Abstract
Objective
This post hoc analysis of the FINCH 1–3 (NCT02889796, NCT02873936 and NCT02886728) studies assessed specific effects of filgotinib on pain control and their relationship with other aspects of efficacy in patients with rheumatoid arthritis (RA).
Methods
Assessments included: residual pain responses of ≤10 and ≤20 mm on a 100 mm visual analogue scale (VAS); the proportion of patients who achieved VAS pain responses in addition to remission or low disease activity by Disease Activity Score-28 with C-reactive protein (DAS28-CRP) or Clinical Disease Activity Index (CDAI) criteria.
Results
Across studies, filgotinib reduced pain from week 2, with responses sustained throughout the studies. In FINCH 1, at week 24, 35.8%, 25.0%, 24.6% and 11.6% of patients in the filgotinib 200 mg, filgotinib 100 mg, adalimumab and placebo arms (each plus methotrexate) achieved VAS pain ≤20 mm in addition to DAS28-CRP remission; 26.3%, 17.9%, 17.2% and 7.6% achieved VAS pain ≤10 mm in addition to DAS28-CRP remission. A similar pattern was seen for CDAI remission. Time during which VAS pain was ≤10 or ≤20 mm was longest with filgotinib 200 mg and comparable between adalimumab and filgotinib 100 mg. Similar findings were reported for filgotinib in FINCH 2 and 3.
Conclusion
In all RA populations studied, pain improvements occurred from week 2 and were sustained over time. In FINCH 1, filgotinib 100 mg provided similar pain amelioration to adalimumab, whereas filgotinib 200 mg resulted in greater pain improvement and higher proportion of patients with residual pain ≤10 or ≤20 mm and meeting DAS28-CRP remission criteria.
Keywords: Antirheumatic Agents; Arthritis, Rheumatoid; Patient Reported Outcome Measures
WHAT IS ALREADY KNOWN ON THIS TOPIC
Patients with active rheumatoid arthritis (RA) commonly experience substantial pain and consider pain control to be an important treatment goal.
WHAT THIS STUDY ADDS
This study provides further information on filgotinib, a Janus kinase (JAK1)-preferential inhibitor, in patients with RA who were treatment naïve or experienced an inadequate response to methotrexate or biological disease-modifying antirheumatic drugs; significantly greater pain reductions were seen with filgotinib versus placebo, adalimumab and methotrexate from as early as week 2, and the time to a 30%, 50%, 70% or 90% pain reduction was generally shorter with filgotinib.
Daily filgotinib 200 mg with methotrexate resulted in a higher proportion of patients achieving low residual pain (≤10 or ≤20 mm) in addition to Disease Activity Score-28 with C-reactive protein (and to a lesser extent Clinical Disease Activity Index) remission than observed in comparator treatment arms.
Over the 52-week study period, patients who received filgotinib 200 mg with methotrexate experienced an additional 3 weeks during which visual analogue scale pain score was ≤10 mm, compared with those on adalimumab with methotrexate; the effects of filgotinib 100 mg plus methotrexate on pain were similar to those seen in the adalimumab plus methotrexate group.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
As recommended for RA clinical trials, it is valuable to assess both disease activity and pain in patients with RA, and extend treat-to-target approaches to include pain or consider integrating other pain management strategies.
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterised by inflammation of the joints, which may result in joint damage and functional disability.1 RA is also often associated with substantial pain that may have a considerable impact on patients and reduce their quality of life.1–3 Pain is considered by patients with RA to be an important symptom and a key target for RA treatment;3–5 it is the most frequently reported initial RA symptom and the most common reason for patients with RA to seek medical attention.6 Multiple mechanisms may contribute to the pain experienced by patients with RA. Pain may be directly connected to disease activity; however, non-inflammatory pathways such as peripheral and central sensitisation may also be involved.4 7 Consequently, patients may continue to experience a significant pain burden even when inflammation is controlled, as assessed by composite scores of disease activity meeting criteria for remission or low disease activity with RA treatment.8 This may even be the case in patients with early RA who have achieved optimal disease control according to treatment guidelines.9 One should consider evaluating pain in addition to disease activity to better understand the patient’s disease burden, as recommended for RA clinical trials.10–13 While strong analgesics may be prescribed to treat pain, opioids are often associated with an unfavourable risk–benefit ratio.14 Ideal RA medications would allow disease activity targets to be achieved, as well as having an added benefit with regards to patient-reported pain.
Many cytokines implicated in both inflammatory and non-inflammatory pain transmission are dependent on Janus kinase (JAK)/signal transducer and activator of transcription signalling.15–17 JAK inhibitors have been found to positively affect pain outcomes compared with placebo or active comparators in different populations of patients with RA. For example, patients with RA with an inadequate response to methotrexate who were treated with the JAK1 and JAK2 inhibitor baricitinib were more likely to achieve relative pain reductions of ≥30%, ≥50% and ≥70% and absolute pain responses of ≤20 or ≤40 mm than those treated with adalimumab or placebo.18 Similarly, in patients with RA with no or limited prior treatment with disease-modifying antirheumatic drugs (DMARDs), baricitinib monotherapy or in combination with methotrexate led to greater and more rapid reductions in pain compared with methotrexate alone.19 Tofacitinib also led to significant decreases in pain compared with placebo (both given with background methotrexate), with a significantly greater proportion of tofacitinib-treated patients achieving a ≥10 mm improvement from baseline in pain.20 In patients with an inadequate response to conventional synthetic DMARDs, upadacitinib, a selective JAK1 inhibitor, led to significant changes from baseline in pain compared with placebo.21 Upadacitinib was also associated with significantly greater improvements from baseline in pain at week 12, compared with adalimumab, in patients with inadequate responses to biologic DMARDs (bDMARDs).22 In each of these examples, the superiority of the JAK inhibitor over placebo or active comparator was based on patient-reported pain, which was assessed using a visual analogue scale (VAS).
Filgotinib is a JAK1 inhibitor approved for the treatment of moderate to severe RA. In the phase 3 FINCH 1–3 studies, filgotinib reduced the signs and symptoms of RA and demonstrated an acceptable safety profile.23–25 The aim of this post hoc analysis of the FINCH 1–3 studies was to assess specific effects of filgotinib on pain control and to explore the relationship between efficacy and pain response.
Methods
Study design
Details of the FINCH studies have been reported previously.23–25 In brief, FINCH 1, 2 and 3 (NCT02889796, NCT02873936 and NCT02886728, respectively) were phase 3, randomised, double-blind trials of filgotinib 100 mg or 200 mg conducted in patients who had an inadequate response to methotrexate (FINCH 1), patients who had an inadequate response to bDMARDs (FINCH 2) or patients who were methotrexate naïve (FINCH 3).23–25 Each study enrolled patients aged ≥18 years with active moderate to severe RA (defined as ≥6 swollen joints and ≥6 tender joints).23–25
All studies were conducted in accordance with the Declaration of Helsinki and were approved by the appropriate institutional review board or ethics committee; all patients provided written informed consent.23–25
Pain assessments
Patients reported pain on a VAS, with responses ranging from 0 mm (no pain) to 100 mm (worst possible pain). Both absolute pain scores and relative reductions in pain score were assessed. Absolute scores of ≤10 mm reflected limited to no pain; scores of ≤20 mm indicated that health status was not negatively affected by pain.18 A relative reduction of 30% in VAS pain score was considered a much improved, meaningful difference, while a reduction of 50% reflected very much improved, substantial improvement.18 Exploratory thresholds of ≥70% and ≥90% relative reduction from baseline were also assessed. In each study, VAS pain score was evaluated at baseline and at weeks 2, 4, 8, 12, 14, 16, 20 and 24; in FINCH 1 and 3, VAS pain score was also evaluated at weeks 30, 36, 44 and 52. Baseline characteristics were assessed in patients according to their pain response by the end of the study (VAS pain score of ≤10, >10 to ≤20 mm and >20 mm). Change from baseline in VAS pain score over time and time to first VAS pain response (absolute VAS pain score of ≤10 or ≤20 mm, or a relative reduction from baseline of ≥30%, ≥50%, ≥70% or ≥90%) were reported. Duration of threshold pain responses achieved over the observation period was evaluated (the mean number of weeks and the mean proportion of the study period during which patients had a VAS pain score of ≤10 or ≤20 mm). The proportion of patients who achieved remission (predefined as Disease Activity Score-28 with C-reactive protein (DAS28-CRP) <2.6 or Clinical Disease Activity Index (CDAI) ≤2.8) or low disease activity (predefined as DAS28-CRP ≥2.6 to ≤3.2 or CDAI >2.8 to ≤10) at week 24 was calculated. Of patients who achieved remission or low disease activity by DAS28-CRP or CDAI, the proportion who also had a VAS pain score of ≤10 or ≤20 mm was determined.
