to allow for early weight-bearing in geriatric patients.40 Finally, dual implants constructs may have a role in the treatment of nonunions.39 Although initial series using these approaches have been favorable, more comparative data are needed to establish clear indications for each technique.
6. Distal Femur Fractures: What Factors Determine Outcomes?
A variety of outcome measures are available to determine success in the management of distal femur fractures including functional outcome scores, prevention of post-traumatic arthritis, malunion, hardware failure/revision rates, and mortality. When evaluating these, it is important to recognize that there are 2 main patient populations that suffer distal femur fractures. These include geriatric, low-energy injuries and are in contrast to younger patients suffering higher-energy injuries. These patient populations have different injuries and demands that influence management and determine which factors are most important in determining outcome.
Lower-energy distal femur fractures tend to occur in geriatric patients after simple falls. These patients are often frail and comorbid. They often have osteoporotic bone and knee arthroplasty prostheses. These patients have low demands and functional goals after surgery are modest. Unfortunately, this group has a high mortality rate which has been estimated at between 13% and 35% at 1 year.44,45 These numbers approximate geriatric hip fracture mortality statistics.46 The treatment of geriatric fracture patients has evolved to encourage robust fixation or arthroplasty constructs to allow early weight-bearing and mobilization.47 Several case series and small randomized controlled trials have examined the safety of early weight-bearing for geriatric distal femoral fracture patients and demonstrated that early weight-bearing can be considered appropriate for most patients.48,49 A recent audit of fragility fracture management in the United Kingdom showed that 96% of patients with hip fracture were prescribed weight-bearing as tolerated after surgery, while only 32% of nonhip fracture patients were similarly prescribed full weight-bearing.47 For patients with distal femur fracture, this varied based on the fixation method used and was noted to be 92% with arthroplasty treatment, 67% with use of an intramedullary nail, and 35% with plate fixation. Early mobilization and early surgery has been shown to reduce mortality rates in the elderly hip fracture population.46 Elderly patients with distal femur fractures are a very similar population, with similar mortality rates and should be treated with the same principles of early mobilization and expedited surgery, with the goal of reducing mortality and maximizing return to function.
Higher-energy distal femur fractures typically occur in younger patients who are generally healthier. These patients have longer life expectancy and higher functional demands than the elderly group. These fractures often present with more biologic challenges due to fracture comminution, open injuries, and soft tissue damage. The most common issues affecting long-term results are treatment factors that lead to complications such as nonunion. This results in prolonged disability, delayed recovery, and the need for repeat procedures. Robust data to guide treatment decisions for distal femur fracture treatment are lacking.50 Owing to the inherent biologic challenges of these high-energy injuries, a robust understanding of bone healing biology and construct biomechanics is required for successful treatment. Understanding of femoral anatomy is also required to avoid hardware irritation, which is very common in the distal femur secondary to symptomatic screws protruding from the medial surface of the distal femur.
In these patients, the accurate restoration of joint surfaces and limb alignment to allow healing and return to function is more critical. Common pitfalls include malreduction in valgus or the “golf club deformity” from inaccurate posterior positioning of modern locking plates.51 Failures are often associated with short plates and overly rigid constructs.52 Technical considerations to optimize biomechanics include appropriately flexible bridging fixation, including longer-length intramedullary nails or plates and their appropriate application to ensure restoration and maintenance of alignment in a challenged biologic environment. In recent years, combination fixation with nail–plate constructs has been proposed to optimize biomechanics for early weight-bearing and in challenging situations such as bone loss or nonunion.53 This technique shows promise for challenging cases, and its usefulness will need to be defined in future studies. Knee joint stiffness postoperatively can be a challenging problem and should be minimized with early range of motion. Outcomes for this higher-energy patient group can be optimized by promoting successful union and return to function.
7. Conclusions
The successful management of distal femur fractures and distal femoral nonunions requires careful consideration of multiple factors. These include biomechanical considerations, attention to factors that minimize the development of post-traumatic osteoarthritis, biological factors, recognition of the relative advantages and disadvantages of nail versus plate versus dual-implant constructs, and finally an understanding of the different populations of patients who present with distal femur fractures and the different priorities they have. A robust understanding of these concepts can significantly improve success rates in the treatment of these challenging injuries.
Footnotes
The authors report no conflicts relevant to this manuscript. No funding was received for the preparation of this manuscript.
The study was deemed exempt from Institutional Review Board and Animal Use Committee Review.
Contributor Information
Justin Haller, Email: justin.haller@hsc.utah.edu.
Peter Augat, Email: biomechanik@bgu-murnau.de.
