Abstract
The subgroup C human adenoviruses induce selective export of newly synthesized viral mRNA from the nucleus to the cytoplasm, with concomitant inhibition of export of the majority of cellular mRNA species. Such posttranscriptional regulation of viral and cellular gene expression in infected cells requires viral E1B and E4 proteins. To facilitate the investigation of parameters that govern selective export in adenovirus-infected cells, we constructed a marked human β-actin minigene under the control of the glucocorticoid-inducible enhancer-promoter of mouse mammary tumor virus and introduced it into the left end of the adenovirus type 5 (Ad5) genome. Transcription of this reporter gene (designated MA) as well as of a sibling, which differed only in the inclusion of a cDNA copy of the Ad2 major late tripartite leader sequence upstream of β-actin sequences (termed MtplA), in recombinant virus-infected cells was strictly dependent on the addition of dexamethasone to the medium. When transcription of the MA gene was induced during the late phase of infection, newly synthesized MA RNA entered the cytoplasm. These transcripts, which contain no viral sequences, therefore reproduce the behavior of exceptional cellular mRNA species observed when transcription of their genes is activated during the late phase of infection (U.-C. Yang, W. Huang, and S. J. Flint, J. Virol. 70:4071–4080, 1996). Unexpectedly, however, higher concentrations of newly synthesized RNA accumulated in the cytoplasm when the tripartite leader sequence was present in the reporter RNA, despite equal rates of transcription of the two reporter genes. Examination of the partitioning of both newly synthesized and steady-state populations of MA and MtplA RNAs between nuclear and cytoplasmic compartments indicated that the tripartite leader sequence did not increase RNA stability in the cytoplasm. Comparison of nuclear and cytoplasmic reporter RNA species by Northern blotting, primer extension, and reverse transcription-PCR provided no evidence for altered processing induced by the tripartite leader sequence. We therefore conclude that the tripartite leader sequence, long known to facilitate the translation of mRNAs during the late phase of adenovirus infection, can also modulate mRNA export from the nucleus.
Infection of permissive cells by human subgroup C adenoviruses such as types 2 and 5 (Ad2 and Ad5) is characterized by the production of very large quantities of viral macromolecules. The efficient expression of viral genes, which is a hallmark of the late phase of the infectious cycle, is the result of both the viral program of transcriptional regulation (see reference 72) and posttranscriptional mechanisms that ensure preferential synthesis of viral proteins.
During the late phase of infection, viral proteins are made in large quantities, while cellular protein synthesis is severely, if not completely, inhibited by 18 to 20 h after infection (2, 7, 83). Neither the stability nor the translatability of cellular mRNAs in vitro is altered by adenovirus infection (3, 75), indicating that viral mRNAs are selectively translated during the late phase of infection. The mechanisms responsible for the efficient synthesis of viral proteins while the translation of cellular mRNA is inhibited are not fully understood (69, 72). However, inhibitory alterations in components of the cellular translational machinery appear to be important. The 5′-untranslated tripartite leader sequences present in all viral mRNAs processed from major late transcripts (8, 18), which enhance translation during the late phase of infection (9, 44, 50), reduce or eliminate the requirement for the cap-binding complex e1F-4F (24). This property of the tripartite leader sequence is believed to contribute to the selective translation of viral mRNAs in infected cells, because the cap-binding protein is underphosphorylated, with concomitant inhibition of the activity of e1F-4F (41, 83; see reference 69). The phosphorylation of the α subunit of e1F-2 by the interferon-inducible DA1 kinase (63), an inhibitory modification that is partially countered by virus-associated VA1, is also likely to contribute to a translationally compromised environment in infected cells (59, 63, 70, 75; see references 51 and 72). Translation of all viral late mRNAs in this environment, but not of early mRNAs, requires the viral L4 100-kDa protein (38). This late protein is an RNA-binding protein (1, 64) whose RNA-binding activity correlates with its ability to support the efficient translation of viral late mRNA species (64). However, this protein exhibits no apparent preference for viral late mRNAs (1, 64), and the mechanism by which it distinguishes this set of mRNAs from both viral early and cellular mRNAs also present in infected cells has not been established.
Within a few hours of the onset of the late phase of adenovirus infection, export of the majority of newly synthesized cellular mRNAs from the nucleus to the cytoplasm is severely inhibited, although viral late mRNAs enter the cytoplasm efficiently (4, 7, 15, 61, 81, 82; reviewed in references 29 and 72). Such selective export of viral late mRNAs requires two early proteins, the E1B 55-kDa and E4 34-kDa proteins (4, 37, 49, 61, 77, 78), which associate within infected cells (68). The complex containing these E1B and E4 proteins appears to induce the alterations in mRNA export characteristic of the late phase of the adenovirus infectious cycle (12, 21). Other viral proteins that promote the export of specific RNA species from the nucleus, notably, the Rev and Rex proteins of the complex human retroviruses human immunodeficiency virus and human T-cell leukemia virus type 1, respectively, select their targets (unspliced and partially spliced viral RNAs) by binding to specific RNA sequences within them (e.g., the Rev-responsive element [RRE] of human immunodeficiency virus type 1 [HIV-1] RNA) (see references 20, 34, and 58). A nuclear export signal in the C-terminal domain of Rev (27) directs the export of RRE-containing RNAs, regardless of whether they have been spliced (28). The primary function of Rev appears to be to induce entry of the RNAs to which it binds into the cellular pathway by which 5S RNA-transcription factor IIIA complexes, certain snRNAs, and specific proteins that contain nuclear export signals related to that of Rev leave the nucleus (27, 31, 32). Like Rev (43, 53), the adenovirus E4 34-kDa protein shuttles between the nucleus and the cytoplasm (23). However, the mechanism by which the adenovirus E1B 55-kDa protein–E4 34-kDa protein complex induces the selective export of adenoviral mRNAs is much less well understood, in part because these proteins influence the posttranscriptional fate of a large set of viral mRNAs that contain no common sequence. Furthermore, the action of these adenovirus proteins, in contrast to that of Rev or Rex, induces inhibition of the export of most mature cellular mRNAs produced in the infected cell nucleus (see above) and of viral early mRNAs (78, 81).
Previous studies have suggested that at least two parameters are important for the export of processed mRNA from the nucleus to the cytoplasm during the late phase of adenovirus infection: the intracellular localization of replicated viral chromosomes and the activation of transcription during the late phase of infection. In Ad2-infected cells, in contrast to uninfected cells, cellular mRNAs fail to chase from a nuclear matrix-associated fraction to a pool extractable in the presence of high salt concentrations, whereas viral mRNAs are recovered in the latter nuclear fraction (22). Furthermore, the ability of viral late mRNAs to chase from a nuclear matrix-associated fraction to a soluble nuclear fraction that appears to represent an intermediate in the normal export pathway is inhibited by a mutation of the E1B 55-kDa protein that impairs the selective export of viral mRNA (49). Such observations, in conjunction with the E4 34-kDa protein-dependent localization of the E1B 55-kDa protein to nuclear viral inclusion bodies in which the viral genome is replicated and transcribed (60), suggest that these viral proteins may localize limiting cellular export factors to the intranuclear sites at which viral late genes are transcribed (48, 60, 61). This model implies that the viral DNA molecules that serve as templates for transcription during the late phase of infection occupy specialized nuclear microenvironments that determine or modulate the posttranscriptional fate of newly transcribed pre-mRNAs, that is, that they are “gated” (10). The reorganization of cellular components that participate in the splicing of both viral and cellular mRNAs, such as snRNPs and the SR family splicing protein in adenovirus-infected cells (11, 14, 62), is consistent with the view that transcription, processing, and export of viral late mRNAs are facilitated by a high degree of organization of the relevant molecular machinery within the nucleus of infected cells. Furthermore, the accumulation of spliced viral late mRNAs in enlarged interchromatin granules that contain cellular splicing components correlates with their efficient export from the nucleus (13).
On the other hand, the most straightforward version of this model, that residence of a gene in the viral chromosome is sufficient to allow selective export of its processed transcripts in adenovirus-infected cells, cannot account for the entry of some newly synthesized cellular mRNAs into the cytoplasm (54, 81). The only property common to all such exceptional cellular mRNAs identified to date is activation of their transcription during the late phase of adenovirus infection (81). Transcription of all viral genes encoding late mRNAs that are selectively exported is also specifically activated following the onset of the late phase of infection (see references 29 and 72), and the RNA products of the early E1A gene, whose transcription is not specifically stimulated during this period, are not selectively exported (81). We have therefore proposed that activation of transcription establishes the molecular couples that determine the efficient export and perhaps the processing of primary transcripts, presumably by directing activated genes (be they viral or cellular) to specialized nuclear microenvironments (81). This variation of the nuclear localization model developed by Shenk and colleagues (48, 60, 61) accounts for all the currently known properties of selective mRNA export in Ad2- or Ad5-infected cells. However, it rests on correlations among the properties of different mRNA species that do or do not leave adenovirus-infected cell nuclei. Here we report the initial results of experiments designed to allow a more rigorous assessment of the importance of the activation of transcription for selective export during the late phase of adenovirus infection, as well as systematic analysis of the parameters that govern the export efficiency of a single mRNA. Unexpectedly, these experiments identified a new parameter that can modulate export efficiency, the presence of the viral major late tripartite leader sequence in processed RNA.
MATERIALS AND METHODS
Construction of inducible human β-actin minigenes.
