Abstract
Objectives
This observational study compares the effectiveness of baricitinib (BARI), a targeted synthetic disease-modifying antirheumatic drug (tsDMARD), with alternative biological DMARDs (bDMARDs) in patients with rheumatoid arthritis (RA), from a prospective, longitudinal cohort.
Methods
We compared patients initiating a treatment course (TC) of BARI, tumour necrosis factor inhibitors (TNFi) or bDMARDs with other modes of action (OMA), during a period when all these DMARDs were available in Switzerland. The primary outcome was drug maintenance; secondary outcomes included discontinuation rates related specifically to ineffectiveness and adverse events. We further analysed rates of low disease activity (LDA) and remission (REM) at 12 months and drug maintenance in bDMARD-naïve and tsDMARD-naïve population.
Results
A total of 1053 TCs were included: 273 on BARI, 473 on TNFi and 307 on OMA. BARI was prescribed to older patients with longer disease duration and more previous treatment failures than TNFi. Compared with BARI, the adjusted drug maintenance was significantly shorter for TNFi (HR for discontinuation: 1.76; 95% CI, 1.32 to 2.35) but not compared with OMA (HR 1.27; 95% CI, 0.93 to 1.72). These results were similar in the b/tsDMARD-naïve population. The higher discontinuation of TNFi was mostly due to increased discontinuation for ineffectiveness (HR 1.49; 95% CI, 1.03 to 2.15), with no significant differences in drug discontinuation for adverse events (HR 1.46; 95% CI, 0.83 to 2.57). The LDA and REM rates at 12 months did not differ significantly between the three groups.
Conclusions
BARI demonstrated a significantly higher drug maintenance compared with TNFi, mainly due to lower drug discontinuations for ineffectiveness. We found no difference in drug maintenance between BARI and OMA. Clinical outcomes did not differ between the three groups. Our results suggest that BARI is an appropriate therapeutic alternative to bDMARDs in the management of RA.
Keywords: RHEUMATOLOGY, EPIDEMIOLOGY, STATISTICS & RESEARCH METHODS
Strengths and limitations of this study.
Use data derived from office-based rheumatologists.
Study period where all alternative medications were available in the market.
Several sensitivity analyses, congruent with the main results.
Not a randomised setting.
Subanalysis in b/tsDMARD-naïve population has limited sample size.
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease leading to widespread inflammation and irreversible joint damage if insufficiently treated. New treatment paradigms have emerged in the last decades, such as ‘early aggressive therapy’ in the so-called ‘window of opportunity’, during which patients are more likely to reach long-term remission (REM).1 A wide panel of biological disease-modifying antirheumatic drugs (bDMARDs) and targeted synthetic DMARDs (tsDMARDs) have been approved in the management of RA, after failure of methotrexate. In clinical trial settings, bDMARDs and tsDMARDs have demonstrated significant reduction of joint inflammation and prevention of joint damage.2–8
Efficacy estimates from placebo-controlled randomised trials often differ from real-world effectiveness estimates, because of patient selection, adherence to therapy and other reasons.9–12 Indeed, drug maintenance of many bDMARDs remains modest in observational analyses, while long-term REMs are rare and secondary loss of efficacy is frequent.13 Furthermore, understanding the clinical effectiveness of bDMARDs or tsDMARDs in specific conditions, such as elderly or multimorbid patients, may become important as we move towards personalised care. Finally, trials provide only limited data on long-term effectiveness and safety because clinical trial follow-up is typically <12 months.
Baricitinib (BARI) has been approved in Switzerland for the treatment of RA in 2017 as well as all around the world. Clinical trials with BARI have established efficacy and demonstrated acceptable adverse event profile, both in combination with methotrexate and in monotherapy.14–20 However, evidence about the effectiveness of BARI compared with tumour necrosis factor inhibitor (TNFi) in real-world settings is scarce. A recently published analysis of registry data from Sweden showed that BARI had higher maintenance as compared with most other bDMARDs.21 Pappas et al, in the USA, also demonstrated that TNFi and non-TNFi drugs had similar outcomes when prescribed in b/tsDMARD-naïve population, an observation replicated in the RA-BE-REAL study.22 23
The aim of our analysis was to compare real-world drug maintenance between BARI and other approved b/tsDMARDs, using data from a European registry.
Methods
Study population
This is a nested cohort study from a prospective, longitudinal cohort of Swiss patients with RA in a real-life setting, the Swiss Clinical Quality Management (SCQM) registry. The SCQM registry was founded in 1997 with the financial support of Swiss regulatory authorities, who recommended a continuous monitoring of all patients receiving new DMARDs. Unlike many other European registries, most patients are enrolled by private office-based rheumatologists (60%), providing a population-based sample of patients with RA in Switzerland. All approved RA treatments are represented in the registry. The data for this analysis were extracted from the SCQM registry on 1st of June 2020.
We used ‘treatment courses’ (TCs) as our denominator of interest, with each new treatment initiation considered as a separate ‘TC’. We included all TCs with the medications of interest initiated between 1st of September 2017 and 1st of June 2020, with at least one follow-up visit, in adult patients with a diagnosis of RA confirmed by a rheumatologist. Thus, a given patient could potentially contribute to several TCs during the study period. To minimise the risk of confounding bias, the time window was selected to include only the period when all the therapies examined were available for prescription and reimbursed (BARI was first reimbursed on the Swiss market in September 2017). We excluded TCs with no follow-up visit at the time of data extraction.
