ABSTRACT
Bispecific antibodies (bsAbs) are a class of antibodies that can mediate novel mechanisms of action compared to monospecific monoclonal antibodies (mAbs). Since the discovery of mAbs and their adoption as therapeutic agents in the 1980s and 1990s, the development of bsAbs has held substantial appeal. Nevertheless, only three bsAbs (catumaxomab, blinatumomab, emicizumab) were approved through the end of 2020. However, since then, 11 bsAbs received regulatory agency approvals, of which nine (amivantamab, tebentafusp, mosunetuzumab, cadonilimab, teclistamab, glofitamab, epcoritamab, talquetamab, elranatamab) were approved for the treatment of cancer and two (faricimab, ozoralizumab) in non-oncology indications. Notably, of the 13 currently approved bsAbs, two, emicizumab and faricimab, have achieved blockbuster status, showing the promise of this novel class of therapeutics. In the 2020s, the approval of additional bsAbs can be expected in hematological malignancies, solid tumors and non-oncology indications, establishing bsAbs as essential part of the therapeutic armamentarium.
KEYWORDS: bsAb, bsADC, CD3ε, CPI, IgG, mab, TCE
Introduction
Since the discovery of monoclonal antibodies, bispecific versions that could be used as therapeutics have been of intense interest.1–5 However, development was slowed due to challenges with the generation and production of bispecific antibodies (bsAbs) for clinical trials and the biological understanding of the more complicated mechanisms of action (MOAs). The first bsAb, the EpCAM/CD3ε T cell engager catumaxomab, was finally approved in 2009 as a treatment of patients with malignant ascites (Figure 1).6 This bsAb was subsequently withdrawn 2013 from the market, likely related to the fact that it could only be administered intraperitoneally, as patients had severe infusion reactions when administered intravenously, and the high immunogenicity due to its rat-mouse chimeric quadrome design with a fully functional Fc portion.6 From 2009 to 2020, only two additional bsAbs were approved: (1) in 2014, the Fc-free tandem single-chain variable fragment (scFv)-based CD19/CD3ε bispecific T cell engager (BiTE) blinatumomab (Amgen) for the treatment of acute lymphoblastic leukemia (ALL),7–9 and (2) in 2017, the humanized hetero-dimeric coagulation factor IXa/X bispecific ART-Ig emicizumab (Roche group), which acts as a Factor VIII mimetic for the treatment of hemophilia A (Figure 1).10,11
Figure 1.
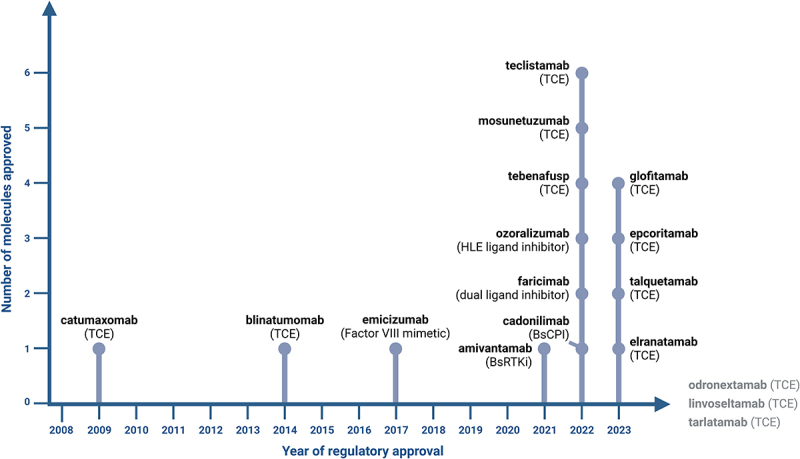
Timeline of regulatory approval of bsAbs with their respective MOA. Linvoseltamab, odronextamab and tarlatamab are currently under regulatory review with a decision anticipated in 2024. Created with Biorender.com.
Due to the intense interest in these molecules and advances in the technologies used to develop them during the past two decades, hundreds of bsAbs have been described, engineered using various different technologies and developed preclinically.1–5 Of these, over 100 bsAbs have reached clinical trials.1,3,5 Based on this major effort in developing novel bsAb formats, novel target combinations and bispecific lead molecules, the landscape has substantially changed recently and bsAb approvals are becoming frequent since 2021. In the past three years alone (2021–2023), 11 novel bsAbs were approved by health authorities in the US, Europe, Japan and/or China for use in patients (Figure 1, Table 1). Of these 11 bsAbs, nine were approved for treatment of cancer: (1) the EGFR/c-MET bsAb amivantamab (Johnson & Johnson (J&J)) for the treatment of non-small cell lung cancer (NSCLC) with EGFR exon 20 insertion mutations,12,13 (2) the gp100-pMHC/CD3ε bsAb tebentafusp (Immunocore) for the treatment of unresectable or metastatic uveal melanoma,14,15 (3) the CD20/CD3ε T cell engager (TCE) mosunetuzumab (Roche group) for the treatment of relapsed/refractory (R/R) follicular lymphoma,16,17 (4) the PD-1/CTLA-4 bsAb cadonilimab (Akeso) for treatment of patients with relapsed or metastatic cervical cancer,18,19 (5) the BCMA/CD3ε TCE teclistamab (J&J) for the treatment of R/R multiple myeloma,20,21 (6) the CD20/CD3ε TCE glofitamab (Roche group) for the treatment of R/R diffuse large B cell lymphoma (DLBCL),22,23 (7) the CD20/CD3ε TCE epcoritamab (AbbVie/Genmab) for the treatment of R/R DLBCL,24,25 (8) the GPRC5D/CD3ε TCE talquetamab (J&J) for the treatment of R/R multiple myeloma26,27 and (9) the BCMA/CD3ε TCE elranatamab (Pfizer) for the treatment of R/R multiple myeloma.28,29 Two additional bsAbs were approved for non-oncology indications, the VEGF-A/Ang-2 bsAb faricimab (Roche group) for the treatment of wet age-related macular degeneration, diabetic macular edema, and macular edema following retinal vein occlusion,30–33 which was the first bsAb approved for use in ophthalmology, and the TNF/human serum albumin (HSA) bsAb ozoralizumab (Taisho) for the treatment of inadequately managed rheumatoid arthritis.34–36 The Roche group with Chugai is currently leading the field with four approved bsAbs, followed by J&J with three approved bsAbs. Notably, of the 13 currently approved bsAbs, two, emicizumab and faricimab, have already achieved blockbuster status with annual sales exceeding four and two billion dollars, respectively, underlining the commercial promise of this novel class of therapeutics.
Table 1.
Approved bsAbs and bsAbs under regulatory review.
