ABSTRACT
Various novel platform technologies have been used for the development of COVID-19 vaccines. In this nested cohort study among healthcare workers in Australia and Brazil who received three different COVID-19-specific vaccines, we (a) evaluated the incidence of adverse events following immunization (AEFI); (b) compared AEFI by vaccine type, dose and country; (c) identified factors influencing the incidence of AEFI; and (d) assessed the association between reactogenicity and vaccine anti-spike IgG antibody responses. Of 1302 participants who received homologous 2-dose regimens of ChAdOx1-S (Oxford-AstraZeneca), BNT162b2 (Pfizer-BioNTech) or CoronaVac (Sinovac), 1219 (94%) completed vaccine reaction questionnaires. Following the first vaccine dose, the incidence of any systemic reaction was higher in ChAdOx1-S recipients (374/806, 46%) compared with BNT162b2 (55/151, 36%; p = 0.02) or CoronaVac (26/262, 10%; p < 0.001) recipients. After the second vaccine dose, the incidence of any systemic reaction was higher in BNT162b2 recipients (66/151, 44%) compared with ChAdOx1-S (164/806, 20%; p < 0.001) or CoronaVac (23/262, 9%; p < 0.001) recipients. AEFI risk was higher in younger participants, females, participants in Australia, and varied by vaccine type and dose. Prior COVID-19 did not impact the risk of AEFI. Participants in Australia compared with Brazil reported a higher incidence of any local reaction (170/231, 74% vs 222/726, 31%, p < 0.001) and any systemic reaction (171/231, 74% vs 328/726, 45%, p < 0.001), regardless of vaccine type. Following a primary course of ChAdOx1-S or CoronaVac vaccination, participants who did not report AEFI seroconverted at a similar rate to those who reported local or systemic reactions. In conclusion, we found that the incidence of AEFI was influenced by participant age and COVID-19 vaccine type, and differed between participants in Australia and Brazil.
KEYWORDS: COVID-19 vaccine, adverse events, antibody responses
Introduction
The ChAdOx1-S (Oxford-AstraZeneca), BNT162b2 (Pfizer-BioNTech) and CoronaVac (Sinovac) COVID-19-specific vaccines are among the most frequently used worldwide to protect against coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1,2
A significant challenge has been vaccine uptake. More than three years after the emergence of COVID-19, nearly one-third of the global population is yet to receive a COVID-19 vaccine dose.3 A contributing factor is hesitancy resulting from concerns about the rapid development of vaccines and the potential for adverse events.4,5 Population-specific data addressing the risk factors for potential adverse events following immunization (AEFI) can contribute to vaccine acceptance and uptake.6
The BRACE (BCG vaccination to reduce the impact of COVID-19 in healthcare workers) trial served as a platform to allow an international study of AEFI, among participants in Australia and Brazil who received intramuscular COVID-19 vaccination (ChAdOx1-S (Oxford-AstraZeneca), BNT162b2 (Pfizer-BioNTech) or CoronaVac (Sinovac)) according to their country-specific vaccine availability. In the first year of the COVID-19 pandemic, a primary series of 2 doses was recommended for ChAdOx1-S (minimum 12-week interval in Australia and Brazil), BNT162b2 (3 and 8 week interval in Australia and Brazil, respectively) and CoronaVac (2 to 4-week interval in Brazil).7,8
In this nested, prospective, cohort study among participants who received homologous 2-dose regimens of three different COVID-19-specific vaccines, we aimed to: (a) determine the incidence of AEFI; (b) compare AEFI by vaccine type, dose and country; (c) identify factors influencing the incidence of AEFI; and (d) assess the association between reactogenicity and vaccine antibody responses.
Materials and methods
Setting and participants
Participants were healthcare workers recruited in Australia and Brazil between March 2020 and April 2021 in the BRACE trial [NCT04327206] who had COVID-19 vaccine responses solicited. The BRACE trial protocol is detailed elsewhere.9 Exclusion criteria in the BRACE trial included previous positive SARS-CoV-2 test or previous receipt of a COVID-19 vaccine. The median interval from blinded BRACE vaccination to the first dose of a COVID-19 vaccine was 94 days (interquartile range 56–156 days).
Participants completed a questionnaire at least one week after each COVID-19 vaccine dose (vaccination administered by their health institution, non-randomized) detailing which vaccine they received and any adverse reactions experienced.
Data collection
Data were collected using REDCap web application,10 including details on demographics and COVID-19 episodes (defined as a symptomatic respiratory or febrile illness with positive COVID-19 PCR or rapid antigen test (RAT)). Information on COVID-19 vaccination (type and timing) and AEFI were collected by telephone interview and web-based questionnaire administered at least seven days after each vaccination dose. The structured questionnaire collected data on local reactions (pain, tenderness, erythema, swelling, itch, regional lymph node swelling), systemic reactions (fever, chills, fatigue, headache, vomiting, diarrhoea, muscle ache, joint ache) and allergic reactions (urticarial, swollen lips, cough/wheeze).
Sample collection and antibody measurement
For a subset of participants in Brazil, peripheral blood was collected prior to first COVID-19 vaccination and 28 (±2) days following first (ChAdOx1-S) or second (ChAdOx1-S, CoronaVac) COVID-19 vaccine dose, and stored at −80°C (as described previously).11 Antibodies against the spike receptor-binding domain of SARS-CoV-2 were measured by chemiluminescent microparticle immunoassays (Abbott, USA), and against nucleocapsid protein (NCP) by Cobas Elecsys anti – SARS-CoV-2 assay (Roche, Switzerland).12 Researchers involved in sample processing, selection and testing were blinded to which COVID-19 vaccine participants had received.