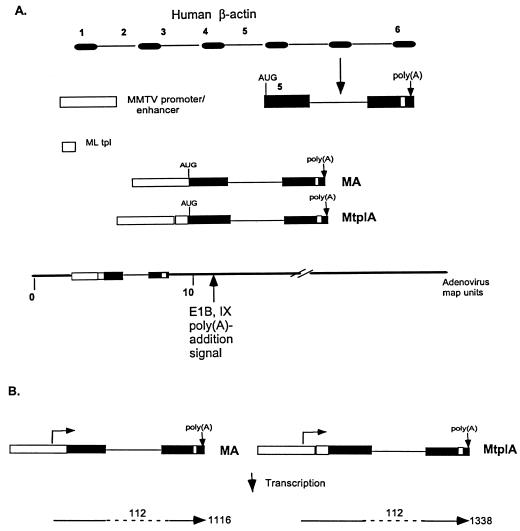
All cloning manipulations and purification of plasmid DNAs were done by standard procedures (66). The human β-actin minigene, which retains both intron 5 and the natural poly(A) addition site, designed for these studies is illustrated in Fig. 1. A 14-kb DNA fragment containing the full-length human β-actin gene obtained by digestion of plasmid p147Tβ-pH17 (47), kindly provided by Larry Kedes, was cloned between the EcoRI and BamHI sites of pUC19. To distinguish transcripts of the β-actin reporter minigene from those of the endogenous gene, a 34-bp oligonucleotide of unrelated sequence and containing an SpeI restriction endonuclease site, designated the SpeI linker, was ligated into a unique NsI site of exon 6 of the β-actin gene (Fig. 1). This marked β-actin gene was used as a template for the synthesis of the minigene by PCR (57). Two PCR products containing the β-actin sequence shown in Fig. 1 were generated with a common 3′ primer, which contained the sequence complementary to positions 3757 to 3739 of β-actin exon 6 followed by a BamHI site and 10 bp of random sequence, and two different 5′ primers. Both contained the sequence corresponding to positions 2778 to 2761 of β-actin exon 5, but this was preceded by either a NotI or an SnaBI site. The PCR was performed for 30 cycles as described previously (57), and products were identified by their predicted length and by restriction endonuclease digestion. A DNA fragment corresponding to positions −1194 to +264 of the glucocorticoid-inducible enhancer-promoter of the long terminal repeat of mouse mammary tumor virus (MMTV) (see references 25, 26, 56, and 65) and flanked by KpnI and SnaBI sites was generated by PCR with plasmid pMMTVmycXhR provided by G. Pendergast. To construct the inducible β-actin minigene, the MMTV fragment, digested with KpnI and SnaBI, and the SnaBI-flanked β-actin DNA fragment, digested with SnaBI and BamHI, were ligated into KpnI- and BamHI-digested DNA of plasmid pDX (a gift from K. Berkner). The desired recombinants were identified by restriction endonuclease digestion. The minigene, which was designated MA (Figure 1), was then sequenced (67) in its entirety to confirm its organization.
FIG. 1.
Organization of glucocorticoid-inducible β-actin minigenes and recombinant viruses that contain them. (A) The organization of the human β-actin gene is shown schematically at the top, with exons and introns represented by black boxes and lines, respectively. As illustrated, the β-actin minigene used in these experiments comprised part of exon 5 and exon 6 and was marked by insertion of a synthetic DNA fragment (white box). The MA and MtplA reporter genes, whose organization is shown, were constructed by ligation of appropriate DNA fragments as described in Materials and Methods. Rescue of these genes into the Ad5 genome generated recombinant viruses with the organization shown for Ad5/MtplA at the bottom of panel A; Ad5/MA differs only in the absence of tripartite leader sequences (tpl) from the reporter gene. (B) The unspliced RNAs predicted for the initiation of transcription from the initiation site of the mouse mammary tumor virus (MMTV) long terminal repeat promoter are shown by arrows. RNA lengths are listed in nucleotides, and intron 5 of the β-actin gene is indicated by broken lines.
A sibling minigene containing the major late tripartite leader sequence between the MMTV enhancer-promoter and β-actin sequences (Fig. 1) was constructed in a similar fashion, except that the MMTV enhancer-promoter was first ligated, via SnaBI sites, to a 180-bp DNA fragment containing tripartite leader sequences flanked by SnaBI and NotI sites. This tripartite leader segment, which contains bp 1 to 172 of the 202-bp complete sequence, corresponds to that used in previous studies of the functions of this 5′-untranslated sequence (9, 44, 50). Ligation of these two DNA fragments was confirmed by Southern blotting (73) with the tripartite leader sequence fragment as a probe. The MMTV tripartite leader sequence DNA segment was then ligated to the β-actin PCR product carrying a 5′ NotI site and plasmid pDX as described above. The structure of this second, inducible reporter gene, designated MtplA, was confirmed by sequencing. The resulting plasmids, containing the MA and MtplA reporter genes, were digested with KpnI, and the KpnI ends were modified by ligation to an oligonucleotide linker containing an XbaI site. The inducible reporter genes were then inserted as XbaI fragments between flanking Ad5 sequences corresponding to the left-hand terminus of the viral genome and E1B sequences present in plasmid pXCX2 (a kind gift from F. Graham) to generate plasmids pXMA and pXMtplA, respectively. The organization of these was confirmed by sequencing across the XbaI junctions.
Construction of recombinant viruses.
The inducible β-actin minigenes flanked by Ad5 E1A sequences were rescued into viruses by the method of McGrory et al. (52). 293 cells, which express the viral E1A and E1B genes and therefore complement the deletions of these genes in the desired recombinants (35), were maintained in Dulbecco’s minimal essential medium supplemented with 5% fetal calf serum, 5% calf serum, and 1% (vol/vol) glutamine. Monolayers of 293 cells were cotransfected by the calcium phosphate precipitation method (16) with either plasmid pXMA or plasmid pXMtplA and plasmid pJM17 (52). Typically, 1 μg of each plasmid was transfected per plate. Salmon sperm DNA was added to bring the total quantity of DNA to 20 μg. After 4 to 6 h, the medium was replaced with fresh medium containing 10% (vol/vol) glycerol for 3 min. The glycerol-shocked cells were washed twice with phosphate-buffered saline and overlaid with 1% (wt/vol) agar in Dulbecco’s minimal essential medium containing 10 mM MgCl2, 4% calf serum, and 7.5% NaHCO3. After 10 to 12 days, individual plaques were picked and suspended in 1 ml of 0.01 M Tris-HCl (pH 7.9) containing 1 mM EDTA and 10 mM NaCl. Following six freeze-thaw cycles, 100-μl portions of each suspension were removed for nucleic acid purification, and the remainder were stored at −80°C. Nucleic acids were purified by digestion for 30 min at 37°C with 20 μg of proteinase K per ml following the addition of an equal volume of 0.02 M Tris-HCl (pH 7.5) containing 40 mM NaCl, 4 mM EDTA, and 0.2% (wt/vol) sodium dodecyl sulfate, phenol-chloroform extraction, and ethanol precipitation with salmon sperm DNA carrier. These plaque lysates were screened by PCR with a 5′ primer corresponding to positions 118 to 140 of Ad5 DNA and a 3′ primer complementary to positions 3335 to 3353 of the viral genome. Recombinant and wild-type viruses were distinguished by the unique sizes of their PCR products following electrophoresis of PCR products in 1.2% gels cast and run in 0.045 M Tris-HCl (pH 7.0) containing 0.044 M boric acid and 0.001 M EDTA (TAE buffer). The viruses present in positive plaques were amplified by passage in 293 cells and subjected to a second round of plaque purification and screening. The recombinant viruses were then amplified to working stocks by repeated low-multiplicity-of-infection (MOI) propagation in 293 cells. Virus titers were determined by plaque assays with 293 cells as described previously (79). Viral DNA was then purified from both Ad5 MA and Ad5 MtplA (30), and the structure of these recombinant viruses was confirmed by sequencing each DNA from a position in 5′-flanking Ad5 sequences across the recombinant gene to a position equivalent to position 3328 of the Ad5 genome (Fig. 1).
Analysis of newly synthesized RNA.
293 cells were infected with 30 PFU of Ad5 MtplA or Ad5 MA per cell, and 3 mM dexamethasone was added to the medium 10 to 14 h after infection. One hour later, the cells were washed twice with phosphate-buffered saline. The cells were incubated at 37°C for 1 to 3 h and labeled with 200 μCi of [3H]uridine (39.70 Ci/mmol; NEN-Dupont) per ml for 15 min, followed by a 15- to 20-min chase in the presence of 10 mM uridine (Sigma). Cytoplasmic and nuclear fractions were separated as described previously (81). The cytoplasmic fractions were incubated with 10 μg of proteinase K per ml for 30 min at 37°C. The cytoplasmic RNA was then extracted twice with phenol-chloroform and ethanol precipitated. Nuclear pellets were dissolved in 4 M guanidinium isothiocyanate in 40 mM Tris-HCl (pH 7.4), containing 20 mM NaCl, and the RNA was sedimented through 5.7 M CsCl cushions by centrifugation for 12 to 16 h at 150,000 × g. Pellets were resuspended in sterile water and dialyzed against 10 mM Tris-HCl (pH 7.4), containing 0.25 M NaCl and 0.005 M EDTA. The 3H-labeled RNA was then hybridized to membrane-bound plasmid DNAs containing viral or cellular genes of interest and to the noncoding strand of the SpeI linker oligonucleotide described above. The Hsp70 RNA constitutively expressed in 293 cells (80) and viral major late (L2) RNA were detected with plasmids described previously (81). Linearized denatured plasmid DNAs were loaded onto membranes with a Minifold II blot apparatus (Schleicher & Schuell, Inc.) as described previously (81). The noncoding strand of the SpeI linker oligonucleotide was mixed with salmon sperm DNA prior to loading onto the membranes. The membranes were cross-linked under UV light and hybridized to [3H]uridine-labeled cytoplasmic and nuclear RNAs in 0.125 M sodium phosphate buffer (pH 6.4) containing 50% formamide, 0.25 M NaCl, 5% (vol/vol) sodium dodecyl sulfate, 10% (vol/vol) PEG 8000, and 1 mM EDTA at 55°C for 12 to 16 h. The membranes were washed, and the radioactivity hybridized to the individual DNAs was determined as described previously (81).
