Notes
Editorial note
This review question is now being addressed according to a new protocol: https://doi.org/10.1002/14651858.CD015719
Abstract
Background
Patients undergoing hematopoietic stem cell transplantation (HSCT) and those with lymphoproliferative disorders (LPD) have a higher incidence of infections due to secondary hypogammaglobulinemia. One approach is the prophylactic administration of intravenous immunoglobulins (IVIG). Randomized controlled trials (RCTs) showed conflicting results in terms of type, schedule, dose and hematological patients benefiting from IVIG. We therefore performed a systematic review and meta‐analysis to evaluate the role of IVIG in these patients.
Objectives
To determine whether prophylaxis with IVIG reduces mortality or affects other outcomes in patients with hematological malignancies.
Search methods
PubMed (January 1966 to December 2007), CENTRAL (The Cochrane Library, up to 2007, issue 1), LILACS and conference proceedings published between 2002‐2007 were searched. The terms "immunoglobulins" or "gammaglobulins" or specific gammaglobulins and similar and the terms "hematologic neoplasms" or "hematologic malignancies" or "transplant" or "autotransplant" or "allotransplant" or "bone marrow transplant" or "peripheral stem cell transplant" and similar were selected. References of all included trials and reviews identified were scanned for additional trials.
Selection criteria
All RCTs comparing prophylaxis of IVIG with placebo, no treatment or another immunoglobulin preparation, different administration schedules or doses for patients with hematological malignancies were included. One author screened all abstracts identified through the search strategy and two reviewers independently inspected each reference identified by the search and applied inclusion criteria.
Data collection and analysis
For each trial, results were expressed as relative risks (RR) with 95% confidence intervals (CI) for dichotomous data and weighted mean differences for continuous data. We conducted meta‐analysis, where enough similar trials were available, using the fixed‐ effects model, unless significant heterogeneity was present. We performed sensitivity analyses to assess the effect of individual methodological quality measures on effect estimates, including allocation generation, concealment and blinding.
Main results
Forty trials were included: thirty included HSCT patients and ten included patients LPD. When polyvalent immunoglobulins or hyperimmune cytomegalovirus (CMV)‐IVIG was compared to control for HSCT, there was no difference in all‐cause mortality. Polyvalent immunoglobulins significantly reduced the risk for interstitial pneumonitis but increased the risk for veno‐occlusive disease and adverse events. In LPD, no benefit in terms of mortality IVIG could be demonstrated but there was a decrease in clinically and microbiologically documented infections.
Authors' conclusions
In patients undergoing HSCT, routine prophylaxis with IVIG is not supported. Its use may be considered in LPD patients with hypogammaglobulinemia and recurrent infections, for reduction of clinically documented infections.
Keywords: Humans; Agammaglobulinemia; Agammaglobulinemia/prevention & control; Bacterial Infections; Bacterial Infections/prevention & control; Bone Marrow Transplantation; Bone Marrow Transplantation/adverse effects; Bone Marrow Transplantation/mortality; Hematologic Neoplasms; Hematologic Neoplasms/mortality; Hematologic Neoplasms/surgery; Hematopoietic Stem Cell Transplantation; Hematopoietic Stem Cell Transplantation/adverse effects; Hematopoietic Stem Cell Transplantation/mortality; Immunoglobulins, Intravenous; Immunoglobulins, Intravenous/therapeutic use; Lymphoproliferative Disorders; Lymphoproliferative Disorders/mortality; Lymphoproliferative Disorders/surgery; Randomized Controlled Trials as Topic
Plain language summary
The role of prophylactic immunoglobulins in hematological malignancies
Patients with hematological malignancies are prone to infections due to defects in their immune system. One of the main defects is a reduction in the level of immunoglobulins. For many years, the notion was that administration of pooled immunoglobulins from healthy donors might reverse this defect. However, randomized controlled trials showed different results in terms of prolongation of survival, reduction of infections and side effects of treatments. We conducted a systematic review assessing the role of administration of immunoglobulins from healthy donors as prophylaxis in patients with hematological malignancies. Our review showed that in the context of bone marrow transplantation the administration of immunoglobulins did not have an effect on survival or other outcomes. On the other hand, in patients with lymphoproliferative disorders like chronic lymphocytic leukemia or multiple myeloma, it reduced substantially the rate of infections. Despite their high cost, prophylactic immunoglobulins might prove cost‐effective in this population.
Background
Description of the condition
Bone marrow transplantation (BMT) and hematopoietic stem cell transplantation (HSCT) compromise the immune system of patients due to the need for high doses of chemoradiotherapy prior to infusion of the donor stem cells. Multiple immunological deficiencies, including T‐ and B‐cell abnormalities and hypogammaglobulinemia (low immunoglobulin levels) predispose patients to a high risk of developing a variety of infections. This period of immunological incompetence usually lasts from six to 12 months. In some subsets of patients [those with chronic graft‐versus‐host disease (GVHD); recipients of unrelated transplants and older patients] persistent T‐ and B‐cell abnormalities may be seen for years, despite normal serum immunoglobulin levels (Siadak 1994). These abnormalities are less prominent in autologous transplantations. Due to the deficient immune function, BMT recipients are highly susceptible to bacterial, viral and fungal infections. In particular, cytomegalovirus (CMV) is a serious problem for these immunocompromised patients. Patients with secondary hypogammaglobulinemia due to underlying low‐grade B‐cell lymphoproliferative disorders (LPD) have a higher incidence of serious bacterial infections, in particular those caused by encapsulated bacteria, when compared with an age‐matched population (Besa 1992; Jim 1956). Infections are the major cause of morbidity and mortality in chronic lymphocytic leukemia (CLL) (Jurlander 1994). The incidence varies, but up to 65% of patients die from infection‐related events (Hansen 1973). Factors underlying the increased susceptibility to infections are many and complex. A major factor is the decreased capacity to produce potent immunoglobulins, often reflected by a certain degree of hypogammaglobulinemia seen in the majority of patients during their disease (Fairley 1961; Jurlander 1994; Rozman 1988). Life threatening infections cause significant morbidity and mortality in patients with multiple myeloma (MM) with a fatality rate of 30% (Chapel 1994a; Perri 1981; Twomey 1973). Since in MM patients host‐defense defects are multifactorial, infections associated with the initial phase of the disease and relapse are of viral, fungal, and bacterial etiology (Perri 1981; Chapel 1994b; Savage 1982). Infections in patients in stable or plateau phase are those typical of depressed humoral immunity, predominantly due to Streptococcus pneumoniae or Haemophilus influenza (Chapel 1994a; Espersen 1984; Savage 1982).
Description of the intervention
Since the defective immune response among hematological cancer patients is due in part to immunoglobulin deficiency, one approach for prevention of infections is the administration of intravenous immunoglobulins (IVIG). Polyvalent human immunoglobulin preparations were one of the first plasma proteins prepared in a purified state as a therapeutic drug. Since the early 1980s safer IVIG preparations became available. IVIG is prepared from pooled human plasma. IVIG contains concentrated IgG with normal plasma ratios of IgG1 and IgG2 but lower percentages of IgG3 and IgG4 and only trace amounts of IgA and IgM. Hyperimmune IVIG is purified from donor plasma selected for high titer toward a specific pathogen like CMV. Several large controlled trials showed that administration of IVIG prevented infection in patients undergoing BMT (Cordonnier 2003; Sullivan 1990; Winston 1987a), especially CMV infection, and interstitial pneumonia (Cordonnier 2003) and reduced the incidence of acute graft versus host disease (GVHD) (Sullivan 1990; Sullivan 1996a; Cordonnier 2003; Abdel‐Mageed 1999; Pirofsky 1984; Winston 1990; Bass 1993; Winston 1987a; Winston 1987b; Wingard 1990; Bowden 1986; Graham‐Pole 1988; Petersen 1987; Wolff 1993; Nasman Bjork 1999). However an improvement in survival was reported in only a few studies (Graham‐Pole 1988; Wolff 1993; Guglielmo 1994) and the type, schedule, dose and patients benefiting form IVIG have not been established. Criteria have been suggested for the use of IVIG among patients with LPD. These include: low levels of protective antibodies to encapsulated organisms, viruses, or bacterial toxins; poor or absent antibody response to immunization or infection; severe hypogammaglobulinemia (serum IgG < 200 mg/dL); and increased rate, severity, and duration of infections, especially infections of the respiratory system or with encapsulated organisms such as Pneumococcus, Haemophilus influenzae, and Meningococcus (Chapel 1994a; Chapel 1994b). However, these criteria are not based on empirical evidence and are not practiced universally. IVIG exposes patients to the potential transmission of new pathogens. It is very expensive in high doses and is not always well tolerated. The incidence of adverse effects is reported to be between 1% and 15%, although fewer than 5% of patients experience clinically significant reactions.
Why it is important to do this review
A meta‐analysis of 12 randomized controlled trials of IVIG prophylaxis in patients undergoing BMT showed a significant reduction in overall mortality, CMV pneumonia and non‐CMV interstitial pneumonia in patients receiving IVIG prophylaxis (Bass 1993). Another meta‐analysis of 18 randomized controlled trials supported the use of IVIG in the prevention of symptomatic CMV disease in transplant recipients including both bone marrow and solid organ transplant recipients (Glowacki 1993; Glowacki 1994). More than 2300 patients were enrolled in the trials included in a systematic review by Sokos at al. based on MEDLINE search and references of all relevant studies and review articles (Sokos 2002). The authors summarized their review by stating that trials examining the utility of IVIG in HSCT show variability in terms of trial design, patients and treatments. According to their search, the role of IVIG in the prevention of CMV infection as well as prophylaxis for GVHD is uncertain. Despite the controversy about the benefit of IVIG in BMT, this agent has been given as part of most transplantation protocols for many years and a National Institutes of Health (NIH) consensus panel endorsed the use of IVIG after allogeneic BMT (Sullivan 1990; Winston 1987a; Bowden 1986; Graham‐Pole 1988; Petersen 1987; Winston 2001; Consensus IVIG 1990; Winston 1993; Condie 1984). No recent meta‐analysis assessed the compiled evidence available to date. The effect of IVIG on overall survival among bone marrow transplant patients and other hemato‐oncological patients is unclear. We therefore performed a systematic review and meta‐analysis of IVIG prophylaxis in hematological malignancies.
Objectives
To determine whether the prophylactic administration of IVIG reduces mortality in patients with hematological malignancies and hematopoietic stem cell transplantation or affect other patient‐related outcomes, including the incidence of infections, hospitalization, GVHD and others in patients with hematological malignancies and hematopoietic stem cell transplantation.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials.
Types of participants
Patients with hematological malignancies ‐ CLL or MM, and patients undergoing BMT or HSCT given IVIG for prophylaxis (and not as treatment of suspected or documented infections). We conducted separate analyses for patients with CLL or MM and patients following allogeneic and autologous HSCT.
Types of interventions
Administration of intravenous or intramuscular immunoglobulins ( polyclonal or monoclonal), hyperimmune immunoglobulin preparations or monoclonal antibodies vs. placebo, no treatment or another immunoglobulin preparation, administration schedule or dose. Pooled immunoglobulins were assessed separately from specific hyperimmune immunoglobulin preparations.
Types of outcome measures
Primary outcomes
All cause mortality
Clinically documented infections, applying standard definitions (Consensus IVIG 1990).
Secondary outcomes
-
Microbiologically documented infections
CMV infections as defined in each study.
Other herpesvirus infections
Bacterial infections
Bacteremia
Infection‐related mortality
Acute and chronic GVHD, veno‐occlusive disease and interstitial pneumonia in allogeneic bone marrow transplants
Disease relapse
-
Adverse events (side effects) and complications ‐ i.e.
Immediate adverse events: allergic reaction, anaphylaxis, fever and chills
Delayed adverse events: renal, pulmonary, aseptic meningitis, arthritis, cerebral infarction, hyperviscosity, hemolysis, and leukopenia
Late adverse events: transmission of infectious agents
For patients with CLL/MM we planned to extract outcomes at end of follow up, since the nature of the disease and its treatment mandate a prolonged follow up. For patients following HSCT we planned to assess the main outcomes at 100 days post transplantation (time of engraftment and recovery from the procedure) and at the longest available follow‐up. In addition, we tried to extract survival data as time to event data.
Search methods for identification of studies
Electronic searches
We conducted a comprehensive search strategy to identify both published and unpublished trials, with no restriction on language or study years. We searched PubMed ; CENTRAL ; LILACS; references of all included studies and major reviews (for search strategy see Appendix 1).
Searching other resources
In addition, we searched conference proceedings published between 2002‐2007 for recently conducted, unpublished trials:
Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC);
European Congress of Clinical Microbiology and Infectious Diseases (available at http://www.akm.ch/eccmid2001‐2004/);
Annual Meeting of the American Society of Hematology (available at http://www.hematology.org/)
and the annual Meeting of the European Hematology Association (available at http://www.ehaweb.org/).
We searched the following trial databases for ongoing and unpublished trials:
UKCCCR Register of Cancer Trials (http://www.ctu.mrc.ac.uk/ukcccr/home.html);
PDQ (Physician Data Query) database of the National Cancer Institute (http://www.cancer.gov/search/clinical_trials/);
and the National Institutes of Health database (http://clinicaltrials.gov/).
We searched the web for new drug application (NDA) documents of the US Food and Drug Administration, which may include unpublished studies.
Data collection and analysis
Study selection One author screened all abstracts identified through our search strategy and selected articles for full‐text inspection (PR). Two authors applied inclusion criteria to all retrieved articles (PR, AGG).
Quality assessment We used an individual component approach, since the use of composite scales has yielded conflicting results. We assessed: allocation concealment (A‐adequate, B ‐ unclear, C‐ inadequate, D ‐ not used), allocation generation (same), blinding, intention to treat analysis, and predetermination of outcome assessment time. We used the definitions detailed in the Cochrane handbook to determine allocation concealment and generation.
Data collection Data from included trials were independently extracted by two reviewers into a data extraction sheet (PR, AGG). Differences in the data extracted were resolved by discussion with a third reviewer (MP, LL, OS or IB). Justification for excluding studies from the review was documented. The first or corresponding author of each included study was contacted for clarifications and further information. Authors of all included trials, and trials in assessment for inclusion, were contacted for clarifications and further information. Data regarding all‐cause mortality and randomization methods were requested primarily.
We extracted the following data from included studies (see table of: "Characteristics of included studies") :
Characteristics of trials:
Study years (years during which the study was conducted)
Location (where the study was conducted)
Setting of trial: outpatient, inpatient, isolation precautions
Multi‐center vs. single institute
Publication status
Duration of follow up: duration of planned IVIG treatment, planned duration of follow up after intervention, actual follow‐up in study
Methodological quality assessment:
-
Allocation concealment:
adequate (A) for example centralized (e.g. allocation by a central office unaware of subject characteristics) or pharmacy‐controlled randomization, pre‐numbered or coded identical containers which are administered serially to participants, on‐site computer system combined with allocations kept in a locked unreadable computer file that can be accessed only after the characteristics of an enrolled participant have been entered, sequentially numbered, sealed, opaque envelopes
unclear (B)
inadequate (C) for example non‐concealed table, non‐opaque envelopes, date of birth, date of admission, hospital numbers or alternation
-
Allocation generation:
adequate (A) for example computer generated, random number table
unclear (B)
inadequate (C) for example date of birth, date of admission, hospital numbers or alternation
-
Blinding:
caregiver
patient
outcome assessor
Characteristics of participants and treatment:
Number of participants in each group
Underlying hematological malignancy
Disease status
Bone marrow transplantation:
none
autologous
allogeneic ‐ sibling, unrelated donor, haplo‐identical
syngeneic
myeloablative vs. non‐myeloablative
T‐cell depletion performed
Type of graft
CMV status of donor and recipient
Lymphoproliferative disorders
Stage of disease
Time from diagnosis
Paraprotein levels
Age and percentage of children <16 years
Baseline immunoglobulin levels
Chemotherapy protocol used in study
Steroid use
Prophylaxis measures used in study other than the intervention and isolation precautions including: G‐CSF, antibacterial, antifungal, anti‐Pneumocystis jirovecii and antiviral prophylaxis and type
Characteristics of interventions:
Type of immunoglobulin
Dose of immunoglobulin
Schedule of administration
Total duration of intervention
Characteristics of outcome measures as defined above. Outcomes were extracted preferentially by intention‐to‐treat, including all individuals randomized in the outcome assessment. Where impossible, data by available case analysis were extracted. In the main analysis all studies were combined. Secondary, per‐protocol analyses were conducted, using the protocol definitions used in each study. We compared the effect estimate obtained from intention to treat studies to that obtained from studies reporting an available case analysis. For trials of IVIG prophylaxis post HSCT we planned to assess all‐cause mortality at two time points, since they reflect different causes for death: mortality at three to four months post SCT ‐ as this is a composite of infections, acute rejection, treatment‐related mortality and mortality at one to two years post SCT ‐ as this is composite of disease‐related mortality, chronic rejection and the above. This was done whenever possible, but in practice, the trials reported on mortality only at a single time point most commonly and this point in time varied between the trials.
Data synthesis Results for dichotomous data were expressed as relative risks (RR) with 95% confidence intervals (CI) and pooled using a fixed effect model, unless significant heterogeneity was present. In this case we considered the appropriateness of a meta‐analysis and pooled trials using a random effects model. As we did not obtain data from the primary studies on time to event data, hazard ratios for mortality or continuous outcomes, we did not perform these meta‐analyses. The number of patients needed to treat (NNT) was calculated as 1/risk difference. The major comparisons were between immunoglobulin vs. placebo / no treatment and pooled vs. monoclonal immunoglobulins.
We assessed for heterogeneity in the results of the trials using a chi‐squared test of heterogeneity (P < 0.1) and the I2 measure of inconsistency. We searched for reasons for heterogeneity assessing the following patient subgroups:
Different IVIG doses and administration schedules
Use of antifungal prophylaxis.
Although pre‐planned, we did not perform subgroup analyses of children, adults, according to CMV sero‐status of bone marrow transplant recipients, or according to CMV screening or pre‐emptive treatment since we did not have enough participants for each subgroup or the necessary data.
We performed sensitivity analyses to assess the effect of individual methodological quality measures on effect estimates, including allocation generation, concealment and blinding. We visually examined funnel plots for mortality and clinically documented infections (1/standard error plotted against RR) in order to estimate potential selection bias (publication or other).
Results
Description of studies
The computerized search strategy identified 855 studies, (including 8 abstracts from conference proceedings) not all relevant for the present review. These were screened for randomized or quasi‐randomized controlled trials according to protocol and their references were searched in order to identify additional references. 73 studies were considered for this review, including 8 abstracts from conference proceedings (for QUORUM diagram see Figure 1).
1.
Flow diagram
We excluded 28 studies for the following reasons (Table of excluded studies): The design of 23 was incompatible with inclusion criteria (13 non‐randomised controlled trials, 2 review articles, 1 meta‐analysis, 1 phase I study which was not a RCT, 3 retrospective studies and 3 reports of subcategories of other studies (Aulitzky 1991; Bunch 1988; Chapel 1992; Chapel 1993; Cortez 2002; Elfenbein 1989; Esperou 2004; Fehir 1989; Gamm 1994; Gerein 1989; Graham Pole 1988; Jurlander 1994; Klaesson 1995; Messori 1994; Nasman Bjork 1999; , Nurnberger 1988; Petersen 1987; Rand 1991; Spitzer 1992; Sullivan 1996; Sullivan 1998; Terada 1980; Vu Van 1985), 3 studies were excluded since they reported on immunoglobulin prophylaxis in patients treated for acute leukemia or solid tumors (Bode 1986, Collins 1991, Gimesi 1992), 1 study reported on patients with primary hypogammaglobulinemia who received standard‐dose immunoglobulin therapy (Eijkhout 2001) and 1 study reported on the results of oral and not parenterally administered immunoglobulins (Copelan 1994 ).
Five reports were identified as duplicate publications and were considered under their primary reference (O'Reilly 1983; Griffiths 1989; Kubaneck 1985; Jackson 1993; Winston 1985). Forty trials were included in our review. Of them 18 studies were multicenter studies (Cordonnier 2003; Winston 2001; Ruutu 1997; Abdel‐Mageed 1999; Boeckh 2001; Sklenar 1993; Salmon 1967; Chapel 1994c; Wolff 1993; Molica 1996; Chapel 1994; Cooperative CLL 1988; Condie 1984; Boughton 1995; Ringden 1987; Jacobsen 1985; Graham Pole 1990; Sullivan 2000) and 22 were single center trials (Zikos 1998; Winston 1982; Meyers 1983; Winston 1993; Poynton 1992; Filipovich 1992; Peltier 1992;Feinstein 1999; Musto 1995; Bowden 1991; Winston 1987; Bordigoni 1987; Winston 1984; Sullivan 1990; Lum 1994; Hargreaves 1992; Serrano 1999; Raiola 2002; Ustun 1998; Bowden 1986; Gluck 1990; Emanuel 1992). These studies randomized 4682 patients, mostly adults, and were published between the years 1967 to 2003.
4223 patients were included in the 30 studies assessing patients receiving prophylaxis after bone marrow or peripheral stem cell transplantation and 459 patients were included in the 10 studies assessing patients with lymphoproliferative disorders. One trial included multiple myeloma patients without specifying either the total number of patients or the number in each arm, therefore we could not include it in the analyses (Gluck 1990).
Of the 30 studies assessing immunoglobulin prophylaxis post transplant, 24 included patients undergoing allogeneic transplantation only (Cordonnier 2003; Winston 2001; Ruutu 1997; Zikos 1998; Abdel‐Mageed 1999; Winston 1982; Meyers 1983; Boeckh 2001; Winston 1993; Feinstein 1999; Bowden 1991; Winston 1987; Condie 1984; Winston 1984; Ringden 1987; Jacobsen 1985; Lum 1994; Graham Pole 1990; Serrano 1999; Raiola 2002; Sullivan 2000; Ustun 1998; Bowden 1986; Emanuel 1992), 5 included patients who either received allogeneic or autologous transplant (Poynton 1992; Filipovich 1992; Peltier 1992; Bordigoni 1987; Sullivan 1990) and 1 evaluated IVIG post‐autologous transplantation only (Wolff 1993). Among these studies, 18 evaluated the use of polyvalent immunoglobulins (Cordonnier 2003; Winston 2001; Abdel‐Mageed 1999; Winston 1993; Poynton 1992; Filipovich 1992; Peltier 1992; Wolff 1993; Feinstein 1999; Winston 1987; Winston 1984; Sullivan 1990; Lum 1994; Graham Pole 1990; Raiola 2002; Sullivan 2000; Ustun 1998; Emanuel 1992), 9 evaluated hyperimmune CMV immunoglobulins (CMV‐IVIG) (Ruutu 1997; Winston 1982; Meyers 1983; Boeckh 2001; Bowden 1991; Bordigoni 1987; Ringden 1987; Serrano 1999; Bowden 1986) and 3 compared between polyvalent immunoglobulins and hyperimmune CMV‐IVIG (Zikos 1998; Condie 1984; Jacobsen 1985). Among the 18 studies evaluating polyvalent immunoglobulins administration, immunoglobulins were compared to placebo in 1 study (Sullivan 2000), to no intervention in 10 trials (Winston 1993; Poynton 1992; Wolff 1993; Feinstein 1999; Winston 1987; Winston 1984; Sullivan 1990; Lum 1994; Ustun 1998; Emanuel 1992), different products and different doses were compared in 2 (Filipovich 1992; Peltier 1992) and in 3 studies respectively (Winston 2001; Abdel‐Mageed 1999; Graham Pole 1990), in 1 study both different doses and placebo were compared (Cordonnier 2003) and in 1 study both different products and different doses were evaluated (Raiola 2002). Among the 9 studies reporting on hyperimmune CMV‐IVIG, immunoglobulins were compared to no intervention in 8 trials (Ruutu 1997; Winston 1982; Meyers 1983; Bowden 1991; Bordigoni 1987; Ringden 1987; Serrano 1999; Bowden 1986) and to placebo in 1 trial (Boeckh 2001). 8 trials included only patients who underwent allogeneic transplantation (Ruutu 1997; Winston 1982; Meyers 1983; Boeckh 2001; Bowden 1991; Ringden 1987; Serrano 1999; Bowden 1986) and in 1 trial either allogeneic or autologous transplant patients were randomized (Bordigoni 1987).
Among the 10 studies of LPD, 4 evaluated patients with CLL (Boughton 1995; Chapel 1994c; Cooperative CLL 1988; Molica 1996), 4 MM patients (Chapel 1994; Musto 1995; Salmon 1967; Hargreaves 1992), 1 both CLL and MM patients (Sklenar 1993) and 1 both MM and low risk non‐Hodgkin lymphoma (Gluck 1990 ). All studies used polyvalent immunoglobulins. In 5 studies immunoglobulins were compared to placebo (Salmon 1967; Chapel 1994; Cooperative CLL 1988; Boughton 1995; Hargreaves 1992), in 3 to no intervention (Molica 1996; Musto 1995; Gluck 1990) and in 2, different doses of immunoglobulins were compared (Sklenar 1993; Chapel 1994c). Two studies in the LPD group were crossover studies (Molica 1996; Musto 1995). These studies were not included in the meta‐analysis since data for the first randomization was not available separately and the combined data could not be used for meta‐analysis. One study comparing various products was composed of two phases: in phase I ‐ 21 patients were randomized to receive one of 3 polyvalent immunoglobulins products weekly and in phase II ‐ 32 patients were randomized to receive the various polyvalent immunoglobulins products every other week. Since the only outcome evaluated in this study was immunoglobulin levels, it was not included in our analyzes (Peltier 1992).
Thirty‐two studies consisted of 2 arms. 5 studies had 3 arms (Winston 2001; Boeckh 2001; Sklenar 1993; Peltier 1992; Condie 1984) and 3 studies had 4 arms (Cordonnier 2003; Filipovich 1992; Bowden 1986). This was mainly due to the use of 3‐4 doses or products of immunoglobulins. Of these, 5 studies involved transplant patients receiving polyvalent immunoglobulins (Cordonnier 2003; Winston 2001; Sklenar 1993; Filipovich 1992; Peltier 1992) and 3 involved transplant patients receiving hyperimmune CMV‐IVIG (Boeckh 2001; Condie 1984; Bowden 1986). None of the studies dealing with lymphoproliferative disorders had more than two arms.
Risk of bias in included studies
Generation of randomization sequence was described as adequate in 11 studies (classified as A). In the remaining 29 studies it was not specified (classified as B). Allocation concealment was adequate by description in 9 studies (classified as A, table of included studies). In the remaining 31 studies allocation concealment was not clear (classified as B, table of included studies). 13 studies were conducted in a double‐blinded fashion. All remaining trials were open. 15 studies reported results per protocol, (Abdel‐Mageed 1999; Bowden 1986; Bowden 1991; Chapel 1994; Cooperative CLL 1988; Feinstein 1999; Graham Pole 1990; Meyers 1983; Poynton 1992; Salmon 1967; Sullivan 1990; Winston 1982; Winston 1984; Winston 1987; Winston 2001). In 3 studies it was unknown whether patients were excluded after randomization (Gluck 1990; Hargreaves 1992). In the remaining 12 studies analysis was performed by intention to treat. 21 studies reported that patients gave their consent to participate in the research. Approval of the ethics committee was reported in 18 of them.
Effects of interventions
BONE MARROW TRANSPLANTATION AND HEMATOPOIETIC STEM CELL TRANSPLANTATION For patients undergoing transplantation (30 studies) prophylaxis was initiated in most studies during conditioning (26 studies) or immediately after transplant (4 studies) and was administered either weekly (15 trials), bi‐weekly (6 trials) or by using a different schedule (9 trials). In most studies immunoglobulin prophylaxis was given for 3 months and the maximum period of administration was 1 year (see table of included studies). In 24 out of the 30 studies, immunoglobulins were administered in the setting of hospitalization in isolation precaution conditions for at least part of the treatment period (Cordonnier 2003; Zikos 1998; Winston 1982; Meyers 1983; Boeckh 2001; Winston 1993; Poynton 1992; Filipovich 1992; Wolff 1993; Feinstein 1999; Bowden 1991; Winston 1987; Bordigoni 1987; Condie 1984; Winston 1984; Ringden 1987; Jacobsen 1985; Sullivan 1990; Lum 1994; Serrano 1999; Raiola 2002; Bowden 1986; Emanuel 1992; Abdel‐Mageed 1999) and the remaining 6 studies did not report on the trial setting (Winston 2001; Ruutu 1997; Peltier 1992; Graham Pole 1990; Sullivan 2000; Ustun 1998).
I.Polyvalent immunoglobulins versus placebo or no intervention (control)
Primary outcome measures1.All cause mortalityEight trials including 1418 patients, out of 12 trials that compared polyvalent immunoglobulins to control reported this outcome (Figure 2). The time of follow up for mortality reporting was heterogeneous in these studies. This comparison includes 5 trials which reported mortality at 100 ‐ 200 days) and 3 trials which reported mortality at more than 2 years (comparison 1.3). Overall, there was no difference in the risk for all‐cause mortality between polyvalent immunoglobulins and placebo or no intervention, RR 0.99 (95% CI 0.88 to 1.12). There was no statistical evidence of heterogeneity (P = 0.4, I2 = 3.3%) (Figure 2). Since there was variability in the time point for reporting mortality, and since some trials reported mortality at 2 different time points, we also divided these analyses to two graphs. The first, (Figure 3 ) with 4 trials including 881 participants reported mortality at 100 days (RR 1.03, 95% CI 0.83 to 1.26) with no statistical evidence of heterogeneity (P = 0.12, I2 = 48%). The second, (Figure 4) with 5 trials including 737 patients reported mortality at 1‐2 years with borderline heterogeneity (RR 1.07, 95% CI 0.94 to 1.21) (P = 0.09, I2 = 51%). We separated the trials according to type of transplant, whether allogeneic or autologous. Our analysis of mortality according to transplant type did not yield a difference in mortality between polyvalent immunoglobulins and control for the allo‐BMT only group, RR 1.07 (95% CI 0.79 to 1.44) and for the allo‐BMT and auto‐BMT group, RR 0.95 (95% CI 0.81 to 1.10) (Figure 5). We also divided the trials according to the use of antifungal prophylaxis. Again, there was no difference in mortality rate between polyvalent immunoglobulins and control in trials in which the patients received or did not receive antifungal prophylaxis RR 1.07 (95% CI 0.74 to 1.53) and RR 0.88 (95% CI 0.76 to 1.02), respectively) (Figure 6). We analyzed separately trials in which the polyvalent immunoglobulins dose was above 500mg/kg, and there was no difference in mortality for patients given high‐dose polyvalent immunoglobulins vs. control, RR 1.06 (95% CI 0.91 to 1.23) (Figure 7)
2.

