Abstract
Objectives
Tuberculosis infection (TBI) is marked by dynamic host–pathogen interactions with persistent low-grade inflammation and is associated with increased risk of cardiovascular diseases (CVD) including acute coronary syndrome, myocardial infarction and stroke. However, few studies assess the relationship between TBI and hypertension, an intermediate of CVD. We sought to determine the association between TBI and hypertension using data representative of the adult US population.
Methods
We performed cross-sectional analyses using data from the 2011–2012 US National Health and Nutrition Examination Survey (NHANES). Eligible participants included adults with valid QuantiFERON-TB Gold In-Tube (QFT-GIT) test results who also had blood pressure measures and no history of TB disease. TBI was defined by a positive QFT-GIT. We defined hypertension by either elevated measured blood pressure levels (ie, systolic ≥130 mm Hg or diastolic ≥80 mm Hg) or known hypertension indications (ie, self-reported previous diagnosis or use of antihypertensive medications). Analyses were performed using robust quasi-Poisson regressions and accounted for the stratified probability sampling design of NHANES.
Results
The overall prevalence of TBI was 5.7% (95% CI 4.7% to 6.7%) and hypertension was present among 48.9% (95% CI 45.2% to 52.7%) of participants. The prevalence of hypertension was higher among those with TBI (58.5%, 95% CI 52.4% to 64.5%) than those without TBI (48.3%, 95% CI 44.5% to 52.1%) (prevalence ratio (PR) 1.2, 95% CI 1.1 to 1.3). However, after adjusting for confounders, the prevalence of hypertension was similar for those with and without TBI (adjusted PR 1.0, 95% CI 1.0 to 1.1). The unadjusted prevalence of hypertension was higher among those with TBI versus no TBI, especially among individuals without CVD risk factors including those with normal body mass index (PR 1.6, 95% CI 1.2 to 2.0), euglycaemia (PR 1.3, 95% CI 1.1 to 1.5) or non-smokers (PR 1.2, 95% CI 1.1 to 1.4).
Conclusions
More than half of adults with TBI in the USA had hypertension. Importantly, we observed a relationship between TBI and hypertension among those without established CVD risk factors.
Summary
The prevalence of hypertension was high (59%) among adults with TBI in the USA. In addition, we found that the prevalence of hypertension was significantly higher among adults with positive QFT without established hypertension risk factors.
Keywords: hypertension, tuberculosis, public health, epidemiology
STRENGTHS AND LIMITATIONS OF THIS STUDY.
These analyses were conducted using data representative of civilian, non-institutionalised US adults, and thus, provide a robust population estimate of the prevalence of latent tuberculosis infection and hypertension in the USA.
Comprehensive definitions and different cut-offs of hypertension were used (ie, measured blood pressure level, previous diagnosis hypertension by healthcare providers) to model the association between latent tuberculosis infection and hypertension.
Our findings may not be representative to other regions with higher burdens of tuberculosis.
The cross-sectional study design of National Health and Nutrition Examination Survey prevented us from assessing the temporal relationship between latent tuberculosis infection and hypertension.
Introduction
About one-quarter of the world’s population (~2 billion) has been infected to Mycobacterium tuberculosis (Mtb).1 Among individuals infected with the bacteria, 5%–10% are at risk of developing TB disease at some point in their life.2 3 Tuberculosis infection (TBI), or most commonly known as latent TBI (LTBI), is increasingly recognised as a heterogeneous clinical state in which some individuals have dynamic host–pathogen interactions with persistent low-grade inflammation. This immune dysregulation has been associated with an increased risk of cardiovascular diseases (CVD) including acute coronary syndromes, myocardial infarction and stroke.1 4–12 This convergence of TBI and CVD risk poses a particular challenge for low-income and middle-income countries where TBI is most prevalent and incidence of chronic non-communicable diseases, including CVD, is increasing rapidly.13 14 Improved understanding of the impact of TBI on CVD risk is vital in settings where TBI and CVD are highly coprevalent in order to design public health intervention programmes aiming to reduce the burden of two diseases.
Epidemiological data from observational cohort studies support an increased risk of CVD among people with TB disease.8–12 Several studies also indicated that hypertension, an established intermediate of CVD, may be more common among patients with TB disease compared with non-TB controls.8 11 14–16 Furthermore, CVD was the leading contributor to post-TB mortality, accounting for 15%–26% of deaths among TB survivors in a recent systematic review and meta-analysis.17 In addition to these associations between TB disease and CVD, recent observational studies have found an association between TBI and various CVDs including acute myocardial infarction and coronary artery disease.9 18 19 However, studies assessing the association between TBI and hypertension remain limited.
To date, few studies have evaluated the relationship between TBI and hypertension. One cohort study from a large metropolitan healthcare system in the USA reported that individuals with TBI had greater incidence of hypertension compared with those without TBI and that rates were highest among those untreated for TBI.5 Furthermore, it is unknown whether the quantitative measures of IGRA, which may indicate the underlying mycobacterial burden and has been associated with increased risks of progression to TB disease,20–23 is associated with hypertension. Improved understanding of the association between TBI, quantitative measures of IGRA and hypertension may clarify the role that TB prevention efforts in reducing the burden of CVD, both in the USA and globally.
Given existing knowledge gaps, we aimed to estimate the association between TBI and hypertension prevalence. We also investigated whether the magnitude of host immune responses to Mtb was associated with hypertension among those with positive IGRA test results.
