Abstract
Background:
Migraine is a group of headache syndromes, with a prevalence of 5%–25%. Migraine is a complex recurrent headache disorder, often unilateral, throbbing or pulsating in nature aggravated by physical activity, bright light, and loud noises. Symptoms associated with migraine without aura are nausea, anorexia, and varying degrees of ophthalmic problems ranging from visual aura, ocular symptoms, and ophthalmoplegia.
Objectives:
The study determined the pattern and ophthalmic manifestations of migraine in Aminu Kano Teaching Hospital, Kano, Nigeria.
Materials and Methods:
This was an observational cross-sectional study. Ethical clearance was obtained from the research ethical committee of Aminu Kano Teaching Hospital (AKTH). A total of 254 patients diagnosed with migraine using international headache society criteria attending neurology clinic in AKTH were interviewed and examined using a structured questionnaire which captured the biodata, pattern of migraine and ocular symptoms associated with migraine. The data obtained were collated and analysed using Statistical Package for the Social Sciences, version 23.
Results:
Of the 254 patients examined, there were 95 (37.4%) males and 159 (62.6%) females, (M:F = 1:2) with mean age of 28.9 ± 9.7 years. Migraine without aura was the commonest type seen in 55.8% of patients. Most patients had unilateral headaches. About 52.8% and 57.5% had headaches that were throbbing in nature. The headache lasted for 72 h in 48.2% and 48 h in 30.3%. It was triggered by physical stress in 49.6% and lack of sleep in 46.5%. The commonest associated symptom was nausea in 44.9%, tinnitus in 39.0%, and vomiting in 29.1%. While the commonest ocular/visual symptom was photophobia in 76% of patients. Most of the patients had family history of migraine (62.8%). Migraine without aura (MWOA) was found to be significantly associated with female gender (P = 0.001; OR = 2.48; 95% CI: 1.45–4.25). Scotoma, fortifying spectra were significantly associated with migraine with aura (MWA; P = 0.0004; OR = 28.46; 95% CI: 11.53–70.35).
Conclusion:
Migraine is one of the most common types of primary headache. There is a female preponderance. Migraine has significant association with visual disturbances and ocular symptoms.
Keywords: Headache, migraine, ocular symptoms, pattern
Introduction
Headaches which do not result from any disorder are termed primary headaches and comprises migraine, tension, and cluster headaches.[1] Whereas secondary headaches result from another disorder.[2] Migraine are a group of headache syndromes, with a prevalence of 5%–25%.[2] It is divided into two main types by the International Headache Society (IHS) in 2004; migraine with aura (old term is classical migraine) and migraine without aura (old term is common migraine).[2]
Migraine is a complex recurrent headache disorder, often unilateral, throbbing or pulsating in nature aggravated by physical activity, bright light, and loud noises.[2] Symptoms associated with migraine without aura are nausea, anorexia, and photophobia.[3,4]
Migraine with aura constitutes 10%–35% of migraines.[2] It consists of aura, headache and post-headache period. The aura is a transient cortical or brain stem dysfunction of gradual onset and can last up to 60 min. Criteria for diagnosis include normal neurological examination and absence of underlying organic disorder.[2]
Aura is divided into visual and neurological symptoms; visual and neurological aura occur simultaneously in 88% of cases,[3] whereas only 25%–35% of migraineurs have visual aura occurring in isolation without neurological symptoms. Neurological auras are paresthesia, motor weakness, numbness, aphasia, alexia, and hemiplegia. Visual auras originate in the striate cortex and range from an enlarging scintillating spot to a complete homonymous hemianopia. Scotoma may be positive or negative. A variant of migraine in young people can present with recurrent transient monocular visual loss without headache and complete recovery. This variant emanates from the retina and optic nerve, known as retinal migraine.[3,4] Lasting neurological symptoms such as visual field defects should prompt neuroimaging. The relationship between glaucoma and migraine has been hypothesised by some authors. Migraine may be a risk factor for normal-tension glaucoma, while studies also showed changes in visual field that are like that seen in early glaucoma.[5] Ophthalmologist should note that there may be increased glaucomatous visual field defects in migraineurs.[6]