Exposure of interest
The exposure of interest was the type of treatment used, namely, BARI, TNFi and bDMARDs with other modes of action (OMA), excluding other tsDMARDs and rituximab. We decided to exclude rituximab a priori because its long-term action impairs a precise estimation of treatment discontinuation. Tofacitinib was excluded because we had insufficient TCs to perform meaningful comparative effectiveness analyses against a single other specific tsDMARD agent. Included TNFi treatments were as follows: adalimumab, etanercept, golimumab, certolizumab and infliximab. Included OMA treatments were as follows: tocilizumab, abatacept, sarilumab and anakinra.
Outcomes
The primary outcome of this analysis was the time to all-cause discontinuation. This outcome, also referred to as ‘drug maintenance’, captures both the drug’s effectiveness and its tolerance.24 The time to all-cause discontinuation was defined as the number of days between treatment initiation and the reported date of discontinuation, or the date of initiation of a new b/tsDMARD, whatever came first. In survival analyses, death and loss to follow-up are censored. We also report discontinuation rates at 12 months. Temporary discontinuations of <6 months (for instance, because of an elective surgery or a pregnancy) were not considered permanent drug discontinuation. Discontinuation reasons are recorded by the clinician when stopping a DMARD treatment, who chooses between four options (‘adverse event’, ‘ineffectiveness’, ‘remission’ or ‘other’).
Preplanned secondary outcomes were time to discontinuation due to ineffectiveness and time to discontinuation due to adverse events. Other secondary outcomes included response rates, namely, the rates of low disease activity (LDA) and REM at 12 months, defined, respectively, as attaining a Clinical Disease Activity Index (CDAI) score ≤ 10 and CDAI score ≤ 2.8 (not mutually exclusive).25 Finally, we performed an exploratory subgroup analysis, restricting the population to b/tsDMARD-naive patients only and reassessing the main outcome in this setting.
Statistical analysis
Analyses were conducted and reported in accordance with European Alliance of Associations for Rheumatology recommendations for comparative effectiveness research.9 Baseline characteristics were compared using generalised linear mixed models to account for repeated treatments within the same patients. For the primary outcomes, Kaplan-Meier survival analyses were used to assess crude drug maintenance, and groups were compared using log-rank tests. Subsequently, missing covariates were imputed using chained equations (see below for details). We then implemented Cox proportional HR models to obtain adjusted estimates. Based on prior subject matter knowledge,26 we adjusted our models for the following potential confounders: age, gender, body mass index, concomitant conventional synthetic DMARDs (csDMARDs) use (yes/no), concomitant prednisone usage (yes/no), CDAI score at baseline, disease duration, smoking status (current, former and never smoker) and line of therapy (1st, 2nd, 3rd, 4th or more), and seropositivity for RA auto-antibodies (yes/no). Detailed definitions for each variable are available in the supplement (online supplemental material 1). The main analysis (survival analysis) accounted for clustering resulting from patients with multiple TCs, inducing correlation within the patient-level data. The cluster term is used to compute a robust variance for the model, by applying the so-called Huber sandwich estimator.27 All conditions of application of the Cox model were verified. One additional sensitivity analysis was conducted for the primary outcome, using augmented inverse probability of treatment weighting (AIPTW).
bmjopen-2023-072300supp001.pdf (763.4KB, pdf)
In secondary analyses, we used the Fine-Gray approach to assess specific reasons for drug discontinuation (ie, ineffectiveness or adverse event) in a competing-risk setting. The Fine-Gray method takes competing risks into account when estimating the cumulative incidence function, modelling the subdistribution hazard without treating competing events as censoring events.28 Other secondary outcomes included response rates (LDA and REM) at 12 months. To avoid overestimations, we computed the response rates using the ‘confounder-adjusted response rate with attrition correction’ (CARRAC) method.29 The latter estimates the response rates using multiple imputations, with a model including both confounders and treatment stop reason. CARRAC thus provides reliable estimates when reasons for treatment discontinuation differ between compared groups.
For all adjusted analyses, missing baseline covariates were imputed using the closest value in a window of −90 days to +30 days. However, this window was reduced to −30 days to +7 days when imputing baseline CDAI. If still missing after this first step, baseline CDAI values were imputed using the linear mixed-effect regression model with quadratic time. We imputed other baseline covariates with a chained equation technique, which provides unbiased estimates if the variables are missing at random.29 Such imputations were performed using 50 data sets with 25 iterations. Imputation was done using the whole data set, before adequately subsetting the data for each group comparison.
We also imputed data required for secondary outcomes, including disease activity. If the CDAI score at 12 months was not available, the closest value in a window of +/-45 days was used (a 3-month-wide window). If still missing, the 12-month CDAI values were imputed using the nearest neighbouring value, as previously described.30
All analyses were conducted using R (V.4.0.3), in particular with packages ‘tableone’, ‘survival’ and ‘mice’.31 Two-tailed p<0.05 was considered significant. We did not adjust the p values for multiple comparisons, as outcomes were prespecified. The final analysis code is shown in the supplement (online supplemental material 2).
bmjopen-2023-072300supp002.pdf (509.9KB, pdf)
Patient and public involvement
Patient involvement is central to the SCQM cohort. Several patients are part of the executive board and involved in the approval of research projects.