Statistical analysis
The cumulative incidence of local, systemic and allergic reactions in the seven days following first and second COVID-19 vaccine doses was calculated among participants who received the same first two doses of COVID-19 vaccine and who provided vaccine safety data after each dose. Reactions following each vaccination dose were compared between participants using Chi square or Fisher exact tests. Reactions were also compared between participants in Australia and those in Brazil, and between those who received ChAdOx1-S or BNT162b2 vaccines. The severity of local and systemic reactions with the highest incidence after any COVID-19 vaccine dose was graded as per the Food and Drug Administration Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials.13
To identify risk factors for the most common adverse reactions, multivariate logistic regression analysis was applied by administered dose among participants who had negative SARS-CoV-2 serology at recruitment, using age, sex, country, vaccine type and prior COVID-19 as explanatory variables. Odds ratio (OR) and 95% confidence intervals (CI) were determined.
To assess the association between reactogenicity and vaccine antibody responses, linear regression of log-transformed antibody data, adjusted for age, sex, presence of cardiovascular disease, diabetes, chronic respiratory disease, workplace COVID-19 direct contact at baseline, was applied among participants who had negative SARS-CoV-2 serology (spike or NCP) prior to their first COVID-19 vaccine. For antibody concentrations, values below the lower limit of detection were assigned a value of half of the lowest detected value, values above the upper limit of detection were assigned a value of 1.5 times the highest detected value. Adjusted geometric mean antibody ratios were compared between participants with and without local/systemic vaccine reactions. BNT162b2 recipients were excluded from this analysis, due to low number of recipients with antibody data. StataIC 17.0 (Statacorp LP, College Station, TX, USA) was used for analyses.
Ethics
Ethical approval was obtained from the Royal Children’s Hospital Human Research Ethics Committee (HREC 62586) with subsequent approvals from all participating sites. All research was done in accordance with relevant guidelines and regulations. All participants provided signed informed consent prior to enrollment.
Results
Of the 1302 participants who received homologous COVID-19 vaccines for the first two doses, 1219 (94%) completed questionnaires providing information on vaccine reactions after both vaccinations (Figure 1). Of these, 988 were in Brazil and 231 in Australia, with an age range of 18 to 73 years (median 41), and the majority (74%) were female. Participants received homologous doses of ChAdOx1-S vaccine (806, 66%; age range 18 to 73 years), BNT162b2 vaccine (151, 12%; 20 to 66 years) or CoronaVac vaccine (262, 21%; 19 to 70 years).
Figure 1.
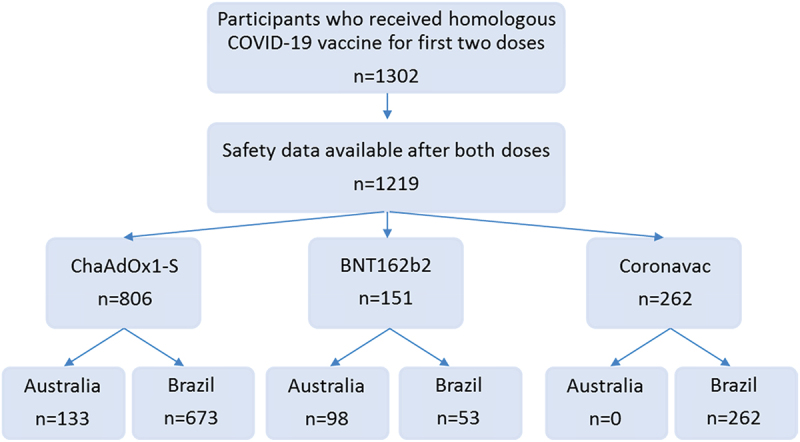
Participants who received homologous COVID-19 vaccine doses.
Incidence of AEFI
In the seven days following first or second dose of COVID-19 vaccine, the incidence of any local reaction differed between vaccines (p < 0.001), and was higher in BNT162b2 vaccine recipients (111/151, 74%), compared with ChaAdOx1 (281/806, 35%; p < 0.001) or CoronaVac (43/262, 16%; p < 0.001) vaccine recipients (Figure 2). The local reaction with the highest incidence after any COVID-19 vaccine dose was injection site pain (ChAdOx1-S 242/806, 30%; BNT162b2 90/151, 60%; CoronaVac 43/262, 16%). Injection site pain after any COVID-19 vaccine dose was mainly mild (ChAdOx1-S 222/242, 92%; BNT162b2 79/90, 88%; CoronaVac 43/43, 100%), with a median duration of two days for any COVID-19 vaccine type (Table S1a-b). The systemic reaction with the highest incidence after any COVID-19 vaccine dose was headache (ChAdOx1-S 239/806, 30%; BNT162b2 48/151, 32%; CoronaVac 22/262, 8%). Headache after any COVID-19 vaccine dose was mainly mild (ChAdOx1-S 178/239, 74%; BNT162b2 33/48, 69%; CoronaVac 22/22, 100%), with a median duration of two days for any COVID-19 vaccine type (except following first CoronaVac dose; median headache duration of one day) (Table S1c-d). The frequency of combinations of reactions also varied by vaccine dose (Table S2).
Figure 2.

Proportion of participants who reported adverse events in the 7 days following COVID-19 vaccination, by vaccine type and dose.
Abbreviations: LN, lymphadenopathy; *p < 0.05; **p < 0.01; ***p < 0.001.
After the first vaccine dose, the incidence of any systemic reaction differed between vaccines (p < 0.001), and was higher in ChAdOx1-S recipients (374/806, 46%) compared with BNT162b2 (55/151, 36%; p = 0.02) or CoronaVac (26/262, 10%; p < 0.001) recipients. After the second vaccine dose, the incidence of any systemic reaction also differed across vaccines (p < 0.001), but was higher in BNT162b2 recipients (66/151, 44%) compared with ChAdOx1-S (164/806, 20%; p < 0.001) or CoronaVac (23/262, 9%; p < 0.001) recipients.
