Abstract
While physical frailty has been recognized as a clinical entity for some time, the concept of cognitive frailty (CF) is now gaining increasing attention in the geriatrics research community. CF refers to the co-occurrence of physical frailty and cognitive impairment in older adults, which has been suggested as a potential precursor to both dementia and adverse physical outcomes. However, this condition represents a challenge for researchers and clinicians, as there remains a lack of consensus regarding the definition and diagnostic criteria for CF, which has limited its utility. Here, using insights from both the physical frailty literature and cognitive science research, we describe emerging research on CF. We highlight areas of agreement as well as areas of confusion and remaining knowledge gaps, and provide our perspective on fine-tuning the current construct, aiming to stimulate further discussion in this developing field.
The relationship between physical frailty and cognitive status in older adults has received increasing attention over the past decade1-4. Physical frailty is a clinical syndrome defined by the presence of three or more of the following criteria: unintentional weight loss, self-reported exhaustion, muscle weakness, slow walking pace and low physical activity5. It is independently associated with cognitive impairment, dementia, loss of function, hospitalization and mortality5-8. While there is no consensus on the definition of CF, it generally refers to a complex construct that involves both physical frailty and cognitive impairment. The cognitive component is defined as a decline in cognitive function that does not meet the criteria for dementia, while the physical component includes factors such as weakness and fatigue. The term CF has been used in over 295 articles on PubMed since 2013, mostly in review and meta-analysis papers. However, there is still a disagreement regarding the exact nature of this phenomenon, and further exploration is needed. The co-occurrence of the two conditions gives rise to the dilemma of having to decide on which condition comes first, with the potential for cognitive-based definitions that rely on cognitive impairment as the primary component of CF, with physical frailty being considered a secondary factor. However, this approach can be criticized for ignoring the potential impact of physical frailty on cognitive functioning. Physical-based definitions, on the other hand, could consider physical frailty as the primary component of CF, with cognitive impairment being considered a secondary factor. However, this approach also ignores the potential impact of cognitive impairment on physical functioning. Research has demonstrated that individuals with CF notably differ from those who have cognitive impairment but not physical frailty. The former group performed more poorly on tests of executive function and attention and exhibited a subcortical–frontal cognitive pattern that differs from that of Alzheimer’s disease (AD)9. In addition, regarding the risk of incident dementia, it has been found that co-occurrence of cognitive impairment and physical frailty carries a higher risk compared to either cognitive impairment or physical frailty alone10. It is quite likely that patients with CF who have AD biomarkers would present different cognitive characteristics compared to patients with CF who have predominantly vascular damages, which necessitates a nuanced and tailored approach to cognitive assessment.
Navigating the intersection of physical and cognitive decline
Several studies11-17 have demonstrated a link between physical frailty and various cognitive traits, including memory, verbal abilities, spatial abilities and processing speed18,19. Individuals with pre-frailty had poorer cognitive performance in both memory and non-memory domains than non-frail individuals20. Similarly, individuals with frailty performed poorly on tests measuring processing speed, verbal fluency and simple reaction time21. As a result, an international consensus group from the International Academy of Nutrition and Aging (IANA) and the International Association of Gerontology and Geriatrics (IAGG) proposed the term CF to describe the simultaneous presence of physical frailty and cognitive impairment without dementia22.
While the original IANA/IAGG definition of CF only included those with a clinical dementia rating (CDR) of 0.5, an extended definition23 has since been proposed, including those with subjective cognitive decline (SCD; CDR < 0.5) and/or positive serum and imaging biomarkers of abnormal amyloid accumulation and neurodegeneration in the presence of pre-frailty or frailty. This updated definition creates a spectrum of CF ranging from SCD + pre-frailty to mild cognitive impairment (MCI) + frailty. SCD is defined as a self-experienced persistent decline in cognitive capacity in comparison to a previously normal status, despite normal performance on standardized cognitive tests (CDR < 0.5). This SCD, considered as pre-MCI, is a critical component of the CF spectrum because it may represent one of the earliest symptomatic manifestations of neurodegenerative disease and may place individuals in the category of being at a greater risk of progressing to MCI and subsequently dementia24. It is, therefore, a key factor in the early identification and reversal of CF.
Motoric cognitive risk (MCR) syndrome represents a pre-dementia syndrome characterized by subjective cognitive complaints and slow gait in older adults without dementia or mobility disability25. Hence, the simultaneous decline in both memory and gait speed was found to be associated with a heightened risk of developing dementia in older individuals26,27. MCR has been proposed as a useful construct to identify older adults at high risk of developing dementia and mobility declines. This syndrome is particularly relevant to the concept of CF, as it represents a state of vulnerability where physical and cognitive impairments intersect, much like CF. Notably, MCR also emphasizes the link between motoric impairment, especially slow gait, and risk of cognitive decline and dementia, and is associated with neurodegenerative and cerebrovascular disease pathologies as we have shown28. Hence, it further underscores the intertwined nature of physical and cognitive declines. In the context of CF, MCR can be seen as a potential early sign or a sub-phenotype of the syndrome. The co-occurrence of subjective cognitive complaints and motoric dysfunction as observed in MCR may well correspond to the early stages of CF. Thus, a detailed examination of MCR and its relationship to CF could provide valuable insights into the identification and management of individuals who may be on the trajectory toward more pronounced cognitive impairment.
Additionally, physio-cognitive decline syndrome has been proposed as the simultaneous presence of cognitive impairment and physical decline and defined as cognitive performance in any domain that is at least 1.5 standard deviations below the mean for age-matched, sex-matched and education-matched norms and the presence of physical weakness and/or slowness29. The 2013 IANA/IAGG consensus report initially aimed to identify cognitive impairment caused by physical conditions using the term CF. However, the report also acknowledged that CF may be a precursor of neurodegenerative processes. This makes it challenging to differentiate between cognitive impairment caused by physical conditions and cognitive impairment resulting from comorbid physical frailty and early/prodromal AD. Cesari et al. recently highlighted the rationale behind this new construct as promoting a holistic approach to the assessment and management of cognitive impairment in older adults and recognizing frailty as a multidimensional phenomenon30.
