Abstract
Objective
Recent data suggest that cerebral amyloid angiopathy (CAA) causes haemorrhagic lesions in cerebellar cortex as well as subcortical cerebral atrophy. However, the potential effect of CAA on cerebellar tissue loss and its clinical implications have not been investigated.
Methods
Our study included 70 non-demented patients with probable CAA, 70 age-matched healthy controls (HCs) and 70 age-matched patients with Alzheimer’s disease (AD). The cerebellum was segmented into percent of cerebellar subcortical volume (pCbll-ScV) and percent of cerebellar cortical volume (pCbll-CV) represented as percent (p) of estimated total intracranial volume. We compared pCbll-ScV and pCbll-CV between patients with CAA, HCs and those with AD. Gait velocity (metres/second) was used to investigate gait function in patients with CAA.
Results
Patients with CAA had significantly lower pCbll-ScV compared with both HC (1.49±0.1 vs 1.73±0.2, p<0.001) and AD (1.49±0.1 vs 1.66±0.24, p<0.001) and lower pCbll-CV compared with HCs (6.03±0.5 vs 6.23±0.6, p=0.028). Diagnosis of CAA was independently associated with lower pCbll-ScV compared with HCs (p<0.001) and patients with AD (p<0.001) in separate linear regression models adjusted for age, sex and presence of hypertension. Lower pCbll-ScV was independently associated with worse gait velocity (β=0.736, 95% CI 0.28 to 1.19, p=0.002) in a stepwise linear regression analysis including pCbll-CV along with other relevant variables.
Interpretation
Patients with CAA show more subcortical cerebellar atrophy than HC or patients with AD and more cortical cerebellar atrophy than HCs. Reduced pCbll-ScV correlated with lower gait velocity in regression models including other relevant variables. Overall, this study suggests that CAA causes cerebellar injury, which might contribute to gait disturbance.
INTRODUCTION
Cerebral amyloid angiopathy (CAA) is the second most common form of sporadic small vessel disease in the elderly and is pathologically associated with deposition of β-amyloid within small-sized to medium-sized cortical and leptomeningeal vessels.1 2 CAA is classically associated with haemorrhagic neuroimaging markers located in supratentorial lobar or cortical regions including lobar intracerebral haemorrhage (ICH), lobar cerebral microbleeds (CMBs) and cortical superficial siderosis (cSS).3 4 Multiple recent studies suggested that strictly superficial cerebellar microbleeds are associated with CAA.5-8 These studies therefore suggest that the classical pathophysiology of CAA in the cerebrum extends to the cerebellum.
A growing body of research shows that CAA is linked with non-haemorrhagic brain lesions such as ischaemic changes, microstructural network alterations and cerebral atrophy.9-12 Two recent studies found significantly lower volumes of cerebral white matter as well as basal ganglia in patients with CAA when compared with both healthy controls (HCs) and patients with Alzheimer’s disease (AD).11 13 In another study, patients with CAA had significantly more cortical thinning as compared with HCs, but cortical atrophy was more predominant in patients with AD as compared with CAA.12 Despite these studies supporting the notion that cerebral subcortical atrophy was more specific to CAA, the potential effect of CAA on cerebellar cortical and subcortical tissues has not been studied yet.
In this current work, we aimed to investigate cerebellar subcortical and cortical volumes in patients with CAA and compared with age-matched HCs and patients with AD. Our hypothesis was that cerebellar subcortical volume would be decreased in patients with CAA compared with age-matched HCs and patients with AD. As the cerebellum is involved in the maintenance of balance, posture and coordination of voluntary movements, it has an important role on gait.14 Previous studies reported an association of white matter integrity in the cerebellar peduncles with gait and mobility scores, suggesting that cerebellar white matter might be critical on gait functions.15 16 Therefore, we also hypothesised that decrease in cerebellar subcortical volume in patients with CAA would correlate with impaired gait velocity.