Methods
Study design and eligible participants
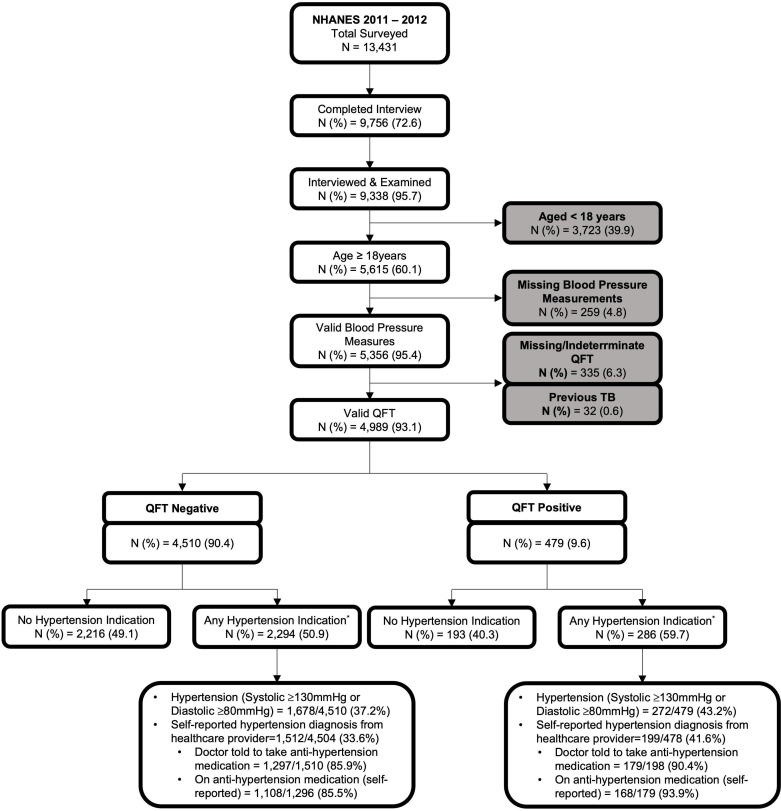
We performed an analysis of cross-sectional data from the 2011–2012 US National Health and Nutrition Examination Survey (NHANES),24 the most recent NHANES cycle released that includes measures of TBI. NHANES is a study led by the US Centers for Disease Control and Prevention, which aims to assess the health and nutritional status of non-institutionalised civilians representative of the US population using a complex, stratified, multistage probability cluster sampling design. NHANES collects demographic and health information using questionnaires administered by trained interviewers and standardised physical examinations performed in mobile examination centres. Eligible NHANES participants for our analyses were adults (≥18 years) with valid TBI test results and blood pressure measurements, and no history of TB disease (figure 1).
Figure 1.
Flow chart depicting unweighted frequencies and percentages of participants included in the final analyses based on the eligibility criteria, NHANES 2011–2012. *Any hypertension was defined as having an average systolic level of ≥130 mm Hg and/or diastolic level of ≥80 mm Hg, or a previous diagnosis of high blood pressure by health providers. This study flow chart provides description of the stepwise exclusion of ineligible participants. From 9338 individuals who completed NHANES interview and medical examination, we included 4989 (53.4%) individuals in our primary analyses after excluding those who are <18 years old or those with a record of previous TB disease, or missing blood pressure data and QuantiFERON results. NHANES, National Health and Nutrition Examination Survey; QFT, QuantiFERON-TB; TB, tuberculosis.
Study measures and definitions
Our primary study outcome, any hypertension, was defined as having either (1) ‘measured hypertension’, defined as an average systolic blood pressure level of ≥130 mm Hg or diastolic blood pressure level of ≥80 mm Hg across three consecutive measurements or (2) a self-reported previous hypertension diagnosis by a healthcare provider or current use of antihypertensive medications (ie, known hypertension). We categorised measured blood pressure levels into ‘normal’ (ie, systolic <120 mm Hg and diastolic <80 mm Hg), ‘borderline hypertension’ (ie, systolic 120–129 mm Hg and diastolic <80 mm Hg), ‘stage 1 hypertension’ (ie, systolic 130–139 mm Hg or diastolic 80–89 mm Hg) and ‘stage 2 hypertension’ (ie, systolic ≥140 mm Hg or diastolic ≥90 mm Hg) according to American College of Cardiology/American Heart Association guidelines.25 Among participants with a prior diagnosis of hypertension, we classified blood pressure as ‘controlled’ (systolic <130 mm Hg and diastolic <80 mm Hg) or ‘uncontrolled’ (systolic ≥130 mm Hg or diastolic ≥80 mm Hg) with or without a self-reported use of antihypertensive medications.
Our primary study exposure, TBI, was defined by a positive QuantiFERON-TB Gold In Tube or QFT test, an in vitro laboratory test to detect TB infection by measuring cell-mediated immune responses to TB-specific antigens.26 27 Individuals with indeterminate test results were excluded from our analyses. For those with a positive QFT, we also extracted the quantitative results and defined the IFN-γ TB antigen response by subtracting TB NIL control values from TB antigen values (ie, Ag-NIL values). To express IFN-γ TB antigen responses, instead of using the traditional manufacturer cut-off of 0.35, we used the 4.00 cut-off as previous studies showed that individuals with Ag-NIL values ≥4.00 are at greater risk from developing TB disease.20 22 23 Thus, in our analyses, Ag-NIL values were categorised as ‘low’ (<4 IU/mL) or ‘high’ (≥4 IU/mL). For a sensitivity analysis, we performed a subgroup analysis of participants with both QFT and tuberculin skin test (TST) results. We defined ‘confirmed TB infection’ when both TST and QFT results were positive and ‘no TB infection’ if both TST and QFT results were negative. Participants with discordant TST and QFT results (ie, TST negative and QFT positive, TST positive and QFT negative) were classified as ‘any discordance’.
Other important covariates, including age, sex, race, educational attainment, income to poverty ratio, country of birth, body mass index (BMI), diabetes mellitus status, HIV status, lipid profile, self-reported smoking behaviour, alcohol consumption, statin prescription and previous diagnosis of coronary heart disease, myocardial infarction or stroke were also abstracted. We classified BMI as ‘underweight’ (BMI<18.5 kg/m2), ‘normal’ (BMI 18.5–24.9 kg/m2), ‘overweight’ (BMI 25–29.9 kg/m2) and obese (BMI≥30 kg/m2).28 As NHANES grouped individuals aged ≥80 years in one category, we divided age into quartile ranges and grouped as ‘quartile 1 (18–31 years)’, ‘quartile 2 (32–47 years)’, ‘quartile 3 (48–62 years)’ and ‘quartile 4 (≥63 years)’ to account for the non-linearity of age in sensitivity analyses.