Northern blotting.
Nuclear and cytoplasmic RNA populations were isolated from infected cells as described above, and poly(A)-containing and poly(A)-lacking RNAs were separated by selection on oligo(dT) bound to magnetic beads (Dynal Company). Both nuclear and cytoplasmic RNA preparations were glyoxylated prior to electrophoresis in 1.2% agarose gels cast and run in 10 mM NaH2PO4 (pH 7.0) and electrophoretic transfer to GeneScreen (Dupont Inc.) membranes as described previously (81). The membranes were hybridized to the noncoding strand of the SpeI linker oligonucleotide or to an oligonucleotide complementary to the sequence from positions +28 to +52 of the MMTV transcription unit 5′ end labeled with T4 polynucleotide kinase and [γ-32P]ATP (3,000 Ci/mmol; NEN-Dupont) and were washed as described previously (19). Hybridization signals were quantified with a Molecular Dynamics PhosphorImager.
Run-on transcription.
Nuclei were isolated from Ad5 MA- or Ad5 MtplA-infected 293 cells and resuspended in 50 mM Tris-HCl (pH 8.3) containing 40% (vol/vol) glycerol, 5 mM MgCl2, and 0.1 mM EDTA at a concentration of 108 nuclei per ml. The elongation of initiated RNA chains was performed at 30°C for 30 min, and the labeled RNA was purified as described previously (42). The labeled RNA was hybridized to the pH 2.3 Hsp70 clone (80) and to the pSP73-ML and SpeI linker oligonucleotide DNAs bound to nylon membranes as described above. Hybridization, washing, and autoradiography of membranes were done as described previously (42). The quantities of RNA hybridized to individual DNAs were determined by scintillation counting of appropriate membrane segments.
Mapping of 5′ ends of β-actin minigene transcripts.
The 5′ ends of nuclear and cytoplasmic transcripts of the MA and MtplA genes shown in Fig. 1 were mapped by primer extension performed as described previously (42) from a primer complementary to positions +28 to +52 of the MMTV transcription unit.
Analysis of processing of β-actin minigene transcripts.
Nuclear and cytoplasmic RNAs were purified from Ad5 MA- or Ad5/MtplA-infected cells and separated into poly(A)-containing and poly(A)-lacking fractions as described above. The RNA preparations were then analyzed by reverse transcription (RT)-PCR (33). RT was from primers that hybridized to a sequence just upstream of the poly(A) addition site of the human β-actin gene or to positions 4014 to 4055 of the Ad5 genome. The latter sequence lies within the portion of the E1B gene that is immediately downstream of the β-actin minigenes in the recombinant viruses (Fig. 1). RT reaction mixtures, which contained 200 U of reverse transcriptase per μl (Gibco-BRL), were as described previously (42). The same 3′ primers and 5′ primers corresponding to positions +21 to +44 of the MMTV transcription unit or to the 5′ end of the ML tripartite leader sequence were used for subsequent PCR with reaction mixtures containing 2.5 U of Taq polymerase (Boehringer Mannheim Biochemicals), 2 mM MgCl2, and 100 μM each deoxynucleotide triphosphate or 100 μM each dATP, dGTP, and TTP and 1 μM dCTP plus 10 μl of [α-32P]dCTP (NEN-Dupont). Following 60 cycles, PCR products were purified by phenol-chloroform extraction and precipitated with 2 volumes of ethanol. They were then resolved by electrophoresis in 1% agarose gels cast and run in TAE buffer and visualized by autoradiography of dried gels or following ethidium bromide staining.
RESULTS
Construction and characterization of recombinant adenoviruses containing an inducible reporter gene.
In order to investigate parameters that might govern export efficiency in adenovirus-infected cells, such as residence of transcription units in the viral genome or activation of transcription (see the introduction), we introduced inducible reporter genes into the E1A and E1B regions of Ad5. The reporter gene we chose to use was derived from the human β-actin gene, because β-actin mRNA has been frequently used in studies of virus-induced inhibition of cellular mRNA export (e.g., see references 5, 61, 79, and 81). To facilitate these experiments and to obtain a small reporter gene that could be readily accommodated in the viral genome, the β-actin minigene comprising exons 5 and 6 plus the intervening intron shown in Fig. 1A was generated with the PCR (see Materials and Methods). This minigene retains the poly(A) addition site of the human β-actin gene, and its predicted spliced product contains an AUG codon in the β-actin reading frame close to its 5′ end (Fig. 1). A 34-bp synthetic oligonucleotide containing an SpeI site was introduced into exon 6 prior to generation of the minigene to allow its transcripts to be distinguished from those of endogenous β-actin genes. The glucocorticoid-inducible transcription control region of MMTV was then ligated to the β-actin gene in the absence or presence of a cDNA copy of the major late tripartite leader sequence as described in Materials and Methods. The MA and MtplA reporter genes thus generated are identical, except for the presence of the tripartite leader sequence in the latter (Fig. 1A). This sequence, whose contribution to efficient translation of major late mRNAs during the late phase of infection is described in the introduction, was included in the MtplA reporter gene to facilitate future investigation of the relationship between mRNA export and translation in infected cells (64). Once the structures and sequences of the reporter genes had been confirmed, they were inserted into a plasmid containing flanking Ad5 sequences from the left end of the viral genome. Recombinant viruses were obtained by in vivo recombination in complementing 293 cells (35) between these plasmids and plasmid pJM17 used by McGrory et al. (52). The recombinant Ad5/MA and Ad5/MtplA viruses (Fig. 1A) were identified, purified, and amplified as described in Materials and Methods. The presence and orientation of the chimeric MA and MtplA genes within the E1A-E1B region (Fig. 1A) were confirmed by sequencing.
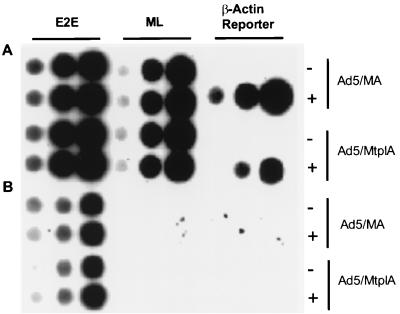
The transcriptional activities of the inducible β-actin minigenes in the absence or presence of the synthetic glucocorticoid dexamethasone were examined by run-on transcription in nuclei isolated from 293 cells infected with either Ad5/MA or Ad5/MtplA. In the experiment shown in Fig. 2, infected cells were incubated in medium containing dexamethasone for 3 h at 11 h after infection or were mock treated prior to isolation of nuclei (see Materials and Methods). Transcripts of the reporter genes, labeled during elongation in isolated nuclei as described previously (42), were detected by hybridization to the noncoding strand of the SpeI oligonucleotide used to mark the β-actin minigene (Fig. 1A) bound to membranes as described in Materials and Methods. The hormone had no discernible effect on the efficiency of transcription from the viral E2E or major late promoters (Fig. 2), as expected. Transcription of the β-actin minigenes could not be detected in the absence of dexamethasone but was strongly induced upon exposure of infected cells to the hormone (Fig. 2). The same results were obtained when dexamethasone was added to infected cells either earlier or later in the viral infectious cycle (data not shown). Dexamethasone-induced transcription was inhibited by low concentrations of α-amanitin (Fig. 2), confirming that the β-actin minigenes were transcribed by RNA polymerase II. The undetectable basal transcription of the chimeric β-actin minigenes carried into cells in the adenovirus genome, but strong induction of transcription by dexamethasone, which was evident within 1 h of hormone treatment and was maintained for several hours following removal of the hormone (data not shown), indicated that transcription of these reporter genes can be experimentally manipulated.
FIG. 2.
Transcription of MMTV β-actin minigenes in Ad5/MA- and Ad5/MtplA-infected cells. 293 cells were infected with 20 PFU of Ad5/MA or Ad5/MtplA per cell and exposed to 3 mM dexamethasone for 3 h at 11 h after infection (+) or mock treated (−). RNA labeled in run-on transcription reaction mixtures (see Materials and Methods) in the absence (A) or presence (B) of 2 μg of α-amanitin per ml, used to inhibit RNA polymerase II, was hybridized to the SpeI oligonucleotide probe specific for the β-actin reporter minigene and to major late (ML) and E2E probes as described in Materials and Methods. For each probe, 0.5, 2.5, or 5.0 μg of DNA (left to right) was loaded on the filter as described in Materials and Methods. Because the E2E probe used in these experiments detects the products of both RNA polymerase II and RNA polymerase III transcription (42), α-amanitin-resistant E2E transcription was observed (E2E probe in panel B).
Increased accumulation of reporter RNA containing the tripartite leader sequence in the cytoplasm of adenovirus-infected cells.