Other visual symptoms include micropsia, macropsia, diplopia, polyopia, halos, autokinesis-(movement of stationary objects), changes in colours, visual hallucinations, increase or decrease of distance from objects, teleopia (objects appearing more distant than they really are), fortification of spectra/teleichopsia (expanding arc in the form of zig-zag lines). Migraine with aura does carry a small risk of ischemic stroke.[6] Despite success of conventional pharmacological therapy of migraine with non-steroidal anti-inflammatory drugs (NSAIDS), caffeine, and calcium blockers, there are reports of treatment with botulinum toxin injections to the cranial musculature and injection of lignocaine with epinephrine to block the branches of ophthalmic nerve.[7]
Other forms of migraine as classified by IHS are retinal migraine and ophthalmoplegic migraine. Retinal migraine presents with visual loss in one eye, 1 h prior or during attacks but resolves within 72 h. The typical clinical presentation of ophthalmoplegic migraine generally involves transient migraine-like headache accompanied by often long-lasting oculomotor, abducent or, rarely, trochlear neuropathy with diplopia and (if oculomotor nerve is involved) pupillary abnormalities and ptosis. Conjunctival injection and pupillary changes are also associated with migraine. It is important to note photophobia is used as a diagnostic criterion for migraine.[8]
The pathophysiology is not completely known but the theory commonly responsible for visual symptoms is cortical spreading depression. Cortical spreading depression (CSD) is a slowly propagated wave of depolarisation followed by suppression of brain activity, a remarkably complex event that involves dramatic changes in neural and vascular function. Since its original description in the 1940s, it has been hypothesised that CSD is the underlying mechanism of the migraine aura, in which there is release of neuro-chemical transmitters that creates depolarisation of adjacent tissue due to potassium release, overall resulting in neuronal excitation of cortical grey matter.
Another theory is the vascular theory explaining that ischemia is caused by vasoconstriction leading to aura then rebound vasodilatation of intracranial vessels triggering perivascular nociception. This ischemia can occur due to vasospasm of the ophthalmic artery or retinal circulation thought to be the cause of ocular migraine. Ischemic optic neuropathy and permanent arcuate scotoma can occur after ocular migraine. Attempts are being made to recreate aura in the laboratory so that a way to abate it is discovered.[9]
A study in Kano reported that migraine without aura is commoner than migraine with aura (58% and 42%, respectively). Nausea, vomiting, and visual impairment were the most commonly associated symptoms and had a significant association with migraine without aura.[10]
Another study in Benin, Nigeria, interviewed patients with neuro-ophthalmic disorders. A total of 76 patients were identified from a total of 1698 patients giving an incidence of 4.47%. The most common disorders were motor nerve palsies (27.6%), optic neuropathies (22.4%) and migraine (14.5%).[11] Migraine triggers were physical stress, lack of sleep and hunger.[12]
An Indian study of 62 patients with ophthalmoplegic migraine showed 48 patients had single attacks, 14 had two or more attacks, fulfilling the IHS criteria for probable and definite ophthalmoplegic migraine, respectively. At presentation, isolated abducent, oculomotor, and trochlear nerve involvement were seen in 35 (56.5%), 21 (33.9%), and 5 (8.1%) of patients, respectively. Almost all the patients had antecedent history of migraine headache before developing ophthalmoplegia (95.2%) while only 4.8% had it within 24 h. Neuro-imaging was normal for all the patients, and steroids hastened recovery.[13]
A study in Norway reported 9.7% of migraine patients had visual disturbances before headache phase, the most frequently scintillating scotoma in 62%, and obscuration of vision in 33%. Rarely they had anopsia (partial defect in visual field), autokinesis (visual illusion of seeing movements of stationary objects), tunnel vision and micropsia.[14] Another study in Hungary found visual auras accompanied headaches in 39% of migraine patients meanwhile 19% had visual disturbance with every attack. The most common visual phenomenon described were phosphenes in 42%, photopsia in 39%, scotoma in 32%, visual snow in 27%, and fortification spectra in 20% of migraine patients. In addition, 65% of them had more than one visual symptoms during migraine which is known as combination phenomenon.[15]
The study aimed to determine the pattern of presentation of migraine and associated ocular symptoms in AKTH, Kano state.
Materials and Methods
This was an observational cross-sectional study carried out on patients already diagnosed with migraine based on the International Classification of Headache disorders criteria in the Neurology clinic of Aminu Kano Teaching Hospital (AKTH), Kano, Nigeria.