METHODS
Study design and participants
We performed a retrospective analysis of data obtained from an ongoing single-centre prospective cohort study of sporadic CAA. Patients diagnosed with probable CAA according to Boston criteria were enrolled between April 2006 and September 2014.3 Demographics, vascular risk factors and clinical data were collected as previously described.12 The inclusion criteria were (1) age of ≥55 years old and (2) multiple macrohaemorrhage/microhaemorrhage restricted to lobar, cortical or corticosubcortical regions; and absence of other cause of haemorrhage. Patients who presented with ischaemic stroke or those who had mild cognitive impairment, dementia or any other neurodegenerative condition were excluded with appropriate clinical and imaging evaluation. All patients were independently mobile and able to function without any limitation in daily activities. In addition, none of the patients had heavy drinking as defined per Centers for Disease Control and Prevention criteria (>14 drinks per week for men and >7 drinks per week for women). Subjects with CAA (n=70) were age-matched to HCs (n=70) and patients with AD (n=70) from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). Enrollment of subjects from the ADNI study was restricted to the same MRI scanner brand and strength as the local CAA cohort to minimise confounding effects from image acquisition; this approach has been used in multiple previous studies.11-13 Age matching was performed by matching each CAA subject to an HC and an AD subject based on the closest age to the first decimal, as previously described.12
Patients with CAA underwent a structural MRI using a Siemens Avanto 1.5T scanner (with a 12-channel head coil). The standardised protocol included T1-weighted multiecho magnetisation-prepared rapid gradient echo (1×1×1 mm voxel size), high-resolution susceptibility-weighted imaging (SWI 0.75×0.75×1.30 mm voxel size) and three-dimensional (3-D) fluid-attenuated inversion recovery MRI (1×1×1 mm voxel size) sequences. All subjects obtained from the ADNI database underwent a similar structural MRI protocol in a 1.5 T Siemens scanner. Further details on the MRI acquisition methods followed by the ADNI study have been previously described.17
3-D cortical and subcortical reconstructions were performed using the FreeSurfer software suite (https://www.surfer.nmr.mgh.harvard.edu).18 The cerebellum was segmented as part of the volume-based stream of FreeSurfer using a subject-independent probabilistic atlas and subject-specific measured values. The probabilistic atlas was built from a training set of brain surfaces and volumes labelled by hand and mapped to the standardised MNI305 brain. The cerebellar subcortical volume pertains to the FreeSurfer segmentation beneath the cerebellar white and grey matter boundary, including cerebellar white matter and deep cerebellar nuclei. Cerebellar cortical volume pertains to the FreeSurfer segmentation from the cerebellar pial surface to the cerebellar white and grey matter boundary. This probabilistic cerebellar labelling scheme of the FreeSurfer atlas is shown in figure 1. Average cortical thickness (CTh), supratentorial white matter volume (WMV), white matter hyperintensity (WMH) volume, supratentorial total brain volume (TBV) and estimated total intracranial volume (eTIV) were also calculated using FreeSurfer. TBV is an automated surface-based calculation of all brain matter excluding cerebellum, brainstem, cerebrospinal fluid and choroid plexus. eTIV is an automated computation exploiting a relationship between intracranial volume and the linear transform to MNI305 to estimate the total volume within the skull.14 In order to compare volumetric measurements across individuals, all volumes were corrected for variability in head size by expressing as percent of eTIV In patients with ICH, the supratentorial volume measures from the ICH-free hemispheres were used and multiplied by 2.
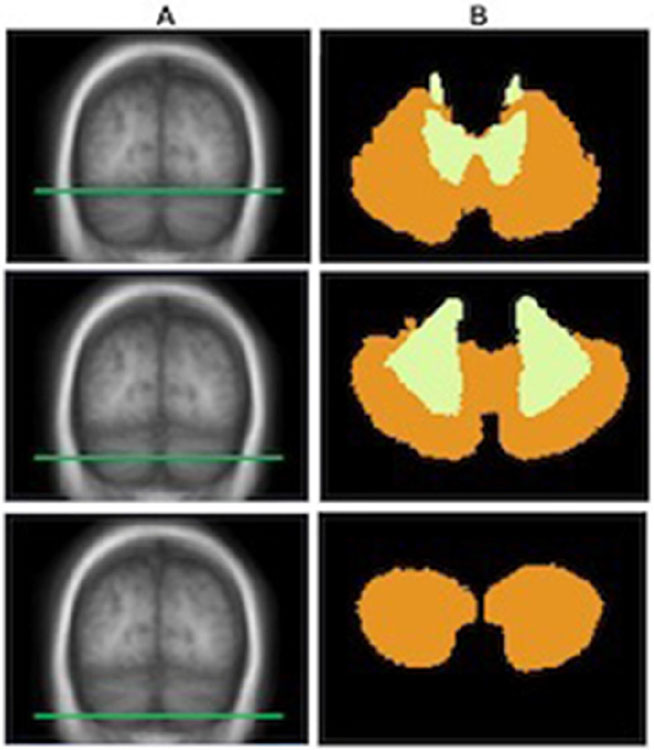
Figure 1.
Probabilistic cerebellar labelling scheme of the FreeSurfer atlas. Cerebellar segmentation into cortical versus subcortical components using the probabilistic FreeSurfer atlas. Three distinct axial slices of the cerebellum as labelled in the probabilistic FreeSurfer atlas in the standardised MNI305 brain. For each axial slice through the T1-weighted image (A), the corresponding parcellation of the cerebellum (B) into cortical (orange) and subcortical (yellow) regions is shown.