Results
Population description
During the study period, 1053 TCs were initiated in 834 different patients, including 273 TCs with BARI, 473 with TNFi and 307 with OMA (online supplemental figures S1 and S2). TNFi were more often given as a second-line therapy after methotrexate failure. Inversely, BARI was prescribed to significantly older patients, with longer disease durations and more previous treatment failures (table 1).
Table 1.
Baseline characteristics of study population, SCQM-RA registry, 2017–2020
| Variable | BARI (TC=273; 273 pts) |
TNFi (TC=473; 408 pts) |
OMA (TC=307; 289 pts) |
p values | |||
| N % of total in group Otherwise: mean (SD) | |||||||
| Patients | Miss. | Miss. | Miss. | ||||
| Female | 78 % | 0 | 74 % | 1 | 73 % | 1 | 0.097 |
| Age (years) | 59 (14) | 0 | 52 (15) | 1 | 59 (13) | 1 | 0.021 |
| Disease duration (years) | 13 (10) | 4 | 8 (9) | 19 | 11 (9) | 5 | 0.027 |
| CDAI baseline (raw data) | 19 (10) | 175 | 18 (10) | 301 | 20 (13) | 204 | 0.34 |
| CDAI baseline (imputed) | 15 (9) | 0 | 14 (9) | 0 | 16 (11) | 0 | 0.06 |
| Obesity (BMI >30) | 16 % | 104 | 14 % | 134 | 13 % | 115 | 0.85 |
| Smoking | 32 | 69 | 26 | ||||
| Current | 17 % | 18 % | 21 % | Ref. | |||
| Former | 28 % | 26 % | 28 % | 0.95 | |||
| Never | 43 % | 41 % | 48 % | 0.98 | |||
| Seropositive (ACPA or RF) | 75 % | 1 | 70 % | 7 | 77 % | 5 | 0.92 |
| TCs | Miss. | Miss. | Miss. | ||||
| Concomitant csDMARD | 40 % | 0 | 61 % | 0 | 46 % | 0 | <0.01 |
| Line of therapy | 0 | 0 | 0 | ||||
| 1st (bio-naive) | 17 % | 48 % | 22 % | Ref. | |||
| 2nd | 20 % | 23 % | 24 % | <0.01 | |||
| 3rd | 19 % | 11 % | 24 % | <0.01 | |||
| 4th or later | 44 % | 18 % | 31 % | <0.01 | |||
| Previous tsDMARD use (non-BARI) | 33 % | 0 | 1 % | 0 | 5 % | 0 | <0.01 |
| Concomitant glucocorticoid (at any time) | 22 % | 0 | 20 % | 0 | 24 % | 0 | 0.35 |
| Mean dose of concomitant glucocorticoid (mg) | 2.0 (4.6) | 0 | 2.1 (5.4) | 0 | 2.2 (5.1) | 0 | 0.95 |
| Dose of BARI (4 mg) | 86 % | 0 | – | – | – | – | – |
In Switzerland, BARI was prescribed to older patients with longer disease duration and more previous treatment failures. Missing values for covariables are reported as absolute numbers. P values are obtained by generalised linear mixed models to account for repeated treatments within the same patients. In TFNi and OMA groups, some patients have contributed several TCs; thus, the total number of TCs exceeds the total number of patients.
ACPA, anticitrullinated peptide antibody; BARI, baricitinib; BMI, body mass index; CDAI, Clinical Disease Activity Index; Miss., number of missing values; OMA, bDMARDs with other modes of action; Ref., reference ; RF, rheumatoid factor; TCs, treatment courses; TNFi, tumour necrosis factor inhibitors; tsDMARD, targeted synthetic DMARDs.
Time to all-cause discontinuation
Crude proportions of treatment discontinuation by reasons are reported in online supplemental table S1, and crude times of observation are represented in online supplemental figure S2.
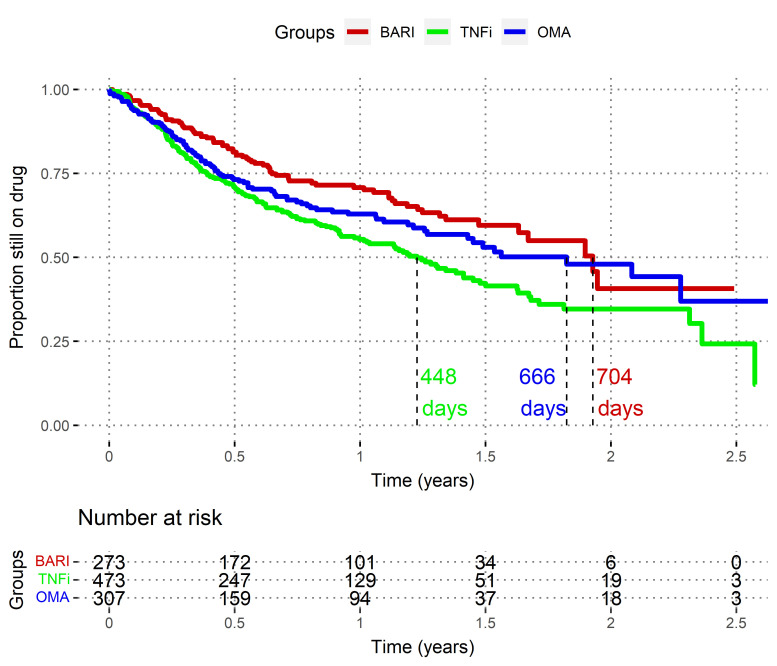
At 12 months, based on the Kaplan-Meier curves (figure 1), the estimated proportions of patients still on therapy were as follows: 71% (95% CI, 65 to 77) in the BARI group, 55% (95% CI, 50 to 61%) in the TNFi group and 63% (95% CI, 57 to 70) in the OMA group.