Statistical analysis
The full analysis set included patients who were randomised and received at least one dose of study drug. Differences in change from baseline for each filgotinib arm versus placebo (FINCH 1 and FINCH 2), adalimumab (FINCH 1) or methotrexate (FINCH 3) were assessed using a mixed-effects model for repeated measures, which included treatment, visit, treatment by visit, stratification factors and baseline value as fixed effects, and patients as the random effect. Least-squares (LS) mean, 95% CI and p value were obtained from the model.
Kaplan–Meier-estimated times to achieve absolute VAS pain score of ≤10 or ≤20 mm, or a relative improvement from baseline of ≥30%, ≥50%, ≥70% or ≥90%, were calculated for each study. HRs were used to compare time to achieve pain responses in the filgotinib arms versus active comparator or placebo arms. HRs were generated from a Cox regression model, stratified by geographic region and anticyclic citrullinated peptide (anti-CCP) or rheumatoid factor (RF) status at screening (and for FINCH 1 and 2, prior exposure to bDMARDs); p values were calculated from a log-rank test with the same stratification factors. Single-variable and multivariable logistic regression analyses with pairwise comparisons were performed to identify predictors of pain response (VAS pain score of ≤10 or ≤20 mm). The single-variable model included treatment group, study, baseline VAS pain score and one additional predictor (either age, anti-CCP or RF positivity, Body Mass Index (BMI), CRP level, CDAI, concurrent oral corticosteroids, DAS28-CRP, duration of RA, ethnicity, Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue score, Health Assessment Questionnaire–Disability Index (HAQ-DI), patient global VAS score, physician global VAS score, prior exposure to bDMARDs, race, ratio of swollen joint count/tender joint count based on 28 joints (SJC28/TJC28), region, Simple Disease Activity Index (SDAI), 36-item short-form health survey (SF-36) mental component summary score (MCS), SF-36 physical component summary score (PCS), sex, smoking status, SJC28 and TJC28). The multivariable model included treatment group, study, baseline pain VAS score and the following additional predictors, which were selected from the single-variable model: anti-CCP or RF positivity, BMI, CDAI, concurrent oral corticosteroids, DAS28-CRP, duration of RA, ethnicity, FACIT-Fatigue score, HAQ-DI, VAS pain score, patient global VAS score, race, region, SDAI, SF-36 MCS, SF-36 PCS, sex, smoking status, TJC28 and treatment. Comparisons were not adjusted for multiplicity; p values are nominal and should be interpreted as exploratory. Each study was assessed separately; for the single-variable and multivariable analyses, pooled data from FINCH 1, 2 and 3 were also assessed.
Results
Baseline characteristics
In each FINCH study, patient characteristics were similar across treatment arms, with mean duration of RA ranging from 1.9 years (in FINCH 3) to 12.6 years (in FINCH 2).23–25 In FINCH 1, 2 and 3, mean baseline VAS pain scores across the treatment arms were 64–66, 66–68 and 64–67 mm out of 100 mm, respectively. Baseline characteristics of patients according to their pain response are shown in online supplemental tables 1–3. In general, patients who achieved the lowest residual VAS pain had numerically lower patient or physician global VAS scores at baseline, although differences between groups were small. For example, in patients who achieved VAS pain scores of ≤10, >10 to ≤20 and >20 mm in FINCH 1, mean (standard deviation (SD)) patient global VAS score at baseline was 60.6 (21.7), 65.8 (18.9) and 72.0 (15.3), respectively (online supplemental table 1).
rmdopen-2023-003839supp002.pdf (169.7KB, pdf)
rmdopen-2023-003839supp001.pdf (4.9MB, pdf)
LS mean change from baseline in VAS pain score over time
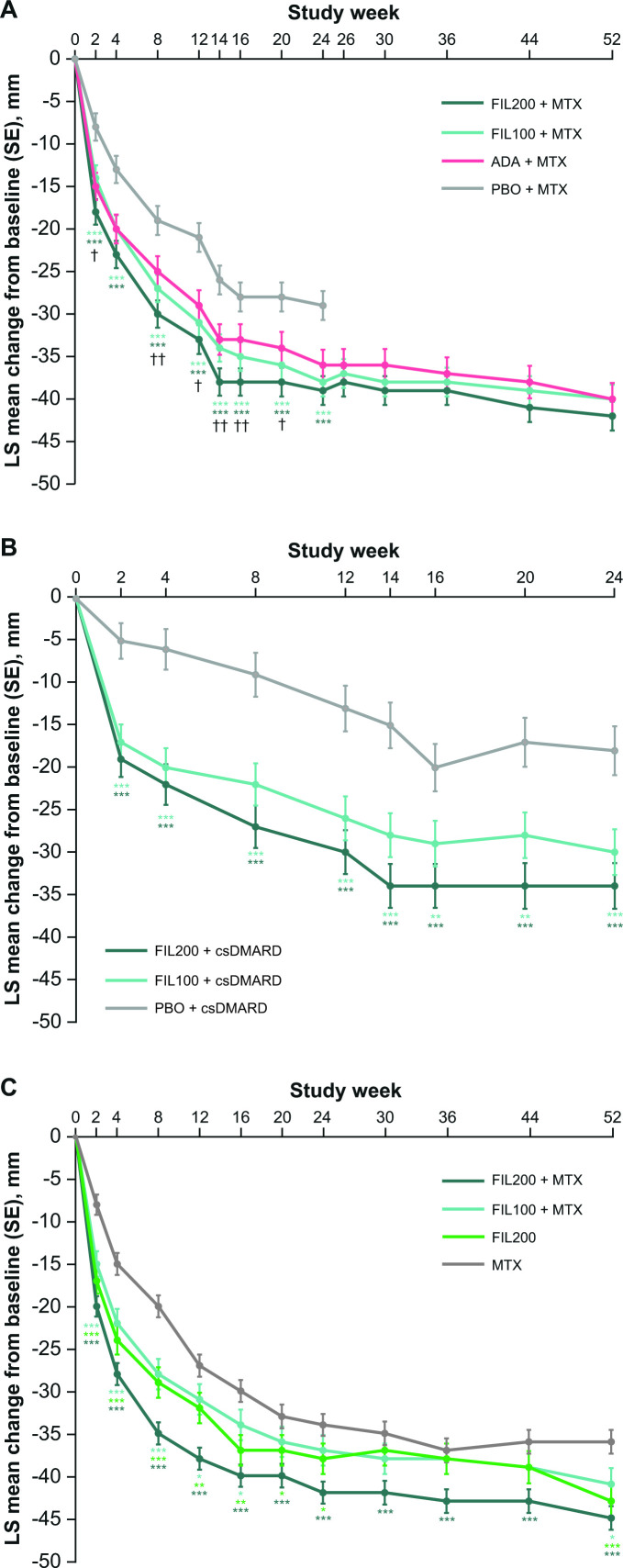
In each study, improvements in VAS pain score were seen as early as week 2 and were sustained over the study duration (up to week 52 in FINCH 1 and FINCH 3, and up to week 24 in FINCH 2) (figure 1). In FINCH 1, improvements from baseline up to week 24 were significantly greater in the filgotinib 200 mg and 100 mg arms (each administered with methotrexate) than in the placebo plus methotrexate arm (p<0.001), and were significantly greater in the filgotinib 200 mg plus methotrexate arm than in the adalimumab plus methotrexate arm at the majority of timepoints assessed (p<0.01 at weeks 8, 14 and 16; p<0.05 at weeks 2, 12 and 20) (figure 1A). In FINCH 2, improvements from baseline were significantly greater with either filgotinib dose than with placebo (each administered with csDMARDs) at all timepoints up to week 24 (p<0.001 for filgotinib 200 mg vs placebo at all timepoints, and for filgotinib 100 mg vs placebo at all timepoints except weeks 16 and 20, when p<0.01; figure 1B). In FINCH 3, filgotinib 200 mg plus methotrexate was the most effective treatment at reducing pain: improvements were significantly greater with filgotinib 200 mg plus methotrexate than with methotrexate alone at all timepoints up to week 52 (p<0.001). Significant differences between filgotinib 100 mg plus methotrexate and methotrexate alone were seen up to week 16 and at week 52 (p<0.001 at weeks 2, 4 and 8; p<0.05 at weeks 12, 16 and 52), and between filgotinib 200 mg monotherapy and methotrexate up to week 24 and at week 52 (p<0.001 at weeks 2, 4, 8 and 52; p<0.01 at weeks 12 and 16; p<0.05 at weeks 20 and 24) (figure 1C).
Figure 1.
