Abstract
Objectives:
Survival of elderly burn patients remains unacceptably poor. The acute phase, defined as the first 96 hours after burn, includes the resuscitation period and influences subsequent outcomes and survival. The aim of this study was to determine if the acute phase response post burn injury is significantly different in elderly patients compared with adult patients and to identify elements contributing to adverse outcomes.
Design:
Cohort study.
Setting:
Tertiary burn center.
Patients:
Adult (< 65 yr old) and elderly (≥ 65 yr old) patients with an acute burn injury.
Interventions:
None.
Measurements and Main Results:
We included all patients with an acute burn injury greater than or equal to 20% total body surface area to our burn center from 2011 to 2016. Clinical and laboratory measures during the acute phase were compared between adult and elderly patients. Outcomes included clinical hemodynamic measurements, organ biomarkers, volume of fluid resuscitation, cardiac agents, and the inflammatory cytokine response in plasma. Data were analyzed using the Student t test, Mann-Whitney U test, and Fisher exact test. A total of 149 patients were included, with 126 adults and 23 elderly. Injury severity was not significantly different among adult and elderly patients. Elderly had significantly lower heart rates (p < 0.05), cardiac index (p < 0.05), mean arterial pressure (p < 0.05), Pao2/Fio2 (p < 0.05), and pH (p < 0.05), along with higher lactate (p < 0.05). Organ biomarkers, particularly creatinine and blood urea nitrogen, showed distinct differences between adults and elderly (p < 0.05). Elderly had significantly lower levels of interleukin-6, monocyte chemotactic protein-1, monocyte chemotactic protein-3, and granulocyte-colony stimulating factor during the acute phase (p < 0.05). Overall mortality was significantly higher in elderly patients (5% vs 52%; p < 0.0001).
Conclusions:
Response to the burn injury during the acute phase response after burn is substantially different between elderly and adult burn patients and is characterized by cardiac depression and hypoinflammation.
Keywords: adults, burns, critical illness, elderly
Overall, the outcomes and survival of burn patients have improved greatly over the last few decades, and the focus of burn care today is to improve the long-term and quality of life rather than survival. However, this is not true for the elderly burn patient population, or those 65 years old and older, as they continue to have poor outcomes. The lethal dose 50, LD50, is the percent total body surface area (TBSA) associated with a 50% percent chance of death. The LD50 is upwards of 90% TBSA burn in the pediatric population and 70–80% TBSA burn in adults, whereas it is approximately 30–35% TBSA for elderly (1–3). Despite the clinical need to improve outcomes of this patient population, little progress has been made over the last 2–3 decades. We recently gained insight into the pathophysiologic responses to burn in elderly patients. Specifically, elderly burn patients do not respond to stress in the way that adult burn patients respond. This study delineated the pathophysiology response of elderly to burn, and it showed that over the hospital course elderly patients have a delayed inflammatory and metabolic responses compared with adults (3). This was confirmed in a study by Stanojcic et al (4), showing that elderly express an altered and reversed inflammatory response indicative that elderly most likely do not have the resources to adequately respond to stress.
The acute phase, defined as the first 96 hours after the injury, is central in determining subsequent clinical outcomes and pathophysiologic responses. We therefore asked whether elderly respond differently to the initial burn trauma in terms of clinical variables, such as resuscitation requirements, and their systemic response compared with adults. This might shed light on an inherent predisposition of an impaired responder phenotype to explain the negative outcomes prevalent in elderly burn patients. Our goal was furthermore to identify central mediators responsible orchestrating poor outcomes. Therefore, this study aimed to identify and compare differences in the acute phase response to burn injury in adult and elderly patients with a burn greater than or equal to 20% TBSA. Specifically, fluid resuscitation requirements, organ function, and the inflammatory responses were all compared. We hypothesized that elderly have a dysregulated response to the initial burn trauma compared with adults who subsequently contributes to a significantly increased mortality.