The images from the CAA cohort were reviewed to visually identify the established neuroimaging markers of CAA. The presence of ICH and CMBs were evaluated according to current consensus criteria.19 The presence of cSS was assessed based on previously published criteria.20 CMBs were counted in supratentorial lobar regions as well as in the cerebellum. Using a previously published topographical template, the cerebellum was anatomically divided into two regions as (1) superficial cerebellar region that includes cerebellar cortex and vermis and (2) deep cerebellar region that includes deep cerebellar grey nuclei and white matter.6 According to this template, microbleeds in the cerebellum were defined as (1) superficial cerebellar microbleeds or (2) deep cerebellar microbleeds.6
Gait velocity (metres/second) was assessed using the speed velocity measure of the Timed Get Up and Go Test in which slower velocity represents worse gait function.21
Statistical analysis
Statistical analyses were performed using Statistical Package for Social Sciences for Windows V.25. Fisher’s exact test was used for categorical variables and the results were presented as counts (%). The independent-samples t-test was applied for continuous variables and the results were presented as mean (±SD). Bivariate correlations were evaluated by Pearson’s correlation test.
The associations between percent of cerebellar subcortical volume (pCbll-ScV) or percent of cerebellar cortical volume (pCbll-CV dependent variable) and diagnosis of CAA were assessed using multiple linear regression analyses adjusted for age, sex and hypertension. Separate linear regression models were also used to assess the associations between pCbll-ScV and gait velocity as well as between pCbll-CV and gait velocity. These models also included age, sex, presence of hypertension, ICH, cSS, total microbleed counts, presence of cerebellar microbleeds, average CTh, pWMV pWMH and pTBV as independent variables. Then, we built a final linear regression model including gait velocity as dependent variable and pCbll-ScV and pCbll-CV as independent variable along with the aforementioned relevant covariates. A stepwise linear regression was applied in this model due to the risk of overfitting the model and potential consequences of multicollinearity between pCbll-ScV and pCbll-CV. The number of microbleeds and pWMH were log-transformed in each regression model due to their non-normal distribution. In all analyses, a p value less than 0.05 was considered statistically significant and all significance tests were two tailed.
RESULTS
This study cohort included 70 patients with probable CAA, 70 age-matched HCs and 70 age-matched patients with AD. Table 1 presents baseline characteristics and volumetric measurements in study groups. Within the CAA cohort, 44 (62.8%) patients had lobar ICH, while the remaining 26 (37.1%) had CMBs only. cSS was present in 30 (42.8%) patients. Fifteen patients with CAA (21.4%) had cerebellar microbleeds, with counts ranging between 1 and 7. All of the cerebellar microbleeds were in the superficial cerebellar regions.
Table 1.
Baseline characteristics and volumetric measurements of all study cohorts
| CAA (n=70) | HCs (n=70) | AD (n=70) | |
|---|---|---|---|
| Age (years), mean±SD | 70.3±6.8 | 70.7±2.5 | 70.7±6.3 |
| Sex (female), n (%) | 16 (22.9)*† | 34 (48.6) | 29 (41.4) |
| Hypertension, n (%) | 43 (61.4)* | 24 (34.3) | 34 (48.6) |
| pCbll-ScV, mean±SD | 1.49±0.1*† | 1.73±0.2 | 1.66±0.2 |
| pCbll-CV, mean±SD | 6.03±0.5* | 6.23±0.6 | 6.03±0.6 |
| pTBV, mean±SD | 54.6±3.89*† | 59.7±3.26 | 55.8±3.02 |
| Average cortical thickness, mean±SD | 2.27±0.1*† | 2.32±0.09 | 2.09±0.1 |
| pWMV, mean±SD | 26.9±2.97*† | 30.2±2.28 | 29.5±1.92 |
| pWMH volume, median (IQR) | 1.66 (0.72–2.32)*† | 0.15 (0.05–0.44) | 0.290 (0.1–0.89) |
Significantly different when compared with patients with AD (p<0.05).
Significantly different when compared with HCs (p<0.05).
AD, Alzheimer’s disease; CAA, cerebral amyloid angiopathy; HC, healthy control; pCbll-CV, percent of cerebellar cortical volume; pCbll-ScV, percent of cerebellar subcortical volume; pTBV, percent of total brain volume; pWMH, percent of white matter hyperintensity; pWMV, percent of white matter volume.
Patients with CAA had significantly lower pCbll-ScV compared with HCs as well as with patients with AD (table 1, p<0.001 for both comparisons). CAA was independently associated with lower pCbll-ScV (β=0.18, 95% CI 0.1 to 0.25, p<0.001) in a linear regression model corrected for age, sex and presence of hypertension. We found similar results comparing CAA to AD, with CAA being independently associated with lower pCbll-ScV (β=0.07, 95% CI 0.03 to 0.1, p<0.001). These results did not change when pTBV was added into the regression models. Within the CAA cohort, pCbll-ScV was not associated with age, sex, hypertension or imaging markers of CAA severity (table 2). pCbll-ScV showed a trend of correlation with pWMV (p=0.088).
Table 2.
