Abstract
Background
Ferroptosis plays an important role in enhancing the efficacy of anti-programmed cell death 1 (PD-1) immunotherapy; however, the molecular mechanisms by which tumor ferroptosis sensitizes melanoma and lung cancer to anti-PD-1 immunotherapy have not been elucidated.
Methods
Cytotoxicity assays, colony formation assays, flow cytometry and animal experiments were used to evaluate the effects of mefloquine (Mef) on survival and ferroptosis in melanoma and lung cancer. RNA sequencing, Real-time quantitative PCR (qRT-PCR), western blotting, chromatin immunoprecipitation-qPCR and flow cytometry were used to determine the molecular mechanisms by which Mef regulates lysophosphatidylcholine acyltransferase 3 (LPCAT3). The relationship between LPCAT3 and the efficacy of anti-PD-1 immunotherapy was verified via a clinical database and single-cell RNA sequencing (ScRNA-Seq).
Results
In this study, we discovered that Mef induces ferroptosis. Furthermore, treatment with Mef in combination with T-cell-derived interferon-γ (IFN-γ) enhanced tumor ferroptosis and sensitized melanoma and lung cancer cells to anti-PD-1 immunotherapy. Mechanistically, Mef upregulated the expression of LPCAT3, a key gene involved in lipid peroxidation, by activating IFN-γ-induced STAT1-IRF1 signaling, and knocking down LPCAT3 impaired the induction of ferroptosis by Mef+IFN-γ. Clinically, analysis of the transcriptome and single-cell sequencing results in patients with melanoma showed that LPCAT3 expression was significantly lower in patients with melanoma than in control individuals, and LPCAT3 expression was positively correlated with the efficacy of anti-PD-1 immunotherapy.
Conclusions
In conclusion, our study demonstrated a novel mechanism by which LPCAT3 is regulated, and demonstrated that Mef is a highly promising new target that can be utilized to enhance the efficacy of anti-PD-1 immunotherapy.
Keywords: Melanoma, Lung Cancer, Immune Checkpoint Inhibitor
WHAT IS ALREADY KNOWN ON THIS TOPIC
Accumulating evidences has shown that ferroptosis is associated with therapeutic resistance. The induction of ferroptosis is an effective approach to reverse therapeutic resistance and enhance the efficacy of immunotherapy.
WHAT THIS STUDY ADDS
Mefloquine (Mef) is a newly identified ferroptosis inducer. Mef upregulated the expression of lysophosphatidylcholine acyltransferase 3 (LPCAT3) by promoting interferon (IFN)-γ-induced STAT1-IRF1 signaling, thereby sensitizing melanoma and lung cancer to anti-programmed cell death 1 (PD-1) immunotherapy.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
These findings reveal the novel mechanism of Mef on IFN-γ-LPCAT3-induced ferroptosis as well as the efficacy of anti-PD-1 immunotherapy, and provide a novel promising strategy to enhance the efficacy of anti-PD-1 immunotherapy in the treatment of melanoma and lung cancer.
Introduction
Ferroptosis is a form of programmed cell death, similar to apoptosis, and is characterized by the accumulation of Reactive oxygen species (ROS) and lipid peroxidation.1 Induction of ferroptosis has been shown to be an effective way to reverse therapeutic resistance in tumors. Several inducers of ferroptosis have been developed. One class of ferroptosis inducers (eg, erastin and sulfasalazine2) reduces intracellular glutathione (GSH) levels by inhibiting the uptake of cystine via the system xc-; another class of ferroptosis inducers (eg, artesunate3 and β-elemene4) acts by inhibiting the reduction of lipid peroxides by GPX4.5 An additional class of inducers (eg, dihydroartemisinin6) increases the cellular labile iron pool to induce ferroptosis. However, even though lipid peroxidation is the central driving mechanism of ferroptosis, no ferroptosis inducers have been developed that target lipid peroxidation.
Immunotherapy is a major breakthrough in melanoma and lung cancer treatment, but treatment resistance remains a great challenge. Recently, ferroptosis has been shown to be involved in tumor resistance to immunotherapy, and the induction of ferroptosis in tumor cells has been shown to help overcome immunotherapeutic resistance.7 Tumor cells undergoing ferroptosis promote phagocytosis by macrophages.8 Ferroptosis cells release damage-associated molecules and promote the phenotypic maturation of dendritic cells.9 In breast cancer, the inhibition of ferroptosis leads to resistance to anti-programmed cell death 1 (PD-1)/programmed death ligand 1 (PD-L1) therapy, whereas the induction of ferroptosis resensitizes resistant tumor cells to PD-1/PD-L1 immunotherapy.10 These studies suggest that the induction of ferroptosis in tumor cells is an effective approach to enhance the efficacy of immunotherapy.
Mefloquine (Mef) is a quinoline ethanol approved by the Food and Drug Administration (FDA) for the treatment of malaria infections caused by Plasmodium falciparum.11 Mef kills Plasmodium by inhibiting protein synthesis through interaction with the Plasmodium 80S ribosomal subunit GTPase-related center.12 Mef has been used in the clinic for 30 years, and in recent years, Mef has shown potential as an old drug that could be investigated for new use and has shown anticancer efficacy in a variety of tumors13; furthermore, Mef does not inhibit the proliferation of normal human fibroblasts, suggesting that its anticancer effect has a certain degree of selectivity.14 However, whether Mef inhibits melanoma and lung cancer cell growth via the immune responses is unclear.
In our study, contrary to the reported induction of tumor cell apoptosis by Mef, we found that Mef is a novel inducer of ferroptosis that promotes ROS-related lipid peroxidation through the augmentation of interferon (IFN)-γ-STAT1-IRF1-induced lysophosphatidylcholine acyltransferase 3 (LPCAT3) expression and enhances tumor killing by T cells and the efficacy of anti-PD-1 immunotherapy.
Methods
Cell culture
The human malignant melanoma cell lines Sk-Mel-28 and A375, the mouse malignant melanoma cell line B16F10, the human melanocyte cell line PIG1, the human non-small cell lung cancer cell line A549 and the mouse Lewis lung cancer cell line LLC were purchased from American Type Culture Collection (USA). the 293T cell line was purchased from Clontech (Mountain View, USA). the PIG1 cell line was cultured in Opti-MEM I Reduced Serum Medium (Gibco, USA); the Sk-Mel-28, A375, and LLC cell lines were cultured in Dulbecco’s modified Eagle’s medium (Gibco, USA); and the B16F10 and A549 cell lines were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium (Gibco, USA) supplemented with 10% fetal bovine serum (Gibco, USA) and 1% penicillin–streptomycin solution (Biological Industries, Israel) at 37°C and 5% CO2.
Animal study
All animal experiments were performed in accordance with protocols approved by the Ethical Review of Experimental Animals Committee at Central South University. B16F10 melanoma cells (5×105 cells in 100 µL of RPMI-1640 medium) were injected into the right flank of 7-week-old female C57BL/6 mice (Hunan SJA Laboratory Animal, China). Tumor-bearing mice were randomly allocated into groups. When the tumor volume reached 50–100 mm3, the mice were treated with IgG2a (Bio X Cell, USA; BE0089)/PD-1 mAb (Bio X Cell, USA; BE0146) (200 µg/mouse, intraperitoneal injection every 3 days), Mef (MCE, USA) (50 mg/kg orally every day), or a combination of both drugs for 9 days. Tumor diameters were measured using a digital caliper every other day. Tumor volume was calculated with the following formula: V=(length×width2)/2, and the relative volume was calculated with the following formula: V=Vn/V1(n is the number of treatment days). When the tumors reached approximately 1 cm3 in size, the tumor-bearing mice were sacrificed. The tumors were immediately collected for flow cytometry analysis or RNA sequencing.