The study adhered to the Tenets of the Helsinki Declaration. The inclusion criteria were patients diagnosed with migraine aged between 18 and 50 years that consented to participate in the study. Patients diagnosed with ocular diseases such as glaucoma those with comorbidity such as hypertension, stroke and diabetes, patients outside the specified age range and those that declined consent were excluded. Individual informed consent were required and signed. The sample size was derived using Fisher’s formula for qualitative variables applied in descriptive studies in which a prevalence study was used.[16]
All patients diagnosed with migraine attending the Neurology clinic constituted the sampling frame. Alternate patients who fulfilled the inclusion criteria were selected until the required sample size was obtained. Selected patients were interviewed using structured modified questionnaires for the study which captured the demographics, migraine history, pattern of migraine, ocular/visual symptoms associated with migraine and history of other relevant eye disorders. Moreover, these symptoms were compared with those at time of first presentation as documented in the patient’s records.
The patients were interviewed using structured modified questionnaire for the study, which captured the demographics, migraine history, pattern of migraine, ocular/visual symptoms associated with migraine and history of other eye disorders. Subsequently, patients were examined by assessing both distant and near visual acuity with ETDRS Log MAR chart/tumbling E chart and near chart. The extraocular muscle motility was examined using six cardinal positions followed by a detailed anterior segment using a pen-torch and slit lamp biomicroscope to examine the conjunctiva, then cornea to identify obvious pathology such as opacity, then the iris and the pupil for any abnormal pupillary light reflex, the lens for opacity or cataract.
A direct ophthalmoscopy was done to document finding of optic disc for colour, outline and cup-to-disc ratio, the retinal vessels, macula and retina. In addition, the intraocular pressure was checked using an applanation tonometer. The findings were recorded, meanwhile, those that had abnormal findings were given appointments to attend the ophthalmology clinic.
The data collated were coded and entered for statistical analysis with Software Package for Social Sciences (SPSS) version 23 (IBM Corp: New York, United States). Data were expressed as mean, ratio, standard deviations, ranges, and frequencies. Chi-square test was used to analyse the association between gender, family history, visual disturbances, and migraine subtype. Those with P value of less than or equal to 0.05, which showed significant association, were subjected to logistic regression.
Results
Of the 254 studied, there were 159 females (62.6%) and 95 males (37.4%) with (M:F = 1:2). The age range was 18–50 years with a mean age of 28.9 ± 9.7 years. Table 1 gives a summary of age and sex distribution of all the patients. They had normal intraocular pressure (10–21 mmHg) with mean of 15.6 ± 2.5 mmHg.
Table 1.
Age and sex distribution of patients with migraine
| Age group (years) | Gender | Total (%) | |
|---|---|---|---|
| Male (%) | Female (%) | ||
| 15–20 | 13 (5.1) | 40 (15.8) | 53 (20.9) |
| 21–25 | 30 (11.8) | 47 (18.5) | 77 (30.3) |
| 26–30 | 20 (7.9) | 8 (3.1) | 28 (11.0) |
| 31–35 | 13 (5.1) | 23 (9.1) | 36 (14.2) |
| 36–40 | 11 (4.3) | 10 (3.9) | 21 (8.2) |
| 41–45 | 4 (1.6) | 12 (4.7) | 16 (6.3) |
| 46–50 | 4 (1.6) | 19 (7.5) | 23 (9.1) |
| Total | 95 (37.4) | 159 (62.6) | 254 (100) |
Migraine type
The commonest type of migraine was migraine without aura (MWOA) which accounted for 55.8% of patients. Migraine with aura was seen in 40.2%. Others were ophthalmoplegic migraine and retinal migraine as shown in Table 2.
Table 2.
Distribution of migraine by type
| Migraine type | Frequency | Percentage (%) |
|---|---|---|
| Migraine without aura | 142 | 55.8 |
| Migraine with aura | 102 | 40.2 |
| Ophthalmoplegic migraine | 6 | 2.4 |
| Retinal migraine | 4 | 1.6 |
| Total | 254 | 100.0 |
Pattern of migraine
About 52.8% of the patients had unilateral headache, 33.5% had bilateral headache, and 13.8% had both types [Table 3]. The nature of headache was mostly throbbing in 55.7% of patients, dull in 28.7%, sharp in 8.7%, and stabbing in 5.1%. The majority of patients (38.6%) had migraine headache more than 3–4 times a month and followed by twice a month in 30.7% and 25.2% of the patients had been diagnosed 6 months earlier.