| Trade name | INN | Targets | MOA | Format | Indications | Year | 1st Approval | Company | |
|---|---|---|---|---|---|---|---|---|---|
| Discontinued | |||||||||
| 1 | Removab | Catumaxomab | EpCAM/CD3ε | T cell engager | 1+1 Quadroma | Ovarian ascites (intraperitonael) | 2009 | Europe | Trion Pharma |
| Approved | |||||||||
| 1 | Blincyto | Blinatumomab | CD19/CD3ε | T cell engager | 1+1 (scFv)2 BiTE | Acute lymphocytic leukemia | 2014 | US | Amgen |
| 2 | Hemlibra | Emicizumab | factor IXa/factor X | Factor VIII mimetic | 1+1 ART-Ig | Haemophilia A | 2017 | US | Roche group |
| 3 | Rybrevant | Amivantamab | EGFR/c-Met | Dual signaling inhibitor + ADCC | 1+1 Duobody | Non-Small Cell Lung Cancer, EGFR exon 20 mutated | 2021 | US | Johnson & Johnson |
| 4 | Kimmtrak | Tebentafusp | gp100-HLA-A*02/CD3ε | T cell engager | 1+1 TCR-scFv | Uveal melanoma | 2022 | US | Immunocore |
| 5 | Vabysmo | Faricimab | Ang-2/VEGF | Dual ligand inhibitor | 1+1 CrossMab | wAMD, DME, RVO | 2022 | US | Roche group |
| 6 | Lunsumio | Mosunetuzumab | CD20/CD3ε | T cell engager | 1+1 IgG-KiH | Relapsed/refractory fNHL | 2022 | Europe | Roche group |
| 7 | Kaitanni | Cadonilimab | PD-1/CTLA-4 | Dual checkpoint inhibitor | 2+2 IgG-scFv Tetrabody | Cervical cancer | 2022 | China | Akeso |
| 8 | Tecvayli | Teclistamab | BCMA/CD3ε | T cell engager | 1+1 Duobody | Relapsed/refractory multiple myeloma | 2022 | Europe | Johnson & Johnson |
| 9 | Nanozora | Ozoralizumab | TNFa/HSA | Ligand inhibitor | 2+1 Nanobody | Rheumatoid arthritis | 2022 | Japan | Taisho Pharmaceutical, Ablynx |
| 10 | Columvi | Glofitamab | CD20/CD3ε | T cell engager | 2+1 CrossMab | Relapsed/refractory DLBCL | 2023 | Canada | Roche group |
| 11 | (T)Epkinly | Epcoritamab | CD20/CD3ε | T cell engager | 1+1 Duobody | Relapsed/refractory DLBCL | 2023 | US | Genmab, AbbVie |
| 12 | Talvey | Talquetamab | GPRC5D/CD3ε | T cell engager | 1+1 Duobody | Relapsed/refractory multiple myeloma | 2023 | US | Johnson & Johnson |
| 13 | Elrexfio | Elranatamab | BCMA/CD3ε | T cell engager | 1+1 bsAb | Relapsed/refractory multiple myeloma | 2023 | US | Pfizer |
| Under regulatory review | |||||||||
| 1 | n.a. | Linvoseltamab | BCMA/CD3ε | T cell engager | 1+1 Veloci-Bi | Relapsed/refractory multiple myeloma | n.a | Pending US | Regeneron |
| 2 | n.a. | Odronextamab | CD20/CD3ε | T cell engager | 1+1 Veloci-Bi | Relapsed/refractory DLBCL | n.a. | Pending US | Regeneron |
| 3 | n.a. | Tarlatamab | DLL3/CD3ε | T cell engager | 1+1 Fc-(scFv)2 Fc-BiTE | Relapsed advanced small cell lung cancer | n.a. | Pending US | Amgen |
| 4 | n.a. | Zanidatamab | HER2/HER2 | Dual signaling inhibitor + ADCC + CDC | 1+1 Azymetric | Biliary tract cancer | n.a. | Pending US | Zymeworks/Jazz Pharmaceuticals |
| 5 | n.a. | Ivonescimab | PD-1/VEGF | Dual checkpoint/ligand inhibitor | 2+2 IgG-scFv Tetrabody | Non-Small Cell Lung Cancer | n.a. | Pending China | Akeso |
ADCC, antibody-dependent cellular cytotoxicity; ART-Ig, bispecific antibody manufacturing technology; BiTE, bispecific T cell engager; CDC, complement-dependent cytotoxicity; DLBCL, diffuse large b cell lymphoma; DME, diabetic macular edema; fNHL, follicular non-Hodgkin’s lymphoma; KIH, knobs-into-holes; n.a., not applicable; RVO, Retinal vein occlusion; scFv, single-chain Fv fragment; TCR, T cell receptor; wAMD, wet age-related macular degeneration.
Bispecific antibody formats
The variety of bispecific antibody formats developed reflects the diversity of technologies applied in these approved bsAbs (Figure 2). The respective bsAb formats cover non-IgG-like bsAbs, with 1) Amgen’s short half-life tandem-scFv-based BiTE format applied in blinatumomab,37,38 2) Immunocore’s T cell receptor (TCR)-scFv fusion-based ImmTac format applied in tebentafusp39,40 and 3) Ablynx’s trivalent bispecific tandem nanobody format with half-life extension via HSA binding applied in ozoralizumab.41 Most bsAbs, however, are IgG-like molecules with pharmacokinetics that are similar to antibodies due to the presence of an Fc. Five asymmetric 1 + 1 IgG-like bsAbs are derived from either controlled Fab arm exchange, based on Duobody technology applied in amivantamab,42 teclistamab,43 epcoritamab,44 and talquetamab,45 or related chain exchange technology by Pfizer46 applied in elranatamab.47 Alternatively, correct chain association is enforced via Chugai’s ART-Ig technology48 applied in the asymmetric 1 + 1 IgG-like bsAb emicizumab49,50 together with common light chains. Correct heavy chain association can also be ensured in asymmetric 1 + 1 IgG-like bsAbs using knobs-into-holes technology applied together with in vitro assembly in mosunetuzumab,51 or together with CrossMab technology to enforce correct light chain association in the 1 + 1 IgG-like bsAb faricimab52 or the trivalent 2 + 1 bsAb glofitamab.53 Finally, Akeso’s cadonilimab comprises a symmetric tetravalent 2 + 2 C-terminal IgG-scFv fusion.54 Among the approved bsAbs, four apply Genmab’s Duobody technology,55,56 three apply Genentech’s knobs-into-holes57,58 and two apply Roche pRED’s CrossMab technology.59–61 The diversity of formats available suggests that “standardized” bsAbs are unlikely to arise, but recent approvals underpin a focus on classical heterodimeric IgG-like bsAb formats. These bsAbs typically show IgG-like pharmacokinetics and low incidence of anti-drug antibodies.
Figure 2.
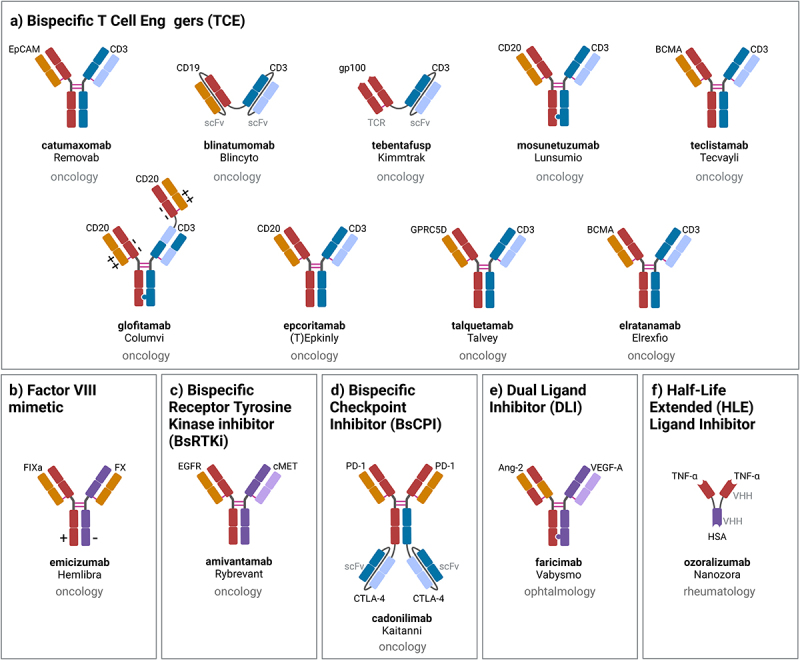
Schematic representation, indication and mechanism of action of approved bsAbs: a) T cell engagers (TCE), b) factor VIII mimetic, dual signaling inhibition: c) bispecific receptor tyrosine kinase (RTK) inhibitor (BsRtki), d) bispecific checkpoint inhibitor (BsCPI), e) dual ligand inhibitor (DLI), f) half-life extended (HLE) ligand inhibitor. Created with Biorender.com.