LS mean change from baseline in VAS pain score in (A) FINCH 1, (B) FINCH 2 and (C) FINCH 3 full analysis set. Baseline value was the last available value collected on or prior to first dose of study drug. ***P<0.001, **p<0.01, *p<0.05 versus placebo (FINCH 1 and 2) or methotrexate (FINCH 3); ††p<0.01, †p<0.05 vs adalimumab (FINCH 1). ADA, adalimumab; csDMARD, conventional synthetic disease-modifying antirheumatic drug; FIL100/200, filgotinib 100/200 mg; LS, least squares; MTX, methotrexate; PBO, placebo; SE, standard error; VAS, visual analogue scale.
Time to first pain response
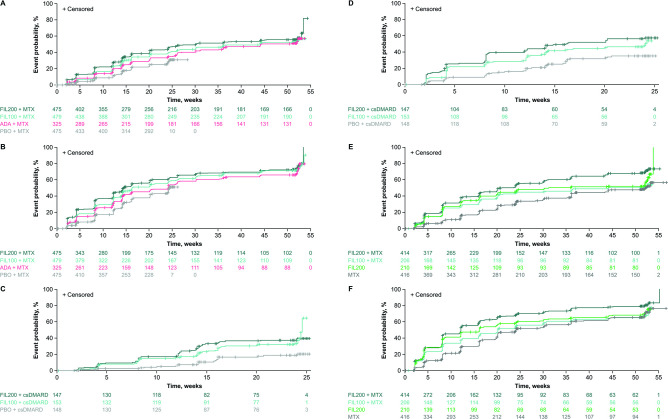
In FINCH 1, a VAS pain score of ≤10 mm was achieved by 53.3% of patients in the filgotinib 200 mg arm versus 48.6% in the adalimumab arm (based on efficacy data through week 52) and 24.8% in the placebo arm (based on efficacy data through week 24). The proportion of patients to achieve a VAS pain score of ≤10 mm was higher in the filgotinib 100 mg arm (50.5%) than in the placebo arm, and it was comparable with that in the adalimumab arm. In FINCH 2, a greater proportion of patients achieved a VAS pain score of ≤10 mm through week 24 with filgotinib 200 mg (36.7%) or filgotinib 100 mg (32.7%) than with placebo (15.5%). In FINCH 3, the proportion of patients with VAS pain scores of ≤10 mm through week 52 was highest with filgotinib 200 mg plus methotrexate (63.5%), and similar with filgotinib 100 mg plus methotrexate (48.1%) and filgotinib 200 mg (53.3%), each of which was higher than that with methotrexate alone (42.8%). The cumulative incidence of patients achieving VAS pain scores of ≤10 mm over time is shown in figure 2A, C and E. Similar results were observed when time to first VAS pain score of ≤20 mm (figure 2B, D and F) or ≥50% reduction in VAS pain score was assessed.
Figure 2.
Cumulative incidence of time to first VAS pain score of ≤10 and ≤20 mm in (A and B) FINCH 1, (C and D) FINCH 2 and (E and F) FINCH 3 full analysis set. The time to event was defined as the time period (weeks) between the first dosing date and the first occurrence of the event of interest. If no event was observed during the study, the patient was censored at the latest visit. Patients with baseline VAS pain score of 0 or missing data were excluded from the analysis. Patients who already had a VAS pain score of ≤10 or ≤20 mm at baseline were censored at baseline, and the time to event was set to 0 weeks. For FINCH 1, efficacy data through week 52 were included for the filgotinib and adalimumab groups; efficacy data through week 24 were included for the placebo group. ADA, adalimumab; csDMARD, conventional synthetic disease-modifying antirheumatic drug; FIL100/200, filgotinib 100/200 mg; MTX, methotrexate; PBO, placebo; VAS, visual analogue scale.
Comparison of time to achieve pain reductions between treatment groups
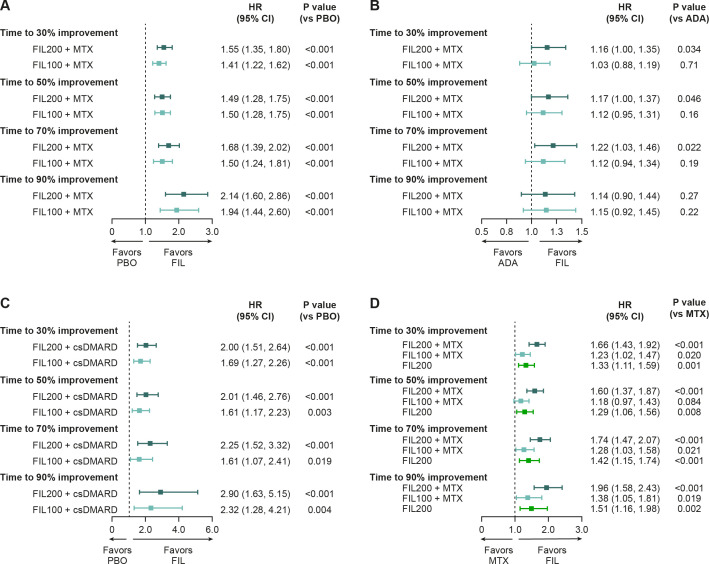
In FINCH 1, time to response was significantly shorter with filgotinib 200 mg than with placebo for a reduction in VAS pain of 30% (HR (95% CI): 1.55 (1.35 to 1.80)), 50% (HR (95% CI): 1.49 (1.28 to 1.75)), 70% (HR (95% CI): 1.68 (1.39 to 2.02)) and 90% (HR (95% CI): 2.14 (1.60 to 2.86)); all p<0.001. Reductions in pain were also reached significantly earlier with filgotinib 100 mg than with placebo (HR (95% CI): 1.41 (1.22 to 1.62) for a reduction of 30%, 1.50 (1.28 to 1.75) for 50%, 1.50 (1.24 to 1.81) for 70% and 1.94 (1.44 to 2.60) for 90%, all p<0.001) (figure 3A). Time to response was significantly shorter with filgotinib 200 mg than with adalimumab for a reduction in VAS pain of 30% (HR (95% CI): 1.16 (1.00 to 1.35); p=0.034), 50% (HR (95% CI): 1.17 (1.00 to 1.37); p=0.046) and 70% (HR (95% CI): 1.22 (1.03 to 1.46); p=0.022) but not 90% (HR (95% CI): 1.14 (0.90 to 1.44); p=0.27); differences between the adalimumab and filgotinib 100 mg arms were not significant (figure 3B). Patients in any of the filgotinib groups achieved a 30%, 50%, 70% and 90% reduction in VAS pain score significantly earlier than those in the placebo (FINCH 2) or methotrexate (FINCH 3) groups (figure 3C, D).
Figure 3.
Time to achieve pain improvement: stratified HR comparison between filgotinib and (A) placebo and (B) adalimumab in FINCH 1, (C) filgotinib and placebo in FINCH 2, and (D) filgotinib and methotrexate in FINCH 3. The time to event was defined as the time period (weeks) between the first dosing date and the first occurrence of the event of interest. If no event was observed during the study, the patient was censored at the latest visit. Subjects with baseline value of 0 or missing data were excluded from analysis. For FINCH 1, efficacy data through week 52 were included for the filgotinib and adalimumab groups; efficacy data through week 24 were included for the placebo group. HRs for the treatment groups were generated from a Cox regression model, stratified by geographic region and presence of anti-CCP antibodies or RF at screening (and prior exposure to bDMARDs for FINCH 1 and 2). P values were obtained from a log-rank test with the same stratification factors. ADA, adalimumab; anti-CCP, anti-cyclic citrullinated peptide; bDMARD, biologic disease-modifying antirheumatic drug; csDMARD, conventional synthetic disease-modifying antirheumatic drug; FIL100/200, filgotinib 100/200 mg; MTX, methotrexate; PBO, placebo; RF, rheumatoid factor.
Duration of time over the observation period during which VAS pain score was ≤10 or ≤20 mm
In FINCH 1, the mean (SD) number of weeks during which VAS pain score was ≤10 mm was 13.2 (17.36) in the filgotinib 200 mg arm, 10.6 (15.32) in the filgotinib 100 mg arm and 10.1 (15.04) in the adalimumab arm (VAS pain score was ≤10 mm for 1.5 (3.77) weeks in the placebo arm; however, data were only included up to week 24, rather than week 52 as for the other treatment arms) (table 1). In FINCH 2, VAS pain score was ≤10 mm for 3.7 (6.07) weeks in the filgotinib 200 mg arm, 2.8 (5.68) weeks in the filgotinib 100 mg arm and 1.0 (3.23) weeks in the placebo arm, and in FINCH 3, for 16.4 (18.37) weeks in the filgotinib 200 mg plus methotrexate arm, 13.0 (17.97) weeks in the filgotinib 100 mg plus methotrexate arm, 12.5 (17.49) weeks in the filgotinib 200 mg arm and 9.0 (14.10) weeks in the methotrexate arm (table 1). Similar patterns were generally seen for VAS pain responses ≤20 mm in each study (table 1).