Forest plot of comparison: 1 Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, outcome: 1.1 All‐cause Mortality.
3.

Forest plot of comparison: 1 Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, outcome: 1.2 All cause mortality 100 days.
4.

Forest plot of comparison: 1 Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, outcome: 1.3 All‐cause Mortality at 1‐2years and more.
5.

Forest plot of comparison: 1 Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, outcome: 1.4 All‐cause Mortality ‐ by type of HSCT.
6.

Forest plot of comparison: 1 Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, outcome: 1.5 All cause mortality ‐by use of antifungal prophylaxis.
7.

Forest plot of comparison: 1 Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, outcome: 1.6 All‐cause Mortality ‐ high dose IVIG.
Sensitivity Analysis There was no difference in all cause mortality between trials of adequate randomization generation (RR 1.40, 95% CI 0.88 to 2.22) (Figure 8) and those in which randomization generation was not clear (RR 0.93, 95% CI 0.83 to 1.05). There was also no difference in all cause mortality between trials which were blinded (RR 0.94, 95% CI 0.76 to 1.17)) and those not blinded (RR 0.99, 95% CI 0.86 to 1.14) (Figure 9). Only one trial reporting all cause mortality of transplant patients receiving polyvalent immunoglobulins had adequate allocation concealment (Cordonnier 2003), thus we did not conduct sensitivity analysis. We conducted sensitivity analysis for mortality also according to ITT versus per protocol. There was no difference in all cause mortality between trials which were analyzed by ITT (RR 1.04, 95% CI 0.87 to 1.24) or per protocol (RR 0.91, 95% CI 0.79 to 1.06) (Figure 10).We did not perform sensitivity analysis according to allocation concealment because only one trial was of adequate allocation concealment (Cordonnier 2003)
8.

Forest plot of comparison: 1 Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, outcome: 1.7 All‐cause Mortality ‐sensitivity analysis by randomization generation.
9.

Forest plot of comparison: 1 Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, outcome: 1.8 All‐cause Mortality ‐sensitivity analysis by double blinding.
10.

Forest plot of comparison: 1 Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, outcome: 1.22 All‐cause Mortality ‐sensitivity analysis by ITT.
The funnel plot for mortality was difficult to interpret due to the small number of studies (additional figures: Figure 11).
11.
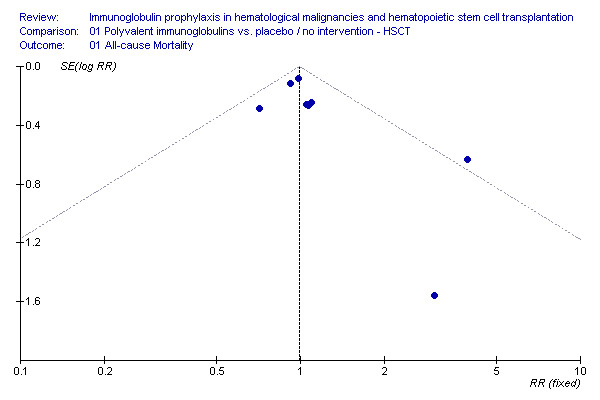
Funnel plot: all cause mortality, IVIG vs. no treatment, HSCT
2.Clinically documented infectionsFive trials which reported clinically documented infections included 688 participants. Polyvalent immunoglobulins administration did not result in a reduction in the occurrence of clinically documented infections, RR 1.00 (95% CI 0.90 to 1.10). There was no evidence for heterogeneity in these comparisons (P = 0.97, I2 = 0%) (Figure 12).
12.

Forest plot of comparison: 1 Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, outcome: 1.9 Clinically documented infections.
Sensitivity Analysis There was no difference in clinically documented infections between trials of adequate randomization generation (RR 0.99, 95% CI 0.86 to 1.14) (Figure 13) and those in which randomization generation was not clear (RR 1.00, 95% CI 0.86 to 1.17). There was also no difference in a clinically documented infections between trials which were blinded (RR 1.01, 95% CI 0.91 to 1.12) and those not blinded (RR 0.99, 95% CI 0.86 to 1.15), (Figure 14).
13.

Forest plot of comparison: 1 Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, outcome: 1.24 Clinically Documented Infections‐ sensitivity analysis by randomization generation.
14.

Forest plot of comparison: 1 Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, outcome: 1.25 Clinically documented infections ‐ sensitivity analysis by blinding.
Secondary outcome measures 3.Microbiologically documented infections (bacterial)Seven trials that reported this outcome included 1186 participants. Polyvalent immunoglobulins prophylaxis did not result in a decrease in the occurrence of microbiologically documented bacterial infections, when analyzed per patient, RR 1.00 (95% CI 0.88 to 1.15 ) (Figure 15), or as episode per patient‐months, RR 0.97 (95% CI 0.82 to 1.16) (Figure 16).
15.

Forest plot of comparison: 1 Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, outcome: 1.10 Microbiologically documented infections ‐ bacterial.
16.

Forest plot of comparison: 1 Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, outcome: 1.11 Microbiologically documented infections ‐ patient months.
4.CMV infection and interstitial pneumonitisSix trials which reported CMV infections included 986 participants. There was no statistically significant difference in the occurrence of CMV infections when analyzed per patient RR 0.84 (95% CI 0.66 to 1.07) (Figure 17) (Figure 18) (Figure 19) or as episodes per patient months RR 0.70 (95% CI 0.49 to 1.02) (Figure 20). Polyvalent immunoglobulins significantly reduced the risk for developing interstitial pneumonitis by 36% (RR 0.64, 95% CI 0.45 to 0.89), 7 trials, 990 patients (Figure 21) (Figure 18) (Figure 19). It should be noted that sensitivity analysis revealed that the significant reduction in interstitial pneumonitis was only in trials of unclear randomization generation (RR 0.52, 95% CI 0.36 to 0.76) and in trials of adequate randomization the effect was not significant (RR 1.66, 95% CI 0.57 to 4.85) (Figure 22). In addition, the significant reduction remained only for trials which were double blind (RR 0.58, 95% CI 0.41 to 0.82) (Figure 23), as opposed to non‐blinded trials (RR 1.56, 95% CI 0.47 to 5.19).
17.

Forest plot of comparison: 1 Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, outcome: 1.12 CMV infections.
18.

Forest plot of comparison: 1 Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, outcome: 1.20 CMV Infections and Interstitial pneumonitis.
19.

Forest plot of comparison: 1 Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, outcome: 1.23 CMV Infections, Interstitial pneumonitis and VOD.
20.

Forest plot of comparison: 1 Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, outcome: 1.13 CMV infections ‐ patient months.
21.

Forest plot of comparison: 1 Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, outcome: 1.14 Interstitial Pneumonitis.
22.

Forest plot of comparison: 1 Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, outcome: 1.28 IP ‐ sensitivity analysis by randomization generation.
23.

Forest plot of comparison: 1 Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, outcome: 1.29 IP ‐ sensitivity analysis by blinding.
5.Infection related mortalityThree trials which compared polyvalent immunoglobulins to placebo or no intervention included 275 participants. Polyvalent immunoglobulins administration did not result in a decrease in the risk of infection‐related death, RR 0.64 (95% CI 0.28 to 1.49) (Figure 24). Although there was a 36% reduction in risk, the 95% confidence was too wide to conclude on benefit.
24.

Forest plot of comparison: 1 Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, outcome: 1.15 Infection‐related Mortality.
6.Acute GVHDSeven trials that reported this outcome included 989 participants. Polyvalent immunoglobulins prophylaxis did not result in a decrease in the occurrence of acute GVHD, RR 0.93 (95% CI 0.83 to 1.04) (Figure 25).
25.

Forest plot of comparison: 1 Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, outcome: 1.16 Acute GVHD.
7.Veno‐occlusive disease (VOD)Four trials including 447 patients reported the occurrence of VOD. When compared to control, polyvalent immunoglobulins prophylaxis resulted in a significantly increased risk for developing VOD, RR 2.73 (95% CI 1.11 to 6.71), (Figure 26) (Figure 19). We separated the trials according to type of transplant, whether allogeneic or autologous. Our analysis of VOD according to transplant type showed increased risk for both groups: the allogeneic only group, RR 2.04 (95% CI 0.76 to 5.49) and the autologous only group, RR 11.8 (95% CI 0.66 to 210.03) (Figure 27). However, these results were not statistically significant, Moreover, since the autologous group included only one trial these results should be interpreted with caution (Wolff 1993). In addition we conducted sensitivity analysis according randomization generation. It should be noted that trials of adequate randomization yielded a significant increase in VOD (RR 3.35, 95% CI 1.19 to 9.47), while for trials of unclear randomization this effect was not significant (Figure 28). Sensitivity analysis according to blinding did not affect results (Figure 29).
26.

Forest plot of comparison: 1 Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, outcome: 1.17 VOD.
27.

Forest plot of comparison: 1 Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, outcome: 1.19 VOD according to type of transplant.
28.

Forest plot of comparison: 1 Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, outcome: 1.26 VOD ‐ sensitivity analysis according to randomization generation.
29.

Forest plot of comparison: 1 Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, outcome: 1.27 VOD ‐ sensitivity analysis by blinding.
8.Adverse eventsFive trials, which included 728 patients (Winston 1984, Winston 1987, Sullivan 1990, Winston 1993, Cordonnier 2003) reported adverse events, with a significant increase for developing adverse events: RR 8.12 (95% CI 3.15 to 20.97) (Figure 30). Only one trial was double‐blind and reported adverse events in the control group (Cordonnier 2003), while the remaining open trials did not report adverse events in the control group. Adverse events were not common (4 of 18 cases (Winston 1984), 9 of 38 cases (Winston 1987), 14 of 184 cases (Sullivan 1990), 7 of 27 cases (Winston 1993), 27 of 150 cases (Cordonnier 2003). Adverse events did not require discontinuation of treatment. They included mainly early adverse events: fever, chills, nausea and vomiting, headaches, myalgias, rash and hypotension without anaphylaxis.
30.

Forest plot of comparison: 1 Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, outcome: 1.18 Adverse Events.
9. Fungal InfectionsFive trials reported this outcome. Polyvalent immunoglobulins prophylaxis did not result in a decrease in the occurrence of fungal infections, RR 0.95 (95% CI 0.72 to 1.25) (Figure 31).
31.

Forest plot of comparison: 1 Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, outcome: 1.30 Fungal Infections.
10. Bacteremia Four trials reported this outcome. Polyvalent immunoglobulins prophylaxis did not result in a decrease in the occurrence of bacteremia, RR 1.03 (95% CI 0.93 to 1.13) (Figure 32).
32.