Table 3.
Localisation of migraine headache
| Laterality of headache | Frequency | Percentage (%) |
|---|---|---|
| Unilateral | 134 | 52.8 |
| Bilateral | 85 | 33.4 |
| Both | 35 | 13.8 |
| Total | 254 | 100.0 |
Presence of other eye disorder not associated with migraine
About 75 patients of the 254 with migraine were diagnosed with an eye disease. Of these, 20.9% of them had refractive error and 8.6% had allergic conjunctivitis. None were sight-threatening
Ethnicity
The majority were Hausa/Fulani accounting for 63.4% of patients, and other tribes such as Nupe and Kanuris accounted for 20.5% of the patients. The Yoruba and Igbo account for 13.4% and 2.7% of the studied population, respectively [Figure 1].
Figure 1.
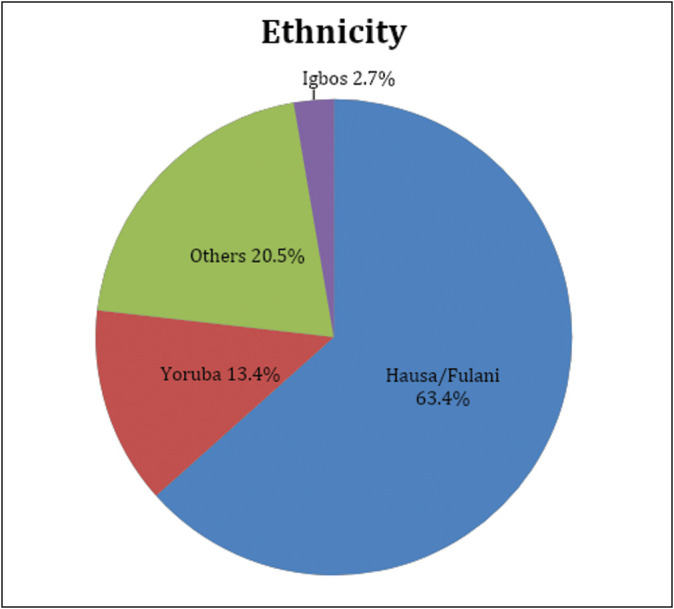
Ethnicity of patients with migraine
The visual disturbances and ocular manifestation of migraine: Of the 254 migraine patients, 204 (81.9%) had visual disturbances/ocular manifestations and 46 (18.1%) of them had no ocular or visual disturbances. Some had one or more ocular/visual disturbances during attacks. The commonest visual disturbance was photophobia reported in 76.0% of migraine patients, and 37.0% had blurred vision. Visual snow/TV static accounted for 29.9%, fortifying spectra in 24.0%, and scotoma in 18.5%. Meanwhile, other symptoms such as redness, photopsia, and lid twitching accounted for 10.6%. Other features are illustrated in Figure 2.
Figure 2.

Ocular symptoms associated with migraine. *Patients could have multiple symptoms
The ocular symptoms associated with migraine headache was found to last for 60 min in 35.8%, 30 min in 30.7% and 24 h in 12.6%. Those that had ocular manifestation lasting for 1 week accounted for 5.9% and had ophthalmoplegic migraine. About 55.9% of patients with migraine associated with visual disturbance/ocular manifestation felt their ocular symptoms were of concern and stopped them from carrying out their daily activities and need further evaluation by an ophthalmologist.
Of the total number examined, 32 (12.6%) patients had refractive errors and their vision improved to 0.0 logMAR (6/6) in both eyes with pinhole/glasses. A total number of 15 patients (5.6%) had subnormal near vision. Those patients were found to be above 40 years of age. Six patients (2.4%) who were diagnosed with ophthalmoplegic migraine had cranial nerve III and cranial nerve IV palsy on examination and were placed on steroids/physiotherapy and said to have improved. All other patients had normal anterior segment examinations and fundoscopy.
Female gender was significantly associated with MWOA (P = 0.001) using chi-square (P-value ≤ 0.05), and females are 2.8 times more likely to have MWOA than MWA (OR = 2.8; 95% CI: 1.45–4.25). Ocular symptoms were significantly associated with MWOA (P = 0.0004) and not with MWA. This is shown in Table 4. Photophobia has no significant association with any of the migraine variants (P = 0.083). Blurring of vision was significantly associated with MWOA (P = 0.001) and it occurs 9.8 times more in MWOA than in MWA. Visual Snow and Micropsia were also associated with MWOA (P = 0.0002) and (P = 0.001), respectively.