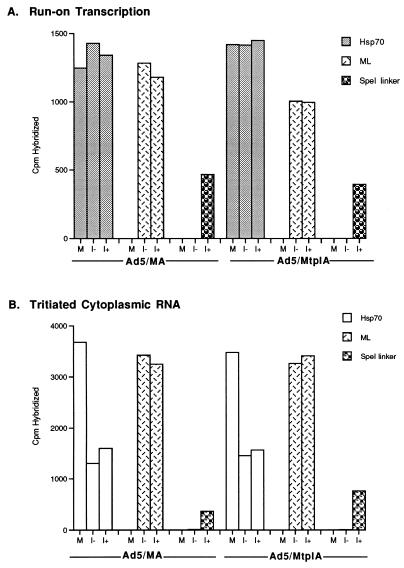
Run-on transcription assays of the kind described above established that the β-actin minigenes present in the Ad5/MA and Ad5/MtplA viruses could be efficiently transcribed in the presence of dexamethasone. To determine whether the transcripts of these genes entered the cytoplasm and thus behaved as mRNA, we first examined the accumulation of newly synthesized reporter RNA in the cytoplasm of recombinant virus-infected cells. Following infection with Ad5/MA or Ad5/MtplA, equal numbers of 293 cells were exposed to dexamethasone for 1 h during the late phase of infection or were mock treated, as were uninfected 293 cells. After 3 h, the cells were labeled with [3H]uridine as described in Materials and Methods. For each condition, a second set of cells infected and treated in parallel was harvested for quantitative run-on transcription assays. The cytoplasmic RNA labeled in vivo and the RNA synthesized during run-on transcription in isolated nuclei were hybridized to the SpeI oligonucleotide described above, as well as to representative cellular and viral genes, the Hsp70 gene constitutively expressed in 293 cells (80), and viral major late sequences, respectively. Typical results of such experiments are illustrated in Fig. 3.
FIG. 3.
Expression of the inducible β-actin minigenes in Ad5/MA- and Ad5/MtplA-infected 293 cells. Equal numbers of human 293 cells infected with 20 PFU of Ad5/MA or Ad5/MtplA per cell or mock infected (M) were exposed to dexamethasone for 1 h at 10 h after infection (I+) as described in Materials and Methods or mock treated (I−). At 14 h after infection, one set of cells was harvested for run-on transcription assays (A), while a duplicate set was used for [3H]uridine pulse-labeling (B). The labeled RNAs were purified and quantified by hybridization to filter-bound DNA as described in Materials and Methods. To control for variations in the number of cells recovered under each condition or in the MOIs of the two viruses, DNA was purified from the nuclear fractions of the [3H]uridine-labeled cells and hybridized to saturating quantities of 32P-labeled human Alu repeat DNA and viral E2E DNA, respectively. ML, major late.
Transcription of the β-actin minigenes in cells infected with either Ad5/MA or Ad5/MtplA could be detected only in dexamethasone-treated cells (Fig. 3A), as reported above. Furthermore, the reporter genes were transcribed with similar efficiencies (Fig. 3A). By 14 h after infection with either recombinant virus, newly synthesized viral late (L2) mRNA efficiently accumulated in the cytoplasm, while the accumulation of newly synthesized cellular (Hsp70) mRNA was inhibited (Fig. 3B), as expected (see the introduction). Newly synthesized MA and Mtpl RNAs could be detected in the cytoplasm of dexamethasone-treated cells infected with the corresponding virus (Fig. 3B). Unexpectedly, however, twice as much newly synthesized reporter RNA containing the tripartite leader sequence accumulated in the cytoplasm (Fig. 3B, Ad5/MtplA). More efficient cytoplasmic accumulation of MtplA RNA, which cannot be attributed to any difference in the rates of transcription of the two reporter genes (Fig. 3A), has been reproducibly observed in many experiments of this kind and is independent of the time during the late phase of infection at which infected cells are treated with dexamethasone (see, for example, Fig. 5).
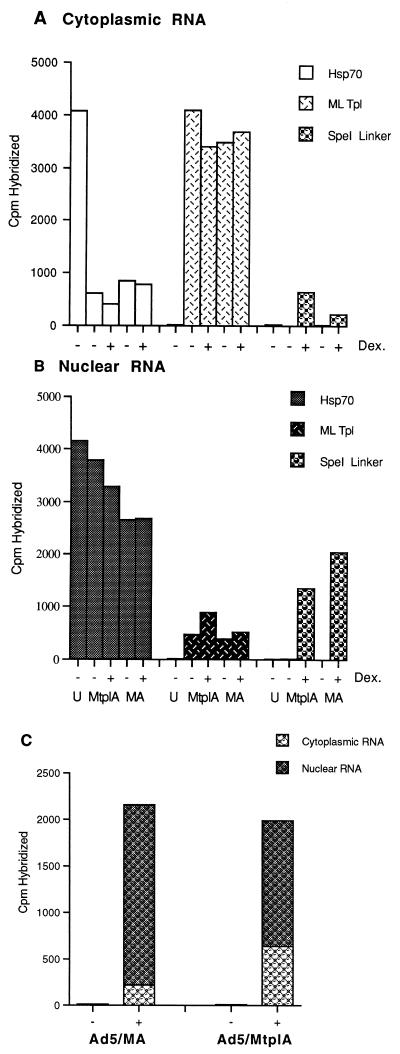
FIG. 5.
Partitioning of newly synthesized MA and MtplA RNAs between nuclear and cytoplasmic fractions of recombinant virus-infected cells. (A and B) The concentrations of the newly synthesized RNA species in cytoplasmic (A) and nuclear (B) fractions of Ad5/MA- and Ad5/MtplA-infected 293 cells treated with dexamethasone (Dex.) for 1 h at 14 h after infection were determined as described in Materials and Methods. U, uninfected; ML Tpl, major late tripartite leader sequence. (C) Total concentrations of newly synthesized MA and MtplA reporter RNAs and their distribution between nuclear and cytoplasmic fractions. In these experiments, the concentrations of cellular and viral DNAs were also examined by blotting with a human Alu repeat sequence and the viral L2 probe, respectively, to monitor variations in the number of cells under each condition and differences in the efficiencies of infection. No major variation was detected.
The tripartite leader sequence alters the intracellular distribution but not the stability of reporter RNA.
The accumulation of newly synthesized RNA in the cytoplasm is determined by the efficiency of each step in the production of the mature mRNA in the nucleus, the efficiency with which the RNA is exported from the nucleus, and the stability of the RNA in nuclear and cytoplasmic compartments. The more efficient cytoplasmic accumulation of the tripartite leader sequence-containing reporter RNA than of its sibling lacking this viral sequence (Fig. 3B) could therefore be the result of differences in the processing, export, or stability of these RNA species. To distinguish among these possibilities, the reporter RNA species and their distribution in infected cells were examined in more detail.
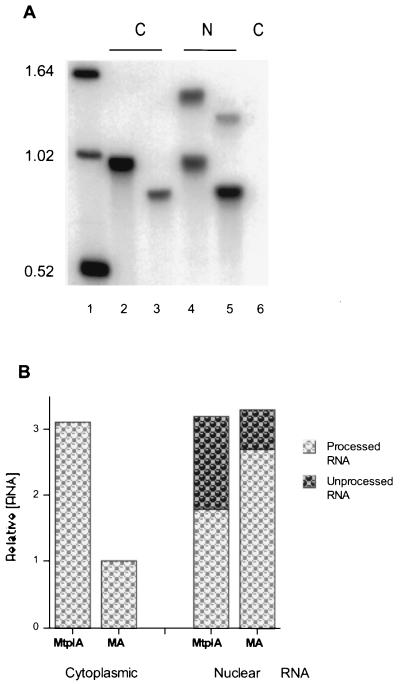
The intracellular distribution of the MA and MtplA reporter RNA species in dexamethasone-treated, Ad5/MA- and Ad5/MtplA-infected 293 cells was initially examined by Northern blotting of poly(A)-containing RNA. Only a single reporter RNA species was detected when cytoplasmic poly(A)-containing RNA isolated from equal numbers of either Ad5/MA- or Ad5/MtplA-infected cells was hybridized to the reporter gene-specific oligonucleotide probe (Fig. 4A, lanes 2 and 3). No corresponding RNA species were detected in cytoplasmic poly(A)-containing RNA prepared from Ad5-infected 293 cells (Fig. 4A, lane 6), confirming that the RNA species detected are specific products of expression of the reporter genes. These same species, and no others, were observed when blots were hybridized to a probe complementary to transcribed MMTV sequences of the chimeric genes (data not shown). The same species, as well as polyadenylated RNA species of the length predicted for unspliced transcripts of the MtplA and MA reporter genes (Fig. 1B), were readily detected in nuclear RNA preparations (Fig. 4A, lanes 4 and 5). Thus, processing of each chimeric reporter gene transcript generated a single polyadenylated RNA species that entered the cytoplasm. However, the distributions of processed MA and MtplA RNAs between nuclear and cytoplasmic fractions were different.
FIG. 4.
Northern blotting of reporter RNAs. (A) Poly(A)-containing cytoplasmic (C) or nuclear (N) RNAs isolated from equal numbers of Ad5/MA (lanes 3 and 5)- and Ad5/MtplA (lanes 2 and 4)-infected 293 cells exposed to dexamethasone for 1 h during the late phase of infection and from Ad5-infected cells (lane 6) were examined by Northern blotting with the reporter gene-specific SpeI oligonucleotide probe as described in Materials and Methods. Glyoxylated DNA markers, whose lengths (in kilobase pairs) are listed on the left, were run in lane 1. (B) The concentrations of the processed and unprocessed reporter RNA species shown in panel A, determined with a Molecular Dynamics PhosphorImager, are expressed relative to those of cytoplasmic processed MA RNA in Ad5/MA-infected cells.
The steady-state cytoplasmic concentration of processed MA RNA was about threefold lower than that of MtplA RNA (Fig. 4A, lanes 2 and 3; Fig. 4B, cytoplasmic RNAs), a difference similar in magnitude to that observed when cytoplasmic concentrations of newly synthesized MA and MtplA RNAs were compared (Fig. 3B). In contrast, nuclear RNA isolated from Ad5/MA-infected cells contained a higher concentration of processed reporter RNA than did nuclear RNA obtained from the same number of Ad5/MtplA-infected cells (Fig. 4A, lanes 4 and 5; Fig. 4B, nuclear RNA samples). These differences in the steady-state cytoplasmic and nuclear concentrations of processed MA and MtplA RNAs have been observed in several independent experiments with both the reporter gene-specific oligonucleotide probe and the MMTV-specific probe described above. The higher concentration of Mtpl RNA than of its tripartite leader sequence-lacking counterpart in the cytoplasm but the lower intranuclear concentration (Fig. 4) and the higher cytoplasmic concentrations of MtplA RNA observed in both steady-state (Fig. 4) and newly synthesized (Fig. 3B) RNA populations suggested that greater stability of MtplA could not account for its increased accumulation in the cytoplasm.