To quantify the number of T cells and T-cell cytokine expression, single-cell suspensions were prepared from fresh tumor tissues. For cell-surface staining, the cells were incubated with specific antibodies for approximately 30 min at 4°C in the dark. For intracellular staining, the cells were restimulated with an eBioscience Flow Intracellular Fixation Permeabilization Buffer (Invitrogen, USA) for 30 min and then incubated with specific antibodies for another 30 min at 4°C in the dark. All samples were analyzed with a flow cytometer and the data were analyzed with FlowJo software. The following antibodies were used: APC/Cyanine7-conjugated anti-mouse CD45 (BioLegend, USA), APC-conjugated anti-mouse CD3 (BioLegend), PerCP/Cyanine5.5-conjugated anti-mouse CD4 (BioLegend), Brilliant Violet 570-conjugated anti-mouse CD8a (BioLegend), Alexa Fluor 488-conjugated anti-mouse IFN-γ (BioLegend), Alexa Fluor 700-conjugated anti-human/mouse Granzyme B recombinant (BioLegend), BV650-conjugated anti-mouse CD366 (TIM-3) (BioLegend), Brilliant Violet 421-conjugated anti-mouse CD279 (PD-1) (BioLegend), PE/Cyanine 7-conjugated anti-mouse CD223 (LAG-3) (BioLegend).
Cytotoxicity assay
Cells were seeded into 96-well plates at 2,000 cells/well and cultured for 0, 24, 48, or 72 hours. Cell viability was measured using a Cell Counting Kit-8 assay (#B34302, Selleck, USA) according to the manufacturer’s instructions. The fluorescence in each plate was measured using a spectrophotometer at an emission wavelength of 450 nm (Beckman, USA).
Colony formation assay
Tumor cells were inoculated into 6-well plates, and after adhesion, different concentrations of drugs were added. After 48 hours of treatment, the medium was changed and the culture lasted for 1–2 weeks. The culture was terminated when visible clones appeared on the bottom of the plates. After washing with phosphate-buffered saline (PBS), the plates were fixed with 4% paraformaldehyde for 15 min and then stained with crystal violet for 20 min. The plates were washed with PBS, allowed to dry naturally, and then photographed and the colonies were counted.
Cell cycle and cell apoptosis
Cell cycle analysis was performed by flow cytometry using a PI/RNase Staining Buffer (BD Pharmingen, USA). The cell cycle distribution was assessed by FlowJo. Apoptosis was assessed with an FITC Annexin V Apoptosis Detection Kit I (BD Pharmingen) by flow cytometry.
qRT-PCR
RNA was extracted with MagZol Reagent (Magen, China). Complementary DNA (cDNA) was generated using HiScript QRT SuperMix (Yeasen Biotechnology, China). Real-time PCR was performed with SYBRGreen qPCR Master Mix (Biake, USA). The PCR primers used are listed in online supplemental table S1.
jitc-2023-008554supp001.pdf (552.2KB, pdf)
Protein preparation and immunoblotting
Cells were lysed in RIPA buffer, and protein concentrations were determined by a BCA Protein Assay Kit (Jiangsu Cowin Biotech, China). A total of 30–50 µg of protein was separated via sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electroblotted onto polyvinylidene fluoride membranes (Millipore, Billerica, Massachusetts, USA). Immunoreactivity was determined by using an imaging system (Bio-Rad, USA).
Antibodies specific for the following proteins were used: rabbit monoclonal anti-phospho-STAT1 (1:1,000; Cell Signaling Technology, USA), rabbit monoclonal anti-IRF1 (1:1,000; Proteintech, USA), mouse monoclonal anti-LPCAT3 (1:1,000; Proteintech), anti-β-tubulin (1:5,000; Proteintech) and anti-GAPDH (1:5,000; Proteintech).
Plasmid and lentiviral vector construction
Lentiviral plasmids containing short hairpin sh-Mock or sh-LPCAT3 (mouse) were purchased from Beijing SyngenTech. First, 293T cells were first transfected with TurboFect transfection reagent (Thermo Scientific, USA) for 18 hours. To generate stable shRNA-expressing cell lines, lentiviruses were used to infect melanoma cells supplemented with polybrene (10 µg/mL). Next, puromycin (2 µg/mL) in a complete medium was used for selection. The knockdown efficiency was quantified by real-time PCR and western blotting.
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) assays were performed using the SimpleChIP Enzymatic Chromatin IP Kit (#9003; CST, USA) according to the manufacturer’s instructions. Sonicated samples were immunoprecipitated with antibodies against IRF1 (#8478; CST, USA) and an IgG control (#2729; CST). After the reversal of the protein-DNA cross-links, the DNA was purified using DNA purification spin columns, and ChIP-enriched chromatin was subjected to real-time PCR. Relative expression levels were normalized to input. The PCR primers used are listed in online supplemental table S1.
Lipid peroxidation
Tumor cells were inoculated into 6-well plates and treated with drugs for 2 hours according to the experimental groups. The BODIPY 581/591-C11 lipid peroxidation fluorescent probe (10 µM; Invitrogen, USA) was added. After the cells were incubated at 37°C for 30 min, flow cytometry analysis was performed. For BODIPY 581/591 C11 staining, the signals from both non-oxidized C11 (the phycoerythrin (PE) channel) and oxidized C11 (the fluorescein isothiocyanate (FITC) channel) were monitored. The ratio of the mean fluorescence intensity (MFI) of FITC to the MFI of PE was calculated for each sample. The data were normalized to control samples.
ROS detection
Tumor cells were inoculated in 6-well plates and treated with drugs for 6 hour according to the experimental groups. The fluorescent probe DCFH-DA (10 µM; Solarbio, China) was diluted in serum-free medium. After incubation at 37°C for 30 min, FITC signals were monitored via flow cytometry.
In vitro T-cell killing assays
Human peripheral blood lymphocytes were extracted with a lymphocyte isolation solution (Sigma, USA). Untouched human T cells from peripheral blood mononuclear cells were isolated with Dynabeads Untouched Human T Cells (Invitrogen, USA) by depleting B cells, natural killer cells, monocytes, platelets, dendritic cells, granulocytes and erythrocytes. Isolated T cells were then stimulated with anti-CD3 and anti-CD28 antibodies (Dynabeads Human T-Activator CD3/CD28, Gibco, USA). Tumor cells were treated with different concentrations of drugs, and the treated tumor cells were mixed with the activated T cells at a ratio of 1:2.5. Anti-PD-1 antibody and homologous control antibody (10 ng/mL) were added to the experimental group and control group, respectively. After 48 hours of treatment, the suspended T cells were removed by washing with PBS, after which the viability of the remaining tumor cells was determined.
RNA sequencing
cDNA library construction, library purification and transcriptome sequencing were performed according to instructions provided by Wuhan Huada Sequencing Company (www.genomics.org.cn, BGI, Shenzhen, China).
Statistical analyses
All the data are presented as the mean (±SD) and were analyzed with GraphPad Prism V.8. A t-test was used for comparisons between two groups. One-way analysis of variance was performed for comparisons among multiple groups. Pearson correlation coefficient was performed for correlation analysis. A p value of <0.05 was considered to indicate statistical significance.