Table 4.
Risk factors of visual symptoms associated with migraine
| Patients’ Characteristics | MWOA | MWA | OR (95% CI) | P-value |
|---|---|---|---|---|
| Gender | ||||
| Male | 62 (65.3%) | 29 (30.5%) | 2.8 (1.45–4.25) | 0.001* |
| Female | 85 (53.4%) | 68 (42.7%) | ||
| Ocular symptoms | ||||
| Present | 105 (50.5%) | 93 (47.3%) | 4.03 (1.85–8.76) | 0.0004* |
| Absent | 37 (80.4%) | 09 (19.6%) | ||
| Photophobia | ||||
| Present | 102 (52.8%) | 81 (42.0%) | 1.70 (0.93–3.10) | 0.083 |
| Absent | 40 (65.6%) | 21 (34.4%) | ||
| Blurring of vision | ||||
| Present | 72 (76.6%) | 22 (23.4%) | 9.82 (5.41–17.87) | 0.001* |
| Absent | 30 (18.8%) | 130 (81.2%) | ||
| Visual snow | ||||
| Present | 14 (32.6%) | 26 (67.4%) | 9.09 (4.80–17.22) | 0.0002* |
| Absent | 128 (60.7%) | 73 (34.6%) | ||
| Micropsia | ||||
| Present | 07 (16.7%) | 35 (31.6%) | 8.77 (3.72–20.68) | 0.001* |
| Absent | 135 (63.7%) | 67 (83.3%) | ||
| Fortifying spectra | ||||
| Present | 06 (9.8%) | 55 (90.2%) | 28.46 (11.53–70.35) | 0.001* |
| Absent | 136 (70.5%) | 47 (24.4%) | ||
| Scotoma | ||||
| Present | 07 (14.9%) | 40 (85.1%) | 13.36 (5.68–31.47) | 0.0004* |
| Absent | 135 (65.2%) | 62 (30.5%) | ||
| Paresthesias | ||||
| Present | 14 (22.2%) | 46 (73.0%) | 7.11 (3.65–13.84) | 0.001* |
| Absent | 128 (67.0%) | 56 (29.3%) | ||
| Nausea | ||||
| Present | 69 (60.5%) | 45 (39.5%) | 0.73 (0.41–1.31) | 0.293 |
| Absent | 73 (52.1%) | 57 (40.7%) | ||
| Phonophobia | ||||
| Present | 02 (7.7%) | 20 (76.9%) | 3.49 (1.16–10.51) | 0.027* |
| Absent | 140 (61.4%) | 82 (36.0%) | ||
| Vomiting | ||||
| Present | 29 (39.2%) | 38 (51.4%) | 2.94 (1.58–4.49) | 0.001* |
| Absent | 113 (62.8%) | 64 (35.6%) | ||
CI: confidence interval, MWA: migraine with aura, MWOA: migraine without aura, OR: odds ratio.
*P value ≤ 0.05 is statistically significant
Fortifying spectra have a strong association with MWA (P = 0.001). It occurs 28 times more in MWA than MWOA. Scotoma and paresthesia were strongly associated with MWA (P = 0.0004) and (P = 0.001), respectively, rather than MWOA as shown in the table. Nausea is not associated with a particular type of migraine (P = 0.293) Based on our findings, it can occur equally in any of migraine subtype without any predilection. Whereas photophobia and vomiting were significantly associated with MWOA (P = 0.027) and (P = 0.001), respectively. This suggests that these accompany MWOA more often than MWA.