For ChAdOx1-S vaccine recipients, there was a higher incidence of any local reaction and any systemic reaction after the first vaccine dose compared with the second dose (local 225/806, 28% vs. 152/806, 19%, p < 0.001; systemic 374/806, 46% vs. 164/806, 20%, p < 0.001). For BNT162b2 vaccine recipients, there was a higher incidence of any local reaction after the first vaccine dose compared with the second dose (101/151, 67% vs. 77/151, 51%, p < 0.01), and weak evidence for a lower incidence of any systemic reaction after the first vaccine dose compared with the second dose (55/151, 36% vs. 66/151, 44%, p = 0.2). For CoronaVac recipients, there was no evidence for a difference in the incidence of any local (28/262, 11% vs. 33/262, 13%, p = 0.5) or systemic reaction (26/262, 10% vs. 23/262, 9%, p = 0.7) after the first compared with the second vaccine dose.
Allergic reactions were the least reported reactions. After the first dose, the incidence of urticaria was 2/806 (0.2%) in ChAdOx1-S recipients, 2/262 (0.8%) in CoronaVac recipients and 0/151 (0%) in BNT162b2 recipients. After the second dose, the incidence of urticaria was 1/806 (0.1%) in ChAdOx1-S recipients, 1/262 (0.4%) in CoronaVac recipients and 0/151 (0%) in BNT162b2 recipients. The two participants who reported urticaria after the second dose also reported this reaction after the first dose.
Participants in Australia compared with Brazil (Figure 3) reported a higher incidence of any local reaction (170/231, 74% vs 222/726, 31%, p < 0.001) and any systemic reaction (171/231, 74% vs 328/726, 45%, p < 0.001), regardless of receiving ChAdOx1-S vaccine or BNT162b2 vaccine, and regardless of dose (first or second).
Figure 3.

Proportion of participants who reported adverse events in the 7 days following COVID-19 vaccination by country and vaccine type for (a) first vaccine dose and (b) second vaccine dose.
Abbreviations: LN, lymphadenopathy; *p < 0.05; **p < 0.01; ***p < 0.001.
Factors associated with any local reaction following COVID-19 vaccination
Following first COVID-19 vaccine dose, in both the univariate and multivariate analysis, any local reaction was more common among younger participants (<50 years old; adjusted OR 2.1, 95%CI 1.4–3.0), participants in Australia (aOR 5.6, 95%CI 3.8–8.2) and varied by COVID-19 vaccine type (BNT162b2 aOR 2.7, 95%CI 1.7–4.4; CoronaVac aOR 0.4, 95%CI 0.2–0.6) (Table 1).
Table 1.
Demographics and factors investigated for association with local reaction.
| Local reaction after first COVID-19 vaccine dose |
Local reaction after second COVID-19 vaccine dose |
||||||
|---|---|---|---|---|---|---|---|
| Total vaccinated | Univariate | Multivariate | Univariate | Multivariate | |||
| Factor | n = 1040 | n (%) | OR (95%CI) | OR (95%CI) | n (%) | OR (95%CI) | OR (95%CI) |
| Sex | |||||||
| Male | 268 | 79 (29.5) | 1 (reference) | – | 46 (17.2) | 1 (reference) | – |
| Female | 772 | 239 (31.0) | 1.1 (0.8–1.5), p = 0.7 | 188 (24.4) | 1.6 (1.1–2.2), p = 0.02 | ||
| Age | |||||||
| ≥50 years | 283 | 72 (25.4) | 1 (reference) | 1 (reference) | 51 (18.0) | 1 (reference) | 1 (reference) |
| 18–49 years | 757 | 246 (32.5) | 1.4 (1.0–1.9), p = 0.03 | 2.1 (1.4–3.0), p < 0.001 | 183 (24.2) | 1.5 (1.0–2.0), p = 0.04 | 2.1 (1.4–3.1), p < 0.001 |
| Country | |||||||
| Brazil | 809 | 164 (20.3) | 1 (reference) | 1 (reference) | 116 (14.3) | 1 (reference) | 1 (reference) |
| Australia | 231 | 154 (66.7) | 7.9 (5.7–10.9), p < 0.001 | 5.6 (3.8–8.2), p < 0.001 | 118 (51.1) | 6.2 (4.5–8.6), p < 0.001 | 5.4 (3.6–8.0), p < 0.001 |
| COVID-19 vaccine | |||||||
| ChAdOx1-S | 713 | 209 (29.3) | 1 (reference) | 1 (reference) | 142 (19.9) | 1 (reference) | 1 (reference) |
| BNT162b2 | 123 | 90 (73.2) | 6.6 (4.3–10.1), p < 0.001 | 2.7 (1.7–4.4), p < 0.001 | 68 (55.3) | 5.0 (3.3–7.4), p < 0.001 | 2.0 (1.2–3.2), p < 0.01 |
| CoronaVac | 204 | 19 (9.3) | 0.2 (0.2–0.4), p < 0.001 | 0.4 (0.2–0.6), p < 0.001 | 24 (11.8) | 0.5 (0.3–0.9), p < 0.01 | 0.8 (0.5–1.4), p = 0.5 |
| Prior COVID-19* | |||||||
| No | 974 | 299 (30.7) | 1 (reference) | – | 227 (22.7) | 1 (reference) | – |
| Yes | 66 | 19 (28.8) | 0.9 (0.5–1.6), p = 0.7 | 7 (18.0) | 0.7 (0.3–1.7), p = 0.5 | ||
Abbreviations: OR, odds ratio.
*COVID-19 prior to dose (for 2nd dose; between 1st and 2nd dose).
There was no significant association in either analysis with participant sex or prior COVID-19 (Table 1).
Following second COVID-19 vaccine dose, in the univariate analysis, any local reaction was more common among females, younger participants, participants in Australia and those vaccinated with BNT162b2. In the multivariate analysis, any local reaction was more common among younger participants (aOR 2.1, 95%CI 1.4–3.1), participants in Australia (aOR 5.4, 95%CI 3.6–8.0) and those vaccinated with BNT162b2 (aOR 2.0, 95%CI 1.2–3.2) (Table 1).
There was no significant association in either analysis with prior COVID-19 (Table 1).