Results
Mefloquine selectively inhibits the proliferation of melanoma and lung cancer cells
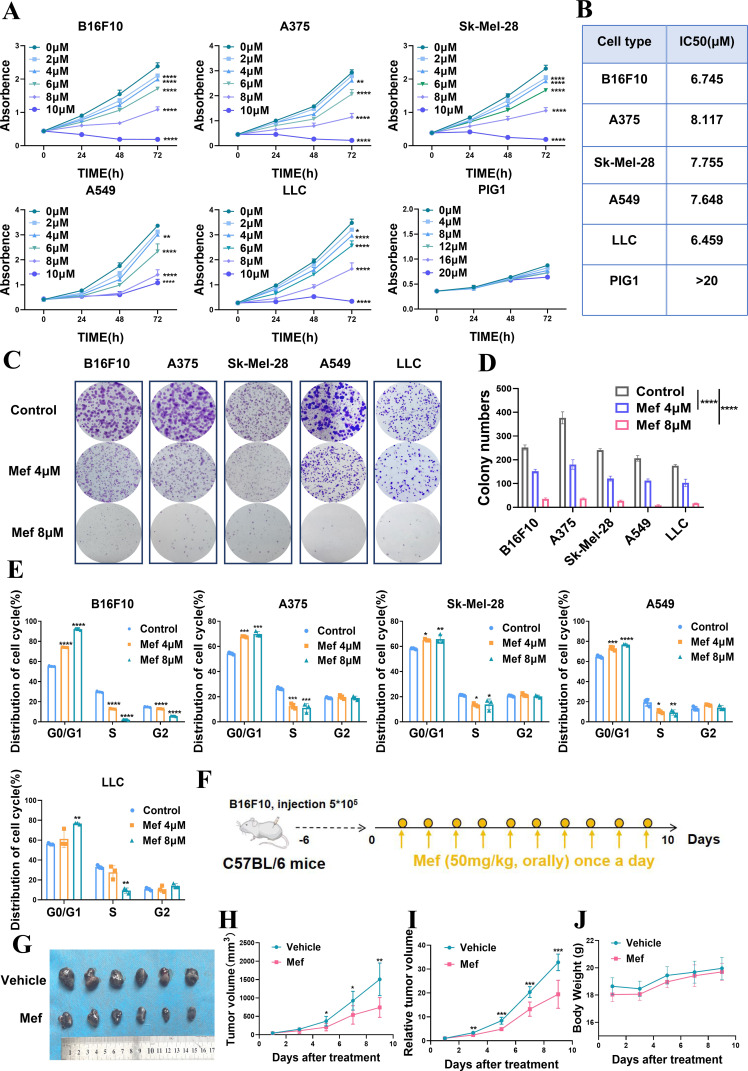
To analyze whether Mef alleviates melanoma and lung cancer, we examined the effect of Mef on the proliferation of melanoma and lung cancer cells and found that Mef could inhibit the proliferation of melanoma cells and lung cancer cells in a time-dependent and dose-dependent manner; and it did not have a significant effect on the proliferation of the human melanocyte cell line PIG1, which indicated that Mef could selectively inhibit the proliferation of tumor cells (figure 1A,B). Mef also significantly inhibited effect on the colony formation ability of melanoma and lung cancer cells (figure 1C,D). Consistent with the observed inhibition of proliferation, Mef induced G0/G1 phase arrest in melanoma and lung cancer cells (figure 1E). Moreover, the results of in vivo experiments confirmed the Mef inhibition of melanoma cell proliferation (figure 1F–J). The above results indicate that Mef inhibits melanoma and lung cancer cell growth and is a highly promising tumor therapeutic agent.
Figure 1.
Mefloquine is preferentially toxic to melanoma and lung cancer cells and impairs tumor growth. (A) The numbers of melanoma cells (A375, Sk-Mel-28, B16F10), lung cancer cells (A549, LLC) and melanocytes (PIG1) in culture supplemented with 0–20 µM Mef for 24, 48, or 72 hours were determined (n=6 replicate cultures for each cell type). (B) The IC50 values for Mef in cultures of different melanoma cell lines, lung cancer cell lines and melanocytes were determined (n=6 replicate cultures for each cell type). (C–D) A colony formation assay was used to assess melanoma and lung cancer cells after the indicated treatment (n=3 replicate cultures for each cell type). (E) Flow cytometry was used to determine the cell cycle distribution of melanoma and lung cancer cells after treatment with 0–8 µM Mef. (F–J) B16F10 tumor-bearing C57BL/6 mice were treated daily with Mef (50 mg/kg, orally) to evaluate the effects on tumor volume and weight (n=6 replicate cultures for each cell type). All the data are presented as the mean (±SD). A t-test was used for comparisons between two groups. One-way analysis of variance was performed for comparisons among multiple groups. Statistical significance was assessed according to statistical methods (*p<0.05; **p<0.01; and ***p<0.001). LLC, Lewis lung cancer; Mef, mefloquine.
Mefloquine upregulates the expression of lipid metabolism-related signals in melanoma and lung cancer
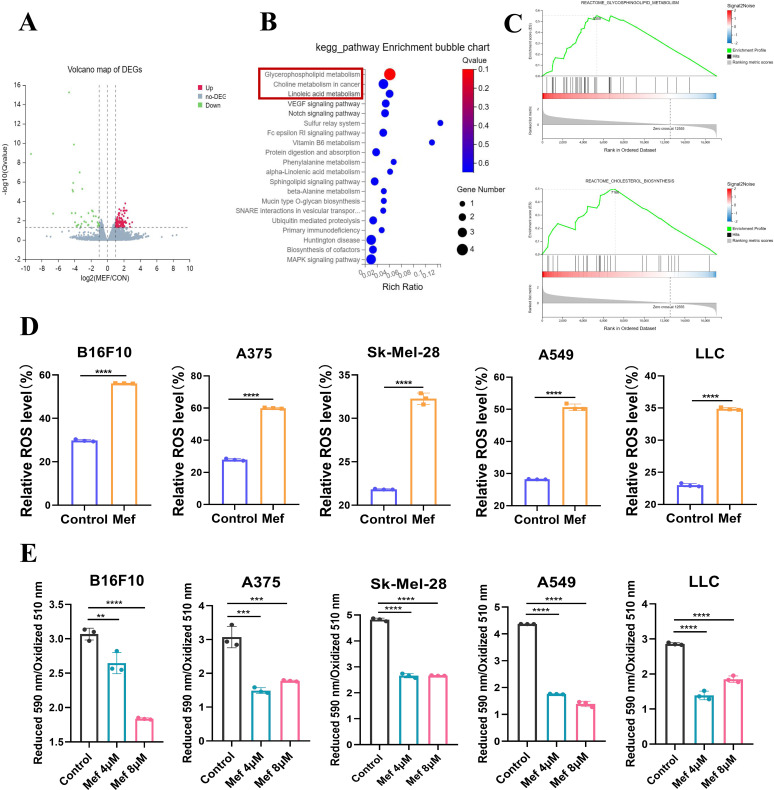
To elucidate the molecular mechanism of tumor suppression by Mef, we used RNA sequencing to sequence the transcriptome of Mef-treated melanoma tissues; the sequencing results showed that 150 genes had upregulated expression and 75 genes had downregulated expression in the Mef-treated group (figure 2A). Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Set Enrichment Analysis (GSEA) signaling enrichment analyses indicated that the 150 genes with upregulated expression were enriched in lipid metabolism-related signaling after Mef treatment (figure 2B,C). Altered lipid metabolism is closely associated with lipid peroxidation-induced ferroptosis in tumor cells,15 and flow cytometry results revealed that Mef did not significantly affect apoptosis by melanoma or lung cancer cells (online supplemental figure S1A). Therefore, we speculated that Mef might induce ferroptosis in melanoma and lung cancer cells by activating intracellular ROS-related lipid peroxidation. Consistent with our hypothesis, Mef significantly increased intracellular ROS levels and lipid peroxidation in melanoma and lung cancer cells (figure 2D,E).
Figure 2.
Mefloquine upregulates the expression of lipid metabolism-related signaling molecules in tumor cells. (A) The volcano plot shows the DEGs after Mef treatment. (B) KEGG enrichment analysis was performed to determine the signaling pathways that were enriched in upregulated DEGs in tumor tissues after Mef treatment. (C) GSEA enrichment analysis was performed to determine the signaling pathways that were enriched in upregulated DEGs in tumor tissues after Mef treatment. (D) Flow cytometric analysis of relative DCFH-DA fluorescence was performed to measure ROS levels in melanoma and lung cancer cells treated with Mef (4 µM) for 6 hours (n=3 replicate cultures for each cell type). (E) Flow cytometric analysis of relative BODIPY 581/591 C11 fluorescence was performed to measure lipid peroxidation levels in melanoma and lung cancer cells treated with Mef for 2 hours. (n=3 replicate cultures for each cell type). All the data are presented as the mean (±SD). A t-test was used for comparisons between two groups. One-way analysis of variance was performed for comparisons among multiple groups. Statistical significance was assessed according to statistical methods (*p<0.05; **p<0.01; and ***p<0.001). DEG,Differentially Expressed Genes; GSEA, Gene Set Enrichment Analysis; KEGG, Kyoto Encyclopedia of Genes and Genomes; LLC, Lewis lung cancer; Mef, mefloquine; ROS, Reactive oxygen species.