Discussion
Migraine is the commonest cause of headache and typically affects patients in their teens till fifth decade, peaks at second to third decade and declines with increase in age except in those with family history of migraine.[17] The ages of the patients in this study are in keeping with findings in other studies.[18,19] Furthermore, the clinical implication for age is that headaches with associated ocular manifestation in patients above 50 years are not common and caution must be taken in evaluating such patients in both ophthalmology and neurology clinic, to rule out other causes such as glaucoma, diabetic/hypertensive retinopathy, retinal vascular diseases, brain tumours, and aneurysms.[20]
Female gender is a risk for migraine as the majority of patients in the study were females similar to worldwide epidemiological studies where 75% of migraineurs are women.[12,14,18,21,22] Migraine is more in women due to female hormones such as estrogen and progesterone responsible for the increase in prevalence of migraine in girls after puberty.[14,22,23] One of the evidence is from epidemiological studies that show a higher female prevalence of migraine after puberty, with lifetime prevalence of 25% compared with 8% in men.[23,24]
The pattern of migraine was used as criteria for diagnosis of migraine using IHS. The most common presentation of headache was unilateral and the typical characteristic of migraine headache was throbbing or pulsating as described which is in keeping with other studies.[12,23,25] The study also shows that bilateral headache does not preclude the diagnosis of migraine. MWOA is of higher proportion in migraine patients whereas ophthalmoplegic and retinal migraine are rare variants of migraine.[2,17,26]
Most of the migraine headaches lasted for 72 h or less without medical intervention. The IHS criteria for diagnosing migraine is headache phase lasting for 4–72 h, and this as obtained in this study and other studies and any headache lasting longer than 72 h should prompt suspicion.[2,27]
Ocular pathologies such as anterior uveitis, angle closure glaucoma, and viral conjunctivitis can present with photophobia, these can easily be ruled out from findings during clinical examinations, whilst systemic diseases such as meningitis have a myriad of other signs and symptoms apart from photophobia.[20]
Other visual disturbances are associated with MWA such as fortifying spectra, phosphenes, and scotoma as in many epidemiological studies.[28,29,30,31] Visual fields are necessary in such patients as complicated migraine can sometimes lead to cortical vascular occlusion with permanent homonymous hemianopia whereas temporary visual field losses after migraine attack are common.[13,32] Modification of lifestyle for those with scotomas and fortifying spectra is necessary such as staying away from driving during attack.[20]
These visual symptoms are found to last on average 30–60 min, the scientific explanation for this is that visual symptoms are due to cortical spreading depression demonstrated by using MRI showed that an electrophysiological event is generated in the human visual cortex due to vasodilatation and vasoconstriction leading to a retinoscopic progression of the visual perception, this event lasts for 20–60 min.[25,28,33]
Some studies have shown a relationship between normal-tension glaucoma and migraine since they share similar pathophysiology which makes visual field testing essential.[15,20]
Ophthalmoplegic migraine is rare as seen in this study and others, this is because presentation is similar to transient ischemic attack and strokes. It is a diagnosis of exclusion, patients must have had two similar events with complete resolution and radiological investigations that are normal. Patients presenting with severe headache diplopia, CN palsy must have a complete workup with neuro-imaging before concluding as migraine because of similar presentation in diabetic ophthalmoplegia, pituitary tumours, berry aneurysms and Tolosa-Hunt syndrome.[13,34]
Retinal migraine was also found in only a few patients and did not have any fundal changes because the loss of vision lasted only for 30–60 min. The changes are usually seen during attack where the retina/optic nerve head appears pale.[15,25,26,35] It is important for such patient to be evaluated by an ophthalmologist to exclude vision-threatening diseases which can present in similar manner such as central retinal artery occlusion, ocular ischemic syndrome and giant cell arteritis. Most patients are seen by the neurologist, and not too many were referred to the eye clinic.[14]
Ophthalmologist needs to be more proactive on the IHS criteria, which makes it easier to diagnose migraine in patients with headaches referred to the eye clinic,[2] especially those who have been refracted several times without amelioration of their symptoms which can mimic asthenopia.[36] The knowledge of migraine pattern, sequence, duration and associated symptoms makes the diagnosis of migraine easier and the ophthalmologist can decide whether the ocular symptoms are attributed to migraine or another disease entity therefore placing the ophthalmologist in the forefront of diagnosing, counselling and management of such patients.
The strength of the study had advantage of using a large sample size relative to migraine prevalence, which gives the desired level of statistical significance.
The limitation of the study is that it was a hospital-based study which will not give us a true representation of those with migraine in the community.
Conclusion
Migraine is a complex disorder which cuts across all gender, more in the working age group. It has associated ocular symptoms making it necessary for the ophthalmologists to diagnose it correctly for appropriate referral and management.