Factors associated with any systemic reaction following COVID-19 vaccination
Following first COVID-19 vaccine dose, in both the univariate and multivariate analysis, any systemic reaction was more likely among younger participants (aOR 3.0, 95%CI 2.1–4.3), participants in Australia (aOR 5.3, 95%CI 3.5–8.2) and varied by COVID-19 vaccine type (BNT162b2 aOR 0.2, 95%CI 0.1–0.4; CoronaVac aOR 0.1, 95%CI 0.1–0.2) (Table 2).
Table 2.
Demographics and factors investigated for association with systemic reaction.
| Systemic reaction after first COVID-19 vaccine dose |
Systemic reaction after second COVID-19 vaccine dose |
||||||
|---|---|---|---|---|---|---|---|
| Total vaccinated | Univariate | Multivariate | Univariate | Multivariate | |||
| TotalFactor | n = 1040 | n (%) | OR (95%CI) | OR (95%CI) | n (%) | OR (95%CI) | OR (95%CI) |
| Sex | |||||||
| Male | 268 | 92 (34.3) | 1 (reference) | – | 40 (14.9) | 1 (reference) | 1 (reference) |
| Female | 772 | 313 (40.5) | 1.3 (1.0–1.7), p = 0.1 | 188 (24.4) | 1.8 (1.3–2.7), p = 0.001 | 1.7 (1.2–2.6), p < 0.01 | |
| Age | |||||||
| ≥50 years | 283 | 77 (27.2) | 1 (reference) | 1 (reference) | 43 (15.2) | 1 (reference) | 1 (reference) |
| 18–49 years | 757 | 328 (43.3) | 2.0 (1.5–2.8), p < 0.001 | 3.0 (2.1–4.3) p < 0.001 | 185 (24.4) | 1.8 (1.3–2.6), p = 0.001 | 2.1 (1.4–3.1) p < 0.001 |
| Study country | |||||||
| Brazil | 809 | 264 (32.6) | 1 (reference) | 1 (reference) | 135 (16.7) | 1 (reference) | 1 (reference) |
| Australia | 231 | 141 (61.0) | 3.2 (2.4–4.4), p < 0.001 | 5.3 (3.5–8.2) p < 0.001 | 93 (40.3) | 3.4 (2.4–4.6), p < 0.001 | 2.3 (1.5–3.5) p < 0.001 |
| COVID-19 vaccine type | |||||||
| ChAdOx1-S | 713 | 339 (47.6) | 1 (reference) | 1 (reference) | 149 (20.9) | 1 (reference) | 1 (reference) |
| BNT162b2 | 123 | 49 (39.8) | 0.7 (0.5–1.1), p = 0.1 | 0.2 (0.1–0.4), p < 0.001 | 61 (49.6) | 3.7 (2.5–5.5), p < 0.001 | 2.3 (1.4–3.6), p = 0.001 |
| CoronaVac | 204 | 17 (8.3) | 0.1 (0.1–0.2), p < 0.001 | 0.1 (0.1–0.2), p < 0.001 | 18 (8.8) | 0.4 (0.2–0.6), p < 0.001 | 0.4 (0.3–0.8), p < 0.01 |
| Prior COVID-19* | |||||||
| No | 974 | 383 (39.3) | 1 (reference) | – | 216 (22.2) | 1 (reference) | – |
| Yes | 66 | 22 (33.3) | 0.8 (0.5–1.3), p = 0.3 | 12 (18.2) | 0.8 (0.3–1.8), p = 0.5 | ||
Abbreviations: OR, odds ratio.
*COVID-19 prior to dose (for 2nd dose; between 1st and 2nd dose).
There was no significant association in either analysis with participant sex or prior COVID-19 (Table 2).
Following second COVID-19 vaccine dose, in both the univariate and multivariate analysis, any systemic reaction was more likely among females (aOR 1.7, 95%CI 1.2–2.6), younger participants (aOR 2.1, 95%CI 1.4–3.1), participants in Australia (aOR 2.3, 95%CI 1.5–3.5) and influenced by COVID-19 vaccine type (BNT162b2 aOR 2.3, 95%CI 1.4–3.6; CoronaVac aOR 0.4, 95%CI 0.3–0.8) (Table 2). There was no significant association in either analysis with prior COVID-19 (Table 2).
Association between reactogenicity and post-vaccine anti-spike IgG level
A subset of 556 participants in Brazil who received a homologous primary COVID-19 vaccination course, completed vaccine reaction questionnaires, and had negative SARS-CoV-2 serology (spike or NCP) prior to their first COVID-19 vaccine dose, also had blood samples available for analysis following first and/or second vaccine doses.
Among first-dose ChAdOx1-S recipients, there was no association between any local or systemic reaction and anti-spike IgG responses (adjusted geometric mean ratio (aGMR) 1.3, 95%CI 0.6–2.7, p = 0.5; aGMR 1.4, 95%CI 0.8–2.4, p = 0.2 respectively) (Figure 4a). Among second-dose ChAdOx1-S recipients, there was weak evidence that any local reaction was associated with lower anti-spike IgG responses (aGMR 0.7, 95%CI 0.5–1.0, p = 0.05) (Figure 4a). There was no association between any systemic reaction and anti-spike IgG responses (aGMR 0.8, 95%CI 0.5–1.1, p = 0.1).
Figure 4.

Geometric mean ratio of anti-spike IgG responses by reaction types following COVID-19 vaccination with (a) ChAdOx1-S and (b) CoronaVac.
*adjusted for age, sex, presence of cardiovascular disease, diabetes, chronic respiratory disease and workplace COVID-19 contact at baseline.
Among 141 second-dose CoronaVac recipients, there was no association between any local or systemic reaction and anti-spike IgG responses (aGMR 0.8, 95%CI 0.5–1.4, p = 0.5; aGMR 1.3, 95%CI 0.7–2.6, p = 0.4 respectively) (Figure 4b).
Among either ChAdOx1-S or CoronaVac recipients, there was no difference in the anti-spike IgG seroconversion rate between participants with and without any local or systemic reactions (Table 3).