Forest plot of comparison: 1 Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, outcome: 1.31 Bacteremia.
II.Hyperimmune CMV‐IVIG versus placebo or no intervention (control)Primary outcome measures
1.All cause mortality
Four trials including 288 patients out of nine trials which compared hyperimmune CMV‐IVIG to control reported this outcome. There was no difference in the risk for all‐cause mortality between hyperimmune CMV‐IVIG and placebo or no intervention, RR 0.86 (95% CI 0.63 to 1.16) (Figure 33). There was no statistical evidence of heterogeneity (P = 0.68, I2 = 0%) (comparison 2.1). Mortality was assessed in these studies between 62 days and 5 years after randomization. Three trials including 234 participants reported mortality at 100 days, RR 0.89 (95% CI 0.64 to 1.24) (Figure 34). Again, there was no statistical evidence of heterogeneity (P = 0.48, I2 = 0%). 2.Clinically documented infectionsThere were no data on this outcome
33.

Forest plot of comparison: 2 Hyperimmune CMV‐IVIG vs. placebo / no intervention ‐ HSCT, outcome: 2.1 All‐cause Mortality.
34.

Forest plot of comparison: 2 Hyperimmune CMV‐IVIG vs. placebo / no intervention ‐ HSCT, outcome: 2.2 All‐cause Mortality ‐ 100d (3‐4mo).
Secondary outcome measures 3.CMV infection and interstitial pneumonitis Eight trials which reported CMV infections included 553 participants and five trials reported on interstitial pneumonitis in 345 patients. Hyperimmune CMV‐IVIG prophylaxis did not result in a decrease in the occurrence of CMV infections, RR 1.02 (95% CI 0.82 to 1.26) (Figure 35) or interstitial pneumonitis (RR 0.95 (95% CI 0.58 to 1.56) (Figure 36).
35.

Forest plot of comparison: 2 Hyperimmune CMV‐IVIG vs. placebo / no intervention ‐ HSCT, outcome: 2.3 CMV infection.
36.

Forest plot of comparison: 2 Hyperimmune CMV‐IVIG vs. placebo / no intervention ‐ HSCT, outcome: 2.4 Interstitial Pneumonitis.
4.Infection related mortalityThree trials which compared polyvalent immunoglobulins CMV‐IVIG to placebo or no intervention included 234 participants. Hyperimmune CMV‐IVIG administration did not result in a statistically significant decrease in the risk of infection‐related death, RR 0.67 (95% CI 0.34 to 1.32) (Figure 37).
37.

Forest plot of comparison: 2 Hyperimmune CMV‐IVIG vs. placebo / no intervention ‐ HSCT, outcome: 2.5 Infection‐related Mortality.
5.Acute GVHDFive trials that reported this outcome included 342 participants. Hyperimmune CMV‐IVIG prophylaxis did not result in a decrease in the occurrence of acute GVHD, RR 1.02 (95% CI 0.72 to 1.44) (Figure 38).
38.

Forest plot of comparison: 2 Hyperimmune CMV‐IVIG vs. placebo / no intervention ‐ HSCT, outcome: 2.6 Acute GVHD.
6.Adverse eventsOnly one study including 54 patients reported adverse effects for hyperimmune CMV‐IVIG as compared to control. Therefore, meta‐analysis could not be done.
7. Fungal InfectionsTwo trials reported this outcome. Hyperimmune CMV‐IVIG prophylaxis did not result in a decrease in the occurrence of fungal infections, RR 1.02 (95% CI 0.54 to 1.93) (Figure 39).
39.

Forest plot of comparison: 2 Hyperimmune CMV‐IVIG vs. placebo / no intervention ‐ HSCT, outcome: 2.8 Fungal Infections.
8. BacteremiaOnly one trial reported this outcome. Therefore, meta‐analysis could not be done.
III.IVIG or anti CMV‐IVIG versus placebo or no intervention (control)Primary outcome measures We pooled all trials that assessed IVIG, whether polyvalent immunoglobulins or specific hyperimmune CMV‐IVIG. 1.All cause mortalityTwelve trials including 1706 patients which compared polyvalent immunoglobulins or specific hyperimmune CMV‐IVIG to control reported this outcome. Pooling all trials showed no difference in mortality, RR 0.97 (95% CI 0.87 to 1.09) (Figure 40). Importantly, there was no statistical evidence of heterogeneity (P = 0.54, I2 = 0%).
40.

Forest plot of comparison: 3 Polyvalent immunoglobulins or hyperimmune CMV‐IVIG vs. placebo / no intervention ‐ HSCT, outcome: 3.1 All‐cause Mortality.
2. All‐cause Mortality ‐ 100d (3‐4 mo) Eight trials including 1178 patients which compared polyvalent immunoglobulins or specific hyperimmune CMV‐IVIG to control reported this outcome. Pooling all trials showed no difference in mortality, RR 0.96 (95% CI 0.82 to 1.14) (Figure 41). Importantly, there was no statistical evidence of heterogeneity (P = 0.26, I2 = 20.8%).
41.

Forest plot of comparison: 3 Polyvalent immunoglobulins or hyperimmune CMV‐IVIG vs. placebo / no intervention ‐ HSCT, outcome: 3.2 All‐cause Mortality ‐ 100d (3‐4 mo).
3. CMV infection and interstitial pneumonitisThirteen trials including 1511 patients which compared polyvalent immunoglobulins or specific hyperimmune CMV‐IVIG to control reported this outcome. Pooling all trials showed no difference in CMV infections, RR 0.90 (95% CI 0.76 to 1.06) (Figure 42). Importantly, there was no statistical evidence of heterogeneity (P = 0.11, I2 = 33.6%). Polyvalent immunoglobulins or hyperimmune anti CMV‐IVIG significantly reduced the risk for developing interstitial pneumonitis by 38% (RR 0.72 (95% CI 0.55 to 0.95) (Figure 43). Importantly, there was no statistical evidence of heterogeneity (P = 0.25, I2 = 19.6%).
42.

Forest plot of comparison: 3 Polyvalent immunoglobulins or hyperimmune CMV‐IVIG vs. placebo / no intervention ‐ HSCT, outcome: 3.3 CMV infection.
43.

Forest plot of comparison: 3 Polyvalent immunoglobulins or hyperimmune CMV‐IVIG vs. placebo / no intervention ‐ HSCT, outcome: 3.4 Interstitial Pneumonitis.
4. Infection‐related MortalitySix trials including 509 patients which compared polyvalent immunoglobulins or specific hyperimmune CMV‐IVIG to control reported this outcome. Pooling all trials showed no difference in infection related mortality, RR 0.66 (95% CI 0.39 to 1.12) (Figure 44). Importantly, there was no statistical evidence of heterogeneity (P = 0.5, I2 = 0%).
44.

Forest plot of comparison: 3 Polyvalent immunoglobulins or hyperimmune CMV‐IVIG vs. placebo / no intervention ‐ HSCT, outcome: 3.5 Infection‐related Mortality.
5. Acute GVHD Twelve trials including 1331 patients which compared polyvalent immunoglobulins or specific hyperimmune CMV‐IVIG to control reported this outcome. Pooling all trials showed no difference in acute GVHD, RR 0.94 (95% CI 0.84 to 1.05) (Figure 45). Importantly, there was no statistical evidence of heterogeneity (P = 0.54, I2 = 0%).
45.

Forest plot of comparison: 3 Polyvalent immunoglobulins or hyperimmune CMV‐IVIG vs. placebo / no intervention ‐ HSCT, outcome: 3.6 Acute GVHD.
6. Adverse EventsSix trials, which included 782 patients reported adverse effects, with a significant increase for developing adverse effects: RR 8.02 (95% CI 3.25 to 19.78) (Figure 46). Importantly, there was no statistical evidence of heterogeneity (P = 0.74, I2 = 0%). IV.Polyvalent immunoglobulins versus hyperimmune CMV‐IVIG Primary outcome measures
46.

Forest plot of comparison: 3 Polyvalent immunoglobulins or hyperimmune CMV‐IVIG vs. placebo / no intervention ‐ HSCT, outcome: 3.7 Adverse Events.
1.All cause mortalityThree trials including 212 patients which compared polyvalent immunoglobulins to hyperimmune CMV‐IVIG reported all cause mortality. Mortality was higher with polyvalent IVIG without statistical significance, RR 1.46 (95% CI 0.92 to 2.32) (Figure 47). There was no statistical evidence of heterogeneity (P = 0.99, I2 = 0%). Mortality was assessed in these studies between 110 days and 4 years after randomization. 2.Clinically documented infectionsOne trial that reported clinically documented infections included 128 participants. Therefore, meta‐analysis could not be done. Secondary outcome measures
47.

Forest plot of comparison: 4 Polyvalent immunoglobulins vs. hyperimmune CMV‐IVIG ‐ HSCT, outcome: 4.1 All‐cause Mortality.
3.CMV infection and interstitial pneumonitisThree trials which reported CMV infections included 212 participants. Polyvalent immunoglobulins prophylaxis was associated with an increased risk to develop CMV infection as compared to hyperimmune CMV‐IVIG prophylaxis, RR 1.42 (95% CI 1.07 to 1.89) (Figure 48) although there was no difference between them regarding the occurrence of interstitial pneumonitis RR 0.83 (95% CI 0.40 to 1.75) (Figure 49).
48.

Forest plot of comparison: 4 Polyvalent immunoglobulins vs. hyperimmune CMV‐IVIG ‐ HSCT, outcome: 4.3 CMV Infection.
49.

Forest plot of comparison: 4 Polyvalent immunoglobulins vs. hyperimmune CMV‐IVIG ‐ HSCT, outcome: 4.4 Interstitial Pneumonitis.
4.Infection related mortalityTwo trials which compared polyvalent immunoglobulins to hyperimmune CMV‐IVIG included 177 participants. Results of the comparison favored hyperimmune CMV‐IVIG without statistical significance, RR 3.28 (95% CI 0.95 to 11.26) (Figure 50).
50.

Forest plot of comparison: 4 Polyvalent immunoglobulins vs. hyperimmune CMV‐IVIG ‐ HSCT, outcome: 4.5 Infection‐related Mortality.
5.Acute GVHDTwo trials which compared polyvalent immunoglobulins to hyperimmune CMV‐IVIG included 163 participants. Results of the comparison favored hyperimmune CMV‐IVIG without statistical significance, RR 1.23 (95% CI 0.87 to 1.75) (Figure 51).
51.

Forest plot of comparison: 4 Polyvalent immunoglobulins vs. hyperimmune CMV‐IVIG ‐ HSCT, outcome: 4.6 Acute GVHD.
6.Adverse eventsNo studies comparing between polyvalent immunoglobulins and hyperimmune CMV‐IVIG prophylaxis reported this outcome
V.Polyvalent immunoglobulins 250 mg/kg versus polyvalent immunoglobulins 500 mg/kg
Primary outcome measures
1.All cause mortalityOne trial including 412 patients which compared these 2 doses of immunoglobulins reported all cause mortality. Therefore, meta‐analysis could not be done.
2.Clinically documented infectionsTwo trials that reported clinically documented infections included 509 participants. There was a slight decrease in the occurrence of clinically documented infections with the lower dose, RR 0.89 (95% CI 0.81 to 0.97) (Figure 52). Secondary outcome measures 3.Microbiologically documented infectionsTwo trials that reported this outcome included 509 participants. There was a slight increase in the occurrence of microbiologically documented infections with the lower dose, RR 1.28 (95% CI 1.04 to 1.57) (Figure 53). This discrepancy between clinically and microbiologically documented infections could stem either from the small number of trials (only two for each comparison) or from the different definitions, i.e. there is not necessarily overlap between the two outcomes.
52.

Forest plot of comparison: 5 Polyvalent immunoglobulins 250mg/kg vs. Polyvalent immunoglobulins or hyperimmune CMV‐IVIG 500mg/kg ‐ HSCT, outcome: 5.2 Clinically documented Infection.
53.

Forest plot of comparison: 5 Polyvalent immunoglobulins 250mg/kg vs. Polyvalent immunoglobulins or hyperimmune CMV‐IVIG 500mg/kg ‐ HSCT, outcome: 5.3 Microbiologically documented Infection.
4.CMV infection and Interstitial pneumonitisOne trial which reported CMV infections included 412 participants. Therefore, meta‐analysis could not be done. Two studies which reported interstitial pneumonitis included 509 participants. There was no difference in the occurrence of interstitial pneumonitis between the two doses, RR 0.98 (95% CI 0.33 to 2.92) (Figure 54).
54.

Forest plot of comparison: 5 Polyvalent immunoglobulins 250mg/kg vs. Polyvalent immunoglobulins or hyperimmune CMV‐IVIG 500mg/kg ‐ HSCT, outcome: 5.5 Interstitial Pneumonitis.
5.Infection related mortalityOne trial which compared polyvalent immunoglobulins to hyperimmune CMV‐IVIG included 412 participants. Therefore, meta‐analysis could not be done.
6.Acute GVHDThree trials which reported acute GVHD included 841 participants. There was a higher rate of acute GVHD with polyvalent immunoglobulins at a dose of 250 mg/kg as compared to 500 mg/kg, RR 1.32 (95% CI 1.13 to 1.55) (Figure 55).
55.

Forest plot of comparison: 5 Polyvalent immunoglobulins 250mg/kg vs. Polyvalent immunoglobulins or hyperimmune CMV‐IVIG 500mg/kg ‐ HSCT, outcome: 5.8 Acute GVHD.
II.LYMPHOPROLIFERATIVE DISORDERS
For patients with LPD (10 trials) polyvalent immunoglobulins were administered for a maximal period of two years. They were administered every two weeks in one trial (Salmon 1967), every three weeks in three trials (Sklenar 1993, Cooperative CLL 1988, Boughton 1995) or every four weeks in five trials (Chapel 1994c, Molica 1996, Musto 1995, Chapel 1994, Gluck 1990) (see table of included studies). In one trial the schedule of immunoglobulins administration was not mentioned (Hargreaves 1992).
I.Polyvalent immunoglobulins versus placebo or no intervention (control)Primary outcome measures 1.All cause mortality Two out of seven trials which compared polyvalent immunoglobulins to control included 163 patients and reported this outcome. There was no difference in the risk for all‐cause mortality between immunoglobulins and control, RR 1.36 (95% CI 0.58 to 3.19) There was no statistical evidence of heterogeneity (P = 0.60, I2 = 0%) (Figure 56). The time period during which mortality was assessed in these studies was 1 year.
56.

Forest plot of comparison: 6 Polyvalent immunoglobulins vs. placebo / no intervention ‐ MM/CLL, outcome: 6.1 All‐cause Mortality.
2.Clinically documented infectionsThree trials that reported clinically documented infections included 205 participants. Immunoglobulins significantly reduced the risk for developing clinically documented infections by 51%, RR 0.49 (95% CI 0.39 to 0.61) There was no statistical evidence of heterogeneity (P = 0.80, I2 = 0%) (Figure 57). The corresponding NNT was 2 patients (95% CI 2 to 3).
57.

Forest plot of comparison: 6 Polyvalent immunoglobulins vs. placebo / no intervention ‐ MM/CLL, outcome: 6.2 Clinically‐documented infections.
Secondary outcome measures 3.Microbiologically documented infections and bacteremiaThree trials that reported this outcome included 205 participants. There was a significant reduction in the occurrence of microbiologically documented infections with the use of immunoglobulins as compared to control, RR 0.71 (95% CI 0.53 to 0.95) (Figure 58). Two studies which included 124 patients, reported the occurrence of bacteremia. There was no difference in reduction in the occurrence of bacteremia RR 0.65 (95% CI 0.14 to 3.07) (Figure 59).
58.

Forest plot of comparison: 6 Polyvalent immunoglobulins vs. placebo / no intervention ‐ MM/CLL, outcome: 6.3 Microbiologically‐documented infections.
59.

Forest plot of comparison: 6 Polyvalent immunoglobulins vs. placebo / no intervention ‐ MM/CLL, outcome: 6.4 Bacteremia.
4.Infection related mortalityOnly one trial compared polyvalent immunoglobulins to control included 82 participants. Therefore, meta‐analysis could not be done.
5.Adverse eventsThree trials, which included 205 patients, reported adverse events. When compared to placebo or no intervention, polyvalent immunoglobulins caused a significant increase in adverse events, RR 2.37 (95% CI 1.74 to 3.24) (Figure 60). When data of the same studies were analyzed according to adverse events requiring discontinuation of prophylaxis, there was no statistically significant difference between polyvalent immunoglobulins and control RR 5.43 (95% CI 0.70 to 42.24) (Figure 61). Side effects were common (16 of 41 cases (Cooperative CLL 1988), 39 of 41 cases (Chapel 1994), 21 of 24 cases (Boughton 1995) but occurred also in the control arm in most studies. In a few cases adverse effects required discontinuation of treatment. These included mainly early side effects‐ fever, chills, nausea and vomiting, headaches, myalgias, rash and hypotension without anaphylaxis.
60.

Forest plot of comparison: 6 Polyvalent immunoglobulins vs. placebo / no intervention ‐ MM/CLL, outcome: 6.6 Adverse Events.
61.