Figure 1.
Non-adjusted time to drug discontinuation analyses (Kaplan-Meier), Swiss Clinical Quality Management registry, 2017–2020. These ‘survival curves’ represent the drug maintenance after initiation, as the estimated proportion of patients still on therapy, by treatment group. Death and loss to follow-up were censored. BARI, baricitinib; OMA, other modes of action bDMARDs; TNFi, tumour necrosis factor inhibitors. Log-rank BARI versus TNFi: p<0.001. Log-rank BARI versus OMA: p=0.11.
Overall, unadjusted time to all-cause discontinuation was significantly longer in the BARI group compared with that in the TNFi group (estimated median prescription survival time of 704 days vs 448 days; log-rank p<0.01; figure 1). These results persisted after adjustment for confounding factors using the multivariable Cox model (HR 1.76; 95% CI, 1.32 to 2.35; p<0.001; online supplemental table 2, figures S3 and S4).
BARI versus OMA time to all-cause discontinuation was not significantly different, even after adjustment (HR 1.27; 95% CI, 0.93 to 1.72; p=0.13; online supplemental table 2, figures S3 and S4).
Sensitivity analyses using AIPTW led to similar conclusions (online supplemental figure S5). Covariates significantly associated with decreased drug maintenance were high baseline CDAI scores and concomitant glucocorticoid usage (online supplemental table S2, figure S3).
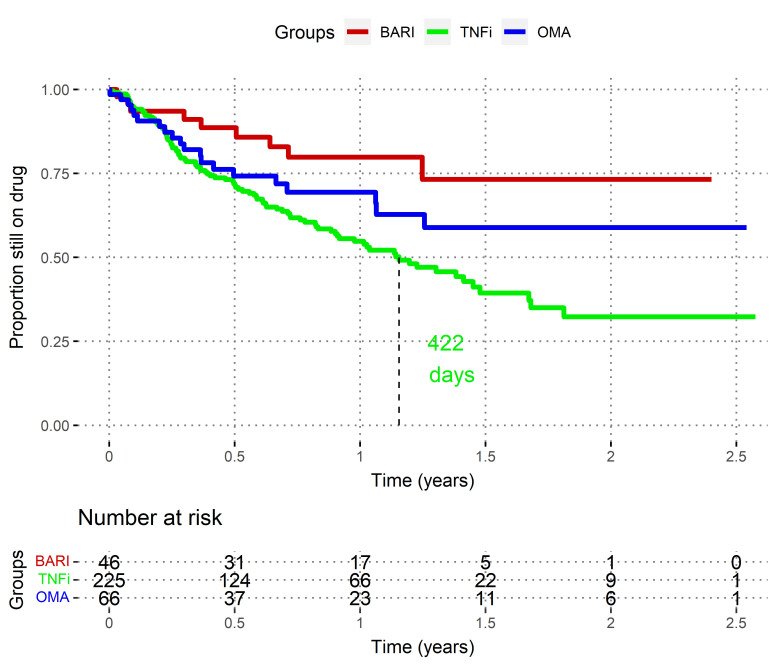
Time to all-cause discontinuation in b/tsDMARD-naïve patients
In this exploratory subgroup analysis, we restricted the population to patients without prior experience of b/tsDMARDs (so-called ‘bio-naïve’ patients, ie first b/tsDMARD prescription after methotrexate failure). In this subpopulation, patient characteristics were more balanced than in the main analysis, except for age, which remained younger in TNFi population, and concomitant csDMARD usage (more frequent in TNFi) (online supplemental table S3). Of note, the sample size was consequently reduced to 46 BARI, 225 TNFi and 66 OMA.
When analysing only these b/tsDMARD-naïve patients, both the non-adjusted (figure 2) and the adjusted differences between BARI and TNFi became larger (HR TNFi vs BARI 2.5; 95% CI, 1.23 to 5.16; p=0.01), but the differences between BARI and OMA group remained not significantly different (HR OMA vs BARI 1.90; 95% CI, 0.71 to 5.1; p=0.2).
Figure 2.
Unadjusted time to drug discontinuation in b/tsDMARD-naïve patients, Swiss Clinical Quality Management registry, 2017–2020. These Kaplan-Meier curves represent the crude ‘survival’ of drug prescription, by treatment group. Death and loss to follow-up are censored. BARI, baricitinib; OMA, other modes of action bDMARDs; TNFi, tumour necrosis factor inhibitors. Log-rank BARI versus TNFi: p=0.003. Log-rank BARI versus OMA: p=0.15.