Comparison of pCbll-ScV with demographic and imaging markers in patients with CAA
| Binary variables | pCbll-ScV (mean±SD) | P value | |
|---|---|---|---|
| Sex | |||
| Male (n=54) | 1.47±0.17 | 0.126 | |
| Female (n=16) | 1.55±0.19 | ||
| Hypertension | |||
| Yes (n=43) | 1.47±0.17 | 0.16 | |
| No (n=27) | 1.53±0.18 | ||
| Presence of ICH | |||
| Yes (n=44) | 1.50±0.17 | 0.475 | |
| No (n=26) | 1.47±0.18 | ||
| Presence of cSS | 0.774 | ||
| Yes (n=30) | 1.48±0.16 | ||
| No (n=40) | 1.50±0.18 | ||
| Presence of cerebellar microbleeds | 0.389 | ||
| Yes (n=15) | 1.46±0.11 | ||
| No (n=55) | 1.50±0.19 | ||
| r | P value | ||
| Correlation of pCbll-ScV with continuous variables | |||
| Age | −0.003 | 0.98 | |
| CMB counts* | −0.049 | 0.689 | |
| pCbll-CV | 0.538 | <0.001 | |
| pWMH volume | −0.052 | 0.67 | |
| pTBV | 0.102 | 0.402 | |
| Average cortical thickness | −0.052 | 0.669 | |
| pWMV | 0.205 | 0.088 | |
Represents logarithmic count of microbleeds.
CMB, cerebral microbleed; cSS, cortical superficial siderosis; ICH, intracerebral haemorrhage; pCbll-CV, percent of cerebellar subcortical volume; pCbll-ScV, percent of cerebellar subcortical volume; pTBV, percent of total brain volume; pWMH, percent of white matter hyperintensity; pWMV, percent of white matter volume.
Patients with CAA also had significantly lower pCbll-CV compared with HCs (6.03±0.5 vs 6.23±0.5, p=0.028). However, there was no difference in pCbll-CV between patients with CAA and those with AD (p=0.957). In regression models, diagnosis of CAA was not associated with pCbll-CV compared with HCs (p=0.145) or patients with AD (p=0.656) corrected for age, sex and hypertension. pCbll-CV correlated with pCbll-ScV (r=0.530, p<0.001). No significant association was found with age, sex, hypertension or imaging markers of CAA severity, but there was a trend towards lower pCbll-CV in patients with cerebellar microbleeds compared with those without (table 3, p=0.091).
Table 3.
Comparison of pCbll-CV with demographic and imaging markers in patients with CAA
| Binary variables | pCbll-CV (mean±SD) | P value | |
|---|---|---|---|
| Sex | |||
| Male (n=54) | 6.00±0.55 | 0.35 | |
| Female (n=16) | 6.13±0.32 | ||
| Hypertension | |||
| Yes (n=43) | 5.98±0.48 | 0.305 | |
| No (n=27) | 6.11±0.54 | ||
| Presence of ICH | |||
| Yes (n=44) | 6.02±0.47 | 0.951 | |
| No (n=26) | 6.03±0.58 | ||
| Presence of cSS | 0.673 | ||
| Yes (n=30) | 6.06±0.47 | ||
| No (n=40) | 6.00±0.53 | ||
| Presence of cerebellar microbleeds | 0.091 | ||
| Yes (n=15) | 5.83±0.54 | ||
| No (n=55) | 6.08±0.49 | ||
| r | P value | ||
| Correlation of pCbll-ScV with continuous variables | |||
| Age | −0.071 | 0.56 | |
| CMB counts* | −0.16 | 0.186 | |
| pCbll-ScV | 0.538 | <0.001 | |
| pWMH volume | −0.064 | 0.601 | |
| pTBV | 0.168 | 0.164 | |
| Average cortical thickness | −0.119 | 0.326 | |
| pWMV | 0.059 | 0.629 | |
Represents logarithmic count of microbleeds.
CMB, cerebral microbleed; cSS, cortical superficial siderosis; ICH, intracerebral haemorrhage; pCbll-CV, percent of cerebellar subcortical volume; pCbll-ScV, percent of cerebellar subcortical volume; pTBV, percent of total brain volume; pWMH, percent of white matter hyperintensity; pWMV, percent of white matter volume.
We also compared demographics, risk factors and imaging markers in patients with cerebellar microbleeds and those without. Patients with cerebellar microbleeds were older, had higher rates of hypertension and higher lobar CMB counts (table 4).
Table 4.
Comparison of demographics and imaging markers between patients with and without cerebellar microbleeds
| Patients with cerebellar microbleeds (n=15) |
Patients without cerebellar microbleeds (n=55) |
P value | |
|---|---|---|---|
| Age (years), mean±SD | 74.2±6.5 | 69.2±6.6 | 0.013 |
| Sex (female), n (%) | 12 (80) | 42 (76.4) | 0.766 |
| Hypertension, n (%) | 13 (86.7) | 30 (54.5) | 0.023 |
| Presence of ICH, n (%) | 12 (80) | 32 (58.2) | 0.121 |
| Lobar CMB counts,* mean±SD | 1.86±0.56 | 1.24±0.73 | 0.003 |
| pWMH, mean±SD | 1.57±0.99 | 1.68±1.11 | 0.727 |
| pCbll-ScV, mean±SD | 1.46±0.11 | 1.50±0.19 | 0.389 |
| pCbll-CV, mean±SD | 5.83±0.54 | 6.08±0.49 | 0.091 |
| pTBV, mean±SD | 56.2±2.9 | 57.71±3.7 | 0.078 |
| Average CTh, mean±SD | 2.26±0.1 | 2.27±0.1 | 0.639 |
| pWMV, mean±SD | 25.8±3.2 | 27.2±2.8 | 0.105 |
Represents logarithmic count of microbleeds.