MATERIALS AND METHODS
Study Design and Participants
Patients (≥ 18 yr old) with an acute burn injury admitted to our provincial burn center between January 2011 and December 2016 were included in this cohort study. Inclusion criteria consisted of admission within 72 hours of injury with burns greater than or equal to 20% TBSA. Patients who died within 96 hours of admission were excluded. The study protocol was approved by the research ethics board (Study number 194–2010). Patients were grouped based on their age status: adult patients (< 65 yr old) were compared with elderly patients (≥ 65 yr old), and the acute phase was defined as 96 hours post burn injury. Ages 65 years old and older were selected as elderly based on a definition by the World Health Organization (5). Preexisting medical conditions included obesity (body mass index ≥ 35 kg/m2), diabetes mellitus, hypertension requiring medication, tobacco smoker, alcohol use/misuse, and drug use/misuse.
Clinical Outcomes
Fluid volumes, colloid administration, cardiac agents, Pao2/Fio2, hemodynamic measurements, and biomarkers during the acute phase were assessed. There was a subset of patients who had hemodynamic monitoring using Pulse Contour Cardiac Output technology, via transpulmonary thermodilution and pulse contour analysis. Cardiac and hemodynamic measurements included heart rate, cardiac index (CI), extravascular lung water index (ELWI), global end-diastolic volume index (GEDI), systemic vascular resistance index (SVRI), central venous pressure (CVP), mean arterial pressure (MAP), and blood pressure (systolic/diastolic). Biomarkers measured were creatinine, blood urea nitrogen (BUN), alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), bilirubin, lactate, and pH. In-hospital complications, length of stay, and mortality were recorded.
Inflammatory Biospecimen Collection and Processing
EDTA-anticoagulated plasma samples were drawn (Percoll-based peripheral blood mononuclear cell isolation) from the periphery during the first 96 hours post burn injury while in hospital. Using the Multiplex platform (Millipore, MA), a panel of cytokines were analyzed that included proinflammatory markers interleukin (IL)–1α, IL-6, IL-1β, tumor necrosis factor (TNF)–α, and interferon (IFN)- γ; antiinflammatory markers IL-10, IL-4, and IL-1 receptor antagonist; immune mediators: granulocyte-colony stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF); and chemokines: growth-related oncogene (GRO), IFN-γ–induced protein 10 (IP-10), monocyte chemotactic protein (MCP)–1, MCP-3, macrophage inflammatory protein (MIP)–1α, and MIP-1β. Experimental kits were all conducted in accordance with manufacturers’ protocol and data analysis was performed using Milliplex Analyst version 5.1 (Vigene Tech Inc., Carlisle, MA).
Statistical Analysis
Data were reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology statement (6). Continuous normally distributed variables were analyzed using the Student t test, and continuous nonnormally distributed variables were analyzed using the Mann-Whitney U test. Categorical data were analyzed using the Fisher exact test. Statistical tests were two-tailed, with a p value of less than 0.05 considered statistically significant. Analyses were performed using SPSS Statistics Version 20.0 (IBM, Armonk, NY).
RESULTS
Patients
Over the 6-year study period 1,583 patients were admitted to the Ross Tilley Burn Centre. Of these, 149 patients met inclusion criteria (Supplemental Fig. 1, Supplemental Digital Content 1, http://links.lww.com/CCM/E151; legend, Supplemental Digital Content 8, http://links.lww.com/CCM/E158). All patients had a percent TBSA burn greater than or equal to 20%, and a summary of demographic and injury characteristics based on age status can be found in Table 1. Mean age overall was 48 ± 16 years, with 114 males (77%). Mean % TBSA burn was 34 ± 13, and 61 patients (41%) had an inhalation injury. The majority of patients (n = 103; 69%) had at least one of the preexisting medical conditions listed: obesity, diabetes mellitus, hypertension requiring medication, tobacco smoker, alcohol use/misuse, and/or drug use/misuse. Age in adults ranged from 19 to 64 years and age in elderly ranged from 65 to 91 years. There were a smaller proportion of males in the elderly group (n = 11; 48%) compared with adults (n = 103; 82%) (p = 0.001). Elderly patients had a percent TBSA burn of 30 ± 10 that was similar to 35 ± 14 in adult patients (p = 0.161). The proportion of inhalation injury was also similar among elderly and adult patients (44% vs 41% respectively; p = 0.820).
TABLE 1.