Understanding the dynamics of CF: potentially reversible versus reversible
CF is often considered a prodromal stage to more serious cognitive impairments such as dementia, and its potential reversibility is a topic of notable interest and controversy. According to Ruan et al.23, CF may be classified into two types based on its course and potential for reversal: ‘potentially reversible’ CF and ‘reversible’ CF. ‘Potentially reversible’ refers to early-stage cases wherein, with timely and effective interventions, further cognitive decline can be halted or slowed down. This type encompasses individuals exhibiting initial signs of cognitive decline that do not yet meet the criteria for dementia. ‘Reversible’, on the other hand, includes cases where implemented interventions can reverse cognitive decline to a considerable extent and restore cognitive functioning. It is crucial to highlight that the ability to reverse CF depends on various factors, including the individual’s overall health status, the severity of cognitive decline and the underlying causes of CF. In addition, Mantovani et al. suggested to use the term ‘reversibility’ for MCI cases when there are identifiable modifiable factors such as polypharmacy, psychiatric conditions (for example, depression), metabolic deficiencies, sleep disturbances or sensory deficits. They cautioned that categorizing MCI as potentially reversible CF, as suggested by Ruan et al., might oversimplify the condition and overlook its multifaceted nature, particularly when the underlying neuropathology is not taken into account31. Furthermore, recent longitudinal studies32,33 have explored both concepts in predicting dementia, highlighting their importance in geriatric clinical care and research. One key point to consider is that, although CF can be a predictor of dementia, it is not invariably or inevitably a step toward dementia. Multiple research studies suggest that changes in an older adults’ overall health status are frequently linked to cognitive impairments. Improving their health could mitigate these cognitive changes, or even reverse them. This is a crucial clinical understanding; without it, any cognitive decline might be misinterpreted as part of a journey toward dementia. While it might not be straightforward to differentiate between reversible and irreversible states, it is critical to consider the possibility of both conditions.
Beyond chicken and egg: debating the directionality of physical frailty and cognitive decline
CF is associated with a higher risk of hospitalization, disability and death34,35. Moreover, CF has been shown to be a predictor of overall dementia, including vascular dementia32. While strong epidemiological evidence supports a relationship between physical frailty and cognitive decline11,36, data have not been consistent on the characterization of this relationship. Some studies suggest that physical frailty may increase the risk of cognitive impairment, MCI and a more rapid rate of cognitive decline12,37. Conversely, cognitive impairment can increase the risk of frailty and adverse health outcomes38. Recent longitudinal studies have shown that cognitive impairment can also be associated with a higher risk of developing frailty in later years among older adults with no frailty at baseline39. A recent study examined the hierarchical development of physical frailty and cognitive impairment, revealing that the occurrence of dementia anytime during the follow-up period, was strongly associated with a high risk of developing cognitive impairment before frailty, and an even higher risk of cognitive impairment and frailty concurrently, as opposed to developing frailty first40.This finding highlights the importance of understanding the interplay between physical frailty and cognitive decline and its potential implications for early diagnosis and intervention.
In exploring the potential links between frailty and cognitive decline, it is crucial to consider the mechanisms underpinning this relationship. Recent evidence suggests a potential correlation between AD pathologies and physical frailty, which raises the possibility of a common underlying factor contributing to both conditions41. A valuable perspective comes from Wallace et al.42, who propose that the severity of frailty could modulate the expression of AD pathology in older adults, potentially influencing the manifestation and progression of cognitive impairments. One potential explanation is that as the damage accumulation underlying frailty occurs, compensatory mechanisms (such as photostatic mechanisms or DNA repair) tend to be hijacked by the frailty status and may allow the AD pathology to progress unchecked. This interaction suggests that addressing frailty in older adults might impact cognitive outcomes, potentially delaying or mitigating cognitive decline. Alternatively, early physical decline and pre-frailty may reflect an early undiagnosed brain pathology. Further longitudinal studies are required to enhance our understanding of the relationship between physical frailty and cognitive function.
Untamed territory: a diverse landscape of CF assessment tools
Estimating the prevalence of CF is challenging due to the ambiguity in its definition, the lack of standardized tools to assess and the limited number of prospective cohort studies available. It is also essential to approach the findings of these studies with caution, as they often differ in sample size and methodology. Research indicates that the identification and documentation of cognitive impairment in primary care is generally inadequate43,44, with less than 25% of patients with mild dementia having it noted in their records45.
In community settings, the prevalence of CF ranges from 1.0% to 4.4%, while clinical-based studies report higher prevalence rates of 10.7% to 22.0%46,47. In Japan, a combined prevalence of frailty and MCI was found to be 2.7%, similar to other studies, with frailty alone at 11.3% and MCI alone at 18.8%48. Various factors, such as age and sex differences in samples, the use of differing CF models and the operationalization of CF’s two components (physical frailty and cognitive impairment), may contribute to varying prevalence estimates across studies47.
The inclusion of SCD, a self-perceived worsening of cognitive function, in CF models not only presents a challenge but also provides an opportunity to identify and possibly intervene with individuals who might be on a trajectory toward cognitive decline and dementia but have not yet reached the threshold of objective cognitive impairment.
Currently, the physical elements of CF are mostly screened using the Cardiovascular Health Study criteria, but there is a lack of consistency in the screening instruments for the cognitive component of this construct47.