Patient and public involvement
None.
Statistical analysis
We estimated weighted prevalence and 95% CIs to determine the burden of TBI and hypertension in the US adult population. Rao-Scott χ2 tests were used to assess the bivariate association between participants’ demographic and clinical characteristics, TBI, Ag-NIL values and hypertension. Multivariable robust Poisson regression with quasi-likelihood was used to estimate the association between TBI and hypertension, expressed in prevalence ratios (PRs) and 95% CI. The same regression approach was used to estimate the association between Ag-NIL responses and hypertension. In addition to PRs, we also estimated prevalence differences (PDs) and their 95%CI. Covariates included in the multivariable models were based on bivariate associations (online supplemental tables S1 and S2), established risk factors reported in previously published studies and directed acyclic graphs (DAGs).29 Briefly, we identified potential confounders using bivariate associations and previously published literature, which then mapped into a DAG to determine inclusion in the final model. To account for the missingness of key covariates in the final adjusted model, we assigned aberrant values to any missing information to avoid deletion. We also assessed interaction between TBI and hypertension by participant characteristics (ie, age, BMI, glycaemic status, smoking status) on the additive (PD) and multiplicative (PR) scales by including the cross-product terms within multivariable models. All analyses were performed by using SAS Survey Analysis PROCs (SAS V.9.4) and survey package in R and accounted for the weighted stratified probability sample design of NHANES by applying weight (WTMEC2YR), cluster (SDMVPSU) and strata (SDMVSTRA) variables (samples of analytic codes are provided in online supplemental table S3). Taylor series linearisation was used to produce design-adjusted standard errors and a two-sided p<0.05 considered statistically significant in all analyses.
bmjopen-2023-075176supp001.pdf (236.7KB, pdf)
Subgroup and sensitivity analyses
Subgroup analyses were conducted using an analytic approach with ‘domain’ variables created to indicate subpopulations of interest.30 31 Subgroup analyses were performed among those with previously diagnosed hypertension to determine the association between TBI (including Ag-NIL values) and controlled hypertension. Sensitivity analyses were performed to quantify systematic errors due to (a) TBI misclassification, (b) hypertension misclassification, (c) covariate misspecification in multivariable models and (d) the classification of age as a confounder. To account for errors resulting from TBI misclassification, we ran additional models with confirmed TB infection as the exposure. To address potential biases due to hypertension misclassification, we ran an additional analysis using the prior hypertension clinical cut-off.25 In this additional model, we defined any hypertension as having (1) an average systolic blood pressure level of ≥140 mm Hg or diastolic blood pressure level of ≥90 mm Hg across three consecutive measurements or (2) a self-reported previous hypertension diagnosis by a healthcare provider or current use of antihypertensive medications. To quantify errors due to covariate misspecification, we ran multiple robust Poisson models with different sets of covariates and observed changes in PRs estimates across models. To account for the confounding effect of age, we ran multiple iterations of robust Poisson models with different forms of age measures (ie, continuous and age quartiles).
Results
Study population
In NHANES 2011–2012, 9338 participants were surveyed and examined (response rate of 69.5%), 60.1% (5615/9338) of whom were ≥18 years old (figure 1). Among included adults, 259 did not have valid blood pressure measurements. Of those with valid blood pressure measurements, 32 had a previous diagnosis of TB disease and 335 had a missing QFT, with 4989 participants meeting eligiblity for this analytical cohort. The weighted prevalence of TBI in the cohort was 5.7% (95% CI 4.7% to 6.7%) and any hypertension was present for 48.9% (95% CI 45.2% to 52.7%) of participants (table 1).
Table 1.
Weighted prevalence and adjusted prevalence ratios (aPRs) of hypertension measures by QuantiFERON-TB Gold In-Tube status among US adults, NHANES 2011–2012
| Hypertension measures | Weighted prevalence of hypertension, % (95%CI) | ||||
| Total N=4989 |
Among QFT (−) 94.3 (93.3, 95.3) |
Among QFT (+) 5.7 (4.7, 6.7) |
Prevalence difference* percentage point (95%CI) |
aPR† (95% CI) | |
| Primary study outcome | |||||
| Any hypertension indication‡ (n=2580/4989) | 48.9 (45.2, 52.7) | 48.3 (44.5, 52.1) | 58.5 (52.4, 64.5) | 10.2 (5.0 to 15.4) | 1.01 (0.97 to 1.06) |
| Measured blood pressure | |||||
| Hypertension§ (n=1885/4989) | 35.0 (32.3, 37.6) | 34.5 (31.8, 37.2) | 43.2 (36.4, 49.9) | 8.7 (1.9 to 15.5) | 1.04 (0.97 to 1.12) |
| Stage one hypertension¶ (n=1273) | 24.5 (22.4, 26.7) | 24.2 (21.9, 26.5) | 30.1 (22.4, 37.9) | 5.9 (−2.3 to 14.2) | 1.13 (0.99 to 1.29) |
| Stage two hypertension** (n=612) | 10.4 (9.1, 11.8) | 10.3 (8.9, 11.7) | 13.0 (9.1, 17.0) | 2.8 (−1.3 to 6.8) | 0.88 (0.75 to 1.02) |
| Hypertension diagnosis | |||||
| Previously diagnosed hypertension†† (n=1711) | 30.8 (27.7, 33.9) | 30.3 (27.1, 33.6) | 38.3 (33.6, 43.1) | 8.0 (2.4 to 13.6) | 0.97 (0.90 to 1.04) |
| Current use of antihypertension medication‡‡ (n=1276) | 86.9 (83.7, 90.1) | 86.3 (82.7, 89.9) | 94.7 (90.9, 98.4) | 8.4 (2.3 to 14.4) | 1.13 (1.02 to 1.09) |
| Undiagnosed hypertension§§ (n=869) | 18.1 (16.1, 20.2) | 18.0 (15.8, 20.2) | 20.2 (14.0, 26.4) | 2.2 (−4.5 to 8.9) | 1.08 (0.91 to 1.28) |
Bold indicates that the finding is significant at α=0.05.