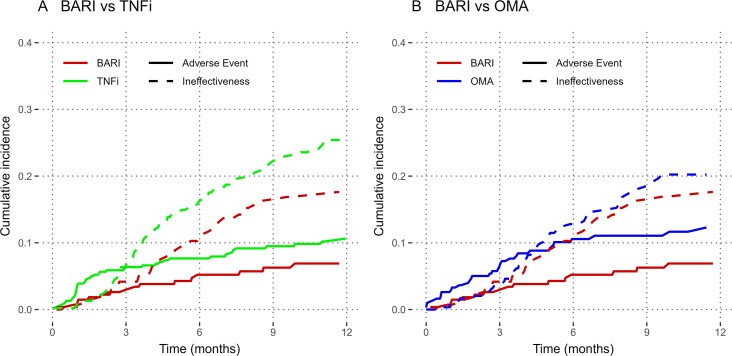
Time to discontinuation for adverse events or ineffectiveness
A secondary outcome was the cumulative incidence of drug discontinuation by specific reasons for discontinuation (ineffectiveness or adverse events, figure 3). Using Fine-Gray adjusted approach, we found no difference in the incidence of adverse event comparing BARI with TNFi (HR 1.46; 95% CI, 0.83 to 2.57; p=0.13) or BARI with OMA (HR 1.34; 95% CI, 0.74 to 2.42; p=0.25). The incidence of drug discontinuation for ineffectiveness was more frequent in TNFi compared with BARI (HR 1.49; 95% CI, 1.03 to 2.15; p=0.01) but similar between OMA and BARI (HR 1.09; 95% CI, 0.72 to 1.64; p=0.69).
Figure 3.
Cumulative incidence of drug discontinuation by stop reason and by type of treatment, Swiss Clinical Quality Management registry, 2017–2020. This figure represents the unadjusted cumulative incidence of drug discontinuation, by group and by reason of discontinuation. BARI, baricitinib; OMA, other modes of action bDMARDs; TNFi, tumour necrosis factor inhibitors.
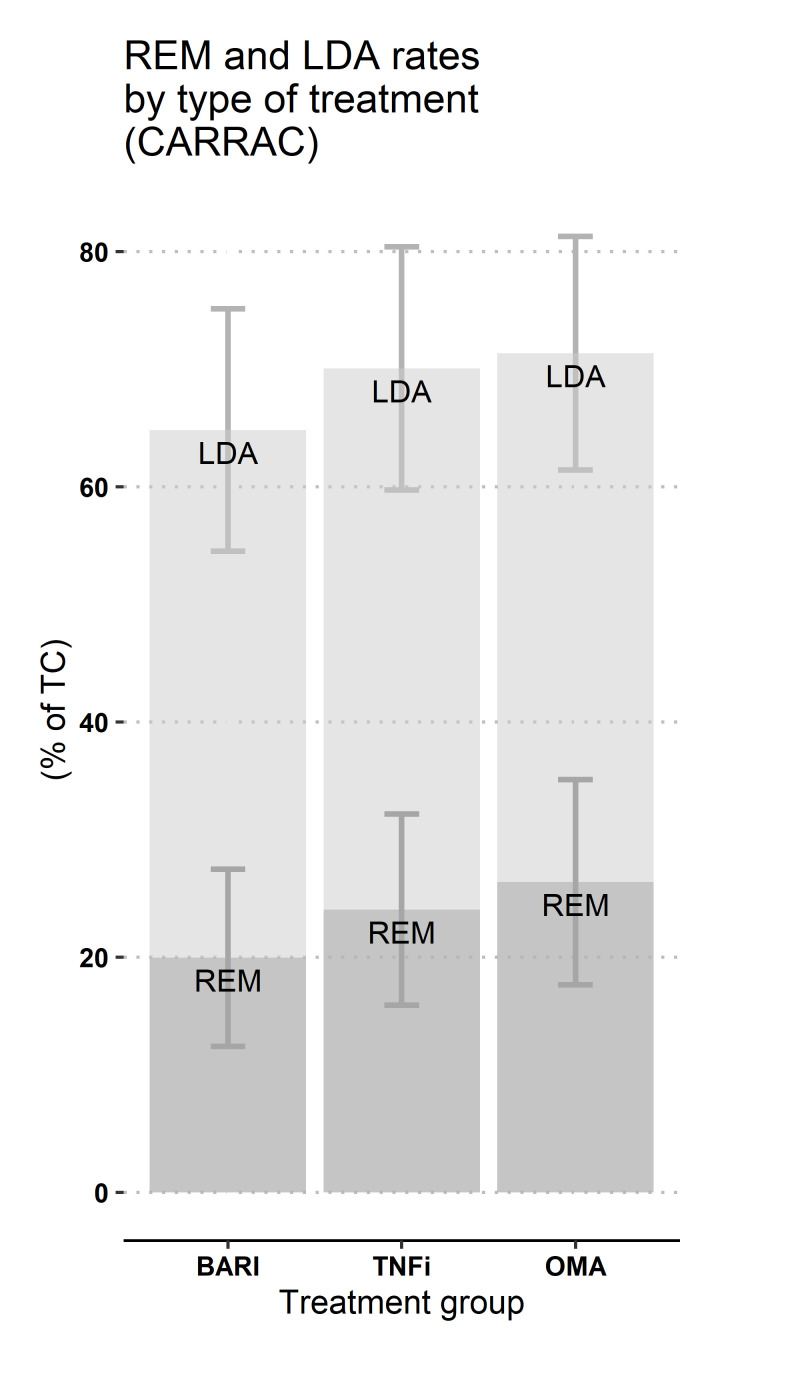
Remission and low disease activity at 12 months
The estimated 12-month rates of REM and LDA, estimated using CARRAC, did not differ significantly between the three groups (figure 4). LDA ranged from 62% to 71%, and REM ranged from 17% to 26%.
Figure 4.