Demographics and Outcomes of Patients By Age Group
| Characteristics | All | Adults | Elderly | p |
|---|---|---|---|---|
| No. of patients | 149 | 126 | 23 | |
| Demographics | ||||
| Age, yr, mean ± SD | 48 ± 16 | 44 ± 12 | 74 ± 6 | < 0.0001 |
| Male, n (%) | 114 (77) | 103 (82) | 11 (48) | 0.001 |
| Preexisting medical conditions, n (%) | ||||
| Obesity (body mass index ≥ kg/m2) | 13 (9) | 10 (8) | 3 (13) | 0.425 |
| Diabetes mellitus | 18 (12) | 13 (10) | 5 (22) | 0.158 |
| Hypertension requiring medication | 31 (21) | 20 (16) | 11 (48) | 0.001 |
| Tobacco smoker | 49 (33) | 42 (33) | 7 (30) | 1.000 |
| Alcohol use/misuse | 39 (26) | 33 (26) | 6 (26) | 1.000 |
| Drug use/misuse | 49 (33) | 47 (37) | 2 (9) | 0.007 |
| Injury characteristics and outcomes | ||||
| Etiology, n (%) | ||||
| Flame | 119 (80) | 98 (78) | 21 (91) | 0.167 |
| Scald | 18 (12) | 16 (13) | 2 (9) | 0.741 |
| Other | 12 (8) | 12 (10) | 0 | 0.214 |
| Inhalation injury, n (%) | 61 (41) | 51 (41) | 10 (44) | 0.820 |
| TBSA, %, mean ± SD | 34 ± 13 | 35 ± 14 | 30 ± 10 | 0.161 |
| Third TBSA, %, median (IQR) | 20 (2–32) | 16 (0–31) | 26 (16–33) | 0.061 |
| LOS, d, median (IQR) a | 30 (20–55) | 30 (20–56) | 37 (19–55) | 0.875 |
| LOS/TBSA, d/%, mean ± SDa | 1.2 ± 0.8 | 1.2 ± 0.8 | 1.5 ± 0.7 | 0.305 |
| Nonsurvivor, n (%) | 18 (12) | 6 (5) | 12 (52) | < 0.0001 |
IQR = interquartile range, LOS = length of stay, TBSA = total body surface area.
Analysis restricted to patients alive until discharge.
Numbers may not add to 100 due to rounding.
Clinical Outcomes
Resuscitation.
Total volume of fluids administered based on Parkland calculation (mL/kg/TBSA) (Supplemental Fig. 2, A and B, Supplemental Digital Content 2, http://links.lww.com/CCM/E152; legend, Supplemental Digital Content 8, http://links.lww.com/CCM/E158) or fluids administered per hour did not significantly differ throughout the acute phase (Supplemental Fig. 2, C and D, Supplemental Digital Content 2, http://links.lww.com/CCM/E152; legend, Supplemental Digital Content 8, http://links.lww.com/CCM/E158). Urine output was significantly increased in elderly patients on day 1 post injury when compared with adult patients (p = 0.036) (Supplemental Fig. 2A, Supplemental Digital Content 2, http://links.lww.com/CCM/E152; legend, Supplemental Digital Content 8, http://links.lww.com/CCM/E158). There was no significant difference in the proportion of patients who received inotropes (Supplemental Fig. 3A, Supplemental Digital Content 3, http://links.lww.com/CCM/E153; legend, Supplemental Digital Content 8, http://links.lww.com/CCM/E158). However, there were a significantly greater proportion of elderly patients who required and received vasopressors on the day of injury (p = 0.014), day 1 post injury (p = 0.028), and day 2 post injury (p = 0.048) (Supplemental Fig. 3B, Supplemental Digital Content 3, http://links.lww.com/CCM/E153; legend, Supplemental Digital Content 8, http://links.lww.com/CCM/E158).
Cardiac and Hemodynamics.