Table 3.
Comparison of the proportion of participants with seroconversion in anti-spike IgG following ChAdOx1-S and CoronaVac vaccination, by reaction type.
| Any local reaction N (%) |
No reaction N (%) |
p-value | Any systemic reaction N (%) |
No reaction N (%) |
p-value | Any reaction N (%) |
No reaction N (%) |
p-value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| ChAdOx1-S | Seroconversion (1st dose) |
31/34 (91%) |
111/115 (97%) |
0.2 | 70/72 (97%) |
111/115 (97%) |
0.6 | 81/86 (94%) |
111/115 (97%) |
0.3 |
| Seroconversion (2nd dose) |
51/51 (100%) |
268/270 (99%) |
0.7 | 67/67 (100%) |
268/270 (99%) |
0.6 | 67/67 (100%) |
268/270v(99%) | 0.5 | |
| CoronaVac | Seroconversion (2nd dose) |
16/16 (100%) |
115/115 (100%) | – | 12/12 (100%) |
115/115 (100%) | – | 26/26 (100%) | 115/115 (100%) | – |
Discussion
In this international study, we found that the reported incidence of adverse reactions following three different COVID-19 vaccines was influenced by both recipient (age, sex) and vaccine (type, dose) factors. In addition, there was a considerable difference between the reported incidence in Australia and Brazil. The most common local (injection pain) and systemic (headache, fatigue, muscle ache) reactions reported were consistent with previous reports.14–22
Our finding that the incidence of systemic reactions was higher following first dose ChAdOx1-S compared with BNT162b2 or CoronaVac, and that the incidence of systemic reactions was higher following second dose BNT162b2 compared with ChAdOx1-S or CoronaVac, are consistent with other studies.18,23–25 Our study is the first to include a comparison with CoronaVac.
Explanations for the differences in incidence of adverse reactions between countries may include: (i) cultural differences in perception of symptoms following vaccination26,27 (ii) the influence of ethnicity on circulating inflammatory cytokine levels28,29; and (iii) the phase of the pandemic. At the time of introduction of COVID-19 vaccines, Brazil had one of the highest (and Australia had one of the lowest) burdens of COVID-19 worldwide.30,31 In Brazil, the high COVID-19 morbidity and mortality led to considerable psychological and physical stress on healthcare workers,32 which may have influenced immunogenicity33,34 and/or perception of vaccine reactions. Moreover, the pandemic-associated strain on the health system may have contributed as healthcare workers in Brazil were not as closely monitored (outside of this study) as those in Australia who had to wait fifteen minutes following vaccination. The fervent communication about AEFIs in the Australian media may have also influenced awareness and reporting.
A lower incidence of both local and systemic AEFI among older adults has been observed in previous studies for seasonal influenza vaccine in Australia,35 the Netherlands,36,37 as well as COVID-19 vaccines (ChAdOx1-S, BNT162b2, mRNA-1273, Ad26.COV2.S) in Germany,38 UK,18,39 Greece,39 the Netherlands40 and US.15 Explanations for the influence of age on AEFI include immunosenescence41,42: older adults produce lower systemic levels of inflammatory cytokines following vaccination.43 Another potential contributing factor is a higher tolerance to pain-like symptoms.44
Inflammatory responses are important for the development of adaptive immunity following vaccination but are likely also responsible for local and systemic adverse reactions.44 The association between vaccine reactogenicity and immunogenicity is gaining increasing research interest. There is some evidence for an association between AEFI and vaccine-induced immune response. A meta-analysis of randomized controlled trials in children showed that prophylactic paracetamol before vaccination (for hepatitis B virus, pneumococcus, Haemophilus influenzae type B and poliovirus) was associated with a decreased incidence of local and systemic AEFI, as well as lower antibody responses after vaccination.45 In adults, inflammation-related AEFI (local reactions and fever) correlated with higher antibody levels following vaccination with recombinant human papillomavirus or hepatitis E virus vaccines.46 The concept that AEFI correlate with a stronger immune response and improved effectiveness is also supported by a study that found that older adults at high risk of cardiovascular disease who experienced mild-moderate AEFI following influenza vaccination, compared with those who did not, were less likely to be hospitalized or die.47
Previous studies suggest an association between reactogenicity and antibody responses following a primary course of BNT162b2 vaccination.48–51 However, other (smaller) studies of BNT162b2 vaccination have shown inconsistent results. Potential explanations for this include sample size and demographics (age, sex, occupation), measures of reactogenicity (presence or severity of single or group of symptoms), prior COVID-19 exposure,52 and the timing and measures of immune response.12 The few studies that have investigated other COVID-19 vaccines have found no correlation. However, their findings are limited by small sample size (ChAdOx1-S n = 42,53 n = 340,51 n = 1625; CoronaVac n = 53).54 Our study, with a larger sample size of participants receiving ChAdOx1-S and CoronaVac vaccination, supports these findings, as there was no strong association between any local or systemic reaction following first or second ChAdOx1-S dose or following second CoronaVac dose, with vaccine antibody response. Importantly, we found that those who did not report AEFI had a similar seroconversion rate to those who reported any local or systemic reaction.
Limitations of our study include that vaccine reactions were self-reported. However, standard questionnaires were used consistent with other studies of reactogenicity. Secondly, participants were healthcare workers, who may have a different perception of adverse reactions than other populations,55 and CoronaVac was only administered in Brazil. Thirdly, the cellular immune response was not evaluated. However, anti-spike antibody responses correlate with COVID-19 protection after vaccination.52,56
Strengths of our study include prospective recruitment of participants in a large international multicentre study, participants with a known COVID-19 history, the ability to compare different COVID-19 vaccine types within the same study, and adjustment of analyses for known potential confounders.
In conclusion, we found that the incidence of local and systemic reactions following COVID-19 vaccination was influenced by participant age and COVID-19 vaccine type, and differed between participants in Australia and Brazil. Following a primary course of ChAdOx1-S or CoronaVac vaccination, participants without any reactions were as likely to seroconvert as those with local and/or systemic reactions.