CMB, cerebral microbleed; cSS, cortical superficial siderosis; CTh, cortical thickness; ICH, intracerebral haemorrhage; pCbll-CV, percent of cerebellar cortical volume; pCbll-ScV, percent of cerebellar subcortical volume; pTBV, percent of total brain volume; pWMH, percent of white matter hyperintensity; pWMV, percent of white matter volume.
Cerebellar microbleed counts significantly correlated with lobar CMB counts (r=0.332, p=0.005). Presence of cerebellar microbleeds was not associated with volumetric parameters except demonstrating a trend towards lower pCbll-CV (p=0.091). In a stepwise logistic regression analysis, only older age (β=1.14, 95%CI 1.01 to 0.28, p=0.027) and higher lobar CMB counts (β=4.55, 95% CI 1.40 to 14.7, p=0.011) were independently associated with the presence of cerebellar microbleeds but not age, sex, presence of HT, ICH, cSS, pWMH, pWMV pCbll-ScV and pCbll-CV.
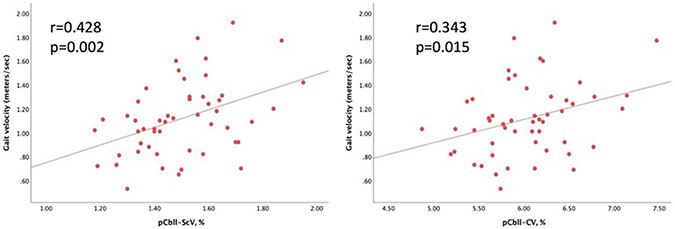
Gait testing was available in 52 patients with CAA (74.2%). Gait velocity significantly correlated with pCbll-ScV and pCbll-CV (figure 2). Gait velocity also correlated with age (r=−0.303, p=0.033), pWMV (r=0.287, p=0.043) and pTBV (r=0.281, p=0.048). There was no association between gait and sex (p=0.820), hypertension (p=0.819), ICH (p=0.747), cSS (p=0.268), CMB counts (p=0.336), pWMH (p=0.687), CTh (p=0.191) or presence of cerebellar microbleeds (p=0.987). In separate linear regression analyses, pCbll-ScV and pCbll-CV were independently associated with gait velocity (β=0.68, 95% CI 0.15 to 1.25, p=0.020, and β=0.206, 95% CI 0.02 to 0.39, p=0.031, respectively) after adjusting for age, sex, presence of hypertension, ICH, cSS, total microbleed counts, presence of cerebellar microbleeds, average CTh, pWMH, pWMV and pTBV. In a final stepwise linear regression, only pCll-ScV remained independently associated with gait velocity (β=0.736, 95% CI 0.28 to 1.19, p=0.002) among covariates including age, sex, presence of hypertension, ICH, cSS, total microbleed counts, presence of cerebellar microbleeds, average CTh, pWMH, pWMV pTBV and pCbll-CV.
Figure 2.
Scatter plots showing correlation of pCbll-ScV and pCbll-CV with gait velocity. pCbll-CV, percent of cerebellar cortical volume; pCbll-ScV, percent of cerebellar subcortical volume.
