Abstract
The cytomegalovirus (CMV) major immediate-early promoter/enhancer is active in many cell culture systems and is considered to be one of the strongest promoters in vitro. However, when this promoter was used in in vivo approaches to gene therapy, it was silenced within a few weeks in several organs including the liver. In this study, we demonstrated transcriptional inactivation of the CMV promoter in mouse liver. In contrast to the CMV promoter, a hybrid promoter consisting of a minimal CMV promoter and the enhancer II of hepatitis B virus was active for at least 11 weeks in mouse liver. While investigating the reason for the shutdown of the CMV promoter, we did not find evidence for methylation of adenovirus DNA in the region of transgene insertion, but we could show that the silenced CMV promoter was reactivated after lipopolysaccharide treatment of mice or partial hepatectomy. Both stimuli are known to activate the transcription factor NFκB, which binds to four sites in the CMV promoter/enhancer. We show that expression from the CMV promoter in hepatocyte-derived cell lines in vitro depends on NFκB. In vivo experiments demonstrate that NFκB, which is not present in mouse hepatocytes in vivo, is activated after infection with recombinant adenoviruses and that the time course of NFκB activation parallels that of CMV promoter-dependent expression. Moreover, adenovirus infection of transgenic mice carrying a CMV promoter-driven lacZ gene leads to strong activation of the expression of this gene in the liver. Thus, NFκB is involved in the activation of the CMV promoter in the liver.
Sufficient expression of transduced genes is a major requirement in somatic gene therapy. To achieve high levels of transgene expression in vivo, strong viral promoters have been widely used. Since the human cytomegalovirus (CMV) major immediate-early promoter/enhancer (8) (hereafter referred to as the CMV promoter) is considered to be one of the strongest promoters in vitro, it has been used for in vivo expression of reporter and therapeutic genes by many investigators. However, although the CMV promoter allowed for a very strong short-term expression of transduced genes in vivo, it became silent within a few weeks after gene transfer in many animal studies. In most cases, expression from the CMV promoter peaked at days 2 to 4 after delivery of the transgene and declined within 4 weeks to barely detectable or background activity. This silencing of the CMV promoter has been reported for both immunocompetent and immunodeficient mice and also seems to be independent of the vector system, the transduction method, and the species used (13, 21, 24, 27, 39, 50, 62). Interestingly, combination of the CMV promoter/enhancer with other regulatory elements allowed for long-term expression in transplanted myoblasts as well as after adenovirus gene transfer to muscle and liver (13, 14, 29), implying that the inhibition of the CMV promoter can be overcome by other transcriptionally active elements. After adenovirus gene transfer to the liver, the CMV promoter was at least 10-fold stronger than any other viral and cellular promoters tested, but its activity declined by several orders of magnitude within a few weeks (21). However, the reason for the short-term nature of the activity of the CMV promoter in liver is unclear, and no laboratory has investigated this phenomenon in detail so far.
The human CMV promoter consists of at least four types of repetitive sequence elements, referred to as the 17-, 18-, 19-, and 21-bp repeats, which are present three to five times within the promoter/enhancer region of the CMV promoter and which form complexes with nuclear proteins (8, 19). The 18- and 19-bp repeats contain consensus binding sites for NFκB and CREB/ATF, respectively, and were shown to mediate the enhancement of CMV promoter activity by these transcription factors (25, 48, 53). The 17-bp repeat was suggested to bind to the transcription factor NF-1 (43). The 21-bp repeat binds to a negative regulator specific for undifferentiated cells as well as to YY1 and was suggested to repress CMV promoter-dependent transcription (31, 52). Other factors which bind to the CMV promoter are AP-1 (48), SP 1 (34), and MDBP (63, 64).
Among transcription factors involved in activation of the CMV promoter, NFκB is of special interest. Transcription factors of the NFκB/Rel family play a central role in the regulation of a variety of cellular and viral genes (for recent reviews, see references 2, 3, 56, and 59). Four NFκB consensus binding sites are present in the CMV promoter, and three of them are identical to the Igκ consensus binding site. Efficient transcription from the CMV promoter was dependent on these sites (6, 46), although the effect of the κB binding site was strongly dependent on the cell type used (43). Furthermore, reactivation of latent CMV infection in transplantation patients was correlated with TNF-α levels, and TNF-α dependent activation of the CMV promoter in the monocytic cell line HL-60 was mediated by NFκB (15, 46). In the context of adenovirus vectors, stimulation of NFκB and/or CREB/ATF by several agents, such as phorbol esters, calcium ionophors, and forskolin, resulted in a strong activation of CMV promoter activity in MRC5 and HeLa cells as well as in primary human vascular smooth muscle cells (10, 57). Therefore, although the CMV promoter is considered to be strong in most cell culture systems, its full activity requires the presence of these transcription factors.
In contrast to the broad activity of the CMV promoter in vitro, experiments with mice transgenic for CMV promoter-driven reporter constructs revealed a different situation in vivo. Although early studies reported CMV promoter-dependent expression in most tissues (18, 51), more detailed studies in both adult and embryonic transgenic mice suggested that the CMV promoter is active only in cell types which are naturally infected by CMV (5, 30). Interestingly, all the studies are consistent insofar as the CMV promoter was silent or very weak in the livers of these transgenic mice.
In the present study, we show that the CMV promoter is specifically silenced in the mouse liver while adenovirus DNA is present at nearly unaltered levels. In contrast, a previously described hepatitis B virus (HBV)-CMV hybrid promoter which is strong and liver specific in vivo (37, 49) retained its activity for at least 11 weeks. The silenced CMV promoter could be reactivated by lipopolysaccharide (LPS) treatment or partial hepatectomy on day 28 after adenovirus infection. In vitro studies showed that NFκB is required for full activity of the CMV promoter in hepatocyte-derived cell lines. In vivo experiments demonstrated that infection with recombinant adenovirus induces NFκB activity in mouse hepatocytes and that activation of this transcription factor follows the same time course as does CMV promoter-dependent expression. Moreover, adenovirus infection strongly stimulated the expression of a CMV promoter-driven β-galactosidase gene in transgenic mice. Our data suggest a critical role for NFκB in CMV promoter activity in mouse liver.
MATERIALS AND METHODS
Viruses.
All the viruses used in this study have been described previously (49). Briefly, adenovirus type 5 (Ad5)-CMVLDLR contains the cDNA for the human low-density lipoprotein (LDL) receptor gene under transcriptional control of the human CMV major immediate-early promoter. In Ad5-EIImCMVLDLR, the expression of the human LDL receptor cDNA is driven by an HBV-CMV hybrid promoter which consists of a basic CMV promoter and the enhancer II of HBV (37). Ad5-RSVBG, in which the Escherichia coli β-galactosidase gene is expressed under the Rous sarcoma virus (RSV) long terminal repeat (LTR) was used as a control virus. Ad-CMVBG was a generous gift from R. Crystal.
Cell culture and reporter gene assays.
HepG2 (human hepatoma) cells and HepSV40 (mouse liver) (45) cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal calf serum (FCS). Transfection was performed using a modified calcium phosphate coprecipitation method as described previously (35). Infection with Ad5-CMVBG was carried out in 24-well plates at low doses (0.2 to 1 PFU/cell) and at a cell density of 105 cells/well. For stimulation experiments, medium was replaced with DMEM containing 1% FCS 12 h after transfection and the cells were stimulated with phorbol myristate acetate (PMA) (50 ng/ml) and/or forskolin (10 μM) (Sigma, Deisenhofen, Germany) for 24 h. Luciferase and β-galactosidase assays were performed as described previously (37).
Animal procedures.
Experiments in this study were performed with 8-week-old female C57BL/6 mice. This mouse strain had been shown to allow for prolonged transgene expression from the RSV LTR in earlier studies (4). The mice were given tail vein injections of 109 PFU of Ad5-CMVLDLR, Ad5-EIImCMVLDLR, or Ad5-RSVBG. At 3, 7, 14, 28 and 77 days after infection, the animals were killed and their livers were frozen in liquid nitrogen immediately. The livers were then pulverized in liquid nitrogen, and the homogenate was used for separate RNA and DNA isolation.
For restimulation experiments, the animals were injected intraperitoneally (i.p.) with 50 μg of LPS (from Escherichia coli serotype O111:B4) (Sigma, Deisenhofen, Germany), 100 μg of LPS, or NaCl on day 28 after infection with 2 × 109 PFU of Ad5-CMVLDLR. At the same time, a second group of mice which had received the same virus dose was subjected to partial hepatectomy as described by Jennings et al. (26). At 16 h after the LPS treatment or partial hepatectomy, the mice were sacrificed and their livers were analyzed for transgene expression.
Transgenic mice of the HCMV-1 line carry the E. coli lacZ gene under transcriptional control of the CMV promoter (−524 to +13) and were a generous gift of P. J. Mitchell (for details, see reference 30). Offspring positive for the lacZ gene were produced by mating male HCMV-1 mice to background strain (Swiss ICR) and checked for transgene expression by 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) staining of ear and tail tissue samples. Stimulation experiments were performed with 8-week-old female animals by tail vein injection of 2 × 109 or 1 × 1010 PFU of Ad5-CMVLDLR.
