Abstract
Background and aims:
Plaque erosion is a common underlying cause of acute coronary syndromes. The role of endothelial shear stress (ESS) and endothelial shear stress gradient (ESSG) in plaque erosion remains unknown. We aimed to determine the role of ESS metrics and maximum plaque slope steepness in plaques with erosion versus stable plaques.
Methods:
This analysis included 46 patients/plaques from TOTAL and COMPLETE trials and Brigham and Women’s Hospital’s database who underwent angiography and OCT. Plaques were divided into those with erosion (n = 24) and matched stable coronary plaques (n = 22). Angiographic views were used to generate a 3-D arterial reconstruction, with centerlines merged from angiography and OCT pullback. Local ESS metrics were assessed by computational fluid dynamics. Among plaque erosions, the up- and down-slope (Δ lumen area/frame) was calculated for each culprit plaque.
Results:
Compared with stable plaque controls, plaques with an erosion were associated with higher max ESS (8.3 ± 4.8 vs. 5.0 ± 1.9 Pa, p = 0.02) and max ESSG any direction (9.2 ± 7.5 vs. 4.3 ± 3.11 Pa/mm, p = 0.005). Proximal erosion was associated with a steeper plaque upslope while distal erosion with a steeper plaque downslope. Max ESS and Max ESSG any direction were independent factors in the development of plaque erosion (OR 1.32, 95%CI 1.06–1.65, p = 0.014; OR 1.22, 95% CI 1.03–1.45, p = 0.009, respectively).
Conclusions:
In plaques with similar luminal stenosis, plaque erosion was strongly associated with higher ESS, ESS gradients, and plaque slope as compared with stable plaques. These data support that ESS and slope metrics play a key role in the development of plaque erosion and may help prognosticate individual plaques at risk for future erosion.
Keywords: Acute coronary syndrome, Plaque erosion, Endothelial shear stress
1. Introduction
Acute coronary syndromes (ACS) remain a challenging and global health problem responsible for an enormous number of major adverse cardiac events (MACE) [1]. The three underlying pathobiologic mechanisms responsible for ACS include plaque rupture, plaque erosion, and calcified nodules [2]. Plaque erosion is defined as a thrombus overlying a plaque with an intact fibrous cap (IFC), caused mainly by endothelial denudation [3,4]. Autopsy and optical coherence tomography (OCT) studies showed that plaque erosion could be the causative mechanism in about 20–40% of patients presenting with ACS [5–9].
The role of endothelial shear stress (ESS), the biomechanical force due to the tangential friction of blood flowing over the arterial endothelium, in the natural history of plaque formation, progression, and destabilization has been studied, but its role in plaque erosion remains unknown [10,11]. Focal areas of high ESS and, in particular, high ESS gradient (ESSG, the ESS value of immediately adjacent plaque endothelial areas) have been hypothesized to promote erosion [12,13]. In addition, no studies examined the detailed mechanistic relationships between longitudinal location of plaque erosion/thrombus, i.e., proximal vs. distal to the minimal luminal area (MLA) of the culprit erosion plaque, and its relationship to the maximum magnitude of upslope vs. downslope of the lumen obstruction topography. Therefore, this study is a hypothesis generating study aimed [1]: to compare ESS and ESSG metrics between plaques with erosion and similar non-culprit control plaques that remained stable and [2], among erosion plaques, to study the mechanistic implications of maximum slope steepness up- and down-stream from the culprit plaque MLA on thrombus location.
2. Patients and methods
2.1. Study design and patients
We retrospectively analyzed imaging data from 46 patients from the OCT sub-studies of both the TOTAL [14] and the COMPLETE [15] trials, as well as a group of similar patients from Brigham & Women’s Hospital’s catheterization laboratory database, who underwent both coronary angiography and OCT imaging during a percutaneous coronary intervention (PCI). The protocols of both TOTAL and COMPLETE trials have been previously described in detail [14,15]; both studies were performed with the subjects’ written informed consent and the institutional committee on human research approved the study protocols. The details of the patient recruitment and exclusion are presented in Online Fig. 1 in the Supplementary Material. We screened 40 patients with plaque erosion from both the TOTAL trial (30 patients) and the Brigham & Women’s Hospital’s database (10 patients) who presented with ACS. Twenty-four plaque erosions satisfied the definition of definite/probable OCT-defined erosion and were included in the plaque erosion group:18 patients presented clinically with STEMI; 2 with NSTEMI; and 4 with unstable angina. For the control plaques, we screened 48 patients (30 patients from the COMPLETE trial and 18 from the Brigham & Women’s Hospital’s database) who underwent coronary angiography for a chronic coronary syndrome. In the COMPLETE trial, definition of non-culprit lesions was “at least 70% stenosis of the vessel diameter on visual estimation or with 50–69% stenosis accompanied by a fractional flow reserve (FFR) measurement of 0.80 or less.” We included 22 control plaques from 22 patients matched for the same MLA and reference luminal area (RLA) as the erosion plaques without features of plaque disruption.
2.2. OCT analysis
OCT analysis was performed at the Vascular Profiling Research Laboratory, Brigham & Women’s Hospital, Harvard Medical School. Quantitative and qualitative analyses were performed using validated offline workstation software, OPTIS ORW E.5.2 (Abbott Vascular, Inc, Temecula, CA, USA). The luminal area was measured in every frame during the OCT pullback in both the erosion and the control groups, and sites of the MLA and RLA were determined for each plaque. The MLA, maximum and minimum luminal diameters, RLA, percent lumen area stenosis, and thrombus were defined based on previously published Expert Review documents on methodology, terminology, and clinical applications of optical coherence tomography [16,17]. Definite erosion was defined as a thrombus overlying a visible plaque with an intact fibrous cap, and probable erosion was defined as [1] luminal endothelial irregularities at the culprit lesion site without thrombus or [2] attenuation of plaque features by an overlying thrombus without superficial lipid or calcified nodules proximal or distal to the thrombus site [18]. The details of OCT and plaque slope calculation are described in the Online Supplementary Materials.
2.3. Computational fluid dynamics (CFD)
Our intracoronary vascular profiling methods have been previously described in detail [11]. The 3D vessel reconstruction, centerline merging, CFD simulation, and post-processing steps were performed according to Expert Consensus Guidelines for patient-specific CFD simulation of blood flow in human coronary arteries [19]. Our CFD methodology is explained in the Online Supplementary Material.
2.4. Statistical analysis
Categorical variables were presented as numbers and proportions, and continuous variables were reported as means and standard deviations. We used the Chi-square statistic to compare categorical variables and the t-test or the Wilcoxon Rank Sum Test to compare continuous variables. To compare the change in lumen area upslope with change in lumen area downslope, we used the Wilcoxon Signed Ranks Test. We also used Spearman correlation to examine the association between maximum ESSG and plaque downslope and plaque upslope. Multivariate analysis was conducted to identify factors associated with plaque erosion: first, least absolute shrinkage and selection operator (LASSO) regression was run to select the variables, and then logistic regression was run to assess their association with erosion development. For the LASSO regression, the following variables were entered in the model: 1) minimum ESS, 2) maximum ESS, 3) average ESS, 4) maximum ESSG any direction, 5) maximum ESSG downslope, 6) maximum ESSG circumferential, and 7) maximum ESSG upslope. For the logistic regression analysis, odds ratios and 95% confidence intervals were reported.
3. Results
A total of 46 plaques were analyzed, one from each of 46 patients (Supplementary Fig. 1). For the erosion group, we selected 24 plaques from 24 patients with an ACS, and for the control group, we selected 22 stable coronary plaques matched for the same MLA and RLA as the erosion plaques without features of plaque disruption. The baseline demographic and clinical data of both the erosion and the control patients are summarized in Table 1.
Table 1.