DISCUSSION
The main findings of this study are that patients with CAA had lower pCbll-ScV when compared with both HCs and patients with AD, and that this volume loss was independently associated with lower gait velocity in patients with CAA. We also confirmed earlier reports that cerebellar microbleeds are typically located in superficial cerebellar regions in patients with CAA.5 6 Previous studies showed that CAA causes significantly more cerebral atrophy in the cortex when compared with HCs and more atrophy in the supratentorial subcortical structures (both white matter and basal ganglia) when compared with both HCs and patients with AD.11 12 Our study supports that subcortical tissue loss is more pronounced in CAA than in AD even in the cerebellum. The pathophysiological mechanism of atrophic changes in CAA is uncertain, but there are likely many factors that can cause tissue loss in CAA including vascular amyloid deposition, vascular dysfunction, global ischaemia or direct effects of CAA-related brain lesions. The classical understanding of CAA pathogenesis is that vascular amyloid accumulates in the supratentorial brain; however, neuropathological studies in patients with AD have reported that amyloid also deposits within the cerebellum.22 23 Although the clinical importance of this cerebellar deposition in CAA is still unknown, there is growing evidence that microbleeds are specifically found in superficial regions of the cerebellum in patients with CAA, a distribution that matches the Boston criteria for the cerebrum.5 6 24 In a study including patients with ICH, it was reported that the superficial cerebellar microbleeds were associated with increased cerebral as well as cerebellar amyloid load as measured by Pittsburgh Compound B–positron emission imaging (PiB-PET), favouring CAA diagnosis.5 Moreover, the association of higher rates of β-amyloid positivity with the presence of cortical cerebellar microbleeds has been reported in a very recent study of memory clinic patients who underwent PiB-PET.25 Our results showing independent association of cerebellar microbleeds with lobar microbleed counts and older age confirm previous reports and support the notion that additional haemorrhagic lesions in the infratentorial region might represent severity of the disease.5 6 On the other hand, absence of relationship with ischaemic markers and tissue loss may be related to the fact that single discernible lesions such as microbleeds and volumetric markers of more global injury such as brain volumes might have different pathophysiological mechanisms in CAA.10 26
Cerebellar atrophy, on the other hand, has been demonstrated in healthy ageing and in patients with AD in several neuropathological as well as neuroimaging studies.23 27-32 Our study, to the best of our knowledge, is the first study exploring cerebellar volume loss in patients with CAA. Our findings demonstrated that patients with CAA had increased volume loss in the subcortical cerebellum as compared with HCs and patients with AD and in the cortical cerebellum as compared with HCs. We did not find any association of pCbll-ScV or pCbll-CV with age, sex, hypertension or any of the CAA-related lesions on neuroimaging. These findings suggest that cerebellar volume loss in patients with CAA is not directly affected by classical vascular risk factors or supratentorial lesions such as ICH, cSS or WMH. A mechanism such as diaschisis where supratentorial pathologies would damage the cerebellum is thus unlikely. The presence of cerebellar microbleeds exclusively in superficial cerebellar regions and the significant correlation between cerebellar and CMB counts support the idea that direct vascular amyloid-related mechanisms might be responsible for both cerebral and cerebellar structural changes. The exact mechanism of the effect of CAA on cerebellar subcortical atrophy is unknown, but extrapolating from the main global ischaemic mechanism of CAA, we found that vascular dysfunction might be an explanation. This hypothesis will need to be tested in appropriate animal models of CAA as well as in humans. Functional MRI can help determine measures of vascular dysfunction in humans, but challenges exist in applying this method to the cerebellum. Future studies including in vivo molecular imaging of cerebellar vascular amyloid and histopathological examination of cerebellum are needed to understand the underlying mechanism of cerebellar structural changes in patients with CAA.
Several studies have reported the relationship of gait function with brain white matter and grey matter loss in patients with small vessel diseases, but the findings in patients with CAA are inconclusive.9 33 34 The role of subcortical cerebellar structures on gait function has been studied with diffusion tensor imaging in an ageing population, and these studies showed that the integrity of the cerebellar peduncles was associated with measures of gait.15 16 We found that lower cerebellar subcortical and cortical volumes were independently associated with gait velocity, supporting the view that cerebellar atrophy in patients with CAA has direct implications on gait function. However, pCbll-ScV but pCbll-CV remained independently associated with gait velocity after a stepwise linear regression analysis suggesting that subcortical cerebellar tissue has more prominent impact on gait. There might be other diseases that might affect gait such as poorly controlled diabetes, or other vascular diseases. However, patients with CAA in our cohort were mobile and independent in daily activities, and they did not have other significant comorbidities affecting their functional abilities. This is ideal to study direct effects of CAA on gait, which was one of our aims for this particular study. Future studies should also use a longitudinal design to test progressive cerebellar volume loss over time and its potential relationship with other imaging markers of CAA and clinical outcome. Such a study requires follow-up imaging after many years as atrophy might be a relatively slow process, but this line of work is ongoing.
Our study has multiple strengths including standardised advanced neuroimaging acquisition and analyses in a large cohort of patients with well-defined CAA, age-matched HC and patients with AD. The standardised gait testing allowed us to confirm the important hypothesis that cerebellar subcortical atrophy might be a direct contributor to gait changes in CAA. We only used one test to evaluate gait but the Timed Get Up and Go Test is an easily interpreted, objective and comprehensive test that incorporates numerous gait tasks including standing up from a seated position, walking, turning, stopping and sitting down. It should also be remembered that a large comparison matrix causes multiple testing problems, so a single outcome measure for gait minimises this problem. The MRIs in HC and AD groups were obtained in different scanners within the USA, but they were performed using machines with the same brand (Siemens) and same field strength (1.5 T) when compared with patients with CAA. Rigorous quality assurance protocols were used for both our patients with CAA scans and ADNI acquisitions, and multiple prior papers used ADNI data as age-matched comparators. To our knowledge, there is no particular concern in pooling scans obtained from the same brand and same field strength scanners for volumetric analyses. Use of 1.5 T MRI scans might also be seen as a limitation of the study; however, resolution of our SWI scans were good enough to easily identify microbleeds, particularly in the cerebellum. Segmentation of the cerebellum might have been improved by using 3 T scans; however, having high-resolution 3D T1-weighted images allowed us to overcome this issue in our study cohorts. We used sex as a biological variable and included it in all regression models together with other relevant covariates. Hypertension was also included in the models as a relevant covariate. Forty-four of our patients had lobar ICHs, while the remaining 26 had lobar CMBs only that might cause heterogeneity in the study cohort. This study sample is not suitable to perform separate regression analyses within patients with ICH and those with CMBs only, but these two groups did not statistically differ in terms of demographics or other imaging markers studied in this work (data not shown). pCbll-ScV and pCbll-CV were not different between patients with ICH (1.50±0.17 and 6.02+0.47) and those with CMBs only (1.47±0.18, 6.03±0.58) either (p=0.475 and 0.951, respectively). We still included presence of ICH as a covariate in every regression model, and it has not been significant in any model.