Table 1.
Duration of threshold pain response achieved over observation period for VAS pain sco
| FINCH 1 | ||||
| FIL200+MTX (n=475) | FIL100+MTX (n=480) | ADA+MTX (n=325) | PBO+MTX (n=475) | |
| Treatment duration* | 52 weeks | 52 weeks | 52 weeks | 24 weeks |
| Duration of VAS pain score ≤10 mm | n=472 | n=475 | n=322 | n=474 |
| Number of weeks: | ||||
| mean (SD) | 13.2 (17.36) | 10.6 (15.32) | 10.1 (15.04) | 1.5 (3.77) |
| median (min, max) | 0.7 (0, 52) | 0.1 (0, 50) | 0.0 (0, 50) | 0.0 (0, 22) |
| % of total duration: | ||||
| mean (SD) | 26.3 (34.18) | 21.6 (30.12) | 20.4 (30.07) | 6.2 (15.81) |
| median (min, max) | 1.6 (0, 100) | 0.3 (0, 96) | 0.0 (0, 96) | 0.0 (0, 93) |
| Duration of VAS pain score ≤20 mm | n=459 | n=467 | n=314 | n=467 |
| Number of weeks: | ||||
| mean (SD) | 20.5 (19.98) | 17.6 (18.27) | 17.1 (18.01) | 3.4 (5.82) |
| median (min, max) | 14.6 (0, 52) | 10.2 (0, 51) | 11.9 (0, 51) | 0.0 (0, 24) |
| % of total duration: | ||||
| mean (SD) | 40.7 (38.82) | 35.6 (35.68) | 34.4 (35.33) | 14.4 (24.54) |
| median (min, max) | 30.1 (0, 99) | 23.5 (0, 100) | 25.3 (0, 99) | 0.0 (0, 99) |
| FINCH 2 | ||||
| FIL200 (n=147) | FIL100 (n=153) | PBO (n=148) | ||
| Treatment duration | 24 weeks | 24 weeks | 24 weeks | |
| Duration of VAS pain score ≤10 mm | n=146 | n=150 | n=146 | |
| Number of weeks: | ||||
| mean (SD) | 3.7 (6.07) | 2.8 (5.68) | 1.0 (3.23) | – |
| median (min, max) | 0.0 (0, 23) | 0.0 (0, 23) | 0.0 (0, 23) | |
| % of total duration: | ||||
| mean (SD) | 16.0 (25.93) | 11.6 (23.26) | 4.6 (14.73) | – |
| median (min, max) | 0.0 (0, 97) | 0.0 (0, 89) | 0.0 (0, 93) | |
| Duration of VAS pain score ≤20 mm | n=143 | n=146 | n=143 | |
| Number of weeks: | ||||
| mean (SD) | 7.3 (8.18) | 5.6 (7.99) | 2.3 (4.89) | – |
| median (min, max) | 2.0 (0, 23) | 0.0 (0, 25) | 0.0 (0, 23) | |
| % of total duration: | ||||
| mean (SD) | 31.3 (34.64) | 23.5 (33.31) | 10.0 (21.29) | – |
| median (min, max) | 13.1 (0, 96) | 0.0 (0, 96) | 0.0 (0, 95) | |
| FINCH 3 | ||||
| FIL200+MTX (n=416) | FIL100+MTX (n=207) | FIL200 (n=210) | MTX (n=416) | |
| Treatment duration | 52 weeks | 52 weeks | 52 weeks | 52 weeks |
| Duration of VAS pain score ≤10 mm | n=407 | n=203 | n=209 | n=411 |
| Number of weeks: | ||||
| mean (SD) | 16.4 (18.37) | 13.0 (17.97) | 12.5 (17.49) | 9.0 (14.10) |
| median (min, max) | 7.4 (0, 52) | 0.0 (0, 51) | 0.6 (0, 51) | 0.0 (0, 50) |
| % of total duration: | ||||
| mean (SD) | 32.9 (35.63) | 26.4 (35.57) | 25.2 (34.32) | 17.8 (27.66) |
| median (min, max) | 16.0 (0, 98) | 0.0 (0, 98) | 1.8 (0, 97) | 0.0 (0, 97) |
| Duration of VAS pain score ≤20 mm | n=394 | n=199 | n=206 | n=397 |
| Number of weeks: | ||||
| mean (SD) | 23.5 (19.77) | 18.3 (19.39) | 18.8 (19.23) | 14.5 (17.08) |
| median (min, max) | 22.1 (0, 52) | 10.9 (0, 52) | 11.8 (0, 52) | 4.4 (0, 52) |
| % of total duration: | ||||
| mean (SD) | 47.8 (38.17) | 37.2 (38.38) | 38.0 (37.62) | 28.9 (33.36) |
| median (min, max) | 49.9 (0, 99) | 21.3 (0, 99) | 24.9 (0, 98) | 10.1 (0, 99) |
For each patient, the total duration of VAS pain score ≤10 or ≤20 mm (weeks) was defined as the sum of all time periods, where VAS score ≤threshold. Linear interpolation between study visits was used to determine the start and endpoints of these periods. For each patient, the percentage of time being ≤threshold was calculated by dividing the total duration of VAS pain score ≤10 or ≤20 mm by the time from first to last completion of VAS pain assessment.
*In FINCH 1, the efficacy data through week 52 were included for the filgotinib and adalimumab groups; the efficacy data through week 24 were included for the placebo group.
ADA, adalimumab; FIL100/200, filgotinib 100/200 mg; MTX, methotrexate; PBO, placebo; VAS, visual analogue scale.
Pain responses and remission/low disease activity by DAS28 or CDAI
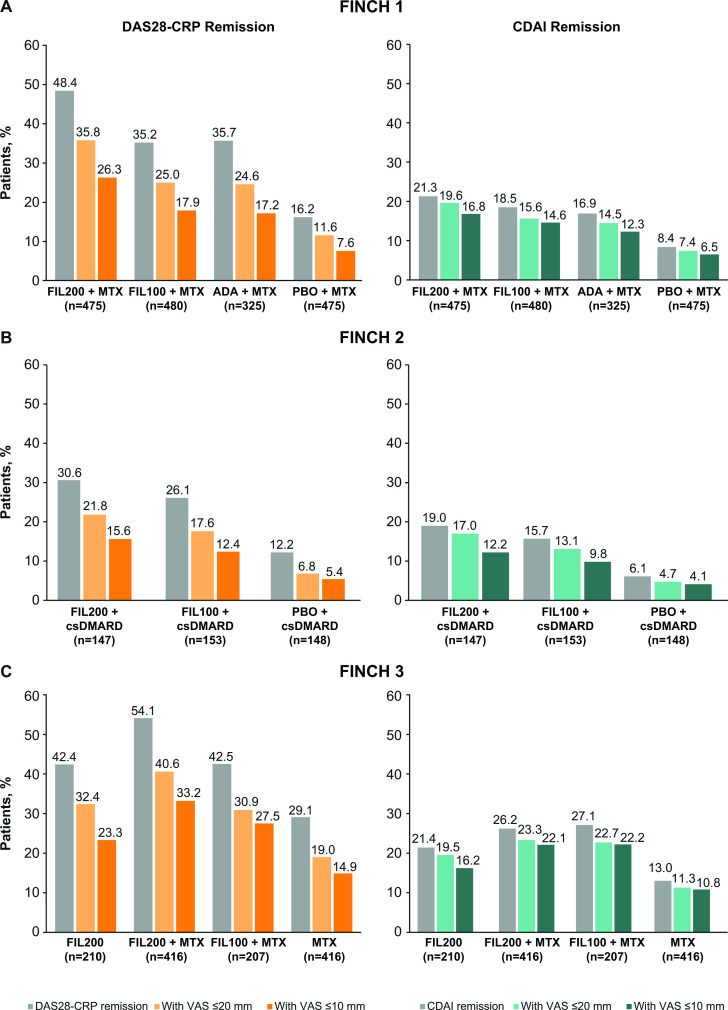
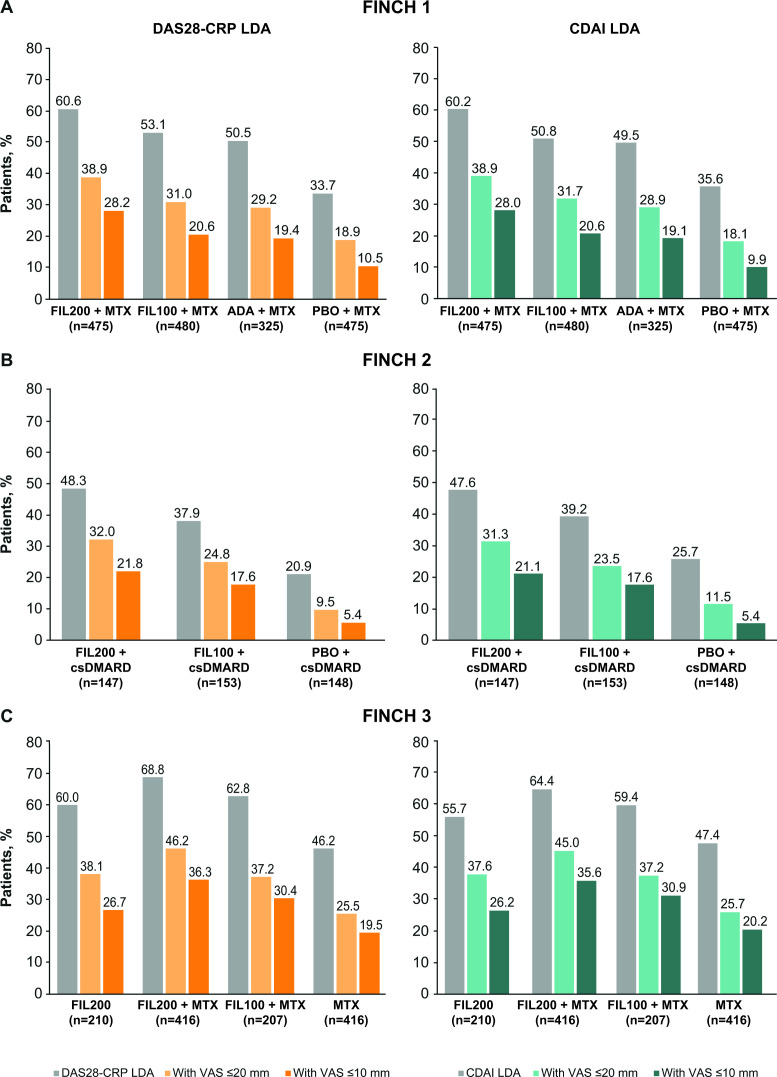