Mefloquine in combination with IFN-γ significantly promoted ferroptosis in melanoma and lung cancer cells
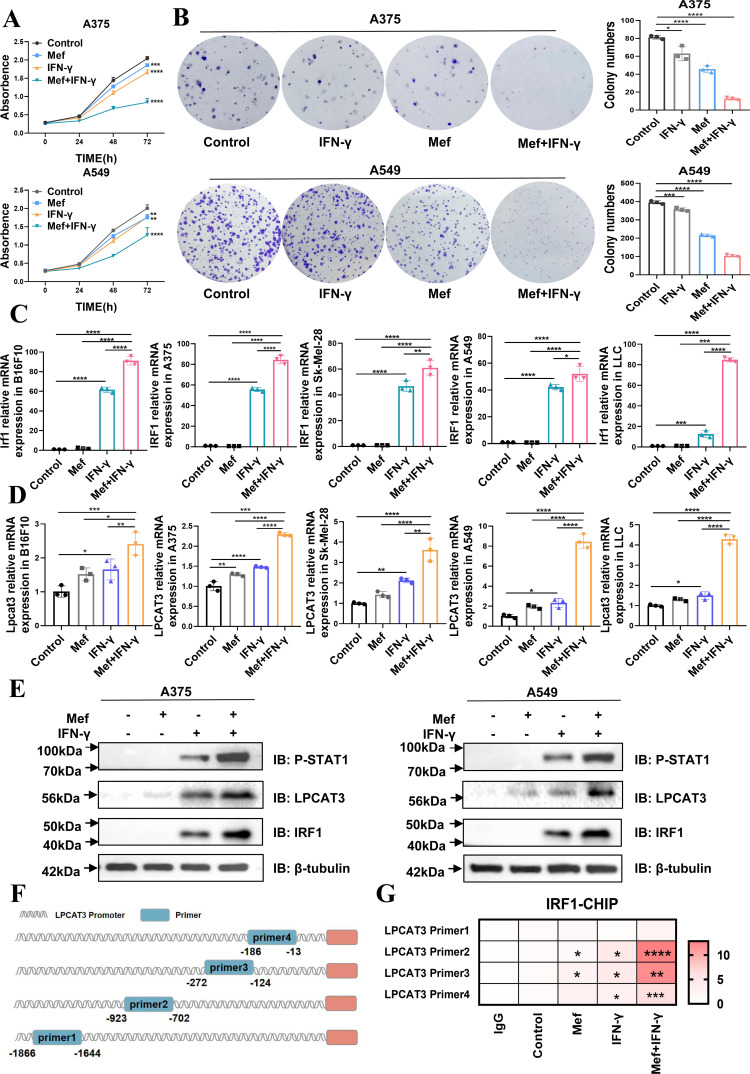
T-cell-derived IFN-γ induces cell ferroptosis; however, the underlying mechanism has not been fully elucidated.16 In our study, we found that Mef enhanced the killing of melanoma and lung cancer cells by T cells (online supplemental figure S2A). Therefore, we speculated that Mef in combination with IFN-γ would enhance lipid peroxidation in melanoma and lung cancer cells and tumor killing by T cells. To investigate our hypothesis, we examined the survival of Mef+IFN-γ-treated tumor cells, and found that melanoma and lung cancer cell proliferation and colony formation were significantly inhibited in the Mef+IFN-γ combination group (figure 3A,B); furthermore, the level of downstream IRF1 was significantly increased in the combination group (figure 3C). Moreover, Mef+IFN-γ significantly upregulated the expression of a key molecule for lipid peroxidation (LPCAT3), and had a lesser effect on another key gene (Acyl coenzyme A synthetase long-chain family member 4 (ACSL4)) (figure 3D,E, online supplemental figure S2B). Additionally, it was shown that arachidonic acid lipoxygenase (ALOX5/15) is known to directly oxidize polyunsaturated fatty acids (PUFAs) in biofilms to mediate ferroptosis.17 18 However, our results showed that Mef+IFN-γ did not affect the expression of ALOX5/15 (online supplemental figure S2C, D), indicating that Mef+IFN-γ affects lipid peroxidation and induces ferroptosis in melanoma and lung cancer cells via LPCAT3. Mechanistically, we found that Mef significantly enhanced the expression of the IFN-γ downstream signaling factors p-STAT1 and IRF1 expression (figure 3E). Therefore, we speculated that Mef enhances tumor cell ferroptosis by activating IFN-γ-STAT1-IRF1-LPCAT3 signaling. We further used the JASPRA website to predict the binding sites for the transcription factor IRF1 on the LPCAT3 promoter and designed four pairs of primers covering the predicted binding sites (figure 3F). ChIP experiments confirmed that IRF1 could directly bind to the LPCAT3 promoter region (−923~−702 bp; −272~−124 bp; and −186~−13 bp), and Mef+IFN-γ treatment enhanced the binding of IRF1 to the LPCAT3 promoter (figure 3G). The above results indicated that Mef+IFN-γ could significantly amplify the induction of ferroptosis by promoting the transcription of LPCAT3.
Figure 3.
Mefloquine in combination with IFN-γ inhibits tumor growth through the activation of Lpcat3. (A) The numbers of A375 and A549 cells in cultures supplemented with Mef (4 µM) or IFN-γ (20 ng/mL) for 24, 48, or 72 hours were determined (n=6 replicate cultures for each cell type). (B) A colony formation assay of A375 and A549 cells in cultures supplemented with Mef (4 µM) or IFN-γ (20 ng/mL) for 48 hours (n=3 replicate cultures for each cell type). (C–D) The relative mRNA expression of LPCAT3 and IRF1 in A375 and A549 cells treated for 48 hours with Mef (4 µM) or IFN-γ (20 ng/mL) was determined (n=3 replicate cultures for each cell type). (E) Immunoblots of LPCAT3, IRF1, and P-STAT1 in A375 and A549 cells after Mef (4 µM) or IFN-γ (20 ng/mL) treatment for 6 hours are shown. (F) Primers were designed for the LPCAT3 promoter region based on JASPAR predictions. (G) IRF1 binding to the promoter of LPCAT3 in A375 cells was validated by ChIP-qPCR (n=3 replicate cultures for each cell type). All the data are presented as the mean (±SD). One-way analysis of variance was performed for comparisons among multiple groups. Statistical significance was assessed according to statistical methods (*p<0.05; **p<0.01; and ***p<0.001). ChIP, chromatin immunoprecipitation; IFN, interferon; LLC, Lewis lung cancer; LPCAT3, lysophosphatidylcholine acyltransferase 3; Mef, mefloquine; mRNA, messenger RNA; qPCR, Quantitative Real-time PCR.