Donald D. Anderson, Email: don-anderson@uiowa.edu.
Michael D. McKee, Email: Michael.McKee@bannerhealth.com.
David Shearer, Email: David.Shearer@ucsf.edu.
Richard Jenkinson, Email: richard.jenkinson@sunnybrook.ca.
Hans-Christoph Pape, Email: Hans-Christoph.Pape@usz.ch.
References
- 1.Henderson CE, Kuhl LL, Fitzpatrick DC, et al. Locking plates for distal femur fractures: is there a problem with fracture healing? J Orthop Trauma. 2011;25(suppl 1):S8–S14. [DOI] [PubMed] [Google Scholar]
- 2.Collinge CA, Reeb AF, Rodriguez-Buitrago AF, et al. Analysis of 101 mechanical failures in distal femur fractures treated with 3 generations of precontoured locking plates. J Orthop Trauma. 2023;37:8–13. [DOI] [PubMed] [Google Scholar]
- 3.von Rüden C, Hungerer S, Augat P, et al. Breakage of cephalomedullary nailing in operative treatment of trochanteric and subtrochanteric femoral fractures. Archives Orthop Trauma Surg. 2015;135:179–185. [DOI] [PubMed] [Google Scholar]
- 4.Kandemir U, Augat P, Konowalczyk S, et al. Implant material, type of fixation at the shaft, and position of plate modify biomechanics of distal femur plate osteosynthesis. J Orthop Trauma. 2017;31:e241–e246. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt U, Penzkofer R, Bachmaier S, et al. Implant material and design alter construct stiffness in distal femur locking plate fixation: a pilot study. Clin Orthop Relat Res. 2013;471:2808–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henschel J, Tsai S, Fitzpatrick DC, et al. Comparison of 4 methods for dynamization of locking plates: differences in the amount and type of fracture motion. J Orthop Trauma. 2017;31:531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffmeier KL, Hofmann GO, Muckley T. Choosing a proper working length can improve the lifespan of locked plates. A biomechanical study. Clin Biomech (Bristol, Avon). 2011;26:405–409. [DOI] [PubMed] [Google Scholar]
- 8.Bottlang M, Lesser M, Koerber J, et al. Far cortical locking can improve healing of fractures stabilized with locking plates. J Bone Joint Surg Am. 2010;92:1652–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofmann-Fliri L, Epari DR, Schwyn R, et al. Biphasic Plating—in vivo study of a novel fixation concept to enhance mechanobiological fracture healing. Injury. 2020;51:1751–1758. [DOI] [PubMed] [Google Scholar]
- 10.Rademakers MV, Kerkhoffs GM, Sierevelt IN, et al. Intra-articular fractures of the distal femur: a long-term follow-up study of surgically treated patients. J Orthop Trauma. 2004;18:213–219. [DOI] [PubMed] [Google Scholar]
- 11.Scott BL, Lee CS, Strelzow JA. Five-year risk of conversion to total knee arthroplasty after operatively treated periarticular knee fractures in patients over 40 years of age. J Arthroplasty. 2020;35:2084–2089.e1. [DOI] [PubMed] [Google Scholar]
- 12.Houdek MT, Watts CD, Shannon SF, et al. Posttraumatic total knee arthroplasty continues to have worse outcome than total knee arthroplasty for osteoarthritis. J Arthroplasty. 2016;31:118–123. [DOI] [PubMed] [Google Scholar]
- 13.Heard BJ, Barton KI, Abubacker S, et al. Synovial and cartilage responsiveness to peri-operative hyaluronic acid ± dexamethasone administration following a limited injury to the rabbit stifle joint. J Orthop Res. 2022;40:838–845. [DOI] [PubMed] [Google Scholar]
- 14.Heard BJ, Barton KI, Chung M, et al. Single intra-articular dexamethasone injection immediately post-surgery in a rabbit model mitigates early inflammatory responses and post-traumatic osteoarthritis-like alterations. J Orthop Res. 2015;33:1826–1834. [DOI] [PubMed] [Google Scholar]
- 15.Jayaram P, Liu C, Dawson B, et al. Leukocyte-dependent effects of platelet-rich plasma on cartilage loss and thermal hyperalgesia in a mouse model of post-traumatic osteoarthritis. Osteoarthr Cartil. 2020;28:1385–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis E, Merghani K, Robertson I, et al. The effectiveness of leucocyte-poor platelet-rich plasma injections on symptomatic early osteoarthritis of the knee: the PEAK randomized controlled trial. Bone Joint J. 2022;104-B:663–671. [DOI] [PubMed] [Google Scholar]