Southern blotting and RNase protection assay.
RNA and genomic DNA were isolated separately from the same piece of tissue by standard methods. Detection of adenovirus DNA in genomic DNA from mouse liver has been described previously (49). Briefly, 10 μg of genomic DNA was digested with NcoI, separated in a 1% agarose gel, blotted on Hybond N+ nylon membrane (Amersham, Little Chalfont, United Kingdom), and probed with a probe specific for a 1,785-bp adenovirus DNA fragment which is released by NcoI digestion of the Ad5 genome. For detection of potential methylation of the CMV promoter, genomic mouse liver DNA harvested on day 3 or 25 after infection of mice with Ad5-CMVLDLR was digested with restriction endonucleases which are blocked by CpG methylation. DNA fragments were processed as described above and probed with a probe specific for the CMV immediate-early promoter.
The RNase protection assays used to specifically detect human LDL receptor RNA in mouse liver and mouse GAPDH RNA have been described elsewhere (49).
Separation of hepatocytes from NPCs.
To separate mouse hepatocytes from nonparenchymal cells (NPCs), liver pefusion was performed as follows. The mice were anesthetized by i.p. injection of 0.1 mg of Ketanest (Parker-Davis, Berlin, Germany) per g of body weight and 4 μl of 0.4% Rompun (Bayer, Leverkusen, Germany) per g of body weight. After laparotomy, the liver was perfused via the portal vein for 5 min with a preperfusion solution (30 mM glucose, 25 mM HEPES [pH 7.5], 300 mM NaCl, 60 mM KCl, 3 mM KH2PO4, 0.5 mM EGTA, 0.5 mM glutamine) and for 8 min with a collagenase solution (30 mM glucose, 25 mM HEPES [pH 7.5], 120 mM NaCl, 48 mM KCl, 1.2 mM KH2PO4, 3 mM CaCl2, 0.5 mM glutamine, 150 ml of DMEM [high glucose] per liter, 0.4% collagenase) (Boehringer Mannheim, Germany) at a flow rate of 3 ml/min. After perfusion, liver cells were dispersed on a petri dish in DMEM and the resulting suspension was filtered through a 100-μm cell strainer (Falcon, Franklin Lakes, N.J.). The suspension was then transferred onto ice and centrifuged for 2 min at 45 × g at 4°C, and the supernatant was removed. The pellet was carefully resuspended in DMEM and recentrifuged. This procedure was performed two more times. The pellet, which contains nearly exclusively hepatocytes, was used for preparation of nuclear extracts.
Nuclear extracts and EMSA.
Nuclear extracts were prepared from whole liver or from isolated hepatocytes, respectively. For extract preparation from whole liver, mice were killed and the liver was removed from each mouse immediately and transferred to ice-cold phosphate-buffered saline (PBS). The whole procedure was performed at 4°C. After three washes in PBS, the liver was minced and transferred to 10 ml of homogenization buffer (sucrose solution 1) (0.32 M sucrose, 10 mM HEPES [pH 7.5], 0.15 mM spermine, 0.5 mM spermidine, 1 mM EGTA, 0.1 mM EDTA, 3 mM CaCl2, 1 mM magnesium acetate, 1 mM dithiothreitol [DTT], 1 mM phenylmethylsulfonyl fluoride [PMSF], 0.1% Triton X-100). For preparation of nuclear extracts from isolated hepatocytes, the hepatocyte pellet was resuspended in 10 ml of homogenization buffer. The cells were homogenized by five strokes in a Wheaton L Dounce homogenizer followed by five strokes in a Wheaton S Dounce homogenizer. The homogenate was mixed with 20 ml of sucrose solution 2 (2 M sucrose, 10 mM HEPES [pH 7.5], 5 mM magnesium acetate, 0.1 mM EDTA, 1 mM DTT, 1 mM PMSF), and the mixture was used to overlay 10 ml of saccharose solution 2 in a Beckman SW28 centrifuge tube. The tubes were centrifuged for 50 min at 25,000 rpm in an SW28 rotor at 4°C, and the nuclear pellet was washed twice in wash solution (10 mM HEPES [pH 7.5], 25 mM KCl, 5 mM magnesium acetate, 0.1 mM EDTA, 1 mM EGTA, 0.15 mM spermine, 0.5 mM spermidine, 1 mM DTT, 0.5 mM PMSF, 20% glycerol). The integrity of nuclei was checked under a microscope, and nuclear proteins were extracted with 100 to 150 μl of nuclear extraction buffer (20 mM HEPES [pH 7.5], 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, 0.5 mM PMSF, 2 μg of aprotinin per ml, 1 μg of leupeptin per ml, 25% glycerol) with gentle stirring for 30 min at 4°C. The nuclear extracts were then centrifuged for 30 min in a Beckman TLA 100.3 rotor at 25,000 rpm at 4°C, the supernatant was dialyzed against a 1,000-fold volume of dialysis buffer (20 mM HEPES [pH 7.5], 100 mM KCl, 0.2 mM EDTA, 0.5 mM DTT, 0.5 mM PMSF, 20% glycerol) for 3 to 4 h at 4°C, and the dialysate was again centrifuged for 30 min at 25,000 × g at 4°C. The protein concentration in the resulting supernatant was measured by the Bradford method, and the supernatant was aliquoted, frozen in liquid nitrogen, and stored at −80°C.
An electrophoretic mobility shift assay (EMSA) for the detection of NFκB was performed as described elsewhere (42) with the addition of 0.1% NP-40. Oligonucleotides containing the H-2K consensus sequence (…GGATTCCCC…) or the κB consensus site present three times in the CMV promoter (…GGGACTTTCC…) were radiolabelled by Klenow fill in and used as probes. The same oligonucleotides were used in competition experiments. For supershift experiments, antibodies against p50 [p50(NLS)-G], p65 [NFκB p65(A)X], Rel-B [Rel B (C-19], and c-Rel [c-Rel(C)-G] (all from Santa Cruz Biotechnology Inc., Santa Cruz, Calif.) were used.
RESULTS
Long-term expression from the CMV immediate-early promoter and HBV-CMV hybrid promoter.
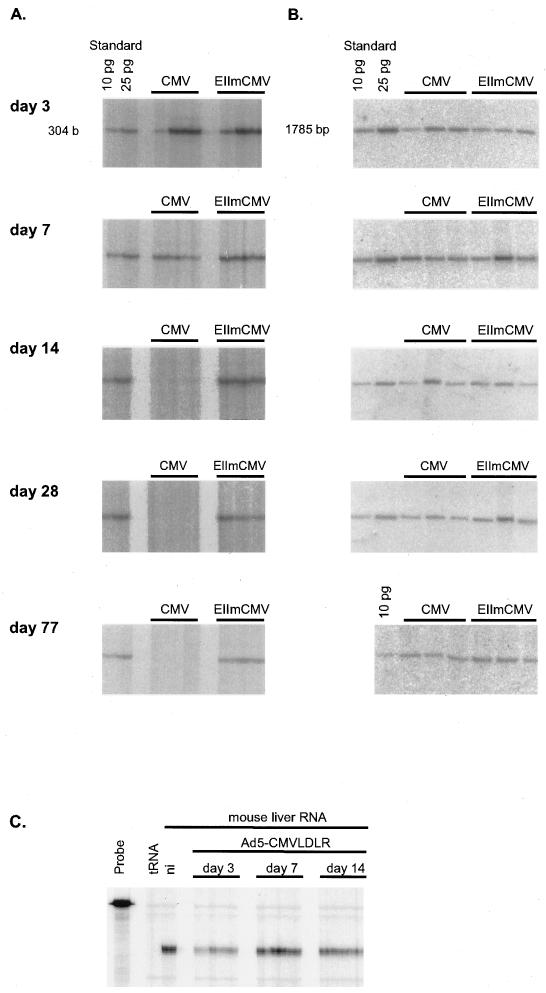
Long-term expression from viral promoters is an important issue in liver gene therapy. Several reports have shown that vectors in which the expression of the transgene is driven by the CMV promoter did not allow for expression in animal liver for longer than about 2 weeks (21, 27, 39). This phenomenon has been observed for other tissues as well as in vitro (1, 13, 50). Since our previously described HBV-CMV hybrid promoter allowed for strong and liver-specific expression in vivo (49), we wished to know if long-term expression from this promoter is influenced by the CMV component of the promoter. Therefore, we monitored expression from the CMV promoter and from the HBV-CMV hybrid promoter for about 11 weeks. Female C57BL/6 mice were infected with 109 PFU of Ad5-CMVLDLR or Ad5-EIImCMVLDLR. The animals in each group were sacrificed on days 3, 7, 14, 28, and 77 after infection, and the presence of viral DNA and the expression of the human LDL receptor were monitored. As shown in Fig. 1, adenovirus DNA was present at each time point at comparable levels in all animals, with a moderate decline on day 77 (Fig. 1B). However, whereas the level of human LDL receptor RNA expressed from the HBV-CMV hybrid promoter did not change markedly from day 3 to day 77 after infection, there was a sharp decline in the level of human LDL receptor RNA expressed from the CMV promoter, with moderately impaired expression on day 7, strongly reduced expression on day 14, and no expression on day 28 (Fig. 1A). The integrity of the RNA was confirmed by an RNase protection assay specific for mouse GAPDH RNA and is shown for the period when CMV promoter-dependent expression was lost (days 3 through 14 after infection [Fig. 1C]). Thus, since Ad5-CMVLDLR DNA is still present at a level comparable to that observed with Ad5-EIImCMVLDLR, we conclude that the CMV promoter is selectively silenced in mouse hepatocytes in vivo.