The Fried frailty phenotype provides a concentrated and specific analysis of the physical aspect of CF because its assessment is based on an extensive set of physical criteria. As such, it is considered a suitable approach for examining physical frailty in relation to cognitive impairment. Beyond the Fried frailty phenotype, several other methods are available for evaluating frailty. One of these methods is the accumulation of deficits approach, also known as the Frailty Index. This approach determines frailty by quantifying the proportion of potential health deficits that an individual exhibits, encompassing symptoms, signs, diseases, disabilities and laboratory abnormalities49. Another alternative is the Clinical Frailty Scale, which rates frailty into nine stages, from very fit to terminally ill, based on a clinician’s evaluation of a patient’s overall health status and degree of frailty. While these frailty tools are proficient at identifying vulnerable older adults, they classify individuals as frail based on a wide range of variables, complicating biological discovery and intervention development within these frameworks50,51.
To identify CF, individuals with frailty should undergo a comprehensive cognitive evaluation that examines their memory and other cognitive abilities, such as executive functions and processing speed. Several cognitive tests and instruments have been suggested, including the speed processing tests, the Montreal Cognitive Assessment (MoCA), the mini–mental state examination, the AD assessment scale cognitive subscale and pre-MCI SCD research criteria22,23. When selecting the appropriate test, it is important to consider that the mini–mental state examination is not sufficient for evaluating executive function and MCI. While the MoCA is an effective overall screening tool for cognitive impairment, the addition of more in-depth assessments that focus on executive function and processing speed can provide a more comprehensive and accurate picture of an individual’s cognitive status31,52. Tools such as the Trail-Making Test, Stroop Test or Digit Symbol Substitution Test can be particularly useful in highlighting these specific cognitive domains.
Delrieu et al. proposed using the Frontal Assessment Battery and the Five-Word Test, as well as diagnostic tests such as the Free and Cued Selective Reminding Test, the Trail-Making Test, Wechsler Adult Intelligence Scale-Revised coding and verbal fluencies to assess cognitive impairment in individuals with frailty9. Won et al. proposed accepting cognitive impairment as 1.5 standard deviations below the mean for age-adjusted, gender-adjusted and education-adjusted norms on any cognitive functioning test to identify CF53.
Exploring the complex interplay between biological mechanisms and risk factors in CF: an unresolved debate
CF is influenced by various biological, environmental and psychosocial factors (Fig. 1). There is a general agreement among experts that the co-occurrence of cognitive decline and physical weakness in older adults is likely to be the result of a complex interplay of biological, environmental and psychosocial factors. However, evidence for direct causality remains lacking, despite the many causes that have been reported in the literature. Several biological mechanisms have been proposed to underlie CF, including chronic inflammation (CI), oxidative stress, neurodegeneration and vascular factors. Although the mechanisms linking frailty and cognitive impairment remain unclear, it is possible that abnormalities in biological processes related to accelerated aging, consistent with the geroscience hypothesis, may be involved54. Moreover, the high prevalence of cardiovascular and metabolic risk factors in persons who develop dual cognitive and mobility impairments or decline may suggest an important role for these factors26. Furthermore, several factors have been linked to CF, including advanced age, lower niacin intake, lack of social support, depression and reduced physical performance55. Cross-sectional studies also reveal associations with older age (over 70 years), lower educational attainment (primary school or lower), poor nutritional status, non-working status, poor self-perceived health and depression56-58.
Fig. 1 ∣. Schematic of the interplay of biological mechanisms, biomarkers and risk factors in CF.
The outer circle highlights that both physical frailty and cognitive decline possess common biological mechanisms, biomarkers and risk factors. These shared elements can interact with each other throughout all stages, forming the foundation of the concept of CF. The middle circle showcases the symptoms that emerge from these biological mechanisms. When there is a sufficient convergence of physical and cognitive symptoms, it leads to the manifestation of physical frailty and/or cognitive decline phenotypes. The presence of both signifies the onset of CF. HPA, hypothalamic–pituitary–adrenal.
CI has been linked to MCI, but there are few studies that have shown direct connections between CI and CF. One study found higher levels of some inflammatory markers in individuals with MCI compared to those with normal cognition59. Additionally, as individuals age, neuroinflammation mediators and pro-inflammatory cytokines are released by glial cells60,61. This buildup of CI in the brain has also been linked to changes at a neuronal level, resulting in neurobehavioral impairments such as reduced ability to learn, to remember and to adapt to changing conditions, as well as higher rates of delirium and depression60,62. Recently, a study found elevated levels of neuroinflammatory cytokines in association with CF63. However, a direct relationship between CI and CF has not been fully examined.
Oxidative stress is characterized by a disturbance in the body’s balance of reactive oxygen species and antioxidants64,65. Multiple studies have hypothesized that oxidative stress and free radicals contribute to the development of cognitive decline and physical frailty66. Participants with cognitive decline and physical frailty were found to have increased levels of reactive oxygen species derivatives and decreased levels of antioxidants67.
Impaired hypothalamic–pituitary–adrenal axis function has also been associated with cognitive decline and physical frailty68. The hypothalamic–pituitary–adrenal axis plays an essential role in maintaining homeostasis in the presence of chronic stressors by producing cortisol69. Higher cortisol levels are associated with lower brain volume, impaired memory in middle-aged adults and a decline in cognitive performance in older adults. Cortisol has also been linked to the vulnerability of patients with frailty47,59,70,71.
Hormonal changes and dysregulation have shown to be indicators of cognitive decline in older adults. Dysregulation of thyroid, androgenic and growth hormones are associated with cognitive decline and physical frailty72. Insulin resistance as a part of endocrine dysregulation68,73-75 may also mediate cognitive decline and physical frailty.
The apolipoprotein E ε4 (APOE4) allele is well established as being associated with cognitive decline76. Evidence suggests that it may also be associated with physical frailty and motor decline in older adults, regardless of cognitive status77,78. Additionally, longitudinal studies have found that physical frailty is a major risk factor for incident vascular dementia in older adults.