In FINCH 1, the proportion of patients achieving DAS28-CRP remission at week 24 was greatest in the filgotinib 200 mg plus methotrexate arm (48.4%), comparable between the filgotinib 100 mg plus methotrexate arm (35.2%) and the adalimumab plus methotrexate arm (35.7%), and lowest in the placebo arm (16.2%) (figure 4A). The proportion of patients who achieved VAS pain scores of ≤20 mm in addition to DAS28-CRP remission was 35.8% in the filgotinib 200 mg plus methotrexate arm, 25.0% in the filgotinib 100 mg plus methotrexate arm, 24.6% in the adalimumab arm and 11.6% in the placebo arm. Correspondingly, the proportion of patients who achieved VAS pain scores of ≤10 mm in addition to DAS28-CRP remission was 26.3%, 17.9%, 17.2% and 7.6% (figure 4A). In FINCH 2, a greater proportion of patients in the filgotinib 200 mg and 100 mg arms than in the placebo arm achieved DAS28-CRP remission (30.6%, 26.1% and 12.2%, respectively) (figure 4B). In the filgotinib 200 mg, filgotinib 100 mg and placebo arms, respectively, the proportion who achieved VAS pain scores of ≤20 mm in addition to DAS28-CRP remission was 21.8%, 17.6% and 6.8%; the proportion to achieve VAS pain scores of ≤10 mm in addition to DAS28-CRP remission was 15.6%, 12.4% and 5.4% (figure 4B). In FINCH 3, DAS28-CRP remission was achieved by 42.4% of the filgotinib 200 mg arm, 54.1% of the filgotinib 200 mg plus methotrexate arm, 42.5% of the filgotinib 100 mg plus methotrexate arm and 29.1% of the methotrexate arm (figure 4C). The proportion of patients to also achieve VAS pain scores of ≤20 or ≤10 mm was greater with filgotinib 200 mg monotherapy than with methotrexate monotherapy (32.4% vs 19.0% and 23.3% vs 14.9%, respectively). The corresponding proportions were 40.6% and 33.2% in the filgotinib 200 mg plus methotrexate group, and 30.9% and 27.5% in the filgotinib 100 mg plus methotrexate group (figure 4C). Similar patterns were observed when remission was assessed using CDAI, although the proportions of patients to achieve remission and pain responses (≤10 or ≤20 mm) in addition to remission were lower than when DAS28-CRP criteria were used (figure 4). In FINCH 1, a higher proportion of patients achieved pain responses in addition to low disease activity (as per DAS28-CRP or CDAI criteria) in the filgotinib 200 mg plus methotrexate arm than in the adalimumab plus methotrexate arm, and in both filgotinib arms (200 and 100 mg) than in the placebo plus methotrexate arm (figure 5A). In FINCH 2 and FINCH 3, the proportion of patients to achieve pain responses in addition to low disease activity was higher in the filgotinib arms than in the placebo plus csDMARD or methotrexate arms, respectively (figure 5B, C).
Figure 4.
Disease response (DAS28-CRP or CDAI remission) and VAS pain score of ≤10 or ≤20 mm in patients with disease response (at week 24) in (A) FINCH 1, (B) FINCH 2 and (C) FINCH 3. ADA, adalimumab; CDAI, Clinical Disease Activity Index; csDMARD, conventional synthetic disease-modifying antirheumatic drug; DAS28-CRP, Disease Activity Score-28 with C-reactive protein; FIL100/200, filgotinib 100/200 mg; MTX, methotrexate; PBO, placebo; VAS, visual analogue scale.
Figure 5.
Disease response (DAS28-CRP or CDAI LDA) and VAS pain score of ≤20 or ≤10 mm in patients with disease response (at week 24) in (A) FINCH 1, (B) FINCH 2 and (C) FINCH 3. ADA, adalimumab; CDAI, Clinical Disease Activity Index; csDMARD, conventional synthetic disease-modifying antirheumatic drug; DAS28-CRP, Disease Activity Score-28 with C-reactive protein; FIL100/200, filgotinib 100/200 mg; LDA, low disease activity; MTX, methotrexate; PBO, placebo; VAS, visual analogue scale.
Predictors of pain response
According to single-variable and multivariable analyses of pooled data from the three FINCH studies, factors that were associated with pain improvement included less impairment at baseline according to the SF-36 PCS and MCS, presence of anti-CCP antibodies or RF and being a former versus never smoker (tables 2 and 3). Increased BMI, increased TJC28 and concurrent use of oral corticosteroids were associated with worse pain outcomes (tables 2 and 3).
Table 2.