FIG. 1.
Time course of human LDL receptor gene expression in livers of adenovirus-transduced mice. C57BL/6 mice were infected with 109 PFU of AdCMV-LDLR (CMV) or Ad5-EIImCMVLDLR (EIImCMV), respectively. The mice were sacrificed at the time points indicated, and RNA and DNA were isolated from the same tissue sample. (A) Expression of human LDL receptor RNA as determined by the RNase protection assay. An in vitro-transcribed human LDL receptor RNA (10 or 25 pg) was used as a size standard and for quantification of the protected fragment. RNA subjected to the RNase protection assay (10 to 35 μg) was related to the amount of virus DNA present in the same tissue sample. (B) Quantification of viral DNA in mouse liver. Total DNA (10 μg) isolated from mouse liver was cleaved with NcoI, subjected to Southern blot analysis, and detected with a probe specific for a 1,785-bp adenovirus DNA fragment. As a standard, 10 and/or 25 pg of the same NcoI fragment obtained from digesting purified Ad5 DNA was used. Each lane represents an individual animal. (C) Integrity of liver RNA from mice infected with Ad5-CMVLDLR. Total RNA (5 μg) from livers of mice infected with Ad5-CMVLDLR and killed 3, 7, and 14 days after infection was subjected to an RNase protection assay specific for mouse glyceraldehyde-3-phosphate dehydrogenase RNA. RNA from the liver of a noninfected animal was used as a control (ni).
There is no evidence for extensive methylation of the CMV promoter region.
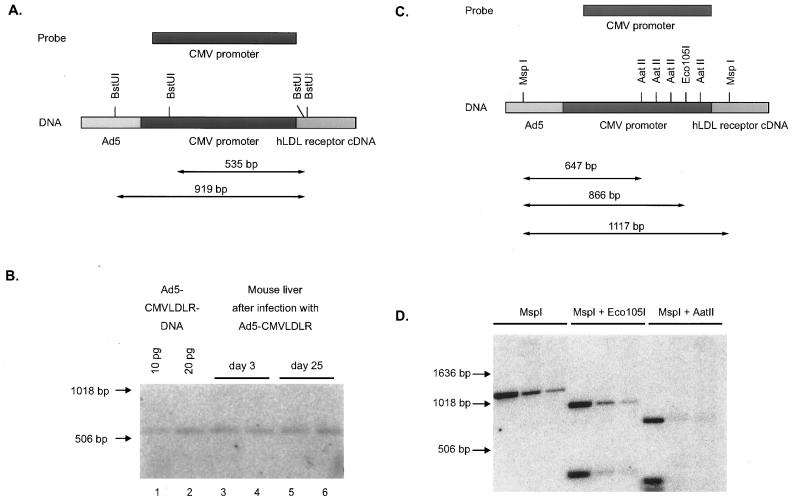
Methylation of DNA sequences is a common mechanism to silence gene expression within the mammalian genome. Prösch et al. (47) have shown that nonspecific methylation of the CMV promoter with the CpG methyltransferase SssI in vitro caused a complete loss of promoter activity in HL-60 cells. Therefore, we tested the CMV promoter sequence present in mouse liver on day 25 after infection with Ad5-CMVLDLR for possible methylation. Since the CMV promoter sequence does not harbor any appropriate site for enzyme pairs such as HpaII-MspI, which allow for discrimination between methylated and unmethylated recognition sites, we performed two cleavage experiments with enzymes known to be blocked by CpG methylation. First, BstUI (FnuDII) recognizes a site distal in the CMV promoter as well as several sites in the flanking sequences of Ad5-CMVLDLR and cleaves only CGCG but not m5CGCG (Fig. 2A). DNA from mouse livers harvested on days 3 and 25 after infection with Ad5-CMVLDLR was digested with BstUI, and the products were subjected to Southern blotting. BstUI cleavage results in the release of a 535-bp fragment from purified Ad5-CMVLDLR DNA (Fig. 2B, lanes 1 and 2). In the case of methylation of the BstUI sites indicated in Fig. 2A, cleavage by this enzyme would be prevented and the intensity of the 535-bp fragment should diminish. As shown in Fig. 2B, the 535-bp fragment signal was present at comparable density on days 3 and 25 after adenovirus infection and no slower-migrating band could be detected. In a second experiment, mouse liver DNA harvested on day 25 after infection was digested with MspI, which cleaves both methylated and unmethylated sequences. Cleavage with MspI releases a 1,117-bp fragment from Ad5-CMVLDLR DNA (Fig. 2C), and the appearance of this fragment is independent of potential methylation of the internal C of its recognition sequence. In contrast, Eco105I and AatII are blocked by CpG methylation in their recognition sites. Simultaneous incubation of liver DNA with MspI and Eco105I or AatII should therefore result in complete degradation of the MspI fragment only if CpG methylation is absent. As seen in Fig. 2D, Eco105I and AatII completely cleave the MspI fragment, indicating that no methylation of adenovirus DNA in the region of transgene insertion had occurred within 25 days after infection of mice with Ad5-CMVLDLR. Although these experiments do not prove the absence of any methylation in the CMV promoter region, they strongly suggest that methylation is not involved in the silencing of CMV promoter-dependent expression.
FIG. 2.
Absence of methylation in the distal region of the human CMV immediate-early promoter. (A) Schematic of the CMV immediate-early promoter region present in Ad5-CMVLDLR and the probe used to detect BstUI fragments. Cleavage of unmethylated DNA results in a 535-bp fragment, whereas methylation of the BstUI sites in the CMV enhancer would result in larger fragments. (B) Southern blot analysis of BstUI-cleaved mouse liver DNA 3 or 25 days after infection. As a standard, 10 and 20 pg of the BstUI fragment obtained from cleavage of purified Ad5-CMVLDLR DNA were used. Size marker positions are indicated at the left. (C) Restriction sites for MspI, Eco105I, and AatII in the CMV promoter region in Ad5-CMVLDLR DNA. The sizes of the major restriction fragments are indicated. (D) Southern blot analysis of DNA from CsCl gradient-purified Ad5-CMVLDLR (lanes 1, 4, and 7) and liver DNA from two mice 25 days after infection with Ad5-CMVLDLR (lanes 2, 5, and 8 and lanes 3, 6, and 9, respectively). DNA was digested with the restriction enzymes indicated and was used in Southern analysis with the CMV promoter probe. Size marker positions are indicated at the left.
LPS treatment restimulates expression from the silenced CMV promoter.
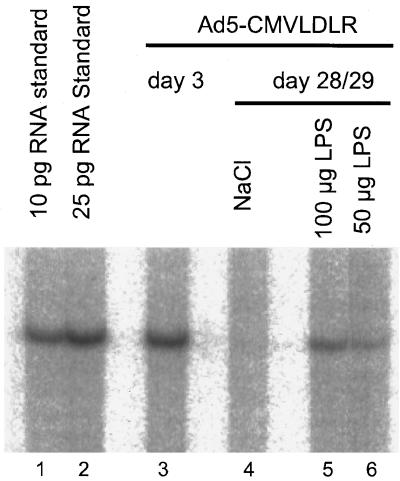
The CMV promoter region harbors four consensus binding sites for the transcription factor NFκB. Although data about the presence of NFκB in rodent liver are partially contradictory, it seems unambiguous that hepatocytes in vivo normally contain no or only very little activated NFκB (11, 16, 17, 55, 60). Therefore, we asked if activation of NFκB in hepatocytes could reactivate expression from the CMV promoter in vivo. Bacterial LPS is a very strong inducer of NFκB and has a strong effect on the activation of this transcription factor in hepatocytes (14a, 17). To test if LPS could stimulate CMV promoter activity in mouse liver in vivo, mice were infected with 2 × 109 PFU of Ad5-CMVLDLR via tail vein injection and were injected i.p. with 100 or 50 μg of LPS on day 28 after infection. The mice were killed 20 h after stimulation with LPS, and their livers were analyzed for expression of the human LDL receptor. As shown in Fig. 3, mice injected with LPS expressed the human LDL receptor RNA at a moderate level (Fig. 3, lanes 5 and 6) whereas the mouse injected with NaCl did not express the transgene (lane 4). Although the level of expression is only about 15 to 20% of that seen on day 3 after injection (lane 3), the expression from the CMV promoter is clearly present after LPS treatment. We conclude that LPS treatment has reactivated the previously silenced CMV promoter.