Patient demographic and clinical characteristics.
| Characteristics | Erosion (N = 24) | Control (N = 22) | p-value |
|---|---|---|---|
| Age (years) | 62.8 ± 11.8 | 66.5 ± 13.9 | 0.33 |
| Male sex n (%) | 18 (75) | 17(77) | 0.86 |
| Hypertension n (%) | 11 (46) | 10 (50) | 0.98 |
| Diabetes n (%) | 4 (16) | 4 (18) | 0.89 |
| Current Smoking n (%) | 11 (46) | 9 (40) | 0.74 |
| Family history n (%) | 4 (16) | 3 (13) | 0.78 |
| Clinical presentation | |||
| STEMI n (%) | 18 (75) | ||
| NSTEMI n (%) | 2 (8) | ||
| UA n (%) | 4 (17) | ||
| Stable angina n (%) | 22 (100) | ||
| Prior MI n (%) | 2 (8) | 2 (9) | 0.93 |
| Prior PCI n (%) | 1 (4) | 1 (4.5) | 0.95 |
| GFR mL/min/1.73m2 | 88.5 | 75.5 ± 26.8 | 0.11 |
| LDL mg/dL | 80.5 ± 27 | 85.9 ± 31 | 0.53 |
| HDL mg/dL | 40.8 ± 6.7 | 51.1 ± 13 | 0.001 |
| Triglyceride mg/dL | 153.16 ± 28.2 | 198.2 ± 61.2 | 0.002 |
| Hemoglobin g/dL | 11.9 ± 3.1 | 12.5 ± 2.3 | 0.46 |
Values are presented as mean ± SD for continuous variables and n (%) for categorical variables. BMI: body mass index; STEMI: ST-segment elevation myocardial infarction; NSTEMI: non-ST-segment elevation myocardial infarction; UA: unstable angina; MI; myocardial infarction; PCI: percutaneous coronary intervention; GFR: estimated glomerular filtration rate calculated using the CKD-EPI equation; LDL: low-density lipoprotein; HDL: high-density lipoprotein.
3.1. OCT plaque characteristics
OCT plaque characteristics are shown in Table 2. There were no statistically significant differences between the two groups of plaques regarding lesion length, MLA, RLA, and the percent of lumen area stenosis. Plaques with erosion have more fibrous components while the control plaques were fibrofatty, having larger lipid arc, calcification, and cholesterol crystals. The minimal fibrous cap thickness was similar in both groups.
Table 2.
Differences in OCT measurements between plaque erosion and control groups.
| Characteristic | Erosion (N = 24) | Control (N = 22) | p-value |
|---|---|---|---|
| Culprit lesion location | <0.0001 | ||
| Proximal LAD n (%) | 7 (29) | 10 (45) | |
| Mid/distal LAD n (%) | 5 (21) | 5 (23) | |
| LCX n (%) | 2(8) | 4 (18) | |
| RCA n (%) | 10 (42) | 3 (14) | |
| Lesion length (mm) | 15.3 ± 5.8 | 15.2 ± 4.8 | 0.98 |
| Minimal lumen area (mm2) | 1.7 ± 0.5 | 2.0 ± 0.6 | 0.08 |
| Reference lumen area (mm2) | 7.8 ± 1.9 | 8.0 ± 2.1 | 0.85 |
| % Lumen area stenosis | 77 ± 7 | 74 ± 8 | 0.15 |
| Plaque type by quadrant a | |||
| Total quadrants at the region of interest | 290 ± 49 | 358 ± 125 | 0.09 |
| Lipid rich | 30.2 ± 16 | 119.1 ± 74.3 | 0.001 |
| % of diseased quadrants | 15.4 ± 9.9 | 49.7 ± 24.7 | 0.001 |
| Fibrous | 130.6 ± 47.8 | 73.9 ± 61.7 | 0.001 |
| % of diseased quadrants | 66.3 ± 39 | 28.8 ± 17 | 0.001 |
| Calcified | 36 ± 11.3 | 51.1 ± 58.2 | 0.11 |
| % of diseased quadrants | 18.3 ± 12 | 21.5 ± 21 | 0.10 |
| Total diseased quadrants | 196.8 ± 61.1 | 244.1 ± 101.7 | 0.19 |
| Maximum lipid arc (°) | 85.4 ± 48.2 | 196.5 ± 61.8 | 0.001 |
| Maximum calcium arc (°) | 75.6 ± 70.0 | 114.3 ± 102.2 | 0.02 |
| Minimal fibrous cap thickness (μm) | 92.3 ± 16 | 93.1 ± 23.6 | 0.90 |
| Cholesterol crystals n (%) | 9 (37%) | 15 (68%) | 0.001 |
Values are presented as mean ± SD for continuous variables and n (%) for categorical variables.
Absolute number of quadrants with each plaque type throughout the lesion, measured at 0.2 mm intervals.
3.2. Endothelial shear stress metrics
ESS data are shown in Table 3. Examples of 3D reconstruction and color-coded ESS metrics for a plaque with erosion vs. a control plaque are shown in Fig. 1.
Table 3.
Differences in ESS metrics between plaque erosion and control groups.
| Characteristic | Erosion (N = 24) | Control (N = 22) | p-value |
|---|---|---|---|
| Mean coronary flow (ml/sec) | 0.7 ± 0.3 | 0.6 ± 0.3 | 0.19 |
| 90° Arc Min ESS/plaque (Pa) | 0.8 ± 0.5 | 0.6 ± 0.4 | 0.40 |
| 90° Arc Max ESS/plaque (Pa) | 8.3 ± 4.8 | 5.0 ± 1.9 | 0.02 |
| 90° Arc Average ESS/plaque (Pa) | 3.9 ± 2 | 3.0 ± 1.29 | 0.18 |
| Max ESSG any direction (Pa/mm) | 9.2 ± 7.5 | 4.3 ± 3.1 | 0.005 |
| Max ESSG plaque upslope (Pa/mm) | 5.0 ± 6.1 | 2.1 ± 2.3 | 0.009 |
| Max ESSG plaque downslope (Pa/mm) | 7.0 ± 7.0 | 3.5 ± 3.1 | 0.16 |
| Max ESSG circumferential (Pa/mm) | 4.0 ± .3.3 | 1.9 ± 1.4 | 0.003 |
| Thrombus location vs. minimal lumen area location in erosion group | |||
| Thrombus location in culprit plaque | Δ Lumen area UPSLOPE (mm2/frame) | Δ Lumen area DOWNSLOPE (mm2/frame) | p-value |
| Proximal to the MLA (n = 10) | 2.2 ± 1.2 | 1.07 ± 0.8 | 0.005 |
| Distal to the MLA (n = 10) | 2.1 ± 0.4 | 3.93 ± 1.8 | 0.005 |
| Across the MLA (n = 4) | 2.5 ± 0.9 | 1.8 ± 0.7 | 0.14 |
| Max Absolute Slope Value | 2.2 ± 1.2 | 3.93 ± 1.8 | 0.007 |
Values are presented as mean ± SD for continuous variables. ESS = endothelial shear stress; ESSG = endothelial shear stress gradient; MLA = minimal lumen area.
Fig. 1.