Ethical approval
Ethical approval was obtained from the ethical review board of Aminu Kano Teaching Hospital.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Antonaci F, Cristina VI, Anna LD, Federica G, Aynur O, Umberto B. The evolution of headache from childhood to adulthood: A review of the literature. J Headache Pain. 2014;15:15. doi: 10.1186/1129-2377-15-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Headache Classification Subcommittee of the International Headache Society. The international classification of headache disorders. Cephalalgia. (2nd ed.) 2004;1:9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 3.Saunders LH, Miller NR, Newman NJ. Walsh and Hoyt’s Clinical Neuro-Ophthalmology. Baltimore: Lippincott Williams and Wilkins; 1998. pp. 3657–723. [Google Scholar]
- 4.Drummond PD. Disturbance in ocular sympathetic function and facial blood flow in unilateral migraine headache. J Neurol Neurosurg Psychiatry. 1990;53:121–5. doi: 10.1136/jnnp.53.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Mannis M, Giraldi JP, De Feo A, Rinalcluzzi S, De Benedetti G, Mollicone A, et al. Migraine and ocular pain in glaucoma suspects. Cephalagia. 1999;19:243–7. doi: 10.1046/j.1468-2982.1999.019004243.x. [DOI] [PubMed] [Google Scholar]
- 6.ComoÔlu S, YarangÜmeli A, KÖz OG. Glaucomatous visual field defects in patients with migraine. J Neurol. 2005;250:201–6. doi: 10.1007/s00415-003-0975-6. [DOI] [PubMed] [Google Scholar]
- 7.Rosengarten B, Sperner J, GÖrgen-pauly U, Kaps M. Cerebrovascular reactivity in adolescents with migraine and tension type headache during headache free interval and attack. Headache. 2003;43:458–63. doi: 10.1046/j.1526-4610.2003.03090.x. [DOI] [PubMed] [Google Scholar]
- 8.Silbertein SD, McCrery DC. Ergotamine and dihydroergotamine, history, pharmacology, and efficacy. Headache. 2005;43:144–66. doi: 10.1046/j.1526-4610.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 9.Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia. 1988;8:1–96. [PubMed] [Google Scholar]
- 10.Owolabi LF, Gwaram B. Clinical profile of primary headache disorders in Kano, Northwestern Nigeria. J Med Tropics. 2012;14:109–15. [Google Scholar]
- 11.Omoti AE, Erameh WJ. Pattern of neuro-ophthalmic disorders in a tertiary eye centre in Nigeria. Niger J Clin Pract. 2007;10:147–51. [PubMed] [Google Scholar]
- 12.Yazdanparast M, Abrishamizadeh AA, Mahboobi H, Omrani A, Ghasemi M, Ghorashi M, et al. Prevalence of and factors associated with migraine in medical students at Bandar Abbas, Southern Iran. Electr Physician. 2012;5:679–84. doi: 10.14661/2013.679-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lal V, Sahota P, Singh P. Ophthalmoplegia with migraine in adults: Is it ophthalmoplegic migraine? Headache. 2009;49:838–50. doi: 10.1111/j.1526-4610.2009.01405.x. [DOI] [PubMed] [Google Scholar]
- 14.Queiroz LP, Rapoport AM, Sheftell FD, Siegel SE, Baskin SM. Character of migraine visual aura. Headache. 2002;8:160–68. doi: 10.1046/j.1526-4610.1997.3703137.x. [DOI] [PubMed] [Google Scholar]
- 15.Russell MB, Olesen J. A nosographic analysis of the migraine aura in a general population. Brain. 2006;119:355–61. doi: 10.1093/brain/119.2.355. [DOI] [PubMed] [Google Scholar]
- 16.Jung SH. Stratified Fisher’s exact test and its sample size calculation. Biom J. 2014;56:129–40. doi: 10.1002/bimj.201300048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu HY, Fuh JL, Lu SR, Chen S-P, Chou C-H, Wang Y-F, et al. Transient visual disturbances in adolescents: Migrainous feature or headache-accompanied? Cephalalgia. 2012;32:1109–15. doi: 10.1177/0333102412460777. [DOI] [PubMed] [Google Scholar]
- 18.Bigal ME, Lipton RB. Excessive acute migraine medication use and migraine progression. Neurology. 2008;71:1821–8. doi: 10.1212/01.wnl.0000335946.53860.1d. [DOI] [PubMed] [Google Scholar]
