Abstract
Background:
Most data on irrigation and debridement with component retention (IDCR) as a treatment for acute periprosthetic joint infections (PJIs) focuses on primary total joint arthroplasties (TJAs). However, the incidence of PJI is greater after revisions. We investigated the outcomes of IDCR with suppressive antibiotic therapy (SAT) following aseptic revision TJAs.
Methods:
Through our total joint registry, we identified 45 aseptic revision TJAs (33 hips, 12 knees) performed from 2000 to 2017 that were treated with IDCR for acute PJI. Acute hematogenous PJI was present in 56%. Sixty four percent of PJIs involved Staphylococcus. All patients were treated with 4 to 6 weeks of intravenous antibiotics with the intention to treat with SAT (89% received SAT). Mean age was 71 years (range, 41 to 90), with 49% being women and a mean body mass index of 30 (range, 16 to 60). Mean follow-up was 7 years (range, 2 to 15).
Results:
The 5-year survivorships free from re-revision for infection and reoperation for infection were 80% and 70%, respectively. Of the 13 reoperations for infection, 46% involved the same species as the initial PJI. The 5-year survivorships free from any revision and any reoperation were 72% and 65%, respectively. The 5-year survivorship free from death was 65%.
Conclusions:
At 5 years following IDCR, 80% of implants were free from re-revision for infection. As the penalty for implant removal is often high in revision TJAs, IDCR with SAT is a viable option for acute infection after revision TJAs in select patients.
Level of Evidence:
IV
Keywords: Periprosthetic joint infection, revision total hip arthroplasty (THA), revision total knee arthroplasty (TKA), Debridement Antibiotics and Implant Retention
INTRODUCTION
Periprosthetic joint infection (PJI) is a common failure mode following total hip arthroplasty (THA) and total knee arthroplasty (TKA), with an incidence of 1 to 2%[1, 2]. The incidence of PJI is higher in revision THAs and TKAs, and because revision rates are increasing, more patients are at risk of PJI[3]. In addition to issues of patient morbidity, PJI places a major burden on healthcare systems and economies. By 2030, the combined annual hospital costs related to PJI of the hip and knee are estimated to reach $1.85 billion[4].
When considering treatment of acute PJI in previously revised hip and knee joints, one must balance the challenges of resecting revision implants with the risk of retaining components and developing chronic PJIs. This is particularly important as revision components are often extensive and well-fixed, while soft tissues are frequently tenuous.
Irrigation and debridement with component retention (IDCR) followed by suppressive antibiotic therapy (SAT) represents a pragmatic treatment for appropriately selected patients who have acute early postoperative or acute hematogenous PJI[5, 6]. This technique typically involves either a single or a planned double IDCR[5–7]. While success rates vary in the literature concerning IDCR for infected primary THAs and TKAs, data on infected revisions treated with IDCR are limited mainly to studies of patients who underwent prior two-stage exchange who then received further surgery[8–10].
As such, the goals of this study were to examine the contemporary results of IDCR with SAT for acute infections after aseptic revision THAs and TKAs, and to identify risk factors for treatment failure, defined explicitly as any reoperation for infection.
PATIENTS AND METHODS
Following Institutional Review Board approval (ID 19–004223), a retrospective review was conducted using our institutional total joint registry (TJR) to identify patients who underwent aseptic revision THA or TKA subsequently complicated by acute PJI and treated with IDCR and SAT between the years January 1, 2000 and December 31, 2016. Patients were eligible if they were ≥18 years of age and diagnosed with acute PJI following aseptic revision THA or TKA.
We identified 45 aseptic revisions (33 revision THAs and 12 revision TKAs) in 44 patients who subsequently developed an acute PJI and were treated with an IDCR and the intention to treat with SAT. There were 22 first time aseptic revisions and 23 were multiply revised. Mean time from primary THA or TKA to the index aseptic revision was 11 years (range, 21 to 39). Among the multiply revised patients, the mean number of aseptic revisions was 3 (range, 2 to 6 revisions). There were no documented infections preceding any of these aseptic revisions. Mean age at time of PJI was 71 years (range, 41 to 90 years) with 49% being women and mean body mass index (BMI) was 30 (range, 16 to 60). Patients were stratified based on the criteria by McPherson et al. [11] which details infection type, host status, and local extremity type (Table 1). There were 27% of patients who were A hosts (7 hips, 5 knees), 49% B hosts (17 hips, 5 knees), and 24% C hosts (9 hips, 2 knees). Patients who did not have local compromising factors were considered local extremity grade 1 (26 hips, 9 knees; 78%), 1 to 2 factors grade 2 (7 hips, 2 knees; 20%), and more than 2 factors grade 3 (1 knee; 2%). Among the 44 patients (45 joints), 7 died and 11 were revised within 2 years. Three were lost to follow-up. Of the remaining 24 joints, the mean follow-up was 7 years (range, 2 to 15 years).