This table shows the prevalence of select hypertension measures in the overall adult cohort of NHANES 2011–2012 as well as stratified by their tuberculosis infection status. The crude measure of association was expressed as prevalence difference, while the adjusted measure of association was expressed as prevalence ratio.
*Mean/prevalence difference was calculated by setting those without TBI (ie, QFT negative) as the referent group.
†Model was adjusted for age, sex, race, education attainment level, country of birth, type 2 diabetes mellitus, body mass index and smoking.
‡Systolic ≥130 mm Hg and/or diastolic ≥80 mm Hg or self-reported previous diagnosis of high blood pressure by health providers or use of antihypertensive medications.
§Including stage 1 and 2 hypertensions (ie, systolic ≥130 mm Hg or diastolic ≥80 mm Hg).
¶Systolic 130–139 mm Hg or diastolic 80–89 mm Hg.
**Systolic ≥140 mm Hg or diastolic ≥90 mm Hg.
††Survey participants answered ‘yes’ to the question ‘(have you/has SP) ever been told by a doctor or other health professional that (you/s/he) had hypertension, also called high blood pressure?’.
‡‡Among those who answered ‘yes’ to ‘because of (your/SP’s) (high blood pressure/hypertension), (have you, has s/he) ever been told to take prescribed medicine?’, survey participants also answered ‘yes’ to the question ‘(are you/is SP) now taking prescribed medicine (for high blood pressure/hypertension)?’.
§§Elevated blood pressure levels (systolic ≥130 mm Hg or diastolic ≥80 mm Hg) with no prior diagnosis of hypertension by healthcare providers.
aPRs, adjusted prevalence ratios; NHANES, National Health and Nutrition Examination Survey; QFT, QuantiFERON-TB.
Associations between TBI and hypertension
The prevalence of any hypertension was higher among those with TBI (58.5%, 95% CI 52.4% to 64.5%) than those without TBI (48.3%, 95% CI 44.5% to 52.1%) (PD 10.2%, 95% CI 5.0% to 15.4%) (table 1). After adjusting for potential confounders including age (continuous), sex, race, educational attainment level (as a proxy of socioeconomic status), country of birth, diabetes mellitus status, BMI and smoking status, the prevalence of any hypertension was similar among those with and without TBI (adjusted PR (aPR) 1.0, 95% CI 1.0 to 1.1). The association between TBI and hypertension was similar when examining the two components used to define our primary outcome (ie, measured hypertension and self-reported hypertension/use of antihypertensive medications) both in the crude and adjusted models (table 1).
Association between Ag-NIL values and hypertension
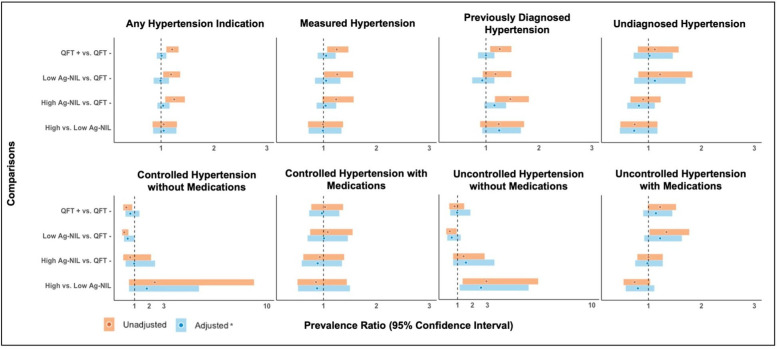
The prevalence of any hypertension was highest among those with TBI and high Ag-NIL values (60.4%, 95%CI 53.0% to 67.7%) compared with those with TBI and low Ag-NIL values (57.6%, 95% CI 48.7% to 66.6%) or those without TBI (48.3%, 95% CI 44.5% to 52.1%) (online supplemental table S4). After adjusting for age and gender, however, the prevalence of any hypertension was similar among the three QFT groups being compared (online supplemental table S5). Similar trends were also observed for the associations between Ag-NIL values and both measured hypertension and self-reported previous diagnosis of hypertension (figure 2).
Figure 2.
Crude and adjusted associations between QuantiFERON-TB Gold In-Tube results (QFT) and select hypertension measures among US adults, NHANES 2011–2012. *Models were adjusted for age and gender. Circles in this panel of figures indicate point estimates from the robust Poisson models, expressed as prevalence ratios with the coloured bands indicating the accompanying 95% CIs. The vertical dashed line on the x-axis value of 1 marks the study null value (ie, β estimates=0 or prevalence ratio=1.00), suggesting no association. The top panel figures were produced from analyses performed among eligible participants (n=4989). The lower panel figures were produced from analyses performed among a subset of participants with known hypertension indication (n=1711). Ag-Nil, antigen tube minus the Nil tube; NHANES, National Health and Nutrition Examination Survey; TB, tuberculosis.
Interaction analyses: established hypertension risk factors and HIV
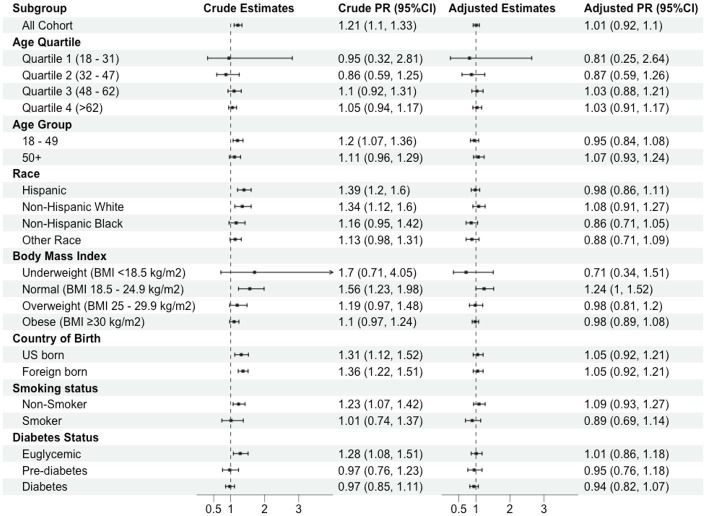
We observed relationships between TBI and hypertension among participants without established hypertension risk factors who would be considered at lower risk for CVD. For example, comparing individuals with and without TBI, the crude PRs (cPRs) of any hypertension was substantially higher among those with normal BMI (cPR 1.6, 95% CI 1.2 to 2.0), euglycaemia (cPR 1.3, 95% CI 1.1 to 1.5) and non-smoking (cPR 1.2, 95% CI 1.1 to 1.5) groups (figure 3) compared with those with or BMI≥25 kg/m2, pre-diabetes/diabetes or smokers. However, after adjusting for age and gender, the association between TBI and hypertension among these groups was attenuated. Additionally, product terms for BMI, glycaemic level and smoking status were non-significant on the aPR scale (p>0.05) (online supplemental table S6).