FIG. 3.
Reactivation of the CMV promoter in mouse liver by LPS. Mice were injected with 2 × 109 PFU of Ad5CMVLDLR. On day 3 after infection, human LDLR RNA is present at a high level (lane 3). On day 28 after infection, mice received a single injection of 200 μl of NaCl (lane 4) or 100 or 50 μg of LPS (lanes 5 and 6). The livers were removed 20 h later, and 10 μg of total liver RNA was analyzed for the expression of human LDL receptor RNA by the RNase protection assay. As a standard, 10 and 25 pg of in vitro-transcribed RNA were used (lanes 1 and 2).
Partial hepatectomy restimulates expression from the silenced CMV promoter.
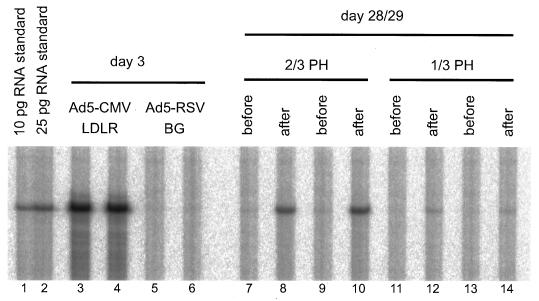
NFκB was found to be strongly activated in the regenerating liver shortly after partial hepatectomy in Fisher rats and C57BL/6 mice (16, 60). We wanted to test if partial hepatectomy could restimulate the silenced CMV promoter in livers of adenovirally transduced mice. To this end, mice were infected with 2 × 109 PFU of Ad5-CMVLDLR and partial hepatectomy was performed on day 28 after infection, when CMV promoter-dependent expression was no longer detectable. In two mice, two-thirds of the liver was removed; in two other mice, only one-third of the liver was resected. The removed liver lobes were frozen immediately, and RNA was prepared. The mice were sacrificed 16 h after the partial hepatectomy, the regenerating livers were harvested, and RNA from livers prior to and after partial hepatectomy from the same animal was analyzed for presence of human LDL receptor transcripts. The outcome of the experiment is shown in Fig. 4. At the time point of liver resection (day 28 after infection), no expression of the human LDL receptor gene is detectable (Fig. 4, lanes 7, 9, 11, and 13). At 16 h later, animals which had received one-third partial hepatectomy expressed the human LDL receptor gene at a low level (lanes 12 and 14) whereas a two-third partial hepatectomy had a marked effect on CMV promoter-dependent transgene expression (lanes 8 and 10). The level of expression of the human LDL receptor was about 30% of that observed in mouse livers on day 3 after infection with Ad5-CMVLDLR (lanes 3 and 4). Southern blot analysis of genomic DNA from livers prior to and after partial hepatectomy did not show any difference in the level of virus DNA (data not shown), confirming that the elevation in the human LDL receptor mRNA level is caused by transcriptional activation. We conclude that partial hepatectomy reactivated the previously silenced expression from the human CMV promoter.
FIG. 4.
Reactivation of the CMV promoter in mouse liver by partial hepatectomy. Mice were injected with 2 × 109 PFU of Ad5CMV-LDLR or Ad5-RSVBG. As determined by the RNase protection assay, human LDL receptor RNA was expressed at a high level in the animals treated with Ad5CMVLDLR on day 3 after infection but not in the animals treated with Ad5-RSVBG (compare lanes 3 and 4 with lanes 5 and 6). On day 28 after infection, each of two animals received a two-third or one-third partial hepatectomy (PH), respectively. The removed liver lobes did not contain human LDL receptor RNA (lanes 7, 9, 11, and 13). At 16 h after the partial hepatectomy, the mice were sacrificed and liver RNA was analyzed for LDL receptor RNA (lanes 8, 10, 12, and 14).
Expression from the CMV promoter in hepatocytes is influenced by NFκB.
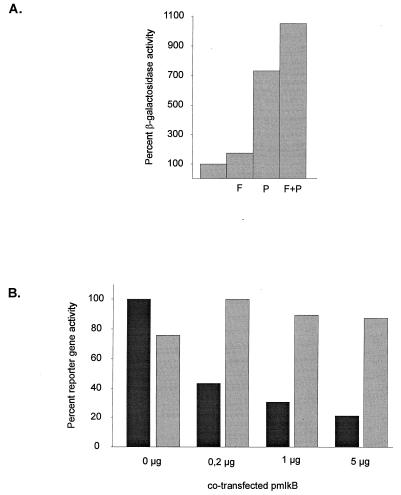
Both LPS and partial hepatectomy stimulate a number of transcription factors which could be involved in reactivating the CMV promoter. To investigate the roles of transcription factor binding to the CMV promoter in hepatocytes in more detail, in vitro experiments were performed with the liver-derived cell lines HepG2 and HepSV40. HepG2 cells have nearly no activated NFκB, whereas the nuclei of HepSV40 cells contain relatively high levels of NFκB (data not shown). First, HepG2 cells were infected with Ad5CMVBG at a multiplicity of infection of 0.2. Infection medium was replaced by DMEM containing 1% FCS, and 10 μM forskolin, 50 ng of PMA per ml, or both were added. PMA is a strong activator of NFκB in many cell lines, whereas forskolin stimulates cyclic AMP-dependent transcription. HepG2 cells are known to contain low nuclear levels of activated NFκB, which is strongly stimulated by treatment with phorbol esters (20, 22). At 24 h after stimulation, the cells were harvested and their β-galactosidase activity was measured. Whereas forskolin caused only a weak stimulation of the CMV promoter, PMA treatment resulted in a 7.3-fold stimulation of promoter activity (Fig. 5A). Combined treatment with forskolin and PMA resulted in a stronger stimulation than activation with either of the single substances (10.5-fold), indicating that the substances might act cooperatively to activate the CMV promoter.
FIG. 5.
NFκB modulates expression from the CMV IE promoter in vitro. (A) HepG2 cells were infected with 0.2 PFU of Ad5-CMVBG for 12 h. The transfection medium was replaced with DMEM containing 1% FCS, and the cells were stimulated with 10 μM forskolin (F), 50 ng of PMA per ml (P), or both (F + P). At 24 h later, the cells were harvested and analyzed for β-galactosidase activity. β-Galactosidase expression was related to the protein content as determined by the Bradford method. (B) HepSV40 cells (3 × 105 cells) were transfected with 2 μg of pCMVluc and increasing amounts of pmIκB. A 1-μg portion of a plasmid expressing the E. coli β-galactosidase gene under the RSV promoter was cotransfected as a transfection control. At 24 h after the end of the transfection, the cells were harvested and luciferase (shaded bars) and β-galactosidase (solid bars) activities were measured.
Apart from NFκB, PMA stimulates other transcription factors involved in CMV promoter-dependent transcription. To test the role of NFκB in CMV promoter activity in hepatocyte-derived cells more specifically, we cotransfected the dominant-negative IκB mutant (IκBΔN [33]) together with a CMV promoter-driven reporter construct into the mouse cell line HepSV40, which has a high basal level of activated NFκB. The phosphorylation-deficient deletion mutant of IκB prevents nuclear transport of NFκB. As shown in Fig. 5B, coexpression of the dominant-negative IκB mutant with a CMV promoter-driven luciferase gene resulted in a moderate repression of CMV promoter activity in HepSV40 cells. This effect was already present at low doses of cotransfected pIκBΔN and was enhanced about twofold when a 25-fold amount of the IκBΔN plasmid was used. In contrast to CMV promoter-driven luciferase gene expression, expression of the lacZ gene from the RSV LTR which does not contain NFκB sites was similar among transfection experiments. Small differences observed in β-galactosidase activity are most probably due to differences in transfection efficiencies. Although it is not clear if the effect of NFκB on the CMV promoter is direct or indirect, we conclude that this transcription factor contributes to the basal activity of the CMV promoter in the HepSV40 mouse liver cell line.
Adenovirus infection results in elevated levels of NFκB in mouse hepatocytes.