Table 1:
McPherson Host Staging System for Periprosthetic Joint Infection
| Category | Grade | Description |
|---|---|---|
|
| ||
| Infection type | I | Acute early postoperative infection |
| II | Acute hematogenous infection | |
| III | Late chronic infection | |
| Systemic host grade | A | Uncompromised |
| B | Compromised (1–2 compromising factors) Severely compromised (>2 compromising factors) or one of the following: Absolute neutrophil count < 1000 |
|
| C | CD4 T cell count < 100 Intravenous drug abuse Chronic active infection, other site Dysplasia or neoplasm of the immune system |
|
| Local extremity grade | 1 | Uncompromised |
| 2 | Compromised (1–2 compromising factors) | |
| 3 | Significant compromise (>2 compromising factors) | |
Table adapted from McPherson et al. [11]
The indications for the index revisions were aseptic loosening in 17 cases (12 hips and 5 knees; 38%), dislocation or instability in 15 (13 hips and 2 knees; 34%), periprosthetic fracture in 6 (4 hips and 2 knees; 13%), osteolysis/ polyethylene wear (5 hips; 11%), and arthrofibrosis (2 knees; 4%). Acetabular components were revised in 7 hips, femoral components in 10 hips, and both acetabular and femoral components in 6 hips. There were ten hips that had isolated head/liner exchanges. In revision TKAs, revision of the tibial component occurred in 2 knees, revision of the femoral component in 4 knees, and both components in 4 knees. There was one case revised for a polyethylene liner alone and another revised a loose patellar component.
There were forty-three (96%) cases that met the 2011 Musculoskeletal Infection Society (MSIS) definition of acute PJI[12] and 2 cases (2 knees; 4%) did not meet full criteria, but were diagnosed with acute PJI necessitating IDCR and SAT by a consulting orthopaedic surgeon and infectious disease specialist. Of these 2 cases, the first had an erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) of 24 mm/hour (mm/h) and 113 mg/liter (mg/L), respectively. For the second, the ESR and CRP were 60 mm/h and 10 mg/L, respectively. Cultures were negative in both cases; however, both had received antibiotics prior to cultures. Synovial cell counts (percentage neutrophils) were 38,000 (85%) and 4,786 (91%) cells/milliliter (mL), respectively.
We selected the 2011 MSIS criteria because we utilized this definition clinically during the study period. Acute early postoperative infections were defined as those occurring within 4 weeks of revision THA or TKA. Acute hematogenous infections were those occurring more than 4 weeks after revision, but with symptoms for less than 3 weeks[6, 13]. There were 25 acute hematogenous infections (14 hips and 11 knees; 56%) and 20 acute early postoperative infections (19 hips and 1 knee; 44%). In the acute early postoperative infection group, the mean time from revision arthroplasty to infection diagnosis was 2.5 weeks (range, 1 to 4 weeks). In the acute hematogenous infection group, mean time from revision to diagnosis of PJI was 62 weeks (range, 4.4 weeks to 12 years). There were six who had definitive infectious etiologies: pneumonia with sepsis (2 hips; 29%); recent nasal or dental procedures (2 knees; 29%); septic arthritis of the ipsilateral 1st metatarsophalangeal joint (1 knee; 14%); and ipsilateral leg cellulitis (1 knee; 14%).
The most common organisms leading to PJI were staphylococcus species (24 hips and 5 knees; 64%) (Table 2). There were nine cases (20%) that were culture negative of which 7 received antibiotics before cultures. Of these, mean ESR and CRP were 55 mm/hour (range, 24 to 106) and 56 mg/L (range, 37 to 113 mg/L), respectively. Mean synovial cell count was 21,024 cells/mL (range, 3,722 to 50,000), and synovial neutrophil percentage was 90% (range, 81 to 98%).
Table 2.
Causative Organism(s)
| Causative Organism | Hips | Knees | Total, n (%) |
|---|---|---|---|
|
| |||
| Staphylococci (monomicrobial and polymicrobial) | 24 | 5 | 29 (64) |
| S. aureus | |||
| MSSA | 5 | 3 | 8 |
| MSSA & enterococcus | 0 | 1 | 1 |
| MSSA & Coagulase-negative staphylococcus | 1 | 0 | 1 |
| MRSA | 4 | 0 | 4 |
| MRSA & Group B Streptococcus | 1 | 0 | 1 |
| Coagulase-negative staphylococcus | 13 | 1 | 14 |
| Staphylococcus lugdunensis | 1 | 0 | 1 |
| Staphylococcus warneri, Corynebacterium striatum | 1 | 0 | 1 |
| Coagulase-negative staphylococcus, Bacteroides Fragilis | 1 | 0 | 1 |
| Staphylococcus simulans, Enterococcus faecalis, Corynebacterium striatum | 1 | 0 | 1 |
| Staphylococcus epidermidis, Corynebacterium striatum, Propionibacterium | 1 | 0 | 1 |
| Culture Negative | 5 | 4 | 9 (20) |
| Streptococcus | 1 | 3 | 4 (9) |
| Strep. mitis | 0 | 1 | 1 |
| Strep. viridans | 0 | 1 | 1 |
| Strep. agalactiae | 0 | 1 | 1 |
| Strep. pneumonia | 1 | 0 | 1 |
| Other | 3 | 0 | 3 (7) |
| Enterococcus faecalis | 1 | 0 | 1 |
| Enterococcus sp., Corynebacterium sp. | 1 | 0 | 1 |
| Bacteroides fragilis | 1 | 0 | 1 |
MSSA: Methicillin-susceptible staphylococcus aureus; MRSA: methicillin-resistant staphylococcus aureus
In 37 cases, a single IDCR was performed. In 8 cases (4 hips & 4 knees), antibiotic beads (3 hips and 3 knees) or an antibiotic-impregnated spacer in place of an arthroplasty insert (1 hip and 1 knee) were implanted followed by a second planned IDCR at a median of 3 days (range, 2 to 39 days) after the first[7, 14]. Following debridement, most IDCR procedures involved irrigation of the joint space with 6 to 9 liters (L) of normal saline solution alone (62%), antibiotic/antiseptic solution along with normal saline (22%), a combination of normal saline with diluted povidone-iodine solution (9%), or a combination of saline, diluted povidone-iodine solution and antibiotic/antiseptic solutions or powders (7%). Components were well-fixed in all cases. Intravenous (IV) antibiotics were used for a mean of 5 weeks (range, 3 to 6).