MOAs for approved bsAbs
TCEs are bispecific antibodies that bind with one specificity to a cell surface tumor antigen, and with the second specificity to a subunit of the TCR complex, so that, upon simultaneous binding to the tumor antigen and the TCR, the T cell is subsequently activated to kill the tumor cell, secrete cytokines and start to proliferate.62 Apparently, T cell engagement strictly relies on bispecificity and cannot be achieved by the combination of two conventional mAbs. For the treatment of cancer, the pre-dominant MOA is T cell engagement, with the largest number of bsAbs approved and in clinical trials being TCEs.5 Figure 2a shows an overview of the evolution of approved TCE bsAb formats. This class of molecules includes TCEs for the treatment of relapsed/refractory hematological cancers: 1) CD19/CD3ε blinatumomab for the treatment of ALL,7–9 2) the CD20/CD3ε TCEs mosunetuzumab for the treatment of R/R non-Hodgkin’s lymphoma (NHL),51 and glofitamab53 and epcoritamab44 for the treatment of R/R DLBCL, and 3) the BCMA/CD3ε TCEs teclistamab43 and elranatamab,47 and GPRC5D/CD3ε talquetamab,45 for the treatment of R/R multiple myeloma. While generally T cell engagement for the treatment of solid tumors appears to be more challenging,63 the soluble gp100-peptideMHC/CD3ε -specific TCR-scFv fusion tebentafusp was the first TCE to be approved for the treatment of uveal melanoma, providing evidence for use outside of hematological tumors.
Hemophilia A is a hereditary bleeding disorder characterized by the lack of blood coagulation Factor VIII. While recombinant Factor VIII can be administered to patients, this is related to challenges such as Factor VIII production, short half-life with frequent administration and the development of neutralizing antibodies.64 For this purpose, Chugai designed and optimized the IXa/X bsAb emicizumab so that it mimics the function of blood coagulation Factor VIII by bringing the enzyme Factor IXa and the substrate Factor X into close proximity while exhibiting IgG-like extended pharmacokinetics49,50 (Figure 2b).
Monospecific receptor tyrosine kinase blocking mAbs, such as anti-EGFR cetuximab and panitumumab or anti-HER2 trastuzumab and pertuzumab, have revolutionized cancer therapy.65 While there has been little progress in targeting additional RTKs, EGFR/c-Met bsAb amivantamab, a first bsAb targeting two RTKs simultaneously, was recently approved for a specific subset of NSCLC patients with EGFR exon 20 insertion mutations.12,13 In addition to its dual signaling inhibitor function, both via blocking the ligand binding sites and via receptor downregulation, amivantamab also contains an afucosylated Fc portion to mediate enhanced antibody-dependent cellular toxicity (ADCC) for NK cell and macrophage/monocyte engagement42 (Figure 2c).
During the past decade, approved immune checkpoint inhibitory antibodies, including those targeting PD-1 (nivolumab, pembrolizumab, cemiplimab), PD-L1 (atezolizumab, avelumab, durvalumab), and CTLA-4 (ipilimumab, tremelimumab), have revolutionized the field of cancer therapy and established cancer immunotherapy.66 Based on this experience, the dual PD-1/CTLA-4 checkpoint inhibitory bsAb cadonilimab was designed with the goal of specifically, and ideally simultaneously, binding and inhibiting PD-1 and CTLA-4 on antigen-specific T cells to overcome checkpoint inhibition54 (Figure 2d).
The pro-angiogenic ligands VEGF-A and Ang-2 contribute to vision loss by fostering retinal angiogenesis and destabilizing blood vessels causing leakiness and subsequent edema and inflammation.67,68 Faricimab was specifically designed to block VEGF-A and Ang-2 in the eye to interfere with angiogenesis, stabilize vessels and reduce leakage and inflammation52,69 (Figure 2e). In order to minimize peripheral activity and abolish FcγR engagement, it was designed with an engineered Fc portion with Triple A FcRn and P329G LALA mutations.52
Notably, bispecificity is a major benefit when considering intraocular administration. For the TNF ligand inhibitor ozoralizumab, it should be noted that functionally, this nanobody construct functions like a monospecific antibody blocking TNF, with the second single domain specificity solely required to enable IgG-like pharmacokinetics by binding to HSA in the absence of an Fc portion, enabling FcRn recycling41 (Figure 2f).
Outlook for the future
In this decade, 11 bsAbs have already been approved, and the approval of additional bsAbs with medical practice-changing potential can be expected in the coming years. In oncology, the development of differentiated dual RTK signaling inhibitors with bsAb co-targeting different cell surface receptors remains an area of active and advanced clinical research. Selected examples are: EGFR/LGR5 like petosemtamab,70 HER2/HER3 like zenocutuzumab71 or bi-paratopic bsAbs targeting HER2 like zanidatamab72 which is currently under regulatory review in the US.73–75 Another very important area to watch in this context is the use of bsAbs for the generation of bispecific antibody-drug conjugates (bsADCs),with several bsADCs being investigated in advanced clinical trials,76–78 including bi-paratopic monospecific bsAbs targeting HER2 like zanidatamab zovodotin (ZW49) or c-MET with REGN5093.79
The development of TCEs for the treatment of different hematological malignancies will remain a major area of research in the field of synthetic immunity approaches, as this class of therapeutics already has proven its benefit. To date, the asymmetric 1 + 1 TCEs odronextamab (CD20/CD3ε)80 and linvoseltamab (BCMA/CD3ε) based on Regeneron’s VelociBi technology are currently under regulatory review that may result in approval in 2024, and various additional TCEs are in advanced clinical development in NHL, multiple myeloma and AML.75 Importantly, emerging data support the idea that TCEs may in the future also find broader application in solid tumors. In fact, recently promising clinical data have been published for Amgen’s 1 + 1 DLL3/CD3ε Fc-BiTE tarlatamab for the treatment of R/R small cell lung cancer,81,82 which is currently under regulatory review, and for Xencor/Amgen’s 2 + 1 STEAP1/CD3ε XmAb xaluritamig for the treatment of R/R prostate cancer.83,84 These data support the view that solid tumor targets that are sufficiently tumor specific to achieve potent anti-tumor efficacy while limiting on-target off-tumor toxicity are available. In this context, tumor activation mechanisms like protease activation aim to increase the therapeutic window of solid tumor-directed TCEs.85–89 Based on the mechanism of action of TCEs that provide the TCR signal 1 to T cells, rapid clinical adoption of CD28- or 4-1BB/CD137-targeted costimulatory bsAbs to provide the signal 2 to T cells, resulting in sustained and more durable T cell responses,90,91 is now occurring.
In the field of boosting endogenous or preexisting immunity, another major area of research remains the development of dual-targeted checkpoint inhibitory bsAbs targeting, for example, PD-1 and CTLA-492,93 or LAG-3 (tebotelimab).94 Several of these dual checkpoint inhibitors are currently in advanced clinical trials and results will show whether they are superior in terms of efficacy and/or safety compared to the respective combinations of PD-1/PD-L1 mAbs with CTLA-4 or LAG-3 mAbs. Notably, the dual-targeted PD-1/VEGF inhibitory bsAb ivonescimab is currently under regulatory review by the National Medicinal Product Administration in China for treatment of NSCLC.75,95
Finally, given the versatility of bsAbs and the potential to mediate completely novel MOAs, the field of bsAbs is poised to see novel emerging approaches and candidates enter the clinic, hopefully providing pivotal data in the years to come, both in oncology and in non-oncology indications, including applications in infection/virology, autoimmunity, metabolism, neurology and ophthalmology. These novel concepts include different approaches as described recently,5 including the development of: 1) effector cell engagers different from TCEs, engaging, e.g., myeloid, NK or γδ-T cells,96–98 2) in situ assembly concepts to specifically activate bsAbs on dual target-expressing cells99,100 or in the tumor microenvironment,101 3) PROTAC-like approaches resulting in internalization and degradation of membrane proteins,102 4) antibody-based cytokine mimetics to trigger cytokine receptors,103,104 and 5) unique solutions for delivery of bsAbs beyond barriers such as the blood-brain-barrier,105 which may have applications for the treatment of neurodegenerative and other diseases.106
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
MS declares employment and patents with Roche, CK declares employment, patents/royalties and stock ownership with Roche.