To assess more rigorously the contribution of differences in the cytoplasmic stability of MA and MtplA RNA species to differences in their cytoplasmic concentrations, the partitioning of newly synthesized reporter RNA between nuclear and cytoplasmic fractions was examined with the pulse-chase labeling protocol described in Materials and Methods. In these experiments, mock- and dexamethasone-treated 293 cells infected in parallel with Ad5/MA or Ad5/MtplA were pulse-labeled with [3H]uridine for 15 min during the late phase of infection. The label was then chased for a short period, 20 min, chosen to allow processing and export of the labeled RNA with minimal turnover in the cytoplasm. The concentrations of MA or MtplA in nuclear and cytoplasmic fractions were determined by hybridization as described above, as were the concentrations of cellular Hsp70 and viral L2 RNAs. The results of one such experiment, in which the rates of transcription of the reporter genes in Ad5/MA- or Ad5/MtplA-infected cells at 14 h after infection were identical, within experimental error, are shown in Fig. 5. The distributions of newly synthesized Hsp70 and viral L2 RNAs between the nucleus and the cytoplasm of recombinant virus-infected cells were as expected: the viral L2 mRNA entered the cytoplasm efficiently, but export of the cellular Hsp70 RNA was inhibited without accumulation of this cellular mRNA in the nucleus, as is characteristic of adenovirus-infected cells (3, 7, 48, 61, 78, 82). A greater concentration of newly synthesized tripartite leader sequence-containing than of tripartite leader sequence-lacking reporter RNA entered the cytoplasm of dexamethasone-treated infected cells (Fig. 5A). This difference was, however, reversed in the nuclear RNA populations of the infected cells (Fig. 5B). Thus, the total concentrations of newly synthesized reporter RNA were very similar in Ad5/MA- and Ad5/MtplA-infected cells, but a larger fraction of the tripartite leader sequence-containing RNA entered the cytoplasm (Fig. 5C). This difference in the distribution of newly synthesized reporter RNAs containing and lacking the tripartite leader sequence between nuclear and cytoplasmic compartments of dexamethasone-treated recombinant virus-infected cells has been reproducibly observed (Table 1). The same total concentrations of the two newly synthesized reporter RNA species (Fig. 5C) together with the increased nuclear concentration of MA RNA (Fig. 5B and C) indicated that the tripartite leader sequence-containing RNA enters the cytoplasm more efficiently than does its sibling, which lacks this adenovirus RNA sequence: increased turnover of the latter compared to the former RNA in the cytoplasm would result in the same nuclear concentrations of the two RNA species but in a decreased total concentration of the tripartite leader sequence-lacking RNA, neither of which was observed (Fig. 5B and C).
TABLE 1.
Efficiency of export of newly synthesized RNA species in recombinant adenovirus-infected cellsa
| Expt | Treatment | Newly synthesized cytoplasmic/nuclear RNA ratio
|
||
|---|---|---|---|---|
| Hsp70 | Major late L2 | β-actin reporter | ||
| 1 | None (uninfected) | 3.1 | ND | ND |
| Ad5/MA + Dex, 11 h | 0.3 | 8.5 | 0.1 | |
| Ad5/MtplA + Dex, 11 h | 0.3 | 8.2 | 0.5 | |
| 2 | None (uninfected) | 3.0 | ND | ND |
| Ad5/MA + Dex, 11 h | 0.5 | 2.5 | 0.3 | |
| Ad5/MtplA + Dex, 11 h | — | 2.3 | 1.0 | |
| 3 | None (uninfected) | 9.7 | ND | ND |
| Ad5/MA + Dex, 12 h | 0.4 | 11.7 | 0.1 | |
| Ad5/MtplA + Dex, 12 h | 0.4 | 12.4 | 0.4 | |
The quantities of newly synthesized Hsp70, major late L2, and β-actin reporter RNAs present in nuclear and cytoplasmic fractions were determined as described in the legend to Fig. 5 and are expressed as the cytoplasmic/nuclear RNA ratio. Neither MA nor MtplA RNA could be detected in Ad5/MA- or Ad5/MtplA-infected cells that were not treated with dexamethasone and that were analyzed in parallel (data not shown, but see Fig. 5). ND, not detectable; —, not determined.
The efficiency with which newly synthesized RNA enters the cytoplasm can be assessed by calculating the ratio of cytoplasmic to nuclear concentrations of newly synthesized RNA from data like those shown in Fig. 5B (81). These values varied for the various RNA species examined among independent infections (Table 1), presumably the result of variations in the time during the infectious cycle and in the pulse-labeling protocol: with the short pulse-chase periods used, the concentrations of newly synthesized RNA entering the cytoplasm would be sensitive to even small differences in parameters such as chase time and temperature among different experiments. For such reasons, the cytoplasmic/nuclear concentration ratios of the β-actin reporter and other RNAs measured in independent infections (Table 1) have not been averaged. It is nevertheless clear that the chimeric reporter RNA that contained the tripartite leader sequence entered the cytoplasm 2.4- to 4.1-fold more efficiently than did its sibling, which lacked this viral RNA sequence (Table 1).
Processing of chimeric reporter RNA species.
The experiments described above could not establish whether inclusion of the tripartite leader sequence in the chimeric reporter gene resulted in a direct increase in the efficiency of export of the RNA from the nucleus to the cytoplasm or altered the processing of reporter gene transcripts within the nucleus, such that the processed RNA entered the cytoplasm more efficiently. The cDNA copy of the tripartite leader sequence included in the chimeric MtplA gene does not include splicing signals, so there is no a priori reason to expect that the processing of its transcript would be different from that of MA gene transcripts. Furthermore, the results of the Northern blotting experiments described above suggested that processed and unprocessed MA and MtplA reporter RNA species differed only in the presence of the tripartite leader sequence in the latter. Further characterization of the reporter RNA species synthesized in Ad5/MA- or Ad5/MtplA-infected cells treated with dexamethasone confirmed this conclusion. For example, RT-PCR of nuclear poly(A)-containing RNA with primers derived from sequences near the poly(A) addition site of the β-actin gene and the initiation site of MMTV transcriptional control yielded single MA and MtplA products of the lengths predicted (Fig. 1B) for unspliced transcripts of the two reporter genes (data not shown). Primer extension of nuclear and cytoplasmic RNAs isolated from Ad5/MA- or Ad5/MtplA-infected cells following exposure to dexamethasone with a primer complementary to positions +27 to +52 of the MMTV transcription unit generated only the predicted cDNAs of 52 nucleotides (data not shown), and no recombinant virus-infected cell-specific RNAs containing viral E1B sequences were detected by either Northern blotting or RT-PCR (data not shown). We can therefore conclude that the chimeric reporter genes were transcribed as predicted (Fig. 1B) and polyadenylated at the β-actin poly(A) addition site. The production of a single processed MA or MtplA RNA in both nuclear and cytoplasmic fractions (Fig. 4A) was also confirmed by RT-PCR (e.g., Fig. 6, lanes 2 and 6).
FIG. 6.
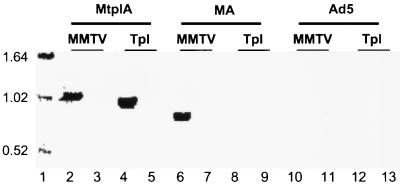
Characterization of MA and MtplA RNA species by RT-PCR. Cytoplasmic poly(A)-containing (lanes 2, 4, 6, 8, 10, and 12) or poly(A)-lacking (lanes 3, 5, 7, 9, 11, and 13) RNAs isolated from Ad5/MtplA-, Ad5/MA-, or Ad5-infected cells following exposure to dexamethasone for 1 h at 11 h after infection were analyzed by RT-PCR with a 3′ primer complementary to sequences near the β-actin poly(A) addition site and a 5′ primer corresponding to positions +21 to +44 of mouse mammary tumor virus (MMTV) transcription unit A or to the 5′ end of the tripartite leader sequence (Tpl). The lengths (in kilobase pairs) of DNA markers (lane 1) are indicated on the left.
Both the unprocessed and processed MtplA RNA species were about 150 to 170 bp longer than the corresponding MA RNA species, as judged by the differences in the lengths of their RT-PCR products (Fig. 6, compare lanes 2 and 6), in good agreement with the 164 bp predicted for the inclusion of the tripartite leader sequence in Mtpl RNA. The presence of the latter sequence in this RNA was confirmed by RT-PCR with a 5′ primer corresponding to the 5′ end of the tripartite leader sequence. A product of 0.91 ± 0.06 kbp was generated from cytoplasmic poly(A)-containing RNA isolated from Ad5/MtplA-infected cells treated with dexamethasone, but no product was obtained from Ad5/MA-infected cell RNA (Fig. 6, lanes 4 and 8). The excellent agreement between the length of this MtplA RT-PCR product and that predicted for spliced reporter RNA from which β-actin intron 6 has been removed (0.91 kbp) indicated that this intron was spliced from reporter transcripts, as expected. However, both MtplA and MA cytoplasmic RNAs differed from the corresponding nucleus-specific RNAs by about 400 nucleotides (e.g., Fig. 4A) rather than the 112 nucleotides of the β-actin intron (Fig. 1B). This difference can be ascribed to splicing within the 5′ MMTV sequences of the reporter RNAs, because the difference in length between the RT-PCR products of processed MtplA RNA obtained with the 5′ MMTV and the 5′ tripartite leader sequence primers was about 85 bp rather than the 243 bp predicted (Fig. 6, lanes 2 and 4). The region of the MMTV transcription unit present in the reporter genes contains a number of sequences that match the 5′ or 3′ splice site consensus sequences which, if active, would result in the removal of from 143 to 207 nucleotides from reporter transcripts. The use of cryptic splice sites is a common result of alteration of splicing signals in RNAs (see references 36, 46, and 71 for reviews), in this case, removal of the MMTV 5′ splice site for env mRNA (56). Splicing at such normally cryptic MMTV splice sites can therefore account for the shorter-than-predicted lengths of processed reporter gene transcripts (e.g., Fig. 4A) and the difference of only 85 bp between the RT-PCR products of processed MtplA RNA generated with the 5′ MMTV and the 5′ tripartite leader sequence primers (Fig. 6B, lanes 2 and 4). Thus, although the processed MA and MtplA RNA species that entered the cytoplasm of recombinant virus-infected cells treated with dexamethasone were spliced more extensively than expected, the only detectable difference between them was the presence of the tripartite leader sequence in the latter (Fig. 4A and 6; data not shown).