After the IDCR, patients were administered IV antibiotics and targeted therapy as directed by cultures and infectious disease specialists following clinical practice guidelines [15]. Patients were recommended to begin SAT following up to 6 weeks of IV, or IV and oral antibiotics (40 patients). Of these 40 patients, 38 received suppressive antibiotics for the life of the implant. Of the 2 remaining patients who halted SAT early, one stopped cefadroxil therapy secondary to clostridium difficile infection, and one prematurely discontinued SAT in anticipation for a planned resection that was delayed. This patient was clinically doing well during this time. There were 15% of patients on SAT who required antibiotic changes at some point postoperatively. There were 14 of the 29 cases involving staphylococcus speciation (12 hips and 2 knees) treated with rifampin.
Harris hip scores (HHS)[16], Knee Society scores (KSS)[17], postoperative complications, and subsequent treatment at outside institutions were recorded at 6 weeks, 3 months, 1 year, 2 years, 5 years, and 5 years thereafter by our TJR. These TJR data were combined with a review of all electronic medical records to ensure accurate capture of outcomes of IDCR for acute PJI.
Data analyses
Data were summarized using means, medians, and standard deviations (SDs) for continuous variables and counts and percentages for categorical variables. The primary outcomes were analyzed as time-to-event using survivorship methodology[18]. Reoperations included any instance where an incision was made, and reoperation for infection included any form of infection, both superficial and deep. Re-revisions encompassed instances when a component was removed. The association between risk factors and outcomes of interest were evaluated using Cox proportional hazards regression. Cox models utilized the robust variance estimate to properly account for patients with bilateral involvement where applicable[19]. Rifampin was studied as a time dependent covariate. All statistical tests were two-sided with statistical significance set at P < 0.05. Analyses were conducted using R version 3.6.2 and Statistical Analysis System 9.4 (SAS Institute, Inc., Cary, North Carolina).
SOURCE OF FUNDING:
No external source of funding was received.
RESULTS
Reoperation and Revision for Infection
The 5-year survivorship free from reoperation for infection was 70% (95% confidence interval (CI) =53–86) (Figure 1). The 5-year survivorships free from reoperation for infection in McPherson Host Types A, B, and C were 83% (95% CI=61–100), 75% (95% CI=50–97), and 36% (95% CI=13–100), respectively. The 5-year survivorships free from reoperation for infection in the single and multiple prior aseptic revision groups was 81% (95% CI=59–100) and 58% (95% CI=36–86), respectively. The 5-year survivorships free from reoperation for infection in the acute hematogenous and acute early PJI groups were 79% (95% CI=58–97) and 57% (95% CI=34–88), respectively. There were 13 reoperations for infection. Of these, 8 were for component resections with or without cement antibiotic spacer insertion, 3 were for unplanned, repeat IDCRs, and 2 were for debridement of superficial wound infections. At the time of reoperation for infection, the infectious organism was the same as during the index IDCR in 6 of 13 patients. Staphylococcus was involved in the initial infection in 9 (69%) of patients who received reoperation for infection.
Figure 1.
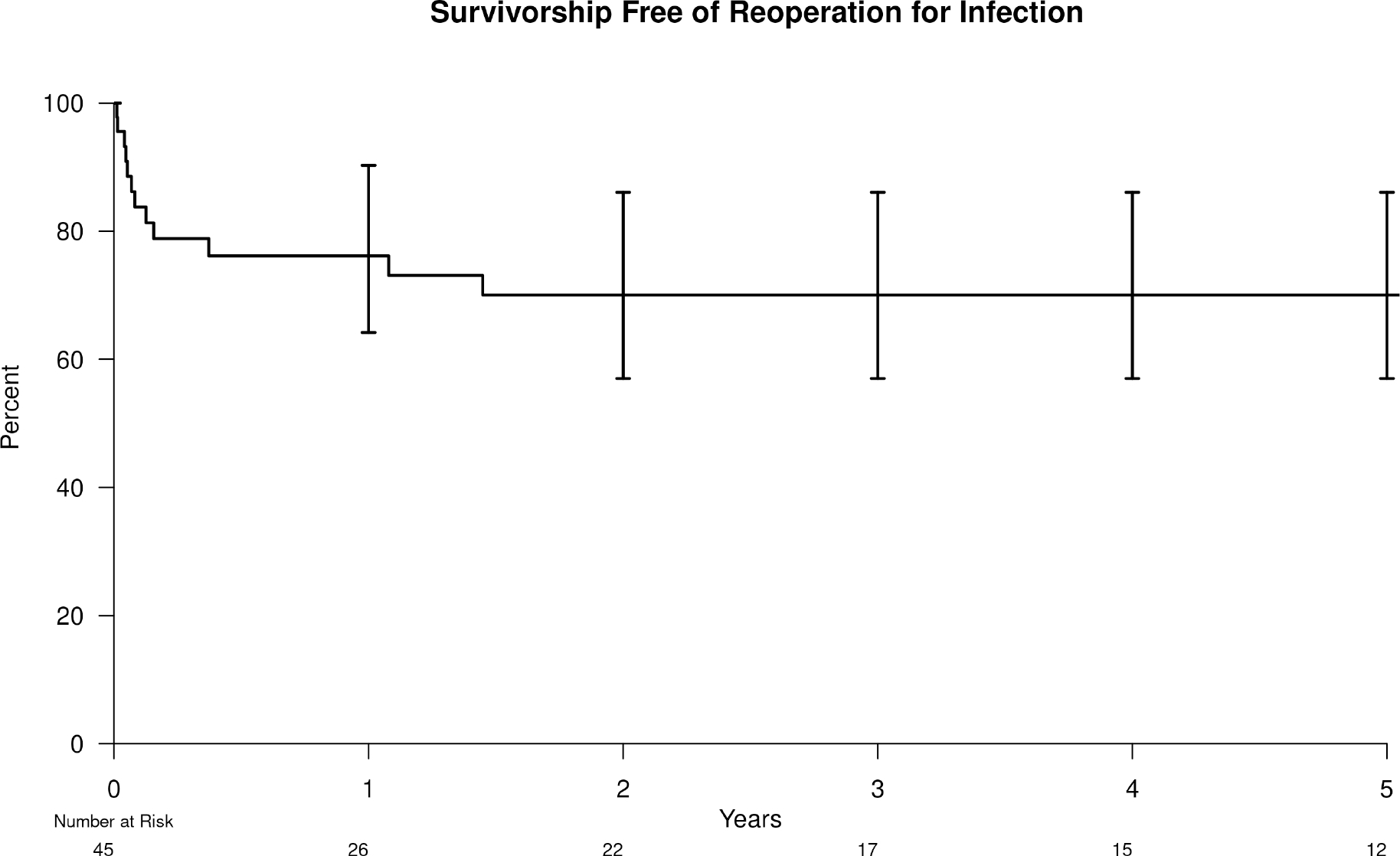
Kaplan-Meier survivorship free from reoperation for infection
The 5-year survivorship free from re-revision for infection was 80% (95% CI=64–94) (Figure 2). The 5-year survivorships free from re-revision for infection in McPherson Host Type A, B, and C were 92% (95% CI=74–100), 80% (95% CI=57–100), and 57% (95% CI=26–100]), respectively. The 5-year survivorships free from re-revision for infection in the single versus multiply revised groups were 91% (95% CI=73–100) and 67% (95% CI=46–94), respectively. The 5-year survivorship free from re-revision for infection in the acute hematogenous and acute early postoperative PJI groups was 87% (95% CI=69–100) and 69% (95% CI=46–97), respectively.
Figure 2.
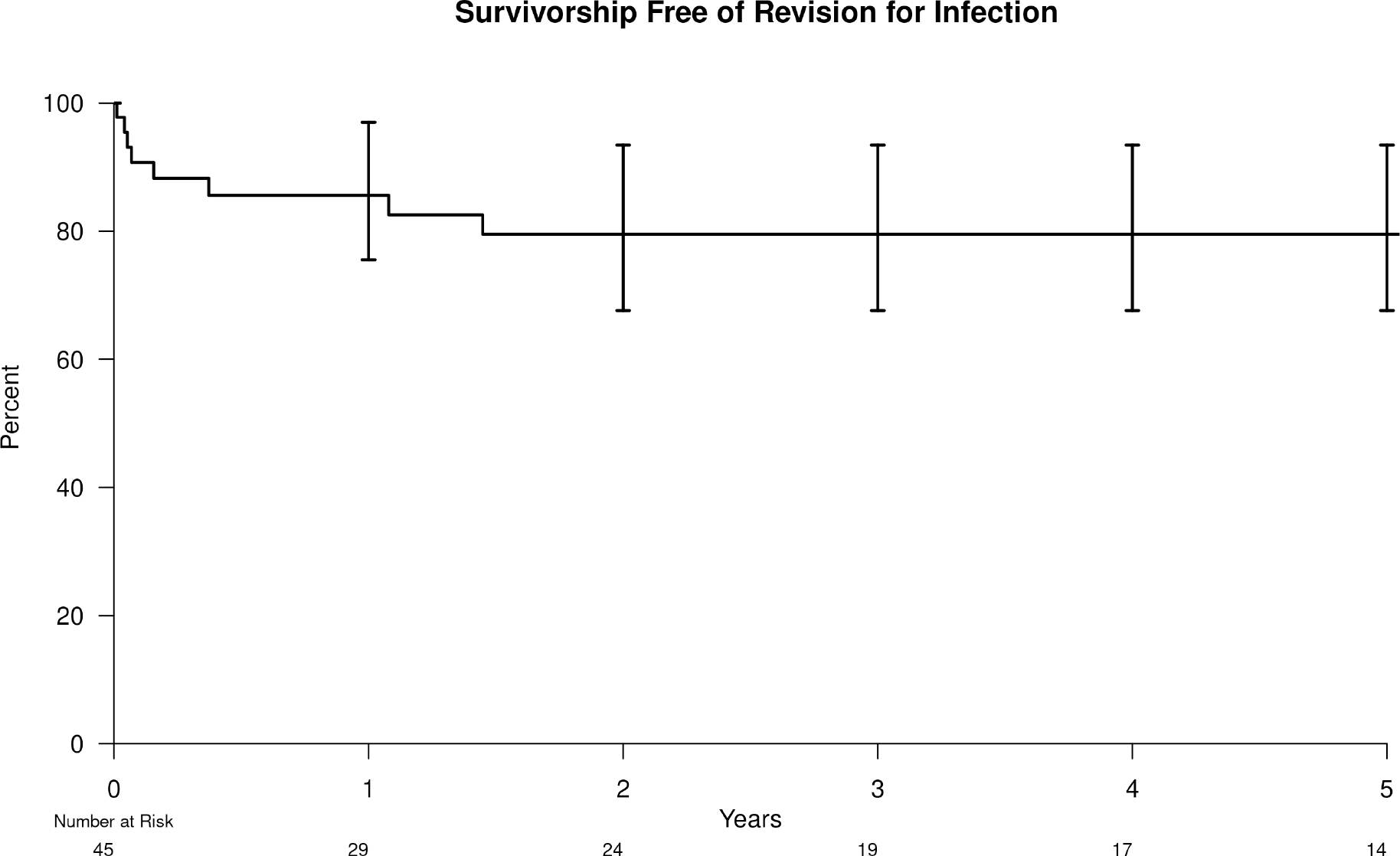
Kaplan-Meier survivorship free from re-revision for infection
The Kaplan-Meier survivorships free from reoperation for infection and re-revision for infection after IDCR for infected revision THAs and revision TKAs are presented in Supplemental Tables 1–4.