- 17.Coleman MC, Goetz JE, Brouillette MJ, et al. Targeting mitochondrial responses to intra-articular fracture to prevent posttraumatic osteoarthritis. Sci Transl Med. 2018;10:eaan5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furman BD, Kimmerling KA, Zura RD, et al. Articular ankle fracture results in increased synovitis, synovial macrophage infiltration, and synovial fluid concentrations of inflammatory cytokines and chemokines. Arthritis Rheumatol. 2015;67:1234–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeKeyser GJ, Epperson R, Zhang C, et al. Articular fragment restoration is critical to mitigate post-traumatic osteoarthritis in a porcine pilon fracture model. Osteoarthr Cartil Open. 2022;4:100266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guilak F, Estes BT, Moutos FT. Functional tissue engineering of articular cartilage for biological joint resurfacing-The 2021 Elizabeth Winston Lanier Kappa Delta Award. J Orthop Res. 2022;40:1721–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niikura T, Lee SY, Sakai Y, et al. Causative factors of fracture nonunion: the proportions of mechanical, biological, patient-dependent, and patient-independent factors. J Orthop Sci. 2014;19:120–124. [DOI] [PubMed] [Google Scholar]
- 22.McKee MD, DiPasquale DJ, Wild LM, et al. The effect of smoking on clinical outcome and complication rates following Ilizarov reconstruction. J Orthop Trauma. 2003;17:663–667. [DOI] [PubMed] [Google Scholar]
- 23.Bergin PF, Rothberg DL, Spitler CA, et al. The prevalence of metabolic and endocrine disturbances on fracture nonunion. Endocr Pract. 2022;28:599–602. [DOI] [PubMed] [Google Scholar]
- 24.Brinker MR, O'Connor DP, Monla YT, et al. Metabolic and endocrine abnormalities in patients with nonunions. J Orthop Trauma. 2007;21:557–570. [DOI] [PubMed] [Google Scholar]
- 25.Wang JW, Weng LH. Treatment of distal femoral nonunion with internal fixation, cortical allograft struts, and autogenous bone-grafting. J Bone Joint Surg Am. 2003;85:436–440. [DOI] [PubMed] [Google Scholar]
- 26.Rajasekaran RB, Jayaramaraju D, Palanisami DR, et al. A surgical algorithm for the management of recalcitrant distal femur nonunions based on distal femoral bone stock, fracture alignment, medial void, and stability of fixation. Arch Orthop Trauma Surg. 2019;139:1057–1068. [DOI] [PubMed] [Google Scholar]
- 27.Redondo-Trasobares B, Sarasa-Roca M, Rosell-Pradas J, et al. Comparative clinical and biomechanical study of different types of osteosynthesis in the treatment of distal femur fractures. Rev Esp Cir Ortop Traumatol. 2023;67:216–225. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez EK, Zurakowski D, Herder L, et al. Mechanical construct characteristics predisposing to non-union after locked lateral plating of distal femur fractures. J Orthop Trauma. 2016;30:403–408. [DOI] [PubMed] [Google Scholar]
- 29.Kim SM, Yeom JW, Song HK, et al. Lateral locked plating for distal femur fractures by low-energy trauma: what makes a difference in healing? Int Orthop. 2018;42:2907–2914. [DOI] [PubMed] [Google Scholar]
- 30.Barber CC, Burnham M, Ojameruaye O, et al. A systematic review of the use of titanium versus stainless steel implants for fracture fixation. OTA Int. 2021;4:e138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aggarwal S, Rajnish RK, Kumar P, et al. Comparison of outcomes of retrograde intramedullary nailing versus locking plate fixation in distal femur fractures: a systematic review and meta-analysis of 936 patients in 16 studies. J Orthop. 2023;36:36–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cone R, Roszman A, Conway Y, et al. Risk factors for nonunion of distal femur fractures. J Orthop Trauma. 2023;37:175–180. [DOI] [PubMed] [Google Scholar]
- 33.Miller MD, Perera J, Smith E, et al. Retrograde intramedullary nailing hardware failure of a supracondylar distal femur fracture with intercondylar extension. Cureus. 2022;14:e26276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bel JC, Court C, Cogan A, et al. Unicondylar fractures of the distal femur. Orthop Traumatol Surg Res. 2014;100:873–877. [DOI] [PubMed] [Google Scholar]
