Abstract
Introduction:
Discordance between gastrointestinal (GI) symptoms and endoscopic inflammation in patients with ulcerative colitis (UC) is known. However, the correlations between symptoms and endoscopic and histologic (endo-histologic) mucosal healing and remains unknown.
Methods:
We performed a secondary analysis of prospectively collected clinical, endoscopic, and histologic data on 254 colonoscopies from 179 unique adults at a tertiary referral center from 2014–2021. Spearman’s rank was used to assess the correlation between patient reported outcomes and objective assessments of disease activity, as measured by validated instruments: 2-item patient-reported outcome measure (PRO-2) for stool frequency and rectal bleeding, the Ulcerative Colitis Endoscopic Index of Severity (UCEIS) for endoscopic inflammation, and the Geboes score for histologic inflammation. The predictive value of objective assessments of inflammation and clinical symptoms was described using sensitivity, specificity, and positive/negative predictive value.
Results:
One-quarter (28%, 72/254) of cases were in endo-histologic remission; of these, 25% (18/72) report GI symptoms (22% diarrhea; 6% rectal bleeding). Endo-histologically active disease had higher sensitivity (95% rectal bleeding; 87% diarrhea) and negative predictive value (94% rectal bleeding, 78% diarrhea) for clinically active disease compared to active disease on endoscopic (77%) or histologic assessment only (80%). The specificity of endo/histologic inflammation for GI symptoms was <65%. PRO-2 was positively correlated with endoscopic disease activity (Spearman’s rank 0.57, 95% CI 0.54–0.60, p<0.0001) and histologic disease activity (Spearman’s rank 0.49, 0.45–0.53, p<0.0001).
Conclusion:
One-quarter of patients with ulcerative colitis in endo-histologic (deep) remission have gastrointestinal symptoms, more commonly with diarrhea than rectal bleeding. Endo-histologic inflammation has high sensitivity (≥87%) for diarrhea/rectal bleeding.
INTRODUCTION
Discordance between gastrointestinal (GI) symptoms and endoscopic inflammation in patients with ulcerative colitis (UC) has been recognized in clinical trial and practice settings.1–3 However, studies that explore the associations between GI symptoms of patients with UC in endoscopic and histologic (endo-histologic) remission are lacking.1,3,4 Addressing this knowledge gap is important because symptomatic remission and mucosal healing are interrelated but distinct treatment targets.5 This is particularly important as the most recently updated Food and Drug Administration (FDA) draft guidance for ulcerative colitis (April 2022) recommends patient-reported outcomes (clinical remission) as the primary endpoint, with endoscopic/histologic outcomes as secondary/exploratory endpoints.6 We herein aim to assess the prevalence of active GI symptoms in patients with mucosal healing of UC, as well as the correlations between symptoms and endo-histologic activity, in a well-defined cohort of patients enrolled in a biobank with prospectively collected clinical, endoscopic, and histologic data.
METHODS
Study Population
We performed a secondary analysis on prospectively collected data from a cohort of adults with UC enrolled in the inflammatory bowel diseases (IBD) biobank at the University of California, San Diego, from June 2014 to November 2022. The diagnosis of UC was based on standard clinical, endoscopic, and histologic assessments.7 Study patients underwent colonoscopy with a gastroenterologist specialized in IBD for endoscopic disease activity assessment and systematically collected segmental colonic biopsies (cecal-ascending colon, transverse colon, descending colon, and rectosigmoid colon). A GI pathologist blinded to the endoscopic and clinical disease activity performed histologic disease assessments on the colonic biopsies. Patient-reported outcomes was collected independently by a study coordinator.
Of the available 452 cases, 198 were excluded due to no standardized histologic disease activity scoring of colonic biopsies (n=57) and no standardized assessment of clinical disease activity within 30 days of the colonoscopy (n=141).