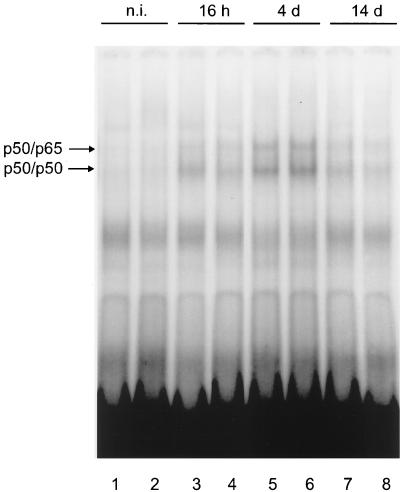
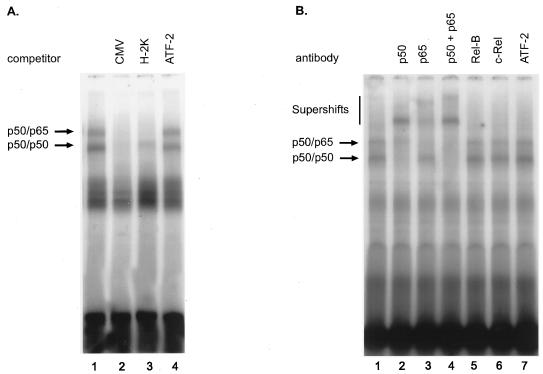
Nuclear extracts of whole rat liver were reported to contain no activated NFκB (11, 55). However, other authors found relatively high levels of NFκB in liver nuclear extracts from untreated rats, but the NFκB activity was located in the NPCs (16, 17, 60). Since NFκB is absent from quiscent hepatocytes, which are the sites of transgene expression in the liver after adenovirus gene transfer, it was interesting to find if infection with recombinant adenovirus could activate this transcription factor in mouse hepatocytes, which, in consequence, would stimulate CMV promoter-dependent transcription. To check for a possible activation of NFκB in hepatocytes following infection with recombinant adenovirus, mice were infected with 2 × 109 PFU of Ad5-CMVLDLR, and at several time points after infection, hepatocyte nuclear extracts were analyzed for the presence of activated NFκB. To this end, livers of animals were perfused with collagenase and hepatocytes were separated from NPCs by several centrifugation steps. To prevent possible contamination by cytoplasmic NFκB, the nuclei were centrifuged through a sucrose cushion before the preparation of nuclear extracts. The hepatocyte nuclear extracts obtained before and at several time points after adenovirus infection were then subjected to EMSA with an H-2K probe which contains the NFκB site of the major histocompatibility complex class I locus. The result of the experiment is shown in Fig. 6. Whereas nuclei of hepatocytes from untreated animals contained only very little NFκB (Fig. 6, lanes 1 and 2), the NFκB level was elevated 16 h after infection (lanes 3 and 4), peaked on day 4 after infection (lanes 5 and 6), and strongly declined on day 14 (lanes 7 and 8). The shift obtained with hepatocyte nuclear extracts from day 4 after infection was also seen with an oligonucleotide probe containing one of the NFκB sites present in the CMV promoter, and specific bands could be competed for with an excess of both the CMV and the H-2K oligonucleotide but not by a nonspecific oligonucleotide (Fig. 7A). Supershift analysis with antisera against NFκB components revealed that the NFκB complexes present in mouse hepatocytes on day 4 after infection consist of p50 and p65 (Fig. 7B). Thus, adenovirus infection results in activation of NFκB in mouse hepatocytes, the nuclear presence of which shows a similar temporal course to the activity of the human CMV promoter.
FIG. 6.
Time course of the presence of NFκB in hepatocytes of adenovirus-infected mice. Mice were infected with 2 × 109 PFU of Ad5-CMVLDLR. At the indicated time points after infection, the livers were perfused, hepatocytes were separated from NPCs, and hepatocyte nuclear extracts were prepared. Nuclear extracts (4 μg) were analyzed for the presence of NFκB by EMSA with the H-2K probe. The positions of NFκB complexes are indicated by arrows. n.i., not infected.
FIG. 7.
Specificity of the NFκB complexes present in mouse hepatocytes after adenovirus infection. (A) Hepatocyte nuclear extract (4 μg) obtained from mouse liver on day 4 after infection was subjected to EMSA with a probe specific for the NFκB site present three times in the CMV promoter. Complex formation was competed for by a 200-fold molar excess of the unlabelled CMV promoter NFκB oligonucleotide (CMV, lane 2) as well as of the unlabelled H-2K oligonucleotide (H-2K, lane 3). No competition was seen with an excess of a nonspecific oligonucleotide (ATF-2, lane 4). (B) Supershifts with antisera specific for NFκB components. Hepatocyte nuclear extract (4 μg) obtained from mouse liver on day 4 after adenovirus infection was hybridyzed to the H-2K probe and incubated with antisera raised against NFκB components after complex formation. The complexes were analyzed by EMSA. The antisera used are indicated at the top of the panel.
Adenovirus infection results in activation of the CMV promoter in the livers of transgenic mice.
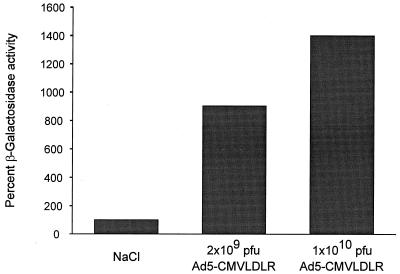
Mice from the CMV-1 line contain the E. coli lacZ gene driven by the CMV promoter and have been described previously (30). To test if adenovirus infection can stimulate CMV promoter-dependent expression in mouse liver, 8-week-old female animals were infected with low and high doses of recombinant adenovirus or treated with sodium chloride, and the β-galactosidase activity in the liver cell extracts was determined luminometrically 4 days after infection. To prevent contamination of liver extract with protein from blood cells, livers were perfused with PBS before being removed. Weak β-galactosidase activity was detected in the livers of untreated animals, which correlated with positive X-Gal staining of fewer than 5% of hepatocytes (data not shown). Tail vein infection of mice with 2 × 109 PFU of Ad5-CMVLDLR resulted in an about 9-fold activation of β-galactosidase activity, and injection of 1 × 1010 PFU of the same virus stimulated β-galactosidase activity 14-fold (Fig. 8). In the latter case, 40 to 50% of hepatocytes stained positive for β-galactosidase whereas no increase in the proportion of stained cells was found in the sodium chloride-treated control group (data not shown). Thus, infection with recombinant adenovirus results in a strong stimulation of the CMV promoter in mouse liver.
FIG. 8.
Stimulation of CMV promoter activity in transgenic mice. Female HCMV-1 mice (8 weeks old) were infected via the tail vein with 2 × 109 or 1 × 1010 PFU of Ad5-CMVLDLR. NaCl-injected animals were used as negative controls. At 4 days after infection, the livers were perfused with PBS and homogenized, and the β-galactosidase activity was determined luminometrically. Basal β-galactosidase activity present in NaCl treated animals was set 100%. Values represent the means of two independent experiments.
DISCUSSION
The inability of the CMV promoter to drive long-term expression in liver has been reported by several authors. However, this phenomenon had not been investigated in detail so far. In this paper, we show (i) that the CMV promoter is specifically silenced in mouse liver although virus DNA is still present at a level comparable to that observed shortly after adenovirus transduction, (ii) that the CMV promoter can be reactivated after being silenced in liver by treatment with LPS or partial hepatectomy, (iii) that the in vitro activity of the CMV promoter in hepatocyte-derived cell lines is modulated by NFκB, (iv) that the CMV promoter activity is paralleled by the activation of NFκB in mouse hepatocytes in vivo after adenovirus transduction, and (v) that transgene expression can be strongly stimulated by adenovirus infection in the livers of mice transgenic for the lacZ gene driven by the CMV promoter.
Two reports have shown that nonspecific in vitro methylation of the CMV promoter results in complete loss of its activity (40, 47). Therefore, we checked the virus DNA present in mouse liver 25 days after infection with Ad5-CMVLDLR for potential methylation and found no evidence of methylation in the region of transgene insertion. Since the absence of methylation was shown for only 6 of about 40 CpG sites present in the CMV promoter region, we cannot preclude methylation of other sites that might be critical for CMV promoter activity. However, specific methylation of single CpGs is rather unlikely to occur in mouse liver, and the reactivatibility of the CMV promoter-dependent expression 28 days after infection rather contradicts a role of DNA methylation in the process of silencing the CMV promoter. Therefore, we suggest that methylation is not involved in silencing of the CMV promoter in mouse liver after adenovirus gene transfer.
At least two further possibilities could explain the silencing and reactivation of the CMV promoter. First, a labile repressor which binds to sequences within the CMV promoter could be present, thereby preventing or impairing transcription from the promoter. Kothari et al. (31) reported a factor called MBF1 whose appearance correlated with a moderate repression of CMV promoter activity in undifferentiated teratocarcinoma T2 and monocytic cells. After differentiation, MBF1 disappeared and CMV promoter activity was enhanced (31, 52). The same group suggested a role for YY1 in downregulation of CMV promoter activity (36). Unfortunately, it is not clear from their data if binding of YY1 to the 21-bp repeat plays a role in CMV promoter repression or if repression by YY1 is due mainly to the dyad symmetry element in the far-upstream modulator region of the CMV promoter which is not present in most CMV promoter expression constructs. However, hepatocytes are highly differentiated cells, which are unlikely to express the same factor(s) responsible for CMV promoter repression in undifferentiated T2 or monocytic cells. Moreover, we did not detect any specific binding of a protein to the 21-bp repeat in mouse liver and hepatocytes before or after adenovirus infection (data not shown). Second, adenovirus infection could elevate the level of transcription factors which are normally not present in differentiated hepatocytes and thereby could enable transcription from the CMV promoter. Until now, changes in the level of transcription factors in the liver following administration of recombinant adenovirus lacking E1 have not been reported. Since both LPS and partial hepatectomy result in the activation of a number of transcriptionally active molecules including NFκB, we suggest that CMV promoter activation by both stimuli is the consequence of activation of such molecules.