- 35.Mittal A, Poole W, Crone D. Interprosthetic femoral fractures managed with modern distal femoral locking plates: 10 years' experience at a UK major trauma centre. Injury. 2021;52:1918–1924. [DOI] [PubMed] [Google Scholar]
- 36.Gerow DE, Ross HL, Bodrogi A, et al. Periprosthetic supracondylar femoral fractures above a total knee replacement: an updated compatibility and technique guide for fixation with a retrograde intramedullary nail. J Orthop Trauma. 2022;36:e92–e97. [DOI] [PubMed] [Google Scholar]
- 37.O'Neill DC, Hakim AJ, DeKeyser GJ, et al. Medial and lateral dual plating of native distal femur fractures: a systematic literature review. OTA Int. 2023;6:e227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeKeyser GJ, Hakim AJ, O'Neill DC, et al. Biomechanical and anatomical considerations for dual plating of distal femur fractures: a systematic literature review. Arch Orthop Trauma Surg. 2022;142:2597–2609. [DOI] [PubMed] [Google Scholar]
- 39.Matelic TM, Monroe MT, Mast JW. The use of endosteal substitution in the treatment of recalcitrant nonunions of the femur: report of seven cases. J Orthop Trauma. 1996;10:1–6. [DOI] [PubMed] [Google Scholar]
- 40.Kontakis MG, Giannoudis PV. Nail plate combination in fractures of the distal femur in the elderly: a new paradigm for optimum fixation and early mobilization? Injury. 2023;54:288–291. [DOI] [PubMed] [Google Scholar]
- 41.Liporace FA, Aneja A, Carroll EA, et al. Maintaining the neutral Axis in the treatment of distal femur fractures via dual plate or nail plate combination technique: when and how? J Orthop Trauma. 2021;35(suppl 5):S38–S40. [DOI] [PubMed] [Google Scholar]
- 42.Wright DJ, DeSanto DJ, McGarry MH, et al. Supplemental fixation of supracondylar distal femur fractures: a biomechanical comparison of dual-plate and plate-nail constructs. J Orthop Trauma. 2020;34:434–440. [DOI] [PubMed] [Google Scholar]
- 43.Passias BJ, Emmer TC, Sullivan BD, et al. Treatment of distal femur fractures with a combined nail-plate construct: techniques and outcomes. J Long Term Eff Med Implants. 2021;31:15–26. [DOI] [PubMed] [Google Scholar]
- 44.Myers P, Laboe P, Johnson KJ, et al. Patient mortality in geriatric distal femur fractures. J Orthop Trauma. 2018;32:111–115. [DOI] [PubMed] [Google Scholar]
- 45.Larsen P, Ceccotti AA, Elsoe R. High mortality following distal femur fractures: a cohort study including three hundred and two distal femur fractures. Int Orthop. 2020;44:173–177. [DOI] [PubMed] [Google Scholar]
- 46.Pincus D, Ravi B, Wasserstein D, et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA. 2017;318:1994–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richardson C, Bretherton CP, Raza M, et al. The Fragility Fracture Postoperative Mobilisation multicentre audit: the reality of weightbearing practices following operations for lower limb fragility fractures. Bone Joint J. 2022;104-B:972–979. [DOI] [PubMed] [Google Scholar]
- 48.Paulsson M, Ekholm C, Jonsson E, et al. Immediate full weight-bearing versus partial weight-bearing after plate fixation of distal femur fractures in elderly patients. A randomized controlled trial. Geriatr Orthop Surg Rehabil. 2021;12:21514593211055889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Consigliere P, Iliopoulos E, Ads T, et al. Early versus delayed weight bearing after surgical fixation of distal femur fractures: a non-randomized comparative study. Eur J Orthop Surg Traumatol. 2019;29:1789–1794. [DOI] [PubMed] [Google Scholar]
- 50.Claireaux HA, Searle HK, Parsons NR, et al. Interventions for treating fractures of the distal femur in adults. Cochrane Database Syst Rev. 2022;10:CD010606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lowe J, Alhandi A, Manoharan A, et al. Axial and rotational malreduction (golf club deformity) in distal femur fractures. J Orthop Trauma. 2022;36:515–518. [DOI] [PubMed] [Google Scholar]
- 52.Harvin WH, Oladeji LO, Della Rocca GJ, et al. Working length and proximal screw constructs in plate osteosynthesis of distal femur fractures. Injury. 2017;48:2597–2601. [DOI] [PubMed] [Google Scholar]
- 53.Liporace FA, Yoon RS. Nail Plate combination technique for native and periprosthetic distal femur fractures. J Orthop Trauma. 2019;33:e64–e68. [DOI] [PubMed] [Google Scholar]