Logistic regression for VAS pain score of ≤10 and ≤20 mm at week 24: single-variable analysis with pairwise comparison
| Baseline parameter | Pairwise comparison | VAS pain score ≤10 mm | VAS pain score ≤20 mm | ||
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Age, years | 1 unit increase | 1.00 (0.99 to 1.00) | 0.322* | 0.99 (0.99 to 1.00) | 0.006 |
| Anti-CCP or RF positive | Yes vs no | 1.52 (1.20 to 1.91) | <0.001 | 1.47 (1.20 to 1.80) | <0.001 |
| BMI, kg/m2 | 1 unit increase | 0.98 (0.97 to 1.00) | 0.028 | 0.98 (0.97 to 0.99) | <0.001 |
| CRP, mg/L | 1 unit increase | 1.00 (1.00 to 1.01) | 0.440* | 1.00 (1.00 to 1.00) | 0.710* |
| CDAI | 1 unit increase | 0.99 (0.98 to 1.00) | 0.001 | 0.99 (0.98 to 0.99) | <0.001 |
| Concurrent oral corticosteroids | Yes vs no | 0.83 (0.70 to 0.99) | 0.040 | 0.82 (0.71 to 0.96) | 0.013 |
| DAS28-CRP | 1 unit increase | 0.88 (0.79 to 0.98) | 0.018 | 0.86 (0.78 to 0.95) | 0.003 |
| Duration of RA, years | 1 unit increase | 0.98 (0.97 to 1.00) | 0.009 | 0.99 (0.98 to 1.00) | 0.027 |
| Ethnicity | Hispanic/Latino vs not Hispanic/Latino | 1.48 (1.19 to 1.84) | <0.001 | 1.23 (1.01 to 1.51) | 0.040 |
| FACIT-Fatigue | 1 unit increase | 1.03 (1.02 to 1.04) | <0.001 | 1.03 (1.02 to 1.04) | <0.001 |
| HAQ-DI | 1 unit increase | 0.65 (0.55 to 0.77) | <0.001 | 0.72 (0.62 to 0.84) | <0.001 |
| Patient global VAS score, mm | 1 unit increase | 0.99 (0.98 to 1.00) | 0.013 | 0.99 (0.98 to 0.99) | <0.001 |
| Physician global VAS score, mm | 1 unit increase | 1.00 (0.99 to 1.00) | 0.140* | 0.99 (0.99 to 1.00) | 0.002 |
| Prior exposure to bDMARDs | Yes vs no | 0.65 (0.31 to 1.37) | 0.258* | 0.63 (0.34 to 1.19) | 0.158* |
| Race | Asian vs White | 0.93 (0.75 to 1.15) | 0.496 | 1.12 (0.93 to 1.35) | 0.221 |
| Black/African American vs White | 0.94 (0.57 to 1.55) | 0.803 | 0.67 (0.42 to 1.06) | 0.084 | |
| Other vs White | 1.65 (1.22 to 2.25) | 0.001 | 1.50 (1.13 to 2.00) | 0.005 | |
| Overall | – | 0.007 | – | 0.006 | |
| SJC28/TJC28 ratio | 1 unit increase | 1.09 (0.98 to 1.20) | 0.099* | 1.12 (0.99 to 1.27) | 0.082* |
| Region | Asia+Southeast Asia vs North America | 0.84 (0.64 to 1.10) | 0.206 | 1.19 (0.94 to 1.52) | 0.154 |
| Eastern Europe vs North America | 0.75 (0.59 to 0.97) | 0.027 | 1.00 (0.80 to 1.26) | 0.974 | |
| South/Central America vs North America | 1.56 (1.16 to 2.12) | 0.004 | 1.72 (1.29 to 2.28) | <0.001 | |
| Western Europe+Other vs North America | 1.09 (0.80 to 1.50) | 0.575 | 1.07 (0.80 to 1.43) | 0.654 | |
| Overall | – | <0.001 | – | <0.001 | |
| North America vs rest of world | 1.06 (0.86 to 1.32) | 0.580* | 0.86 (0.71 to 1.05) | 0.136* | |
| SDAI | 1 unit increase | 0.99 (0.98 to 1.00) | 0.003 | 0.99 (0.98 to 0.99) | <0.001 |
| SF-36 MCS | 1 unit increase | 1.02 (1.02 to 1.03) | <0.001 | 1.02 (1.01 to 1.03) | <0.001 |
| SF-36 PCS | 1 unit increase | 1.05 (1.03 to 1.06) | <0.001 | 1.04 (1.03 to 1.06) | <0.001 |
| Sex | Female vs male | 0.77 (0.63 to 0.94) | 0.012 | 0.92 (0.76 to 1.11) | 0.370* |
| Smoking status | Current vs never | 1.28 (1.00 to 1.63) | 0.050 | 1.14 (0.92 to 1.43) | 0.239 |
| Former vs never | 1.78 (1.40 to 2.25) | <0.001 | 1.53 (1.22 to 1.91) | <0.001 | |
| Overall | – | <0.001 | – | <0.001 | |
| SJC28 | 1 unit increase | 1.00 (0.98 to 1.01) | 0.724* | 0.99 (0.98 to 1.01) | 0.206* |
| TJC28 | 1 unit increase | 0.97 (0.96 to 0.98) | <0.001 | 0.97 (0.96 to 0.98) | <0.001 |
The model included treatment group, study, baseline pain VAS score and one of the additional predictors shown.
*Predictor ineligible for multivariable model (p≥0.05).
Anti-CCP, anti-cyclic citrullinated peptide; bDMARD, biologic disease-modifying antirheumatic drug; BMI, body mass index; CDAI, Clinical Disease Activity Index; CRP, C-reactive protein; DAS28-CRP, Disease Activity Score-28 with C-reactive protein; FACIT-Fatigue, Functional Assessment of Chronic Illness Therapy–Fatigue; HAQ-DI, Health Assessment Questionnaire–Disability Index; MCS, mental component summary score; PCS, physical component summary score; RF, rheumatoid factor; SDAI, Simple Disease Activity Index; SF-36, 36-item short-form health survey; SJC28, swollen joint count based on 28 joints; TJC28, tender joint count based on 28 joints; VAS, visual analogue scale.
Table 3.
Logistic regression for VAS pain score of ≤10 and ≤20 mm at week 24: multivariable analysis with pairwise comparison
| Baseline parameter | Pairwise comparison | VAS pain score ≤10 mm | VAS pain score ≤20 mm | ||
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Age, years | 1 unit increase | – | – | 0.99 (0.99 to 1.00) | 0.016 |
| Anti-CCP or RF positive | Yes vs no | 1.46 (1.14 to 1.88) | 0.003 | 1.35 (1.09 to 1.67) | 0.007 |
| BMI, kg/m2 | 1 unit increase | 0.98 (0.96 to 0.99) | 0.009 | 0.98 (0.96 to 0.99) | 0.002 |
| CDAI | 1 unit increase | 1.03 (0.97 to 1.10) | 0.283 | 1.04 (0.99 to 1.10) | 0.145 |
| Concurrent oral corticosteroids | Yes vs no | 0.79 (0.66 to 0.95) | 0.014 | 0.78 (0.66 to 0.92) | 0.003 |
| DAS28-CRP | 1 unit increase | 1.54 (1.06 to 2.24) | 0.024 | 1.69 (1.20 to 2.37) | 0.002 |
| Duration of RA, years | 1 unit increase | 0.98 (0.97 to 0.99) | 0.007 | 0.99 (0.98 to 1.00) | 0.100 |
| Ethnicity | Hispanic/Latino vs not Hispanic/Latino | 1.04 (0.68 to 1.59) | 0.851 | 0.79 (0.54 to 1.16) | 0.233 |
| FACIT-Fatigue | 1 unit increase | 0.99 (0.98 to 1.01) | 0.497 | 1.00 (0.99 to 1.01) | 0.914 |
| HAQ-DI | 1 unit increase | 0.95 (0.75 to 1.19) | 0.629 | 1.07 (0.87 to 1.31) | 0.528 |
| Pain VAS score, mm | 1 unit increase | 1.00 (0.99 to 1.00) | 0.340 | 1.00 (0.99 to 1.01) | 0.511 |
| Patient global VAS score, mm | 1 unit increase | 0.99 (0.98 to 1.00) | 0.170 | 0.99 (0.98 to 1.00) | 0.014 |
| Physician global VAS score, mm | 1 unit increase | – | – | 1.00 (0.99 to 1.00) | 0.635 |
| Race | Asian vs White | 0.95 (0.55 to 1.64) | 0.865 | 1.15 (0.57 to 2.34) | 0.701 |
| Black/African American vs White | 0.95 (0.55 to 1.64) | 0.848 | 0.67 (0.40 to 1.11) | 0.117 | |
| Other vs White | 1.13 (0.75 to 1.70) | 0.548 | 0.99 (0.67 to 1.46) | 0.970 | |
| Region | Asia+Southeast Asia vs North America | 0.70 (0.30 to 1.59) | 0.390 | 0.69 (0.33 to 1.47) | 0.337 |
| Eastern Europe vs North America | 0.84 (0.61 to 1.16) | 0.287 | 0.92 (0.69 to 1.23) | 0.569 | |
| South/Central America vs North America | 1.62 (1.00 to 2.61) | 0.050 | 1.88 (1.21 to 2.94) | 0.005 | |
| Western Europe+Other vs North America | 0.99 (0.69 to 1.42) | 0.971 | 0.86 (0.62 to 1.20) | 0.385 | |
| SDAI | 1 unit increase | 0.97 (0.91 to 1.04) | 0.381 | 0.95 (0.89 to 1.01) | 0.078 |
| SF-36 MCS | 1 unit increase | 1.03 (1.02 to 1.04) | <0.001 | 1.03 (1.01 to 1.04) | <0.001 |
| SF-36 PCS | 1 unit increase | 1.05 (1.03 to 1.07) | <0.001 | 1.04 (1.03 to 1.06) | <0.001 |
| Sex | Female vs male | 0.90 (0.72 to 1.14) | 0.383 | – | – |
| Smoking status | Current vs never | 1.22 (0.93 to 1.59) | 0.148 | 1.15 (0.91 to 1.46) | 0.237 |
| Former vs never | 1.72 (1.32 to 2.24) | <0.001 | 1.63 (1.28 to 2.08) | <0.001 | |
| TJC28 | 1 unit increase | 0.93 (0.89 to 0.96) | <0.001 | 0.95 (0.91 to 0.98) | 0.002 |
| Treatment | Adalimumab vs placebo | 1.90 (1.30 to 2.79) | <0.001 | 1.81 (1.33 to 2.48) | <0.001 |
| FIL100 vs placebo | 2.38 (1.74 to 3.24) | <0.001 | 2.04 (1.59 to 2.62) | <0.001 | |
| FIL200 vs placebo | 3.18 (2.34 to 4.32) | <0.001 | 2.78 (2.16 to 3.56) | <0.001 | |
The model included treatment group, study, baseline VAS pain score and all of the additional predictors shown.