Forest plot of comparison: 6 Polyvalent immunoglobulins vs. placebo / no intervention ‐ MM/CLL, outcome: 6.7 Adverse Events requiring discontinuation.
6.Fungal InfectionsOne trial reported this outcome. Therefore, meta‐analysis could not be done.
7. BacteremiaOne trial reported this outcome. Therefore, meta‐analysis could not be done.
Two trials were cross‐over studies and were not included in our meta‐analysis (Musto 1995, Molica 1996). No washout period was reported in these two trials. The first trial reported on 25 patients with multiple myeloma with low levels of immunoglobulins or a recent history of recurrent infections (Musto 1995). Patients received polyvalent immunoglobulins every 4 to 6 weeks or no treatment for 12 months and were then switched to observation or polyvalent immunoglobulins for another year. A total of 30 serious infections occurred in 250 patient‐months during the observation period compared with 10 infections in 261 patient‐months during the IVIG period. The second study enrolled 42 CLL patients with hypogammaglobulinemia and/or a history of at least one severe infection during the previous 6 months (Molica 1996). Patients received immunoglobulins every 4 to 6 weeks or no treatment for 6 months and were then switched to observation or immunoglobulins for another 12 months. The incidence of infections was significantly lower among the patients who completed the first 6 months of immunoglobulins as compared to observation. Similarly, the rate of infections was significantly lower among the patients who completed the 12 months of immunoglobulins as compared to observation.
Discussion
BONE MARROW AND STEM CELL TRANSPLANTATIONOur review included 30 trials evaluating the use of immunoglobulins as prophylaxis in patients undergoing BMT or PBSCT. We demonstrated that prophylaxis with polyvalent immunoglobulins or specific hyperimmune CMV‐ IVIG did not have an effect on mortality. Polyvalent immunoglobulins were associated with a decrease in interstitial pneumonitis. Polyvalent immunoglobulins or hyperimmune CMV‐IVIG had no influence on the other (infection‐related) outcomes or GVHD. The administration of polyvalent immunoglobulins increased the risk for VOD.
Our results are different from those of the meta‐analysis conducted by Bass 1993 which showed a reduction of all cause mortality with the use of immunoglobulins. When we checked the three studies that indicated that immunoglobulin reduced mortality in Bass' review we found that one of them was excluded from our analysis since it reported on the total number of deaths in the study but not separately on the number in the two arms (Graham Pole 1990); and one described two pilot studies compared to historical controls (Vu Van 1985). The third study compared polyvalent immunoglobulins to hyperimmune CMV‐IVIG and therefore was not included in our analysis comparing polyvalent immunoglobulins to placebo/ no treatment (Condie 1984). We found a borderline influence on the occurrence of CMV infections with the use of polyvalent immunoglobulins and no effect at all with the use of hyperimmune CMV‐IVIG. Similarly, Bass 1993 also found only a borderline significant reduction in symptomatic CMV infections with the use of immunoglobulins but when analyzed separately they found the rate of fatal CMV infections to be significantly reduced by the administration of immunoglobulins. Unlike us and Bass, Glowacki 1994 in their meta‐analysis showed a clear significant beneficial effect of immunoglobulins in terms of CMV infections. This difference might have been attributed to the difference in the study populations (bone marrow and solid organ recipients).
The most significant beneficial outcome in our review was the reduction of 36% in the occurrence of interstitial pneumonitis by polyvalent immunoglobulins, RR 0.64 (95% CI 0.45 to 0.89). This could not be confirmed with the use of hyperimmune CMV‐IVIG. Interestingly, a direct comparison between them did not yield any difference regarding the occurrence of interstitial pneumonitis, RR 0.83 (95% CI 0.40 to 1.75). This could be explained by the small number of trials. Our data did not allow us to separate between CMV and non‐CMV interstitial pneumonitis. Bass 1993 analyzed separately the occurrence of CMV and of non‐CMV interstitial pneumonia and found a reduction in both with IVIG.
We could not show such an effect of immunoglobulin therapy in prevention and treatment of GVHD in our meta‐analysis. This applied to prophylaxis with polyvalent immunoglobulins as well as hyperimmune CMV‐IVIG and for various preparations or doses. Concerns about increased incidence of hepatic VOD in patients receiving IVIG have been raised. This was mainly due to the study published by Cordonnier et al. (Cordonnier 2003). An interesting finding in our meta‐analysis was that pooled IVIG prophylaxis resulted in a significantly increased risk for developing VOD, RR 2.73 (95% CI 1.11 to 6.71) (Figure 9), although these results should be interpreted cautiously due to the small number of studies evaluating this outcome. Several possibilities might explain this increased risk. One explanation is attack of liver cells by of the immunoglobulins which contain high levels of antibodies in a similar way to another antibody, Gemtuzumab ozogamicin, which is associated with VOD through receptor mediated targeting of CD33 cells in the liver (Wadleigh 2003). Another mechanism suggested is through induction of hyperviscosity affecting the circulation in the small hepatic venules by pooled IVIG. The last option explaining this result is through the effects of cytokines triggered by immunoglobulin administration (Cordonnier 2003).
The number of trials comparing between different doses of IVIG in our meta‐analysis was small. When the dose of 500 mg/kg was compared to 250 mg/kg there was no difference in all cause mortality, infection related mortality, the occurrence of CMV infections, interstitial pneumonitis and VOD. Slight changes were noted regarding the rate of clinically documented infections, microbiologically documented infections and the rate of acute GVHD but the number of trials and the differences were too small to draw any conclusions. The lack of effect on mortality and of difference between the different preparations and doses of polyvalent immunoglobulins do not support a biological effect of immunoglobulins in the context of transplant.
Adverse effects associated with IVIG infusion can be divided into three types ‐ immediate, delayed and late. Usually fewer than 5% of patients experience clinically significant reactions in routine practice. In our review the absolute risk for adverse events with IVIG was higher (49/415, 11.8% unadjusted) and as expected the five studies assessing side effects showed a significant increase for developing adverse effects with IVIG as compared to control: RR 8.12 (95% CI 3.15 to 20.97).
In summary, since IVIG are associated with side effects, a higher rate of VOD and are highly expensive, current information does not support their use as routine prophylaxis for patients undergoing HSCT. LPD: CLL AND MULTIPLE MYELOMA
Only few randomized controlled studies evaluated the role of polyvalent immunoglobulins in MM and CLL in our systematic review. We therefore combined trials regarding both LPDs. There was lack of evidence of effect of prophylaxis with pooled IVIG on all cause mortality assessed after one year of treatment. Only two small studies reported on this outcome. The most significant finding was that polyvalent immunoglobulins reduced the risk for developing clinically documented infections by 51%, with a small number of patients needed to treat (NNT 2, 95% CI 2 to 3). All 3 trials evaluating this outcome and included in the analysis favored treatment with IVIG over control (Cooperative CLL 1988; Chapel 1994; Boughton 1995). We also found a reduction in the occurrence of microbiologically documented infections with the use of polyvalent immunoglobulins as compared to control, RR 0.71 (95% CI 0.53 to 0.95). We did not have enough data to do subgroup analysis according to immunoglobulin levels or previous infections. Therefore, conclusions as to which patients might benefit most from IVIG prophylaxis cannot be drawn. The trials included in our meta‐analysis included patients with hypogammaglobulinemia and recurrent infections and thus our results apply to these patients. Our findings support the recommendations of the NIH consensus paper stating that in hypogammaglobulinemia associated with CLL, polyvalent immunoglobulins can decrease the number of infections significantly although it has no effect on mortality. However, since the number of patients in these trials is rather small, only three trials including 205 patients actually registering the endpoints of clinically and microbiologically documented infections, conclusions regarding the benefit with regard to these outcomes should be regarded with some scepticism. Furthermore, conclusions concerning all cause mortality should be regarded even more cautiously since this outcome was evaluated in only two trials including 163 patients.
Limitations of this review
The major limitation is that the majority of the studies are old, with many of them reporting on patients treated in the 80's and 90's. The techniques and supportive treatments for patients undergoing transplantation for hematological malignancies have changed considerably during the last two decades which might have influence on the results. These changes include modifications of transplant regimens ‐ i.e. myeloablative vs. reduced intensity conditioning, the use of bone marrow vs. stem cell grafts, detection, prevention and treatment of infections, mainly gancyclovir for CMV and prophylaxis and treatment for bacterial infections and prevention and treatment of complications associated with BMT, mainly GVHD and VOD. In addition, in most studies the donors were HLA‐ matched siblings while the growing number of matched unrelated, haploidentical and cord blood transplants are not reflected in the older studies and could modify our results. The other limitation of our study is the relatively small number of prospective, randomized, controlled trials reported for the group of LPDs. Only two trials reported on all cause mortality in LPD. Most studies were of uncertain methodological quality with unclear randomization generation and allocation concealment. However, when we performed a sensitivity analysis of all cause mortality by randomization generation there was no difference in all cause mortality between trials of adequate randomization generation and those in which randomization generation was not clear.
Authors' conclusions
Implications for practice.
In patients undergoing HSCT, current information does not support the use of IVIG as routine prophylaxis, for the following reasons: prophylaxis with polyvalent immunoglobulins or hyperimmune CMV‐IVIG does not affect overall survival. The only outcome for which pooled IVIG was protective was interstitial pneumonitis. It was not proven as protective against acute GVHD, clinically documented infections, CMV infections or bacterial infections and was associated with a higher rate of VOD. Hyperimmune CMV‐IVIG did not affect any of the outcomes examined including all cause mortality, interstitial pneumonia, CMV infections, acute GVHD or VOD. Since these agents are associated with significant side effects, a higher rate of VOD and are highly expensive, current information does not support their use as routine prophylaxis for patients undergoing transplant. This is even more so, in view of the modern transplant techniques and the use of gancyclovir and broad spectrum antibiotic for prophylaxis and treatment. The use of IVIG may be considered in patients with hypogammaglobulinemia associated with CLL or MM and recurrent infections. This is since IVIG can decrease the number of infections significantly. Since the absolute risk reduction of clinically documented infections was high and the number needed to treat was small (2 patients), it might prove cost effective to use prophylactic IVIG in these patients especially in view of the costs of antibiotics, hospitalization days, loss of working days and other prophylactic measures.
Implications for research.
Among HSCT recipients trials comparing polyclonal or hyperimmune anti‐CMV IVIG are probably not warranted. More controlled trials on IVIG prophylaxis are needed in LPD patients with hypogammaglobulinemia and / or recurrent infections in order to assess the effect on overall survival and the best schedule of IVIG administration. Future trials should adhere more to adequate randomization, allocation concealment and blinding, Data on all‐cause mortality should be reported, even if not as a primary outcome.
What's new
| Date | Event | Description |
|---|---|---|
| 5 March 2024 | Amended | This review question is now being addressed according to a new protocol: https://doi.org/10.1002/14651858.CD015719 |
History
Protocol first published: Issue 2, 2007 Review first published: Issue 4, 2008
Acknowledgements
We thank the authors who responded to our requests: Prof. M. Boeckh, Prof. P Bordigoni, Prof. C. Cordonnier, Prof. H. Chapel, Prof. J. Englund, Prof. Stefan Gluck, Prof. L.G. Lum, Prof. S. Molica, Prof. P. Musto, Prof. K. Sullivan, Prof. S.N. Wolff
Appendices
Appendix 1. Search strategy
The following search strategy was modified to search the different databases:
Part 1 "Immunoglobulins"[MeSH] OR "Immunoglobulins, Intravenous"[MeSH] OR "gamma‐Globulins"[MeSH] OR immunoglobulin* OR gammaglobulin* OR gamma globulin* OR immune globulin* OR immune globulin, intravenous OR intravenous immune globulin OR omrigam OR sandoglobulin* OR ivig OR hyperimmune* OR Alphaglobin OR Endobulin OR Gamimune OR Gamimmune OR Gamimune N OR Gamimmune N OR Intraglobin F OR Venimmune OR Venoglobulin‐I OR Venoglobulin I OR VenoglobulinI OR Venoglobulin OR Iveegam OR Intraglobin OR Gammagard OR Gammonativ OR Globulin‐N OR Globulin N OR GlobulinN OR (cytomegalovirus AND "Antibodies, Monoclonal"[MESH])
Part 2: (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR random* OR placebo) NOT (animals [mh] NOT humans [mh])
Part 3: hematologic neoplasms [mh] OR (hematological OR hematologic) malignan*[tw] OR (hematological OR hematologic) neoplas*[tw] OR myeloma OR leukemia OR lymphoma
Part 4: autograft* OR autotransplant* OR allograft* or allotransplant* OR homograft* OR homotransplant* OR bone marrow transplant* OR stem cell transplant* OR peripheral blood stem cell transplant* OR ((autologous OR allogeneic OR allogenic) AND (transplant* OR graft*)).
(Part 3 OR part 4) AND part 1 AND part 2
We did not limit the search strategy for prophylaxis and by outcome to enhance the sensitivity of our search.
Data and analyses
Comparison 1. Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 All‐cause Mortality | 8 | 1418 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.88, 1.12] |
| 1.1.1 2 years and more | 3 | 474 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.87, 1.15] |
| 1.1.2 100‐200 days | 5 | 944 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.81, 1.20] |
| 1.2 All cause mortality 100 days | 4 | 881 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.83, 1.26] |
| 1.3 All‐cause Mortality at 1‐2years and more | 5 | 737 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.94, 1.21] |
| 1.4 All‐cause Mortality ‐ by type of HSCT | 6 | 907 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.89, 1.18] |
| 1.4.1 allogeneic transplant | 3 | 305 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.79, 1.44] |
| 1.4.2 autologous and allo transplant | 2 | 432 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.81, 1.10] |
| 1.4.3 autologous alone | 1 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.93 [1.14, 13.61] |
| 1.5 All cause mortality ‐by use of antifungal prophylaxis | 5 | 758 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.79, 1.04] |
| 1.5.1 Use of oral polyene | 2 | 251 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.74, 1.53] |
| 1.5.2 no antifungal prophylaxis | 3 | 507 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.76, 1.02] |
| 1.6 All‐cause Mortality ‐ high dose IVIG | 3 | 590 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.91, 1.23] |
| 1.7 All‐cause Mortality ‐sensitivity analysis by randomization generation | 8 | 1445 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.86, 1.09] |
| 1.7.1 randomization generation A | 2 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.88, 2.22] |
| 1.7.2 randomization generation B | 6 | 1075 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.83, 1.05] |
| 1.8 All‐cause Mortality ‐sensitivity analysis by double blinding | 8 | 1445 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.86, 1.09] |
| 1.8.1 double blinding | 2 | 697 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.76, 1.17] |
| 1.8.2 no blinding | 6 | 748 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.86, 1.14] |
| 1.9 Clinically documented infections | 5 | 688 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.90, 1.10] |
| 1.10 Microbiologically documented infections ‐ bacterial | 7 | 1186 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.88, 1.15] |
| 1.11 Microbiologically documented infections ‐ patient months | 6 | 3542 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.82, 1.16] |
| 1.12 CMV infections | 6 | 986 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.66, 1.07] |
| 1.13 CMV infections ‐ patient months | 4 | 2082 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.49, 1.02] |
| 1.14 Interstitial Pneumonitis | 7 | 990 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.45, 0.89] |
| 1.15 Infection‐related Mortality | 3 | 275 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.28, 1.49] |
| 1.16 Acute GVHD | 7 | 989 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.83, 1.04] |
| 1.17 VOD | 4 | 447 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.73 [1.11, 6.71] |
| 1.18 Adverse Events | 5 | 728 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.12 [3.15, 20.97] |
| 1.19 VOD according to type of transplant | 4 | 447 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.73 [1.11, 6.71] |
| 1.19.1 allo | 3 | 277 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.76, 5.49] |
| 1.19.2 auto | 1 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.80 [0.66, 210.03] |
| 1.20 CMV Infections and Interstitial pneumonitis | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.20.1 CMV infections | 6 | 986 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.66, 1.07] |
| 1.20.2 Interstitial pneumonitis | 7 | 990 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.45, 0.89] |
| 1.21 acute GVHD and VOD | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.21.1 Acute GVHD | 7 | 989 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.83, 1.04] |
| 1.21.2 VOD | 4 | 447 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.73 [1.11, 6.71] |
| 1.22 All‐cause Mortality ‐sensitivity analysis by ITT | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.22.1 ITT | 6 | 986 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.87, 1.24] |
| 1.22.2 PER PROTOCOL | 3 | 473 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.79, 1.06] |
| 1.23 CMV Infections, Interstitial pneumonitis and VOD | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.23.1 CMV infections | 6 | 986 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.66, 1.07] |
| 1.23.2 Interstitial pneumonitis | 7 | 990 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.45, 0.89] |
| 1.23.3 VOD | 4 | 447 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.73 [1.11, 6.71] |
| 1.24 Clinically Documented Infections‐ sensitivity analysis by randomization generation | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.24.1 randomization generation A | 2 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.86, 1.14] |
| 1.24.2 Randomization Generation B | 3 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.86, 1.17] |
| 1.25 Clinically documented infections ‐ sensitivity analysis by blinding | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.25.1 Double blind | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.91, 1.12] |
| 1.25.2 no blinding | 4 | 488 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.86, 1.15] |
| 1.26 VOD ‐ sensitivity analysis according to randomization generation | 4 | 447 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.73 [1.11, 6.71] |
| 1.26.1 Randomization A | 2 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.35 [1.19, 9.47] |
| 1.26.2 Randomization B | 2 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.16, 7.51] |
| 1.27 VOD ‐ sensitivity analysis by blinding | 4 | 447 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.73 [1.11, 6.71] |
| 1.27.1 double blind | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.44 [0.76, 7.82] |
| 1.27.2 no blinding | 3 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.27 [0.78, 13.59] |
| 1.28 IP ‐ sensitivity analysis by randomization generation | 6 | 898 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.43, 0.87] |
| 1.28.1 Randomization generation A | 2 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.57, 4.85] |
| 1.28.2 Randomization generation B | 4 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.36, 0.76] |
| 1.29 IP ‐ sensitivity analysis by blinding | 7 | 990 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.45, 0.89] |
| 1.29.1 double blind | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.47, 5.19] |
| 1.29.2 no blinding | 6 | 790 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.41, 0.82] |
| 1.30 Fungal Infections | 5 | 1031 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.72, 1.25] |
| 1.31 Bacteremia | 4 | 653 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.93, 1.13] |
1.1. Analysis.

Comparison 1: Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, Outcome 1: All‐cause Mortality
1.2. Analysis.

Comparison 1: Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, Outcome 2: All cause mortality 100 days
1.3. Analysis.

Comparison 1: Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, Outcome 3: All‐cause Mortality at 1‐2years and more
1.4. Analysis.

Comparison 1: Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, Outcome 4: All‐cause Mortality ‐ by type of HSCT
1.5. Analysis.

Comparison 1: Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, Outcome 5: All cause mortality ‐by use of antifungal prophylaxis
1.6. Analysis.

Comparison 1: Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, Outcome 6: All‐cause Mortality ‐ high dose IVIG
1.7. Analysis.

Comparison 1: Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, Outcome 7: All‐cause Mortality ‐sensitivity analysis by randomization generation
1.8. Analysis.

Comparison 1: Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, Outcome 8: All‐cause Mortality ‐sensitivity analysis by double blinding
1.9. Analysis.

Comparison 1: Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, Outcome 9: Clinically documented infections
1.10. Analysis.

Comparison 1: Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, Outcome 10: Microbiologically documented infections ‐ bacterial
1.11. Analysis.

Comparison 1: Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, Outcome 11: Microbiologically documented infections ‐ patient months
1.12. Analysis.

Comparison 1: Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, Outcome 12: CMV infections
1.13. Analysis.

Comparison 1: Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, Outcome 13: CMV infections ‐ patient months
1.14. Analysis.

Comparison 1: Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, Outcome 14: Interstitial Pneumonitis
1.15. Analysis.

Comparison 1: Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, Outcome 15: Infection‐related Mortality
1.16. Analysis.

Comparison 1: Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, Outcome 16: Acute GVHD
1.17. Analysis.

Comparison 1: Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, Outcome 17: VOD
1.18. Analysis.

Comparison 1: Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, Outcome 18: Adverse Events
1.19. Analysis.

Comparison 1: Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, Outcome 19: VOD according to type of transplant
1.20. Analysis.

Comparison 1: Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, Outcome 20: CMV Infections and Interstitial pneumonitis
1.21. Analysis.

Comparison 1: Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, Outcome 21: acute GVHD and VOD
1.22. Analysis.

Comparison 1: Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, Outcome 22: All‐cause Mortality ‐sensitivity analysis by ITT
1.23. Analysis.

Comparison 1: Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, Outcome 23: CMV Infections, Interstitial pneumonitis and VOD
1.24. Analysis.

Comparison 1: Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, Outcome 24: Clinically Documented Infections‐ sensitivity analysis by randomization generation
1.25. Analysis.

Comparison 1: Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, Outcome 25: Clinically documented infections ‐ sensitivity analysis by blinding
1.26. Analysis.

Comparison 1: Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, Outcome 26: VOD ‐ sensitivity analysis according to randomization generation
1.27. Analysis.

Comparison 1: Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, Outcome 27: VOD ‐ sensitivity analysis by blinding
1.28. Analysis.

Comparison 1: Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, Outcome 28: IP ‐ sensitivity analysis by randomization generation
1.29. Analysis.

Comparison 1: Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, Outcome 29: IP ‐ sensitivity analysis by blinding
1.30. Analysis.

Comparison 1: Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, Outcome 30: Fungal Infections
1.31. Analysis.

Comparison 1: Polyvalent immunoglobulins vs. placebo / no intervention ‐ HSCT, Outcome 31: Bacteremia
Comparison 2. Hyperimmune CMV‐IVIG vs. placebo / no intervention ‐ HSCT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 All‐cause Mortality | 4 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.63, 1.16] |
| 2.2 All‐cause Mortality ‐ 100d (3‐4mo) | 3 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.64, 1.24] |
| 2.3 CMV infection | 8 | 553 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.82, 1.26] |
| 2.4 Interstitial Pneumonitis | 5 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.58, 1.56] |
| 2.5 Infection‐related Mortality | 3 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.34, 1.32] |
| 2.6 Acute GVHD | 5 | 342 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.72, 1.44] |
| 2.7 Adverse Events | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.00 [0.38, 129.34] |
| 2.8 Fungal Infections | 2 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.54, 1.93] |
| 2.9 Bacteremia | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [1.23, 2.52] |
2.1. Analysis.

Comparison 2: Hyperimmune CMV‐IVIG vs. placebo / no intervention ‐ HSCT, Outcome 1: All‐cause Mortality
2.2. Analysis.

Comparison 2: Hyperimmune CMV‐IVIG vs. placebo / no intervention ‐ HSCT, Outcome 2: All‐cause Mortality ‐ 100d (3‐4mo)
2.3. Analysis.

Comparison 2: Hyperimmune CMV‐IVIG vs. placebo / no intervention ‐ HSCT, Outcome 3: CMV infection
2.4. Analysis.

Comparison 2: Hyperimmune CMV‐IVIG vs. placebo / no intervention ‐ HSCT, Outcome 4: Interstitial Pneumonitis
2.5. Analysis.

Comparison 2: Hyperimmune CMV‐IVIG vs. placebo / no intervention ‐ HSCT, Outcome 5: Infection‐related Mortality
2.6. Analysis.

Comparison 2: Hyperimmune CMV‐IVIG vs. placebo / no intervention ‐ HSCT, Outcome 6: Acute GVHD
2.7. Analysis.

Comparison 2: Hyperimmune CMV‐IVIG vs. placebo / no intervention ‐ HSCT, Outcome 7: Adverse Events
2.8. Analysis.

Comparison 2: Hyperimmune CMV‐IVIG vs. placebo / no intervention ‐ HSCT, Outcome 8: Fungal Infections
2.9. Analysis.