References
- 1.Labrijn AF, Janmaat ML, Reichert JM, Parren P.. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov. 2019;18(8):585–9. doi: 10.1038/s41573-019-0028-1. PMID: 31175342. [DOI] [PubMed] [Google Scholar]
- 2.Brinkmann U, Kontermann RE. The making of bispecific antibodies. MAbs. 2017;9(2):182–212. doi: 10.1080/19420862.2016.1268307. PMID: 28071970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brinkmann U, Kontermann RE. Bispecific antibodies. Science. 2021;372(6545):916–17. doi: 10.1126/science.abg1209. PMID: 34045345. [DOI] [PubMed] [Google Scholar]
- 4.Kontermann RE, Brinkmann U. Bispecific antibodies. Drug Discov Today. 2015;20(7):838–47. doi: 10.1016/j.drudis.2015.02.008. PMID: 25728220. [DOI] [PubMed] [Google Scholar]
- 5.Klein C, Brinkmann U, Reichert JM, Kontermann RE. The present and future of bispecific antibodies for cancer therapy. Nat Rev Drug Discov. 2024. in press. doi: 10.1038/s41573-024-00896-6. [DOI] [PubMed] [Google Scholar]
- 6.Seimetz D, Lindhofer H, Bokemeyer C. Development and approval of the trifunctional antibody catumaxomab (anti-EpCAM×anti-CD3) as a targeted cancer immunotherapy. Cancer Treat Rev. 2010;36(6):458–67. doi: 10.1016/j.ctrv.2010.03.001. PMID: 20347527. [DOI] [PubMed] [Google Scholar]
- 7.Sanford M. Blinatumomab: first global approval. Drugs. 2015;75(3):321–27. doi: 10.1007/s40265-015-0356-3. PMID: 25637301. [DOI] [PubMed] [Google Scholar]
- 8.Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, Noppeney R, Viardot A, Hess G, Schuler M. et al. Tumor regression in cancer patients by very low doses of a T cell–engaging antibody. Science. 2008;321(5891):974–77. doi: 10.1126/science.1158545. PMID: 18703743. [DOI] [PubMed] [Google Scholar]
- 9.Kantarjian H, Stein A, Gokbuget N, Fielding AK, Schuh AC, Ribera JM, Wei A, Dombret H, Foa R, Bassan R. et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836–47. doi: 10.1056/NEJMoa1609783. PMID: 28249141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott LJ, Kim ES. Emicizumab-kxwh: First Global Approval. Drugs. 2018;78(2):269–74. doi: 10.1007/s40265-018-0861-2. PMID: 29357074. [DOI] [PubMed] [Google Scholar]
- 11.Mahlangu J, Oldenburg J, Paz-Priel I, Negrier C, Niggli M, Mancuso ME, Schmitt C, Jimenez-Yuste V, Kempton C, Dhalluin C. et al. Emicizumab prophylaxis in patients who have hemophilia a without inhibitors. N Engl J Med. 2018;379(9):811–22. doi: 10.1056/NEJMoa1803550. PMID: 30157389. [DOI] [PubMed] [Google Scholar]
- 12.Syed YY. Amivantamab: First Approval. Drugs. 2021;81(11):1349–53. doi: 10.1007/s40265-021-01561-7. PMID: 34292533. [DOI] [PubMed] [Google Scholar]
- 13.Zhou C, Tang KJ, Cho BC, Liu B, Paz-Ares L, Cheng S, Kitazono S, Thiagarajan M, Goldman JW, Sabari JK. et al. Amivantamab plus chemotherapy in NSCLC with EGFR Exon 20 insertions. N Engl J Med. 2023;389(22):2039–51. doi: 10.1056/NEJMoa2306441. PMID: 37870976. [DOI] [PubMed] [Google Scholar]
- 14.Dhillon S. Tebentafusp: First Approval. Drugs. 2022;82(6):703–10. doi: 10.1007/s40265-022-01704-4. PMID: 35364798. [DOI] [PubMed] [Google Scholar]
- 15.Nathan P, Hassel JC, Rutkowski P, Baurain JF, Butler MO, Schlaak M, Sullivan RJ, Ochsenreither S, Dummer R, Kirkwood JM. et al. Overall survival benefit with Tebentafusp in metastatic uveal melanoma. N Engl J Med. 2021;385(13):1196–206. doi: 10.1056/NEJMoa2103485. PMID: 34551229. [DOI] [PubMed] [Google Scholar]
- 16.Kang C. Mosunetuzumab: First Approval. Drugs. 2022;82(11):1229–34. doi: 10.1007/s40265-022-01749-5. PMID: 35947358. [DOI] [PubMed] [Google Scholar]
- 17.Budde LE, Sehn LH, Matasar M, Schuster SJ, Assouline S, Giri P, Kuruvilla J, Canales M, Dietrich S, Fay K. et al. Safety and efficacy of mosunetuzumab, a bispecific antibody, in patients with relapsed or refractory follicular lymphoma: a single-arm, multicentre, phase 2 study. Lancet Oncol. 2022;23:1055–65. doi: 10.1016/S1470-2045(22)00335-7. PMID: 35803286. [DOI] [PubMed] [Google Scholar]
- 18.Keam SJ. Cadonilimab: First Approval. Drugs. 2022;82(12):1333–39. doi: 10.1007/s40265-022-01761-9. PMID: 35986837. [DOI] [PubMed] [Google Scholar]
- 19.Frentzas S, Gan HK, Cosman R, Coward J, Tran B, Millward M, Zhou Y, Wang W, Xia D, Wang ZM. et al. A phase 1a/1b first-in-human study (COMPASSION-01) evaluating cadonilimab in patients with advanced solid tumors. Cell Rep Med. 2023;4(11):101242. doi: 10.1016/j.xcrm.2023.101242. PMID: 37852261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang C. Teclistamab: First Approval. Drugs. 2022;82(16):1613–19. doi: 10.1007/s40265-022-01793-1. PMID: 36352205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hindie E. Teclistamab in Relapsed or Refractory Multiple Myeloma. N Engl J Med. 2022;387:1721. doi: 10.1056/NEJMc2211969. PMID: 36322859. [DOI] [PubMed] [Google Scholar]
- 22.Shirley M. Glofitamab: First Approval. Drugs. 2023;83(10):935–41. doi: 10.1007/s40265-023-01894-5. PMID: 37285013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dickinson MJ, Carlo-Stella C, Morschhauser F, Bachy E, Corradini P, Iacoboni G, Khan C, Wrobel T, Offner F, Trneny M. et al. Glofitamab for relapsed or refractory diffuse large B-Cell lymphoma. N Engl J Med. 2022;387(24):2220–31. doi: 10.1056/NEJMoa2206913. PMID: 36507690. [DOI] [PubMed] [Google Scholar]
- 24.Frampton JE. Epcoritamab: First Approval. Drugs. 2023;83(14):1331–40. doi: 10.1007/s40265-023-01930-4. PMID: 37597091. [DOI] [PubMed] [Google Scholar]
- 25.Thieblemont C, Phillips T, Ghesquieres H, Cheah CY, Clausen MR, Cunningham D, Do YR, Feldman T, Gasiorowski R, Jurczak W. et al. Epcoritamab, a Novel, Subcutaneous CD3xCD20 Bispecific T-Cell–Engaging Antibody, in Relapsed or Refractory Large B-Cell Lymphoma: Dose Expansion in a Phase I/II Trial. JCO. 2022;41(12):2238–47. doi: 10.1200/JCO.22.01725. PMID: 36548927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chari A, Minnema MC, Berdeja JG, Oriol A, van de Donk N, Rodriguez-Otero P, Askari E, Mateos MV, Costa LJ, Caers J. et al. Talquetamab, a T-Cell–Redirecting GPRC5D Bispecific Antibody for Multiple Myeloma. N Engl J Med. 2022;387(24):2232–44. doi: 10.1056/NEJMoa2204591. PMID: 36507686. [DOI] [PubMed] [Google Scholar]
- 27.Keam SJ. Talquetamab: First Approval. Drugs. 2023;83(15):1439–45. doi: 10.1007/s40265-023-01945-x. PMID: 37792138. [DOI] [PubMed] [Google Scholar]