Assessments of GI Symptoms and Mucosal Healing
We quantified GI symptoms using the two-item patient-reported outcome measure (PRO-2) for stool frequency (SF) and rectal bleeding (RB).5,8 Mucosal healing was defined as the lack of inflammation (remission) on endoscopy (UC Endoscopic Index of Severity, UCEIS=0),5,9 histology (Geboes score <2B.0),10 and endoscopy plus histology (endo-histologic remission: UCEIS=0 plus Geboes <2B.0). Histologic remission (Geboes score <2B.0) corresponds to the absence of neutrophils/eosinophils in the lamina propria and epithelium, crypt destruction, erosions, and ulcerations on colonic biopsies.10
Statistical Analysis
Our primary aim was to determine was to assess the correlations between symptoms and endo-histologic disease activity. We used Spearman’s rank correlation coefficient to assess for associations between clinical (PRO-2) and endoscopic (UCEIS) and histologic (Geboes score) disease activity. Fisher’s transformation was used to obtain the 95% confidence interval (CI). We assessed the predictive value of active objective endoscopic, histologic, or endo-histologic disease activity (predictor) for the presence of GI symptoms (outcome) using sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). We investigated the associations between gender, ethnicity, smoking status, UC disease extent, and UC therapies on the discordance between the presence of clinical symptoms in the setting of mucosal healing using univariate logistic regression, reported as odd ratios (OR) with 95%-CI. We performed sensitivity analyses on only the first colonoscopy in patients with >1 colonoscopies and UC extent (limited vs extensive). Limited colitis includes patients with UC involvement distal to the splenic flexure, including proctitis, proctosigmoiditis, and left-sided colitis.
Statistical analysis was performed using JMP®, Version 16. SAS Institute Inc., Cary, NC, 1989–2022. P-values of <0.05 were considered statistically significant.
Ethical Considerations
This human research study was performed in accordance with the Declaration of Helsinki and received Institutional Review Board approval from the University of California, San Diego. All patients provided informed consent.
RESULTS
Patient Demographics
A total of 254 colonoscopies (cases) from 179 unique patients were analyzed, with 51% females (92/179) and a median age of 41 years (IQR 29–55) (Supplemental Table 1). The majority of cases had extensive UC (61%, 154/254), and the majority of patients were treated with advanced therapies (59%, 105/179) including biologic therapies and/or small molecules (Supplemental Table 1). Less than half of the cases were in remission clinically (SF0/RB0 remission: 45%, 113/254; median PRO-2 = 1, IQR 0–2), endoscopically (40%, 102/254; median UCEIS = 1, IQR 0–4), or histologically (37%, 95/254; median Geboes score = 7, IQR 2–12), or endo-histologically (28%, 72/254) (Table 1).
Table 1:
Distribution of Clinical, Endoscopic, and Histologic Disease Activity from 254 Colonoscopies in 179 Adults with Ulcerative Colitis
| Clinical Disease Activity n (%) | Endoscopic-Histologic Disease Activity | Endoscopic Disease Activity | Histologic Disease Activity | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Remission n=72 | Active n=182 | Remission n=102 | Active n=152 | Remission n=95 | Active n=159 | |
|
| ||||||
| Clinical Disease Activity | ||||||
| Remission (n=113) | 54 (75.0) | 59 (32.4) | 70 (68.6) | 43 (28.3) | 67 (70.5) | 46 (28.9) |
| Active (n=141) | 18 (25.0) | 123 (67.6) | 32 (31.4) | 109 (71.7) | 28 (29.5) | 113 (71.1) |
|
| ||||||
| Stool Frequency | ||||||
| Normal (n=130) | 56 (77.8) | 74 (40.7) | 75 (73.5) | 55 (36.2) | 70 (73.7) | 60 (37.7) |
| Increased (n=124) | 16 (22.2) | 108 (59.3) | 27 (26.5) | 97 (63.8) | 25 (26.3) | 99 (62.2) |
| SF score 0.1–1.0 (n=49) | 10 (13.9) | 39 (21.4) | 19 (18.6) | 30 (19.7) | 15 (15.8) | 34 (21.4) |
| SF score 1.1–2.0 (n=32) | 6 (8.3) | 26 (14.3) | 7 (6.9) | 25 (16.4) | 6 (6.3) | 26 (16.4) |