As a first step toward the investigation of factors involved in the temporal activity of the CMV promoter in mouse liver in vivo, we focused on NFκB for several reasons. First, activation of NFκB is an early event in CMV infection, and reactivation of this promoter in persisting CMV infection depends on tumor necrosis factor alpha (TNFα)-mediated induction of NFκB (32, 46, 61). Therefore, NFκB was likely to play a role in CMV promoter-dependent transcription in the liver, which is not a target organ for CMV infection. Second, activated NFκB is not present in hepatocytes of rats and mice in vivo, and several lines of mice transgenic for CMV promoter-driven reporter genes exhibited no or only little expression of the transgene in the liver. In contrast, cultured rodent hepatocytes, which usually allow for CMV promoter-dependent expression, contain activated NFκB (16, 17, 23). Third, the presence of four NFκB binding sites in the CMV promoter is the most striking characteristic of this promoter, and efficient expression from the CMV promoter has been shown to depend on the presence of these NFκB sites in certain cell types such as lymphocytes and VSMCs in vitro. However, in other cell types, for instance HeLa cells, CMV promoter activity was shown to be less dependent on the NFκB sites (6, 43, 48).
In our experiments, the activity of the CMV promoter was shown to depend on NFκB in hepatocyte-derived cell lines. PMA strongly stimulated the activity of this promoter in HepG2 cells, which are negative for activated NFκB, and prevention of nuclear transport of NFκB by coexpression of a dominant-negative IκBα mutant inhibited CMV promoter activity in HepSV40 cells, whose nuclei harbor p50/p65 complexes. However, the effect of the dominant-negative mutant was only moderate (fourfold inhibition), which is in agreement with our hypothesis that NFκB might be only one of several factors involved in CMV promoter activation in vitro. For example, both AP1 and CREB/ATF binding activities are present in HepSV40 cells and could partially compensate for the depletion of NFκB.
The dependence of the in vivo activity of the CMV promoter in mouse liver on NFκB was supported by three lines of evidence. First, partial hepatectomy and LPS both stimulated the previously silenced CMV promoter-driven transcription in mouse liver. Both partial hepatectomy and LPS have been shown to activate NFκB in hepatocytes (11, 12, 16, 17, 55, 60). Second, infection with recombinant adenovirus led to activation of NFκB in mouse hepatocytes, and the observed time course of NFκB activation in mouse hepatocytes paralleled the expression from the CMV promoter in mouse liver. Third, infection of mice transgenic for a CMV promoter-driven lacZ gene with recombinant adenovirus resulted in strong activation of transgene expression in the liver. Although no separation of hepatocytes and NPCs was performed in this experiment, activation of β-galactosidase expression clearly occurred in hepatocytes, as was observed after X-Gal staining (data not shown). Moreover, partial hepatectomy in these mice also stimulated the CMV promoter (data not shown). The fact that the same dose of recombinant adenovirus both strongly activates CMV promoter-dependent expression in mouse liver and induces NFκB activity in hepatocytes strongly suggests that CMV promoter activity in hepatocytes after infection with recombinant adenovirus is dependent on NFκB.
We are aware that other transcription factors, like AP1 and CREB/ATF, which bind to the CMV promoter, might also play a role in the reactivation as well in the short-term activity of the CMV promoter in hepatocytes. After partial hepatectomy, several transcription factors are activated in a characteristic temporal manner (for a recent review, see reference 54). For instance, the AP1 level in liver is strongly elevated after partial hepatectomy (12, 60). Therefore, AP1 might contribute to the reactivation of the CMV promoter. However, we did not detect an activity binding to the CMV promoter AP1 site after adenovirus infection in hepatocyte nuclear extracts (data not shown), which would imply that induction of this transcription factor is not involved in the short-term activity of the CMV promoter in liver. Thus, certain transcription factors might contribute to the temporal activity and reactivation of the CMV promoter to different extents. We think that NFκB is critical for full activity of the CMV promoter in mouse liver. This idea is supported by our in vitro results, which show that inhibition of NFκB translocation represses expression from the CMV promoter without completely silencing it.
The mechanism of NFκB activation in mouse hepatocytes following infection with recombinant adenovirus remains unknown. It is possible that an immune response to adenovirus infection is responsible for the elevation in NFκB level observed in hepatocytes. NFκB is efficiently induced by interleukin-1 (IL-1), IL-6 and TNFα. The main sources of IL-1 and TNFα are activated mononuclear monocytes in the liver, namely, Kupffer cells. However, depletion of macrophages from the liver still allowed for expression from the CMV promoter (58). IL-6, which plays an important part in liver regeneration and activation of acute-phase proteins, acts via activation of NFκB in hepatocytes. Although increases in the IL-6 level after systemic administration of low doses of recombinant adenovirus (1 × 109 to 2 × 109 PFU/mouse) have not been reported so far, it is possible that this cytokine is involved. In the lungs, local administration of E1-deficient virus at minimal doses between 107 and 108 PFU resulted in elevation of IL-6 levels in serum (38). Thus, cytokine response to virus infection could stimulate NFκB and thereby CMV promoter-dependent transcription. This mechanism can also account for the temporal activity of the CMV promoter in the context of other gene transfer systems.
It was recently shown that recombinant E1-deficient adenovirus by itself can stimulate NFκB in the infected cell. For instance, Bruder and Kovesdi (9) showed that adenovirus infection of HeLa cells resulted in rapid activation of the Raf/MAPK pathway. Raf-1 was shown to be involved in the activation of NFκB (7). However, this mechanism cannot account for the time course of NFκB activation observed in our experiments. Transfection experiments by Pahl et al. (44) showed that retention of the adenovirus E3/19K protein in the endoplasmic reticulum and subsequent endoplasmic reticulum overload activated NFκB in vitro. The adenovirus vector used in our study theoretically can produce this E3 protein, although efficient transcription of E3 genes requires E1A gene products. Since transfection of low doses of the E3 gene was sufficient to induce strong NFκB activation in the experiments of Pahl et al. (44), it is possible that despite low expression of E3 genes, this mechanism plays a part in the in vivo induction of NFκB. The idea that an intrinsic property of the adenovirus vector might play a role in CMV promoter activation is supported by the X-Gal staining pattern in the livers of transgenic mice after adenovirus infection, which is similar to patterns observed after systemic infection of mice with Ad5-RSVBG (data not shown).
While our experiments were in progress, Armentano et al. (1) reported an unaltered expression from the CMV promoter in the lungs of nude mice for 21 days. In their study, sustained expression was observed only in immunodeficient animals and was dependent on the integrity of the adenovirus E4 region. Since in their study coinfection with E4-positive vectors could reconstitute CMV promoter-driven transgene expression from E4-defective vectors, a role of E4 products in CMV promoter activity was suggested. However, it is possible that this phenomenon refers to the specific situation in the lungs of immunodeficient animals, since we and others (21) observed silencing of the CMV promoter in the livers of immunocompetent animals when using vectors that contain a complete E4 region. We also cannot exclude that differences in the adenovirus serotype and subtle differences in the vector backbone, apart from E4, account for the observed differences.
In summary, we have shown that the activity of a previously silenced CMV promoter can be restored in the liver, and we suggest a role for NFκB in the temporal activity of this promoter in vivo. It will be interesting to analyze additional factors activated in the liver after infection with low doses of adenovirus vectors with E1 deleted. Moreover, alternative stimuli of CMV promoter-dependent transcription in transgenic mice will be tested and could provide further insight into the mechanisms involved in the frequently observed silencing of this strong promoter in vivo.
ACKNOWLEDGMENTS
We thank Heidemarie Riedel and Alexandra-V. Bohne for expert technical assistance, and we are grateful to Grit Sandig and Vigo Heissmeyer for helpful suggestions. We thank Susanna Prösch, Manal Morsy, and Claus Scheidereit for critical readings of the manuscript. We are particularly grateful to Pamela J. Mitchell for providing the CMV-1 transgenic mice and to Daniel Krappmann and Claus Scheidereit for their generous gift of the dominant-negative IκB mutant.