- 28.Lesokhin AM, Tomasson MH, Arnulf B, Bahlis NJ, Miles Prince H, Niesvizky R, Rodrίguez-Otero P, Martinez-Lopez J, Koehne G, Touzeau C. et al. Elranatamab in relapsed or refractory multiple myeloma: phase 2 MagnetisMM-3 trial results. Nat Med. 2023;29(9):2259–67. doi: 10.1038/s41591-023-02528-9. PMID: 37582952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dhillon S. Elranatamab: First Approval. Drugs. 2023;83(17):1621–27. doi: 10.1007/s40265-023-01954-w. PMID: 37924427. [DOI] [PubMed] [Google Scholar]
- 30.Heier JS, Khanani AM, Quezada Ruiz C, Basu K, Ferrone PJ, Brittain C, Figueroa MS, Lin H, Holz FG, Patel V. et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet. 2022;399(10326):729–40. doi: 10.1016/S0140-6736(22)00010-1. PMID: 35085502. [DOI] [PubMed] [Google Scholar]
- 31.Shirley M. Faricimab: First Approval. Drugs. 2022;82(7):825–30. doi: 10.1007/s40265-022-01713-3. PMID: 35474059. [DOI] [PubMed] [Google Scholar]
- 32.Wykoff CC, Abreu F, Adamis AP, Basu K, Eichenbaum DA, Haskova Z, Lin H, Loewenstein A, Mohan S, Pearce IA. et al. Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): two randomised, double-masked, phase 3 trials. Lancet. 2022;399(10326):741–55. doi: 10.1016/S0140-6736(22)00018-6. PMID: 35085503. [DOI] [PubMed] [Google Scholar]
- 33.Hattenbach LO, Abreu F, Arrisi P, Basu K, Danzig CJ, Guymer R, Haskova Z, Heier JS, Kotecha A, Liu Y. et al. BALATON and COMINO: phase III randomized clinical trials of Faricimab for retinal vein occlusion: study design and rationale. Ophthalmol Sci. 2023;3:100302. doi: 10.1016/j.xops.2023.100302. PMID: 37810589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka Y, Kawanishi M, Nakanishi M, Yamasaki H, Takeuchi T. Efficacy and safety of the anti-TNF multivalent NANOBODY® compound ozoralizumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: a 52-week result of a phase II/III study (OHZORA trial). Mod Rheumatol. 2023;33(5):883–90. doi: 10.1093/mr/roac119. PMID: 36197757. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka Y, Kawanishi M, Nakanishi M, Yamasaki H, Takeuchi T. Efficacy and safety of anti-TNF multivalent NANOBODY® compound ‘ozoralizumab’ without methotrexate co-administration in patients with active rheumatoid arthritis: a 52-week result of phase III, randomised, open-label trial (NATSUZORA trial). Mod Rheumatol. 2023;33(5):875–82. doi: 10.1093/mr/roac126. PMID: 36201360. [DOI] [PubMed] [Google Scholar]
- 36.Keam SJ. Ozoralizumab: first approval. Drugs. 2023;83(1):87–92. doi: 10.1007/s40265-022-01821-0. PMID: 36509938. [DOI] [PubMed] [Google Scholar]
- 37.Dreier T, Baeuerle PA, Fichtner I, Grun M, Schlereth B, Lorenczewski G, Kufer P, Lutterbuse R, Riethmuller G, Gjorstrup P. et al. T cell costimulus-independent and very efficacious inhibition of tumor growth in mice bearing subcutaneous or leukemic human B cell lymphoma xenografts by a CD19-/CD3- bispecific single-chain antibody construct. J Immunol. 2003;170(8):4397–402. doi: 10.4049/jimmunol.170.8.4397. PMID: 12682277. [DOI] [PubMed] [Google Scholar]
- 38.Kufer P, Lutterbuse R, Baeuerle PA. A revival of bispecific antibodies. Trends Biotechnol. 2004;22:238–44. doi: 10.1016/j.tibtech.2004.03.006. PMID: 15109810. [DOI] [PubMed] [Google Scholar]
- 39.Berman DM, Bell JI. Redirecting polyclonal T cells against cancer with soluble T cell receptors. Clin Cancer Res. 2022;29(4):697–704. doi: 10.1158/1078-0432.CCR-22-0028. PMID: 36255733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liddy N, Bossi G, Adams KJ, Lissina A, Mahon TM, Hassan NJ, Gavarret J, Bianchi FC, Pumphrey NJ, Ladell K. et al. Monoclonal TCR-redirected tumor cell killing. Nat Med. 2012;18(6):980–87. doi: 10.1038/nm.2764. PMID: 22561687. [DOI] [PubMed] [Google Scholar]
- 41.Ishiwatari-Ogata C, Kyuuma M, Ogata H, Yamakawa M, Iwata K, Ochi M, Hori M, Miyata N, Fujii FY. Ozoralizumab, a humanized anti-TNFα NANOBODY® compound, exhibits efficacy not only at the onset of Arthritis in a human TNF transgenic mouse but also during secondary failure of administration of an Anti-TNFα IgG. Front Immunol. 2022;13:853008. doi: 10.3389/fimmu.2022.853008. PMID: 35273620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neijssen J, Cardoso RMF, Chevalier KM, Wiegman L, Valerius T, Anderson GM, Moores SL, Schuurman J, Parren P, Strohl WR. et al. Discovery of amivantamab (JNJ-61186372), a bispecific antibody targeting EGFR and MET. J Biol Chem. 2021;296:100641. doi: 10.1016/j.jbc.2021.100641. PMID: 33839159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pillarisetti K, Powers G, Luistro L, Babich A, Baldwin E, Li Y, Zhang X, Mendonca M, Majewski N, Nanjunda R. et al. Teclistamab is an active T cell–redirecting bispecific antibody against B-cell maturation antigen for multiple myeloma. Blood Adv. 2020;4(18):4538–49. doi: 10.1182/bloodadvances.2020002393. PMID: 32956453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Engelberts PJ, Hiemstra IH, de Jong B, Schuurhuis DH, Meesters J, Beltran Hernandez I, Oostindie SC, Neijssen J, van den Brink EN, Horbach GJ. et al. DuoBody-CD3xCD20 induces potent T-cell-mediated killing of malignant B cells in preclinical models and provides opportunities for subcutaneous dosing. EBioMedicine. 2020;52:102625. doi: 10.1016/j.ebiom.2019.102625. PMID: 31981978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verkleij CPM, Broekmans MEC, van Duin M, Frerichs KA, Kuiper R, de Jonge AV, Kaiser M, Morgan G, Axel A, Boominathan R. et al. Preclinical activity and determinants of response of the GPRC5DxCD3 bispecific antibody talquetamab in multiple myeloma. Blood Adv. 2021;5:2196–215. doi: 10.1182/bloodadvances.2020003805. PMID: 33890981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strop P, Ho WH, Boustany LM, Abdiche YN, Lindquist KC, Farias SE, Rickert M, Appah CT, Pascua E, Radcliffe T. et al. Generating bispecific human IgG1 and IgG2 antibodies from any antibody pair. J Mol Biol. 2012;420:204–19. doi: 10.1016/j.jmb.2012.04.020. PMID: 22543237. [DOI] [PubMed] [Google Scholar]
- 47.Panowski SH, Kuo TC, Zhang Y, Chen A, Geng T, Aschenbrenner L, Kamperschroer C, Pascua E, Chen W, Delaria K. et al. Preclinical efficacy and safety comparison of CD3 bispecific and ADC modalities targeting BCMA for the treatment of multiple myeloma. Mol Cancer Ther. 2019;18:2008–20. doi: 10.1158/1535-7163.MCT-19-0007. PMID: 31434693. [DOI] [PubMed] [Google Scholar]