Supplementary Material
Acknowledgments
We thank the BRACE trial participants for making this study possible. We also thank the researchers involved in establishing the BRACE trial (see Supplementary Material 3 for the BRACE trial Consortium Group), in particular: Ms Veronica Abruzzo, Ms Sonja Elia, Ms Casey Goodall, Ms Ann Krastev, Ms Grace Gell.
The Murdoch Children’s Research Institute (MCRI) leads the BRACE trial across five countries. It is supported by the Victorian Government’s Operational Infrastructure Support Programme. The trial is also supported by the Bill & Melinda Gates Foundation [INV-017302], the Minderoo Foundation [COV-001], Sarah and Lachlan Murdoch, the Royal Children’s Hospital Foundation [2020-1263 BRACE Trial], Health Services Union NSW, the Peter Sowerby Foundation, the Ministry of Health Government of South Australia, the NAB Foundation, the Calvert-Jones Foundation, the Modara Pines Charitable Foundation, the UHG Foundation Pty Ltd, Epworth Healthcare and individual donors. The sponsors had no role in the collection, analysis and interpretation of data or in the preparation, review or approval of the manuscript.
L.F.P. is supported by the Swiss National Science Foundation [Early Postdoc Mobility Grant, P2GEP3_178155]. N.C. is supported by a National Health and Medical Research Council Investigator Grant [GNT1197117].
Funding Statement
This work was supported by the National Health and Medical Research Council [Investigator Grant GNT1197117].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Deidentified participant data and data dictionary are available to others on request and on completion of a signed data access agreement. Requests can be made in writing to braceresearch@mcri.edu.au.
Author contributions
Conceptualisation or design of the work: N.C., N.M, P.V, L.P. Acquisition of data: all authors. Analysis, or interpretation of data: P.V. and L.P. Original drafting: P.V. Revising, editing, and final approval of the manuscript: all authors.
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2323853.
References
- 1.World Health Organization . Coronavirus disease (COVID-19) pandemic. 2023. [accessed 2023 May 25]. https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- 2.World Health Organization . COVID-19 vaccine tracker. 2022. Dec 2 [accessed 2023 Aug 28]. https://covid19.trackvaccines.org/agency/who/.
- 3.Mathieu E, Ritchie H, Rodés-Guirao L, Appel C, Giattino C, Hasell J, Macdonald B, Dattani S, Beltekian D, O-O E. et al. Our world in data: coronavirus (COVID-19) vaccinations. 2023. [accessed 2023 Aug 28]. https://ourworldindata.org/coronavirus.
- 4.Lazarus JV, Wyka K, White TM, Picchio CA, Rabin K, Ratzan SC, Parsons Leigh J, Hu J, El-Mohandes A. Revisiting COVID-19 vaccine hesitancy around the world using data from 23 countries in 2021. Nat Commun. 2022;13(1):3801. doi: 10.1038/s41467-022-31441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Troiano G, Nardi A. Vaccine hesitancy in the era of COVID-19. Public Health. 2021;194:245–10. doi: 10.1016/j.puhe.2021.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azarpanah H, Farhadloo M, Vahidov R, Pilote L. Vaccine hesitancy: evidence from an adverse events following immunization database, and the role of cognitive biases. BMC Public Health. 2021;21(1):1686. doi: 10.1186/s12889-021-11745-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Australian Government . Australian technical advisory group on immunisation. [accessed 2023 May 24]. https://www.health.gov.au/committees-and-groups/australian-technical-advisory-group-on-immunisation-atagi.
- 8.World Health Organization . The Sinovac-CoronaVac COVID-19 vaccine: what you need to know. 2022. [accessed 2023 May 24]. https://www.who.int/news-room/feature-stories/detail/the-sinovac-covid-19-vaccine-what-you-need-to-know.
- 9.Pittet LF, Messina NL, Gardiner K, Orsini F, Abruzzo V, Bannister S, Bonten M, Campbell JL, Croda J, Dalcolmo M. et al. BCG vaccination to reduce the impact of COVID-19 in healthcare workers: protocol for a randomised controlled trial (BRACE trial). BMJ Open. 2021;11(10):e052101. doi: 10.1136/bmjopen-2021-052101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Messina NL, Sperotto MG, Puga MAM, da Silva PV, de Oliveira RD, Moore CL, Pittet LF, Jamieson T, Dalcolmo M, Dos Santos G. et al. Impact of vaccine platform and BCG vaccination on antibody responses to COVID-19 vaccination. Front Immunol. 2023;14:1172851. doi: 10.3389/fimmu.2023.1172851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bond KA, Williams E, Nicholson S, Lim S, Johnson D, Cox B, Putland M, Gardiner E, Tippett E, Graham M. et al. Longitudinal evaluation of laboratory-based serological assays for SARS-CoV-2 antibody detection. Pathology. 2021;53(6):773–779. doi: 10.1016/j.pathol.2021.05.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US Food and Drug Administration . Guidance for industry: toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. 2007. [DOI] [PubMed]
- 14.Alqahtani S, Jokhdar H, Al-Tawfiq JA, Al-Otaibi S, Assiri A, Almudarra S, Alabdulkareem K, Haji A. Adverse events following administration of COVID-19 vaccines in Saudi Arabia. Sci Rep. 2022;12:19551. doi: 10.1038/s41598-022-23471-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapin-Bardales J, Gee J, Myers T. Reactogenicity following receipt of mRNA-based COVID-19 vaccines. JAMA. 2021;325(21):2201–2202. doi: 10.1001/jama.2021.5374. [DOI] [PubMed] [Google Scholar]