Estimated response rates at 12 months (CARRAC), Swiss Clinical Quality Management registry, 2017–2020. BARI, baricitinib; LDA, low disease activity (ie, CDAI score ≤ 10), in light grey; CARRAC, confounder-adjusted response rate with attrition correction; OMA, other modes of action bDMARDs; REM, remission (ie, CDAI score ≤ 2.8), in dark grey; TNFi, tumour necrosis factor inhibitors. 95% CI, are represented. This method does not allow computing p values. Nb: two estimates were obtained in the BARI group and averaged to display only one representative value on the plot. Actual row output was 68% (95%CI 55% to 80%) (BARI vs TNFi model) or 62% (95% CI, 54 to 70) (BARI vs OMA model) for LDA and 23% (95% CI, 14 to 31) (BARI vs TNFi model) or 17% (95% CI, 10 to 24) (BARI vs OMA model) for REM.
Discussion
In this study, the overall drug maintenance of BARI was significantly longer compared with TNFi, despite the fact that it was prescribed to older patients with longer disease duration and more previous treatment failures similar to what was observed in RA-BE-REAL, another real-world study.23 However, the adjusted 12-month response rates in terms of LDA and REM did not differ significantly between BARI, TNFi and OMA groups. The difference in drug discontinuation owes mainly to more treatment discontinuations for ineffectiveness in the TNFi group compared with the BARI group, while drug discontinuation due to adverse event did not differ significantly between the groups.
Our results are in line with previous findings comparing other Janus-kinase inhibitors (JAKi) (ie, tofacitinib as well as BARI) to TNFi and OMA medications,22 32 which reported longer drug maintenance of tsDMARD compared with TNFi and similar maintenance to other bDMARDs. Of note, Lauper et al, using data from 19 national registers, found no difference in retention time between JAKi and TNFi.33 Still, Lauper et al grouped all JAKi together in their study; thus, it is not clear if these observations remain true for BARI alone, which might differ from other JAKi. For instance, Barbulescu et al reported a higher drug maintenance for BARI compared with tofacitinib.21
It was previously shown that BARI is more efficient in relieving pain compared with adalimumab therapy,34 and some molecular mechanisms relevant to Janus kinases and its signal transducer and activator of transcription proteins (JAK-STATs) signalling have been hypothesised.35 This observation has been suggested to result in antinociceptive effect independent from inflammation.35 This faster pain relief could partially explain why BARI has increased maintenance than other medications in our study, even though having similar 12-months LDA and REM rates. An alternative hypothesis is that the more convenient oral administration encourages patients to stay on medication longer. Yet, a third possible interpretation is that patients who experienced numerous treatment failures tend to stay on their latest therapy; however, our study accounts for this potential bias, by performing a sensitivity analysis in a subgroup of b/tsDMARD-naïve patients, which showed a similar result. Finally, given the recent discussion regarding tofacitinib safety,36 future research needs to clarify whether a class effect for JAKi-related adverse events exist. In this analysis, we found no indication of an increased incidence of adverse-related treatment discontinuation with BARI compared with alternative bDMARDs. Randomised controlled trials are ongoing to further compare safety profile of BARI versus TNFi (NCT04086745 and NCT03915964).
Limitations and strengths
This work has several limitations, mostly inherent to the observational setting. First, as this is a non-randomised study, we cannot formally exclude unmeasured confounding between the groups. The available baseline variables were, in most cases, adequately balanced, except for age. When we restricted the analysis to the subgroup of b/tsDMARD-naïve patients, we found largely similar results. Despite being limited by the small sample size, this exploratory subgroup analysis suggests that confounding by line of treatment was adequately accounted for in the adjusted analysis.
Second, the average length of follow-up was only approximatively 200 days per TC (online supplemental figure S2). Indeed, our study covers about 2 and a half years, and we only included TC newly initiated during this time window. Also, because of the study setting, as much as 65% of TCs did not have CDAI scores recorded at the date of initiation, and many were missing at the 12-month exact timepoint (online supplemental figure S6). Hence, our analysis of response rates relied heavily on linear interpolation techniques, using other available timepoints, which results in large confidence intervals for estimated response rates.30
The main strength of the study is that it relies on real-world data and includes a relatively large number of patients providing adequate statistical power (online supplemental figure S7). As these patients are mostly treated by office-based rheumatologists, our study population is representative of routine clinical practice. Also, subgroup analyses and sensitivity analyses were consistent with the main results.
Conclusions
In this non-randomised cohort study, drug maintenance of BARI was significantly higher than TNFi. However, we found no difference in drug maintenance when comparing BARI with other bDMARDs. Based on available data, the estimated 12-month response rates did not significantly differ between BARI, TNFi and OMA groups. We found no difference in treatment discontinuation for adverse event between the three groups. Overall, our results are in line with findings from randomised trials.
Supplementary Material
Acknowledgments
Almut Scherer, from the SCQM. Also, all of the participating physicians of the SCQM in rheumatic diseases. A complete list of rheumatology offices and hospitals that are contributing to the SCQM registries can be found on www.scqm.ch/institutions.
Footnotes
Contributors: BTPG is the guarantor for this publication. He contributed to data management, data analysis, figures and manuscript drafting. DM contributed to data management, data analysis, figures and manuscript revision. RA contributed to data analysis (in particular, sensitivity analyses) and manuscript revision. KL took part in data analysis and manuscript revision. CL was involved in the study design and manuscript revision. CP was involved in the study design and manuscript revision. RM contributed to the study design and manuscript revision. DSC was involved in the study design, data analysis and interpretation, and manuscript revision. AF was in charge of the study design (principal investigator), data analysis, data interpretation and manuscript revision.