Figure 3.
Relationship between positive QuantiFERON-TB result and hypertension: Stratified by demographic and clinical characteristics among US adults, NHANES 2011–2012. This figure shows results from the analyses with statistical interaction term included in the robust Poisson models to evaluate the joint effect between tuberculosis infection and other key risk factors on hypertension. We selected these ‘moderator’ variables by identifying common risk factors for cardiovascular diseases from published studies (eg, age, race, body mass index, country of birth, smoking status and diabetes status). NHANES, National Health and Nutrition Examination Survey; PR, prevalence ratio; TB, tuberculosis.
We also found that the association between TBI and hypertension may be different across HIV status. For instance, the crude PD of any hypertension comparing those with TBI to those without TBI was 4.1 percentage points (95% CI −4.3 to 12.5) among those without HIV infection and 81.6 percentage points (95%CI 61.0 to 100.0) among those with HIV infection.
Subgroup and sensitivity analyses
From subgroup analyses conducted among those with known hypertension, the prevalence of controlled hypertension without medications was significantly lower among those with positive QFT (5.2%, 95% CI 2.0% to 8.3%) compared with those with negative QFT (11.8%, 95% CI 9.5% to 14.0%), although the association was no longer significant after adjusting for key confounders (aPR 0.6, 95% CI 0.4 to 1.1) (table 2). Conversely, the prevalence of uncontrolled hypertension with medications, the more severe form of hypertension, although non-significant, were slightly higher among those with positive QFT compared with those with negative QFT (figure 2).
Table 2.
Weighted prevalence and adjusted prevalence (aPR) ratios of controlled and uncontrolled hypertension by QuantiFERON-TB Gold In-Tube status among US adults with known hypertension, NHANES 2011–2012
| Hypertension controls | Weighted prevalence of hypertension, % (95%CI) | ||||
| Total N=1711 |
Among QFT (−) 94.3 (93.3, 95.3) |
Among QFT (+) 5.7 (4.7, 6.7) |
Mean/prevalence difference* percentage point (95%CI) |
aPR† (95% CI) | |
| Controlled without medications‡ (n=308) | 11.3 (9.2, 13.3) | 11.8 (9.5, 14.0) | 5.2 (2.0, 8.3) | −6.6 (−10.5 to to 2.7) | 0.62 (0.36 to 1.09) |
| Controlled with medications§ (n=838) | 33.9 (29.1, 38.8) | 33.9 (28.8, 40.0) | 34.8 (25.5, 44.1) | 0.9 (−9.0 to 10.9) | 1.10 (0.84 to 1.45) |
| Uncontrolled without medications¶ (n=127) | 15.0 (12.0, 18.1) | 15.2 (12.0, 18.5) | 12.2 (5.5, 18.9) | −3.1 (−10.5 to 4.4) | 0.80 (0.41 to 1.59) |
| Uncontrolled with medications** (n=438) | 39.8 (36.7, 42.8) | 39.1 (35.7, 42.6) | 47.8 (40.1, 55.6) | 8.7 (−1.0, 18.4) | 1.16 (0.94 to 1.43) |
Bold indicates that the finding is significant at α=0.05.
This table summarises findings on whether latent tuberculosis infection is associated with severe clinical manifestation of hypertension, indicated by elevated measured blood pressure levels with the use of antihypertensive medications among individuals with known hypertension indications (n=1711).
*Mean/prevalence difference was calculated by setting those without TBI (ie, QFT negative) as the referent group.
†Model was adjusted for age, sex, race, education attainment level, country of birth, type-2 diabetes mellitus, body mass index and smoking.
‡Having systolic blood pressure <130 mm Hg and a diastolic blood pressure <80 mm Hg without a record of taking medications to lower blood pressure levels.
§Having systolic blood pressure <130 mm Hg and a diastolic blood pressure <80 mm Hg with a record of taking medications to lower blood pressure levels.
¶Having systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥80 mm Hg without a record of taking medications to lower blood pressure levels.
**Having systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥80 mm Hg with a record of taking medications to lower blood pressure levels.
NHANES, National Health and Nutrition Examination Survey; QFT, QuantiFERON-TB; TBI, tuberculosis infection.
In models with confirmed TB infection (ie, positive QFT and positive TST) as the study exposure, the prevalence of any hypertension was highest among those with confirmed TB infection (60.8%, 95% CI 51.4% to 70.3%) compared with those with no TB infection (49.6%, 95% CI 45.7% to 53.5%) or those with discordant TST and QFT results (52.7%, 95% CI 43.9% to 61.6%) (p=0.12) (online supplemental table S7). We observed similar trends in the crude and adjusted associations between TBI and hypertension when we used both QFT and TST (online supplemental table S8) versus QFT alone to define TBI. Results from models that used prior clinical cut-offs to define hypertension (systolic blood pressure level of ≥140 mm Hg or diastolic blood pressure level of ≥90 mm Hg) were similar to results from models with current hypertension definitions (aPRprior 1.01, 95% CI 0.97 to 1.06 vs aPRcurrent 0.94, 95% CI 0.89 to 1.00) (data not shown). Results from sensitivity analyses to quantify bias due to covariate misspecification in the multivariable models indicated that PRs of any hypertension comparing those with positive QFT to those with negative QFT were similar when age was treated continuously or grouped in quartiles (ranged from 1.0 to 1.1) (online supplemental table S9).