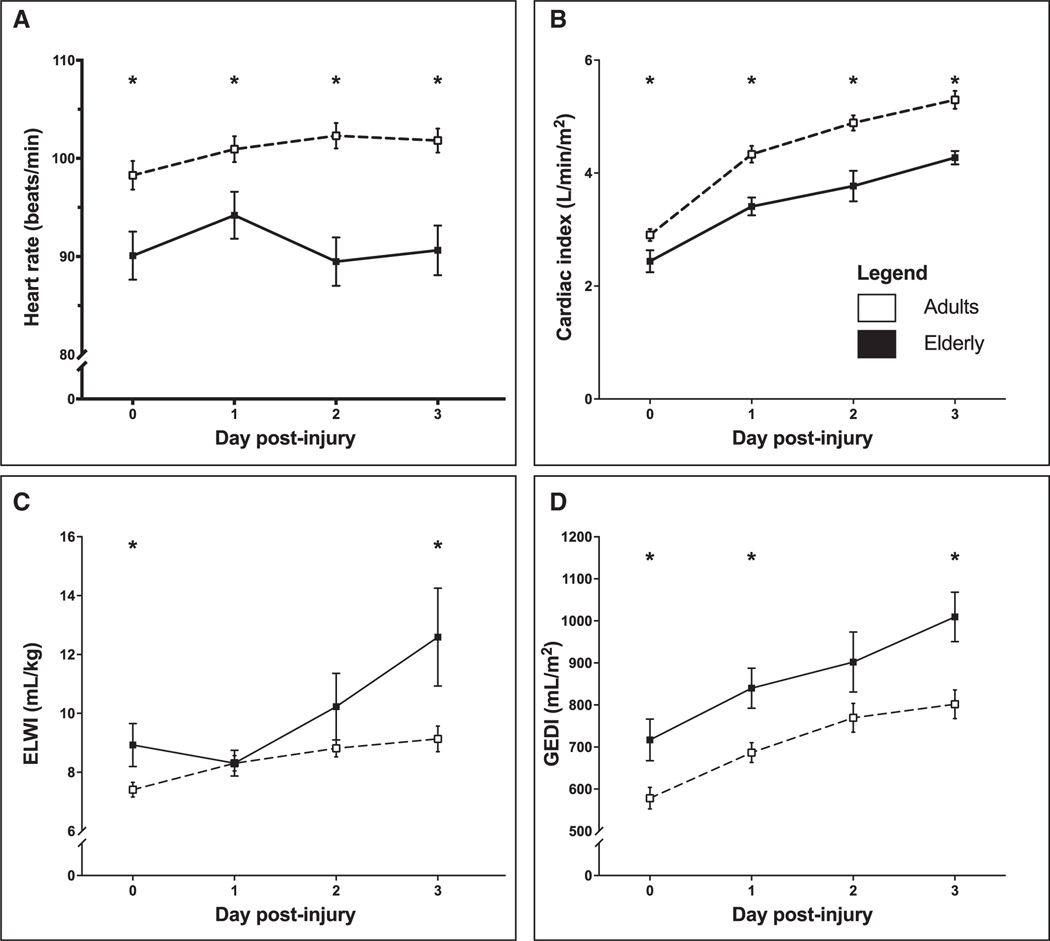
Cardiac and hemodynamic measurements showed signs of decreased perfusion in elderly. The mean heart rate in elderly patients was significantly lower than adult patients on the day of injury (p = 0.018), day 1 post injury (p = 0.036), day 2 post injury (p < 0.0001), and day 3 post injury (p < 0.001) (Fig. 1A). Similarly, CI was significantly lower on day 0 (p = 0.043) and continued to be lower on days 1 (p = 0.003), 2 (p < 0.001), and 3 (p = 0.008) in elderly patients (Fig. 1B).
Figure 1.
Mean heart rate (A), cardiac index (B), extravascular lung water index (ELWI) (C), and global end-diastolic volume index (GEDI) (D). Error bars indicate SEM. *p < 0.05.
ELWI was significantly higher in elderly patients on the day of injury (p = 0.017) and day 3 post injury (p = 0.006) (Fig. 1C). GEDI (Fig. 1D) and SVRI (Fig. 2A) were also significantly higher at various time points throughout the acute phase (p < 0.05). There were no differences in CVP (Fig. 2B); however, MAP (Fig. 2C) and diastolic blood pressure (Fig. 2D) were significantly lower in elderly patients (p < 0.05). Taken together, it appears that elderly have a decreased cardiac efficiency, associated with increased preload, increased resistance leading to decreased output, and hence hypoperfusion as found by decreased MAP and diastolic blood pressure.
Figure 2.
Mean systemic vascular resistance index (SVRI) (A), central venous pressure (B), mean arterial pressure (C), and blood pressure (systolic/diastolic) (D). Error bars indicate SEM. *p < 0.05.