DISCUSSION
The chimeric reporter genes described here exhibit a number of properties that should facilitate investigation of the parameters that govern the export of processed mRNA from the nucleus in adenovirus-infected cells. When these chimeric genes were built into the E1A-E1B region of the viral chromosome and introduced into complementing 293 cells by infection, their transcription was activated by the synthetic glucocorticoid dexamethasone (Fig. 2). The strict dependence of reporter gene transcription on exogenous hormone (Fig. 2), which activates the MMTV transcriptional control region via GREs present in its enhancer (25, 26, 56, 65), and the initiation of transcription from the MMTV start site (data not shown) established that the transcriptional activity of the reporter gene was not influenced by flanking adenoviral sequences, such as E1A enhancers located upstream (39, 40). Reporter gene transcription continued for several hours following the removal of exogenous dexamethasone (e.g., Fig. 3), a property ideal for investigation of the influence of the time during the adenovirus infectious cycle at which transcription is activated upon export of processed transcripts.
When transcription of either of the two reporter genes examined here was activated during the late phase of infection, reporter RNA was readily detected in the cytoplasmic fraction upon examination of either newly synthesized (Fig. 3 and 5) or steady-state (Fig. 4A) RNA populations. The complete absence of nucleus-specific MA or MtplA RNAs with the properties predicted for unspliced transcripts from cytoplasmic fractions (Fig. 4A) indicated that these cytoplasmic reporter RNAs were specifically exported. Such export of MA RNA, which contains no viral sequences, following transcriptional activation of the MA gene occurred when export of typical cellular mRNAs was inhibited (Fig. 5 and Table 1). Thus, this reporter RNA will allow systematic assessment of the contributions of parameters such as transcriptional activation during the late phase of infection and residence of a transcription unit in the viral chromosome to selective export in adenovirus-infected cells. Indeed, the results of preliminary experiments suggest that MA RNA synthesized during the late phase of infection following the activation of transcription prior to the onset of viral DNA synthesis is exported markedly less efficiently than when transcription is activated during the late phase of infection (41a).
Although both reporter RNAs were exported to the cytoplasm following activation of transcription of the MA or MtplA gene, larger quantities of the tripartite leader sequence-containing, but otherwise identical, reporter RNA were detected in cytoplasmic fractions (Fig. 3, 4, and 5). This difference cannot be attributed to more efficient transcription of the MtplA reporter gene (Fig. 3). The total quantities of MA and MtplA synthesized in recombinant virus-infected cells treated with dexamethasone in a short period (40 min) were identical (within experimental error), but a greater fraction of the tripartite leader sequence-containing RNA than of its sibling lacking this sequence entered the cytoplasm (Fig. 5). This difference in the partitioning of the two RNAs labeled during a short period and the preferential cytoplasmic accumulation of MtplA observed in steady-state RNA populations (Fig. 4) indicated that the tripartite leader sequence does not stabilize cytoplasmic reporter RNA. Nor could any influence of the tripartite leader sequence on the pathways by which polyadenylated reporter RNA species are processed within the nucleus be detected (Fig. 4A and 6; data not shown). We therefore conclude that the tripartite leader sequence, whose presence in processed MtplA RNA (Fig. 6) was the only unique feature of this RNA compared to MA RNA, stimulates export of RNA from the nucleus. As in all experiments of this kind, export is defined in a purely operational sense as all essential reactions up to and including translocation of the RNA through nuclear pore complexes. As judged by estimation of the efficiencies with which newly synthesized MA and MtplA RNAs entered the cytoplasm (Table 1), the tripartite leader sequence increased export efficiency by a factor of 2.4 to 4. Although not dramatic, this increase has been observed reproducibly in independent infections (Table 1) and is apparently not influenced by the time during the late phase of infection at which transcription of the reporter gene is activated by exogenous dexamethasone (Table 1; data not shown).
The adenovirus major late mRNAs to which the tripartite leader sequence is attached encode essential virion proteins or proteins that regulate the assembly of virus particles or late protein synthesis (72). The functions of the tripartite leader sequence during adenovirus infection have therefore invariably been investigated with artificial constructs. Initial experiments, in which cDNA copies of the tripartite leader sequence were linked to E1A (50) or mouse dihydrofolate reductase (9) sequences built into the E1A region of the Ad5 genome, established that the tripartite leader sequence stimulates the translation of mRNAs to which it is linked in cis. No differences in steady-state cytoplasmic concentrations of mRNAs that contained or lacked the tripartite leader sequence were observed in these experiments (9, 50), but export was not directly examined. On the other hand, the addition of the tripartite leader sequence to the 5′ end of the herpes simplex virus type 1 thymidine kinase mRNA both stabilized the mRNA in the cytoplasm and decreased its nuclear half-life in adenovirus-infected cells (55). The latter property could be the result of more efficient export, although this possibility was not investigated. As we have now demonstrated, the tripartite leader sequence can modulate the export of mRNA from the nucleus, in this case when present in a chimeric mRNA that contains MMTV and human β-actin sequences. Whether the tripartite leader sequence also stimulates translation of this mRNA in adenovirus-infected cells has not yet been investigated. The differences among the results reported here and in previous investigations of the function of the tripartite leader sequence indicate that this sequence can govern mRNA metabolism at multiple posttranscriptional steps, including export from the nucleus, cytoplasmic stability, and translation. Furthermore, the principal effect of this sequence appears to be context dependent, determined by the RNA sequences to which it is linked. From the point of view of export, for example, other sequences present in a processed mRNA might override the export function of the tripartite leader sequence or render export so efficient that the contribution of this sequence cannot be detected. The different ways in which the tripartite leader sequence can influence the metabolism of mRNAs and the possibility that its function is context dependent raise the questions of whether this sequence regulates multiple properties of the adenovirus major late mRNAs in which it normally resides and whether all members of this large set of mRNAs are modulated in identical fashion by the tripartite leader sequence. It will therefore be important to compare the production and translation in adenovirus-infected cells of bona fide major late mRNAs containing and lacking the tripartite leader sequence.
The most straightforward mechanism by which the tripartite leader sequence might stimulate export is through its specific recognition by viral or cellular proteins that mediate export in adenovirus-infected cells, an interaction that would facilitate entry of the RNA into the export pathway. In this model, a sequence within the tripartite leader sequence would be analogous to the RRE of HIV-1 mRNAs, the viral sequence whose recognition by the HIV-1 Rev protein allows the export of unspliced and partially spliced viral mRNAs (see the introduction). Obvious candidates for proteins that bind specifically to the adenovirus tripartite leader sequence are the E1B 55-kDa and E4 34-kDa proteins (see the introduction). However, these viral early proteins allow the selective export of both viral and certain cellular mRNAs that lack the tripartite leader sequence (see the introduction), indicating that their primary function cannot be specific recognition of a putative tripartite leader sequence “export” signal. Whether viral or cellular proteins other than translation initiation factors bind to the tripartite leader sequence is not known. As this is the first adenovirus RNA sequence influencing export efficiency in infected cells to be identified, identification of any nuclear proteins that recognize it specifically might provide valuable clues about the molecular mechanisms responsible for RNA export and its regulation. On the other hand, it is also possible that the stimulation of export by the tripartite leader sequence is not the result of an interaction with components of the export machinery but rather is related to, or is a direct consequence of, the role of this sequence in translation. It is well established that, in mammalian cells, nonsense codons lead to reduced concentrations of mRNA via effects on nuclear rather than, or in addition to, on cytoplasmic RNA (5, 6, 17, 45, 74, 76). The mechanisms by which the translatability of an mRNA determines its nuclear concentration or splicing have not yet been elucidated, but models under consideration include translational translocation, in which initiation of the translation of mRNA in the cytoplasm facilitates its translocation through the nuclear pore complex (45, 76), and nuclear scanning of the mRNA for translatability (76). Thus, the ability of the tripartite leader sequence to induce efficient translation of mRNA in the poor translational environment of adenovirus-infected cells could facilitate export by mechanisms analogous to those that normally couple the accumulation of cytoplasmic cellular mRNA to its translatability. As stimulation of translation by the tripartite leader sequence requires its presence at the 5′ end of mRNA (50), whereas its direct recognition by proteins that mediate or stimulate export should be independent of its position within mRNA, it should be possible to distinguish such direct and indirect mechanisms by which the tripartite leader sequence might stimulate export.
ACKNOWLEDGMENTS
We thank Georgia Guan for expert technical assistance, K. Berkner, F. Grahm, G. Pendergast, L. Kedes, and R. Morimoto for gifts of plasmids, and Tom Shenk and Renée Finnen for critical reading of the manuscript.
This work was supported by a grant from the National Institutes of Health to S. J. Flint.