Any Re-revision and Any Reoperation
The 5-year survivorship free from any re-revision was 72% (95% CI=56–88) (Figure 3). In addition to the above re-revisions and reoperations for infection, there were 2 additional aseptic revisions. Both were acetabular component revisions for recurrent dislocation. The 5-year survivorship free from any reoperation was 65% (95% CI=48–82) (Figure 4). In addition to the above re-revisions and septic reoperations, there was 1 additional aseptic reoperation. This patient received debridement of a recurrent gluteal hematoma secondary to a gluteal artery pseudoaneurysm after a fall.
Figure 3.
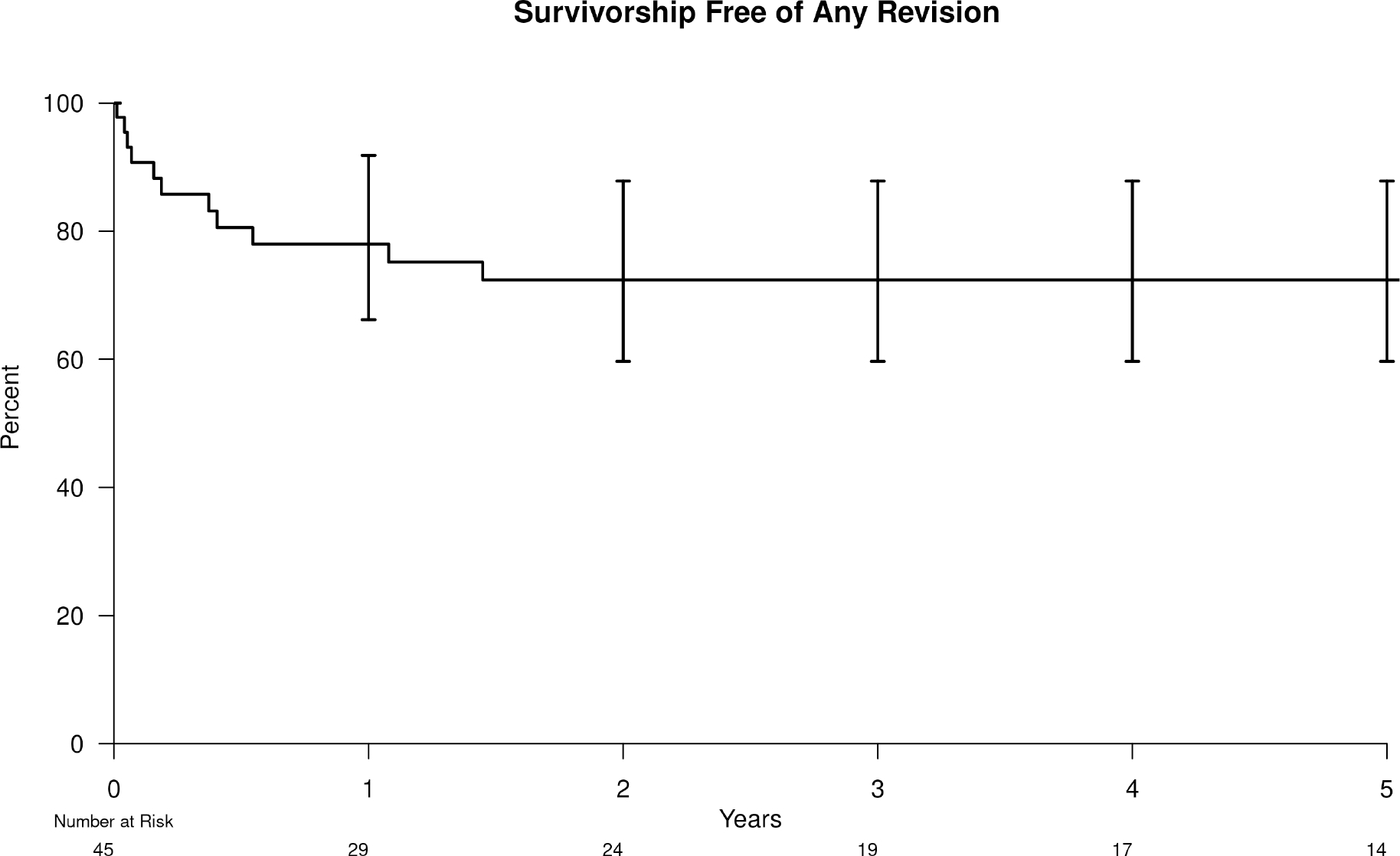
Kaplan-Meier survivorship free from any reoperation
Figure 4.