Organ and Inflammatory Biomarkers
Organ Biomarkers.
Elderly and adult patients had similar creatinine on the day of injury and day 1 post injury. However, creatinine was significantly elevated during days 2 (p = 0.019) and 3 post injury (p = 0.003) in elderly burn patients (Fig. 3A). BUN was significantly increased for the majority of the acute phase, including the day of injury (p = 0.021), and days 1 (p = 0.009) and 2 post injury (p = 0.011) in elderly patients (Fig. 3B). Extending this analysis to liver function, we found that liver function measures, including ALP, AST, ALT, and bilirubin, were not significantly different among elderly and adult burn patients (data not shown). Lactate was significantly higher in elderly patients during the day of injury (p = 0.007) (Fig. 3C). Elderly patients were slightly more acidotic than adults during day 3 post injury (p = 0.016) (Fig. 3D).
Figure 3.
Mean creatinine (A), blood urea nitrogen (B), lactate (C), and pH (D) of burn patients. Error bars indicate SEM. *p < 0.05.
Although there was no difference in the number of patients with inhalation injury between the two groups, elderly patients had a significantly decreased Pao2/Fio2 ratio upon admission, indicating that elderly patients may have decreased or impaired lung function (Supplemental Fig. 4, Supplemental Digital Content 4, http://links.lww.com/CCM/E154; legend, Supplemental Digital Content 8, http://links.lww.com/CCM/E158).
Inflammatory Biomarkers.
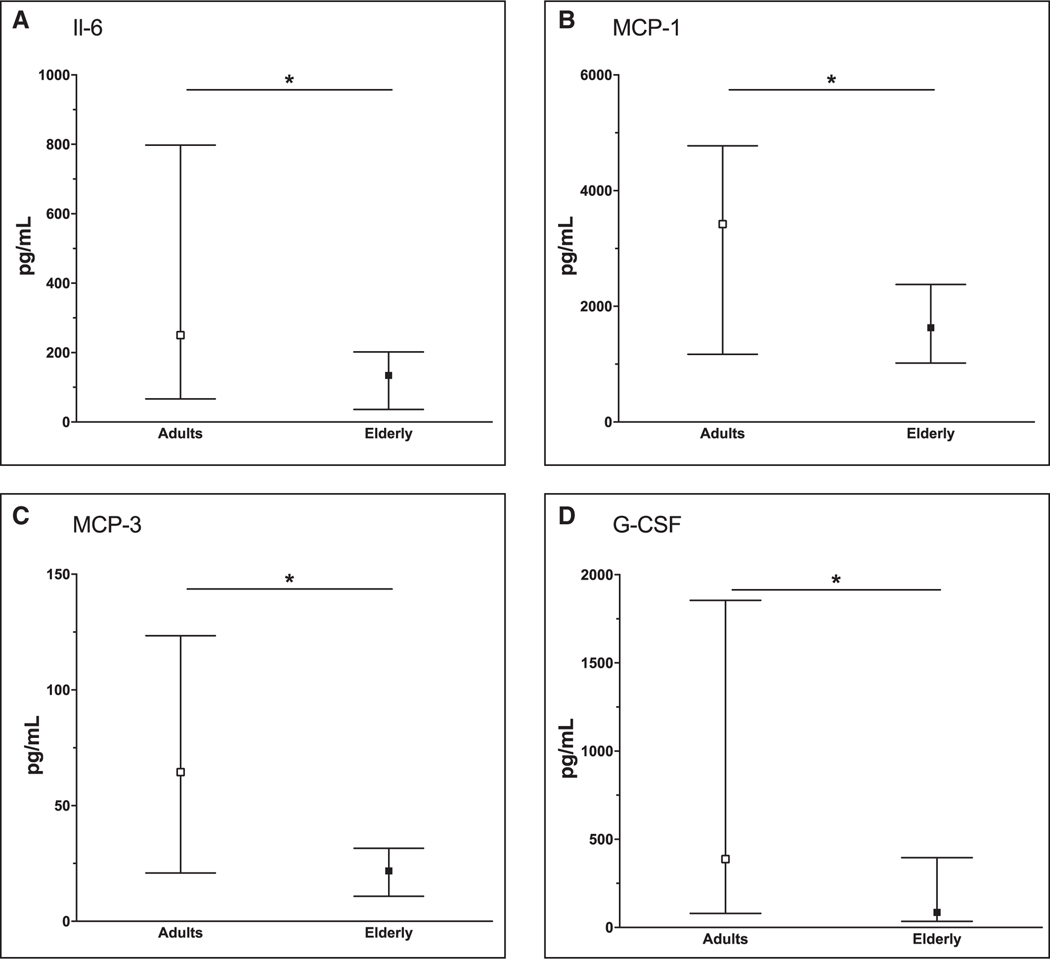
As a hallmark of systemic inflammation, IL-6 was significantly lower in elderly burn patients during the acute phase after injury (p = 0.016) (Fig. 4A). Decreased chemokine expression was present for MCP-1 (p = 0.030) (Fig. 4B) and MCP-3 (p = 0.032) (Fig. 4C). Cytokine expression was also decreased for G-CSF elderly patients (p = 0.020) (Fig. 4D). A similar signal was observed for IL-1β, MIP-1α, MIP-1β, IFN-γ, GRO, and IP-10 (p > 0.05) (Supplemental Fig. 5, Supplemental Digital Content 5, http://links.lww.com/CCM/E155; legend, Supplemental Digital Content 8, http://links.lww.com/CCM/E158). All other cytokine proportions (IL-10, IL-4, IL-1RA, IL-1α, TNF, and GM-CSF) during the acute phase case be found in Supplemental Figure 6 (Supplemental Digital Content 6, http://links.lww.com/CCM/E156; legend, Supplemental Digital Content 8, http://links.lww.com/CCM/E158).
Figure 4.