Anti-CCP, anti-cyclic citrullinated peptide; BMI, Body Mass Index; CDAI, Clinical Disease Activity Index; DAS28-CRP, Disease Activity Score-28 with C-reactive protein; FACIT-Fatigue, Functional Assessment of Chronic Illness Therapy–Fatigue; HAQ-DI, Health Assessment Questionnaire–Disability Index; MCS, Mental Component Summary score; PCS, Physical Component Summary score; RF, rheumatoid factor; SDAI, Simple Disease Activity Index; SF-36, 36-item short-form health survey; TJC28, tender joint count based on 28 joints; VAS, visual analogue scale.
Discussion
The results of this analysis indicate that filgotinib reduced pain, reflected by the VAS pain score, in patients with active RA who had an inadequate response to methotrexate or bDMARDs, or who were methotrexate naïve. Filgotinib had a rapid onset on action—reductions in VAS pain score were seen as early as week 2, with responses sustained over time (up to week 52 in FINCH 1 and 3 and up to week 24 in FINCH 2). Improvements from baseline were significantly greater with filgotinib than with placebo, with the greatest improvements observed in those who received filgotinib 200 mg plus methotrexate. Reductions in pain (of 30%, 50%, 70% and 90%) were generally reached earlier with filgotinib 200 mg than with adalimumab, and with either filgotinib 200 mg or 100 mg than with placebo or methotrexate. For example, the HR (95% CI) for a 30% reduction in pain for filgotinib 200 mg versus adalimumab was 1.16 (1.00, 1.35), p=0.034. Similarly, the mean time during which VAS pain score was ≤10 or ≤20 mm was approximately 3 weeks longer with filgotinib 200 mg than with adalimumab, and was longer with either filgotinib dose than with placebo or methotrexate. The improvement observed with filgotinib 200 mg over adalimumab is likely clinically relevant and meaningful for patients. In addition, the finding that filgotinib 100 mg and adalimumab give rise to comparable pain outcomes reinforces the value of the lower dose of filgotinib in patients achieving disease activity control that is inclusive of satisfactory pain amelioration.
Current treatment guidelines advocate treat-to-target approaches for the management of RA, whereby treatments are modified until disease remission or low disease activity is achieved.26 27 Pain in RA is initially driven by inflammation, but other non-inflammatory causes may also contribute to pain experience, including, for example, mechanical issues and involvement of the central nervous system pain regulatory pathways.28 JAK 1 and 2 inhibition has been shown to ameliorate pain in patients with RA.29 As JAK inhibitors are reported not to cross the blood–brain barrier, their effects on pain may be elicited via pain-mediating cytokines, such as granulocyte-macrophage colony-stimulating factor and interleukin 6,29 rather than through direct effects on the central nervous system. Baricitinib was shown to improve pain to a greater extent than adalimumab, even though both therapies had a similar effect on clinical measures of inflammation,29 suggesting that JAK inhibition may offer potential added value for pain amelioration when treat-to-target goals are otherwise met. Similarly, in this analysis, in patients with active RA who were methotrexate inadequate responders, a greater proportion of those treated with filgotinib 200 mg versus adalimumab and a similar proportion of those treated with filgotinib 100 mg versus adalimumab, achieved stringent pain responses in addition to clinical responses (remission or low disease activity). When remission was assessed, this finding was more apparent when DAS28-CRP rather than CDAI criteria were used—an observation reflected in the results of the multivariable analyses, which showed that baseline DAS28-CRP, but not CDAI, predicted VAS pain scores of ≤10 or ≤20 mm being achieved. These observations may reflect differences in scoring these outcomes. While there is unequivocal evidence for the value of treating to target, physicians will recognise that not all patients will achieve the more stringent disease activity targets and, whether or not such targets are achieved, some patients will continue to report troublesome pain. In such instances, it may be beneficial to extend the treat-to-target principle to include an adjunctive low residual pain target,10 or to consider integrating alternative pain management strategies as part of a multidisciplinary approach.
Our analysis has some limitations. The inclusion of different treatment arms in each study makes it difficult to draw conclusions on the relative effectiveness of filgotinib among different RA patient populations. Also, the single-variable and multivariable analyses indicated that smoking status was associated with pain improvement, with former smokers more likely to show improvement than those who never smoked. This finding may be related to the relationship between smoking and increased risk of anti-CCP antibody or RF positivity.30 31 However, specific information regarding smoking, in terms of quantity or duration, was not collected for the former-smoking group, limiting the extent to which this finding can be interpreted.
In conclusion, this analysis of the FINCH studies indicates that filgotinib has a rapid and long-lasting effect on pain, greater than or comparable to that achieved with active comparators, across RA patient populations. Compared with adalimumab, effects on pain were generally favourable with filgotinib 200 mg and similar to those with filgotinib 100 mg.
Acknowledgments
We thank the physicians and patients who participated in the studies. Medical writing support was provided by Debbie Sherwood, BSc, CMPP (Aspire Scientific, Bollington, UK) and funded by Galapagos NV. Publication coordination was provided by Jessica Naddafy-Clark (Galapagos NV).
Footnotes
Twitter: @drpnash
Presented at: Work reported in this manuscript was previously presented at ACR 2023: Taylor P, Kavanaugh A, Nash P, et al. The impact of filgotinib on disease activity outcomes with concomitant pain control in the Phase 3 FINCH studies [abstract]. Arthritis Rheumatol 2023;75 (suppl 9).
Correction notice: This article has been corrected since it was first published online. In Figure 3D the label PBO should read MTX.
Contributors: PCT, CW and P-JS were involved in the concept or design or work; RA, PCT, DdV, CW and P-JS were involved with the acquisition or analysis of data for the work. CW acts as a guarantor of the study. All authors were involved with the interpretation of the data and read and approved the final version of the manuscript.
Funding: The FINCH studies were funded by Gilead Sciences, Inc. (Foster City, CA, USA) and Galapagos NV (Mechelen, Belgium).
Competing interests: PCT reports speaker fees from AbbVie; consultancy fees from AbbVie, Biogen, Fresenius, Galapagos, Gilead, GlaxoSmithKline, Janssen, Lilly, Nordic Pharma, Pfizer, Sanofi and UCB Pharma; and grant/research support from Galapagos. AK reports consultancy fees from AbbVie, Amgen, BMS, Janssen, Novartis, Pfizer and UCB. PN reports speaker fees, consultancy fees and grant/research support from AbbVie, Amgen, BMS, Celgene, Gilead/Galapagos, Janssen, Lilly, Novartis and Pfizer. JP has nothing to disclose. GP reports speaker fees from AbbVie, Boehringer, Lilly, Pfizer, Roche and Sanofi; consultancy fees from AbbVie, Boehringer, Galapagos, Lilly, Pfizer and Roche; and has been a paid instructor for Lilly and Roche. BF reports consultancy fees from AbbVie, Amgen, Biogen, BMS, Celltrion, Fresenius Kabi, Janssen, Lilly, Medac, MSD, Mylan, Nordic Pharma, Novartis, Pfizer, Roche, Sandoz, Sanofi-Genzyme, SOBI, UCB and Viatris and grant/research support from AbbVie, Lilly, MSD and Pfizer. RA reports consultancy fees from AbbVie, Amgen, Biogen, BMS, Celltrion, Gilead, Janssen, Lilly, Medac, MSD, Mylan, Novartis, Pfizer, Roche, Sandoz, Sanofi-Genzyme, UCB and Viatris. KH is an employee of, and a shareholder in, Gilead. SR is a former employee of Gilead. DdV and CW are employees of, and shareholders in, Galapagos. P-JS is a former employee of Galapagos. RW reports speaker fees and consultancy fees from Celltrion, Galapagos and Gilead.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Anonymised individual patient data will be shared upon request for research purposes, dependent upon the nature of the request, the merit of the proposed research and the availability of the data and their intended use. The full data sharing policy for Gilead Sciences can be found at https://www.gilead.com/about/ethics-and-code-of-conduct/policies. The data sharing policy for Galapagos NV can be found at https://www.clinicaltrials-glpg.com/us/en/data-transparency.html
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study involves human participants. This is a post hoc analysis of previously reported studies. Approval by ethics committees has been previously published in the primary paper. Participants gave informed consent to participate in the study before taking part.