- 19.Woldeamanuel YW, Andreou AP, Cowan RP. Prevalence of migraine headache and its weight on neurological burden in Africa: A 43-year systematic review and meta-analysis of community based studies. J Neurol Sci. 2014;342:1–15. doi: 10.1016/j.jns.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 20.Broadway DC, Drance SM. Glaucoma and vasospasm. Br J Ophthalmol. 2000;82:862–70. doi: 10.1136/bjo.82.8.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stovner LY, Zwart JA, Hagen K, Terwindt GM, Pascual J. Epidemiology of headache in Europe. Euro J Neurol. 2006;13:333–45. doi: 10.1111/j.1468-1331.2006.01184.x. [DOI] [PubMed] [Google Scholar]
- 22.Ezeala-Adikaibe BA, Onyekonwu C, Okudo G, Onodugo O, Ekenze S, Orjioke C, et al. Prevalence of primary headache in urban slum in Enugu state, south east Nigeria: A door to door survey. Headache. 2014;54:160–10. doi: 10.1111/head.12465. [DOI] [PubMed] [Google Scholar]
- 23.Steiner TJ, Scher AI, Stewart WF, Kolodner K, Liberman J, Lipton RB. The prevalence and disability burden of adult migraine in England and their relationship to age, gender and ethnicity. Cephalalgia. 2003;23:519–27. doi: 10.1046/j.1468-2982.2003.00568.x. [DOI] [PubMed] [Google Scholar]
- 24.Lipton RB, Stewart WF, Celentano DD, Reed ML. Undiagnosed migraine headaches. A comparison of symptoms based and reported physician diagnosis. Arch Intern Med. 1992;152:1273–8. doi: 10.1001/archinte.152.6.1273. [DOI] [PubMed] [Google Scholar]
- 25.Jogi V, Mehta S, Gupta A, Singh P, Lal V. More clinical observations on migraine associated with mono-ocular visual symptoms in an Indian population. Ann Indian Acad Neurol. 2016;19:63–8. doi: 10.4103/0972-2327.168628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pierre G, Dominque V, Michel LM, et al. Is migraine with cranial palsy an ophthalmoplegic migraine. Headache. 2007;8:119–22. doi: 10.1007/s10194-007-0371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders. Cephalalgia. (3rd ed. (Beta version)) 2013;33:629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- 28.Cologno D, Torrelli P, Manzoni GC. Transient visual disturbance during migraine without aura attacks. Headache. 2002;42:930–3. doi: 10.1046/j.1526-4610.2002.02216.x. [DOI] [PubMed] [Google Scholar]
- 29.Eriksen MK, Thomsen LL, Olesen J. Sensitivity and specificity of the new international diagnostic criteria for migraine with aura. J Neurol Neurosurg Psychiatry. 2005;76:212–7. doi: 10.1136/jnnp.2004.037853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schankin CJ, Maniyar FH, Digre KB, Goadsby PJ. Visual snow: A disorder distinct from persistent migraine aura. Brain. 2014;137:1419–28. doi: 10.1093/brain/awu050. [DOI] [PubMed] [Google Scholar]
- 31.Simpson JC, Goadsby PJ, Prabhakar P. Positive persistent visual symptoms (visual snow) presenting as migraine variant in 12-year-old girl. Pediatr Neurol. 2013;49:361–3. doi: 10.1016/j.pediatrneurol.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Drummon PD, Anderson M. Visual loss after attack of migraine with aura. Cephalagia. 2000;12:349–52. doi: 10.1111/j.1468-2982.1992.00349.x. [DOI] [PubMed] [Google Scholar]
- 33.Rasmausen BK, Olesen J. Migraine with aura and migraine without aura: An epidemiological study. Cephalalgia. 2000;12:221–8. doi: 10.1046/j.1468-2982.1992.1204221.x. [DOI] [PubMed] [Google Scholar]
- 34.Kirschmann M, Thomsen LL, Olesen J. Basilar type migraine, clinical, epidemiologic and genetic features. Neurology. 2006;66:880–86. doi: 10.1212/01.wnl.0000203647.48422.dd. [DOI] [PubMed] [Google Scholar]
- 35.Ambar C, Angshuman M. Ophthalmoplegic migraine: A critical analysis and a new proposal. Ann Indian Acad Neurol. 2012;15:2–5. doi: 10.4103/0972-2327.99985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mattson P, Lundberg PO. Characteristic and prevalence of transient visual disturbances of visual aura. Cephalagia. 1999;19:478–84. doi: 10.1046/j.1468-2982.1999.019005479.x. [DOI] [PubMed] [Google Scholar]