- 48.Igawa T. Next generation antibody therapeutics using bispecific antibody technology. Yakugaku Zasshi. 2017;137:831–36. doi: 10.1248/yakushi.16-00252-3. PMID: 28674296. [DOI] [PubMed] [Google Scholar]
- 49.Kitazawa T, Igawa T, Sampei Z, Muto A, Kojima T, Soeda T, Yoshihashi K, Okuyama-Nishida Y, Saito H, Tsunoda H. et al. A bispecific antibody to factors IXa and X restores factor VIII hemostatic activity in a hemophilia a model. Nat Med. 2012;18:1570–74. doi: 10.1038/nm.2942. PMID: 23023498. [DOI] [PubMed] [Google Scholar]
- 50.Sampei Z, Igawa T, Soeda T, Okuyama-Nishida Y, Moriyama C, Wakabayashi T, Tanaka E, Muto A, Kojima T, Kitazawa T. et al. Identification and multidimensional optimization of an asymmetric bispecific IgG antibody mimicking the function of factor VIII cofactor activity. PloS One. 2013;8(2):e57479. doi: 10.1371/journal.pone.0057479. PMID: 23468998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun LL, Ellerman D, Mathieu M, Hristopoulos M, Chen X, Li Y, Yan X, Clark R, Reyes A, Stefanich E. et al. Anti-CD20/CD3 T cell–dependent bispecific antibody for the treatment of B cell malignancies. Sci Transl Med. 2015;7(287):287ra270. doi: 10.1126/scitranslmed.aaa4802. PMID: 25972002. [DOI] [PubMed] [Google Scholar]
- 52.Regula JT, Lundh von Leithner P, Foxton R, Barathi VA, Gemmy Cheung CM, Bo Tun SB, Wey YS, Iwata D, Dostalek M, Moelleken J. et al. Targeting key angiogenic pathways with a bispecific CrossMAb optimized for neovascular eye diseases. EMBO Mol Med. 2017;9(7):985. doi: 10.15252/emmm.201707895. PMID: 28533211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bacac M, Colombetti S, Herter S, Sam J, Perro M, Chen S, Bianchi R, Richard M, Schoenle A, Nicolini V. et al. CD20-TCB with obinutuzumab pretreatment as next-generation treatment of hematologic malignancies. Clin Cancer Res. 2018;24:4785–97. doi: 10.1158/1078-0432.CCR-18-0455. PMID: 29716920. [DOI] [PubMed] [Google Scholar]
- 54.Pang X, Huang Z, Zhong T, Zhang P, Wang ZM, Xia M, Li B. Cadonilimab, a tetravalent PD-1/CTLA-4 bispecific antibody with trans-binding and enhanced target binding avidity. MAbs. 2023;15(1):2180794. doi: 10.1080/19420862.2023.2180794. PMID: 36872527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gramer MJ, van den Bremer ET, van Kampen MD, Kundu A, Kopfmann P, Etter E, Stinehelfer D, Long J, Lannom T, Noordergraaf EH. et al. Production of stable bispecific IgG1 by controlled Fab-arm exchange: scalability from bench to large-scale manufacturing by application of standard approaches. MAbs. 2013;5(6):962–73. doi: 10.4161/mabs.26233. PMID: 23995617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Labrijn AF, Meesters JI, de Goeij BE, van den Bremer ET, Neijssen J, van Kampen MD, Strumane K, Verploegen S, Kundu A, Gramer MJ. et al. Efficient generation of stable bispecific IgG1 by controlled Fab-arm exchange. Proc Natl Acad Sci U S A. 2013;110:5145–50. doi: 10.1073/pnas.1220145110. PMID: 23479652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ridgway JB, Presta LG, Carter P. ‘Knobs-into-holes’ engineering of antibody CH3 domains for heavy chain heterodimerization. Protein Eng. 1996;9(7):617–21. doi: 10.1093/protein/9.7.617. PMID: 8844834. [DOI] [PubMed] [Google Scholar]
- 58.Zhu Z, Presta LG, Zapata G, Carter P. Remodeling domain interfaces to enhance heterodimer formation. Protein Sci. 1997;6:781–88. doi: 10.1002/pro.5560060404. PMID: 9098887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Regula JT, Imhof-Jung S, Molhoj M, Benz J, Ehler A, Bujotzek A, Schaefer W, Klein C. Variable heavy–variable light domain and Fab-arm CrossMabs with charged residue exchanges to enforce correct light chain assembly. Protein Eng Des Sel. 2018;31(7–8):289–99. doi: 10.1093/protein/gzy021. PMID: 30169707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schaefer W, Regula JT, Bahner M, Schanzer J, Croasdale R, Durr H, Gassner C, Georges G, Kettenberger H, Imhof-Jung S. et al. Immunoglobulin domain crossover as a generic approach for the production of bispecific IgG antibodies. Proc Natl Acad Sci U S A. 2011;108:11187–92. doi: 10.1073/pnas.1019002108. PMID: 21690412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Surowka M, Schaefer W, Klein C. Ten Years in the making: application of CrossMab technology for the development of therapeutic bispecific antibodies and antibody fusion proteins. MAbs. 2021;13(1):1967714. doi: 10.1080/19420862.2021.1967714. PMID: 34491877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van de Donk N, Zweegman S. T-cell-engaging bispecific antibodies in cancer. Lancet. 2023;402(10396):142–58. doi: 10.1016/S0140-6736(23)00521-4. PMID: 37271153. [DOI] [PubMed] [Google Scholar]
- 63.Arvedson T, Bailis JM, Britten CD, Klinger M, Nagorsen D, Coxon A, Egen JG, Martin F. Targeting solid tumors with bispecific T cell engager immune therapy. Annu Rev Cancer Biol. 2022;6(1):17–34. doi: 10.1146/annurev-cancerbio-070620-104325. PMID: WOS:000789891800002. [DOI] [Google Scholar]
- 64.Lenting PJ, Denis CV, Christophe OD. Emicizumab, a bispecific antibody recognizing coagulation factors IX and X: how does it actually compare to factor VIII? Blood. 2017;130(23):2463–68. doi: 10.1182/blood-2017-08-801662. PMID: 29042366. [DOI] [PubMed] [Google Scholar]
- 65.Fauvel B, Yasri A. Antibodies directed against receptor tyrosine kinases: current and future strategies to fight cancer. MAbs. 2014;6(4):838–51. doi: 10.4161/mabs.29089. PMID: 24859229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sharma P, Goswami S, Raychaudhuri D, Siddiqui BA, Singh P, Nagarajan A, Liu J, Subudhi SK, Poon C, Gant KL. et al. Immune checkpoint therapy—current perspectives and future directions. Cell. 2023;186(8):1652–69. doi: 10.1016/j.cell.2023.03.006. PMID: 37059068. [DOI] [PubMed] [Google Scholar]
- 67.Cao Y, Langer R, Ferrara N. Targeting angiogenesis in oncology, ophthalmology and beyond. Nat Rev Drug Discov. 2023;22(6):476–95. doi: 10.1038/s41573-023-00671-z. PMID: 37041221. [DOI] [PubMed] [Google Scholar]