References
- 1. Shams S, Martinez JM, Dawson JRD, et al. The therapeutic landscape of rheumatoid arthritis: current state and future directions. Front Pharmacol 2021;12:680043. 10.3389/fphar.2021.680043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kosinski M, Kujawski SC, Martin R, et al. Health-related quality of life in early rheumatoid arthritis: impact of disease and treatment response. Am J Manag Care 2002;8:231–40 [PubMed] [Google Scholar]
- 3. Taylor P, Manger B, Alvaro-Gracia J, et al. Patient perceptions concerning pain management in the treatment of rheumatoid arthritis. J Int Med Res 2010;38:1213–24. 10.1177/147323001003800402 [DOI] [PubMed] [Google Scholar]
- 4. Walsh DA, McWilliams DF. Mechanisms, impact and management of pain in rheumatoid arthritis. Nat Rev Rheumatol 2014;10:581–92. 10.1038/nrrheum.2014.64 [DOI] [PubMed] [Google Scholar]
- 5. van der Elst K, Meyfroidt S, De Cock D, et al. Unraveling patient-preferred health and treatment outcomes in early rheumatoid arthritis: a longitudinal qualitative study. Arthritis Care Res (Hoboken) 2016;68:1278–87. 10.1002/acr.22824 [DOI] [PubMed] [Google Scholar]
- 6. De Cock D, Van der Elst K, Stouten V, et al. The perspective of patients with early rheumatoid arthritis on the journey from symptom onset until referral to a rheumatologist. Rheumatol Adv Pract 2019;3:rkz035. 10.1093/rap/rkz035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Taylor PC. Pain in the joints and beyond; the challenge of rheumatoid arthritis. Lancet Rheumatol 2023;5:e351–60. 10.1016/S2665-9913(23)00094-2 [DOI] [PubMed] [Google Scholar]
- 8. Ishida M, Kuroiwa Y, Yoshida E, et al. Residual symptoms and disease burden among patients with rheumatoid arthritis in remission or low disease activity: a systematic literature review. Mod Rheumatol 2018;28:789–99. 10.1080/14397595.2017.1416940 [DOI] [PubMed] [Google Scholar]
- 9. Van der Elst K, Verschueren P, De Cock D, et al. One in five patients with rapidly and persistently controlled early rheumatoid arthritis report poor well-being after 1 year of treatment. RMD Open 2020;6:e001146. 10.1136/rmdopen-2019-001146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pazmino S, Lovik A, Boonen A, et al. Does including pain, fatigue, and physical function when assessing patients with early rheumatoid arthritis provide a comprehensive picture of disease burden J Rheumatol 2021;48:174–8. 10.3899/jrheum.200758 [DOI] [PubMed] [Google Scholar]
- 11. Pazmino S, Lovik A, Boonen A, et al. New indicator for discordance between patient-reported and traditional disease activity outcomes in patients with early rheumatoid arthritis. Rheumatology (Oxford) 2022;62:108–15. 10.1093/rheumatology/keac213 [DOI] [PubMed] [Google Scholar]
- 12. Felson DT, Anderson JJ, Boers M, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. Arthritis Rheumatism 1993;36:729–40. 10.1002/art.1780360601 [DOI] [PubMed] [Google Scholar]
- 13. Boers M, Tugwell P, Felson DT, et al. World Health Organization and International League of Associations for Rheumatology core endpoints for symptom modifying antirheumatic drugs in rheumatoid arthritis clinical trials. J Rheumatol Suppl 1994;41:86–9. [PubMed] [Google Scholar]
- 14. Pazmino S, Verschueren P, Westhovens R. Does opioid-based pharmacotherapy have a place in rheumatoid arthritis therapy Expert Opin Pharmacother 2021;22:1945–7. 10.1080/14656566.2021.1940136 [DOI] [PubMed] [Google Scholar]
- 15. Guida F, De Gregorio D, Palazzo E, et al. Behavioral, biochemical and electrophysiological changes in spared nerve injury model of neuropathic pain. Int J Mol Sci 2020;21:3396. 10.3390/ijms21093396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guo Y-J, Li H-N, Ding C-P, et al. Red nucleus interleukin-1beta evokes tactile allodynia through activation of JAK/STAT3 and JNK signaling pathways. J Neurosci Res 2018;96:1847–61. 10.1002/jnr.24324 [DOI] [PubMed] [Google Scholar]
- 17. Fang D, Kong L-Y, Cai J, et al. Interleukin-6-mediated functional upregulation of TRPV1 receptors in dorsal root ganglion neurons through the activation of JAK/PI3K signaling pathway: roles in the development of bone cancer pain in a rat model. Pain 2015;156:1124–44. 10.1097/j.pain.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 18. Taylor PC, Lee YC, Fleischmann R, et al. Achieving pain control in rheumatoid arthritis with baricitinib or adalimumab plus methotrexate: results from the RA-BEAM trial. J Clin Med 2019;8:831. 10.3390/jcm8060831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Taylor PC, Alten R, Álvaro Gracia JM, et al. Achieving pain control in early rheumatoid arthritis with baricitinib monotherapy or in combination with methotrexate versus methotrexate monotherapy. RMD Open 2022;8:e001994. 10.1136/rmdopen-2021-001994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Strand V, van der Heijde D, Tanaka Y, et al. Tofacitinib in combination with methotrexate in patients with rheumatoid arthritis: patient-reported outcomes from the 24-month phase 3 ORAL scan study. Clin Exp Rheumatol 2020;38:848–57. 10.1136/rmdopen-2016-000308 [DOI] [PubMed] [Google Scholar]
- 21. Strand V, Pope J, Tundia N, et al. Upadacitinib improves patient-reported outcomes in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying antirheumatic drugs: results from SELECT-NEXT. Arthritis Res Ther 2019;21:272. 10.1186/s13075-019-2037-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bergman M, Tundia N, Martin N, et al. Patient-reported outcomes of upadacitinib versus abatacept in patients with rheumatoid arthritis and an inadequate response to biologic disease-modifying antirheumatic drugs: 12- and 24-week results of a phase 3 trial. Arthritis Res Ther 2022;24:155. 10.1186/s13075-022-02940-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Combe B, Kivitz A, Tanaka Y, et al. Filgotinib versus placebo or adalimumab in patients with rheumatoid arthritis and inadequate response to methotrexate: a phase III randomised clinical trial. Ann Rheum Dis 2021;80:848–58. 10.1136/annrheumdis-2020-219214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Genovese MC, Kalunian K, Gottenberg J-E, et al. Effect of filgotinib vs placebo on clinical response in patients with moderate to severe rheumatoid arthritis refractory to disease-modifying antirheumatic drug therapy: the FINCH 2 randomized clinical trial. JAMA 2019;322:315–25. 10.1001/jama.2019.9055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Westhovens R, Rigby WFC, van der Heijde D, et al. Filgotinib in combination with methotrexate or as monotherapy versus methotrexate monotherapy in patients with active rheumatoid arthritis and limited or no prior exposure to methotrexate: the phase 3 randomised controlled FINCH 3 trial. Ann Rheum Dis 2021;80:727–38. 10.1136/annrheumdis-2020-219213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685–99. 10.1136/annrheumdis-2019-216655 [DOI] [PubMed] [Google Scholar]
- 27. Fraenkel L, Bathon JM, England BR, et al. American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res (Hoboken) 2021;73:924–39. 10.1002/acr.24596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boyden SD, Hossain IN, Wohlfahrt A, et al. Non-inflammatory causes of pain in patients with rheumatoid arthritis. Curr Rheumatol Rep 2016;18:30. 10.1007/s11926-016-0581-0 [DOI] [PubMed] [Google Scholar]
- 29. Simon LS, Taylor PC, Choy EH, et al. The JAK/STAT pathway: a focus on pain in rheumatoid arthritis. Semin Arthritis Rheum 2021;51:278–84. 10.1016/j.semarthrit.2020.10.008 [DOI] [PubMed] [Google Scholar]
- 30. van Wesemael TJ, Ajeganova S, Humphreys J, et al. Smoking is associated with the concurrent presence of multiple autoantibodies in rheumatoid arthritis rather than with anti-citrullinated protein antibodies per se: a multicenter cohort study. Arthritis Res Ther 2016;18:285. 10.1186/s13075-016-1177-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sparks JA, Karlson EW. The roles of cigarette smoking and the lung in the transitions between phases of preclinical rheumatoid arthritis. Curr Rheumatol Rep 2016;18:15. 10.1007/s11926-016-0563-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2023-003839supp002.pdf (169.7KB, pdf)
rmdopen-2023-003839supp001.pdf (4.9MB, pdf)
Data Availability Statement
Data are available upon reasonable request. Anonymised individual patient data will be shared upon request for research purposes, dependent upon the nature of the request, the merit of the proposed research and the availability of the data and their intended use. The full data sharing policy for Gilead Sciences can be found at https://www.gilead.com/about/ethics-and-code-of-conduct/policies. The data sharing policy for Galapagos NV can be found at https://www.clinicaltrials-glpg.com/us/en/data-transparency.html