Comparison 2: Hyperimmune CMV‐IVIG vs. placebo / no intervention ‐ HSCT, Outcome 9: Bacteremia
Comparison 3. Polyvalent immunoglobulins or hyperimmune CMV‐IVIG vs. placebo / no intervention ‐ HSCT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 All‐cause Mortality | 12 | 1706 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.87, 1.09] |
| 3.1.1 Polyvalent IVIG | 8 | 1418 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.88, 1.12] |
| 3.1.2 CMV‐IVIG | 4 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.63, 1.16] |
| 3.2 All‐cause Mortality ‐ 100d (3‐4 mo) | 8 | 1178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.82, 1.14] |
| 3.2.1 Polyvalent IVIG | 5 | 944 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.81, 1.20] |
| 3.2.2 CMV‐IVIG | 3 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.64, 1.24] |
| 3.3 CMV infection | 13 | 1511 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.76, 1.06] |
| 3.3.1 Polyvalent IVIG | 6 | 986 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.66, 1.07] |
| 3.3.2 CMV‐IVIG | 7 | 525 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.78, 1.21] |
| 3.4 Interstitial Pneumonitis | 12 | 1335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.55, 0.95] |
| 3.4.1 Polyvalent IVIG | 7 | 990 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.45, 0.89] |
| 3.4.2 CMV‐IVIG | 5 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.58, 1.56] |
| 3.5 Infection‐related Mortality | 6 | 509 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.39, 1.12] |
| 3.5.1 Polyvalent IVIG | 3 | 275 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.28, 1.49] |
| 3.5.2 CMV‐IVIG | 3 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.34, 1.32] |
| 3.6 Acute GVHD | 12 | 1331 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.84, 1.05] |
| 3.6.1 Polyvalent IVIG | 7 | 989 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.83, 1.04] |
| 3.6.2 CMV‐IVIG | 5 | 342 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.72, 1.44] |
| 3.7 Adverse Events | 6 | 782 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.02 [3.25, 19.78] |
| 3.7.1 Polyvalent IVIG | 5 | 728 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.12 [3.15, 20.97] |
| 3.7.2 CMV‐IVIG | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.00 [0.38, 129.34] |
3.1. Analysis.

Comparison 3: Polyvalent immunoglobulins or hyperimmune CMV‐IVIG vs. placebo / no intervention ‐ HSCT, Outcome 1: All‐cause Mortality
3.2. Analysis.

Comparison 3: Polyvalent immunoglobulins or hyperimmune CMV‐IVIG vs. placebo / no intervention ‐ HSCT, Outcome 2: All‐cause Mortality ‐ 100d (3‐4 mo)
3.3. Analysis.

Comparison 3: Polyvalent immunoglobulins or hyperimmune CMV‐IVIG vs. placebo / no intervention ‐ HSCT, Outcome 3: CMV infection
3.4. Analysis.

Comparison 3: Polyvalent immunoglobulins or hyperimmune CMV‐IVIG vs. placebo / no intervention ‐ HSCT, Outcome 4: Interstitial Pneumonitis
3.5. Analysis.

Comparison 3: Polyvalent immunoglobulins or hyperimmune CMV‐IVIG vs. placebo / no intervention ‐ HSCT, Outcome 5: Infection‐related Mortality
3.6. Analysis.

Comparison 3: Polyvalent immunoglobulins or hyperimmune CMV‐IVIG vs. placebo / no intervention ‐ HSCT, Outcome 6: Acute GVHD
3.7. Analysis.

Comparison 3: Polyvalent immunoglobulins or hyperimmune CMV‐IVIG vs. placebo / no intervention ‐ HSCT, Outcome 7: Adverse Events
Comparison 4. Polyvalent immunoglobulins vs. hyperimmune CMV‐IVIG ‐ HSCT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 All‐cause Mortality | 3 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.92, 2.32] |
| 4.2 Clinically documented Infection | 1 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.89, 2.79] |
| 4.3 CMV Infection | 3 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.07, 1.89] |
| 4.4 Interstitial Pneumonitis | 2 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.40, 1.75] |
| 4.5 Infection‐related Mortality | 2 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.28 [0.95, 11.26] |
| 4.6 Acute GVHD | 2 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.87, 1.75] |
4.1. Analysis.

Comparison 4: Polyvalent immunoglobulins vs. hyperimmune CMV‐IVIG ‐ HSCT, Outcome 1: All‐cause Mortality
4.2. Analysis.

Comparison 4: Polyvalent immunoglobulins vs. hyperimmune CMV‐IVIG ‐ HSCT, Outcome 2: Clinically documented Infection
4.3. Analysis.

Comparison 4: Polyvalent immunoglobulins vs. hyperimmune CMV‐IVIG ‐ HSCT, Outcome 3: CMV Infection
4.4. Analysis.

Comparison 4: Polyvalent immunoglobulins vs. hyperimmune CMV‐IVIG ‐ HSCT, Outcome 4: Interstitial Pneumonitis
4.5. Analysis.

Comparison 4: Polyvalent immunoglobulins vs. hyperimmune CMV‐IVIG ‐ HSCT, Outcome 5: Infection‐related Mortality
4.6. Analysis.

Comparison 4: Polyvalent immunoglobulins vs. hyperimmune CMV‐IVIG ‐ HSCT, Outcome 6: Acute GVHD
Comparison 5. Polyvalent immunoglobulins 250mg/kg vs. Polyvalent immunoglobulins or hyperimmune CMV‐IVIG 500mg/kg ‐ HSCT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 All‐cause Mortality | 1 | 412 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.84, 1.25] |
| 5.2 Clinically documented Infection | 2 | 509 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.81, 0.97] |
| 5.3 Microbiologically documented Infection | 2 | 509 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.04, 1.57] |
| 5.4 CMV Infection | 1 | 412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.34, 1.41] |
| 5.5 Interstitial Pneumonitis | 2 | 509 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.33, 2.92] |
| 5.6 Infection related Mortality | 1 | 412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.47, 1.43] |
| 5.7 Acute GVHD | 3 | 841 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.13, 1.55] |
5.1. Analysis.

Comparison 5: Polyvalent immunoglobulins 250mg/kg vs. Polyvalent immunoglobulins or hyperimmune CMV‐IVIG 500mg/kg ‐ HSCT, Outcome 1: All‐cause Mortality
5.2. Analysis.

Comparison 5: Polyvalent immunoglobulins 250mg/kg vs. Polyvalent immunoglobulins or hyperimmune CMV‐IVIG 500mg/kg ‐ HSCT, Outcome 2: Clinically documented Infection
5.3. Analysis.

Comparison 5: Polyvalent immunoglobulins 250mg/kg vs. Polyvalent immunoglobulins or hyperimmune CMV‐IVIG 500mg/kg ‐ HSCT, Outcome 3: Microbiologically documented Infection
5.4. Analysis.

Comparison 5: Polyvalent immunoglobulins 250mg/kg vs. Polyvalent immunoglobulins or hyperimmune CMV‐IVIG 500mg/kg ‐ HSCT, Outcome 4: CMV Infection
5.5. Analysis.

Comparison 5: Polyvalent immunoglobulins 250mg/kg vs. Polyvalent immunoglobulins or hyperimmune CMV‐IVIG 500mg/kg ‐ HSCT, Outcome 5: Interstitial Pneumonitis
5.6. Analysis.

Comparison 5: Polyvalent immunoglobulins 250mg/kg vs. Polyvalent immunoglobulins or hyperimmune CMV‐IVIG 500mg/kg ‐ HSCT, Outcome 6: Infection related Mortality
5.7. Analysis.

Comparison 5: Polyvalent immunoglobulins 250mg/kg vs. Polyvalent immunoglobulins or hyperimmune CMV‐IVIG 500mg/kg ‐ HSCT, Outcome 7: Acute GVHD
Comparison 6. Polyvalent immunoglobulins vs. placebo / no intervention ‐ MM/CLL.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 All‐cause Mortality | 2 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.58, 3.19] |
| 6.2 Clinically‐documented infections | 3 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.39, 0.61] |
| 6.3 Microbiologically‐documented infections | 3 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.53, 0.95] |
| 6.4 Bacteremia | 2 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.14, 3.07] |
| 6.5 Infection‐related Mortality | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.95] |
| 6.6 Adverse Events | 3 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.37 [1.74, 3.24] |
| 6.7 Adverse Events requiring discontinuation | 3 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.43 [0.70, 42.24] |
| 6.8 Clinically and microbiologically documented infections | 3 | 410 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.48, 0.69] |
| 6.8.1 Clinically‐documented infections | 3 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.39, 0.61] |
| 6.8.2 Microbiologically‐documented infections | 3 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.53, 0.95] |
| 6.9 Fungal infections | 1 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.26, 8.30] |
| 6.10 Bacteremia | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.68] |
6.1. Analysis.

Comparison 6: Polyvalent immunoglobulins vs. placebo / no intervention ‐ MM/CLL, Outcome 1: All‐cause Mortality
6.2. Analysis.

Comparison 6: Polyvalent immunoglobulins vs. placebo / no intervention ‐ MM/CLL, Outcome 2: Clinically‐documented infections
6.3. Analysis.

Comparison 6: Polyvalent immunoglobulins vs. placebo / no intervention ‐ MM/CLL, Outcome 3: Microbiologically‐documented infections
6.4. Analysis.

Comparison 6: Polyvalent immunoglobulins vs. placebo / no intervention ‐ MM/CLL, Outcome 4: Bacteremia
6.5. Analysis.

Comparison 6: Polyvalent immunoglobulins vs. placebo / no intervention ‐ MM/CLL, Outcome 5: Infection‐related Mortality
6.6. Analysis.

Comparison 6: Polyvalent immunoglobulins vs. placebo / no intervention ‐ MM/CLL, Outcome 6: Adverse Events
6.7. Analysis.

Comparison 6: Polyvalent immunoglobulins vs. placebo / no intervention ‐ MM/CLL, Outcome 7: Adverse Events requiring discontinuation
6.8. Analysis.

Comparison 6: Polyvalent immunoglobulins vs. placebo / no intervention ‐ MM/CLL, Outcome 8: Clinically and microbiologically documented infections
6.9. Analysis.

Comparison 6: Polyvalent immunoglobulins vs. placebo / no intervention ‐ MM/CLL, Outcome 9: Fungal infections
6.10. Analysis.