Funding: This analysis has been made possible by the financial support of Eli Lilly (Suisse) SA to the Geneva University Hospitals. Also, the SCQM is financially supported by pharmaceutical industries and donors, including Eli Lilly. A list of financial supporters can be found on www.scqm.ch/sponsors.
Competing interests: BTPG has been once a paid speaker (Eli Lilly) and participated in the advisory board (Janssen). CP is employed by Eli Lilly and holds stock options (Eli Lilly and Company). CL is employed by Eli Lilly and holds stock options (Eli Lilly and Novartis). AF has received grants or contracts (Eli Lilly, Pfizer, AbbVie, Gilead and BMS), consulting fees (AstraZeneca, AbbVie, Pfizer and Gilead) and honorary payments (BMIS, AbbVie, Eli Lilly, Pfizer and MSD) and participated in advisory boards (Astra-Zeneca, Gilead, Novartis, AbbVie, Eli Lilly, Pfizer, J&J, Mylan and UCB). DM, RA, RM and DSC have no conflicts of interest to disclose.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Data may be obtained from a third party and are not publicly available. Data may be obtained after approval and permission from this license holder (SCQM). Contact information for data request: scqm@hin.ch.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and has been approved by the Geneva Ethical Review Boards as required by local law (Project ID: 2019-00930; approval date 28 May 2019). Every participant has signed an information and consent form for inclusion in the SCQM registry. Hence, this study has been conducted in accordance with the ethical principles of the Declaration of Helsinki and is consistent with Good Pharmacoepidemiology Practices. Participants gave informed consent to participate in the study before taking part.
References
- 1. Emery P, Seto Y. Role of biologics in early arthritis. Clin Exp Rheumatol 2003;21:S191–4. [PubMed] [Google Scholar]
- 2. Lipsky PE, van der Heijde DM, St Clair EW, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-tumor necrosis factor trial in rheumatoid arthritis with concomitant therapy study group. N Engl J Med 2000;343:1594–602. 10.1056/NEJM200011303432202 [DOI] [PubMed] [Google Scholar]
- 3. Breedveld FC, Emery P, Keystone E, et al. Infliximab in active early rheumatoid arthritis. Ann Rheum Dis 2004;63:149–55. 10.1136/ard.2003.013961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Klareskog L, van der Heijde D, de Jager JP, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet 2004;363:675–81. 10.1016/S0140-6736(04)15640-7 [DOI] [PubMed] [Google Scholar]
- 5. Weinblatt ME, Kremer JM, Bankhurst AD, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:FC fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med 1999;340:253–9. 10.1056/NEJM199901283400401 [DOI] [PubMed] [Google Scholar]
- 6. Maini R, St Clair EW, Breedveld F, et al. Infliximab (chimeric anti-tumour necrosis factor a monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. Lancet 1999;354:1932–9. 10.1016/S0140-6736(99)05246-0 [DOI] [PubMed] [Google Scholar]
- 7. Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum 2006;54:26–37. 10.1002/art.21519 [DOI] [PubMed] [Google Scholar]
- 8. Moreland LW, Schiff MH, Baumgartner SW, et al. Etanercept therapy in rheumatoid arthritis. Ann Intern Med 1999;130:478. 10.7326/0003-4819-130-6-199903160-00004 [DOI] [PubMed] [Google Scholar]
- 9. Dixon WG, Carmona L, Finckh A, et al. EULAR points to consider when establishing, analysing and reporting safety data of biologics registers in rheumatology. Ann Rheum Dis 2010;69:1596–602. 10.1136/ard.2009.125526 [DOI] [PubMed] [Google Scholar]
- 10. Wolfe F, Michaud K. The Hawthorne effect, sponsored trials, and the overestimation of treatment effectiveness. J Rheumatol 2010;37:2216–20. 10.3899/jrheum.100497 [DOI] [PubMed] [Google Scholar]
- 11. Wolfe F, Michaud K. The loss of health status in rheumatoid arthritis and the effect of biologic therapy: a longitudinal observational study. Arthritis Res Ther 2010;12:R35. 10.1186/ar2944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ostergaard M, Unkerskov J, Linde L, et al. Low remission rates but long drug survival in rheumatoid arthritis patients treated with infliximab or etanercept: results from the nationwide danish DANBIO database. Scand J Rheumatol 2007;36:151–4. 10.1080/03009740601089267 [DOI] [PubMed] [Google Scholar]
- 13. Souto A, Maneiro JR, Gómez-Reino JJ. Rate of discontinuation and drug survival of biologic therapies in rheumatoid arthritis: a systematic review and meta-analysis of drug registries and health care databases. Rheumatology 2016;55:kev374. 10.1093/rheumatology/kev374 [DOI] [PubMed] [Google Scholar]
- 14. Taylor PC, Keystone EC, van der Heijde D, et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med 2017;376:652–62. 10.1056/NEJMoa1608345 [DOI] [PubMed] [Google Scholar]
- 15. Fleischmann R, Schiff M, van der Heijde D, et al. Baricitinib, methotrexate, or combination in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment. Arthritis Rheumatol 2017;69:506–17. 10.1002/art.39953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dougados M, van der Heijde D, Chen Y-C, et al. Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: results from the RA-BUILD study. Ann Rheum Dis 2017;76:88–95. 10.1136/annrheumdis-2016-210094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Genovese MC, Kremer J, Zamani O, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med 2016;374:1243–52. 10.1056/NEJMoa1507247 [DOI] [PubMed] [Google Scholar]