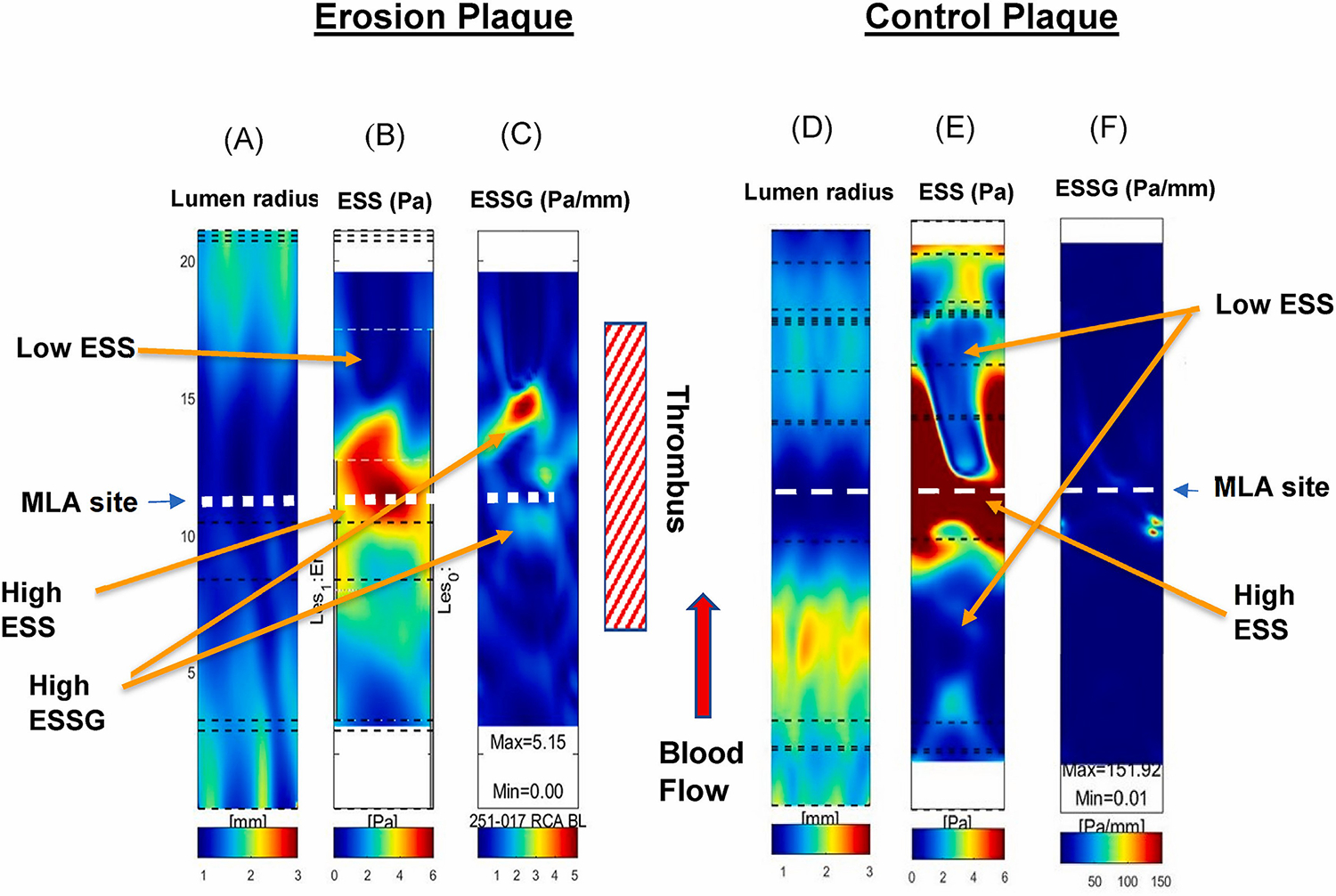
Representative images of 3D reconstruction and color-coded lumen radius (A), endothelial shear stress (ESS) (B), and endothelial shear stress gradient (ESSG) (C) values from a patient with plaque erosion; and a patient with a control plaque (D), (E), and (F), respectively.
Location of thrombus noted in the patient with plaque erosion. Arrows identify the location of severe lumen obstruction (MLA), high ESS (throat of obstruction), low ESS (up-, down-stream from the MLA), and high ESSG (differences in the magnitude of ESS in immediately adjacent endothelial cells, located at the site of abrupt change in lumen area and ESS).
Mean coronary flow was similar in both erosion and control plaques. Compared to the control stable plaques, plaques with an erosion were associated with significantly higher max ESS (8.3 ± 4.8 vs. 5.0 ± 1.9 Pa, p = 0.027), Max ESSG any direction (9.2 ± 7.5 vs. 4.3 ± 3.11 Pa/mm, p = 0.005), Max ESSG circumferential direction (4.0 ± 3.3 vs. 1.9 ± 1.4 Pa/mm, p = 0.003) and Max ESSG along the plaque upslope (5.0 ± 6.1 vs. 2.1 ± 2.3 Pa/mm, p = 0.009) (Fig. 2). Erosion plaques exhibited a numerically higher Max ESSG along the plaque downslope compared to Control plaques (7.0 ± 7.0 vs. 3.5 ± 3.1 Pa/mm), although this difference was not statistically significant (p = 0.16).
Fig. 2.
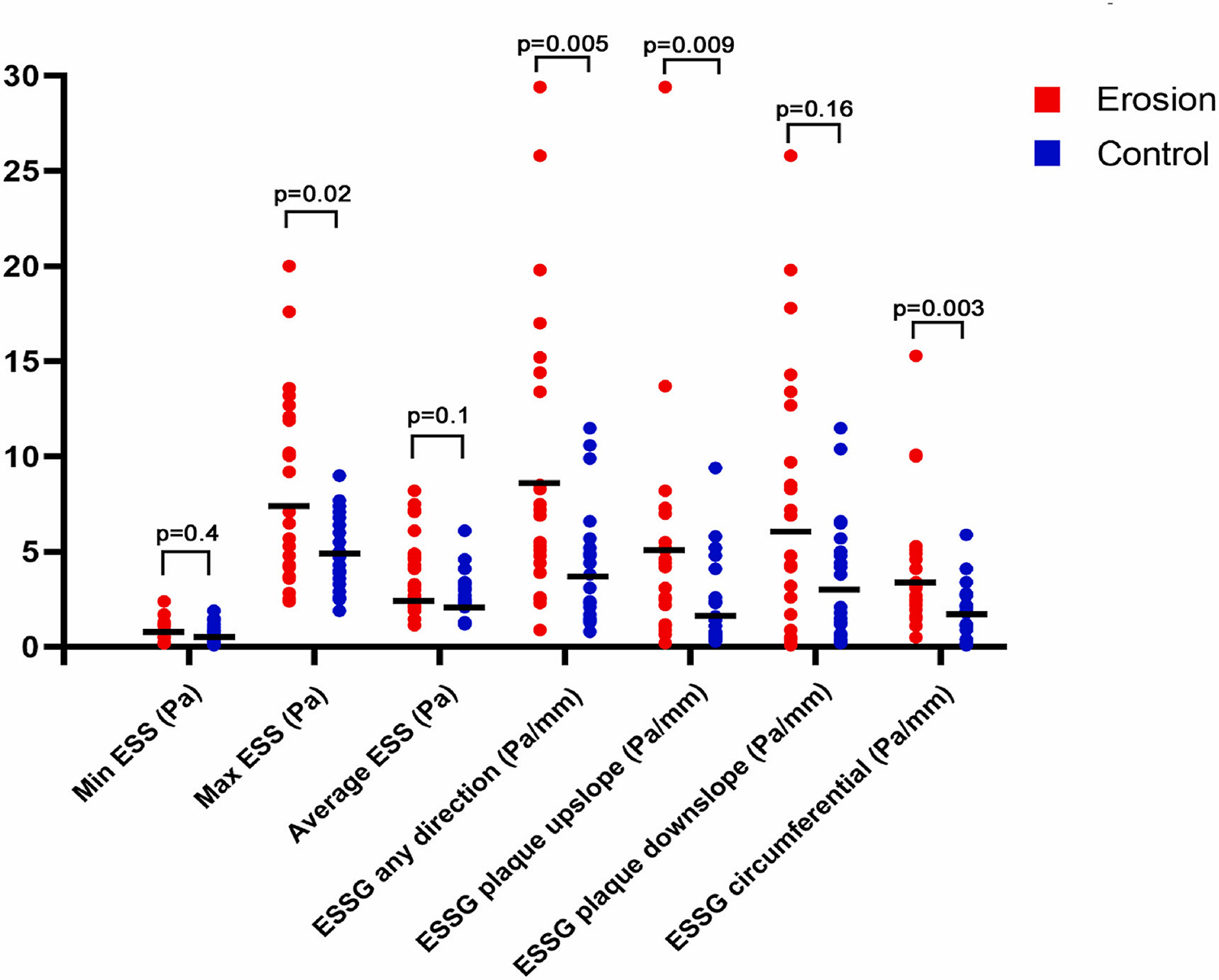
Differences in endothelial shear stress metrics between the erosion group (n = 24) and the control group (n = 22).