REFERENCES
- 1.Armentano D, Zabner J, Sacks C, Sookdeo C C, Smith M P, St. George J A, Wadsworth S C, Smith A E, Gregory R. Effect of the E4 region on the persistence of transgene expression from adenovirus vectors. J Virol. 1997;71:2408–2416. doi: 10.1128/jvi.71.3.2408-2416.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeuerle P A, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 3.Baeuerle P A, Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 4.Barr D, Tubb J, Ferguson D, Scaria A, Lieber A, Wilson C, Perkins J, Kay M A. Strain related variations in adenovirally mediated transgene expression from mouse hepatocytes in vivo: comparisons between immunocompetent and immunodeficient inbred strains. Gene Ther. 1995;2:151–155. [PubMed] [Google Scholar]
- 5.Baskar J F, Smith P P, Nilaver G, Jupp R A, Hoffmann S, Peffer N J, Tenney D J, Colberg-Poley A M, Ghazal P, Nelson J A. The enhancer domain of the human cytomegalovirus immediate-early promoter determines cell type-specific expression in transgenic mice. J Virol. 1996;70:3207–3214. doi: 10.1128/jvi.70.5.3207-3214.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellas R E, Lee J S, Sonenshein G E. Expression of a constitutive NF-κB-like activity is essential for proliferation of cultured bovine vascular smooth muscle cells. J Clin Invest. 1995;96:2521–2527. doi: 10.1172/JCI118313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertrand F, Philippe C, Antoine P J, Baud L, Groyer A, Capeau J, Cherqui G. Insulin activates nuclear factor NFκB in mammalian cells through a Raf-1-mediated pathway. J Biol Chem. 1995;270:24435–24441. doi: 10.1074/jbc.270.41.24435. [DOI] [PubMed] [Google Scholar]
- 8.Boshart M, Weber F, Jahn G, Dorsch-Häsler K, Fleckenstein B, Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985;41:521–530. doi: 10.1016/s0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- 9.Bruder J T, Kovesdi I. Adenovirus infection stimulates the Raf/MAPK signaling pathway and induces interleukin-8 expression. J Virol. 1997;71:398–404. doi: 10.1128/jvi.71.1.398-404.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clesham G J, Brown H, Efstathiou S, Weissberg P L. Enhancer stimulation unmasks latent gene transfer after adenovirus-mediated gene delivery to human vascular smooth muscle cells. Circ Res. 1996;79:1188–1195. doi: 10.1161/01.res.79.6.1188. [DOI] [PubMed] [Google Scholar]
- 11.Cressman D E, Greenbaum L E, Haber B A, Taub R. Rapid activation of post-hepatectomy factor/nuclear factor κB in hepatocytes, a primary response in the regenerating liver. J Biol Chem. 1994;269:30429–30435. [PubMed] [Google Scholar]
- 12.Cressman D E, Greenbaum L E, DeAngelis R A, Ciliberto G, Furth E E, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379–1383. doi: 10.1126/science.274.5291.1379. [DOI] [PubMed] [Google Scholar]
- 13.Dai Y, Roman M, Naviaux R K, Verma I M. Gene therapy via primary myoblasts: long-term expression of factor IX protein following transplantation in vivo. Proc Natl Acad Sci USA. 1992;89:10892–10895. doi: 10.1073/pnas.89.22.10892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai Y, Schwarz E M, Gu D, Zhang W-W, Sarvetnick N, Verma I M. Cellular and humoral immune response to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allow for long-term expression. Proc Natl Acad Sci USA. 1995;92:1401–1405. doi: 10.1073/pnas.92.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14a.Essani N A, McGuire G M, Manning A M, Jaeschke H. Endotoxin-induced activation of the nuclear transcription factor κB and expression of E-selectin messanger RNA in hepatocytes, Kupffer cells, and endothelial cells in vivo. J Immunol. 1996;156:2956–2963. [PubMed] [Google Scholar]
- 15.Fietze E, Prösch S, Reike P, Stein J, Döcke W-D, Staffa G, Löning S, Devaux S, Emmerich F, von Baehr R, Krüger D H, Volk H-D. Cytomegalovirus infection in transplantation patients. Transplantation. 1994;58:675–680. [PubMed] [Google Scholar]
- 16.FitzGerald M J, Webber E M, Donovan J R, Fausto N. Rapid DNA binding by nuclear factor κB in hepatocytes at the start of liver regeneration. Cell Growth Differ. 1995;6:417–427. [PubMed] [Google Scholar]
- 17.Freedman A R, Sharma R J, Nabel G J, Emerson S G, Griffin G E. Cellular distribution of nuclear factor κB binding activity in rat liver. Biochem J. 1992;287:645–649. doi: 10.1042/bj2870645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furth P A, Hennighausen L, Baker C, Beatty B, Woychick R. The variability in activity of the universally expressed human cytomegalovirus immediate early gene 1 enhancer/promoter in transgenic mice. Nucleic Acids Res. 1991;19:6205–6208. doi: 10.1093/nar/19.22.6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghazal P, Lubon H, Fleckenstein B, Hennighausen L. Binding of transcription factors and creation of a large nucleoprotein complex on the human cytomegalovirus enhancer. Proc Natl Acad Sci USA. 1987;84:3658–3662. doi: 10.1073/pnas.84.11.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gruber P J, Torres-Rosado A, Wolak M L, Leff T. Apo Ciii gene transcription is regulated by a cytokine inducible NF-κB element. Nucleic Acids Res. 1994;22:2417–2422. doi: 10.1093/nar/22.12.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo Z S, Wang L-H, Eisensmith R C, Woo S C L. Evaluation of promoter strength for hepatic gene expression in vivo following adenovirus-mediated gene transfer. Gene Ther. 1996;3:802–810. [PubMed] [Google Scholar]
- 22.Hansen S K, Baeuerle P A, Blasi F. Purification, reconstitution, and IkB association of the c-Rel-p65 (RelA) complex, a strong activator of transcription. Mol Cell Biol. 1994;14:2593–2603. doi: 10.1128/mcb.14.4.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hattori M, Tugores A, Westwick J K, Veloz L, Leffert H L, Karin M, Brenner D A. Activation of activating protein 1 during hepatic acute phase response. Am J Physiol. 1993;264:G95–G103. doi: 10.1152/ajpgi.1993.264.1.G95. [DOI] [PubMed] [Google Scholar]
- 24.Hickman A E, Malone R W, Lehmann-Bruinsma K, Sih T R, Knoell D, Szoka F C, Walzem R, Carlson D M, Powell J S. Gene expression following direct injection of DNA into liver. Hum Gene Ther. 1994;5:1477–1483. doi: 10.1089/hum.1994.5.12-1477. [DOI] [PubMed] [Google Scholar]
- 25.Hunninghake G W, Monick M M, Liu B, Stinski M F. The promoter-regulatory region of the major immediate-early gene of human cytomegalovirus responds to T-lymphocyte stimulation and contains functional cyclic AMP response elements. J Virol. 1989;63:3026–3033. doi: 10.1128/jvi.63.7.3026-3033.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jennings, G. S., J. Bartkova, S. Herwig, J. Lukas, J. Bartek, and M. Strauss. Submitted for publication.