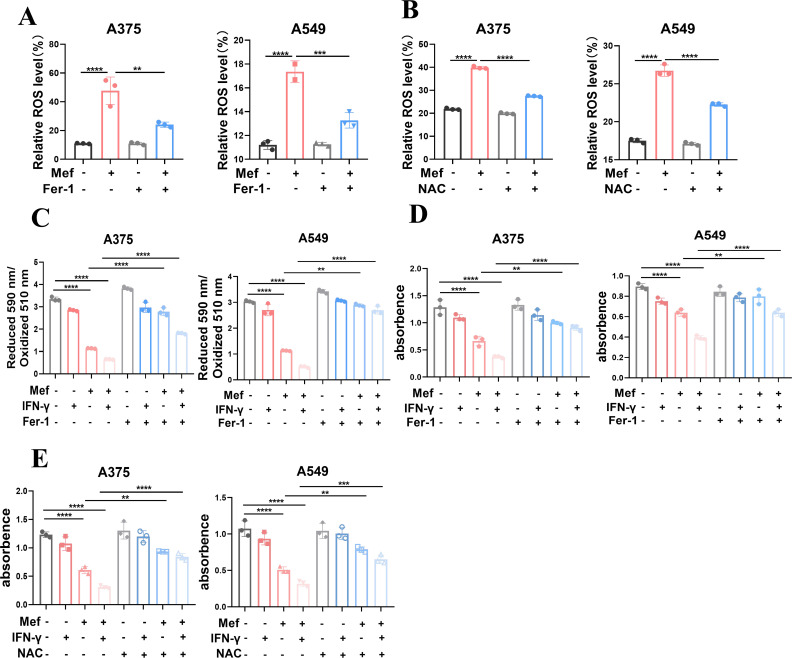
To confirm that Mef-induced cell death in melanoma and lung cancer cells occurred mainly by ferroptosis, we treated tumor cells with a ferroptosis inhibitor (ferrostatin-1, Fer-1) or an ROS inhibitor (N-acetyl-L-cysteine, NAC) and found that Fer-1 and NAC significantly inhibited Mef-induced ROS production (figure 4A,B). The Fer-1 reversed the change in lipid peroxidation levels induced by Mef+IFN-γ (figure 4C). Moreover, the Fer-1 partially reversed the tumor cell death induced by Mef+IFN-γ (figure 4D). Similarly, co-administration of NAC reduced tumor cell death (figure 4E). These results demonstrated that the combination of Mef with IFN-γ mainly led to ferroptosis in melanoma and lung cancer cells and that Mef was a potent ferroptosis inducer.
Figure 4.
Inhibitors of ROS and ferroptosis suppress mefloquine+IFN-γ-induced ferroptosis. (A) The ROS levels in A375 and A549 cells after Mef stimulation (4 µM, 6 hours) alone or in the presence of a ferroptosis inhibitor (ferrostatin-1, Fer-1; 1 µM, 6 hours) were determined (n=3 replicate cultures for each cell type). (B) The ROS levels in A375 and A549 cells after Mef stimulation (4 µM, 6 hours) alone or in the presence of an ROS inhibitor (N-acetylcysteine (NAC), 2.5 µM, 6 hours) were determined (n=3 replicate cultures for each cell type). (C) The lipid peroxidation levels in A375 and A549 cells after Mef stimulation (4 µM, 2 hours) alone or in the presence of Fer-1 (1 µM, 2 hours) were determined (n=3 replicate cultures for each cell type). (D) The numbers of A375 and A549 cells in cultures supplemented with Mef (4 µM), IFN-γ (20 ng/mL) or Fer-1 (1 µM) for 48 hours are shown (n=3 replicate cultures for each cell type). (E) The numbers of A375 and A549 cells in culture supplemented with Mef (4 µM), IFN-γ (20 ng/mL) or NAC (2.5 µM) for 48 hours are shown (n=3 replicate cultures for each cell type). NAC and Fer-1 will treat the cells 8 hours in advance before adding Mef. All the data are presented as the mean (±SD). One-way analysis of variance was performed for comparisons among multiple groups. Statistical significance was assessed according to statistical methods (*p<0.05; **p<0.01; and ***p<0.001). IFN, interferon; Mef, mefloquine; ROS, Reactive oxygen species.
Mefloquine+IFN-γ induces ferroptosis in melanoma and lung cancer cells by regulating LPCAT3
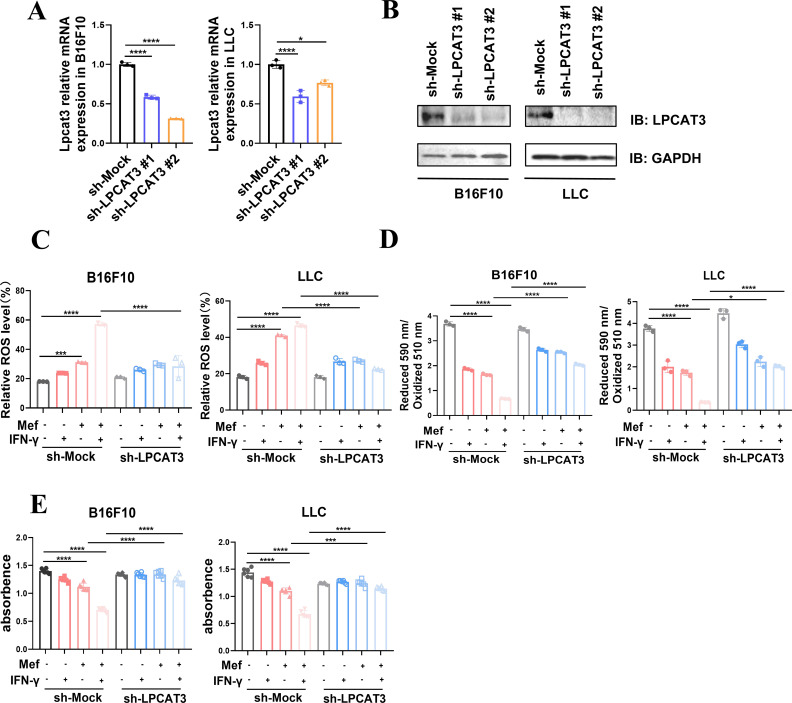
The above results demonstrated that the expression of the key gene LPCAT3, which promotes lipid peroxidation-associated ferroptosis, was significantly upregulated in the Mef+IFN-γ group (figure 3D); thus, we speculated that LPCAT3 was a key factor in Mef+IFN-γ-induced ferroptosis in melanoma and lung cancer cells. To investigate this theory, we knocked down LPCAT3 in melanoma and lung cancer cells (figure 5A,B) and found that knockdown of LPCAT3 inhibited the induction of ROS and lipid peroxidation by Mef+IFN-γ in melanoma and lung cancer cells (figure 5C,D). Moreover, the knockdown of LPCAT3 inhibited the killing of melanoma and lung cancer cells by Mef+IFN-γ (figure 5E), suggesting that Mef+IFN-γ promotes lipid peroxidation and ferroptosis in melanoma and lung cancer cells by upregulating LPCAT3 expression.
Figure 5.
Knockdown of LPCAT3 suppressed mefloquine+IFN-γ-induced ferroptosis. (A) The relative mRNA expression of LPCAT3 in B16F10 and LLC cells expressing sh-Mock or two independent sh-LPCAT3 was determined (n=3 replicate cultures for each cell type). (B) Immunoblots of LPCAT3 in B16F10 and LLC cells expressing sh-Mock or two independent sh-LPCAT3 are shown. (C) The ROS levels in B16F10 and LLC cells expressing sh-Mock or two independent sh-LPCAT3 constructs treated with Mef (4 µM) or IFN-γ (20 ng/mL) were determined (n=3 replicate cultures for each cell type). (D) The lipid peroxidation levels in B16F10 and LLC cells expressing Mock or two independent shLPCAT3 constructs treated with Mef (4 µM) or IFN-γ (20 ng/mL) were determined (n=3 replicate cultures for each cell type). (E) The numbers of B16F10 and LLC cells expressing Mock or two independent shLPCAT3 constructs treated with Mef (4 µM) or IFN-γ (20 ng/mL) (n=6 replicate cultures for each cell type) were determined. All the data are presented as the mean (±SD). One-way analysis of variance was performed for comparisons among multiple groups. Statistical significance was assessed according to statistical methods (*p<0.05; **p<0.01; and ***p<0.001). IFN, interferon; LLC, Lewis lung cancer; LPCAT3, lysophosphatidylcholine acyltransferase 3; Mef, mefloquine; mRNA, messenger RNA; ROS, Reactive oxygen species.