The Wilcoxon Rank test was used for statistical comparisons. Plaques with an erosion were associated with higher max ESS (8.3 ± 4.8 vs. 5.0 ± 1.9 Pa, p = 0.027), Max ESSG any direction (9.2 ± 7.5 vs. 4.3 ± 3.11 Pa/mm, p = 0.005), Max ESSG circumferential direction (4.0 ± 3.3 vs. 1.9 ± 1.4 Pa/mm, p = 0.003) and Max ESSG along the plaque upslope (5.0 ± 6.1 vs 2.1 ± 2.3 Pa/mm, p = 0.009) compared to the control plaques. There was no difference in min ESS between erosion and control plaques (0.8 ± 0.5 vs 0.6 ± 0.4, p = 0.40).
3.3. Location of erosion thrombus and plaque topographical slope
The thrombus location in culprit plaques was heterogeneously distributed both proximal to the MLA site in 41% of cases and distal to the MLA site in 41% of cases, while the thrombus straddled the MLA site in 16% of cases (Table 3). There was no difference in clinical presentation of patients with plaque erosion in the upslope vs downslope location of the thrombus: erosion thrombus located in the plaque upslope (10 cases) presented clinically with STEMI in 7 cases and with unstable angina in 3 cases, while erosion thrombus located in the plaque downslope presented with STEMI in 8 cases and NSTEMI in 2 cases.
There was a moderate correlation between the Max ESSG and plaque downslope (r =0.38, p =0.001) as well as Max ESSG and plaque upslope (r = 0.27, p = 0.001).
By multivariant analysis, proximal plaque erosion was significantly associated with steeper plaque upslope vs. plaque downslope (Δ lumen area upslope vs. Δ lumen area downslope 2.2 ± 1.2 mm2/frame vs. 1.07 ± 0.8 mm2/frame, p = 0.005). Similarly, distal plaque erosion was associated with steeper plaque downslope vs. upslope (Δ lumen area upslope vs. Δ lumen area downslope 2.1 ± 0.4 mm2/frame vs. 3.93 ± 1.8 mm2/frame, p = 0.005). In addition, the Max absolute slope value was significantly higher in the downslope of the eroded plaque compared to the upslope, 3.93 ± 1.8 mm2/frame vs. 2.2 ± 1.2 mm2/frame, p = 0.007.
Multivariate analysis showed that both the Max ESS and Max ESSG any direction were independent factors in the development of plaque erosion, the odds ratios and 95% confidence intervals were 1.32 (1.06–1.65), p = 0.014 and 1.22 (1.03–1.45), p = 0.009, respectively. The odds of a plaque manifesting an erosion increased 32% with each unit increase in Max ESS and 22% with each unit increase in ESSG any direction compared to a control plaque.
Examples of erosion location and the hemodynamic effect of the geometry of plaque slope are shown in Figs. 3 and 4.
Fig. 3.
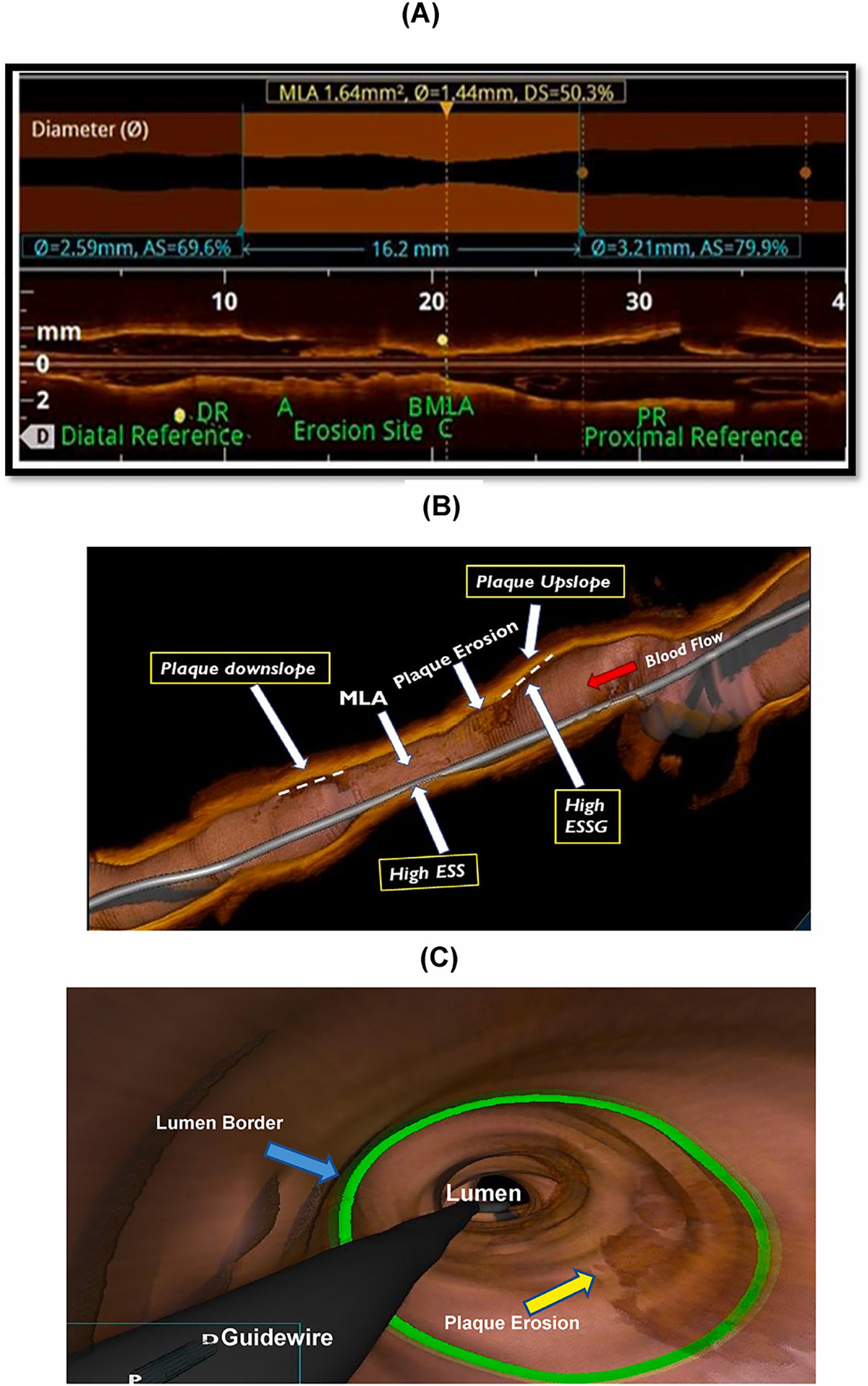
(A) Representative image of erosion site as it appears in a longitudinal OCT pullback, which enables assessment of erosion location and plaque topography (quantitative shape); (B) three-dimensional OCT view of plaque erosion showing the relationship between plaque slope steepness and ESS metrics: (C) three-dimensional OCT flythrough view of the plaque erosion.
Fig. 4.