Several studies on CF have reported varying patterns of cognitive impairment, with some emphasizing deficits in frontally mediated cognitive functions, such as executive functions, while others report more general cognitive changes, including memory impairment4,12. This variability may reflect differences in the specific cognitive domains assessed, the tools used for assessment and the characteristics of the study participants4. For example, studies focusing on global cognitive changes might have not looked at specific cognitive domains in detail or did not exclude individuals with dementia from their samples, potentially biasing results toward more general cognitive changes. Additionally, the specific etiology of CF in different individuals, which can be influenced by a multitude of factors including vascular health, neurodegenerative processes and systemic health, may also contribute to these different patterns of cognitive impairment31. Given these considerations, it is important for future research in CF to apply comprehensive and standardized cognitive assessments that allow for detailed analysis of different cognitive domains. Furthermore, careful sample selection and characterization, including the exclusion of individuals with established dementia, are crucial for reducing bias and enhancing the validity of findings.
The road ahead: embracing the unknown in CF prevention and delaying progression
Over the years, an increasing number of studies have suggested that interventions focusing on improving physical activity can also benefit cognitive health by reducing cognitive decline. A 24-month structured, moderate-intensity physical activity program has been shown to decrease CF in sedentary older adults. The participants in the physical activity group demonstrated a 21% lower chance of worsening CF compared to those in a health education group79. Furthermore, incorporating a multicomponent exercise routine can enhance functional capacity and executive function, while moderate-intensity activities can reduce CF and promote healthy aging. These multidomain interventions often combine physical exercise prescription (resistance, aerobic, balance and flexibility training), cognitive training, dietary counseling and promotion of psychosocial support79,80.
There are additional factors that impact both the physical frailty and cognitive status of older adults, such as sleep quality and social isolation47,81,82. Recently, studies have found a correlation between poor sleep quality, including difficulty in falling asleep, and CF81. Frailty status has been found to improve more substantially in individuals participating in both a structured exercise program and bimonthly group reading activities compared to those who did not participate. Social activities that promote interactions have been linked to favorable outcomes in adults with frailty and with cognitive impairment83-85. In addition, while there is no specific food or supplement that has been conclusively shown to directly impact physical frailty and cognitive impairment, following a lifelong balanced diet, such as the Mediterranean diet, along with engaging in regular physical and mental exercise, is considered an effective preventive approach against cognitive decline in older age86. To mitigate the development of CF, it is imperative to prioritize the development of interventions that address these specific variables and aim to prevent their negative impact on cognitive health in older individuals.
Research has also investigated dual-task interventions, which involve performing two tasks (for example, walking and memory tests) simultaneously. Aging individuals, especially those with cognitive impairment, may experience a decline in their ability to perform dual tasks. These complex tasks demand more cognitive and motor resources and could potentially prevent or reverse frailty in older adults. However, there is currently no evidence to support additional benefits from simultaneous cognitive training87.
Evidence supports the notion that higher education enhances resilience against cognitive impairment. Indeed, research shows that, given equivalent degrees of brain pathology, individuals with more education display fewer cognitive issues than expected88. Moreover, factors such as higher socioeconomic status, complex occupations, low stress levels and active participation in mental, physical and social activities probably bolster resilience and decelerate cognitive decline89.
However, despite the evidence, the mechanisms underlying these associations are still not well understood. Furthermore, the role of these elements in clinical evaluations of older individuals remains undefined. The exploration of these issues is of paramount importance and warrants additional research both for understanding the mechanisms and developing pharmacological interventions for CF prevention.
CF demystified: integrating concepts for clarity
CF represents the intersection between cognitive impairment and physical vulnerability, impacting an individual’s ability to perform activities of daily living and increasing their risk for adverse health outcomes, such as disability, falls, hospitalization and mortality.
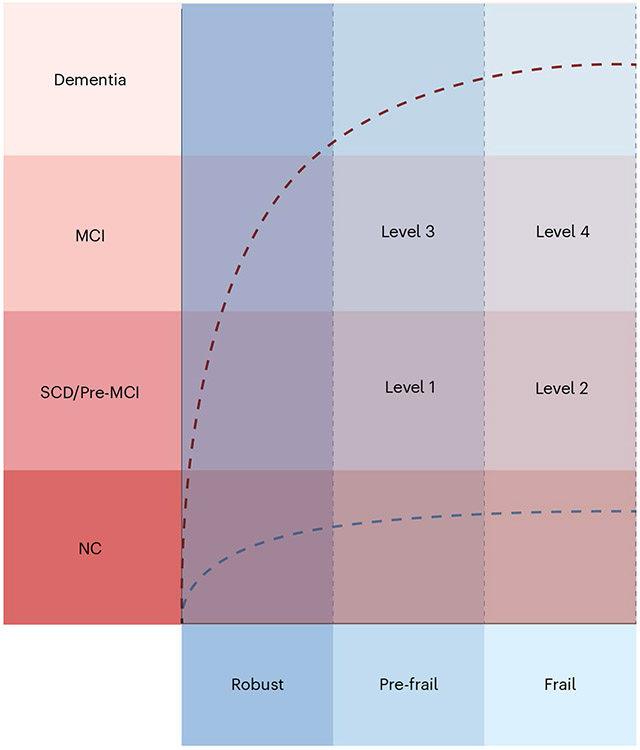
CF can be defined as the co-occurrence of cognitive decline and physical frailty in older adults, resulting from a complex interplay of biological, environmental and psychosocial factors (Fig. 2). It is characterized by a cognitive impairment that does not meet the criteria for dementia, manifesting in difficulties with memory, attention, language and other cognitive abilities. The physical component encompasses a decline in physical function, including weakness, fatigue and decreased mobility, ultimately impairing activities of daily living. This condition is thought to originate from a combination of physical frailty and cognitive decline. However, determining whether cognitive decline is an inherent part of the CF syndrome or an early symptom of a concurrent neurodegenerative disease can be challenging. The relationship between physical and cognitive impairments is not hierarchical, and there is no consensus on whether physical or cognitive impairments should be considered primary or secondary components of CF. Additionally, in the realm of aging, there exists a spectrum of older adults with varying degrees of cognitive and physical conditions. Some may be physically frail but display no notable cognitive impairments, while others might exhibit cognitive deficits yet maintain physical robustness. This heterogeneity highlights the importance of understanding the interplay between cognitive and physical domains when evaluating and addressing CF in geriatric populations. We suggest categorizing CF (Fig. 3) based on varying levels of severity, rather than stages, with each level characterized by its unique combination of physical frailty and cognitive impairment. Given the distinct negative outcomes associated with each scenario90,91, we propose the following severity levels for CF: level 1, physical pre-frailty + SCD; level 2, physical frailty + SCD; level 3, physical pre-frailty + MCI; and level 4, physical frailty + MCI.