- 16.Graña C, Ghosn L, Evrenoglou T, Jarde A, Minozzi S, Bergman H, Buckley BS, Probyn K, Villanueva G, Henschke N. et al. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst Rev. 2022;12:Cd015477. doi: 10.1002/14651858.CD015477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klugar M, Riad A, Mekhemar M, Conrad J, Buchbender M, Howaldt HP, Attia S. Side effects of mRNA-based and viral vector-based COVID-19 vaccines among German healthcare workers. Biology (Basel). 2021;10(8):752. doi: 10.3390/biology10080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menni C, Klaser K, May A, Polidori L, Capdevila J, Louca P, Sudre CH, Nguyen LH, Drew DA, Merino J. et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID symptom study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21(7):939–949. doi: 10.1016/S1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orebi HA, Emara HE, Alhindi AA, Shahin MR, Hegazy AH, Kabbash IA, Saied SM. Perceptions and experiences of COVID-19 vaccines’ side effects among healthcare workers at an Egyptian university hospital: a cross-sectional study. Trop Med Health. 2022;50(1):37. doi: 10.1186/s41182-022-00427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, Han W, Chen Z, Tang R, Yin W. et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181–92. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, Bellamy D, Bibi S, Bittaye M, Clutterbuck EA. et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C. et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amaro C, Monteiro C, Duarte AP. COVID-19 vaccines adverse reactions reported to the pharmacovigilance unit of Beira interior in portugal. J Clin Med. 2022;11(19):5591. doi: 10.3390/jcm11195591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paczkowska A, Hoffmann K, Michalak M, Hans-Wytrychowska A, Bryl W, Kopciuch D, Zaprutko T, Ratajczak P, Nowakowska E, Kus K. Safety profile of COVID-19 vaccines among healthcare workers in Poland. Vaccines. 2022;10(3):434. doi: 10.3390/vaccines10030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryan FJ, Norton TS, McCafferty C, Blake SJ, Stevens NE, James J, Eden GL, Tee YC, Benson SC, Masavuli MG. et al. A systems immunology study comparing innate and adaptive immune responses in adults to COVID-19 mRNA and adenovirus vectored vaccines. Cell Rep Med. 2023;4(3):100971. doi: 10.1016/j.xcrm.2023.100971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peacock S, Patel S. Cultural influences on pain. Rev Pain. 2008;1(2):6–9. doi: 10.1177/204946370800100203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rupe A, Ahlers-Schmidt CR, Wittler R. A comparison of perceptions of fever and fever phobia by ethnicity. Clin Pediatr (Phila). 2009;49:172–6. doi: 10.1177/0009922809336208. [DOI] [PubMed] [Google Scholar]
- 28.Mayr FB, Spiel AO, Leitner JM, Firbas C, Kliegel T, Jilma B. Ethnic differences in plasma levels of interleukin-8 (IL-8) and granulocyte colony stimulating factor (G-CSF). Transl Res. 2007;149(1):10–14. doi: 10.1016/j.trsl.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Delaney NL, Esquenazi V, Lucas DP, Zachary AA, Leffell MS. TNF-alpha, TGF-beta, IL-10, IL-6, and INF-gamma alleles among African Americans and Cuban Americans. Report of the ASHI minority workshops: part IV. Hum Immunol. 2004;65:1413–19. doi: 10.1016/j.humimm.2004.07.240. [DOI] [PubMed] [Google Scholar]
- 30.Gebru AA, Birhanu T, Wendimu E, Ayalew AF, Mulat S, Abasimel HZ, Kazemi A, Tadesse BA, Gebru BA, Deriba BS. et al. Global burden of COVID-19: situational analyis and review. Hum Antibodies. 2021;29(2):139–148. doi: 10.3233/HAB-200420. [DOI] [PubMed] [Google Scholar]
- 31.Bigoni A, Malik AM, Tasca R, Carrera MBM, Schiesari LMC, Gambardella DD, Massuda A. Brazil’s health system functionality amidst of the COVID-19 pandemic: an analysis of resilience. Lancet Reg Health – Am. 2022;10:100222. doi: 10.1016/j.lana.2022.100222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato TO, de Faria BSF, Albuquerque BB, Silva FLD, Rohwedder LS, de Azevedo RT, Gonçalves JS, Vieira L, Triches MI, de Sousa RA. et al. Poor health conditions among Brazilian healthcare workers: the study design and baseline characteristics of the HEROES cohort. Healthcare (Basel). 2022;10:2096. doi: 10.3390/healthcare10102096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130(4):601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marsland AL, Walsh C, Lockwood K, John-Henderson NA. The effects of acute psychological stress on circulating and stimulated inflammatory markers: a systematic review and meta-analysis. Brain Behav Immun. 2017;64:208–219. doi: 10.1016/j.bbi.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alexis JP, Catherine G, Peter J, Helen EQ, Parveen F, Patrick C, Alan L, Christopher CB, Michael SG, Thomas S. et al. Active surveillance of 2017 seasonal influenza vaccine safety: an observational cohort study of individuals aged 6 months and older in Australia. BMJ Open. 2018;8:e023263. doi: 10.1136/bmjopen-2018-023263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smetana J, Chlibek R, Shaw J, Splino M, Prymula R. Influenza vaccination in the elderly. Hum Vaccin Immunother. 2018;14:540–9. doi: 10.1080/21645515.2017.1343226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Govaert TM, Dinant GJ, Aretz K, Masurel N, Sprenger MJ, Knottnerus JA. Adverse reactions to influenza vaccine in elderly people: randomised double blind placebo controlled trial. BMJ. 1993;307(6910):988–990. doi: 10.1136/bmj.307.6910.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warkentin L, Werner F, Zeschick N, Kühlein T, Steininger P, Überla K, Kaiser I, Sebastião M, Hueber S. Reactogenicity and safety of COVID-19 primary immunisation and booster vaccination regimens: a comparative observational cohort study. BMC Med. 2023;21:218. doi: 10.1186/s12916-023-02924-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mathioudakis AG, Ghrew M, Ustianowski A, Ahmad S, Borrow R, Papavasileiou LP, Petrakis D, Bakerly ND. Self-reported real-world safety and reactogenicity of COVID-19 vaccines: a vaccine recipient survey. Life (Basel). 