- 18. Smolen JS, Genovese MC, Takeuchi T, et al. Safety profile of Baricitinib in patients with active rheumatoid arthritis with over 2 years median time in treatment. J Rheumatol 2019;46:7–18. 10.3899/jrheum.171361 [DOI] [PubMed] [Google Scholar]
- 19. Keystone EC, Taylor PC, Tanaka Y, et al. Patient-reported outcomes from a phase 3 study of baricitinib versus placebo or adalimumab in rheumatoid arthritis: secondary analyses from the RA-BEAM study. Ann Rheum Dis 2017;76:1853–61. 10.1136/annrheumdis-2017-211259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taylor PC, Takeuchi T, Burmester GR, et al. Safety of baricitinib for the treatment of rheumatoid arthritis over a median of 4.6 and up to 9.3 years of treatment: final results from long-term extension study and integrated database. Ann Rheum Dis 2022;81:335–43. 10.1136/annrheumdis-2021-221276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barbulescu A, Askling J, Chatzidionysiou K, et al. Effectiveness of baricitinib and tofacitinib compared with bDMARDs in RA: results from a cohort study using nationwide Swedish register data. Rheumatology (Oxford) 2022;61:3952–62. 10.1093/rheumatology/keac068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pappas DA, St John G, Etzel CJ, et al. Comparative effectiveness of first-line tumour necrosis factor inhibitor versus non-tumour necrosis factor inhibitor biologics and targeted synthetic agents in patients with rheumatoid arthritis: results from a large US registry study. Ann Rheum Dis 2021;80:96–102. 10.1136/annrheumdis-2020-217209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alten R, Burmester GR, Matucci Cerinic M, et al. Pos0666 a multinational, prospective, observational study in patients with rheumatoid arthritis receiving baricitinib, targeted synthetic or biologic disease-modifying therapies: 12 month time to discontinuation. Ann Rheum Dis 2022;81:606–7. 10.1136/annrheumdis-2022-eular.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Du Pan SM, Scherer A, Gabay C, et al. Differential drug retention between anti-TNF agents and alternative biological agents after inadequate response to an anti-TNF agent in rheumatoid arthritis patients. Ann Rheum Dis 2012;71:997–9. 10.1136/annrheumdis-2011-200882 [DOI] [PubMed] [Google Scholar]
- 25. Aletaha D, Smolen J. The simplified disease activity index (SDAI) and the clinical disease activity index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol 2005;23:S100–8. [PubMed] [Google Scholar]
- 26. Lauper K, Finckh A. Predictive factors of treatment persistence in rheumatoid arthritis. Jt Bone Spine 2020;87:531–4. 10.1016/j.jbspin.2020.03.006 [DOI] [PubMed] [Google Scholar]
- 27. White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica 1980;48:817. 10.2307/1912934 [DOI] [Google Scholar]
- 28. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 29. Mongin D, Lauper K, Finckh A, et al. Accounting for missing data caused by drug cessation in observational comparative effectiveness research: a simulation study. Ann Rheum Dis 2022;81:729–36. 10.1136/annrheumdis-2021-221477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mongin D, Lauper K, Turesson C, et al. Imputing missing data of function and disease activity in rheumatoid arthritis registers: what is the best technique RMD Open 2019;5:e000994. 10.1136/rmdopen-2019-000994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van Buuren S, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Soft 2011;45. 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 32. Finckh A, Tellenbach C, Herzog L, et al. Comparative effectiveness of antitumour necrosis factor agents, Biologics with an alternative mode of action and tofacitinib in an observational cohort of patients with rheumatoid arthritis in Switzerland. RMD Open 2020;6:e001174. 10.1136/rmdopen-2020-001174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lauper K, Iudici M, Mongin D, et al. Effectiveness of TNF-inhibitors, abatacept, Il6-inhibitors and JAK-inhibitors in 31 846 patients with rheumatoid arthritis in 19 registers from the ‘JAK-pot’ collaboration. Ann Rheum Dis 2022;81:1358–66. 10.1136/annrheumdis-2022-222586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Taylor PC, Lee YC, Fleischmann R, et al. Achieving pain control in rheumatoid arthritis with baricitinib or adalimumab plus methotrexate: results from the RA-BEAM trial. J Clin Med 2019;8:831. 10.3390/jcm8060831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Busch-Dienstfertig M, González-Rodríguez S. JAK-STAT signaling, and pain. JAKSTAT 2013;2:e27638. 10.4161/jkst.27638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Álvaro-Gracia JM, García-Llorente JF, Valderrama M, et al. Update on the safety profile of tofacitinib in rheumatoid arthritis from clinical trials to real-world studies: a narrative review. Rheumatol Ther 2021;8:17–40. 10.1007/s40744-020-00258-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-072300supp001.pdf (763.4KB, pdf)
bmjopen-2023-072300supp002.pdf (509.9KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data may be obtained from a third party and are not publicly available. Data may be obtained after approval and permission from this license holder (SCQM). Contact information for data request: scqm@hin.ch.