Median interleukin (IL)–6 (A), monocyte chemotactic protein (MCP)–1 (B), MCP-3 (C), and granulocyte-colony stimulating factor (G-CSF) (D) of burn patients by age status. Error bars indicate the interquartile range. *p < 0.05.
Mortality
Half of elderly patients (n = 12; 52%) died in-hospital in comparison with 5% (n = 6) of adult patients (p < 0.0001) (Table 1). Survival analysis, assessed using the Kaplan-Meier method, found that the cumulative probability of survival at 60 days for elderly patients was 26% in comparison with 96% for adults (p < 0.0001) (Supplemental Fig. 7, Supplemental Digital Content 7, http://links.lww.com/CCM/E157; legend, Supplemental Digital Content 8, http://links.lww.com/CCM/E158). The difference begins at 10 days in-hospital, where survival rapidly decreases in elderly patients (Supplemental Fig. 7, Supplemental Digital Content 7, http://links.lww.com/CCM/E157; legend, Supplemental Digital Content 8, http://links.lww.com/CCM/E158).
DISCUSSION
This study aimed to identify differences in the acute phase response to burn injury that might contribute to mortality and poor outcomes in elderly patients. We hypothesized that elderly have a substantially different response to burn trauma during the first 96 hours relative to adult patients. We saw distinct differences in cardiac function and blood pressure, resulting in decreased perfusion pressure in elderly patients. This was also consistent with elderly receiving greater amounts of inotropes and vasopressors compared with adults. Even with these vasoactive agents, elderly have lower heart rates, MAP, and organ perfusion. Acutely, organ dysfunction is likely a result of the ischemia or hypoperfusion that progresses over time to manifest organ failure that subsequently leads to increased mortality.
Elderly burn patients expressed lower heart rates, lower CI, higher right-sided filling pressure, increased SVRI, but lower MAP. The consequence of cardiac dysfunction includes decreased organ perfusion, manifested by increased organ damage biomarkers, and by lack of lactate clearance and decreased pH. Thus, the present results are indicative of the inability of the elderly patient to respond to the increased cardiac demand or stress response required to maintain adequate organ perfusion. With this pathologically altered response in elderly delineated, the challenge remains to improve cardiac function and organ perfusion.