Fig. 2 ∣. Integrating CF concepts for clarity as a complex, multidimensional geriatric syndrome resulting from the complex interplay of biological, environmental, and psychosocial factors.
Psychosocial, environmental and age-related biological factors intertwine with observed declines in physical and cognitive abilities. Although these age-influenced biological changes may vary in their effect, they collectively play a part in the decline. The culmination of these factors leads to a condition known as CF, which underscores the interplay between cognitive and physical deterioration. Despite physical and cognitive decline potentially evolving separately, these shared influences can shape their progression concurrently.
Fig. 3 ∣. Schematic of CF categories.
Level 1 represents the combination of physical pre-frailty and subjective cognitive decline (SCD); level 2 represents physical frailty and SCD; level 3 represents physical pre-frailty and mild cognitive impairment (MCI); and level 4 represents physical frailty and MCI. NC, normal cognition.
It is important to note that there is no implied expectation of progression between severity levels in a linear fashion. Instead, the categorization is intended to help to assess and address the varying levels of negative outcomes experienced by individuals with pre-frailty or frailty.
To accurately assess physical frailty and cognitive function in older adults, we recommend using the Fried frailty phenotype for physical frailty assessment, combined with a multistep approach for cognitive assessment to provide a consistent measurement approach. The concept of CF acknowledges that cognitive and physical vulnerabilities are not mutually exclusive, and that their combined presence can lead to unique challenges for affected individuals. This recognition underscores the necessity for comprehensive and multidisciplinary approaches in assessment, prevention and management strategies to promote overall well-being and quality of life for older adults experiencing CF.
For the initial screening of level 1 and level 2 (pre-MCI SCD), we propose a multistep process:
First, general practitioners or other primary care professionals could administer the SCD questionnaire92, which is designed to detect individuals who experience subjective memory complaints. The SCD questionnaire is relatively easy to implement and has shown good reliability and validity in identifying individuals at risk for cognitive decline.
If the individual reports cognitive complaints, the addition of more specific assessments focusing on executive function and processing speed can provide a more comprehensive and accurate picture of an individual’s cognitive status. Tools such as the Trail-Making Test, Stroop Test or Digit Symbol Substitution Test can be particularly useful in highlighting these specific cognitive domains.
For cognitive impairment, the practitioner could then administer more detailed neuropsychological tests to evaluate cognitive performance in multiple domains. This could include tests such as the MoCA, which is more sensitive to early cognitive changes compared to the AD assessment scale cognitive subscale93.
Finally, SCD alone is not enough to establish a diagnosis of CF; its presence along with physical frailty could potentially signal CF. The introduction of select biomarkers could further bolster the accuracy and reliability of diagnosing and monitoring this condition. Such biomarkers, although still in the validation phase, may encompass inflammatory markers, neurodegenerative markers, indicators of oxidative stress, markers of metabolic conditions and cardiovascular biomarkers. Their inclusion in a comprehensive CF assessment could enhance the overall precision and robustness of the diagnosis. The concept of reversibility is another critical aspect of CF that warrants further investigation. Some studies suggest that CF could be potentially reversible, especially when interventions are implemented early. This highlights the importance of early detection and intervention strategies and underscores the urgency for more research in this area.
In conclusion, although there has been uncertainty primarily centered around the exact nature, definition and screening instruments of CF, our understanding of CF has improved over the past decade. CF describes the intersection of cognitive decline and physical frailty in older adults, characterized by a combination of cognitive impairment, physical weakness, and a reduced ability to perform daily activities. The co-occurrence of cognitive impairment and physical frailty carries a higher risk of developing dementia, as well as increased morbidity and mortality, when compared to either cognitive impairment or physical frailty alone. Some reversibility has been observed, but the extent and sustainability of this reversal remain unknown. Future research may further elucidate the heterogeneity of physical frailty and use innovative tools, such as AI-enabled devices, to characterize physical, social and cognitive functions in older adults. This may help us to subclassify frailty based on specific physical, cognitive and social domains and identify novel biomarkers to understand the mechanisms and relationships between these domains that constitute CF.