| SF score 2.1–3.0 (n=43) | 0 (0) | 43 (23.6) | 1 (1.0) | 42 (27.6) | 4 (4.2) | 39 (24.5) |
|
| ||||||
| Rectal Bleeding | ||||||
| Absent (n=181) | 68 (94.4) | 113 (62) | 94 (92.1) | 87 (57.2) | 89 (93.7) | 92 (57.9) |
| Present (n=73) | 4 (5.6) | 69 (37.9) | 8 (7.8) | 65 (42.8) | 6 (6.3) | 67 (42.1) |
| RB score 0.1–1.0 (n=41) | 4 (5.6) | 37 (20.3) | 8 (7.8) | 33 (2.2) | 5 (5.3) | 36 (22.6) |
| RB score 1.1–2.0 (n=28) | 0 (0) | 28 (15.9) | 0 (0) | 28 (18.4) | 1 (1.0) | 27 (17.0) |
| RB score 2.1–3.0 (n=4) | 0 (0) | 4 (2.2) | 0 (0) | 4 (2.6) | 0 (0) | 4 (2.5) |
Clinical, endoscopic, and histologic disease activity on 254 colonoscopies from 179 unique adults with ulcerative colitis (UC). Clinical disease activity was assessed by the two-item patient-reported outcome (PRO-2) measure for stool frequency and rectal bleeding; clinical remission was defined as normal stool frequency without rectal bleeding. PRO-2 quantifies stool frequency (SF) and rectal bleeding (RB), averaged over 3 days. Stool frequency was scored from 0–3 for normal number of stools for the patient (0), 1–2 stools/day more than normal (1), 3–4 stools per day more than normal (2), and 5 or more stools/day than normal (3). Rectal bleeding was scored from 0–3 for no blood seen (0), streaks blood with stool less than half the time (1), obvious blood with stool most of the time (2), and passing blood alone (3). Endoscopic activity for UC is assessed by the UC Endoscopic Index of Severity (UCEIS) with remission defined as UCEIS score of 0. Histologic activity is assessed by the Gehoes score with remission defined as the absence of neutrophils/eosinophils in the lamina propria and epithelium, crypt description, erosions, and ulcerations (Geboes score <2B.0). Abbreviations: RB, rectal bleeding; SF, stool frequency
Associations between Patient-Reported and Objective Disease Activity Measures
One-quarter (25%, 18/72) of patients reported diarrhea/rectal bleeding despite endoscopic-histologic remission (Table 1), with increased SF (22%, 16/72) more common than RB (6%, 4/72) (Table 1). None of the patients in endo-histologic remission reported significant rectal bleeding (i.e., obvious blood with stool most of the time or passing blood alone) (Table 1).
The Spearman’s rank correlation coefficient for clinical disease activity and endoscopic disease activity was 0.57 (95% CI 0.54–0.60, p<0.0001) versus clinical disease activity and histologic disease activity was 0.49 (0.45–0.53, p<0.0001), respectively. The Spearman’s rank correlation coefficients were similar on the sensistivity analyses for UC disease extent (limited vs extensive) and using only the first colonoscopy per patient (Supplemental Tables 2 and 3).
The sensitivity of inflammation on endo-histologic assessment was higher (87%) than endoscopic inflammation alone (77%) or histological inflammation alone (80%) for the presence of diarrhea/rectal bleeding (Figure 1). The sensitivity of endo-histologic inflammation was higher for rectal bleeding (95%) than diarrhea (87%), particularly for the highest scores (SF: 94%, RB: 100%) vs the lowest scores (SF: 77%, RB: 90%) (Supplemental Table 4). The presence of endo-histologically active disease had 100% sensitivity for obvious passage of blood and 94% sensitivity for >2 stools per day more than normal. The PPV (68–71%) and NPV (69–75%) of objective inflammation for patient-reported GI symptoms were similar between endoscopic-, histologic-, and endo-histologic assessments (Figure 1).
Figure 1:
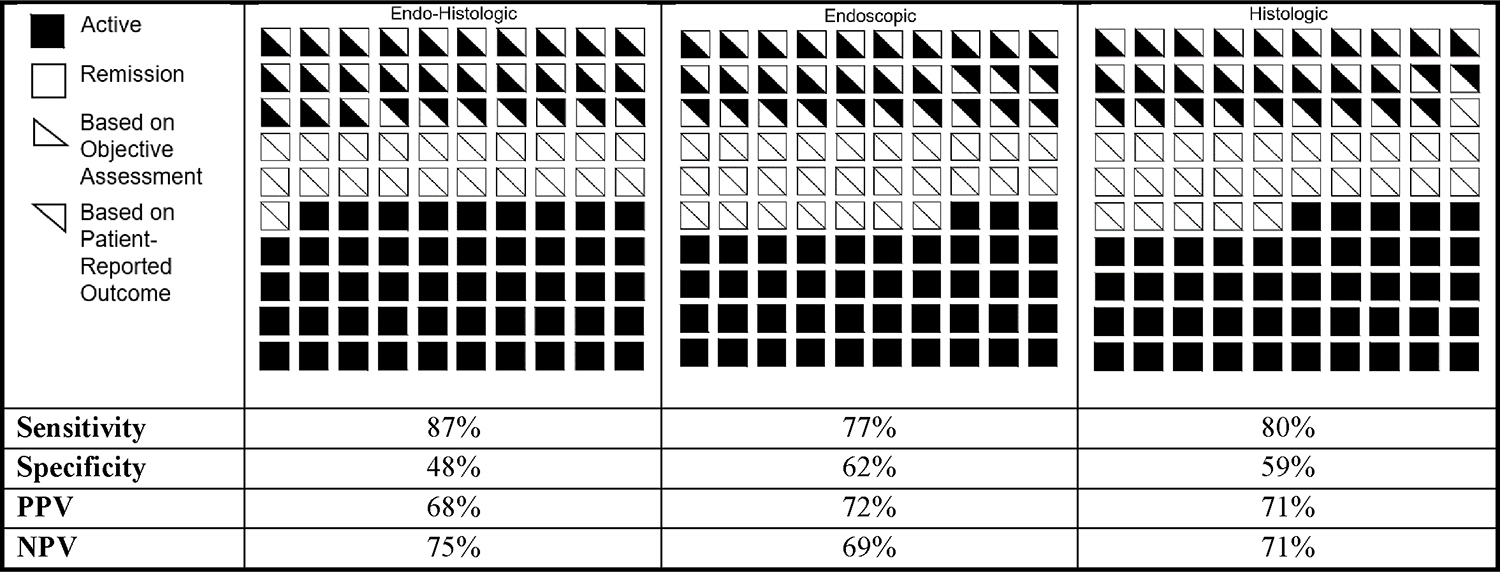
Distribution of Disease Activity by Clinical, Endoscopic, and Histologic Assessments in Adults with Ulcerative Colitis (n=254)
Distribution of disease activity as assessed by clinical (top triangle) and objective (endoscopy/histology; bottom triangle) on 254 colonoscopies from 179 unique patients with ulcerative colitis. Clinical, endoscopic, and histologic disease activity was quantified by the 2-item patient-reported outcome measure (PRO-2; remission = 0), UC Endoscopic Index of Severity (UCEIS; remission = 0), and Geboes score (remission < 2B.0). Abbreviations: NPV, negative predictive value; PPV, positive predictive value.