Organs that also appear to be substantially affected beside the heart are the kidneys and the lung. We hypothesize that the lung is affected by decreased Pao2/Fio2 ratio due to increased pulmonary edema that is likely due to decreased cardiac function and right heart failure. Of concern however is that decreased oxygenation is adding to the acidotic and hypoperfusion state of the elderly burn patient. The kidneys are considered first responders in terms of ischemia reperfusion injury. We found in our study that both BUN and creatinine are significantly increased during the acute phase in elderly burn patients. The increased renal markers could be increased not only due to decreased blood flow but also due to renal damage or acute kidney injury (AKI). AKI has been recently recognized as a major contributor to morbidity and mortality. A systematic review and meta-analysis found that in burn patients, AKI is associated with a median mortality rate of 35% and in those that required renal replacement therapy, median mortality was 80% (7). Whether AKI is the sole culprit of increased mortality or whether it is symptomatic of a more complex situation leading to poor outcome is not entirely clear, but we can clearly identify renal impairment and potentially renal damage in elderly burn patients. A possible approach to improve the outcome of elderly burn patients could be the early initiation of hemofiltration. Recent studies by Chung et al (8, 9) indicate that outcomes, including mortality, can be improved by early initiation of hemofiltration post burn injury. Burned elderly patients could potentially benefit from such an approach because hemofiltration is more tolerable in patients with premorbid conditions or cardiac compromised situations.
It appears that elderly patients survive at least the first week of burn, as we excluded patients who died within the first 96 hours; however, a substantial difference in the mortality curve or Kaplan-Meier starts in week 2 post injury. These findings suggest that potential consequences of the first 96 hours are not associated with an immediate mortality. The initial phase of hypoperfusion of the organs and inadequate stress response potentiates the risk of failure to heal with decreased resources. We also have evidence that the inflammatory profile initially after burn in elderly is hypoinflammation rather than hyper-inflammation. Important early immune and inflammatory stimulators such as IL-6, MCP-1, and MIP-1 are significantly decreased in elderly. Others and we speculate that an early adequate immune and inflammatory response is required for recovery and failure to respond lead to poor outcome. It seems evident that elderly who are failing to respond have poor outcomes. The immune failure can be due to two factors: the CNS or the immune system. It is well documented that the CNS stimulates and modulates inflammatory and immune responses (10, 11). Alteration in the CNS could lead to an inadequate stress response. Another possibility could be that it is the immune system with an inadequate stress response. Immune organs include bone marrow, spleen, liver, and residual immune cells. These immune-modulating and immune cell producing organs might simply be unable to augment their production leading to immune paralysis or an impaired immune response in elderly (4). Future studies investigating whether improving MAP and keeping elderly less acidotic with better organ perfusion may reduce ischemia and hypoperfusion injury leading to improved outcomes after burns are warranted. This research might identify potentially modifiable features of a particularly vulnerable population.
Our study has several limitations. First, this study is limited to a single academic hospital. However, it is informative about the adult and elderly population at an institution without systemic change over the study period. Additionally, this is set in a high-volume burn speciality center that follows the American Burn Association criteria for admission (12) and, as such, has a population that is generalizable to other similar centers. Second, elderly patients might have comorbidities that could affect their acute phase response and overall mortality (3). To decrease bias, we excluded patients who died within the first 96 hours. Third, we recognize that chronologic age is not necessarily representative of biological age and does not address frailty (13), lifestyle, or genetics. The age status of elderly was based on the conventional chronologic age of 65 years old and older as defined by the World Health Organization (5). Last, the proportion of adult (< 65 yr old) and elderly (≥ 65 yr old) patients were not equal, (85% adults vs 15% elderly); however, injury severity was not significantly different between the two age groups. Furthermore, this proportion is reflective of the overall burn population at our center (84% adults vs 16% elderly) (3) and comparable with the 2017 general population in Canada (79% adults vs 21% elderly) (14).