In conclusion, patients with CAA show significant subcortical cerebellar atrophy when compared with both HCs and patients with AD and cortical cerebellar atrophy when compared with HCs. These changes do not appear to be impacted by supratentorial structural lesions such as ICH, cSS or WMH. pCbll-ScV outperformed pCbll-CV along with other relevant variables on predicting gait velocity, supporting the view that subcortical cerebellar tissue has implications on gait in CAA.
Key messages.
What is already known on this topic
Previous studies on cerebral amyloid angiopathy (CAA) demonstrated lower volumes of cerebral white matter and basal ganglia compared with healthy subjects and patients with Alzheimer’s disease (AD) and thinner cortices as compared with healthy subjects. Multiple lines of evidence also show that CAA is also associated with subcortical cerebellar microbleeds. However, the potential effect of CAA on cerebellar cortical and subcortical tissues have not been studied yet.
What this study adds
In this study, we found that patients with CAA show significant subcortical cerebellar atrophy compared with both healthy subjects and patients with AD and cortical cerebellar atrophy when compared with healthy controls, and subcortical cerebellar tissue loss correlates with worse gait velocity in CAA.
How this study might affect research, practice and/or policy
This study suggests that CAA is a contributor to cerebellar tissue loss that might lead to implications on gait.
Funding
This study was supported by National Institutes of Health (NINDS R01NS114526 (MEG) and NINDS AG26484 (SMG)).
Footnotes
Competing interests None declared.
Ethics approval The study was performed with the approval of and in accordance with the guidelines of the institutional review board of Massachusetts General Hospital. The participants gave written informed consent to participate in the study before taking part. There were no photographs, videos or other information of any recognisable person.
Data availability statement
Data are available upon reasonable request. Data for this study were gathered from two sources: (1) data obtained as part of an approved study by the institutional review board of Massachusetts General Hospital; these data are not publicly available but may be available upon reasonable request; and (2) data collected from the Alzheimer’s Disease Neuroimaging Initiative and is available in their public open-access repository.
REFERENCES
- 1.Gurol M, Greenberg S. Uncommon Causes of Stroke. In: Caplan LR, Biller J, ed. Cerebral amyloid angiopathies. 3rd edition. Cambridge, UK: Cambridge University Press, 2018: 534–44. [Google Scholar]
- 2.Banerjee G, Carare R, Cordonnier C, et al. The increasing impact of cerebral amyloid angiopathy: essential new insights for clinical practice. J Neurol Neurosurg Psychiatry 2017;88:982–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knudsen KA, Rosand J, Karluk D, et al. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology 2001;56:537–9. [DOI] [PubMed] [Google Scholar]
- 4.Linn J, Halpin A, Demaerel P, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology 2010;74:1346–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsai H-H, Pasi M, Tsai L-K, et al. Superficial cerebellar microbleeds and cerebral amyloid angiopathy: a magnetic resonance Imaging/Positron emission tomography study. Stroke 2020;51:202–8. [DOI] [PubMed] [Google Scholar]
- 6.Pasi M, Pongpitakmetha T, Charidimou A, et al. Cerebellar microbleed distribution patterns and cerebral amyloid angiopathy. Stroke 2019;50:1727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Itoh Y, Yamada M, Hayakawa M, et al. Cerebral amyloid angiopathy: a significant cause of cerebellar as well as lobar cerebral hemorrhage in the elderly. J Neurol Sci 1993;116:135–41. [DOI] [PubMed] [Google Scholar]
- 8.Cuny E, Loiseau H, Rivel J, et al. Amyloid angiopathy-related cerebellar hemorrhage. Surg Neurol 1996;46:235–9. [DOI] [PubMed] [Google Scholar]