Mefloquine enhances the immunotherapeutic efficacy of PD-1 mAb
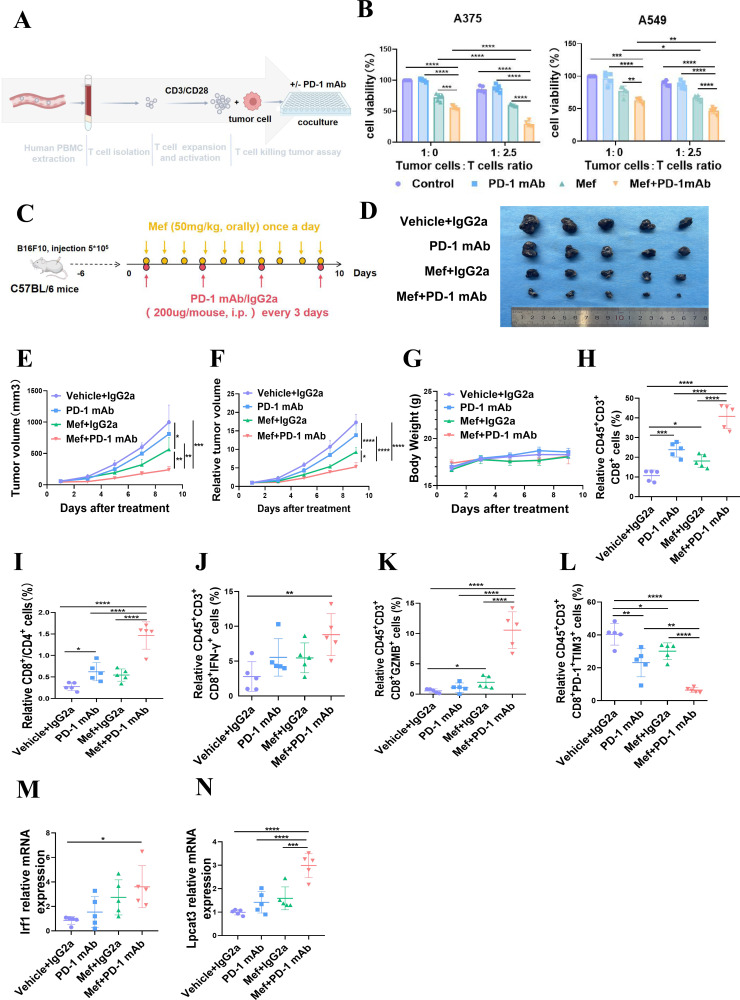
Based on the results of in vivo animal experiments shown in figure 1F-J, we found that Mef inhibited the growth of tumors from B16f10 tumor-bearing mice with intact immune systems (figure 1F–J) and enhanced the killing effect of tumor cells by T cells (online supplemental figure S2A), suggesting that the tumor-killing effect mediated by Mef is closely related to the immunity of T cells. Anti-PD-1 enhances tumor killing by increasing IFN-γ secretion from T cells; hence, we co-cultured T cells with tumor cells in vitro. The co-culture results showed that Mef significantly enhanced the killing of tumor cells by T cells, and the killing effect on melanoma and lung cancer cells was further enhanced after the addition of the anti-PD-1 (figure 6A,B). Furthermore, we validated our results in vivo and demonstrated that Mef significantly enhanced the immunotherapeutic efficacy of the anti-PD-1 without affecting the body weight of mice (figure 6C–G). Analysis of the tumor immune microenvironment using multicolor flow cytometry revealed a significant increase in the proportion of CD8+ T cells and a significant improvement in T-cell function in the combination therapy group (figure 6H–L and online supplemental figure S3A–D). As shown in figure 6J–L and online supplemental figure S3A–D, the expression of IFN-γ and granzyme B (GZMB), which are functional indicators of T cells, was significantly increased in the combination therapy group, while Mef+PD-1 mAb administration also significantly reduced the proportion of exhausted T cells. Consistent with the cellular results, LPCAT3 and IRF1 expression was significantly upregulated in tumors in the Mef+PD-1 mAb group (figure 6M,N). These results indicated that Mef enhanced the efficacy of anti-PD-1 immunotherapy by promoting T cells killing.
Figure 6.
Mefloquine enhances the efficacy of anti-PD-1 immunotherapy. (A) A schematic diagram of in vitro assays in which immune cells kill tumor cells is shown. (B) The killing of tumor cells by different proportions of patient-derived T cells treated with or without anti-PD-1 (10 ng/mL) for 48 hours was evaluated. (C) The schematic diagram shows the treatment of melanoma with Mef combined with PD-1 mAb. (D–G) B16F10 tumor-bearing C57BL/6 mice were treated with Mef (50 mg/kg, orally, daily) or IGg2a/anti-PD-1 mAb (200 µg/mouse, i.p. injection, every 3 days) to evaluate the effects on tumor volume and weight (n=5). (H–L) The Percentages of CD8+CD3+CD45+ T cells (H) CD8+/CD4+ T cells (I) IFN-γ+CD8+CD3+CD45+ T cells (J) GZMB+CD8+CD3+CD45+ T cells (K) and PD-1+TIM3+CD8+CD3+CD45+ T cells (L) in B16F10 tumors were determined (n=5). (M–N) The relative mRNA expression levels of lysophosphatidylcholine acyltransferase 3 (M) and IRF1 (N) in B16F10 tumors were determined (n=5). All the data are presented as the mean (±SD). One-way analysis of variance was performed for comparisons among multiple groups. Statistical significance was assessed according to statistical methods (*p<0.05; **p<0.01; and ***p<0.001). i.p., intraperitoneal; IFN, interferon; Mef, mefloquine; mAb, monoclonal antibody; mRNA, messenger RNA; PBMCs, peripheral blood mononuclear cells; PD-1, programmed cell death 1.
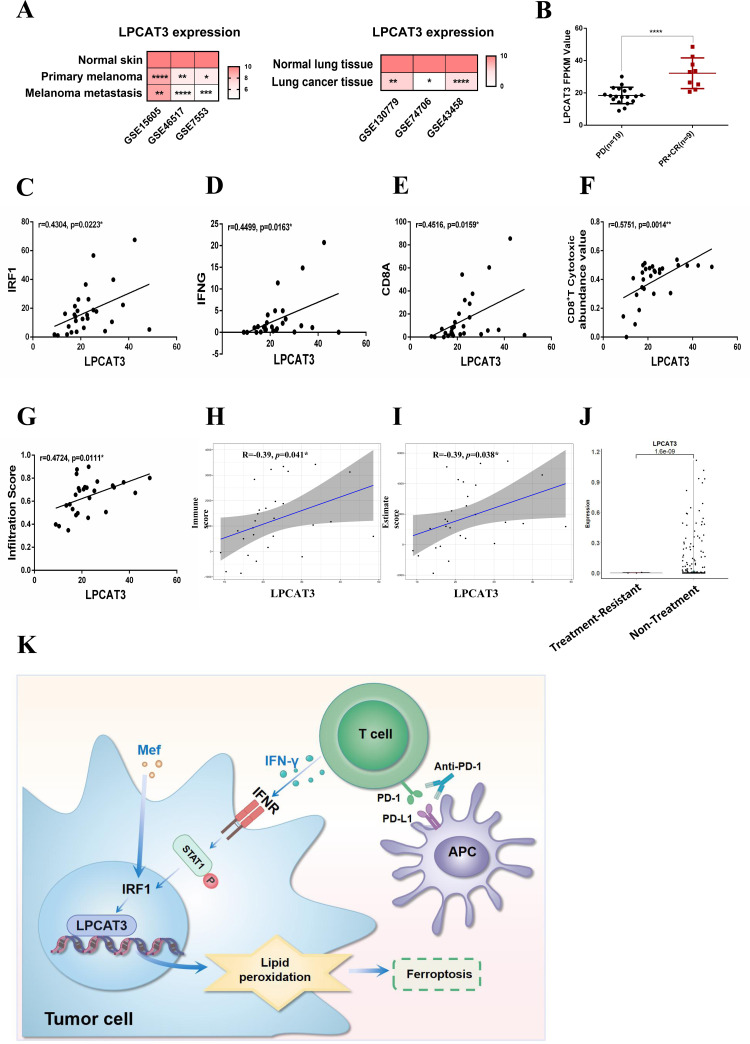
LPCAT3 expression is positively correlated with the prognosis of patients with melanoma receiving anti-PD-1 immunotherapy
The analysis of clinical data from patients with melanoma/lung cancer in the Gene Expression Omnibus (GEO) database revealed that LPCAT3 was expressed at lower levels in tumors than in normal tissues (figure 7A). Moreover, LPCAT3 had significantly lower expression in three melanoma cell lines compared with that in melanocytes (PIG1) (online supplemental figure S4A). Analysis of the immune microenvironment of samples from the three GEO data sets using ImmucellAI revealed that LPCAT3 expression was positively correlated with cytotoxic T-cell levels (online supplemental figure S4B–D). Further analysis of the clinical data of patients receiving anti-PD-1 immunotherapy revealed that LPCAT3 expression was greater in the anti-PD-1-responsive group (PR+CR; PR, partial response; CR, complete response) than in the anti-PD-1-resistant group (PD, progressive disease) (figure 7B). Consistent with the results of the in vitro experiments, the expression of LPCAT3 was proportional to that of IRF1, IFN-γ and CD8a, thus confirming that the expression of LPCAT3 was closely related to IFN-γ-IRF1 signaling (figure 7C–E). Further analysis of the immune microenvironment of anti-PD-1 antibody-treated patients using ImmucellAI revealed that LPCAT3 expression was positively correlated with the enrichment of cytotoxic CD8+ T cells in the immune microenvironment and was positively correlated with the immune cell infiltration scores (figure 7F,G). ESTIMATE analysis revealed that LPCAT3 expression was positively correlated with the immune score and ESTIMATE score (figure 7H,I), indicating that LPCAT3 was positively associated with an improved immune microenvironment. Similarly, single-cell analysis revealed that LPCAT3 expression in melanoma cells was reduced in patients with anti-PD-1 treatment-resistant melanoma (figure 7J and online supplemental figure S4E), suggesting that LPCAT3 plays an important role in the efficacy of immunotherapy against melanoma cells.