Acknowledgements
This work was supported by the Bright Focus Foundation Research Award (to P.M.A.); and the Johns Hopkins University Claude D. Pepper Older Americans Independence Center, which is funded by the National Institute on Aging of the National Institutes of Health under award number P30AG021334. The funding source had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. We gratefully acknowledge J. E. Fairman from The Johns Hopkins Department of Arts as Applied to Medicine for contributing to the creation of figures.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Godin J, Armstrong JJ, Rockwood K & Andrew MK Dynamics of frailty and cognition after age 50: why it matters that cognitive decline is mostly seen in old age. J. Alzheimers Dis 58, 231–242 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Avila-Funes JA et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J. Am. Geriatr. Soc 57, 453–461 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Robertson DA, Savva GM & Kenny RA Frailty and cognitive impairment—a review of the evidence and causal mechanisms. Ageing Res. Rev 12, 840–851 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Brigola AG et al. Relationship between cognition and frailty in elderly: a systematic review. Dement. Neuropsychol 9, 110–119 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fried LP et al. The physical frailty syndrome as a transition from homeostatic symphony to cacophony. Nat. Aging 1, 36–46 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyd CM, Xue Q-L, Simpson CF, Guralnik JM & Fried LP Frailty, hospitalization, and progression of disability in a cohort of disabled older women. Am. J. Med 118, 1225–1231 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Kulmala J, Nykänen I, Mänty M & Hartikainen S Association between frailty and dementia: a population-based study. Gerontology 60, 16–21 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Bandeen-Roche K. et al. Phenotype of frailty: characterization in the women’s health and aging studies. J. Gerontol. A Biol. Sci. Med. Sci 61, 262–266 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Delrieu J. et al. Neuropsychological profile of ‘cognitive frailty’ subjects in MAPT study. J. Prev. Alzheimers Dis 3, 151–159 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grande G. et al. Co-occurrence of cognitive impairment and physical frailty, and incidence of dementia: systematic review and meta-analysis. Neurosci. Biobehav. Rev 107, 96–103 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Buchman AS, Boyle PA, Wilson RS, Tang Y & Bennett DA Frailty is associated with incident Alzheimer’s disease and cognitive decline in the elderly. Psychosom. Med 69, 483–489 (2007). [DOI] [PubMed] [Google Scholar]
- 12.Boyle PA, Buchman AS, Wilson RS, Leurgans SE & Bennett DA Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J. Am. Geriatr. Soc 58, 248–255 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samper-Ternent R, Al Snih S, Raji MA, Markides KS & Ottenbacher KJ Relationship between frailty and cognitive decline in older Mexican Americans. J. Am. Geriatr. Soc 56, 1845–1852 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Auyeung TW, Lee JSW, Kwok T & Woo J Physical frailty predicts future cognitive decline—a four-year prospective study in 2737 cognitively normal older adults. J. Nutr. Health Aging 15, 690–694 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Jacobs JM, Cohen A, Ein-Mor E, Maaravi Y & Stessman J Frailty, cognitive impairment and mortality among the oldest old. J. Nutr. Health Aging 15, 678–682 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Gray SL et al. Frailty and incident dementia. J. Gerontol. A Biol. Sci. Med. Sci 68, 1083–1090 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitnitski A, Fallah N, Rockwood MRH & Rockwood K Transitions in cognitive status in relation to frailty in older adults: a comparison of three frailty measures. J. Nutr. Health Aging 15, 863–867 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Yassuda MS et al. Frailty criteria and cognitive performance are related: data from the FIBRA study in Ermelino Matarazzo, São Paulo, Brazil. J. Nutr. Health Aging 16, 55–61 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Sternäng O. et al. Grip strength and cognitive abilities: associations in old age. J. Gerontol. B Psychol. Sci. Soc. Sci 71, 841–848 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lorenzo-López L. et al. Clinical and neuropsychological correlates of prefrailty syndrome. Front. Med https://www.frontiersin.org/articles/10.3389/fmed.2020.609359 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bunce D, Batterham PJ & Mackinnon AJ Long-term associations between physical frailty and performance in specific cognitive domains. J. Gerontol. B Psychol. Sci. Soc. Sci 74, 919–926 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Kelaiditi E. et al. Cognitive frailty: rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. J. Nutr. Health Aging 17, 726–734 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Ruan Q. et al. Cognitive frailty, a novel target for the prevention of elderly dependency. Ageing Res. Rev 20, 1–10 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Arvanitakis Z. et al. Memory complaints, dementia, and neuropathology in older blacks and whites. Ann. Neurol 83, 718–729 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verghese J. et al. Motoric Cognitive Risk syndrome: multicenter incidence study. Neurology 83, 2278–2284 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian Q. et al. Dual cognitive and mobility impairments and future dementia—setting a research agenda. Alzheimers Dement. 10.1002/alz.12905 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian Q et al. Association of dual decline in memory and gait speed with risk for dementia among adults older than 60 years: a multicohort individual-level meta-analysis. JAMA Netw. Open 3, e1921636 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buchman AS et al. Correlated decline of cognitive and motor phenotypes and ADRD pathologies in old age. Alzheimers Dement. 10.1002/alz.13347 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen L-K & Arai H Physio-cognitive decline as the accelerated aging phenotype. Arch. Gerontol. Geriatr 88, 104051 (2020). [DOI] [PubMed] [Google Scholar]
- 30.Cesari M, Sloane PD & Zimmerman S The controversial condition of cognitive frailty: what it is, what it should be. J. Am. Med. Dir. Assoc 21, 146–148 (2020). [DOI] [PubMed] [Google Scholar]