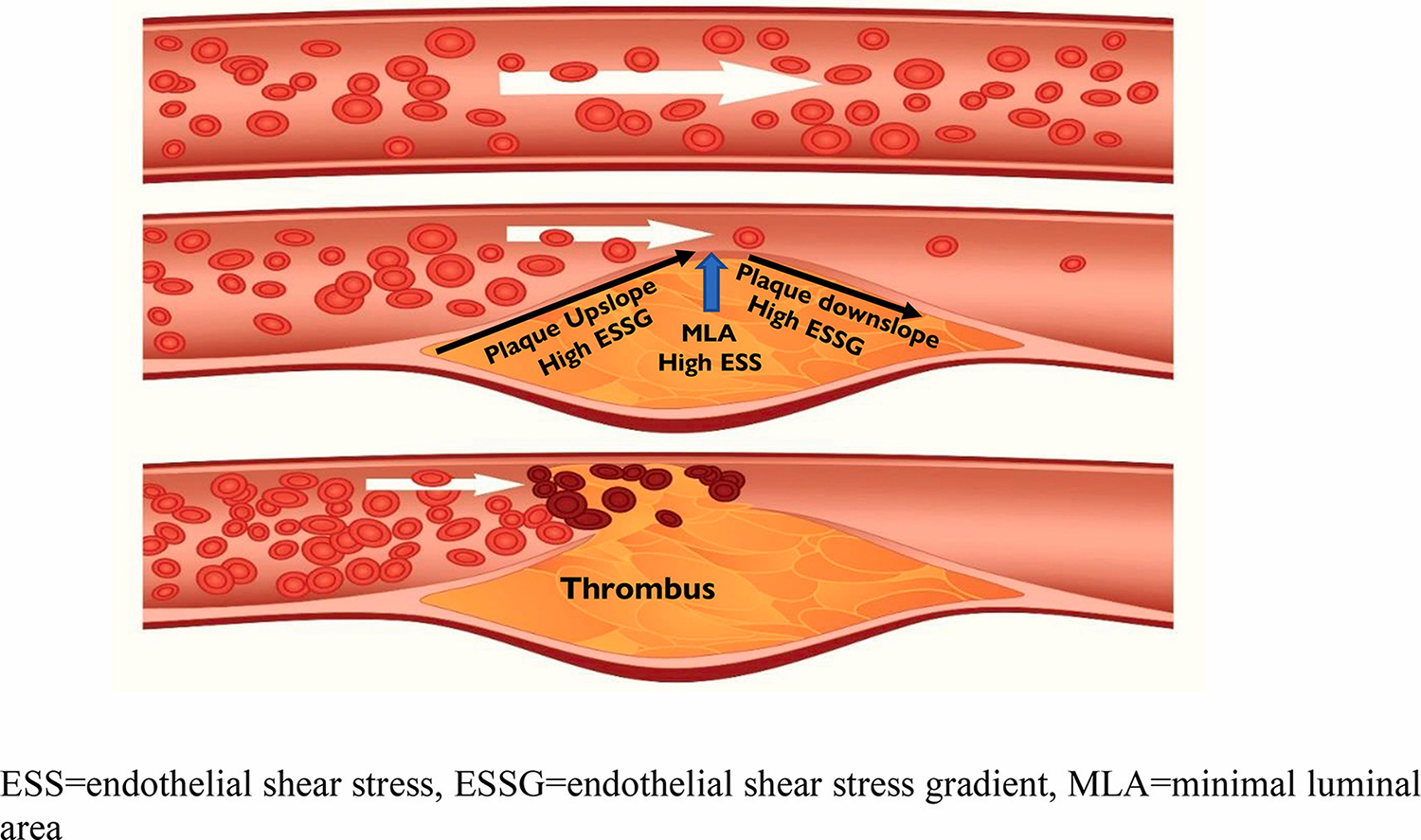
The geometry of plaque lumen obstruction (slope) influences local blood flow surrounding plaque and may biomechanically contribute to the nature and severity of plaque destabilization and thrombus formation (red arrow).
4. Discussion
The present study investigated the pathobiologic mechanisms responsible for plaque erosion by focusing on differences in ESS and ESSG between atherosclerotic plaques with erosion causing an ACS compared to stable control plaques of similar minimal and reference lumen dimensions; and, among erosion plaques, the effect of maximum topography slope steepness up- and down-stream from the culprit plaque MLA on the location of the thrombus (Fig. 5). Our main findings were [1]: plaques that exhibited erosion and thrombus formation were associated with high shear stress metrics of Max ESS and ESSG vs. similar plaques without destabilization. There was no difference in Min ESS between erosion and stable plaques. There were significant and substantial differences in local ESSG measurements in all directions in erosion plaques compared to control plaques. Multivariate analysis showed that the two independent factors associated with plaque erosion were Max ESS and Max ESSG any direction. The odds of manifesting a plaque erosion increased by 32% per each Pa increase in Max ESS and 22% per each Pa/mm increase in Max ESSG any direction [2]. Among the plaques with erosion, the site of erosion and thrombus was distributed both proximally and distally to the culprit plaque MLA site, and there were important plaque topographical characteristics that contributed to the location of the thrombus: proximal location of the plaque erosion was associated with a steeper plaque upslope vs. downslope, while distal location of plaque erosion was associated with steeper plaque downslope vs. upslope. There was no difference in the clinical manifestations of patients with an upslope versus a downslope location of plaque erosion and thrombus. There was a moderate correlation between the metrics of Max ESSG and plaque slope, both of which represent the plaque topographical shape encroaching into the lumen.
Fig. 5.
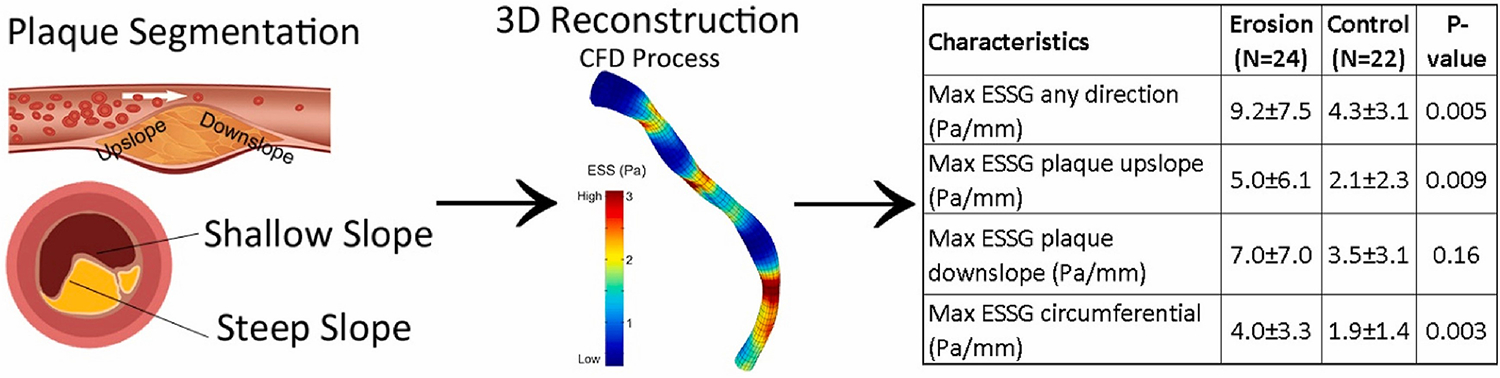
Schematic presentation of the study’s main points showing the effect of plaque slope steepness on erosion development (left part of the panel), the process of 3-D vessel reconstruction and CFD, and the 3-D color map of the ESS (middle part of the panel), and a table summarizing the main study results concerning the differences in ESS metrics (ESS and ESSG) between the plaque erosion group and the control group (right side of the panel).
4.1. The role of ESS metrics in plaque erosion
Given the increasingly important role of plaque erosion as a culprit mechanism responsible for ACS, multiple investigations have begun to explore the pathobiologic molecular and biomechanical processes that may be mechanistically contributing to the erosion process and the subsequent thrombus formation [20–23]. Low ESS upregulates pro-inflammatory, pro-atherogenic, and pro-thrombotic properties of the endothelium, and is associated with plaque initiation and progression [24], while high ESS may be associated with increased vulnerability [25–27]. Plaques exposed to either low or high ESS environments have been associated with plaque destabilization and new MACE [28–30]. An early in-vivo OCT and CFD study of 18 ACS patients presenting with plaque erosion showed that in 94% of cases the thrombus involved the MLA and peak ESS/ESSG, and in 78% of cases thrombus began close to the area of high ESS/high ESSG at the MLA and extended distally to the area of oscillatory shear stress index (focal blood flow recirculation, OSI) or low ESS at the distal shoulder [31], underscoring the synergistic and complex roles of plaque topography, local fluid dynamics, and ESS in initiating the cascade of events that result in erosion and subsequent thrombus formation. The study did not include stable “control” plaques of similar lumen obstruction and coronary blood flow without erosion/thrombus, however, to determine if the observed hemodynamic conditions were specifically associated with the development of the erosion/thrombus pathobiology per se or simply reflected the nature of blood flow patterns through a severe luminal obstruction.