Kaplan-Meier survivorship free from any revision
Risk Factors for Failure
The risk of failure, defined as reoperation for infection, was significantly worse in patients who had Host Type C vs. Host Type A (HR 5.3, 95% CI=1.1–26; P=0.04). Given the small sample size, no other subfactors significantly contributed to failure (Table 3).
Table 3.
Univariate Cox Regression Risk Analysis
| Variable | Reoperation for Infection | Any Reoperation | Re-revision for Infection | Any Re-revision | Death | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |
|
| ||||||||||
| Men (vs. women) | 1.5 (0.5, 4.6) | 0.45 | 1.3 (0.49, 3.4) | 0.6 | 1.9 (0.48, 7.3) | 0.37 | 1.2 (0.4, 3.4) | 0.77 | 1.2 (0.5, 2.6) | 0.68 |
| BMI ≥ 30 kg/m2 (vs. BMI < 30 kg/m2) | 1.2 (0.4, 3.7) | 0.73 | 1.4 (0.51, 3.7) | 0.52 | 2.2 (0.56, 8.9) | 0.26 | 1.9 (0.62, 5.5) | 0.27 | 0.78 (0.36, 1.7) | 0.53 |
| Age at IDCR < 70 years (vs. age ≥ 70) | 1.9 (0.64, 5.8) | 0.25 | 1.6 (0.6, 4.2) | 0.35 | 4.4 (0.92, 21) | 0.06 | 2.1 (0.7, 6.2) | 0.18 | 0.48 (0.22, 1) | 0.06 |
| Host B (vs. A) | 1.8 (0.32, 10) | 0.51 | 1.8 (0.47, 7.1) | 0.38 | 3.1 (0.31, 32) | 0.33 | 2.6 (0.55, 12) | 0.23 | 5.7 (1.1, 29) | 0.035 |
| Host C (vs. A) | 5.3 (1.1, 26.1) | 0.04 | 4.2 (1.14, 15.7) | 0.03 | 7.5 (0.74, 75) | 0.09 | 5.7 (1.3, 26) | 0.025 | 13 (2.3, 76) | 0.004 |
| Host C (vs. B) | 2.9 (0.96, 8.9) | 0.06 | 2.3 (0.84, 6.4) | 0.11 | 2.4 (0.66, 8.7) | 0.19 | 2.3 (0.72, 7) | 0.16 | 2.4 (1.1, 5.3) | 0.038 |
| Acute early infection (vs. acute hematogenous) | 2 (0.67, 5.7) | 0.22 | 1.6 (0.59, 4.3) | 0.36 | 2.1 (0.57, 7.4) | 0.27 | 1.8 (0.61, 5.5) | 0.28 | 0.92 (0.42, 2) | 0.83 |
| Multiple prior aseptic revisions (vs. single) | 1.7 (0.56, 5.3) | 0.35 | 1.9 (0.68, 5.1) | 0.22 | 2.2 (0.54, 8.8) | 0.28 | 2.6 (0.82, 8.3) | 0.10 | 0.95 (0.45, 2) | 0.9 |
| Single IDCR (vs. double) | 0.71 (0.23, 2.2) | 0.56 | 0.85 (0.28, 2.6) | 0.77 | 0.75 (0.19, 3.1) | 0.69 | 1.1 (0.28, 4.2) | 0.92 | 1.7 (0.63, 4.5) | 0.29 |
| Rifampin treated (vs. no rifampin) | 0.77 (0.2, 2.9) | 0.7 | 0.95 (0.27, 3.4) | 0.94 | 2.24 (0.4, 13) | 0.36 | 1.9 (0.43, 7.9) | 0.41 | ---- | ---- |
| Staphylococcus organism (vs. not) | 1.4 (0.45, 4.2) | 0.58 | 0.99 (0.38, 2.5) | 0.98 | 1.2 (0.33, 4.5) | 0.77 | 0.92 (0.33, 2.6) | 0.88 | 1.7 (0.63, 4.5) | 0.29 |
HR = Hazard Ratio; CI = Confidence Interval; BMI = Body Mass Index; IDCR = Irrigation and Debridement with Component Retention
Mortality
The 5-year survivorship free from death was 65% (95% CI=51–83) and was 91% (95% CI=72–100), 66% (95% CI=47–93), and 41% (95% CI=19–86) for Host Types A, B, and C, respectively. Death was significantly associated with Host Type C versus Type A (HR 13.4, 95% CI=2.3–76; P=0.004), Type B versus Type A (HR 5.7, 95% CI=1.1–29; P=0.035), and Type C versus Type B (HR 2.4, 95% CI=1.1–5.3; P=0.038). No deaths were attributed to SAT.