This study is significant as it identifies differences in the acute phase response to burn injury in adult and elderly patients with a burn greater than or equal to 20% TBSA. The most prominent difference was impaired and depleted cardiac function in elderly burn patients when compared with adult burn patients. Impaired cardiovascular function most likely leads to hypoperfusion with subsequent physiologic adverse alterations including organ failure. How can these finding be translated into the clinical setting to improve outcomes? We suggest that individually monitoring and improving cardiac function might improve organ perfusion, maintain metabolic and inflammatory responses, and stimulate cell recovery in elderly burn patients. Based on our data, we encourage clinicians to consider of (1) cardiac monitoring by using noninvasive cardiac monitoring devices to give real-time information on fluid status, cardiac function, and systemic vascular resistance. This could optimize how clinicians resuscitate or provide additional information for use of vasopressors (2). Dobutamine maybe an effective adjunct to improve contractility and cardiac function. We would suggest that if a patient has a low CI or low cardiac output that dobutamine may be the agent of choice to improve cardiac function, therefore improving perfusion of vital organs and increase lactate clearance (3). Furthermore, we believe that impaired cardiac function associated with hypotension may lead to overresuscitation as hypotension is falsely interpreted as hypovolemia. We suggest that overresuscitation should be avoided in elderly burns, as this will worsen cardiovascular function and in fact increase hypoperfusion (4). If overresuscitation occurs, we suggest initiating hemofiltration early which may improve clinical outcomes. But we would like to mention that these suggestions are speculative as there are elderly burn patients who have preexisting cardiac issues who cannot be optimized in terms of cardiovascular function. It is necessary that robust clinical trials are undertaken to determine how to target therapy to improve clinical outcomes in an ever-growing elderly population.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank the Ross Tilley Burn Centre staff for their support and patients for their participation.
Supported, in part, by Canadian Institutes of Health Research number 123336, Canadian Institutes of Health Research CMA151725, National Institutes of Health number R01GM087285.
Dr. Shahrokhi received funding from Acelity and Smith & Nephew.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Klein MB, Goverman J, Hayden DL, et al. ; Inflammation and Host Response to Injury, and Large-Scale Collaborative Research Program: Benchmarking outcomes in the critically injured burn patient. Ann Surg 2014; 259:833–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeschke MG, Peck MD: Burn care of the elderly. J Burn Care Res 2017; 38:e625–e628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeschke MG, Patsouris D, Stanojcic M, et al. : Pathophysiologic response to burns in the elderly. EBioMedicine 2015; 2:1536–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanojcic M, Chen P, Xiu F, et al. : Impaired immune response in elderly burn patients: New insights into the immune-senescence phenotype. Ann Surg 2016; 264:195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kowal P, Dowd JE: Definition of an Older Person. Proposed Working Definition of an Older Person in Africa for the MDS Project. Geneva, Switzerland, World Health Organization, 2001 [Google Scholar]
- 6.von Elm E, Altman DG, Egger M, et al. ; STROBE Initiative: The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007; 370:1453–1457 [DOI] [PubMed] [Google Scholar]
- 7.Brusselaers N, Monstrey S, Colpaert K, et al. : Outcome of acute kidney injury in severe burns: A systematic review and meta-analysis. Intensive Care Med 2010; 36:915–925 [DOI] [PubMed] [Google Scholar]
- 8.Chung KK, Coates EC, Smith DJ Jr, et al. ; Randomized controlled Evaluation of high-volume hemofiltration in adult burn patients with Septic shoCk and acUte kidnEy injury (RESCUE) Investigators: High-volume hemofiltration in adult burn patients with septic shock and acute kidney injury: A multicenter randomized controlled trial. Crit Care 2017; 21:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung KK, Lundy JB, Matson JR, et al. : Continuous venovenous hemofiltration in severely burned patients with acute kidney injury: A cohort study. Crit Care 2009; 13:R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chavan SS, Pavlov VA, Tracey KJ: Mechanisms and therapeutic relevance of neuro-immune communication. Immunity 2017; 46:927–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pavlov VA, Tracey KJ: Neural regulation of immunity: Molecular mechanisms and clinical translation. Nat Neurosci 2017; 20:156–166 [DOI] [PubMed] [Google Scholar]
- 12.American Burn Association: Burn Center Referral Criteria, 2017. Available at: https://ameriburn.org/public-resources/burn-center-referral-criteria/. Accessed March 10, 2018 [Google Scholar]
- 13.Romanowski KS, Barsun A, Pamlieri TL, et al. : Frailty score on admission predicts outcomes in elderly burn injury. J Burn Care Res 2015; 36:1–6 [DOI] [PubMed] [Google Scholar]
- 14.Statistics Canada: Table 051–0001 - Estimates of Population, By Age Group and Sex For July 1, Canada, Provinces and Territories, Annual (Persons Unless Otherwise Noted; ), CANSIM: (Database), 2017. Available at: https://www150.statcan.gc.ca/t1/tbl1/en/cv.action?pid=1710000501. Accessed March 10, 2018 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.