The pathobiologic significance and role of abrupt changes in local fluid hemodynamics (i.e., ESS gradient) remains unclear. Thondapu et al. [32], analyzed the OCT images in 37 patients with an ACS caused by either plaque rupture (19 patients) or plaque erosion (18 patients), and performed arterial 3-D reconstruction and CFD calculations. They observed that plaque erosion was independently associated with high ESSG, high ESS, and high OSI while plaque rupture was independently associated with high ESSG. Their results suggested that ESSG was higher at rupture sites than erosion sites and that OSI was higher at erosion sites, while ESS was similar in plaque rupture and erosion. These studies did not, however, include a comparator of a separate control group of stable plaques with similar MLA, RLA, and coronary blood flow, as we included, to confirm the association of the abnormal ESS metrics on the pathobiology of erosion/thrombus formation. Our study is unique in that we utilized “control” stable plaques with the same MLA, RLA, and coronary blood flow as the erosion plaques and confirmed that the abnormal ESS patterns associated with plaque erosion are related to the adverse ESS patterns related to the nuanced features of the plaque morphology, as we are able to demonstrate, and not simply related to a severe obstruction.
While ESS represents the biomechanical force on endothelial cells that regulates their shape and function and varies enormously along the longitudinal course and around the circumference of a coronary artery, the ESS gradient represents differences in the magnitude of ESS in immediately adjacent endothelial cells. Areas of sharp acceleration or deceleration manifest a high ESS gradient regardless of the absolute value of ESS. Thus, areas of low ESS, such as distal to an obstruction, or areas of high ESS, such as at an MLA, or even areas of circumferential or lateral ESS heterogeneity, may still manifest a high ESSG despite the differences in absolute ESS magnitude and direction [33]. In the present study, we uniquely confirm, by including control plaques similar to the erosion plaques, that the ESSG is an essential and independent, mechanistic factor associated with the presence of plaque erosion.
High ESSG promotes biomechanical and pathobiological consequences that adversely affect coronary endothelium and uniquely predispose plaques to develop erosion and subsequent thrombus formation. In-vitro and in-vivo studies indicate that ESSG can modulate endothelial cells via several mechanisms independent from the absolute value of the ESS that involve activation of endothelial Toll-like receptor 2, neutrophil accumulation, macrophage recruitment with the subsequent elaboration of cytotoxic substances, apoptosis, and endothelial denudation, the critical step in thrombus formation [34,35]. Alternatively, high ESSG may be associated with other mechanisms that affect cellular junction proteins such as connexin43, cell adhesion molecules (PECAM)-1, inflammatory cells migration, activation of metalloproteinases with increased extracellular matrix degradation, increased cell turnover, and loss of cellular architecture and continuity [36–43]. Furthermore, in areas of high ESSG, abrupt changes in the magnitude and direction of shear stress might lead to compression or expansion of the endothelial surface, which may affect the integrity and function of the endothelial cells [37,39,44]. These ESSG-induced mechanisms of plaque erosion may work in concert with, or independently from, other mechanisms that involve ESS-induced destabilization pathways [39,40,43,44].
There are important and broad implications from our observation that the significantly increased magnitude of ESSG is different in all directions in the erosion plaques versus the control plaques. These multiple directions of increased ESS gradients do not necessarily reflect a severe obstruction, but rather a sudden change in the shear force on the endothelial surface that may contribute to plaque destabilization even in the absence of severe luminal obstruction. These observations may mechanistically explain why some MACE outcomes, including plaque erosion and thrombus formation, may occur in the setting of nonobstructive CAD (MINOCA) [45].
4.2. The role of plaque topography and slope in plaque erosion
Our results support and extend previous data correlating the biomechanical slope of plaque topography as a pathobiologic mechanism contributing to plaque destabilization. In the present study, we found that plaque erosion proximal to the MLA site is associated with steeper plaque upslope versus downslope, while erosion located distal to the MLA site is associated with steeper plaque downslope versus upslope. The dominant ACS clinical presentation for both plaque upslope and plaque downslope erosion were STEMI. An early CCTA study explored the biomechanical relationship between longitudinal lesion geometry, i. e., plaque slope upstream versus downstream, and the resultant external biomechanical force (axial plaque stress, APS) exerted on that plaque in 114 lesions from 81 patients with plaque rupture [46]. They observed that lesion topography with the steepest slope located upstream manifested significantly higher APS in that upstream portion of the plaque, while a lesion with the steepest slope located downstream manifested significantly higher APS in that downstream portion of the plaque. These elevations of external biomechanical APS operated independently of stenosis severity, drop in perfusion pressure, or local ESS.
A subsequent IVUS study investigated the clinical impact of longitudinal lesion geometry (APS) on ACS manifestations in 125 patients with plaque rupture based on the location of the steepest slope of plaque contour and found that 84% of cases of plaque destabilization occurred up- or downstream from the MLA and, furthermore, observed that plaques with the steepest slope proximal to the MLA predominantly were associated with acute MI while plaques with the steepest slope distal to the MLA were associated with non-MI ACS [47]. In addition, plaques with the steepest slope proximal to the MLA more frequently developed a STEMI while plaques with the steepest slope distal to the MLA primarily manifested a NSTEMI. This observation shed the light on the plaque slope as a possible mechanistic trigger in the development of plaque erosion. More experience will be necessary to assess the role of APS/plaque steepness on the specific pathobiology responsible for plaque erosion.
4.3. Clinical implications of the pathobiological and biomechanical foundations of plaque erosion and thrombus formation
The growing concordance of information from basic science investigations of the vascular biology of coronary atherosclerosis, as well as intravascular and non-invasive imaging, point to a broader and more nuanced understanding of the pathobiologic and biomechanical factors that are mechanistically responsible for plaque erosion/thrombus formation and MACE outcomes. Areas of low or high ESS are associated with plaque development, progression, and destabilization, but our understanding of plaque behavior is expanded by the growing appreciation that the external surface of plaque topography and slope (ESSG and APS) provide another important adverse biomechanical influence. This enhanced multifactorial understanding may enable us to identify high-risk plaques with characteristics that indicate particular risk for future erosion/thrombus and may ultimately provide justification for preemptive focal therapeutic intervention to the culprit portion of that plaque to avert the adverse natural history and future MACE.
4.4. Study limitations
The present study has several limitations. The sample size was relatively small. The diagnosis and segmentation of OCT-defined plaque erosion were challenging, especially in the cases defined as probable erosion where attenuation can obscure the plaque characteristics and lumen borders. Per the TOTAL trial’s protocol, thrombus aspiration using a thrombectomy catheter was performed prior to OCT imaging which could have altered the vascular wall. We excluded all the cases in which the vascular wall could have been injured during thrombus aspiration prior to OCT imaging. Bifurcation sites, where both ESS and ESSG may fluctuate substantially, are a frequent site for atherosclerosis development, but for technical reasons, we did not calculate local ESS in those areas or in the side branches, similar to most other CFD studies.
4.5. Conclusions
Compared to stable plaques with similarly severe obstruction, plaque erosion is associated with higher Max ESS and Max ESSG. Proximal erosion is associated with a steeper plaque upslope while distal erosion is associated with a steeper plaque downslope. Maximum ESS and Max ESS gradients are independent factors associated with plaque erosion development. These data support the key role that ESS plays in the development of plaque erosion and may help prognostically to determine whether an individual plaque is at risk for future erosion.
Supplementary Material
Acknowledgments
We acknowledge the team of the Vascular Profiling Laboratory at Brigham and Women’s Hospital and the co-investigators of both the TOTAL and the COMPLETE trials for their invaluable contribution to this research.