REFERENCES
- 1.Adam S, Dreyfuss G. Adenovirus proteins associated with mRNA and hnRNA in infected HeLa cells. J Virol. 1989;61:3276–3283. doi: 10.1128/jvi.61.10.3276-3283.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson C W, Baum P R, Gesteland R F. Processing of adenovirus 2-induced proteins. J Virol. 1973;12:241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babich A, Feldman C T, Nevins J R, Darnell J E, Weinberger C. Effect of adenovirus on metabolism of specific host mRNAs: transport and specific translational discrimination. Mol Cell Biol. 1983;3:1212–1221. doi: 10.1128/mcb.3.7.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babiss L E, Ginsberg H S, Darnell J E. Adenovirus E1B proteins are required for accumulation of late viral mRNA and for effects on cellular mRNA translation and transport. Mol Cell Biol. 1985;5:2552–2558. doi: 10.1128/mcb.5.10.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baserga S J, Benz E J. Beta-globin nonsense mutation: deficient accumulation of mRNA occurs despite normal cytoplasmic stability. Proc Natl Acad Sci USA. 1992;89:2935–2939. doi: 10.1073/pnas.89.7.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belgrader P, Cheng J, Zhau X, Stephenson L S, Marquat L Y. Mammalian nonsense codons can be cis effectors of nuclear mRNA half-life. Mol Cell Biol. 1994;14:8219–8228. doi: 10.1128/mcb.14.12.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beltz G A, Flint S J. Inhibition of HeLa cell protein synthesis during adenovirus infection: restriction of cellular messenger RNA sequences to the nucleus. J Mol Biol. 1979;131:353–373. doi: 10.1016/0022-2836(79)90081-0. [DOI] [PubMed] [Google Scholar]
- 8.Berget S M, Moore C, Sharp P A. Spliced segments at the 5′ terminus of adenovirus 2 late mRNA. Proc Natl Acad Sci USA. 1977;74:3171–3175. doi: 10.1073/pnas.74.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berkner K L, Sharp P A. Effect of tripartite leader on synthesis of a non-viral protein in an adenovirus 5 recombinant. Nucleic Acids Res. 1985;13:841–857. doi: 10.1093/nar/13.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blobel G. Gene gating: a hypothesis. Proc Natl Acad Sci USA. 1985;82:8527–8529. doi: 10.1073/pnas.82.24.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bridge E, Carmo-Fonseca M, Lamond A, Pettersson U. Nuclear organization of splicing small nuclear ribonucleoproteins in adenovirus-infected cells. J Virol. 1993;67:5792–5802. doi: 10.1128/jvi.67.10.5792-5802.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bridge E, Ketner G. Interaction of adenoviral E4 and E1b products in late gene expression. Virology. 1990;174:345–353. doi: 10.1016/0042-6822(90)90088-9. [DOI] [PubMed] [Google Scholar]
- 13.Bridge E, Riedel K U, Johansson B M, Pettersson U. Spliced exons of adenovirus late RNAs colocalize with snRNP in a specific nuclear domain. J Cell Biol. 1996;135:303–314. doi: 10.1083/jcb.135.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bridge E, Xia D X, Carmo-Fonesca M, Cardinali B, Lamond A I, Pettersson U. Dynamic organization of splicing factors in adenovirus-infected cells. J Virol. 1995;69:281–290. doi: 10.1128/jvi.69.1.281-290.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castiglia C L, Flint S J. Effects of adenovirus infection on rRNA synthesis and maturation in HeLa cells. Mol Cell Biol. 1983;3:662–671. doi: 10.1128/mcb.3.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng J, Maquat L E. Nonsense codons can reduce the abundance of nuclear mRNA without affecting the abundance of pre-mRNA or the half-life of cytoplasmic mRNA. Mol Cell Biol. 1993;13:1892–1902. doi: 10.1128/mcb.13.3.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chow L T, Gelinas R E, Broker T R, Roberts R J. An amazing sequence arrangement at the 5′ ends of adenovirus 2 messenger RNA. Cell. 1977;12:1–8. doi: 10.1016/0092-8674(77)90180-5. [DOI] [PubMed] [Google Scholar]
- 19.Church G M, Gilbert W. Genomic sequencing. Proc Natl Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cullen B R, Malim M H. The HIV-1 Rev protein: prototype of a novel class of eukaryotic post-transcriptional regulators. Trends Biochem Sci. 1991;16:346–350. doi: 10.1016/0968-0004(91)90141-h. [DOI] [PubMed] [Google Scholar]
- 21.Cutt J R, Shenk T, Hearing P. Analysis of adenovirus early region 4-encoded polypeptides synthesized in productively infected cells. J Virol. 1987;61:543–552. doi: 10.1128/jvi.61.2.543-552.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denome R M, Werner E A, Patterson R J. RNA metabolism in nuclei: adenovirus and heat shock alter intranuclear RNA compartmentalization. Nucleic Acids Res. 1989;17:2081–2098. doi: 10.1093/nar/17.5.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dobbelstein, M., J. Roth, W. T. Kimberly, A. J. Levine, and T. Shenk. Nuclear export of the E1B 55-kDa and E4 34-kDa adenoviral oncoproteins mediated by a rev-like signal sequence. EMBO J., in press. [DOI] [PMC free article] [PubMed]
- 24.Dolph P J, Racaniello V, Villamarin A, Pallodino F, Schneider R J. The adenovirus tripartite leader may eliminate the requirement of cap-binding protein complex during translation initiation. J Virol. 1988;62:2059–2066. doi: 10.1128/jvi.62.6.2059-2066.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donehower L A, Huang A L, Hager G L. Regulatory and coding potential of the mouse mammary tumor virus long terminal redundancy. J Virol. 1981;37:226–238. doi: 10.1128/jvi.37.1.226-238.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fasel N, Pearson K, Buetti E, Diggelmann H. The region of mouse mammary tumor virus DNA containing the long terminal repeat includes a long coding sequence and signals for hormonally regulated transcription. EMBO J. 1982;1:3–7. doi: 10.1002/j.1460-2075.1982.tb01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischer A, Huber J, Boulens W C, Mattaj I W, Lührmann R. The HIV-1 Rev activation domain is a nuclear export signal that accesses an export pathway used by specific cellular RNAs. Cell. 1995;82:475–483. doi: 10.1016/0092-8674(95)90436-0. [DOI] [PubMed] [Google Scholar]
- 28.Fischer A, Meyer S, Teufel M, Heckel C, Lührmann R, Rautmann G. Evidence that HIV-1 Rev directly promotes the nuclear export of unspliced RNA. EMBO J. 1994;13:4105–4112. doi: 10.1002/j.1460-2075.1994.tb06728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flint S J. Regulation of adenovirus mRNA formation. Adv Virus Res. 1986;31:169–228. doi: 10.1016/s0065-3527(08)60264-x. [DOI] [PubMed] [Google Scholar]
- 30.Flint S J, Gallimore P H, Sharp P A. Comparison of viral RNA sequences in adenovirus 2-transformed and lytically infected cells. J Mol Biol. 1975;96:47–68. doi: 10.1016/0022-2836(75)90181-3. [DOI] [PubMed] [Google Scholar]
- 31.Fridell R A, Bogerd H P, Cullen B R. Nuclear export of late HIV-1 mRNAs occurs via a cellular protein export pathway. Proc Natl Acad Sci USA. 1996;93:4421–4424. doi: 10.1073/pnas.93.9.4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fritz C C, Green M R. HIV Rev uses a conserved cellular protein export pathway for the nucleocytoplasmic transport of RNA. Curr Biol. 1996;6:848–854. doi: 10.1016/s0960-9822(02)00608-5. [DOI] [PubMed] [Google Scholar]
- 33.Frohman M A, Dush M K, Martin G R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci USA. 1988;85:8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorlich D, Mattaj I W. Nucleocytoplasmic transport. Science. 1996;271:1513–1518. doi: 10.1126/science.271.5255.1513. [DOI] [PubMed] [Google Scholar]
- 35.Graham F L, Smiley J, Russell W C, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–72. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 36.Green M R. Pre-mRNA splicing. Annu Rev Genet. 1986;20:671–708. doi: 10.1146/annurev.ge.20.120186.003323. [DOI] [PubMed] [Google Scholar]
- 37.Halbert D N, Cutt J R, Shenk T. Adenovirus early region 4 encodes functions required for efficient DNA replication, late gene expression, and host cell shutoff. J Virol. 1985;56:250–257. doi: 10.1128/jvi.56.1.250-257.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayes B W, Telling G C, Myat M M, Williams J F, Flint S J. The adenovirus L4 100-kilodalton protein is necessary for efficient translation of viral late mRNA species. J Virol. 1990;64:2732–2742. doi: 10.1128/jvi.64.6.2732-2742.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hearing P, Shenk T. The adenovirus type 5 enhancer contains two functionally distinct domains: one is a duplicated enhancer element. Cell. 1986;45:229–236. doi: 10.1016/0092-8674(86)90387-9. [DOI] [PubMed] [Google Scholar]
- 40.Hearing P, Shenk T. Functional analysis of the nucleotide sequence surrounding the cap site for adenovirus type 5 region E1A messenger RNAs. J Mol Biol. 1983;167:809–822. doi: 10.1016/s0022-2836(83)80112-0. [DOI] [PubMed] [Google Scholar]
- 41.Huang J, Schneider R J. Adenovirus inhibition of cellular protein synthesis involves inactivation of cap-binding protein. Cell. 1991;65:271–280. doi: 10.1016/0092-8674(91)90161-q. [DOI] [PubMed] [Google Scholar]
- 41a.Huang, W. Unpublished observations.