Discussion
Using data representative of US adult population, we found a high prevalence of hypertension (ie, nearly one out of two) in the 2011–2012 NHANES cycle. We reported similar adjusted prevalence of hypertension among individuals with or without TBI. In our study, individuals with positive QFT and high Ag-NIL values were more likely to have any hypertension, but less likely to have the more severe form of hypertension (ie, uncontrolled hypertension without medications). We also observed that the association between TBI and hypertension was more common among individuals without established hypertension risk factors. Collectively, our results provide preliminary epidemiological evidence suggesting that hypertension, a well-established intermediate for CVD, was more common among individuals with TBI than those without TBI in the US populations.
Our finding suggesting that hypertension is more common among individuals with TBI than those without TBI is consistent with previous studies, although the prevalence was similar after adjusting for key confounders. Our null adjusted findings may indicate that the association between TBI and hypertension among NHANES cohort were confounded by demographic characteristics (eg, age, sex). In contrast, a retrospective cohort study conducted among 5185 individuals with TBI and healthy controls using data from a large metropolitan healthcare system in the US reported a higher hazard rates of hypertension incidence (defined by International Classification of Diseases [ICD]-9 codes) among those with TBI (defined by ICD-9 codes and tuberculin skin test/IFN-γ release assay) compared with healthy controls without TBI (HR 2.0, 95% CI 1.6 to 2.5).5 In addition, a cross-sectional study conducted among 2,351 TST-positive individuals in South India reported a slightly higher prevalence of hypertension (defined as systolic >130 mm Hg) among those with confirmed TBI (defined as TST and QFT positive) (15%) compared with those latent TB negative (12%) (aOR 1.18, 95% CI 1.0 to 1.56).32 Unlike the two studies mentioned above, we used a more comprehensive definition of hypertension by combining objectively measured blood pressure levels (systolic and diastolic) and known hypertension indications (ie, previous hypertension diagnosis or self-reported use of antihypertensive medications) to avoid potential misclassification.
Despite our null findings, we identified several plausible mechanisms that may explain how TBI may be associated with hypertension. First, underlying pathophysiology related to chronic inflammation, even at relatively low levels, is linked to hypertension, and therefore, the proinflammatory state that accompanies TBI may increase blood pressure.33 34 Second, TBI may be a proxy of other key factors related to social position which in turn impact hypertension risk. Hypertension is known to be multifactorial spanning from the group or community to the individual. Several physical, social, political and environments risk factors that may influence hypertension were not fully accounted for in our analyses (eg, stress, family history, diet, lifestyle, physical activity, geographical delineation, illicit drug use, access to healthcare or insurance coverage). If some of these variables are associated with TBI, it is plausible that our reported estimates are distorted due to residual confounding effects. Further studies using social ecological models and longitudinal designs are warranted to better understand the true effect of TBI on hypertension.
Furthermore, we also reported that the prevalence of hypertension was highest among individuals with positive QFT and high Ag-NIL values, but we observed no dose–response relationship nor statistical significance after adjusting for key risk factors. TB infection has been associated with enhanced levels of systemic inflammation and immune activation, including increased expression of tumour necrosis factor-α, interferons and interlukin-6 (IL-6).4–7 These chemokines and dysfunctional immune responses play an important role in the pathogenesis of hypertension and CVD.35 36 Individuals with positive QFT and higher Ag-NIL values are more likely to develop to active TB22 37 as they may have higher mycobacterial burden,20 and thus, could potentially have higher degree of inflammation or immune responses to the bacterial infection. Interestingly, among those with previously diagnosed hypertension, we found that individuals with TBI may have more severe hypertension manifestation compared with those without TBI. This was indicated by the higher prevalence of uncontrolled hypertension without medications among those with TBI. However, the available data do not allow us to discern if these differences are due to clinical differences or access to care.
Our cross-sectional study design may not be the appropriate design to observe the expected associations or dose–response relationship between TBI, IFN-γ TB antigen responses and hypertension. Furthermore, the time of TBI in the life course may have different implications on TBI and hypertension association. In this NHANES cohort, the majority (>90%) of foreign born with positive QFT have stayed in the US for ≥5 years, and thus, we postulated that TBI happened before arriving in the USA. It is plausible that these individuals are either in the latent or incipient stage where there is no to minimum bacteria replication, and thus, minimum proinflammatory responses.38 Newly arrived immigrants may face higher level of stress with acculturation and other social-environmental pressures which could impact systemic inflammation, immune responses and/or increased risks of hypertension. Prospective studies to follow individuals with recent TBI diagnosis are still warranted to determine the hypertension and CVD risk trajectories.
Interestingly, we observed associations between TBI and hypertension among those with normal BMI, euglycaemic and non-smokers without adjusting for potential confounders. These groups may be considered at lower risk of CVD. Although the associations were attenuated and non-significant after controlling for potential confounders, the prevalence of hypertension remained higher when comparing those with TBI than those without TBI among these groups. This further reinforces the premise that there are likely to be differing effects of TBI on hypertension risk within subgroups. While the significant TBI–hypertension associations observed among those with lower risk of CVD may be due to the larger sample sizes in NHANES, these preliminary results suggest the need for mechanistic studies. Further clinical investigations and modelling studies are needed to determine whether targeted TB preventive treatment is effective to reduce the global burden of CVD among these groups.