Gender, ethnicity, smoking, UC disease extent, and treatment with advanced therapies were not predictors for the presence or absence of diarrhea/rectal bleeding of patients with UC in endo-histologic remission (Supplemental Table 5).
DISCUSSION
The concordance/discordance between patient-reported outcomes and objective disease activity reported int his study is important in the contemporaneous treat-to-target paradigm where clinical, endoscopic, and histologic remission are recommended as the respective primary, secondary, and exploratory treatment endpoints in the updated FDA guidance for UC (2022).6 In this secondary analysis of prospectively collected standardized clinical, endoscopic, and endoscopic disease activity in a cohort of adults with UC participating in a biobank, increased stool frequency persisted in 22% of cases in endo-histologic remission. Only 6% had rectal bleeding (none with more than streaks of blood in bowel movements). Endo-histologically active disease had higher sensitivity (95% rectal bleeding; 87% diarrhea) and negative predictive value (94% rectal bleeding, 78% diarrhea) for clinically active disease compared to active disease on endoscopic or histologic assessment only, especially if the symptoms are moderate-to-severe (rather than mild).
In post-hoc analyses of UC clinicals, half of the patients with UC with endoscopic remission in clinical trials reported increased stool frequency (SF), although the prevalence of rectal bleeding (RB) was much lower.1,2 The stronger correlations between symptoms and more active disease were also reported in previous post-hoc analyses of clinical trials that assessed endoscopic disease activity only (mild-to-moderate2 vs moderate-to-severe1). A systematic review of 15 randomized placebo-controlled controlled induction UC trials found that histologic remission was not less sensitive than endoscopic remission for clinical disease activity.4 This study adds to the existing literature with prospective data captured in the clinical setting.
A limitation of this study is that it may not be generalizable to all patients with UC. Nearly 60% of the patients in our study are on advanced therapy. Our study setting is at a tertiary referral center with a dedicated IBD practice, and the study population comprises of patients enrolled in a biobank. Another limitation of this study is the small sample size of patients with endo-histologic remission (n=72), nonetheless this sample size is double of the previously published sample (largest n=30 in the EMBARK multicenter observational study).3
Strengths of this study include the prospective, protocolized (systematic) collection of segmental colonic biopsies and histologic evaluation in a well-defined cohort of patients. This methodology can increase the accuracy of objective disease activity assessment compared to non-standardized sampling or from limited assessment from flexible sigmoidoscopy. Moreover, this study may better reflect the spectrum of disease activity in the clinical setting compared to clinical trials which are predominately limited to carefully selected patients with moderate-to-severe disease activity.1
In conclusion, one-quarter of patients in deep remission report GI symptoms. Increased stool frequency is common (22%) in adults with UC in endo-histologic remission, though rectal bleeding is rare (3%). The combination of endoscopy and histology provides higher sensitivity (87%) to clinical disease activity than endoscopy alone (77%). Future studies to better characterize secondary causes of persistent GI symptoms despite UC in endo-histologic remission (e.g. bile acid diarrhea, celiac disease, disorders of the gut-brain axis) will be important to understand the etiologies and treatments of persistent GI symptoms despite mucosal healing of UC.