- 31.Mantovani E. et al. Towards a redefinition of cognitive frailty. J. Alzheimers Dis 76, 831–843 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solfrizzi V. et al. Reversible cognitive frailty, dementia, and all-cause mortality. The Italian longitudinal study on aging. J. Am. Med. Dir. Assoc 18, 89.e1–89.e8 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Solfrizzi V. et al. Additive role of a potentially reversible cognitive frailty model and inflammatory state on the risk of disability: the Italian longitudinal study on aging. Am. J. Geriatr. Psychiatry 25, 1236–1248 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Lee W-J, Peng L-N, Liang C-K, Loh C-H & Chen L-K Cognitive frailty predicting all-cause mortality among community-living older adults in Taiwan: a 4-year nationwide population-based cohort study. PLoS ONE 13, e0200447 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.John PD, Tyas SL, Griffith LE & Menec V The cumulative effect of frailty and cognition on mortality—results of a prospective cohort study. Int. Psychogeriatr 29, 535–543 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Han ES, Lee Y & Kim J Association of cognitive impairment with frailty in community-dwelling older adults. Int. Psychogeriatr 26, 155–163 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Borges MK, Canevelli M, Cesari M & Aprahamian I Frailty as a predictor of cognitive disorders: a systematic review and meta-analysis. Front. Med 6, 26 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen S. et al. Physical frailty is associated with longitudinal decline in global cognitive function in non-demented older adults: a prospective study. J. Nutr. Health Aging 22, 82–88 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Yu R. et al. The effects of combinations of cognitive impairment and pre-frailty on adverse outcomes from a prospective community-based cohort study of older chinese people. Front. Med https://www.frontiersin.org/article/10.3389/fmed.2018.00050 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chu NM et al. Hierarchical development of frailty and cognitive impairment: clues into etiological pathways. J. Gerontol. A Biol. Sci. Med. Sci 74, 1761–1770 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buchman AS, Schneider JA, Leurgans S & Bennett DA Physical frailty in older persons is associated with Alzheimer disease pathology. Neurology 71, 499–504 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wallace LMK et al. Investigation of frailty as a moderator of the relationship between neuropathology and dementia in Alzheimer’s disease: a cross-sectional analysis of data from the Rush Memory and Aging Project. Lancet Neurol. 18, 177–184 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mackin RS & Areán PA Incidence and documentation of cognitive impairment among older adults with severe mental illness in a community mental health setting. Am. J. Geriatr. Psychiatry 17, 75–82 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mitchell AJ, Meader N & Pentzek M Clinical recognition of dementia and cognitive impairment in primary care: a meta-analysis of physician accuracy. Acta Psychiatr. Scand 124, 165–183 (2011). [DOI] [PubMed] [Google Scholar]
- 45.Ólafsdóttir M, Skoog I & Marcusson J Detection of dementia in primary care: the Linköping study. Dement. Geriatr. Cogn. Disord 11, 223–229 (2000). [DOI] [PubMed] [Google Scholar]
- 46.Sugimoto T. et al. Epidemiological and clinical significance of cognitive frailty: a mini review. Ageing Res. Rev 44, 1–7 (2018). [DOI] [PubMed] [Google Scholar]
- 47.Panza F. et al. Different cognitive frailty models and health- and cognitive-related outcomes in older age: from epidemiology to prevention. J. Alzheimers Dis 62, 993–1012 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shimada H. et al. Combined prevalence of frailty and mild cognitive impairment in a population of elderly Japanese people. J. Am. Med. Dir. Assoc 14, 518–524 (2013). [DOI] [PubMed] [Google Scholar]
- 49.Mitnitski AB, Mogilner AJ & Rockwood K Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal 1, 323–336 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walston J, Buta B & Xue Q-L Frailty screening and interventions: considerations for clinical practice. Clin. Geriatr. Med 34, 25–38 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walston J. et al. Moving frailty toward clinical practice: nia intramural frailty science symposium summary. J. Am. Geriatr. Soc 67, 1559–1564 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Apostolo J. et al. Mild cognitive decline. a position statement of the cognitive decline group of the european innovation partnership for active and healthy ageing (EIPAHA). Maturitas 83, 83–93 (2016). [DOI] [PubMed] [Google Scholar]
- 53.Won CW et al. Modified criteria for diagnosing ‘cognitive frailty’. Psychiatry Investig. 15, 839–842 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fabrício D, de M, Chagas MHN & Diniz BS Frailty and cognitive decline. Transl. Res 221, 58–64 (2020). [DOI] [PubMed] [Google Scholar]
- 55.Rivan NFM et al. Cognitive frailty among Malaysian older adults: baseline findings from the LRGS TUA cohort study. Clin. Interv. Aging 14, 1343–1352 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Das S. Cognitive frailty among community-dwelling rural elderly population of West Bengal in India. Asian J. Psychiatry 70, 103025 (2022). [DOI] [PubMed] [Google Scholar]
- 57.Wongtrakulruang P. et al. The prevalence of cognitive frailty and pre-frailty among older people in Bangkok metropolitan area: a multicenter study of hospital-based outpatient clinics. J. Frailty Sarcopenia Falls 05, 62–71 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Navarro-Pardo E, Facal D, Campos-Magdaleno M, Pereiro AX & Juncos-Rabadán O Prevalence of cognitive frailty, do psychosocial-related factors matter? Brain Sci. 10, 968 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sartori AC, Vance DE, Slater LZ & Crowe M The impact of inflammation on cognitive function in older adults: implications for health care practice and research. J. Neurosci. Nurs 44, 206–217 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Godbout JP & Johnson RW Age and neuroinflammation: a lifetime of psychoneuroimmune consequences. Neurol. Clin 24, 521–538 (2006). [DOI] [PubMed] [Google Scholar]