Comparison 6: Polyvalent immunoglobulins vs. placebo / no intervention ‐ MM/CLL, Outcome 10: Bacteremia
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdel‐Mageed 1999.
| Study characteristics | ||
| Methods | Randomization generation: not specified; Allocation concealment: unclear; Blinding: none; Excluded: 18 pts. | |
| Participants | 350 patients with acute leukemia or CML who had allogeneic stem cell transplantation from sibling donors; Multi‐center ‐ 10 centers, USA Setting: hospitalization in isolation precautions with laminar air flow | |
| Interventions | IV polyvalent Immunoglobulin in 2 doses. The trial included 2 arms: (50 mg/kg; 250 mg/kg; 500 mg/kg ) Schedule: weekly from day ‐8 to day +111 posttransplant (18 doses) | |
| Outcomes | All cause mortality; CDI; CMV infections; relapse; | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Boeckh 2001.
| Study characteristics | ||
| Methods | Randomization generation: computer generated; Allocation concealment: sealed envelopes; Blinding: double blind Excluded: none | |
| Participants | 179 patients who had myeloablative allogeneic stem cell transplantation from sibling donors and unrelated donors; Multi‐center ‐ 3 centers, USA; Setting: hospitalization in isolation precautions and then outpatients | |
| Interventions | IV anti CMV specific monoclonal ab (MSL‐109) in 2 doses. The trial included 2 arms: 60 mg/kg and 15 mg/kg. Schedule: every 14 days from day ‐1 to day +84 post‐ransplant | |
| Outcomes | All cause mortality; MDI; bacterial infections; fungal infections; bacteremia; CMV disease; hospitalization; IRM; acute GVHD; engraftment; relapse; adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Bordigoni 1987.
| Study characteristics | ||
| Methods | Randomization generation: random numbers; Allocation concealment: opaque envelopes; Blinding: none; Excluded:none | |
| Participants | 60 patients who had myeloablative allogeneic and autologous bone marrow transplantation; Single center, France, Europe; Setting: hospitalization in isolation precautions and then outpatients | |
| Interventions | IV anti CMV IgG (Nancy) enriched plasma 4 ml/kg vs control. The trial included 2 arms: anti CMV IgG (Nancy) enriched plasma and control; Schedule: day ‐7, ‐3, 0 and then every 15 days to day 90 posttransplant | |
| Outcomes | All cause mortality; CMV infection; IRM; IP; engraftment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Boughton 1995.
| Study characteristics | ||
| Methods | Randomization generation: not specified; Allocation concealment: central ‐ from a reference center; Blinding: double blind Excluded: none | |
| Participants | 42 patients with CLL; Multi‐center ‐ 20 centers in UK, Europe; Setting: outpatients | |
| Interventions | IV polyvalent Immunoglobulin (Sandoglobulin) vs placebo. The trial included 2 arms: IVIG 18 and placebo Schedule: every 3 weeks for 12 months | |
| Outcomes | All cause mortality; CDI; MDI; bacterial infections; viral infections; bacteremia; immunoglobulin levels; adverse effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Bowden 1986.
| Study characteristics | ||
| Methods | Randomization generation: protocol registrar using a table of random permutations; Allocation concealment: adequate measures to conceal allocation protocol registrar; Blinding: none; Excluded: 2 pts. | |
| Participants | 99 patients who had myeloablative allogeneic bone marrow transplantation, all recipients CMV negative; single center, USA; Setting: hospitalization in isolation precautions and then outpatients | |
| Interventions | CMV immune globulin vs. control. The trial included 4 arms: CMV immune globulin (150 mg/kg) + seronegative blood products vs. seronegative blood products alone vs. CMV immune globulin alone (150 mg/kg) vs. control; Schedule: days ‐5, ‐1 , +6,+20, +34 ‐ dose of 150 mg/kg; days +48; +62 ‐ dose of 100 mg/kg posttransplant |
|
| Outcomes | All cause mortality; CMV infection; CMV disease; | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Bowden 1991.
| Study characteristics | ||
| Methods | Randomization generation: adequate‐protocol registrar using assignment from a table of random permutations; Allocation concealment:: unclear; Blinding: none; Excluded: 3 pts. | |
| Participants | 123 patients who had myeloablative allogeneic bone marrow transplantation for ALL, AML, CML, aplastic anemia, lymphoma and other; Single center: USA; Setting: hospitalization in isolation precautions and then outpatients | |
| Interventions | CMV IVIG vs placebo. The trial included 2 arms: CMV IVIG (Cutter Biological) 200 mg/kg vs. placebo; Schedule: days ‐8; ‐6 pretransplant; +1; +7; +14; +21; +28; +42; +56; +70 posttransplant (10 doses) | |
| Outcomes | All cause mortality; CMV infection; CMV disease; hospitalization; IRM; acute GVHD; IP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Chapel 1994.
| Study characteristics | ||
| Methods | Randomization generation: randomization table for each study site; Allocation concealment: randomization code kept by pharmacy; Blinding: double blind Excluded: 1 pt. | |
| Participants | 83 multiple myeloma plateau phase patients; Multi‐center ‐9 centers, UK, Europe; Setting: Outpatients | |
| Interventions | IVIG polyvalent Immunoglobulin (Gammagard) vs placebo. The trial included 2 arms: IVIG 0.4 g/kg vs. placebo Schedule: every 4 weeks for 12 months | |
| Outcomes | All cause mortality; CDI; MDI; bacterial infections; bacteremia; IRM; adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Chapel 1994c.
| Study characteristics | ||
| Methods | Randomization generation: table of random numbers; Allocation concealment: allocated centrally; Blinding: double blind Excluded: none | |
| Participants | 34 B‐CLL patients; Multi‐center‐ USA + Europe Setting: outpatients | |
| Interventions | IVIG polyvalent Immunoglobulin (Gammagard) in 2 doses. The trial included 2 arms: (250 mg/kg; 500 mg/kg ) Schedule: every 4 weeks for 1 year | |
| Outcomes | All cause mortality; CDI; MDI; bacterial infections; fungal infections; viral infection; immunoglobulin levels; adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Condie 1984.
| Study characteristics | ||
| Methods | Randomization generation: not specified; Allocation concealment: unclear; Blinding: none; Excluded: none | |
| Participants | 55 patients who had myeloablative allogeneic myeloablative bone marrow transplantation for acute leukemia (AML + ALL) ; Multi‐center ‐ 2 centers; USA; Setting: hospitalization in isolation precautions and then outpatients | |
| Interventions | Hyperimmune CMV IVIG vs. CMV deficient IVIG vs. control. The trial included 3 arms: Hyperimmune CMV IVIG 200 mg/kg vs. CMV deficient IVIG 200 mg/kg vs. control ‐ not randomized, matched control; Schedule: posttransplant days: +25; +50; +75 | |
| Outcomes | All cause mortality; CMV infection; CMV disease; acute GVHD; IP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Cooperative CLL 1988.
| Study characteristics | ||
| Methods | Randomization generation: table of random numbers; Allocation concealment: allocated centrally; Blinding: double blind; Excluded: 3 pts. | |
| Participants | 84 CLL patients; Multi‐center ‐ 10 centers, USA & Europe Setting: outpatient | |
| Interventions | IVIG polyvalent Immunoglobulin (Gammagard) vs placebo. The trial included 2 arms: IVIG 0.4 g/kg vs. placebo; Schedule: every 4 weeks for 12 months | |
| Outcomes | All cause mortality; CDI; MDI; bacterial infections; IRM; adverse events bacteremia; | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Cordonnier 2003.
| Study characteristics | ||
| Methods | Randomization generation: computer generated; Allocation concealment: central randomization; Blinding: double blind Excluded:none | |
| Participants | 200 patients who had myeloablative allogeneic stem cell transplantation from HLA identical sibling donors; Multi‐center ‐ 19 centers; France,Europe Setting: hospitalization in isolation precautions and then outpatients | |
| Interventions | IV polyvalent Immunoglobulin vs placebo. The trial included 4 arms: IVIG in 3 different doses (50 mg/kg; 250 mg/kg; 500 mg/kg ) and placebo Schedule: 16 weekly doses from ‐7d to day 100 post‐transplant | |
| Outcomes | All cause mortality; CDI; MDI; bacterial infections; fungal infections; viral infections; CMV infections; CMV disease; TRM; acute GVHD; chronic GVHD; VOD; IP; engraftment; relapse; adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Emanuel 1992.
| Study characteristics | ||
| Methods | Randomization generation: not specified; Allocation concealment: unclear; Blinding: none; Excluded: none | |
| Participants | 92 patients who had myeloablative allogeneic bone marrow transplantation single center; USA; Setting: hospitalization in isolation precautions and then outpatients | |
| Interventions | IV polyvalent Immunoglobulin (Gammagard) vs control. The trial included 2 arms: IVIG 500 mg/kg then 250 mg/kg and control; Schedule: 500 mg/kg every 2 weeks from day ‐7 to day +100 then 250 mg/kg from day +100 to day +180 | |
| Outcomes | All cause mortality; MDI; bacterial infections; CMV infections; CMV disease; IP; engraftment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Feinstein 1999.
| Study characteristics | ||
| Methods | Randomization generation: not specified; Allocation concealment: unclear; Blinding: none Excluded: 19 pts. | |
| Participants | 260 patients who had myeloablative allogeneic bone marrow transplantation from HLA identical sibling donors; single center, USA; Setting: hospitalization in isolation precautions and then outpatients | |
| Interventions | IV polyvalent Immunoglobulin (Gamimmune) vs control. The trial included 2 arms: IVIG 500 mg/kg and control; Schedule: daily from days ‐6 to ‐1 then every 3rd day posttransplant from day +3 to day +90 | |
| Outcomes | All cause mortality; CDI; MDI; bacterial infections; fungal infections; viral infections; CMV infections; hospitalization; acute GVHD; engraftment; immunoglobulin levels; adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Filipovich 1992.
| Study characteristics | ||
| Methods | Randomization generation: not specified; Allocation concealment: database personnel and pharmacy personnel; Blinding: double blind Excluded: none | |
| Participants | 42 patients who had autoBMT and alloBMT for acute leukemia, CML, other malignancy or non‐malignant disease; Single center, USA; Setting: inpatient | |
| Interventions | IVIG polyvalent immunoglobulin products at a dose of 500 mg/kg. The trial included 4 arms: Gamimmune, Gammagard, Sandoglobulin, immune Globulin Intravenous; Schedule: every other week for 3 doses (week ‐1 to week +3); | |
| Outcomes | CMV infections; immunoglobulin levels | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Gluck 1990.
| Study characteristics | ||
| Methods | Randomization generation: not specific; Allocation concealment: unclear; Blinding: none; Excluded: unknown | |
| Participants | Unknown number of patients with multiple myeloma and low risk non‐Hodgkin lymphoma; single center, Europe, Germany; Setting: outpatients | |
| Interventions | IV polyvalent Immunoglobulin vs placebo. The trial included 2 arms: IVIG (IVIG 0.5 ‐0.7 g/kg) and placebo Schedule: monthly for 6 months | |
| Outcomes | Descriptive results on outcomes and not the exact numbers of patients who developed infections in each arm | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Graham Pole 1990.
| Study characteristics | ||
| Methods | Randomization generation: not specified; Allocation concealment: unclear; Blinding: none Excluded: 57 pts. | |
| Participants | 150 patients who had alloBMT from HLA matched and mismatched donors; Multi‐center ‐ 8 centers; USA; Setting: not reported | |
| Interventions | IV polyvalent Immunoglobulin in 2 doses. The trial included 2 arms: 2 different doses (250 mg/kg; 500 mg/kg); Schedule: weekly from 1 week before to 16 weeks after BMT posttransplant | |
| Outcomes | Authors reported on the total number of deaths in the study but not separately on the numbers in the 2 arms | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hargreaves 1992.
| Study characteristics | ||
| Methods | Randomization generation: table of random numbers; Allocation concealment: allocated centrally; Blinding: double blind Excluded: unknown | |
| Participants | 39 patients with multiple myeloma patients; single center, UK, Europe; Setting: outpatients | |
| Interventions | IVIG polyvalent immunoglobulin (Gammagard) vs. placebo. The trial included 2 arms: IVIG and placebo | |
| Outcomes | Authors reported on the total rate of infections in both the treatment and the placebo arms | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Jacobsen 1985.
| Study characteristics | ||
| Methods | Randomization generation: not specified; Allocation concealment: unclear; Blinding: none; Excluded: none | |
| Participants | 49 patients who had myeloablative allogeneic bone marrow transplantation for ALL, AML, AUL, CML, lymphoma, SAA; Multi‐center ‐ 4 centers; Germany, Europe Setting: hospitalization in isolation precautions and then outpatients | |
| Interventions | Hyperimmune CMV IVIG vs. control IG. The trial included 2 arms: Hyperimmune CMV IVIG 0.1 G protein/kg vs. control IG 0.1 G protein/kg; Schedule: day ‐7; day +13; day +33; day +53 ; day +79; day + 93 | |
| Outcomes | All cause mortality; CMV disease; IRM; acute GVHD; IP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Lum 1994.
| Study characteristics | ||
| Methods | Randomization generation: not specified; Allocation concealment: unclear; Blinding: none; Excluded:none | |
| Participants | 54 patients who had myeloablative allogeneic bone marrow transplantation; single center, USA; Setting: hospitalization in isolation precautions and then outpatients | |
| Interventions | IVIG polyvalent immunoglobulin vs. control. The trial included 2 arms: IVIG 400 mg/kg vs. control;Schedule: 10 weekly infusions from day +14 to day +79 | |
| Outcomes | All cause mortality; IRM; acute GVHD; chronic GVHD | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Meyers 1983.
| Study characteristics | ||
| Methods | Randomization generation: not specified; Allocation concealment: unclear; Blinding: none; Excluded: 6 pts. | |
| Participants | 68 patients with myeloablative allogeneitic BMT for acute leukemia and CML; Sincle‐center USA; Setting: hospitalization in isolation precautions and then outpatients | |
| Interventions | IM anti‐CMV hyperimmune globulin vs. control. The trial included 2 arms: anti‐CMV hyperimmune globulin and control; Schedule: on days ‐4 and ‐2 before BMT, then weekly to day +77 posttransplant | |
| Outcomes | CMV infections; IRM; immunoglobulin levels | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Molica 1996.
| Study characteristics | ||
| Methods | Randomization generation:not specified; Allocation concealment: unclear; Blinding: none; Excluded: none | |
| Participants | 42 CLL patients; single center, Italy, Europe Setting: Outpatients | |
| Interventions | IV polyvalent Immunoglobulin (Vena‐N) vs. control. The trial included 4 arms: Patients were randomly allocated to receive an infusion of IVIG every 4 weeks for at least 6 months or no treatment. Then they were switched to observation or IVIG for another 12 months; finally, they received IVIG or no therapy for 6 more months. | |
| Outcomes | All cause mortality; CDI; MDI; IRM | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Musto 1995.
| Study characteristics | ||
| Methods | Randomization generation:not specified; Allocation concealment: unclear; blinding: none; Excluded: none | |
| Participants | 25 Multiple myeloma patients | |
| Interventions | IV polyvalent Immunoglobulin (Vena‐N) vs. control. The trial included 8 arms: IVIG then no therapy then IVIG; IVIG then no therapy then no therapy; IVIG then IVIG then IVIG; IVIG then IVIG then no therapy; No therapy then no therapy then IVIG; No therapy then no therapy then no therapy No therapy then IVIG then IVIG No therapy then IVIG then no therapy Schedule: every 4 weeks for 6 months, then 4 weeks for 12 months then 4 weeks for 6 months | |
| Outcomes | All cause mortality; CDI; IRM | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Peltier 1992.
| Study characteristics | ||
| Methods | Randomization generation:not specified; Allocation concealment: unclear ; Blinding: double blind Excluded: none | |
| Participants | 53 CMV ‐ seronegative patients who had undergone AutoBMT and AlloBMT for acute leukemia, CML, other malignancy or non‐malignant disease; Single ‐ center USA; Setting: not mentioned | |
| Interventions | IVIG polyvalent immunoglobulin products at a dose of 500 mg/kg. The trial included 3 arms: Gammagard, Sandoglobulin, Gamimmune; Schedule: weekly starting the week before BMT, total 6 doses | |
| Outcomes | No relevant outcomes (immunoglobulin levels) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Poynton 1992.
| Study characteristics | ||
| Methods | Randomization generation: not specified; Allocation concealment: unclear; Blinding: none; Excluded: 9 pts. | |
| Participants | 72 patients who had autologous or allogeneic bone marrow transplantation; Single center; UK, Europe Setting: hospitalization in isolation precautions and then outpatients | |
| Interventions | IgM and IgA enriched IVIG Immunoglobulin vs control. The trial included 2 arms: IgM and IgA enriched IVIG and control; Schedule: days 0, +3, +7, then weekly until neutrophil > 0.5 x 109/l | |
| Outcomes | All cause mortality; CDI; MDI; bacterial infections; fungal infections; bacteremia; CMV disease; hospitalization; antibiotics; IRM; VOD; engraftment; relapse; immunoglobulin levels; | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Raiola 2002.
| Study characteristics | ||
| Methods | Randomization generation:not specified; Allocation concealment:: unclear; Blinding: none Excluded:none | |
| Participants | 89 patients who had allogenetic alternative donor bone marrow transplantation Single center ‐ Italy, Europe; Setting: hospitalization in isolation precautions and then outpatients |
|
| Interventions | IVIG polyvalent immunoglobulin (Pentaglobulin) in 2 doses. The trial included 2 arms: IVIG 200 mg/kg and 400 mg/kg; Schedule: every 2 weeks from day ‐7 to day +100 | |
| Outcomes | All cause mortality; IRM; GVHD; relapse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Ringden 1987.
| Study characteristics | ||
| Methods | Randomization generation: not specified; Allocation concealment:: unclear; Blinding: none; Excluded:none | |
| Participants | 54 patients who had allogenetic myeloablative bone marrow transplantation; Multi center; 5 centers Scandinavia Europe; Setting: hospitalization in isolation precautions and then outpatients |
|
| Interventions | CMV hyperimmune plasma vs control. The trial included 2 arms: CMV hyperimmune plasma 30 ml/kg and control; Schedule: 4 times 2 days period (each period total 30 ml/kg): days 3 & 4; days 25 & 26; days 50 & 51; days 75 & 76 |
|
| Outcomes | All cause mortality; CMV infection; CMV disease; IRM; acute GVHD; chronic GVHD; IP; immunoglobulin levels; adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Ruutu 1997.
| Study characteristics | ||
| Methods | Randomization generation: not specified; Allocation concealment: unclear; Blinding: none; Excluded: none | |
| Participants | 28 patients who had myeloablative allogeneic bone marrow transplantation from HLA identical sibling or unrelated donors; Multi‐center ‐ number not mentioned; Scandinavia, Europe Setting: not specified | |
| Interventions | Anti‐CMV hyperimmune globulin vs control. The trial included 2 arms: anti‐CMV hyperimmune globulin 400 mg/kg and control Schedule: 0.4g/kg on day ‐8, then 0.2g/kg on day ‐1,+7,+14,+21,+28,+35,+42,+56,+70 | |
| Outcomes | All cause mortality; CMV infections; CMV disease; acute GVHD; chronic GVHD; IP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Salmon 1967.
| Study characteristics | ||
| Methods | Randomization generation: adequate‐randomized card selection; Allocation concealment: unclear; Blinding: double blind Excluded: 7 pts | |
| Participants | 48 patients with active multiple myeloma; multi‐center‐ 5 centers; USA (ECOG); Setting: outpatients | |
| Interventions | Cohn fraction II gamma globulin IM vs placebo. The trial included 2 arms: 20 ml of Cohn fraction II gamma globulin 16.5% and 10 ml of Human serum albumin 5% Schedule: every 2 weeks for at least 6 weeks | |
| Outcomes | All cause mortality; CDI; bacterial infections; IRM; adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Serrano 1999.
| Study characteristics | ||
| Methods | Randomization generation: not specified; Allocation concealment: unclear; Blinding: none; Excluded:none | |
| Participants | 92 patients who had myeloablative allogeneic bone marrow transplantation from HLA identical sibling donors; single center, Spain, Europe; Setting: hospitalization in isolation precautions and then outpatients | |
| Interventions | Hyperimmune CMV IVIG vs control. The trial included 2 arms: Hyperimmune CMV IVIG at a dose of 150 mg/kg and control. Schedule: every 2 weeks from day 2 to day +86, then monthly till 1 yr post BMT | |
| Outcomes | All cause mortality; CDI; bacterial infections; fungal infections; bacteremia; CMV infection; CMV disease; IRM; acute GVHD; chronic GVHD; IP; engraftment; relapse; immunoglobulin levels | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Sklenar 1993.
| Study characteristics | ||
| Methods | Randomization generation: not specified; Allocation concealment: unclear; Blinding: only patient blinded Excluded: none | |
| Participants | 62 CLL and multiple myeloma patients; Multi‐center ‐ 10 centers, USA and Europe; Setting: Outpatients | |
| Interventions | IV polyvalent Immunoglobulin in 3 doses. The trial included 3 arms: (100 mg/kg; 400 mg/kg; 800 mg/kg ) Schedule: every 3 weeks total 6 doses (week 0, 3, 6, 9, 12, 15) | |
| Outcomes | All cause mortality; immunoglobulin levels; adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Sullivan 1990.
| Study characteristics | ||
| Methods | Randomization generation: not specified; Allocation concealment: unclear; Blinding: none; Excluded: 14 pts. | |
| Participants | 383 patients who had myeloablative allogeneic BMT or autologous BMT or syngeneic BMT for SAA or acute leukemia or CML or lymphoma; single center ‐ USA; Setting: hospitalization in isolation precautions and then outpatients | |
| Interventions | IV polyvalent Immunoglobulin (Gamimune N) vs control. The trial included 2 arms: IVIG 500 mg/kg and control; Schedule: 15 weekly infusions from day ‐7 to day +90 | |
| Outcomes | All cause mortality; CDI; MDI; bacterial infections; fungal infections; viral infections; bacteremia; acute GVHD; IP; relapse; immunoglobulin levels; adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Sullivan 2000.
| Study characteristics | ||
| Methods | Randomization generation: not specified; Allocation concealment: unclear; Blinding: double blind; Excluded: none | |
| Participants | 497 patients who had myeloablative allogeneic BMT from unrelated donor; Multi‐center‐ USA and Europe; Setting: hospitalization in isolation precautions and then outpatients | |
| Interventions | IV polyvalent immunoglobulin (Gammagard) vs. placebo. The trial included 2 arms: IVIG 500 mg/kg and placebo; Schedule: day ‐7 and ‐1 and then weekly from day +6 to day +90 | |