DISCUSSION
While efforts have been made to understand the outcomes of IDCR for acute PJI of a primary THA or TKA, the outcomes of IDCR for acute infections after aseptic revision TJAs remain poorly understood. In our series, the 5-year survivorships free from re-revision and reoperation for infection were 80% and 70%, respectively. McPherson Host Type C was predictive of failure compared to host Types A and B. Furthermore, strong trends were observed suggesting single prior aseptic revisions had a lower rate of recurrent infection and multiply revised patients and acute hematogenous PJI had better outcomes after IDCR than acute early postoperative PJI. Finally, staphylococcus was the predominant organism associated with failed IDCRs.
There are limited data studying IDCR following PJI of revision TJAs[8], [20]. Barry et al.[8] recently compared outcomes between patients with extensive fixation of revision TKA constructs who underwent either IDCR or two-stage exchange, regardless of prior infection status. They determined a 5-year survivorship free from reoperation for infection of 60%. In our series, we found that the 5-year survivorships free from re-revision and reoperation for infection were 80% and 70%, respectively. Re-revision-free survival is possible following IDCR for an acutely infected previously revised THA or TKA, however careful patient selection is critical.
Patient selection and risk factor mitigation are important in optimizing outcomes after IDCR. The McPherson Host Classification system predicted failure in our series, with Host Type C patients sustaining significantly more reoperations for infection, revisions, and mortality at 5 years compared to Host Type A (P<0.05). Furthermore, our series suggested that having multiply revised (vs. single revision) TJAs and acute early postoperative infections (vs. acute hematogenous PJI) portended worse prognoses. However, these results did not achieve statistical significance given our sample size. These findings are consistent with evidence from prior studies investigating infected primary revision arthroplasties[5].
Studies have suggested that staphylococcal speciation leads to a worse prognosis[6]. In one multicenter investigation, rifampin use was identified as a key determinate to improved outcomes following acute PJI due to staphylococcus treated with IDCR[21]. In our study, most failures were secondary to staphylococcus species (64%), with mono and polymicrobial infections with staphylococcal involvement considered as one group. However, no significant association was seen with the small number of patients who received rifampin. Prospective study using standardized antibiotic durations and rifampin-based regimens for treating staphylococcus PJI remains of value as retrospective investigations studying rifampin therapy are often challenged by survival and indications biases[21, 22].
Suppressive antibiotic therapy is another important consideration. A recent systematic review of primary TJAs treated with IDCR and SAT found that the rate of antibiotic-related adverse effects leading to cessation occurred in 4% of patients[23]. In our series, 15% of patients required cessation or exchange of their antibiotics at some point in their postoperative course, and no single standard regimen was observed. Given this heterogeneity, and the fact that 89% of patients in our series were started on SAT (despite an intention to treat), we cannot compare results between patients treated with and without suppression, or results following specific antibiotic regimens. Future study is warranted to prospectively evaluate standardized suppression plans for safety and efficacy.
This study is not without its potential limitations. Its modest sample size limited statistical power for more robust comparative analyses. Additionally, as the study population represented a rare cohort, revision THAs and TKAs receiving IDCR were combined into one group. Furthermore, as this study spanned a collection period of nearly two decades, there was a lack of standardized postoperative protocols which many consider important factors in treating acute PJI. Also, the study has inherent limitations to its retrospective design, including its reliance on available clinical documentation from surgeons and infectious disease consultants to rule out infection in all preceding revision arthroplasties. Despite these limitations, to our knowledge we present one of the largest series studying outcomes of acutely infected revision TJAs treated with IDCR and SAT with midterm follow-up.
In conclusion, we demonstrated that in a cohort of IDCR for acute PJI following aseptic revision THA or TKA, 80% of components were unrevised for infection at 5 years. Furthermore, McPherson Host Type A demonstrated superior outcomes, and single prior aseptic revisions and acute hematogenous PJI performed clinically better than multiply revised joints and acute early postoperative PJI, respectively. However, these trends were not statistically significant given our small numbers. As the penalty for implant removal is high, select patients may benefit from IDCR with SAT following acute infection of aseptic revision TJAs.
Supplementary Material
Supplemental Table 2. Kaplan-Meier survivorships free of revision for infection following IDCR for acute PJI of revision THA
Supplemental Table 3. Kaplan-Meier survivorships free from reoperation for infection following IDCR for acute PJI of revision TKA
Supplemental Table 4. Kaplan-Meier survivorships free from revision for infection following IDCR for acute PJI of revision TKA
Supplemental Table 1. Kaplan-Meier survivorships free of reoperation for infection following IDCR for acute PJI of revision THA
ACKNOWLEDGMENTS
The authors would like to acknowledge Ms. Kristin Fruth, B.S. and Mr. Dirk R. Larson, M.S. for their statistical contributions.
The authors would like to acknowledge the Andrew A. and Mary S. Sugg Professorship in Orthopedic Research for its philanthropic support that made such research possible.