Our study is subject to limitations. First, our TBI definition (ie, according to QFT positivity) may include a broad spectrum of individuals who may have cleared the infection, have latent TB, incipient TB or subclinical TB since no further clinical assessment was made (eg, symptom screening, chest X-ray, culture test).39 40 Second, we could not determine the temporal relationship between TBI and hypertension with the cross-sectional study design used in the present paper. Third, we did not account for any record of hypertension prescription, or other commonly prescribed medications that could potentially affect blood pressure levels. Fourth, we defined some of our key variables (including hypertension status and hypertension medication intake) with self-reported information that may be prone to recall bias and likely included some misclassification. However, if misclassification of hypertension was non-differential with respect to TBI, we expect any misclassification in our results would likely biased towards the null.41 Fourth, we did not estimate (a) stratum specific PRs for HIV and (b) effects of HIV clinical information (eg, CD4 counts) in our stratified models due to the small, unweighted frequency of individuals with HIV infection. The wide CIs reported around our PDs among HIV and non-HIV group also needs to be interpreted with caution considering the low prevalence of HIV infection in the 2011–2012 NHANES cycle. Further clinical studies with larger sample size are still warranted to fully assess the joint effect between HIV (including HIV clinical characteristics) and TBI, and its association with hypertension. Last, this study was conducted using survey data representative of US adult population but may not be generalisable to other regions with higher TB burdens. Furthermore, we used datafrom NHANES 2011–2012 and were not able to determine whether the prevalence of TB infection and hypertension reported in this study cycle is reflective of the current US population. An updated analysis to assess trends in the association across multiple NHANES cycles is warranted.24
In conclusion, we reported a higher prevalence of hypertension among individuals with positive QFT, although the association was non-significant after adjusting for key confounders, particularly age. To determine the direction of the association between TBI and hypertension, a prospective study following hypertension-free individuals at TBI diagnosis is warranted and would help establish the biological pathways regarding how TBI might increase the risk of CVD. Future prospective work should address the question whether individuals treated for LTBI have lower risk of hypertension. Importantly, our results underscore the need to screen for hypertension and other metabolic disorders among those with TBI, especially among those without traditional CVD risk factors; doing so may help prevent premature deaths attributed to TB and CVD.
Supplementary Material
Footnotes
Twitter: @argitadsalindri
MAH and MJM contributed equally.
Contributors: MAH, MJM and ADS conceived the study design. ADS performed the analyses. ADS, MAH and MJM wrote the first draft of the manuscript. SA, UG, EU and JRA assisted with further drafting and revisions of manuscripts. All authors reviewed and approved the final version of the manuscript. Guarantors: ADS and MJM.
Funding: This work was supported in part by the National Center for Advancing Translational Sciences (grant numbers R03TR004097 to MAH), the National Institute of Allergy and Infectious Diseases (grant number UM1AI069501; grant numbers R01AI153152 and R21AI156161 to MJM; grant number K23AI134182 to SA) and the National Heart, Lung and Blood Institute (grant number R01HL156779 to MAH) at the National Institutes of Health.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data sharing not applicable as no datasets generated and/or analysed for this study. This work used publicly available data of the US National Health and Nutrition Examination Survey (NHANES) 2011–2012 that can be downloaded directly from CDC’s webpage.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Following federal regulations, this work was determined as 'non-human subject research' by the Institutional Review Boards (IRB) at Emory University, and thus, does not require IRB review.
References
- 1. Behr MA, Kaufmann E, Duffin J, et al. Latent tuberculosis: two centuries of confusion. Am J Respir Crit Care Med 2021;204:142–8. 10.1164/rccm.202011-4239PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Houben R, Dodd PJ. The global burden of latent tuberculosis infection: A re-estimation using mathematical Modelling. PLoS Med 2016;13:e1002152. 10.1371/journal.pmed.1002152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . Tuberculosis Fact Sheet 2016, Available: http://www.who.int/mediacentre/factsheets/fs104/en
- 4. Feria MG, Chang C, Ticona E, et al. Pro-inflammatory alterations of circulating monocytes in latent tuberculosis infection. Open Forum Infect Dis 2022;9:ofac629. 10.1093/ofid/ofac629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mandieka E, Saleh D, Chokshi AK, et al. Latent tuberculosis infection and elevated incidence of hypertension. J Am Heart Assoc 2020;9:e019144. 10.1161/JAHA.120.019144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dixon DL, Wohlford GF, Abbate A. Inflammation and hypertension. Hypertension 2020;75:297–8. 10.1161/HYPERTENSIONAHA.119.14195 [DOI] [PubMed] [Google Scholar]
- 7. Kiazyk S, Ball TB. Latent tuberculosis infection: an overview. Can Commun Dis Rep 2017;43:62–6. 10.14745/ccdr.v43i34a01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chung W-S, Lin C-L, Hung C-T, et al. Tuberculosis increases the subsequent risk of acute coronary syndrome: a nationwide population-based cohort study. Int J Tuberc Lung Dis 2014;18:79–83. 10.5588/ijtld.13.0288 [DOI] [PubMed] [Google Scholar]
- 9. Huaman MA, Ticona E, Miranda G, et al. The relationship between latent tuberculosis infection and acute myocardial infarction. Clin Infect Dis 2018;66:886–92. 10.1093/cid/cix910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huaman MA, Kryscio RJ, Fichtenbaum CJ, et al. Tuberculosis and risk of acute myocardial infarction: a propensity score-matched analysis. Epidemiol Infect 2017;145:1363–7. 10.1017/S0950268817000279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sheu J-J, Chiou H-Y, Kang J-H, et al. Tuberculosis and the risk of ischemic stroke: a 3-year follow-up study. Stroke 2010;41:244–9. 10.1161/STROKEAHA.109.567735 [DOI] [PubMed] [Google Scholar]