| Outcomes | All cause mortality; TRM; relapse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Ustun 1998.
| Study characteristics | ||
| Methods | Randomization generation: not specified; Allocation concealment: unclear; Blinding: none; Excluded: none | |
| Participants | 14 patients who had alloSCT from HLA identical siblings for acute leukemia, CML, MDS; single center‐ Turkey, Europe; Setting: not mentioned | |
| Interventions | IV polyvalent Immunoglobulin vs control. The trial included 2 arms: IVIG 0.5 g/kg and control; Schedule: day ‐4 then weekly until ANC>0.5 x10(9)/l | |
| Outcomes | All cause mortality; CDI; acute GVHD; VOD; engraftment; | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Winston 1982.
| Study characteristics | ||
| Methods | Randomization generation:not specified; Allocation concealment: unclear; Blinding: none Excluded: 6 pts | |
| Participants | 54 patients who had myeloablative allogeneic bone marrow transplantation from HLA identical sibling donors; single center; USA Setting: hospitalization in isolation precautions and then outpatients | |
| Interventions | Anti‐CMV hyperimmune plasma at a dose of 10 ml/kg vs. control; Schedule: before conditioning, then on day +3; +30; +45; +60; +75; +90; +120 | |
| Outcomes | All cause mortality; CMV infection; CMV disease; IRM; TRM; acute GVHD; IP; relapse; adverse events | |
| Notes | Excluded patients died (ITT) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Winston 1984.
| Study characteristics | ||
| Methods | Randomization generation: not specified; Allocation concealment: unclear; Blinding: none Excluded: 5 pts. | |
| Participants | 41 patients who had myeloablative allogeneic BMT for acute leukemia in remission or relapse and aplastic anemia from HLA identical sibling donors; Single‐center, USA; setting: hospitalization in isolation precautions and then outpatients | |
| Interventions | IV polyvalent Immunoglobulin 5% in 10% maltose vs control. The trial included 2 arms: IVIG 5% in 10% maltose 20 cc/kg and control; Schedule: before initiation of conditioning and then every week until day +120 | |
| Outcomes | All cause mortality; viral infections; CMV infections; CMV disease; acute GVHD; IP; adverse events | |
| Notes | Results from an ongoing study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Winston 1987.
| Study characteristics | ||
| Methods | Randomization generation: not specified; allocation concealment: unclear; Blinding: none Excluded: 14 pts. | |
| Participants | 89 patients who had alloBMT T‐ cell depleted from sibling for acute leukemia in remission or relapse and for aplastic anemia; Single‐center, USA; setting: hospitalization in isolation precautions and then outpatients | |
| Interventions | IV polyvalent Immunoglobulinvs control. The trial included 2 arms: IVIG 20 cc/kg and control; Schedule: before initiation of conditioning and then every week until day +120 | |
| Outcomes | all cause mortality; CMV infection; CMV disease; acute GVHD; IP; adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Winston 1993.
| Study characteristics | ||
| Methods | Randomization generation: not specified; allocation concealment: unclear; Blinding: none Excluded: none | |
| Participants | 51 patients who had myeloablative allogeneic BMT for acute leukemia nd aplastic anemia and CML and MDS from HLA sibling or unrelated donors; Single‐center, USA; setting: hospitalization in isolation precautions and then outpatients | |
| Interventions | IV polyvalent ImmunoglobulinI(Sandoglobulin) vs control. The trial included 2 arms: IVIG 1 g/kg and control; Schedule: before initiation of conditioning and then every week until day +120 | |
| Outcomes | all cause mortality ; bacterial infections; fungal infections; viral infections; bacteremia; CMV infections; CMV disease; IRM; acute GVHD; IP; relapse; adverse events | |
| Notes | in both arms seronegative blood products were administered | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Winston 2001.
| Study characteristics | ||
| Methods | Randomization generation: not specified; allocation concealment: unclear; Blinding: double blind Excluded: 9 pts. | |
| Participants | 627 patients with acute leukemia, CML, lymphoma, aplastic anemia who had myeloablative allogeneic bone marrow transplantation from sibling or matched unrelated donors; Multi‐center ‐ 13 centers; USA Setting: not specified | |
| Interventions | IV polyvalent Immunoglobulin in 3 doses. The trial included 3 arms: 100 mg/kg; 250 mg/kg; 500 mg/kg Schedule: day ‐2, then weekly from day 0 to day +90, then monthly from day +90 to day +360 | |
| Outcomes | all cause mortality; CDI; MDI; bacterial infections; fungal infections; viral infections; bacteremia; CMV infection; CMV disease; IRM; TRM; acute GVHD; chronic GVHD; VOD; IP; relapse; adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wolff 1993.
| Study characteristics | ||
| Methods | Randomization generation:computer generated; allocation concealment: unclear‐ computer generated scheme at each study center; Blinding: none; Excluded:none | |
| Participants | 170 patients who had myeloablative autologous BMT or myelosuppressive Rx. for acute leukemia or other disease; Multi‐center ‐ 3 centers; USA; setting: hospitalization in isolation precautions | |
| Interventions | IV polyvalent immunoglobulin (Sandoglobulin) vs. control. The trial included 2 arms: IIVIG Sandoglobulin 500 mg/kg and control; Schedule: weekly dose beginning at start of chemotherapy and discontinued when severe side effects occurred or when neutropenia resolved (>500 PMNs) | |
| Outcomes | all cause mortality; CDI; fungal infections; bacteremia; IRM; TRM; VOD; IP; adverse event | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Zikos 1998.
| Study characteristics | ||
| Methods | Randomization generation: not specified; allocation concealment: unclear; Blinding: none; Excluded:none | |
| Participants | 128 patients who had myeloablative allogeneic BMT + peripheral stem cell transplant from from HLA identical sibling donor for acute leukemia or CML or SAA and others; single ‐ center ‐ Italy, Europe Setting: hospitalization in isolation precautions and then outpatients | |
| Interventions | anti‐CMV hyperimmune globulin ( CMV IgG) vs. polyvalent Immunoglobulin. The trial included arms: anti‐CMV hyperimmune globulin 100mg/kg (CMV IgG) and polyvalent Immunoglobulin (IVIG) 400 mg/kg Schedule: weekly from day ‐7 to day +100d | |
| Outcomes | all cause mortality; CDI; fungal infections; CMV infections; CMV disease; IRM; TRM; acute GVHD; chronic GVHD; IP; | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
CDI=clinically documented infections; MDI=microbiologically documented infections (all pathogens included, ie bacterial, fungal, viral, other); IRM=infection related mortality; TRM=transplant related mortality; GVHD=graft versus host disease; VOD=veno‐occlusive disease; IP=interstitial pneumonia
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aulitzky 1991 | Phase I study. The safety and pharmacokinetics of two neutralizing human IgG1 monoclonal antibodies to CMV in BMT recipients was assessed in an open phase I trial. |
| Bode 1986 | Children with leukemia (ALL) and solid tumors reported together as a single cohort (not CLL or MM or BMT) |
| Bunch 1988 | Reports the study by the cooperative CLL group |
| Chapel 1992 | Not an RCT. The study describes the kinetics of immunoglobulin levels in 15 patients given IVIG in a different RCT included in this review (see reference Co‐operative group for the study of CLL) |
| Chapel 1993 | Review |
| Collins 1991 | Reports a study of immunoglobulin prophylaxis in patients with acute leukemia (not CLL or MM or BMT) |
| Copelan 1994 | Oral and not parenteral immunoglobulins. This is a controlled trial of orally administered immunoglobulin following bone marrow transplantation where 72 patients were randomized to oral Ig vs. placebo |
| Cortez 2002 | Not an RCT. Also the intervention was the administration of RSV (respiratory syncitial virus) immunoglobulins and not polyvalent IG or anti CMV IG |
| Eijkhout 2001 | This is a crossover study dealing with 43 patients with primary hypogammaglobulinemia who received standard‐dose immunoglobulin therapy for 9 months, followed by a 3‐month washout period, and high‐dose intravenous immunoglobulin therapy for 9 months, or vice versa. |
| Elfenbein 1989 | Not an RCT |
| Esperou 2004 | This is a subcategory of Cordonnier 2003 dealing with costs only |
| Fehir 1989 | Not RCT. This is a publication reporting on Immune globulin (GAMMAGARD) prophylaxis of CMV infections in patients undergoing organ transplantation and allogeneic bone marrow transplantation |
| Gamm 1994 | The study itself is not an RCT and quotes the RCT by Chapel 1994 (which is already included in the review) |
| Gerein 1989 | All pts. received first hyperimmune CMVIG and only those who became negative were randomized |
| Gimesi 1992 | Reports a study of immunoglobulin prophylaxis in children with acute lymphoblastic leukemia (not CLL or MM or BMT) |
| Graham Pole 1988 | Not an RCT |
| Jurlander 1994 | No RCT. 15 patients with CLL and hypogammaglobulinaemia and a history of recurrent infections received a fixed dose of 10 grams of gammaglobulin intravenously every 3 weeks. |
| Klaesson 1995 | Not RCT. Forty‐five recipients of bone marrow from HLA‐identical siblings were given intravenous immune globulin once a week during the first 3 months after transplantation. Fifty‐three consecutive previously transplanted HLA‐identical siblings were included as controls. |
| Messori 1994 | Not RCT, meta‐analysis. This is an analysis of the RCT's published on the effectiveness of hyperimmune immunoglobulins for prevention of CMV infection or disease in CMV‐seronegative recipients of allogeneic bone marrow transplantation (BMT). The clinical trials were identified by searching a number of computerized literature databases, by reviewing bibliographies of the paper examined and by consulting experts. |
| Nasman Bjork 1999 | Retrospective study. Sera obtained from 13 IVIG‐treated and 31 non‐IVIG‐treated patients before and at different time points after BMT, ranging from 3 days to 3 years, and from 18 healthy controls, were analyzed using a quantitative immunoblot system. |
| Nurnberger 1988 | Not an RCT |
| Petersen 1987 | This report represents a retrospective analysis of bacterial and fungal bacteremia of the same patients from another RCT (see reference Meyers in included studies) |
| Rand 1991 | No RCT. The pharmacokinetics of an IVIG, Gammagard were measured in 31 CMV antibody negative BMT patients as part of a multicenter efficacy trial of 2 weekly dose regimens. |
| Spitzer 1992 | Not an RCT. Historical controls. Continuous infusion intravenous immunoglobulin was compared to intermittent infusion following bone marrow transplantation |
| Sullivan 1996 | includes 383 pts. from Sullivan 1990 & 94 pts. from Bowden 1986. Outcomes reported only for 100 pts |
| Sullivan 1998 | Retrospective analysis. A retrospective analysis of two randomized clinical trials conducted Iin one center to determine the effect of IVIg infusions on the development and severity of VOD. Patients were randomized to receive or not to receive IVIG prophylaxis after allogeneic BMT. To determine the relationship of IVIG to the development and severity of VOD, a single observer reviewed data displays created for each patient for grading VOD without knowledge of patient IVIG use. |
| Terada 1980 | Not an RCT |
| Vu Van 1985 | Not an RCT, a description of 2 pilot studies compared to a historical control |
Contributions of authors
Pia Raanani ‐ Conception and design, provision of study material, protocol development, searching for trials, data extraction, analysis and interpretation of data, writing of review and final approval, clinical and scientific advice
Anat Gafter‐Gvili ‐ Provision of study material, protocol development, searching for trials, data extraction, eligibility and quality assessment, analysis and interpretation of data, writing of review and final approval, methodological advice
Mical Paul ‐ Provision of study material, protocol development, data extraction, eligibility and quality assessment, analysis and interpretation of data, writing of review and final approval, methodological advice
Isaac Ben‐Bassat ‐Protocol development, writing of review and final approval, clinical and scientific advice
Leonard Leibovici ‐ Conception and design, protocol development, writing of review and final approval, clinical and scientific advice
Ofer Shpilberg ‐ Conception and design, protocol development, writing of review and final approval, clinical and scientific advice
Sources of support
Internal sources
No sources of support provided
External sources
No sources of support provided
Declarations of interest
No potential conflict of interest
Edited (no change to conclusions)
References
References to studies included in this review
Abdel‐Mageed 1999 {published data only}
- Abdel-Mageed A, Graham-Pole J, Del Rosario ML, Longmate J, Ochoa S, Amylon M, Elfenbein GJ, Janiec J, Jansen J, Lazarus HM. Comparison of two doses of intravenous immunoglobulin after allogeneic bone marrow transplants. Bone marrow transplantation 1999;23(9):929-32. [DOI] [PubMed] [Google Scholar]
Boeckh 2001 {published data only}
- Boeckh, M, Bowden, RA, Storer, B, Chao, NJ, Spielberger, R, Tierney, DK, Gallez-Hawkins, G, Cunningham, T, Blume, KG, Levitt, D, Zaia, JA. Randomized, placebo-controlled, double-blind study of a cytomegalovirus-specific monoclonal antibody (MSL-109) for prevention of cytomegalovirus infection after allogeneic hematopoietic stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2001;1(7):343-51. [DOI] [PubMed] [Google Scholar]
Bordigoni 1987 {published data only}
- Bordigoni, P, Janot, C, Aymard, JP, Witz, F, Bene, MC, Legras, B, Schoonemann, F, Olive, D, Streiff, F. [Clinical and biological evaluation of the preventive role of anti-cytomegalovirus specific immunoglobulins in bone marrow grafts. Randomized study of 60 patients] [Evaluation clinique et biologique du role prphylatctyque des immunoglobulines specifiques anto-cytomegalovirus dans les greffes medullaires]. Nouvelle revue fran?aise d'hematologie 1987;29(5):289-93. [PubMed] [Google Scholar]
Boughton 1995 {published data only}
- Boughton, BJ, Jackson, N, Lim, S, Smith, N. Randomized trial of intravenous immunoglobulin prophylaxis for patients with chronic lymphocytic leukaemia and secondary hypogammaglobulinaemia. Clinical and laboratory haematology 1995;17(1):75-80. [DOI] [PubMed] [Google Scholar]
Bowden 1986 {published data only}
- Bowden RA, Sayers M, Flournoy N, Newton B, Banaji M, Thomas ED, Meyers JD. ytomegalovirus immune globulin and seronegative blood products to prevent primary cytomegalovirus infection after marrow transplantation. The New England journal of medicine 1986;314(16):1006-10. [DOI] [PubMed] [Google Scholar]
Bowden 1991 {published data only}
- Bowden, RA, Fisher, LD, Rogers, K, Cays, M, Meyers, JD. Cytomegalovirus (CMV)-specific intravenous immunoglobulin for the prevention of primary CMV infection and disease after marrow transplant. The Journal of infectious diseases 1991;164(3):483-7. [DOI] [PubMed] [Google Scholar]
Chapel 1994 {published data only}
- Chapel, HM, Lee, M, Hargreaves, R, Pamphilon, DH, Prentice, AG. Randomised trial of intravenous immunoglobulin as prophylaxis against infection in plateau-phase multiple myeloma. The UK Group for Immunoglobulin Replacement Therapy in Multiple Myeloma. Lancet 1994;343(8905):1059-63. [DOI] [PubMed] [Google Scholar]
Chapel 1994c {published data only}
- Chapel, H, Dicato, M, Gamm, H, Brennan, V, Ries, F, Bunch, C, Lee, M. Immunoglobulin replacement in patients with chronic lymphocytic leukaemia: a comparison of two dose regimes. British journal of haematology 1994;88:209-12. [DOI] [PubMed] [Google Scholar]
Condie 1984 {published data only}
- Condie RM, O'Reilly RJ. Prevention of cytomegalovirus infection by prophylaxis with an intravenous, hyperimmune, native, unmodified cytomegalovirus globulin. Randomized trial in bone marrow transplant recipients. The American journal of Medicine 1984;76(3A):134-141. [DOI] [PubMed] [Google Scholar]
- O'Reilly, RJ, Reich, L, Gold, J, Kirkpatrick, D, Dinsmore, R, Kapoor, N, Condie, R. A randomized trial of intravenous hyperimmune globulin for the prevention of CMV infections following marrow transplantation: preliminary results. Transplantation proceedings 1983;XV(1):1405-1411. [Google Scholar]
Cooperative CLL 1988 {published data only}
- Cooperative Group for the Study of Immunoglobulin in Chronic Lymphocytic Leukemia. Intravenous immunoglobulin for the prevention of infection in chronic lymphocytic leukemia. A randomized, controlled clinical trial. Cooperative Group for the Study of Immunoglobulin in Chronic Lymphocytic Leukemia. The New England journal of medicine 1988;319(14):902-7. [DOI] [PubMed] [Google Scholar]
- Griffiths, H, Brennan, V, Lea, J, Bunch, C, Lee, M, Chapel, H. Crossover study of immunoglobulin replacement therapy in patients with low-grade B-cell tumors. Blood 1989;73(2):366-8. [PubMed] [Google Scholar]
Cordonnier 2003 {published data only}
- Cordonnier C, Chevret S, Legrand M, Rafi H, Dhedin N, Lehmann B, Bassompierre F, Gluckman E. Should immunoglobulin therapy be used in allogeneic stem-cell transplantation? A randomized, double-blind, dose effect, placebo-controlled, multicenter trial. Annals of internal medicine 2003;139(1):8-18. [DOI] [PubMed] [Google Scholar]
Emanuel 1992 {published data only}
- Emanuel D, Taylor J, Brochstein J, Kernan N, Boulad F, Small TGillo A, Laver J, Castro-Malaspina H, Young J, Papadopoulos E, Carabasi M, Childs B, Flomenberg M, O'Reilly R. The use of intravenous immune globulin as prophylaxis for the infectious complications of allogeneic marrow transplantation. In: Blood. Vol. 80. 1992:271a.
Feinstein 1999 {published data only}
- Feinstein, LC, Seidel, K, Jocum, J, Bowden, RA, Anasetti, C, Deeg, HJ, Flowers, ME, Kansu, E, Martin, PJ, Nash, RA, Storek, J, Etzioni, R, Applebaum, FR, Hansen, JA, Storb, R, Sullivan, KM. Reduced dose intravenous immunoglobulin does not decrease transplant-related complications in adults given related donor marrow allografts. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 1999;5(6):369-78. [DOI] [PubMed] [Google Scholar]
Filipovich 1992 {published data only}
- Filipovich, AH, Peltier, MH, Bechtel, MK, Dirksen, CL, Strauss, SA, Englund, JA. Circulating cytomegalovirus (CMV) neutralizing activity in bone marrow transplant recipients: comparison of passive immunity in a randomized study of four intravenous IgG products administered to CMV-seronegative patients. Blood 1992;80:2656-60. [PubMed] [Google Scholar]
Gluck 1990 {published data only}
- Gluck, S, Schnutenhaus, W, Schneider, W. Prophylactic intravenous immunoglobulin (IVIG) application to patients with recurrent infections and secondary antibody deficiency due to B-cell neoplasia. In: Blut. Vol. 61. 1990:140.
Graham Pole 1990 {published data only}
- Graham Pole, J, Amylon, M, Elfenbein, G, Gallo, J, Klempere, M, Janssen, J, Lazarus, H, Pick, T, Fand, K, Spruce, W. Preventing systemic infections with IVIG: a randomized multicenter trial. Bone Marrow Transplantation 1990;5(suppl 2):132. [Google Scholar]
Hargreaves 1992 {published data only}
- Hagreaves RM, Lea JR, Holt J, Bunch C, Reid C, Griffiths H, Chapel, H. Infection, immune response and intravenous immunoglobulin infection prophylaxis in myeloma. British journal of cancer 1992;66(suppl XVII Abstract O40):11.
Jacobsen 1985 {published data only}
- Jacobsen, N, Schafer, U, Ostendorf, P, Kubaneck, B, Wolf, H. Intravenous hyperimmune globulin prophylaxis against cytomegalovirus interstitial pneumonitis after allogenic bone marrow transplantation. The Tokai journal of experimental and clinical medicine 1985;10(2-3):193-5. [PubMed] [Google Scholar]
- Kubaneck B, Ernst P, Ostendorf P, Schafer U, Wolf H. Preliminary data of a controlled trial of interavenous hyperimmune globulin in the prevention of cytomegalovirus infection in bone marrow transplant recipients. Transplantation proceedings 1985;XVII:468-9. [Google Scholar]
Lum 1994 {published data only}
- Lum L, Bitonti O, Galoforo S, Walker A, Lum C, Abella E, Babington R, Karenes C, Momim F, Uberti J, Ratanatharathorn V, Sensenbrenner L. Intravenous gammaglobulin (IVIG) does not alter immune parameters in a randomized trial after bone marrow transplantation (BMT). In: Experimental hematology. Vol. 22. 1994:680.
Meyers 1983 {published data only}
- Meyers, JD, Leszczynski, J, Zaia, JA, Flournoy, N, Newton, B, Snydman, DR, Wright, GG, Levin, MJ, Thomas, ED. Prevention of cytomegalovirus infection by cytomegalovirus immune globulin after marrow transplantation. Annals of internal medicine 1983;98:442-6. [DOI] [PubMed] [Google Scholar]
Molica 1996 {published data only}
- Molica S, Musto P, Chiurazzi F, Specchia G, Brugiatelli M, Cicoira L, Levato D, Nobile F, Carotenuto M, Liso V, Rotoli B. Prophylaxis against infections with low-dose intravenous immunoglobulins (IVIG) in chronic lymphocytic leukemia. Results of a crossover study. Haematologica 1996;81(2):121-6. [PubMed] [Google Scholar]
Musto 1995 {published data only}
- Musto, P, Brugiatelli, M, Carotenuto, M. Prophylaxis against infections with intravenous immunoglobulins in multiple myeloma. British journal of haematology 1995;89(4):945-6. [DOI] [PubMed] [Google Scholar]
Peltier 1992 {published data only}
- Peltier MK, Filipovich AH, Bechtel M, Dirksen CL, Englund JA. Randomized double-blinded comparison of three intravenous immunoglobulin products in bone marrow transplantation. Seminars in hematology 1992;29:112-5. [PubMed] [Google Scholar]
Poynton 1992 {published data only}
- Jackson, SK, Parton, J, Barnes, RA, Poynton, CH, Fegan, C. Effect of IgM-enriched intravenous immunoglobulin (Pentaglobin) on endotoxaemia and anti-endotoxin antibodies in bone marrow transplantation. European journal of clinical investigation 1993;23(9):540-5. [DOI] [PubMed] [Google Scholar]
- Poynton CH, Jackson S, Fegan C, Barnes RA, Whittaker JA. Use of IgM enriched intravenous immunoglobulin (Pentaglobin) in bone marrow transplantation. Bone marrow transplantation 1992;9:451-7. [PubMed] [Google Scholar]
Raiola 2002 {published data only}
- Raiola AM, Di Grazia C, Dominietto A, Bregante S, Gualandi F, Lamparelli T, Occhini D, Van Lint MT, Bacigalupo A. Prevention of graft versus host disease with IgM enriched immunoglobulins: a preliminary analysis of a randomized study [abstract]. In: Bone Marrow Transplantation. Vol. 29. 2002:S188.
Ringden 1987 {published data only}
- Ringden, O, Pihlstedt, P, Volin, L, Nikoskelainen, J, Lonnqvist, B, Ruutu, P, Ruutu, T, Toivanen, A, Wahren, B. Failure to prevent cytomegalovirus infection by cytomegalovirus hyperimmune plasma: a randomized trial by the Nordic Bone Marrow Transplantation Group. Bone Marrow Transplantation 1987;2(3):299-305. [PubMed] [Google Scholar]
Ruutu 1997 {published data only}
- Ruutu T, Ljungman P, Brinch L, Lenhoff S, Lonnqvist B, Ringden O, Ruutu P Volin L, Albrechtsen D, Sallerfors B, Ebeling F, Myllyla G. No prevention of cytomegalovirus infection by anti-cytomegalovirus hyperimmune globulin in seronegative bone marrow transplant recipients. The Nordic BMT Group. Bone marrow transplantation 1997;19(3):233-6. [DOI] [PubMed] [Google Scholar]