- 68.Sharma A, Kumar N, Kuppermann BD, Bandello F, Loewenstein A. Faricimab: expanding horizon beyond VEGF. Eye (Lond). 2020;34(5):802–04. doi: 10.1038/s41433-019-0670-1. PMID: 31695160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Foxton RH, Uhles S, Gruner S, Revelant F, Ullmer C. Efficacy of simultaneous VEGF-A/ANG-2 neutralization in suppressing spontaneous choroidal neovascularization. EMBO Mol Med. 2019;11(5). doi: 10.15252/emmm.201810204. PMID: 31040126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Herpers B, Eppink B, James MI, Cortina C, Canellas-Socias A, Boj SF, Hernando-Momblona X, Glodzik D, Roovers RC, van de Wetering M. et al. Functional patient-derived organoid screenings identify MCLA-158 as a therapeutic EGFR × LGR5 bispecific antibody with efficacy in epithelial tumors. Nat Cancer. 2022;3(4):418–36. doi: 10.1038/s43018-022-00359-0. PMID: 35469014. [DOI] [PubMed] [Google Scholar]
- 71.Schram AM, Odintsov I, Espinosa-Cotton M, Khodos I, Sisso WJ, Mattar MS, Lui AJW, Vojnic M, Shameem SH, Chauhan T. et al. Zenocutuzumab, a HER2xHER3 Bispecific Antibody, Is Effective Therapy for Tumors Driven by NRG1 Gene Rearrangements. Cancer Discov. 2022;12:1233–47. doi: 10.1158/2159-8290.CD-21-1119. PMID: 35135829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weisser NE, Sanches M, Escobar-Cabrera E, O’Toole J, Whalen E, Chan PWY, Wickman G, Abraham L, Choi K, Harbourne B. et al. An anti-HER2 biparatopic antibody that induces unique HER2 clustering and complement-dependent cytotoxicity. Nat Commun. 2023;14(1):1394. doi: 10.1038/s41467-023-37029-3. PMID: 36914633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meric-Bernstam F, Beeram M, Hamilton E, Oh DY, Hanna DL, Kang YK, Elimova E, Chaves J, Goodwin R, Lee J. et al. Zanidatamab, a novel bispecific antibody, for the treatment of locally advanced or metastatic HER2-expressing or HER2-amplified cancers: a phase 1, dose-escalation and expansion study. Lancet Oncol. 2022;23:1558–70. doi: 10.1016/S1470-2045(22)00621-0. PMID: 36400106. [DOI] [PubMed] [Google Scholar]
- 74.Harding JJ, Fan J, Oh DY, Choi HJ, Kim JW, Chang HM, Bao L, Sun HC, Macarulla T, Xie F. et al. Zanidatamab for HER2-amplified, unresectable, locally advanced or metastatic biliary tract cancer (HERIZON-BTC-01): a multicentre, single-arm, phase 2b study. Lancet Oncol. 2023;24:772–82. doi: 10.1016/S1470-2045(23)00242-5. PMID: 37276871. [DOI] [PubMed] [Google Scholar]
- 75.Crescioli S, Kaplon H, Chenoweth A, Wang L, Visweswaraiah J, Reichert JM. Antibodies to watch in 2024. MAbs. 2024;16(1):2297450. doi: 10.1080/19420862.2023.2297450. PMID: 38178784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dumontet C, Reichert JM, Senter PD, Lambert JM, Beck A. Antibody–drug conjugates come of age in oncology. Nat Rev Drug Discov. 2023;22(8):641–61. doi: 10.1038/s41573-023-00709-2. PMID: 37308581. [DOI] [PubMed] [Google Scholar]
- 77.Tsuchikama K, Anami Y, Ha SYY, Yamazaki CM. Exploring the next generation of antibody-drug conjugates. Nat Rev Clin Oncol. 2024. doi: 10.1038/s41571-023-00850-2. PMID: 38191923. [DOI] [PubMed] [Google Scholar]
- 78.Gu Y, Wang Z, Wang Y. Bispecific antibody drug conjugates: Making 1+1>2. Acta Pharm Sin B. 2024. doi: 10.1016/j.apsb.2024.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Perez Bay AE, Faulkner D, DaSilva JO, Young TM, Yang K, Giurleo JT, Ma D, Delfino FJ, Olson WC, Thurston G. et al. A bispecific METxMET antibody–drug conjugate with cleavable linker is processed in recycling and late endosomes. Mol Cancer Ther. 2023;22(3):357–70. doi: 10.1158/1535-7163.MCT-22-0414. PMID: 36861363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bannerji R, Arnason JE, Advani RH, Brown JR, Allan JN, Ansell SM, Barnes JA, O’Brien SM, Chavez JC, Duell J. et al. Odronextamab, a human CD20×CD3 bispecific antibody in patients with CD20-positive B-cell malignancies (ELM-1): results from the relapsed or refractory non-Hodgkin lymphoma cohort in a single-arm, multicentre, phase 1 trial. Lancet Haematol. 2022;9(5):e327–39. doi: 10.1016/S2352-3026(22)00072-2. PMID: 35366963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ahn MJ, Cho BC, Felip E, Korantzis I, Ohashi K, Majem M, Juan-Vidal O, Handzhiev S, Izumi H, Lee JS. et al. Tarlatamab for patients with Previously treated small-cell lung cancer. N Engl J Med. 2023;389(22):2063–75. doi: 10.1056/NEJMoa2307980. PMID: 37861218. [DOI] [PubMed] [Google Scholar]
- 82.Paz-Ares L, Champiat S, Lai WV, Izumi H, Govindan R, Boyer M, Hummel HD, Borghaei H, Johnson ML, Steeghs N. et al. Tarlatamab, a first-in-class DLL3-targeted bispecific T cell engager, in recurrent small-cell lung cancer: an open-label, phase 1 study. JCO. 2023;41(16):2893–903. doi: 10.1200/JCO.22.02823. PMID: 36689692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kelly WK, Danila DC, Lin CC, Lee JL, Matsubara N, Ward PJ, Armstrong AJ, Pook D, Kim M, Dorff TB. et al. Xaluritamig, a STEAP1 × CD3 XmAb 2+1 immune therapy for metastatic castration-resistant prostate cancer: results from dose exploration in a first-in-human study. Cancer Discov. 2023;14(1):OF1–14. doi: 10.1158/2159-8290.CD-23-0964. PMID: 37861461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nolan-Stevaux O, Li C, Liang L, Zhan J, Estrada J, Osgood T, Li F, Zhang H, Case R, Murawsky CM. et al. AMG 509 (Xaluritamig), an Anti-STEAP1 XmAb 2+1 T-cell Redirecting Immune Therapy with Avidity-Dependent Activity Against Prostate Cancer. Cancer Discov. 2023. doi: 10.1158/2159-8290.CD-23-0984. PMID: 37861452. [DOI] [PubMed] [Google Scholar]
- 85.Panchal A, Seto P, Wall R, Hillier BJ, Zhu Y, Krakow J, Datt A, Pongo E, Bagheri A, Chen TT. et al. COBRA™: a highly potent conditionally active T cell engager engineered for the treatment of solid tumors. MAbs. 2020;12(1):1792130. doi: 10.1080/19420862.2020.1792130. PMID: 32684124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Geiger M, Stubenrauch KG, Sam J, Richter WF, Jordan G, Eckmann J, Hage C, Nicolini V, Freimoser-Grundschober A, Ritter M. et al. Protease-activation using anti-idiotypic masks enables tumor specificity of a folate receptor 1-T cell bispecific antibody. Nat Commun. 2020;11(1):3196. doi: 10.1038/s41467-020-16838-w. PMID: 32581215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dettling DE, Kwok E, Quach L, Datt A, Degenhardt JD, Panchal A, Seto P, Krakow JL, Wall R, Hillier BJ. et al. Regression of EGFR positive established solid tumors in mice with the conditionally active T cell engager TAK-186. J Immunother Cancer. 