Supplementary Material
Funding:
This study is funded by the AGA Research Foundation’s AGA-Bristol Myers Squibb Pilot Research Award in Inflammatory Bowel Disease Health Disparities (AGA2022-21-03; Dr. Tse). Dr. Singh is supported by NIDDK K23DK117058 and R03DK129631, Litwin Pioneers in IBD grant, and PCORI Contract CER-2020C3-21024. Dr. Boland is supported by NIH K23 DK123406 and NIH P30 DK120515. Dr. Dulai is supported by a Digestive Diseases Research Center grant NIH DK12051.
Footnotes
Conflicts of Interests: CST has received research grants from the American Gastroenterological Association and the Crohn’s and Colitis Foundation. SS has received research grants from AbbVie, Janssen, and Pfizer, and personal fees from Pfizer (for ad hoc grant review). BSB has received research grants from Prometheus Biosciences, Gilead, and has served as a consultant for Bristol Myers Squibb, Pfizer, and Takeda. AEC has received consulting fees from AbbVie, Bristol Myers Squibb, Janssen, and Takeda. TD is an employee at Prometheus Laboratories. PSD has received research support and consultation funding from Takeda, AbbVie, Janssen, Pfizer, and Gilead. JN and HL have no conflicts of interest to declare.
Contributor Information
Chung Sang Tse, Division of Gastroenterology, University of Pennsylvania, Philadelphia, PA.
Hang P. Nguyen, Yale School of Medicine, New Haven, CT.
Siddharth Singh, Division of Gastroenterology, University of California, San Diego, La Jolla, CA.
Parambir S. Dulai, Division of Gastroenterology and Hepatology, Northwestern Medicine Digestive Health Center, Chicago, IL.
Jennifer Neill, Division of Gastroenterology, University of California, San Diego, La Jolla, CA.
Helen Le, Division of Gastroenterology, University of California, San Diego, La Jolla, CA.
Mark Valasek, Laboratory Director, Pathology Institute, 3201 University Drive East, Suite 330 Bryan, TX 77802, USA
Thierry Dervieux, Prometheus Laboratories, San Diego, California.
Angelina E. Collins, Division of Gastroenterology, University of California, San Diego, La Jolla, CA.
Brigid Sweeney Boland, Division of Gastroenterology, University of California, San Diego, La Jolla, CA.
References
- 1.Narula N, Alshahrani AA, Yuan Y, Reinisch W, Colombel JF. Patient-Reported Outcomes and Endoscopic Appearance of Ulcerative Colitis: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2019;17(3):411–418.e413. [DOI] [PubMed] [Google Scholar]
- 2.Ma C, Sandborn WJ, D’Haens GR, et al. Discordance Between Patient-Reported Outcomes and Mucosal Inflammation in Patients With Mild to Moderate Ulcerative Colitis. Clin Gastroenterol Hepatol. 2020;18(8):1760–1768.e1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colombel JF, Keir ME, Scherl A, et al. Discrepancies between patient-reported outcomes, and endoscopic and histological appearance in UC. Gut. 2017;66(12):2063–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sedano R, Hogan M, Zou G, et al. Comparison of the Relative Sensitivity of Clinical, Endoscopic, and Histologic Remission for Detection of Treatment Efficacy in Ulcerative Colitis Trials. Inflammatory Bowel Diseases. 2022. [DOI] [PubMed] [Google Scholar]
- 5.Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology. 2021. [DOI] [PubMed] [Google Scholar]
- 6.Food and Drug Administration. Developing Drugs for Treatment Guidance for Industry: Draft Guidance. In: Services USDoH, Human, eds. Silver Spring, M: Food and Drug Administration; 2022. [Google Scholar]
- 7.Satsangi J, Silverberg MS, Vermeire S, Colombel J-F. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55(6):749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jairath V, Khanna R, Zou GY, et al. Development of interim patient-reported outcome measures for the assessment of ulcerative colitis disease activity in clinical trials. Aliment Pharmacol Ther. 2015;42(10):1200–1210. [DOI] [PubMed] [Google Scholar]
- 9.Travis SP, Schnell D, Krzeski P, et al. Developing an instrument to assess the endoscopic severity of ulcerative colitis: the Ulcerative Colitis Endoscopic Index of Severity (UCEIS). Gut. 2012;61(4):535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geboes K, Riddell R, Öst A, Jensfelt B, Persson T, Löfberg R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut. 2000;47(3):404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