Financial support
This work was funded in part by National Institute of Health (US) grants R01 HL146144-01A1; RO1 HL140498. We gratefully acknowledge the generous support of the Schaubert Family.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Diaa Hakim: Formal analysis, OCT and Coronary angiography Image analysis, writing the manuscript. Natalia Pinilla-Echeverri: Revision of the manuscript. Ahmet U. Coskun: CFD calculation. Zhongyue Pu: Formal analysis, OCT Image analysis. Olli A. Kajander: Revision of the manuscript. Deborah Rupert: Formal analysis, OCT Image analysis. Charles Maynard: Formal analysis, Statistical analysis. Nicholas Cefalo: CFD calculation. Gerasimos Siasos: Revision of the manuscript. Michail I. Papafaklis: Revision of the manuscript. Stefanu Kostas: Revision of the manuscript. Lampros K. Michalis: Revision of the manuscript. Sanjit Jolly: Revision of the manuscript. Shamir R. Mehta: Revision of the manuscript. Tej Sheth: Revision of the manuscript. Kevin Croce: Revision of the manuscript. Peter H. Stone: Supervision, Research idea, research supervision, Formal analysis, data analysis, mentoring and manuscript revision.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.atherosclerosis.2023.05.013.
References
- [1].Haider A, Bengs S, Luu J, Osto E, Siller-Matula JM, Muka T, et al. , Sex and gender in cardiovascular medicine: presentation and outcomes of acute coronary syndrome, Eur. Heart J. 41 (13) (2020. Apr 1) 1328–1336. [DOI] [PubMed] [Google Scholar]
- [2].Falk E, Nakano M, Bentzon JF, Finn AV, Virmani R, Update on acute coronary syndromes: the pathologists’ view, Eur. Heart J. 34 (10) (2013. Mar) 719–728. [DOI] [PubMed] [Google Scholar]
- [3].Farb A, Burke AP, Tang AL, Liang TY, Mannan P, Smialek J, et al. , Coronary plaque erosion without rupture into a lipid core. A frequent cause of coronary thrombosis in sudden coronary death, Circulation 93 (7) (1996. Apr 1) 1354–1363. [DOI] [PubMed] [Google Scholar]
- [4].Durand E, Scoazec A, Lafont A, Boddaert J, Al Hajzen A, Addad F, et al. , In vivo induction of endothelial apoptosis leads to vessel thrombosis and endothelial denudation: a clue to the understanding of the mechanisms of thrombotic plaque erosion, Circulation 109 (21) (2004. Jun 1) 2503–2506. [DOI] [PubMed] [Google Scholar]
- [5].Jia H, Abtahian F, Aguirre AD, Lee S, Chia S, Lowe H, et al. , In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography, J. Am. Coll. Cardiol. 62 (19) (2013. Nov 5) 1748–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Higuma T, Soeda T, Abe N, Yamada M, Yokoyama H, Shibutani S, et al. , A combined optical coherence tomography and intravascular ultrasound study on plaque rupture, plaque erosion, and calcified nodule in patients with ST-segment elevation myocardial infarction: incidence, morphologic characteristics, and outcomes after percutaneous coronary intervention, JACC Cardiovasc. Interv. 8 (9) (2015. Aug 17) 1166–1176. [DOI] [PubMed] [Google Scholar]
- [7].Niccoli G, Montone RA, Di Vito L, Gramegna M, Refaat H, Scalone G, et al. , Plaque rupture and intact fibrous cap assessed by optical coherence tomography portend different outcomes in patients with acute coronary syndrome, Eur. Heart J. 36 (22) (2015. Jun 7) 1377–1384. [DOI] [PubMed] [Google Scholar]
- [8].Saia F, Komukai K, Capodanno D, Sirbu V, Musumeci G, Boccuzzi G, et al. , Eroded versus ruptured plaques at the culprit site of STEMI: in vivo pathophysiological features and response to primary PCI, JACC Cardiovasc Imaging 8 (5) (2015. May) 566–575. [DOI] [PubMed] [Google Scholar]
- [9].Wang L, Parodi G, Maehara A, Valenti R, Migliorini A, Vergara R, et al. , Variable underlying morphology of culprit plaques associated with ST-elevation myocardial infarction: an optical coherence tomography analysis from the SMART trial, Eur Heart J Cardiovasc Imaging 16 (12) (2015. Dec) 1381–1389. [DOI] [PubMed] [Google Scholar]
- [10].Wentzel JJ, Chatzizisis YS, Gijsen FJH, Giannoglou GD, Feldman CL, Stone PH, Endothelial shear stress in the evolution of coronary atherosclerotic plaque and vascular remodelling: current understanding and remaining questions, Cardiovasc. Res. 96 (2) (2012. Nov 1) 234–243. [DOI] [PubMed] [Google Scholar]
- [11].Stone PH, Saito S, Takahashi S, Makita Y, Nakamura S, Kawasaki T, et al. , Prediction of progression of coronary artery disease and clinical outcomes using vascular profiling of endothelial shear stress and arterial plaque characteristics: the PREDICTION Study, Circulation 126 (2) (2012. Jul 10) 172–181. [DOI] [PubMed] [Google Scholar]
- [12].Dolan JM, Meng H, Singh S, Paluch R, Kolega J, High fluid shear stress and spatial shear stress gradients affect endothelial proliferation, survival, and alignment, Ann. Biomed. Eng. 39 (6) (2011. Jun) 1620–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Giannopoulos AA, Antoniadis AP, Croce K, Chatzizisis YS, Erosion of thin-cap fibroatheroma in an area of low endothelial shear stress: anatomy and local hemodynamic environment dictate outcomes, JACC Cardiovasc. Interv. 9 (8) (2016. Apr 25) e77–e78. [DOI] [PubMed] [Google Scholar]
- [14].Jolly SS, Cairns J, Yusuf S, Meeks B, Shestakovska O, Thabane L, et al. , Design and rationale of the TOTAL trial: a randomized trial of routine aspiration ThrOmbecTomy with percutaneous coronary intervention (PCI) versus PCI ALone in patients with ST-elevation myocardial infarction undergoing primary PCI, Am. Heart J. 167 (3) (2014. Mar) 315–321, e1. [DOI] [PubMed] [Google Scholar]
- [15].Mehta SR, Wood DA, Storey RF, Mehran R, Bainey KR, Nguyen H, et al. , Complete revascularization with multivessel PCI for myocardial infarction, N. Engl. J. Med. 381 (15) (2019. Oct 10) 1411–1421. [DOI] [PubMed] [Google Scholar]
- [16].Prati F, Regar E, Mintz GS, Arbustini E, Di Mario C, Jang IK, et al. , Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis, Eur. Heart J. 31 (4) (2010. Feb) 401–415. [DOI] [PubMed] [Google Scholar]
- [17].Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, et al. , Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation, J. Am. Coll. Cardiol. 59 (12) (2012. Mar 20) 1058–1072. [DOI] [PubMed] [Google Scholar]
- [18].Jia H, Abtahian F, Aguirre AD, Lee S, Chia S, Lowe H, et al. , In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography, J. Am. Coll. Cardiol. 62 (19) (2013. Nov 5) 1748–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gijsen F, Katagiri Y, Barlis P, Bourantas C, Collet C, Coskun U, et al. , Expert recommendations on the assessment of wall shear stress in human coronary arteries: existing methodologies, technical considerations, and clinical applications, Eur. Heart J. 40 (41) (2019. Nov 1) 3421–3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Vergallo R, Papafaklis MI, Yonetsu T, Bourantas CV, Andreou I, Wang Z, et al. , Endothelial shear stress and coronary plaque characteristics in humans: combined frequency-domain optical coherence tomography and computational fluid dynamics study, Circ Cardiovasc Imaging 7 (6) (2014. Nov) 905–911. [DOI] [PubMed] [Google Scholar]