2021;11(3):249. doi: 10.3390/life11030249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rolfes L, Härmark L, Kant A, van Balveren L, Hilgersom W, van Hunsel F. COVID-19 vaccine reactogenicity - a cohort event monitoring study in the Netherlands using patient reported outcomes. Vaccine. 2022;40:970–6. doi: 10.1016/j.vaccine.2022.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Müller L, Andrée M, Moskorz W, Drexler I, Walotka L, Grothmann R, Ptok J, Hillebrandt J, Ritchie A, Rabl D. et al. Age-dependent immune response to the biontech/Pfizer BNT162b2 coronavirus disease 2019 vaccination. Clin Infect Dis. 2021;73(11):2065–2072. doi: 10.1093/cid/ciab381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crooke SN, Ovsyannikova IG, Poland GA, Kennedy RB. Immunosenescence and human vaccine immune responses. Immun Ageing. 2019;16:25. doi: 10.1186/s12979-019-0164-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.El Yousfi M, Mercier S, Breuillé D, Denis P, Papet I, Mirand PP, Obled C. The inflammatory response to vaccination is altered in the elderly. Mech Ageing Dev. 2005;126(8):874–881. doi: 10.1016/j.mad.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 44.Hervé C, Laupèze B, Del Giudice G, Didierlaurent AM, Tavares Da Silva F. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines. 2019;4:39. doi: 10.1038/s41541-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Das RR, Panigrahi I, Naik SS, Quach C. The effect of prophylactic antipyretic administration on post-vaccination adverse reactions and antibody response in children: a systematic review. PloS One. 2014;9(9):e106629. doi: 10.1371/journal.pone.0106629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhuang C-L, Lin Z-J, Bi Z-F, Qiu L-X, Hu F-F, Liu X-H, Lin B-Z, Su Y-Y, Pan H-R, Zhang T-Y. et al. Inflammation-related adverse reactions following vaccination potentially indicate a stronger immune response. Emerging Microbes Infect. 2021;10(1):365–375. doi: 10.1080/22221751.2021.1891002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peikert A, Claggett BL, Kim K, Udell JA, Joseph J, Desai AS, Farkouh ME, Hegde SM, Hernandez AF, Bhatt DL. et al. Association of post-vaccination adverse reactions after influenza vaccine with mortality and cardiopulmonary outcomes in patients with high-risk cardiovascular disease: the INVESTED trial. Eur J Heart Fail. 2023;25(2):299–310. doi: 10.1002/ejhf.2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uwamino Y, Kurafuji T, Sato Y, Tomita Y, Shibata A, Tanabe A, Yatabe Y, Noguchi M, Arai T, Ohno A. et al. Young age, female sex, and presence of systemic adverse reactions are associated with high post-vaccination antibody titer after two doses of BNT162b2 mRNA SARS-CoV-2 vaccination: an observational study of 646 Japanese healthcare workers and university staff. Vaccine. 2022;40(7):1019–1025. doi: 10.1016/j.vaccine.2022.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jubishi D, Okamoto K, Hamada K, Ishii T, Hashimoto H, Shinohara T, Yamashita M, Wakimoto Y, Otani A, Hisasue N. et al. The association between adverse reactions and immune response against SARS-CoV-2 spike protein after vaccination with BNT162b2 among healthcare workers in a single healthcare system: a prospective observational cohort study. Hum Vaccin Immunother. 2022;18:2048559. doi: 10.1080/21645515.2022.2048559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Debes AK, Xiao S, Colantuoni E, Egbert ER, Caturegli P, Gadala A, Milstone AM. Association of vaccine type and prior SARS-CoV-2 infection with symptoms and antibody measurements following vaccination among health care workers. JAMA Intern Med. 2021;181(12):1660–1662. doi: 10.1001/jamainternmed.2021.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bannister WP, Raben D, Valentiner-Branth P, Tolstrup M, Larsen L, Tarp B, Brouw Iversen M, Schmeltz Søgaard O, Rye Ostrowski S, Breinholt Stærke N. et al. Association of self-reported systemic reactions following SARS-CoV-2 vaccination with immunological response in the Danish national cohort study of effectiveness and safety of SARS-CoV-2 vaccines (ENFORCE). Open Forum Infect Dis. 2023;10. doi: 10.1093/ofid/ofad248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wei J, Pouwels KB, Stoesser N, Matthews PC, Diamond I, Studley R, Rourke E, Cook D, Bell JI, Newton JN. et al. Antibody responses and correlates of protection in the general population after two doses of the ChAdOx1 or BNT162b2 vaccines. Nat Med. 2022;28(5):1072–1082. doi: 10.1038/s41591-022-01721-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hwang YH, Song KH, Choi Y, Go S, Choi SJ, Jung J, Kang CK, Choe PG, Kim NJ, Park WB. et al. Can reactogenicity predict immunogenicity after COVID-19 vaccination? Korean J Intern Med. 2021;36(6):1486–1491. doi: 10.3904/kjim.2021.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang R, Leung KY, Liu D, Fan Y, Lu L, Chan PC, To KK, Chen H, Yuen KY, Chan KH. et al. Correlation of immunogenicity and reactogenicity of BNT162b2 and CoronaVac SARS-CoV-2 vaccines. mSphere. 2022;7(2):e0091521. doi: 10.1128/msphere.00915-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rau K, von Heeringen E, Bühler N, Wagenpfeil S, Becker SL, Schneitler S. Recipient-reported reactogenicity of different SARS-CoV-2 vaccination regimens among healthcare professionals and police staff in Germany. Vaccines. 2023;11(7):1147. doi: 10.3390/vaccines11071147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feng S, Phillips DJ, White T, Sayal H, Aley PK, Bibi S, Dold C, Fuskova M, Gilbert SC, Hirsch I. et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27(11):2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified participant data and data dictionary are available to others on request and on completion of a signed data access agreement. Requests can be made in writing to braceresearch@mcri.edu.au.