Figure 7.
LPCAT3 is associated with an improved immune microenvironment and immunotherapeutic efficacy. (A) Data from the GEO database were used to assess LPCAT3 expression in patient-derived primary melanoma, metastatic melanoma, normal skin, lung cancer tissue and normal lung tissue. (B) Data from the GEO database (GSE 91061 (PD (n=19), PR+CR (n=9))) were used to assess LPCAT3 expression in patients with anti-PD-1-treated melanoma. (C–E) Data from the GEO database (GSE91061) was used to analyze the correlation between LPCAT3 and IRF1 (C), IFN-γ (D) and CD8a (E) after anti-PD-1 therapy. (F–G) ImmuCellAI was used to analyze the correlation between LPCAT3 expression and CD8+ T-cell enrichment (F) and LPCAT3 expression and immune cell infiltration (G) in the immune microenvironment. (H–I) The ESTIMATE algorithm was used to analyze the correlation between LPCAT3 expression and immune score (H) and LPCAT3 expression and ESTIMATE score (I) in PD and PR CR patients from the GSE91061 data set. (J) Single-cell sequencing was used to analyze the expression of LPCAT3 in melanoma cells resistant to anti-PD-1 immunotherapy. (K) A diagram of the molecular mechanism described in the article is shown. All the data are presented as the mean (±SD). A t-test was used for comparisons between two groups. One-way analysis of variance was performed for comparisons among multiple groups. Pearson correlation coefficient was performed for correlation analysis. Statistical significance was assessed according to statistical methods (*p<0.05; **p<0.01; and ***p<0.001). CR, complete response; GEO, Gene Expression Omnibus; IFN, interferon; LPCAT3, lysophosphatidylcholine acyltransferase 3; Mef, mefloquine; PR, partial response; PD, progressive disease; PD-1, programmed cell death 1; PD-L1, programmed death ligand 1.
In summary, our results suggest that promoting ferroptosis in tumor cells by targeting LPCAT3 is a highly promising therapeutic modality for application. In addition, the results showed that Mef enhances ferroptosis and the therapeutic efficacy of anti-PD-1 by promoting IFN-γ-IRF1-LPCAT3 signaling, suggesting that the FDA-approved agent Mef is a promising new inducer of ferroptosis, as well as a promising new sensitizer for anti-PD-1 immunotherapy.
Discussion
Research on the potential of Mef in the treatment of tumors is emerging, and Mef inhibits the progression of many tumors through different signaling pathways.13 In prostate cancer, Mef inhibits tumor progression by activating the ROS-mitogen-activated protein kinase/AMP-activated protein kinase (MAPK/AMPK) signaling pathway.14 In gastric cancer and cervical cancer, Mef induces apoptosis by inhibiting PI3K-Akt signaling and reduces mitochondrial respiration and function by inhibiting the mammalian target of rapamycin (mTOR) pathway, which ultimately triggers tumor cell apoptosis.19 Mef also stimulates apoptosis in breast and colorectal cancer cells by inhibiting autophagy20 21; mechanistically, Mef prevents mitochondrial autophagic degradation in colorectal cancer stem cells (CSCs) through the inhibition of RAB5/7, LAMP1/2, and PINK1/PARKIN, which induces tumor cell apoptosis.20 The vast majority of existing studies have shown that Mef causes tumor cell death by apoptosis, whereas in melanoma and lung cancer, we found that the proportion of Mef-induced apoptotic cells was less than 6%, and Mef-induced apoptosis was not the main form of cell death in melanoma/lung cancer. Mef-induced ferroptosis was the main form of death in melanoma and lung cancer cells. Our results provide evidence of a new mechanism of Mef-induced cell death and provide a new theoretical basis for the use of Mef in melanoma and lung cancer treatment.
Ferroptosis is coregulated by lipid metabolism, ROS and iron ions.22 Of these, the peroxidation of specific lipid components is the central driving mechanism of ferroptosis.15 ACSL4 and LPCAT3 are two key enzymes that are involved in the core driving mechanism of ferroptosis.15 23 ACSL4 catalyzes the ligation of long-chain PUFAs to coenzyme A, and LPCAT3 facilitates the esterification and incorporation of these products into membrane phospholipids, which consequently activates ferroptosis.16 24 One report showed that ACSL4 promotes T-cell tumor immunity,16 and in our study, we identified an important role for LPCAT3 in promoting T-cell immunotherapy efficacy in melanoma and lung cancer. We revealed that Mef significantly enhanced IFN-γ-STAT1-IRF1-induced LPCAT3 expression but limited the induction of ACSL4, thus demonstrating that Mef+IFN-γ induces ferroptosis in tumor cells and sensitizes tumors to T-cell killing mainly through the upregulation of LPCAT3 expression.
LPCAT3, also known as MBOAT5, belongs to the O-acyltransferase (MBOAT) family that regulates cellular ferroptosis by modulating the abundance of polyunsaturated phospholipids in the cell membrane.25 26 The transcription factors liver X receptor,27 Peroxisome Proliferator Activated Receptor α (PPARα) 28 and PPARδ29 have been reported to directly regulate LPCAT3 transcription in non-tumorigenic diseases. In lung adenocarcinoma cells, the E1A-binding protein p300 (EP300) binds to both YAP and ZEB and induces LPCAT3 transcription via H3K27Ac, which promotes lipid peroxidation-associated ferroptosis in tumor cells.26 However, the regulatory mechanism of LPCAT3 in tumors has rarely been studied, and additional studies will be needed to elucidate the molecular mechanisms that regulate LPCAT3. In our study, a novel molecular regulatory mechanism of LPCAT3 was identified. Our results demonstrated that Mef enhanced IFN-γ-STAT1-IRF1-induced LPCAT3 expression in melanoma and lung cancer. Additionally, the results of clinical data analysis showed that LPCAT3 expression was elevated in patients with melanoma and was positively correlated with a better immune microenvironment and anti-PD-1 immunotherapy efficacy, suggesting that LPCAT3 may serve as a target to predict anti-PD-1 immunotherapy efficacy.
Conclusions
Taken together, our study revealed that Mef promotes tumor cell ferroptosis and sensitizes tumors to anti-PD-1 immunotherapy by increasing the IFN-γ-STAT1-IRF1-induced LPCAT3 (figure 7K). We elucidated the novel molecular mechanism by which the FDA-approved Mef sensitizes cells to anti-PD-1 immunotherapy by modulating ferroptosis, thus providing a new therapeutic strategy for tumor immunotherapy.