Funding:
Dr. Daniel J Berry is funded by grants from the National Institutes of Health (R01AR73147, R01HL147155), NIAMS (P30AR76312). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Delanois RE, Mistry JB, Gwam CU, Mohamed NS, Choksi US, Mont MA: Current Epidemiology of Revision Total Knee Arthroplasty in the United States. J Arthroplasty 32(9):2663, 2017 [DOI] [PubMed] [Google Scholar]
- 2.Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J: Economic burden of periprosthetic joint infection in the United States. J Arthroplasty 27(8 Suppl):61, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Mortazavi SMJ, Schwartzenberger J, Austin MS, Purtill JJ, Parvizi J: Revision total knee arthroplasty infection: incidence and predictors. Clinical orthopaedics and related research 468(8):2052, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Premkumar A, Kolin DA, Farley KX, Wilson JM, McLawhorn AS, Cross MB, Sculco PK: Projected Economic Burden of Periprosthetic Joint Infection of the Hip and Knee in the United States. J Arthroplasty 36(5):1484, 2021 [DOI] [PubMed] [Google Scholar]
- 5.Bryan AJ, Abdel MP, Sanders TL, Fitzgerald SF, Hanssen AD, Berry DJ: Irrigation and Debridement with Component Retention for Acute Infection After Hip Arthroplasty: Improved Results with Contemporary Management. J Bone Joint Surg Am 99(23):2011, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Weston JT, Watts CD, Mabry TM, Hanssen AD, Berry DJ, Abdel MP: Irrigation and debridement with chronic antibiotic suppression for the management of infected total knee arthroplasty: A Contemporary Analysis. Bone Joint J 100-b(11):1471, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Chung AS, Niesen MC, Graber TJ, Schwartz AJ, Beauchamp CP, Clarke HD, Spangehl MJ: Two-Stage Debridement With Prosthesis Retention for Acute Periprosthetic Joint Infections. J Arthroplasty 34(6):1207, 2019 [DOI] [PubMed] [Google Scholar]
- 8.Barry JJ, Geary MB, Riesgo AM, Odum SM, Fehring TK, Springer BD: Irrigation and Debridement with Chronic Antibiotic Suppression Is as Effective as 2-Stage Exchange in Revision Total Knee Arthroplasty with Extensive Instrumentation. J Bone Joint Surg Am 103(1):53, 2021 [DOI] [PubMed] [Google Scholar]
- 9.Fehring KA, Abdel MP, Ollivier M, Mabry TM, Hanssen AD: Repeat Two-Stage Exchange Arthroplasty for Periprosthetic Knee Infection Is Dependent on Host Grade. J Bone Joint Surg Am 99(1):19, 2017 [DOI] [PubMed] [Google Scholar]
- 10.Kheir MM, Tan TL, Gomez MM, Chen AF, Parvizi J: Patients With Failed Prior Two-Stage Exchange Have Poor Outcomes After Further Surgical Intervention. J Arthroplasty 32(4):1262, 2017 [DOI] [PubMed] [Google Scholar]
- 11.McPherson EJ, Woodson C, Holtom P, Roidis N, Shufelt C, Patzakis M: Periprosthetic total hip infection: outcomes using a staging system. Clin Orthop Relat Res (403):8, 2002 [PubMed] [Google Scholar]
- 12.New definition for periprosthetic joint infection. J Arthroplasty 26(8):1136, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Laffer RR, Graber P, Ochsner PE, Zimmerli W: Outcome of prosthetic knee-associated infection: evaluation of 40 consecutive episodes at a single centre. Clin Microbiol Infect 12(5):433, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Estes CS, Beauchamp CP, Clarke HD, Spangehl MJ: A two-stage retention débridement protocol for acute periprosthetic joint infections. Clinical orthopaedics and related research 468(8):2029, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR: Executive summary: diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 56(1):1, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Harris WH: Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am 51(4):737, 1969 [PubMed] [Google Scholar]
- 17.Insall JN, Dorr LD, Scott RD, Scott WN: Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res (248):13, 1989 [PubMed] [Google Scholar]
- 18.Dinse GE, Lagakos SW: Nonparametric estimation of lifetime and disease onset distributions from incomplete observations. Biometrics 38(4):921, 1982 [PubMed] [Google Scholar]
- 19.Austin PC: Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med 35(30):5642, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sukhonthamarn K, Tan TL, Strony J, Brown S, Nazarian D, Parvizi J: The Fate of Periprosthetic Joint Infection Following Megaprosthesis Reconstruction. JB JS Open Access 6(4), 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becker A, Kreitmann L, Triffaut-Fillit C, Valour F, Mabrut E, Forestier E, Lesens O, Cazorla C, Descamps S, Boyer B, Chidiac C, Lustig S, Montbarbon E, Batailler C, Ferry T: Duration of rifampin therapy is a key determinant of improved outcomes in early-onset acute prosthetic joint infection due to Staphylococcus treated with a debridement, antibiotics and implant retention (DAIR): a retrospective multicenter study in France. J Bone Jt Infect 5(1):28, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Becker A, Kreitmann L, Ferry T: Reply to Scheper and de Boer’s comment on “Duration of rifampin therapy is a key determinant of improved outcomes in early-onset acute prosthetic joint infection due to Staphylococcus treated with a debridement, antibiotics and implant retention (DAIR): a retrospective multicenter study in France” by Becker et al. (2020). J Bone Jt Infect 6(1):19, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malahias MA, Gu A, Harris EC, Adriani M, Miller AO, Westrich GH, Sculco PK: The Role of Long-Term Antibiotic Suppression in the Management of Peri-Prosthetic Joint Infections Treated With Debridement, Antibiotics, and Implant Retention: A Systematic Review. J Arthroplasty 35(4):1154, 2020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 2. Kaplan-Meier survivorships free of revision for infection following IDCR for acute PJI of revision THA
Supplemental Table 3. Kaplan-Meier survivorships free from reoperation for infection following IDCR for acute PJI of revision TKA
Supplemental Table 4. Kaplan-Meier survivorships free from revision for infection following IDCR for acute PJI of revision TKA
Supplemental Table 1. Kaplan-Meier survivorships free of reoperation for infection following IDCR for acute PJI of revision THA