- 12. Salindri AD, Wang J-Y, Lin H-H, et al. Post-tuberculosis incidence of diabetes, myocardial infarction, and stroke: retrospective cohort analysis of patients formerly treated for tuberculosis in Taiwan, 2002 - 2013. Int J Infect Dis 2019;84:127–30. 10.1016/j.ijid.2019.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Magee MJ, Salindri AD, Gujral UP, et al. Convergence of non-communicable diseases and tuberculosis: a two-way street Int J Tuberc Lung Dis 2018;22:1258–68. 10.5588/ijtld.18.0045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seegert AB, Rudolf F, Wejse C, et al. Tuberculosis and hypertension—a systematic review of the literature. Int J Infect Dis 2017;56:54–61. 10.1016/j.ijid.2016.12.016 [DOI] [PubMed] [Google Scholar]
- 15. Shen T-C, Huang K-Y, Chao C-H, et al. The risk of chronic kidney disease in tuberculosis: a population-based cohort study. QJM 2015;108:397–403. 10.1093/qjmed/hcu220 [DOI] [PubMed] [Google Scholar]
- 16. Marak B, Kaur P, Rao SR, et al. Non-communicable disease Comorbidities and risk factors among tuberculosis patients, Meghalaya, India. Indian J Tuberc 2016;63:123–5. 10.1016/j.ijtb.2015.07.018 [DOI] [PubMed] [Google Scholar]
- 17. Romanowski K, Baumann B, Basham CA, et al. Long-term all-cause mortality in people treated for tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis 2019;19:1129–37. 10.1016/S1473-3099(19)30309-3 [DOI] [PubMed] [Google Scholar]
- 18. Khoufi EAA. Association between latent tuberculosis and ischemic heart disease: a hospital-based cross-sectional study from Saudi Arabia. Pan Afr Med J 2021;38:362. 10.11604/pamj.2021.38.362.28110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alsayed Hasanain AF, El-Maghraby KM, H Zayed AA, et al. Latent tuberculosis infection among patients with coronary artery stenosis: A case-control study. Int J Mycobacteriol 2018;7:143–7. 10.4103/ijmy.ijmy_34_18 [DOI] [PubMed] [Google Scholar]
- 20. Gupta RK, Lipman M, Jackson C, et al. Quantitative IFN-Γ release assay and Tuberculin skin test results to predict incident tuberculosis. A prospective cohort study. Am J Respir Crit Care Med 2020;201:984–91. 10.1164/rccm.201905-0969OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zellweger J-P, Sotgiu G, Block M, et al. Risk assessment of tuberculosis in contacts by IFN-Γ release assays. A tuberculosis network European trials group study. Am J Respir Crit Care Med 2015;191:1176–84. 10.1164/rccm.201502-0232OC [DOI] [PubMed] [Google Scholar]
- 22. Andrews JR, Nemes E, Tameris M, et al. Serial Quantiferon testing and tuberculosis disease risk among young children: an observational cohort study. Lancet Respir Med 2017;5:282–90. 10.1016/S2213-2600(17)30060-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Winje BA, White R, Syre H, et al. Stratification by interferon-Γ release assay level predicts risk of incident TB. Thorax 2018:thoraxjnl-2017-211147. 10.1136/thoraxjnl-2017-211147 [DOI] [PubMed] [Google Scholar]
- 24. CDC . National Health and Nutrition Examination Survey 2011 - 2012. 2012. [Google Scholar]
- 25. Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/Apha/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American college of cardiology/American heart Association task force on clinical practice guidelines. Hypertension 2018;71:e13–115. 10.1161/HYP.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 26. Mazurek GH, Jereb J, Lobue P, et al. Guidelines for using the Quantiferon-TB gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep 2005;54:49–55. [PubMed] [Google Scholar]
- 27. Banaei N, Gaur RL, Pai M. Interferon gamma release assays for latent tuberculosis: what are the sources of variability J Clin Microbiol 2016;54:845–50. 10.1128/JCM.02803-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weir CB, Jan A. BMI Classification Percentile And Cut Off Points. Treasure Island, FL: StatPearls, 2022. [PubMed] [Google Scholar]
- 29. Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology 1999;10:37–48. [PubMed] [Google Scholar]
- 30. CDC . NHANES Survey Methods and Analytic Guidelines, Available: https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx#analytic-guidelines
- 31. CDC . NHANES Sample Code, Available: https://wwwn.cdc.gov/nchs/nhanes/tutorials/samplecode.aspx
- 32. Munisankar S, Rajamanickam A, Balasubramanian S, et al. Prevalence of proximate risk factors of active tuberculosis in latent tuberculosis infection: A cross-sectional study from South India. Front Public Health 2022;10:1011388. 10.3389/fpubh.2022.1011388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huaman MA, Henson D, Ticona E, et al. Tuberculosis and cardiovascular disease: linking the epidemics. Trop Dis Travel Med Vaccines 2015;1:10. 10.1186/s40794-015-0014-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huaman MA, De Cecco CN, Bittencourt MS, et al. Latent tuberculosis infection and Subclinical coronary Atherosclerosis in Peru and Uganda. Clin Infect Dis 2021;73:e3384–90. 10.1093/cid/ciaa1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. De Miguel C, Rudemiller NP, Abais JM, et al. Inflammation and hypertension: new understandings and potential therapeutic targets. Curr Hypertens Rep 2015;17:507. 10.1007/s11906-014-0507-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tanase DM, Gosav EM, Radu S, et al. Arterial hypertension and Interleukins: potential therapeutic target or future diagnostic marker Int J Hypertens 2019;2019:3159283. 10.1155/2019/3159283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ledesma JR, Ma J, Zheng P, et al. Interferon-gamma release assay levels and risk of progression to active tuberculosis: a systematic review and dose-response meta-regression analysis. BMC Infect Dis 2021;21. 10.1186/s12879-021-06141-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Migliori GB, Ong CWM, Petrone L, et al. The definition of tuberculosis infection based on the spectrum of tuberculosis disease. Breathe (Sheff) 2021;17:210079. 10.1183/20734735.0079-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dowdy DW, Behr MA. Are we Underestimating the annual risk of infection with Mycobacterium tuberculosis in high-burden settings. Lancet Infect Dis 2022;22:e271–8. 10.1016/S1473-3099(22)00153-0 [DOI] [PubMed] [Google Scholar]
- 40. Anwar A, Hamdan A-J, Salim B, et al. Diagnostic utility of Quantiferon-TB gold (QFT-G) in active pulmonary tuberculosis. J Glob Infect Dis 2015;7:108–12. 10.4103/0974-777X.162231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lash TL, Fox MP, Fink AK. Applying Quantitative Bias Analysis to Epidemiologic Data: Statistics for Biology and Health. New York: Springer Science and Business Media, 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-075176supp001.pdf (236.7KB, pdf)
Data Availability Statement
Data sharing not applicable as no datasets generated and/or analysed for this study. This work used publicly available data of the US National Health and Nutrition Examination Survey (NHANES) 2011–2012 that can be downloaded directly from CDC’s webpage.