Footnotes
QT and NL contributed equally.
Contributors: QT and NL carried out in vitro and in vivo animal experiments. QT and JC analyzed the sequencing data and performed statistical analysis. QT, NL and JW obtained and/or analyzed human data. XC and CP supervised the study. NL and CP conceptualized the study and wrote the manuscript. CP is responsible for the overall content of the paper as a guarantor. All authors read and approved the final manuscript.
Funding: This work was supported by National Natural Science Foundation of China, Grant No. 82073458, 82203024, 82221002, and by National Natural Science Foundation of Hunan Province, Grant No. 2023JJ40918, by the science and technology innovation Program of Hunan Province (2021RC4013), the Program of Introducing Talents of Discipline to Universities (111 Project, No. B20017).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The data sets analyzed during the current study are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
All experiments were approved by the Ethics Committee of Xiangya Hospital (Central South University, China, Animal ethics code: 2022020130).
References
- 1. Yang WS, SriRamaratnam R, Welsch ME, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014;156:317–31. 10.1016/j.cell.2013.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roh J-L, Kim EH, Jang HJ, et al. Induction of ferroptotic cell death for overcoming cisplatin resistance of head and neck cancer. Cancer Lett 2016;381:96–103. 10.1016/j.canlet.2016.07.035 [DOI] [PubMed] [Google Scholar]
- 3. Markowitsch SD, Schupp P, Lauckner J, et al. Artesunate inhibits growth of Sunitinib-resistant renal cell carcinoma cells through cell cycle arrest and induction of ferroptosis. Cancers (Basel) 2020;12:12. 10.3390/cancers12113150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen P, Li X, Zhang R, et al. Combinative treatment of Β-Elemene and Cetuximab is sensitive to KRAS mutant colorectal cancer cells by inducing ferroptosis and inhibiting epithelial-mesenchymal transformation. Theranostics 2020;10:5107–19. 10.7150/thno.44705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang C, Liu X, Jin S, et al. Ferroptosis in cancer therapy: a novel approach to reversing drug resistance. Mol Cancer 2022;21:47. 10.1186/s12943-022-01530-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Du J, Wang X, Li Y, et al. DHA exhibits synergistic therapeutic efficacy with cisplatin to induce ferroptosis in pancreatic ductal adenocarcinoma via modulation of iron metabolism. Cell Death Dis 2021;12:705. 10.1038/s41419-021-03996-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang W, Green M, Choi JE, et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 2019;569:270–4. 10.1038/s41586-019-1170-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Luo X, Gong H-B, Gao H-Y, et al. Oxygenated phosphatidylethanolamine navigates phagocytosis of ferroptotic cells by interacting with TLR2. Cell Death Differ 2021;28:1971–89. 10.1038/s41418-020-00719-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tang D, Kepp O, Kroemer G. Ferroptosis becomes immunogenic: implications for anticancer treatments. Oncoimmunology 2020;10:1862949. 10.1080/2162402X.2020.1862949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jiang Z, Lim S-O, Yan M, et al. TYRO3 induces anti-PD-1/PD-L1 therapy resistance by limiting innate immunity and tumoral ferroptosis. J Clin Invest 2021;131:e139434. 10.1172/JCI139434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Trenholme CM, Williams RL, Desjardins RE, et al. Mefloquine (WR 142,490) in the treatment of human malaria. Science 1975;190:792–4. 10.1126/science.1105787 [DOI] [PubMed] [Google Scholar]
- 12. Wong W, Bai X-C, Sleebs BE, et al. Mefloquine targets the plasmodium falciparum 80s ribosome to inhibit protein synthesis. Nat Microbiol 2017;2:17031. 10.1038/nmicrobiol.2017.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mudassar F, Shen H, O’Neill G, et al. Targeting tumor hypoxia and mitochondrial metabolism with anti-parasitic drugs to improve radiation response in high-grade gliomas. J Exp Clin Cancer Res 2020;39:208. 10.1186/s13046-020-01724-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yan K-H, Yao C-J, Hsiao C-H, et al. Mefloquine exerts anticancer activity in prostate cancer cells via ROS-mediated modulation of AKT, ERK, JNK and AMPK signaling. Oncol Lett 2013;5:1541–5. 10.3892/ol.2013.1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol 2021;22:266–82. 10.1038/s41580-020-00324-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liao P, Wang W, Wang W, et al. CD8(+) T cells and fatty acids orchestrate tumor ferroptosis and immunity via ACSL4. Cancer Cell 2022;40:365–78. 10.1016/j.ccell.2022.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kuhn H, Banthiya S, van Leyen K. Mammalian lipoxygenases and their biological relevance. Biochim Biophys Acta 2015;1851:308–30. 10.1016/j.bbalip.2014.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li K, Wang M, Huang Z-H, et al. ALOX5 inhibition protects against dopaminergic neurons undergoing ferroptosis. Pharmacol Res 2023;193:106779. 10.1016/j.phrs.2023.106779 [DOI] [PubMed] [Google Scholar]
- 19. Liu Y, Chen S, Xue R, et al. Mefloquine effectively targets gastric cancer cells through phosphatase-dependent inhibition of PI3K/AKT/mTOR signaling pathway. Biochem Biophys Res Commun 2016;470:350–5. 10.1016/j.bbrc.2016.01.046 [DOI] [PubMed] [Google Scholar]
- 20. Takeda M, Koseki J, Takahashi H, et al. Disruption of endolysosomal RAB5/7 efficiently eliminates colorectal cancer stem cells. Cancer Res 2019;79:1426–37. 10.1158/0008-5472.CAN-18-2192 [DOI] [PubMed] [Google Scholar]
- 21. Sharma N, Thomas S, Golden EB, et al. Inhibition of autophagy and induction of breast cancer cell death by mefloquine, an antimalarial agent. Cancer Lett 2012;326:143–54. 10.1016/j.canlet.2012.07.029 [DOI] [PubMed] [Google Scholar]
- 22. Tang D, Chen X, Kang R, et al. Ferroptosis: molecular mechanisms and health implications. Cell Res 2021;31:107–25. 10.1038/s41422-020-00441-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lei P, Bai T, Sun Y. Mechanisms of ferroptosis and relations with regulated cell death: a review. Front Physiol 2019;10:139. 10.3389/fphys.2019.00139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sha W, Hu F, Xi Y, et al. Mechanism of ferroptosis and its role in type 2 diabetes mellitus. J Diabetes Res 2021;2021:9999612. 10.1155/2021/9999612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shao G, Qian Y, Lu L, et al. Research progress in the role and mechanism of LPCAt3 in metabolic related diseases and cancer. J Cancer 2022;13:2430–9. 10.7150/jca.71619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cui J, Wang Y, Tian X, et al. LPCAT3 is transcriptionally regulated by YAP/ZEB/EP300 and collaborates with ACSL4 and YAP to determine ferroptosis sensitivity. Antioxid Redox Signal 2023;39:491–511. 10.1089/ars.2023.0237 [DOI] [PubMed] [Google Scholar]
- 27. Rong X, Albert CJ, Hong C, et al. LXRs regulate ER stress and inflammation through dynamic modulation of membrane phospholipid composition. Cell Metab 2013;18:685–97. 10.1016/j.cmet.2013.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhao Y, Chen Y-Q, Bonacci TM, et al. Identification and characterization of a major liver lysophosphatidylcholine acyltransferase. J Biol Chem 2008;283:8258–65. 10.1074/jbc.M710422200 [DOI] [PubMed] [Google Scholar]
- 29. Singh AB, Liu J. Identification of hepatic lysophosphatidylcholine acyltransferase 3 as a novel target gene regulated by peroxisome proliferator-activated receptor δ. J Biol Chem 2017;292:884–97. 10.1074/jbc.M116.743575 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2023-008554supp001.pdf (552.2KB, pdf)
Data Availability Statement
Data are available upon reasonable request. The data sets analyzed during the current study are available from the corresponding author on reasonable request.