Salmon 1967 {published data only}
- Salmon, SE, Samal, BA, Hayes, DM, Hosley, H, Miller, SP, Schilling, A. Role of gamma globulin for immunoprophylaxis in multiple myeloma. The New England journal of medicine 1967;277:1336-40. [DOI] [PubMed] [Google Scholar]
Serrano 1999 {published data only}
- J Serrano, M Falcon, JA Castillejo, JA Navarro, A Pascual, I Gracia-Rubio, C Martin, P Gomez, F Martinez, A Torres. A randomized trial to determine the utility of intravenous hyperimmune immunoglobulin in antimicrobial prophylaxis and its immunomodulatory effect in allogeneic bone marrow transplantation hyperimmune immunoglobulin in antimicrobial prophylaxis and its immunomodulatory effect in allogeneic bone marrow transplantation. Transplantology (Madrid) 1999;10(1):1-10. [Google Scholar]
Sklenar 1993 {published data only}
- Sklenar, I, Schiffman, G, Jonsson, V, Verhoef, G, Birgens, H, Boogaerts, M, Ferrant, A, Christensen, BE, Hasle, H, Drivsholm, A. Effect of various doses of intravenous polyclonal IgG on in vivo levels of 12 pneumococcal antibodies in patients with chronic lymphocytic leukaemia and multiple myeloma. Oncology 1993;50:466-477. [DOI] [PubMed] [Google Scholar]
Sullivan 1990 {published data only}
- Sullivan, KM, Kopecky, KJ, , Jocom , J, Fisher, L, Buckner, CD, Meyers, JD, Counts, GW, Bowden, RA, Peterson, FB, Witherspoon, RP. Immunomodulatory and antimicrobial efficacy of intravenous immunoglobulin in bone marrow transplantation. The New England journal of Medicine 1990;323(11):705-12. [DOI] [PubMed] [Google Scholar]
Sullivan 2000 {published data only}
- Sullivan, K, Seidel, K, Jocom, J, Anasetti, C, Hnasen, J, Storb, R, Messner, H, Ndemanee, A, Goldman, J, Kolb, H, Beatty, P, Gluckman, E. Intravenous immunoglobulin (IVIG) prophylaxis in unrelated ddonor bone marrow transplantation(BMT): a phase III double-blind, Placebo-controlled multi-institutional trial. In: ASCO annual meeting abstract book. 2000:abstract 181.
Ustun 1998 {published data only}
- Ustun, C, Ozcan, M, Beksac, M, Ilhan, O, Cakir Gurman, G, Akan, H, Demirer, T, Arat, M, Celebi, H, Konuk, N, et al. Clinical effects of intravenous immune globulin (IVIG)treatment in patients with allogenetic peripheral stem cell transplantation. Blood 1998;92(10 Suppl1 (Pt 2)):355b, abstract 4528. [Google Scholar]
Winston 1982 {published data only}
- Winston, DJ, Pollard, RB, Ho, WG, Gallagher, JG, Rasmussen, LE, Huang, SN, Lin, CH, Gossett, TG, Merigan, TC, Gale, RP. Cytomegalovirus immune plasma in bone marrow transplant recipients. Annals of internal medicine 1982;97(1):11-8. [DOI] [PubMed] [Google Scholar]
Winston 1984 {published data only}
- Winston, DJ, Ho, WG, Lin, CH, Budinger, MD, Champlin, RE, Gale, RP. Intravenous immunoglobulin for modification of cytomegalovirus infections associated with bone marrow transplantation. Preliminary results of a controlled trial. The American journal of medicine 1984;76(3A):128-33. [DOI] [PubMed] [Google Scholar]
Winston 1987 {published data only}
- Winston DJ, Ho WG, Lin GH et al Winston DJ, Ho WG, Lin GH et al [Use of a polyvalent intravenous immunoglobulin or specific cytomegalovirus hyperimmunoglobulin for modification of cytomegalovirus infections and prevention of interstitial pneumonias following bone marrow transplantation] Immun Infekt 1985, 13:296-301 Winston DJ, Ho WG, Lin GH et al. Use of a polyvalent intravenous immunoglobulin or specific cytomegalovirus hyperimmunoglobulin for modification of cytomegalovirus infections and prevention of interstitial pneumonias following bone marrow transplantation. Immunitat und Infektion 1985;13:296-301. [PubMed] [Google Scholar]
- Winston, DJ, Ho, WG, Lin, CH, Bartoni, K, Budinger, MD, Gale, RP, Champlin, RE. Intravenous immune globulin for prevention of cytomegalovirus infection and interstitial pneumonia after bone marrow transplantation. Annals of internal medicine 1987;106(1):12-8. [DOI] [PubMed] [Google Scholar]
Winston 1993 {published data only}
- Winston, DJ, Ho, WG, Bartoni, K, Champlin, RE. Intravenous immunoglobulin and CMV-seronegative blood products for prevention of CMV infection and disease in bone marrow transplant recipients. Bone marrow transplantation 1993;12(3):283-8. [PubMed] [Google Scholar]
Winston 2001 {published data only}
- Winston, DJ, Antin, JH, Wolff, SN, Bierer, BE, Small, T, Miller, KB, Linker, C, Kaizer, H, Lazarus, HM, Petersen, FB, Cowan, MJ, Ho, WG, Wingard, JR, Schiller, GJ, Territo, MC, Jiao, J, Petrarca, MA, Tonetta, SA. A multicenter, randomized, double-blind comparison of different doses of intravenous immunoglobulin for prevention of graft-versus-host disease and infection after allogeneic bone marrow transplantation. Bone marrow transplantation 2001;28(2):187-96. [DOI] [PubMed] [Google Scholar]
Wolff 1993 {published data only}
- Wolff SN, Fay JW, Herzig RH, Greer JP, Dummer S, Brown RA, Collins RH, Stevens DA, Herzig GP. High-dose weekly intravenous immunoglobulin to prevent infections in patients undergoing autologous bone marrow transplantation or severe myelosuppressive therapy. A study of the American Bone Marrow Transplant Group. Annals of internal medicine 1993;118(12):937-42. [DOI] [PubMed] [Google Scholar]
Zikos 1998 {published data only}
- Zikos, P, Van Lint, MT, Lamparelli, T, Gualandi, F, Occhini, D, Mordini, N, Berisso, G, Bregante, S, Bacigalupo, A. A randomized trial of high dose polyvalent intravenous immunoglobulin (HDIgG) vs. Cytomegalovirus (CMV) hyperimmune IgG in allogeneic hemopoietic stem cell transplants (HSCT). Haematologica 1998;83(2):132-7. [PubMed] [Google Scholar]
References to studies excluded from this review
Aulitzky 1991 {published data only}
- Aulitzky, WE, Schulz, TF, Tilg, H, Niederwieser, D, Larcher, K, Ostberg, L, Scriba, M, Martindale, J, Stern, AC, Grass, P. Human monoclonal antibodies neutralizing cytomegalovirus (CMV) for prophylaxis of CMV disease: report of a phase I trial in bone marrow transplant recipients. The Journal of infectious diseases 1991;163(6):1344-7. [DOI] [PubMed] [Google Scholar]
Bode 1986 {published data only}
- Bode, U, Erps, M, Liappis, N. Immunoglobulin administration as infection prevention in oncologic patients [Immunglobulingabe als infektionprophylaxe onkologischer patienten?]. Klinische P?diatrie 1986;198(6):476-8. [DOI] [PubMed] [Google Scholar]
Bunch 1988 {published data only}
- Bunch, C. Immunoglobulin replacement in chronic lymphocytic leukaemia. Nouvelle Revue Francaise d'Hematologie 1988;30(5-6):419-22. [PubMed] [Google Scholar]
Chapel 1992 {published data only}
- Chapel HM, Lee M. Immunoglobulin replacement in patients with chronic lymphocytic leukemia (CLL):kinetics of immunoglobulin metabolism. Journal of clinical immunology 1992;12(1):17-20. [DOI] [PubMed] [Google Scholar]
Chapel 1993 {published data only}
- Chapel, HM, Hargreaves, R, Lee, M, Pamphilon, D. Intravenous immunoglobulin therapy in patients with multiple myeloma. Immunodeficiency 1993;4(1-4):77-8. [PubMed] [Google Scholar]
Collins 1991 {published data only}
- Collins, PW, Slocombe, G, Wainwright, A, Newland, AC. A pilot study using intravenous immunoglobulin for the prevention of infection during remission induction in acute leukaemia. Leukemia & lymphoma 1991;6:25-30. [DOI] [PubMed] [Google Scholar]
- Collins, PW, Solocombe, G, Wainwright, A, Newland, AC. Intravenous immunoglobulin for infection prophylaxis in acute leukemia. In: British Journal of Haemaology. Vol. 77. 1991:75.
Copelan 1994 {published data only}
- Copelan, EA, Bechtel, TP, Klein, JP, Klein, JL, Tutschka, P, Kapoor, N, Featheringham, NC, Avalos, BR. Controlled trial of orally administered immunoglobulin following bone marrow transplantation. Bone marrow transplantation 1994;13:87-91. [PubMed] [Google Scholar]
Cortez 2002 {published data only}
- Cortez, K, Murphy, BR, Almeida, KN, Beeler, J, Levandowski, RA, Gill, VJ, Childs, RW, Barrett, AJ, Smolskis, M, Bennett, JE. Immune-globulin prophylaxis of respiratory syncytial virus infection in patients undergoing stem-cell transplantation. The Journal of infectious diseases 2002;186:834-8. [DOI] [PubMed] [Google Scholar]
Eijkhout 2001 {published data only}
- Eijkhout, HW, Der Meer, JW, Kallenberg, CG, Weening, RS, van Dissel, JT, Sanders, LA, Strengers, PF, Nienhuis, H, Schellekens, PT. The effect of two different dosages of intravenous immunoglobulin on the incidence of recurrent infections in patients with primary hypogammaglobulinemia. A randomized, double-blind, multicenter crossover trial. Annals of internal medicine 2001;135:165-74. [DOI] [PubMed] [Google Scholar]
Elfenbein 1989 {published data only}
- Elfenbein, G, Krischer, J, Babington, R, Hong, R, Jansen, J, Lazarus, H, Winton, E, Rand, K. Interim results of a multicenter trial to prevent cytomegalovirus pneumonia after allogeneic bone marrow transplantation. Transplantation proceedings 1989;21(1):3099-100. [PubMed] [Google Scholar]
Esperou 2004 {published data only}
- Esperou, H, Brunot, A, Roudot-Thoraval, F, Buzyn, A, Dhedin, N, Rio, B, Chevret, S, Bassompierre, F, Gluckman, E, Cordonnier, C, Durand-Zaleski, I. Predicting the costs of allogeneic sibling stem-cell transplantation: results from a prospective, multicenter, French study. Transplantation 2004;77:1854-8. [DOI] [PubMed] [Google Scholar]
Fehir 1989 {published data only}
- Fehir, KM, Decker, WA, Samo, T, Young, JB, Lederer, E, Lawrence, EC. Immune globulin (GAMMAGARD) prophylaxis of CMV infections in patients undergoing organ transplantation and allogeneic bone marrow transplantation. Transplantation proceedings 1989;21(1 Pt 3):3107-9. [PubMed] [Google Scholar]
Gamm 1994 {published data only}
- Gamm, H, Huber, C, Chapel, H, Lee, M, Ries, F, Dicato, MA. Intravenous immune globulin in chronic lymphocytic leukaemia. Clinical and experimental immunology 1994;97 Suppl 1:17-20. [PMC free article] [PubMed] [Google Scholar]
Gerein 1989 {published data only}
- Gerein, V, Kropp, H, Schwabe, D, Gussetis, E, Langer, B, Kornhuber, B. [Prevention of cytomegalic inclusion disease in leukemia patients with a cytomegalovirus hyperimmune globulin preparation] [Zytomegalie-prophylaxe bei leukamierpatienten mit einem zytomegalie-hyperimmunglobulinpraparat]. Klinische P?diatrie 1989;201(4):323-9. [DOI] [PubMed] [Google Scholar]
Gimesi 1992 {published data only}
- Gimesi, A, Eibl, M, Koos, R, Somlo, P, Magyarossy, E, Kardos, G, Fazekas, E, Schmidt, M, Borsi, J, Schuler, D. Immunoglobulin prophylaxis during intensive treatment of acute lymphoblastic leukemia in children. Acta Paediatrica Hungarica 1992;32(2):115-25. [PubMed] [Google Scholar]
Graham Pole 1988 {published data only}
- Graham-Pole, J, Camitta, B, Casper, J, Elfenbein, G, Gross, S, Herzig, R, Koch, P, Mahoney, D, Marcus, R, Munoz, L, Munoz, L, Pick, T, Spruce, W, Steuber, P, Weiner, R. Intravenous immunoglobulin may lessen all forms of infection in patients receiving allogeneic bone marrow transplantation for acute lymphoblastic leukemia: a pediatric oncology group study. Bone marrow transplantation 1988;3:559-66. [PubMed] [Google Scholar]
Jurlander 1994 {published data only}
- Jurlander J, Geisler CH, Hansen MM. Treatment of hypogammaglobulinaemia in chronic lymphocytic leukaemia by low-dose intravenous gammaglobulin. European journal of haematology 1994;53(2):114-8. [DOI] [PubMed] [Google Scholar]
Klaesson 1995 {published data only}
- Klaesson, S, Ringden, O, Markling, L, Tammik, L. Intravenous immunoglobulin: immune modulatory effects in vitro and clinical effects in allogeneic bone marrow transplant recipients. Transplantation proceedings 1995;27(6):3536-7. [PubMed] [Google Scholar]
Messori 1994 {published data only}
- Messori, A, Rampazzo, R, Scroccaro, G, Martini, N. Efficacy of hyperimmune anti-cytomegalovirus immunoglobulins for the prevention of cytomegalovirus infection in recipients of allogeneic bone marrow transplantation: a meta-analysis. Bone marrow transplantation 1994;13(2):163-7. [PubMed] [Google Scholar]
Nasman Bjork 1999 {published data only}
- Nasman B, I, Fesel C, Brissac C, Lundkvist I. Prophylactic intravenous immunoglobulin treatment influences serum immunoglobulin M repertoire development after allogeneic bone marrow transplantation. Scandinavian journal of immunology 1999;50(1):73-82. [DOI] [PubMed] [Google Scholar]
Nurnberger 1988 {published data only}
- Nurenberger, W, Willers, R, Schroten, H, Bunning, G, Gobel, U, Whan, V. Preventive long term intravenous immunoglobulin infusion in children with acute lymphatic leukemia. II. Zymosan opsonization is decreased and is not increased by IgG infusions [Prophylaktische Langzeit-i.v.-Immunoglobulin-Infusion bei Kindern mot akuter lymphatischer Leulamie. II. Die Zymosan-Opsonisierung ist erniedrigt und wird durch IgG-infusionen nicht gesteigert]. Monatsschroft Kinderheilkunde: Organ der Deutschen Gesellschaft fur Kinderheilkunde 1988;136(4):181-185. [PubMed] [Google Scholar]
Petersen 1987 {published data only}
- Petersen FB, Bowden RA, Thornquist M, Meyers JD, Buckner CD, Counts GW, Nelson N, Newton BA, Sullivan KM, McIver J. The effect of prophylactic intravenous immune globulin on the incidence of septicemia in marrow transplant recipients. Bone marrow transplantation 1987;2(2):141-7. [PubMed] [Google Scholar]
Rand 1991 {published data only}
- Rand, KH, Gibbs, K, Derendorf, H, Graham-Pole, J. Pharmacokinetics of intravenous immunoglobulin (Gammagard) in bone marrow transplant patients. Journal of clinical pharmacology 1991;31(12):1151-4. [DOI] [PubMed] [Google Scholar]
Spitzer 1992 {published data only}
- Spitzer, TR, Cottler-Fox, M, Sullivan, P, Lynch, M, Tefft, MC, Pickle, LW, Cirenza, E, Cahill, R, Deeg, HJ, Sacher, R. Continuous infusion intravenous immunoglobulin is associated with a reduced incidence of infection and achieves higher serum immunoglobulin G levels than intermittent infusion following bone marrow transplantation. Seminars in hematology 1992;29(3 Suppl 2):123-6. [PubMed] [Google Scholar]
Sullivan 1996 {published data only}
- Sullivan, KM, Storek, J, Kopecky, KJ, Jocom, J, Longton, G, Flowers, M, Siadak, M, Nims, J, Witherspoon, RP, Anasetti, C, Appelbaum, FR, Bowden, RA, Buckner, CD, Crawford, SW, Deeg, HJ, Hansen, JA, McDonald, GB, Sanders, JE, Storb, R. A controlled trial of long-term administration of intravenous immunoglobulin to prevent late infection and chronic graft-vs.-host disease after marrow transplantation: clinical outcome and effect on subsequent immune recovery. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 1996;2(1):44-53. [PubMed] [Google Scholar]
Sullivan 1998 {published data only}
- Sullivan, KM, Kansu, E, Storer, B, Jocom, J, Emerson, G, Reagan, T, Emerson, V, Siadak, MF, Davis, C, Appelbaum, FR, Buckner, CD, Hansen, JA, Shulman, HM, Storb, R, McDonald, GB. Intravenous immunoglobulin and the risk of hepatic veno-occlusive disease after bone marrow transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 1998;4(1):20-6. [DOI] [PubMed] [Google Scholar]
Terada 1980 {published data only}
- Terada, H, Matsuno, K. Clinical effects of gamma globulin in severe infections associated with leukemia and malignant lymphoma. [Rinsho ketsueki] The Japanese journal of clinical hematology 1980;21(9):1328-32. [PubMed] [Google Scholar]
Vu Van 1985 {published data only}
- Vu Van, H, Fiere, D, Chomel, J, Thouvenot, D, Guyotat, D, Dutou, L, Ginestet, C, Lacroze, M, Aymard, M. Prophylaxis against cytomegalovirus infection after BMT with gammaglobulins. Experimental hematology (suppl.17) 1985;13:105. [Google Scholar]
Additional references
Bass 1993
- Bass EB, Powe NR, Goodman SN, Graziano SL, Griffiths RI, Kickler TS, Wingard JR. Efficacy of immune globulin in preventing complications of bone marrow transplantation: a meta-analysis.. Bone marrow transplantation 1993;12(3):273-82. [PubMed] [Google Scholar]
Besa 1992
- Besa EC. Recent advances in the treatment of chronic lymphocytic leukemia: defining the role of intravenous immunoglobulin. Seminars in hematology 1992;29(3 Suppl 2):14-23. [PubMed] [Google Scholar]
Chapel 1994a
- Chapel HM, Lee M, Hargreaves R, Pamphilon DH, Prentice AG. Randomised trial of intravenous immunoglobulin as prophylaxis against infection in plateau-phase multiple myeloma. The UK Group for Immunoglobulin Replacement Therapy in Multiple Myeloma. Lancet 1994;343(8905):1059-63. [DOI] [PubMed] [Google Scholar]
Chapel 1994b
- Chapel HM, Lee M. The use of intravenous immune globulin in multiple myeloma. Clinical and experimental immunology 1994;97 Suppl 1:21-4. [PMC free article] [PubMed] [Google Scholar]
Consensus IVIG 1990
- No authors listed. Consensus on IVIG. Lancet 1990;336(8713):470-2. [PubMed] [Google Scholar]
Espersen 1984
- Espersen F, Birgens HS, Hertz JB, Drivsholm A. Current patterns of bacterial infection in myelomatosis. Scandinavian journal of infectious diseases 1984;16(2):169-73. [DOI] [PubMed] [Google Scholar]
Fairley 1961
- Fairley GH, Scott RB. Hypogammaglobulinaemia in chronic lymphatic leukaemia. British medical journal 1961;5257:920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glowacki 1993
- Glowacki LS, Smaill FM. Meta-analysis of immune globulin prophylaxis in transplant recipients for the prevention of symptomatic cytomegalovirus disease. Transplantation proceedings 1993;25(1 Pt 2):1408-10. [PubMed] [Google Scholar]
Glowacki 1994
- Glowacki LS, Smaill FM. Use of immune globulin to prevent symptomatic cytomegalovirus disease in transplant recipients--a meta-analysis. Clinical transplantation 1994;8(1):10-18. [PubMed] [Google Scholar]
Graham‐Pole 1988
- Graham-Pole J, Camitta B, Casper J, Elfenbein G, Gross S, Herzig R, Koch P, Mahoney D, Marcus R, Munoz L. Intravenous immunoglobulin may lessen all forms of infection in patients receiving allogeneic bone marrow transplantation for acute lymphoblastic leukemia: a pediatric oncology group study. Bone marrow transplantation 1988;3(6):559-66. [PubMed] [Google Scholar]
Griffiths 1989
- Griffiths, H, Brennan, V, Lea, J, Bunch, C, Lee, M, Chapel, H. Crossover study of immunoglobulin replacement therapy in patients with low-grade B-cell tumors. Blood 1989;73(2):366-8. [PubMed] [Google Scholar]
Guglielmo 1994
- Guglielmo BJ, Wong-Beringer A, Linker CA. Immune globulin therapy in allogeneic bone marrow transplant: a critical review. Bone marrow transplantation 1994;13(5):499-510. [PubMed] [Google Scholar]
Hansen 1973
- Hansen MM. Chronic lymphocytic leukaemia. Clinical studies based on 189 cases followed for a long time. Scandinavian journal of haematology. Supplementum 1973;18:3-286. [PubMed] [Google Scholar]
Jackson 1993
- Jackson, SK, Parton, J, Barnes, RA, Poynton, CH, Fegan, C. Effect of IgM-enriched intravenous immunoglobulin (Pentaglobin) on endotoxaemia and anti-endotoxin antibodies in bone marrow transplantation. European journal of clinical investigation 1993;23(9):540-5. [DOI] [PubMed] [Google Scholar]
Jim 1956
- Jim RT, Reinhard EH. Agammaglobulinemia and chronic lymphocytic leukemia. Annals of internal medicine 1956;44(4):790-6. [DOI] [PubMed] [Google Scholar]
Kubaneck 1985
- Kubaneck B, Ernst P, Ostendorf P, Schafer U, Wolf H. Preliminary data of a controlled trial of interavenous hyperimmune globulin in the prevention of cytomegalovirus infection in bone marrow transplant recipients. Transplantation proceedings 1985;XVII:468-9. [Google Scholar]
O'Reilly 1983
- O'Reilly, RJ, Reich, L, Gold, J, Kirkpatrick, D, Dinsmore, R, Kapoor, N, Condie, R. A randomized trial of intravenous hyperimmune globulin for the prevenetion of CMV infections following marrow transplantation: preliminary results. Transplantation proceedings 1983;XV(1):1405-11. [Google Scholar]
Perri 1981
- Perri RT, Hebbel RP, Oken MM. Influence of treatment and response status on infection risk in multiple myeloma. The American journal of medicine 1981;71(6):935-40. [DOI] [PubMed] [Google Scholar]
Pirofsky 1984
- Pirofsky B. Intravenous immune globulin therapy in hypogammaglobulinemia. A review. The American journal of medicine 1984;76(3A):53-60. [DOI] [PubMed] [Google Scholar]
Rozman 1988
- Rozman C, Montserrat E, Vinolas N. Serum immunoglobulins in B-chronic lymphocytic leukemia. Natural history and prognostic significance. Cancer 1988;61(2):279-83. [DOI] [PubMed] [Google Scholar]
Savage 1982
- Savage DG, Lindenbaum J, Garrett TJ. Biphasic pattern of bacterial infection in multiple myeloma. Annals of internal medicine 1982;96(1):47-50. [DOI] [PubMed] [Google Scholar]
Siadak 1994
- Siadak MF, Kopecky K, Sullivan KM. Reduction in transplant-related complications in patients given intravenous immuno globulin after allogeneic marrow transplantation. Clinical and experimental immunology 1994;97(Suppl 1):53-7. [PMC free article] [PubMed] [Google Scholar]
Sokos 2002
- Sokos DR, Berger M, Lazarus HM. Intravenous immunoglobulin: appropriate indications and uses in hematopoietic stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2002;8(3):117-30. [DOI] [PubMed] [Google Scholar]
Sullivan 1996a
- Sullivan KM. Immunomodulation in allogeneic marrow transplantation: use of intravenous immune globulin to suppress acute graft-versus-host disease. Clinical and experimental immunology 1996;104 Suppl 1:43-8. [PubMed] [Google Scholar]
Twomey 1973
- Twomey JJ. Infections complicating multiple myeloma and chronic lymphocytic leukemia. Archives of internal medicine 1973;132(4):562-5. [PubMed] [Google Scholar]
Wadleigh 2003
- Wadleigh M, Richardson PG, Zahrieh D, Lee SJ, Cutler C, Ho V, Alyea EP, Antin JH, Stone RM, Soiffer RJ, DeAngelo DJ. Prior gemtuzumab ozogamicin exposure significantly increases the risk of veno-occlusive disease in patients who undergo myeloablative allogeneic stem cell transplantation. Blood 2003;102(5):1578-82. [DOI] [PubMed] [Google Scholar]
Wingard 1990
- Wingard JR. Advances in the management of infectious complications after bone marrow transplantation. Bone marrow transplantation 1990;6(6):371-83. [PubMed] [Google Scholar]
Winston 1985
- Winston DJ, Ho WG, Lin GH et al Winston DJ, Ho WG, Lin GH et al [Use of a polyvalent intravenous immunoglobulin or specific cytomegalovirus hyperimmunoglobulin for modification of cytomegalovirus infections and prevention of interstitial pneumonias following bone marrow transplantation] Immun Infekt 1985, 13:296-301 Winston DJ, Ho WG, Lin GH et al. Use of a polyvalent intravenous immunoglobulin or specific cytomegalovirus hyperimmunoglobulin for modification of cytomegalovirus infections and prevention of interstitial pneumonias following bone marrow transplantation. Immunit?t und Infektion 1985;13:296-301. [PubMed] [Google Scholar]
Winston 1987a
- Winston DJ, Ho WG, Lin CH, Bartoni K, Budinger MD, Gale RP, Champlin RE. Intravenous immune globulin for prevention of cytomegalovirus infection and interstitial pneumonia after bone marrow transplantation. Annals of internal medicine 1987;106(1):12-8. [DOI] [PubMed] [Google Scholar]
Winston 1987b
- Winston DJ, Ho WG, Lin CH, Bartoni K, Budinger MD, Gale RP, Champlin RE. Intravenous immune globulin for prevention of cytomegalovirus infection and interstitial pneumonia after bone marrow transplantation. Annals of internal medicine 1987;106(1):12-8. [DOI] [PubMed] [Google Scholar]
Winston 1990
- Winston DJ, Ho WG, Champlin RE. Cytomegalovirus infections after allogeneic bone marrow transplantation. Reviews of infectious diseases 1990;12 Suppl 7:S776-S792. [DOI] [PubMed] [Google Scholar]