- [21].Vergallo R, Papafaklis MI, D’Amario D, Michalis LK, Crea F, Porto I, Coronary plaque erosion developing in an area of high endothelial shear stress: insights from serial optical coherence tomography imaging, Coron. Artery Dis. 30 (1) (2019. Jan) 74–75. [DOI] [PubMed] [Google Scholar]
- [22].Mullick AE, Tobias PS, Curtiss LK, Modulation of atherosclerosis in mice by Toll-like receptor 2, J. Clin. Invest. 115 (11) (2005. Nov) 3149–3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mullick AE, Soldau K, Kiosses WB, Bell TA, Tobias PS, Curtiss LK, Increased endothelial expression of Toll-like receptor 2 at sites of disturbed blood flow exacerbates early atherogenic events, J. Exp. Med. 205 (2) (2008. Feb 18) 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH, Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior, J. Am. Coll. Cardiol. 49 (25) (2007. Jun 26) 2379–2393. [DOI] [PubMed] [Google Scholar]
- [25].Gijsen FJH, Mastik F, Schaar JA, Schuurbiers JCH, van der Giessen WJ, de Feyter PJ, et al. , High shear stress induces a strain increase in human coronary plaques over a 6-month period, EuroIntervention J Eur Collab Work Group Interv Cardiol Eur Soc Cardiol 7 (1) (2011. May) 121–127. [DOI] [PubMed] [Google Scholar]
- [26].Samady H, Eshtehardi P, McDaniel MC, Suo J, Dhawan SS, Maynard C, et al. , Coronary artery wall shear stress is associated with progression and transformation of atherosclerotic plaque and arterial remodeling in patients with coronary artery disease, Circulation 124 (7) (2011. Aug 16) 779–788. [DOI] [PubMed] [Google Scholar]
- [27].White SJ, Hayes EM, Lehoux S, Jeremy JY, Horrevoets AJG, Newby AC, Characterization of the differential response of endothelial cells exposed to normal and elevated laminar shear stress, J. Cell. Physiol. 226 (11) (2011. Nov) 2841–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bourantas CV, Zanchin T, Torii R, Serruys PW, Karagiannis A, Ramasamy A, et al. , Shear stress estimated by quantitative coronary angiography predicts plaques prone to progress and cause events, JACC Cardiovasc Imaging 13 (10) (2020. Oct) 2206–2219. [DOI] [PubMed] [Google Scholar]
- [29].Stone PH, Maehara A, Coskun AU, Maynard CC, Zaromytidou M, Siasos G, et al. , Role of low endothelial shear stress and plaque characteristics in the prediction of nonculprit major adverse cardiac events: the PROSPECT study, JACC Cardiovasc Imaging 11 (3) (2018. Mar) 462–471. [DOI] [PubMed] [Google Scholar]
- [30].Kumar A, Thompson EW, Lefieux A, Molony DS, Davis EL, Chand N, et al. , High coronary shear stress in patients with coronary artery disease predicts myocardial infarction, J. Am. Coll. Cardiol. 72 (16) (2018. Oct 16) 1926–1935. [DOI] [PubMed] [Google Scholar]
- [31].Yamamoto E, Thondapu V, Poon E, Sugiyama T, Fracassi F, Dijkstra J, et al. , Endothelial shear stress and plaque erosion: a computational fluid dynamics and optical coherence tomography study, JACC Cardiovasc Imaging 12 (2) (2019. Feb) 374–375. [DOI] [PubMed] [Google Scholar]
- [32].Thondapu V, Mamon C, Poon EKW, Kurihara O, Kim HO, Russo M, et al. , High spatial endothelial shear stress gradient independently predicts site of acute coronary plaque rupture and erosion, Cardiovasc. Res. 117 (8) (2021. Jul 7) 1974–1985. [DOI] [PubMed] [Google Scholar]
- [33].Schirmer CM, Malek AM, Wall shear stress gradient analysis within an idealized stenosis using non-Newtonian flow, Neurosurgery 61 (4) (2007. Oct) 853–863, discussion 863–864. [DOI] [PubMed] [Google Scholar]
- [34].Franck G, Mawson T, Sausen G, Salinas M, Masson GS, Cole A, et al. , Flow perturbation mediates neutrophil recruitment and potentiates endothelial injury via TLR2 in mice: implications for superficial erosion, Circ. Res. 121 (1) (2017. Jun 23) 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Quillard T, Araújo HA, Franck G, Shvartz E, Sukhova G, Libby P, TLR2 and neutrophils potentiate endothelial stress, apoptosis and detachment: implications for superficial erosion, Eur. Heart J. 36 (22) (2015. Jun 7) 1394–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Davies PF, Remuzzi A, Gordon EJ, Dewey CF, Gimbrone MA, Turbulent fluid shear stress induces vascular endothelial cell turnover in vitro, Proc. Natl. Acad. Sci. U. S. A. 83 (7) (1986. Apr) 2114–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].LaMack JA, Friedman MH, Individual and combined effects of shear stress magnitude and spatial gradient on endothelial cell gene expression, Am. J. Physiol. Heart Circ. Physiol. 293 (5) (2007. Nov) H2853–H2859. [DOI] [PubMed] [Google Scholar]
- [38].DePaola N, Davies PF, Pritchard WF, Florez L, Harbeck N, Polacek DC, Spatial and temporal regulation of gap junction connexin43 in vascular endothelial cells exposed to controlled disturbed flows in vitro, Proc. Natl. Acad. Sci. U. S. A. 96 (6) (1999. Mar 16) 3154–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Dolan JM, Meng H, Sim FJ, Kolega J, Differential gene expression by endothelial cells under positive and negative streamwise gradients of high wall shear stress, Am. J. Physiol. Cell Physiol. 305 (8) (2013. Oct 15) C854–C866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Nagel T, Resnick N, Dewey CF, Gimbrone MA, Vascular endothelial cells respond to spatial gradients in fluid shear stress by enhanced activation of transcription factors, Arterioscler. Thromb. Vasc. Biol. 19 (8) (1999. Aug) 1825–1834. [DOI] [PubMed] [Google Scholar]
- [41].Sakamoto N, Saito N, Han X, Ohashi T, Sato M, Effect of spatial gradient in fluid shear stress on morphological changes in endothelial cells in response to flow, Biochem. Biophys. Res. Commun. 395 (2) (2010. Apr 30) 264–269. [DOI] [PubMed] [Google Scholar]
- [42].Tardy Y, Resnick N, Nagel T, Gimbrone MA, Dewey CF, Shear stress gradients remodel endothelial monolayers in vitro via a cell proliferation-migration-loss cycle, Arterioscler. Thromb. Vasc. Biol. 17 (11) (1997. Nov) 3102–3106. [DOI] [PubMed] [Google Scholar]
- [43].Phelps JE, DePaola N, Spatial variations in endothelial barrier function in disturbed flows in vitro, Am. J. Physiol. Heart Circ. Physiol. 278 (2) (2000. Feb) H469–H476. [DOI] [PubMed] [Google Scholar]
- [44].DePaola N, Gimbrone MA, Davies PF, Dewey CF, Vascular endothelium responds to fluid shear stress gradients, Arterioscler Thromb J Vasc Biol 12 (11) (1992. Nov) 1254–1257. [DOI] [PubMed] [Google Scholar]
- [45].Reynolds HR, Maehara A, Kwong RY, Sedlak T, Saw J, Smilowitz NR, et al. , Coronary optical coherence tomography and cardiac magnetic resonance imaging to determine underlying causes of myocardial infarction with nonobstructive coronary arteries in women, Circulation 143 (7) (2021. Feb 16) 624–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Choi G, Lee JM, Kim HJ, Park JB, Sankaran S, Otake H, et al. , Coronary artery axial plaque stress and its relationship with lesion geometry: application of computational fluid dynamics to coronary CT angiography, JACC Cardiovasc Imaging 8 (10) (2015. Oct) 1156–1166. [DOI] [PubMed] [Google Scholar]
- [47].Lee JM, Choi G, Hwang D, Park J, Kim HJ, Doh JH, et al. , Impact of longitudinal lesion geometry on location of plaque rupture and clinical presentations, JACC Cardiovasc Imaging 10 (6) (2017. Jun) 677–688. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