- 42.Huang W, Pruzan R, Flint S J. In vivo transcription from the adenovirus E2E promoter by RNA polymerase III. Proc Natl Acad Sci USA. 1994;4:1265–1269. doi: 10.1073/pnas.91.4.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalland K H, Szilvay A M, Brokstad K A, Saetrevik W, Haukenes G. The human immunodeficiency virus type 1 Rev protein shuttles between the cytoplasm and nuclear compartments. Mol Cell Biol. 1994;14:7436–7444. doi: 10.1128/mcb.14.11.7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaufman R J. Identification of the components necessary for adenovirus translational control and their utilization in cDNA expression vectors. Proc Natl Acad Sci USA. 1985;82:689–693. doi: 10.1073/pnas.82.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kessler O, Chasin L A. Effects of nonsense mutations on nuclear and cytoplasmic adenine phosphoribosyltransferase RNA. Mol Cell Biol. 1996;16:4426–4435. doi: 10.1128/mcb.16.8.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krawczak M, Reis J, Cooper D N. The mutational spectrum of single base-pair substitutions in mRNA splice junctions of human genes: causes and consequences. Hum Genet. 1992;90:41–54. doi: 10.1007/BF00210743. [DOI] [PubMed] [Google Scholar]
- 47.Leavitt J, Gunning P, Porreca P, Ng S-Y, Lin C-S, Kedes L. Molecular cloning and characterization of mutant and wild-type human β-actin genes. Mol Cell Biol. 1984;4:1961–1969. doi: 10.1128/mcb.4.10.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leppard K, Shenk T. The adenovirus E1B 55kD protein influences mRNA transport via intranuclear effects on RNA metabolism. EMBO J. 1989;8:2329–2336. doi: 10.1002/j.1460-2075.1989.tb08360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leppard N. Selective effects on adenovirus late gene expression of deleting the E1B 55K protein. J Gen Virol. 1993;74:575–582. doi: 10.1099/0022-1317-74-4-575. [DOI] [PubMed] [Google Scholar]
- 50.Logan J, Shenk T. Adenovirus tripartite leader sequence enhances translation of mRNAs late after infection. Proc Natl Acad Sci USA. 1984;81:3655–3659. doi: 10.1073/pnas.81.12.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mathews M B, Shenk T E. Adenovirus virus-associated RNA and translation control. J Virol. 1991;65:5657–5662. doi: 10.1128/jvi.65.11.5657-5662.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McGrory W J, Bautista D S, Graham F L. A simple technique for the rescue of early region mutations into infectious human adenovirus type 5. Virology. 1988;163:614–617. doi: 10.1016/0042-6822(88)90302-9. [DOI] [PubMed] [Google Scholar]
- 53.Meyer B, Malim M. The HIV-1 Rev trans-activator shuttles between the nucleus and the cytoplasm. Genes Dev. 1994;8:1538–1547. doi: 10.1101/gad.8.13.1538. [DOI] [PubMed] [Google Scholar]
- 54.Moore M, Schaack J, Baim S R, Morimoto R I, Shenk T. Induced heat shock mRNAs escape the nucleocytoplasmic transport block in adenovirus-infected HeLa cells. Mol Cell Biol. 1987;7:4505–4512. doi: 10.1128/mcb.7.12.4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moore M, Shenk T. The adenovirus tripartite leader sequence can alter nuclear and cytoplasmic metabolism of a non-adenovirus mRNA within infected cells. Nucleic Acids Res. 1988;16:2247–2262. doi: 10.1093/nar/16.5.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moore R, Dixon M, Smith R, Peters G, Dickson C. Complete nucleotide sequence of a milk-transmitted mouse mammary tumor virus: two frameshift suppression events are required for translation of gag and pol. J Virol. 1986;61:480–490. doi: 10.1128/jvi.61.2.480-490.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mullis K, Faloona F, Scharf S, Saiki R, Horn G, Erlich H. Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harbor Symp Quant Biol. 1986;51:263–273. doi: 10.1101/sqb.1986.051.01.032. [DOI] [PubMed] [Google Scholar]
- 58.Nigg E A. Nucleocytoplasmic transport: signals, mechanisms and regulation. Nature. 1997;386:779–787. doi: 10.1038/386779a0. [DOI] [PubMed] [Google Scholar]
- 59.O’Malley R P, Duncan R F, Hershey J W B, Mathews M B. Modification of protein synthesis initiation factors and the shut-off of host protein synthesis in adenovirus-infected cells. Virology. 1989;168:112–118. doi: 10.1016/0042-6822(89)90409-1. [DOI] [PubMed] [Google Scholar]
- 60.Ornelles D, Shenk T. Location of the adenovirus early region 1B 55-kilodalton protein during lytic infection: association with nuclear viral inclusions requires the early region 4 34-kilodalton protein. J Virol. 1991;65:424–439. doi: 10.1128/jvi.65.1.424-429.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pilder S, Moore M, Logan J, Shenk T. The adenovirus E1B-55K transforming polypeptide modulates transport or cytoplasmic stabilization of viral and host cell mRNAs. Mol Cell Biol. 1986;6:470–476. doi: 10.1128/mcb.6.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rebelo L, Almeida F, Ramos C, Bohmann K, Lamond A I, Carmo-Fonesca M. The dynamics of coiled bodies in the nucleus of adenovirus-infected cells. Mol Biol Cell. 1996;7:1137–1151. doi: 10.1091/mbc.7.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reichel P A, Merrick W C, Siekierka J, Mathews M B. Regulation of a protein synthesis initiation factor by adenovirus virus-associated RNA. Nature. 1985;313:196–200. doi: 10.1038/313196a0. [DOI] [PubMed] [Google Scholar]
- 64.Riley D, Flint S J. RNA-binding properties of a translational activator, the adenovirus L4 100-kilodalton protein. J Virol. 1993;67:3586–3595. doi: 10.1128/jvi.67.6.3586-3595.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ringold G M. Regulation of mouse mammary tumor virus gene expression by glucocorticoid hormones. Curr Top Microbiol Immunol. 1979;106:79–103. doi: 10.1007/978-3-642-69357-1_4. [DOI] [PubMed] [Google Scholar]
- 66.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. pp. 7.12–7.16. [Google Scholar]
- 67.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sarnow P, Hearing P, Anderson C W, Halbert D N, Shenk T, Levine A J. Adenovirus early region 1B 58,000-dalton tumor antigen is physically associated with an early region 4 25,000-dalton protein in productively infected cells. J Virol. 1984;49:692–700. doi: 10.1128/jvi.49.3.692-700.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schneider R J. Cap independent translation in adenovirus infected cells. Curr Top Microbiol Immunol. 1995;203:117–129. doi: 10.1007/978-3-642-79663-0_6. [DOI] [PubMed] [Google Scholar]
- 70.Schneider R J, Safer B D, Munemitsu S M, Samuel C E, Shenk T. Adenovirus VAI RNA prevents phosphorylation of the eukaryotic initiation factor 2 alpha subunit subsequent to infection. Proc Natl Acad Sci USA. 1985;82:4321–4325. doi: 10.1073/pnas.82.13.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sharp P A. Splicing of messenger RNA precursors. Science. 1987;235:766–771. doi: 10.1126/science.3544217. [DOI] [PubMed] [Google Scholar]
- 72.Shenk T. Adenoviridae and their replication. In: Fields B, Howley P, Knipe D, editors. Virology. New York, N.Y: Raven Press; 1996. pp. 2111–2148. [Google Scholar]
- 73.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 74.Takeshita K, Forget B G, Scarpa A, Benz E J. Intranuclear defect in beta-globin mRNA accumulation due to a premature translation termination codon. Blood. 1984;64:13–22. [PubMed] [Google Scholar]
- 75.Thimmappaya B, Weinberger C, Schneider R J, Shenk T. Adenovirus VAI RNA is required for efficient translation of viral mRNA at late times after infection. Cell. 1982;31:543–551. doi: 10.1016/0092-8674(82)90310-5. [DOI] [PubMed] [Google Scholar]
- 76.Urlaub G, Mitchell P J, Ciudad C J, Chasin L A. Nonsense mutations in the dihydrofolate reductase gene affect mRNA processing. Mol Cell Biol. 1989;9:2868–2880. doi: 10.1128/mcb.9.7.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weinberg D H, Ketner G. Adenoviral early region 4 is required for efficient viral DNA replication and for late gene expression. J Virol. 1986;57:833–838. doi: 10.1128/jvi.57.3.833-838.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Williams J, Karger B D, Ho Y S, Castiglia C L, Mann T, Flint S J. The adenovirus E1B 495R protein plays a role in regulating the transport and stability of the viral late messages. Cancer Cells. 1986;4:275–284. [Google Scholar]
- 79.Williams J F. Enhancement of adenovirus plaque formation on HeLa cells by magnesium chloride. J Gen Virol. 1970;9:251–253. doi: 10.1099/0022-1317-9-3-251. [DOI] [PubMed] [Google Scholar]
- 80.Wu B J, Hunt C, Morimoto R I. Structure and expression of the human gene encoding major heat shock protein Hsp70. Mol Cell Biol. 1985;5:330–341. doi: 10.1128/mcb.5.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang U-C, Huang W, Flint S J. mRNA export correlates with activation of transcription in human subgroup C adenovirus-infected cells. J Virol. 1996;70:4071–4080. doi: 10.1128/jvi.70.6.4071-4080.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yoder S S, Robberson B L, Leys E J, Hook A G, Al-Ubaidi M, Yeung K Y, Kellems R E, Berget S M. Control of cellular gene expression during adenovirus infection: induction and shutoff of dihydrofolate reductase gene expression by adenovirus type 2. Mol Cell Biol. 1983;3:819–828. doi: 10.1128/mcb.3.5.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang Y, Feigenblum D, Schneider R J. A late adenovirus factor induces e1F-4B dephosphorylation and inhibition of cellular protein synthesis. J Virol. 1994;68:7040–7050. doi: 10.1128/jvi.68.11.7040-7050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]