- 61.Leonoudakis D. et al. Anti-inflammatory and neuroprotective role of natural product securinine in activated glial cells: implications for Parkinson’s disease. Mediators Inflamm. 2017, 8302636 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sikora E. et al. Cellular senescence in brain aging. Front. Aging Neurosci 13, 646924 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sargent L. et al. Shared mechanisms for cognitive impairment and physical frailty: a model for complex systems. Alzheimers Dement. 6, e12027 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Betteridge DJ What is oxidative stress? Metabolism 49, 3–8 (2000). [DOI] [PubMed] [Google Scholar]
- 65.Pahwa R, Goyal A & Jialal I Chronic inflammation. StatPearls http://www.ncbi.nlm.nih.gov/books/NBK493173/ (accessed 28 December 2022). [Google Scholar]
- 66.Mulero J, Zafrilla P & Martinez-Cacha A Oxidative stress, frailty and cognitive decline. J. Nutr. Health Aging 15, 756–760 (2011). [DOI] [PubMed] [Google Scholar]
- 67.Sargent L. et al. Shared biological pathways for frailty and cognitive impairment: a systematic review. Ageing Res. Rev 47, 149–158 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ma L & Chan P Understanding the physiological links between physical frailty and cognitive decline. Aging Dis. 11, 405–418 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stephens MAC & Wand G Stress and the HPA axis. Alcohol Res. 34, 468–483 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee BK et al. Associations of salivary cortisol with cognitive function in the Baltimore memory study. Arch. Gen. Psychiatry 64, 810–818 (2007). [DOI] [PubMed] [Google Scholar]
- 71.Echouffo-Tcheugui JB et al. Circulating cortisol and cognitive and structural brain measures: the Framingham Heart Study. Neurology 91, e1961–e1970 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maggio M et al. The hormonal pathway to cognitive impairment in older men. J. Nutr. Health Aging 16, 40–54 (2012). [DOI] [PubMed] [Google Scholar]
- 73.Leng SX et al. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin. Exp. Res 16, 153–157 (2004). [DOI] [PubMed] [Google Scholar]
- 74.Sanz B. et al. Serum adiponectin is associated with body composition and cognitive and psychological status in older adults living in long-term nursing homes. Exp. Gerontol 121, 1–9 (2019). [DOI] [PubMed] [Google Scholar]
- 75.Nagasawa M. et al. High plasma adiponectin levels are associated with frailty in a general old-old population: the septuagenarians, octogenarians, nonagenarians investigation with centenarians study. Geriatr. Gerontol. Int 18, 839–846 (2018). [DOI] [PubMed] [Google Scholar]
- 76.Hsiung G-YR, Sadovnick AD & Feldman H Apolipoprotein E ε4 genotype as a risk factor for cognitive decline and dementia: data from the canadian study of health and aging. CMAJ 171, 863–867 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Buchman AS et al. Apolipoprotein E ε4 allele is associated with more rapid motor decline in older persons. Alzheimer Dis. Assoc. Disord 23, 63–69 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mourtzi N. et al. Apolipoprotein ε4 allele is associated with frailty syndrome: results from the hellenic longitudinal investigation of ageing and diet study. Age Ageing 48, 917–921 (2019). [DOI] [PubMed] [Google Scholar]
- 79.Liu Z. et al. Effect of 24-month physical activity on cognitive frailty and the role of inflammation: the LIFE randomized clinical trial. BMC Med. 16, 185 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lauretani F. et al. Comprehensive model for physical and cognitive frailty: current organization and unmet needs. Front. Psychol 10.3389/fpsyg.2020.569629 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Griffiths J, Seesen M, Sirikul W & Siviroj P Malnutrition, depression, poor sleep quality, and difficulty falling asleep at night are associated with a higher risk of cognitive frailty in older adults during the COVID-19 restrictions. Nutrients 15, 2849 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hajek A, Riedel-Heller SG & König H-H Perceived social isolation and cognitive functioning. Longitudinal findings based on the German Ageing Survey. Int. J. Geriatr. Psychiatry 35, 276–281 (2020). [DOI] [PubMed] [Google Scholar]
- 83.Murukesu RR, Singh DKA, Shahar S, Subramaniam P A multi-domain intervention protocol for the potential reversal of cognitive frailty: ‘WE-RISE’ randomized controlled trial. Front. Public Health https://www.frontiersin.org/articles/10.3389/fpubh.2020.00471 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gallucci M. et al. ‘Camminando e leggendo… ricordo’ (walking and reading… I remember): prevention of frailty through the promotion of physical activity and reading in people with mild cognitive impairment. Results from the TREDEM registry. J. Alzheimers Dis 77, 689–699 (2020). [DOI] [PubMed] [Google Scholar]
- 85.Romera-Liebana L. et al. Effects of a primary care-based multifactorial intervention on physical and cognitive function in frail, elderly individuals: a randomized controlled trial. J. Gerontol. A Biol. Sci. Med Sci 73, 1688–1674 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dominguez LJ & Barbagallo M The relevance of nutrition for the concept of cognitive frailty. Curr. Opin. Clin. Nutr. Metab. Care 20, 61–68 (2017). [DOI] [PubMed] [Google Scholar]
- 87.Rezola-Pardo C. et al. Comparison between multicomponent and simultaneous dual-task exercise interventions in long-term nursing home residents: the Ageing-ONDUAL-TASK randomized controlled study. Age Ageing 48, 817–823 (2019). [DOI] [PubMed] [Google Scholar]
- 88.Xu W. et al. Education and risk of dementia: dose-response meta-analysis of prospective cohort studies. Mol. Neurobiol 53, 3113–3123 (2016). [DOI] [PubMed] [Google Scholar]
- 89.Fratiglioni L, Marseglia A & Dekhtyar S Ageing without dementia: can stimulating psychosocial and lifestyle experiences make a difference. Lancet Neurol. 19, 533–543 (2020). [DOI] [PubMed] [Google Scholar]
- 90.Gale C, Ritchie SJ, Starr JM & Deary IJ Physical frailty and decline in general and specific cognitive abilities: the Lothian Birth Cohort 1936. J. Epidemiol. Community Health 74, 108–113 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Feng L. et al. Physical frailty, cognitive impairment, and the risk of neurocognitive disorder in the Singapore longitudinal ageing studies. J. Gerontol. A Biol. Sci. Med. Sci 72, 369–375 (2017). [DOI] [PubMed] [Google Scholar]
- 92.Rami L. et al. The subjective cognitive decline questionnaire (SCD-Q): a validation study. J. Alzheimers Dis 41, 453–466 (2014). [DOI] [PubMed] [Google Scholar]
- 93.Nasreddine ZS et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc 53, 695–699 (2005). [DOI] [PubMed] [Google Scholar]