- 9.Reijmer YD, Fotiadis P, Martinez-Ramirez S, et al. Structural network alterations and neurological dysfunction in cerebral amyloid angiopathy. Brain 2015;138:179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reijmer YD, van Veluw SJ, Greenberg SM. Ischemic brain injury in cerebral amyloid angiopathy. J Cereb Blood Flow Metab 2016;36:40–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fotiadis P, Reijmer YD, Van Veluw SJ, et al. White matter atrophy in cerebral amyloid angiopathy. Neurology 2020;95:e554–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fotiadis P, van Rooden S, van der Grond J, et al. Cortical atrophy in patients with cerebral amyloid angiopathy: a case-control study. Lancet Neurol 2016;15:811–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fotiadis P, Pasi M, Charidimou A, et al. Decreased basal ganglia volume in cerebral amyloid angiopathy. J Stroke 2021;23:223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernard JA, Seidler RD. Moving forward: age effects on the cerebellum underlie cognitive and motor declines. Neurosci Biobehav Rev 2014;42:193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavallari M, Moscufo N, Skudlarski P, et al. Mobility impairment is associated with reduced microstructural integrity of the inferior and superior cerebellar peduncles in elderly with no clinical signs of cerebellar dysfunction. Neuroimage Clin 2013;2:332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kafri M, Sasson E, Assaf Y, et al. High-Level gait disorder: associations with specific white matter changes observed on advanced diffusion imaging. J Neuroimaging 2013;23:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jack CR, Bernstein MA, Fox NC, et al. The Alzheimer’s disease neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging 2008;27:685–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischl B FreeSurfer. Neuroimage 2012;62:774–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charidimou A, Boulouis G, Roongpiboonsopit D, et al. Cortical superficial siderosis multifocality in cerebral amyloid angiopathy: a prospective study. Neurology 2017;89:2128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Podsiadlo D, Richardson S. The timed "Up & Go": a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142–8. [DOI] [PubMed] [Google Scholar]
- 22.Catafau AM, Bullich S, Seibyl JP, et al. Cerebellar amyloid-β plaques: how frequent are they, and do they influence 18F-Florbetaben SUV ratios? J Nucl Med 2016;57:1740–5. [DOI] [PubMed] [Google Scholar]
- 23.Braak H, Braak E, Bohl J, et al. Alzheimer’s disease: amyloid plaques in the cerebellum. J Neurol Sci 1989;93:277–87. [DOI] [PubMed] [Google Scholar]
- 24.Gavriliuc P, Molad J, Yaghmour N, et al. Cerebellar hemorrhages in patients with cerebral amyloid angiopathy. J Neurol Sci 2019;405:116418. [DOI] [PubMed] [Google Scholar]
- 25.Jung YH, Jang H, Park SB, et al. Strictly lobar microbleeds reflect amyloid angiopathy regardless of cerebral and cerebellar compartments. Stroke 2020;51:3600–7. [DOI] [PubMed] [Google Scholar]
- 26.Gokcal E, Horn MJ, van Veluw SJ, et al. Lacunes, microinfarcts, and vascular dysfunction in cerebral amyloid angiopathy. Neurology 2021;96:e1646–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sjöbeck M, Englund E. Alzheimer’s disease and the cerebellum: a morphologic study on neuronal and glial changes. Dement Geriatr Cogn Disord 2001;12:211–8. [DOI] [PubMed] [Google Scholar]
- 28.Wegiel J, Wisniewski HM, Dziewiatkowski J, et al. Cerebellar atrophy in Alzheimer’s disease-clinicopathological correlations. Brain Res 1999;818:41–50. [DOI] [PubMed] [Google Scholar]
- 29.Tabatabaei-Jafari H, Walsh E, Shaw ME, et al. The cerebellum shrinks faster than normal ageing in Alzheimer’s disease but not in mild cognitive impairment. Hum Brain Mapp 2017;38:3141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomann PA, Schläfer C, Seidl U, et al. The cerebellum in mild cognitive impairment and Alzheimer’s disease - a structural MRI study. J Psychiatr Res 2008;42:1198–202. [DOI] [PubMed] [Google Scholar]
- 31.Toniolo S, Serra L, Olivito G, et al. Patterns of cerebellar gray matter atrophy across Alzheimer’s disease progression. Front Cell Neurosci 2018;12:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han S, An Y, Carass A, et al. Longitudinal analysis of regional cerebellum volumes during normal aging. Neuroimage 2020;220:117062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim YJ, Kwon HK, Lee JM, et al. Gray and white matter changes linking cerebral small vessel disease to gait disturbances. Neurology 2016;86:1199–207. [DOI] [PubMed] [Google Scholar]
- 34.de Laat KF, Reid AT, Grim DC, et al. Cortical thickness is associated with gait disturbances in cerebral small vessel disease. Neuroimage 2012;59:1478–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request. Data for this study were gathered from two sources: (1) data obtained as part of an approved study by the institutional review board of Massachusetts General Hospital; these data are not publicly available but may be available upon reasonable request; and (2) data collected from the Alzheimer’s Disease Neuroimaging Initiative and is available in their public open-access repository.