2022;10(6):e004336. doi: 10.1136/jitc-2021-004336. PMID: 35728872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boustany LM, LaPorte SL, Wong L, White C, Vinod V, Shen J, Yu W, Koditek D, Winter MB, Moore SJ. et al. A probody T cell–engaging bispecific antibody targeting EGFR and CD3 inhibits colon cancer growth with limited toxicity. Cancer Res. 2022;82(22):4288–98. doi: 10.1158/0008-5472.CAN-21-2483. PMID: 36112781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cattaruzza F, Nazeer A, To M, Hammond M, Koski C, Liu LY, Pete Yeung V, Rennerfeldt DA, Henkensiefken A, Fox M. et al. Precision-activated T-cell engagers targeting HER2 or EGFR and CD3 mitigate on-target, off-tumor toxicity for immunotherapy in solid tumors. Nat Cancer. 2023;4(4):485–501. doi: 10.1038/s43018-023-00536-9. PMID: 36997747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Claus C, Ferrara C, Xu W, Sam J, Lang S, Uhlenbrock F, Albrecht R, Herter S, Schlenker R, Husser T. et al. Tumor-targeted 4-1BB agonists for combination with T cell bispecific antibodies as off-the-shelf therapy. Sci Transl Med. 2019;11(496):11. doi: 10.1126/scitranslmed.aav5989. PMID: 31189721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Skokos D, Waite JC, Haber L, Crawford A, Hermann A, Ullman E, Slim R, Godin S, Ajithdoss D, Ye X. et al. A class of costimulatory CD28-bispecific antibodies that enhance the antitumor activity of CD3-bispecific antibodies. Sci Transl Med. 2020;12(525):12. doi: 10.1126/scitranslmed.aaw7888. PMID: 31915305. [DOI] [PubMed] [Google Scholar]
- 92.Berezhnoy A, Sumrow BJ, Stahl K, Shah K, Liu D, Li J, Hao SS, De Costa A, Kaul S, Bendell J. et al. Development and preliminary clinical activity of PD-1-Guided CTLA-4 blocking bispecific DART molecule. Cell Rep Med. 2020;1:100163. doi: 10.1016/j.xcrm.2020.100163. PMID: 33377134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dovedi SJ, Elder MJ, Yang C, Sitnikova SI, Irving L, Hansen A, Hair J, Jones DC, Hasani S, Wang B. et al. Design and efficacy of a monovalent bispecific PD-1/CTLA4 antibody that enhances CTLA4 blockade on PD-1(+) activated T cells. Cancer Discov. 2021;11(5):1100–17. doi: 10.1158/2159-8290.CD-20-1445. PMID: 33419761. [DOI] [PubMed] [Google Scholar]
- 94.Luke JJ, Patel MR, Blumenschein GR, Hamilton E, Chmielowski B, Ulahannan SV, Connolly RM, Santa-Maria CA, Wang J, Bahadur SW. et al. The PD-1- and LAG-3-targeting bispecific molecule tebotelimab in solid tumors and hematologic cancers: a phase 1 trial. Nat Med. 2023;29(11):2814–24. doi: 10.1038/s41591-023-02593-0. PMID: 37857711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang L, Luo Y, Ren S, Zhang Z, Xiong A, Su C, Zhou J, Yu X, Hu Y, Zhang X. et al. A phase 1b study of ivonescimab, a programmed cell death protein-1 and vascular endothelial growth factor bispecific antibody, as first- or second-line therapy for advanced or metastatic immunotherapy-naive NSCLC. J Thorac Oncol. 2023. doi: 10.1016/j.jtho.2023.10.014. PMID: 37879536. [DOI] [PubMed] [Google Scholar]
- 96.Whalen KA, Rakhra K, Mehta NK, Steinle A, Michaelson JS, Baeuerle PA. Engaging natural killer cells for cancer therapy via NKG2D, CD16A and other receptors. MAbs. 2023;15(1):2208697. doi: 10.1080/19420862.2023.2208697. PMID: 37165468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mensurado S, Blanco-Dominguez R, Silva-Santos B. The emerging roles of γδ T cells in cancer immunotherapy. Nat Rev Clin Oncol. 2023;20(3):178–91. doi: 10.1038/s41571-022-00722-1. PMID: 36624304. [DOI] [PubMed] [Google Scholar]
- 98.Sewnath CA, Behrens LM, van Egmond M. Targeting myeloid cells with bispecific antibodies as novel immunotherapies of cancer. Expert Opin Biol Th. 2022;22(8):983–95. doi: 10.1080/14712598.2022.2098675. PMID: WOS:000828289800001. [DOI] [PubMed] [Google Scholar]
- 99.Dickopf S, Buldun C, Vasic V, Georges G, Hage C, Mayer K, Forster M, Wessels U, Stubenrauch KG, Benz J. et al. Prodrug-Activating Chain Exchange (PACE) converts targeted prodrug derivatives to functional bi- or multispecific antibodies. Biol Chem. 2022;403:495–508. doi: 10.1515/hsz-2021-0401. PMID: 35073465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Banaszek A, Bumm TGP, Nowotny B, Geis M, Jacob K, Wolfl M, Trebing J, Kucka K, Kouhestani D, Gogishvili T. et al. On-target restoration of a split T cell-engaging antibody for precision immunotherapy. Nat Commun. 2019;10(1):5387. doi: 10.1038/s41467-019-13196-0. PMID: 31772172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lucchi R, Bentanachs J, Oller-Salvia B. The masking game: design of activatable antibodies and mimetics for selective therapeutics and cell control. ACS Cent Sci. 2021;7(5):724–38. doi: 10.1021/acscentsci.0c01448. PMID: 34079893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wells JA, Kumru K. Extracellular targeted protein degradation: an emerging modality for drug discovery. Nat Rev Drug Discov. 2023;23(2):126–40. doi: 10.1038/s41573-023-00833-z. PMID: 38062152. [DOI] [PubMed] [Google Scholar]
- 103.Yen M, Ren J, Liu Q, Glassman CR, Sheahan TP, Picton LK, Moreira FR, Rustagi A, Jude KM, Zhao X. et al. Facile discovery of surrogate cytokine agonists. Cell. 2022;185(8):1414–30 e1419. doi: 10.1016/j.cell.2022.02.025. PMID: 35325595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Harris KE, Lorentsen KJ, Malik-Chaudhry HK, Loughlin K, Basappa HM, Hartstein S, Ahmil G, Allen NS, Avanzino BC, Balasubramani A. et al. A bispecific antibody agonist of the IL-2 heterodimeric receptor preferentially promotes in vivo expansion of CD8 and NK cells. Sci Rep. 2021;11(1):10592. doi: 10.1038/s41598-021-90096-8. PMID: 34011961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhao P, Zhang N, An Z. Engineering antibody and protein therapeutics to cross the blood–brain barrier. Antib Ther. 2022;5(4):311–31. doi: 10.1093/abt/tbac028. PMID: 36540309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Grimm HP, Schumacher V, Schäfer M, Imhof-Jung S, Freskgård PO, Brady K, Hofmann C, Rüger P, Schlothauer T, Göpfert U. et al. Delivery of the Brainshuttle™ amyloid-beta antibody fusion trontinemab to non-human primate brain and projected efficacious dose regimens in humans. MAbs. 2023;15(1):2261509. doi: 10.1080/19420862.2023.2261509. PMID: WOS:001084988100001. [DOI] [PMC free article] [PubMed] [Google Scholar]