- 27.Kay M A, Li Q, Liu T-J, Leland F, Toman C, Finegold M, Woo S. Hepatic gene therapy: persistent expression of human α1-antitrypsin in mice after direct gene delivery in vivo. Hum Gene Ther. 1992;3:641–647. doi: 10.1089/hum.1992.3.6-641. [DOI] [PubMed] [Google Scholar]
- 28.Kay M A, Rothenberg S, Landen C N, Bellinger D A, Leland F, Toman C, Finegold M, Thompson A R, Read M S, Brinkhous K M, Woo S L C. In vivo correction of hemophelia B: sustained partial correction in factor IX-deficient dogs. Science. 1993;262:117–119. doi: 10.1126/science.8211118. [DOI] [PubMed] [Google Scholar]
- 29.Kiwaki K, Kanegae Y, Saito I, Komaki S, Nakamura K, Miyazaki J-I, Endo F, Matsuda I. Correction of ornithine transcarbamylase deficiency in adult spffash mice and in OTC-deficient human hepatocytes with recombinant adenovirus bearing the CAG promoter. Hum Gene Ther. 1996;7:821–830. doi: 10.1089/hum.1996.7.7-821. [DOI] [PubMed] [Google Scholar]
- 30.Koedodd M, Fichtel A, Meier P, Mitchell P J. Human cytomegalovirus (HCMV) immediate-early enhancer/promoter specificity during embryogenesis defines target tissues of congenital HCMV infection. J Virol. 1995;69:2194–2207. doi: 10.1128/jvi.69.4.2194-2207.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kothari S, Baullie J, Sissons J G P, Sinclair J H. The 21 bp repeat element of the human cytomegalovirus major immediate early enhancer is a negative regulator of gene expression in undifferentiated cells. Nucleic Acids Res. 1991;29:1767–1771. doi: 10.1093/nar/19.8.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kowalik T F, Wing B, Haskill J S, Azizkhan J C, Baldwin A S, Jr, Huang E S. Multiple mechanisms are implicated in the regulation of NF-kappa B activity during human cytomegalovirus infection. Proc Natl Acad Sci USA. 1993;90:1107–1111. doi: 10.1073/pnas.90.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krappmann D, Wulczyn F G, Scheidereit C. Different mechanisms control signal-induced degradation and basal turnover of the NF-κB inhibitor IkBα in vivo. EMBO J. 1996;15:6716–6726. [PMC free article] [PubMed] [Google Scholar]
- 34.Lang D, Fickenscher H, Stamminger T. Analysis of proteins binding to the proximal promoter region of the human cytomegalovirus IE-1/2 enhancer/promoter reveals both consensus and aberrant recognition sequences for transcription factors Sp1 and CREB. Nucleic Acids Res. 1992;20:3287–3295. doi: 10.1093/nar/20.13.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lieber A, Sandig V, Strauss M. A mutant T7 phage promoter is specifically transcribed by T7-RNA polymerase in mammalian cells. Eur J Biochem. 1993;217:387–394. doi: 10.1111/j.1432-1033.1993.tb18257.x. [DOI] [PubMed] [Google Scholar]
- 36.Liu R, Baillie J, Sissons J G P, Sinclair J H. The transcription factor YY1 binds to negative regulatory elements in the human cytomegalovirus major immediate early enhancer/promoter and mediates repression in non-permissive cells. Nucleic Acids Res. 1994;22:2453–2459. doi: 10.1093/nar/22.13.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Löser P, Sandig V, Kirillova I, Strauss M. Evaluation of HBV promoters for use in hepatic gene therapy. Biol Chem Hoppe-Seyler. 1996;377:187–193. doi: 10.1515/bchm3.1996.377.3.187. [DOI] [PubMed] [Google Scholar]
- 38.McElvaney N G, Crystal R G. IL-6 release and airway administration of human CFTR cDNA adenovirus vector. Nat Med. 1995;1:182–184. doi: 10.1038/nm0395-182b. [DOI] [PubMed] [Google Scholar]
- 39.Miyanohara A, Johnson P A, Elam R L, Dai Y, Witztum J L, Verma I M, Friedmann T. Direct gene transfer to the liver with herpes simplex virus type 1 vectors: transient production of physiologically relevant levels of circulating factor IX. New Biol. 1992;4:238–246. [PubMed] [Google Scholar]
- 40.Muiznieks I, Doerfler W. The impact of 5′-CG-3′ methylation on the activity of different eukaryotic promoters: a comparative study. FEBS Lett. 1994;344:251–254. doi: 10.1016/0014-5793(94)00394-7. [DOI] [PubMed] [Google Scholar]
- 41.Müller U, Kleinberger T, Shenk T. Adenovirus E4orf4 protein reduces phosphorylation of c-Fos and E1A proteins while simultaneously reducing the level of AP-1. J Virol. 1992;66:5867–5878. doi: 10.1128/jvi.66.10.5867-5878.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naumann M, Scheidereit C. Activation of NF-kappa B in vivo is regulated by multiple phosphorylations. EMBO J. 1994;13:4597–4607. doi: 10.1002/j.1460-2075.1994.tb06781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niller H H, Hennighausen L. Formation of several specific nucleoprotein complexes on the human cytomegalovirus immediate early enhancer. Nucleic Acids Res. 1991;19:3715–3721. doi: 10.1093/nar/19.13.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pahl H L, Sester M, Burgert H-G, Baeuerle P A. Activation of transcription factor NF-κB by the adenovirus E3/19K protein requires its ER retention. J Cell Biol. 1996;132:511–522. doi: 10.1083/jcb.132.4.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paul D. Immortalized differenciated hepatocyte lines derived from transgenic mice harbouring SV40-T-antigen. Exp Cell Res. 1988;175:354–362. doi: 10.1016/0014-4827(88)90199-1. [DOI] [PubMed] [Google Scholar]
- 46.Prösch S, Staak K, Stein J, Liebenthal C, Stamminger T, Volk H-D, Krüger D H. Stimulation of the human cytomegalovirus IE enhancer/promoter in HL-60 cells by TNFα is mediated via induction of NF-κB. Virology. 1995;208:197–206. doi: 10.1006/viro.1995.1143. [DOI] [PubMed] [Google Scholar]
- 47.Prösch S, Stein J, Staak K, Liebenthal C, Volk H-D, Krüger D H. Inactivation of the very strong HCMV immediate early promoter by DNA CpG methylation in vitro. Biol Chem Hoppe-Seyler. 1996;377:195–201. doi: 10.1515/bchm3.1996.377.3.195. [DOI] [PubMed] [Google Scholar]
- 48.Sambucetti L C, Cherrington J M, Wilkinson G W G, Mocarski E S. NF-κB activation of the cytomegalovirus enhancer is mediated by a viral transactivator and by T cell stimulation. EMBO J. 1989;8:4251–4258. doi: 10.1002/j.1460-2075.1989.tb08610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sandig V, Löser P, Lieber A, Kay M A, Strauss M. HBV-derived promoters direct liver-specific expression of an adenovirally transduced LDL receptor gene. Gene Ther. 1996;3:1002–1009. [PubMed] [Google Scholar]
- 50.Scharfmann R, Axelrod H, Verma I M. Long-term in vivo expression of retrovirus-mediated gene transfer in mouse fibroblast implants. Proc Natl Acad Sci USA. 1991;88:4626–4630. doi: 10.1073/pnas.88.11.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmidt E V, Christoph G, Zeller R, Leder P. The cytomegalovirus enhancer: a pan-active control element in transgenic mice. Mol Cell Biol. 1990;10:4406–4411. doi: 10.1128/mcb.10.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sinclair J H, Baillie J, Byrant L A, Taylor-Wiedeman J A, Sissons J G P. Repression of human cytomegalovirus major immediate early gene expression in a monocytic cell line. J Gen Virol. 1992;73:433–435. doi: 10.1099/0022-1317-73-2-433. [DOI] [PubMed] [Google Scholar]
- 53.Stamminger, T., H. Fickenscher, and B. Fleckenstein. Cell type-specific induction of the major immediate early enhancer of human cytomegalovirus by cyclic AMP. J. Gen. Virol. 71:105–113. [DOI] [PubMed]
- 54.Taub R. Transcriptional control of liver regeneration. FASEB J. 1996;10:413–427. [PubMed] [Google Scholar]
- 55.Tewari M, Dobrzanski P, Mohn K L, Cressman D E, Hsu J-C, Bravo R, Taub R. Rapid induction in regenerating liver of RL/IF-1 (an IκB that inhibits NF-κB, RelB-p50, and c-Rel-p50) and PHF, a novel κB site-binding factor. Mol Cell Biol. 1992;12:2898–2908. doi: 10.1128/mcb.12.6.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verma I M, Stevenson J K, Schwarz E M, van Antwerp D, Miyamoto S. Rel/NF-κB/IκB family: intimate tales of association and dissociation. Genes Dev. 1996;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 57.Wilkinson G W G, Akrigg A. Constitutive and enhanced expression from the CMV major IE promoter in a defective adenovirus vector. Nucleic Acids Res. 1992;20:2233–2239. doi: 10.1093/nar/20.9.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wolff G, Worgall S, van Rooijen N, Song W-R, Harvey B-G, Crystal R G. Enhancement of in vivo adenovirus-mediated gene transfer and expression by prior depletion of tissue macrophages in the target organ. J Virol. 1997;71:624–629. doi: 10.1128/jvi.71.1.624-629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wulczyn F G, Krappmann D, Scheidereit C. The NF-κB/Rel and IκB gene families: mediators of immune response and inflammation. J Mol Med. 1996;74:749–769. doi: 10.1007/s001090050078. [DOI] [PubMed] [Google Scholar]
- 60.Yamada Y, Kirillova I, Peschon J P, Fausto N. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc Natl Acad Sci USA. 1997;94:1441–1446. doi: 10.1073/pnas.94.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yurochko A D, Kowalik T F, Huong S M, Huang E S. Human cytomegalovirus upregulates NF-kappa B activity by transactivating the NF-kappa B p105/p50 and p65 promoters. J Virol. 1995;69:5391–5400. doi: 10.1128/jvi.69.9.5391-5400.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zabner J, Wadsworth S C, Smith A E, Welsh M J. Adenovirus-mediated generation of cAMP-stimulated Cl− transport in cystic fibrosis airway epithelia in vitro: effect of promoter and administration method. Gene Ther. 1996;3:458–465. [PubMed] [Google Scholar]
- 63.Zhang X-Y, Inamdar N M, Supakar P C, Wu K, Ehrlich K C, Ehrlich M. Three MDBP sites in the immediate-early enhancer-promoter region of human cytomegalovirus. Virology. 1991;182:865–869. doi: 10.1016/0042-6822(91)90631-k. [DOI] [PubMed] [Google Scholar]
- 64.Zhang X-Y, Ni J-I, Saifudeen Z, Asiedu C K, Supakar P C, Ehrlich M. Increasing binding of a transcription factor immediately downstream of the cap site of a cytomegalovirus gene represses expression. Nucleic Acids Res. 1995;23:3026–3033. doi: 10.1093/nar/23.15.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]